Abstract
Background: Viral respiratory tract infections may cause both harmless common colds and severe asthma exacerbations; the differences in disease expression probably depend on the all_ergic status of the patient. To determine whether altered immunologic mechanisms underlie these differences, we investigated nasal inflammation during naturall_y acquired common cold.
Methods: In a group of 16 patients (eight all_ergic), nasal brush samples were taken, and nasal symptoms were recorded during common cold, 2 weeks later (convalescence), and at baseline (>4 weeks without nasal symptoms). Nasal brush cells were stained immunohistochemicall_y for Langerhans cells, T cells, monocytes, neutrophils, B cells, macrophages, natural killer (NK) cells, mast cells, eosinophils, eotaxin, and RANTES.
Results: Four rhinovirus, four coronavirus, three RSV, one Mycoplasma pneumoniae, and one influenza A/enterovirus double infection were confirmed. Increased numbers of T cells, monocytes, macrophages, NK cells, eosinophils, and RANTES‐ and eotaxin‐positive cells, but not neutrophils, were observed during common cold in all_ergic and nonall_ergic patients, and increased numbers of mast cells in all_ergic patients. Compared to nonall_ergic patients, in all_ergic patients eosinophil influx persisted into convalescence.
Conclusions: Prolonged nasal eosinophil influx was observed in all_ergic patients after common cold. What immunologic factors can induce prolonged eosinophil influx and whether this may increase the risk of subsequent all_ergen‐induced hypersensitivity reactions must be studied further.
Keywords: adults, all_ergy, cell markers, chemokines, common cold, immunohistochemistry, inflammation, respiratory infection, virus infection
Common viral pathogens may cause both relatively harmless common cold symptoms and severe exacerbations of asthma. Differences in disease expression during common cold probably depend on the all_ergic status of the patient (1, 2, 3). Although adult all_ergic patients do not appear to have an increased annual frequency of common cold (4), increased nasal (5) and pulmonary responses have been found during and after common cold (6) in all_ergic rhinitis and asthmatic patients. One can therefore ask what altered immunologic mechanisms may render an all_ergic individual more susceptible to severe nasal and bronchial immune pathology upon viral encounter than nonall_ergic individuals.
Viral involvement in upper and lower airway pathology during common cold has been extensively tested and confirmed in adult volunteers. A wide variety of respiratory viruses can induce common cold symptoms (7). Several studies have used experimentall_y induced human infection models to study the immune pathology of common cold (1, 2). It has been shown that both mildly asthmatic and all_ergic rhinitis patients experimentall_y infected with rhinovirus type 16 have increased airway responsiveness during and after infection compared to healthy volunteers (1, 3).
Nasal and bronchial sampling studies during viral infection generall_y show increased numbers of lymphocytes, eosinophils, and neutrophils (8, 9, 10, 11, 12). Elevated levels of several cytokines and chemokines such as eotaxin, TNF‐α, IL‐1β, IL‐5, IL‐6, IL‐8, and IFN‐γ have also been detected during common cold in nasal and bronchial tissue (13, 14). When immunologic responses in asthmatic and nonasthmatic patients were compared during common cold, an enhanced eosinophilic response was observed in asthmatic patients during the infection, which tended to be prolonged until convalescence (9, 14, 15). With the exception of increased neutrophil and fibrinogen levels in atopics during common cold (9), no other immunologic differences, such as differences in T helper 1 and 2 cytokine balances, have been observed so far between atopic and nonatopic patients.
Only a few studies have investigated the immunologic mechanisms of naturall_y acquired common cold (11, 13). To clarify the immunologic mechanisms underlying virall_y induced immune pathology in all_ergic patients, we examined differences in both inflammatory cell types and eosinophil‐specific chemokines in all_ergic and nonall_ergic patients in nasal brush specimens (16) during naturall_y acquired common cold.
