Abstract
Introduction: Two species of Aedes (Ae.) mosquitoes (Ae. aegypti and Ae. albopictus) are primary vectors for emerging arboviruses that are a significant threat to public health and economic burden worldwide. Distribution of these vectors and the associated arboviruses, such as dengue virus, chikungunya virus, yellow fever virus, and Zika virus, was for a long time restricted by geographical, ecological, and biological factors. Presently, arbovirus emergence and dispersion are more rapid and geographically widespread, largely due to expansion of the range for these two mosquitoes that have exploited the global transportation network, land perturbation, and failure to contain the mosquito population coupled with enhanced vector competence. Ae. aegypti and Ae. albopictus may also sustain transmission between humans without having to depend on their natural reservoir forest cycles due to arthropod adaptation to urbanization. Currently, there is no single strategy that is adequate to control these vectors, especially when managing arbovirus outbreaks.
Objective: This review aimed at presenting the characteristics and abilities of Ae. aegypti and Ae. albopictus, which can drive a global public health risk, and suggests strategies for prevention and control.
Methods: This review presents the geographic range, reproduction and ecology, vector competence, genetic evolution, and biological and chemical control of these two mosquito species and how they have changed and developed over time combined with factors that may drive pandemics and mitigation measures.
Conclusion: We suggest that more efforts should be geared toward the development of a concerted multidisciplinary approach.
Keywords: mosquitoes, arboviruses, vector control, pandemic risk
Introduction
Members of the mosquito genus Aedes (Ae.) are associated with transmission of many arboviruses. Presently, Ae. mosquitoes, specifically Ae. (Stegomyia) aegypti (Linnaeus 1762) and Ae. (Stegomyia) albopictus (Skuse 1894), are of main interest due to their association with emerging and reemerging infectious diseases with serious public health consequences (Weaver and Reisen 2010). These two mosquito vectors are competent vectors of four major arboviruses, for example, dengue virus, chikungunya virus, yellow fever virus, and Zika virus, which cause heavy health burden and economic losses globally. For many years, these vectors and viruses have been endemic and restricted to particular regions, but now they are spreading into new tropical, subtropical, and temperate areas, thus expanding the global coverage, a situation that may escalate large-scale epidemics (Kraemer et al. 2015). A fundamental question is whether it is plausible for these globally spread Ae. species to trigger a global epidemic comprising of simultaneous outbreaks of these arboviruses. In addition, is there a multinational response and containment strategy?
By this review, we present some basic characteristics and abilities of Ae. aegypti and Ae. albopictus to colonize and establish populations in new geographical areas along with their associated arboviruses, expose the potential risk to global health, and give some recommendations for vector control and risk minimization. In essence, there is a need for a combined effective strategy to manage arbovirus epidemics worldwide.
Geographic Range of Ae. aegypti and Ae. albopictus
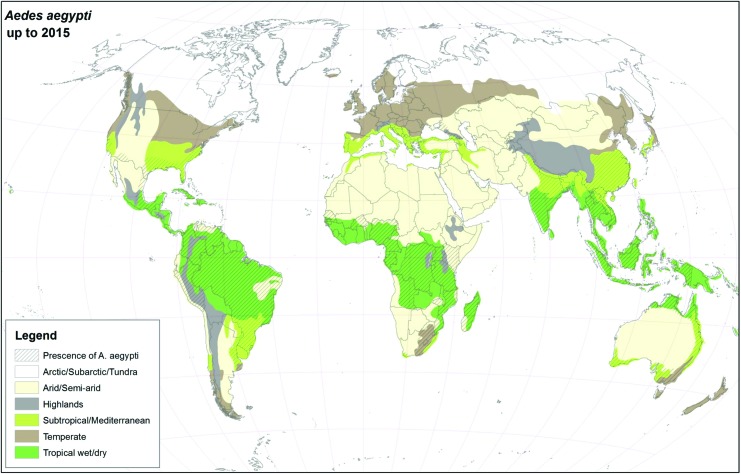
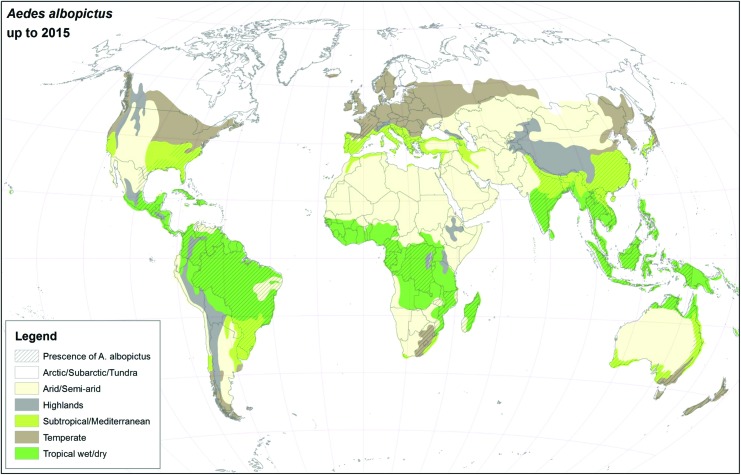
For many years, Ae. aegypti and Ae. albopictus were geographically restricted to the African continent and Southeast Asia, respectively (Womack 1993, Mousson et al. 2005, Scholte and Schaffner 2007), but presently, they have colonized almost all continents (Kraemer et al. 2015), see Figs. 1 and 2. As suggested in this article, the major drivers for geographic expansion of invasive mosquito species are globalization and changes in the environment (including climate change).
FIG. 1.
Global distribution of Ae. aegypti using climatic and surveillance data collected up to 2015. Color images are available online.
FIG. 2.
Global distribution of Ae. albopictus using climatic and surveillance data collected up to 2015. Color images are available online.
To our knowledge, there are only a few geostatistical methods to explore sympatric Ae. aegypti and Ae. albopictus populations. In an article by Duncombe et al. (2013), they found that although Aedes mosquitoes reside sympatrically, they prefer different locations (Duncombe et al. 2013). In biology, two related species or populations are considered sympatric when they exist in the same geographic area and frequently encounter one another. It is important to remember that cryptic species may be medically important in vector-borne disease transmission, vector ecology, and evolutionary biology.
An initially interbreeding population that splits into two or more distinct species sharing a common range exemplifies sympatric speciation. Such speciation may be a product of reproductive isolation—which prevents hybrid offspring from being viable or able to reproduce, thereby reducing gene flow—that results in genetic divergence. Sympatric speciation does not imply secondary contact, which is speciation or divergence in allopatry, followed by range expansions, leading to an area of sympatry. Sympatric species or taxa in secondary contact may or may not interbreed.
To exemplify, the introduction of Ae. aegypti to Asia in the 19th century led to this species becoming the dominant dengue fever vector in cities in which it was better adapted for than native Ae. albopictus (Lounibos 2002, Gratz 2004). Since then, Ae. albopictus has adjusted to urban environments, although it still favors areas of dense vegetation, and the two species reside sympatrically throughout Asia. Generally, wherever the density of Ae. aegypti was found to be high, the density of Ae. albopictus was low, and vice versa. However, it would be interesting to foresee whether the global distribution of Ae. aegypti and Ae. albopictus species leads to sympatric coexistence or competitive exclusion.
