Abstract
This review focuses on recent advances in the field of cytomegalovirus (CMV). The 2 main strategies for CMV prevention are prophylaxis and preemptive therapy. Prophylaxis effectively prevents CMV infection after solid organ transplantation (SOT) but is associated with high rates of neutropenia and delayed-onset postprophylaxis disease. In contrast, preemptive therapy has the advantage of leading to lower rates of CMV disease and robust humoral and T-cell responses. It is widely used in hematopoietic cell transplant recipients but is infrequently utilized after SOT due to logistical considerations, though these may be overcome by novel methods to monitor CMV viremia using self-testing platforms. We review recent developments in CMV immune monitoring, vaccination, and monoclonal antibodies, all of which have the potential to become part of integrated strategies that rely on viral load monitoring and immune responses. We discuss novel therapeutic options for drug-resistant or refractory CMV infection, including maribavir, letermovir, and adoptive T-cell transfer. We also explore the role of donor factors in transmitting CMV after SOT. Finally, we propose a framework with which to approach CMV prevention in the foreseeable future.
Keywords: cytomegalovirus, transplant, prophylaxis, preemptive therapy, resistant CMV
Cytomegalovirus (CMV) is among the most significant pathogens after solid organ transplantation (SOT) and hematopoietic cell transplantation (HCT), particularly in seronegative recipients of seropositive donors (D+/R–) and seropositive recipients (R+), respectively. These patients are defined as “high risk” for CMV infection. CMV-seropositive SOT recipients possess preexisting CMV-specific cell-mediated immunity and are thus at an intermediate risk for CMV infection, with D+/R+ recipients being at a higher risk than D–/R+ recipients due to the potential for superinfection with donor-derived virus. Despite these classification systems, an individual patient’s risk of CMV infection is nuanced and multifaceted, due to a complex interplay of not only classic CMV risk factors but also elements of the innate and adaptive immune system (eg, Toll-like receptor and interleukin gene polymorphisms, complement levels, natural killer cell activity, T-cell kinetics, and other variables) [1].
Without antiviral prophylaxis, CMV infection develops in most high-risk SOT recipients and can lead to viremia, disease, and end-organ damage. Its immunomodulatory effects may precipitate graft rejection and predispose patients to other opportunistic infections. While antiviral prophylaxis with ganciclovir or valganciclovir has been the standard of care for high-risk SOT recipients for decades, these drugs are associated with increased cost, neutropenia, and high rates of postprophylaxis disease. Preemptive therapy, on the other hand, which involves targeted screening and initiation of antivirals upon the detection of viremia, has been successfully used in HCT recipients for years and avoids the myelosuppressive toxicities of ganciclovir/valganciclovir prophylaxis. Despite long being recognized as a major clinical problem, there are to this day no safe or effective antivirals for the treatment of ganciclovir-resistant or refractory CMV. Finally, little work has been done to elucidate the biology of CMV in the premortem donor and the transmission of CMV from organ donor to recipient.
In this review, we summarize the topical findings that have emerged in the past few years, with a focus on CMV prevention, management of drug-resistant or refractory disease, and the variables surrounding the role of the donor in primary CMV infection. Throughout this review, CMV infection is defined in accordance with international guidelines [2], which is the presence of CMV replication in tissue, blood, or other bodily fluids regardless of symptomatology. This is distinct from “latent CMV.” CMV disease is defined as CMV infection accompanied by clinical signs and symptoms, while asymptomatic CMV infection is defined as CMV replication without signs and symptoms of disease.
CMV PREVENTION
Overview of Prophylaxis Versus Preemptive Therapy
The 2 strategies for the prevention of CMV in transplant recipients are antiviral prophylaxis and preemptive therapy, which are each considered the preferred prevention approaches after SOT and HCT, respectively (Table 1). Each strategy has its own specific advantages and disadvantages. Prophylaxis after SOT has been shown to reduce not only CMV infection and disease while patients are receiving antiviral therapy, but also graft loss, mortality, and opportunistic infections [2]. All CMV D+/R– or R+ SOT recipients being managed with prophylaxis typically receive oral valganciclovir for a defined duration, usually 3–6 months, but up to 12 months after lung transplantation [1, 3, 4]. In contrast, the overall benefit of prophylaxis after HCT has been difficult to assess [5]. Thus, HCT recipients have traditionally been managed with preemptive therapy, whereby they are monitored for the development of asymptomatic CMV replication and are only given antivirals upon the detection of viremia, with the goal of preventing progression to disease. The long-term benefits of preemptive therapy are postulated to occur as a result of the development of CMV-specific immunity due to controlled viremia [1, 2]. As discussed below, however, there are emerging data demonstrating the benefits of preemptive therapy even after SOT, though this approach is not currently standard of care at most transplant centers, in part due to logistical considerations. A hybrid strategy whereby initial antiviral prophylaxis is followed by surveillance for CMV viremia with preemptive therapy is often used in SOT recipients [6], though its benefit remains unproven. To date, the optimal approach for the prevention of CMV remains a controversial issue.
Table 1.
