Abstract
The mechanisms that regulate the balance between stem cell duplication and differentiation in adult tissues remain in debate. Using a combination of genetic lineage tracing and marker-based assays, the quantitative statistical analysis of clone size and cell composition has provided insights into the patterns of stem cell fate across a variety of tissue types and organisms. These studies have emphasized the role of niche factors and environmental cues in promoting stem cell competence, fate priming, and stochastic renewal programs. At the same time, evidence for injury-induced “cellular reprogramming” has revealed the remarkable flexibility of cell states, allowing progenitors to reacquire self-renewal potential during regeneration. Together, these findings have questioned the nature of stem cell identity and function. Here, focusing on a range of canonical tissue types, we review how quantitative modeling–based approaches have uncovered conserved patterns of stem cell fate and provided new insights into the mechanisms that regulate self-renewal.
Stem cells are defined by their ability to self-renew while giving rise to more differentiated progeny (Watt and Hogan 2000). In the adult, stem cells are responsible for the maintenance of renewing tissues, constantly replenishing differentiated cells lost through damage or exhaustion. Understanding how stem cells function to regulate the balance between cell duplication and differentiation in homeostasis, and regenerate tissue following injury, represents a defining question in stem cell biology.
Traditionally, efforts to define stem cell identity have focused on the search for marker-based characterizations. However, functional assays based on genetic lineage tracing (Kretzschmar and Watt 2012) and intravital imaging (Hara et al. 2014; Ritsma et al. 2014) have challenged the concept of discrete stem cell states residing at the apex of an “invariant one-way” hierarchy (Donati and Watt 2015; Clevers and Watt 2018; Ge and Fuchs 2018; Tai et al. 2019). By quantifying the fate behavior of labeled cells and their progeny (clones) in undisturbed tissue, the potency and renewal activity of targeted cell populations have been assessed over the long term. Through quantitative modeling–based approaches, statistical characterizations of cell lineage–tracing data have provided insight into the “patterns” of self-renewal, often challenging prevailing models of stem cell behavior (Klein and Simons 2011).
By addressing a selection of renewing tissue types, the aim of this review is to reflect on how statistical approaches and theoretical insights (Box 1) have reshaped, and at times revised, our understanding of stem cell identity and function. By resolving—often conserved—patterns of stem cell fate across different tissue types, such modeling-based schemes are providing a quantitative platform to frame targeted questions about the molecular mechanisms that regulate self-renewal. In the following sections, we have highlighted the narrative in which quantitative modeling–based reasoning is invoked, and set in bold key concepts that have emerged in stem cell biology.
Box 1. Quantitative Models in Biology.
The application of theoretical modeling–based methods in biology has a long and somewhat checkered history (Goldstein 2018). Reviled by some, models can be seen as an unnecessary, simplistic, and unreliable instrument to describe phenomena that may otherwise be readily apparent without the need for mathematical or computational abstraction. So, what is their value and when should they be invoked?
In stem cell biology, as in other areas of science, interest usually lies in deriving mechanistic understanding from experimental measures. However, the meaning of “mechanism” is open to different definitions and interpretations. In a given experimental data set, one might identify a structure, pattern, or correlate, such as statistical scaling behavior (see main text), which constitutes a “phenomenon.” Here, when we speak of “mechanism,” we have in mind a “phenomenology”: a minimal abstraction of the experimental system in the form of a hypothesis or “model” that affords both a faithful description of the phenomenon and, crucially, falsifiable predictions. When bootstrapped, this program represents the basis of the scientific method: experiment → model (i.e., mechanism) → prediction → experiment → (adjustment or refinement of the) model → prediction → experiment → and so on.
When dealing with quantitative phenomena, it is often useful to articulate a mechanism through the language of mathematics, questioning what is the minimal theoretical, or sometimes computational, model that is capable of capturing the experimental phenomena, and what are its predictions. Importantly, whether heuristic (a gene regulatory network) or mathematical, such a modeling framework does not, and should not, involve all, or sometimes even any(!), of the “microscopic” degrees of freedom. A biological mechanism does not have to be anchored in the language of genes and gene products. If this seems irregular, one might reflect on an example such as the phenomenon of flight in birds or insects, and question whether its mechanism lies in the metabolism or contractility of muscle cells or the aerodynamics of lift.
So when is a theoretical or modeling-based approach advantageous? In biology, we are often confronted with phenomena involving large assemblies of equivalent “agents,” be they molecules, cells, or organisms, subject to the same statistical (intrinsic and environmental) stimuli. Despite their inherent complexity, often robust and conserved collective, emergent behaviors can (and usually do) arise (Anderson 1972). It is in this context that theoretical modeling–based schemes can provide a framework to derive mechanistic understanding.
LESSONS FROM GERMLINE STEM CELL MAINTENANCE: FROM INVARIANT TO POPULATION ASYMMETRIC RENEWAL
Historically, studies of germline maintenance in the Drosophila melanogaster have provided fundamental insights into the regulation of stem cell self-renewal (Fuller and Spradling 2007; Lehmann 2012). In Drosophila testes, sperm production relies on the activity of germline stem cells (GSCs) that lie anchored by adherens junctions to specialized somatic hub cells (Fig. 1A). By providing a polarized source of “fate determinants” (including the ligands of the Unpaired family [Upd] and Decapentaplegic [Dpp]), the hub functions as a closed niche, maintaining GSC competence. During division, the anchoring of the mother centrosome to the adherens junction orients the spindle, positioning daughter cells away from the hub, and facilitating their entry into a differentiation pathway that begins in the gonialblast state (Fig. 1B). Through the combination of force-mediated spindle orientation and the localization of fate determinants, the niche provides a basis to regulate asymmetric GSC fate (Venkei and Yamashita 2018). But how generic and robust is such a mechanism of invariant asymmetric stem cell self-renewal, in which each and every stem cell division results in an asymmetric fate outcome?
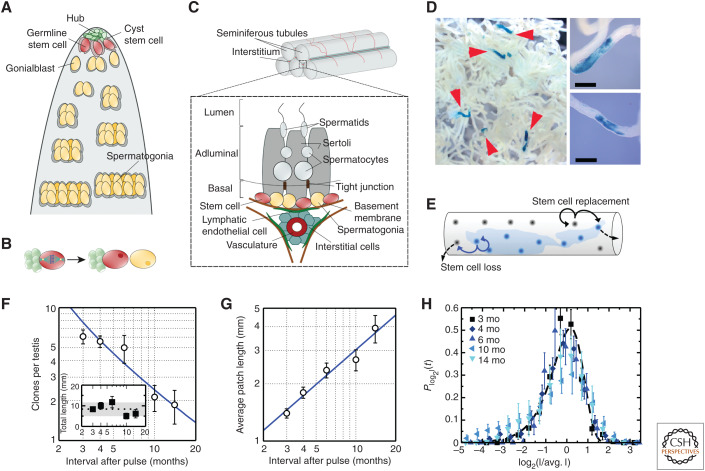
Figure 1.
Germline maintenance in the Drosophila and mouse testis. (A) Schematic of the Drosophila testis showing germline stem cells (GSCs) anchored by adherens junctions to somatic hub cells and ensheathed by cyst stem cells. The hub functions as a closed niche, supplying factors that maintain stem cell competence. (B) During GSC division, spindle orientation by the hub positions daughter cells away from the niche, leaving cells primed for differentiation and loss. (C) Schematic of the mouse testis showing spermatogonia roaming freely on the basement membrane of the seminiferous tubules. In homeostasis, spermatogonia expand through serial rounds of incomplete mitotic division before entry into meiosis when they translocate across tight junctions toward the lumen. GSCs are contained within a subpopulation of undifferentiated cells and are characterized by heterogeneous expression of markers. During the periodic seminiferous cycle, cells positive for the expression of retinoic acid receptor (RAR)γ are transferred by retinoic acid signaling into a differentiated (Kit+) cell compartment. With GSCs sharing the basement membrane with their differentiating progeny, the mouse testis provides an example of an open or facultative niche. (D) Patches of clonally labeled cells in the seminiferous tubules induced by a Ngn3 promoter at 3 months postinduction. Scale bars, 0.2 mm. (E–G) Schematic of the neutral drift model (E) showing stem cell loss through differentiation compensated by the duplication of neighbors leading to continual clonal loss (F) compensated by expansion of neighbors (G), as depicted in F (inset). Lines show prediction of neutral drift model. (H) When plotted against the rescaled patch length, the clone size distribution shows a collapse onto the scaling dependence predicted by the neutral drift model (shown as a dashed line). For further details, see Klein et al. (2010). (A and B are from Amoyel and Bach 2015; adapted, with permission, from Elsevier © 2015; C is from Kitadate et al. 2019; adapted under the terms of the Creative Commons Attribution License [CC BY]; D–H are from Klein et al. 2010; adapted, with permission, from Elsevier © 2010.)
In mouse, sperm production relies on the proliferation of spermatogonial stem cells (SSCs) that “roam” freely and actively among their differentiating progeny on the basement membrane of the seminiferous tubules in the testis (see Yoshida 2019). In homeostasis, SSCs self-renew, giving rise to differentiating progeny that transit through some 10 to 11 rounds of incomplete division before entering into meiosis, when they translocate across tight junctions separating the basal layer from the adluminal compartment (Fig. 1C). Further progression is then directed through distinct stages of maturation by periodic cycles of retinoic acid (RA) signaling that propagate as “phase” waves—like a “Mexican wave”—along the length of the tubules (de Rooij and Russell 2000). But where does stem cell function reside? Is self-renewal potential limited, as in Drosophila, to a defined subpopulation of singly isolated (Asingle) spermatogonia that undergo rounds of complete division, giving rise to Asingle cells committed to differentiation, the “As model” (Huckins 1971; Oakberg 1971)? Or is renewal potential distributed more widely to include syncytial pairs (Apair) or “aligned” chains (Aaligned-4, etc.)?
