Sir,
The WHO has recommended reverse transcription-polymerase chain reaction (RT-PCR) for the confirmation of coronavirus disease 2019 (COVID-19) diagnosis. Real-time RT-PCR assays with automated extraction systems are required to process large numbers of specimens. Corman et al1 have reported three real-time RT-PCR assays [based on the RNA-dependent RNA polymerase (RdRp) gene, envelope (E) gene and nucleocapsid (N) gene] for detecting beta coronaviruses, including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)1, and additionally, Chu et al2 have reported two real-time RT-PCR assays based on ORF 1b and N gene that are highly conserved among Sarbeco viruses.
At the Indian Council of Medical Research-National Institute of Virology (ICMR-NIV), Pune, we adopted a real-time RT-PCR assay for screening (E gene assay) and confirmation (RdRp, N and ORF gene)1 along with housekeeping Rnase P gene. Sequences and source of primers and probes used in this study are given in the Table, and the performance of screening assay was assessed using in vitro transcribed (IVT) RNA for SARS-CoV-2 targeting E gene, whereas the confirmatory RdRp assay used purified RNA of SARS-coronavirus Frankfurt 1 strain. Limited supply of positive controls was available from the WHO from Charité Laboratories, Berlin, via European Virus Archive Global (EVAg). A need was sensed to provide positive control to all laboratories in the national network of Viral Research and Diagnostic Laboratories (VRDLs) for real-time RT-PCR. Thus, positive controls for the screening and confirmatory assays were generated in-house.
Table.
Primer and probe sets used in this study
| Assay type | Name | Sequence (5’- 3’) |
|---|---|---|
| E gene screening assay | E_Sarbeco_F1 | ACAGGTACGTTAATAGTTAATAGCGT† |
| E_Sarbeco_R2 | ATATTGCAGCAGTACGCACACA† | |
| E_Sarbeco_P1 | FAM-ACACTAGCATCCTTACTGCGCTTCG-BHQ‡ | |
| RNase P gene (internal control) screening assay | RNaseP -F1 | AGATTTGGACCTGCGAGCG† |
| RNaseP -R1 | GAGCGGCTGTCTCCACAAGT† | |
| RNaseP -P1 | FAM-TTCTGACCTGAAGGCTCTGCGCG-BHQ‡ | |
| RdRp gene confirmatory assay | RdRP_SARSr-F2 | GTGARATGGTCATGTGTGGCGG† |
| RdRP_SARSr-R1 | CARATGTTAAASACACTATTAGCATA† | |
| RdRP_SARSr-P2 (Specific for Wuhan-CoV) | FAM-CAGGTGGAACCTCATCAGGAGATGC-QSY‡ | |
| HKU ORF gene confirmatory assay | HKU-ORF1b-nsp14F | TGGGGYTTTACRGGTAACCT† |
| HKU-ORF1b-nsp14 R | AACRCGCTTAACAAAGCACTC† | |
| HKU-ORF1b-nsp14 P | FAM-TAGTTGTGATGCWATCATGACTAG-QSY‡ |
Source: †Eurofins Genomics India Pvt. Ltd., Bengaluru; ‡Invitrogen, USA
Using whole-genome sequence of the first Indian COVID-19 case, forward primers with T7 promoter tag at the 5´ end, were designed to amplify full-length E gene, N gene and partial RdRp and ORF 1b regions. Gene-specific PCR was carried out to amplify the desired PCR product. Amplicons were purified using Qiagen direct PCR purification kit (Qiagen, Hilden, Germany). IVT was synthesized using T7 Riboprobe (Promega, USA) as per the kit protocol. Ten-fold serial dilutions of each transcribed RNA products were tested with respective gene primer probe sets for specific detection and limit of detection.
