Abstract
STUDY QUESTION
Are intrauterine insemination (IUI) performance characteristics and post-processing total motile sperm count (TMC) related to live birth rate in couples with unexplained infertility?
SUMMARY ANSWER
Patient discomfort with IUI and lower inseminate TMC were associated with a reduced live birth rate, while time from hCG injection to IUI, sperm preparation method and ultrasound guidance for IUI were not associated with live birth success.
WHAT IS ALREADY KNOWN
We previously determined that some baseline characteristics of couples with unexplained infertility, including female age, duration of infertility, history of prior loss and income, were related to live birth rate across a course of ovarian stimulation and IUI treatment. However, the relationship between treatment outcomes and per-cycle characteristics, including ultrasound guidance for IUI, timing of IUI relative to hCG injection, difficult or painful IUI and inseminate TMC, are controversial, and most prior investigations have not evaluated live birth outcome.
STUDY DESIGN, SIZE, DURATION
This was a secondary analyses of 2462 cycles from the Assessment of Multiple Intrauterine Gestations from Ovarian Stimulation (AMIGOS) clinical trial. This prospective, randomised, multicentre clinical trial determined live birth rates following IUI after ovarian stimulation with clomiphene citrate, letrozole or gonadotropins in 854 couples with unexplained infertility. It was conducted between 2011 and 2014, and couples could undergo up to four consecutive treatment cycles.
PARTICIPANTS/MATERIALS, SETTING, METHODS
AMIGOS was an NIH-sponsored Reproductive Medicine Network trial conducted at 12 clinical sites. Participants were women with unexplained infertility who were between 18 and 40 years of age. Cluster-weighted generalised estimating equations (GEE), which account for informative clustering of multiple IUI treatment cycles within the same patient, were used to determine associations between IUI performance characteristics, including inseminate TMC, and live birth rate. Efficiency curves were also generated to examine the relationship between inseminate TMC and live birth rate.
MAIN RESULTS AND THE ROLE OF CHANCE
After adjustment for treatment group and baseline factors previously associated with live birth across a course of OS-IUI treatment, patient discomfort during the IUI procedure was associated with a reduction in live birth rate (aRR 0.40 (0.16–0.96)). Time from hCG trigger injection to IUI was not significantly associated with outcome. Higher TMC was associated with greater live birth rate (TMC 15.1–20.0 million (14.8%) compared to ≤5 million (5.5%)) (aRR 2.09 (1.31–3.33)). However, live births did occur with TMC ≤ 1 million (5.1%).
LIMITATIONS, REASONS FOR CAUTION
This investigation is a secondary analysis, and AMIGOS was not designed to address the present question. Since timed intercourse was allowed as part of the AMIGOS trial, we cannot rule out the possibility that any given pregnancy resulted from intercourse rather than IUI.
WIDER IMPLICATIONS OF THE FINDINGS
Most factors associated with the performance of IUI were not significantly related to obtaining live birth. Our findings suggest that higher TMC inseminated leads to an increase in live birth rate up to TMC ~20 million. However, there may be no reasonable threshold below which live birth is not possible with IUI.
STUDY FUNDING/COMPETING INTEREST(S)
Funding was received through grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD): U10 HD077680, U10 HD39005, U10 HD38992, U10 HD27049, U10 HD38998, U10 HD055942, HD055944, U10 HD055936 and U10 HD055925. This research was made possible by funding by the American Recovery and Reinvestment Act. Dr Hansen reports grants from NIH/NICHD and Yale University during the conduct of the study, grants from Roche Diagnostics and grants from Ferring International Pharmascience Center US outside the submitted work. Dr Peck reports support from Ferring Pharmaceuticals outside the submitted work. Dr Coward has nothing to disclose. Dr Wild reports grants from NICHD during the conduct of the study. Dr Trussell has nothing to disclose. Dr Krawetz reports grants from NICHD during the conduct of the study, grants from Merck and support from Taylor and Frances and from Springer, outside the submitted work. Dr Diamond reports grants from NIH/NICHD, Yale University, during the conduct of the study and support from Advanced Reproductive Care AbbVie, Bayer and ObsEva, outside the submitted work. Dr Legro reports support from Bayer, Kindex, Odega, Millendo and AbbVie and grants and support from Ferring, outside the submitted work. Dr Coutifaris reports grants from NICHD/NIH and personal fees from American Society for Reproductive Medicine, outside the submitted work. Dr Alvero has nothing to disclose. Dr Robinson reports grants from NIH during the conduct of the study. Dr Casson has nothing to disclose. Dr Christman reports grants from NICHD during the conduct of the study. Dr Santoro reports grants from NIH during the conduct of the study. Dr Zhang reports grants from NIH during the conduct of the study and support from Shangdong University outside the submitted work.
TRIAL REGISTRATION NUMBER
n/a
Keywords: unexplained infertility, intrauterine insemination, sperm total motile count, live birth, ultrasound guidance
Introduction
Ovarian stimulation with intrauterine insemination (IUI) is a commonly utilised treatment in couples with unexplained infertility (Guzick 1998; Guzick et al., 1999; Berker et al., 2011). Factors reported to be associated with treatment outcomes include the age of the female partner (Brzechffa et al., 1998; Sahakyan et al., 1999), the response to ovarian stimulation (Ghesquiere et al., 2007; Park et al., 2007) and the post-processing total motile sperm count (TMC) for IUI (Guzick et al., 1999; Miller et al., 2002; Lemmens et al., 2016; Thijssen et al., 2017). Although previous investigations have suggested thresholds for the TMC below which pregnancy and live-birth rates are decreased following treatment, this threshold varies significantly between studies (Brasch et al., 1994; Campana et al., 1996; Van Voorhis et al., 2001; Miller et al., 2002). Other factors that may be related to treatment outcomes, including performance characteristics of the IUI itself, have been poorly investigated. Ultrasound guided embryo transfers (Buckett, 2003), easier transfers (Schoolcraft et al., 2001) and the lack of bleeding during transfers (Alvero et al., 2003) have all been associated with improved pregnancy rates in IVF. However, the relationship between these performance characteristics and IUI outcomes is uncertain. Similarly, the relationships between semen preparation methods and IUI outcomes are poorly characterised. Although a meta-analysis concluded that there was no significant difference in pregnancy rates following IUI with different semen preparation techniques (Boomsma et al., 2007), other investigations have suggested that sperm quality, with respect to DNA integrity, may be superior with certain preparation techniques (Jackson et al., 2010; Zhang et al., 2011). However, none of these investigations have reported the outcome of live birth.