Key Differences Between Prophylaxis Versus Preemptive Therapy for Cytomegalovirus Prevention Among Solid Organ Versus Hematopoietic Cell Transplant Recipients
| Transplant Type | Solid Organ Transplantation | Hematopoietic Cell Transplantation | ||
|---|---|---|---|---|
| Prophylaxis | Preemptive | Prophylaxis | Preemptive | |
| Clinical practice | Commonplace | Uncommon | Uncommon | Commonplace |
| Positives | Large evidence base Ease of coordination Prevents CMV infection/disease and indirect effects of CMV (graft loss, opportunistic infections) | Simulates natural CMV immunity Prevents delayed-onset CMV Less neutropenia Possibly more cost-effective | Reduction in mortality among CMV-seropositive recipients | Large evidence base Limited toxicity Acceleration of immune constitution Possible protective effect on leukemia relapse |
| Negatives | Postprophylaxis disease High cost Neutropenia Use of antivirals in some patients who will not develop CMV infection | Logistical difficulties Small evidence base Unknown impact on indirect effects of CMV Viral thresholds not defined Unknown frequency of testing Rapid doubling time of CMV viral loads in some patients | Exacerbation of cytopenias with ganciclovir/valganciclovir Poor efficacy of acyclovir/valacyclovir Low bioavailability of oral antivirals in persons with GVHD Use of antivirals in some patients who will not develop CMV infection | Logistical difficulties Rapid doubling time of CMV viral loads in some patients |
Abbreviations: CMV, cytomegalovirus; GVHD, graft-vs-host disease.
Prophylaxis, Delayed-Onset CMV Disease, and Neutropenia
Prophylaxis after SOT prevents CMV infection during the period when patients are receiving antivirals [3, 4], but is associated with high rates of postprophylaxis delayed-onset CMV disease, with resultant increased mortality during the first year after transplantation [7]. Extending the duration of prophylaxis, while adopted by many transplant centers, does not curtail the risk of postprophylaxis disease and leads to significant myelotoxicity [8, 9]. Many transplant centers monitor patients for the development of CMV viremia for a fixed duration upon discontinuation of prophylaxis. While intuitively appealing, this practice only detects a small proportion of patients with CMV infection, as most either develop infection after the monitoring period has ended, clear their viremia spontaneously, or progress to CMV disease prior to initiation of antivirals because of rapid viral load doubling [10]. Valganciclovir prophylaxis is also associated with high rates of neutropenia after SOT. In a study of 304 liver transplant recipients, neutropenia developed in 40% of patients receiving valganciclovir prophylaxis vs 16% of those not on prophylaxis and was an independent predictor of overall mortality [11]. To reduce the risk of neutropenia, many centers use low-dose valganciclovir prophylaxis, but this unfortunately leads to breakthrough viremia, particularly due to ganciclovir-resistant CMV [12].
A recent meta-analysis evaluated CMV prophylaxis in HCT recipients [5], although this approach remains infrequently used due to the myelotoxicity of ganciclovir/valganciclovir. In addition, while there are randomized trial data demonstrating the efficacy of acyclovir and valacyclovir for CMV prophylaxis after HCT [13–16], the use of these drugs is not widespread due to the need for intravenous administration of acyclovir and extremely high doses of valacyclovir, as well as the modest anti-CMV activity of these agents [17]. However, since recipient CMV seropositivity is associated with poor overall outcomes after HCT [18] and detectable plasma viremia is associated with overall mortality in seropositive HCT recipients [19], prophylaxis with a nontoxic drug or prevention with an effective vaccine may be beneficial.
Letermovir, a novel terminase complex inhibitor with no hematological toxicities, has been shown to reduce clinically significant CMV infection after HCT and is licensed for CMV prophylaxis among high-risk HCT recipients [20]. Limited clinical experience exists with the use of letermovir in the SOT setting, and resistance may develop earlier than with valganciclovir or foscarnet [21]. While it may not reduce the risk of postprophylaxis disease after SOT, it is not myelosuppressive and thus may be an option for patients with dose-limiting toxicity due to valganciclovir. A clinical trial of letermovir prophylaxis among CMV D+/R– kidney transplant recipients is under way (ClinicalTrials.gov identifier NCT03443869) [16].
Preemptive Therapy
CMV viremia is inherent with preemptive therapy but occurs in a carefully controlled manner, which allows for the rapid initiation of antivirals. The major benefit of preemptive therapy compared to prophylaxis after SOT is the lower frequency of delayed-onset CMV disease [22, 23]. The biological basis of this finding is that controlled CMV viremia simulates immunity as a natural CMV vaccine, resulting in greater antibody neutralization and CD8+ T-cell responses compared with prophylaxis [24]. Most trials comparing prophylaxis and preemptive therapy after SOT have focused on kidney transplant recipients and comprised a small number of D+/R– patients [22, 25–27]. More recently, a randomized trial in 205 high-risk D+/R– liver transplant recipients documented that preemptive therapy compared to prophylaxis for 100 days was efficacious and cost-effective for the prevention of CMV disease within 12 months posttransplant (NCT01552369) [28].
Nonetheless, published guidelines have generally recommended prophylaxis as the CMV prevention modality of choice after SOT [29], in part because of the large evidence base supporting the use of prophylaxis, its beneficial impact on the indirect effects of CMV, and the logistical difficulties of implementing a preemptive protocol at transplant centers. In addition, the frequency and duration of CMV monitoring, the viral load thresholds at which to start antivirals when viremia develops, and the optimal duration of antiviral therapy to treat controlled asymptomatic viremia have not been rigorously defined after SOT.
HCT recipients, in contrast, have been managed using preemptive therapy for years, allowing for the continuous optimization of this approach in HCT patients. Indeed, results in the standard-of-care preemptive therapy groups from recent randomized trials suggest excellent prevention of CMV disease by using this strategy (Table 2). However, while late monitoring after HCT is recommended for high-risk patients, adherence to weekly monitoring schedules is poor, and late disease continues to occur [19]. Nonetheless, in addition to the benefits of preemptive therapy in limiting drug toxicity and possibly accelerating immune reconstitution, the permissive CMV reactivation that occurs in this context may also have a protective effect on leukemic relapse [30, 31]. However, the issue remains controversial as the data are inconsistent, and the effect of CMV reactivation on nonrelapse mortality appears to outweigh any possible antileukemic effect. Larger analyses assessing the effect of preemptive therapy on all outcomes after HCT will be needed to answer this question.
