Abstract
Arterial inflammation is a hallmark of atherosclerosis and appropriate management of this inflammation represents a major unmet therapeutic need for cardiovascular disease (CVD) patients. Here, we review the diverse contributions of immune cells to atherosclerosis, including mechanisms of activation in this context and the cytokine circuits that underlie disease progression, and the recent application of these insights in the form immunotherapy to treat cardiovascular disease. Recent studies on the cardiovascular comorbidity that arises in autoimmunity are revealing additional roles for cytokines in atherosclerosis. We discuss these findings and highlight data pointing to interleukin (IL)-1b, tumor necrosis factor, and IL-17 as cytokines that, at least in some settings, may present effective targets to reduce cardiovascular disease progression.
ETOC
The appropriate management of arterial inflammation, a hallmark of atherosclerosis, represents an unmet therapeutic need for cardiovascular disease patients. Randolph and colleagues review the cytokine circuits that underlie the diverse contributions of immune cells to atherosclerosis and discuss the recent application of these insights in the form immunotherapy to treat cardiovascular disease.
Introduction
Atherosclerosis is a ubiquitous pathology in humans, being observed in ancient populations for at least the last 4000 years (Allam et al., 2009). Its slow natural progression amplifies during aging and can lead to acute myocardial infarction, typically beyond the 4th decade of life. Anatomically and histologically, atherosclerosis is characterized by the development of a pronounced chronic inflammatory response in the intimal layer of artery walls (such as coronary arteries in human). The arterial intima is the layer between the arterial endothelium and the first band of elastic lamina in arteries, such that the intima is positioned on top of the smooth muscle cell rich medial layer and the outer arterial layer known as the adventitia. The progression of inflammation increases the size of the intima, forming an inflamed structure called plaque, which narrows the volume of space for blood flow through the vessel (stenosis). The inflammatory response can also result in sudden rupture of intimal plaque integrity that can trigger episodic occlusion of the vessel (often a coronary artery supplying the heart) giving rise to rapid ischemia and consequent myocardial infarction. As discussed in more detail below, atherosclerosis arises from two intersecting pathophysiological developments: (i) overwhelmed or defective cholesterol handling and (ii) low-level constitutive activation of the arterial vasculature due to oscillatory blood flow, such that vascular permeability appears to increase enough to allow cholesterol to access the artery wall in the first place. That is, despite low level inflammation being a natural feature of vessels characterized by oscillatory flow, atherosclerosis will typically not take hold if plasma cholesterol is low because it must begin to accumulate in the artery wall to advance disease. Because oscillatory blood flow is a feature of curved or branching arteries, plaques tend not to form continuously along the arterial intima, but rather at focal points around branches and curves of arteries.
Atherosclerosis has historically been the leading cause of cardiovascular disease. However, major advances in treatment, especially the use of statins, and improvements in diet and lifestyle over the past several decades have markedly reduced atherosclerosis as a cause of cardiovascular mortality, giving way to heart failure as the cardiovascular condition most starkly on the rise (Benjamin et al., 2018). Nonetheless, because of the ubiquitous tendency for atherosclerosis to develop in human subjects, there remains a need to find new ways to combat atherosclerosis. That is, some populations remain at high risk as relative non-responders to frontline lipid-lowering therapy (statins), while others may benefit from additional drugs that act in concert with standard-of-care approaches. In particular, as we discuss herein, autoimmune and chronic inflammatory diseases including lupus, rheumatoid arthritis, psoriasis, and, more recently, inflammatory bowel disease have been linked to increased cardiovascular comorbidity and potential premature mortality in these patients.
Besides drugs that target lipid management, there is now emerging evidence that targeting inflammation, particularly the cytokines that orchestrate inflammation, can further lower atherosclerosis. Therapeutically targeting soluble cytokines indeed has yielded dramatic benefits in a wide variety of inflammatory diseases, including in many of the autoimmune diseases listed above. However, translation of these powerful approaches into patients for the directed treatment of established cardiovascular disease is in its infancy. Here, we review the basic pathophysiology of cardiovascular inflammation, discuss the current status of antiinflammatory therapy in human atherosclerosis, followed by in-depth analysis of the underlying cytokine networks. We then focus on what is known and what remains unknown about the risk of cardiovascular disease in autoimmunity, with a view toward the possibility that treatments will emerge to combat not just the underlying autoimmunity but also its coincidence with cardiovascular disease.
Genesis, evolution and cellular composition of the atherosclerotic plaque
The Nobel Prize-winning, pioneering work of J. Goldstein and M. Brown on the cell biology of low density lipoprotein and its major receptor led to, in their own words, the “inescapable conclusion that the LDL pathway functions in man to protect against atherosclerosis”(Goldstein and Brown, 1977). Cholesterol is an essential molecule in animal cells that is required for appropriate membrane integrity and to facilitate signaling within membranes. It is also the precursor of various metabolites like vitamin D, bile acids, oxysterol intermediates that signal in immunity, and steroids, including sex hormones and hormones of the adrenal gland (Cyster et al., 2014). As such, cholesterol is synthesized in the liver and is distributed to cells in the body through secretion into plasma of esterified cholesterol packaged within the lipoprotein particles very low density lipoproteins (VLDL) and LDL, in which apolipoprotein B100 serves as the key amphipathic protein that assembles the lipoprotein. Alternatively, cholesterol is absorbed from the diet, packaged within intestinal epithelial cells along with triglycerides in particles called chylomicrons that rely on the alternatively spliced form of apolipoprotein B, apoB48, for assembly. These lipoprotein particles deliver cholesterol to tissues after passage through the endothelial barrier via transcytosis mechanisms involving a range of different receptors (Zhang et al., 2018), or via reduced junctional integrity associated with increased vascular permeability (Essler et al., 1999; Mundi et al., 2018).
Much of what we now know about cholesterol trafficking and metabolism, and associated atherosclerosis, has been learned using experimental models of the disease, especially from mouse models. The rate of LDL-cholesterol transcytosis across the endothelium is affected by conditions that impact cardiovascular susceptibility; for instance, estrogen, with an established protective role against atherosclerosis, limits transcytosis rates (Ghaffari et al., 2018; Sessa, 2018). Once LDL-cholesterol has entered the artery wall, it fails to broadcast deeply and does not readily pass out of tissues (Bremmelgaard et al., 1986; Stender and Hjelms, 1984). This entrapment of LDL in the subendothelial space is the basis for the widely held “response-to-retention” hypothesis for the genesis of atherosclerotic plaque (Williams and Tabas, 1995). The “response” of greatest importance is the ensuing inflammatory response, the subject of the present review.
