Abstract
Aims/hypothesis
Coronavirus disease-2019 (COVID-19) is a life-threatening infection caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) virus. Diabetes has rapidly emerged as a major comorbidity for COVID-19 severity. However, the phenotypic characteristics of diabetes in COVID-19 patients are unknown.
Methods
We conducted a nationwide multicentre observational study in people with diabetes hospitalised for COVID-19 in 53 French centres in the period 10–31 March 2020. The primary outcome combined tracheal intubation for mechanical ventilation and/or death within 7 days of admission. Age- and sex-adjusted multivariable logistic regressions were performed to assess the prognostic value of clinical and biological features with the endpoint. ORs are reported for a 1 SD increase after standardisation.
Results
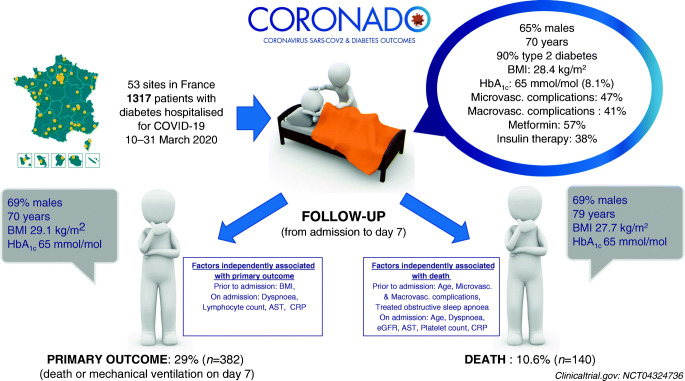
The current analysis focused on 1317 participants: 64.9% men, mean age 69.8 ± 13.0 years, median BMI 28.4 (25th–75th percentile: 25.0–32.7) kg/m2; with a predominance of type 2 diabetes (88.5%). Microvascular and macrovascular diabetic complications were found in 46.8% and 40.8% of cases, respectively. The primary outcome was encountered in 29.0% (95% CI 26.6, 31.5) of participants, while 10.6% (9.0, 12.4) died and 18.0% (16.0, 20.2) were discharged on day 7. In univariate analysis, characteristics prior to admission significantly associated with the primary outcome were sex, BMI and previous treatment with renin–angiotensin–aldosterone system (RAAS) blockers, but not age, type of diabetes, HbA1c, diabetic complications or glucose-lowering therapies. In multivariable analyses with covariates prior to admission, only BMI remained positively associated with the primary outcome (OR 1.28 [1.10, 1.47]). On admission, dyspnoea (OR 2.10 [1.31, 3.35]), as well as lymphocyte count (OR 0.67 [0.50, 0.88]), C-reactive protein (OR 1.93 [1.43, 2.59]) and AST (OR 2.23 [1.70, 2.93]) levels were independent predictors of the primary outcome. Finally, age (OR 2.48 [1.74, 3.53]), treated obstructive sleep apnoea (OR 2.80 [1.46, 5.38]), and microvascular (OR 2.14 [1.16, 3.94]) and macrovascular complications (OR 2.54 [1.44, 4.50]) were independently associated with the risk of death on day 7.
Conclusions/interpretations
In people with diabetes hospitalised for COVID-19, BMI, but not long-term glucose control, was positively and independently associated with tracheal intubation and/or death within 7 days.
Trial registration
Electronic supplementary material
The online version of this article (10.1007/s00125-020-05180-x) contains peer-reviewed but unedited supplementary material, which is available to authorised users.
Keywords: BMI, COVID-19, Death, Diabetes, HbA1c, Hypertension, Mechanical ventilation
Introduction
Since the first case in China in December 2019, the epidemic of coronavirus disease-2019 (COVID-19), a disease caused by the severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) virus, rapidly spread worldwide and was declared a pandemic by the World Health Organization on 11 March 2020 [1, 2].
It is well known that people with diabetes have increased infection risk, especially for influenza and pneumonia [3, 4]. Moreover, diabetes was previously reported as a major risk factor for mortality in people infected with the 2009 H1N1 pandemic influenza and, more recently, with the Middle East respiratory syndrome-related coronavirus (MERS-CoV) [5, 6]. Epidemiological studies have quickly and consistently pointed out diabetes as one of the major comorbidities associated with COVID-19 and affecting its severity.
The prevalence of diabetes in patients with COVID-19 was first reported to range from 5% to 20% in Chinese studies, increasing with the severity of the disease [7]. More recently, Grasselli et al have reported a diabetes prevalence of 17% in patients admitted to intensive care units (ICUs) for severe COVID-19 infection in Lombardy, Italy [8]. Furthermore, the COVID-19-Associated Hospitalisation Surveillance Network (COVID-NET) reported a diabetes prevalence of 28.3% in hospitalised patients in the USA [9].
More importantly, all studies published so far have reported a two- to threefold higher prevalence of diabetes in patients in ICUs compared with those with less severe disease and an increased mortality in people with diabetes [10–14]. For instance, in a retrospective study from Wuhan, diabetes was present in 19% of 191 COVID-19 inpatients but its prevalence raised to 31% in deceased people compared with 14% in those who survived [12]. A recent meta-analysis further demonstrated that diabetes was associated with a more than doubled risk for ICU admission and a more than tripled risk for death [14].
In this context, patients with diabetes have been listed as people at higher risk for severe illness from COVID-19 by several health authorities and learned medical societies [15]. However, precise data regarding diabetes characteristics in hospitalised people with COVID-19 are still lacking. Moreover, the relationship between diabetes-related phenotypes and the severity of COVID-19 remains unknown. CORONADO (Coronavirus SARS-CoV-2 and Diabetes Outcomes) is a nationwide multicentre observational study that aims to identify the clinical and biological features associated with disease severity and mortality risk in people with diabetes hospitalised for COVID-19.
Methods
Study oversight
The CORONADO study was launched in all French hospitals volunteering to share data on hospitalised COVID-19 patients with diabetes. The study was sponsored by CHU (centre hospitalier universitaire) Nantes, designed in accordance with the declaration of Helsinki and conducted in accordance with French legislation with approval obtained from the local ethics committee (Institutional Review Board/Institutional Ethics Committee – GNEDS [groupe nantais d'éthique dans le domaine de la santé]; Ref. CORONADOV2), the CEREES (comité d'expertise pour les recherches, les études et les évaluations dans le domaine de la santé; n° INDS [institut national des données de santé]:1544730) and the CNIL (commission nationale de l'informatique et des libertés; DR-2020-155/920129). In light of the purely non-interventional design of this observational study and the emergency situation related to the COVID-19 pandemic, the CNIL and the GNEDS have repealed the systematic collection of written informed consent. They recommended that we collect an ‘oral non-opposition to participate’ as far as possible (in particular by publishing study information via posters in the hospitals). Living patients who were unable to give consent on admission all received information about their inclusion in the CORONADO study before discharge, and therefore had a clear and free choice to object to the use of their clinical data. Any patient declining to participate in the study or expressing his or her opposition to data collection from hospital information systems, even after hospital discharge, was excluded from the study.
Study design and participants
The aim of the CORONADO study was to describe the phenotypic characteristics and prognosis of individuals admitted to hospital with COVID-19 between 10 March and 10 April 2020. Inclusion criteria were (1) hospitalisation in a dedicated COVID-19 unit with COVID-19 diagnosis confirmed biologically (by SARS-CoV-2 PCR test) and/or clinically/radiologically (i.e. as ground-glass opacity and/or crazy paving on chest computed tomography [CT] scan); (2) personal history of diabetes or newly diagnosed diabetes on admission (i.e. HbA1c ≥48 mmol/mol [6.5%] during hospitalisation).
