Abstract
Background
Since its first description, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), formerly known as 2019-nCoV, has attracted tremendous attention in a short period of time as the death toll and number of confirmed cases grows unceasingly.
Methods
To provide a better understanding of the importance of abnormal laboratory findings in COVID-19 diagnosis and prognosis, we searched the Scopus, PubMed, and Web of Science medical databases and selected 19 articles (totaling 2988 patients, 484 of whom [16.1%] had severe disease) that reported panels of laboratory examinations in patients with COVID-19.
Results
Although in vitro diagnostics, primarily using PCR- and ELISA-based methods, efficiently contribute to the etiological identification of SARS-CoV-2 infection, we suggest that laboratory medicine may also be of significant assistance when differentiating between severe and non-severe COVID-19.
Conclusion
When we wrote this article, our ability to provide a definitive conclusion may have been adversely affected by some limitations, such as the low sample size, differently applied methods, dissimilar reference ranges, non-synchronized representations of results, and variety of the patients’ panels. Despite the limitations, the analysis of the current scientific literature demonstrates the value of laboratory parameters as simple, rapid, and cost-effective biomarkers in COVID-19 patients.
Keywords: SARS-CoV-2, Coronavirus, COVID-19, Laboratory findings, Prognosis, Diagnosis
1. Introduction
Over the past two decades, the SARS (severe acute respiratory syndrome) and MERS (Middle East respiratory syndrome) outbreaks have garnered public health emergencies against coronaviruses (CoV) in 2002 and 2012, respectively. At the end of 2019, an outbreak of pneumonia with unknown etiology was reported in Wuhan, the most populous city in central China with more than 11 million residents [1]. SARS-CoV-2, formerly known as 2019-nCoV, is a newly emerging virus belonging to the Coronaviridae family, presumably derived from a bat SARS-like coronavirus and transmitted to humans after the emergence of mutations in the spike glycoprotein (protein S) and nucleocapsid N protein [2]. This zoonotic pathogen, called coronavirus disease 2019 (COVID-19) by the World Health Organization (WHO), is assumed to be the latest global biological human hazard. Although its mortality rate is lower than SARS and MERS, the long incubation period (up to 2 weeks) together with the relatively low pathogenicity increases the risk of SARS-CoV-2 contagion and facilitates its spread [3]. According to the WHO’s data, COVID-19 has already spread worldwide, with over 8,900,000 diagnosed cases in more than 210 different countries, causing more than 465,000 related deaths as of June 20, 2020 (https://www.who.int/). COVID-19 is a mysterious respiratory and systemic syndrome primarily presenting clinical symptoms of dry cough, dyspnea, and fever, which in some cases (8–15% depending on the geographical setting and individual characteristics) lead to a critical condition necessitating specialized management at intensive care units (ICU) [4].
This is not the first nor will it be the last time that a viral pneumonia pandemic has been described as a global health emergency by the WHO. Timely identification of virus carriers is vital not only to prevent their spread but also to more efficiently control disease progression. To the best of our knowledge, while most published articles have discussed the clinical features and imaging findings of COVID-19, few studies have addressed the diagnostic and prognostic value of abnormal laboratory findings [5]. Irrespective of its inherent definition [6], the contributory role of laboratory medicine is far beyond etiological detection and it is now almost undeniable that this branch of medical science is effectively involved in epidemiologic surveillance, determination of prognosis, patient follow-up, and, last but not least, therapeutic monitoring of the wide range of human diseases, including COVID-19 [7]. To better represent how abnormal laboratory findings are important in COVID-19 diagnosis and prognosis, we searched the Scopus, PubMed, and Web of Science medical databases using the keywords “COVID-19,” “2019-nCoV,” or “coronavirus 2019.” We ultimately selected 19 articles (totaling 2988 patients, 484 of whom (16.1%) were severely affected) that provided a panel of laboratory examinations in COVID-19 patients. Although when we wrote this article, some limitations such as low sample size, different applied methods, dissimilar reference ranges, non-synchronized methods of representing the results, and variety in the panels conducted may have adversely affected the ability to draw a clear conclusion, analyzing the current scientific literature will definitively shed light on the value of these patients’ laboratory parameters.
2. Diagnostic value of laboratory findings
The main routine tests requested for COVID-19 patients include complete blood count (CBC), assays investigating coagulation and fibrinolysis cascades (PT, aPTT, and D-dimers), and inflammation-related parameters (ESR, CRP, ferritin, and procalcitonin). Due to the potential ability of the virus to severely impair several vital organs such as the heart, liver, and kidneys [8], analyzing the biochemical factors is an appropriate way for clinicians to evaluate the functional activities of these organs.
2.1. Diagnostic value of CBC, coagulation tests, and inflammation-related parameters
Data from 15 published articles reflecting the value of blood cell count and differential percentages of lymphocytes and neutrophils from patients with severe/non-severe COVID-19 are summarized in Table 1 . As presented, while lymphopenia is a prominent finding in most patients, some studies have reported an increased number of neutrophils. Notably, the total leukocyte count varies among patients, which may reflect the dominance of either lymphopenia or neutrophilia. Taken together, decreased lymphocytes accompanied by mild thrombocytopenia are among the most common abnormal findings attracting attention in CBC of COVID-19 patients. It has also been reported that some COVID-19 patients have increased prothrombin time (PT) together with prolonged activated partial thromboplastin time (aPTT). Adding to these abnormalities, elevated D-dimers further support the occurrence of coagulopathy and as we will discuss later, it is an important indicator of disease progression. It was previously established that inflammation-related parameters are highly elevated in acute phases. COVID-19 makes no exception to this rule, whereby the erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and procalcitonin are increased in the sera of these patients, albeit with different values. It is worth mentioning that while the diagnostic value of CRP is superior to procalcitonin (Table 1), the latter may potentially be of greater value than the former for predicting disease progression. Li et al. detected ferritin levels above the upper limit of the reference range in 49 out of 54 (90.7%) COVID-19 patients and showed that the ferritin levels decreased as hs-CRP decreased, but they were significantly higher than the upper reference range for at least 5 days after hs-CRP returned to normal [9]. Previous studies have shown that soluble CD-163 (sCD-163), which represents the activation of macrophages, increases parallel to ferritin during the acute inflammation stage [10], suggesting that ferritin measurement may provide diagnostic value and can be used for diagnostic purposes in COVID-19 [11]. In this vein, the results of a study screening ferritin in hemodialysis patients revealed that COVID-19 patients displayed a mean increase of 275% after viral infection. They showed that ferritin levels remained stable or slowly decreased during the period of sickness in most patients [12].
