Abstract
Previously, we reported the establishment of cells with persistent SARS-CoV infection after apoptotic events and showed that both JNK and PI3K/Akt signaling pathways are important for persistence by treatment with inhibitors at the early stages of SARS-CoV infection. However, the mechanisms of establishment of persistent infection are still unclear. In this study, we investigated which signaling pathways play important roles in escape from apoptosis in cells infected with SARS-CoV. In persistently infected cells at 50 h.p.i., PI3K/Akt, JNK, p38 MAPK and Bcl-2 were phosphorylated and the protein levels of Bcl-2 and Bcl-xL were increased. When surviving cells were treated with the JNK-specific inhibitor, SP600125, at 50 h.p.i., all cells died, suggesting that the JNK signaling pathway is necessary for maintenance of persistently infected cells. Among the signaling pathways in persistently infected cells, Akt and JNK were phosphorylated in SARS-CoV-nucleocapsid (N) protein-expressing Vero E6 cells using vaccinia viral vector (DIs), strongly suggesting that N protein-induced phosphorylation of Akt and JNK are necessary to establish persistence. These results indicated that at least four proteins, Akt, JNK, Bcl-2 and Bcl-xL, are necessary for survival of persistently SARS-CoV-infected cells.
Keywords: SARS, JNK, Bcl-2, Bcl-xL, Nucleocapsid protein
Severe acute respiratory syndrome (SARS) is a newly discovered infectious disease with atypical pneumonia caused by SARS coronavirus (SARS-CoV). SARS became a global health threat due to its rapid transmission and high fatality rate [1], [2].
Vero E6 is a cell line derived from African green monkey kidney cells and is sensitive to SARS-CoV. Many laboratories use this cell line to study SARS-CoV. Infection of Vero E6 cells with SARS-CoV induces apoptosis via activation of caspase-3 [3]. Akt and mitogen-activated protein kinases (MAPKs), including c-Jun N-terminal protein kinase (JNK), extracellular signal-related kinase (ERK) 1/2, and p38 MAPK, are phosphorylated in SARS-CoV-infected Vero E6 cells [3], [4], [5]. Especially, activation of p38 and inactivation of Akt by SARS-CoV infection induce cytopathic effects and apoptosis in virus-infected cells, respectively. Phosphorylation of p38 MAPK is known to regulate signal transducer and activator of transcription 3 and 90 kDa ribosomal S6 kinases [5], [6]. Although the majority of virus-infected cells die by apoptosis, we found that a small population of virus-infected cells remained alive and these cells grew with production of virus [7]. Four groups, including ours, independently reported persistent infection of cultured cells by SARS-CoV [8], [9], [10]. We found that JNK and PI3K/Akt signaling pathways are important for the establishment of persistent SARS-CoV infection in Vero E6 cells when these specific inhibitors were added soon after viral adsorption.
In this study, we further analyzed the mechanisms of establishment of persistent SARS-CoV infection in Vero E6 cells. We found that anti-apoptotic proteins, Bcl-2 and Bcl-xL, are important for persistent infection. Nucleocapsid (N) protein of SARS-CoV was suggested to play important roles in phosphorylation of Akt and JNK.
Materials and methods
Cells and virus. Vero E6 cells were subcultured routinely in 75-cm3 flasks in Dulbecco’s modified Eagle’s medium (DMEM; Sigma, St. Louis, MO, USA) supplemented with 0.2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 5% (v/v) fetal bovine serum (FBS), and maintained at 37 °C in an atmosphere of 5% CO2. The medium was changed to 2% FBS DMEM before virus infection. SARS-CoV, which was isolated as Frankfurt 1 and kindly provided by Dr. J. Ziebuhr, was used in the present study. Infection was usually performed at a multiplicity of infection (m.o.i.) of 5. DIs-N expressing N RNA of SARS-CoV and DIs-GFP expressing GFP RNA were described in our previous study [11]. Confluent Vero E6 cells were infected with DIs-N and -GFP at 5 m.o.i.
