Summary
Background
The ongoing COVID-19 pandemic is a global threat. Identification of markers for symptom onset and disease progression is a pressing issue. We described the clinical features of people infected on board the Diamond Princess cruise ship who were diagnosed with asymptomatic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection or mild or severe COVID-19, on admission to the Self-Defense Forces Central Hospital (Tokyo, Japan) and at the end of observation.
Methods
This retrospective, single-centre study included participants with laboratory-detected SARS-CoV-2 infection who were admitted to the Self-Defense Forces Central Hospital from Feb 11 to Feb 25, 2020. Clinical records, laboratory data, and radiological findings were analysed. Clinical outcomes were followed up until discharge or Feb 26, 2020, whichever came first. We defined asymptomatic infection as SARS-CoV-2 infection with no history of clinical signs and symptoms, severe COVID-19 as clinical symptoms of pneumonia (dyspnoea, tachypnoea, peripheral capillary oxygen saturation <93%, and need for oxygen therapy), and mild COVID-19 as all other symptoms. Clinical features on admission were compared among patients with different disease severity, including asymptomatic infection, at the end of observation. We used univariable analysis to identify factors associated with symptomatic illness among asymptomatic people infected with SARS-CoV-2 and disease progression in patients with COVID-19.
Findings
Among the 104 participants included in the final analysis, the median age was 68 years (IQR 47–75) and 54 (52%) were male. On admission, 43 (41%) participants were classified as asymptomatic, 41 (39%) as having mild COVID-10, and 20 (19%) as having severe COVID-19. At the end of observation, 33 (32%) participants were confirmed as being asymptomatic, 43 (41%) as having mild COVID-19, and 28 (27%) as having severe COVID-19. Serum lactate hydrogenase concentrations were significantly higher in the ten participants who were asymptomatic on admission but developed symptomatic COVID-19 compared with the 33 participants who remained asymptomatic throughout the observation period (five [50%] vs four [12%] participants; odds ratio 7·25, 95% CI 1·43–36·70; p=0·020). Compared with patients with mild disease at the end of observation, patients with severe COVID-19 were older (median age 73 years [IQR 55–77] vs 60 years [40–71]; p=0·028) and had more frequent consolidation on chest CT (13 [46%] of 28 vs nine [21%] of 43; p=0·035) and lymphopenia (16 [57%] vs ten [23%]; p=0·0055) on admission.
Interpretation
Older age, consolidation on chest CT images, and lymphopenia might be risk factors for disease progression of COVID-19 and contribute to improved clinical management.
Funding
None.
Introduction
COVID-19 is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The first case was reported in Wuhan, China, in December, 2019,1 and the ongoing outbreak has been characterised as a pandemic by WHO. Because no definitive treatment for COVID-19 is available yet, identification of patients at high risk for severe illness is crucial to prepare and provide sufficient supportive therapy. Additionally, a massive increase in case numbers has led to the collapse of health-care systems in several countries. Therefore, triage of patients and their transportation to medical facilities with dedicated intensive-care capabilities are necessary. The clinical characteristics of COVID-19 and the pathogenicity of SARS-CoV-2 are still being investigated; most studies published at the beginning of the outbreak focused on patients with severe disease,2, 3, 4, 5 although more recent studies have considered patients with mild or moderate COVID-19.6, 7 In Japan, many infections occurred among the passengers and crew members on board the Diamond Princess cruise ship in February, 2020. By March 1, 2020, there were approximately 700 individuals with laboratory-detected SARS-CoV-2 infection.8, 9 The 3711 passengers and crew members on the cruise ship were tested for SARS-CoV-2 by RT-PCR, and all symptomatic and asymptomatic individuals were referred to medical institutions designated for infectious diseases in accordance with the Infectious Diseases Control Law of Japan.8, 9 Approximately 15% of all patients with laboratory-detected infection from the Diamond Princess were admitted to the Self-Defense Forces Central Hospital in Tokyo, Japan.
Research in context.
Evidence before this study
We searched PubMed from its inception until March 1, 2020, for articles published in English using the keywords “novel coronavirus”, “2019 novel coronavirus”, “2019-nCoV”, “severe acute respiratory syndrome coronavirus 2”, “SARS-CoV-2”, “COVID-19”, “mass infection”, “herd infection”, “cruise ship”, “Diamond Princess”, “asymptomatic”, and “subclinical”. There were no published clinical studies featuring COVID-19 as a result of mass infection on board a cruise ship. We found two articles that compared people with asymptomatic infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) or mild or severe COVID-19. However, neither described potential predictors of symptomatic illness among people infected with SARS-CoV-2 and markers for disease progression in patients with COVID-19.