Material and methods
Subjects
During the winters of 1998 and 1999, eight all_ergic patients (mean age 28 years) and eight nonall_ergic patients (mean age 31 years) with clinical symptoms of common cold participated in this study (Table 1). Within 4 days of the onset of a naturall_y acquired common cold, patients recorded the severity of nasal symptoms on a visual analog scale (VAS) ranging from mild or absent (0) to severe (100). The total nasal symptom score was calculated as the sum of five individual nasal symptom scores recorded (runny nose, nasal blockage, sneezing, nasal itching, and eye watering and irritation, ranging together from 0 to 500). Patients were considered to have common cold and were included in the study when the total nasal symptom score was higher than 100 and when patients presented with at least symptoms of a runny nose and nasal blockage. Allergic sensitivity was confirmed by a positive skin prick test reaction with a wheal diameter of at least 2+ (Vivodiagnost; ALK Benelux BV, Groningen, The Netherlands) or detection of specific serum IgE (Phadiatop, Pharmacia CAP System, Uppsala, Sweden) for house‐dust mite, grass pollen, birch pollen, or cat and dog all_ergens. All sensitized patients had mild rhinitis symptoms without asthma. None of the patients had used topical and systemic corticosteroids for the previous 4 weeks. Each patient gave informed consent, and the Rotterdam University medical ethics committee approved this nasal brush study.
Table 1.
Patient characteristics
| Patient | Age (years) | Type of infection | Sensitization | |
|---|---|---|---|---|
| Nonall_ergic | 1 | 32 | Coronavirus OC43 | – |
| 2 | 26 | Coronavirus 229E | – | |
| 3 | 33 | Influenza A virus/enterovirus | – | |
| 4 | 24 | Rhinovirus | – | |
| 5 | 39 | Rhinovirus | – | |
| 6 | 35 | RSV | – | |
| 7 | 29 | ND | – | |
| 8 | 26 | ND | – | |
| Allergic | 1 | 31 | Coronavirus OC43 | HDM, grass and birch pollen |
| 2 | 23 | Coronavirus OC43 | HDM, grass pollen, cat | |
| 3 | 26 | Mycoplasma pneumoniae | HDM, cat | |
| 4 | 37 | Rhinovirus | HDM | |
| 5 | 29 | Rhinovirus | HDM | |
| 6 | 26 | RSV | Grass pollen | |
| 7 | 30 | RSV | Cat, dog | |
| 8 | 24 | ND | HDM, grass and birch pollen, cat |
RSV: respiratory syncytial virus; HDM: house‐dust mite; ND: not detectable.
Study design
Nasal brush samples were taken during the acute phase of the common cold and during convalescence (2–3 weeks later). Baseline samples were taken between 1 and 12 months after the common cold and when no nasal symptoms had been present for at least 4 weeks. At each sampling moment, patients recorded the severity of several nasal symptoms reported previously and general malaise on a VAS. All samples were taken outside the pollen season.
Nasal brushes
Cells harvested from nasal brush samples were collected as previously described by Godthelp et al. (16). Cells were washed in 7 ml of RPMI 1640 medium (Life Technologies). The supernatant was collected and stored at −80°C until viral and Mycoplasma RNA was isolated for PCR amplification. Cells were pelleted, placed in Tissue‐tek II OCT compound (Miles, Inc, Diagnostics Division, Terrytown, NY, USA), and snap‐frozen in liquid nitrogen. Frozen sections (6 µm) were transferred to 10% poly‐l‐lysine (Sigma)‐coated microscope slides and stored at −80°C until use.
Virus detection
The type of infection was confirmed in nasal brush samples within the first few days of common cold. Respiratory syncytial virus (RSV), adenovirus, influenza virus types A and B, enterovirus, and parainfluenza virus types 1 and 2 infections were detected by immunofluorescent staining of nasal brush cells with antiviral antibodies or by viral isolation from 4 ml nasal brush supernatant. Rhinovirus, coronavirus, and Mycoplasma pneumoniae infections were detected by amplification of viral RNA from 0.5 ml nasal brush supernatant by RT‐PCR, followed by hybridization with either rhinovirus, coronavirus, or M. pneumoniae‐specific radiolabeled probes (17).