We previously reported the relationship between couple baseline characteristics and treatment outcomes following 4 cycles of OS-IUI in the Assessment of Multiple Intrauterine Gestations from Ovarian Stimulation (AMIGOS) multicenter clinical trial (Diamond et al., 2015a, Hansen et al., 2016). Important predictors of live birth included female age, duration of infertility, income level and history of prior pregnancy loss (Hansen et al., 2016). Other factors, including female and male BMI, race and ethnicity, smoking status, alcohol use and baseline serum concentration of AMH, were not related to treatment outcome. The purpose of the present study was to determine predictors of live birth on a per cycle basis. Specifically, we were interested in whether the quality and the preparation of the inseminate and the IUI performance characteristics were related to live birth outcome in women with unexplained infertility undergoing IUI after ovarian stimulation. We hypothesised that factors associated with a difficult IUI (e.g. bleeding and multiple attempts to perform the IUI) would be associated with lower pregnancy rates than those observed with an unremarkable IUI. Furthermore, we hypothesised that live-birth rates would be higher with ultrasound guided IUI and with a greater TMC, compared with no ultrasound guidance and a lower TMC.
Materials and Methods
Study design
This is a secondary analysis of 2695 OS-IUI cycles from 900 participants in the AMIGOS clinical trial (Diamond et al., 2015a). The trial design, analysis plan, baseline characteristics of the participating couples, inclusion and exclusion criteria and methods, as well as the trial outcomes, have previously been published (Diamond et al., 2015a; Diamond et al., 2015b). Briefly, the AMIGOS trial was a prospective, multicentre randomised clinical trial which evaluated the outcomes of conception, clinical pregnancy, live birth and multiple gestations associated with ovarian stimulation and IUI in couples with unexplained infertility. The trial was conducted at 12 clinical locations in the United States (clinicaltrials.gov NCT01044862), with registration on 8 January 2010 and first enrolment on 2 July 2010. Treatment arms included clomiphene citrate (300 couples), letrozole (299 couples) and gonadotropin (Menopur®, [hMG] Ferring Pharmaceuticals; 301 couples). Couples underwent IUI treatment in the assigned arm until up to 4 cycles were completed or pregnancy occurred. Participating women were ≥18 to ≤40 years with regular menses and had a normal uterine cavity with at least one patent fallopian tube, no evidence of advanced endometriosis and a male partner with a semen specimen with at least 5 million TMC in the ejaculate. Institutional Review Board approval was obtained at each study site, and all participants provided written informed consent. The investigation was monitored by a Data and Safety Monitoring Board. The criteria for human chorionic gonadotropin (hCG) administration and cycle cancelation in the IUI cycles have previously been reported (Diamond et al., 2015a; Hansen et al., 2016). IUI was performed 0–44 h after administration of hCG. Live birth was defined as the delivery of a viable infant. Catheter type, sperm preparation method and the use of ultrasound guidance for IUI were not specified in the protocol, but accommodated to reflect local practices at the 12 participating sites.
Data analyses
Data from the AMIGOS trial was obtained from the Data Coordinating Center (DCC) Collaborative Center for Statistics in Science at Yale University in New Haven, Connecticut. The DCC was responsible for data management in AMIGOS (including data entered at each participating centre). A total of 2695 cycles were recorded in the AMIGOS trial. Following the exclusion of cancelled cycles, duplicate entries and records with data entry errors or missing values for covariate data, data from 2462 cycles for 854 women were available for analysis: 793 (32.2%) first cycles, 654 (26.6%) second cycles, 529 (21.5%) third cycles, 436 (17.7%) and 50 (2.0%) fifth cycles among some patients who had a prior cycle cancelled. Due to the higher number of missing values for one of the characteristics of interest, time from hCG to insemination (n = 123), these cycles were retained for analyses evaluating other IUI performance and sperm characteristics. Baseline characteristics for the 854 women were compared by live birth outcome with Wilcoxon rank sum test and chi-square test as appropriate.
The outcome of interest was live birth, defined as delivery of a viable infant. IUI characteristics considered in these analyses included the use of ultrasound guidance, type of catheter (firm or soft), clinician’s report of IUI difficulty, requirement for multiple insemination attempts, bleeding with IUI, patient-reported discomfort with IUI, type of sperm preparation (density gradient, wash or other), hours from hCG trigger injection to IUI and post-preparation TMC in millions.
The distribution of live births was compared by categories of IUI characteristics, TMC and time from hCG to IUI. Beginning with counts <5 million, TMC was grouped into increasing categories of 5 million (≤5.0, 5.1–10.0, 10.1–15.0, 15.1–20.0, ≥20.1). Time from hCG to IUI was categorised by using the 90th percentile of the distribution to identify the upper range (>39 h) and grouping the remaining values into four groups of equal time increments (<19, 19–25, 26–32, 33–39, >39 h), truncating the lowest category at <19 due to small numbers. An efficiency curve was also produced to display the percentage of live births (y-axis) among all cycles at or below increasing integer values for TMC (x-axis). Thus, cycles with TMC above the specified value were omitted from the calculated percentage of live births at each value. This approach examines low values at which live births do not occur and upper values at which birth rates do not continue to increase with accumulation to higher levels. For patients who had second, third or fourth cycles that resulted in a live birth and, thus, had a prior cycle available for comparison, we also evaluated the median difference in TMC values between the cycle resulting in a live birth and the prior cycle using the Wilcoxon signed rank test for paired data.
The data including multiple IUI cycles per patient were analysed using a generalised estimating equations (GEE) methodology to fit a Poisson regression model with robust standard errors to account for correlation of multiple IUI treatment cycles within the same patient. The models estimated risk ratios (RR) and 95% confidence intervals for associations between the IUI characteristics and live birth. Because the number of IUI cycles completed per patient is influenced by the outcome of prior cycles (i.e. informative clustering), a cluster-weighted model was fit to appropriately account for cycle number (Yland et al., 2019) by weighting the GEE score equation by the inverse of the number of IUI cycles completed for each couple (Williamson et al., 2003; Williamson et al., 2007). Based on characteristics previously identified to be associated with live birth in the AMIGOS study population (Hansen et al., 2016), adjusted models controlled for age (continuous), income (<$50 000, ≥$50 000, wish not to answer), duration of infertility (continuous), history of pregnancy loss (yes/no) and ovarian stimulation treatment group (clomiphene citrate, letrozole or gonadotropin). Adjustment for unilateral tubal patency, history of ectopic pregnancy and number of follicles 16 mm or greater that developed during the ovarian stimulation-IUI cycle were also examined, but these factors were not retained in the adjusted models due to unchanged results. Statistical analyses were performed using SAS 9.4 software (SAS Institute Inc., Cary, NC).