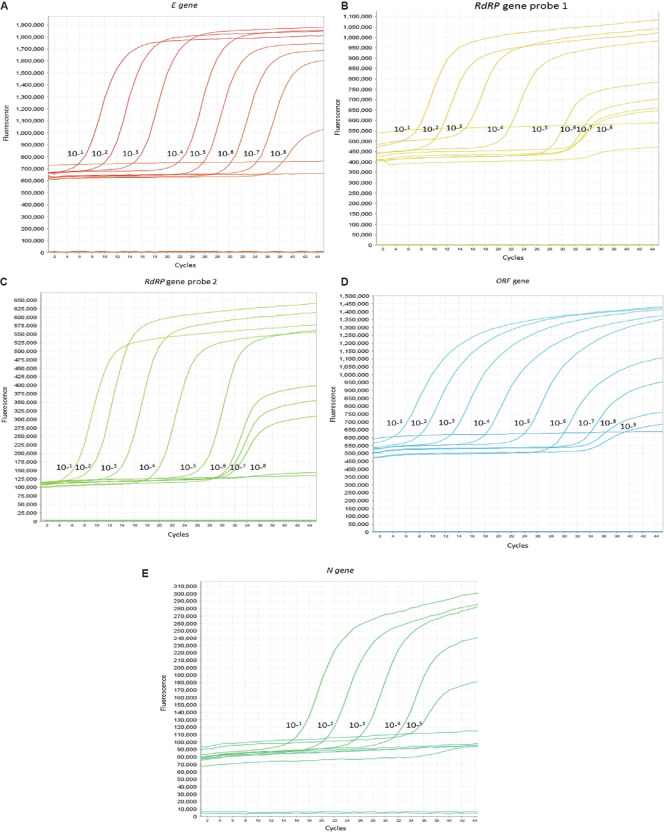
Gene-specific desired amplification was also observed in conventional RT-PCR (E: 550 bp, N: 1254 bp, RdRP: 344, ORF: 388) (Fig. 1). Further, the IVT RNA of each gene was serially diluted 10-fold (101 to 1010), and the performance was tested with gene-specific primer probe by real-time RT-PCR. All the transcribed RNA showed amplification with specific primer probe. The limit of detection for E gene was 106 yielding a cycle threshold (Ct) at cycle 29, RdRP (p1) was 105 with 27 Ct, RdRp (p2) was 106 with 29 Ct and ORF 1b106 with 28 Ct, whereas N gene showed 103 with 25 Ct (Fig. 2A-E).
Fig. 1.
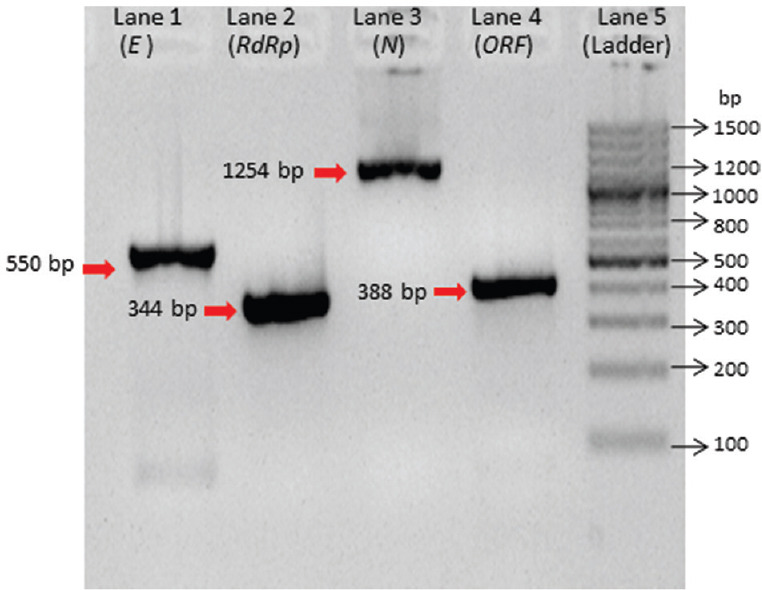
Positive DNA amplification of envelope (E), RNA-dependent RNA polymerase (RdRp), nucleocapsid (N) and open reading frame (ORF) 1b-nsp14 for in vitro transcribed preparation. Lane 1: E gene (550 bp), lane 2: RdRp gene (344 bp), lane 3: N gene (1254 bp) positive, lane 4: ORF (388 bp), lane 5: 100 bp DNA ladder.