Added value of this study
To our knowledge, this is the first study showing the predictors of symptomatic illness in people infected with SARS-CoV-2. We present a summary of the clinical characteristics of 104 participants with laboratory-detected SARS-CoV-2 infection as a result of mass infection on the Diamond Princess cruise ship who were treated at Self-Defense Forces Central Hospital, Japan, from Feb 11 to Feb 25, 2020. Serum lactate dehydrogenase concentrations were significantly higher in patients who were initially asymptomatic on admission to the hospital and developed symptomatic COVID-19 during the observation period than in those who remained asymptomatic throughout. Older age, consolidation on chest CT images, and lymphopenia on admission were more frequent in patients with severe COVID-19 than those with mild COVID-19 at the end of observation, and thus could be potential risk factors for disease progression.
Implications of all the available evidence
More than 70% of our patients with SARS-CoV-2 infection had asymptomatic or mild disease. However, massive increase in case numbers has led to the collapse of health-care systems. Combining clinical and laboratory findings with chest CT imaging could help to identify people who are at risk of symptom onset and clinical deterioration.
In clinical settings, identifying people who are at risk of symptom onset and clinical deterioration is essential. Therefore, understanding the clinical characteristics of patients with COVID-19 of different severity is necessary. To that end, we retrospectively analysed the detailed clinical characteristics of hospitalised people with SARS-CoV-2 infection as a result of the Diamond Princess outbreak, including those with asymptomatic infection and mild or severe COVID-19, who were treated in the Self-Defense Forces Central Hospital.
Methods
Study design and participants
We did a retrospective review of the medical records of passengers and crew members with laboratory-detected SARS-CoV-2 infection who were aboard the Diamond Princess and were admitted to the Self-Defense Forces Central Hospital in Tokyo, Japan, between Feb 11 and Feb 25, 2020. The 3711 passengers and crew members on the Diamond Princess were examined by quantitative RT-PCR or nested RT-PCR for SARS-CoV-2 at the National Institute of Infectious Diseases and Regional Institutes of Public Health using pharyngeal swabs or sputum specimens according to the recommended protocol by the Japanese National Institute of Infectious Disease.8, 9, 10, 11, 12 This study was reviewed and approved by the Japan Self-Defense Forces Central Hospital (approval number 01–011). Informed consent, both written and oral, was obtained from all enrolled participants.
Procedures
Participant information was retrospectively obtained from the hospital medical records and included nationality, clinical records, laboratory findings, and chest CT images. Participants' clinical history, physical examination, and chest CT scans were done on the day of admission, and any blood tests were completed within the first 2 days following admission. Geographical regions for nationalities were classified according to the standard country or area codes for statistical use by the UN Statistics Division. All data were obtained and reviewed by two study investigators (ST and KI). The observation period was defined as the time interval between the date of admission and the date of discharge or Feb 26, 2020, whichever was earliest.
Asymptomatic cases were defined as people with SARS-CoV-2 infection presenting with no clinical signs and symptoms from medical interviews and physical examinations from the start of quarantine (Feb 3) to end of hospitalisation. Severe symptomatic cases were defined as participants showing clinical symptoms of pneumonia (dyspnoea, tachypnoea, peripheral capillary oxygen saturation <93%, and need for oxygen therapy).9 Participants with other symptoms were classified as mild cases. Radiographical findings were not used to classify cases, meaning asymptomatic cases could still present with radiographical abnormalities.
Statistical analysis
Continuous variables with a normal distribution were expressed as mean (SD) and with a non-normal distribution as median (IQR) and compared using Student's t test for parametric data and Wilcoxon rank-sum test for non-parametric data. Odds ratios (ORs) and a two-sided 95% CIs for ORs were calculated. Categorical variables were compared using the χ2 test or, if the cells had expected frequencies of less than ten, using Fisher's exact test. A two-sided p value of less than 0·05 was considered significant. All statistical analyses were done using Stata 13.
Role of the funding source
There was no funding source for this study. The corresponding author had full access to all study-related data and had final responsibility for the decision to submit the study for publication.
Results
Between Feb 11 and Feb 25, 2020, 107 people with laboratory-detected SARS-CoV-2 infection on board the Diamond Princess were hospitalised at the Self-Defense Forces Central Hospital in Japan. Three people were excluded from the study because they withdrew consent and 104 participants were included in the final analysis. Participants were aged 25–93 years, with a median age of 68 years (IQR 47–75), and 54 (52%) were male (table 1 ). Most participants were from east Asia, predominantly from Japan and China. The observation period ranged from 3 to 15 days (median 10 days, IQR 7–10). Half of the participants had comorbidities (table 1). On the basis of their presentation on the day of admission, 43 (41%) participants who did not have any clinical signs or symptoms were classified as asymptomatic, whereas 41 (39%) were classified as having mild COVID-19 and 20 (19%) as having severe COVID-19. At the end of the observation period, 33 (32%) participants were confirmed as being asymptomatic (table 2 ), 43 (41%) as having mild COVID-19, and 28 (27%) as having severe COVID-19 (table 3 ).
Table 1.