Table 2.
Cytomegalovirus Disease Incidence Rates in Standard-of-Care Groups in Recent Randomized Controlled Multicenter Trials Using Preemptive Polymerase Chain Reaction–Guided Therapy in Hematopoietic Cell Transplantation
| Author [Reference] | Year | No. of Patients | Period Posttransplantation | CMV Disease Incidence, Early/Late |
|---|---|---|---|---|
| Marty et al [32] | 2013 | 227 | Early/late | 2.4%/4.8% |
| Kharfan-Dabaja et al [33] | 2012 | 34 | Day 365 | 8.8% |
| Chemaly et al [34] | 2014 | 33 | Early | 0% |
| Boeckh et al [35] | 2015 | 89 | Late | 2.2% |
| Marty et al [20] | 2017 | 170 | Early/late | 1.2%/1.8% |
| Marty et al [36] | 2019 | 149 | Early/late | 2.0%/3.4% |
Abbreviation: CMV, cytomegalovirus.
Novel Approaches to Diagnose CMV Viremia
CMV surveillance in the late period after SOT and HCT has been advocated as part of extended monitoring or hybrid strategies of prophylaxis and preemptive therapy. However, adherence to the optimal weekly monitoring schedule has been a formidable obstacle due to logistical challenges in the late posttransplant period, preventing many patients from having regular office-based blood draws. The dried blood spot (DBS) technology has been used to diagnose CMV by polymerase chain reaction in the congenital CMV setting and has recently been adopted to design a self-testing platform, which accurately quantitates the CMV viral load in finger stick–collected DBSs [37]. A multicenter randomized controlled trial is currently ongoing in HCT recipients at high risk for late CMV complications to determine whether a mobile device–assisted home-based DBS self-testing method will result in higher adherence rates for late CMV surveillance (NCT03910478) [38].
Immune Monitoring
Monitoring of CMV-specific immunity has emerged as a promising new modality that may be able to shift the field of CMV prevention toward the paradigm of precision medicine. The largest study to date of the clinical utility of CMV immune monitoring comprised 537 kidney transplant recipients (260 D+/R– and 277 R+) from 43 international centers, who were enrolled either pre- or posttransplantation and prospectively followed for 12 months after transplantation. Antiviral prophylaxis was administered as standard of care (generally 6 vs 3 months for the D+/R– and R+ groups, respectively), and all patients had a T-SPOT.CMV assay checked within 5 days of completing antiviral prophylaxis and at 1, 2, 3, 4, and 6 months after prophylaxis. The assay measured interferon-γ release from CD4+ and CD8+ cells in response to CMV antigen stimulation. Among the 368 patients who were eligible for analysis, the presence of a positive cell-mediated immune assay at the end of prophylaxis predicted freedom from CMV events [39]. In contrast, patients with low levels of CMV cell-mediated immunity were more likely to experience CMV infection and disease. Additionally, pretransplant cell-mediated immunity was a predictor of future CMV events, whereas immunosuppression was not. However, these differences were most pronounced among CMV R+ kidney transplant recipients and not the “high risk” CMV D+/R– patients, a minority of whom actually developed CMV-specific immunity by the end of antiviral prophylaxis. Thus, while the investigators were able to find a high negative predictive value for CMV infection among the few CMV D+/R– who developed CMV-specific cell-mediated immunity, the performance characteristics of this test were overall extremely poor in this cohort. Nonetheless, these data suggest that such assays performed in both the pre- and posttransplant settings may be able to risk-stratify patients, particularly those who are CMV R+.
It remains to be determined how the use of CMV immune monitoring will be incorporated into clinical practice. Among the challenges that must be overcome are the lack of routine availability, high cost, slow turnaround time, and lack of standardization due to the multitude of assays available. Indeed, several commercial tests exist that measure interferon-γ production by CD4+ and/or CD8+ T cells in response to stimulation with CMV-specific antigens or peptides (eg, pp65 and intermediate-early 1 [IE-1] antigen) [6, 39, 40]. However, other markers may also accurately predict CMV-specific immunity, such as the T-box transcription factor (“T-bet”) or even complex polyfunctional T-cell and cytokine signatures [41, 42]. Furthermore, none of these platforms have incorporated the assessment of humoral immunity, which is important for the control of primary CMV infection and may also be able to predict postprophylaxis CMV disease risk [24]. In the current era, these technologies may be used, but clinicians will need to interpret their results with caution.
Monoclonal Antibodies
While the field of CMV prevention and immunity has largely focused on T-cell responses, neutralizing antibodies may also play a role in controlling CMV, particularly primary infection as previously mentioned. This has led to interest in the study of monoclonal antibodies as a method of CMV prevention. Unlike polyclonal antibodies (such as intravenous immunoglobulin), which were once commonly used for prophylaxis [43], monoclonal antibodies are more specific and less toxic, and may allow for the administration of higher doses of drug. A recent phase 2 trial of D+/R– kidney transplant recipients demonstrated that RG7667, a combination of 2 anti-CMV monoclonal antibodies that inhibit entry of CMV into host cells, reduced the proportion of patients with CMV viremia within 24 weeks (but not 12 weeks) posttransplantation, significantly delayed the time to CMV viremia, and reduced the incidence of CMV disease [44]. Notably, although RG7667 did not eliminate the need for antivirals, the use of preemptive valganciclovir was lower in the RG7667 group compared to the placebo group, suggesting that its role may be as an adjunct to preemptive therapy among high-risk SOT recipients.