Table 1.
Main features and laboratory findings of the selected reports.
| Chen et al.[61] | Yang et al.[19] | Liu et al.[15] | Cao et al.[41] | Wu et al.[62] | Zhou et al.[16] | Chen et al.[63] | Guan et al.[17] | Wan et al.[43] | Huang et al.[14] | Xu et al.[64] | Zhang et al.[42] | Shi et al.[65] | Zhang et al.[49] | Chen et al.[66] | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Features | ||||||||||||||||
|
No. cases (Severe) |
99 (17) |
149 (0) |
12 (6) |
198 (19) |
80 (N/R) |
191 (54) |
29 (14) |
1099 (173) |
135 (40) |
41 (13) |
62 (1) |
221 (55) |
81 (N/R) |
140 (58) |
9 pregnant | |
| Age (year) | 56 | 45 | 54 | 50 | 44 | 56 | 56 | 47 | 40 | 49 | 41 | 55 | 49.5 | 57 | 30 | |
| Female (%) | 32% | 45.6% | 33% | 49% | 48% | 38% | 28% | 41.9% | 46.7% | 27% | 44% | 51% | 48% | 49% | 100% | |
| CBC | ||||||||||||||||
| Leukocytes | ↑24%; ↓9% | ↑1.34% ↓24.1% | ↑8% | ↑10.4% ↓15.5% | ↑10%; ↓9% | ↑21%; ↓17% | ↑21%; ↓21% | ↑5.9%; ↓33.7% | ↑6.6%; ↓20.7% | ↑30%; ↓25% | ↑2%; ↓31% | ↑10.4%; ↓33% |
↑32%; | ↑12%; ↓20% | ↑22% | |
| Lymphocytes | ↓35% | ↓35.5% | ↓55% | ↓8.9% ↑37.5% |
↓43% | ↓40% | ↓69% | ↓83.2% | ↓50% | ↓63% | ↓42% ↑58% |
↓73.8% ↑26.2% |
↓33% | ↓75% | ↓56% | |
| Neutrophils | ↑38% | ↑4% | ↑17% | ↑6.2% | ↑20%; | |||||||||||
| Platelets | ↓12% | ↓13.4% | ↓8% | ↓17.6% | ↓7% | ↓17% | ↓36.2% | ↓17% | ↓5% | ↓5% | ||||||
| Hemoglobin | ↓51% | ↓15% | ↓41% | |||||||||||||
| Coagulation | ||||||||||||||||
| D-dimer | ↑36% | ↑14% | ↑32.8% | ↑42% | ↑46.4% | |||||||||||
| PT | ↑5% | ↑11.4% | ↑9.2% | ↑6% | ||||||||||||
| aPTT | ↑6% | ↑26.8% | ↑17.9% | |||||||||||||
| Inflammation | ||||||||||||||||
| CRP | ↑86% | ↑55% | ↑83% | ↑78.4% | ↑46% | ↑93% | ↑60.7% | ↑91% | ↑75% | |||||||
| Procalcitonin | ↑6% | ↑8% | ↑28.4% | ↑40% | ↑9% | ↑0% | ↑5.5% | ↑3% | ↑8% | ↑11% | ↑5.9% | ↑35% | ||||
| ESR | ↑85% | ↑86.8% | ||||||||||||||
2.2. Diagnostic value of biochemical parameters
Table 2 summarizes the changes in biochemical parameters reported by 12 articles. As presented, increased levels of lactate dehydrogenase (LDH), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total bilirubin (Bili) and decreased levels of albumin are among the most common abnormal laboratory findings in COVID-19 patients. Changes were not limited to the indicated parameters since elevated creatine kinase (CK) and increased creatinine (Cr) were also demonstrated in earlier studies. Knowing that the primary site of the SARS-CoV-2 attack is the lower respiratory tract together with the fact that LDH is an important marker of lung damage [13] may explain, at least partly, why this enzyme’s level is elevated in most COVID-19 patients [14], [15], [16]. The emergence of severe disease due to the injury of non-pulmonary organs may also instigate abnormal values of kidney- and liver-related biochemical parameters. Guan et. al. reported that ALT and AST levels in COVID-19 patients were elevated in 21.3% and 22.2% of cases, respectively [17], which may mirror virus-mediated liver impairment. The results of a recent study also revealed that 2–11% of COVID-19 patients had liver comorbidities and 14–53% had abnormal levels of ALT and AST [18]. Analysis of creatinine in 149 cases demonstrated that 28.8% of COVID-19 patients had an increased levels, representing SARS-CoV-2’s ability to induce kidney injury [19]. The results of multiple lines of evidence have indicated that measuring the biochemical parameters not only retains a specific diagnostic significance in this infection but their abnormalities may also correlate with unfavorable outcomes, as we will discuss later.
Table 2.