Results
Characteristics of women undergoing OS-IUI
Baseline characteristics and the primary outcome of the AMIGOS trial have been previously reported (Diamond et al., 2015a; Diamond et al., 2015b). Baseline characteristics of women contributing cycle-specific data to the present investigations are shown in Table I. Age, duration of infertility and income were significantly different between women achieving a live birth compared to those who did not (Table I). BMI, race, history of pregnancy loss and history of live birth were not significantly different between live birth and non-live birth groups (Table I).
Table I.
Baseline characteristics of 854 patients undergoing intrauterine insemination by live birth.
| Live birth (n = 213) | No live birth (n = 641) | ||
|---|---|---|---|
| Median (IQR) | Median (IQR) | P a | |
| Age (years) | 31.0 (5.0) | 32.0 (7.0) | 0.007 |
| Duration of infertility (months) | 24.0 (20.0) | 24.0 (30.0) | 0.0005 |
| BMI | 25.2 (9.0) | 25.0 (8.0) | 0.86 |
| n (%) | n (%) | P b | |
| Race | 0.39 | ||
| White | 182 (85.5) | 516 (80.5) | |
| Black | 12 (5.6) | 59 (9.2) | |
| Asian | 10 (4.7) | 42 (6.6) | |
| American Indian | 2 (0.9) | 7 (1.1) | |
| More than one race | 7 (3.3) | 17 (2.7) | |
| Income | 0.03 | ||
| <$50 000 | 22 (10.3) | 116 (18.1) | |
| ≥$50 000 | 151 (70.9) | 414 (64.6) | |
| Wish not to answer | 40 (18.8) | 111 (17.3) | |
| History of pregnancy loss | 0.46 | ||
| Yes | 49 (23.0) | 132 (20.6) | |
| No | 164 (77.0) | 509 (79.4) | |
| History of live birth | 0.61 | ||
| Yes | 45 (21.1) | 125 (19.5) | |
| No | 168 (78.9) | 516 (80.5) |
Abbreviations: IQR, interquartile range.
aWilcoxon rank sum test.bChi-square test for independence.
Characteristics of cycles by live birth outcome
Characteristics of the ovarian stimulation-IUI cycles stratified by live birth outcome are presented in Table II. For all cycle characteristics examined, the unadjusted RR for live birth outcomes were similar to adjusted associations when controlling for age, duration of infertility, income, history of pregnancy loss and treatment group. Live births were no more common in cycles with ultrasound guidance compared to cycles without guidance (aRR 1.02, 95% CI 0.73–1.43). IUIs performed with firm catheters had fewer live birth rates than those performed with soft catheters; however, the difference was not significant (aRR 0.77, 95% CI 0.54–1.11). IUI performance characteristics associated with a more difficult IUI, including ‘difficult IUI’, ‘multiple IUI attempts’, ‘bleeding with IUI’ and ‘discomfort with IUI’ had lower live birth rates than IUI cycles without these characteristics (Table II). However, only patient reported discomfort with the IUI procedure was associated with a significantly lower chance of live birth (aRR 0.40, 95% CI 0.16–0.96, Table II).
Table II.
IUI performance characteristics and their associations with live birth.
| Live birth | ||||
|---|---|---|---|---|
|
Yes
213 cycles n (%) |
No
2249 cycles n (%) |
Unadjusted RR
a
(95% CI) |
Adjusted RR
b
(95% CI) |
|
| Ultrasound guidance | ||||
| Yes | 54 (9.4) | 521 (90.6) | 1.01 (0.75–1.38) | 1.02 (0.73–1.43) |
| No | 159 (8.4) | 1728 (91.6) | ||
| Catheter type | ||||
| Firm | 33 (6.9) | 447 (93.1) | 0.78 (0.54–1.13) | 0.77 (0.54–1.11) |
| Soft | 180 (9.1) | 1802 (90.9) | ||
| Difficult IUI | ||||
| Yes | 10 (6.0) | 156 (94.0) | 0.67 (0.35–1.26) | 0.68 (0.36–1.29) |
| No | 203 (8.8) | 2093 (91.2) | ||
| Multiple IUI attempts | ||||
| Yes | 11 (5.4) | 194 (94.6) | 0.59 (0.32–1.11) | 0.63 (0.34–1.17) |
| No | 202 (9.0) | 2055 (91.1) | ||
| Bleeding with IUI | ||||
| Yes | 17 (7.9) | 199 (92.1) | 0.68 (0.41–1.13) | 0.73 (0.44–1.20) |
| No | 196 (8.7) | 2050 (91.3) | ||
| Discomfort with IUI | ||||
| Yes | 5 (6.3) | 74 (93.7) | 0.37 (0.15–0.88) | 0.40 (0.16–0.96) |
| No | 208 (8.7) | 2175 (91.3) | ||
| Sperm prep type | ||||
| Density gradient | 135 (9.2) | 1332 (90.8) | Ref | Ref |
| Wash | 71 (7.7) | 856 (92.3) | 0.92 (0.69–1.22) | 0.88 (0.67–1.16) |
| Other | 7 (10.3) | 61 (89.7) | 1.08 (0.53–2.23) | 1.19 (0.60–2.35) |
| Time from hCG to IUIc | ||||
| <19 h | 16 (10.7) | 133 (89.3) | 1.32 (0.66–2.62) | 1.34 (0.69–2.59) |
| 19 to 25 h | 21 (6.6) | 297 (93.4) | 0.77 (0.40–1.52) | 0.79 (0.41–1.53) |
| 26 to 32 h | 15 (6.4) | 219 (93.6) | 0.70 (0.33–1.46) | 0.73 (0.35–1.55) |
| 33 to 39 h | 137 (9.4) | 1325 (90.6) | 1.19 (0.70–2.05) | 1.21 (0.71–2.06) |
| ≥39 h | 14 (8.0) | 162 (92.1) | Ref | Ref |
| Post-prep total motile sperm count (millions) | ||||
| ≤5.0 | 26 (5.5) | 450 (94.5) | Ref | Ref |
| 5.1–10.0 | 35 (8.1) | 400 (92.0) | 1.28 (0.77–2.12) | 1.23 (0.75–2.03) |
| 10.1–15.0 | 31 (9.3) | 301 (90.7) | 1.26 (0.74–2.12) | 1.22 (0.73–2.04) |
| 15.1–20.0 | 32 (14.8) | 184 (85.2) | 2.28 (1.39–3.72) | 2.09 (1.31–3.33) |
| >20.0 | 89 (8.9) | 914 (91.1) | 1.46 (0.95–2.27) | 1.38 (0.90–2.11) |
aRisk ratios (RR) and 95% confidence intervals (CI) were calculated using cluster-weighted generalised estimating equations method to estimate modified Poisson regression models with robust standard errors to account for multiple IUI cycles in the same patient.
