Abstract
Maternal preconceptional cytomegalovirus (CMV) immunity does not protect the fetus from acquiring congenital CMV infection (cCMV). Nonprimary infections due to recurrence of latent infections or reinfection with new virus strains during pregnancy can result in fetal infection. Because the prevalence of cCMV increases with increasing maternal CMV seroprevalence, the vast majority of the cases of cCMV throughout the world follow nonprimary maternal infections and is more common in individuals of lower socioeconomic background. Horizontal exposures to persons shedding virus in bodily secretions (young children, sexual activity, household crowding, low income) probably increase the risk of acquisition of an exogenous nonprimary CMV infection and fetal transmission. In addition, more frequent acquisition of new antibody reactivities in transmitter mothers suggest that maternal reinfection by new viral strains could be a major source of congenital infection in such populations. However, the exact frequency of CMV nonprimary infection in seroimmune women during pregnancy and the rate of intrauterine transmission in these women are yet to be defined. Usually, the birth prevalence of cCMV is high (≥7:1000) in highly seropositive populations. There is increasing evidence that the frequency and severity of the clinical and laboratory abnormalities in infants with congenital CMV infection born to mothers with nonprimary CMV infection are similar to infants born after a primary maternal infection. This is particularly true for sensorineural hearing loss, which contributes to one third of all early-onset hearing loss in seropositive populations. This brief overview will discuss the need for more research to better clarify the natural history of cCMV in highly seropositive populations, which, in almost all populations, remains incompletely defined.
Keywords: CMV seropositive mothers, congenital infection, cytomegalovirus, highly CMV seropositive populations, natural history
Congenital cytomegalovirus infection (cCMV) is the most common intrauterine viral infection in humans. To limit damaging infants from this congenital infection using preventive and/or therapeutic interventions, the natural history of cCMV must be completely understood. After 4 decades of investigation, challenging questions about the complex dynamics of the mother-to-fetus transmission of this infection and prediction of its consequences to infant’s health remain unanswered.
In stark contrast to other congenital infections such as rubella and toxoplasmosis, maternal preconceptional cytomegalovirus (CMV) immunity does not protect the fetus from acquiring cCMV. Thus, cCMV can be either a result of primary or nonprimary CMV infections during pregnancy. In addition, the prevalence of cCMV infection increases as maternal CMV seroprevalence increases with maternal nonprimary infections, denoting a significant burden of cCMV infection.
More important, although primary maternal infection during pregnancy represents a significant risk for virus transmission to the fetus and disease, nonprimary (recurrent) infections and transmission to the fetus in women with prior existing immunity to this virus is frequent. Although early reports reasoned that infants with cCMV born to mothers with prior immunity would be protected from symptomatic cCMV infection, more recent data indicate that nonprimary maternal infections also lead to disease and its long-term consequences.
This brief overview will discuss existing data of the human natural history of cCMV in highly seropositive populations focusing on available reports on epidemiological characteristics of mothers and infants affected with cCMV.
MATERNAL CYTOMEGALOVIRUS SEROPREVALENCE
Cytomegalovirus acquisition in a population is typically an age-dependent event [1]. Cytomegalovirus seroprevalence rates likely serve as a marker for the size of the reservoir of the virus as well as variations in the host, environmental, behavioral, social, and cultural characteristics within different populations. Most women of childbearing age from highly seropositive populations are usually no longer susceptible to primary CMV infections. In a representative age-stratified sample of unselected pregnant women from an urban Brazilian low-income maternal population, we have detected that CMV seroprevalence is almost universal (97%) and is found at similar levels in pregnant women of ages ranging from 12 to 46 years, suggesting that the majority of primary CMV infections occur early, in infancy or childhood [2]. In addition, the overall relative stability of CMV antibody levels detected in this study was likely a consequence of the ability of CMV to cause recurrent infections and the frequent viral exposure inside this population.
In highly industrialized countries, maternal seroprevalence is usually relatively low (≤50%) to intermediate (50%–70%) [3]. However, variable rates of seropositivity have been reported from mothers from different ethnic and socioeconomic backgrounds within the same geographic region. As an example, seroprevalence rates in the United States were higher for (1) nonwhites than whites and (2) individuals from lower socioeconomic backgrounds compared with higher socioeconomic ones [4]. In addition, in France seroprevalence rates are lower for women whose origin is Metropolitan France (43.7%) compared with those from other backgrounds such as sub-Saharan and North Africa (94.6%) [5].
In a recent systematic review and meta-analysis of available data, Zuhair et al [6] have estimated the worldwide CMV seroprevalence. These estimates have fortified that there is a socioeconomic link with CMV showing that, at any given age, CMV prevalence is higher in individuals of lower socioeconomic groups. According to these authors, the global estimated mean seroprevalence among women of reproductive age was 86% (95% confidence interval [CI], 83–89). The highest prevalence rates were estimated for the less developed regions of the world such as Eastern Mediterranean (88%–95%), Western Pacific (86%–94%), and Southeast Asia (82%–94%), whereas the lowest seroprevalences were found in the Americas (69%–87%), the European region (63%–76%), and Ireland (22%–66%).