Fixing and staining of cells. The cells in 24-well plates were fixed with 10% formaldehyde for at least 24 h, and stained with 0.1% naphthol blue-black for 30 min. This convenient method was described by Everitt and Wohlfart for determination of the actual or relative number of cells in anchorage culture [12]. After washing out with water, the plates were scanned with a GT-9400UF scanner (Epson, Tokyo, Japan). The dye–protein complexes were released hydrolytically with 0.1 M NaOH and measured spectrophotometrically at 660 nm. When cells and the supernatant contained infectious SARS-CoV, the cell number was counted using this method. The number of cells that did not contain SARS-CoV was counted using the WST-1 cell proliferation assay system (Takara, Shiga, Japan).
Inhibitors. The JNK inhibitor, SP600125, and PI3K/Akt inhibitor, LY294002, were purchased from Calbiochem (San Diego, CA, USA) and Cell Signaling Technology Inc. (Beverly, MA, USA), respectively. These inhibitors were dissolved in dimethyl sulfoxide (DMSO) at a concentration of 10 mM. The same volume of DMSO alone was used as a control.
Western blotting. The whole-cell extracts were electrophoresed on 5–20% gradient polyacrylamide gels, and transferred electrophoretically onto PVDF membranes (Immobilon-P; Millipore, Bedford, MA, USA). In the present study, we applied two sets of samples to polyacrylamide gels, and the membranes were divided into two halves after blotting, or membranes were examined once using a LumiGLO Elite chemiluminescent system (Kirkegaard and Perry Laboratories, Gaithersburg, ML, USA), and then stripped using Restore Western blot stripping buffer (Pierce, Rockford, IL, USA) for the second detection. The following antibodies, obtained from Cell Signaling Technology Inc., were used in the present study at a dilution of 1:1000: rabbit anti-phospho Akt (Ser473) antibody, rabbit anti-Akt antibody, rabbit anti-phospho ERK (Thr202/Tyr204) antibody, rabbit anti-ERK antibody, rabbit anti-p38 MAPK (Thr180/Tyr182) antibody, rabbit anti-p38 MAPK antibody, rabbit anti-phospho SAPK/JNK (Thr183/Tyr185) antibody, rabbit anti-SAPK/JNK antibody, rabbit anti-phospho Bcl-2 (Ser 70) antibody, and anti-Bcl-xL antibody. Mouse anti-Bcl-2 antibody was purchased from BD Biosciences (Franklin Lakes, NJ, USA) and used at a dilution of 1:500. Mouse anti-β-actin antibody was purchased from Sigma and used at a dilution of 1:5000. Rabbit anti-SARS Nucleocapsid protein antibody was described previously [3].
Results
Phosphorylation of signaling pathways in cells persistently infected by SARS-CoV
As indicated in our previous studies, apoptotic signals, cleaved caspase-3 and DNA fragmentation, are detected at 18 and 24 h post-infection (h.p.i.) in SARS-CoV-infected Vero E6 cells [3]. At 24 h.p.i., cells begin to show rounding and persistently infected cells are observed after 48 h.p.i. To investigate which signaling pathways are phosphorylated in persistently SARS-CoV-infected cells, protein samples were obtained from these cells at 50 h.p.i. Vero E6 cells were prepared at confluency in T-25 flasks with 2% fetal bovine serum (FBS) containing Dulbecco’s modified Eagle’s medium (DMEM), and infected with SARS-CoV at 5 m.o.i. On the other hand, Vero E6 cells were prepared in T-25 flasks at several concentrations with 2% FBS containing DMEM as controls because surviving cell number is different (less than 5% of total cells) in each experiment. At 50 h.p.i., surviving cells and controls were washed with 2% FBS containing DMEM 5 times (with pipetting 25 times). Although most dead cells were washed out, a fraction of dead cells were attached to surviving cells and could not be removed completely by washing. As phosphorylation status of signaling pathways sometimes changes following trypsinization and centrifugation, sample buffer for Western blotting analysis was added directly to the washed cells. We obtained a protein sample from mock-infected cells, with a similar cell number to persistently infected cells. The protein samples seemed to contain a maximum of 50% proteins from surviving cells. Western blotting analysis was performed using antibodies to phosphorylated proteins of signaling pathways. As shown in Fig. 1 , Akt, JNK, and p38 MAPK were phosphorylated in surviving cells. On the other hand, Akt was phosphorylated in control cells, while JNK and p38 MAPK were not. As Akt was dephosphorylated in both confluent and subconfluent cells after 18 h.p.i. as shown in our previous studies [4], [13], detection of strongly phosphorylated Akt was suggested to reflect a feature of surviving cells that had escaped from cell death. In addition, the phosphorylated Akt in surviving cells indicated anti-apoptotic activity. The levels of the anti-apoptotic proteins, Bcl-2 and Bcl-xL, and phosphorylated Bcl-2, were also increased in surviving cells. The anti-apoptotic Bcl-2 family proteins, Bcl-2 and Bcl-xL, play important roles in inhibiting mitochondria-dependent cell death pathways [14]. This result suggested that Akt and JNK are important to establish persistent infection as indicated in our previous study, and that Bcl-xL and Bcl-2 are important for survival. Interestingly, p38 MAPK was strongly phosphorylated in surviving cells, suggesting that persistently infected cells consist of a balance between cell death and survival.