bAdjusted models control for age (continuous), income (<$50 000, ≥$50 000, wish not to answer), treatment group (clomiphene citrate, letrozole, gonadotropin), duration of infertility (continuous) and history of pregnancy loss (yes/no)
cTime from hCG to insemination is missing 10 cycles that resulted in a live birth and 113 cycles that did not result in a live birth
The IUI sperm preparation method was not associated with live birth rate, nor was time from hCG injection to IUI (Table II, Fig. 1). Time from hCG injection to IUI was also not associated with live birth rate when stratified by treatment group (data not shown). Post-processing TMC for IUI was associated with live birth rate, with a significant increase in live birth rate when comparing IUIs with TMC 15.1–20.0 million (14.8%) to TMC ≤ 5 million (5.5%) (aRR 2.09, 95% CI 1.31–3.33, Table II, Fig. 2a). However, live birth rates for IUIs with TMC 5.1 to 10 million (8.1%), 10.1 to 15 million (9.3%) or >20 million (8.9%) did not significantly differ from that with TMC ≤ 5 million. Live births did occur in IUI cycles with TMC ≤ 1 million (4/79, 5.1%), the rate of which was not significantly lower than the live birth rate in cycles with TMC >1–5 million (22/397, 5.5%, P = 0.49). Inseminate TMC for the 4 cycles resulting in live birth with TMC ≤ 1 million were 170 000, 324 000, 696 000 and 792 000. Efficiency curve analysis (Fig. 2b) demonstrated that the live birth rate increased in IUI cycles up to a TMC of ~20 million. Among the 112 patients who conceived in the second, third or fourth IUI cycles and had a prior cycle for comparison, the median change in TMC values between the cycle resulting in a live birth and the previous cycle was not significantly different to zero (P = 0.93).
Figure 1.
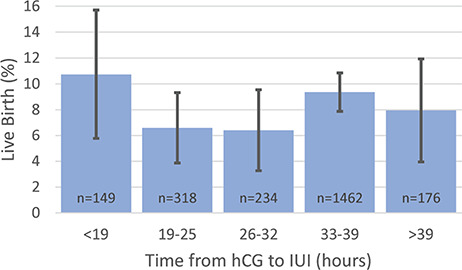
Percentage of live births and 95% confidence intervals by hours elapsed between hCG injection and intrauterine insemination.
Figure 2.
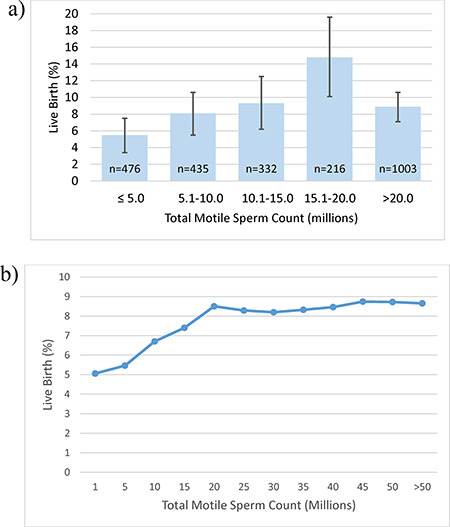
Live birth in relation to total motile sperm count. (a) Percentage of live births and 95% confidence intervals by post-processing total motile sperm count. (b) Efficiency curve for live birth by total motile sperm count. The curve displays the live birth rate on the y-axis at or below the integer value for post-processing total motile sperm count on the x-axis. Thus, cycles with total motile sperm counts above the given value are omitted from the calculated percentage of live births at each value.
Discussion
Common treatment strategies for couples with unexplained infertility include a course (typically 3–4 cycles) of ovarian stimulation-IUI, followed by IVF for those couples unsuccessful with IUI. Since many couples undergo IUI prior to more costly and complex ART treatments, an understanding of the factors associated with treatment outcomes is important in counselling patients and making treatment recommendations. The AMIGOS trial affords an important opportunity to identify predictors of success given its large size, multicentre design and thorough characterisation of participants and treatment cycles. We have previously reported predictors of treatment outcomes in AMIGOS following a course of ovarian stimulation-IUI treatment (Hansen et al., 2016). In the present study, we explore predictors of live birth in the AMIGOS trial on a per cycle basis, with a focus on performance of the IUI and post-processing sperm TMC.
After adjustment for treatment group, female age, duration of infertility, income, history of pregnancy loss and cycle number, we determined that most IUI performance characteristics were not significantly related to the outcome of live birth, including ultrasound guidance, time from hCG injection to IUI, sperm preparation type and IUI catheter type. Most characteristics associated with a difficult IUI procedure, such as bleeding during the procedure and/or physician-reported difficulty with IUI, were associated with lower point estimates of live birth compared to unremarkable IUI procedures; however, these differences were not significant. Only patient-reported discomfort with the IUI procedure was associated with a statistically significant and clinically meaningful decrease in the live-birth rate. The TMC available for IUI was significantly associated with the live birth rate, with an increase from a TMC of ~5 million to ~20 million. Above a TMC of 20 million, the live birth rate per IUI did not increase. Importantly, live births still occurred following IUI procedures when the TMC was ≤1 million, with the percentage of live births in this group (5.1%) similar to IUI cycles with TMC >1–5 million (5.5%), although confidence intervals were wide in the ≤1 million IUI group due to small sample size.