Main features and values of the biochemical parameters in the selected reports.
| Yang et al.[19] | Chen et al.[61] | Liu et al.[15] | Chen et al.[63] | Guan et al.[17] | Cao et al.[41] | Zhou et al.[16] | Wan et al.[43] | Chen et al.[67] | Huang et al.[14] | Xu et al.[64] | Chen et al.[66] | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Features | |||||||||||||
|
No. cases (Severe) |
149 (0) |
99 (17) |
12 (6) |
29 (14) |
1099 (173) |
198 (19) |
191 (54) |
135 (40) |
175 (N/R) |
41 (13) |
62 (1) |
9 pregnant |
|
| Age (year) | 45 | 56 | 54 | 56 | 47 | 50 | 56 | 40 | 46 | 49 | 41 | 30 | |
| Female (%) | 45.6% | 32% | 33% | 28% | 41.9% | 49% | 38% | 46.7% | 53% | 27% | 44% | 100% | |
| Biochemical | |||||||||||||
| LDH | ↑30% | ↑76% | ↑92% | ↑69% | ↑41% | ↑67% | ↑43% | ↑33% | ↑73% | ↑27% | |||
| ALT | ↑12% | ↑28% | ↑17% | ↑17% | ↑21.3% | ↑10.8% | ↑31% | ↑19% | ↑33% | ||||
| AST | ↑18% | ↑35% | ↑8% | ↑24% | ↑22.2% | ↑17.4% | ↑22% | ↑16% | ↑37% | ↑16% | ↑33% | ||
| Bilirubin | ↑2.7% | ↑18% | ↑0% | ↑3% | ↑10.5% | ↑2.6% | |||||||
| Albumin | ↓6% | ↓98% | ↓50% | ↓52% | ↓40% | ||||||||
| CK | ↑8% | ↑13% | ↑17% | ↑13.7% | ↑13% | ↑7.4% | ↑14% | ↑33% | |||||
| Creatinine | ↑28.8% | ↑3% | ↑17% | ↑7% | ↑5.3% | ↑4% | ↑4.4% | ↑10% | ↑5% | ||||
2.3. Diagnostic value of laboratory parameters in cerebrospinal fluid (CSF)
Albeit lung tissue is the most common organ that is affected by SARS-CoV-2, the emergence of atypical clinical manifestations such as headache, nausea, vomiting, myalgia, dizziness, impaired consciousness, and the loss of the sense of smell and taste have demonstrated the neurotropism potential of this virus. This suggests that the virus may affect the nervous system of some COVID-19 patients, either through a direct or an indirect mechanism [20], [21], [22]. As the first evidence of SARS-CoV-2’s direct invasion of the nervous system, Zhou L. et al. detected SARS-CoV-2 in the CSF of a 56-year-old patient who was diagnosed with viral encephalitis [23]. Filatov A. et al. reported a 74-year-old patient who was positive for SARS-CoV-2 and presented with encephalopathy [24]. In a strange case of COVID-19 in a 24-year-old man, while the molecular identification of SARS-CoV-2 was negative in a nasopharynx sample, it was positive in the CSF [25]. In a report on two patients with COVID-19 and concurrent neurological symptoms, Al Saiegh et al. showed that the patients’ CSF may have been devoid of viral particles even when they tested positive for COVID-19 via nasal swab. They concluded that whether SARS- CoV-2 is present in the CSF may depend on the systemic disease severity and degree of the virus’ nervous tissue tropism [26].
CSF analyses of a 60-year-old SARS-CoV-2 patient who developed akinetic mutism due to encephalitis showed that he was positive for pleocytosis and hyperproteinorrachia and displayed increased concentrations of IL-8 and TNF-α. Notably, applying high-dose steroid treatment results in progressive clinical improvement along with a reduction in CSF parameters, thus supporting inflammatory-mediated involvement within the brain in COVID-19 [27]. Intriguingly, a CSF analysis of a 41-year-old obese female with a history of diabetes showed 65 red blood cells and 70 white blood cells, 100% of which were lymphocytes. CSF protein was 100 and glucose was 120 (with serum glucose of 200) [28]. Examination of CSF samples obtained from seven COVID-19 patients who underwent brain MRI and electroencephalography showed that oligoclonal bands were present in 2 patients, and the protein and IgG levels were elevated only in 1 patient. Notably, the RT-PCR results of the CSF samples were negative for SARS-CoV-2 in all 7 patients [29]. The CSF sample of a 30-year-old COVID-19 patient who was admitted to the neurology emergency room with generalized tonic-clonic seizure showed normal protein and glucose levels with five cell counts (all lymphocytes) [30].
3. Prognostic value of laboratory findings
Comparing the number of deaths to the total number of cases demonstrated that most COVID-19 patients recover; however, the increasing number of global fatalities is a reminder that SARS-CoV-2 continues to take its toll [31]. Notwithstanding atypical pneumonia being the primary symptom [32], the occurrence of severe disease, mainly resulting from immune-mediated hyper-inflammation, may lead to death in some cases. Introducing potent biomarkers to timely predict disease outcomes is an essential field of research in a wide range of diseases from simple infections to human malignancies. Since the first description of COVID-19, several articles introduced some laboratory findings as valuable prognostic factors, which we will discuss in the next sections.