Immunohistochemical staining procedures
Slides with frozen nasal brush cells were defrosted and fixed in acetone for 10 min, rinsed in phosphate‐buffered saline (PBS, pH 7.4), and placed in a semiautomatic stainer (Sequenza, Shandon, Amsterdam, The Netherlands). To block nonspecific antibody binding, slides were incubated for 10 min at room temperature with a 10% normal goat serum (CLB) diluted in PBS supplemented with 3% bovine serum albumin (Sigma) and 0.1% azide (PBS/BSA). Subsequently, the slides were incubated for 60 min with mouse antihuman monoclonal antibodies for the cell markers and chemokines mentioned in Table 2 (diluted in PBS/BSA). The slides were rinsed in PBS, and incubated for 30 min with biotinylated goat antimouse Ig serum (1:50 in PBS/BSA plus 10% human serum), rinsed in PBS, and incubated either with streptavidin alkaline phosphatase (1:50 in PBS/BSA plus 10% human serum; Biogenex, Klinipath, Duiven, The Netherlands) for cell type staining, or with polyclonal goat antibiotin antibody (1:50 in PBS/BSA plus 10% human serum; Sigma) for chemokine staining for 30 min at room temperature. Sections were rinsed with distilled water and TRIS buffer (pH 8.5) and incubated for 30 min with New Fuchsin substrate (Chroma, Kongen, Germany). Finall_y, the sections were counterstained with Gill's hematoxylin and mounted in glycerin‐gelatin. Control staining was performed by the substitution of primary monoclonal antibody by an isotypic control antibody.
Table 2.
Monoclonal antibodies used for immunohistochemical staining
| Antibody | Specificity | Cell type/chemokine | Titer | Source |
|---|---|---|---|---|
| OKT6 | CD1a | Langerhans cells | 1:25 | Dept. of Immunology, EMCR, Netherlands |
| T3‐4B5 | CD3 | Total T‐cell pool | 1:100 | DAKO, Denmark |
| Leu2a | CD8 | Cytotoxic T cells | 1:100 | B & D, Dorset, UK |
| Mon/1 | CD14 | Monocytes | 1:600 | CLB, Amsterdam, Netherlands |
| 80H5 | CD15 | Neutrophils | 1:25 | Immunotech, Marseille, France |
| IOB4a | CD19 | B cells | 1:200 | Immunotech, Marseille, France |
| EBM11 | CD68 | Macrophages | 1:300 | DAKO, Denmark |
| HP‐3B1 | CD94 | Natural killer (NK) cells | 1:50 | Coulter, Netherlands |
| G3 | tryptase | Mast cells | 1:100 | Chemicon, UK |
| BMK‐13 | MBP | Eosinophils | 1:100 | Sanbio, Uden, Netherlands |
| αEotaxin | Eotaxin | Chemokine | 1:15 | R & D systems, UK |
| αRANTES | RANTES | Chemokine | 1:50 | Chemicon, UK |
MBP: major basic protein.
Light microscope evaluation
For every nasal brush sample, at least 2000 cells with purple‐blue‐stained nuclei were counted. The number of positively stained cells was calculated as a percentage of the 2000 cells present. Positively stained cells had a bright red‐stained cell membrane, red‐stained cytoplasm, or both, depending on the cell type or chemokine evaluated. Cells were counted at a magnification of ×400.
Statistical analysis
The statistical analysis of cell numbers was performed with SPSS. Differences in cell counts between the three sampling moments were analyzed with the nonparametric Friedman test for related samples. Differences between sampling moments were considered statisticall_y significant when the P value was ≤0.05. Subsequently, differences between two sampling moments were analyzed with the Wilcoxon signed rank test for paired samples. Differences between all_ergic and nonall_ergic patients at each sampling moment were measured with the Mann‐Whitney U‐test. Correlations between numbers of cell types, chemokines, and clinical parameters were tested with Spearman's correlation coefficient. Differences between groups and sampling moments were considered to be statisticall_y significant when P≤0.05.
