Abstract
M2 (tumor-supportive) macrophages may upregulate growth differentiation factor 15 (GDF15), which is highly expressed in prostate tumors, but the combined utility of these markers as prognostic biomarkers are unclear. We retrospectively studied 90 prostate cancer cases that underwent radical prostatectomy as their primary treatment and were followed for biochemical recurrence (BCR). These cases also had a benign prostate biopsy at least 1 year or more before their prostate cancer surgery. Using computer algorithms to analyze digitalized immunohistochemically stained slides, GDF15 expression and the presence of M2 macrophages based on the relative density of CD204- and CD68-positive macrophages were measured in prostate: (i) benign biopsy, (ii) cancer and (iii) tumor-adjacent benign (TAB) tissue. Both M2 macrophages (P = 0.0004) and GDF15 (P < 0.0001) showed significant inter-region expression differences. Based on a Cox proportional hazards model, GDF15 expression was not associated with BCR but, in men where GDF15 expression differences between cancer and TAB were highest, the risk of BCR was significantly reduced (hazard ratio = 0.26; 95% confidence interval = 0.09–0.94). In addition, cases with high levels of M2 macrophages in prostate cancer had almost a 5-fold increased risk of BCR (P = 0.01). Expression of GDF15 in prostate TAB was associated with M2 macrophage levels in both prostate cancer and TAB and appeared to moderate M2-macrophage-associated BCR risk. In summary, the relationship of GDF15 expression and CD204-positive M2 macrophage levels is different in a prostate tumor environment compared with an earlier benign biopsy and, collectively, these markers may predict aggressive disease.
Growth differentiation factor 15 (GDF15) expression and M2 macrophages levels could play a role in prostate carcinogenesis. Biochemical recurrent disease is associated with high M2 macrophages levels in cancer and GDF15 expression that is lower in cancer compared to adjacent benign prostate.
Introduction
Inflammation is critical in tumor development and progression and can be both tumor promoting and antitumorigenic (1). In the prostate gland, chronic prostatitis is thought to increase the risk of cancer by as much as 60% (2,3). However, a more in-depth examination of prostatic inflammation on the histologic level suggests that histologic inflammation in the benign prostate may decrease the risk for cancer (4–6). Pre-clinical studies show that pathologic mechanisms associated with the activation of a chronic inflammatory response play an important role in the pathogenesis and progression of prostate cancer (7). Although the infiltration of inflammatory cells might be associated with the proliferation of prostatic glandular cells (8), eventually leading to cancer development, it remains unclear what types of inflammatory cells infiltrate and how these cells affect malignant transformation.
Tumor-associated factors can polarize macrophages toward either the M1 (tumor-suppressive) or M2 (tumor-supportive) direction. M1 ‘classically activated’ macrophages are associated with acute inflammation and T-cell immunity and their function is to kill and phagocytose target cells (9). These macrophages express high levels of major histocompatibility complex class II, the CD68 protein and CD80 and CD86 costimulatory molecules. M2 macrophages, also known as ‘alternatively activated’ macrophages, are associated with tumor growth and are key regulators of the link between inflammation and cancer. These macrophages function in removing parasites, dampening the immune response and wound healing. M2 macrophages are identifiable by cell-surface markers, such as hemoglobin-scavenger receptor (CD163), mannose receptor C type 1 (CD206) and macrophage-scavenger receptor 1 (MSR1 or CD204) (10). In prostate, the mechanisms and factors that induce polarization of macrophages to the M2 state remains an active area of investigation (11).
MSR1 or CD204, is a multifunctional cell membrane scavenger receptor that recognizes many different types of negatively charged macromolecules (12) and is reported to be overexpressed on M2 macrophages (13). CD204-positive macrophages display a large amount of plasticity and can have many different states along a continuum ranging between the extremes of the M1 and M2 phenotypes depending upon the signals they receive from their environment and can be ‘repolarized’ in vitro to their opposite state (14). CD204-positive macrophages have been reported to correlate with tumor progression and poor patient outcome in glioma, ovarian epithelial tumors, lung, pancreatic, gastric, renal, oral and esophageal cancers (15–20). In prostate cancer, two studies have shown that decreased numbers of CD204-positive cells are associated with a higher likelihood of cancer and cancer progression (21,22), but a third study found that, in men with prostate cancer, CD204 expression is higher in cancer than in adjacent normal cells (8).
Growth differentiation factor 15 (GDF15), also known as macrophage inhibitory cytokine 1 (MIC-1), a divergent member of the transforming growth factor-β (TGF-β) superfamily of cytokines, appears to have a key role in regulating inflammatory pathways in prostate, exhibiting both tumor-suppressing and tumor-promoting functions (23). GDF15 suppresses the activity of nuclear factor-kappaB, indicating a tumor-suppressing quality, but has also been shown to preferentially inhibit M1 macrophage formation, indicating a pro-tumorigenic quality (24). In a recent study of esophageal squamous cell carcinomas, M2 macrophages appeared to upregulate GDF15 expression with a strong positive correlation between GDF15 expression and CD204-positive cells (25). While this finding is intriguing, overall not much is known about the interplay between GDF15 and M2 polarized macrophages in cancer development.
In the present study, we characterized the prostate inflammatory environment in ‘benign’ biopsy tissue, as well as overt malignant tumor tissue in paired prostate specimens taken before diagnosis and after the development of tumor. By measuring how GDF15 expression levels and location in the prostate tumor environment compares to benign prostate of the same individual, as well as the dynamics of GDF15 expression in relation to the presence of the CD204-positive M2 macrophage phenotype, and how the associations of these two markers relate to disease outcome, a clearer understanding of the role of GDF15 in relation to the M1–M2 transition in prostate carcinogenesis should start to emerge.
Materials and methods
Study sample
After obtaining appropriate approval from the Henry Ford Health System Institutional Review Board, we ascertained 90 prostate cancer cases with primary treatment of radical prostatectomy occurring between 1999 and 2012 who also had at least one previous benign prostate biopsy 1 or more years before their surgery (Table 1). Surgical and benign biopsy specimens were on average 1504.8 days (or 4.1 years) apart with a range of 368 days to 16.3 years. Cases were 38% African American (AA) and 59% White with an average age of 63.5 and 65.1 years at the time of diagnosis, respectively. About 30% of tumors were considered high grade (Gleason grade group 3 and above). To determine biochemical recurrence (BCR) for our study sample, we electronically retrieved all prostate-specific antigen (PSA) test results from the date of surgery forward. A total of 1423 PSA test results were retrieved, with the men in this sample having a median of 10 PSA tests and the number of tests ranging from 2 to 29. A BCR event was defined as having two consecutive detectable rising PSA levels (>0.2 ng/ml) 4 weeks or more after surgery. A total of 26% of cases experienced BCR with the median follow-up of men who did not biochemically recur 7.1 years. The study is a retrospective surgical sample review and a blanket consent for research studies are obtained at the time of surgery from the patients.