It is generally believed that the first incursion of Ae. aegypti from Africa to the Americas occurred during the slave trade era in the 1600s (Tabachnick 1991, Eisen and Moore 2013, Brown et al. 2014). The spread to Australia, Europe, and Southeast Asia took place later during the 20th century (Halstead 1966, 2006, Guzman and Kouri 2003, Gratz 2004, Hapuarachchi et al. 2010, Dick et al. 2012, Brown Evans et al. 2014). Inherently, Ae. aegypti is less tolerant to stressful climatic conditions such as temperatures below 10°C, implying that present and future incursion and establishment into subtropical and temperate regions are linked to climate change (Almeida et al. 2007, Liu-Helmersson et al. 2016). On the other hand, factors driving the disappearance of Ae. aegypti in the 1970s from the Mediterranean, Black Sea, and Macaronesian and Atlantic Ocean regions (i.e., Madeira, Canary Islands, and the Azores) have been connected to establishment of appropriate water infrastructure and improved hygiene through development of piped water systems (Saliternik 1958, Holstein 1967). Furthermore, the use of insecticides such as dichlorodiphenyltrichloroethane (DDT), as part of malaria control, has significantly contributed to elimination of Ae. aegypti. It has also been suggested that the vector has undergone natural extinction (Holstein 1967). Since then, Ae. aegypti has reestablished in southern Russia, Madeira, Georgia, and northeastern Turkey (Almeida et al. 2007, Scholte et al. 2009, Akiner et al. 2016).
Ae. albopictus is an indigenous species of Southeast Asia that has successively expanded its presence to Africa, Europe, Australia, the Americas, and Middle East (Gratz 2004, Benedict et al. 2007). Currently, Ae. albopictus is more geographically widespread than Ae. aegypti in Southeast Asia and Southeastern United States (Medlock et al. 2015). The first reported observations of Ae. albopictus outside Asia were in Albania, Texas, and Brazil in 1979, 1985, and 1986, respectively (Forattini 1986, Sprenger and Wuithiranyagool 1986, Adhami and Reiter 1998). Later, it became established also in other parts of the Americas, West and Central Africa, the Pacific and Indian Ocean islands, Australia, New Zealand, and the Caribbean islands (Gratz 2004, Derraik 2006, Paupy et al. 2009). However, in some countries such as New Zealand, Barbados, and Trinidad, the vector is presently absent, perhaps due to effective entomological surveillance programs situated at the ports of entry (Lambrechts et al. 2010). Currently, Ae. albopictus is considered as one of the most invasive mosquito species (Bonizzoni et al. 2013).
Reproduction and Ecology of Ae. aegypti and Ae. albopictus
Ae. aegypti and Ae. albopictus thrive in warm and humid climate and they are predominantly day feeders (Van Kleef et al. 2010). Ae. albopictus undergoes estivation, and in some cases, the eggs may survive temperatures below 0°C, whereas Ae. aegypti is intolerant to lower temperatures, especially freezing temperatures (Romi et al. 2006, Gould and Higgs 2009, Rios and Maruniak 2011, Thomas et al. 2012). Ae. aegypti females lay multiple batches of eggs, whereas Ae. albopictus lay single batches after a bloodmeal. Although these two mosquito species are container-inhabiting mosquitoes, Ae. aegypti has a preference for human settlements, whereas Ae. albopictus is inclined to periurban and rural environments (Christophers 1960, Chan et al. 1971, Braks et al. 2003, Tsuda et al. 2006). During favorable conditions, especially at high temperatures and flooding, eggs of both Ae. aegypti and Ae. albopictus hatch within a few days into larvae. The larvae undergo thereafter four molts, which may take between 9 and 13 days. The male mosquitoes develop faster than the females and molt earlier into pupae. After a period of ∼2 days, the pupae develop further into adult mosquitoes; see Fig. 3.
FIG. 3.
Ae. aegypti (left) and Ae. albopictus (right) adult mosquitoes. Morphologically, both are dark in color with white strips on their backs and legs. However, Ae. albopictus is smaller, with a single, longitudinal, silvery dorsal stripe, while Ae. aegypti has a silvery, lyre-shaped dorsal pattern on its scutum (photo, Anders Lindström).
Research based on bloodmeal analysis of Ae. aegypti and Ae. albopictus demonstrates that both mosquito species feed on a wide range of vertebrate hosts (Eritja et al. 2005, Barrera et al. 2012). However, Ae. aegypti is more anthropophilic than Ae. albopictus, hence a more efficient vector in urban areas (Paupy et al. 2009, Valerio et al. 2010). Some studies have indicated a decline of the Ae. aegypti population and a simultaneous increase of the Ae. albopictus population, especially in regions where they coexist (Christophers 1960, O'meara et al. 1995, Lounibos et al. 2016). This observation could be attributed to interspecies competition beside the control Ae. aegypti population by intensive vector control methods such as environmental sanitation and population reduction using Ae aegypti-targeted traps, mainly the sentinel autocidal gravid ovitraps in urban settings (Barrera et al. 2018). A suggested explanation for the competitive displacement of Ae. aegypti by Ae. albopictus is satyrization, a mating interference method where males of one species mate with females of another species, hence decreasing their fitness without generating hybrids (Tripet et al. 2011).
Vector Competence
Vector competence (ability for infection, dissemination, and transmission of virus) differs between mosquitoes of different species and among virus strains. Ae. aegypti and Ae. albopictus are known to transmit all four dengue virus serotypes, yellow fever virus, chikungunya virus, and Zika virus and suggested to be potential vectors of Venezuelan equine encephalitis virus (Larsen and Ashley 1971, Fontenille et al. 1997, Gratz 2004, de Lamballerie et al. 2008, da Moura et al. 2015, Ferreira-de-Brito et al. 2016, Seixas et al. 2018). Ae. albopictus feeds on a wide range of hosts and is known to be a significant biting irritant with the potential to become a serious health threat as a bridge vector for many zoonotic pathogens to humans (Benedict et al. 2007). This mosquito vector is able to transmit at least 22 arboviruses, including dengue, yellow fever, chikungunya, Rift Valley fever, Japanese encephalitis, West Nile, and Sindbis viruses (Gubler and Rosen 1976, Savage et al. 1992, Mitchell 1995, Moore and Mitchell 1997, Schaffner et al. 2013, Medlock et al. 2015, Xia et al. 2018). Furthermore, Potosi virus, Cache Valley virus, La Crosse virus, Eastern equine encephalitis virus, Venezuelan equine encephalitis virus, and Mayaro virus may also be transmitted by these two mosquito species (Larsen and Ashley 1971, Turell et al. 2005, Long et al. 2011). In addition, Ae. albopictus has been shown experimentally to transmit other arboviruses as well, such as Ross River, Western equine encephalitis, Oropouche, Jamestown Canyon, San Angelo, and Trivittatus viruses (Moore and Mitchell 1997).