Fig. 1.
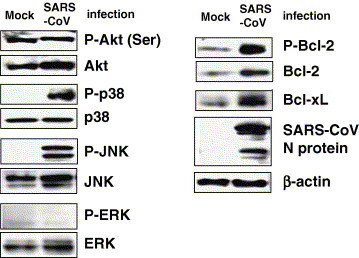
Phosphorylation status of signaling pathways in persistently SARS-CoV-infected cells. Vero E6 cells were prepared at confluency in T-25 flasks with 2% fetal bovine serum (FBS) containing Dulbecco’s modified Eagle’s medium (DMEM), and infected with SARS-CoV at 5 m.o.i. At 50 h.p.i., surviving cells and controls were washed 5 times with 2% FCS containing DMEM (pipetting a total of 25 times). Mock-infected subconfluent cells, similar in number to surviving cells that had escaped from apoptosis by SARS-CoV infection, were also washed in the same manner. Western blotting analysis was performed using these protein samples.
Importance of PI3K/Akt and JNK for establishment of persistently virus-infected cells
In our previous study, we showed that treatment of Vero E6 cells with the JNK inhibitor, SP600125, and PI3K/Akt inhibitor, LY294002, 1 h after inoculation with SARS-CoV prevented persistent SARS-CoV infection [7]. Therefore, we concluded that activation of JNK and PI3K/Akt by SARS-CoV infection is important for the establishment of persistence. To investigate whether these inhibitors affect the establishment of viral persistence in cells at the late stage of SARS-CoV infection, cells were treated with inhibitors at 50 h.p.i. As shown in Fig. 2 A and B, SP600125 killed the cells completely, while LY294002 did not. As LY294002 has an inhibitory effect on cell proliferation, as indicated in Fig. 2C and in our previous study [13], the growth rate of persistently infected cells was slow. On the other hand, treatment with SP600125 in the absence of SARS-CoV infection did not affect cell proliferation. As Akt in virus-infected cells was dephosphorylated after 18 h.p.i., as shown in our previous studies [4], [13], phosphorylation of Akt at the early stage of infection may be important for preventing apoptosis. However, this result strongly suggested that once persistence is established, phosphorylation of Akt is not necessary for survival. Therefore, we concluded that activation of PI3K/Akt is essential for the establishment of persistent infection with SARS-CoV at time points before cell death, whereas activation of JNK is required at the time of establishment of persistence.
Fig. 2.