Baseline characteristics of patients with SARS-CoV-2 infection
| All patients (n=104) | ||
|---|---|---|
| Age, years | 68 (47–75) | |
| Sex | ||
| Female | 50 (48%) | |
| Male | 54 (52%) | |
| Nationality | ||
| East Asia | 55 (53%) | |
| Southeast Asia | 21 (20%) | |
| Europe | 4 (4%) | |
| North America | 14 (13%) | |
| Oceania | 4 (4%) | |
| Other | 6 (6%) | |
| Observation period, days | 10 (7–10) | |
| Time from onset to admission, days | 5 (2–7) | |
| Current smoker | 18 (17%) | |
| Comorbidities | ||
| Any | 52 (50%) | |
| Cardiovascular disease | 31 (30%) | |
| Endocrine disorder | 9 (9%) | |
| Diabetes | 7 (7%) | |
| Respiratory disorder | 7 (7%) | |
| Malignancy | 4 (4%) | |
| Severity on admission | ||
| Asymptomatic | 43 (41%) | |
| Mild | 41 (39%) | |
| Severe | 20 (19%) | |
Data are n (%) or median (IQR). SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
Table 2.
Clinical characteristics of 43 patients with SARS-CoV-2 infection who were asymptomatic on admission
|
All patients (n=43) |
At the end of observation |
p value |
|||
|---|---|---|---|---|---|
| Asymptomatic (n=33) | Symptomatic (n=10) | ||||
| Age, years | 69 (61–75) | 70 (57–75) | 68 (56–74) | 0·966 | |
| Sex | .. | .. | .. | 1·000 | |
| Female | 24 (56%) | 18 (55%) | 6 (60%) | .. | |
| Male | 19 (44%) | 15 (45%) | 4 (40%) | .. | |
| Current smoker | 7 (16%) | 5 (15%) | 2 (20%) | 0·656 | |
| Comorbidities | |||||
| Any | 21 (49%) | 15 (45%) | 6 (60%) | 0·488 | |
| Cardiovascular disease | 12 (28%) | 9 (27%) | 3 (30%) | 1·000 | |
| Endocrine disorder | 3 (7%) | 2 (6%) | 1 (10%) | 0·558 | |
| Diabetes | 5 (12%) | 5 (15%) | 0 | 0·320 | |
| Respiratory disorder | 4 (9%) | 2 (6%) | 2 (20%) | 0·226 | |
| Malignancy | 2 (5%) | 1 (3%) | 1 (10%) | 0·415 | |
| Signs and symptoms | |||||
| Fever | 3 (7%) | 0 | 3 (30%) | .. | |
| Cough | 7 (16%) | 0 | 7 (70%) | .. | |
| Malaise | 2 (5%) | 0 | 2 (20%) | .. | |
| Headache | 2 (5%) | 0 | 2 (20%) | .. | |
| Sore throat | 0 | 0 | 0 | .. | |
| Runny nose | 0 | 0 | 0 | .. | |
| Diarrhoea | 1 (2%) | 0 | 1 (10%) | .. | |
| Dyspnoea | 3 (7%) | 0 | 3 (30%) | .. | |
| Tachypnoea | 3 (7%) | 0 | 3 (30%) | .. | |
| SpO2 <93% | 3 (7%) | 0 | 3 (30%) | .. | |
| Radiographical findings on admission | |||||
| Any abnormal lung findings | 25 (58%) | 17 (52%) | 8 (80%) | 0·153 | |
| Lateral | 13 (30%) | 10 (30%) | 3 (30%) | 1.000 | |
| Bilateral | 12 (28%) | 7 (21%) | 5 (50%) | 0·111 | |
| Ground-glass opacities | 15 (35%) | 11 (33%) | 4 (40%) | 0·719 | |
| Multifocal ground-glass opacities | 12 (28%) | 9 (27%) | 3 (30%) | 1·000 | |
| Consolidation | 9 (21%) | 6 (18%) | 3 (30%) | 0·413 | |
| Crazy-paving appearance | 5 (12%) | 2 (6%) | 3 (30%) | 0·073 | |
| Nodular lesion | 3 (7%) | 2 (6%) | 1 (10%) | 0·558 | |
| Laboratory findings on admission | |||||
| AST >38 IU/L | 4 (9%) | 3 (9%) | 1 (10%) | 1·000 | |
| ALT >45 IU/L | 5 (12%) | 4 (12%) | 1 (10%) | 1·000 | |
| LDH >230 IU/L | 9 (21%) | 4 (12%) | 5 (50%) | 0·020 | |
| C-reactive protein >0·3 mg/dL | 18 (42%) | 11 (33%) | 7 (70%) | 0·067 | |
| White blood cell count <4000 cells per μL | 6 (14%) | 4 (12%) | 2 (20%) | 0·611 | |
| Platelet count <15 × 104 cells per μL | 2 (5%) | 1 (3%) | 1 (10%) | 0·415 | |
| Lymphocyte count <1200 cells per μL | 13 (30%) | 9 (27%) | 4 (40%) | 0·458 | |
Data are n (%) or median (IQR), unless otherwise specified. SpO2=peripheral oxygen saturation. AST=aspartate aminotransferase. ALT=alanine aminotransferase. LDH=lactate dehydrogenase. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
Table 3.