Vector competence studies infer that Ae. albopictus has become a more effective vector for CHIKV and is currently able to cause disease within a few days postingestion of infected blood (Moutailler et al. 2009, Rohani et al. 2009). Interestingly, a single amino acid change in the envelope 1 gene (E1), at position 226 (A226V), was found to be responsible for the increased adaptability and improved transmission of CHIKV by Ae. albopictus compared with Ae. aegypti (Tsetsarkin et al. 2007). Studies also demonstrate that Ae. albopictus and Ae. aegypti, when coinfected with DENV and CHIKV, are able to replicate and disseminate both viruses independently (Vazeille et al. 2010, Nuckols et al. 2015).
In essence, increased knowledge of vector competence and transmission of mosquito-borne infections improves the possibilities to predict, prevent, and respond to emerging arbovirus threats and develop novel early warning systems and accordingly vector control.
Genetic Evolution of Ae. aegypti and Ae. albopictus
Mitochondrial genes of mosquitoes within the Ae. genus are relatively conserved and maternally inherited (Arctander 1995, Hudson and Turelli 2003, Hlaing et al. 2009, Behura et al. 2011). However, some coding genes have been shown to undergo a more rapid evolution, which is informative when characterizing relationships between populations. Certain genes, such as the mitochondrial cytochrome c oxidase subunit 1 (COI) gene, are frequently used for species identification of mosquitoes (Helmersson 2013, Engdahl et al. 2014). Genetic variations found in mitochondrial genes of Ae. aegypti and Ae. albopictus indicate that both species have haplotypes spatially distributed across the world. Population genetic analyses of Ae. aegypti of Thailand and North America indicated the presence of several haplotypes also in the nicotinamide adenine dinucleotide (NADH) and dehydrogenase subunit 4 mitochondrial DNA gene sequence (ND4) (Bosio et al. 2005, Mousson et al. 2005). Ae. albopictus populations have undergone genetic changes in the region encoding the sodium dehydrogenase subunit 5 (ND5), leading to distinct haplotypes (Avise 1994). This gene is a useful marker when studying the spatiotemporal evolution of Ae. albopictus populations. Moreover, the mitochondrial gene ND5 has been shown to be the most variable protein-coding sequence within the genus of Anopheles mosquitoes (Besansky et al. 1997).
One interesting observation from population genetics is the inferred linkage between genetic markers and vector competence, for example, the susceptibility of subpopulations from Ae. aegypti to DENV serotype 2 (Failloux et al. 2002). This discovery may lead to further understanding of the establishment of mosquito populations and appropriate prevention and control strategies considering that mosquitoes may belong to different genetic lineages. A population dynamic study on Ae. aegypti involving genetically divergent strains with varying insecticide resistance levels, genetic markers, and vector competence for DENV revealed the possibility of transmitting characteristics from one mosquito population to another by creating new genetic variants through random mating (Ocampo and Wesson 2004). These variants may pose challenges to insecticide resistance, which directly impact the control of mosquito populations, since it results in a reduction in the susceptibility of vector populations to insecticides (Vontas et al. 2012).
Forecast of Ae. aegypti and Ae. albopictus as Pandemic Drivers
Arboviruses constitute a global health risk when novel viruses arise, acquire new characteristics, or are deliberately released. Globalization has significantly increased the vulnerability of human and animal populations to emerging arbovirus diseases. In recent years, we have noticed the occurrence of traditional tropical diseases also in temperate areas, for example, chikungunya fever in Italy in 2007, outbreaks of West Nile fever in the United States in 1999 and Greece and Romania in 2010, and local transmission of dengue fever in France and Croatia in 2010, (Tsai et al. 1998, Hubálek and Halouzka 1999, Lanciotti et al. 1999, Papa et al. 2011, Lwande et al. 2015).
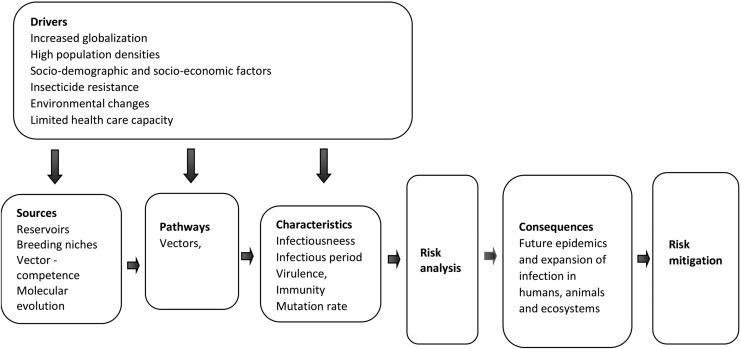
A wide range of risk drivers are known to exacerbate the emergence and spread of arbovirus diseases. The major drivers are globalization and changes in the environment (including climate change). Other components are sociodemographic factors (population aging, urbanization, social inequality, and lifestyle), insecticide resistance, health care capacity, animal health, and food safety. Intensive agriculture, population density, and inadequate infrastructure, such as improper water storage, are other risk factors that affect the impact of an outbreak. Dissemination and expansion of Ae. aegypti and Ae. albopictus also involve human activities such as trade of used tires and water storage in open containers (O'meara et al. 1995). The mutual ability of Ae. aegypti and Ae. albopictus to transmit similar pathogens and even become coinfected with viral pathogens may have consequences for the severity of the disease in susceptible hosts. Figure 4 illustrates an overview of risk drivers affecting local and/or global spread of mosquito vectors and their accompanying arboviruses. These risk factors exemplify topics that could be useful when raising awareness and suggest focus areas for a comprehensive risk analysis, which then can be used for a more holistic risk mitigation strategy.
FIG. 4.
A schematic outline of tasks and individual components to be considered for risk analysis and risk mitigation.
Societal factors, for example, health care resources and capacities and travel or transportation restrictions, can be adapted for emerging and suspected events to mitigate or limit effects. Increased risk for diseases caused by arboviruses can necessitate reorganization of care facilities in hospitals or new arrangements for patient handling. Increased prevalence of arbovirus infections in specific geographical areas can impose the need for travel restrictions or relocation of larger social events, for example, sporting events.
Strategy for Prevention and Control of Ae. aegypti and Ae. albopictus
So far, there is no single vector prevention and control method that is effective in controlling mosquito populations in all settings. Therefore, a combination of vector control methods, including biological, chemical, and genetic methods, is needed. Biological procedures are an attractive and environmentally friendly way to control vector populations. The use of fish feeding on mosquito larvae can be applied in containers with stagnant water, especially when targeting Ae. aegypti (Martinez-Ibarra et al. 2002), or micro-organisms such as Bacillus thuringiensis israelensis can act as biological larvicides (de Melo-Santos et al. 2009). Sterile insect techniques, satyrization, and Wolbachia interfere with the reproductive mechanisms of vectors and consequently with virus replication, with Wolbachia being the most important (Iturbe-Ormaetxe et al. 2011, Bargielowski and Lounibos 2016, Dutra et al. 2016, Rainey et al. 2016, Chung et al. 2018, Ritchie et al. 2018, van den Hurk 2018).
