Summary
Middle East respiratory syndrome (MERS) is a respiratory disease caused by MERS coronavirus. Because of lack of vaccination, various studies investigated the therapeutic efficacy of antiviral drugs and supportive remedies. A systematic literature search from 10 databases was conducted and screened for relevant articles. Studies reporting information about the treatment of MERS coronavirus infection were extracted and analyzed. Despite receiving treatment with ribavirin plus IFN, the case fatality rate was as high as 71% in the IFN‐treatment group and exactly the same in patients who received supportive treatment only. Having chronic renal disease, diabetes mellitus and hypertension increased the risk of mortality (P < .05), and chronic renal disease is the best parameter to predict the mortality. The mean of survival days from onset of illness to death was 46.6 (95% CI, 30.5‐62.6) for the IFN group compared with 18.8 (95% CI, 10.3‐27.4) for the supportive‐only group (P = .001). Delay in starting treatment, older age group, and preexisting comorbidities are associated with worse outcomes. In conclusion, there is no difference between IFN treatment and supportive treatment for MERS patients in terms of mortality. However, ribavirin and IFN combination might have efficacious effects with timely administration and monitoring of adverse events. Large‐scale prospective randomized studies are required to assess the role of antiviral drugs for the treatment of this high mortality infection.
Keywords: interferon, MERS‐CoV, Middle East respiratory syndrome, systematic review
List of abbreviations
- CART
the classification and regression tree model
- CHF
congestive heart failure
- CI
confidential intervals
- CRD
chronic renal disease
- DM
diabetes mellitus
- ECMO
extracorporeal membrane oxygenation
- MD
mean difference
- MERS‐CoV
Middle East respiratory syndrome coronavirus
- MERS
Middle East respiratory syndrome
- NPV
negative prediction value
- PPV
positive prediction value
- SARS
severe acute respiratory syndrome
- SPSS
Statistical Package for Social Sciences
1. INTRODUCTION
Middle East respiratory syndrome (MERS) is a viral respiratory disease caused by MERS coronavirus (MERS‐CoV), and it is relatively new to humans.1 The first report took place in Saudi Arabia in 2012.2 Afterwards, MERS cases were reported across a wide geographic distribution around the globe. However, the vast majority of these cases were directly or indirectly linked to traveling or residing in the Middle East.2 There is no vaccine against MERS‐CoV, and current recommendations advise people to only adhere to general preventive measures such as handwashing, frequently disinfecting touched surfaces, and avoiding personal contact with infected populations.2
The clinical picture of the infection ranges from asymptomatic or mild respiratory symptoms to severe acute respiratory disease and death.3 Most infected patients present with flu‐like symptoms (cough, shortness of breath, fever, and chills) then rapidly progress into severe illness with pneumonia and acute respiratory distress symptoms, for which many of them require mechanical ventilation (72%).4 Furthermore, the MERS can be complicated by acute renal failure that requires hemodialysis and by disseminated intravascular coagulation with multiorgan failure contributing to the high mortality of the disease.2, 4, 5, 6, 7, 8, 9
Currently, there is no reliable remedy for MERS, and all interventions range from supportive to antiviral therapy. Two in vitro studies suggested a possible efficacious effect of interferon alpha‐2b and ribavirin in the treatment of MERS infection.10, 11 Consequently, the investigators further examined the efficacy of these drugs in an animal study.12 Potential benefits of these antiviral treatments in both in vitro and animal studies persuaded clinicians to question the feasibility and applicability of such an approach in humans. Therefore, we aimed to recapitulate the evidence from all human published data about MERS clinical management in a systematic review and meta‐analysis, to summarize the efficacy and safety of current applied therapeutics and define risk factors associated with outcomes. As a result, we may provide a better approach to more compatible management of fatal consequences of MERS infections and the risk imposed by recent outbreaks in densely populated areas.
2. METHODS
2.1. Search strategy and selection criteria
Our study was performed according to the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses.13 We developed a protocol of methods and registered it in the international prospective register of systematic reviews (PROSPERO) (reference, CRD42015024819). In June 2015, we conducted a systematic search of 10 databases including PubMed, Scopus, Web of Science, Google Scholar, WHO Global Health Library, Virtual Health Library (containing Cochrane, MEDLINE, LILACS, IBECS, and SciELO), POPLINE, New York Academy of Medicine Grey Literature Report (NYAMGLR), SIGLE, and ClinicalTrials.gov. Search results were limited to references published since January 1, 2012, when MERS was first detected. In addition, the human filter was applied in the case of PubMed.
After removing duplicates, three trained reviewers were assigned to independently screen the titles and abstracts of all references generated from the aforementioned search strategy on the basis of the following inclusion and exclusion criteria. Inclusion criteria were (a) any study that gives information about the treatment of MERS‐CoV infection, (b) all types of study designs were included, and (c) no restriction was made with respect to language, age, and area. Exclusion criteria were (a) data that could not be reliably extracted, (b) data sets considered as overlapping, (c) studies published before 1/1/2012, (d) book chapter, thesis, letter, conference paper, poster, or editorial, and (e) animal or in vitro studies. Three reviewers compared their screening results and discussed the differences. A consensus was reached through discussion.