To assign stem cell identity, emphasis is often placed on finding signature patterns of gene expression. However, markers may overlap between functionally distinct populations, whereas expression levels may fluctuate or adjust in response to environmental cues (Graf and Stadtfeld 2008; Morrison and Spradling 2008; Tang 2012; Donati and Watt 2015). As a result, it is often instructive to focus on a functional definition of stem cell identity based on fate behavior, calling for a “dynamic” measure (LeBlond 1965). Using tritiated thymidine incorporation as a primitive clonal mark, early tracing studies by LeBlond and colleagues provided key insights into the functional identity of stem cells in cycling epithelial tissues (Clermont and LeBlond 1953). However, it was not until the advent of genetic lineage-tracing methods that the fate of targeted cell populations could be assessed in vivo over the long term. In this approach, the activation of one or many fluorescent reporter genes following the administration of an inducing agent confers a hereditary mark allowing the fate of individual clones to be traced in undisturbed tissue over a defined time course (Kretzschmar and Watt 2012).
But how can such “static” lineage-tracing measures be used to recover information on individual cell fate decisions? For a given clone, the history of fate decisions following cell division may be ambiguous, with multiple combinations and permutations of duplicative, asymmetric, and terminal divisions leading to the same outcome. Yet, from features of the statistical distribution of clone size and cell composition, and its evolution over time, quantitative information on the “rules” of cell fate can be recovered by—often straightforward—mathematical reasoning. In particular, if SSC fate were characterized by rounds of invariant asymmetric division, as predominates in Drosophila testis, the distribution of clone sizes would simply become fixed at the “unit size” of cells supported by an individual SSC. However, if fate asymmetry was enforced only at the level of the population, so that chance SSC loss through differentiation is compensated by duplication of others, a steady increase in the average size of surviving clones would be compensated by a proportionate reduction in clone density. Such processes, involving the balanced stochastic expansion, contraction, and loss of clones, constitute a dynamics known as “neutral drift” (Klein and Simons 2011).
Clone dynamics based on population asymmetric self-renewal finds a signature in the convergence of the resulting size distribution toward statistical “scaling” behavior in which the probability Cn(t) of finding a clone at a time t postinduction with a cell number n larger than some multiple of the average n(t) becomes fixed,
Notably, from the form of the “scaling function,” f(x), insight into the pattern of self-renewal can be inferred. In particular, when the balance between stem cell loss and replacement is correlated locally in space, the scaling function takes the form f(x) = exp[ − πx2/4] in the “quasi” one-dimensional geometry of the seminiferous tubules, whereas the average clone size grows as , where λ denotes the effective average stem cell loss/replacement rate.
Applied to the mouse testis, Yoshida and colleagues used such a quantitative lineage-tracing strategy to trace the long-term fate of cells marked by the expression of Neurogenin 3 (Ngn3), a gene enriched in undifferentiated spermatogonia “primed” for differentiation (Nakagawa et al. 2007). Consistently, following an initial large-scale loss of differentiation-primed clones, only a minority of clones were found to persist over the longer term, representing those rooted in the SSC population (Fig. 1D). Consistent with populational asymmetric fate, convergence of clone sizes onto the hallmark scaling clone size dependence (Fig. 1E–H) showed that SSC maintenance involves frequent and stochastic stem cell loss compensated by the duplication of neighbors along the seminiferous tubules (Klein et al. 2010).
Although this finding provided insight into the functional fate behavior of SSCs, it did not reveal their morphological identity (viz. Asingle, Apair, etc.) nor the underlying “mechanism” of fate balance. Indeed, the resolution of such fate “rules” posed a conundrum: With SSCs separated by numerous differentiating progenies on the basement membrane of the seminiferous tubules, how does SSC duplication and differentiation become coordinated locally to maintain density homeostasis? This is the challenge faced by all renewing tissues supported by an open or “facultative” niche in which stem cells lie dispersed among their differentiating progeny (Morrison and Spradling 2008).
Recently, insight into the basis of stochastic SSC renewal has been obtained. In a mechanism that generalizes the closed niche–based regulation of GSC fate in Drosophila testis, Yoshida and colleagues have argued that SSC maintenance relies on a self-organizing feedback mechanism involving competition for “fate determinants” (Kitadate et al. 2019). By correlating the reception and consumption (internalization) of secreted factors (including fibroblast growth factor [FGF] family members), released by (lymphatic endothelial) niche cells, with the inhibition of differentiation licensing factors (including retinoic acid receptor [RAR]-γ), SSCs are able to sense their local density and adjust their fate bias in response (Fig. 2A). When the local SSC density levels are low, FGF levels are high and SSCs are biased for renewal; when the density is high, FGF levels are low and SSCs become licensed (primed) toward differentiation and loss.
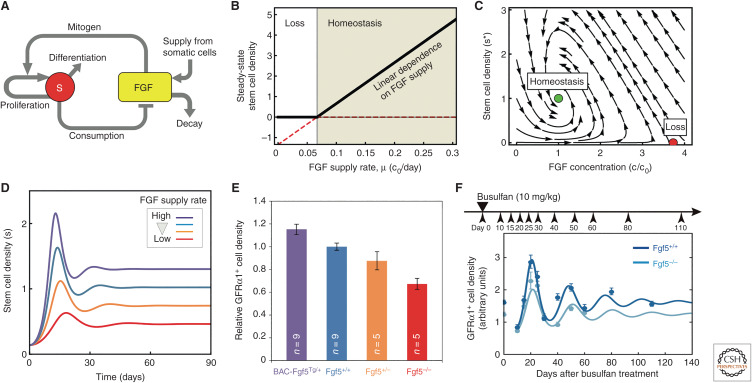
Figure 2.
Competition for fate determinants—a mechanism of population asymmetric self-renewal. (A) Model of the feedback competition mechanism showing the mutual regulation of the stem cell density and abundance of fate determinant (fibroblast growth factors [FGFs]). By correlating the inhibition of differentiation licensing factors (retinoic acid receptor [RAR]γ) with the reception and consumption of niche factors (FGFs) secreted by lymphatic endothelial cells, germline stem cells (GSCs) are able to sense their density and adjust their fate bias in response. (B) Above threshold, steady-state stem cell density is predicted to rise linearly with FGF concentration. (C) Phase portrait depicted the corresponding dynamics of stem cell density and FGF concentration. The system shows two fixed points: a homeostatic state (green dot) and a loss state (red dot). For the given parameter set, only the homeostatic state is stable, as all trajectories obtained by following the arrows converge toward this state. (D) When perturbed from homeostasis, the feedback model predicts an oscillatory phase of recovery back to steady state (as indicated in B). (E) Consistently, measurements of the spermatogonial stem cell (SSC) density, as assessed by the expression of the marker growth factor receptor (GFR)α1, shows that the stem cell density scales linearly with the allele fraction of the FGF5. (F) Moreover, following depletion using a chemical agent (busulfan), the SSC density shows an oscillation phase of recovery. For further details, see Kitadate et al. (2019). (A–F are from Kitadate et al. 2019; adapted under the terms of the Creative Commons Attribution License [CC BY].)
Once again, evidence for the density-dependent feedback mechanism was found through the development of a minimal modeling–based scheme in which temporal changes in the local stem cell density, s(t), depend on the concentration of fate determinant, c(t), through the kinetic equation
where λ denotes the SSC division rate, and the Hill-type function h(x) = xm/(1 + xm) interpolates smoothly between unity (viz. cell duplication) at high concentration, c ≫ c0, and zero (differentiation) at low concentration, c ≪ c0. At the same time, the concentration of fate determinant varies as
where μ denotes the effective production rate by (endothelial) niche cells, k is the degradation rate, and k′ is the consumption rate by SSCs, moderated by the factor 2h(c/c0), which accounts for the limiting effect of receptor concentration. In steady state, the model predicts an SSC density, , that depends linearly on the production rate of fate determinant (Fig. 2B), whereas displacement from steady state predicts an oscillatory return to homeostasis (Fig. 2C,D). Consistent with this, the analysis of genetic mouse mutants confirmed that SSC density varied linearly with the allele fraction of FGFs, whereas the recovery of SSC density following large-scale chemical ablation followed the predicted oscillatory dynamics (Fig. 2E,F; Kitadate et al. 2019).
As a basis to regulate stem cell fate, such a “Malthusian-like” feedback mechanism of density regulation, reminiscent of “quorum sensing” in bacterial populations (Miller and Bassler 2001), has many advantages. As well as providing a basis to regulate stem cell density both in homeostasis and in response to injury, it can adjust straightforwardly to heterogeneity or spatial variation in the concentration of niche factors, interpolating smoothly between an open and closed niche organization (Jörg et al. 2019). Although the model relies on the secretion and reception of local signaling factors, the feedback mechanism is easily generalized to accommodate mechanical cues based on cell crowding (Shraiman 2005; Hannezo et al. 2016; Yamaguchi et al. 2017; Yamaguchi and Kawaguchi 2019) or signals from differentiating progeny (Rodriguez-Brenes et al. 2013). Indeed, parallel studies have shown that a reciprocal feedback mechanism, in which the fate of each cell population is correlated with the reception of fate determinants secreted by the other, provides a robust and stable mechanism to regulate the proportion of tissue components (Zhou et al. 2018; Varahan et al. 2019).
Note that the competition mechanism bears similarities with the “chalone hypothesis” introduced in the 1960s as a framework to explain the potential regulation of proliferative activity in epithelial tissues (Bullough 1962). In this model, secreted factors, chalones, from differentiating cells were thought to regulate cell-cycle progression, so that the production rate of differentiated cells can be matched to their demand. Such a “mitogen” competition-like mechanism, which might function in concert with competition for niche factors, acts through proliferative activity and not through affecting changes in fate outcome. Intriguingly, support for such a mechanism has been found in the regulation of the hair follicle, acting to ensure the “episodic” regulation of the hair cycle (Plikus et al. 2009).