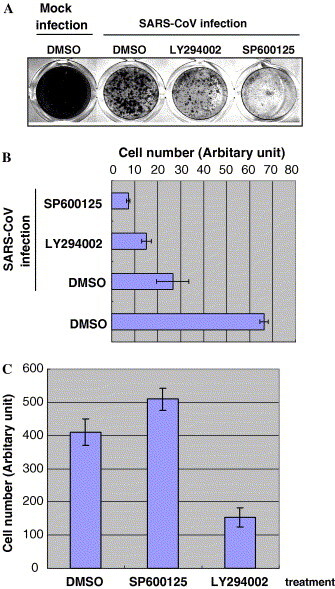
Effects of JNK and PI3K/Akt inhibitors on cell viability of persistently SARS-CoV-infected cells. (A) Confluent Vero E6 cells in 24-well plates were infected with SARS-CoV for 50 h, and then LY294002 (10 μM) and SP600125 (20 μM) were added to the cells. All wells contained the same volume of DMSO. After incubation for 7 days, the cells were fixed with 10% formaldehyde and stained with 0.1% naphthol blue-black. (B) Stained cells were quantified by measuring the absorbance at OD660 with addition of NaOH. (C) Subconfluent cells were treated with inhibitors for 5 days, and then cells were counted using the WST-1 cell proliferation assay system [6].
Phosphorylation of signaling pathways by SARS-CoV-nucleocapsid protein
Next, we investigated which signaling pathways are phosphorylated by nucleocapsid (N) protein of SARS-CoV because several reports indicated that expression of N protein induces phosphorylation of signaling pathways. Surjit et al. reported that ERK, phosphorylated Akt and Bcl-2 are down-regulated, whereas JNK, p38 MAPK activation, activated caspase-3 and -7 are up-regulated in COS-1 cells in the absence of growth factors [15]. They suggested that N protein is able to induce apoptosis under stress conditions. To understand which signaling pathways are phosphorylated by N protein in Vero E6 cells persistently infected with SARS-CoV, we made an N expression plasmid. Transfection of the N expression plasmid was performed using transfection reagents, Magnetfection and VeroFect (OZ Biosciences, Marseille, France), which our screen of transfection reagents suggested to be the best transfection systems for Vero E6 cells, which have low transfection efficiency (data not shown). However, levels of expression of N protein by these two reagents were far lower than those in SARS-CoV-infected Vero E6 cells. Therefore, we next used the vaccinia virus expression system (DIs-N) [11]. Vero E6 cells were infected with DIs-N at 5 m.o.i. and protein samples were obtained at 18 h.p.i. We used DIs-GFP, which expresses GFP protein in infected cells, as a control at the same m.o.i. As shown in Fig. 3 , both Akt and JNK were phosphorylated in DIs-N-infected cells as compared with DIs-GFP-infected cells. There was no significant difference in the amount of Bcl-2, Bcl-xL, and phosphorylated p38 MAPK. This result suggested that phosphorylation of Akt and JNK induced by N protein in SARS-CoV-infected Vero E6 cells plays important roles for the establishment of persistence.
Fig. 3.
Modulation of signaling pathways by N expression. Confluent Vero E6 cells in 24-well plates were infected with DIs-N and DIs-GFP at 5 m.o.i. Protein samples were obtained at 18 h.p.i. and Western blotting analysis was performed.
Discussion
In our previous study, the signaling pathways of JNK and Akt were shown to be important for establishment of persistent SARS-CoV infection, when these inhibitors were added soon after SARS-CoV infection [7]. Approximately 95% of confluent Vero E6 cells died 2 days after infection with SARS-CoV. The remaining 5% of cells that survived grew with persistent virus infection. When 24-well plates were used for experiments, the persistently infected cells reached confluence by 7 days. Interestingly, the PI3K/Akt inhibitor, LY294002, permitted cell survival when added after apoptotic events, but activation of JNK was also necessary for survival after apoptotic events. Our previous study demonstrated the importance of Akt activation for proliferation of SARS-CoV-infected cells [4]. Phosphorylation of Akt was down-regulated in subconfluent cells by SARS-CoV infection [13]. Nevertheless, LY294002-treated surviving cells that had escaped from SARS-CoV-induced apoptosis could still grow slowly. One of the reasons for this is that PI3K/Akt inhibitor needs 3 days after treatment to inhibit cell proliferation [13]. Therefore, persistent cell colonies may grow slightly in the presence of LY294002. SARS-CoV replicates in surviving cells, and these cells are still alive in the presence of LY294002, suggesting that the signaling pathway of PI3K/Akt is not necessary to prevent apoptosis in cells with persistent virus infection. The apoptotic signaling pathways may be blocked independent of PI3K/Akt in surviving cells. In this study, we demonstrated that the anti-apoptotic proteins Bcl-2 and Bcl-xL were present at elevated levels in persistently infected cells. Because Bcl-2 is slightly increased and phosphorylated at acute infection (24 h.p.i.), but not Bcl-xL (data not shown), Bcl-xL may be more important than Bcl-2 for survival. These results indicated that the PI3K/Akt signaling pathway is important for cell survival in the early stages of SARS-CoV-induced apoptosis, whereas the JNK, Bcl-2, and Bcl-xL pathways are important after apoptotic events. We found SP600125 that slightly prevented SARS-CoV-induced apoptosis (unpublished data). The JNK signaling pathway is one of the key factors for understanding persistence of SARS-CoV.