Table 1.
Demographic and clinical characteristics of study sample by prostate cancer biochemical recurrence (BCR) status (n = 90)
| Characteristic | Disease free (n = 67) | BCR (n = 23) | P value |
|---|---|---|---|
| Race | 0.55 | ||
| White | 42 (63%) | 13 (57%) | |
| AA | 24 (36%) | 10 (43%) | |
| Other | 1 (1%) | 0 | |
| Mean age at diagnosis | 63.9 ± 6.5 | 66.2 ± 5.9 | 0.148 |
| PSA at time of biopsy | 4.4 ± 13.5 | 7.2 ± 5.4 | 0.35 |
| Mean duration between benign biopsy and surgery dates | 4.2 ± 3.3 | 4.5 ± 3.0 | 0.68 |
| Grade group | |||
| 1 | 25 (37.5%) | 2 (8.7%) | 0.001a |
| 2 | 28 (42%) | 8 (34.8%) | |
| 3 | 9 (13.5%) | 10 (43.5%) | |
| 4 | 3 (4%) | 0 | |
| 5 | 2 (3%) | 3 (13%) | |
| Pathological stage | |||
| 2A | 9 (13.4%) | 0 | 0.15b |
| 2B | 18 (26.9%) | 8 (34.8%) | |
| 2C | 25 (37.3%) | 6 (26.1%) | |
| 3A | 13 (19.4%) | 6 (26.1%) | |
| 3B | 2 (3%) | 3 (13%) |
aGrade groups 1 and 2 and 3–5 combined for chi-square test.
bStages 2A–2C and 3A and 3B combined for chi-square test.
Pathological assessment of inflammation
All biopsy and prostatectomy specimens were reviewed by two independent pathologists to exclude malignancy in the benign biopsy specimens and score inflammation in the tissue. Location (acinar, peri acinar and stromal), extent (focal, multifocal and diffuse) and grade (mild, moderate and severe) of inflammation were assessed using the Nickel criteria (26); when inflammatory infiltrate varied, the highest grade was recorded. Acute and chronic inflammations were evaluated by the distribution of polymorpho-nuclear leukocytes and mononuclear cells, respectively (27) with each specimen graded on a four-tier scale for both cell types. In each prostate surgical section, regions of tumor and tumor-adjacent benign glands were demarcated and scored separately for the primary tumor focus. These same regions were also used for immunohistochemistry (IHC) analyses described below.
Specimen processing, IHC and imaging
Surgical prostatectomy and biopsy specimens preserved as formalin-fixed, paraffin-embedded blocks were procured from the Henry Ford Hospital biorepository. For biopsy specimens used for analysis, we randomly selected a subset of blocks for each case from blocks with available tissue cores. For surgical prostatectomy specimen, one block per case with tumor was selected. Serial sections at 5 μm thickness were cut from both the biopsy and the surgical prostatectomy specimen. The middle section was Hematoxylin and Eosin stained and used to confirm the tumor/benign status of the specimen and for histopathological assessment of inflammation. The remaining sections were stained for a set of immune cell markers using a standard IHC protocol. Slides were dried for 60 min in a 60°C drying oven, de-paraffinized and hydrated. Antigens were retrieved by boiling the slide in citrate buffer for 10 min and cooling to room temperature. All slides were analyzed with the Dako Autostainer Link 48 (Dako North America, Carpinteria, CA). The following antibodies and concentrations were used to differentiate immune cell types: macrophages (anti-CD68+, IR613 from Dako) at 1:200 dilution, tumor-associated macrophages (TAMs; MSR1 or CD204+, HPA000272 from Sigma Life Science) at 1:200 dilution and cytokine growth differentiation factor 15 (MIC-1 or GDF15, AMAb90687 from Atlas Antibodies) at 1:1500 dilution. The expression of CD68 (pan macrophage), CD204 (M2 TAM) and GDF15 were analyzed as single IHC staining on adjacent sections of the surgical and biopsy specimens. In order to maintain similar experimental conditions within the pair, the matched surgical and biopsy specimens were processed together for IHC.
Whole slide scanning of CD68, CD204 and GDF15 stained matched benign biopsy and prostatectomy surgical slides were performed using a Ventana iScan HT slide scanner (Roche Diagnostics) at ×40 magnification. Three glandular regions of interest that also included the surrounding stromal regions were delineated by the study pathologist on slides stained for CD68: (i) benign glands in the biopsy slides in prostatectomy slides, (ii) prostate cancer and 3) tumor-adjacent benign (TAB) glands. Additional information about the processing and analysis of whole slide-scanned images is provided in the Supplementary Materials (available at Carcinogenesis online).
Statistical analysis
For comparisons of patients with and without BCR, paired t-tests were used for continuous variables and chi-square tests for categorical variables. An analysis of variance was used to test for heterogeneity of mean levels of CD68- and CD204-positive macrophages and GDF15 expression across the three prostate tissue regions. P values for differences between pairs of means were computed with a Tukey multiple comparison adjustment. Intra- and inter-region comparisons of macrophage density and GDF15 expression were also analyzed with Pearson correlation coefficients. Macrophage density and GDF15 expression values were modeled both on a continuous and categorical scale—the latter as groups divided into tertiles or at the median. Analyses that considered time to BCR included log-rank tests and Cox proportional hazard models that analyzed the marker level differences with respect to time to BCR. Time to BCR was defined as the duration between the date of surgery and the second PSA test that defined the recurrence event or censored at the last post-operative PSA test for men that did not recur. Both unadjusted (only a covariate for the marker) and adjusted models were tested—the latter adjusted for advanced tumor stage, advanced tumor grade and PSA level at cancer diagnosis. Model outliers were assessed through the examination of deviance residuals and assumptions of linearity through the examination of Martingale residuals. To determine whether the proportional hazards assumption was met, models were run where the singular and joint significance of each covariate by time interaction term was tested.
Results
Demographic and clinical characteristics of the study cohort
Among our study sample of 90 prostate cancer surgical cases, most could be classified as either White or AA (Table1). Study subjects had a median follow-up after surgery of over 7 years with 75% of subjects followed up for at least 3 years. A higher percentage of AA men had biochemically recurrent disease (29 versus 24%), but racial differences were not statistically significant. The mean age at diagnosis was not significantly different between men who biochemically recurred (66.2 ± 5.9 years) and those who did not (63.9 ± 6.5 years). PSA at the time of benign biopsy was higher in men who had BCR (7.2 ± 5.4 ng/ul) compared to men who did not (4.4 ± 13.5 ng/ul), but the difference was not statistically different (P ≤ 0.35). Men who had BCR also appeared to have more advanced Gleason grade with biochemically recurrent cases more often having a Gleason grade group of 3 or higher (P ≤ 0.001).