Genetic vector control methods have been used to limit mosquito populations, yet they are too slow to affect populations and require too much infrastructure to ever be useful for outbreaks. For example, genetic modification of Ae. aegypti has been applied using self-limiting genes such as OX513A that interfere with the survival of its offspring (Entwistle and Dhang 2014, Carvalho et al. 2015). The OX513A gene could also be used in combination with fluorescent markers for monitoring (Wallace 2013, Gabrieli et al. 2014). This latter method has been utilized for visualization of Ae. aegypti in Brazil, Malaysia, Cayman Islands, and the United States, and it could potentially be applicable in the control of Ae. albopictus mosquitoes as well (Harris et al. 2012, Subramaniam et al. 2012, Carvalho et al. 2015, Nimmo and Beech 2015). Release of genetically modified mosquitoes in nature might become feasible in arbovirus-endemic settings, for example, transgenic mosquitoes, to suppress the occurrence of vector-competent mosquitoes and hence reduce transmission. In addition, the application of CRISPR/Cas9 enables genetic modification of mosquitoes to block genes responsible for virus transmission and reduces vector competence in mosquito populations (Gantz et al. 2015). However, this method needs to be coupled with caution and involvement with relevant stakeholders, experts, and communities to avoid side effects (Knols et al. 2007).
Since the 20th century, a number of mosquitoes have developed resistance to insecticides, including natural (pyrethrum, nicotine, and neem extracts), organic chemical compounds (organophosphates and carbonates), and inorganic material (metals). Presently, insecticide resistance has become a large public health problem, especially when controlling mosquito vectors that transmit arboviruses to humans. Resistance to DDT was first demonstrated in the Ae. nigromaculis mosquito species by Bohart and Murray (1950). Ae. aegypti resistance against DDT was detected in Puerto Rico in the 1960s (Fox 1961), followed by other countries and states within the Americas (Burton 1964, Entwistle 1964, Flynn et al. 1964, Klassen and Brown 1964). Data from the Arthropod Pesticide Resistance Database (APRD) indicate that Ae. aegypti is resistant to 24 insecticides across 41 countries. Since then, subsequent reports of insecticide resistance in other disease vectors such as Anopheles gambiae and Culex pipiens have been described.
Prevention and control of Ae. aegypti and Ae. albopictus by biological or chemical procedures, as well as the lack of efficacious vaccines for preventing arbovirus transmission, are great challenges for the future and extremely important in our efforts to avoid large-scale epidemics or pandemics.
Insights for Tackling Epidemics
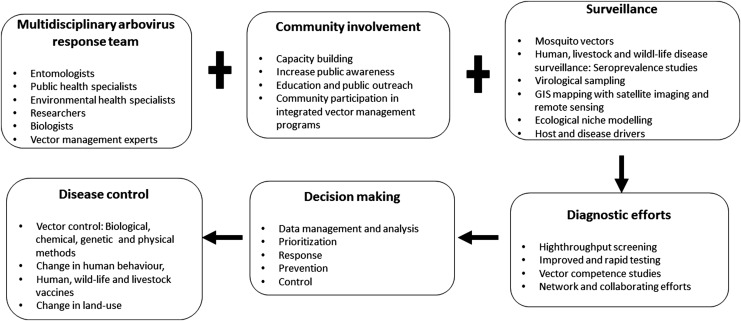
The review provides an overview of existing capabilities that (if combined properly) could greatly improve the preparedness to combat mosquito-borne diseases in the future. The solution to these problems seems not to be exclusively dependent on new sophisticated techniques, new innovated research findings, or more money, but to use what is existing, combine techniques, collaborate across borders with disease monitoring, vector surveillance, vector and pathogen identification, information sharing, and use standardized guidelines and procedures (SOPs) for sampling, preparation, and transportation. Visualization of the relationship or interplay between different resources and tasks has been demonstrated in Fig. 5, which exemplifies relationships between resources and tasks and how to connect for better preparedness and strength to fight epidemics. This is highly cost-effective and efficient. It is most important to reach out with relevant information to the community, national authorities, and decision makers. For that purpose, funded national and international programs and research communication through workshops, conferences, stakeholder meetings, and media are important to spread this urgent information and prepare for awareness and capability building against large epidemics and/or pandemics.
FIG. 5.
Insights for tackling epidemics. The information in the rectangular boxes indicates how different components involved in tackling arboviruses epidemics linked to Ae. aegypti and Ae. albopictus relate with each other for better preparedness and strength to fight epidemics. The plus sign designates joint efforts, whereas the arrows indicate the key activities that will help to link research outputs to early warning and response capacity outcomes and disease control.
In addition, surveillance tools for computerized real-time collection of data from different geographical regions and sources are urgently needed to provide a platform for rapid detection and response against arboviruses causing large epidemics. The data management and data analysis platform(s) must allow cross-border sharing of genomic and disease information and links to international, regional, and local requests. Such a common system will constitute a powerful platform for systematic collection of surveillance data and guide decision makers with better insights for a rapid response and quick recovery. This tool will also allow for improved prioritization, risk planning, and policy making.
The risk drivers shown in Fig. 4 form the basis for understanding and analyzing effects of emerging infectious diseases and serve as inputs for risk analysis, which then can be used for risk mitigation. Methods and standards for information exchange should be developed with the aim of increasing coverage and awareness of potential risk and eventualities pertaining to arbovirus outbreaks. Building capacity and institutional development will enhance a common basis for training and education of people at different professional levels, such as (1) training aids as manuals and tutorials for exercises; (2) gamed-based training tools for first responders and health care workers; (3) accredited reference laboratories supporting reference samples to ensure delivery of reliable results that will foster better patient management and treatment; (4) rapid diagnostic tests that need to be developed, optimized, and incorporated as part of routine diagnostics, especially in hospital settings and community health care centers located in endemic and risk areas; and (5) application of high-throughput sequencing platforms, as part of the point-of-care diagnostics, to enable detection of known and suspected emerging diseases in risk populations outside epidemics. Through next-generation sequencing (NGS), population genetics of both vectors and emerging arbovirus diseases will be understood. Discovery of new pathogens by NGS will provide greater insights into pathogen, vector, and host dynamics and will over time support risk planning and priority setting. To ensure effective control of mosquito vectors, a number of factors should be considered, including the safety of the environment, health of humans, animals and other living creatures, vector capacity, and social acceptance by relevant authority of the community in question.
An outline of possible interventions that affect the impact of an arbovirus outbreak is given in a phase-dependent manner in Table 1.
Table 1.