2.2. Data extraction
Extracted data included publication year, year of research, country and city of the patients, year of subject recruitment, study design, participant enrollment, data collection method, baseline characteristics before treatment, diagnostic method of MERS‐CoV, time from admission to treatment start, treatment for MERS‐CoV, and outcome survival. This work was conducted by 2 investigators evaluating the references independently, and all disagreements were discussed to reach a consensus from supervisors.
2.3. Quality assessment
The quality of included clinical data was assessed using (CARE) statement for case reports14 and the 9 metrics tool for nonrandomized studies.15 Three reviewers were assigned to assess each included reference independently.
2.4. Statistical analysis
Mortality rates were treated as dichotomous variables with their respective 95% confidential intervals (CI). Statistical heterogeneity was assessed using the I 2 statistic16, 17 and assumed to be influential when I 2 was greater than 50% or P ≤ .1.18, 19 A fixed‐effect model was used because there was no evidence of heterogeneity between studies. Meta‐analysis was performed using data analysis and statistical software (STATA) that was developed by StataCorp. Fisher exact (or chi‐square, as appropriate) and Mann‐Whitney U tests were used for the categorical and continuous variables, respectively. The classification and regression tree (CART) model was used to identify independent variables that predict mortality outcome.20 Invasive ventilation and renal replacement therapy were also chosen as the outcomes in the CART model because of direct correlation with severity and mortality.20 All possible variables were extracted to build CART (Table S4, Found in the Supporting Information). The performance parameters are accuracy, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV).20 Results were considered to have statistical significance if the P value was <.05. For evaluating the survival rate over time between 2 groups of all‐type IFN and supportive‐only treatment, log‐rank test and Kaplan‐Meier survival analysis were performed. We performed 2 sets of Kaplan‐Meier survival curves. The first set described the survival time from hospital admission to death while the second set reported the survival time from onset of symptoms to death. The mean survival days was calculated for both sets. Patients who were still on admission while the original study ended were included in Kaplan‐Meier curves but considered as expired patients from final analysis. Data were analyzed using statistical computing and graphics (R) software version 3.3.2 developed by R Foundation for Statistical Computing. Statistical Package for Social Sciences (SPSS) released by International Business Machines Corporation was used for analysis as well.
3. RESULTS
3.1. Search results
A total of 1095 references were retrieved. Upon screening them regarding inclusion and exclusion criteria from Section 2, eleven references were included for data extraction and analysis.21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31 Additionally, 5 references were identified through manual search.7, 8, 9, 32, 33 So a total of 16 studies were eligible for selection as shown in Figure 1.
Figure 1.
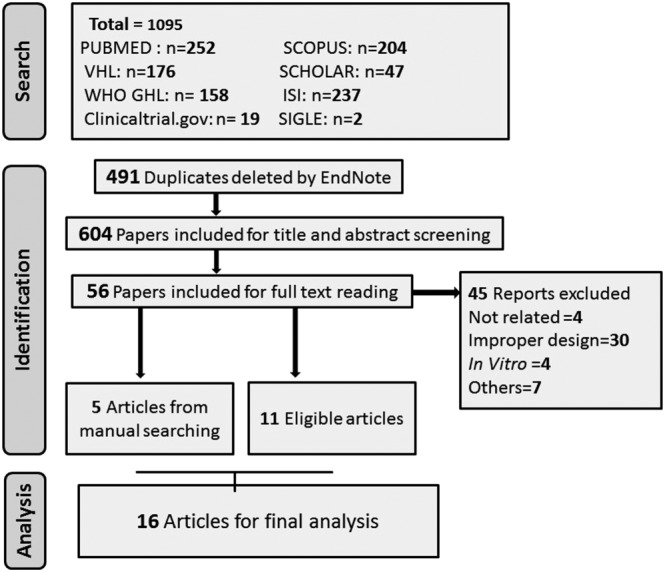
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses flow diagram of studies' screening and selection according to inclusion and exclusion criteria
3.2. Characteristics of included studies
Of the 16 studies including 116 patients, 10 were case reports,7, 8, 9, 21, 24, 25, 28, 30, 31, 33 2 were case series,23, 26 and 4 were observational studies.22, 27, 29, 32 Saudi Arabia was the country of origin for patients in 13 studies, and the rest were from France, Greece, and Qatar. Detailed characteristics of patients in our included articles are presented in Table 1.
Table 1.