Clinical characteristics of patients with mild or severe COVID-19 at the end of observation
| All patients (n=71) |
At the end of observation |
p value | |||
|---|---|---|---|---|---|
| Mild (n=43) | Severe (n=28) | ||||
| Age, years | 67 (44–75) | 60 (40–71) | 73 (55–77) | 0·028 | |
| Sex | .. | .. | .. | 0·585 | |
| Female | 32 (45%) | 21 (49%) | 11 (39%) | .. | |
| Male | 39 (55%) | 22 (51%) | 17 (61%) | .. | |
| Current smoker | 13 (18%) | 6 (14%) | 7 (25%) | 0·347 | |
| Comorbidities | |||||
| Any | 37 (52%) | 19 (44%) | 18 (64%) | 0·157 | |
| Cardiovascular disease | 22 (31%) | 11 (26%) | 11 (39%) | 0·295 | |
| Endocrine disorder | 7 (10%) | 3 (7%) | 4 (14%) | 0·422 | |
| Diabetes | 2 (3%) | 1 (2%) | 1 (4%) | 1·000 | |
| Respiratory disorder | 5 (7%) | 2 (5%) | 3 (11%) | 0·376 | |
| Malignancy | 3 (4%) | 2 (5%) | 1 (4%) | 1·000 | |
| Paracetamol on admission | 4 (6%) | 4 (9%) | 0 | 0·148 | |
| Signs and symptoms on admission | |||||
| Fever | 30 (42%) | 19 (44%) | 11 (39%) | 0·807 | |
| Cough | 29 (41%) | 16 (37%) | 13 (46%) | 0·599 | |
| Malaise | 12 (17%) | 6 (14%) | 6 (21%) | 0·521 | |
| Headache | 10 (14%) | 8 (19%) | 2 (7%) | 0·296 | |
| Sore throat | 11 (15%) | 7 (16%) | 4 (14%) | 1·000 | |
| Runny nose | 16 (23%) | 8 (19%) | 8 (29%) | 0·389 | |
| Diarrhoea | 8 (11%) | 5 (12%) | 3 (11%) | 1·000 | |
| Dyspnoea | 7 (10%) | 0 | 7 (25%) | .. | |
| Tachypnoea | 16 (23%) | 0 | 16 (57%) | .. | |
| SpO2 <93% | 3 (4%) | 0 | 3 (11%) | .. | |
| Radiographical findings on admission | |||||
| Any abnormal lung finding | 52 (73%) | 28 (65%) | 24 (86%) | 0·062 | |
| Lateral | 21 (30%) | 13 (30%) | 8 (29%) | 1·000 | |
| Bilateral | 31 (44%) | 15 (35%) | 16 (57%) | 0·109 | |
| Ground-glass opacities | 37 (52%) | 18 (42%) | 19 (68%) | 0·057 | |
| Multifocal ground-glass opacities | 34 (48%) | 17 (40%) | 17 (61%) | 0·088 | |
| Consolidation | 22 (31%) | 9 (21%) | 13 (46%) | 0·035 | |
| Crazy-paving appearance | 19 (27%) | 8 (19%) | 11 (39%) | 0·098 | |
| Nodular lesion | 5 (7%) | 4 (9%) | 1 (4%) | 0·642 | |
| Laboratory findings on admission | |||||
| AST >38 IU/L | 15 (21%) | 6 (14%) | 9 (32%) | 0·081 | |
| ALT >45 IU/L | 13 (18%) | 6 (14%) | 7 (25%) | 0·347 | |
| LDH >230 IU/L | 23 (32%) | 13 (30%) | 10 (36%) | 0·796 | |
| C-reactive protein >0·3 mg/dL | 42 (59%) | 23 (53%) | 19 (68%) | 0·339 | |
| White blood cell count <4000 cells per μL | 15 (21%) | 9 (21%) | 6 (21%) | 1·000 | |
| Platelet count <15 × 104 cells per μL | 7 (10%) | 5 (12%) | 2 (7%) | 0·696 | |
| Lymphocyte count <1200 cells per μL | 26 (37%) | 10 (23%) | 16 (57%) | 0·0055 | |
Data are n (%) or median (IQR), unless otherwise specified. SpO2=peripheral oxygen saturation. AST=aspartate aminotransferase. ALT=alanine aminotransferase. LDH=lactate dehydrogenase.
Fever and cough were the most common clinical signs and symptoms on admission among the 71 patients who were symptomatic at the end of the observation period (table 3), and these remained the most common at the end of observation (appendix p 1). Of the 43 participants who were asymptomatic on admission, ten (23%) developed symptomatic COVID-19 during the observation period, including three (7%) participants who developed severe COVID-19 and required supplemental oxygen therapy (table 2). Of the 41 participants with mild disease on admission, five (12%) developed severe COVID-19 and required supplemental oxygen therapy, leading to a total of eight (10%) of 84 participants with asymptomatic or mild presentation on admission developing severe COVID-19. Additionally, of the 71 participants who were symptomatic at the end of the observation period, 14 (20%) required oxygen therapy and one (1%) required mechanical ventilation, while ten (14%) were treated with antibiotics and five (7%) with lopinavir–ritonavir (appendix p 1). There were no reports of complications or death during the observation period. 30 (29%) patients had been discharged by Feb 26.