The “response-to-retention” hypothesis was generated as a refinement of the “response-to-injury” hypothesis originally proposed by Russell Ross (Ross et al., 1977). The latter importantly underscores that the status of plaque endothelium is critical. While it is not necessarily overtly injured, the arterial endothelium at sites prone to plaque development (arterial branches and areas of nonlaminar blood flow) displays constitutive activation of NFkB and expression of adhesion molecules like Vascular Cell Adhesion Molecule 1 (VCAM1), which is rather normally observed in postcapillary venules activated by master proinflammatory cytokines TNFα or IL-1β. This state of activation in the endothelium is not sufficient to initiate plaque formation unless cholesterol levels in plasma rise (Hajra et al., 2000). That is, even C57BL/6 mice display activated arterial endothelium, but atherosclerosis does not develop in standard C57BL/6 mice. Mouse strains must be engineered to elevate plasma cholesterol, such as via loss of LDL receptor or VLDL receptor cofactors like apoE (Ishibashi et al., 1993; Zhang et al., 1992) following consumption of a cholesterol-enriched diet (Lichtman et al., 1999). Thus, the core elements of both the response-to-injury (initial endothelial activation) and response-to-retention (cholesterol accumulation) hypothesis appear to apply and are not mutually exclusive but rather complementary.
From an immunological perspective, it is fascinating to note that a specialized population of mononuclear phagocytes, sometimes referred to as vascular dendritic cells (Choi et al., 2009; Jongstra-Bilen et al., 2006; Millonig et al., 2001; Paulson et al., 2010), characterizes the normal arterial intima. So far as is known, these cells are present from birth onward in humans (Bobryshev and Lord, 1995, 1998; Waltner-Romen et al., 1998) and mice (Jongstra-Bilen et al., 2006; Liu et al., 2008), independent of the presence of atherosclerosis, possibly evolving to carry microbes out of the artery wall upon infection (Roufaiel et al., 2016). Cybulsky and colleagues have demonstrated that initiation of atherosclerosis is linked to the activity of these cells (Cybulsky and Jongstra-Bilen, 2010; Paulson et al., 2010) (Williams and Randolph, unpublished data). However, how they initiate the inflammatory cascades that promote the genesis of atherosclerotic plaque is not well understood. Paulson et al. make a case for these phagocytes being the first to acquire and accumulate cholesterol. Indeed, these cells may participate in the import and retention of cholesterol into the artery wall itself (Paulson et al., 2010), while simultaneously fostering the inflammatory triggers that subsequently recruit monocytes steadily throughout the course of the ensuing chronic disease (Swirski et al., 2006).
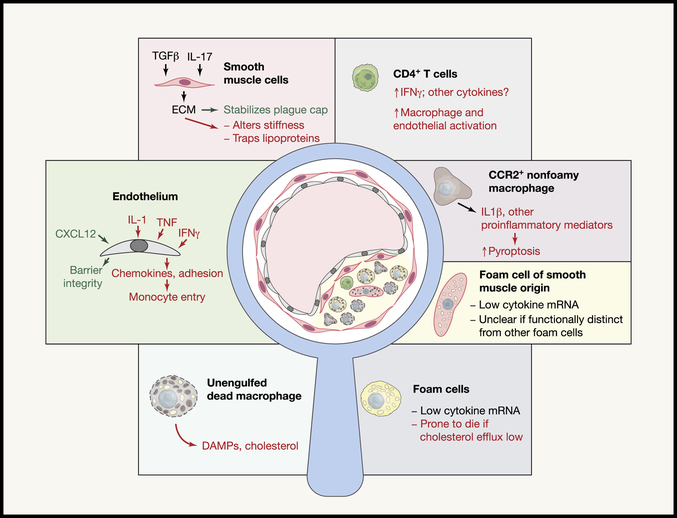
Cholesterol taken up by these vascular resident phagocytes and by monocytes that begin to swarm in, in concert with cholesterol retention in the artery wall, is readily esterified and stored in cytoplasmic lipid droplets. Such a phagocyte heavily burdened with lipid droplets is referred to as a foam cell. One long-held concept thought to link foam cells to the progression of atherosclerosis was the idea that esterified cholesterol could be not contained in esterified form efficiently enough, such that some excess level of free cholesterol would occur to drive signaling changes and cellular toxicity. In this model, the foam cell was considered the central driver of inflammation in plaques. However, in the last few years, a new concept emerged that, even early on in plaques, cholesterol crystals are formed and these, in turn, activate the inflammasome and lead to the local production of IL-1β (Duewell et al., 2010). This concept is currently one of the most compelling in the field and can be associated with recent clinical studies (discussed below) as well as much current research activity in the field. In concurrence with this concept, recent work illustrates that foam cells in plaques are not expressing inflammatory cytokine mRNA for IL-1β or other proinflammatory cytokines or chemokines, but instead such expression is observed in CCR2+ nonfoamy macrophages within plaques that are more akin to recently recruited monocytes (Kim et al., 2018) (Figure. 1). These data fit with observations that foam cell formation is not inherently pro-inflammatory, but rather antiinflammatory due to activation of the transcription factor Liver X Receptor (LXR) via binding cholesterol synthesis intermediate desmosterol as a ligand (Spann et al., 2012). Collectively, these new findings raise important questions for the future. Are the IL-1β+ inflammatory macrophages recovered from plaque an end-stage macrophage that has gone down a distinct and nonoverlapping differentiation pathway from that of foam cells (Figure 2)? Or are the proinflammatory CCR2+ cells monocyte-like cells part way through the process of differentiating into foam cells (Figure 2)? Since it’s known that newly recruited monocytes have limited ability to penetrate deeply within plaques (Williams et al., 2018), the presence of CCR2+ inflammatory macrophages are nearer the luminal surface, which may give these macrophages better access to immunomodulatory drugs.
Figure 1. A close look at the cellular composition of atherosclerotic plaques and their connection to cytokine circuits.
Like a mysterious crime thriller, the complex interactions of cells and cytokines within the atherosclerotic plaque promoting dysfunction are still being recognized. This figure schematizes an atherosclerotic plaque within a magnifying glass. The various cell types in the plaque are delineated around the outer margins. Cytokine circuits that promote adverse pathology are shown in red text, favorable pathways in green text, and neutral pathways in black.
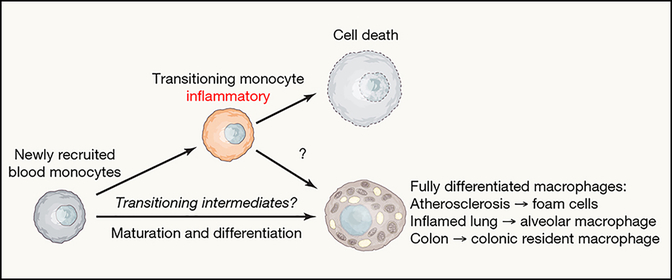
Figure 2. Monocyte – macrophage differentiation pathways in atherosclerotic disease.
