Abstract
Background
Cell entry of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) depends on binding of the viral spike (S) proteins to angiotensin-converting enzyme 2 and on S protein priming by TMPRSS2. Inhibition of TMPRSS2 may work to block or decrease the severity of SARS-CoV-2 infections. Intriguingly, TMPRSS2 is an androgen-regulated gene that is up-regulated in prostate cancer where it supports tumor progression and is involved in a frequent genetic translocation with the ERG gene. First- or second-generation androgen-deprivation therapies (ADTs) decrease the levels of TMPRSS2. Here we put forward the hypothesis that ADTs may protect patients affected by prostate cancer from SARS-CoV-2 infections.
Materials and methods
We extracted data regarding 9280 patients (4532 males) with laboratory-confirmed SARS-CoV-2 infection from 68 hospitals in Veneto, one of the Italian regions that was most affected by the coronavirus disease 2019 (COVID-19) pandemic. The parameters used for each COVID-19-positive patient were sex, hospitalization, admission to intensive care unit, death, tumor diagnosis, prostate cancer diagnosis, and ADT.
Results
There were evaluable 9280 SARS-CoV-2-positive patients in Veneto on 1 April 2020. Overall, males developed more severe complications, were more frequently hospitalized, and had a worse clinical outcome than females. Considering only the Veneto male population (2.4 million men), 0.2% and 0.3% of non-cancer and cancer patients, respectively, tested positive for SARS-CoV-2. Comparing the total number of SARS-CoV-2-positive cases, prostate cancer patients receiving ADT had a significantly lower risk of SARS-CoV-2 infection compared with patients who did not receive ADT (OR 4.05; 95% CI 1.55–10.59). A greater difference was found comparing prostate cancer patients receiving ADT with patients with any other type of cancer (OR 4.86; 95% CI 1.88–12.56).
Conclusion
Our data suggest that cancer patients have an increased risk of SARS-CoV-2 infections compared with non-cancer patients. However, prostate cancer patients receiving ADT appear to be partially protected from SARS-CoV-2 infections.
Key words: androgen-deprivation therapy, COVID-19, prostate cancer
Highlights
-
•
SARS-CoV-2-infected men have a worse clinical outcome than women.
-
•
Cancer patients have an increased risk of SARS-CoV-2 infection.
-
•
Prostate cancer patients receiving androgen-deprivation therapies appear to be partially protected from the infection.
Introduction
In December 2019, a previously unknown coronavirus (SARS-CoV-2) causing severe acute respiratory syndrome emerged in the city of Wuhan in the Hubei province of China.1, 2, 3 The full spectrum of the disease, termed COVID-19 (coronavirus disease 2019), ranges from mild, self-limiting respiratory tract illness to severe progressive pneumonia, multiorgan failure, and death.4 By 22 April 2020, the virus spread to over 150 countries and affected more than 2.5 million individuals, causing over 175 000 deaths.5
TMPRSS2 is a member of the family of type II transmembrane serine proteases that are involved in multiple physiological and pathological processes, including cancer and viral infections.6, 7, 8 In particular, TMPRSS2 has been implicated as a critical host cell factor for the spread of several clinically relevant viruses, including influenza A viruses, SARS-CoV, and MERS-CoV coronaviruses.9, 10, 11, 12, 13, 14 Recent studies report that the new SARS-CoV-2 binds to angiotensin-converting enzyme 2 (ACE2) for cell entry, followed by proteolytic cleavage of the S protein by TMPRSS2 allowing the fusion of viral and cellular membranes.15 , 16 Moreover, in vitro evidence indicates that TMPRSS2 inhibition by camostat mesylate may be beneficial to prevent the infection of SARS-CoV-2.15
TMPRSS2 is highly expressed in both localized and metastatic prostate cancers17 , 18 and its transcription is regulated by the androgen receptor (AR).17 Intriguingly, it has been shown that ARs regulate TMPRSS2 expression also in non-prostatic tissues, including lung. In vitro and in vivo results show that androgen administration induces TMPRSS2 expression in human lung epithelial cells and that androgen deprivation reduces TMPRSS2 transcription in murine lung.19 The androgen-dependent regulation of TMPRSS2 expression in the lung may explain the increased susceptibility of men to develop SARS-CoV-2 severe infections when compared with women.