Among the ten participants who were asymptomatic on admission and developed symptomatic COVID-19 during observation, the most frequent symptom was cough (table 2). Conversely, 17 (52%) of the 33 participants who did not develop symptoms during the observation period had abnormal lung findings with various patterns on CT images (table 2; figure 1 ). Univariable analysis showed no significant differences in the distribution of age, sex, and comorbidities or the prevalence of abnormal lung findings and CT patterns between symptomatic and asymptomatic participants. The prevalence of increased lactate dehydrogenase (LDH) concentrations was significantly higher in participants who were initially asymptomatic but developed symptomatic COVID-19 during the observation period (OR 7·25, 95% CI 1·43–36·70; p=0·020; appendix p 4).
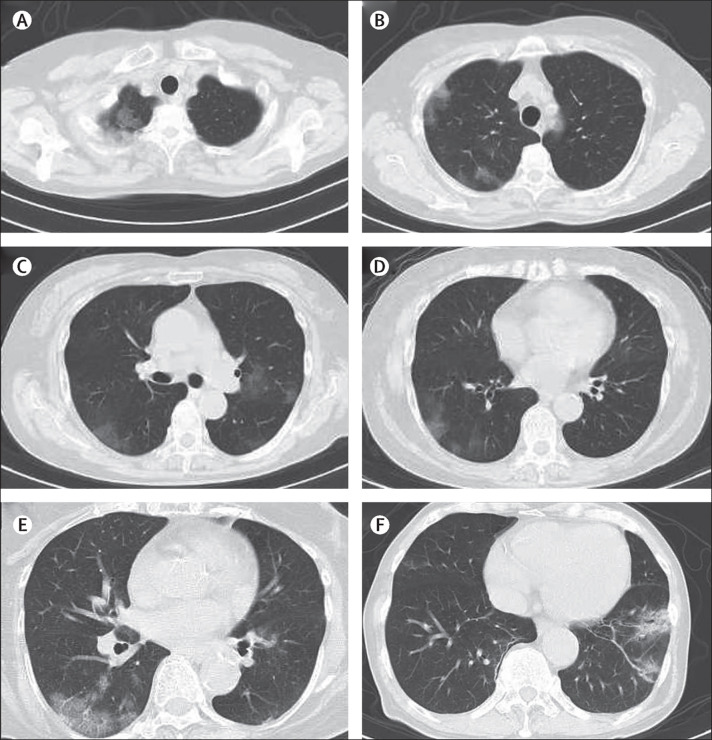
Figure 1.
CT patterns of abnormal lung findings among asymptomatic patients with SARS-CoV-2 infection
Images (A) to (D) are from a 73-year-old woman. Patchy non-segmental ground-glass opacities are observed adjacent to the parietal pleura in the right upper lobe (A, B) and in both lower lobes (C, D). Image (E) shows ground-glass opacities with interlobular septal thickening (crazy-paving appearance) adjacent to the parietal pleura in the right lower lobe of a 70-year-old woman. Image (F) shows ground-glass opacities with consolidation, bronchial wall thickening, and bronchiectasis in left lower lobe of a 76-year-old man. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
The eight participants who developed severe COVID-19 during the observation period were aged 42–87 years (median 75 years, IQR 72–77) and five (63%) were male (appendix pp 2–3). The period from symptom onset to the reclassification of disease presentation as severe COVID-19 ranged from 1 to 5 days (median 4 days, IQR 3–5). Additionally, all eight participants developed radiographical abnormalities on CT before the emergence of symptoms of typical pneumonia such as tachypnoea, dyspnoea, and hypoxaemia (median 4 days [IQR 2–5] before symptoms; range 1–8 days; figure 2 ). Four (50%) of these participants had no symptoms of cough and three (38%) had no symptoms of fever at study end. When comparing these eight participants who developed severe COVID-19 with the 76 participants who remained asymptomatic or had mild COVID-19 at the end of the observation period, we found no significant differences in the distribution of sex and comorbidities between the two groups (appendix pp 2–3). Compared with the 76 participants who were asymptomatic or had mild disease at the end of the observation period, the eight participants who developed severe COVID-19 were significantly older (median 75 years [IQR 72–77] vs 67 years [45–74]; p=0·037) and had significantly higher prevalence of any abnormal lung findings (eight [100%] vs 45 [59%]; OR not estimable; p=0·023), bilateral abnormalities (six [75%] vs 22 [29%]; OR 7·36 [95% CI 1·38–39·33]; p=0·015), consolidation (six [75%] vs 15 [20%]; 12·20 [2·23–66·59]; p=0·0026), elevation of C-reactive protein (seven [88%] vs 34 [45%]; 8·65 [1·01–73·75]; p=0·028), and lymphopenia (five [63%] vs 19 [25%]; 5·00 [1·10–22·92]; p=0·039) on the day of admission (appendix pp 2–3).