TYPE OF MATERNAL INFECTION, RISK FACTORS, AND OVERALL BURDEN OF CONGENITAL CYTOMEGALOVIRUS INFECTION
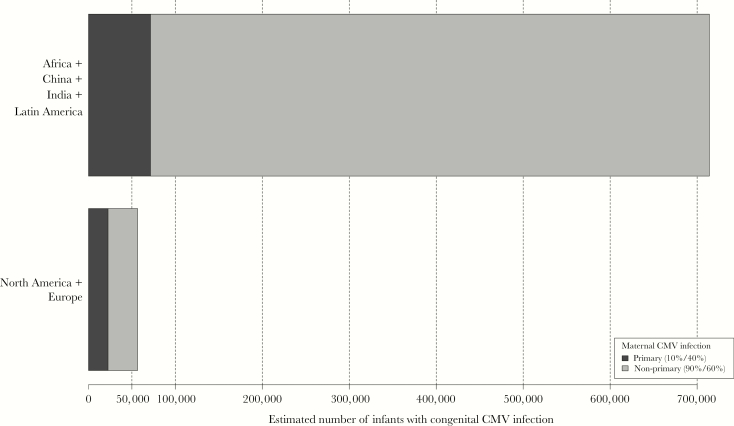
As has been observed in studies of maternal CMV seroprevalence, the birth prevalence of cCMV is population-dependent. Estimates for geographic regions with the lowest mean seroprevalences rates from available systematic reviews and modeling data have indicated that one half to two thirds of the infections occurring in the United States and Northern Europe result from nonprimary infections rather than primary maternal infections [7, 8]. Reinforcing these figures, recent data from a large newborn screening in French maternities showed that approximately 50% of cCMV infections that were identified were products of immune mothers [9]. On the other hand, considering the high CMV seroprevalence in women of childbearing age in populations of the less developed regions of the world, the absolute majority of cCMV in these regions very likely results from nonprimary maternal CMV infections, as we have already demonstrated in a Brazilian population with 98% maternal seroprevalence rate in which 90% of the congenital infections were associated with nonprimary infections in approximately 2000 women-infant pairs observed from first trimester gestation until 1 month after delivery [10]. Taken these data together, as outlined in Figure 1, maternal nonprimary infections contribute to the highest global burden of cCMVs.
Figure 1.
Estimates of the number of infants with congenital cytomegalovirus infection (cCMV) infection born in regions of high maternal cytomegalovirus (CMV) seroprevalence (Africa, China, India, Latin America) compared with the number of infants with cCMV born in areas of low to intermediate maternal CMV seroprevalence (North America, Europe) according to the expected proportion of cCMV resulting from maternal primary or nonprimary CMV infections. Calculation were based on population estimates for childbearing age females (15–35) and birth rates in each geographic region [11]. Based on available data specific for countries that are located in the regions noted above, mean birth prevalence rates of cCMV were estimated to be 6:1000 for North America and Europe and 9:1000 for Africa, China, India, and Latin America. The cCMV rate resulting from primary maternal infection was estimated to be 10% for the highly seropositive populations and 40% for those in low to intermediate seropositive populations with the remainder of cases of cCMV resulting from nonprimary maternal infection.
Nonprimary infections in seropositive women can occur due to reactivation/recurrence of an existing latent endogenous virus or after the acquisition of new virus (reinfection) [12]. An early study [13] based on viral genetic analyses has suggested that endogenous reactivation would be the principal mechanism involved in the transmission of the virus from mothers to infants based on demonstration of identical or very closely related virus strains in mothers and their infected infants. However, this conclusion has not been validated by investigations utilizing more definitive technologies because these early studies relied on a relatively insensitive technology for the detection of genetic variation compared with contemporary methodologies. Results from recent studies, which have described significant genomic diversity of human CMV from congenitally infected infants and their mothers, have been inconsistent with this older paradigm, suggesting the possibility of intrauterine infection with more than 1 viral strain [14, 15]. Furthermore, utilizing a serological assay to detect infection with new strains of CMV based on the appearance of new antibody specificity against 1 of 4 polymorphic epitopes in a virion protein, it has been shown that seropositive women are frequently reinfected with serologically distinct strains of CMV [16–18]. Considering that the used assay to detect strain-specific antibodies is limited in sensitivity for identifying all possible new serological reactivities, the annualized rate of reinfection has been estimated to be at least 10%–30% in the urban, low-income US and Brazilian seropositive women. More important, it has been demonstrated that acquisition of new antibody reactivities during pregnancy was more frequent among mothers who transmitted fetal infection than in nontransmitters [16]. Most (86%) infected infants had CMV-deoxyribonucleic acid (DNA) recovered from blood, saliva, or urine with sequences encoding the antigenic determinants detected by maternal antibody reactivity that followed seroconversion during pregnancy [18]. These data, taken together, argue that maternal reinfection by new strains of CMV is a major source of congenital infection in such populations.