3.1. Prognostic value of CBC, coagulation tests, and inflammation-related parameters
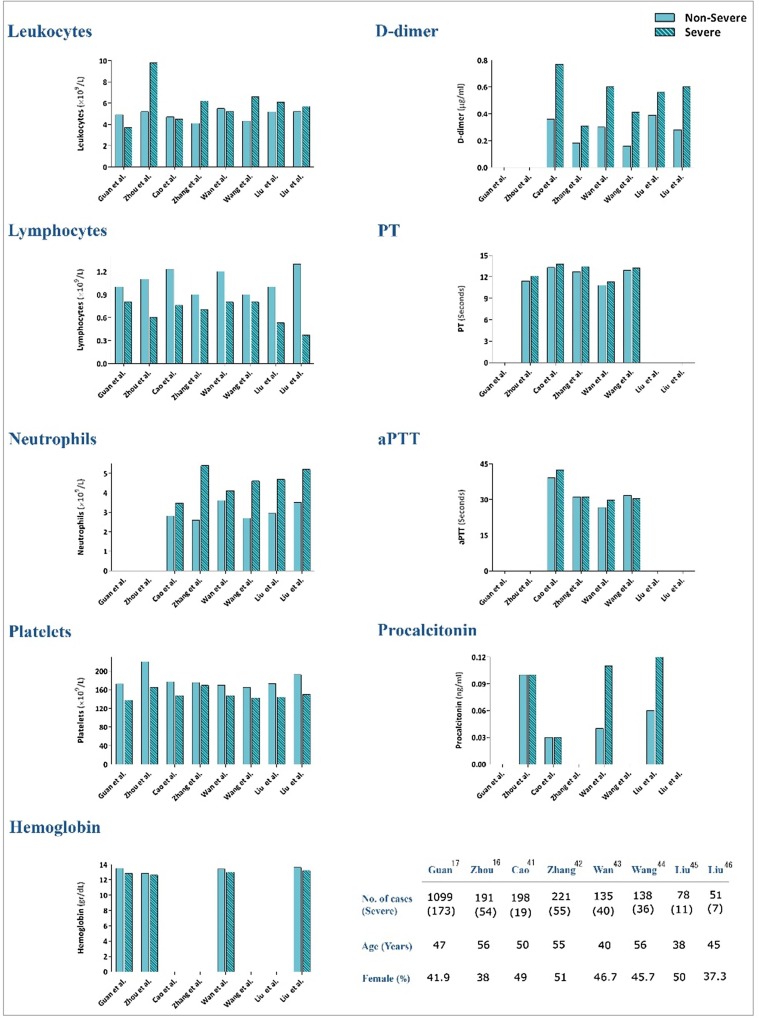
The lymphocyte count is an important parameter to directly discriminate between COVID-19 patients with and without severe disease [8], [33]. Given that most COVID19 fatalities experienced greater lymphopenia, it is reasonable to assume that the lymphocyte count is a rapid and commonly available laboratory parameter that can predict disease severity in COVID19 [33], [34]. Leukocytes and neutrophils were also significantly higher in a severe group in a study conducted on 94 patients at Shenzhen Third People’s Hospital [35]. In agreement with these studies, Wang et al. reported that ICU patients had fewer lymphocytes and more leukocytes and neutrophils than non-ICU patients [36]. Huang et al. reported similar findings, noting that ICU patients experienced a higher frequency of leukocytosis, neutrophilia, and lymphopenia than non-ICU patients [14]. As represented in Fig. 1 , while the lymphocyte counts lower than 0.8 × 109/L may be associated with COVID-19 severity, number of neutrophils higher than 3.5 × 109/L may reflect a poor clinical outcome. Yang et al. reported that the elevated neutrophil-to-lymphocyte ratio (NLR) may predict COVID-19 prognosis [37]. The results of a meta-analysis of six studies demonstrated that an increased NLR level may suggest a poor prognosis in patients with SARS-CoV-2 infection [38]. Also, the results of a recent study revealed that the incidence of critical illness in COVID-19 patients aged more than 50 was 9.1% (1/11) for patients having NLR < 3.13, while it was 50% (7/14) for those with NLR ≥ 3.13 [39]. A meta-analysis of 9 studies totaling 1779 COVID-19 patients with 399 (22.4%) severe cases reported that a low platelet count was associated with an increased risk of severe disease and mortality. The authors proposed that thrombocytopenic COVID-19 patients will experience disease with a higher risk of adverse outcomes during hospitalization [40]. Based on the results represented in Fig. 1, we suggest 150 × 109/L as a cut-off level for platelet count to predict poor prognosis. Fig. 1 demonstrates the results of 8 articles [16], [17], [41], [42], [43], [44], [45], [46] comparing CBC parameters between severe and non-severe groups.
Fig. 1.
Main features and laboratory findings in the selected reports between severe and non-severe groups.
The prognostic significance of laboratory tests is not limited to the valuable data represented by simple CBC examinations, as increased PT and D-dimer values may be indicative of a worse prognosis [14], [47]. Tang et al. reported a significant difference in the occurrence rate of coagulopathy in terms of disseminated intravascular coagulation (DIC) between COVID-19 patients who died of the disease compared to those who survived (71.4% vs 0.6%) [47]. Although the results of our recent meta-analysis revealed that the estimated pooled means of PT and aPTT were higher in severe cases, their mean values were not significantly higher as compared with non-severe patients. On the other hand, the mean value of D-dimers in severe patients was significantly higher than non-severe cases (P = 0.01), highlighting that elevation of D-dimers may effectively contribute to mirror the progression of disease toward an unfavorable clinical picture [48]. Based on the results represented in Fig. 1, we suggest 0.4 µg/mL as a cut-off level for D-dimers to predict poor prognosis. In a study reporting the results of 13 severe 140 COVID-19 patients, increased procalcitonin values were 25% vs 0% in ICU patients compared to non-ICU patients, respectively [14]. As CRP was significantly elevated in the progression group compared to the improvement/stabilization group (38.9 vs 10.6 mg/l, P = 0.024) [45], follow-up of these inflammation-related proteins seems to provide valuable data. A study by Zhang et al. on 140 COVID-19 patients, including 58 severe cases, further supported the previous data. They reported that increased levels of D-dimer along with elevated procalcitonin and CRP levels could help clinicians effectively discriminate between severe and non-severe COVID-19 cases [49]. Taken together, analyzing PT and D-dimer values coupled with follow-up of altered patterns of procalcitonin and CRP levels may provide a simple and rapid method of predicting disease prognosis (Fig. 1).
Secondary hemophagocytic lymphohistiocytosis (sHLH), an immune disorder that causes uncontrolled systemic inflammation, is most commonly elicited by viral infections [50] and is characterized by an overwhelming secretion of inflammatory cytokines leading to multi-organ failure [51]. Acute respiratory distress syndrome (ARDS), fewer blood cells, lower fibrinogen levels, fever, and increased levels of serum aspartate aminotransferase and ferritin are among the most common features of sHLH [52], similar to COVID-19, especially in symptomatic patients [53]. Dysregulation of the inflammatory cytokine expression profile is not limited to the novel coronavirus, since a significant correlation between cytokine storm and disease severity was reported in both SARS-CoV and MERS-CoV infections [54]. In a study investigating the contributory role of cytokines in COVID-19 severity, Yang et al. examined 48 cytokines in plasma samples from 53 COVID-19 patients (34 were severe cases) and reported that 14 cytokines were significantly elevated upon admission. Of these cytokines, IL-1ra, IP-10 (interferon-gamma induced protein 10), and MCP3 (monocyte chemotactic protein-3) were independent predictors of the progression of COVID-19, and the combination of these cytokines showed the highest area under the curve (AUC) of the receiver-operating characteristic (ROC) calculations [55]. In agreement, transcriptome sequencing of RNAs isolated from the bronchoalveolar lavage fluid (BALF) and peripheral blood mononuclear cell (PBMC) specimens of COVID-19 patients highlighted the association between COVID-19 pathogenesis and excessive cytokine release, such as CCL2/MCP-1, CXCL10/IP-10, CCL3/MIP-1A, and CCL4/MIP1B [56]. Examination of inflammatory cytokines (including IL-1, soluble IL-2 receptor [sIL-2R], IL-6, IL-8, IL-10, and tumor necrosis factor-α [TNF-α]) within 24 h of admission of 47 COVID-19 patients revealed that the concentrations of sIL-2R and IL-6 in critically ill patients were significantly higher than those with severe disease, while the IL-10 and TNF-α levels were not statistically different between the two groups [9].