Results
Nasal symptoms and patient characteristics
All the patients investigated had a significantly elevated total nasal symptom score (Fig. 1) and increased symptoms of general malaise during the acute phase of common cold as compared to convalescence and baseline. In 13 patients (81%), a virus or M. pneumoniae infection was confirmed (Table 1). During the acute and convalescent phase, no differences in systemic and local nasal symptoms were observed between all_ergic and nonall_ergic patients. At baseline, all_ergic patients reported slightly higher total nasal symptom scores (P=0.05) than nonall_ergic patients.
Figure 1.
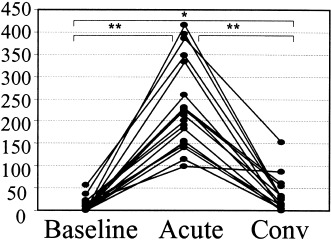
Total nasal symptom score during baseline, acute phase (acute), and convalescent phase (conv) of common cold (*P<0.05, **P<0.01).
Microscopic evaluation
The number of positively stained cells was counted per 2000 nasal brush cells of which the nuclei were stained dark purple‐blue. Positively stained cells had a red cell membrane (CD1a, CD3, CD8, CD19, and CD94) or cytoplasm (major basic protein [MBP], tryptase, eotaxin, and RANTES) or both (CD14, CD15, and CD68). Fig. 2 shows representative sections of nasal brush cells stained for eosinophils, and CD3‐, CD68‐, and eotaxin‐positive cells.
Figure 2.
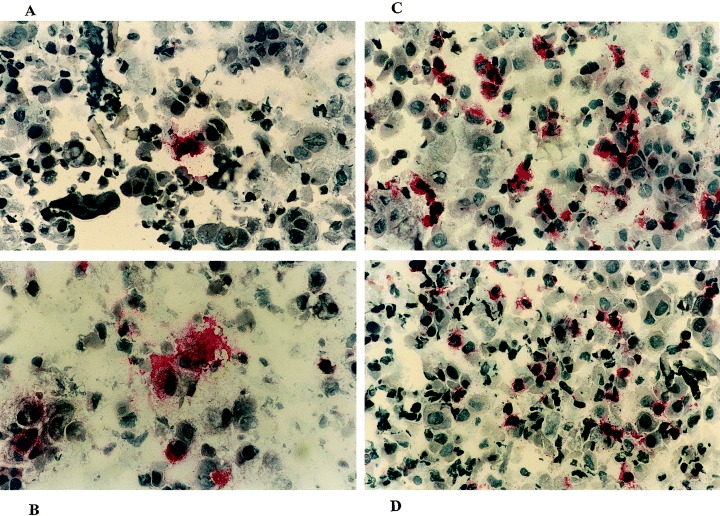
Nasal brush samples stained for A) MBP (eosinophils), B) eotaxin, C) CD68 (macrophages), and D) CD3 (T cells).
Inflammatory cell influx
During common cold, significantly increased numbers of cells positive for CD3, CD14, CD68, and CD94 were observed as compared to convalescence and baseline samples (Fig. 3). During baseline and convalescence generall_y, fewer than 5% monocytes, macrophages, and natural killer cells (NK cells) were detected. This figure increased sharply during common cold to levels of up to 20% of cells present in the nasal brush samples. Baseline levels of CD3‐ positive T cells varied considerably between 0 and 6%. This increased to a maximum of 13% of the cells present during common cold. Fewer CD8‐positive T cells than CD3‐positive T cells were detected in nasal brush samples (range 0–6.6%), but there was a slight trend toward increased numbers during common cold (P=0.1; Friedman test) (Fig. 3). High numbers of neutrophils (CD15‐positive cells) were observed (range 0.7–62.7%), but no significant differences were observed between the three sampling moments (Fig. 3). Langerhans cells (CD1a positive) (median; range: 0; 0–0.3% positive) and B cells (CD19 positive) (median; range: 0; 0–0.4% positive) were detected in only a few nasal brush samples. No differences in the cell types mentioned were observed between all_ergic and nonall_ergic patients during the acute and convalescent phases of common cold. At baseline, we observed significantly more macrophages in nonall_ergic than in all_ergic patients (P=0.02).
Figure 3.