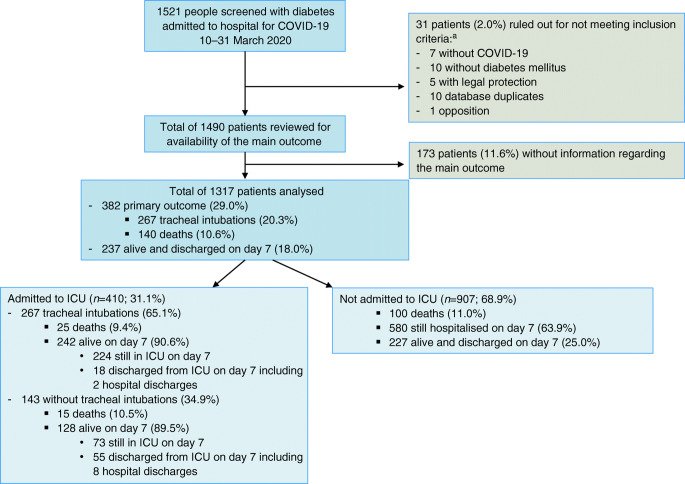
Owing to the rapid recruitment rate and in order to make clinically relevant findings available as quickly as possible, the scientific committee, on 5 April 2020, suggested a premature database lock on 18 April 2020 for participants admitted in the period 10–31 March 2020, and continuation of recruitment with no further modification. A first set of analyses was performed in 1317 participants selected according to the following criteria: (1) meeting the eligibility criteria; (2) available information on the main outcome, recorded on day 7 following admission; (3) available data on age and sex (see flowchart in Fig. 1).
Fig. 1.
Study flowchart. aTwo patients ruled out for not meeting inclusion criteria were in two categories
Patient follow-up and clinical outcomes
The composite primary endpoint combined tracheal intubation for mechanical ventilation and death within 7 days of admission. Secondary outcomes included death on day 7, tracheal intubation on day 7, admission to ICUs and discharge on day 7. Participants discharged before day 7 were systematically contacted to check for the non-occurrence of these events on day 7.
Data collection
Data collection was performed by clinical research associates and physicians in participating centres. They were instructed to systematically review the medical files of all COVID-19 inpatients, select those with diabetes, extract data from their medical files and, if needed, contact the patient’s general and/or specialist practitioners, regular pharmacist or biomedical laboratory. Collected data included clinical data (age, sex, ethnicity, BMI), classification of diabetes as noted in the medical file by the physician in charge of the patient, duration of diabetes, recent glycaemic control (i.e. two most recent HbA1c levels determined before admission), microvascular and macrovascular complications and comorbidities. HbA1c considered in the analysis was determined locally in the 7 days following admission or, if not available, was the result of a routine determination in the previous 6 months. Microvascular complications were defined as severe diabetic retinopathy (proliferative retinopathy and/or laser photocoagulation and/or clinically significant macular oedema requiring laser and/or intra-vitreal injections) and/or diabetic kidney disease (proteinuria [AER ≥300 mg/24 h; urinary albumin/creatinine ratio ≥300 mg/g; urinary albumin/creatinine ratio ≥30 mg/mmol creatinine; proteinuria ≥500 mg/24 h] and/or eGFR equal to or lower than 60 ml min−1 [1.73 m]−2, using the Chronic Kidney Disease Epidemiology Collaboration [CKD-EPI] formula) and/or history of diabetic foot ulcer. Macrovascular complications were defined as ischaemic heart disease (acute coronary syndrome and/or coronary artery revascularisation) and/or cerebrovascular disease (stroke and/or transient ischaemic attack) and/or peripheral artery disease (amputation owing to ischaemic disease and/or lower limb artery revascularisation). In addition, COVID-19-related clinical, radiological and biological characteristics were collected at admission as well as their clinical evolution during the hospital stay.
Statistical analysis
Quantitative data were expressed as mean ± SD or median [25th –75th percentile]. Categorical variables were given as number (percentage) of participants. As prespecified in the protocol, the main objective of the study was descriptive. We calculated that a population size of 300 participants with an attrition of 20% for missing data, and a percentage of 16% of our main outcome, would give us a 95% CI equal to 11.7–21.1% using the Clopper–Pearson estimate.
Univariate logistic regression models were used to calculate OR associated with primary outcome or death on day 7. Natural log transformation was consistently considered in cases of skewed distribution, which applied to BMI and biological features.
Age- and sex-adjusted ORs for the primary outcome were plotted for BMI, HbA1c and admission plasma glucose using degree 2 fractional polynomial approaches [16].
Multivariable logistic regression models were used to separately assess the association of the primary outcome and death on day 7 with clinical and biological features. A standardisation process was also applied using z scores for the purpose of direct comparison. In our initial statistical analysis plan, four covariates were systematically forced in the models: age, sex, BMI and HbA1c. However, since HbA1c did not contribute to the risk of either the primary outcome or death on day 7, and owing to a significant number of missing data for HbA1c and BMI, our multivariable models ultimately took only age and sex into account. Other variables were considered only if associated with the main outcome in univariate analysis (threshold: two-sided p value ≤0.10) and selected in the final model after a stepwise backward/forward selection process. In the event of obvious collinearity (such as alanine aminotransferase [ALT] with aspartate aminotransferase [AST], or white cell count with lymphocyte count), only the variable associated with the smaller p value was considered for multivariable analysis. In the final model, interactions were checked between all pairs of covariates.
We built two distinct multivariable models both separately for the main outcome and for the risk of death: (1) the first included covariates related to patient history prior to admission (chronic diabetes complications and other comorbidities) and routine medications; (2) the second included covariates related to medical presentation on admission, such as COVID-19 symptoms and biological determinations. This corresponds to the situation of a physician in an emergency room or department, assessing the prognosis of his/her patient.
All statistical tests were two-sided with a type 1 error set at 5%. All analyses were performed on available data, without imputation, and using statistical software R version 3.6.2 (https://cran.r-project.org/bin/windows/base/old/3.6.2/).
Results
Population and clinical outcomes
The present analysis focused on 1317 participants with diabetes and confirmed COVID-19 admitted to 53 French hospitals during the period 10–31 March 2020.
A total of 382 patients (29.0%; 95% CI 26.6, 31.5) met the primary outcome. Overall, 410 patients (31.1%; 95% CI 28.6, 33.7) were admitted to ICUs within 7 days of hospital admission, including 267 individuals who required tracheal intubation for mechanical ventilation (20.3%; 95% CI 18.1, 22.5). One hundred and forty deaths (10.6%; 95% CI 9.0, 12.4) were recorded on day 7. In contrast, 237 participants (18.0%; 95% CI 16.0, 20.2) were discharged on day 7 (see flowchart in Fig. 1).
Demographic and diabetes-related characteristics
The clinical characteristics of the whole population are shown in Table 1. Mean (± SD) age was 69.8 ± 13.0 years and 64.9% were men. The classification of diabetes cases mainly included type 2 diabetes (88.5%), and less frequently type 1 diabetes (3.0%) or other aetiologies (5.4%). In addition, 3.1% of the participants were newly diagnosed with diabetes on admission (HbA1c ≥48 mmol/mol [6.5%]). The median BMI was 28.4 (25th–75th percentile 25.0–32.7) kg/m2. The mean HbA1c value was 65 ± 21 mmol/mol (8.1 ± 1.9%). A medical history of hypertension and dyslipidaemia were found in 77.2% and 51.0% of the participants, respectively. Microvascular and macrovascular complications were reported in 46.8% and 40.8% of individuals, respectively. Regarding routine glucose-lowering medications, 38.3% of the participants were on insulin therapy while 56.6% received metformin and 21.6% dipeptidyl peptidase 4 (DPP-4) inhibitors. Moreover, treatment with renin–angiotensin–aldosterone system (RAAS) blockers (ACE inhibitors and/or angiotensin II receptor blockers [ARBs] and/or mineralocorticoid-receptor antagonists [MRAs]) and statins was used by 57.1% and 47.6% of the participants, respectively.
Table 1.