Outline of Topics for Surveillance and Response During and After Arbovirus Epidemics
| Intervention | Before outbreak | During outbreak | After outbreak |
|---|---|---|---|
| Surveillance | Assemble retrospective data from earlier outbreaks. Extract past and present records for vector/arbovirus occurrence. Record relevant clinical cases in hospitals and collect data from nearby communities with earlier disease experiences. |
Determine incidence and specific characteristics of the outbreak. Use valid case definition criteria. Identify key vectors, climate, and seasonal patterns during the outbreak. Use of satellite imaging and GIS tools to follow the situation and identify potential risk areas. |
Clarify the transmission dynamics of vectors and the pathogen. Identify hot spot areas for pathogens and their vectors. Reconsider criteria for screening and identification of clinical cases. |
| Research | Develop standard operating procedures (SOPs) and evaluate rapid diagnostic tests at risk areas and local hospitals. Establish screening platforms for virus/vector identification. Establish GIS and satellite imaging platforms for identification of risk areas. |
Early start of sampling, transportation screening, and documentation of samples. Open a high-throughput platform for screening pathogens in obtained samples. Perform an in-depth identification and characterization of pathogens and vectors at the genetic level. Create risk maps of the present epidemic. Release trustful communication of the situation through established channels. |
Evaluate available diagnostic tools. Experiences, information exchange, and lessons learned are noted. Assess identification procedures of pathogens and vector species. Identify and reduce possible risk factors for future epidemics. Evaluate vaccines and/or antiviral treatments. |
| Response | Launch a global emergency surveillance and response system for vector-borne diseases and establish cross-border information- and data-sharing platforms. Develop risk maps for vectors and virus and assess hot spots. |
Dispatch multidisciplinary teams to the site of the outbreak to handle the situation. Use applicable case identification procedures, confirmatory diagnosis, and treatment procedures. |
Use of suitable visualization tools for analysis, presentation, and communication of entomological and arbovirus surveillance data. Prediction of future outbreaks through satellite imagery of spatial-temporal data. |
| Capacity building | Initiate training courses in: Vector control and surveillance. Sampling procedures, including transport and documentation. Rapid diagnostics, (field) tests, and insights into bioinformatics. Risk assessment/mitigation and information sharing. |
Construction of competent professional teams of personnel with different competences capable of crisis management at diverse situations during outbreaks. | Improve information and communication pathways for a more rapid and efficient response to the epidemic situation. |
| Institutional development | Provide tools for automatic collection and data sharing. Establish efficient handling and investigation of pathogens. Access of reference samples from key laboratories. |
Availability to high-risk laboratories, workplace, equipment, and laboratory. Staff and infrastructure. | Organize for future accessibility of quality equipment, data collection, and management tools. Reconsider laboratory safety and security procedures. |
| Policy | Invest in public standby capability and allocate resources for mitigation of future epidemics or pandemics. Educate laboratory personnel and response groups in safety/security guidelines. |
Establish a command and control group capable of receiving large quantities of information, evaluating, and delivering a response to the epidemic situation. Organize groups of first responders for detection, identification, and handling of critical situations and provide assistance at the scene of the epidemic. |
Bring together lessons learned, resulting in better awareness and faster response against an epidemic. Review risk assessments and dynamics of future epidemics. Reconsider prioritization, risk planning, and policy making. |
Conclusions
We foresee the capability of Ae. aegypti and Ae. albopictus in causing future epidemics and pandemics by virtue of their extensive distribution and ability to spread different arboviruses. Despite concerns addressing the global distribution of these vectors, a comprehensive global strategy to understand the implications of global occurrence—in terms of public health (pandemic threat), population genetics of the responsible vectors and corresponding arboviruses, and the lack of overt prevention and response—is urgently needed. A multidisciplinary approach is desired for prevention and control of these two key players associated with large arbovirus epidemics.
Acknowledgment
Dr. Per Wikström at FOI is greatly acknowledged for the graphics.
Authors' Contributions
O.W.L., G.B., and J.N. conceived the study and wrote the first draft of the manuscript. V.O., A.L., C.A., and M.E. participated in discussions affecting the contents and direction of the manuscript. All authors have read and approved the final manuscript.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported by the Swedish Defence Research Agency, project A495718, and Swedish Research Council Formas (grant no.221-2014-1556).
References
- Adhami J, Reiter P. Introduction and establishment of Aedes (Stegomyia) albopictus skuse (Diptera: Culicidae) in Albania. J Am Mosquito Control Assoc 1998; 14:340–343 [PubMed] [Google Scholar]
- Akiner MM, Demirci B, Babuadze G, Robert V, et al. Spread of the invasive mosquitoes Aedes aegypti and Aedes albopictus in the Black Sea region increases risk of chikungunya, dengue, and Zika outbreaks in Europe. PLoS Negl Trop Dis 2016; 10:e0004664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida A, Gonçalves Y, Novo M, Sousa C, et al. Vector monitoring of Aedes aegypti in the Autonomous Region of Madeira, Portugal. Euro Surveill 2007; 12:E071115. [DOI] [PubMed] [Google Scholar]
- Arctander P. Comparison of a mitochondrial gene and a corresponding nuclear pseudogene. Proc Biol Sci 1995; 262:13–19 [DOI] [PubMed] [Google Scholar]
- Avise J. Molecular Marker. Natural History and Evolution. New York: Chapman and Hall, 1994: 511 [Google Scholar]
- Bargielowski IE, Lounibos LP. Satyrization and satyrization-resistance in competitive displacements of invasive mosquito species. Insect Sci 2016; 23:162–174 [DOI] [PubMed] [Google Scholar]