Well described risks for acquiring CMV infections in seronegative women include exposure to young children [3, 19] and sexual activity [20]. As in seronegative women, horizontal exposures to persons shedding virus in bodily secretions probably increases the risk of acquisition of an exogenous nonprimary CMV infection [21]. However, in contrast to seronegative women for whom the diagnosis of CMV infection acquisition can be definitively made by demonstration of seroconversion or presumably by the presence of CMV low-avidity immunoglobulin (Ig)G and specific IgM in the first trimester of gestation [22], no definitive/simple way of confirming a nonprimary infection, either due to reinfection or reactivation, is available. Few studies on the risk factors of maternal nonprimary infections in highly seropositive populations have indirectly approached markers of viral reactivation/reinfection in these women, using intrauterine transmission and viral shedding as endpoints. In a US young urban predominantly black population for whom no definition of the type of maternal infection was provided, caring for young children, recent onset of sexual activity, and household crowding contributed to an increased risk for cCMV in the offspring of young women [23]. In addition, black race [24] and/or low socioeconomic status were identified as risk factors for cCMV in the United States [3]. Younger age has been commonly reported as more frequent among mothers of infected neonates than in those of the uninfected ones in studies from countries with seroprevalence rates ≥90% [25] such as The Gambia [26], China [27], and Mexico [28]. Likewise, unemployment and younger age (<25 years) were 5- to 8-fold more likely in French mothers with nonprimary CMV infection delivering infants with cCMV than in nontransmitters [9]. These features suggest that analogous factors influence the force of nonprimary infections among seropositive women in diverse geographic regions such as exposure to individuals shedding CMV and reinfection; however, genetic and/or immunological characteristics among younger women or those with different ethnic and racial backgrounds have not yet been studied.
Using CMV-DNA shedding in different bodily compartments (urine, saliva, vaginal secretions, and blood) as a marker of viral replication and nonprimary infection, 35% of Brazilian pregnant seropositive women tested periodically from the first trimester gestation until 1 month after delivery shed virus at least once [29]. It is notable that maternal age, ethnicity, education level, sexual activity, parity, and marital status were not associated with viral shedding, but mothers living with or providing daily care to young children (3–6 years) were twice as likely to shed CMV compared with those not providing care or living with children. Likewise, living in crowded households (≥2 people per room) was associated with higher frequency of viral shedding. These findings reinforce the concept that exposure to CMV play an important role in nonprimary CMV infection in this population,
MOTHER-TO-FETUS TRANSMISSION OF CYTOMEGALOVIRUS
Congenital CMV infection is a result of the mother-to-fetus transmission through the placenta, which can occur any time during pregnancy. Existing estimates indicate that the risk of fetal transmission after primary infection ranges from 14.2% to 52.4% (average of 32.4%) with an increased risk in the third trimester [30]. In contrast, transmissions due to nonprimary infection have been reported in only 1.4% (1.1%–1.7%) of cases, which usually has been taken as evidence for partial protection of intrauterine transmission by maternal immunity. However, although assessments for the risk of transmission after primary maternal infection have been derived from data obtained in relatively small cohorts of women with serologically/virologically proven primary infection during pregnancy, estimates for transmission after nonprimary maternal infection have been generally based on the occurrence of cCMV among women who were seropositive before or at the beginning of gestation [3, 9]. Because it is not certain that all seropositive women experienced reactivation or were reinfected, the available approximations could be underestimating the true risk of intrauterine transmission after a nonprimary maternal infection. Therefore, the exact frequency of CMV nonprimary infection in seroimmune women during pregnancy and the rate of intrauterine transmission in these women are yet to be defined [21].
BIRTH PREVALENCE OF CONGENITAL CYTOMEGALOVIRUS INFECTION AND DISEASE
A realistic estimate of the overall birth prevalence of cCMV infection is approximately 6/1000 live births [3]. It is usually higher (≥7:1000) in low-resource countries where maternal seroprevalence is high (>95%), including South American [31], Asian [27], and African countries [32].
In the past, it was believed that maternal immunity prevented or at least reduced the virulence of fetal infection based on data showing that symptomatic cCMV occurred almost exclusively after primary CMV infection during pregnancy [33, 34]. However, this view has been challenged since the reports from Ahlfors et al [35] in the Swedish population describing the occurrence of congenital CMV symptomatic damaging disease after nonprimary maternal infection. Later, these outcomes were also identified in the US population [36]. Subsequently, other studies confirmed that the frequency and severity of the clinical and laboratory abnormalities in infants with cCMV born to mothers with nonprimary CMV infection are similar to infants born after a primary maternal infection.