In a recent study investigating the clinical predictors of COVID-19 mortality, Ruan et al. suggested that COVID-19 mortality might be due to virus-activated cytokine storm syndrome. They reported higher levels of C-reactive protein (126.6 in fatal cases vs 34.1 in discharged cases, P < 0.001), IL-6 (11.4 in fatal cases vs 6.8 in discharged cases, P < 0.001), and serum ferritin (1297 in fatal cases vs 614 in discharged cases, P < 0.001) [33], all suggesting that uncontrolled systemic inflammation can be considered one of the major causes of disease severity in SARS-CoV-2 infection. In a recent meta-analysis of IL-6 serum levels in COVID-19, Coomes et al. reported that the mean IL-6 concentrations were 2.9-fold higher in patients with complicated COVID-19 compared to those with non-complicated disease [57]. In another study, COVID-19 patients who progressed to ARDS had significantly increased IL-6 levels (median 7.39 pg/mL vs median 6.29 pg/mL, P = 0.03) [58]. Xu et al. conducted a non-randomized clinical trial to investigate the therapeutic effects of IL-6 pathway blockade using tocilizumab. Notably, they reported that 15 of 20 patients (75%) had lower oxygen intake within 5 days after tocilizumab [50], further highlighting the fact that increased secretion of inflammatory cytokines may effectively contribute to COVID-19 pathogenesis [51]. In a study by Zhou et al., IL-6 and serum ferritin levels were elevated in non-survivors compared to survivors throughout the clinical course and increased with illness deterioration [16]. Notably, it has been reported that hyperferritinemia can activate macrophages [59], [60], which increases the secretion of pro-inflammatory cytokines, and the subsequent inflammation is mainly responsible for organ damage. Although ferritin is a positive acute phase reactant and serum level of this intracellular protein increases during inflammation, dying cells may also release ferritin. Thus, it is reasonable to assume that higher serum ferritin levels in severely affected COVID-19 patients might indicate a greater extent of organ damage.
3.2. Prognostic value of biochemical parameters
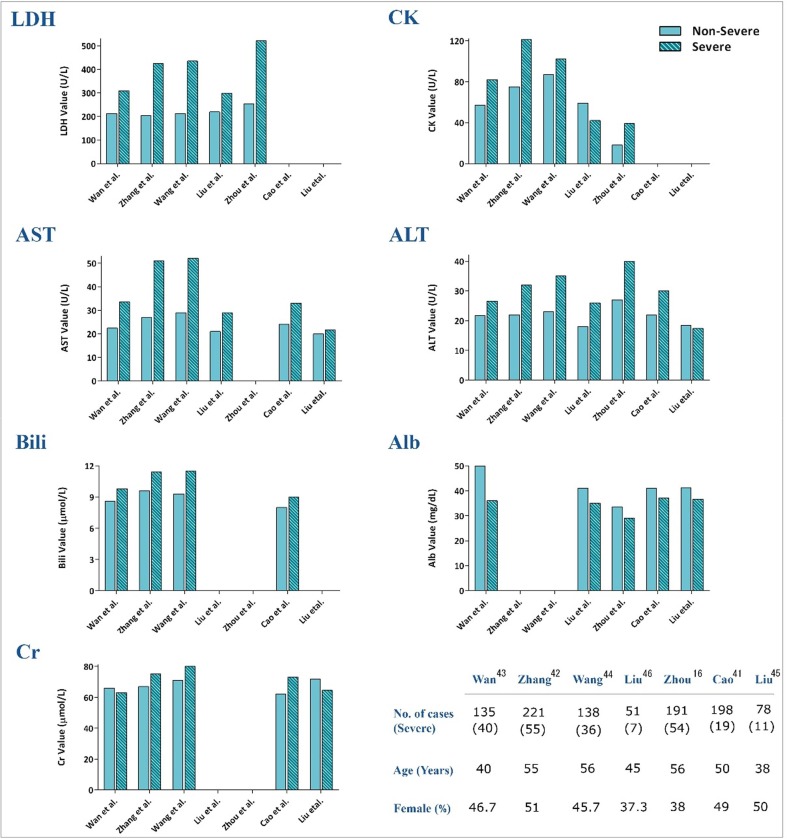
Consistent with hematological and inflammatory parameters, some studies proposed several biochemical factors as commonly available laboratory determinants to predict COVID-19 severity. The results of our recent meta-analysis revealed that while severe COVID-19 cases displayed higher values of ALT, AST, and total bilirubin compared to non-severe patients (mean differences of 7.48, 12.07, 3.07), value of albumin was significantly lower in severe cases (mean differences of −6.15); highlighting that abnormal values of liver-related examinations may contribute to reflect the progression of the disease toward an unfavorable outcome [accepted article]. Based on the results represented in Fig. 2 , we suggest 280 U/L as a cut-off level for LDH to predict poor prognosis. Yuan et al. screened the changes in patterns of serum biochemical factors during hospitalization in 94 discharged COVID-19 patients. They found a direct correlation between the decline of LDH and CK with viral mRNA elimination, suggesting that a constitutive decrease in LDH or CK levels probably predicts a favorable response to the course of COVID-19 patients [35]. Liu et al. also reported that albumin was significantly lower in a progression group than an improvement/stabilization group (36.62 vs 41.27 g/l, P = 0.006) [45]. In agreement, Huang et al. introduced decreased albumin along with increased LDH, ALT, and total bilirubin levels as appropriate biomarkers with the ability to discriminate between severe and non-severe groups [14]. In a large cohort of 1099 patients from 552 hospitals, Guan et al. reported a higher degree of abnormal liver aminotransferase levels in patients with severe disease than non-severe subjects [17]. In a comment on COVID-19 liver injury, Zhang et al. reported that 14–53% of patients had increased ALT and AST levels during disease progression [18]. Fig. 2 shows the results of 7 articles [16], [41], [42], [43], [44], [45], [46] reflecting the prognostic values of COVID-19 biochemical factors.