- Barrera R, Amador M, Munoz J, Acevedo V. Integrated vector control of Aedes aegypti mosquitoes around target houses. Parasit Vectors 2018; 11:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera R, Bingham AM, Hassan HK, Amador M, et al. Vertebrate hosts of Aedes aegypti and Aedes mediovittatus (Diptera: Culicidae) in rural Puerto Rico. J Med Entomol 2012; 49:917–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behura SK, Lobo NF, Haas B, deBruyn B, et al. Complete sequences of mitochondria genomes of Aedes aegypti and Culex quinquefasciatus and comparative analysis of mitochondrial DNA fragments inserted in the nuclear genomes. Insect Biochem Mol Biol 2011; 41:770–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict MQ, Levine RS, Hawley WA, Lounibos LP. Spread of the tiger: Global risk of invasion by the mosquito Aedes albopictus. Vector Borne Zoonotic Dis 2007; 7:76–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besansky NJ, Lehmann T, Fahey GT, Fontenille D, et al. Patterns of mitochondrial variation within and between African malaria vectors, Anopheles gambiae and An. arabiensis, suggest extensive gene flow. Genetics 1997; 147:1817–1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohart R, Murray W. DDT resistance in Aedes nigromaculis larvae. Proc Calif Mosq Contr Assoc 1950; 18:22–23 [Google Scholar]
- Bonizzoni M, Gasperi G, Chen X, James AA. The invasive mosquito species Aedes albopictus: Current knowledge and future perspectives. Trends Parasitol 2013; 29:460–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosio CF, Harrington LC, Jones JW, Sithiprasasna R, et al. Genetic structure of Aedes aegypti populations in Thailand using mitochondrial DNA. Am J Trop Med Hyg 2005; 72:434–442 [PubMed] [Google Scholar]
- Braks MA, Honório NA, Lourenço-De-Oliveira R, Juliano SA, et al. Convergent habitat segregation of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in southeastern Brazil and Florida. J Med Entomol 2003; 40:785–794 [DOI] [PubMed] [Google Scholar]
- Brown JE, Evans BR, Zheng W, Obas V, et al. Human impacts have shaped historical and recent evolution in Aedes aegypti, the dengue and yellow fever mosquito. Evolution 2014; 68:514–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton GJ. Results of insecticide resistance tests against Aedes aegypti adults and larvae in British Guiana. Mosq News 1964; 24:200–202 [Google Scholar]
- Carvalho DO, McKemey AR, Garziera L, Lacroix R, et al. Suppression of a field population of Aedes aegypti in Brazil by sustained release of transgenic male mosquitoes. PLoS Negl Trop Dis 2015; 9:e0003864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan YC, Chan KL, Ho BC. Aedes aegypti (L.) and Aedes albopictus (Skuse) in Singapore City. 1. Distribution and density. Bull World Health Organ 1971; 44:617–627 [PMC free article] [PubMed] [Google Scholar]
- Christophers S. Aedes aegypti (L.) the Yellow Fever Mosquito: Its Life History, Bionomics and Structure. New York: Cambridge University Press, 1960 [Google Scholar]
- Chung HN, Rodriguez SD, Gonzales KK, Vulcan J, et al. Toward Implementation of Mosquito Sterile Insect Technique: The Effect of Storage Conditions on Survival of Male Aedes aegypti Mosquitoes (Diptera: Culicidae) During Transport. J Insect Sci 2018; 18:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Moura AJ, de Melo Santos MA, Oliveira CM, Guedes DR, et al. Vector competence of the Aedes aegypti population from Santiago Island, Cape Verde, to different serotypes of dengue virus. Parasit Vectors 2015; 8:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lamballerie X, Leroy E, Charrel RN, Ttsetsarkin K, et al. Chikungunya virus adapts to tiger mosquito via evolutionary convergence: A sign of things to come? Virol J 2008; 5:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Melo-Santos MAV, de Araújo AP, Rios EMM, Regis L. Long lasting persistence of Bacillus thuringiensis serovar. israelensis larvicidal activity in Aedes aegypti (Diptera: Culicidae) breeding places is associated to bacteria recycling. Biol Control 2009; 49:186–191 [Google Scholar]
- Derraik JG. A scenario for invasion and dispersal of Aedes albopictus (Diptera: Culicidae) in New Zealand. J Med Entomol 2006; 43:1–8 [DOI] [PubMed] [Google Scholar]
- Dick OB, San Martín JL, Montoya RH, del Diego J, et al. The history of dengue outbreaks in the Americas. Am J Trop Med Hyg 2012; 87:584–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncombe J, Espino F, Marollano K, Velazco A, et al. Characterising the spatial dynamics of sympatric Aedes aegypti and Aedes albopictus populations in the Philippines. Geospat Health 2013; 8:255–265 [DOI] [PubMed] [Google Scholar]
- Dutra HLC, Rocha MN, Dias FBS, Mansur SB, et al. Wolbachia blocks currently circulating Zika virus isolates in Brazilian Aedes aegypti mosquitoes. Cell Host Microbe 2016; 19:771–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen L, Moore CG. Aedes (Stegomyia) aegypti in the continental United States: A vector at the cool margin of its geographic range. J Med Entomol 2013; 50:467–478 [DOI] [PubMed] [Google Scholar]
- Engdahl C, Larsson P, Näslund J, Bravo M, et al. Identification of Swedish mosquitoes based on molecular barcoding of the COI gene and SNP analysis. Mol Ecol Resour 2014; 14:478–488 [DOI] [PubMed] [Google Scholar]
- Entwistle J, Dhang P. 3 Emerging Technologies for Urban Mosquito Management. In: Dhang P, ed. Urban Insect Pests: Sustainable Management Strategies. CABI, United Kingdom, 2014; 23 [Google Scholar]
- Entwistle P. The distribution of mirid species and of resistant mirids in Nigeria. Proc. Conf. Mirids and Other Pests of Cocoa, Ibadan, Nigeria. 1964:9–17 [Google Scholar]
- Eritja R, Escosa R, Lucientes J, Marques E, et al. Worldwide invasion of vector mosquitoes: Present European distribution and challenges for Spain. Issues Bioinvasion Sci 2005; 7:87–97 [Google Scholar]
- Failloux A-B, Vazeille M, Rodhain F. Geographic genetic variation in populations of the dengue virus vector Aedes aegypti. J Mol Evol 2002; 55:653–663 [DOI] [PubMed] [Google Scholar]
- Ferreira-de-Brito A, Ribeiro IP, Miranda RMd, Fernandes RS, et al. First detection of natural infection of Aedes aegypti with Zika virus in Brazil and throughout South America. Mem Inst Oswaldo Cruz 2016; 111:655–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn A, Schoof H, Morlan H, Porter J. Susceptibility of seventeen strains of Aedes aegypti (L.) from Puerto Rico and the Virgin Islands to DDT, dieldrin, and malathion. Mosq News 1964; 24:118–123 [Google Scholar]
- Fontenille D, Diallo M, Mondo M, Ndiaye M, et al. First evidence of natural vertical transmission of yellow fever virus in Aedes aegypti, its epidemic vector. Trans R Soc Trop Med Hyg 1997; 91:533–535 [DOI] [PubMed] [Google Scholar]
- Forattini OP. Identificação de Aedes (Stegomyia) albopictus (Skuse) no Brasil. Rev Saúde Pública 1986; 20:244–245 [DOI] [PubMed] [Google Scholar]
- Fox I. Resistance of Aedes aegypti to certain chlorinated hydrocarbon and organo-phosphorus insecticides in Puerto Rico. Bull World Health Organ 1961; 24:489. [PMC free article] [PubMed] [Google Scholar]