Clinical characteristics prior to admission of CORONADO participants, according to primary outcome (tracheal intubation and/or death within 7 days of admission), and death on day 7
| Clinical features | Number of people with available data | All | Primary outcome (n = 382) OR (95% CI) |
Death (n = 140) OR (95% CI) |
|---|---|---|---|---|
| Sex (female/male) | 1317 | 462/1317 (35.1) | 0.77 (0.60, 0.99) | 0.80 (0.55, 1.17) |
| Age (years)a | 1317 | 69.8 ± 13.0 | 1.00 (0.99, 1.01) | 1.09 (1.07, 1.11) |
| Age class (years) | 1317 | |||
| <55 | 159/1317 (12.1) | 1 | 1 | |
| 55–64 | 266/1317 (20.2) | 0.58 (0.38, 0.90) | 1.00 (0.23, 4.23) | |
| 65–74 | 394/1317 (29.9) | 0.89 (0.60, 1.31) | 3.22 (0.95, 10.1) | |
| ≥75 | 498/1317 (37.8) | 0.85 (0.58, 1.24) | 14.6 (4.56, 46.6) | |
| Type of diabetes | 1317 | |||
| Type 2 | 1166/1317 (88.5) | 1 | 1 | |
| Type 1 | 39/1317 (3.0) | 0.73 (0.35, 1.56) | 0.44 (0.11, 1.86) | |
| Other | 71/1317 (5.4) | 1.33 (0.80, 2.20) | 1.50 (0.77, 2.93) | |
| Diagnosed on admission | 41/1317 (3.1) | 0.79 (0.38, 1.63) | – | |
| Ethnicity | 1035 | |||
| EU | 641/1035 (61.9) | 1 | 1 | |
| MENA | 196/1035 (18.9) | 0.98 (0.69, 1.40) | 0.87 (0.52, 1.47) | |
| AC | 174/1035 (16.8) | 0.96 (0.66, 1.40) | 0.78 (0.44, 1.37) | |
| AS | 24/1035 (2.3) | 1.51 (0.65, 3.52) | – | |
| BMI (kg/m2)a | 1117 | 28.4 [25.0–32.7] | 1.25 (1.09, 1.42) | 0.95 (0.78, 1.16) |
| BMI class | 1117 | |||
| <25 kg/m2 | 279/1117 (25) | 1 | 1 | |
| 25–29.9 kg/m2 | 410/1117 (36.7) | 1.33 (0.93, 1.89) | 0.70 (0.42, 1.16) | |
| 30–39.9 kg/m2 | 359/1117 (32.1) | 1.71 (1.20, 2.43) | 0.76 (0.45, 1.27) | |
| ≥40 kg/m2 | 69/1117 (6.2) | 1.28 (0.70, 2.32) | 0.74 (0.29, 1.84) | |
| Diabetes duration (years) | 772 | 13.6 ± 10.9 | 1.00 (0.98, 1.01) | 1.01 (0.99, 1.04) |
| HbA1c (mmol/mol)a | 846 | 65.4 ± 21.2 | 0.99 (0.99, 1.00) | 1.00 (0.99, 1.02) |
| HbA1c (%)a | 846 | 8.1 ± 1.9 | 0.94 (0.86, 1.03) | 1.02 (0.87, 1.19) |
| HbA1c (categories) | 846 | |||
| <53 mmol/mol (7%) | 245/846 (29.0) | 1 | 1 | |
| 53–63 mmol/mol (7–7.9%) | 228/846 (27.0) | 0.84 (0.55, 1.27) | 1.55 (0.82, 2.93) | |
| 64–74 mmol/mol (8–8.9%) | 164/846 (19.4) | 0.92 (0.59, 1.45) | 1.09 (0.52, 2.28) | |
| ≥75 mmol/mol (9%) | 209/846 (24.7) | 0.78 (0.51, 1.21) | 0.84 (0.40, 1.75) | |
| Hypertension | 1299 | 1003/1299 (77.2) | 1.23 (0.92, 1.65) | 1.82 (1.11, 2.98) |
| Dyslipidaemia | 1255 | 640/1255 (51.0) | 1.07 (0.84, 1.37) | 1.21 (0.84, 1.74) |
| Tobacco use | 1029 | |||
| Never | 582/1029 (56.6) | 1 | 1 | |
| Former | 390/1029 (37.9) | 1.21 (0.91, 1.61) | 1.00 (0.64, 1.57) | |
| Current | 57/1029 (5.5) | 1.54 (0.87, 2.74) | 1.20 (0.49, 2.93) | |
| Long, term diabetes complications | ||||
| Microvascular complications | 883 | 413/883 (46.8) | 1.28 (0.94, 1.73) | 5.25 (3.03, 9.10) |
| Severe diabetic retinopathy | 954 | 66/954 (6.9) | 1.22 (0.71, 2.11) | 2.05 (1.03, 4.07) |
| Diabetic kidney disease | 1066 | 355/1066 (33.3) | 1.03 (0.78, 1.37) | 3.19 (2.09, 4.87) |
| History of diabetic foot ulcer | 1232 | 76/1232 (6.2) | 0.67 (0.38, 1.18) | 1.53 (0.79, 2.99) |
| Macrovascular complications | 1189 | 485/1189 (40.8) | 1.18 (0.91, 1.52) | 3.58 (2.41, 5.31) |
| Ischaemic heart disease (ACS/CAR) | 1251 | 336/1251 (26.9) | 1.04 (0.79, 1.37) | 2.65 (1.84, 3.82) |
| Cerebrovascular disease (stroke or TIA) | 1267 | 163/1267 (12.9) | 1.02 (0.71, 1.47) | 2.19 (1.4, 3.42) |
| Peripheral artery disease (major amputation/LLAR) | 1285 | 145/1285 (11.3) | 0.91 (0.61, 1.34) | 1.97 (1.23, 3.17) |
| Comorbidities | ||||
| Heart failure | 1206 | 140/1206 (11.6) | 0.78 (0.52, 1.17) | 2.28 (1.42, 3.66) |
| NAFLD or liver cirrhosis | 1107 | 119/1107 (10.7) | 1.23 (0.81, 1.86) | 0.70 (0.34, 1.41) |
| Active cancer | 1282 | 194/1282 (15.1) | 1.08 (0.77, 1.50) | 1.55 (0.99, 2.42) |
| COPD | 1278 | 133/1278 (10.4) | 0.96 (0.64, 1.43) | 1.36 (0.80, 2.32) |
| Treated OSA | 1189 | 144/1189 (12.1) | 1.44 (0.99, 2.08) | 1.81 (1.12, 2.93) |
| Organ graft | 1302 | 38/1302 (2.9) | 1.14 (0.57, 2.28) | 0.46 (0.11, 1.93) |
| End stage renal failure | 831 | 60/831 (7.2) | 0.66 (0.35, 1.27) | 0.62 (0.24, 1.60) |
| Routine treatment before admission | ||||
| Metformin | 1317 | 746/1317 (56.6) | 0.95 (0.75, 1.21) | 0.59 (0.42, 0.84) |
| Sulfonylurea/glinides | 1317 | 367/1317 (27.9) | 1.03 (0.79, 1.34) | 0.74 (0.49, 1.13) |
| DPP-4 inhibitors | 1317 | 285/1317 (21.6) | 1.01 (0.75, 1.34) | 0.85 (0.55, 1.32) |
| GLP1-RA | 1317 | 123/1317 (9.3) | 1.36 (0.92, 2.01) | 0.64 (0.32, 1.29) |
| Insulin | 1317 | 504/1317 (38.3) | 1.01 (0.79, 1.29) | 1.71 (1.20, 2.43) |
| Loop diuretics | 1317 | 252/1317 (19.1) | 1.10 (0.81, 1.48) | 2.49 (1.70, 3.64) |
| Thiazide diuretics | 1317 | 267/1317 (20.3) | 1.08 (0.81, 1.45) | 0.98 (0.63, 1.52) |
| Potassium-sparing diuretics | 1317 | 59/1317 (4.5) | 1.17 (0.67, 2.05) | 1.77 (0.88, 3.58) |
| MRA | 1317 | 53/1317 (4.0) | 0.96 (0.52, 1.78) | 2.03 (1.00, 4.13) |
| β-blockers | 1317 | 442/1317 (33.6) | 1.03 (0.80, 1.32) | 1.84 (1.29, 2.62) |
| ACE inhibitors | 1317 | 354/1317 (26.9) | 1.17 (0.90, 1.52) | 1.43 (0.99, 2.08) |
| ARBs | 1317 | 389/1317 (29.5) | 1.22 (0.94, 1.57) | 1.15 (0.79, 1.67) |
| ARBs and/or ACE inhibitors | 1317 | 737/1317 (56.0) | 1.32 (1.03, 1.68) | 1.58 (1.09, 2.28) |
| ARBs and/or ACE inhibitors and/or MRA | 1317 | 752/1317 (57.1) | 1.29 (1.01, 1.65) | 1.67 (1.15, 2.43) |