Fig. 2.
(A-E) Multicomponent amplification graph for 10-fold dilutions of in vitro transcribed RNA: (A) E gene, (B) RdRp gene probe 1, (C) RdRp gene probe 2, (D) ORF gene, (E) N gene. The X axis represents number of cycles and Y axis represents amount of fluorescence.
When the assay was first set up at the National Influenza Centre of ICMR-NIV, Pune, the IVT RNA for E and SARS coronavirus Frankfurt 1 strain were received from EVAg. The real-time PCR screening assay (E gene) was also established at the 13 VRDLs as part of ICMR's efforts to expand testing to VRDLs closer to major airports3. However, due to screening of low number of samples, the repeated use of positive controls was made. The supply of IVT RNA as positive control for E gene from EVAg was limited and had non-consistent performance when diluted further. In addition, the control for RdRp assay was from a SARS coronavirus Frankfurt 1 isolate, which yielded weak signal with RdRp Wuhan-specific probe. This necessitated the development of an indigenous IVT RNA for E and RdRp. In addition, majority of the WHO screening protocols (5 of 6) are based on N gene targeting different nucleotide positions and require multiple specific positive controls4. Hence, an IVT RNA was designed for entire N gene which would be compatible for multiple protocols.
We demonstrated the successful use of IVT RNA for N gene recommended in various protocols available on WHO site. The partial RdRp IVT RNA worked well with both the RdRp probes described in Charité, Berlin, Germany5, especially Wuhan-specific RdRp probe 2, which could be used as confirmatory test. All the IVT RNA had good yield and performed well with specific primer probe.
In conclusion, gene-specific IVT RNA was synthesized for all the gene targets used in real time PCR. These IVTs were used effectively by all VRDLs as positive control. Successful establishment of diagnostic system including in-house positive control was beneficial to provide timely diagnosis and accelerate clinical management and isolation of SARS-CoV-2 patients and to control further spread.
Acknowledgment
Authors acknowledge the National Influenza Centre staff: Drs S. Bharadwaj, R. Ghug, Ms U. Saha, Servshri H. Kengle, A. Awhale, Dr V. Malik, Ms A. Jagtap, Shri A. Gondhalikar, Ms S. Digraskar, Ms P. Malsane, Shri V. Awatade, Ms S. Bhorekar, Dr S. Salve, Ms P. Shinde and Dr B. Nimhas.
Footnotes
Financial support & sponsorship: Authors acknowledge the Department of Health Research, Ministry of Health & Family Welfare, Government of India, New Delhi, for financial support
Conflicts of Interest: None.
References
- 1.Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DKW, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020:25. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. pii: 2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chu DKW, Pan Y, Cheng SMS, Hui KPY, Krishnan P, Liu Y, et al. Molecular diagnosis of a novel coronavirus (2019-nCoV) causing an outbreak of pneumonia. Clin Chem. 2020 doi: 10.1093/clinchem/hvaa029. pii: Hvaa02920200117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Department of Health Research, Ministry of Health & Family Welfare, Government of India. Establishment of a network of laboratories for managing epidemics and natural calamities (VRDL) [accessed on February 29, 2020]. Available from: https://dhr.gov.in/schemes/esablishment-network-laboratories-managing-epidemicsand-natural-calamities .
- 4.World Health Organization. Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases. WHO; 2020. [accessed on January 17, 2020]. Available from: https://wwwwhoint/publications-detail/laboratory-testing-for-2019-novel-coronavirus-in-suspected-human-cases-20200117 . [Google Scholar]
- 5.World Health Organization. Coronavirus disease (COVID-19) technical guidance: Laboratory testing for 2019-nCoV in humans. WHO; 2020. [accessed on January 17, 2020]. Available from: https://wwwwhoint/emergencies/diseases/novel-coronavirus-2019/technical-guidance/laboratory-guidance . [Google Scholar]