Reliable estimates of the birth prevalence of clinically apparent (symptomatic) cCMV after nonprimary maternal infections from studies with adequate sample size are still scarce. In a systematic review, Dollard et al [37] examined data from 15 studies encompassing a total of 117 986 neonates screened for cCMV. Of these, 810 (7:1000) were identified as infected, and 103 (12.7%) were symptomatic, resulting in a birth prevalence of cCMV disease of 0.9 of 1000 (95% CI, 0.7–1.1). Among these, 36.5% were born to mothers from populations with lower income and higher cCMV infection rates, and the proportion of symptomatic cCMV did not differ between these infants (13.4%) and the remaining (11.6%) ones born in more privileged populations with lower cCMV rates. To summarize available data for high seroprevalence populations, Table 1 shows 12 prospective studies that adequately screened (viral or CMV-DNA detection in saliva and/or urine within 3 weeks of birth) neonates in ≥80% CMV seroprevalence populations from different countries. All but 4 studies [26, 38–40] screened more than 1000 neonates. Although the average estimates are similar to the previously mentioned review, it is noticeable that the birth prevalence rates of cCMV infection varied widely (from 3:1000 to 61:1000) as well as symptomatic cCMV birth prevalence rates (from 0 to 14.6:1000). Overall, these data support that symptomatic infection does occur in highly seropositive populations, but they also suggest that factors other than the population seroprevalence might influence the occurrence of symptomatic cCMV disease.
Table 1.
Congenital CMV Infection Birth Prevalence Rates and Symptomatic Disease Estimates in Neonates Born in Highly Seropositive Populations
| Country, Author(s) (Year) [Ref.] | Maternal CMV Seroprevalence (%) | cCMV Infection Birth Prevalence Rate (cCMV/Screened) | Symptomatic cCMV Prevalence Rate (Symptomatic/Screened) | Proportion of Symptomatic cCMV |
|---|---|---|---|---|
| Ivory Coast, Schopfer et al (1978) [32] | 99.7 | 14:1000 (28/2132) | 0:1000 | 0.0% |
| The Gambia, van der Sande et al (2007) [26] | 100.0 | 54:1000 (40/741) | 0:1000 | 0.0% |
| Nigeria, Olusanya et al (2015) [39] | 97.2 | 38:1000 (10/263) | 7.6:1000 | 20.0% (2/10) |
| Israel, Schlesinger et al (2003) [41] | 80.5–85.0 | 7:1000 (14/2000) | 0.5:1000 | 9.0% (1/14) |
| Mexico, Noyola et al (2003) [40] | 91.6 | 9:1000 (5/599) | 0:1000 | 0.0% |
| Japan, Numazaki and Fujikawa (2004) [42] | 85.0 | 3:1000 (37/11 938) | 0.4:1000 | 13.5% (5/37) |
| Japan, Kamada et al (1983) [43] | 93.7 | 5:1000 (11/2070) | 0:1000 | 0.0% |
| USA, Fowler et al (1993) [44] | Low incomea | 12:1000 (215/17 163) | 0.9:1000 | 7.4% (16/215) |
| Brazil, Mussi-Pinhata et al [31, 45, 46] | 96.7 | 8:1000 (189/24 095) | 0.9:1000 | 11.1% (12/121) |
| India, Dar et al (2008) [38] | 99.0 | 21:1000 (9/419) | 2.3:1000 | 11.1% (1/9) |
| China, Zhang et al (2007) [47] | 93.1 | 61:1000 (71/1159) | 14.6:1000 | 23.9% (17/71) |
| China, Wang et al (2017) [27] | 96.2% | 7:1000 (75/10 933) | 0.0:1000 | 0.0% |
Abbreviations: cCMV, congenital cytomegalovirus infection; CMV, cytomegalovirus; Ref., reference.
aNot provided.
SPECTRUM OF THE CONGENITAL CYTOMEGALOVIRUS DISEASE
Congenital CMV infection is classified at birth based on the presence (symptomatic) or absence (asymptomatic) of clinical findings of cCMV disease. The severity of cCMV disease is related to the long-term prognosis and it is used for indication of antiviral treatment [48]. More recently, cCMV disease has been categorized in mild or moderate to severe, which is characterized by multiple manifestations including thrombocytopenia, petechiae, hepatomegaly, splenomegaly, neonatal hepatitis, and neurologic involvement with or without microcephaly [49].