| Statins | 1317 | 627/1317 (47.6) | 1.03 (0.81, 1.31) | 1.19 (0.84, 1.68) |
Data are presented as numbers (%) and mean ± SD, or median [25th–75th percentile] if not normally distributed
aFor quantitative variables, OR corresponds to an increase of 1 SD. Only BMI was natural log transformed before OR calculation
Ethnicity: EU (Europid), MENA (Middle East North Africa); AC (African or Caribbean), AS (Asian)
HBA1c corresponds to the HBA1c value determined in the 6 months prior to or in the first 7 days following hospital admission
Diabetic kidney disease defined as eGFR ≤60 ml min−1 [1.73 m]−2 and/or proteinuria
MRAs include spironolactone and eplerenone
ACS, acute coronary syndrome; CAR, coronary artery revascularisation; COPD, chronic obstructive pulmonary disease; GLP1-RA, glucagon-like peptide 1-receptor agonist; LLAR, lower limb artery revascularisation; NAFLD, non-alcoholic fatty liver disease; TIA, transient ischaemic attack
Characteristics of COVID-19 on admission
Characteristics of COVID-19 on admission are provided in Table 2. The median duration of symptoms before admission was 5 days (25th–75th percentile, 2–8 days). As expected, the most common signs were fever (77.9%), cough (68.7%), fatigue (62.4%), dyspnoea (61.8%) and digestive disorders (34.5%). SARS-CoV-2 PCR testing was performed in 1268 participants, with a positive result in 96.8%. Thoracic CT imaging demonstrated typical ground-glass opacity and/or crazy paving in 818 individuals (90.0%). Biological findings were consistent with obvious infection as illustrated by a median C-reactive protein (CRP) at 77.8 (38.4–132.7) mg/l. Median plasma glucose at admission was 9.20 (6.80–12.62) mmol/l.
Table 2.
COVID-19-related clinical, radiological and biological characteristics on admission in CORONADO participants, according to primary outcome (tracheal intubation and/or death within 7 days of admission), and death on day 7
| Characteristic | Number of people with available data | All | Primary outcome (n = 382) OR (95% CI) |
Death (n = 140) OR (95% CI) |
|---|---|---|---|---|
| COVID-19 symptoms | 1313 | 1237/1313 (94.2) | 3.20 (1.58, 6.49) | 2.21 (0.79, 6.13) |
| Time between symptom onset and hospital admission (days) | 1302 | 5 [2–8] | 1.01 (0.99, 1.03) | 0.96 (0.92, 0.99) |
| Clinical presentation | ||||
| Fever | 1288 | 1003/1288 (77.9) | 1.07 (0.80, 1.44) | 0.73 (0.49, 1.10) |
| Fatigue | 1239 | 773/1239 (62.4) | 1.15 (0.89, 1.49) | 1.13 (0.77, 1.65) |
| Cough | 1270 | 872/1270 (68.7) | 0.99 (0.76, 1.29) | 0.87 (0.59, 1.28) |
| Cephalalgia | 1193 | 157/1193 (13.2) | 0.85 (0.58, 1.25) | 0.44 (0.21, 0.92) |
| Dyspnoea | 1292 | 798/1292 (61.8) | 2.56 (1.95, 3.36) | 2.29 (1.51, 3.47) |
| Rhinitis and/or pharyngeal symptoms | 1178 | 111/1178 (9.4) | 0.78 (0.49, 1.23) | 0.39 (0.16, 0.99) |
| Ageusia and/or anosmia | 1073 | 136/1073 (12.7) | 0.73 (0.47, 1.12) | 0.34 (0.14, 0.85) |
| Digestive disorders | 1236 | 427/1236 (34.5) | 0.83 (0.64, 1.08) | 0.88 (0.60, 1.30) |
| Chest CT imaging | ||||
| Abnormal chest CT | 896 | 844/896 (94.2) | 1.16 (0.61, 2.21) | – |
| Ground-glass opacity/crazy paving | 818 | 736/818 (90.0) | 1.83 (1.02, 3.28) | 1.70 (0.66, 4.32) |
| Biological findings | ||||
| Positive SARS-CoV-2 PCR | 1268 | 1227/1268 (96.8) | 2.44 (1.02, 5.85) | 1.54 (0.47, 5.06) |
| Admission plasma glucose (mmol/l)a | 940 | 9.20 [6.80–12.62] | 1.28 (1.12, 1.48) | 1.20 (0.98, 1.46) |
| Plasma creatinine (μmol/l)a | 1196 | 91 [69–133] | 1.24 (1.10, 1.40) | 1.56 (1.33, 1.82) |
| eGFR (ml min−1 [1.73 m]−2)a | 1196 | 69 [41.7–89.5] | 0.82 (0.73, 0.93) | 0.61 (0.52, 0.71) |
| ALT (%ULN)a | 1068 | 0.62 [0.41–0.99] | 1.25 (1.10, 1.42) | 0.84 (0.67, 1.06) |
| AST (%ULN)a | 1053 | 1.05 [0.75–1.51] | 1.78 (1.54, 2.06) | 1.34 (1.14, 1.59) |
| GGT (%ULN)a | 983 | 0.94 [0.56–1.73] | 1.25 (1.10, 1.43) | 0.97 (0.78, 1.20) |
| Haemoglobin (g/l)a | 1276 | 129 [114–143] | 0.95 (0.84, 1.07) | 0.96 (0.81, 1.14) |
| White cell count (103/mm3)a | 1269 | 6440 [4930–8610] | 1.27 (1.12, 1.44) | 1.43 (1.19, 1.70) |
| Lymphocyte count (103/mm3)a | 1211 | 990 [685–1400] | 0.69 (0.60, 0.80) | 0.75 (0.60, 0.92) |
| Platelet count (103/mm3)a | 1273 | 193 [151–246] | 0.86 (0.76, 0.97) | 0.86 (0.73, 1.02) |
| D-dimers (μg/l)a | 397 | 830 [350–1571] | 0.93 (0.76, 1.15) | 1.25 (0.84, 1.86) |
| CRP (mg/l)a | 1208 | 77.8 [38.4–132.7] | 1.99 (1.69, 2.34) | 1.49 (1.20, 1.84) |
| LDH (UI/l)a | 566 | 351 [268–496] | 2.43 (1.85, 3.18) | 1.62 (1.10, 2.39) |
| CPK (UI/l)a | 549 | 145 [72–319] | 1.56 (1.30, 1.88) | 1.68 (1.31, 2.17) |
| Fibrinogen (g/l)a | 658 | 6.0 [4.8–7.2] | 1.32 (1.09, 1.58) | 1.05 (0.84, 1.31) |
Data are presented as numbers (%) or median [25th–75th percentile] if not normally distributed
aAll biological quantitative variables were natural log transformed. OR corresponds to an increase of 1 SD
eGFR was calculated according to the CKD-EPI formula
GGT, γ-glutamyl transferase; LDH, lactate dehydrogenase; ULN, upper limit of normal
Of interest, diabetes-related disorders were reported in 11.1% of the participants on admission with 132 episodes of severe hyperglycaemia, including 40 of ketosis, of which 19 were ketoacidosis, as well as 14 hypoglycaemic events, while severe anorexia was reported in 83 participants (6.3%).