The diversity of myeloid cells within atherosclerotic plaque was recently studied by single-cell RNA-sequencing. Furthermore, literature in other organ systems, as discussed in the text, highlights evidence that intermediate stages of monocytes differentiating to tissue resident macrophages pass through a transient pro-inflammatory phase. However, inflammatory and less inflammatory resident states may be distinct pathways. On the right, we depict a predicted cell fate map based on Monocle analysis of the work by Kim et al. (Kim et al., 2018)
Examples are readily found in the literature that illustrate that monocytes can go through a highly inflammatory transitional stage as they differentiate during inflammation. First, monocytes in the colon continuously differentiate to resident colonic macrophages that have antiinflammatory roles. However, in experimental colitis, they are arrested in the transitional phase of differentiation that coincides with a highly inflammatory state (Bain et al., 2013) (Figure 2). In a model of lung fibrosis, monocytes in transition to alveolar macrophages are inflammatory and pro-fibrotic, but ultimately differentiate to anti-inflammatory alveolar macrophages resembling resident macrophages observed in homeostasis (Misharin et al., 2017) (Figure 2). However, from single cell RNA sequencing (scRNAseq) data (Kim et al., 2018), an algorithm to predict differentiation pathways can be applied. The results suggest that there may be little or no cross-over in the pathways that lead to the IL-1β+ inflammatory signature versus that of the foam cell populations (unpublished observations). This issue is important to settle experimentally because if the inflammatory macrophage in plaque is on a continuum toward a non-inflammatory state, one scenario therapeutically might be to accelerate progression through the transient inflammatory period. However, if the inflammatory state is a distinct pathway that involves a common precursor with the foam cell (like the monocyte), but otherwise does not overlap with pathways leading to foam cells, therapeutics might be envisioned that would block the inflammatory trajectory altogether without affecting the cholesterol removal function of foam cells.
Besides monocyte recruitment expanding the pool of foam cells, plaque macrophages may also expand by proliferation (Robbins et al., 2013). The relative balance of macrophage proliferation versus recruitment from blood remains rather unclear. Beyond macrophages, there is growing appreciation for a diversity of cell types and phenotypes that also are found in plaques, including lymphocytes and dendritic cells (Choi et al., 2011; Frostegard et al., 1999; Macritchie et al., 2012; Sage et al., 2014; Yun et al., 2016). As discussed in detail elsewhere (Hansson and Hermansson, 2011; Wolf and Ley, 2019), antigen-specific T cells (primarily Th1/Th17) may promote plaque inflammation, whereas regulatory T cells may reign in plaque inflammation (Ait-Oufella et al., 2006; Subramanian et al., 2013). Recent advances using tetramers to identify regulatory T cells (Treg) recognizing a peptide in apoB, the major protein in LDL, supports the immune-modulatory potential of targeting these cells in mouse and man (Kimura et al., 2018) and the possibility that vaccination could be used to expand them (Kimura et al., 2015; Virmani et al., 2006). B lymphocytes are also implicated in local and systemic influences on disease progression, with pending clinical studies on the horizon (Sage et al., 2018). Innate B cell-derived antibodies have a disease-protective function, in part by clearing and modifying cellular responses to oxidized LDL (Sage et al., 2018). Furthermore, the arterial adventitia contains a diverse and rich immune cell milieu, including a variety of macrophages with a distinct life cycle and function from those in the intima (Ensan et al., 2016; Lim et al., 2018). As plaque advances in mice, especially aging mice, the adjacent adventitial compartment can develop a robust tertiary lymphoid compartment with disease-modulating activity (Grabner et al., 2009; Hu et al., 2015). It is beyond the scope of this review to discuss the biology and cell types of the adventitia, but it seems quite likely that the growing research on this topic will affect our understanding of plaque genesis and progression. We furthermore emphasize the technical problem that the adventitial network of cells creates for the study of intimal phagocytes: even in plaque-bearing arteries, single cell suspensions of the artery typically are comprised in majority of adventitial cells, such that studies of atherosclerosis by flow cytometry requires one to proceed with caution.
Finally, beyond hematopoietic cells, it has been long appreciated that smooth muscle cells comprise key components of advancing plaques (Schwartz et al., 1986; Schwartz et al., 2018). Indeed, very early work in the field highlighted the clonal proliferation of smooth muscle cells as a crucial feature of plaque (Benditt, 1974). It is now universally appreciated that the presence of smooth muscle cells and the matrix they produce near the plaque lumen (typically called the “fibrous cap”) serves to physically stabilize the plaque by separating the deeper areas in plaque, which in advanced stages are necrotic and highly prothrombotic, from the bloodstream at the arterial lumen (Figure 1). In humans, the thickness of the fibrous cap is inversely linked to the probability that a plaque will rupture to initiate myocardial infarction (Virmani et al., 2006). Smooth muscle cells have a complex origin, with intimal plaque smooth muscle cells invading from the medial and adventitial layers, but also sometimes arising during endothelial to mesenchymal transitions (Kovacic et al., 2012). Strikingly, smooth muscles also have the capacity to transdifferentiate into macrophage-like cells that contribute to the foam cells present in plaque (Cherepanova et al., 2016; Rong et al., 2003; Shankman et al., 2015). At least for foam cells that still bear enough features of smooth muscle cells to discern them as such (eg., cells expressing residual smooth muscle actin mRNA), these foam cells share with the more classical macrophage foam cells the feature of being relatively noninflammatory in gene expression profile (Kim et al., 2018) (Figure 1).
While we have emphasized that living foam cells are not the cells in plaque actively producing cytokines (Kim et al., 2018), this finding does not imply that foam cells do not strongly promote progression of plaque or inflammation indirectly (Figure 1). One major role they play in disease progression is linked to their death, either by necroptosis (Lin et al., 2013), which is directly connected to inflammation and release of danger-associated molecular patterns (DAMPs), or apoptosis (Feng et al., 2003). Pyroptosis that is, by definition, connected to inflammasome activation may occur in plaque, given the role of NLRP3 in disease progression (Duewell et al., 2010). However, based on the expression profile of plaque macrophages, this mode of death is more likely relevant to nonfoamy macrophages. In contrast to necroptosis and pyroptosis, apoptosis is not an inflammatory process, but if apoptotic cells are not readily removed through a process called efferocytosis, inflammation can nonetheless ensue. Efferocytosis is well documented to be defective in atherosclerosis (Thorp et al., 2008; Thorp and Tabas, 2009). Consequently, dead cell debris accumulates within plaques, with the accumulated material forming acellular pockets of debris referred to as necrotic core. Expansion of necrotic core is destabilizing and, in man, increases the probability of clinically significant plaques vulnerable to rupture. Recently, blockade of CD47, “the don’t eat me signal,” was shown to restore efferocytosis in experimental atherosclerosis (Kojima et al., 2016). That atherosclerosis progression was blunted strongly by anti-CD47 treatment indicates that restoring efferocytosis guards against the accumulation of necrotic core that drives plaque vulnerability, even as it also underscores the important role that failed efferocytosis has in driving plaque inflammation and progression. The study implicated the well-characterized proinflammatory cytokine tumor necrosis factor (TNF) in the blockade of efferocytosis (Kojima et al., 2016). It also seems possible that the lingering of necrotic debris derived from foam cells, with accumulated DAMPs and extracellular cholesterol crystals (Katz et al., 1976), is ultimately linked to the recruitment and activation of the nonfoamy CCR2+ IL-1β+ macrophages identified in plaques, tying the two macrophage types together in a positive feedback loop that promotes disease.