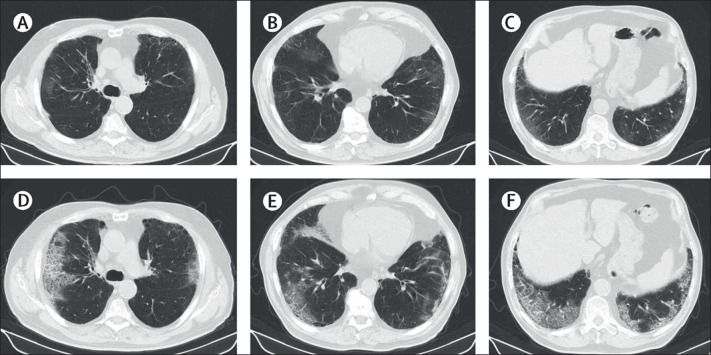
Figure 2.
Progression of CT findings in a 75-year-old man who developed severe COVID-19
The patient was asymptomatic on the day of admission. On the fourth day of admission, he developed tachypnoea and hypoxaemia and was administered oxygen therapy. (A–C) Chest CT images on the day of admission show multifocal ground-glass opacities adjacent to the parietal pleura in multiple lobes with emphysematous changes. (D–F) Follow-up chest CT images on the tenth day of admission show an increase in the extent of ground-glass opacities with crazy-paving appearance.
Finally, when comparing clinical characteristics between participants with mild and severe COVID-19 at the end of the observation period, we found that patients in the severe COVID-19 group were significantly older than those in the mild COVID-19 group (table 3). There were no significant differences in the distribution of sex, comorbidities, clinical signs, and symptoms between the two groups. However, there was a greater prevalence of consolidation as a CT pattern in participants with severe disease than in those with mild disease (OR 3·27, 95% CI 1·15–9·30; table 3). Additionally, the prevalence of lymphopenia was higher in participants who had severe COVID-19 at the end of the observation period than in those with mild COVID-19 (4·40, 1·57–12·32; table 3).
Discussion
This retrospective, single-centre study provides a summary of the clinical features of people with SARS-CoV-2 infection and COVID-19 of varying severity as a result of mass infection on board a cruise ship. LDH concentrations were significantly higher in the ten participants who were initially asymptomatic on admission to the hospital and developed symptomatic COVID-19 during the observation period than in the 33 participants who remained asymptomatic throughout. Older age, consolidation on chest CT images, and lymphopenia on admission were significantly more frequent among the 28 participants with severe COVID-19 at the end of the observation period than among the 43 participants with mild COVID-19. Older age, consolidation, and lymphopenia on admission were also more frequent among the eight patients who developed severe COVID-19 during observation compared with those who were asymptomatic or had mild COVID-19 at the end of the observation period. Overall, these findings suggest that serum LDH concentration might be a predictor of symptomatic illness among people infected with SARS-CoV-2 and that older age, consolidation on chest CT images, and lymphopenia are potential risk factors for disease progression in patients with COVID-19.
The predictors of symptomatic illness among people infected with SARS-CoV-2 and risk factors for disease progression in COVID-19 are still partially unclear because people who are asymptomatic or with mild symptoms do not necessarily visit a hospital, and in many instances are even actively discouraged from doing this. During the COVID-19 outbreak on the Diamond Princess, 3711 individuals on board were tested for SARS-CoV-2 by RT-PCR, and all individuals found positive for SARS-CoV-2 infection were referred to hospital. This unique situation allowed us to retrospectively observe the clinical course of asymptomatic people with SARS-CoV-2 infection, as well as those with mild and severe COVID-19 symptoms.
Previously published findings of COVID-19 in China suggest that old age, male sex, and presence of comorbidities are potential risk factors for disease progression and poor prognosis.1, 12 Zhou and colleagues13 indicated that older age, high sequential organ failure assessment score, and increased D-dimer concentrations were potential risk factors for death in patients with COVID-19, whereas Qin and colleagues14 reported that lymphopenia and an increased neutrophil–lymphocyte ratio were frequently observed in patients with severe COVID-19 compared with those with mild disease. Our findings support those of previous reports suggesting older age and lymphopenia as potential risk factors for severe COVID-19. Lymphopenia, which is a common feature of symptomatic COVID-19,15, 16 could be a direct consequence of SARS-CoV-2 or might be mediated by inflammatory cytokines and chemokines. Qin and colleagues14 also suggested that SARS-CoV-2 infection might affect T lymphocytes and dysregulate the immune system, consequently leading to secondary bacterial infections. They also suggested that in severe cases, lymphopenia could be induced by inflammatory cytokines and chemokines, including tumor necrosis factor-α, interleukin (IL)-2 receptor, IL-6, IL-8, and IL-10, and could lead to lymphocyte deficiency and migration.14 However, in the present study, we observed lymphopenia in 27% of participants who were asymptomatic at the end of the observation period. Further studies are warranted to determine the mechanism of lymphopenia for the prevention of COVID-19 exacerbation. Comparing the concentrations of inflammatory cytokines and chemokines between patients with symptomatic and asymptomatic COVID-19 might help to understand the mechanism of lymphopenia.