Given that TMPRSS2 levels are under the control of androgens not only in the prostate but also in the lung, we put forward the hypothesis that androgen deprivation therapies (ADTs) may protect patients affected by prostate cancer from SARS-CoV-2 infections.
Materials and methods
Details of patients with a diagnosis of SARS-CoV-2 infection in the Italian region of Veneto, with or without cancer, were obtained from the following data sources: (i) the Veneto Archive of COVID-19-positive subjects, updated on 1 April 2020, (ii) the Tumor Registry Archive, and (iii) the Regional Medicines Technical Commission. The parameters used for each patient positive to COVID-19 were: sex, hospitalized (yes/no), admission to an intensive care unit (ICU) (yes/no), death, tumor diagnosis, diagnosis of prostate cancer, and ADT. The primary end point of the study was to assess the frequency of SARS-CoV-2 infection in: (i) patients affected by cancer, (ii) patients affected by prostate cancer, (iii) patients affected by prostate cancer in therapy with or without ADT, and (iv) to assess the severity of SARS-CoV-2 infection on the categories above based on patients' hospitalization, admission to an ICU, or death. Statistical evaluation of the strength of the association between SARS-CoV-2 cases and different types of tumor patients in the male population of the Veneto Region was obtained by means of odds ratio (OR). Data were considered also after stratification for the severity of the disease. The 95% confidence interval (CI) for OR was obtained using the Miettinen-Nurminen method.20 The P value was calculated according to Sheskin.21 Comparisons among frequencies were obtained with the chi-square test. Statistical significance was considered for P < 0.05.
Results
We extracted data regarding 9280 patients with laboratory-confirmed SARS-CoV-2 infection from 68 hospitals in the Veneto Region. The average age of patients was 73 years for hospitalized, 67 years for ICU-hospitalized, and 81 years for deceased patients.
Although women were infected at a higher prevalence than men (44% men; 56% women), male patients developed more severe forms of the disease (Figure 1 ). Men were more frequently hospitalized (60% men; 40% women), represented the vast majority of ICU-hospitalized patients (78% men; 22% women), and accounted for more deaths (62% men; 38% women) (Figure 1). These data are in line with recent results from another study, reporting a more severe outcome for men infected by SARS-CoV-2.22
Figure 1.
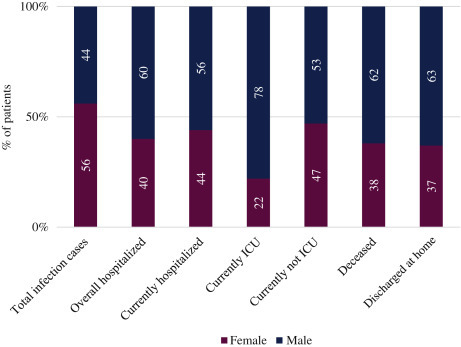
Percentage of patients infected with SARS-CoV-2 divided by sex showing an increased severity of COVID-19 in males.
Among the patients for which clinical data were available (N = 9280), 8.5% had a diagnosis of cancer (n = 786) and 1.3% had prostate cancer (n = 118). Considering only the male population (n = 4532), cancer patients represent 9.5% (n = 430) and those with prostate cancer 2.6% (n = 118). The distribution of tumor types in SARS-CoV-2-infected male cancer patients is shown in supplementary Table S1, available at Annals of Oncology online. Prostate cancer patients accounted for 28% of the total tumor cases, followed by kidney/bladder cancer (17%), colorectal cancer (15%), and leukemia/lymphoma (11%). The prevalence of some types of cancer (e.g. lung cancer) in the population of SARS-CoV-2-positive patients was different from those of noninfected cancer patients in Europe.23
The overall distributions by age of SARS-CoV-2-positive cases in non-cancer and cancer patients are indicated in Table 1 . Of note, SARS-CoV-2-positive patients with cancer were slightly older than patients without cancer (Table 1). Overall, 68% (n = 3085) of all SARS-CoV-2 males were less than 70 years old, while only 36% (n = 153 cancer patients, n = 43 prostate cancer) of cancer patients were in this age group. On the contrary, no relevant age differences were observed between patients with a history of prostate cancer compared with the total number of cancer patients. Overall, in the male population, cancer patients had a higher risk of SARS-CoV-2 infection (OR 1.79; CI 1.62–1.98) (Table 2 ).