M1/M2 macrophage density and GDF15 expression in different prostate regions and age effects
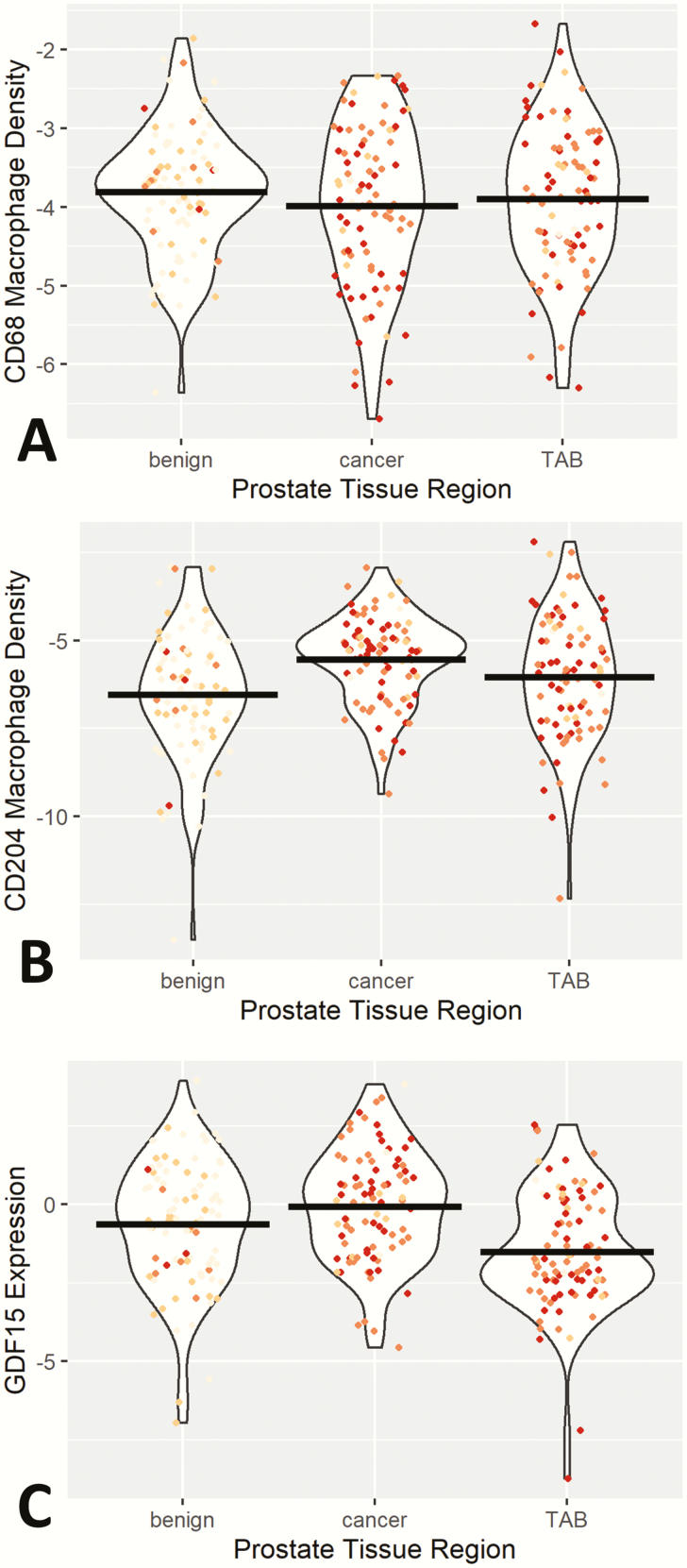
Macrophage density as measured by the area of CD68-expressing cells was not significantly different between the three different regions of the prostate specimens examined (Figure 1A). However, the density of CD204 expressing macrophages was significantly different (P ≤ 0.0001) between benign biopsies and the two prostate regions examined in prostatectomy specimens (cancer and TAB; Figure 1B). CD204 macrophage density was lowest in benign biopsy, intermediate in TAB and highest in cancer. GDF15 expression significantly (P ≤ 0.0001) varied across these three regions of the prostate as well (Figure 1C). The highest GDF15 expression levels were in cancer and lowest expression in TAB with GDF15 expression in TAB significantly lower than expression in either cancer (P ≤ 0.0001) or benign (P ≤ 0.007) prostate. In general, age was positively associated with CD68 levels and negatively associated with GDF15 expression. The age association with M2 CD204 marker was tissue dependent, with age negatively associated with CD204 density in prostate cancer and TAB but positively associated with age in prostate benign biopsy. All these associations were relatively weak, and none reached statistical significance (data not shown).
Figure 1.
Violin plot of CD68-positive macrophage density (A), CD204-positive macrophage density (B) and GDF15 expression (C) across prostate regions of benign biopsy, cancer and TAB. For (A) and (B), y-axis macrophage density levels represent natural log transformed percentage of positive expression within tissue area. GDF15 expression values on y-axis of (C) are the natural log-transformed percentage of positive expression within tissue area multiplied by the pixel intensity values. Individual data points are also plotted with intensity of red dots representing the level of inflammation in each specimen. Mean levels of marker by prostate region are represented by black bars.
Correlation of M1/M2 macrophage density and GDF15 expression in different prostate regions
Interestingly, GDF15 expression in TAB was significantly correlated with the CD204/CD68 macrophage density level in both the TAB (r = 0.27; P ≤ 0.009) and cancer (r = 0.26; P ≤ 0.01). Other intra- and inter-region correlations of these two markers were not as strong (Supplementary Figure 2A and Supplementary Table 1, available at Carcinogenesis online) with the CD204/CD68 macrophage density level in benign prostate biopsy having a negative, albeit non-significant, correlation with GDF15 expression across all three prostate tissue regions. Intra-region correlations of CD204/CD68 macrophage density and GDF15 expression in benign prostate biopsy and cancer were much lower than the correlation in TAB. In terms of intra-marker correlations across regions, CD204/CD68 macrophage density levels were strongly correlated between cancer and TAB (Supplementary Figure 2B and Supplementary Table 1, available at Carcinogenesis online), but levels in neither region were correlated with benign prostate levels. GDF15 expression was not correlated between cancer and TAB regions (Supplementary Figure 2C and Supplementary Table 1, available at Carcinogenesis online), but there was a weak nominally significant correlation between GDF15 expression in benign prostate biopsy and TAB regions (r = 0.20; P ≤ 0.05).