The cytokine IL-1β in cardiovascular disease – from bench to bedside
The aforementioned work suggests that targeting cytokines like TNF and IL-1 might comprise a viable therapeutic strategy to reduce disease burden. The success of statins as standard-of-care therapy naturally means that new therapeutics would most typically be add-ons to statin treatment. Thus, it is important to recognize that statin treatment is among the disease management changes in recent history that has significantly altered the clinical presentation of atherosclerosis. For instance, based on analysis of human endarterectomy specimens, plaque macrophage content has declined, while collagen content has risen (van Lammeren et al., 2014). Similarly showing a trend toward features less likely to prompt plaque rupture is the increase in calcified burden and decrease in noncalcified burden (which would include inflammatory cells and necrotic core) in response to statin therapy (Lee et al., 2018). This shift has led to a de-emphasis on the problem of plaque rupture, with an increasing recognition that erosion of the plaque surface rather than acute rupture is becoming more common (Libby and Pasterkamp, 2015). One might then wonder if mouse models of experimental atherosclerosis currently in use still mimic therapeutically relevant atherosclerosis in the modern era, as the features of mouse plaque (like high macrophage content) more closely resemble human disease as it appeared in the past than the present. However, considering the success of recent therapeutic approaches that arose from research in mouse models, it is reasonable to cautiously argue that the mouse models remain highly relevant and valuable. A major example of recent success in translating work from experimental models to the clinic is the work that led to the successful identification of IL-1β as a viable therapeutic target.
As its name suggests, interleukin 1 (originally called human leukocytic pyrogen) is a long-established mediator of inflammation first purified in 1977 (Dinarello et al., 1977) and cloned in 1984 (Auron et al., 1984). The cytokine was first pursued for its functional connection to fever and the acute phase response, and this work paved the way for the concept that there were, in fact, “hormones of the immune system”. It was also quickly recognized that IL-1, along with TNF, acts as a central regulator of endothelial cell activation. Through activation of NFkB that in turn programs upregulation of adhesion molecules and chemoattractants, IL-1 orchestrates inflammatory cell recruitment to sites of inflammation (Bussolino et al., 1988; Furie and McHugh, 1989; Huber et al., 1991; Moser et al., 1989; Pober et al., 1986), an activity especially relevant to atherosclerosis. It is now clear that IL-1 is part of a family of cytokines, with family members being broadly linked to many inflammatory and developmental diseases. In addition to the two majors of IL-1, IL-1α and IL1β, family members include IL-18, IL-33, IL-36α, IL-36β, IL-36γ, IL-37, and IL-38 (Dinarello, 2018).
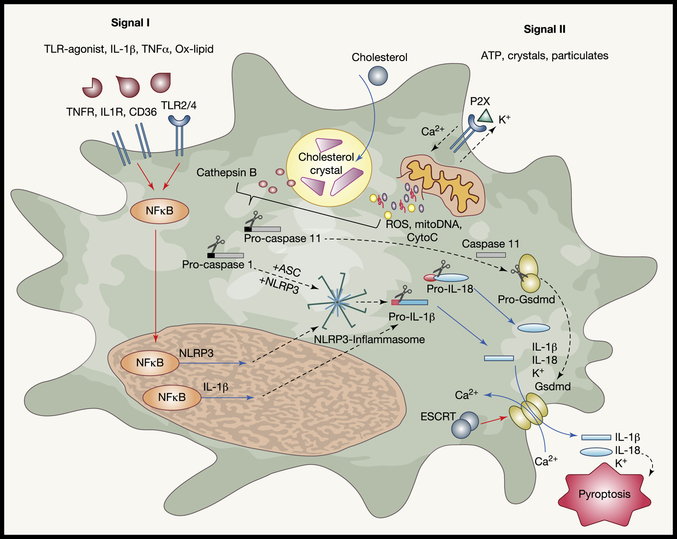
IL-1β is generated as an inactive propeptide in the cell cytoplasm, and activation requires cleavage of the pro-form of the molecule. Such cleavage can occur either intra- or extra-cellularly. IL-1β is typically cleaved by caspase-1 (interleukin-1 converting enzyme), an intracellular cysteine protease that also requires its own cleavage for activation (Figure 3). IL-1β propeptide can be expressed and the inflammasome can be activated in a variety of cells, including endothelial cells, but is especially relevant in macrophages (Libby et al., 1986; Xiao et al., 2013). Expression can be stimulated downstream of any one of a multitude of stimuli, including recognition of microbes, binding of extracellular ATP, hypoxia, and phagocytosis of non-degradable particles. The NLRP3 (nucleotide oligomerization domain (NOD), leucine rich repeat (LRR), and pyrin domain (PYD) containing protein 3)-mediated inflammasome is broadly associated with a variety of disease and represents the best studied mechanism for IL-1β activation (Rajamaki et al., 2010) (Folco et al., 2014). The NLR family consists of approximately 2 dozen family members in human and in excess of 30 in mice, many of which share the NLR and Pyrin domains that are active components of the inflammasome complex (Guo et al., 2015; Ting et al., 2008).
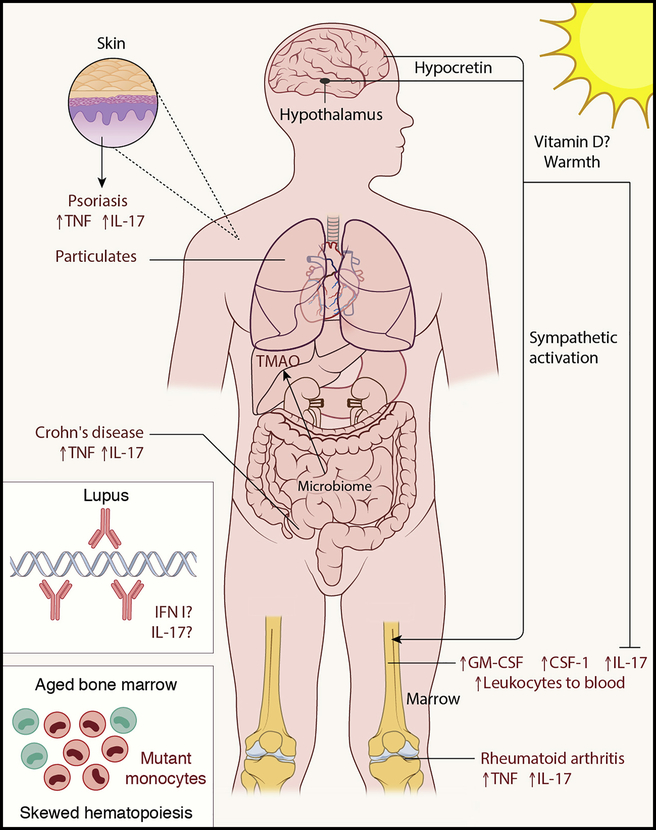
Figure 3. Systemic modifiers of atherosclerosis.