Table 1.
Percentage of SARS-CoV-2 male cases divided by age in non-cancer, cancer, and prostate cancer patients
| Age (years) |
SARS-CoV-2-positive |
Cancer |
Prostate cancer |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| <70 | 3085 (68.1) | 153 (35.6) | 43 (36.4) |
| 70–79 | 743 (16.4) | 139 (32.3) | 43 (36.4) |
| 80+ | 704 (15.5) | 138 (32.1) | 32 (27.1) |
| Total | 4532 (100) | 430 (100) | 118 (100) |
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Table 2.
Prevalence of SARS-CoV-2 positive cases in the male population of the Veneto Region, concerning cancer patients
| Exposed population | SARS-CoV-2-positive | OR (95% CI) | |
|---|---|---|---|
| Total male population | 2 399 783a | 4532 (0.2%) | — |
| Prevalent cancer patients | 127 368b | 430 (0.3%) | 1.79 (1.62–1.98) P < 0.0001 |
Data from the total male population were considered as the reference for OR calculation.
CI, confidence interval; OR, odds ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Male resident population in the Veneto region as of January 1st, 2019 (source: National Health Statistics Institute).
As of January 1st, 2016 (source: Veneto Tumor Registry. Available at https://gecoopendata.registrotumoriveneto.it/prevalenza.php?lang=EN).
Analyzing disease outcomes in male patients, we observed that patients with a tumor diagnosis, including prostate cancer, develop more severe disease conditions in males (Figure 2 ). Indeed, 47.0% (n = 2131) of male patients were hospitalized and 6.9% (n = 312) died, while among patients with cancer 67.9% (n = 292) were hospitalized and 17.4% (n = 75) died. No significant differences were observed between prostate cancer patients and those affected by other types of cancers. These differences were not due to the age of patients. As shown in supplementary Table S2, available at Annals of Oncology online, when cancer and non-cancer patients were grouped according to age, cancer patients infected by SARS-CoV-2 still had higher hospitalization rates and mortality than non-cancer patients, particularly in the group younger than 70 years (P = 0.0055). In summary, our data indicate that male cancer patients have an increased risk of SARS-CoV-2 infection and develop more severe forms of COVID-19, in line with a recent study.24
Figure 2.
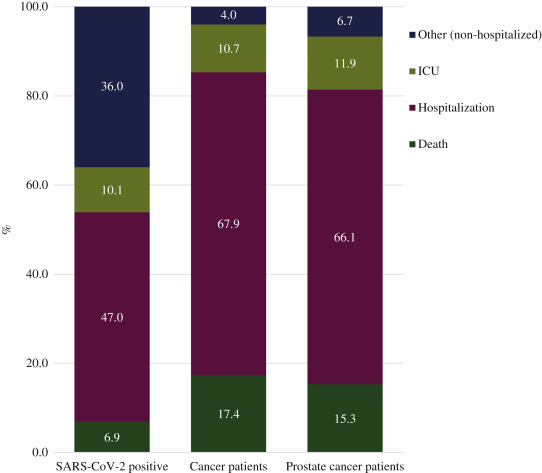
Percentage of male patients infected with SARS-CoV-2 in Veneto divided by hospitalization, ICU, and death.
Finally, we divided the population of prostate cancer patients according to two treatment modalities, namely ADT and non-ADT. Strikingly, only 4 out of 5273 patients receiving ADT in Veneto developed SARS-CoV-2 infection and none of these patients died (Table 3 ). Comparing the total number of SARS-CoV-2-positive cases, patients with prostate cancer receiving ADT had a significantly lower risk of SARS-CoV-2 infection compared with patients who did not receive ADT (OR 4.05; 95% CI 1.55–10.59). An even greater difference was found comparing prostate cancer patients receiving ADT with patients with any other type of cancer (OR 4.86; 95% CI 1.88–12.56). Altogether, these data indicate that androgen deprivation in prostate cancer patients is associated with a reduced probability to develop SARS-CoV-2 infections and with more positive infection outcomes.
Table 3.