Vaccines
An ideal CMV vaccine should be able to prevent or modulate CMV replication and disease. Unfortunately, trials of CMV vaccines have yielded conflicting results [45]. A study from the 1980s of a live attenuated vaccine given to kidney transplant candidates found no reduction in the incidence of CMV infection, though infections were milder in patients who received the vaccine [46]. A proof-of-concept phase 2 trial of kidney and liver transplant candidates found that a CMV glycoprotein B (gB) vaccine with an MF59 adjuvant resulted in increased levels of gB antibody titers, with shorter duration of viremia and less ganciclovir requirement among vaccinated individuals [47]. In contrast, another phase 2 trial of a CMV plasmid vaccine including gB and pp65 did not prevent CMV infection in D+/R– kidney transplant recipients, but this was thought to be due to its administration 30 days after transplantation [48]. A trial of a bivalent recombinant RNA viral vector subunit CMV vaccine that expresses pp65 and a truncated isoform of gB (dubbed HB101) against CMV infection among D+/R– living-donor kidney transplant candidates (administered prior to transplant) is currently recruiting patients (NCT03629080) [45].
CMV vaccines have also been evaluated in seropositive HCT recipients. Preliminary results of a phase 3 trial using the gB/pp65 DNA vaccine showed a failure to prevent CMV disease or mortality [49]. A pp65 peptide vaccine showed promising results in a phase 1 trial [50], and recently reported results using a modified vaccinia Ankara peptide vaccine also showed a reduction in CMV viral loads in an early-phase clinical trial [51].
ADVANCES IN CMV TREATMENT
New Drugs for Refractory and Resistant CMV Infection
Ganciclovir-resistant CMV infection remains associated with poor outcomes, due in part to the significant toxicities of foscarnet and cidofovir [1, 52]. Similarly, outcomes of refractory CMV infection, defined as progressive CMV disease without the detection of UL97 or UL54 resistance mutations, remain suboptimal [1]. However, drug development for these indications has stalled. A phase 2 study of maribavir, a direct UL97 inhibitor, demonstrated efficacy against refractory or resistant CMV infections in transplant recipients [53], and a phase 3 trial is currently enrolling patients (NCT02931539) [50]. Unfortunately, there are currently no plans to further develop brincidofovir (an oral lipid conjugate form of cidofovir with no nephrotoxicity) due to its failure to meet the primary endpoints in prophylaxis studies of HCT recipients [1].
Until the ongoing maribavir trial is completed, few good options exist for the management of resistant and refractory CMV infection [54]. One currently available consideration is the off-label use of letermovir. This is an appealing drug because of its favorable side-effect profile, its availability in both oral and intravenous formulations, and its activity against CMV strains with UL97 and UL54 mutations. Unlike most other anti-CMV antivirals, it has multiple CYP system-mediated interactions [20]. However, reports of its use for the treatment (as opposed to prevention) of CMV infection in both SOT and HCT remain sparse, and the treatment dose remains undefined.
In a case series of 4 lung transplant recipients and 1 HCT recipient receiving letermovir, 4 of 5 patients experienced reductions in CMV viremia, but 1 patient experienced a rapid increase in viral load [55]. The latter treatment failure was thought to be due to the use of the lower 240-mg dose of letermovir as opposed to the standard 480-mg dose recommended for prophylaxis. Only 2 patients received letermovir monotherapy, with the rest receiving combination therapy with ganciclovir or foscarnet. Another case series of 2 lung and 2 heart transplant recipients with CMV retinitis showed that all patients had funduscopic improvement on letermovir, though 3 of them received concurrent intravitreal medications [56]. In addition, despite the fact that doses of 720–960 mg were used, 2 patients had persistent low-level viremia, and 1 had high-grade viremia. Most ominously, genotyping confirmed the presence of treatment-related UL56 resistance mutations, which have been shown to rapidly develop during serial viral passage experiments in vitro [57]. Clearly, large clinical trials evaluating the efficacy of dose-optimized letermovir, possibly in combination with other drugs, for the treatment of resistant and refractory CMV infection must be conducted.
Adoptive T-Cell Transfer
The use of adoptive CMV-specific T-cell therapy is a potential alternative to classic antiviral therapy for patients with refractory or resistant CMV infection. There are studies to support the use of this treatment modality among HCT recipients with viral infections [58, 59]. However, data in SOT recipients are sparse. In 2 CMV D+/R– lung transplant recipients with ganciclovir-resistant CMV disease, adoptive T-cell therapy effectively cured the infection, provided long-term CMV-specific immunity, and resulted in no untoward consequences to the allograft [60, 61]. More recently, outcomes were reported for 13 SOT recipients (8 D+/R–, 3 D+/R+, and 2 D–/R–) with drug-resistant or refractory CMV disease who prospectively received expanded autologous CMV-specific T cells [38]. Eleven of these 13 patients responded to therapy, with resolution or reduction of viremia, improvement in end-organ disease, and discontinuation or reduced use of antivirals. In addition, 4 patients had an increase in CMV-specific T-cell immunity, even after cessation of T-cell therapy.
There are challenges that must be overcome before such technologies can become sustainable for mainstream use, particularly the issues of limited donor availability (autologous vs allogeneic human leukocyte antigen–matched donors) and prolonged product generation time. The advent of third-party virus-specific T-cell banks for “off the shelf” administration of products is a promising new development in the field and will result in the on-demand availability of T-cell products [58]. This treatment modality will need to be explored in future studies.