A few of the systemic modifiers of atherosclerosis are depicted here and discussed in the text. Text in red depict adverse signals that worsen cardiovascular disease and text in blue depict mechanisms that suppress cardiovascular disease, although recent studies have not found a benefit for vitamin D, as once thought.
Canonical activation of the inflammasome involves engagement of two independent signaling pathways (sometimes termed signal I and signal II) (Figure 3). In-depth analysis of the signaling pathways responsible for inflammasome activation were recently reviewed (Baldrighi et al., 2017; Grebe et al., 2018; He et al., 2016). In brief, signal I, or priming, is the step that induces expression of IL-1b and NLRP3 polypeptides. It occurs via activation of classic pattern recognition receptors, such as TLR2 or TLR4, or inflammation sensors like TNFR or IL1R (Franchi et al., 2009). These pathways converge on the common downstream transcription factor NFκB signaling pathway. NFκB activation prompts the expression of NLRP3 and IL-1β. Signal 2 then involves assembly of the NLRP3 inflammasome macromolecular complex, which in turn activates caspase 1 to cleave pro-IL1b to its active form. Crystals are often associated with the activation and progression of this second signal. In atherosclerosis, cholesterol crystals form in plaques (Small et al., 1984), including early plaques (Duewell et al., 2010), and even in cultures of cholesterol with macrophages (Kellner-Weibel et al., 1999), and these are thought to be vital to activation of NLRP3 and IL-1b in plaques (Figure 3). Mechanistically, crystal particulates can rupture the phagolysosome within macrophages, leading to spillage of cathepsin enzymes that in turn trigger NLPR3 activation and assembly. In late plaques, even during disease regression, extracellular crystals are common.
IL-1β and IL-18 lack N-terminal tags to promote their entry into the endoplasmic reticulum and subsequent export through conventional secretion pathways. As proteins generated in the cytoplasm, their secretion requires the formation of a specialized pore formed by the gasdermin d (Gsdmd) protein, which is cleaved downstream of active capase-11 (Kayagaki et al., 2015; Shi et al., 2015a). Following cleavage, Gsdmd oligomerizes at the cell membrane creating a pore for the release of cellular contents, including inflammatory molecules IL-1β and IL-18. This release also includes loss of membrane potential (K+), and calcium entry and, at least often, leads to the form of cell death called pyroptosis. However, whether this pathway inevitably leads to cellular death, or whether cells are able to release IL-1β and persist in the tissue is under great scrutiny, and this question is highly relevant to links between macrophage death, impaired efferocytosis, and inflammation in atherosclerotic plaque, as discussed above. Gsdmd-mediated pore formation was recently found to be controlled by the molecule called Ca2+-dependent recruitment of the endosomal sorting complexes required for transport (ESCRT), which functions to repair damaged membranes and thereby sparing death as a consequence of gasdermin pore formation (Evavold and Kagan, 2019; Ruhl et al., 2018).
Treatment of pigs chronically with exogenous IL-1β augments atherosclerosis progression (Shimokawa et al., 1996), whereas loss of IL-1β in apoE−/− atherogenic mice decreases plaque burden and endothelial activation (Kirii et al., 2003). Fitting with the latter, atherosclerosis in mice depends on the MyD88 signaling adaptor relevant to IL-1R signaling (Michelsen et al., 2004) and NLRP3 inflammasome (Duewell et al., 2010). Subsequently, in humans, IL-1β and NLRP3 were associated with the risk of coronary artery disease (Afrasyab et al., 2016), and showed elevated expression in the aorta of patients with atherosclerotic plaque formation (Paramel Varghese et al., 2016; Shi et al., 2015b; Zheng et al., 2013).
The canakinumab anti-inflammatory thrombosis outcome study (CANTOS) trial was the first anti-inflammatory trial to treat atherosclerosis (Ridker et al., 2017), and the target chosen for evaluation was IL-1β. Specifically, the trial used a fully humanized monoclonal antibody, canakinumab, previously approved for the treatment of rheumatoid arthritis (Ruperto et al., 2012; Schett et al., 2016) and cryopyrin-associated periodic syndrome (Lachmann et al., 2009). This drug to neutralize IL-1β was tested in high-risk cardiovascular disease patients who had a history of myocardial infarction and elevated acute phase protein C-reactive protein (CRP). In addition to being randomized for receipt of canakinumab, patients were maintained on standard-of-care therapy, which typically included aggressive statin use. A dose of 150 mg every 3 months showed a significant benefit in the primary endpoint that surveyed the number of cardiovascular events occurring between 3–4 years later. This IL-1β-targeted therapy effectively reduced circulating cytokine IL-6 and CRP, independent of any changes in cholesterol. While not a primary outcome of the study, canakinumab was also associated with reduced cancer mortality(Ridker et al., 2017), which is consistent with previous studies (Apte et al., 2006).
CANTOS established that inflammation can be targeted in atherosclerosis to reduce clinically significant cardiovascular disease. However, targeting of IL-1β has its own inherit risks. Patients receiving canakinumab experienced elevated fatal infections, which led to an overall no change in “all-cause” mortality associated with the study (Ridker et al., 2017). The inclusion of only patients with elevated CRP levels of >2 mg/L, shows the potential for targeting the inflammatory arm of atherosclerosis, but it leaves open the question of whether this approach will be effective for the general population that may have local generation of IL-1β in plaques but not signs of systemic inflammation. Importantly, patients that showed the most dramatic reduction in serum inflammatory markers correlated with those having least occurrence of cardiovascular events. Thus, overall, this study represents an important cornerstone in the field as it suggests that targeting the inflammatory arm of atherosclerosis can provide clinical benefit in atherosclerosis, even in the context of statin therapy. It remains uncertain, however, exactly where and how canakinumab conferred protection. Did it make its way into plaque to bind and neutralize IL-1b inside of the plaque milieu? Or did it largely fail to access the dense and complex plaque interstitium but instead critically act at the endothelial surface of the affected artery walls by inhibiting endothelial activation and thus further recruitment of inflammatory cells? Or, given that responders had evidence of systemic inflammation that was reduced by the drug, was its main effect on a systemic target that may be anatomically distinct from plaque? If a systemic target was the most important target, what systemic target was pivotal? Finally, although its net effect was beneficial, were there detrimental effects of the blockade that suppressed an even greater benefit that might have otherwise occurred, given that IL-1b also has roles that are atheroprotective (Gomez et al., 2018)? Answering these questions that aim to better understand the mechanism of action might pave the way for a therapeutic approach to be devised in the future that has similar or greater reductions in atherosclerotic inflammation without heightened risk in host defense.