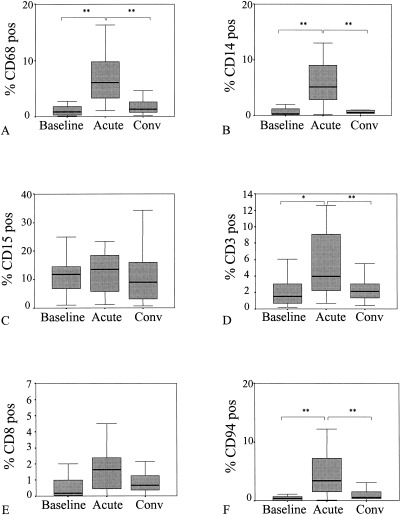
Percentage of A) macrophages (CD68), B) monocytes (CD14), C) neutrophils (CD15), D) CD3‐positive T cells, E) CD8‐positive T cells, and F) natural killer cells (CD94) during baseline, acute phase (acute), and convalescent phase (conv) of common cold (*P<0.05, **P<0.01).
Eosinophils, mast cells, and eotaxin‐ and RANTES‐positive cells
During the acute phase of common cold in all_ergic and nonall_ergic patients, significantly increased numbers of eosinophils (MBP‐positive cells) were detected as compared to baseline. No differences between all_ergic and nonall_ergic patients were observed during common cold and at baseline. However, during convalescence, all_ergic patients had significantly higher eosinophil levels than nonall_ergic patients (P=0.03) (Fig. 4a).
Figure 4.
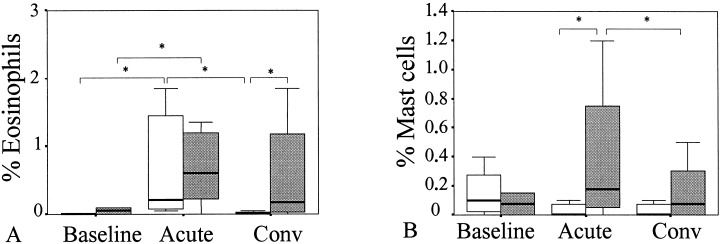
Percentage of A) eosinophils (MBP) and B) mast cells (tryptase) during baseline, acute phase (acute), and convalescent phase (conv) of common cold in all_ergic (gray bars) and nonall_ergic patients (white bars) (*P<0.05).
In all_ergic patients during the acute phase of common cold, increased numbers of mast cells (tryptase‐positive cells) were found as compared to convalescence and baseline (Fig. 4b), while no differences were found in nonall_ergic patients between the three sampling moments. The numbers of mast cells during common cold were significantly higher in all_ergic than nonall_ergic patients (P=0.04).
Numbers of RANTES‐ and eotaxin‐positive cells increased during common cold as compared to baseline in all_ergic and nonall_ergic patients (Fig. 5). Although the numbers of eotaxin‐positive cells decreased after common cold, still higher numbers were detected during convalescence than baseline. The numbers of RANTES‐positive cells also remained elevated after common cold. No differences between all_ergic and nonall_ergic patients in numbers of eotaxin‐ and RANTES‐positive cells were observed at all_ three sampling moments.
Figure 5.
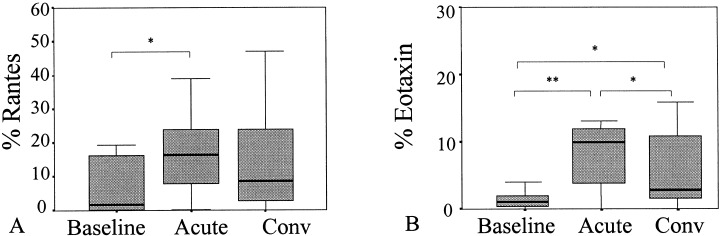
Percentage of A) RANTES‐ and B) eotaxin‐positive cells present in nasal brushes during baseline, acute phase (acute), and convalescent phase (conv) of common cold (*P<0.05, **P<0.01).