ROLE OF DONOR IN TRANSMITTING CMV AFTER SOT
Classical risk factors for CMV, such as D+/R– serostatus, the use of T-cell–depleting induction, net state of immunosuppression, lung or small intestinal transplantation, and factors related to innate/adaptive immunity have mostly focused on the transplant recipient [2]. In contrast, little attention has been given to the role of the donor in transmitting CMV and to the initial steps that lead to primary infection. Even among CMV D+/R– (high-risk) SOT recipients, CMV transmission is not universal, though donor characteristics that promote primary infection are largely unknown [62]. Data from the 1980s suggest that the first step for primary CMV infection after transplantation is the reactivation of latent donor virus found in small numbers of donor cells, which occurs due to antigenic stimulation or immunosuppression. These donor viruses subsequently infect activated host lymphocytes that have already infiltrated the allograft as part of the host-vs-graft response, which in turn disseminate throughout the recipient [63]. Alternatively, primary CMV infection may occur once CMV-infected donor lymphocytes or monocytes in the graft are activated, express the virus, then spread into the bloodstream and infect circulating recipient mononuclear cells.
More recently, it has become apparent that CMV transmission after SOT is a complex and dynamic process, with multiple CMV strains being transmitted from donors to recipients, and with the simultaneous occurrence of both donor-derived CMV viremia and reactivation of latent CMV infection among R+ recipients in some instances [64]. The time required for primary donor-derived infection to occur once the organ has been transplanted is unknown, though in 1 study, CMV was transmitted from a seropositive liver allograft to a seronegative recipient even though the seropositive organ was in situ for only 28 hours, as the patient underwent an allograft hepatectomy and subsequent retransplant from a CMV-seronegative donor [65]. Older donors may also be more likely to transmit CMV. For instance, CMV was detected at greater frequency in blood donated by individuals >60 years of age compared with that donated by younger individuals [66]. Additionally, repeated episodes of CMV reactivation may occur in older adults, as evidenced by the presence of increased anti-CMV antibody titers and detection of CMV in the urine of 91% of elderly individuals [62].
Overall, transmission from CMV-seropositive donors is likely influenced by the complex conditions that influence CMV latency in deceased donors, persistent or intermittent free viral DNAemia in the donor, an unknown critical viral inoculum of CMV in the allograft, and the function of CMV-specific T cells in the allograft, which may be impaired around the time of death. However, the kinetics of CMV and of anti-CMV T cells once death of the donor is imminent are unknown. Should future studies identify high-risk seropositive premortem donors, these individuals may be targets for interventions that reduce their burden of CMV prior to organ donation.
FUTURE DIRECTIONS
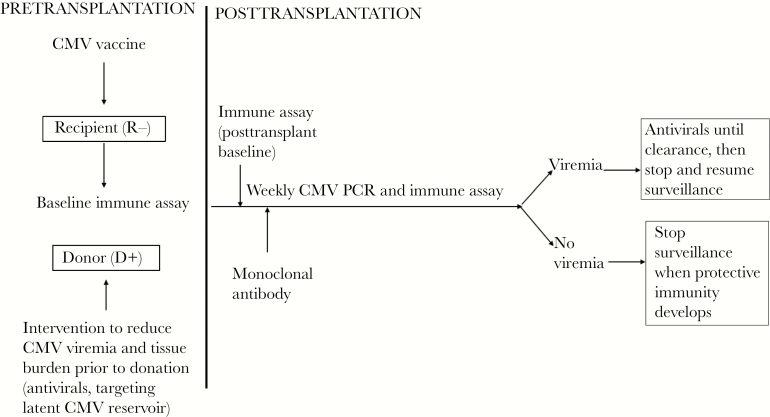
Our understanding of the risks, natural history, and management of CMV has dramatically changed since the availability of ganciclovir nearly 3 decades ago in 1989. The coming 3 decades will undoubtedly witness new revolutions in our approach to CMV infection after transplantation. It is imperative that we move beyond the concept of universal prophylaxis in SOT. Thus, we envision the framework outlined in Figure 1 for CMV prevention after SOT in the future. In HCT, where a detectable viral load per se is associated with overall mortality, prophylaxis or vaccination, possibly with optimized long-term surveillance, may reduce overall mortality. As the field steadfastly shifts into the era of precision medicine, it will become critical to learn how to integrate data from new CMV prevention studies, cell-mediated immune assays, monoclonal antibodies, vaccines, adoptive T-cell technologies, and innovative testing methods and biomarkers to optimize the care of high-risk transplant recipients.
Figure 1.
Potential future paradigm for cytomegalovirus (CMV) prevention after solid organ transplantation. By targeting both donor and recipient (before and after transplantation), this approach would combine knowledge gained from studies of prophylaxis, preemptive therapy, vaccines, monoclonal antibodies, and CMV-specific immunity to optimize prevention of CMV. Abbreviations: CMV, cytomegalovirus; PCR, polymerase chain reaction.
Notes
Acknowledgments. The authors thank Charles B. Wessel, MLS, head of Research Initiatives at the University of Pittsburgh’s Health Sciences Library System, for his assistance with the scientific literature review.
Supplement sponsorship. This supplement was sponsored by NIAID and NICHD.