Systemic modifiers of atherosclerosis – lessons from autoimmunity
The possibility that therapeutics like canakinumab act at least in part systemically to quell atherosclerosis progression is not far-fetched, given the growing appreciation for systemic regulators of cardiovascular disease. So far, we have discussed atherosclerosis as a local disease, and indeed, lesions are local, chronic foci of inflammation. However, it is clear that there are many systemic modifiers of disease. Such modifiers include several well known risk factors like smoking, diet, and sedentary life style, as well as others like inhaled environment particulates (Bai and Sun, 2016) (Figure 4), with many of these common risk factors still not fully understood mechanistically. Dietary interventions like a shift to a “Mediterranean diet” strongly reduced cardiovascular events, even in a population of subjects also treated with statins (Estruch et al., 2018). Diet, of course, in turn affects the intestinal microbiome that, in the presence of the right substrates, can promote the generation of pro-atherogenic metabolites like trimethyl amine oxide (TMAO) from dietary intermediates (Tang and Hazen, 2014) (Figure 4). The mechanism by which TMAO promotes atherosclerosis is also not fully understood.
Figure 4. Inflammasome activation in atherosclerosis progression.
The two-step signaling sequence in activation of the inflammasome is schematized here and discussed in the text. We emphasize the concept that, in atherosclerosis, cholesterol crystals may be the disease-driving signal that links cholesterol and inflammatory pathways in atherosclerosis.
A growing list of systemic modifiers mechanistically act to affect the bone marrow’s production of myeloid cells. It has been documented that high neutrophil and monocyte counts in the blood are an independent risk factor for cardiovascular disease (Chapman et al., 2004; Coller, 2005; Danesh et al., 1998; Nasir et al., 2005), perhaps in part by increasing the supply of cells able to become recruited to atherosclerotic plaques. Mouse models of have begun to shed light on systemic modifiers that control myeloid cell output. Atherosclerosis is elevated after an event like myocardial infarction due to the elevation in β3-adrenergic signaling in a brain-bone marrow axis that sends more monocytes into the bloodstream (Dutta et al., 2012) (Figure 4), which in turn is linked to GM-CSF production (Anzai et al., 2017). The GM-CSF receptor also accounts for elevated myeloid cell output from the bone marrow driven by hypercholesterolemia (Wang et al., 2014). Atherosclerosis is also elevated by a disrupted sleep cycle that results in a reduction in the sleep-wake cycle mediator hypocretin. Hypocretin normally suppresses myelopoiesis through limiting production of colony stimulating factor 1 (CSF-1) in the bone marrow (McAlpine et al., 2019). Conversely, warm ambient temperature is associated with reduced cardiovascular risk and is linked in mice and man to a reduced output of monocytes from the marrow (Williams et al., 2017) (Fig. 4). Warm ambient temperature is often associated with increased sun exposure, potentially affecting vitamin D levels, which has been argued in observational studies to be linked to cardiovascular risk. However, a recent large clinical trial of vitamin D supplementation fail to indicate a key role for vitamin D in affecting cardiovascular outcomes (Manson et al., 2019), raising questions about the validity of the link (Fig. 4). Finally, in a phenomenon called clonal hematopoiesis, somatic mutations that occur in hematopoietic stem cells during aging can allow for selection and outgrowth of clones that express disease-adverse mutations. Some of these appear to negatively affect myelopoiesis or cardiovascular disease (Jaiswal et al., 2014) (Fig. 4). Thus, many lifestyle features, from diet to sleep to ambient temperature and aging, seem to act on atherosclerosis by regulating the bone marrow output of myeloid cells.
Another major class of systemic risk in cardiovascular disease may be unrelated to direct effects on myeloid cell output from the bone marrow – autoimmunity (Figure 4). It is striking that a number of diseases classified as autoimmune diseases carry substantially elevated risk of cardiovascular events and mortality. Chief among them is lupus with an elevated risk of atherosclerosis, where the presence autoantibodies in the disease increases the odds of cardiovascular events by more than 5 times (Ballocca et al., 2015). Well documented, increased risk is also observed in rheumatoid arthritis (Young et al., 2006) and psoriasis (Hu and Lan, 2017). More recently, data pointing to elevated cardiovascular risk has emerged in the inflammatory bowel disease, Crohn’s disease (Aniwan et al., 2018; Wu et al., 2017) and type I diabetes (Lee et al., 2015; Subramanian and Hirsch, 2018). If the reports in Crohn’s disease hold up in further investigation, the finding will be especially interesting, because Crohn’s disease is associated with reductions in plasma LDL-cholesterol (Aarestrup et al., 2018; Levy et al., 2000; Romanato et al., 2009), thus highlighting the possibility that increased cardiovascular disease can be sufficiently driven by inflammatory triggers like cytokines to overcome, or counterbalance, the protective effect that lowered LDL would confer.
Among all the diseases listed in the paragraphs above, only type I diabetes has a defined antigen discovered to date, with autoreactive T cells directed against specific insulin peptides, leading to T cell-mediated destruction of pancreatic islet β cells (Wan et al., 2018). One model related to the antigenic targets in autoimmunity that may be especially relevant in lupus is that human endogenous retroviruses, which are ubiquitous and active in the genome, elicit autoimmune T cells (Suntsova et al., 2015; Tokuyama et al., 2018). Recent data suggest that lupus patients overexpressed certain endogenous retroviral products due to loss of negative regulators that would otherwise suppressed expression; this was also true in mice (Treger et al., 2019). Perhaps with fundamental understanding of most autoimmune diseases still under investigation, it is not surprising that the mechanistic basis of heightened cardiovascular disease risk is scarcely understood. However, given that the consequence of elevated cardiovascular risk is decreased overall mortality, it is quite important that clinical care of autoimmune patients take elevated cardiovascular risk into account.
A reasonably straightforward concept that might account for heightened cardiovascular disease in autoimmunity is that circulating cytokines produced at the site of autoimmune reactions are made in enough abundance to spill into the circulation, and then act on distal arteries prone to atherosclerosis to promote vascular cell activation and dysfunction. We call this hypothesis cytokine spillover. To take this hypothesis into account, we will focus on recent efforts in the literature to understand the cardiovascular comorbidity in psoriasis, as now cardiovascular drivers in this autoimmune disease, while still primitive, are a bit better understood in psoriasis than the others. First, it is quite clear that there are systemic vascular changes in psoriasis. Recent transcriptional profiling shows evidence of venous endothelial activation to express adhesion molecules and chemokines, and expression of IL-1β mRNA and other cytokines by circulating leukocytes (Garshick et al., 2019). Circulating TNF and IL-17 have been documented in psoriasis (Hu and Lan, 2017). Drugs that neutralize TNF not only reduce the skin disease in some patients, but also effective at reducing cardiovascular events, as shown in numerous studies (Hu and Lan, 2017). One might assume that anti-TNF blockade acts by quelling the action of its target in the systemic circulation, which has a number of redundant effects with IL-1 on endothelium and other cellular targets.