Characteristics of the included studies
| Study Name | Study Design | Country | Age | Male | Types of Treatment | Total Cases | Number of Deaths |
|---|---|---|---|---|---|---|---|
| AlGhamdi et al21 | Case report | Saudi Arabia | 44 | 1 | Interferon | 1 | 1 |
| Al‐Tawfiq et al22 | Retrospective observational study | Saudi Arabia | 57.6 | 3 | Interferon | 5 | 5 |
| Shalhoub et al29 | Retrospective cohort | Saudi Arabia | 65.9 | 14 | Interferon | 24 | 18 |
| Khalid et al26 | Case series | Saudi Arabia | 58.8 | 5 | Interferon | 6 | 3 |
| Khalid et al25 | Case report | Saudi Arabia | 47 | 1 | Interferon | 2 | 0 |
| Spanakis et al30 | Case report | Greece | 69 | 1 | Interferon | 1 | 1 |
| Omrani et al27 | Retrospective cohort | Saudi Arabia | 65.5 | 32 | Interferon (cases) and supportive treatment only (control) | 44 | 34 |
| Shalhoub et al28 | Case report | Saudi Arabia | 51 | 1 | Interferon | 1 | 0 |
| Al‐Hameed et al32 | Prospective cohort | Saudi Arabia | 56.5 | 6 | Interferon | 8 | 6 |
| Arabi et al23 | Case series | Saudi Arabia | 59 | 8 | Supportive treatment only | 12 | 7 |
| Thabet et al31 | Case report | Saudi Arabia | 9‐month‐old | 1 | Supportive treatment only | 1 | 1 |
| Guberina et al24 | Case report | Qatar | 45 | 1 | Supportive treatment only | 1 | 0 |
| Omrani et al33 | Case report | Saudi Arabia | 43.3 | 3 | Supportive treatment only | 3 | 2 |
| Guery et al7 | Case report | France | 57.5 | 2 | Supportive treatment only | 2 | 1 |
| Memish et al8 | Case report | Saudi Arabia | 39 | 4 | Supportive treatment only | 4 | 2 |
| Zaki et al9 | Case report | Saudi Arabia | 60 | 1 | Supportive treatment only | 1 | 1 |
Eight studies used specific antiviral treatment21, 22, 25, 26, 28, 29, 30, 32 while 7 studies used supportive treatment (including invasive ventilation, prone position, renal replacement therapy, vasopressors, corticosteroids, immunoglobulins, and oseltamivir).7, 8, 9, 23, 24, 31, 33 Omrani et al27 used both specific antiviral treatment and supportive treatment. The specific antiviral treatments were IFN (alpha‐2a, alpha‐2b, and beta‐1a), ribavirin, and several others including tenofovir, emtricitabine, lopinavir, and ritonavir. Among 116 patients recruited, 29 were reported with detailed information regarding baseline characteristics, comorbidities, treatments, outcomes, and some with survival time, which was subsequently used to perform univariable analysis and Kaplan‐Meier survival curves (Table S4, Found in the Supporting Information).
Hemodialysis dependency appeared in higher frequency in the IFN group than in the supportive‐only group (P = .025). Conversely, renal replacement therapy and vasopressors were used more often in the supportive‐only group than in the IFN group (P = .019) (Table 2).
Table 2.
Comparison between IFN and comparator groups (no antiviral/IFN)
| Factor | Treatment with IFN (n = 68) | Comparator Group (n = 48) | P Value |
|---|---|---|---|
| Age (mean) | 62.2 | 57.3 | |
| Gender (males) | 48 (71) | 40 (83) | .114 |
| Mortality | 48 (71) | 34 (71) | 1 |
| Diabetes | 38 (56) | 26 (54) | .862 |
| Hypertension | 26 (38) | 9 (19) | .024 |
| Chronic renal impairment | 22 (32) | 11 (23) | 0.27 |
| Dialysis dependent | 10 (15) | 1 (2) | .025 a |
| Congestive heart failure | 17 (25) | 10 (21) | .603 |
| Other comorbidities | 23 (34) | 3 (6) | .0005 a |
| Number of comorbidities | 136 | 60 | |
| Invasive ventilation | 52 (76) | 43 (90) | .071 |
| Renal replacement | 17 (25) | 22 (46) | .019 |
| Corticosteroids | 28 (41) | 28 (58) | .068 |
| Oseltamivir | 31 (46) | 31 (65) | .043 |
| Vasopressors | 25 (37) | 32 (67) | .002 |
| Prone position | 4 (6) | 6 (12.5) | .315a |
| ECMO | 3 (3) | 5 (10.4) | .272 a |
Abbreviation: ECMO, extracorporeal membrane oxygenation; IFN, interferon.
Significant values are in bold.
Fisher exact test.
3.3. Quality assessment
The quality of 10 case reports7, 8, 9, 21, 24, 25, 28, 30, 31, 33 and 2 case series23, 26 was assessed using the CARE checklist (Table S1, Found in the Supporting Information). All of them contained an introduction and described the importance of the case in the abstract, main introduction section, demographics and symptoms of the patients, significant clinical findings, timeline for patients' history organization, diagnostic methods, type of treatment, clinical outcomes, discussion of the relevant medical literature, rationale for conclusion, and the primary take away lessons from these case reports. However, none of them described the diagnostic challenges, prognostic characteristics, or patients' perspective. Only Guery et al7 reported obtaining informed consent from patients.
The quality of 4 observational studies22, 27, 29, 32 was assessed using the 9 metrics tool15 (Table S2, Found in the Supporting Information). Three of them had a score23, 27, 29 of 5 of 9 as they described study design, characteristics of the patient population, inclusion criteria, method quality, and MERS diagnosis. However, none of the 3 studies described data collection method, assignment method of patients, exclusion criteria, and interpretation.
3.4. Treatment with antiviral drugs
Treatment with antiviral drugs, other than oseltamivir, was reported in 9 studies. IFNs (IFN alpha‐2a, alpha‐2b, or beta‐1a) in combination with ribavirin were the common remedies used in all 9 studies.