Interestingly, when we used the N expression system of vaccinia virus (DIs-N), Akt and JNK were phosphorylated in Vero E6. The differences in the results between our study and that reported by Surjit et al. using COS-1 are most likely due to the use of different cell cultures and different expression systems [15]. It is not yet clear whether N protein alone is able to induce phosphorylation of these signaling pathways in Vero E6 cells because we have no useful system for plasmid transfection of these cells. Both Akt and JNK were phosphorylated in our system due to additional stress by expression of vaccinia viral proteins. Because Bcl-2 and Bcl-xL were not increased by N protein expression, these anti-apoptotic proteins may not be down-stream of Akt and JNK signaling pathways.
In this paper, we showed possible mechanisms of establishment of persistent SARS-CoV infection. Further investigations are necessary to determine signaling pathways, which are able to up-regulate Bcl-2 and Bcl-xL levels.
Acknowledgments
We thank Drs. S. Harada (National Institute of Infectious Diseases, Japan) and M. Funaba (Azabu University, Japan) for helpful suggestions. We also thank Ms. M. Ogata (National Institute of Infectious Diseases, Japan) for her assistance. This work was supported in part by the Japan Health Science Foundation and, Japan Society for the Promotion of Science, Tokyo, Japan.
References
- 1.Marra M.A., Jones S.J., Astell C.R., Holt R.A., Brooks-Wilson A., Butterfield Y.S., Khattra J., Asano J.K., Barber S.A., Chan S.Y., Cloutier A., Coughlin S.M., Freeman D., Girn N., Griffith O.L., Leach S.R., Mayo M., McDonald H., Montgomery S.B., Pandoh P.K., Petrescu A.S., Robertson A.G., Schein J.E., Siddiqui A., Smailus D.E., Stott J.M., Yang G.S., Plummer F., Andonov A., Artsob H., Bastien N., Bernard K., Booth T.F., Bowness D., Czub M., Drebot M., Fernando L., Flick R., Garbutt M., Gray M., Grolla A., Jones S., Feldmann H., Meyers A., Kabani A., Li Y., Normand S., Stroher U., Tipples G.A., Tyler S., Vogrig R., Ward D., Watson B., Brunham R.C., Krajden M., Petric M., Skowronski D.M., Upton C., Roper R.L. The genome sequence of the SARS-associated coronavirus. Science. 2003;300:1399–1404. doi: 10.1126/science.1085953. [DOI] [PubMed] [Google Scholar]
- 2.Rota P.A., Oberste M.S., Monroe S.S., Nix W.A., Campagnoli R., Icenogle J.P., Penaranda S., Bankamp B., Maher K., Chen M.H., Tong S., Tamin A., Lowe L., Frace M., DeRisi J.L., Chen Q., Wang D., Erdman D.D., Peret T.C., Burns C., Ksiazek T.G., Rollin P.E., Sanchez A., Liffick S., Holloway B., Limor J., McCaustland K., Olsen-Rasmussen M., Fouchier R., Gunther S., Osterhaus A.D., Drosten C., Pallansch M.A., Anderson L.J., Bellini W.J. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–1399. doi: 10.1126/science.1085952. [DOI] [PubMed] [Google Scholar]