Epidemiological characteristics of prostate cancer patients with confirmed SARS-CoV-2 infection in Veneto, treated with or without androgen-deprivation therapy
| PCa in ADT | PCa non-ADT | OR (95% CI) | Other tumors | OR (95% CI) | |
|---|---|---|---|---|---|
| Cancer patients in Veneto Region population | 5273 | 37 161a | 84934a | ||
| Total no. of SARS-CoV-2-positive | 4 | 114 | 4.05 (1.55–10.59) P = 0.0043 |
312 | 4.86 (1.88–12.56) P = 0.0011 |
| Mild disease | 3 | 83 | 3.93 (1.31–11.77) P = 0.0144 |
223 | 4.62 (1.56–13.69) P = 0.0057 |
| Non-hospitalized | 1 | 7 | 9 | ||
| Hospitalized | 2 | 76 | 214 | ||
| Severe disease | 1 | 31 | 4.40 (0.76–25.50) P = 0.0982 | 89 | 5.53 (0.97–31.58) P = 0.0544 |
| Intensive care (ICU) | 1 | 13 | 32 | ||
| Deceased | 0 | 18 | 57 | ||
| Estimated total SARS-CoV-2-positive cases/100 000 | 76 | 307 | 367 |
PCa patients with ADT were considered as reference for OR calculation. Patients are presented referring to the degree of infection severity.
ADT, androgen-deprivation therapy; CI, confidence interval; OR, odds ratio; PCa, prostate cancer; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Prevalent cancer patients’ data are as of January 1st, 2016 (source: Veneto Tumor Registry. Available at https://gecoopendata.registrotumoriveneto.it/prevalenza.php?lang=EN).
Discussion
Among patients with severe atypical pneumonia, the mortality rate is very high.25 , 26 Two coronaviruses have already caused severe respiratory syndromes previously in this century: the severe acute respiratory syndrome coronavirus (SARS-CoV)27 , 28 and the Middle-East respiratory syndrome coronavirus (MERS-CoV).29 However, besides containment measures, we do not have any effective treatment or vaccine against the deadly respiratory syndrome caused by SARS-CoV-2.
TMPRSS2 transcription is regulated by the AR.17 This feature contributes to the high expression of TMPRSS2 in the prostate gland18 and the high frequency of genomic rearrangements involving the TMPRSS2 promoter and members of the ETS gene family, particularly ERG, which places this oncogene under the control of the AR.30 In vitro studies demonstrate that TMPRSS2 inhibition may be beneficial to prevent infection with SARS-CoV-2.15 However, few specific TMPRSS2 inhibitors are currently in preclinical/clinical development.
The evidence that ARs regulate TMPRSS2 expression in non-prostatic tissues, including lung,19 may explain the increased susceptibility of men to SARS-CoV-2 severe infections. This suggests novel potential therapeutic interventions to treat COVID-19-affected people. ADT is known to decrease the levels of TMPRSS2,31 and AR antagonists could be used to block or decrease the severity of SARS-CoV-2 infection in male patients, potentially in combination with other inhibitors of viral entry or replication. In addition, other drugs that interfere with positive regulation of TMPRSS2 by ARs, AR co-regulatory factors, and transcription factors (e.g., ETS, SP1) could be used to suppress TMPRSS2 expression and activity in lung and other tissues.32, 33, 34, 35, 36 Besides the regulation of TMPRSS2, androgens could also increase the severity of SARS-CoV-2 infection in males by modulating the immune response. Androgens can increase the number and function of circulating neutrophils, increase the production of interleukin 1β (IL-1β), IL-10, IL-2, and transforming growth factor-β (TGF-β) by immune cells, and decrease the antibody response to viral infections.37 In this respect, it is worth noticing that recent evidence demonstrates that neutrophils are responsible for the cytokine storm syndrome, observed in patients with severe COVID-19.37 , 38 Besides androgens, other steroid hormones (e.g. estrogen, glucocorticoids) may enhance TMPRSS2 expression through binding of their respective nuclear receptors to responsive elements (e.g. ERE, GRE) in the gene promoter, thereby explaining the severity of infections in some cases of female patients.39 , 40 Therefore, inhibition of these regulatory pathways through already available therapies may also decrease the severity of SARS-CoV-2 infections. Our data are in line with recent reports from China that demonstrated similar trends in male patients and those with cancer.24
Our study may have some limitations. SARS-CoV-2-infected cancer patients may have been tested at a greater rate than non-cancer patients, since these patients are more often hospitalized. This may explain the higher prevalence of infected individuals in the cancer patient population. Prostate cancer patients with ADT may also practice more social distancing than prostate cancer patients with non-ADT and the totality of cancer patients.