A further feature, implicit in the feedback mechanism, is the manifestation of fate priming: When deprived of access to fate determinants by cell crowding, the up-regulation of differentiation licensing factors leaves SSCs primed for differentiation, becoming committed only when exposed to the periodic RA signal. If SSCs regain access to fate determinants before commitment, they may readjust their fate bias toward renewal, a capability evidenced by the massively increased persistence of Ngn3-targeted clones following SSC loss (Nakagawa et al. 2007). Such behavior undermines the traditional concept of an invariant equipotent stem cell state defined by a discrete gene expression signature. Instead, SSCs are characterized by variable and changing survival potential, responding reversibly to changes in local niche factor concentration, reflected in a heterogenous and dynamic gene expression pattern (see Yoshida 2019). But are invertebrates so different? Indeed, when traced over the longer term, a similar pattern of “cell state flexibility” is found in both the male (Sheng and Matunis 2011) and the female (Kronen et al. 2014) Drosophila germline, suggesting that such behavior may be a conserved feature of stem cell regulation in gonads and potentially other tissue types (see below).
Finally, the feedback mechanism also provides a basis to explain the phenomenon of “selfish selection” in human spermatogenesis (Maher et al. 2014). By promoting a competitive advantage, activating mutations in Fgf receptor genes or Ras family confers a survival advantage on SSCs, driving nonneutral mutant clone expansion (Jörg et al. 2019) leading to progressive field transformation, similar to the process of field cancerization (Curtius et al. 2018), a vulnerability evidenced by the increased prevalence of certain congenital diseases in the offspring of aging fathers.
NEUTRAL DRIFT DYNAMICS AND CELL STATE FLEXIBILITY IN THE INTESTINAL CRYPT
We turn now to consider a second canonical stem cell system: the mammalian small intestine. The small intestine is composed of glands, crypts of Lieberkühn, that invaginate into the stroma and villi that protrude into the gut lumen (Peterson and Artis 2014). The large intestine (colon) has a similar organization but lacks villi. The small intestine is lined by a columnar epithelium comprising intestinal stem cells (ISCs), lineage-restricted progenitors, and differentiated absorptive and secretory cells (Grün et al. 2015). In homeostasis, ISCs positioned at the crypt base self-renew, giving rise to progenitors and their progeny that actively move in migration streams along the axis of the crypt and onto the villus (Krndija et al. 2019), where eventually they shed (Fig. 3A).
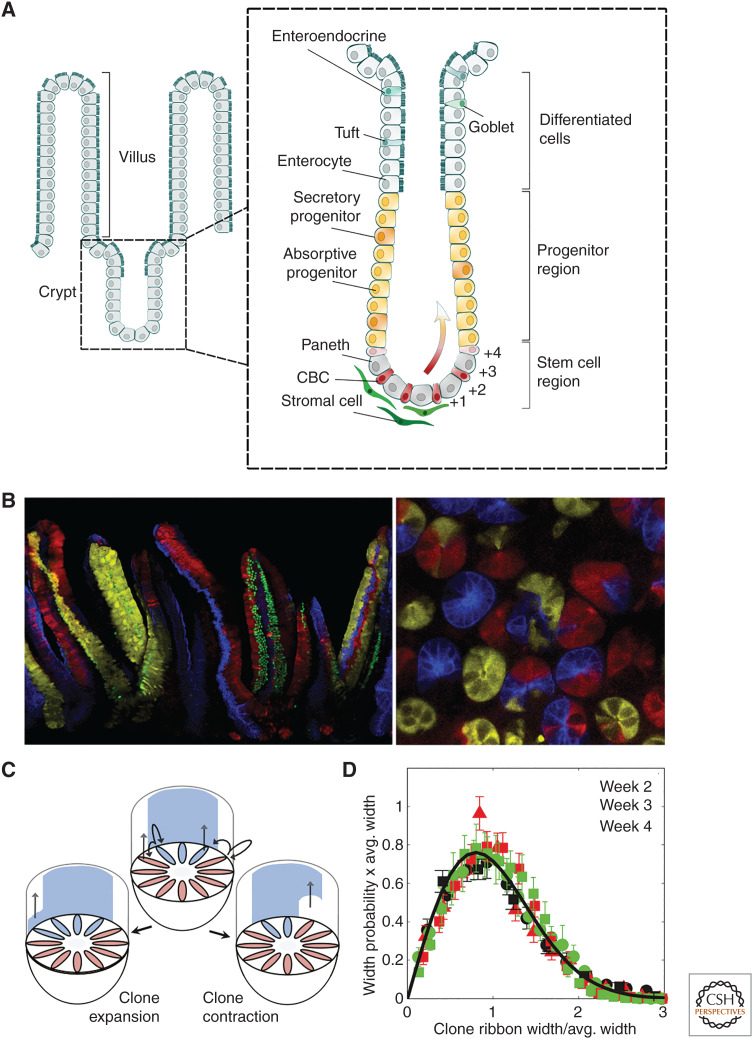
Figure 3.
Maintenance of the mouse intestinal crypt. (A) The columnar epithelium of the small intestine is maintained by intestinal stem cells (ISCs), identified as crypt base columnar cells (CBCs), which localize around the base of glandular invaginations known as crypts. ISCs give rise to sublineage-restricted progenitors that differentiate into secretory and absorptive cells that, together, move in migration streams along the axis of the crypt and onto the villus, where they shed. Factors secreted by secretory (Paneth) cells as well as stromal cells provide a niche environment that maintain stem cell competence. As ISCs divide, some become displaced from the niche and enter into a differentiation program. (B) Lineage tracing using the multicolor R26R-Confetti reporter system induced at high (mosaic) labeling density at 8 wk (left) and 4 wk (right) postinduction. The left-hand image is a vertical section showing ribbons of lineage-labeled cells moving along the axis of the crypts (bottom) and onto the villi (top). The right-hand image is a horizontal section near the base of the crypt showing clusters of lineage-labeled cells expanding around the circumference of the crypt. (C) Following genetic labeling, ISC-derived clones undergo a process of neutral drift in which clones expand and contract around the crypt base circumference until the clone is lost or the crypt becomes monoclonally fixed. (D) Neutral drift dynamics of clone widths is evidenced by convergence of their size distribution onto statistical scaling behavior at intermediate times (see main text). This behavior masks a more refined organization in which ISCs positioned near the base of the crypt are biased toward duplication, whereas those at the niche border (defined by the range Lgr5 expression) are primed, but not committed, for differentiation (see main text). (A is based on data in Gehart and Clevers 2019; B is from Snippert et al. 2010; adapted, with permission, from Elsevier © 2010; C and D are from Lopez-Garcia et al. 2010; adapted, with permission, from The American Association for the Advancement of Science © 2010.)
The identity and fate behavior of ISCs has been long in debate (Gehart and Clevers 2019): Early studies by Cheng and LeBlond (1974) placed emphasis on slender crypt-base columnar cells (CBCs), whereas, later, attention switched to “labeling-retaining” cells positioned near the +4 cell position along the crypt axis (Potten et al. 1974). However, it was not until the advent of genetic labeling techniques that the dynamics of renewing cells could be traced over the long term (Barker et al. 2007). Using a lineage-tracing strategy based on a leucine-rich repeat-containing G-protein-coupled receptor 5 (Lgr5) promoter, the monoclonal conversion of individual crypts confirmed that epithelial cell types were maintained by multipotent ISCs. But what is their multiplicity and pattern of renewal?
Once again, using a static lineage-tracing approach, evidence for the fate behavior of ISCs was sought in the statistical distribution of clone sizes, indexed by the circumferential width of labeled “ribbons” of cells that migrate along the axis of the crypt (Fig. 3B). As in the mouse testis, convergence of the clone size distribution onto the same hallmark statistical scaling behavior supported a model in which stochastic ISC loss through differentiation was compensated by the duplication of neighbors (Fig. 3C,D), leading to neutral drift of clones around the crypt base until clones are lost or the crypt becomes monoclonally fixed (Williams et al. 1992; Lopez-Garcia et al. 2010; Snippert et al. 2010). But what is the multiplicity of ISCs and their rate of loss and replacement, λ? From a fit to the average clone size dependence, it was possible to obtain only an estimate of the ratio of N2/λ, where N denotes an “effective” ISC number. However, later, these parameters were disentangled in a clever strategy based on continuous clone induction using measurements of the ratio of partially labeled to monoclonal crypts (Kozar et al. 2013). Based on this analysis, estimates of the effective stem cell number—both in mouse and human tissue (Nicholson et al. 2018; Stamp et al. 2018)—ranged from ∼N = 4 to 6, depending on the region of the intestine, a figure notably smaller than the number of Lgr5+ CBCs, taken by many to be a proxy for ISC identity.
But what is the meaning of the effective ISC number, and what is the basis of the stochastic renewal program? Although the minimal one-dimensional model, depicted in Figure 3C, predicts the medium- to long-term clone dynamics of ISCs, it represents only a caricature of a more complex cellular organization at the crypt base. To gain deeper insight into the cellular dynamics and fate, intravital live imaging was used to reconstruct in time-lapse lineages of individual Lgr5-targeted clones at the crypt base over a several-day time course (Ritsma et al. 2014). Extending the one-dimensional model, quantitative statistical analysis of the lineage data showed that the emergence of statistical scaling behavior in the medium term masks a more refined process in which ISCs positioned at the crypt base experience a bias toward renewal (i.e., are more likely to persist over the long term), whereas those positioned near the border of the niche (defined by the range of Lgr5 expression) are primed, but not yet committed, for differentiation (i.e., are more likely to be lost through differentiation over the long term).
Taken together, this behavior echoes that resolved in mouse testis. ISC competence is mediated by access to “fate determinants,” including Wnt, Notch, and other signaling factors derived from secretory (Paneth) cells and stromal components (Tan and Barker 2014; Santos et al. 2018). As ISCs divide, others become displaced, losing access to niche factors and leaving them poised (licensed) for differentiation, with commitment occurring in response to secondary signals. Consistently, manipulation of the levels of R-spondin, an activator of canonical Wnt signaling, showed that the size of the functional ISC pool (and range of Lgr5 expression) adjusts rapidly to changes in the niche (Yan et al. 2017b). When viewed from this perspective, a large number of intestinal cells—characterized by a dynamic and heterogeneous expression pattern linked to niche location—maintain self-renewal potential, whereas only a limited, effective number (abstracted from the mapping of the clone dynamics to the minimal one-dimensional neutral drift model) function to renew over the intermediate term, and only one, by chance, “succeeds” in the long term.