Correlation between cells, cytokines, and nasal symptom severity
During the acute phase of common cold, several statisticall_y significant positive correlations were found between different cell markers, chemokines, and clinical parameters. The numbers of macrophages, monocytes, T cells, and NK cells all_ correlated well with each other (data not shown). The number of eotaxin‐positive cells correlated well with T cells and NK cells (r=0.7, P=0.003 and r=0.8, P=0.001, respectively). There were also positive correlations between the numbers of eotaxin‐positive cells and both upper respiratory symptoms and the feeling of general malaise (r=0.6, P=0.015, and r=0.6, P=0.02, respectively).
Discussion
To clarify the immunologic mechanisms underlying immune pathology during common cold in all_ergic patients, we examined differences in inflammatory cell types and eosinophil‐specific chemokines between all_ergic and nonall_ergic patients in nasal brush specimens during naturall_y acquired common cold. Both all_ergic and nonall_ergic patients showed an initial response of macrophages, monocytes, T cells, NK cells, and eosinophils in the nasal epithelium. However, a mast‐cell response was observed during the acute phase only in all_ergic patients. In addition, the increase in numbers of eosinophils during the acute phase persisted only into convalescence in all_ergic patients. These different immunologic responses may underlie different disease expression during common cold in all_ergic and nonall_ergic patients.
A wide variety of respiratory viruses can induce common cold symptoms (7, 18) and can induce airway hyperresponsiveness in all_ergic patients (1, 3). However, until now, almost all_ common cold studies used experimentall_y induced infection models with rhinovirus type 16 in man (2, 19). Allergen provocation studies (20) have shown that immunologic data derived from experimental studies can be very different from data acquired in naturall_y occurring disease, stressing the importance of studying natural disease. Little is known about the role of viruses other than rhinovirus in inducing inflammatory responses related to common cold.
The influx of inflammatory cells such as T cells, monocytes, macrophages, and NK cells found in this study is comparable to what has been found in common cold studies in all_ergic patients and in controls (10, 11, 12). In contrast to these studies, we did not observe a nasal influx of neutrophils (8, 9). Since high numbers of neutrophils are found in the nose during all_ sampling moments but no differences were observed between all_ergic and nonall_ergic patients, it would not seem very likely that neutrophils play a role in differentiating the two patient groups. However, functional differences, such as in cytokine production, may explain differences in airway hyperreactivity.
The mast‐cell response in this study, and studies showing increased levels of histamine in lavage samples during common cold in all_ergic subjects (21) indicate the role of mast cells in airway hyperresponsiveness. In addition, mast cells after all_ergen chall_enge have been shown to produce mediators and cytokines which attract and activate eosinophils, leading to priming of subjects and induction of airway hyperresponsiveness. However, mediators such as histamine production alone do not result in priming phenomena. Therefore, this is not a mast‐cell product which is very likely to explain airway hyperresponsiveness after common cold in all_ergic disease.
The most likely candidates for inducing immune pathology seem to be the eosinophils. Although increased numbers of eosinophils were detected in the nose of all_ergic and nonall_ergic patients during common cold, it was only in all_ergic patients that this eosinophilia persisted into convalescence. In asthmatic patients also, prolonged eosinophilia has been found after induced common cold in bronchial (9, 15) and nasal samples (14). To our knowledge, these observations are the first which show persistent nasal eosinophilic responses in relatively mildly all_ergic subjects after naturall_y acquired common cold. There have been reports of increased nasal and bronchial hyperreactivity in subjects with all_ergic rhinitis (1). Influx of mast cells, release of mast‐cell products, and persistence of eosinophils in the airway mucosa may cause an increase in airway hyperreactivity in all_ergic patients compared to nonall_ergic controls.
Contrary to what we expected on the basis of the findings above, no differences were found between all_ergic and nonall_ergic patients in eosinophil‐specific chemokine positive cells. An increase in RANTES‐ and eotaxin‐positive cells was observed during and after common cold in both groups. However, eosinophils may display enhanced susceptibility in all_ergic individuals for the chemokines mentioned; for example, through altered chemokine receptor expression (22, 23). Other mechanisms, such as increased expression of ICAM‐1 in inflammatory and epithelial cells (11, 24, 25, 26), or decreased apoptosis of eosinophils, may account for the prolonged eosinophilia in the nose after common cold (27). The next step will be to measure the actual quantities of chemokines produced by nasal brush cells in both all_ergic and nonall_ergic patients.