Fig. 2.
Main features and values of the biochemical parameters in the selected reports between severe and non-severe groups.
4. Conclusion
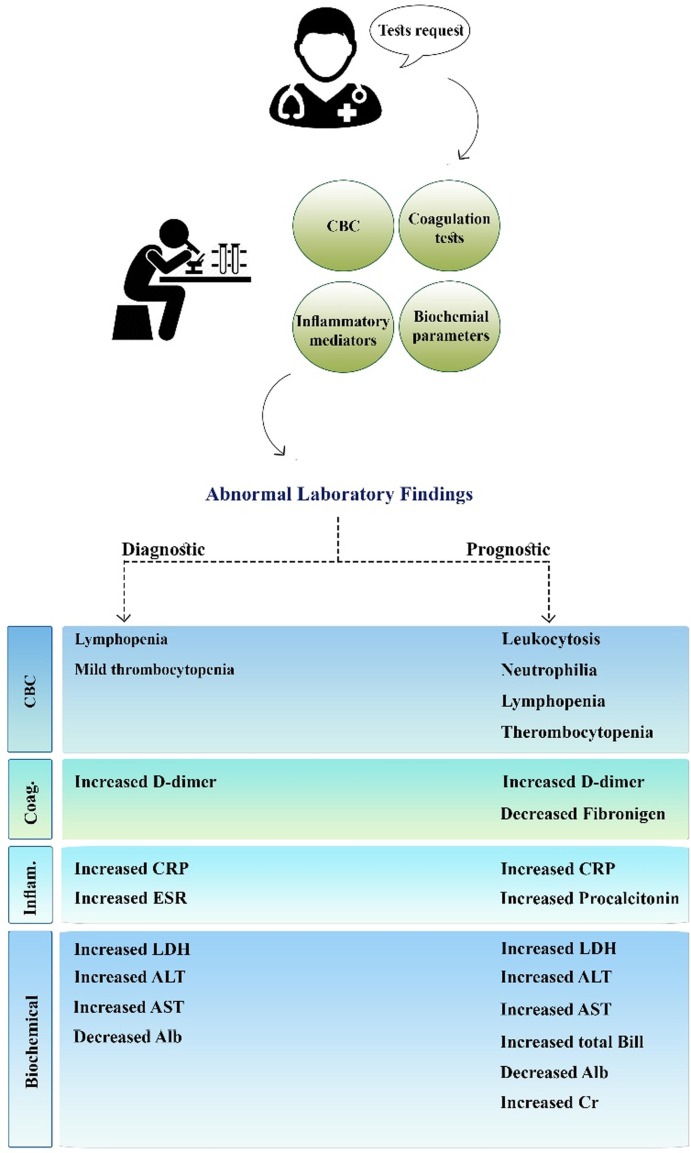
Although in vitro diagnostics efficiently contribute to the early identification of SARS-CoV-2 infection, there is evidence that laboratory medicine may also provide essential assistance to discriminate between severe and non-severe COVID-19. The large variations in the clinical features of the disease, spanning from asymptomatic to fatal, necessitates the identification and application of novel laboratory biomarkers to rapidly and economically predict COVID-19 prognosis. Fig. 3 presents a summary of the laboratory findings with diagnostic and prognostic values.
Fig. 3.
Summary of laboratory findings with diagnostic and prognostic values.
Declaration of Competing Interest
The authors declare no potential conflicts of interest concerning the research, authorship, and/or publication of this article.
Acknowledgments
The authors thank Shahid Beheshti University of Medical Sciences for supporting this study (Grant No.: 23604).