Our findings of no association between ultrasound guidance and live birth rate in IUI treatments is consistent with two small RCTs (Ramon et al., 2009; Polat et al., 2015) and contrasts with a third RCT (Oruc et al., 2014) and with a small retrospective study (Oztekin et al., 2013), although the present study is the first to report the outcome of live birth. In the only prospective study to suggest an improvement in the pregnancy rate associated with ultrasound guidance (Oruc et al., 2014), provider experience was associated with the pregnancy rate rather than ultrasound guidance per se. Our findings are in contrast to the observed relationship between ultrasound guided embryo transfers and improved outcomes in IVF cycles (Buckett, 2003). It seems highly likely that an IUI with (in most cases) millions of sperm inseminated in a substantially larger fluid volume would be much less position-dependent than the transfer of one or two embryos into the uterine cavity.
Prior RCTs have investigated the timing of IUI relative to the hCG trigger and, consistent with our findings, showed no significant difference in the pregnancy rate between 0 and 34–36 h (Aydin et al., 2013), 32–34 and 38–40 h (Claman et al., 2004) or in the live birth rate between 24 and 36 h after hCG (Rahman et al., 2011). A recent structured review determined that there is moderate evidence that IUI can be performed between 24 and 40 h post hCG injection without compromising pregnancy rates (Cohlen et al., 2018). In contrast to the pre-specified intervals in the above RCTs, the AMIGOS protocol gave investigators the flexibility to perform IUIs at any point between 0 and 44 h after the hCG injection in order to accommodate clinic schedules at the 12 sites conducting the study. As a result, we were able to assess live birth rates following IUI across a wide time interval after the hCG injection. While AMIGOS was not designed to evaluate this relationship specifically, our findings, together with those from the above RCTs, provide reassurance that successful outcomes of treatment are likely unrelated to the timing of IUI following hCG injection.
Prior investigations of the relationship between catheter type and IVF outcomes have demonstrated an improved live birth rate with the use of soft catheters (Schoolcraft et al., 2001; ASRM Practice Committee 2017). However, the relationship between catheter type and IUI outcomes is uncertain. While we observed fewer live births in IUI cycles conducted with a firm as opposed to soft catheter, the findings were not statistically significant. Several RCTs have investigated the relationship between soft versus firm catheters and success with IUI treatment, although few have included the outcome of live birth (reviewed in van der Poel et al., 2010). A systematic review of these trials suggested no improvement in live birth outcome in IUI cycles conducted with soft compared to firm catheters (van der Poel et al., 2010). However, the quality of the evidence was considered low. Unlike the RCTs comparing two different types of catheters, the AMIGOS protocol was pragmatic and allowed investigators to use the IUI catheter of their choice. As a result, multiple different types of catheters were used in AMIGOS, making direct comparisons with other RCTs problematic. Additionally, because selection of catheter type was not random, it is possible that firm catheters were utilised in the setting of a difficult IUI, which in our study was associated with a lower (although not significantly lower) probability of live birth. Thus, lower point estimates of live birth with firm catheters may be a reflection of a difficult IUI rather than the use of a firm catheter, per se.
To our knowledge, our finding of a negative impact of patient-reported discomfort with IUI on live birth rate is novel. Although all of the parameters associated with a more traumatic IUI, including a difficult IUI, multiple IUI attempts and bleeding with IUI, were associated with a lower point estimate of success, only patient discomfort was statistically significant. However, it is important to interpret this finding with caution. Only 79 (of 2462) IUI procedures were associated with discomfort, and we cannot rule out the possibility that this association is a consequence of multiple comparisons. While other investigations have assessed IUI-related factors such as discomfort during the procedure, discomfort was associated with the catheter type rather than pregnancy outcomes (reviewed in van der Poel et al., 2010). The overall low live birth rate in IUI cycles in general limits the ability to detect significant differences even in a large, multicentre clinical trial like AMIGOS. Additional studies are needed to further characterise the relationship between difficult IUI procedures and treatment outcomes.
Prior investigations have suggested that sperm preparation methods such as density gradient with ‘swim-up’ (Jackson et al., 2010) or density gradient alone are associated with lower rates of sperm DNA fragmentation compared to other methods such as ‘simple wash’ techniques (Zhang et al., 2011). Additionally, a lower rate of sperm DNA fragmentation has been associated with improved clinical pregnancy rate with IUI procedures (Duran et al., 2002; Castilla et al., 2010). However, a systematic review of RCTs, none of which reported live birth rate, found no evidence of a difference between pregnancy rates for swim-up, density gradient or simple wash techniques in IUI treatment cycles (Boomsma et al., 2007). Consistent with this systematic review, we also found no relationship between the sperm preparation method and live birth outcome in AMIGOS. Additional RCTs are needed to address the role of sperm preparation methods in specific populations such as those with high DNA fragmentation index and HPV-positive status.
Multiple prior studies have addressed the relationship between post-processing sperm TMC and treatment outcomes (Campana et al., 1996; Guzick et al., 1999; Miller et al., 2002; Merviel et al., 2010; Dinelli et al., 2014; Lemmens et al., 2016; Thijssen et al., 2017), with lower-limit thresholds for offering or recommending IUI treatments ranging from 1 to 5 million TMC. In the majority of these studies, the live birth rate success was not reported. While we observed the highest live birth rates with inseminate TMC between 15.1 and 20 million, other investigations have suggested peak pregnancy rates with TMC between 5 and 10 million (Lemmens et al., 2016; Thijssen et al., 2017). These observed differences are likely related to different patient populations, treatment protocols and inclusion criteria between investigations. Interestingly, we found that live birth rates with TMC > 20 million were not similarly elevated when compared to inseminates with ≤5 million. It should be noted, however, that estimates for 15.1–20 million were less precise due to the smaller number of cycles in this TMC category. Other investigations have reported a similar curvilinear relationship between inseminate TMC and outcomes (Lemmens et al., 2016; Thijssen et al., 2017). While speculative, it is possible that couples with inseminates with high TMC represent a subgroup of patients with unexplained infertility who are less amenable to IUI treatment.