IFN alpha‐2a was used in 4 studies (n = 35 patients),21, 27, 28, 29 all of which used a dose of 180 μg/wk for treatment. IFN alpha‐2b was used in 5 studies (n = 22)22, 25, 26, 30, 32 with a dose ranging between 100 and 180 μg/wk. IFN alpha‐2b dose was not reported in Al‐Hameed et al.32 IFN beta‐1a was used in 2 studies (n = 12), both of which used a dose of 44 μg/wk for treatment.28, 29
Ribavirin administration was started with a loading dose followed by subsequent doses in all 9 studies. The loading dose was 2000 mg for all studies while 400 mg in one study of AlGhamdi et al.21 The subsequent doses, however, were variable among studies and ranged between 400 and 3600 mg/d. The frequency of administration of ribavirin was 3 doses per day in 2 studies22, 30 and 2 doses per day in another 3 studies.21, 28, 29 Duration of treatment with ribavirin was also variable and ranged between 5 and 26 days. The subsequent oral ribavirin dose was adjusted according to the calculated creatinine clearance in 3 studies.25, 26, 27 However, the duration of treatment (8‐10 days) and the loading dose (2000 mg) used in the 3 studies were similar, regardless of creatinine clearance (Table S3, Found in the Supporting Information).
In addition to treatment with IFN and ribavirin, the treatment regimen in Shalhoub et al28 included treatment with tenofovir/emtricitabine (TDF/FTC) 300/200 mg orally once daily, in combination with ritonavir‐boosted atazanavir (atazanavir 300 mg plus ritonavir 100 mg) orally once daily. Similarly, triple therapy with IFN alpha‐2a, ribavirin, and lopinavir/ritonavir (400/100 mg twice daily) was used to treat the Greek patient in Spanakis et al.30
It is worth mentioning that there was a significant lag period between presentation and initiation of the antiviral treatment. The period from the admission of patients to the initiation of antiviral (IFNs and ribavirin) was reported in 13 patients and had a mean of 12 days and ranged between 1 day in Khalid et al26 and 21 days in Al‐Tawfiq et al (Table S3, Found in the Supporting Information).22
3.5. Supportive treatment
Supportive treatment alone was used in 7 studies (n = 24 patients), whereas supportive treatment along with antiviral medications was used in another 8 studies (n = 48) and one study compared supportive/antiviral medications against supportive treatment alone (n = 20 and 24, respectively). Different modalities of treatments were used to support the patients, including invasive ventilation, extracorporeal membrane oxygenation, corticosteroids, renal replacement therapy, vasopressors, oseltamivir, and prone positioning. Most patients (95 of 116, 81.9%) required the use of invasive mechanical ventilation: 76.4% of patients in IFNs treatment group (52 of 68) compared with 90% of patients in supportive treatment–only group (43 of 48).
3.6. Therapeutic outcomes
Regarding mortality, studies with more than 2 cases were included in the meta‐analysis. The pooled proportion of mortality was 0.714 (0.618‐0.795) from 8 studies including 106 MERS patients (Figure 2). In 68 patients received IFN treatment, the mortality rate was high (71%) in spite of receiving treatment with ribavirin plus IFN (alpha‐2a, alpha‐2b, or beta‐1a). Likewise, the mortality rate was high in patients who received supportive treatment only (71%, n = 48). There was no statistical significant mortality difference when comparing mortality of both groups (P = 1) (Table 2). The same insignificant mortality difference was shown when comparing 3 types of IFN treatments: IFN alpha‐2a, IFN alpha‐2b, and IFN beta‐1a (P = .65) (Table 3).
Figure 2.
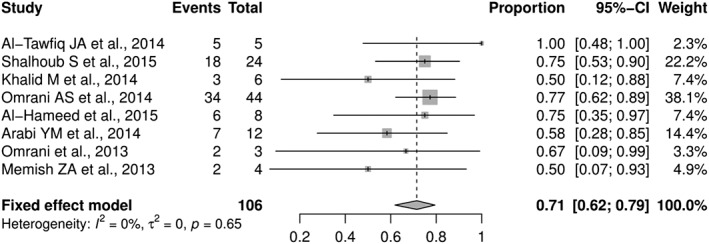
Forest plot meta‐analysis of 8 studies regarding the mortality rate of patients with MERS. CI, Confidence interval; P, probability value
Table 3.
Comparison between types of IFNs
| Factor | IFN Alpha‐2a (n = 34) | IFN Alpha‐2b (n = 22) | IFN Beta‐1a (n = 11) | P Value |
|---|---|---|---|---|
| Age (mean) | 65.8 | 57.1 | 67 | |
| Gender (males) | 27 (79) | 16 (73) | 4 (36) | .024 |
| Mortality | 26 (76) | 15 (68) | 7 (64) | .65 |
| Diabetes | 24 (71) | 9 (41) | 5 (45) | .065 |
| Hypertension | 11 (32) | 8 (36) | 7 (64) | .173 |
| Chronic renal impairment | 11 (32) | 8 (36) | 3 (27) | .869 |
| Dialysis dependent | 4 (12) | 4 (18) | 2 (18) | .723a |
| Congestive heart failure | 10 (29) | 5 (23) | 2 (18) | .715 |
| Other comorbidities | 13 (3) | 10 (9) | 0 | .0027 |
| Invasive ventilation | 30 (85) | 16 (73) | 6 (55) | .052 |
| Renal replacement therapy | 12 (35) | 5 (23) | … | .317 |
| Corticosteroids | 11 (32) | 16 (73) | … | .003 |
| Oseltamivir | 18 (53) | 13 (59) | … | .655 |
| Vasopressors | 14 (41) | 11 (50) | … | .517 |
| Prone position | 4 (12) | 0 | … | .146a |
| ECMO | 2 (6) | 1 (5) | … | 1a |
Abbreviation: ECMO, extracorporeal membrane oxygenation; IFN, interferon.