Notably, such a renewal mechanism provides a framework to understand how mutations that stimulate and/or recruit local niche factors, or activate signaling receptors, can promote nonneutral cell competition and mutant clone expansion, driving accelerated drift to monoclonality. Consistently, analysis of lineage-tracing data following the activation of Kras or deletion of adenomatous polyposis coli (APC) shows that the dynamics of mutant clones can be captured quantitatively through a minimal refinement of the one-dimensional model (Vermeulen et al. 2013; Snippert et al. 2014; Huels et al. 2018).
As well as showing evidence for reversible fate priming under normal homeostatic conditions, lineage-tracing studies based on injury models show that, echoing the behavior in the germline, intestinal progenitors normally committed to differentiation into either secretary or absorptive lineages are able to reacquire long-term self-renewal potential in response to injury, pointing to cell state flexibility (van Es et al. 2012; Buczacki et al. 2013; Roche et al. 2015; Tetteh et al. 2016; Jadhav et al. 2017; Yan et al. 2017a; Tomic et al. 2018). Although the mechanism of damage-induced “cellular reprogramming” remains in debate, one study based on single-cell profiling analysis proposes the role of a “revival” stem cell, a distinct quiescent cell that repopulates the adult stem cell compartment in response to injury (Ayyaz et al. 2019), whereas other studies based on injury models (Yui et al. 2018) and organoid assays (Serra et al. 2019) suggest the reacquisition of a developmental-like state. Yet, the manifestation of cell state flexibility, emerging as a conserved feature of multiple epithelial tissue types (Donati and Watt 2015; Clevers and Watt 2018; Ge and Fuchs 2018; Tai et al. 2019), further undermines a rigid classification of stem cell identity.
Despite the success of the neutral drift theory as a model of clonal dynamics in the intestinal crypt, puzzling features still remain. Among these is the observation of a seemingly low rate of clonal drift in the human colonic crypt, with clone fixation times measured in years (Nicholson et al. 2018; Stamp et al. 2018). Whether this behavior reflects a preponderance of asymmetric ISC divisions or betrays the existence of a lineage hierarchy characterized by a quiescent ISC subpopulation at its apex remains an intriguing and important open question.
As with the intestine, the stomach is compartmentalized into anatomically distinct regions (Mills and Shivdasani 2011) comprising the proximal forestomach, which forms a squamous epithelium, the glandular corpus, and the distal glandular pylorus, which lies adjacent to the duodenum. The glandular compartments are organized into units comprising a gland base, a neck, and an isthmus domain connecting to a pit compartment that opens out onto the gastric surface epithelium. Once again, quantitative clonal lineage-tracing studies show that maintenance of the pyloric glands mimics the organization of the intestinal crypt, with turnover of the epithelium supported by the neutral competition of Lgr5+ stem cells at the gland base (Leushacke et al. 2016). However, maintenance of the corpus epithelium is distinct.
Recently, tracing studies based on targeted reporters show that the base and isthmus regions of the corpus epithelium are maintained by distinct stem cell pools. The base region is replenished by slow-cycling Lgr5/Troy+ targeted chief cells, which function as a reserve population capable of regenerating the entire gland in response to damage (Stange et al. 2013; Leushacke et al. 2017). By contrast, the upper region is maintained by a pool of rapidly cycling isthmus-localized stem cells that give rise to lineage-restricted progeny that segregate bilaterally along the axis of the crypt, giving rise to distinct differentiated cell types. In a generalization of the intestinal crypt dynamics, quantitative lineage-tracing analysis reveals an isthmus stem cell renewal program involving “punctuated” neutral drift in which long-lived parietal cell barriers restrict the lateral expansion of clones around the gland circumference (Han et al. 2019), a behavior resonant with the slow dynamics seen in the human colonic crypt. Once again, analysis of single-cell expression profiling data suggests that, in common with ISCs, isthmus stem cells are not characterized by a single gene expression signature, but are heterogeneous, with survival potential linked to the exposure to, as yet uncharacterized, localized niche factors.
MAINTENANCE OF SQUAMOUS EPITHELIA
Staying with the theme of epithelial tissues, we now turn to skin. In mammals, the interfollicular skin epidermis (IFE) comprises a squamous stratified tissue interspersed with hair follicles, sebaceous glands, and sweat glands (Rognoni and Watt 2018). The esophagus comprises a similar structure but lacks most of the skin appendages. In both cases, the constant turnover of tissue is supported by stem cells confined to the basal layer (Fig. 4A). As some cells divide, others detach, entering into a differentiation program that leads to the formation of dead flattened squames that eventually slough from the surface.
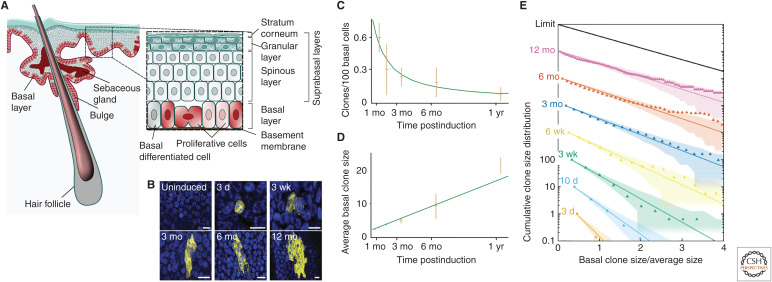
Figure 4.
Skin interfollicular epidermis, a squamous epithelial tissue. (A) The mouse skin interfollicular epidermis (IFE) comprises a stratified squamous epithelium interspersed with hair follicles, sweat glands, and sebaceous glands. All cell division in IFE takes place in the basal layer. Following commitment to terminal differentiation, cells delaminate from the basal layer and enter the suprabasal layers, where they mature into functional differentiated cell types before being shed from the skin surface. The mouse esophagus shows a similar organization as skin, but lacks most of the skin appendages. (B) Section through the basal layer of mouse esophagus showing typical clones induced using a ubiquitous promoter at a range of time points. Note that, despite expansion, clones remain roughly cohesive over time. (Cell nuclei marked by DAPI are blue. Scale bar, 10 μm.) In homeostasis, cells lost from the basal layer through differentiation are replenished by neighbors, leading to neutral drift dynamics of the clonal population. (C–E) During this process, continual loss of basal clones (C) is compensated by a near-linear increase in the average size of surviving clones (D), whereas the size distribution (E) converges to the hallmark exponential scaling dependence. (A is from Jones and Simons 2008; adapted, with permission, from the authors who retained the copyright to reuse their own work; B–E are from Doupé et al. 2012; adapted, with permission, from The American Association for the Advancement of Science © 2012.)
Despite decades of investigation, the basis of epidermal homeostasis remains controversial (Rognoni and Watt 2018). Based on tritiated thymidine incorporation, Marques-Pereira and LeBlond proposed that maintenance of the rodent esophageal epithelium involved a stochastic program in which chance cell differentiation delamination is compensated by division (Marques-Pereira and Leblond 1965). Later, in epidermis, emphasis was placed on a hierarchical model in which IFE is compartmentalized into a mosaic of “epidermal proliferative units,” each supported by a stem cell and its transit-amplifying (TA) cell progeny, the latter having limited proliferative potential (Mackenzie 1970; Potten 1974). However, it was only through the advent of genetic labeling that the fate of renewing cells could be traced over the long term.
Once again, applied to mouse tail epidermis, quantitative analysis of genetic lineage-tracing data revealed progressive clonal loss allied with a linear-like increase in the average size of surviving clones (Clayton et al. 2007). At the same time, convergence of the clone size distribution onto statistical scaling behavior, Cn(t) = f(n/〈n(t)〉), with a scaling function f(x) = exp[ − x], suggested a pattern of maintenance involving the balanced stochastic loss and replacement of a single renewing “progenitor” compartment. Later, similar behavior was reported in mouse ear (Doupé et al. 2010), paw (Lim et al. 2013), oral mucosa (Jones et al. 2019), and esophagus (Fig. 4B–E; Doupé et al. 2012), pointing to a conserved pattern of self-renewal in squamous epithelia. However, in contrast to the one-dimensional dynamics of the seminiferous tubules and intestinal crypt, in the two-dimensional arrangement of the skin epidermis, it is not possible to determine whether cell duplication is correlated locally with differentiation delamination, as both translate to the same scaling dependence (Klein and Simons 2011). Elegant short-term intravital live imaging by Greco and colleagues later argued in favor of a program in which cell delamination promotes the division of neighbors, echoing the model of Marques-Pereira and LeBlond (Rompolas et al. 2013; Mesa et al. 2018).
Despite the appeal of this simple “one-progenitor cell” paradigm—a manifestation of “voter model” dynamics in statistical physics (Klein and Simons 2011)—it remains controversial. First, culture assays based on primary human keratinocytes provide compelling evidence in support of a proliferative hierarchy, with some cells showing long-term colony-forming capacity (holoclones)—used therapeutically in transplantation—whereas others show only limited proliferative potential (paraclones) (Barrandon and Green 1987; see De Rosa et al. 2019). Moreover, tracing studies based on targeted promoters (Mascre et al. 2012) provide evidence for proliferative heterogeneity in mouse tail IFE, findings supported by the identification of two distinct IFE differentiation programs in the tail (Gomez et al. 2013) and the differential tumor-initiating capacity of targeted populations (Sánchez-Danés et al. 2016).
Further work is required to resolve the question of proliferative heterogeneity in squamous epithelia. As with germline and intestinal maintenance, the ubiquity of long-term scaling behavior of clone size exposes a strength and limitation of the modeling-based method. Because multiple cell-based models of renewal translate in the long term to the same statistical scaling dynamics, fine-scale structure of the renewing population and its progeny are difficult to resolve. For example, if differentiation occurred via an intermediate subpopulation of TA-like basal cells, the long-term dynamics would be characterized by the same exponential scaling behavior. Further progress will require emphasis on the mechanisms that underpin stochastic renewal programs. Indeed, one possible explanation for the conflicting reports in skin is that the basal cell layer may constitute a facultative niche environment in which proliferative activity is regulated by local mechanical cues, but fate outcome is informed by access to signaling factors from dermal components and/or neighboring epithelial cells (Miroshnikova et al. 2018; Wickström and Niessen 2018; Mobasseri et al. 2019). By correlating the inhibition of differentiation licensing factors with reception of fate determinants, the regulation of density homeostasis may mirror the dynamics of SSCs. Variations in the local concentration or diversity of niche components, which correlate with changes in gene expression of epithelial cells, could explain the observed heterogeneity in the long-term survival potential—and tumor-initiating capacity—of targeted basal populations. Such factors could also explain variations in proliferative activity (Jones et al. 1995) and/or prime the in vitro clonogenic capacity of keratinocytes in human IFE.