Some studies have suggested incorporating strict morphology criteria into the decision to recommend for or against offering ovarian stimulation-IUI treatment, and in some cases recommending against IUI treatment with a post-processing TMC of <1 million combined with strict morphology <4% (reviewed in Ombelet et al., 2014). In AMIGOS, we could not evaluate strict morphology on a per cycle basis, as morphology was only obtained at the baseline screening semen analysis. Nevertheless, there is increasing debate as to the utility of strict morphology in recommending for IUI versus IVF. A recent meta-analysis has suggested no relationship between pregnancy outcomes and low versus normal morphology, even when the threshold for normal morphology was reduced to 1% (Kohn et al., 2018).
In AMIGOS, we found significant differences in live birth rate when comparing IUI outcomes between cycles with a TMC <5 million to those cycles with a TMC between 15.1 and 20.0 million. However, we also found the live birth rate in IUI cycles with a TMC ≤ 1 million (4 live births among 79 cycles (5.1%)) to be similar to those cycles with TMC >1–5 million (22 live births among 397 cycles (5.5%)). While the birth rate in cycles with TMC ≤ 1 million is based on sparse data and comparisons should be interpreted cautiously due to limited power and precision, this finding calls into question the use of a threshold of 1 million TMC in order to offer IUI treatment or cancel an IUI cycle. Our findings are consistent with a recent review that determined it was not possible to define a post-processing cut-off point below which IUI should be withheld (Cohlen et al., 2018). Additional studies are needed to define the lower end of the TMC range to offer IUI, particularly given the cost and invasiveness of IVF.
It should be noted that men participating in AMIGOS were required to have >5 million total motile sperm in the ejaculate on the screening semen analysis, and inseminates with ≤1 million TMC that resulted in a live birth were not representative of other samples from the same participants. As a result, our findings may not extend to men who chronically produce specimens with TMC ≤1 million. Additionally, we cannot rule out the possibility that a live birth that occurred following an IUI cycle with an inseminate with ≤1 million TMC was the result of timed intercourse, as participating couples were allowed to have timed intercourse as part of the protocol. It should also be noted that ~19% of post-processing IUI specimens had a TMC ≤5 million. This finding, together with the relatively low TMC for participation in AMIGOS, suggests that the study included men with, what many would consider, mild male factor infertility. While live births occurred in IUI cycles with TMC ≤5 million, success rates were significantly lower than in those cycles with TMC between 15.1 and 20.0 million.
Strengths and limitations of the present investigation should be noted. The AMIGOS clinical trial was a large, prospective, multicentre, randomised controlled trial. The participants and their individual treatment cycles were extensively characterised, the treatment was standardised and live births rates were reported. Our statistical analyses took into account multiple treatment cycles for the same couple and made adjustments for baseline characteristics previously shown to be associated with the live-birth rate in the AMIGOS trial. However, the AMIGOS trial was not designed to address the questions posed in this secondary analysis. Thus, we cannot rule out unmeasured confounding as a potential explanation for the associations observed. Additionally, we could not assess the relationship between some sperm parameters that have been associated with treatment outcomes, such as DNA fragmentation (Cho and Agarwal, 2018) and HPV status (Foresta et al., 2015), as these were not assessed in the AMIGOS trial.
In summary, in this large, prospective clinical trial evaluating live birth rates following ovarian stimulation-IUI treatment in couples with unexplained infertility, we identified patient-reported discomfort with the IUI procedure as a negative predictor for a live birth. Most other performance-related characteristics of the IUI, including time from hCG to IUI, ultrasound guidance and catheter type were not related to success. Sperm preparation type was also not related to the live-birth rate. These findings should provide reassurance to physicians and patients that technologies such as ultrasound guidance of IUI are not necessary and that there is no need for anxiety when multiple IUI attempts are required, if there is IUI-associated bleeding or if there is variability in timing of IUI post hCG injection. Our finding that the live birth rate following IUI with TMC ≤1 million was similar to that seen with TMC >1–5 million suggests that there may be no reasonable threshold below which live birth is not possible with IUI. Additional investigations are needed to readdress thresholds for offering ovarian stimulation-IUI treatment.
Acknowledgements
The authors would like to acknowledge the important contributions of Dr Esther Eisenberg, who served as Project Scientist for the NICHD-sponsored Reproductive Medicine Network (RMN) and was a member of the RMN Steering Committee during the conduct of the AMIGOS trial. Dr Eisenberg did not have any role in the funding decisions nor oversight of the grants that funded the RMN or RMN investigators.
Authors’ roles
K.R.H.: AMIGOS investigator, study concept, design of analysis, interpretation, manuscript preparation, revision and approval of final version. J.P.: statistical analyses, interpretation, manuscript preparation and approval of final version. M.C.: manuscript preparation, interpretation, revision and approval of final version. R.A.W.: statistical analyses, interpretation, manuscript preparation and approval of final version. J.C.T.: manuscript preparation, interpretation, revision and approval of final version. S.A.K.: AMIGOS investigator, manuscript preparation, interpretation, revision and approval of final version. M.P.D.: primary investigator for AMIGOS, interpretation of results, manuscript revision and approval of final version. R.S.L.: AMIGOS investigator, interpretation of results and manuscript revision for intellectual content and approval of final version. C.C.: AMIGOS investigator, manuscript revision for intellectual content and approval of final version. R.A.: data collection for AMIGOS, manuscript revision and approval of final version. R.R.: data collection for AMIGOS, manuscript revision and approval of final version. P.C.: data collection for AMIGOS, manuscript revision and approval of final version. G.M.C.: data collection for AMIGOS, manuscript revision and approval of final version. N.S.: study design for AMIGOS, interpretation of results, manuscript revision and approval of final version. H.Z.: data coordination for AMIGOS, interpretation of results, manuscript revision and approval of final version.
Funding
Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD, U10 HD077680, U10 HD39005, U10 HD38992, U10 HD27049, U10 HD38998, U10 HD055942, HD055944, U10 HD055936, U10 HD055925). This research was made possible by funding by the American Recovery and Reinvestment Act. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD or NIH.