Significant values are in bold.
Fisher exact test.
The duration from hospitalization to death was reported in 78 of 82 deceased patients. All 78 reported patients died within 3 months of hospitalization. Moreover, 59% of the 82 patients died within the first 2 weeks of admission, and 79% died within 1 month of admission.
In a case series of 6 patients from Saudi Arabia by Khalid et al,26 3 patients had comorbid conditions and needed mechanical ventilation before dying, while the other 3 patients who did not require mechanical ventilation survived. A case report from Greece described one patient with MERS‐CoV infection who died despite receiving IFN alpha‐2a, ribavirin, and lopinavir. The patient had a multiorgan failure and was later diagnosed with colon cancer. MERS‐CoV was not detectable in his respiratory tract until several days before death.30
Regarding the adverse effects of interferon and ribavirin combination therapy, 2 patients had pancreatic enzyme elevation, and one had significant hemolysis when being treated with IFN alpha‐2a plus ribavirin.22 In addition, the reduction in hemoglobin level was 4.32 g/L in 20 MERS patients treated with IFN alpha‐2b plus ribavirin, whereas Omrani et al27 reported only 2.14 g/L of hemoglobin reduction in 24 patients treated with supportive‐only treatment (P = .002).
3.7. Risk factors associated with outcomes
There was a significant difference between death and survival in patients with chronic renal disease (CRD). Nine CRD patients died of 17 deceased cases, and no patient had CRD in the survival group. In addition, diabetes mellitus (DM) and hypertension showed similar significant variation, suggesting that having CRD, hypertension, and/or DM increased the risk of mortality (P < .05). There was no significant difference between death and survival regarding gender, ribavirin, corticosteroid, oseltamivir, IFN beta‐1a, IFN alpha‐2b, IFN alpha‐2a, congestive heart failure, and other comorbidities (P > .05). All 17 deceased patients required mechanical ventilation before dying compared with only 2 patients of 12 survived patients (P < .001). For continuous variables, there was a significant difference between death and survival in age, where the dead patients were older, and time from onset of illness to initiation of antiviral treatment, being longer in the deceased patients (P < .05) (Table 4).
Table 4.
Associated factors with cases' outcomes
| Variables | Deaths (n = 17)a | Survivors (n = 12)a | MD (95% CI) | OR (95% CI) | P Value |
|---|---|---|---|---|---|
| Age, mean (SD) | 56.4 (21.6) years | 39.8 (13.3) years | 16.6 (3.3‐30.0) | … | .014 |
| Time from admission to antiviral treatment start, mean (SD) | 15.1 (4.4) days | 1.7 (0.6) days | 13.4 (10.2‐16.6) | … | .003 |
| Gender (male), % | 15 (88) | 10 (83) | 1.5 (0.18‐12.46) | 1 | |
| DM | 7 | 0 | … | .023 | |
| HTN | 6 | 0 | … | .028 | |
| CRD | 9 | 0 | … | .003 | |
| DD | 4 | 0 | … | .12 | |
| CHF | 2 | 0 | … | .498 | |
| Other comorbidities | 10 | 3 | 4.29 (0.84‐21.76) | .13 | |
| IFN alpha‐2a | 1 | 1 | 0.69 (0.04‐12.20) | 1 | |
| IFN alpha‐2b | 9 | 5 | 1.58 (0.35‐7.00) | .71 | |
| IFN beta‐1a | 1 | 0 | … | .414 | |
| Ribavirin | 10 | 6 | 1.43 (0.32‐6.32) | .716 | |
| Ventilation | 17 | 2 | … | <.001 | |
| Corticosteroid | 8 | 6 | 0.89 (0.20‐3.90) | 1 | |
| Oseltamavir | 10 | 4 | 2.86 (0.61‐13.34) | .264 | |
| Inotropes | 6 | 1 | 6.00 (0.62‐58.43) | .187 | |
| Renal therapy | 8 | 1 | 9.80 (1.02‐93.50) | .043 |
Abbreviations: CHF, congestive heart failure; CI, confidential interval; CRD, chronic renal diseases; DD, dialysis dependent; DM, diabetes mellitus; HTN, hypertension; MD, mean difference; SD, standard deviation.
Other comorbidities: colon adenocarcinoma, HIV, renal transplant, asthma, sleep apnea, coronary artery disease, atrial fibrilation, ischemic heart disease, right bundle branch block, cardiomyopathy, MI, dyslipidemia, histamine induced angioedema, multiple myeloma, and obesity.
Significant values are in bold.
Numbers are the frequency and percent (%) otherwise stated.
3.8. CART model
The modeling tool, CART, identified CRD as the best parameter to predict mortality (Figure 3A). The performance of the decision tree tool that classified mortality outcome was at an accuracy of 72.4%, sensitivity of 52.9%, specificity of 100%, PPV of 100%, and NPV of 60%. In addition, treatment with inotropes was exhibited as the best parameter to predict renal replacement therapy outcome (Figure 3B). The performance of the decision tree tool that classified renal replacement therapy outcome was at an accuracy of 86.2%, sensitivity of 66.67%, specificity of 95%, PPV of 85.7%, and NPV of 86.4%. No significant results were detected regarding the invasive ventilation outcome.