References
- 1.Lu H., Stratton C.W., Tang Y.W. The Wuhan SARS-CoV-2–What's Next for China. J. Med. Virol. 2020 doi: 10.1002/jmv.25738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benvenuto D., Giovanetti M., Ciccozzi A., Spoto S., Angeletti S., Ciccozzi M. The 2019-new coronavirus epidemic: evidence for virus evolution. J. Med. Virol. 2020;92(4):455–459. doi: 10.1002/jmv.25688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 4.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. LancetRespiratory Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lippi G., Plebani M. Laboratory abnormalities in patients with COVID-2019 infection. Clin. Chem. Lab. Med. (CCLM) 2020;1(ahead-of-print) doi: 10.1515/cclm-2020-0198. [DOI] [PubMed] [Google Scholar]
- 6.Lippi G., Plebani M. A modern and pragmatic definition of Laboratory Medicine. Clin. Chem. Lab. Med. (CCLM) 2020;1(ahead-of-print) doi: 10.1515/cclm-2020-0114. [DOI] [PubMed] [Google Scholar]
- 7.Lippi G., Plebani M. The critical role of laboratory medicine during coronavirus disease 2019 (COVID-19) and other viral outbreaks. Clin. Chem. Lab. Med. CCLM. 2020;1(ahead-of-print) doi: 10.1515/cclm-2020-0240. [DOI] [PubMed] [Google Scholar]
- 8.Wang T., Du Z., Zhu F., Cao Z., An Y., Gao Y., Jiang B. Comorbidities and multi-organ injuries in the treatment of COVID-19. The Lancet. 2020 doi: 10.1016/S0140-6736(20)30558-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y., Hu Y., Yu J., Ma T. Retrospective analysis of laboratory testing in 54 patients with severe-or critical-type 2019 novel coronavirus pneumonia. Lab. Invest. 2019;2020:1–7. doi: 10.1038/s41374-020-0431-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colafrancesco S., Priori R., Alessandri C., Astorri E., Perricone C., Blank M., Agmon-Levin N., Shoenfeld Y., Valesini G. sCD163 in AOSD: a biomarker for macrophage activation related to hyperferritinemia. Immunol. Res. 2014;60(2–3):177–183. doi: 10.1007/s12026-014-8563-7. [DOI] [PubMed] [Google Scholar]
- 11.Shoenfeld Y. Corona (COVID-19) time musings: Our involvement in COVID-19 pathogenesis, diagnosis, treatment and vaccine planning. Autoimmun. Rev. 2020 doi: 10.1016/j.autrev.2020.102538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bataille S., Pedinielli N., Bergougnioux J.-P. Could ferritin help the screening for COVID-19 in hemodialysis patients? Kidney Int. 2020 doi: 10.1016/j.kint.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jurisic V., Radenkovic S., Konjevic G. The actual role of LDH as tumor marker, biochemical and clinical aspects. Adv. Cancer Biomarkers Springer. 2015:115–124. doi: 10.1007/978-94-017-7215-0_8. [DOI] [PubMed] [Google Scholar]
- 14.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J., Wang Z., Li J., Li J., Feng C. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020;63(3):364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020 doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X., Liu L., Shan H., Lei C.-L., Hui D.S. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang C., Shi L., Wang F.-S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol. Hepatol. 2020 doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang W., Cao Q., Qin L., Wang X., Cheng Z., Pan A., Dai J., Sun Q., Zhao F., Qu J. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): A multi-center study in Wenzhou city, Zhejiang. J. Infect. China. 2020 doi: 10.1016/j.jinf.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panciani P.P., Saraceno G., Zanin L., Renisi G., Signorini L., Battaglia L., Fontanella M.M. SARS-CoV-2:“Three-steps” infection model and CSF diagnostic implication. Brain. Behav. Immun. 2020 doi: 10.1016/j.bbi.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.I.P. Bandeira, M.A.M. Schlindwein, L.C. Breis, J. Pierre, S. Peron, M.V.M. Gonçalves, Neurological complications of pandemic COVID-19: What have we got so far? (2020). [DOI] [PubMed]
- 22.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., Chang J., Hong C., Zhou Y., Wang D. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou L., Zhang M., Wang J., Gao J. Sars-Cov-2: Underestimated damage to nervous system. Travel Med. Infect. Dis. 2020 doi: 10.1016/j.tmaid.2020.101642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Filatov A., Sharma P., Hindi F., Espinosa P.S. Neurological complications of coronavirus disease (COVID-19): encephalopathy. Cureus. 2020;12(3) doi: 10.7759/cureus.7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moriguchi T., Harii N., Goto J., Harada D., Sugawara H., Takamino J., Ueno M., Sakata H., Kondo K., Myose N. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis. 2020 doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al Saiegh F., Ghosh R., Leibold A., Avery M.B., Schmidt R.F., Theofanis T., Mouchtouris N., Philipp L., Peiper S.C., Wang Z.-X. Status of SARS-CoV-2 in cerebrospinal fluid of patients with COVID-19 and stroke. J. Neurol. Neurosurg. Psychiatry. 2020 doi: 10.1136/jnnp-2020-323522. [DOI] [PubMed] [Google Scholar]
- 27.Pilotto A., Odolini S., Stefano Masciocchi S., Comelli A., Volonghi I., Gazzina S., Nocivelli S., Pezzini A., Focà E., Caruso A. Steroid-responsive encephalitis in Covid-19 disease. Ann. Neurol. 2020 doi: 10.1002/ana.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duong L., Xu P., Liu A. Meningoencephalitis without respiratory failure in a young female patient with COVID-19 infection in Downtown Los Angeles, early April 2020. Brain. Behav. Immun. 2020 doi: 10.1016/j.bbi.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C., Collange O., Boulay C., Fafi-Kremer S., Ohana M. Neurologic features in severe SARS-CoV-2 infection. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karimi N., Sharifi Razavi A., Rouhani N. Frequent Convulsive Seizures in an Adult Patient with COVID-19: A Case Report. Iran. Red Crescent Med. J. 2020;22(3) doi: 10.5812/ircmj.102828. [DOI] [Google Scholar]
- 31.Baud D., Qi X., Nielsen-Saines K., Musso D., Pomar L., Favre G. Real estimates of mortality following COVID-19 infection. Lancet. Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30195-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cascella M., Rajnik M., Cuomo A., Dulebohn S.C., Di Napoli R. StatPearls Publishing; 2020. Features, evaluation and treatment coronavirus (COVID-19), StatPearls [Internet] [PubMed] [Google Scholar]