- 3.Mizutani T., Fukushi S., Saijo M., Kurane I., Morikawa S. Phosphorylation of p38 MAPK and its downstream targets in SARS coronavirus-infected cells. Biochem. Biophys. Res. Commun. 2004;319:1228–1234. doi: 10.1016/j.bbrc.2004.05.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mizutani T., Fukushi S., Saijo M., Kurane I., Morikawa S. Importance of Akt signaling pathway for apoptosis in SARS-CoV-infected Vero E6 cells. Virology. 2004;327:169–174. doi: 10.1016/j.virol.2004.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mizutani T., Fukushi S., Murakami M., Hirano T., Saijo M., Kurane I., Morikawa S. Tyrosine dephosphorylation of STAT3 in SARS coronavirus-infected Vero E6 cells. FEBS Lett. 2004;577:187–192. doi: 10.1016/j.febslet.2004.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mizutani T., Fukushi S., Saijo M., Kurane I., Morikawa S. Regulation of p90RSK phosphorylation by SARS-CoV infection in Vero E6 cells. FEBS Lett. 2006;580:1417–1424. doi: 10.1016/j.febslet.2006.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mizutani T., Fukushi S., Saijo M., Kurane I., Morikawa S. JNK and PI3k/Akt signaling pathways are required for establishing persistent SARS-CoV infection in Vero E6 cells. Biochem. Biophys. Acta. 2005;1741:4–10. doi: 10.1016/j.bbadis.2005.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan P.K., To K.F., Lo A.W., Cheung J.L., Chu I., Au F.W., Tong J.H., Tam J.S., Sung J.J.J., Ng H.K. Persistent infection of SARS coronavirus in colonic cells in vitro. J. Med. Virol. 2004;74:1–7. doi: 10.1002/jmv.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palacios G., Jabado O., Renwick N., Briese T., Lipkin W.I. Severe acute respiratory syndrome coronavirus persistence in Vero cells. Chin. Med. J. (Engl.) 2005;118:451–459. [PubMed] [Google Scholar]
- 10.Yamate M., Yamashita M., Goto T., Tsuji S., Li Y.G., Warachit J., Yunoki M., Ikuta K. Establishment of Vero E6 cell clones persistently infected with severe acute respiratory syndrome coronavirus. Microbes Infect. 2005;7:1530–1540. doi: 10.1016/j.micinf.2005.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.K. Ishii, H. Hasegawa, N. Nagata, M. Mizutani, S. Morikawa, T. Suzuki, F. Taguchi, M. Tashiro, T. Takemori, T. Miyamura, Y. Tsunetsugu-Yokota, Induction of protective immunity against severe acute respiratory syndrome coronavirus (SARS-CoV) infection using highly attenuated recombinant vaccinia virus DIs, Virology (in press). [DOI] [PMC free article] [PubMed]
- 12.Everitt E., Wohlfart C. Spectrophotometric quantitation of anchorage-dependent cell numbers using extraction of naphthol blue-black-stained cellular protein. Anal. Biochem. 1987;162:122–129. doi: 10.1016/0003-2697(87)90016-9. [DOI] [PubMed] [Google Scholar]
- 13.Mizutani T., Fukushi S., Iizuka D., Inanami O., Kuwabara M., Takashima H., Yanagawa H., Saijo M., Kurane I., Morikawa S. Inhibition of cell proliferation by SARS-CoV infection in Vero E6 cells. FEMS Immunol. Med. Microbiol. 2006;46:236–243. doi: 10.1111/j.1574-695X.2005.00028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim R. Unknotting the roles of Bcl-2 and Bcl-xL in cell death. Biochem. Biophys. Res. Commun. 2005;333:336–343. doi: 10.1016/j.bbrc.2005.04.161. [DOI] [PubMed] [Google Scholar]
- 15.Surjit M., Liu B., Jameel S., Chow V.T., Lal S.K. The SARS coronavirus nucleocapsid protein induces actin reorganization and apoptosis in COS-1 cells in the absence of growth factors. Biochem. J. 2004;383:13–18. doi: 10.1042/BJ20040984. [DOI] [PMC free article] [PubMed] [Google Scholar]