Figure 3.
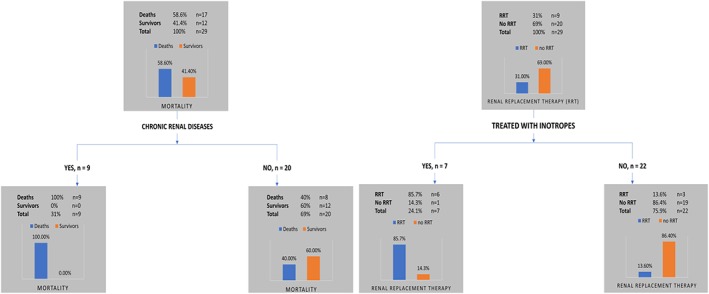
The classification and regression tree model of 29 cases with individual data. A, Chronic renal disease was the best prediction variable for mortality rate. B, Inotropes was best prediction variable for renal replacement therapy
3.9. Survival rate over time
Survival time from admission to death was compared for IFN‐treated (n = 30) and for non‐IFN (supportive care only, n = 14) patients. All 44 cases died within 80 days after hospital admission (Figure 4A). Only one female case treated with IFN was eliminated from analysis because the patient remained intubated when the original study ended. However, she had met death criteria; thus, she was included with deceased patients in the final outcome analysis. The mean survival days was 21.3 days (95% CI, 14.1‐28.5) for IFN group and 21.4 days (95% CI, 12.4‐30.4) for the supportive‐only group, P = .977. Overall, mean of survival days for both groups was 21.3 (95% CI, 15.7‐26.9).
Figure 4.
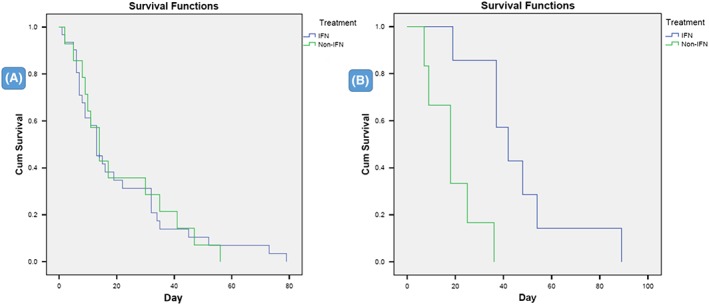
Kaplan‐Meier survival curves showing death days over time in IFN‐treated patients and supportive‐only groups. A, Death days from hospital admission to death for 44 cases (P = .977) and, B, death days from onset of illness to death for 13 cases (P = .001)
Only 7 cases in the IFN group and 6 cases in the supportive‐only group reported information about time from onset of symptoms to death. A second Kaplan‐Meier survival analysis was performed and showed that all of them died within 89 days from onset of illness. The mean of survival days was 46.6 (95% CI, 30.5‐62.6) for the IFN group compared with 18.8 (95% CI, 10.3‐27.4) for the supportive‐only group. The difference between the 2 groups was statistically significant (P = .001) (Figure 4B). The longer survival period from onset to death was simply attributed to the duration between onset and admission.
The mean time from onset of illness to the admission of 23 patients in IFN group was 6.52 days compared with the same number of patients in the supportive‐only group who had a mean of 2.43 days. The mean difference (MD) of time between 2 groups was also calculated: MD (95% CI) = 4.09 (2.71‐5.47), P < .05.
Further specific comparison of death versus nondeath cases in both IFN and supportive treatments was conducted to get the mean days from onset to admission. The mean of days for 10 IFN‐treated dead patients and 5 IFN‐treated survivals was 7.79 and 4.40, respectively, with MD (95% CI) = 3.39 (−3.48 to 10.26), P = .3. Moreover, the mean of days for 13 supportively treated dead patients and 10 supportively treated survivals was 2.69 and 2.1, respectively, with MD (95% CI) = .59 (−1.35 to 2.53), P = .53. The mean of days from onset of illness to admission for pooled total cases of death and survived was 4.07 and 2.27, respectively, with MD (95% CI) = 1.8 (−0.81 to 4.41), P = .17.
4. DISCUSSION
Our systematic review highlights the significance of age and period between the illness onset and start of antiviral therapy in MERS cases' prognostic assessment. Our results revealed that younger age and more rapid initiation of antiviral therapy are associated with higher chances of survival. We also found that patients who require ventilation are more likely to die compared with patients who do not require it. Furthermore, there is no difference between IFN treatment and supportive treatment in terms of mortality rate and no significant effect on the duration of survival in infected patients' different groups.
Several factors can affect the clinical outcomes of MERS therapy. In our data, there was a significantly higher number of patients with hypertension and dialysis dependence in the IFN group compared with the supportive‐only group. The survival time of patients receiving antiviral therapy and the efficacy of the drugs were likely reduced because of hypertension and dialysis dependence. Nevertheless, upon investigating the outcomes of 13 patients under treatment for MERS, we found that, despite the higher number of comorbidities in the IFN group compared with the supportive‐only group, patients on IFN survived considerably longer from onset of symptoms than those on supportive therapy. This result highlights the potential efficacy of antiviral treatment in MERS‐CoV infection. However, the number of cases is small to draw any conclusions, and further studies are definitely required.