In conclusion, increased numbers of mast cells during infection and enhanced nasal eosinophilia after common cold in all_ergic patients may explain nasal and bronchial hyperreactivity and asthma exacerbations after viral infection. Further studies are needed to clarify why eosinophils tend to persist longer in the noses of all_ergic patients than in those of nonall_ergic patients after common cold.
Acknowledgments
This study was supported by The Netherlands Asthma Foundation and The Netherlands Organization for Scientific Research.
References
- 1. Gern JE, Calhoun W, Swenson C, Shen G, Busse WW. Rhinovirus infection preferentiall_y increases lower airway responsiveness in all_ergic subjects. Am J Respir Crit Care Med 1997;155:1872–1876. [DOI] [PubMed] [Google Scholar]
- 2. Grunberg K, Smits HH, Timmers MC, et al Experimental rhinovirus 16 infection. Effects on cell differentials and soluble markers in sputum in asthmatic subjects. Am J Respir Crit Care Med 1997;156(2 Pt 1):609–616. [DOI] [PubMed] [Google Scholar]
- 3. Grunberg K, Timmers MC, Smits HH, et al Effect of experimental rhinovirus 16 colds on airway hyperresponsiveness to histamine and interleukin‐8 in nasal lavage in asthmatic subjects in vivo . Clin Exp Allergy 1997;27:36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hinriksdottir I & Melen I. Allergic rhinitis and upper respiratory tract infections. Acta Otolaryngol Suppl 1994;515:30–32. [DOI] [PubMed] [Google Scholar]
- 5. Doyle WJ, Skoner DP, Fireman P, et al Rhinovirus 39 infection in all_ergic and nonall_ergic subjects. J Allergy Clin Immunol 1992;89:968–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Johnston SL, Pattemore PK, Sanderson G, et al Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. BMJ 1995;310(6989):1225–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Makela MJ, Puhakka T, Ruuskanen O, et al Viruses and bacteria in the etiology of the common cold. J Clin Microbiol 1998;36:539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Levandowski RA, Weaver CW, Jackson GG. Nasal‐secretion leukocyte populations determined by flow cytometry during acute rhinovirus infection. J Med Virol 1988;25:423–432. [DOI] [PubMed] [Google Scholar]
- 9. Pizzichini MM, Pizzichini E, Efthimiadis A, et al Asthma and natural colds. Inflammatory indices in induced sputum: a feasibility study. Am J Respir Crit Care Med 1998;158:1178–1184. [DOI] [PubMed] [Google Scholar]
- 10. Winther B, Farr B, Turner RB, Hendley JO, Gwaltney JM Jr, Mygind N. Histopathologic examination and enumeration of polymorphonuclear leukocytes in the nasal mucosa during experimental rhinovirus colds. Acta Otolaryngol Suppl (Stockh) 1984;413:19–24. [DOI] [PubMed] [Google Scholar]
- 11. Trigg CJ, Nicholson KG, Wang JH, et al Bronchial inflammation and the common cold: a comparison of atopic and nonatopic individuals. Clin Exp Allergy 1996;26:665–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thomas LH, Fraenkel DJ, Bardin PG, Johnston SL, Holgate ST, Warner JA. Leukocyte responses to experimental infection with human rhinovirus. J Allergy Clin Immunol 1994;94(6 Pt 2):1255–1262. [DOI] [PubMed] [Google Scholar]
- 13. Roseler S, Holtappels G, Wagenmann M, Bachert C. Elevated levels of interleukins IL‐1 beta, IL‐6 and IL‐8 in naturall_y acquired viral rhinitis. Eur Arch Otorhinolaryngol Suppl 1995;1:S61–63. [DOI] [PubMed] [Google Scholar]