In summary, these data need to be further validated in additional large cohorts of SARS-CoV-2-infected patients and corrected for multiple variables. ADT, based on luteinizing hormone-releasing hormone (LHRH) agonist/antagonists or AR inhibitors, may be considered to reduce SARS-CoV-2 infections or complications in high-risk male populations. Given that the effects of these compounds are reversible, they could be used transiently (e.g. 1 month) in patients affected by SARS-CoV-2, thereby reducing the risk of side-effects due to long-term administration.
Acknowledgements
We thank N. D'Elia for the help to draft and revise the manuscript and Dr F. Russo of the Veneto Region for providing data on Sars-CoV-2-infected patients.
Funding
This work was supported by the following grants to AA: European Research Council Consolidator Grant [grant number 683136]; Swiss Cancer League [grant number 4267]; Swiss National Science Foundation (SNSF) [grant number 176045], Prostate Cancer Foundation [grant number 19CHAL08]; and Italian Association for Cancer Research Investigator Grant [grant number 22030].
Disclosure
The authors have declared no conflicts of interest.
Supplementary data
References
- 1.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou P., Yang X.L., Wang X.G. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu N., Zhang D., Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johns Hopkins Coronavirus Resource Center COVID-19 Map. https://coronavirus.jhu.edu/map.html Available at:
- 6.Bugge T.H., Antalis T.M., Wu Q. Type II transmembrane serine proteases. J Biol Chem. 2009;284:23177–23181. doi: 10.1074/jbc.R109.021006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi S.Y., Bertram S., Glowacka I., Park Y.W., Pohlmann S. Type II transmembrane serine proteases in cancer and viral infections. Trends Mol Med. 2009;15:303–312. doi: 10.1016/j.molmed.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Shin W.J., Seong B.L. Type II transmembrane serine proteases as potential target for anti-influenza drug discovery. Expert Opin Drug Discov. 2017;12:1139–1152. doi: 10.1080/17460441.2017.1372417. [DOI] [PubMed] [Google Scholar]
- 9.Glowacka I., Bertram S., Muller M.A. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J Virol. 2011;85:4122–4134. doi: 10.1128/JVI.02232-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iwata-Yoshikawa N., Okamura T., Shimizu Y., Hasegawa H., Takeda M., Nagata N. TMPRSS2 contributes to virus spread and immunopathology in the airways of murine models after coronavirus infection. J Virol. 2019;93:e01815–e01818. doi: 10.1128/JVI.01815-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsuyama S., Nagata N., Shirato K., Kawase M., Takeda M., Taguchi F. Efficient activation of the severe acute respiratory syndrome coronavirus spike protein by the transmembrane protease TMPRSS2. J Virol. 2010;84:12658–12664. doi: 10.1128/JVI.01542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen L.W., Mao H.J., Wu Y.L., Tanaka Y., Zhang W. TMPRSS2: a potential target for treatment of influenza virus and coronavirus infections. Biochimie. 2017;142:1–10. doi: 10.1016/j.biochi.2017.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shulla A., Heald-Sargent T., Subramanya G., Zhao J., Perlman S., Gallagher T. A transmembrane serine protease is linked to the severe acute respiratory syndrome coronavirus receptor and activates virus entry. J Virol. 2011;85:873–882. doi: 10.1128/JVI.02062-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tarnow C., Engels G., Arendt A. TMPRSS2 is a host factor that is essential for pneumotropism and pathogenicity of H7N9 influenza A virus in mice. J Virol. 2014;88:4744–4751. doi: 10.1128/JVI.03799-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffmann M., Kleine-Weber H., Schroeder S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuyama S., Nao N., Shirato K. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc Natl Acad Sci U S A. 2020;117:7001–7003. doi: 10.1073/pnas.2002589117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lucas J.M., Heinlein C., Kim T. The androgen-regulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microenvironment and promotes prostate cancer metastasis. Cancer Discov. 2014;4:1310–1325. doi: 10.1158/2159-8290.CD-13-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lucas J.M., True L., Hawley S. The androgen-regulated type II serine protease TMPRSS2 is differentially expressed and mislocalized in prostate adenocarcinoma. J Pathol. 2008;215:118–125. doi: 10.1002/path.2330. [DOI] [PubMed] [Google Scholar]