Level of inflammation as measured by either grade or extent was moderately associated with the density of M2 macrophages expressing CD204—with macrophage density increasing with both inflammation grade (P ≤ 0.03) and extent (P ≤ 0.05). However, after adjusting for the prostate tissue region these associations were no longer observed (data not shown). On the other hand, adjusting for prostate tissue region resulted in a stronger negative association of both inflammation grade and extent with GDF15 expression. CD68-positive macrophages were strongly positively associated with the extent of inflammation (P ≤ 0.0002), but not inflammation grade, irrespective of whether the prostate tissue region was considered. In general, trends of marker expression with inflammation grade or extent were consistent across prostate tissue regions.
M1/M2 macrophage density and GDF15 expression in different prostate regions and prostate cancer recurrence
Whether the amount and/or proportion of M1–M2 macrophages and/or GDF15 expression were related to prostate cancer outcome was tested in Cox proportional hazards models of time to BCR after surgery (Supplementary Table 2, available at Carcinogenesis online). The density of CD68-positive M1 macrophages in different prostate regions or differences in CD68 macrophage density between regions were not significantly associated with BCR. A modest increase in BCR risk was associated with increasing CD68-positive macrophage density in cancer compared with the adjacent benign cells (Δ cancer/TAB), but this risk did not reach statistical significance [hazard ratio (HR) = 1.37; 95% confidence interval (CI) = 0.84, 2.25]. Adjusting for PSA at diagnosis, Gleason grade and pathologic stage resulted in nominal risk estimate changes. Alternatively, the density of the M2 macrophage marker CD204 in cancer was associated with an increased BCR risk (HR = 1.98, P ≤ 0.002)—with the adjusted risk estimate remaining statistically significant. Also associated with BCR was the unadjusted risk for Δ cancer/benign for CD204-positive macrophages (HR = 1.26, P ≤ 0.01). Further analysis to determine the significance of CD204-postive macrophage density was done using a CD204/CD68 ratio to standardize the M2 macrophage measure. The ratio of CD204/CD68 macrophages in both TAB and cancer were significantly associated with BCR with an approximately 35% increased risk in unadjusted models. Only the association in cancer remained significant after adjustment for clinical factors (HR = 1.35; P ≤ 0.04). Associations with BCR were not observed for the CD204/CD68 macrophage ratio in benign prostate biopsy or differences in CD204/CD68 macrophage ratio between regions of the prostate.
Potential threshold effects of either CD204/CD68 macrophage density and/or GDF15 expression on BCR risk were examined by categorizing expression levels into tertiles—low, middle and high—and comparing risk associated with the middle and highest tertile to the lowest tertile (Table 2). For CD204/CD68 macrophage density in cancer, the highest tertile was associated with a statistically significant (P ≤ 0.01) increased risk of BCR of over 4-fold in the adjusted model. Although not statistically significant, there appeared to be an increasing trend in the risk of BCR with increasing CD204/CD68 density in the prostate TAB region. Models of the difference in CD204/CD68 density between the prostate tumor and benign adjacent regions (Δ cancer/TAB) also had HRs around three in the two highest tertiles in the adjusted model, although neither HR reached statistical significance. The highest tertile of the Δ cancer/TAB for GDF15 expression was associated with the risk of BCR in the opposite direction even after adjusting for clinical covariates (HR = 0.29; 95% CI = 0.09, 0.94). None of the other models of GDF15 expression had HRs that approached statistical significance.
Table 2.
Hazard ratios of prostate cancer biochemical recurrence associated with CD204/CD68 ratio density and GDF15 expression in prostate pre- and post-diagnosis
| Unadjusted HRs | Adjusted HRa | |||||||
|---|---|---|---|---|---|---|---|---|
| Levelb | Middle tertile | Highest tertile | Middle tertile | Highest tertile | ||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| CD204/CD68 ratio density | ||||||||
| Benign | 0.64 (0.22, 1.84) | 0.40 | 0.90 (0.34, 2.34) | 0.82 | 0.82 (0.27, 2.53) | 0.73 | 1.15 (0.43, 3.13) | 0.78 |
| TAB | 3.10 (0.97, 9.93) | 0.06 | 3.52 (1.07, 11.59) | 0.04 | 2.95 (0.91, 9.58) | 0.07 | 2.24 (0.60, 8.42) | 0.23 |
| Cancer | 1.60 (0.48, 5.27) | 0.44 | 3.97 (1.38, 11.43) | 0.01 | 1.78 (0.52, 6.12) | 0.36 | 4.82 (1.51, 15.43) | 0.01 |
| Δ TAB/benign | 1.30 (0.43, 3.88) | 0.64 | 3.15 (1.10, 9.05) | 0.30 | 1.33 (0.44, 4.00) | 0.61 | 1.96 (0.59, 6.55) | 0.27 |
| Δ Cancer/benign | 2.46 (0.81, 7.46) | 0.12 | 2.63 (0.88, 7.89) | 0.08 | 2.47 (0.80, 7.58) | 0.11 | 2.18 (0.70, 6.77) | 0.18 |
| Δ Cancer/TAB | 1.66 (0.59, 4.67) | 0.34 | 1.41 (0.49, 4.05) | 0.53 | 2.88 (0.88, 9.37) | 0.08 | 3.09 (0.89, 10.69) | 0.07 |
| GDF15 expression | ||||||||
| Benign | 0.81 (0.28, 2.33) | 0.69 | 1.27 (0.49, 3.31) | 0.62 | 1.48 (0.47, 4.59) | 0.51 | 1.19 (0.43, 3.28) | 0.74 |
| TAB | 0.98 (0.34, 2.81) | 0.97 | 1.55 (0.57, 4.17) | 0.39 | 0.97 (0.33, 2.85) | 0.96 | 1.32 (0.47, 3.72) | 0.60 |
| Cancer | 1.11 (0.43, 2.84) | 0.84 | 0.52 (0.18, 1.52) | 0.23 | 1.18 (0.45, 3.10) | 0.73 | 0.52 (0.18, 1.55) | 0.24 |
| Δ TAB/benign | 0.44 (0.14, 1.43) | 0.17 | 1.28 (0.52, 3.17) | 0.59 | 0.60 (0.18, 2.01) | 0.41 | 1.34 (0.53, 3.35) | 0.44 |
| Δ Cancer/benign | 1.35 (0.53, 3.42) | 0.53 | 0.58 (0.19, 1.73) | 0.33 | 1.33 (0.52, 3.45) | 0.55 | 0.57 (0.19, 1.70) | 0.31 |
| Δ Cancer/TAB | 0.51 (0.20, 1.29) | 0.15 | 0.28 (0.09, 0.88) | 0.03 | 0.57 (0.22, 1.50) | 0.26 | 0.29 (0.09, 0.94) | 0.04 |
aAdjusted for PSA at diagnosis, Gleason grade and pathologic stage.
bReferent category is lowest tertile of expression.