Conflict of interest
Dr Hansen reports grants from NIH/NICHD and Yale University during the conduct of the study, grants from Roche Diagnostics and grants from Ferring International Pharmascience Center US outside the submitted work. Dr Peck reports support from Ferring Pharmaceuticals outside the submitted work. Dr Coward has nothing to disclose. Dr Wild reports grants from NICHD during the conduct of the study. Dr Trussell has nothing to disclose. Dr Krawetz reports grants from NICHD during the conduct of the study, grants from Merck and support from Taylor and Frances and from Springer, outside the submitted work. Dr Diamond reports grants from NIH/NICHD, Yale University, during the conduct of the study and support from Advanced Reproductive Care AbbVie, Bayer and ObsEva, outside the submitted work. Dr Legro reports support from Bayer, Kindex, Odega, Millendo and AbbVie and grants and support from Ferring, outside the submitted work. Dr Coutifaris reports grants from NICHD/NIH and personal fees from American Society for Reproductive Medicine, outside the submitted work. Dr Alvero has nothing to disclose. Dr Robinson reports grants from NIH during the conduct of the study. Dr Casson has nothing to disclose. Dr Christman reports grants from NICHD during the conduct of the study. Dr Santoro reports grants from NIH during the conduct of the study. Dr Zhang reports grants from NIH during the conduct of the study and support from Shangdong University outside the submitted work.
References
- Alvero R, Hearns-Stokes RM, Catherino WH, Leondires MP, Segars JH. The presence of blood in the transfer catheter negatively influences outcome at embryo transfer. Hum Reprod 2003;18:1848–1852. [DOI] [PubMed] [Google Scholar]
- ASRM Practice Committee. Performing the embryo transfer: a guideline. Fertil Steril 2017;107:882–896. [DOI] [PubMed] [Google Scholar]
- Aydin Y, Hassa H, Oge T, Tokgoz VY. A randomized study of simultaneous hCG administration with intrauterine insemination in stimulated cycles. Eur J Obstet Gynecol Reprod Biol 2013;170:444–448. [DOI] [PubMed] [Google Scholar]
- Berker B, Kahraman K, Taskin S, Sukur YE, Sonmezer M, Atabekoglu CS. Recombinant FSH versus clomiphene citrate for ovarian stimulation in couples with unexplained infertility and male subfertility undergoing intrauterine insemination: a randomized trial. Arch Gynecol Obstet 2011;284:1561–1566. [DOI] [PubMed] [Google Scholar]
- Boomsma CM, Heineman MJ, Cohlen BJ, Farquhar C. Semen preparation techniques for intrauterine insemination. Cochrane Database Syst Rev 2007;Cd004507. [DOI] [PubMed] [Google Scholar]
- Brasch JG, Rawlins R, Tarchala S, Radwanska E. The relationship between total motile sperm count and the success of intrauterine insemination. Fertil Steril 1994;62:150–154. [DOI] [PubMed] [Google Scholar]
- Brzechffa PR, Daneshmand S, Buyalos RP. Sequential clomiphene citrate and human menopausal gonadotrophin with intrauterine insemination: the effect of patient age on clinical outcome. Hum Reprod 1998;13:2110–2114. [DOI] [PubMed] [Google Scholar]
- Buckett WM. A meta-analysis of ultrasound-guided versus clinical touch embryo transfer. Fertil Steril 2003;80:1037–1041. [DOI] [PubMed] [Google Scholar]
- Campana A, Sakkas D, Stalberg A, Bianchi PG, Comte I, Pache T, Walker D. Intrauterine insemination: evaluation of the results according to the woman’s age, sperm quality, total sperm count per insemination and life table analysis. Hum Reprod 1996;11:732–736. [DOI] [PubMed] [Google Scholar]
- Castilla JA, Zamora S, Gonzalvo MC, Luna Del Castillo JD, Roldan-Nofuentes JA, Clavero A, Bjorndahl L, Martinez L. Sperm chromatin structure assay and classical semen parameters: systematic review. Reprod Biomed Online 2010;20:114–124. [DOI] [PubMed] [Google Scholar]
- Cho CL, Agarwal A. Role of sperm DNA fragmentation in male factor infertility: a systematic review. Arab J Urol 2018;16:21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claman P, Wilkie V, Collins D. Timing intrauterine insemination either 33 or 39 hours after administration of human chorionic gonadotropin yields the same pregnancy rates as after superovulation therapy. Fertil Steril 2004;82:13–16. [DOI] [PubMed] [Google Scholar]
- Cohlen B, Bijkerk A, Van der Poel S, Ombelet W. IUI: review and systematic assessment of the evidence that supports global recommendations. Hum Reprod Update 2018;24:300–319. [DOI] [PubMed] [Google Scholar]
- Diamond MP, Legro RS, Coutifaris C, Alvero R, Robinson RD, Casson P, Christman GM, Ager J, Huang H, Hansen KR et al. Letrozole, gonadotropin, or clomiphene for unexplained infertility. N Engl J Med 2015a;373:1230–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond MP, Legro RS, Coutifaris C, Alvero R, Robinson RD, Casson P, Christman GM, Ager J, Huang H, Hansen KR et al. Assessment of multiple intrauterine gestations from ovarian stimulation (AMIGOS) trial: baseline characteristics. Fertil Steril 2015b;103:962–973.e964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinelli L, Courbiere B, Achard V, Jouve E, Deveze C, Gnisci A, Grillo JM, Paulmyer-Lacroix O. Prognosis factors of pregnancy after intrauterine insemination with the husband’s sperm: conclusions of an analysis of 2,019 cycles. Fertil Steril 2014;101:994–1000. [DOI] [PubMed] [Google Scholar]
- Duran EH, Morshedi M, Taylor S, Oehninger S. Sperm DNA quality predicts intrauterine insemination outcome: a prospective cohort study. Hum Reprod 2002;17:3122–3128. [DOI] [PubMed] [Google Scholar]
- Foresta C, Noventa M, De Toni L, Gizzo S, Garolla A. HPV-DNA sperm infection and infertility: from a systematic literature review to a possible clinical management proposal. Andrology 2015;3:163–173. [DOI] [PubMed] [Google Scholar]