However, there is some cause for questioning the cytokine spillover model. First, the extent of organ damage in lupus, a change that would be expected to correlate with the magnitude of cytokine release, is a lesser risk factor for cardiovascular events than the presence of autoantibodies (Ballocca et al., 2015) (Fig. 4). Furthermore, a particular observation arising in human subjects and mouse models of psoriasis related to the use of IL-23 targeted therapeutics is hard to explain in the context of the cytokine spillover concept. The cytokine IL-23 plays a role in the induction of Th17 immunity, although putative nonpathogenic Th17 cells can develop in the absence of IL-23 signaling (Meyer Zu Horste et al., 2016). Both IL-23 and IL-17 appear to have strong roles in psoriatic lesions in man (Nestle et al., 2009). This insight led to the more recent development of therapeutics targeting IL-23 and IL-17 after anti-TNF was already in clinical use. Therapeutics targeting IL-23 are effective at reducing skin lesion manifestations, and thus would certainly have been expected to reduce cytokine spillover. However, such treatment has not prevented enhanced cardiovascular disease in several studies (Hu and Lan, 2017), including a recent observational study with a prospective design that examined plaque burden and characteristics over a 1-year endpoint in psoriasis subjects using plaque imaging (Elnabawi et al., 2019).
One might argue that these studies suggest that the IL-23 – IL-17 circuit itself is not relevant to cardiovascular disease burden, in contrast to the effects of blocking broadly pro-inflammatory cytokines like IL-1 and TNF. However, in an exciting additional arm of the recent plaque imaging study, the impact of IL-17 blockade was able, in contrast to the therapeutic targeting IL-23, to not only prevent plaque expansion in the 1-year analysis, but disease burden actually declined (Elnabawi et al., 2019). Thus, it may be the case that IL-17, even if not IL-23, is a viable target to reduce cardiovascular risk in psoriasis. The mechanism of action remains unclear. In a mouse model of psoriasis initiated by the use of the TLR7 agonist imiquimod, we have recently reported that chronic experimental psoriasis leads to changes in the extracellular matrix that are dependent upon IL-17 but, like the clinical studies, not prevented by targeting IL-23 p40 (Huang et al., 2019). We argue that the changes in extracellular matrix have the capacity to promote lipoprotein retention and vascular stiffness, such that IL-17 might be linked to both enhanced atherosclerosis and hypertension. In other models of hypertension in mice, IL-17 has been established to be a critical player and may act by targeting the matrix-producing capacity of vascular smooth muscle cells (Wu et al., 2014). What remains rather unclear in these model systems is whether IL-17 is produced within the artery wall by T cells recruited therein (the models are dependent on T cells (Guzik et al., 2007; Huang et al., 2019) or if systemically circulating IL-17 was operative. Given the argument that multiple autoimmune diseases converge on common features like a role for nucleic acid sensors (Theofilopoulos et al., 2017), including TLR7, it seems possible that the data in the context of psoriasis will be relevant to other autoimmune diseases beyond psoriasis. IL-17 is elevated in rheumatoid arthritis (Kotake et al., 1999) (Figure 4) and lupus (Speeckaert et al., 2016), even though lupus is particularly associated with a strong type I interferon signature (Obermoser and Pascual, 2010).
Might neutralizing IL-17 might be an attractive approach to treating atherosclerosis in the general population, beyond individuals affected by autoimmunity? There is reason to consider that the answer may be indeed be yes. First, returning to the theme of myeloid cell supply from the bone marrow, the effector functions of IL-17 include regulation of the recruitment of neutrophils to some infections (Korn et al., 2009). Connected to this effector role, IL-17 promotes granulopoiesis (Forlow et al., 2001; Liu et al., 2010; Parsa et al., 2016; Stark et al., 2005; von Vietinghoff and Ley, 2009) and, thus, here we return to the theme of systemic mediators that affect output from the bone marrow. Second, most studies examining deficiency or blockade of IL-17 in mouse models of atherosclerosis are associated with reduced disease progression (Erbel et al., 2014; Erbel et al., 2009; Madhur et al., 2011; Smith et al., 2010), including models combining atherosclerosis with renal impairment (Ge et al., 2013; Nordlohne et al., 2018), which may be relevant to renal complications in lupus. On the other hand, the role of IL-17 in promoting collagen deposition not only might exacerbate disease by trapping lipoprotein (Huang et al., 2019), it may also promote the favorable deposition of collagen that creates the protective fibrous cap at the atherosclerotic lesion lumen (Gistera et al., 2013). These data collectively indicate that IL-17 may have some protective roles in atherosclerosis like favoring development of the fibrous cap, but overall it appears to be primarily linked to detrimental effects that drive disease progression.
We have implied that lessons from the links between autoimmunity and cardiovascular disease might yield insight into approaches to treat the inflammatory aspect of atherosclerosis in the general population. However, we would be remiss not to mention that there is reason for caution in this line of logic, related to similar logic being applied in the recent past to the use of methotrexate as a treatment to combat inflammation in atherosclerosis. Methotrexate has long been considered an anti-inflammatory intervention used clinically. Although the antiinflammatory impact of the drug methotrexate seems complex and not fully understood, it is thought to act through modulation of folate metabolism (Brown et al., 2016). Used widely and effectively in rheumatoid arthritis, methotrexate lowers accompanying cardiovascular risk (Roubille et al., 2015). It is also effective at reducing cardiovascular risk in psoriasis (Hu and Lan, 2017). These favorable outcomes served as part of the motivation for a second trial, the Cardiovascular Inflammation Reduction Trial (CIRT), that was developed side-by-side with the CANTOS trial discussed above. Unfortunately, methotrexate failed to reduce serum inflammatory markers IL-6, IL-1β, and CRP, and no significant changes in cardiovascular events during the study period were observed (Ridker et al., 2019). These findings raise strong caution about the possibility that effective targets to treat cardiovascular manifestations in autoimmunity can be extended to the general population. However, a major caveat with methotrexate is its nonclassical mechanism of action with respect to inflammation, as it may simply be unable to effect anti-inflammatory shifts in the general population.
Concluding remarks
In this review, we covered emerging concepts in the basic understanding of atherosclerosis and turned to consideration of the role that cytokines play in the disease and whether targeting cytokines has therapeutic potential in the general population or in patients with autoimmunity that carry heightened cardiovascular risk. We underscore that, even though the disease characteristics are evolving in humans as treatments improve, mouse models of atherosclerosis remain fundamental tools that have great utility in understanding the disease and evaluating targets for new therapy. A major point of emphasis is our recent understanding that there are two types of macrophages in plaques, foamy and nonfoamy macrophages, that we propose work in synergy to drive disease progression. This synergy likely revolves around the generation of crystalline cholesterol to spark IL-1β expression and activation in the nonfoamy population, with the foamy cells facilitating, likely through death in the plaque and impaired efferocytosis, the availability of extracellular cholesterol in the plaque. Fitting with this concept is the success of anti-IL1β as a treatment target. However, it remains unclear if the treatment was effective because it acted within plaques or acted systemically. Indeed, whether and how systemic cytokines distal to plaque might affect disease, even in conditions like autoimmunity, remain to be clearly delineated. What is very clear is that some modifiers of cardiovascular risk target production of myeloid cells in the bone marrow, cells that may go on to enter plaque and promote its progression. Indeed, a wide variety of life style modifiers act by affecting myelopoiesis in the bone marrow.