Similar patterns of stochastic self-renewal have been defined from lineage-tracing studies of other “extended” epithelial tissues, from the pseudostratified columnar epithelium of mouse trachea (Watson et al. 2015) to (the structurally similar) Drosophila midgut (De Navascués et al. 2012). Indeed, in the context of trachea, differentiated (club) cells have been shown to undergo damage-induced cellular reprogramming following stem cell loss (Tata et al. 2013), similar to the cell state flexibility reported in the intestinal crypt, whereas reciprocal feedback mechanisms have been proposed as a mechanism to balance the size of the epithelial cell compartments (Pardo-Saganta et al. 2015).
HEMATOPOIESIS
A survey of the mechanisms of stem cell self-renewal would be incomplete without a discussion of blood. The blood system is composed of two major lineages, the myeloid and lymphoid (Boisset and Robin 2012). Using bone marrow transplantation to rescue lethally irradiated mice, early pioneering work by Till and McCulloch showed that these lineages are derived from a single multipotent stem cell (McCulloch and Till 1960; Siminovitch et al. 1963). Since then, transplantation assays have been widely used to study hematopoietic stem cell (HSC) behavior and lineage relationships. Combined with in vitro colony-forming assays (Iscove et al. 1970), these studies established a classic hierarchical organization in which slow-cycling HSCs, with long-term clonogenic capacity, give rise to more rapidly cycling HSCs with limited proliferative potential. These cells then give rise to cycling multipotent progenitors (MPPs) that, in turn, differentiate into the myeloid and lymphoid sublineages. But, to what extent do culture and transplantation assays reflect the dynamics of renewing cell in unperturbed tissue (a key question pertinent to other tissue types)?
Recently, advances in lineage-tracing technology have begun to allow the fate of HSCs and their progeny to be traced in undisturbed mouse tissue (Sun et al. 2014; Pei et al. 2017; Rodriguez-Fraticelli et al. 2018). Challenging the prevailing hierarchical model, these studies suggest that hematopoiesis relies largely on the renewal activity of MPPs, whereas HSCs appear to make only a minimal contribution during normal homeostasis. Indeed, without preconditioning, the most primitive HSCs (as assessed by transplantation potential) rarely contribute even during regeneration after targeted stem and progenitor cell depletion (Schoedel et al. 2016). As well as challenging the function of HSCs, these findings also question how actively proliferating MPPs are able to renew in the facultative environment of the bone marrow niche. One possibility is that, once again, in common with germline, density regulation of actively migrating MPPs may be controlled by competition for fate determinants, whereas slow-cycling HSCs contribute only infrequently to the MPP pool, protecting genomic integrity over the long term. Indeed, preliminary evidence for such behavior is revealed in the nonmonotonic pattern of recovery of MPP density following fluorouracil (5-FU) treatment of mice (Schoedel et al. 2016), a hallmark of density-dependent feedback (Jörg et al. 2019). Further support for such a model is provided by long-term clonal lineage tracing using lentiviral labeling of HSCs transplanted into rhesus macaques (Goyal et al. 2015).
In a model in which asymmetric HSC divisions at rate μ contribute infrequently and stochastically to a population of MPPs that renew through competition for fate determinants, the clone size distribution is predicted to converge to a negative binomial form, formally a “birth–death process with immigration” (Goyal et al. 2015; Simons 2016),
where λ denotes the effective MPP loss/replacement rate and n0 denotes the average output of a clonally labeled MPP. Notably, serial measurements of clone size over a 4- to 12-yr time course provide evidence for the convergence of clone size to a stationary form with statistical size distribution that matches well with that predicted by the model (Goyal et al. 2015).
BEYOND HOMEOSTASIS
So far, our focus has been on the dynamics of adult tissues in which stem cells must self-renew. By contrast, the proliferative potential and potency of progenitors may change during development, to ensure that tissues are specified with the correct size, pattern, and composition. These contrasting demands suggest that the programs that regulate the changing balance between cell duplication and differentiation of progenitors may be different from those that control adult stem cell fate. These differences are emphasized in the context of mouse cortical development, in which quantitative analysis of clonal fate data shows that radial–glial progenitors follow an unfolding and “deterministic”-like fate program, transferring sequentially within and between “competence states” (Gao et al. 2014). Such behavior echoes that found in invertebrates, where temporal changes in neural progenitor fate are mediated by cascades of transcription factor expression (Holguera and Desplan 2018).
However, in later-stage developmental processes, the recursive output of differentiating cell types may involve the “renewal” activity of precursors, invoking fate programs similar to those seen in adult. The situation is exemplified by the development of ductal tissues, such as the mammary gland epithelium. In mouse, the mammary gland initiates embryonically as a placode-like structure along the ventral epidermis. At birth, epithelial cells form a rudimentary tree-like structure that invades into an adipocyte-rich stroma known as the fat pad. Then, during puberty, tip-localized mammary stem cells (MaSCs), restricted to the myoepithelial basal and luminal sublineages, act cooperatively to drive serial rounds of ductal elongation and bifurcation, leaving behind a ramified ductal network. But what regulates the fate behavior of MaSCs, coordinating the large-scale patterning of tissue?
Using a combination of lineage tracing and whole gland reconstruction, statistical modeling–based analyses have linked network growth to a “self-organizing” process based on a minimal set of local rules (Hannezo et al. 2017; Scheele et al. 2017). Within this framework, tips—known as terminal end buds (TEBs)—constitute a crypt-like niche environment, supporting the renewal activity of MaSCs driving ductal growth. By correlating ductal termination (signaled by collective cell-cycle exit) with exposure to factors secreted from maturing ducts, the network develops as a “branching-annihilating random walk.” Within this framework, active TEBs self-organize into a (soliton-like) pulse at the edge of the growing network. Density-dependent feedback drives the system to a critical state in which ductal bifurcation is balanced by termination. Remarkably, evidence for the same self-organizing mechanism is found in the development of other ductal tissue types including mouse pancreas (Sznurkowska et al. 2018) and kidney (Hannezo et al. 2017). Although these studies define the basis for the large-scale organization and patterning of tissue, they do not identify the mechanisms that ensure MaSC renewal. However, given the stochastic nature of individual fate decisions, niche-based competition mechanisms are likely to be relevant.
OUTLOOK
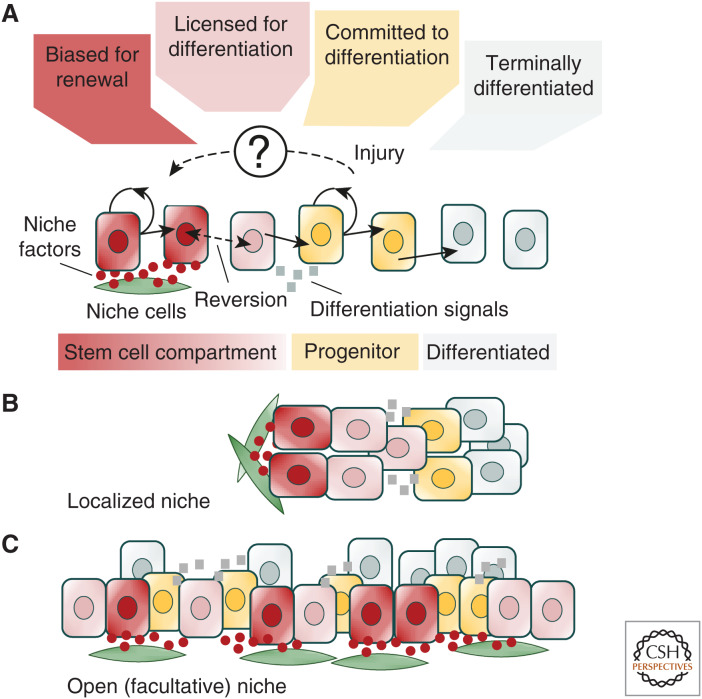
Over the past decade, understanding of the cellular mechanisms that govern tissue maintenance and repair have advanced. At the same time, perspectives on the identity and functional fate behavior of stem cells have been revised. At the turn of the century, tissue stem cells were widely thought to be discrete, individually long-lived, and defined by signature expression of molecular markers. Twenty years later, functional lineage-tracing studies have shown that stem cell potential is not “invariant” but, through exposure to local niche factors and environmental cues, cells may switch reversibly between states biased for renewal or primed for differentiation. At the same time, progenitors normally fated for cell differentiation and loss may reacquire long-term self-renewal potential following injury (see Rajagopal 2019). Through the quantitative statistical analysis of long-term static lineage tracing and short-term intravital live-imaging data, conserved “principles” of stem cell fate have emerged. Together, these studies emphasize that self-renewal potential is not invested in individual cells but rests within a “community” in which interactions with the environment allow stem cells to sense their density and adjust continuously their fate bias in response (Fig. 5). Although these “rules” of cell fate do not, in themselves, disclose the underlying molecular mechanisms, they provide a quantitative platform to frame targeted questions into the programs that regulate cell fate decision-making and the basis of cell commitment.
Figure 5.
Niche-based model of stem cell regulation. (A) Schematic summarizing the regulation of stem cell density homeostasis based on the competition for niche factors. The reception of factors secreted from niche cells inhibits stem cell differentiation, leaving cells biased for renewal. When deprived of these factors, the up-regulation of differentiation licensing factors leaves stem cells primed, but not committed to differentiation. Reexposure to niche factors allows stem cells to reverse their fate bias. However, following exposure to secondary differentiation cues, released either by differentiating progeny or extrinsic signals, stem cells enter into a program that leaves them committed to differentiation. Following injury, progenitors may reprogram, reacquiring stem cell competence either directly or via some intermediate state. (B) In a discrete or localized niche, such as that found in the Drosophila germline or intestinal crypt, stem cells become spatially segregated from their differentiating progeny. (C) By contrast, in an open or facultative niche, such as that found in mouse testis, stem cells lie intermingled among their differentiating progenies.