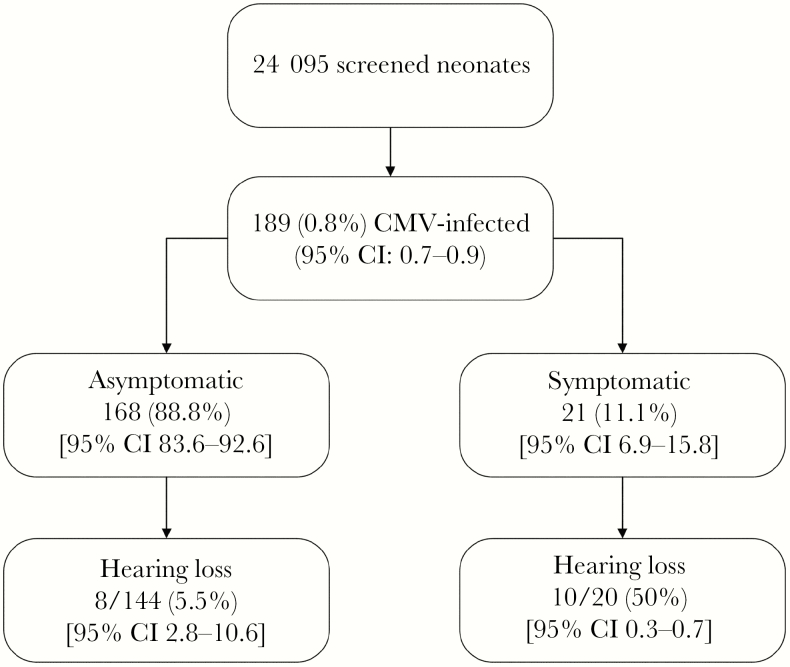
The true frequency of mild or moderate to severe congenital CMV disease detected in the neonatal period in highly seroprevalence populations is unknown. In our experience, after prospectively screening 24 095 neonates in a highly seropositive population (Figure 2) [31, 45, 46], of the 21 neonates with symptomatic congenital infection, most (76%) had moderate or severe disease, whereas the remaining had isolated and mild findings detected after birth. In addition, intrauterine growth restriction (IUGR), which has been infrequently reported and although not usually considered as a CMV-related finding, was associated with cCMV. Intrauterine growth restriction was 2-fold and severe IUGR was 3-fold more likely in infants with cCMV than in the uninfected ones, even after adjusting for maternal age, parity, marital status, and schooling, suggesting that cCMV contributes significantly to the burden of IUGR in these populations.
Figure 2.
Combined data obtained in cohorts of Brazilian neonates screened for congenital cytomegalovirus infection (cCMV) showing neonatal characteristics of infected infants and occurrence of hearing loss in symptomatic and asymptomatic infants with cCMV (data based on results from the references [31, 45, 46]). CI, confidence interval; CMV, cytomegalovirus.
The most common sequela of cCMV is sensorineural hearing loss (SNHL) in both asymptomatic and symptomatic infants, detected at birth or later in childhood. The overall prevalence of cCMV-related SNHL varies from 22% to 65% in newborn infants with symptomatic infection and from 6% to 23% in the asymptomatic ones [50–52]. To define the contribution of the CMV to hearing loss and its characteristics, infected infants identified in our studies were submitted to hearing screening and comprehensive audiological follow-up. Overall, cCMV infection was estimated to be responsible for one third of all cases of permanent hearing loss in children in this population [46]. As shown in Figure 2, similar to other populations of lower or high CMV seroprevalence rates, hearing loss was detected in approximately 10% of the infants with cCMV, with a greater incidence in those symptomatic (55.0%) than in the asymptomatic cCMV infections (5.5%). Among symptomatic infants, hearing loss was not associated with the clinical presentation of the disease because it occurred in 8 of 15 (53.3%) infants with moderate to severe findings and in 2 of 5 (40.0%) of the ones with mild disease. In a subset of these infants, we were able to demonstrate that they were born to mothers with nonprimary CMV infection during gestation. Our data strongly reinforce that permanent hearing loss frequency and severity in offspring of women with nonprimary infections during pregnancy are similar to those reported for infants born to women with primary infection, a finding also previously observed by Ross et al [50].
It has been shown that hearing loss detected early in life can progress or fluctuate during infancy. In addition, there are data supporting that 5% to 38% of both symptomatic and asymptomatic infants with normal hearing tests at birth could develop hearing impairment during follow-up [53, 54]. There are limited data of long-term hearing follow-up in cCMV-infected infants from highly seropositive populations. However, delayed-onset SNHL seems to be an infrequent event in children with asymptomatic cCMV from the studied Brazilian population because none of 116 infants with normal hearing at baseline who were followed-up (49 for 18–48 months, and 67 for ≥48 months) were found to have SNHL.
No long-term studies have evaluated the impact of cCMV on neurodevelopmental outcomes in highly seropositive maternal populations. In general, it is estimated that half of the infants with symptomatic cCMV will develop long-term neurodevelopmental impairment [36, 55, 56]. In a recent report, 2 large population-based studies following children with cCMV up to age 5 or more found that all moderate and severe CMV-related sequelae occurred in the first year of life [56]. The authors highlighted that nonprimary maternal infections were involved in half of the burden of cCMV disease even in these developed countries with low maternal CMV seroprevalence. On the other hand, most of the studies have shown that children with asymptomatic cCMV had similar performance on neurodevelopmental evaluation than uninfected controls [47, 57]. More recently, Lopez et al [58], who studied 91 asymptomatic CMV-infected infants and 42 controls from medium and high socioeconomic backgrounds through 18 years of age, reported that those with normal hearing by age 2 years did not differ in full-scale intelligence, vocabulary, or academic performance scores compared with uninfected children. Considering the parallels of the impact of cCMV on hearing loss in populations with low and high maternal seroprevalences, one could hypothesize that the consequences on neurodevelopmental would be similar in frequency and manifestations, but additional studies are needed.