In terms of different supportive remedies, corticosteroids did not show any promising effects on the survival of infected patients, which is also consistent with previous studies.8, 23, 27, 33 Patients with signs of severe respiratory distress, shock, or hypoxemia should be given oxygen therapy immediately, and those who cannot maintain a SpO2 ≥ 90% with oxygen therapy should be considered for intubation and mechanical ventilation. However, noninvasive ventilation should be avoided because of the high risk of spreading the infection.34 Patients who have an indication for ventilation were more likely to have an accompanying serious condition compared with other patients,34 which could explain the higher risk of mortality among those patients as shown in our results.
Because of high case fatality rate and faster progression to respiratory failure, it will be pressing to distinguish MERS from severe acute respiratory syndrome for timely management. However, it is considered a diagnostic challenge for health care professionals due to similar clinical features.35 Our results obtained with CART modeling suggest that chronic respiratory disease can be the best predictor of death in patients with MERS. Therefore, it is of utmost necessity to thoroughly assess and continuously monitor the respiratory functions of patients with suspected MERS‐CoV infection. Also, health care agencies in high risk areas are recommended to bridge any gaps in their medical facilities and personnel. They should get their staff familiar with the precautious measures that are required to face any future outbreaks.
Our study is limited by the small number of included articles and patients, which hindered our ability to validate our CART model. In addition, there are no published clinical trials, and even only one ongoing clinical trial has been found, testing the combination of lopinavir/ritonavir and IFN beta‐1b therapy on MERS patients (NCT02845843). Another recent trial has been registered but not yet started recruitment, to investigate the safety and immunogenicity of MERS‐CoV vaccine (NCT03399578). Nevertheless, further investigations are required to objectively assess the effect of antiviral therapy on the survival duration and overall outcomes of MERS‐CoV infections.
5. CONCLUSION
There is no evidence of any difference between IFN treatment and supportive treatment for MERS patients in terms of mortality. The ribavirin and IFN combination might have promising effects where therapy can be started promptly and adverse effects monitored carefully; a randomized controlled trial is required to assess this possibility. Concerning prognostic factors, delayed treatment, older age, and accompanying comorbidities such as hypertension, DM, chronic kidney disease, and dialysis dependence are associated with worse outcomes. Because of high fatality, the seriousness of this newly emerging disease, and a limited number of available cases, we believe there is an urgent need for large‐scale clinical trials on the efficacy of antiviral treatment of MERS‐CoV infections.
CONFLICT OF INTEREST
The authors declare no competing interests.
FUNDING
This work was supported in part by a “Grant‐in‐Aid for Scientific Research (B)” (16H05844, 2016‐2019, for Nguyen Tien Huy) from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan and by the Japan Initiative for Global Research Network on Infectious Diseases (J‐GRID) for Kenji Hirayama. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
AUTHOR CONTRIBUTIONS
N.T.H., A.A.G., M.E.M., and K.H. participated in the design of the study. L.V.T., L.M.D., M.G.K., N.L.V., and A.M.A.A. performed in the analysis and interpreted it. All authors contribute the screening and data extraction, wrote the manuscript, read, and approved the final manuscript.
Supporting information
Table S1: CARE checklist for case reports.
Table S2: Quality assessment using nine metrics tool for non‐randomized studies.
Table S3: Protocol of treatment.
Table S4: Basic characteristics of patients and mortality.
Morra ME, Van Thanh L, Kamel MG, et al. Clinical outcomes of current medical approaches for Middle East respiratory syndrome: A systematic review and meta‐analysis. Rev Med Virol. 2018;28:e1977 10.1002/rmv.1977
Mostafa Ebraheem Morra, Le Van Thanh, and Mohamed Gomaa Kamel equally contributed in the work.
REFERENCES
- 1. Kupferschmidt K. Infectious diseases. MERS surges again, but pandemic jitters ease. Science. 2015;347(6228):1296‐1297. 10.1126/science.347.6228.1296 [DOI] [PubMed] [Google Scholar]
- 2. WHO . Middle East respiratory syndrome coronavirus (MERS‐CoV). May 2017.