Factors prior to admission associated with study outcomes
In univariate analysis considering the primary outcome, male sex was more frequent (69.1% vs 63.2%, p = 0.0420) and BMI was significantly higher (median 29.1 [25.9–33.6] vs 28.1 [24.8–32.0] kg/m2, p = 0.0009) in patients who met the primary outcome compared with the others, as was the use of RAAS blockers (61.5% vs 55.3%, p = 0.0386) (Table 1 and electronic supplementary material [ESM] Table 1).
Furthermore, several characteristics prior to admission were associated with the risk of death on day 7 including age, hypertension, micro- and macrovascular diabetic complications and comorbidities such as heart failure or treated obstructive sleep apnoea (OSA). Among prior medications, metformin use was lower in people who died. In contrast, insulin therapy, RAAS blockers, β-blockers, loop diuretics and MRAs were found to be associated with death on day 7 (Table 1 and ESM Table 1).
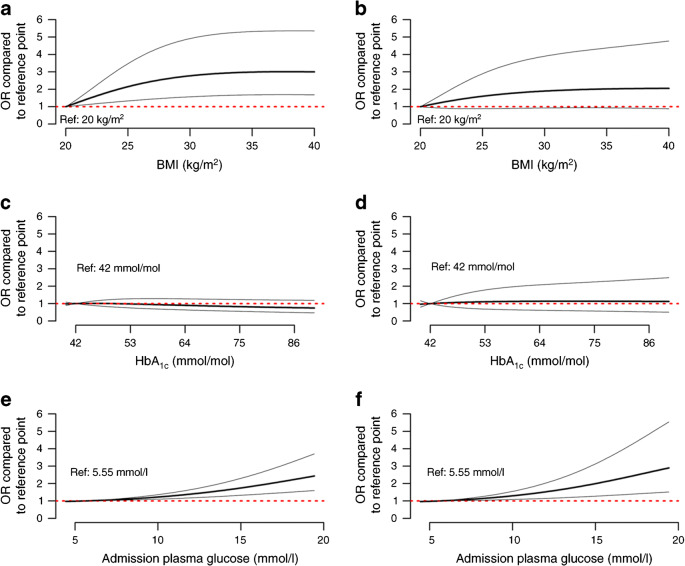
When using age- and sex-adjusted nonlinear models, BMI was significantly and positively associated with the primary outcome (p = 0.0001) but not with death on day 7 (p = 0.1488) (Fig. 2). In contrast, HbA1c level was neither associated with the primary outcome nor with death on day 7.
Fig. 2.
Sex- and age-adjusted ORs for the main outcome and for death, using logistic regression models with degree 2 multiple fractional polynomials. (a, b) OR for BMI for the primary outcome (a; p = 0.0001) and for death (b; p = 0.1488) on day 7 (reference value 20 kg/m2; n = 1117). (c, d) OR for HbA1c for the primary outcome (c; p = 0.2897) and for death (d; p = 0.9129) on day 7 (reference value 42 mmol/mol; n = 846). (e, f) OR for admission plasma glucose for the primary outcome (e; p = 0.0001) and for death (f; p = 0.0059) on day 7 (reference value 5.55 mmol/l; n = 940). The thick black line gives the OR compared with the reference point, the thin grey lines are the 95% CI, and the red dotted red line (OR = 1) corresponds to a similar risk-level as the reference point
Multivariable analyses were then conducted with characteristics prior to admission. BMI remained associated with the primary outcome in a model where sex and age were forced into models. When comorbidities and routine treatment were entered in an adjusted model with stepwise selection, BMI was the only independent factor associated with the primary outcome, with an adjusted OR of 1.28 (95% CI 1.10, 1.47) (Table 3). Finally, age, history of microvascular or macrovascular complications, and treated OSA were found to be independently associated with the risk of death on day 7 (Table 4). A sensitivity analysis conducted only in patients with a positive SARS-CoV-2 PCR test found similar results for both the primary outcome and death (data not shown).
Table 3.
Multivariable analysis of the primary outcome in CORONADO participants: covariables prior to admission
| Model ‘prior to admission’: fully adjusted |
Model ‘prior to admission’: stepwise selection with age and sex forced |
|||
|---|---|---|---|---|
| Patient characteristics | OR (95% CI) | p value | OR (95% CI) | p value |
| Age (+1 SD) | 1.05 (0.90, 1.21) | 0.5495 | 1.06 (0.92, 1.22) | 0.4448 |
| Sex (female/male) | 0.76 (0.57, 1.03) | 0.0777 | 0.75 (0.56, 1.01) | 0.0559 |
| BMI (+1 SD) | 1.24 (1.06, 1.44) | 0.0064 | 1.28 (1.10, 1.47) | 0.0010 |
| Treated OSA | 1.15 (0.76, 1.73) | 0.5036 | – | – |
| ARBs and/or ACE inhibitors and/or MRAs | 1.15 (0.86, 1.54) | 0.3493 | – | – |
Models were applied in 1020 participants yielding 281 primary outcomes (27.5%)
BMI was natural log transformed. For quantitative variables, OR corresponds to an increase of 1 SD after standardisation
MRAs include spironolactone and eplerenone
Table 4.
Multivariable analysis of the risk of death on day 7 in CORONADO participants: covariables prior to admission
| Model ‘prior to admission’: fully adjusted |
Model ‘prior to admission’: stepwise selection with age and sex forced |
|||
|---|---|---|---|---|
| Patient characteristics | OR (95% CI) | p value | OR (95% CI) | p value |
| Age (+1 SD) | 2.39 (1.67, 3.42) | <0.0001 | 2.48 (1.74, 3.53) | <0.0001 |
| Sex (female/male) | 0.78 (0.43, 1.40) | 0.4023 | 0.78 (0.44, 1.38) | 0.4007 |
| Hypertension | 0.76 (0.34, 1.70) | 0.5087 | – | – |
| Microvascular complications | 1.78 (0.92, 3.44) | 0.0846 | 2.14 (1.16, 3.94) | 0.0153 |
| Macrovascular complications | 2.26 (1.25, 4.08) | 0.0069 | 2.54 (1.44, 4.50) | 0.0013 |
| Heart failure | 1.08 (0.54, 2.15) | 0.8249 | – | – |
| Active cancer | 1.45 (0.77, 2.73) | 0.2458 | – | – |
| Treated OSA | 2.65 (1.36, 5.19) | 0.0044 | 2.80 (1.46, 5.38) | 0.0020 |
| β-Blockers | 1.19 (0.69, 2.06) | 0.5321 | – | – |
| Metformin | 0.80 (0.45, 1.43) | 0.4532 | – | – |
| Insulin | 1.26 (0.72, 2.22) | 0.4130 | – | – |
| Loop diuretics | 1.39 (0.76, 2.55) | 0.2806 | – | – |
| ARBs and/or ACE inhibitors and/or MRAs | 1.22 (0.68, 2.20) | 0.5069 | – | – |
Models were applied to 758 participants yielding 74 deaths (9.8%)
The OR for age corresponds to an increase of 1 SD after standardisation
MRAs include spironolactone and eplerenone
Factors on admission associated with study outcomes
Regarding COVID-19 symptoms on admission, dyspnoea was positively associated with the primary outcome and with death on day 7, whereas cephalalgia, upper respiratory tract symptoms (rhinitis and/or pharyngeal symptoms), and ageusia/anosmia, as well as time between symptom onset and admission, were negatively associated with death on day 7 (Table 2 and ESM Table 2). Several biological parameters reflecting the severity of the infection were also associated with both primary outcome and death on day 7, such as CRP, creatine phosphokinase (CPK) and lymphopaenia. Reduced kidney function, assessed by admission eGFR, and increased plasma AST level, was associated with both outcomes (Table 2 and ESM Table 2). In age- and sex-adjusted nonlinear models, admission plasma glucose was significantly and positively associated with the primary outcome (p = 0.0001) and with death on day 7 (p = 0.0059) (Fig. 2).