CONCLUSIONS
Ideally, future studies should explore more definitive ways of defining nonprimary infections in seropositive women either by reactivation or reinfection, identifying epidemiological and immunological characteristics of women who acquire nonprimary infections as well as the risk of transmission of CMV infection to the fetus in these women. These data, taken together with the understanding of to what degree and how maternal immunity could protect the fetus from infection or disease, have the potential to guide appropriate ways of preventing neurodevelopment consequences that can follow this congenital infection. Furthermore, and perhaps less frequently discussed, there is a need for better understanding of the impact of CMV-related hearing loss and neurodevelopmental outcome at older ages in asymptomatic children of seropositive mothers from highly seropositive populations.
Notes
Acknowledgments. We are very grateful to William J. Britt who provided us with a Figure 1 draft.
Supplement sponsorship. This supplement was sponsored by NIAID and NICHD.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Manicklal S, Emery VC, Lazzarotto T, Boppana SB, Gupta RK. The “silent” global burden of congenital cytomegalovirus. Clin Microbiol Rev 2013; 26:86–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yamamoto AY, Castellucci RA, Aragon DC, Mussi-Pinhata MM. Early high CMV seroprevalence in pregnant women from a population with a high rate of congenital infection. Epidemiol Infect 2013; 141:2187–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol 2007; 17:253–76. [DOI] [PubMed] [Google Scholar]
- 4. Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol 2010; 20:202–13. [DOI] [PubMed] [Google Scholar]
- 5. N’Diaye DS, Yazdanpanah Y, Krivine A, et al. Predictive factors of cytomegalovirus seropositivity among pregnant women in Paris, France. PLoS One 2014; 9:e89857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zuhair M, Smit GS, Wallis G, et al. Estimation of the worldwide seroprevalence of cytomegalovirus: a systematic review and meta-analysis. Rev Med Virol 2019; e2034. [DOI] [PubMed] [Google Scholar]
- 7. de Vries JJ, van Zwet EW, Dekker FW, Kroes AC, Verkerk PH, Vossen AC. The apparent paradox of maternal seropositivity as a risk factor for congenital cytomegalovirus infection: a population-based prediction model. Rev Med Virol 2013; 23:241–9. [DOI] [PubMed] [Google Scholar]
- 8. Wang C, Zhang X, Bialek S, Cannon MJ. Attribution of congenital cytomegalovirus infection to primary versus non-primary maternal infection. Clin Infect Dis 2011; 52:e11–3. [DOI] [PubMed] [Google Scholar]
- 9. Leruez-Ville M, Magny JF, Couderc S, et al. Risk factors for congenital cytomegalovirus infection following primary and nonprimary maternal infection: a prospective neonatal screening study using polymerase chain reaction in saliva. Clin Infect Dis 2017; 65:398–404. [DOI] [PubMed] [Google Scholar]
- 10. Mussi-Pinhata MM, Yamamoto AY, Aragon DC, et al. Seroconversion for cytomegalovirus infection during pregnancy and fetal infection in a highly seropositive population: “The BraCHS Study”. J Infect Dis 2018; 218:1200–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. United Nations. DESA/Population Division. World Population Prospects, 2019. Available at: https://population.un.org/wpp/DataQuery/. Accessed 23 August 2019. [Google Scholar]
- 12. Britt W. Controversies in the natural history of congenital human cytomegalovirus infection: the paradox of infection and disease in offspring of women with immunity prior to pregnancy. Med Microbiol Immunol 2015; 204:263–71. [DOI] [PubMed] [Google Scholar]
- 13. Huang ES, Alford CA, Reynolds DW, Stagno S, Pass RF. Molecular epidemiology of cytomegalovirus infections in women and their infants. N Engl J Med 1980; 303:958–62. [DOI] [PubMed] [Google Scholar]
- 14. Ross SA, Novak Z, Pati S, et al. Mixed infection and strain diversity in congenital cytomegalovirus infection. J Infect Dis 2011; 204:1003–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pokalyuk C, Renzette N, Irwin KK, et al. Characterizing human cytomegalovirus reinfection in congenitally infected infants: an evolutionary perspective. Mol Ecol 2017; 26:1980–90. [DOI] [PubMed] [Google Scholar]
- 16. Boppana SB, Rivera LB, Fowler KB, Mach M, Britt WJ. Intrauterine transmission of cytomegalovirus to infants of women with preconceptional immunity. N Engl J Med 2001; 344:1366–71. [DOI] [PubMed] [Google Scholar]