ACKNOWLEDGMENTS
We acknowledge discussions with past and present members of the Simons laboratory, including P. Greulich, E. Hannezo, A. Klein, and S. Rulands, as well as M. Alcolea, C. Blanpain, H. Clevers, K. Jensen, J. van Rheenen, and S. Yoshida. B.D.S acknowledges funding from the Royal Society through the EP Abraham Research Professorship (RP\R1\180165) and the Wellcome Trust (098357/Z/12/Z). L.C. acknowledges the support of the Herchel Smith Fund.
Footnotes
Editors: Cristina Lo Celso, Kristy Red-Horse, and Fiona M. Watt
Additional Perspectives on Stem Cells: From Biological Principles to Regenerative Medicine available at www.cshperspectives.org
REFERENCES
*Reference is also in this collection.
- Amoyel M, Bach EA. 2015. MT-nanotubes: lifelines for stem cells. Cell Stem Cell 17: 133–134. 10.1016/j.stem.2015.07.004 [DOI] [PubMed] [Google Scholar]
- Anderson PW. 1972. More is different. Science 177: 393–396. 10.1126/science.177.4047.393 [DOI] [PubMed] [Google Scholar]
- Ayyaz A, Kumar S, Sangiorgi B, Ghoshal B, Gosio J, Ouladan S, Fink M, Barutcu S, Trcka D, Shen J, et al. 2019. Single-cell transcriptomes of the regenerating intestine reveal a revival stem cell. Nature 569: 121–125. 10.1038/s41586-019-1154-y [DOI] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, et al. 2007. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007. 10.1038/nature06196 [DOI] [PubMed] [Google Scholar]
- Barrandon Y, Green H. 1987. Three clonal types of keratinocyte with different capacities for multiplication. Proc Natl Acad Sci 84: 2302–2306. 10.1073/pnas.84.8.2302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisset J-C, Robin C. 2012. On the origin of hematopoietic stem cells: progress and controversy. Stem Cell Res 8: 1–13. 10.1016/j.scr.2011.07.002 [DOI] [PubMed] [Google Scholar]
- Buczacki SJA, Zecchini HI, Nicholson AM, Russell R, Vermeulen L, Kemp R, Winton DJ. 2013. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature 495: 65–69. 10.1038/nature11965 [DOI] [PubMed] [Google Scholar]
- Bullough WS. 1962. The control of mitotic activity in adult mammalian tissues. Biol Rev 37: 307–342. 10.1111/j.1469-185X.1962.tb01615.x [DOI] [PubMed] [Google Scholar]
- Cheng H, Leblond CP. 1974. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V: Unitarian theory of the origin of the four epithelial cell types. Am J Anat 141: 537–561. [DOI] [PubMed] [Google Scholar]
- Clayton E, Doupé DP, Klein AM, Winton DJ, Simons BD, Jones PH. 2007. A single type of progenitor cell maintains normal epidermis. Nature 446: 185–189. 10.1038/nature05574 [DOI] [PubMed] [Google Scholar]
- Clermont Y, LeBlond C. 1953. Renewal of spermatogonia in the rat. Am J Anat 93: 475–501. 10.1002/aja.1000930308 [DOI] [PubMed] [Google Scholar]
- Clevers H, Watt FM. 2018. Defining adult stem cells by function, not by phenotype. Annu Rev Biochem 87: 1015–1027. 10.1146/annurev-biochem-062917-012341 [DOI] [PubMed] [Google Scholar]
- Curtius K, Wright NA, Graham TA. 2018. An evolutionary perspective on field cancerization. Nat Rev Cancer 18: 19–32. 10.1038/nrc.2017.102 [DOI] [PubMed] [Google Scholar]
- De Navascués J, Perdigoto CN, Bian Y, Schneider MH, Bardin AJ, Martínez-Arias A, Simons BD. 2012. Drosophila midgut homeostasis involves neutral competition between symmetrically dividing intestinal stem cells. EMBO J 31: 2473–2485. 10.1038/emboj.2012.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rooij DG, Russell LD. 2000. All you wanted to know about spermatogonia but were afraid to ask. J Androl 21: 776–798. [PubMed] [Google Scholar]
- *.De Rosa L, Latella MC, Secone Seconetti A, Cattelani C, Bondanza S, De Luca M. 2019. Toward combined cell and gene therapy for genodermatoses. Cold Spring Harb Pespect Biol. 10.1101/cshperspect.a035667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donati G, Watt FM. 2015. Stem cell heterogeneity and plasticity in epithelia. Cell Stem Cell 16: 465–476. 10.1016/j.stem.2015.04.014 [DOI] [PubMed] [Google Scholar]
- Doupé DP, Klein AM, Simons BD, Jones PH. 2010. The ordered architecture of murine ear epidermis is maintained by progenitor cells with random fate. Dev Cell 18: 317–323. 10.1016/j.devcel.2009.12.016 [DOI] [PubMed] [Google Scholar]
- Doupé DP, Alcolea MP, Roshan A, Zhang G, Klein AM, Simons BD, Jones PH. 2012. A single progenitor population switches behavior to maintain and repair esophageal epithelium. Science 337: 1091–1093. 10.1126/science.1218835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller MT, Spradling AC. 2007. Male and female Drosophila germline stem cells: two versions of immortality. Science 316: 402–404. 10.1126/science.1140861 [DOI] [PubMed] [Google Scholar]
- Gao P, Postiglione MP, Krieger TG, Hernandez L, Wang C, Han Z, Streicher C, Papusheva E, Insolera R, Chugh K, et al. 2014. Deterministic progenitor behavior and unitary production of neurons in the neocortex. Cell 159: 775–788. 10.1016/j.cell.2014.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y, Fuchs E. 2018. Stretching the limits: from homeostasis to stem cell plasticity in wound healing and cancer. Nat Rev Genet 19: 311–325. 10.1038/nrg.2018.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehart H, Clevers H. 2019. Tales from the crypt: new insights into intestinal stem cells. Nat Rev Gastroenterol Hepatol 16: 19–34. 10.1038/s41575-018-0081-y [DOI] [PubMed] [Google Scholar]
- Goldstein RE. 2018. Are theoretical results “results”? eLife 7: 1–9. 10.7554/eLife.40018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez C, Chua W, Miremadi A, Quist S, Headon DJ, Watt FM. 2013. The interfollicular epidermis of adult mouse tail comprises two distinct cell lineages that are differentially regulated by Wnt, Edaradd, and Lrig1. Stem Cell Reports 1: 19–27. 10.1016/j.stemcr.2013.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal S, Kim S, Chen ISY, Chou T. 2015. Mechanisms of blood homeostasis: lineage tracking and a neutral model of cell populations in rhesus macaques. BMC Biol 13: 85 10.1186/s12915-015-0191-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf T, Stadtfeld M. 2008. Heterogeneity of embryonic and adult stem cells. Cell Stem Cell 3: 480–483. 10.1016/j.stem.2008.10.007 [DOI] [PubMed] [Google Scholar]
- Grün D, Lyubimova A, Kester L, Wiebrands K, Basak O, Sasaki N, Clevers H, van Oudenaarden A. 2015. Single-cell messenger RNA sequencing reveals rare intestinal cell types. Nature 525: 251–255. 10.1038/nature14966 [DOI] [PubMed] [Google Scholar]
- Han S, Fink J, Jörg DJ, Lee E, Yum MK, Chatzeli L, Merker SR, Josserand M, Trendafilova T, Andersson-Rolf A, et al. 2019. Defining the identity and dynamics of adult gastric isthmus stem cells. Cell Stem Cell 25: 342–356.e7. 10.1016/j.stem.2019.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannezo E, Coucke A, Joanny JF. 2016. Interplay of migratory and division forces as a generic mechanism for stem cell patterns. Phys Rev E 93: 22405 10.1103/PhysRevE.93.022405 [DOI] [PubMed] [Google Scholar]
- Hannezo E, Scheele CLGJ, Moad M, Drogo N, Heer R, Sampogna RV, van Rheenen J, Simons BD. 2017. A unifying theory of branching morphogenesis. Cell 171: 242–255.e27. 10.1016/j.cell.2017.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K, Nakagawa T, Enomoto H, Suzuki M, Yamamoto M, Simons BD, Yoshida S. 2014. Mouse spermatogenic stem cells continually interconvert between equipotent singly isolated and syncytial states. Cell Stem Cell 14: 658–672. 10.1016/j.stem.2014.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holguera I, Desplan C. 2018. Neuronal specification in space and time. Science 362: 176–180. 10.1126/science.aas9435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckins C. 1971. The spermatogonial stem cell population in adult rats. I. Their morphology, proliferation and maturation. Anat Rec 169: 533–557. 10.1002/ar.1091690306 [DOI] [PubMed] [Google Scholar]
- Huels DJ, Bruens L, Hodder MC, Cammareri P, Campbell AD, Ridgway RA, Gay DM, Faller WJ, Nixon C, Zeiger LB, et al. 2018. Wnt ligands influence tumour initiation by controlling the number of intestinal stem cells. Nat Commun 9: 1132 10.1038/s41467-018-03426-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iscove NN, Till JE, McCulloch EA. 1970. The proliferative states of mouse granulopoietic progenitor cells. Exp Biol Med 134: 33–36. 10.3181/00379727-134-34721 [DOI] [PubMed] [Google Scholar]
- Jadhav U, Saxena M, O'Neill NK, Saadatpour A, Yuan GC, Herbert Z, Murata K, Shivdasani RA. 2017. Dynamic reorganization of chromatin accessibility signatures during dedifferentiation of secretory precursors into Lgr5+ intestinal stem cells. Cell Stem Cell 21: 65–77.e5. 10.1016/j.stem.2017.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P, Simons BD. 2008. Epidermal homeostasis: do committed progenitors work while stem cells sleep? Nat Rev Mol Cell Biol 9: 82–88. 10.1038/nrm2292x [DOI] [PubMed] [Google Scholar]
- Jones PH, Harper S, Watt FM. 1995. Stem cell patterning and fate in human epidermis. Cell 80: 83–93. 10.1016/0092-8674(95)90453-0 [DOI] [PubMed] [Google Scholar]