The prognostic value of characteristics on admission was finally investigated in multivariable models (Table 5). Among clinical symptoms, dyspnoea was the only predictor of the primary outcome. Regarding biological parameters, lymphopaenia on admission was independently associated with the primary outcome, as were increased AST and CRP concentrations. Dyspnoea and plasma levels of both AST and CRP were also independently associated with the risk of death on day 7, as well as decrease in platelet count and eGFR (Table 6). Once again, similar results were obtained for both the primary outcome and death on day 7 when considering only the patients with a positive PCR test (data not shown).
Table 5.
Multivariable analysis of the primary outcome in CORONADO participants: covariables on admission
| Model ‘on admission’: fully adjusted |
Model ‘on admission’: stepwise selection with age and sex forced |
|||
|---|---|---|---|---|
| Patient characteristics | OR (95% CI) | p value | OR (95% CI) | p value |
| Age (+1 SD) | 0.98 (0.77, 1.25) | 0.8569 | 1.02 (0.81, 1.28) | 0.8981 |
| Sex (female/male) | 1.46 (0.88, 2.41) | 0.1410 | 1.44 (0.89, 2.32) | 0.1417 |
| BMI (+1 SD) | 1.13 (0.89, 1.43) | 0.3297 | – | – |
| Dyspnoea | 2.17 (1.34, 3.50) | 0.0015 | 2.10 (1.31, 3.35) | 0.0020 |
| Admission plasma glucose (+1 SD) | 1.14 (0.92, 1.42) | 0.2391 | – | – |
| eGFR (+1 SD) | 0.81 (0.64, 1.01) | 0.0643 | – | – |
| AST (+1 SD) | 2.19 (1.65, 2.90) | <0.0001 | 2.23 (1.70, 2.93) | <0.0001 |
| Lymphocyte count (+1 SD) | 0.70 (0.53, 0.94) | 0.0161 | 0.67 (0.50, 0.88) | 0.0050 |
| Platelet count (+1 SD) | 0.80 (0.63, 1.01) | 0.0623 | – | – |
| CRP (+1 SD) | 2.00 (1.47, 2.73) | <0.0001 | 1.93 (1.43, 2.59) | <0.0001 |
Models were applied to 619 participants yielding 177 primary outcomes (28.6%)
For quantitative variables, OR corresponds to an increase of 1 SD after natural log transformation and standardisation, except for age, which was not natural log transformed
eGFR was calculated according to the CKD-EPI formula
Table 6.
Multivariable analysis of the risk of death on day 7 in CORONADO participants: covariables on admission
| Model ‘clinical and biological’: fully adjusted |
Model ‘clinical and biological’: stepwise selection with age and sex forced |
|||
|---|---|---|---|---|
| Patient characteristics | OR (95% CI) | p value | OR (95% CI) | p value |
| Age (+1 SD) | 4.28 (2.64, 6.94) | <0.0001 | 4.12 (2.59, 6.55) | <0.0001 |
| Sex (female/male) | 0.92 (0.44, 1.92) | 0.8237 | 1.02 (0.50, 2.09) | 0.9523 |
| Cephalalgia | 1.55 (0.43, 5.58) | 0.5029 | – | – |
| Dyspnoea | 2.73 (1.30, 5.73) | 0.0079 | 2.80 (1.37, 5.72) | 0.0049 |
| Rhinitis and/or pharyngeal signs | 0.46 (0.11, 1.96) | 0.2957 | – | – |
| Ageusia and/or anosmia | 1.31 (0.43, 4.01) | 0.6403 | – | – |
| Admission plasma glucose (log, +1 SD) | 1.30 (0.94, 1.82) | 0.1148 | – | – |
| eGFR (log, +1 SD) | 0.51 (0.37, 0.69) | <0.0001 | 0.51 (0.38, 0.69) | <0.0001 |
| AST (log, +1 SD) | 1.93 (1.38, 2.71) | 0.0001 | 1.85 (1.33, 2.56) | 0.0003 |
| White cell count (log, +1 SD) | 1.29 (0.86, 1.92) | 0.2126 | – | – |
| Platelet count (log, +1 SD) | 0.66 (0.47, 0.92) | 0.0144 | 0.71 (0.53, 0.97) | 0.0292 |
| CRP (log, +1 SD) | 1.70 (1.09, 2.66) | 0.0202 | 1.87 (1.20, 2.89) | 0.0052 |
Models were applied to 612 participants yielding 59 primary outcomes (9.6%)
For quantitative variables, OR corresponds to an increase of 1 SD after natural log transformation and standardisation, except for age, which was also standardised but not natural log transformed
eGFR was calculated according to the CKD-EPI formula
Discussion
CORONADO is the first study specifically dedicated to people with diabetes infected with SARS-CoV-2 and admitted to hospital. CORONADO was designed to address three main goals: (1) assess the phenotypic characteristics of patients with diabetes hospitalised for COVID-19; (2) estimate the prevalence of the primary outcome, which combines death and tracheal intubation for mechanical ventilation within the first 7 days following admission; (3) identify in this specific population certain prognostic factors associated with early severity of COVID-19. When considering variables prior to admission, our results support no independent association between a severe course of COVID-19 and age, sex, long-term glucose control, chronic complications, hypertension or usual medications, including RAAS blockers and DPP-4 inhibitors. Only BMI turned out to be independently associated with the primary outcome. When considering variables on admission, dyspnoea, lymphopaenia, and increased AST and CRP levels were independent prognostic factors for severe course of COVID-19.
To our knowledge, CORONADO is the first study that provides precise information regarding the characteristics of diabetes in the severe forms of COVID-19. The study population roughly resembles the French population of people living with diabetes, except for HbA1c, which was clearly higher in our study (65 mmol/mol [8.1%]) compared with the nationwide ENTRED-2 survey participants older than 65 years (54 mmol/mol [7.1%]) [17]. Of note, there was no overrepresentation of declared type 1 diabetes (only 3.0% of participants) in people with diabetes hospitalised for COVID-19.
The primary outcome occurred in 29.0% of CORONADO participants. While the design of the present study did not enable comparison of the severity of COVID-19 in people with or without diabetes, 20.3% of the study population required tracheal intubation for mechanical ventilation with a mortality rate of 10.6% as early as 7 days after admission. The severity of the prognosis of COVID-19 observed in people with diabetes in the present study is in accordance with previous epidemiological studies [10–13, 18, 19], and meta-analyses [14, 20]. An important issue is the choice of our primary endpoint, which combines death (an unequivocal outcome) with tracheal intubation for mechanical ventilation. It should be emphasised that the latter outcome can result from different factors, which were impossible to standardise in all centres, such as (1) clinical deterioration, (2) refusal to be intubated, or (3) futility (i.e. a medical decision not to intubate), leading to potentially fewer patients actually intubated compared with those meeting intubation criteria.
Regarding the clinical characteristics of COVID-19 in CORONADO participants, there was a high prevalence of fever and respiratory symptoms (cough, dyspnoea) and, to a lesser extent, digestive disorders. In addition to symptoms directly related to COVID-19, people with diabetes can also require management of acute metabolic disorders. In particular, physicians should be warned not only of the risk of ketoacidosis but also of hypoglycaemia, probably favoured by COVID-19-induced anorexia without concomitant adaptation of glucose-lowering drugs.
With the aim of providing clinicians with criteria to evaluate the risk of severe COVID-19 on an individual level in people with diabetes, we performed multivariable analyses to identify pre-admission and on-admission prognostic factors. Since some preclinical studies previously highlighted potential mechanistic links between glucose control, immune response and MERS-CoV infection [21], we were particularly interested in studying the relationship between long-term glucose control and COVID-19 prognosis. In fact, we failed to find any association between HbA1c (even with the highest values, >75 mmol/mol [9.0%]) and either the primary outcome or death on day 7. On the basis of this result and in order to increase the sample size for our analyses, we decided not to force HbA1c in the multivariate models.