Potential conflicts of interest. M. B. has received research support and consulting fees from Merck, Chimerix, Shire (now Takeda), Astellas, and Gilead; research support from Lophius Bioscience; and consulting fees from Helocyte, GSK, Oxford Immunotec, and Artemis Therapeutics. All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Haidar G, Singh N. Viral infections in solid organ transplant recipients: novel updates and a review of the classics. Curr Opin Infect Dis 2017; 30:579–88. [DOI] [PubMed] [Google Scholar]
- 2. Razonable RR, Humar A. Cytomegalovirus in solid organ transplant recipients-guidelines of the American Society of Transplantation Infectious Diseases community of practice. Clinical Transplant 2019:e13512. [DOI] [PubMed] [Google Scholar]
- 3. Palmer SM, Limaye AP, Banks M, et al. Extended valganciclovir prophylaxis to prevent cytomegalovirus after lung transplantation: a randomized, controlled trial. Ann Intern Med 2010; 152:761–9. [DOI] [PubMed] [Google Scholar]
- 4. Humar A, Limaye AP, Blumberg EA, et al. Extended valganciclovir prophylaxis in D+/R– kidney transplant recipients is associated with long-term reduction in cytomegalovirus disease: two-year results of the IMPACT study. Transplantation 2010; 90:1427–31. [DOI] [PubMed] [Google Scholar]
- 5. Chen K, Cheng MP, Hammond SP, Einsele H, Marty FM. Antiviral prophylaxis for cytomegalovirus infection in allogeneic hematopoietic cell transplantation. Blood Adv 2018; 2:2159–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kotton CN, Kumar D, Caliendo AM, et al. The Transplantation Society International CMV Consensus Group The third international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation 2018; 102:900–31. [DOI] [PubMed] [Google Scholar]
- 7. Limaye AP, Bakthavatsalam R, Kim HW, et al. Impact of cytomegalovirus in organ transplant recipients in the era of antiviral prophylaxis. Transplantation 2006; 81:1645–52. [DOI] [PubMed] [Google Scholar]
- 8. Beam E, Lesnick T, Kremers W, Kennedy CC, Razonable RR. Cytomegalovirus disease is associated with higher all-cause mortality after lung transplantation despite extended antiviral prophylaxis. Clin Transplant 2016; 30:270–8. [DOI] [PubMed] [Google Scholar]
- 9. Wiita AP, Roubinian N, Khan Y, et al. Cytomegalovirus disease and infection in lung transplant recipients in the setting of planned indefinite valganciclovir prophylaxis. Transpl Infect Dis 2012; 14:248–58. [DOI] [PubMed] [Google Scholar]
- 10. Lisboa LF, Preiksaitis JK, Humar A, Kumar D. Clinical utility of molecular surveillance for cytomegalovirus after antiviral prophylaxis in high-risk solid organ transplant recipients. Transplantation 2011; 92:1063–8. [DOI] [PubMed] [Google Scholar]
- 11. Alraddadi B, Nierenberg NE, Price LL, et al. Characteristics and outcomes of neutropenia after orthotopic liver transplantation. Liver Transpl 2016; 22:217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stevens DR, Sawinski D, Blumberg E, Galanakis N, Bloom RD, Trofe-Clark J. Increased risk of breakthrough infection among cytomegalovirus donor-positive/recipient-negative kidney transplant recipients receiving lower-dose valganciclovir prophylaxis. Transpl Infect Dis 2015; 17:163–73. [DOI] [PubMed] [Google Scholar]
- 13. Meyers JD, Reed EC, Shepp DH, et al. Acyclovir for prevention of cytomegalovirus infection and disease after allogeneic marrow transplantation. N Engl J Med 1988; 318:70–5. [DOI] [PubMed] [Google Scholar]
- 14. Prentice HG, Gluckman E, Powles RL, et al. Impact of long-term acyclovir on cytomegalovirus infection and survival after allogeneic bone marrow transplantation. European Acyclovir for CMV Prophylaxis Study Group. Lancet 1994; 343:749–53. [DOI] [PubMed] [Google Scholar]
- 15. Winston DJ, Yeager AM, Chandrasekar PH, Snydman DR, Petersen FB, Territo MC; Valacyclovir Cytomegalovirus Study Group Randomized comparison of oral valacyclovir and intravenous ganciclovir for prevention of cytomegalovirus disease after allogeneic bone marrow transplantation. Clin Infect Dis 2003; 36:749–58. [DOI] [PubMed] [Google Scholar]
- 16. Ljungman P, de La Camara R, Milpied N, et al. Valacyclovir International Bone Marrow Transplant Study Group Randomized study of valacyclovir as prophylaxis against cytomegalovirus reactivation in recipients of allogeneic bone marrow transplants. Blood 2002; 99:3050–6. [DOI] [PubMed] [Google Scholar]
- 17. Boeckh M, Ljungman P. How we treat cytomegalovirus in hematopoietic cell transplant recipients. Blood 2009; 113:5711–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Teira P, Battiwalla M, Ramanathan M, et al. Early cytomegalovirus reactivation remains associated with increased transplant-related mortality in the current era: a CIBMTR analysis. Blood 2016; 127:2427–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Green ML, Leisenring W, Xie H, et al. Cytomegalovirus viral load and mortality after haemopoietic stem cell transplantation in the era of pre-emptive therapy: a retrospective cohort study. Lancet Haematol 2016; 3:e119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marty FM, Ljungman P, Chemaly RF, et al. Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. N Engl J Med 2017; 377:2433–44. [DOI] [PubMed] [Google Scholar]
- 21. Cherrier L, Nasar A, Goodlet KJ, Nailor MD, Tokman S, Chou S. Emergence of letermovir resistance in a lung transplant recipient with ganciclovir-resistant cytomegalovirus infection. Am J Transplant 2018; 18:3060–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Khoury JA, Storch GA, Bohl DL, et al. Prophylactic versus preemptive oral valganciclovir for the management of cytomegalovirus infection in adult renal transplant recipients. Am J Transplant 2006; 6:2134–43. [DOI] [PubMed] [Google Scholar]