- Jones KB, Furukawa S, Marangoni P, Ma H, Pinkard H, D'Urso R, Zilionis R, Klein AM, Klein OD. 2019. Quantitative clonal analysis and single-cell transcriptomics reveal division kinetics, hierarchy, and fate of oral epithelial progenitor cells. Cell Stem Cell 24: 183–192.e8. 10.1016/j.stem.2018.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jörg DJ, Kitadate Y, Yoshida S, Simons BD. 2019. Competition for stem cell fate determinants as a mechanism for tissue homeostasis. arXiv 190103903v2 [Google Scholar]
- Kitadate Y, Jo DJ, Tokue M, Takahashi S, Simons BD, Yoshida S, Tokue M, Maruyama A, Ichikawa R, Tsuchiya S, et al. 2019. Competition for mitogens regulates spermatogenic stem cell homeostasis in an open niche. Cell Stem Cell 24: 79–92.e6. 10.1016/j.stem.2018.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein AM, Simons BD. 2011. Universal patterns of stem cell fate in cycling adult tissues. Development 138: 3103–3111. 10.1242/dev.060103 [DOI] [PubMed] [Google Scholar]
- Klein AM, Nakagawa T, Ichikawa R, Yoshida S, Simons BD. 2010. Mouse germ line stem cells undergo rapid and stochastic turnover. Cell Stem Cell 7: 214–224. 10.1016/j.stem.2010.05.017 [DOI] [PubMed] [Google Scholar]
- Kozar S, Morrissey E, Nicholson AM, van der Heijden M, Zecchini HI, Kemp R, Tavaré S, Vermeulen L, Winton DJ. 2013. Continuous clonal labeling reveals small numbers of functional stem cells in intestinal crypts and adenomas. Cell Stem Cell 13: 626–633. 10.1016/j.stem.2013.08.001 [DOI] [PubMed] [Google Scholar]
- Kretzschmar K, Watt FM. 2012. Lineage tracing. Cell 148: 33–45. 10.1016/j.cell.2012.01.002 [DOI] [PubMed] [Google Scholar]
- Krndija D, El Marjou F, Guirao B, Richon S, Leroy O, Bellaiche Y, Hannezo E, Matic Vignjevic D. 2019. Active cell migration is critical for steady-state epithelial turnover in the gut. Science 365: 705–710. 10.1126/science.aau3429 [DOI] [PubMed] [Google Scholar]
- Kronen MR, Schoenfelder KP, Klein AM, Nystul TG. 2014. Basolateral junction proteins regulate competition for the follicle stem cell niche in the Drosophila ovary. PLoS ONE 9: e101085 10.1371/journal.pone.0101085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlond CP. 1965. The time dimension in histology. Am J Anat 116: 1–27. 10.1002/aja.1001160102 [DOI] [PubMed] [Google Scholar]
- Lehmann R. 2012. Germline stem cells: origin and destiny. Cell Stem Cell 10: 729–739. 10.1016/j.stem.2012.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leushacke M, Barker N, Pin C. 2016. Quantifying Lgr5-positive stem cell behaviour in the pyloric epithelium. Sci Rep 6: 21923 10.1038/srep21923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leushacke M, Tan SH, Wong A, Swathi Y, Hajamohideen A, Tan LT, Goh J, Wong E, Denil SLIJ, Murakami K, et al. 2017. Lgr5-expressing chief cells drive epithelial regeneration and cancer in the oxyntic stomach. Nat Cell Biol 19: 774–786. 10.1038/ncb3541 [DOI] [PubMed] [Google Scholar]
- Lim X, Tan SH, Koh WLC, Chau RMW, Yan K, Kuo CJ, Van Amerongen R, Klein AM, Nusse R. 2013. Interfollicular epidermal stem cells self-renew via autocrine Wnt signaling. Science 342: 1226–1230. 10.1126/science.1239730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Garcia C, Klein AM, Simons BD, Winton DJ. 2010. Intestinal stem cell replacement follows a pattern of neutral drift. Science 330: 822–825. 10.1126/science.1196236 [DOI] [PubMed] [Google Scholar]
- Mackenzie IC. 1970. Relationship between mitosis and the ordered structure of the stratum corneum in mouse epidermis. Nature 226: 653–655. 10.1038/226653a0 [DOI] [PubMed] [Google Scholar]
- Maher GJ, Goriely A, Wilkie AOM. 2014. Cellular evidence for selfish spermatogonial selection in aged human testes. Andrology 2: 304–314. 10.1111/j.2047-2927.2013.00175.x [DOI] [PubMed] [Google Scholar]
- Marques-Pereira JP, Leblond CP. 1965. Mitosis and differentiation in the stratified squamous epithelium of the rat esophagus. Am J Anat 117: 73–87. 10.1002/aja.1001170106 [DOI] [PubMed] [Google Scholar]
- Mascre G, Dekoninck S, Drogat B, Youssef KK, Brohe S, Sotiropoulou PA, Simons BD, Blanpain C. 2012. Distinct contribution of stem and progenitor cells to epidermal maintenance. Nature 489: 257–262. 10.1038/nature11393 [DOI] [PubMed] [Google Scholar]
- McCulloch EA, Till JE. 1960. The radiation sensitivity of normal mouse bone marrow cells, determined by quantitative marrow transplantation into irradiated mice. Radiat Res 13: 115–125. 10.2307/3570877 [DOI] [PubMed] [Google Scholar]
- Mesa KR, Kawaguchi K, Cockburn K, Gonzalez D, Boucher J, Xin T, Klein AM, Greco V. 2018. Homeostatic epidermal stem cell self-renewal is driven by local differentiation. Cell Stem Cell 23: 677–686.e4. 10.1016/j.stem.2018.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MB, Bassler BL. 2001. Quorum sensing in bacteria. Annu Rev Microbiol 55: 165–199. 10.1146/annurev.micro.55.1.165 [DOI] [PubMed] [Google Scholar]
- Mills JC, Shivdasani RA. 2011. Gastric epithelial stem cells. Gastroenterology 140: 412–424. 10.1053/j.gastro.2010.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miroshnikova YA, Le HQ, Schneider D, Thalheim T, Rübsam M, Bremicker N, Polleux J, Kamprad N, Tarantola M, Wang I, et al. 2018. Adhesion forces and cortical tension couple cell proliferation and differentiation to drive epidermal stratification. Nat Cell Biol 20: 69–80. 10.1038/s41556-017-0005-z [DOI] [PubMed] [Google Scholar]
- Mobasseri SA, Zijl S, Salameti V, Walko G, Stannard A, Garcia-Manyes S, Watt FM. 2019. Patterning of human epidermal stem cells on undulating elastomer substrates reflects differences in cell stiffness. Acta Biomater 87: 256–264. 10.1016/j.actbio.2019.01.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Spradling AC. 2008. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell 132: 598–611. 10.1016/j.cell.2008.01.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Nabeshima Y, Yoshida S. 2007. Functional identification of the actual and potential stem cell compartments in mouse spermatogenesis. Dev Cell 12: 195–206. 10.1016/j.devcel.2007.01.002 [DOI] [PubMed] [Google Scholar]
- Nicholson AM, Olpe C, Hoyle A, Thorsen AS, Rus T, Colombé M, Brunton-Sim R, Kemp R, Marks K, Quirke P, et al. 2018. Fixation and spread of somatic mutations in adult human colonic epithelium. Cell Stem Cell 22: 909–918.e8. 10.1016/j.stem.2018.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakberg EF. 1971. Spermatogonial stem-cell renewal in the mouse. Anat Rec 169: 515–531. 10.1002/ar.1091690305 [DOI] [PubMed] [Google Scholar]
- Pardo-Saganta A, Tata PR, Law BM, Saez B, Chow RDW, Prabhu M, Gridley T, Rajagopal J. 2015. Parent stem cells can serve as niches for their daughter cells. Nature 523: 597–601. 10.1038/nature14553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei W, Feyerabend TB, Rössler J, Wang X, Postrach D, Busch K, Rode I, Klapproth K, Dietlein N, Quedenau C, et al. 2017. Polylox barcoding reveals haematopoietic stem cell fates realized in vivo. Nature 548: 456–460. 10.1038/nature23653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson LW, Artis D. 2014. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol 14: 141–153. 10.1038/nri3608 [DOI] [PubMed] [Google Scholar]
- Plikus MV, Widelitz RB, Maxson R, Chuong CM. 2009. Analyses of regenerative wave patterns in adult hair follicle populations reveal macro-environmental regulation of stem cell activity. Int J Dev Biol 53: 857–868. 10.1387/ijdb.072564mp [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten CS. 1974. Epidermal proliferative unit—the possible role of the central basal-cell. Cell Tissue Kinet 7: 77–88. [DOI] [PubMed] [Google Scholar]
- Potten CS, Kovacs L, Hamilton E. 1974. Continuous labeling studies on mouse skin and intestine. Cell Prolif 7: 271–283. 10.1111/j.1365-2184.1974.tb00907.x [DOI] [PubMed] [Google Scholar]
- *.Rajagopal J. 2019. Stem cell plasticity. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a035733. [DOI] [Google Scholar]
- Ritsma L, Ellenbroek SIJ, Zomer A, Snippert HJ, De Sauvage FJ, Simons BD, Clevers H, Van Rheenen J. 2014. Intestinal crypt homeostasis revealed at single-stem-cell level by in vivo live imaging. Nature 507: 362–365. 10.1038/nature12972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche KC, Gracz AD, Liu XF, Newton V, Akiyama H, Magness ST. 2015. SOX9 maintains reserve stem cells and preserves radioresistance in mouse small intestine. Gastroenterology 149: 1553–1563.e10. 10.1053/j.gastro.2015.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Brenes IA, Wodarz D, Komarova NL. 2013. Stem cell control, oscillations, and tissue regeneration in spatial and non-spatial models. Front Oncol 3: 1–10. 10.3389/fonc.2013.00082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Fraticelli AE, Wolock SL, Weinreb CS, Panero R, Patel SH, Jankovic M, Sun J, Calogero RA, Klein AM, Camargo FD. 2018. Clonal analysis of lineage fate in native haematopoiesis. Nature 553: 212–216. 10.1038/nature25168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognoni E, Watt FM. 2018. Skin cell heterogeneity in development, wound healing, and cancer. Trends Cell Biol 28: 709–722. 10.1016/j.tcb.2018.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompolas P, Mesa KR, Greco V. 2013. Spatial organization within a niche as a determinant of stem-cell fate. Nature 502: 513–518. 10.1038/nature12602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Danés A, Hannezo E, Larsimont JC, Liagre M, Youssef KK, Simons BD, Blanpain C. 2016. Defining the clonal dynamics leading to mouse skin tumour initiation. Nature 536: 298–303. 10.1038/nature19069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos AJM, Lo Y, Mah AT, Kuo CJ. 2018. The intestinal stem cell niche: homeostasis and adaptations. Trends Cell Biol 28: 1062–1078. 10.1016/j.tcb.2018.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele CLGJ, Hannezo E, Muraro MJ, Zomer A, Langedijk NSM, Van Oudenaarden A, Simons BD, Van Rheenen J. 2017. Identity and dynamics of mammary stem cells during branching morphogenesis. Nature 542: 313–317. 10.1038/nature21046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoedel KB, Morcos MNF, Zerjatke T, Roeder I, Grinenko T, Voehringer D, Göthert JR, Waskow C, Roers A, Gerbaulet A. 2016. The bulk of the hematopoietic stem cell population is dispensable for murine steady-state and stress hematopoiesis. Blood 128: 2285–2296. 10.1182/blood-2016-03-706010 [DOI] [PubMed] [Google Scholar]