- 19.Mikkonen L., Pihlajamaa P., Sahu B., Zhang F.P., Janne O.A. Androgen receptor and androgen-dependent gene expression in lung. Mol Cell Endocrinol. 2010;317:14–24. doi: 10.1016/j.mce.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 20.Miettinen O., Nurminen M. Comparative analysis of two rates. Stat Med. 1985;4:213–226. doi: 10.1002/sim.4780040211. [DOI] [PubMed] [Google Scholar]
- 21.Sheskin D. 3rd ed. Chapman & Hall/CRC; Boca Raton: 2004. Handbook of Parametric and Nonparametric Statistical Procedures. [Google Scholar]
- 22.Cai H. Sex difference and smoking predisposition in patients with COVID-19. Lancet Respir Med. 2020;8:e20. doi: 10.1016/S2213-2600(20)30117-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferlay J., Colombet M., Soerjomataram I. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 2018;103:356–387. doi: 10.1016/j.ejca.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Liang W., Guan W., Chen R. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lazzerini M., Putoto G. COVID-19 in Italy: momentous decisions and many uncertainties. Lancet Glob Health. 2020;8:e641–e642. doi: 10.1016/S2214-109X(20)30110-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Remuzzi A., Remuzzi G. COVID-19 and Italy: what next? Lancet. 2020;395:1225–1228. doi: 10.1016/S0140-6736(20)30627-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drosten C., Gunther S., Preiser W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 28.Ksiazek T.G., Erdman D., Goldsmith C.S. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 29.Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 30.Tomlins S.A., Rhodes D.R., Perner S. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 31.Shaw G.L., Whitaker H., Corcoran M. The early effects of rapid androgen deprivation on human prostate cancer. Eur Urol. 2016;70:214–218. doi: 10.1016/j.eururo.2015.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asangani I.A., Dommeti V.L., Wang X. Therapeutic targeting of BET bromodomain proteins in castration-resistant prostate cancer. Nature. 2014;510:278–282. doi: 10.1038/nature13229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faivre E.J., Wilcox D., Lin X. Exploitation of castration-resistant prostate cancer transcription factor dependencies by the novel bet inhibitor ABBV-075. Mol Cancer Res. 2017;15:35–44. doi: 10.1158/1541-7786.MCR-16-0221. [DOI] [PubMed] [Google Scholar]
- 34.Ianculescu I., Wu D.Y., Siegmund K.D., Stallcup M.R. Selective roles for cAMP response element-binding protein binding protein and p300 protein as coregulators for androgen-regulated gene expression in advanced prostate cancer cells. J Biol Chem. 2012;287:4000–4013. doi: 10.1074/jbc.M111.300194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gordon V., Bhadel S., Wunderlich W. CDK9 regulates AR promoter selectivity and cell growth through serine 81 phosphorylation. Mol Endocrinol. 2010;24:2267–2280. doi: 10.1210/me.2010-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shinde D., Albino D., Zoma M. Transcriptional reprogramming and inhibition of tumor-propagating stem-like cells by EC-8042 in ERG-positive prostate cancer. Eur Urol Oncol. 2019;2:415–424. doi: 10.1016/j.euo.2018.08.024. [DOI] [PubMed] [Google Scholar]
- 37.Klein S.L., Flanagan K.L. Sex differences in immune responses. Nat Rev Immunol. 2016;16:626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 38.Barnes B.J., Adrover J.M., Baxter-Stoltzfus A. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J Exp Med. 2020;217:e20200652. doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arora V.K., Schenkein E., Murali R. Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell. 2013;155:1309–1322. doi: 10.1016/j.cell.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu Z., Wang Y., Xiao Z.G. Nuclear receptor ERRalpha and transcription factor ERG form a reciprocal loop in the regulation of TMPRSS2:ERG fusion gene in prostate cancer. Oncogene. 2018;37:6259–6274. doi: 10.1038/s41388-018-0409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