Survival analyses for time to recurrence for M1/M2 Macrophage density and GDF15 expression in different prostate regions
Prostate cancer BCR with regard to levels of CD204/CD68 macrophage density in cancer and the difference in GDF15 expression between cancer and TAB prostate regions was further explored with Kaplan–Meier survival plots. Men in the lowest tertile of CD204/CD68 macrophage density tended to have less biochemically recurrent disease over time than men in the middle or highest tertile (Supplementary Figure 3A, available at Carcinogenesis online). The survival curves for the latter two groups did not appear to separate, and the log-rank statistic for this survival plot stratification was not statistically significant (P ≤ 0.11). In plotting out the survival curves based on the distribution of the difference in GDF15 expression in cancer and TAB regions (Supplementary Figure 3B, available at Carcinogenesis online), there was a clear trend for better outcomes when the amount of GDF15 expression in cancer exceeded that in the TAB (log-rank P value ≤ 0.004). Men in the tertile with the greatest difference in GDF15 expression between cancer and TAB regions had the best outcomes compared with men in the other two tertiles, where the GDF15 expression in cancer was the same or less than in TAB having the highest BCR rate.
M1/M2 macrophage density and GDF15 expression within and between different prostate regions and association with prostate cancer recurrence
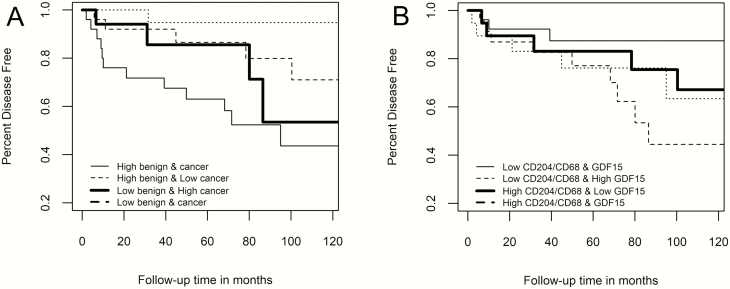
Further analysis was done to determine whether a different level of GDF15 expression and M2 macrophages in benign biopsy and malignant prostate and/or a different level of both markers within the same prostate region were associated with the risk of BCR. Table 3 shows models with interaction hazard ratios (IHRs) that address the question of whether the joint effect of each marker/region combination is less or greater than would be expected based on combined effects of individual risk estimates. One IHR showed a 17% significant (P ≤ 0.009) increased risk for the joint effect of CD204/CD68 ratio density in benign prostate biopsy and cancer, indicating a positive interaction between the density of CD204-positive relative to CD68-positive macrophages in these two prostate regions. Another IHR showed a 22% significant (P ≤ 0.008) decreased risk for the joint effect of GDF15 expression and CD204/CD68 ratio density in prostate cancer, indicating a negative interaction between these two factors. Exploring these interactions more in depth in Table 4, a high CD204/CD68 ratio density in both benign and cancer regions was associated with an almost 5-fold increased risk of BCR compared with men who had low levels of CD204/CD68 ratio density in these two regions. Furthermore, men who had a high CD204/CD68 ratio density in benign prostate biopsy but a low level in cancer had a much-reduced risk of biochemical recurrence compared with men whose CD204/CD68 ratio density was low in both prostate regions. For the prostate TAB region, the highest risk of prostate cancer BCR was observed for men with a high CD204/CD68 ratio density and low GDF15 expression. And while increased GDF15 expression in TAB appeared to increase the risk of prostate cancer BCR when CD204/CD68 ratio density was low, when both the CD204/CD68 ratio density and GDF15 expression was high in the TAB, risk of prostate BCR remained about the same. Figure 2A and B show the corresponding Kaplan–Meier survival plots for prostate cancer BCR for these two stratifications. The log-rank statistic for both the CD204/CD68 density levels in benign prostate biopsy and cancer (P ≤ 0.01) and the CD204/CD68 density and GDF15 expression levels in TAB (P ≤ 0.05) were statistically significant.
Table 3.
Modeling main effects and interactions of CD204/CD68 ratio density and GDF15 expression in prostate pre- and post-diagnosis on prostate cancer biochemical recurrence risk
| Model | Region(s) | First main effect HR | Second main effect HR | IHR | 95% CI | P value |
|---|---|---|---|---|---|---|
| CD204/CD68 density in different prostate tissue regions | Benign–TAB | 1.10 | 1.27 | 1.02 | (0.89–1.17) | 0.73 |
| Benign–cancer | 1.28 | 2.51 | 1.17 | (1.04–1.32) | 0.009 | |
| TAB–cancer | 0.86 | 1.42 | 0.98 | (0.87–1.10) | 0.72 | |
| GDF15 expression in different prostate tissue regions | Benign–TAB | 0.96 | 1.12 | 0.98 | (0.87–1.10) | 0.68 |
| Benign–cancer | 0.93 | 1.03 | 1.01 | (0.91–1.12) | 0.89 | |
| TAB–cancer | 1.17 | 0.90 | 0.98 | (0.88–1.10) | 0.76 | |
| CD204/CD68 density and GDF15 expression in the same prostate tissue region | Benign | 1.08 | 1.09 | 1.03 | (0.94–1.14) | 0.53 |
| TAB | 0.74 | 0.84 | 0.78 | (0.64–0.94) | 0.008 | |
| Cancer | 0.77 | 1.32 | 0.88 | (0.74–1.05) | 0.15 |
HR, hazard ratio; IHR, interaction hazards ratio.
Table 4.
Hazard ratios of prostate cancer BCR associated with multiregion combinations of CD204/CD68 ratio density and GDF15 expression in prostate pre- and post-diagnosis
| Marker and prostate region combinationa | HR | 95% CI | P value |
|---|---|---|---|
| Low CD204/CD68 in benign prostate and cancer | 1.00 | Reference | |
| Low CD204/CD68 in benign prostate and high CD204/CD68 in cancer | 2.35 | (0.55–9.92) | 0.25 |
| High CD204/CD68 in benign prostate and low CD204/CD68 in cancer | 0.27 | (0.03–2.54) | 0.25 |
| High CD204/CD68 in benign prostate and cancer | 4.79 | (1.40–16.44) | 0.01 |
| Low CD204/CD68 and low GDF15 in TAB | 1.00 | Reference | |
| Low CD204/CD68 and high GDF15 in TAB | 2.39 | (0.56–10.17) | 0.24 |
| High CD204/CD68 and low GDF15 in TAB | 3.71 | (0.89–15.38) | 0.07 |
| High CD204/CD68 and high GDF15 in TAB | 2.67 | (0.64–11.34) | 0.18 |
aLow and high expression levels are defined as below and above the median level of the marker expression for the defined region
Figure 2.
Kaplan–Meier survival plots of prostate cancer BCR with respect to levels of CD204/CD68 Macrophage density in prostate cancer and benign biopsy (A) and the combined CD204/CD68 macrophage density and GDF15 expression in prostate TAB (B).