In this study, the prevalence of consolidation detected by chest CT was significantly higher in severe cases than in mild cases in patients who were symptomatic at the end of observation. Consolidation was also frequently detected in participants who developed severe COVID-19 during the observation period. Pan and colleagues17 and Shi and colleagues18 reported that abnormal lung findings varied from ground-glass opacities to consolidation during the course of COVID-19 and that the appearance of consolidation was more frequent within 1–3 weeks after symptom onset. Previous studies have found that 55% of patients with COVID-19 developed dyspnoea in a median of 8 days (IQR 5–13) after symptom onset;1 thus, the development of consolidation on CT is associated with disease deterioration. Generally, consolidation is easily detectable by chest x-ray, which therefore might be useful for the evaluation of risk for disease progression in patients with COVID-19 in settings with few resources.
Additionally, CT imaging showed a high prevalence of lung abnormalities in patients with COVID-19. The various patterns of abnormal lung findings were observed in asymptomatic (52%) as well as in symptomatic (73%) participants at the end of the observation period. Previous reports focusing on CT imaging findings of COVID-19 also noted the presence of abnormal lung findings in asymptomatic individuals.18, 19 Bai and colleagues20 investigated the common radiological characteristics of COVID-19 pneumonia and reported that chest CT had a diagnostic sensitivity of 73–93% and a specificity of 93–100% in distinguishing COVID-19 from viral pneumonia. Therefore, chest CT might also be a useful diagnostic tool for asymptomatic and symptomatic people with SARS-CoV-2 infection. In the present study, all eight patients who developed severe COVID-19 during the observation period presented with CT abnormalities before the appearance of typical pneumonia symptoms such as dyspnoea, tachypnoea, and hypoxaemia. Of these eight patients, four had no cough and three had no fever at the end of observation. These findings highlight that COVID-19 pneumonia can develop without any notable symptoms. Conversely, most asymptomatic participants with radiological abnormalities on CT imaging did not develop severe pneumonia. The differences observed in clinical outcomes might be associated with patient background characteristics as well as the genetic and pathogenic diversity of SARS-CoV-2. Understanding the relevance of CT abnormalities to the duration of virus shedding and the incidence of secondary bacterial pneumonia is important to improve the clinical management of COVID-19. Further investigation is needed to clarify the clinical impact of CT findings in people with asymptomatic SARS-CoV-2 infection or mild COVID-19.
Notably, we found a significant difference in the prevalence of increased LDH concentrations between patients who developed symptomatic COVID-19 during the observation period and those who remained asymptomatic throughout, although there was no significant difference in the prevalence of lymphopenia between the two groups. Increased serum LDH concentrations are associated with lung tissue damage,21 and serum LDH is considered a marker for disease activity and progression in pneumonia from various causes.22, 23 Given that abnormal lung findings are frequently observed in people with asymptomatic SARS-CoV-2 infection, our results indicate that increased LDH concentrations might also reflect lung tissue damage by COVID-19 and might be considered a predictor of symptomatic illness among people infected with SARS-CoV-2. Patient assessment with determination of serum LDH and chest CT imaging might facilitate the successful dedication of medical resources to patients who are at increased risk for disease progression in the setting of mass infections, as well as in normal clinical settings.
The major limitations of this study were selection bias and the small population size. We enrolled a homogeneous cluster of relatively healthy individuals who remained on board a cruise ship for an extended period of time. Therefore, the patient population was not an exact representation of normal clinical settings. Triage on the Diamond Princess before emergency transportation also introduced a sampling bias with an atypically large number of participants. The Self-Defense Forces Central Hospital in Tokyo where symptomatic individuals were referred to is close to the Yokohama port where the cruise ship was docked. However, critical cases were preferentially referred to neighbouring designated hospitals in Yokohama that were equipped to handle infectious diseases. Likewise, some clinical signs and symptoms could have occurred and resolved before people were referred to our hospital, and thus might have been missed by our study. Additionally, laboratory tests and CT images were obtained only once for most patients, and thus we were unable to determine changes in lymphocyte count and radiological findings over the course of observation.
In conclusion, our study highlights clinical features of people infected with SARS-CoV-2 with various degrees of severity and shows that serum LDH, age, consolidation on CT scan, and lymphopenia might be predictors of disease severity. Multicentre, multinational cohort studies with large population sizes that use multivariable analysis should be done to more accurately determine predictive markers for symptomatic illness among people infected with SARS-CoV-2 and risk factors for disease progression.
Data sharing
The raw data used in this study are available from the corresponding author upon reasonable request.