An interesting finding is the association of BMI with study outcomes. Indeed, in our study, BMI was positively and independently associated with the primary outcome, which is largely driven by tracheal intubation. Interestingly, a recent report on COVID-19 patients in ICU showed an association between BMI and the requirement for mechanical ventilation, irrespective of diabetic status [22]. However, such an association with BMI was no longer statistically significant when considering death on day 7. It should also be noted that the increased risk for the primary outcome appears to be less pronounced in patients with morbid obesity (grade 3, BMI ≥40 kg/m2) compared with those who were overweight or with grade 1–2 obesity, a situation previously described as the ‘obesity paradox’ in ICUs [23]. Additional studies are clearly warranted to decipher the link between obesity, metabolic complications and COVID-19 severity with specific attention to fat mass distribution, insulin resistance and inflammatory/immune profiles.
While hypertension was previously reported as the most prevalent comorbidity in the general population with severe COVID-19 [2, 9, 12], it was not independently associated with the severity of the disease in the study. In addition, RAAS blockers (ACE inhibitors, ARBs and MRAs) were not independently associated with the main outcome, supporting the recent recommendation not to discontinue RAAS blockade [24]. Moreover, we found no association between glucose-lowering drugs, including DPP-4 inhibitors, that have been suggested to potentially interfere with coronavirus infection and COVID-19 prognosis [21, 25].
Our complementary multivariable approach was suitable for the identification of characteristics on admission associated with COVID-19 prognosis, of particular relevance for the management of people with diabetes in the setting of an emergency room. Notably, we found an age- and sex-independent association between increased admission plasma glucose levels and the severity of COVID-19, as previously reported in critically ill patients [26]. However, we speculate that this observation is rather the consequence of the severity of the infection than a causal primary factor.
Another important result concerns the identification of the prognostic factors of early death in people with diabetes and COVID-19. Compared with the primary outcome, which reflects aggressive management in ICUs with tracheal intubation, death on day 7 was more prevalent in elderly participants with an OR >14 for people older than 75 years, compared with younger individuals. In addition, these individuals also very frequently exhibited complications of diabetes (microvascular and macrovascular complications, mainly coronary heart disease) as well as pulmonary diseases (such as OSA). As expected, they were also more frequently on insulin therapy and taking multiple drugs (such as diuretics). Conversely, metformin use was associated with a reduced risk of early death, probably reflecting a less advanced stage of diabetes with fewer comorbidities (such as severe chronic kidney disease) that contraindicate its use. In multivariable analyses, age, diabetic complications and treated OSA remained significantly and independently associated with death on day 7. In addition, dyspnoea, reduced eGFR and platelet count, and increased AST and CRP on admission were independent markers of early death.
The discrepancy between the primary combined outcome (mainly driven by tracheal intubation) and death on day 7 could be explained by the fact that there were medical decisions not to pursue aggressive therapy in this frail population. In contrast, our data can be considered reassuring for the majority of people living with type 1 diabetes. Indeed, there was no death in participants with type 1 diabetes younger than 65 years. Additional data collection is currently ongoing to provide a precise picture of the rare individuals with type 1 diabetes hospitalised for COVID-19.
Some limitations must be acknowledged in the current analysis. We focused on hospitalised COVID-19 cases and our results cannot be generalised to all people with COVID-19 and diabetes, especially those with a less severe form of the disease. A secondary limitation is the size of our study population and the large proportion (i.e. 35.7%) of patients without available HbA1c. This is in accordance with the observation that only 55% of the people with diabetes had had three or more HbA1c determinations in the previous year according to French national registry data [27]. Finally, the present report focuses only on short-term prognosis (i.e. 7 days after admission) and one cannot exclude the possibility that diabetes characteristics prior to admission could be associated with severe COVID-19 outcomes in the longer term. However, strengths must be acknowledged such as the originality of the medical question leading to the CORONADO initiative and the inclusion of participants on a national basis. In addition, a large majority (>93%) of COVID-19 cases were confirmed with a positive PCR test, with few cases diagnosed from medical and/or radiological observations only. We also structured data collection in order to obtain a precise and standardised recording of phenotypic characteristics of the diabetic study population.
In conclusion, the CORONADO study refined the phenotypes of COVID-19 individuals with diabetes admitted to hospital and showed that chronic glycaemic control did not impact the immediate severity of COVID-19. Elderly populations with long-term diabetes with advanced diabetic complications and/or treated OSA were particularly at risk of early death, and might require specific management to avoid contamination with SARS-CoV-2. BMI also appears as an independent prognostic factor for COVID-19 severity in the population living with diabetes, requiring hospital admission.
Electronic supplementary material
(PDF 405 kb)
Acknowledgements
We thank the sponsor (DRCI [délégation à la recherche clinique et à l'innovation] CHU Nantes), the Clinical Project Manager (M. Saignes, CHU Nantes, Nantes, France) and assistant (J. Saunier, CHU Nantes, Nantes, France), Clinical Research Associates (S. El Andaloussi [CHU Nantes, Nantes, France], J. Martin-Gauthier [CHU Nantes, Nantes, France], E. Rebouilleau, [CHU Nantes, Nantes, France]) and data managers (B. Guyomarch [CHU Nantes, Nantes, France], T. Roman [CHU Nantes, Nantes, France]). We are grateful to P. Tucker (Trets, France) for editing the manuscript. We acknowledge all medical staff involved in the diagnosis and treatment of patients with COVID-19 in participating centres. We thank all general practitioners, specialists, pharmacists and biological laboratories responsible for hospitalised patients for providing additional medical information to investigators. We thank the Société Francophone du Diabète (SFD) and Société Française d’Endocrinologie (SFE) for disseminating the study design and organisation, and the Fédération Française des Diabétiques (FFD) for participating in the study organisation.
Authors’ relationships and activities
BC reports grants and personal fees from Amgen, personal fees from AstraZeneca, personal fees from Akcea, personal fees from Genfit, personal fees from Gilead, personal fees from Eli Lilly, personal fees from Novo Nordisk, personal fees from MSD, grants and personal fees from Sanofi, and grants and personal fees from Regeneron. SH reports personal fees and non-financial support from AstraZeneca, grants and personal fees from Bayer, personal fees from Boehringer Ingelheim, grants from Dinno Santé, personal fees from Eli Lilly, non-financial support from LVL, personal fees and non-financial support from MSD, personal fees from Novartis, grants from Pierre Fabre Santé, personal fees and non-financial support from Sanofi, personal fees and non-financial support from Servier, and personal fees from Valbiotis. MP reports personal fees and non-financial support from Novo Nordisk, non-financial support from Sanofi, and non-financial support from Amgen. SB reports personal fees from Novo Nordisk, personal fees from Sanofi, personal fees from Eli Lilly, personal fees from Medtronic, and personal fees from Abbott. PD reports personal fees from Novo Nordisk, personal fees from Sanofi, personal fees from Eli Lilly, personal fees from MSD, personal fees from Novartis, personal fees from Abbott, personal fees from AstraZeneca, personal fees from Boehringer Ingelheim, and personal fees from Mundipharma. BG reports personal fees from Eli Lilly, personal fees from Novo Nordisk, personal fees from Mellitus Care, personal fees from Isis Diabetes, and grants from Boehringer Ingelheim. MJ reports personal fees and non-financial support from Sanofi, personal fees and non-financial support from Eli Lilly, personal fees and non-financial support from Novo Nordisk, grants and personal fees from Boehringer Ingelheim, grants, personal fees and non-financial support from Medtronic, personal fees and non-financial support from Abbott, personal fees and non-financial support from BMS, personal fees and non-financial support