Discussion
Prostate tumors are rich in TAMs, which can be either the cytotoxic M1 or protumorigenic M2 phenotype. During the course of prostate carcinogenesis, M2 TAMs contribute to the immunosuppressive microenvironment that promotes tumor growth and metastasis (28). Among the various cytokines that may play a role in prostate carcinogenesis, one of the most intriguing is GDF15 that appears to have pleiotropic functions in the early and late stages of carcinogenesis (29). Recent evidence suggests that macrophages that acquire M2-like characteristics may upregulate GDF15 expression in human cancer (25). In a study of men with prostate cancer treated with surgery and then followed for BCR, we measured the co-expression of M1/M2 macrophages and GDF15 in paired prostate benign biopsy and tumor surgical specimens. Both M2 macrophage levels and GDF15 expression were increased in prostate tumor specimens compared with benign prostate biopsy; however, GDF15 expression showed a pattern where the lowest mean levels were observed in the prostate TAB and intermediate GDF15 expression levels were observed in benign prostate biopsy. M2 macrophages were less prevalent in benign prostate biopsy compared with prostate TAB. These differences in M2 macrophages and GDF15 expression in the benign biopsy and malignant prostate were not uniform and, upon close examination of the expression changes and co-expression of these two markers, we found associations with risk of prostate cancer BCR after surgery.
In our study population, the risk of developing biochemically recurrent disease in men with prostate cancer was associated with the difference in expression levels of GDF15 between the tumor and normal-appearing regions adjacent to the prostate tumor. Specifically, GDF15 expression was generally found to be higher in prostate tumor compared with normal adjacent regions but, when GDF15 expression levels in the two regions were reversed, the risk of prostate cancer BCR was increased. Our results are similar to those of Karan et al. (30), who showed higher staining for GDF15 in tumor regions compared with normal benign regions in 15 prostate adenocarcinomas. These authors did not report any clinical or demographic information on their samples, nor was it clear whether the benign regions of the prostate assayed were directly adjacent to the prostate tumor. A similar study of 28 prostate cancer cases also found GDF15 expression to be higher in prostate tumor versus benign regions; however, the differential expression of GDF15 diminished in cases with higher Gleason grade (31). More recently, Jones et al. (32) in a slightly smaller sample of 16 prostate cancer cases showed elevated MIC-1 (GDF15) expression in both prostate tumors and structurally intact adjacent tissues—the latter region appearing to have nominally higher expression levels. Interestingly, another recent study of prostate intra-tumor heterogeneity found that GDF15 expression was elevated but with a high degree of variation, suggesting complex interactions between tumor cells and the tumor microenvironment via GDF15 (33).
Our results suggest that GDF15 expression as a biomarker for prostate cancer BCR should be analyzed relative to its expression in the prostate tumor and adjacent benign regions. In a large prospective cohort study of men with a pathologically verified diagnosis of prostate cancer, the authors found that elevated levels of GDF15 in serum taken at the time of diagnosis was predictive of prostate cancer mortality (34). Nakamura et al. (35) extensively analyzed the tumors of 66 men who underwent prostatectomy and found upregulation of MIC-1 in prostate cancer and in advanced and more aggressive prostatic tumors based on Gleason grade, suggesting that the MIC-1 protein should be evaluated as a potential diagnostic and prognostic biomarker. In the largest study to date of GDF15 expression in over 600 prostate tumors, we found that GDF15 expression was elevated in prostate cancer compared to benign, but only in AA men did prostate tumors show altered expression of GDF15 according to pathologic stage or grade (36). To our knowledge, the expression of GDF15 in prostate as a marker for prostate cancer outcomes has not been investigated previously, and our finding that the difference in GDF15 between the tumor and adjacent benign region is the strongest predictor of BCR is consistent with the suspected diverse role of GDF in prostate carcinogenesis and suggests that GDF15 can either suppress or promote cancer depending on whether it is expressed in tumor or the surrounding adjacent benign regions.
We have also shown that macrophages expressing the M2 marker CD204 are at an increased concentration in the tumor and adjacent benign regions of the prostate compared with an earlier benign prostate biopsy. Moreover, when this M2 marker is elevated in either tumor or adjacent benign regions of the prostate, the risk of prostate cancer BCR is also increased. Elevated levels of M2 macrophages has been shown to be a marker of worse prognosis for several cancers, including breast (37,38), bladder (39), colon (40), lung (41,42) and pancreas (43). In prostate cancer, Fujii et al. (8) found that the ratio of the M2 marker CD204 to the pan-macrophage marker CD68 increased steadily from benign prostate to high-grade prostatic intra-epitheleal neoplasia to prostate cancer, which is consistent with the differences we found between benign prostate biopsy and prostate tumors. In terms of prognosis, a recent study showed that men with high numbers of M2 macrophages in the prostate tumor environment, as measured by the M2 maker CD163, had increased odds of dying of prostate cancer (44). Another study of 93 prostate cancer surgical patients using CD163 found that the M2 phenotype was significantly associated with extracapsular tumor extension but not biochemcial recurrence (45). In our study, men in the highest tertile of CD204 positive macrophage density in either prostate tumor or the adjacent normal tissue had a 4-fold increased risk of prostate cancer BCR.
M2 phenotype macrophages appear to increase in a stepwise fashion from normal (i.e., no inflammation) prostate tissue, to primary untreated carcinomas, to hormone-naïve regional lymph node metastases, to metastatic castrate-resistant prostate cancer (46). Interestingly, Nonomura et al. (21) found that the count of M2 positive macrophages in benign biopsy was predictive of a later positive prostate cancer biopsy. In our study, M2 macrophage density in benign prostate biopsy was not predictive of prostate cancer BCR, but M2 macrophage density in benign prostate was predictive of prostate cancer outcome when taking into account the M2 macrophage density in prostate tumor. Specifically, a low M2 macrophage density in benign prostate biopsy and a high M2 macrophage density in prostate tumor were associated with an increased risk of prostate cancer BCR, but a high M2 macrophage density observed in benign prostate biopsy but not in prostate tumor was associated with a lower risk of prostate BCR. This demonstrates the dynamic state of the M2 phenotype in prostate and raises the possibility of devising treatments to manipulate this phenotype for better prostate cancer outcomes (47).