Acknowledgments
Acknowledgments
We thank everyone involved in the COVID-19 management and treatment team from the Self-Defense Forces Central Hospital in Japan and members who were assembled from other institutes of Japan Self-Defense Forces. Particularly, we thank Koji Kameda, Takayuki Yamamoto, Daishi Higashiyama, Yoshitaka Imoto, Masataka Miyama, Tsukasa Mizuno, Kento Kato, Mamoru Honda, Shoji Takeda, Masumi Ogawa, Shingo Tanaka, Hisashi Sasaki, Mitsuki Yamaga, Shinichiro Ota, and Hiroaki Taniguchi for supporting the data collection, and Akira Fujikawa and Shohei Inui for the interpretation of chest CT images. We also thank Shingo Tamaki (School of Tropical Medicine and Global Health, Nagasaki University, Japan) for the supporting statistical analysis, Kazuhiro Nakaya (Medical Department, Ground Staff Office, Ministry of Defense) for the scientific advice, and Enago (Tokyo, Japan) for the English language review.
Contributors
TI and KT contributed to study conception and design. ST and KI contributed to data collection and analysis. ST, KI, MI, and KM contributed to drafting and editing the manuscript. TKoda, HO, SK, TKode, MK, and SM revised the manuscript. MS, SS, and YU provided study supervision. All authors read and approved the final manuscript.
Declaration of interests
We declare no competing interests.
Supplementary Material
References
- 1.Huang C, Wang Y, Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang JJ, Dong X, Cao YY. Clinical characteristics of 140 patients infected by SARS-CoV-2 in Wuhan, China. Allergy. 2020 doi: 10.1111/all.14238. published online Feb 19. [DOI] [PubMed] [Google Scholar]
- 3.Chen N, Zhou M, Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D, Hu B, Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li K, Wu J, Wu F. The clinical and chest ct features associated with severe and critical COVID-19 pneumonia. Invest Radiol. 2020;55:327–331. doi: 10.1097/RLI.0000000000000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lechien JR, Chiesa-Estomba CM, Place S. Clinical and epidemiological characteristics of 1,420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med. 2020 doi: 10.1111/joim.13089. published online April 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim GU, Kim MJ, Ra SH. Clinical characteristics of asymptomatic and symptomatic patients with mild COVID-19. Clin Microbiol Infect. 2020 doi: 10.1016/j.cmi.2020.04.040. published online April 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kakimoto K, Kamiya H, Yamagishi T, Matsui T, Suzuki M, Wakita T. Initial investigation of transmission of COVID-19 among crew members during quarantine of a cruise ship—Yokohama, Japan, February 2020. MMWR Morb Mortal Wkly Rep. 2020;69:312–313. doi: 10.15585/mmwr.mm6911e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Institute of Infectious Diseases Field briefing: Diamond Princess COVID-19 cases. Feb 19, 2020. https://www.niid.go.jp/niid/en/2019-ncov-e/9407-covid-dp-fe-01.html
- 10.Nao N, Shirato K, Katano H, Matsuyam S, Takeda M. Detection of second case of 2019-nCoV infection in Japan. National Institute of Infectious Diseases. Jan 25, 2020. https://www.niid.go.jp/niid/en/2019-ncov-e/9334-ncov-vir3-2.html
- 11.Zhang J, Zhou L, Yang Y, Peng W, Wang W, Chen X. Therapeutic and triage strategies for 2019 novel coronavirus disease in fever clinics. Lancet Respir Med. 2020;8:e11–e12. doi: 10.1016/S2213-2600(20)30071-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang J, Zheng Y, Gou X. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou F, Yu T, Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qin C, Zhou L, Hu Z. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa248. published online March 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guan W-j, Ni Z-y, Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen H, Guo J, Wang C. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan F, Ye T, Sun P. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. 2020;295:715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi H, Han X, Jiang N. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inui S, Fujikawa A, Jitsu M. Chest CT findings in cases from the cruise ship “Diamond Princess” with coronavirus disease 2019 (COVID-19) Radiol Cardiothorac Imaging. 2020;2 doi: 10.1148/ryct.2020200110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bai HX, Hsieh B, Xiong Z. Performance of radiologists in differentiating COVID-19 from viral pneumonia on chest CT. Radiology. 2020 doi: 10.1148/radiol.2020200823. published online March 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drent M, Cobben N, Henderson R, Wouters E, van Dieijen-Visser M. Usefulness of lactate dehydrogenase and its isoenzymes as indicators of lung damage or inflammation. Eur Respir J. 1996;9:1736–1742. doi: 10.1183/09031936.96.09081736. [DOI] [PubMed] [Google Scholar]
- 22.Quist J, Hill AR. Serum lactate dehydrogenase (LDH) in Pneumocystis carinii pneumonia, tuberculosis, and bacterial pneumonia. Chest. 1995;108:415–418. doi: 10.1378/chest.108.2.415. [DOI] [PubMed] [Google Scholar]
- 23.Lu A, Wang C, Zhang X, Wang L, Qian L. Lactate dehydrogenase as a biomarker for prediction of refractory mycoplasma pneumoniae pneumonia in children. Respir Care. 2015;60:1469–1475. doi: 10.4187/respcare.03920. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data used in this study are available from the corresponding author upon reasonable request.