- 17. Ross SA, Arora N, Novak Z, Fowler KB, Britt WJ, Boppana SB. Cytomegalovirus reinfections in healthy seroimmune women. J Infect Dis 2010; 201:386–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yamamoto AY, Mussi-Pinhata MM, Boppana SB, et al. Human cytomegalovirus reinfection is associated with intrauterine transmission in a highly cytomegalovirus-immune maternal population. Am J Obstet Gynecol 2010; 202:297.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stagno S, Cloud G, Pass RF, Britt WJ, Alford CA. Factors associated with primary cytomegalovirus infection during pregnancy. J Med Virol 1984; 13:347–53. [DOI] [PubMed] [Google Scholar]
- 20. Hyde TB, Schmid DS, Cannon MJ. Cytomegalovirus seroconversion rates and risk factors: implications for congenital CMV. Rev Med Virol 2010; 20:311–26. [DOI] [PubMed] [Google Scholar]
- 21. Fowler KB, Boppana SB. Congenital cytomegalovirus infection. Semin Perinatol 2018; 42:149–54. [DOI] [PubMed] [Google Scholar]
- 22. Prince HE, Lape-Nixon M. Role of cytomegalovirus (CMV) IgG avidity testing in diagnosing primary CMV infection during pregnancy. Clin Vaccine Immunol 2014; 21:1377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fowler KB, Pass RF. Risk factors for congenital cytomegalovirus infection in the offspring of young women: exposure to young children and recent onset of sexual activity. Pediatrics 2006; 118:e286–92. [DOI] [PubMed] [Google Scholar]
- 24. Fowler KB, Ross SA, Shimamura M, et al. Racial and ethnic differences in the prevalence of congenital cytomegalovirus infection. J Pediatr 2018; 200:196–201 e1. [DOI] [PubMed] [Google Scholar]
- 25. Lanzieri TM, Dollard SC, Bialek SR, Grosse SD. Systematic review of the birth prevalence of congenital cytomegalovirus infection in developing countries. Int J Infect Dis 2014; 22:44–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van der Sande MA, Kaye S, Miles DJ, et al. Risk factors for and clinical outcome of congenital cytomegalovirus infection in a peri-urban West-African birth cohort. PLoS One 2007; 2:e492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang S, Wang T, Zhang W, et al. Cohort study on maternal cytomegalovirus seroprevalence and prevalence and clinical manifestations of congenital infection in China. Medicine (Baltimore) 2017; 96:e6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Noyola DE, Matienzo-Serment L, Rodriguez-Vidal SO, Ochoa-Perez UR, Pina-Granja JM, Garcia-Sepulveda CA. [Congenital cytomegalovirus infection in newborn infants from the state of San Luis Potosi, Mexico]. Salud Publica Mex 2011; 53:513–5. [DOI] [PubMed] [Google Scholar]
- 29. Barbosa NG, Yamamoto AY, Duarte G, et al. Cytomegalovirus shedding in seropositive pregnant women from a high-seroprevalence population: the Brazilian Cytomegalovirus Hearing and Maternal Secondary Infection Study. Clin Infect Dis 2018; 67:743–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Enders G, Daiminger A, Bäder U, Exler S, Enders M. Intrauterine transmission and clinical outcome of 248 pregnancies with primary cytomegalovirus infection in relation to gestational age. J Clin Virol 2011; 52:244–6. [DOI] [PubMed] [Google Scholar]
- 31. Mussi-Pinhata MM, Yamamoto AY, Moura Brito RM, et al. Birth prevalence and natural history of congenital cytomegalovirus infection in a highly seroimmune population. Clin Infect Dis 2009; 49:522–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schopfer K, Lauber E, Krech U. Congenital cytomegalovirus infection in newborn infants of mothers infected before pregnancy. Arch Dis Child 1978; 53:536–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stagno S, Pass RF, Dworsky ME, et al. Congenital cytomegalovirus infection: the relative importance of primary and recurrent maternal infection. N Engl J Med 1982; 306:945–9. [DOI] [PubMed] [Google Scholar]
- 34. Fowler KB, Stagno S, Pass RF, Britt WJ, Boll TJ, Alford CA. The outcome of congenital cytomegalovirus infection in relation to maternal antibody status. N Engl J Med 1992; 326:663–7. [DOI] [PubMed] [Google Scholar]
- 35. Ahlfors K, Ivarsson SA, Harris S, et al. Congenital cytomegalovirus infection and disease in Sweden and the relative importance of primary and secondary maternal infections. Preliminary findings from a prospective study. Scand J Infect Dis 1984; 16:129–37. [DOI] [PubMed] [Google Scholar]
- 36. Boppana SB, Fowler KB, Britt WJ, Stagno S, Pass RF. Symptomatic congenital cytomegalovirus infection in infants born to mothers with preexisting immunity to cytomegalovirus. Pediatrics 1999; 104:55–60. [DOI] [PubMed] [Google Scholar]
- 37. Dollard SC, Grosse SD, Ross DS. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol 2007; 17:355–63. [DOI] [PubMed] [Google Scholar]