- Ghesquiere SL, Castelain EG, Spiessens C, Meuleman CL, D'Hooghe TM. Relationship between follicle number and (multiple) live birth rate after controlled ovarian hyperstimulation and intrauterine insemination. Am J Obstet Gynecol 2007;197:589.e581–589.e585. [DOI] [PubMed] [Google Scholar]
- Guzick D. Polycystic ovary syndrome: symptomatology, pathophysiology, and epidemiology. Am J Obstet Gynecol 1998;179:S89–s93. [DOI] [PubMed] [Google Scholar]
- Guzick DS, Carson SA, Coutifaris C, Overstreet JW, Factor-Litvak P, Steinkampf MP, Hill JA, Mastroianni L, Buster JE, Nakajima ST et al. Efficacy of superovulation and intrauterine insemination in the treatment of infertility. National Cooperative Reproductive Medicine Network. N Engl J Med 1999;340:177–183. [DOI] [PubMed] [Google Scholar]
- Hansen KR, He AL, Styer AK, Wild RA, Butts S, Engmann L, Diamond MP, Legro RS, Coutifaris C, Alvero R et al. Predictors of pregnancy and live-birth in couples with unexplained infertility after ovarian stimulation-intrauterine insemination. Fertil Steril 2016;105:1575–1583.e1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson RE, Bormann CL, Hassun PA, Rocha AM, Motta EL, Serafini PC, Smith GD. Effects of semen storage and separation techniques on sperm DNA fragmentation. Fertil Steril 2010;94:2626–2630. [DOI] [PubMed] [Google Scholar]
- Kohn TP, Kohn JR, Ramasamy R. Effect of sperm morphology on pregnancy success via intrauterine insemination: a systematic review and meta-analysis. J Urol 2018;199:812–822. [DOI] [PubMed] [Google Scholar]
- Lemmens L, Kos S, Beijer C, Brinkman JW, Horst FA, Hoven L, Kieslinger DC, Vrouwerff NJ, Wolthuis A, Hendriks JC et al. Predictive value of sperm morphology and progressively motile sperm count for pregnancy outcomes in intrauterine insemination. Fertil Steril 2016;105:1462–1468. [DOI] [PubMed] [Google Scholar]
- Merviel P, Heraud MH, Grenier N, Lourdel E, Sanguinet P, Copin H. Predictive factors for pregnancy after intrauterine insemination (IUI): an analysis of 1038 cycles and a review of the literature. Fertil Steril 2010;93:79–88. [DOI] [PubMed] [Google Scholar]
- Miller DC, Hollenbeck BK, Smith GD, Randolph JF, Christman GM, Smith YR, Lebovic DI, Ohl DA. Processed total motile sperm count correlates with pregnancy outcome after intrauterine insemination. Urology 2002;60:497–501. [DOI] [PubMed] [Google Scholar]
- Ombelet W, Dhont N, Thijssen A, Bosmans E, Kruger T. Semen quality and prediction of IUI success in male subfertility: a systematic review. Reprod Biomed Online 2014;28:300–309. [DOI] [PubMed] [Google Scholar]
- Oruc AS, Yilmaz N, Gorkem U, Inal HA, Seckin B, Gulerman C. Influence of ultrasound-guided artificial insemination on pregnancy rates: a randomized study. Arch Gynecol Obstet 2014;289:207–212. [DOI] [PubMed] [Google Scholar]
- Oztekin D, Ozcinar E, Kose C, Gulhan I, Ozeren M, Tinar S. The use of ultrasound during intrauterine insemination in unexplained infertility may improve pregnancy outcomes. Med Princ Pract 2013;22:291–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SJ, Alvarez JR, Weiss G, Von Hagen S, Smith D, McGovern PG. Ovulatory status and follicular response predict success of clomiphene citrate-intrauterine insemination. Fertil Steril 2007;87:1102–1107. [DOI] [PubMed] [Google Scholar]
- Polat I, Ekiz A, Yildirim G, Sahin O, Ulker V, Alkis I, Tekirdag AI. Ultrasound-guided intrauterine insemination versus blind intrauterine insemination: a randomized controlled trial. Clin Exp Obstet Gynecol 2015;42:657–662. [PubMed] [Google Scholar]
- Rahman SM, Karmakar D, Malhotra N, Kumar S. Timing of intrauterine insemination: an attempt to unravel the enigma. Arch Gynecol Obstet 2011;284:1023–1027. [DOI] [PubMed] [Google Scholar]
- Ramon O, Matorras R, Corcostegui B, Meabe A, Burgos J, Exposito A, Crisol L. Ultrasound-guided artificial insemination: a randomized controlled trial. Hum Reprod 2009;24:1080–1084. [DOI] [PubMed] [Google Scholar]
- Sahakyan M, Harlow BL, Hornstein MD. Influence of age, diagnosis, and cycle number on pregnancy rates with gonadotropin-induced controlled ovarian hyperstimulation and intrauterine insemination. Fertil Steril 1999;72:500–504. [DOI] [PubMed] [Google Scholar]
- Schoolcraft WB, Surrey ES, Gardner DK. Embryo transfer: techniques and variables affecting success. Fertil Steril 2001;76:863–870. [DOI] [PubMed] [Google Scholar]
- Thijssen A, Creemers A, Van der Elst W, Creemers E, Vandormael E, Dhont N, Ombelet W. Predictive value of different covariates influencing pregnancy rate following intrauterine insemination with homologous semen: a prospective cohort study. Reprod Biomed Online 2017;34:463–472. [DOI] [PubMed] [Google Scholar]
- Poel N, Farquhar C, Abou-Setta AM, Benschop L, Heineman MJ. Soft versus firm catheters for intrauterine insemination. Cochrane Database Syst Rev 2010;Cd006225. [DOI] [PubMed] [Google Scholar]
- Van Voorhis BJ, Barnett M, Sparks AE, Syrop CH, Rosenthal G, Dawson J. Effect of the total motile sperm count on the efficacy and cost-effectiveness of intrauterine insemination and in vitro fertilization. Fertil Steril 2001;75:661–668. [DOI] [PubMed] [Google Scholar]
- Williamson JM, Datta S, Satten GA. Marginal analyses of clustered data when cluster size is informative. Biometrics 2003;59:36–42. [DOI] [PubMed] [Google Scholar]
- Williamson JM, Kim HY, Warner L. Weighting condom use data to account for nonignorable cluster size. Ann Epidemiol 2007;17:603–607. [DOI] [PubMed] [Google Scholar]
- Yland J, Messerlian C, Minguez-Alarcon L, Ford JB, Hauser R, Williams PL. Methodological approaches to analyzing IVF data with multiple cycles. Hum Reprod 2019;34:549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XD, Chen MY, Gao Y, Han W, Liu DY, Huang GN. The effects of different sperm preparation methods and incubation time on the sperm DNA fragmentation. Hum Fertil (Camb) 2011;14: 187–191. [DOI] [PubMed] [Google Scholar]