Our study provided the opportunity to evaluate the potential effect of GDF15 expression in relation to the density of M2 macrophages in prostate—a marker indicative of a more tumorigenic state. Examination of the expression profile of the M2 phase macrophages and GDF15 in prostate tumor regions suggested that GDF15 may moderate the effects of M2 macrophages on prostate cancer progression. We showed that men with the highest level of M2 macrophages in prostate tumor had almost a 5-fold increased risk of prostate cancer BCR. An increased risk of BCR was also observed for M2 macrophages in benign glands adjacent to tumor but, when GDF15 expression levels in these benign glands were also high, the increased risk of BCR-associated elevated M2 macrophages appeared to be dampened. This suggests an antagonistic effect of GDF15 on M2-driven carcinogenisis. In fact, GDF15 expression in benign glands adjacent to tumor was more strongly correlated with M2 macrophage levels in tumor and benign glands adjacent to tumor than it was with GDF15 expression in tumor. A possible explanation for this observation is that GDF15 is produced in response to M2 macrophages in benign prostate, but this response is diminished upon malignant transformation. In those instances when GDF15 is still abundant in tumor relative to the surrounding benign glands, the cancer progression appears to be slowed. A study in esophageal cancer suggests that M2 phenotype macrophages may upregulate GDF15 and that TAMs may express GDF15 (25). In prostate cancer, another cytokine, pigment epithelium-derived factor, has been shown to act directly on monocytes/macrophages by inducing their migration and differentiation into M1-type cells (48). Whether GDF15 is expressed by M2 macrophages and/or suppresses these tumorigenic macrophages by some type of feedback loop will require additional basic research. However, our results suggest that GDF15 may have some therapeutic potential in M2-driven prostate cancers.
Our study has several unique features that allowed us to investigate the dynamics of inflammatory environment in prostate carcinogenesis and also make inferences about how the change in prostate histology may affect prostate cancer outcomes. In analyzing markers of inflammation in paired prostate benign biopsies and subsequent prostate tumor and adjacent benign tissue, we had observation windows of both the pre- and post-malignant prostate and could investigate how changing levels of M2 macrophage and GDF15 might influence subsequent cancer progression. Because our study was observational, both the timing of the benign prostate biopsy and the follow-up after prostate cancer surgery were driven by medical cause and, therefore, not systematic. However, most of our study population had robust follow-up and benign biopsies had to occur at least 1 year before the surgery date, which should have excluded biopsies that were more likely to be tissue samples from normal tissue adjacent to a tumor missed on biopsy. Biopsies of all potential study cases were reviewed by one or more pathologists and those with any evidence of malignancy were excluded from the study. Our study is also limited by the amount of tissue specimen available for immunohistochemical analysis, especially with regard to biopsies, which precluded having a complete picture of the inflammatory environment in prostate. However, all tissue specimens were systematically selected and, while the number of analyzable biopsy cores was variable, we found no association of marker expression with the number of cores analyzed. Our study sample size was modest and, therefore, generally precluded making precise risk estimates. Most previous studies of GDF15 expression in prostate have had even smaller sample sizes (30,32,35), and our study is the first to examine the association of prostate GDF15 expression in relation to prostate cancer BCR, as well as the joint effects of GDF15 expression and M2 macrophage density. Using a computer-generated algorithm for measuring marker expression provided an unbiased quantitative expression score. In addition, our racially heterogenous sample increased the generalizability of our findings.
In summary, we have shown that, in men with prostate cancer and with a previous benign biopsy, an emerging M2 macrophage environment increases the risk for biochemically recurrent disease. Furthermore, GDF15 may have modifying effects on the carcinogenic potential of the M2 macrophage phenotype, particularly when it is expressed to a greater degree in normal-appearing glands adjacent to tumor compared with the tumor region. As M2 macrophage markers are developed into clinical tests for risk prediction of aggressive cancers, incorporation of GDF15 expression can potentially help such risk prediction tools. GDF15 should also be further investigated for its therapeutic potential in M2-driven prostate cancer. Further evaluation of these dynamics in the prostate immune cellular profile in the pre- and post-malignant prostate may offer insight into inflammatory-mediated prostate carcinogenesis.
Supplementary Material
Acknowledgements
The authors would like to acknowledge HFCI histology core members LaToya Jackson and Sarah Beste for their assistance with histology work and IHC stain optimizations and Scott Maresh and Aly Gonzalez for their critical input in study data collection.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- AA
African American
- BCR
biochemical recurrence
- CI
confidence interval
- GDF15
growth differentiation factor 15
- HR
hazard ratio
- IHC
immunohistochemistry
- MIC-1
macrophage inhibitory cytokine 1
- MSR1
macrophage-scavenger receptor 1
- PSA
prostate-specific antigen
- TAB
tumor-adjacent benign
- TAM
tumor-associated macrophage
- TGF-β
transforming growth factor-β
Funding
This study was supported by the National Institutes of Health (5R01-ES011126) and Henry Ford Health System internal research funds.
References
- 1. Grivennikov S.I., et al. (2010) Immunity, inflammation, and cancer. Cell, 140, 883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jiang J., et al. (2013) The role of prostatitis in prostate cancer: meta-analysis. PLoS One, 8, e85179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dennis L.K., et al. (2002) Epidemiologic association between prostatitis and prostate cancer. Urology, 60, 78–83. [DOI] [PubMed] [Google Scholar]
- 4. Karakiewicz P.I., et al. (2007) Chronic inflammation is negatively associated with prostate cancer and high-grade prostatic intraepithelial neoplasia on needle biopsy. Int. J. Clin. Pract., 61, 425–430. [DOI] [PubMed] [Google Scholar]
- 5. Moreira D.M., et al. (2014) Baseline prostate inflammation is associated with a reduced risk of prostate cancer in men undergoing repeat prostate biopsy: results from the REDUCE study. Cancer, 120, 190–196. [DOI] [PubMed] [Google Scholar]
- 6. Kryvenko O.N., et al. (2012) Inflammation and preneoplastic lesions in benign prostate as risk factors for prostate cancer. Mod. Pathol., 25, 1023–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gandaglia G., et al. (2017) The role of prostatic inflammation in the development and progression of benign and malignant diseases. Curr. Opin. Urol., 27, 99–106. [DOI] [PubMed] [Google Scholar]
- 8. Fujii T., et al. (2013) Immunohistochemical analysis of inflammatory cells in benign and precancerous lesions and carcinoma of the prostate. Pathobiology, 80, 119–126. [DOI] [PubMed] [Google Scholar]
- 9. Mosser D.M., et al. (2008) Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol., 8, 958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Martinez F.O., et al. (2006) Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J. Immunol., 177, 7303–7311. [DOI] [PubMed] [Google Scholar]