- 33.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020:1–3. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan L., Wang Q., Zhang D., Ding J., Huang Q., Tang Y.-Q., Wang Q., Miao H. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduction Targeted Therapy. 2020;5(1):1–3. doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuan J., Zou R., Zeng L., Kou S., Lan J., Li X., Liang Y., Ding X., Tan G., Tang S. The correlation between viral clearance and biochemical outcomes of 94 COVID-19 infected discharged patients. Inflamm. Res. 2020;1–8 doi: 10.1007/s00011-020-01342-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.D. Wang, B. Hu, C. Hu, F. Zhu, X. Liu, J. Zhang, B. Wang, H. Xiang, Z. Cheng, Y. Xiong, Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA [Internet]. 2020 Feb [cited 2020 Mar 23]; 323 (11): 1061-9, 2020. [DOI] [PMC free article] [PubMed]
- 37.Yang A.-P., Liu J., Tao W., Li H.-M. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int. Immunopharmacol. 2020:106504. doi: 10.1016/j.intimp.2020.106504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lagunas-Rangel F.A. Neutrophil-to-lymphocyte ratio and lymphocyte-to-C-reactive protein ratio in patients with severe coronavirus disease 2019 (COVID-19): A meta-analysis. J. Med. Virol. 2020 doi: 10.1002/jmv.25819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu J., Liu Y., Xiang P., Pu L., Xiong H., Li C., Zhang M., Tan J., Xu Y., Song R. Neutrophil-to-lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. J. Transl. Med. 2019;18(2020):1–12. doi: 10.1186/s12967-020-02374-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lippi G., Plebani M., Henry B.M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A meta-analysis. Clin. Chim. Acta. 2020 doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cao M., Zhang D., Wang Y., Lu Y., Zhu X., Li Y., Xue H., Lin Y., Zhang M., Sun Y. Clinical features of patients infected with the 2019 novel coronavirus (COVID-19) in Shanghai, China. medRxiv. 2020 [Google Scholar]
- 42.Zhang G.-Q., Hu C., Luo L.-J., Fang F., Chen Y.-F., Li J.-G., Peng Z.-Y., Pan H. Clinical features and treatment of 221 patients with COVID-19 in Wuhan, China (2/27/2020) China. 2020 doi: 10.1016/j.jcv.2020.104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wan S., Xiang Y., Fang W., Zheng Y., Li B., Hu Y., Lang C., Huang D., Sun Q., Xiong Y. Clinical features and treatment of COVID-19 patients in Northeast Chongqing. J. Med. Virol. 2020 doi: 10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.D. Wang, B. Hu, C. Hu, F. Zhu, X. Liu, J. Zhang, B. Wang, H. Xiang, Z. Cheng, Y. Xiong, Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China, JAMA (2020). [DOI] [PMC free article] [PubMed]
- 45.Liu W., Tao Z.-W., Lei W., Ming-Li Y., Kui L., Ling Z., Shuang W., Yan D., Jing L., Liu H.-G. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Med. J. Chin. 2019:2020. doi: 10.1097/CM9.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jian-ya G. Clinical characteristics of 51 patients discharged from hospital with COVID-19 in Chongqing, China. medRxiv. 2020 [Google Scholar]
- 47.Tang N., Li D., Wang X., Sun Z. Abnormal Coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bashash D., Abolghasemi H., Salari S., Olfatifar M., Eshghi P., Akbari M.E. Elevation of D-Dimer, But Not PT and aPTT, Reflects the Progression of COVID-19 Toward an Unfavorable Outcome: A Meta-Analysis. IJBC. 2020;12(2):47–53. [Google Scholar]
- 49.Zhang J.-J., Dong X., Cao Y.-Y., Yuan Y.-D., Yang Y.-B., Yan Y.-Q., Akdis C.A., Gao Y.-D. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020 doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 50.Ramos-Casals M., Brito-Zerón P., López-Guillermo A., Khamashta M.A., Bosch X. Adult haemophagocytic syndrome. The Lancet. 2014;383(9927):1503–1516. doi: 10.1016/S0140-6736(13)61048-X. [DOI] [PubMed] [Google Scholar]
- 51.Ye Q., Wang B., Mao J. The pathogenesis and treatment of theCytokine Storm'in COVID-19. J. Infect. 2020 doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. The Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Siracusano G., Pastori C., Lopalco L. Humoral immunity in COVID-19 patients: a window on the state of the art. Front. Immunol. 2020;11:1049. doi: 10.3389/fimmu.2020.01049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol., Springer. 2017:529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang Y., Shen C., Li J., Yuan J., Yang M., Wang F., Li G., Li Y., Xing L., Peng L. Exuberant elevation of IP-10, MCP-3 and IL-1ra during SARS-CoV-2 infection is associated with disease severity and fatal outcome. MedRxiv. 2020 [Google Scholar]
- 56.Xiong Y., Liu Y., Cao L., Wang D., Guo M., Jiang A., Guo D., Hu W., Yang J., Tang Z. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerging Microbes Infect. 2020;9(1):761–770. doi: 10.1080/22221751.2020.1747363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coomes E.A., Haghbayan H. Interleukin-6 in COVID-19: a systematic review and meta-analysis. MedRxiv. 2020 doi: 10.1002/rmv.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu C., Chen X., Cai Y., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C., Zhang Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Int. Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kernan K.F., Carcillo J.A. Hyperferritinemia and inflammation. Int. Immunol. 2017;29(9):401–409. doi: 10.1093/intimm/dxx031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.R. Piero, B. Onorina, C. Paolo, I. Annamaria, S. Yehuda, Severe hyper-inflammatory COVID-19, another piece in the puzzle of the“ htperferritinemic syndrome, Rheumatol Point View (2020).
- 61.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu J., Wu X., Zeng W., Guo D., Fang Z., Chen L., Huang H., Li C. Chest CT Findings in Patients With Coronavirus Disease 2019 and Its Relationship With Clinical Features. Invest. Radiol. 2020;55(5):257–261. doi: 10.1097/RLI.0000000000000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen L., Liu H., Liu W., Liu J., Liu K., Shang J., Deng Y., Wei S. Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia. Zhonghua jie he he hu xi za zhi= Zhonghua jiehe he huxi zazhi= Chinese Journal Of Tuberculosis And Respiratory Diseases. 2020;43:E005. doi: 10.3760/cma.j.issn.1001-0939.2020.0005. [DOI] [PubMed] [Google Scholar]
- 64.Xu X.-W., Wu X.-X., Jiang X.-G., Xu K.-J., Ying L.-J., Ma C.-L., Li S.-B., Wang H.-Y., Zhang S., Gao H.-N. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368 doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shi H., Han X., Jiang N., Cao Y., Alwalid O., Gu J., Fan Y., Zheng C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet. Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen H., Guo J., Wang C., Luo F., Yu X., Zhang W., Li J., Zhao D., Xu D., Gong Q. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. The Lancet. 2020;395(10226):809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li X., Hu C., Su F., Dai J. Hypokalemia and Clinical Implications in Patients with Coronavirus Disease 2019 (COVID-19) medRxiv. 2020 doi: 10.1001/jamanetworkopen.2020.11122. [DOI] [PMC free article] [PubMed] [Google Scholar]