- Serra D, Mayr U, Boni A, Lukonin I, Rempfler M, Meylan LC, Stadler MB, Strnad P, Papasaikas P, Vischi D, et al. 2019. Self-organization and symmetry breaking in intestinal organoid development. Nature 569: 66–72. 10.1038/s41586-019-1146-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng XR, Matunis E. 2011. Live imaging of the Drosophila spermatogonial stem cell niche reveals novel mechanisms regulating germline stem cell output. Development 138: 3367–3376. 10.1242/dev.065797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shraiman BI. 2005. Mechanical feedback as a possible regulator of tissue growth. Proc Natl Acad Sci 102: 3318–3323. 10.1073/pnas.0404782102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siminovitch L, McCulloch EA, Till JE. 1963. The distribution of colony-forming cells among spleen colonies. J Cell Comp Physiol 62: 327–336. 10.1002/jcp.1030620313 [DOI] [PubMed] [Google Scholar]
- Simons BD. 2016. Deep sequencing as a probe of normal stem cell fate and preneoplasia in human epidermis. Proc Natl Acad Sci 113: 128–133. 10.1073/pnas.1516123113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snippert HJ, van der Flier LG, Sato T, van Es JH, van den Born M, Kroon-Veenboer C, Barker N, Klein AM, van Rheenen J, Simons BD, et al. 2010. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 143: 134–144. 10.1016/j.cell.2010.09.016 [DOI] [PubMed] [Google Scholar]
- Snippert HJ, Schepers AG, van Es JH, Simons BD, Clevers H. 2014. Biased competition between Lgr5 intestinal stem cells driven by oncogenic mutation induces clonal expansion. EMBO Rep 15: 62–69. 10.1002/embr.201337799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamp C, Zupanic A, Sachdeva A, Stoll EA, Shanley DP, Mathers JC, Kirkwood TBL, Heer R, Simons BD, Turnbull DM, et al. 2018. Predominant asymmetrical stem cell fate outcome limits the rate of niche succession in human colonic crypts. EBioMedicine 31: 166–173. 10.1016/j.ebiom.2018.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stange DE, Koo BK, Huch M, Sibbel G, Basak O, Lyubimova A, Kujala P, Bartfeld S, Koster J, Geahlen JH, et al. 2013. Differentiated troy+ chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell 155: 357–368. 10.1016/j.cell.2013.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Ramos A, Chapman B, Johnnidis JB, Le L, Ho YJ, Klein A, Hofmann O, Camargo FD. 2014. Clonal dynamics of native haematopoiesis. Nature 514: 322–327. 10.1038/nature13824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sznurkowska MK, Hannezo E, Azzarelli R, Rulands S, Nestorowa S, Hindley CJ, Nichols J, Göttgens B, Huch M, Philpott A, et al. 2018. Defining lineage potential and fate behavior of precursors during pancreas development. Dev Cell 46: 360–375.e5. 10.1016/j.devcel.2018.06.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai K, Cockburn K, Greco V. 2019. Flexibility sustains epithelial tissue homeostasis. Curr Opin Cell Biol 60: 84–91. 10.1016/j.ceb.2019.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan DW-M, Barker N. 2014. Intestinal stem cells and their defining niche. In Stem cells in development and disease (ed. Rendl M), Vol. 107, pp. 77–107. Academic, Orlando, FL. [DOI] [PubMed] [Google Scholar]
- Tang DG. 2012. Understanding cancer stem cell heterogeneity and plasticity. Cell Res 22: 457–472. 10.1038/cr.2012.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata PR, Mou H, Pardo-Saganta A, Zhao R, Prabhu M, Law BM, Vinarsky V, Cho JL, Breton S, Sahay A, et al. 2013. Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature 503: 218–223. 10.1038/nature12777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetteh PW, Basak O, Farin HF, Wiebrands K, Kretzschmar K, Begthel H, van den Born M, Korving J, de Sauvage F, van Es JH, et al. 2016. Replacement of lost Lgr5-positive stem cells through plasticity of their enterocyte-lineage daughters. Cell Stem Cell 18: 203–213. 10.1016/j.stem.2016.01.001 [DOI] [PubMed] [Google Scholar]
- Tomic G, Morrissey E, Kozar S, Ben-Moshe S, Hoyle A, Azzarelli R, Kemp R, Chilamakuri CSR, Itzkovitz S, Philpott A, et al. 2018. Phospho-regulation of ATOH1 is required for plasticity of secretory progenitors and tissue regeneration. Cell Stem Cell 23: 436–443.e7. 10.1016/j.stem.2018.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Es JH, Sato T, van de Wetering M, Lyubimova A, Yee Nee AN, Gregorieff A, Sasaki N, Zeinstra L, van den Born M, Korving J, et al. 2012. Dll1+ secretory progenitor cells revert to stem cells upon crypt damage. Nat Cell Biol 14: 1099–1104. 10.1038/ncb2581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varahan S, Walvekar A, Sinha V, Krishna S, Laxman S. 2019. Metabolic constraints drive self-organization of specialized cell groups. eLife 8: e46735 10.7554/eLife.46735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkei ZG, Yamashita YM. 2018. Emerging mechanisms of asymmetric stem cell division. J Cell Biol 217: 3785–3795. 10.1083/jcb.201807037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen L, Morrissey E, van der Heijden M, Nicholson AM, Sottoriva A, Buczacki S, Kemp R, Tavaré S, Winton DJ. 2013. Defining stem cell dynamics in models of intestinal tumor initiation. Science 342: 995–998. 10.1126/science.1243148 [DOI] [PubMed] [Google Scholar]
- Watson JK, Rulands S, Wilkinson AC, Wuidart A, Ousset M, Van Keymeulen A, Göttgens B, Blanpain C, Simons BD, Rawlins EL. 2015. Clonal dynamics reveal two distinct populations of basal cells in slow-turnover airway epithelium. Cell Rep 12: 90–101. 10.1016/j.celrep.2015.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt FM, Hogan BLM. 2000. Out of Eden: Stem cells and their niches. Science 287: 1427–1430. 10.1126/science.287.5457.1427 [DOI] [PubMed] [Google Scholar]
- Wickström SA, Niessen CM. 2018. Cell adhesion and mechanics as drivers of tissue organization and differentiation: local cues for large scale organization. Curr Opin Cell Biol 54: 89–97. 10.1016/j.ceb.2018.05.003 [DOI] [PubMed] [Google Scholar]
- Williams ED, Lowes AP, Williams D, Williams GT. 1992. A stem cell niche theory of intestinal crypt maintenance based on a study of somatic mutation in colonic mucosa. Am J Pathol 141: 773–776. [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H, Kawaguchi K. 2019. Universal voter model emergence in genetically labeled homeostatic tissues. arXiv 1903.02985 [Google Scholar]
- Yamaguchi H, Kawaguchi K, Sagawa T. 2017. Dynamical crossover in a stochastic model of cell fate decision. Phys Rev E 96: 12401 10.1103/PhysRevE.96.012401 [DOI] [PubMed] [Google Scholar]
- Yan KS, Gevaert O, Zheng GXY, Anchang B, Probert CS, Larkin KA, Davies PS, Cheng ZF, Kaddis JS, Han A, et al. 2017a. Intestinal enteroendocrine lineage cells possess homeostatic and injury-inducible stem cell activity. Cell Stem Cell 21: 78–90.e6. 10.1016/j.stem.2017.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan KS, Janda CY, Chang J, Zheng GXY, Larkin KA, Luca VC, Chia LA, Mah AT, Han A, Terry JM, et al. 2017b. Non-equivalence of Wnt and R-spondin ligands during Lgr5+ intestinal stem-cell self-renewal. Nature 545: 238–242. 10.1038/nature22313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Yoshida S. 2019. Sperm stem cells. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a036186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yui S, Azzolin L, Maimets M, Pedersen MT, Fordham RP, Hansen SL, Larsen HL, Guiu J, Alves MRP, Rundsten CF, et al. 2018. YAP/TAZ-dependent reprogramming of colonic epithelium links ECM remodeling to tissue regeneration. Cell Stem Cell 22: 35–49.e7. 10.1016/j.stem.2017.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Franklin RA, Adler M, Jacox JB, Bailis W, Shyer JA, Flavell RA, Mayo A, Alon U, Medzhitov R. 2018. Circuit design features of a stable two-cell system. Cell 172: 744–757.e17. 10.1016/j.cell.2018.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]