- Gabrieli P, Smidler A, Catteruccia F. Engineering the control of mosquito-borne infectious diseases. Genome Biol 2014; 15:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantz VM, Jasinskiene N, Tatarenkova O, Fazekas A, et al. Highly efficient Cas9-mediated gene drive for population modification of the malaria vector mosquito Anopheles stephensi. Proc Natl Acad Sci USA 2015; 112:E6736–E6743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould EA, Higgs S. Impact of climate change and other factors on emerging arbovirus diseases. Trans R Soc Trop Med Hyg 2009; 103:109–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz NG. Critical review of the vector status of Aedes albopictus. Med Vet Entomol 2004; 18:215–227 [DOI] [PubMed] [Google Scholar]
- Gubler DJ, Rosen L. Variation among geographic strains of Aedes albopictus in suceptibility to infection with dengue viruses. Am J Trop Med Hyg 1976; 25:318–325 [DOI] [PubMed] [Google Scholar]
- Guzman G, Kouri G. Dengue and dengue hemorrhagic fever in the Americas: Lessons and challenges. J Clin Virol 2003; 27:1–13 [DOI] [PubMed] [Google Scholar]
- Halstead SB. Mosquito-borne haemorrhagic fevers of South and South-East Asia. Bull World Health Organ 1966; 35:3. [PMC free article] [PubMed] [Google Scholar]
- Halstead SB. Dengue in the Americas and Southeast Asia: Do they differ? Rev Panam Salud Publica 2006; 20:407–415 [DOI] [PubMed] [Google Scholar]
- Hapuarachchi H, Bandara K, Sumanadasa S, Hapugoda M, et al. Re-emergence of Chikungunya virus in South-east Asia: Virological evidence from Sri Lanka and Singapore. J Gener Virol 2010; 91:1067–1076 [DOI] [PubMed] [Google Scholar]
- Harris AF, McKemey AR, Nimmo D, Curtis Z, et al. Successful suppression of a field mosquito population by sustained release of engineered male mosquitoes. Nat Biotechnol 2012; 30:828–830 [DOI] [PubMed] [Google Scholar]
- Helmersson E. Molecular Identification of Mosquito Species: Evaluation of a Rapid DNA Extraction Method Together with DNA Barcoding as a Tool for Identification of Species. Uppsala University, Sweden, 2013 [Google Scholar]
- Hlaing T, Tun-Lin W, Somboon P, Socheat D, et al. Mitochondrial pseudogenes in the nuclear genome of Aedes aegypti mosquitoes: Implications for past and future population genetic studies. BMC Genet 2009; 10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstein M. Dynamics of Aedes aegypti distribution, density and seasonal prevalence in the Mediterranean area. Bull World Health Organ 1967; 36:541–543 [PMC free article] [PubMed] [Google Scholar]
- Hubálek Z, Halouzka J. West Nile fever—a reemerging mosquito-borne viral disease in Europe. Emerg Infect Dis 1999; 5:643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson RR, Turelli M. Stochasticity overrules the “three-times rule”: Genetic drift, genetic draft, and coalescence times for nuclear loci versus mitochondrial DNA. Evolution 2003; 57:182–190 [DOI] [PubMed] [Google Scholar]
- Iturbe-Ormaetxe I, Walker T, O'Neill SL. Wolbachia and the biological control of mosquito-borne disease. EMBO Rep 2011; 12:508–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klassen W, Brown A. Genetics of insecticide-resistance and several visible mutants in Aedes aegypti. Can J Genet Cytol 1964; 6:61–73 [DOI] [PubMed] [Google Scholar]
- Knols BG, Bossin HC, Mukabana WR, Robinson AS. Transgenic mosquitoes and the fight against malaria: Managing technology push in a turbulent GMO world. Am J Trop Med Hyg 2007; 77(6_Suppl):232–242 [PubMed] [Google Scholar]
- Kraemer MU, Sinka ME, Duda KA, Mylne AQ, et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife 2015; 4:e08347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrechts L, Scott TW, Gubler DJ. Consequences of the expanding global distribution of Aedes albopictus for dengue virus transmission. PLoS Neglect Trop Dis 2010; 4:e646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciotti R, Roehrig J, Deubel V, Smith J, et al. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science 1999; 286:2333–2337 [DOI] [PubMed] [Google Scholar]
- Larsen JR, Ashley RF. Demonstration of Venezuelan equine encephalomyelitis virus in tissues of Aedes aegypti. Am J Trop Med Hyg 1971; 20:754–760 [DOI] [PubMed] [Google Scholar]
- Liu-Helmersson J, Quam M, Wilder-Smith A, Stenlund H, et al. Climate change and Aedes vectors: 21st century projections for dengue transmission in Europe. EBioMedicine 2016; 7:267–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long KC, Ziegler SA, Thangamani S, Hausser NL, et al. Experimental transmission of Mayaro virus by Aedes aegypti. Am J Trop Med Hyg 2011; 85:750–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lounibos LP. Invasions by insect vectors of human disease. Annu Rev Entomol 2002; 47:233–266 [DOI] [PubMed] [Google Scholar]
- Lounibos LP, Bargielowski I, Carrasquilla MC, Nishimura N. Coexistence of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in peninsular florida two decades after competitive displacements. J Med Entomol 2016; 53:1385–1390 [DOI] [PubMed] [Google Scholar]
- Lwande OW, Mosomtai G, Symekher S. 2015. West nile virus, a reemerging 571 virus. Precision Medicine 2:e604. doi: 10.14800/pm.604 [DOI] [Google Scholar]
- Martinez-Ibarra J, Guillén YG, Arredondo-Jimenez J, Rodriguez-Lopez M. Indigenous fish species for the control of Aedes aegypti in water storage tanks in Southern Mexico. BioControl 2002; 47:481–486 [Google Scholar]
- Medlock J, Hansford K, Versteirt V, Cull B, et al. An entomological review of invasive mosquitoes in Europe. Bull Entomol Res 2015; 105:637–663 [DOI] [PubMed] [Google Scholar]
- Mitchell C. Geographic spread of Aedes albopictus and potential for involvement in arbovirus cycles in the Mediterranean basin. J Vector Ecol 1995; 20:44–58 [Google Scholar]
- Moore CG, Mitchell CJ. Aedes albopictus in the United States: Ten-year presence and public health implications. Emerg Infect Dis 1997; 3:329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousson L, Dauga C, Garrigues T, Schaffner F, et al. Phylogeography of Aedes (Stegomyia) aegypti (L.) and Aedes (Stegomyia) albopictus (Skuse)(Diptera: Culicidae) based on mitochondrial DNA variations. Genet Res 2005; 86:1–11 [DOI] [PubMed] [Google Scholar]
- Moutailler S, Barre H, Vazeille M, Failloux AB. Recently introduced Aedes albopictus in Corsica is competent to Chikungunya virus and in a lesser extent to dengue virus. Trop Med Int Health 2009; 14:1105–1109 [DOI] [PubMed] [Google Scholar]
- Nimmo DD, Beech C. Genetically engineered mosquitoes in the US. Outlooks Pest Manage 2015; 26:207–210 [Google Scholar]
- Nuckols J, Huang Y-J, Higgs S, Miller A, et al. Evaluation of simultaneous transmission of chikungunya virus and dengue virus type 2 in infected aedes aegypti and aedes albopictus (Diptera: Culicidae). J Med Entomol 2015; 52:447–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'meara GF, Evans LF, Gettman AD, Cuda JP. Spread of Aedes albopictus and decline of Ae. aegypti (Diptera: Culicidae) in Florida. J Med Entomol 1995; 32:554–562 [DOI] [PubMed] [Google Scholar]