- 14. Fleming HE, Little FF, Schnurr D, et al Rhinovirus‐16 colds in healthy and in asthmatic subjects: similar changes in upper and lower airways. Am J Respir Crit Care Med 1999;160:100–108. [DOI] [PubMed] [Google Scholar]
- 15. Fraenkel DJ, Bardin PG, Sanderson G, Lampe F, Johnston SL, Holgate ST. Lower airways inflammation during rhinovirus colds in normal and in asthmatic subjects. Am J Respir Crit Care Med 1995;151(3 Pt 1):879–886. [DOI] [PubMed] [Google Scholar]
- 16. Godthelp T, Holm AF, Fokkens WJ, et al Dynamics of nasal eosinophils in response to a nonnatural all_ergen chall_enge in patients with all_ergic rhinitis and control subjects: a biopsy and brush study. J Allergy Clin Immunol 1996;97:800–811. [DOI] [PubMed] [Google Scholar]
- 17. Andeweg AC, Bestebroer TM, Huybreghs M, Kimman TG, De Jong JC. Improved detection of rhinoviruses in clinical samples by using a newly developed nested reverse transcription‐PCR assay. J Clin Microbiol 1999;37:524–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arruda E, Pitkaranta A, Witek TJ Jr, Doyle CA, Hayden FG. Frequency and natural history of rhinovirus infections in adults during autumn. J Clin Microbiol 1997;35:2864–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Calhoun WJ, Dick EC, Schwartz LB, Busse WW. A common cold virus, rhinovirus 16, potentiates airway inflammation after segmental antigen bronchoprovocation in all_ergic subjects. J Clin Invest 1994;94:2200–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fokkens WJ, Godthelp T, Holm AF, Klein‐Jan A. Local corticosteroid treatment: the effect on cells and cytokines in nasal all_ergic inflammation. Am J Rhinol 1998;12:21–26. [DOI] [PubMed] [Google Scholar]
- 21. Igarashi Y, Skoner DP, Doyle WJ, White MV, Fireman P, Kaliner MA. Analysis of nasal secretions during experimental rhinovirus upper respiratory infections. J Allergy Clin Immunol 1993;92:722–731. [DOI] [PubMed] [Google Scholar]
- 22. Yawalkar N, Uguccioni M, Scharer J, et al Enhanced expression of eotaxin and CCR3 in atopic dermatitis. J Invest Dermatol 1999;113:43–48.DOI: 10.1046/j.1523-1747.1999.00619.x [DOI] [PubMed] [Google Scholar]
- 23. Ying S, Robinson DS, Meng Q, et al Enhanced expression of eotaxin and CCR3 mRNA and protein in atopic asthma. Association with airway hyperresponsiveness and predominant co‐localization of eotaxin mRNA to bronchial epithelial and endothelial cells. Eur J Immunol 1997;27:3507–3516. [DOI] [PubMed] [Google Scholar]
- 24. Vignola AM, Campbell AM, Chanez P, et al HLA‐DR and ICAM‐1 expression on bronchial epithelial cells in asthma and chronic bronchitis. Am Rev Respir Dis 1993;148:689–694. [DOI] [PubMed] [Google Scholar]
- 25. Ciprandi G, Pronzato C, Ricca V, Bagnasco M, Canonica GW. Evidence of intercellular adhesion molecule‐1 expression on nasal epithelial cells in acute rhinoconjunctivitis caused by pollen exposure. J Allergy Clin Immunol 1994;94:738–746. [DOI] [PubMed] [Google Scholar]
- 26. Bentley AM, Durham SR, Robinson DS, et al Expression of endothelial and leukocyte adhesion molecules interacellular adhesion molecule‐1, E‐selectin, and vascular cell adhesion molecule‐1 in the bronchial mucosa in steady‐state and all_ergen‐induced asthma. J Allergy Clin Immunol 1993;92:857–868. [DOI] [PubMed] [Google Scholar]
- 27. Simon H & Alam R. Regulation of eosinophil apoptosis: transduction of survival and death signals. Int Arch Allergy Immunol 1999;118:7–14. [DOI] [PubMed] [Google Scholar]