- 23. Sun HY, Wagener MM, Singh N. Prevention of posttransplant cytomegalovirus disease and related outcomes with valganciclovir: a systematic review. Am J Transplant 2008; 8:2111–8. [DOI] [PubMed] [Google Scholar]
- 24. Limaye AP, Green ML, Edmison B, et al. Prospective assessment of cytomegalovirus immunity in high-risk donor-seropositive/recipient-seronegative liver transplant recipients receiving either preemptive therapy or prophylaxis. J Infect Dis 2019; 220:752–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reischig T, Jindra P, Hes O, Svecova M, Klaboch J, Treska V. Valacyclovir prophylaxis versus preemptive valganciclovir therapy to prevent cytomegalovirus disease after renal transplantation. Am J Transplant 2008; 8:69–77. [DOI] [PubMed] [Google Scholar]
- 26. Kliem V, Fricke L, Wollbrink T, Burg M, Radermacher J, Rohde F. Improvement in long-term renal graft survival due to CMV prophylaxis with oral ganciclovir: results of a randomized clinical trial. Am J Transplant 2008; 8:975–83. [DOI] [PubMed] [Google Scholar]
- 27. Witzke O, Hauser IA, Bartels M, Wolf G, Wolters H, Nitschke M; VIPP Study Group Valganciclovir prophylaxis versus preemptive therapy in cytomegalovirus-positive renal allograft recipients: 1-year results of a randomized clinical trial. Transplantation 2012; 93:61–8. [DOI] [PubMed] [Google Scholar]
- 28. Singh N, Winston D, Razonable RR, et al. Preemptive therapy (PET) versus prophylaxis for prevention of cytomegalovirus (CMV) disease in high-risk donor seropositive/recipient seronegative (D+R–) liver transplant recipients (LTR): a NIH-sponsored, randomized, controlled, multicenter trial In: IDWeek, San Francisco, CA: 2018. [Google Scholar]
- 29. Razonable RR, Humar A; AST Infectious Diseases Community of Practice Cytomegalovirus in solid organ transplantation. Am J Transplant 2013; 13(Suppl 4):93–106. [DOI] [PubMed] [Google Scholar]
- 30. Elmaagacli AH, Steckel NK, Koldehoff M, et al. Early human cytomegalovirus replication after transplantation is associated with a decreased relapse risk: evidence for a putative virus-versus-leukemia effect in acute myeloid leukemia patients. Blood 2011; 118:1402–12. [DOI] [PubMed] [Google Scholar]
- 31. Litjens NHR, van der Wagen L, Kuball J, Kwekkeboom J. Potential beneficial effects of cytomegalovirus infection after transplantation. Front Immunol 2018; 9:389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Marty FM, Winston DJ, Rowley SD, et al. CMX001-201 Clinical Study Group CMX001 to prevent cytomegalovirus disease in hematopoietic-cell transplantation. N Engl J Med 2013; 369:1227–36. [DOI] [PubMed] [Google Scholar]
- 33. Kharfan-Dabaja MA, Boeckh M, Wilck MB, et al. A novel therapeutic cytomegalovirus DNA vaccine in allogeneic haemopoietic stem-cell transplantation: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis 2012; 12:290–9. [DOI] [PubMed] [Google Scholar]
- 34. Chemaly RF, Ullmann AJ, Ehninger G. CMV prophylaxis in hematopoietic-cell transplantation. N Engl J Med 2014; 371:576–7. [DOI] [PubMed] [Google Scholar]
- 35. Boeckh M, Nichols WG, Chemaly RF, et al. Valganciclovir for the prevention of complications of late cytomegalovirus infection after allogeneic hematopoietic cell transplantation: a randomized trial. Ann Intern Med 2015; 162:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Marty FM, Winston DJ, Chemaly RF, et al. SUPPRESS Trial Clinical Study Group A randomized, double-blind, placebo-controlled phase 3 trial of oral brincidofovir for cytomegalovirus prophylaxis in allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2019; 25:369–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Limaye AP, Santo Hayes TK, Huang ML, Magaret A, Boeckh M, Jerome KR. Quantitation of cytomegalovirus DNA load in dried blood spots correlates well with plasma viral load. J Clin Microbiol 2013; 51:2360–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Smith C, Beagley L, Rehan S, et al. Autologous adoptive T-cell therapy for recurrent or drug-resistant cytomegalovirus complications in solid organ transplant recipients: a single-arm open-label phase I clinical trial. Clin Infect Dis 2019; 68:632–40. [DOI] [PubMed] [Google Scholar]
- 39. Kumar D, Chin-Hong P, Kayler L, et al. A prospective multicenter observational study of cell-mediated immunity as a predictor for cytomegalovirus infection in kidney transplant recipients. Am J Transplant 2019; 19:2505–16. [DOI] [PubMed] [Google Scholar]
- 40. Gliga S, Korth J, Krawczyk A, et al. T-Track-CMV and QuantiFERON-CMV assays for prediction of protection from CMV reactivation in kidney transplant recipients. J Clin Virol 2018; 105:91–6. [DOI] [PubMed] [Google Scholar]
- 41. Pipeling MR, John ER, Orens JB, Lechtzin N, McDyer JF. Primary cytomegalovirus phosphoprotein 65-specific CD8+ T-cell responses and T-bet levels predict immune control during early chronic infection in lung transplant recipients. J Infect Dis 2011; 204:1663–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Snyder LD, Chan C, Kwon D, et al. Polyfunctional T-cell signatures to predict protection from cytomegalovirus after lung transplantation. Am J Respir Crit Care Med 2016; 193:78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Grossi P, Mohacsi P, Szabolcs Z, Potena L. Cytomegalovirus immunoglobulin after thoracic transplantation: an overview. Transplantation 2016; 100(Suppl 3):S1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ishida JH, Patel A, Mehta AK, et al. Phase 2 randomized, double-blind, placebo-controlled trial of RG7667, a combination monoclonal antibody, for prevention of cytomegalovirus infection in high-risk kidney transplant recipients. Antimicrob Agents Chemother 2017; 61. doi:10.1128/AAC.01794-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Griffiths P. New vaccines and antiviral drugs for cytomegalovirus. J Clin Virol 2019; 116:58–61. [DOI] [PubMed] [Google Scholar]