- 38. Dar L, Pati SK, Patro AR, et al. Congenital cytomegalovirus infection in a highly seropositive semi-urban population in India. Pediatr Infect Dis J 2008; 27:841–3. [DOI] [PubMed] [Google Scholar]
- 39. Olusanya BO, Slusher TM, Boppana SB. Prevalence of congenital cytomegalovirus infection in Nigeria: a pilot study. Pediatr Infect Dis J 2015; 34:322–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Noyola DE, Mejía-Elizondo AR, Canseco-Lima JM, Allende-Carrera R, Hernánsez-Salinas AE, Ramírez-Zacarías JL. Congenital cytomegalovirus infection in San Luis Potosi, Mexico. Pediatr Infect Dis J 2003; 22:89–90. [DOI] [PubMed] [Google Scholar]
- 41. Schlesinger Y, Halle D, Eidelman AI, et al. Urine polymerase chain reaction as a screening tool for the detection of congenital cytomegalovirus infection. Arch Dis Child Fetal Neonatal Ed 2003; 88:F371–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Numazaki K, Fujikawa T. Chronological changes of incidence and prognosis of children with asymptomatic congenital cytomegalovirus infection in Sapporo, Japan. BMC Infect Dis 2004; 4:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kamada M, Komori A, Chiba S, Nakao T. A prospective study of congenital cytomegalovirus infection in Japan. Scand J Infect Dis 1983; 15:227–32. [DOI] [PubMed] [Google Scholar]
- 44. Fowler KB, Stagno S, Pass RF. Maternal age and congenital cytomegalovirus infection: screening of two diverse newborn populations, 1980–1990. J Infect Dis 1993; 168:552–6. [DOI] [PubMed] [Google Scholar]
- 45. Yamamoto AY, Mussi-Pinhata MM, Isaac Mde L, et al. Congenital cytomegalovirus infection as a cause of sensorineural hearing loss in a highly immune population. Pediatr Infect Dis J 2011; 30:1043–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yamamoto AY, Anastasio AR, Massuda ET, et al. Contribution of congenital cytomegalovirus (cCMV) to permanent hearing loss in a highly seropositive population: “The BraCHS study” [Epub ahead of print May 17, 2019]. Clin Infect Dis 2019; pii:ciz413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang XW, Li F, Yu XW, Shi XW, Shi J, Zhang JP. Physical and intellectual development in children with asymptomatic congenital cytomegalovirus infection: a longitudinal cohort study in Qinba mountain area, China. J Clin Virol 2007; 40:180–5. [DOI] [PubMed] [Google Scholar]
- 48. Kimberlin DW, Jester PM, Sanchez PJ, et al. Valganciclovir for symptomatic congenital cytomegalovirus disease. N Engl J Med 2015; 372:933–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rawlinson WD, Boppana SB, Fowler KB, et al. Congenital cytomegalovirus infection in pregnancy and the neonate: consensus recommendations for prevention, diagnosis, and therapy. Lancet Infect Dis 2017; 17:e177–88. [DOI] [PubMed] [Google Scholar]
- 50. Ross SA, Fowler KB, Ashrith G, et al. Hearing loss in children with congenital cytomegalovirus infection born to mothers with preexisting immunity. J Pediatr 2006; 148:332–6. [DOI] [PubMed] [Google Scholar]
- 51. Goderis J, De Leenheer E, Smets K, Van Hoecke H, Keymeulen A, Dhooge I. Hearing loss and congenital CMV infection: a systematic review. Pediatrics 2014; 134:972–82. [DOI] [PubMed] [Google Scholar]
- 52. Fowler KB. Congenital cytomegalovirus infection: audiologic outcome. Clin Infect Dis 2013; 57(Suppl 4):S182–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dahle AJ, Fowler KB, Wright JD, Boppana SB, Britt WJ, Pass RF. Longitudinal investigation of hearing disorders in children with congenital cytomegalovirus. J Am Acad Audiol 2000; 11:283–90. [PubMed] [Google Scholar]
- 54. Foulon I, Naessens A, Foulon W, Casteels A, Gordts F. A 10-year prospective study of sensorineural hearing loss in children with congenital cytomegalovirus infection. J Pediatr 2008; 153:84–8. [DOI] [PubMed] [Google Scholar]
- 55. Nishida K, Morioka I, Nakamachi Y, et al. Neurological outcomes in symptomatic congenital cytomegalovirus-infected infants after introduction of newborn urine screening and antiviral treatment. Brain Dev 2016; 38:209–16. [DOI] [PubMed] [Google Scholar]
- 56. Townsend CL, Forsgren M, Ahlfors K, Ivarsson SA, Tookey PA, Peckham CS. Long-term outcomes of congenital cytomegalovirus infection in Sweden and the United Kingdom. Clin Infect Dis 2013; 56:1232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Conboy TJ, Pass RF, Stagno S, et al. Intellectual development in school-aged children with asymptomatic congenital cytomegalovirus infection. Pediatrics 1986; 77:801–6. [PubMed] [Google Scholar]
- 58. Lopez AS, Lanzieri TM, Claussen AH, et al. Intelligence and academic achievement with asymptomatic congenital cytomegalovirus infection. Pediatrics 2017; 140:e20171517. [DOI] [PMC free article] [PubMed] [Google Scholar]