- 11. Taddei M.L., et al. (2014) Senescent stroma promotes prostate cancer progression: the role of miR-210. Mol. Oncol., 8, 1729–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Platt N., et al. (2001) Is the class A macrophage scavenger receptor (SR-A) multifunctional? The mouse’s tale. J. Clin. Invest., 108, 649–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mantovani A., et al. (2002) Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol., 23, 549–555. [DOI] [PubMed] [Google Scholar]
- 14. Heusinkveld M., et al. (2011) Identification and manipulation of tumor associated macrophages in human cancers. J. Transl. Med., 9, 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Komohara Y., et al. (2008) Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. J. Pathol., 216, 15–24. [DOI] [PubMed] [Google Scholar]
- 16. Ohtaki Y., et al. (2010) Stromal macrophage expressing CD204 is associated with tumor aggressiveness in lung adenocarcinoma. J. Thorac. Oncol., 5, 1507–1515. [DOI] [PubMed] [Google Scholar]
- 17. Kawamura K., et al. (2009) Detection of M2 macrophages and colony-stimulating factor 1 expression in serous and mucinous ovarian epithelial tumors. Pathol. Int., 59, 300–305. [DOI] [PubMed] [Google Scholar]
- 18. Komohara Y., et al. (2011) Macrophage infiltration and its prognostic relevance in clear cell renal cell carcinoma. Cancer Sci., 102, 1424–1431. [DOI] [PubMed] [Google Scholar]
- 19. Kurahara H., et al. (2011) Significance of M2-polarized tumor-associated macrophage in pancreatic cancer. J. Surg. Res., 167, e211–e219. [DOI] [PubMed] [Google Scholar]
- 20. Lin C.N., et al. (2015) The significance of the co-existence of osteopontin and tumor-associated macrophages in gastric cancer progression. BMC Cancer, 15, 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nonomura N., et al. (2010) Decreased infiltration of macrophage scavenger receptor-positive cells in initial negative biopsy specimens is correlated with positive repeat biopsies of the prostate. Cancer Sci, 101, 1570–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Takayama H., et al. (2009) Decreased immunostaining for macrophage scavenger receptor is associated with poor prognosis of prostate cancer. BJU Int., 103, 470–474. [DOI] [PubMed] [Google Scholar]
- 23. Wang X., et al. (2013) The diverse roles of nonsteroidal anti-inflammatory drug activated gene (NAG-1/GDF15) in cancer. Biochem. Pharmacol., 85, 597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lambert J.R., et al. (2015) Reduced expression of GDF-15 is associated with atrophic inflammatory lesions of the prostate. Prostate, 75, 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Urakawa N., et al. (2015) GDF15 derived from both tumor-associated macrophages and esophageal squamous cell carcinomas contributes to tumor progression via Akt and Erk pathways. Lab. Invest., 95, 491–503. [DOI] [PubMed] [Google Scholar]
- 26. Nickel J.C., et al. (2001) Consensus development of a histopathological classification system for chronic prostatic inflammation. BJU Int., 87, 797–805. [DOI] [PubMed] [Google Scholar]
- 27. Okada K., et al. (2000) Correlation of histological inflammation in needle biopsy specimens with serum prostate- specific antigen levels in men with negative biopsy for prostate cancer. Urology, 55, 892–898. [DOI] [PubMed] [Google Scholar]
- 28. Hayashi T., et al. (2019) Main Inflammatory cells and potentials of anti-inflammatory agents in prostate cancer. Cancers (Basel), 11, 1153–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vaňhara P., et al. (2012) Growth/differentiation factor-15: prostate cancer suppressor or promoter? Prostate Cancer Prostatic Dis., 15, 320–328. [DOI] [PubMed] [Google Scholar]
- 30. Karan D., et al. (2003) Dysregulated expression of MIC-1/PDF in human prostate tumor cells. Biochem. Biophys. Res. Commun., 305, 598–604. [DOI] [PubMed] [Google Scholar]
- 31. Patrikainen L., et al. (2007) Expression profiling of PC-3 cell line variants and comparison of MIC-1 transcript levels in benign and malignant prostate. Eur. J. Clin. Invest., 37, 126–133. [DOI] [PubMed] [Google Scholar]
- 32. Jones A.C., et al. (2015) Prostate field cancerization: deregulated expression of macrophage inhibitory cytokine 1 (MIC-1) and platelet derived growth factor A (PDGF-A) in tumor adjacent tissue. PLoS One, 10, e0119314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guo T., et al. (2018) Multi-region proteome analysis quantifies spatial heterogeneity of prostate tissue biomarkers. Life Sci. Alliance, 1, e201800042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brown D.A., et al. (2009) Macrophage inhibitory cytokine 1: a new prognostic marker in prostate cancer. Clin. Cancer Res., 15, 6658–6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nakamura T., et al. (2003) Quantitative analysis of macrophage inhibitory cytokine-1 (MIC-1) gene expression in human prostatic tissues. Br. J. Cancer, 88, 1101–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Iczkowski K.A., et al. (2020) Racial disparities in expression of GDF15 and NFκB in prostate cancer and benign prostatic epithelium. Cancer Health Dispar, in press. [Google Scholar]
- 37. Miyasato Y., et al. (2017) High density of CD204-positive macrophages predicts worse clinical prognosis in patients with breast cancer. Cancer Sci., 108, 1693–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tiainen S., et al. (2015) High numbers of macrophages, especially M2-like (CD163-positive), correlate with hyaluronan accumulation and poor outcome in breast cancer. Histopathology, 66, 873–883. [DOI] [PubMed] [Google Scholar]
- 39. Lima L., et al. (2014) The predominance of M2-polarized macrophages in the stroma of low-hypoxic bladder tumors is associated with BCG immunotherapy failure. Urol. Oncol., 32, 449–457. [DOI] [PubMed] [Google Scholar]
- 40. Herrera M., et al. (2013) Cancer-associated fibroblast and M2 macrophage markers together predict outcome in colorectal cancer patients. Cancer Sci., 104, 437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jackute J., et al. (2018) Distribution of M1 and M2 macrophages in tumor islets and stroma in relation to prognosis of non-small cell lung cancer. BMC Immunol., 19, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sumitomo R., et al. (2019) M2 tumor-associated macrophages promote tumor progression in non-small-cell lung cancer. Exp. Ther. Med., 18, 4490–4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hu H., et al. (2016) The M2 phenotype of tumor-associated macrophages in the stroma confers a poor prognosis in pancreatic cancer. Tumour Biol., 37, 8657–8664. [DOI] [PubMed] [Google Scholar]
- 44. Erlandsson A., et al. (2019) M2 macrophages and regulatory T cells in lethal prostate cancer. Prostate, 79, 363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lanciotti M., et al. (2014) The role of M1 and M2 macrophages in prostate cancer in relation to extracapsular tumor extension and biochemical recurrence after radical prostatectomy. Biomed Res. Int., 2014, 486798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zarif J.C., et al. (2019) Mannose receptor-positive macrophage infiltration correlates with prostate cancer onset and metastatic castration-resistant disease. Eur. Urol. Oncol., 2, 429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Seif F., et al. (2019) A review of preclinical experiments toward targeting M2 macrophages in prostate cancer. Curr. Drug Targets, 20, 789–798. [DOI] [PubMed] [Google Scholar]
- 48. Nelius T., et al. (2013) Positive correlation between PEDF expression levels and macrophage density in the human prostate. Prostate, 73, 549–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.