from MSD, and grants, personal fees and non-financial support from AstraZeneca. LP reports personal fees and non-financial support from Sanofi, personal fees and non-financial support from Eli Lilly, personal fees and non-financial support from Novo Nordisk, and personal fees and non-financial support from MSD. RR reports grants, personal fees and non-financial support from Sanofi, grants, personal fees and non-financial support from Novo Nordisk, personal fees and non-financial support from Eli Lilly, personal fees from Mundipharma, personal fees from Janssen, personal fees from Servier, grants and personal fees from AstraZeneca, personal fees from MSD, personal fees from Medtronic, personal fees from Abbott, grants from Diabnext, and personal fees from Applied Therapeutics. JFG reports personal fees and non-financial support from Eli Lilly, personal fees and non-financial support from Novo Nordisk, personal fees and non-financial support from Gilead, and personal fees and non-financial support from AstraZeneca. PG reports personal fees from Abbott, personal fees from Amgen, personal fees from AstraZeneca, personal fees from Boehringer Ingelheim, personal fees from Eli Lilly, personal fees from MSD, personal fees from Mundipharma, grants and personal fees from Novo Nordisk, personal fees from Sanofi, and personal fees from Servier. All other authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Abbreviations
- ALT
Alanine aminotransferase
- ARB
Angiotensin II receptor blocker
- AST
Aspartate aminotransferase
- CKD-EPI
Chronic Kidney Disease Epidemiology Collaboration
- CORONADO
Coronavirus SARS-CoV-2 and Diabetes Outcomes
- COVID-19
Coronavirus disease-2019
- CPK
Creatine phosphokinase
- CRP
C-reactive protein
- CT
Computed tomography
- DPP-4
Dipeptidyl peptidase 4
- ICU
Intensive care unit
- MERS-CoV
Middle East respiratory syndrome-related coronavirus
- MRA
Mineralocorticoid-receptor antagonist
- OSA
Obstructive sleep apnoea
- RAAS
Renin–angiotensin–aldosterone system
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus-2
Contribution statement
BC, SH and MW had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. BC, SH and MW contributed to the work equally and should be regarded as co-first authors. Concept and design: BC, JFG, PG, SH, MP, RRoussel, MW. Acquisition, analysis, or interpretation of data: BB, DB, BC, FC-R, CC, J-FG, PG, SH, VK, BL, MP, RRobert, RRoussel, J-FT, CT, MW on behalf of the scientific committee of the study (the list of scientific committee is available in the ESM). Statistical analysis: MW, SC, PJS. Patient recruitment: AA-S, IA, CA, GA, FB, SB, MB-G, OB, BC, EC, PD, ED, AD-B, BG, J-FG, PG, SH, MJ, VK, LMarchand, LMeyer, LP, GP, J-PR, RRoussel, AS, CT, BT, CV. Fundraising: BC, PG, SH, MP and BB. Drafting the manuscript: BC, PG, SH, MP, MW. Critical revision of the manuscript for important intellectual content: all co-authors. All authors have approved the final version of the manuscript.
Funding
This study received the following funding: the Fondation Francophone de Recherche sur le Diabète (FFRD), supported by Novo Nordisk, MSD, Abbott, AstraZeneca, Lilly and FFD (Fédération Française des Diabétiques) – CORONADO initiative emergency grant; Société Francophone du Diabète (SFD) – CORONADO initiative emergency grant; Air Liquide Health Care international. All research facilities are acknowledged for providing research associates and research technicians for clinical investigations pro bono. The funders of the study had no role in study design, data collection, data analysis, data interpretation or writing of the report. The corresponding authors (BC and SH) had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Data availability
A data-sharing statement is available in the ESM.
Footnotes
A complete list of the CORONADO trial investigators is provided in the Electronic supplementary material (ESM).
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Bertrand Cariou, Samy Hadjadj and Matthieu Wargny contributed equally to this article.
Change history
11/22/2021
The link in reference 15 has been corrected.
Change history
7/2/2020
A Correction to this paper has been published: 10.1007/s00125-020-05207-3
Contributor Information
Bertrand Cariou, Email: bertrand.cariou@univ-nantes.fr.
Samy Hadjadj, Email: samy.hadjadj@univ-nantes.fr.
References
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah BR, Hux JE. Quantifying the risk of infectious diseases for people with diabetes. Diabetes Care. 2003;26(2):510–513. doi: 10.2337/diacare.26.2.510. [DOI] [PubMed] [Google Scholar]
- 4.Muller LM, Gorter KJ, Hak E, et al. Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin Infect Dis. 2005;41(3):281–288. doi: 10.1086/431587. [DOI] [PubMed] [Google Scholar]
- 5.Yang JK, Feng Y, Yuan MY. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet Med. 2006;23(6):623–628. doi: 10.1111/j.1464-5491.2006.01861.x. [DOI] [PubMed] [Google Scholar]
- 6.Alqahtani FY, Aleanizy FS, Ali El Hadi Mohamed R, et al. Prevalence of comorbidities in cases of Middle East respiratory syndrome coronavirus: a retrospective study. Epidemiol Infect. 2018;147:1–5. doi: 10.1017/S0950268818002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcome of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323(16):1674–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garg S, Kim L, Whitaker M, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 – COVID-NET, 14 States, March 1–30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Onder G, Rezza G, Brusaferro S (2020) Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 10.1001/jama.2020.4683 [DOI] [PubMed]
- 11.Bhatraju PK, Ghassemieh BJ, Nichols M et al (2020) Covid-19 in critically ill patients in the Seattle region – case series. N Engl J Med. 10.1056/NEJMoa2004500 [DOI] [PMC free article] [PubMed]
- 12.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu C, Chen X, Cai Y et al (2020) Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed]
- 14.Roncon L, Zuin M, Rigatelli G, Zuliani G. Diabetic patients with COVID-19 infection are at higher risk of ICU admission and poor short-term outcome. J Clin Virol. 2020;127:104354. doi: 10.1016/j.jcv.2020.104354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention (2020) Coronavirus Disease 2019 (COVID-19): groups at higher risk for severe illness. Available from https://aimvein.com/blogs/news/covid-19-measure-for-high-risk-groups. Accessed 21 April 2020
- 16.Sauerbrei W, Meier-Hirmer C, Benner A, Royston P. Multivariable regression model building by using fractional polynomials: description of SAS, STATA and R programs. Comput Stat Data Anal. 2006;50(12):3464–3485. doi: 10.1016/j.csda.2005.07.015. [DOI] [Google Scholar]
- 17.Pornet C, Bourdel-Marchasson I, Lecomte P, et al. Trends in the quality of care for elderly people with type 2 diabetes: the need for improvements in safety and quality (the 2001 and 2007 ENTRED Surveys) Diabetes Metab. 2011;37(2):152–161. doi: 10.1016/j.diabet.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.CDC COVID-19 response team Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 – United States, February 12–March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(13):382–386. doi: 10.15585/mmwr.mm6913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang I, Anthonius M, Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia – a systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr. 2020;14(4):395–403. doi: 10.1016/j.dsx.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kulcsar J, Coleman CM, Beck SE, Frieman MD. Comorbid diabetes results in immune dysregulation and enhanced disease severity following MERS-CoV infection. JCI Insight. 2019;4(20):e131774. doi: 10.1172/jci.insight.13177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simonnet A, Chetboun M, Poissy J et al (2020) High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring). 10.1002/oby.22831 [DOI] [PMC free article] [PubMed]
- 23.Schetz M, De Jong A, Deane AM, et al. Obesity in the critically ill: a narrative review. Intensive Care Med. 2019;45(6):757–769. doi: 10.1007/s00134-019-05594-1. [DOI] [PubMed] [Google Scholar]
- 24.Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin–angiotensin–aldosterone system inhibitors in patients with COVID-19. N Engl J Med. 2020;382(17):1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drucker DJ. Coronavirus infection and type 2 diabetes shared-pathways with therapeutic implications. Endocr Rev. 2020;41(3):bnaa011. doi: 10.1210/endrev/bnaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345(19):1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 27.Fosse-Edorh S, Mandereau-Bruno L, Piffaretti C (2018). Le poids du diabète en France en 2016 Synthèse épidémiologique. Santé publique France:8p; available from https://www.santepubliquefrance.fr/maladies-et-traumatismes/diabete/documents/rapport-synthese/le-poids-du-diabete-en-france-en-2016.-synthese-epidemiologique. Accessed 21 April 2020 [Article in French]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 405 kb)
Data Availability Statement
A data-sharing statement is available in the ESM.