- Ocampo CB, Wesson DM. Population dynamics of Aedes aegypti from a dengue hyperendemic urban setting in Colombia. Am J Trop Med Hyg 2004; 71:506–513 [PubMed] [Google Scholar]
- Papa A, Xanthopoulou K, Gewehr S, Mourelatos S. Detection of West Nile virus lineage 2 in mosquitoes during a human outbreak in Greece. Clin Microbiol Infect 2011; 17:1176–1180 [DOI] [PubMed] [Google Scholar]
- Paupy C, Delatte H, Bagny L, Corbel V, et al. Aedes albopictus, an arbovirus vector: From the darkness to the light. Microbes Infect 2009; 11:1177–1185 [DOI] [PubMed] [Google Scholar]
- Rainey SM, Martinez J, McFarlane M, Juneja P, et al. Wolbachia blocks viral genome replication early in infection without a transcriptional response by the endosymbiont or host small RNA pathways. PLoS Pathog 2016; 12:e1005536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios L, Maruniak JE (2011). Asian Tiger Mosquito, Aedes albopictus (Skuse)(Insecta: Diptera: Culicidae). Publication No. EENY-319. University of Florida Institute of Food and Agricultural Services and Florida Department of Agriculture and Consumer Services, Gainesville. Available at http://entnemdept ufl. edu/creatures/aquatic/asian_tiger.htm
- Ritchie SA, van den Hurk AF, Smout MJ, Staunton KM, et al. Mission accomplished? We need a guide to the ‘post release’ world of Wolbachia for Aedes-borne disease control. Trends Parasitol 2018; 34:217–226 [DOI] [PubMed] [Google Scholar]
- Rohani A, Potiwat R, Zamree I, Lee H. Refractoriness of Aedes aegypti (Linnaeus) to dual infection with dengue and chikungunya virus. Southeast Asian J Trop Med Public Health 2009; 40:443. [PubMed] [Google Scholar]
- Romi R, Severini F, Toma L. Cold acclimation and overwintering of female Aedes albopictus in Roma. J Am Mosq Control Assoc 2006; 22:149–151 [DOI] [PubMed] [Google Scholar]
- Saliternik Z. The Problems of Mosquitoes in Israel and their Control. Proc N J Mosq Exterm Assoc 1958; 45:70–79 [Google Scholar]
- Savage HM, Ezike VI, Nwankwo A. First record of breeding populations of Aedes albopictus in continental Africa: Implications for arboviral transmission. J Am Mosq Control Assoc 1992; 8:101–103 [PubMed] [Google Scholar]
- Schaffner F, Medlock J, Van Bortel W. Public health significance of invasive mosquitoes in Europe. Clin Microbiol Infect 2013; 19:685–692 [DOI] [PubMed] [Google Scholar]
- Scholte E-J, Schaffner F. 14. Waiting for the tiger: Establishment and spread of the Aedes albopictus mosquito in Europe. In: Takken W, Knols BGJ, eds. Emerging Pests and Vector-Borne Diseases in Europe. Wageningen, Netherlands: Wageningen Academic Publishers, 2007:241–260 [Google Scholar]
- Scholte E, Den Hartog W, Dik M, Schoelitsz B, et al. Introduction and control of three invasive mosquito species in the Netherlands, July-October 2010. Euro Surveill 2009; 15:1817–1824 [PubMed] [Google Scholar]
- Seixas G, Jupille H, Yen PS, Viveiros B, et al. Potential of Aedes aegypti populations in Madeira Island to transmit dengue and chikungunya viruses. Parasit Vectors 2018; 11:509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprenger D, Wuithiranyagool T. The discovery and distribution of Aedes albopictus in Harris County, Texas. J Am Mosq Control Assoc 1986; 2:217. [PubMed] [Google Scholar]
- Subramaniam T, Lee HL, Ahmad NW, Murad S. Genetically modified mosquito: The Malaysian public engagement experience. Biotechnol J 2012; 7:1323–1327 [DOI] [PubMed] [Google Scholar]
- Tabachnick WJ. Evolutionary genetics and arthropod-borne disease: The yellow fever mosquito. Am Entomol 1991; 37:14–26 [Google Scholar]
- Thomas SM, Obermayr U, Fischer D, Kreyling J, et al. Low-temperature threshold for egg survival of a post-diapause and non-diapause European aedine strain, Aedes albopictus (Diptera: Culicidae). Parasites Vectors 2012; 5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripet F, Lounibos LP, Robbins D, Moran J, et al. Competitive reduction by satyrization? Evidence for interspecific mating in nature and asymmetric reproductive competition between invasive mosquito vectors. Am J Trop Med Hyg 2011; 85:265–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai TF, Popovici F, Cernescu C, Campbell GL, et al. West Nile encephalitis epidemic in southeastern Romania. Lancet 1998; 352:767–771 [DOI] [PubMed] [Google Scholar]
- Tsetsarkin KA, Vanlandingham DL, McGee CE, Higgs S. A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog 2007; 3:e201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda Y, Suwonkerd W, Chawprom S, Prajakwong S, et al. Different spatial distribution of Aedes aegypti and Aedes albopictus along an urban-rural gradient and the relating environmental factors examined in three villages in northern Thailand. J Am Mosq Control Assoc 2006; 22:222–228 [DOI] [PubMed] [Google Scholar]
- Turell MJ, Dohm DJ, Sardelis MR, O'guinn ML, et al. An update on the potential of North American mosquitoes (Diptera: Culicidae) to transmit West Nile virus. J Med Entomol 2005; 42:57–62 [DOI] [PubMed] [Google Scholar]
- Valerio L, Marini F, Bongiorno G, Facchinelli L, et al. Host-feeding patterns of Aedes albopictus (Diptera: Culicidae) in urban and rural contexts within Rome province, Italy. Vector Borne Zoonotic Dis 2010; 10:291–294 [DOI] [PubMed] [Google Scholar]
- Wallace H. Genetically Modified Mosquitoes: Ongoing Concerns. Malaysia: Third World Network; 2013 [Google Scholar]
- van den Hurk AF. From Incriminating Stegomyia fasciata to Releasing Wolbachia pipientis: Australian Research on the Dengue Virus Vector, Aedes aegypti, and Development of Novel Strategies for Its Surveillance and Control. Trop Med Infect Dis 2018; 3: pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kleef E, Bambrick H, Hales S. The geographic distribution of dengue fever and the potential influence of global climate change. TropIKA. net(AHEAD): 2010; 1–22 [Google Scholar]
- Vazeille M, Mousson L, Martin E, Failloux A-B. Orally co-Infected Aedes albopictus from La Reunion Island, Indian Ocean, can deliver both dengue and chikungunya infectious viral particles in their saliva. PLoS Negl Trop Dis 2010; 4:e706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver SC, Reisen WK. Present and future arboviral threats. Antiviral Res 2010; 85:328–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack M. The yellow fever mosquito, Aedes aegypti. Wing Beats 1993; 5:4 [Google Scholar]
- Vontas J, Kioulos E, Pavlidi N, Morou E, et al. Insecticide resistance in the major dengue vectors Aedes albopictus and Aedes aegypti. Pestic Biochem Physiol 2012; 104:126–131 [Google Scholar]
- Xia H, Wang Y, Atoni E, Zhang B, et al. Mosquito-associated viruses in China. Virol Sin 2018; 33:5–20 [DOI] [PMC free article] [PubMed] [Google Scholar]