- 3. Memish ZA, Al‐Tawfiq JA, Makhdoom HQ, et al. Screening for Middle East respiratory syndrome coronavirus infection in hospital patients and their healthcare worker and family contacts: a prospective descriptive study. Clin Microbiol Infect. 2014;20(5):469‐474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Assiri A, Al‐Tawfiq JA, Al‐Rabeeah AA, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13(9):752‐761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Assiri A, McGeer A, Perl TM, et al. Hospital outbreak of Middle East respiratory syndrome coronavirus. New England Journal of Medicine. 2013;369(5):407‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Drosten C, Seilmaier M, Corman VM, et al. Clinical features and virological analysis of a case of Middle East respiratory syndrome coronavirus infection. Lancet Infect Dis. 2013;13(9):745‐751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guery B, Poissy J, el Mansouf L, et al. Clinical features and viral diagnosis of two cases of infection with Middle East respiratory syndrome coronavirus: a report of nosocomial transmission. The Lancet. 2013;381(9885):2265‐2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Memish ZA, Zumla AI, Al‐Hakeem RF, Al‐Rabeeah AA, Stephens GM. Family cluster of Middle East respiratory syndrome coronavirus infections. N Engl J Med. 2013;368(26):2487‐2494. [DOI] [PubMed] [Google Scholar]
- 9. Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814‐1820. [DOI] [PubMed] [Google Scholar]
- 10. de Wilde AH, Raj VS, Oudshoorn D, et al. MERS‐coronavirus replication induces severe in vitro cytopathology and is strongly inhibited by cyclosporin A or interferon‐α treatment. J Gen Virol. 2013;94(8):1749‐1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Falzarano D, de Wit E, Martellaro C, Callison J, Munster VJ, Feldmann H. Inhibition of novel β coronavirus replication by a combination of interferon‐α2b and ribavirin. Sci Rep. 2013;3:1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Falzarano D, De Wit E, Rasmussen AL, et al. Treatment with interferon‐[alpha] 2b and ribavirin improves outcome in MERS‐CoV‐infected rhesus macaques. Nat Med. 2013;19(10):1313‐1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Case report guidelines [http://www.care-statement.org/]
- 15. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta‐analyses [http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp] [DOI] [PubMed]
- 16. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7(3):177‐188. [DOI] [PubMed] [Google Scholar]
- 17. Higgins J, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses [journal article as teaching resource, deposited by John Flynn]. Br Med J. 2003;327:557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Munafò MR, Flint J. Meta‐analysis of genetic association studies. Trends Genet. 2004;20(9):439‐444. [DOI] [PubMed] [Google Scholar]
- 19. Zintzaras E, Lau J. Synthesis of genetic association studies for pertinent gene‐disease associations requires appropriate methodological and statistical approaches. J Clin Epidemiol. 2008;61(7):634‐645. [DOI] [PubMed] [Google Scholar]
- 20. Kamel MG, Nam NT, Han NHB, et al. Post‐dengue acute disseminated encephalomyelitis: a case report and meta‐analysis. PLoS Negl Trop Dis. 2017;11(6):e0005715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. AlGhamdi M, Mushtaq F, Awn N, Shalhoub S. MERS CoV infection in two renal transplant recipients: case report. Am J Transplant. 2015;15(4):1101‐1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Al‐Tawfiq JA, Momattin H, Dib J, Memish ZA. Ribavirin and interferon therapy in patients infected with the Middle East respiratory syndrome coronavirus: an observational study. Int J Infect Dis. 2014;20:42‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Arabi YM, Arifi AA, Balkhy HH, et al. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann Intern Med. 2014;160(6):389‐397. [DOI] [PubMed] [Google Scholar]
- 24. Guberina H, Witzke O, Timm J, et al. A patient with severe respiratory failure caused by novel human coronavirus. Infection. 2014;42(1):203‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Khalid M, Al Rabiah F, Khan B, Al Mobeireek A, Butt TS, Al Mutairy E. Ribavirin and interferon‐alpha2b as primary and preventive treatment for Middle East respiratory syndrome coronavirus: a preliminary report of two cases. Antivir Ther. 2015;20(1):87‐91. [DOI] [PubMed] [Google Scholar]
- 26. Khalid M, Khan B, Al Rabiah F, et al. Middle Eastern respiratory syndrome corona virus (MERS CoV): case reports from a tertiary care hospital in Saudi Arabia. Ann Saudi Med. 2014;34(5):396‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Omrani AS, Saad MM, Baig K, et al. Ribavirin and interferon alfa‐2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect Dis. 2014;14(11):1090‐1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shalhoub S, AlZahrani A, Simhairi R, Mushtaq A. Successful recovery of MERS CoV pneumonia in a patient with acquired immunodeficiency syndrome: a case report. J Clin Virol. 2015;62:69‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shalhoub S, Farahat F, Al‐Jiffri A, et al. IFN‐alpha2a or IFN‐beta1a in combination with ribavirin to treat Middle East respiratory syndrome coronavirus pneumonia: a retrospective study. J Antimicrob Chemother. 2015;70(7):2129‐2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Spanakis N, Tsiodras S, Haagmans BL, et al. Virological and serological analysis of a recent Middle East respiratory syndrome coronavirus infection case on a triple combination antiviral regimen. Int J Antimicrob Agents. 2014;44(6):528‐532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thabet F, Chehab M, Bafaqih H, Al Mohaimeed S. Middle East respiratory syndrome coronavirus in children. Saudi Med J. 2015;36(4):484‐486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Al‐Hameed F, Wahla AS, Siddiqui S, et al. Characteristics and outcomes of Middle East respiratory syndrome coronavirus patients admitted to an intensive care unit in Jeddah, Saudi Arabia. J Intensive Care Med. 2016;31(5):344‐348. [DOI] [PubMed] [Google Scholar]
- 33. Omrani AS, Matin MA, Haddad Q, Al‐Nakhli D, Memish ZA, Albarrak AM. A family cluster of Middle East respiratory syndrome coronavirus infections related to a likely unrecognized asymptomatic or mild case. Int J Infect Dis. 2013;17(9):e668‐e672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. CDC . Infection prevention/control and management guidelines for patients with Middle East respiratory syndrome coronavirus (MERS CoV) infection. 2014. [PubMed]
- 35. Hui DS, Memish ZA, Zumla A. Severe acute respiratory syndrome vs. the Middle East respiratory syndrome. Curr Opin Pulm Med. 2014;20(3):233‐241. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: CARE checklist for case reports.
Table S2: Quality assessment using nine metrics tool for non‐randomized studies.
Table S3: Protocol of treatment.
Table S4: Basic characteristics of patients and mortality.