- 46. Plotkin SA, Smiley ML, Friedman HM, et al. Towne-vaccine-induced prevention of cytomegalovirus disease after renal transplants. Lancet 1984; 1:528–30. [DOI] [PubMed] [Google Scholar]
- 47. Griffiths PD, Stanton A, McCarrell E, et al. Cytomegalovirus glycoprotein-B vaccine with MF59 adjuvant in transplant recipients: a phase 2 randomised placebo-controlled trial. Lancet 2011; 377:1256–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vincenti F, Budde K, Merville P, et al. A randomized, phase 2 study of ASP0113, a DNA-based vaccine, for the prevention of CMV in CMV-seronegative kidney transplant recipients receiving a kidney from a CMV-seropositive donor. Am J Transplant 2018; 18:2945–54. [DOI] [PubMed] [Google Scholar]
- 49. Maertens J, Bermúdez A, Logan A, et al. A randomised, placebo-controlled Phase 3 study to evaluate the efficacy and safety of ASP0113, a first-in-class, DNA-based vaccine in CMV-seropositive allogeneic haematopoietic cell transplant recipients. In: 45th Annual Meeting of the European Society for Blood and Marrow Transplantation, Frankfurt, Germany, 24–27 March 2019. [Google Scholar]
- 50. Nakamura R, La Rosa C, Longmate J, et al. Viraemia, immunogenicity, and survival outcomes of cytomegalovirus chimeric epitope vaccine supplemented with PF03512676 (CMVPepVax) in allogeneic haemopoietic stem-cell transplantation: randomised phase 1b trial. Lancet Haematol 2016; 3:e87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Aldoss I, Baden L, Ariza-Heredia E, et al. Multi-antigen vaccine (TRIPLEX) based on attenuated poxvirus prevents cytomegalovirus viremia in a multi-center placebo-controlled, double line, randomized phase 2 clinical trial in allogeneic HCT recipients. In: 45th Annual Meeting of the European Society for Blood and Marrow Transplantation, Frankfurt, Germany, 24–27 March 2019. [Google Scholar]
- 52. Avery RK, Arav-Boger R, Marr KA, et al. Outcomes in transplant recipients treated with foscarnet for ganciclovir-resistant or refractory cytomegalovirus infection. Transplantation 2016; 100:e74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Papanicolaou GA, Silveira FP, Langston AA, et al. Maribavir for refractory or resistant cytomegalovirus infections in hematopoietic-cell or solid-organ transplant recipients: a randomized, dose-ranging, double-blind, phase 2 study. Clin Infect Dis 2019; 68:1255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. El Chaer F, Shah DP, Chemaly RF. How I treat resistant cytomegalovirus infection in hematopoietic cell transplantation recipients. Blood 2016; 128:2624–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Phoompoung P, Ferreira VH, Tikkanen J, et al. Letermovir as salvage therapy for CMV infection in transplant recipients [manuscript published online ahead of print 15 May 2019]. Transplantation 2019. doi:10.1097/TP.0000000000002785. [DOI] [PubMed] [Google Scholar]
- 56. Turner N, Strand A, Grewal DS, et al. Use of letermovir as salvage therapy for drug-resistant cytomegalovirus retinitis. Antimicrob Agents Chemother 2019; 63. doi:10.1128/AAC.02337-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chou S. Rapid in vitro evolution of human cytomegalovirus UL56 mutations that confer letermovir resistance. Antimicrob Agents Chemother 2015; 59:6588–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Houghtelin A, Bollard CM. Virus-specific T cells for the immunocompromised patient. Front Immunol 2017; 8:1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tzannou I, Leung KS, Martinez C, et al. Safety and preliminary efficacy of “ready to administer” cytomegalovirus (CMV)-specific T cells for the treatment of patients with refractory CMV infection. Blood 2016; 128:388. [Google Scholar]
- 60. Brestrich G, Zwinger S, Fischer A, et al. Adoptive T-cell therapy of a lung transplanted patient with severe CMV disease and resistance to antiviral therapy. Am J Transplant 2009; 9:1679–84. [DOI] [PubMed] [Google Scholar]
- 61. Holmes-Liew CL, Holmes M, Beagley L, et al. Adoptive T-cell immunotherapy for ganciclovir-resistant CMV disease after lung transplantation. Clin Transl Immunology 2015; 4:e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Stowe RP, Kozlova EV, Yetman DL, Walling DM, Goodwin JS, Glaser R. Chronic herpesvirus reactivation occurs in aging. Exp Gerontol 2007; 42:563–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Forbes BA. Acquisition of cytomegalovirus infection: an update. Clin Microbiol Rev 1989; 2:204–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Manuel O, Pang XL, Humar A, Kumar D, Doucette K, Preiksaitis JK. An assessment of donor-to-recipient transmission patterns of human cytomegalovirus by analysis of viral genomic variants. J Infect Dis 2009; 199:1621–8. [DOI] [PubMed] [Google Scholar]
- 65. Lumgair HA, Rolando N, O’Beirne J, Sharma D, Griffiths PD. Transient residence of a seropositive organ is sufficient to transfer human cytomegalovirus to a seronegative recipient. Transpl Infect Dis 2014; 16:501–4. [DOI] [PubMed] [Google Scholar]
- 66. Furui Y, Satake M, Hoshi Y, Uchida S, Suzuki K, Tadokoro K. Cytomegalovirus (CMV) seroprevalence in Japanese blood donors and high detection frequency of CMV DNA in elderly donors. Transfusion 2013; 53:2190–7. [DOI] [PubMed] [Google Scholar]