Novel therapeutics to neutralize cardiovascular risk are especially needed in populations at higher risk, such as those with autoimmunity, but more information on mechanisms underlying the cardiovascular comorbidity is needed. Overall, large reductions in risk can be achieved by changes in lifestyle (here we highlighted diet (Estruch et al., 2018), with mechanisms of action just now coming into focus (eg., (McAlpine et al., 2019)) and much more work needed. In a closing remark that converges science with modern society, the New York Times Magazine recently reported on how those in our society most at risk for cardiovascular disease are in the bottom earning bracket, working perhaps two full-time minimum wage jobs such that substantial risk factors accumulate like loss of sleep, depression and isolation, lack of time for exercise, consumption of inexpensive but unhealthy food, and the desire to smoke tobacco as a stimulus to get through it all (Dollars on the Margin, Matthew Desmond). Ongoing studies suggest that an increase in minimum wage reduces psychosocial stress and promotes healthier lifestyle related to most of these factors, including the decision and ability to quit smoking (Du and Leigh, 2015). Effective strategies to combat cardiovascular disease are, thus, to be found both inside and outside of the laboratory.
Acknowledgements
We gratefully acknowledge the help of Konstantin Zaitsev (Washington University PhD Program in Immunology) in generating the Monocle lineage analysis described as unpublished data and to Dr. Bernd Zinselmeyer for assistance with illustrations. We are also grateful to Drs. Parrakal Deepak (Washington University) and Jaehoon Choi (Hanyang University) for scientific interactions and recent discussions that have helped to shape parts of this article. The authors are funded by NIH grants National Institutes of Health (NIH), R37 AI049653 and DP1DK109668 (to G.J.R.); NIH K99 HL138163 (J. W. W.), and a Career Development Award from the American Heart Association (L. H.). As of March 1, 2019, J. W. W. is affiliated with the Department of Integrative Biology and Physiology, and the Center for Immunology, University of Minnesota, Minneapolis, MN.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarestrup J, Jess T, Kobylecki CJ, Nordestgaard BG, and Allin KH (2018). Cardiovascular risk profile among patients with inflammatory bowel disease: a population-based study of >100,000 individuals. J Crohns Colitis. [DOI] [PubMed] [Google Scholar]
- Afrasyab A, Qu P, Zhao Y, Peng K, Wang H, Lou D, Niu N, and Yuan D (2016). Correlation of NLRP3 with severity and prognosis of coronary atherosclerosis in acute coronary syndrome patients. Heart Vessels 31, 1218–1229. [DOI] [PubMed] [Google Scholar]
- Ait-Oufella H, Salomon BL, Potteaux S, Robertson AK, Gourdy P, Zoll J, Merval R, Esposito B, Cohen JL, Fisson S, et al. (2006). Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med 12, 178–180. [DOI] [PubMed] [Google Scholar]
- Allam AH, Thompson RC, Wann LS, Miyamoto MI, and Thomas GS (2009). Computed tomographic assessment of atherosclerosis in ancient Egyptian mummies. JAMA 302, 2091–2094. [DOI] [PubMed] [Google Scholar]
- Aniwan S, Pardi DS, Tremaine WJ, and Loftus EV Jr. (2018). Increased Risk of Acute Myocardial Infarction and Heart Failure in Patients With Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol 16, 1607–1615 e1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzai A, Choi JL, He S, Fenn AM, Nairz M, Rattik S, McAlpine CS, Mindur JE, Chan CT, Iwamoto Y, et al. (2017). The infarcted myocardium solicits GM-CSF for the detrimental oversupply of inflammatory leukocytes. J Exp Med 214, 3293–3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apte RN, Dotan S, Elkabets M, White MR, Reich E, Carmi Y, Song X, Dvozkin T, Krelin Y, and Voronov E (2006). The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor-host interactions. Cancer Metastasis Rev 25, 387–408. [DOI] [PubMed] [Google Scholar]
- Auron PE, Webb AC, Rosenwasser LJ, Mucci SF, Rich A, Wolff SM, and Dinarello CA (1984). Nucleotide sequence of human monocyte interleukin 1 precursor cDNA. Proc Natl Acad Sci U S A 81, 7907–7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y, and Sun Q (2016). Fine particulate matter air pollution and atherosclerosis: Mechanistic insights. Biochim Biophys Acta 1860, 2863–2868. [DOI] [PubMed] [Google Scholar]
- Bain CC, Scott CL, Uronen-Hansson H, Gudjonsson S, Jansson O, Grip O, Guilliams M, Malissen B, Agace WW, and Mowat AM (2013). Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol 6, 498–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldrighi M, Mallat Z, and Li X (2017). NLRP3 inflammasome pathways in atherosclerosis. Atherosclerosis 267, 127–138. [DOI] [PubMed] [Google Scholar]
- Ballocca F, D’Ascenzo F, Moretti C, Omede P, Cerrato E, Barbero U, Abbate A, Bertero MT, Zoccai GB, and Gaita F (2015). Predictors of cardiovascular events in patients with systemic lupus erythematosus (SLE): a systematic review and meta-analysis. Eur J Prev Cardiol 22, 1435–1441. [DOI] [PubMed] [Google Scholar]
- Benditt EP (1974). Evidence for a monoclonal origin of human atherosclerotic plaques and some implications. Circulation 50, 650–652. [DOI] [PubMed] [Google Scholar]
- Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, et al. (2018). Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 137, e67–e492. [DOI] [PubMed] [Google Scholar]
- Bobryshev YV, and Lord RS (1995). Ultrastructural recognition of cells with dendritic cell morphology in human aortic intima. Contacting interactions of Vascular Dendritic Cells in athero-resistant and athero-prone areas of the normal aorta. Arch Histol Cytol 58, 307–322. [DOI] [PubMed] [Google Scholar]
- Bobryshev YV, and Lord RS (1998). Mapping of vascular dendritic cells in atherosclerotic arteries suggests their involvement in local immune-inflammatory reactions. Cardiovasc Res 37, 799–810. [DOI] [PubMed] [Google Scholar]
- Bremmelgaard A, Stender S, Lorentzen J, and Kjeldsen K (1986). In vivo flux of plasma cholesterol into human abdominal aorta with advanced atherosclerosis. Arteriosclerosis 6, 442–452. [DOI] [PubMed] [Google Scholar]
- Brown PM, Pratt AG, and Isaacs JD (2016). Mechanism of action of methotrexate in rheumatoid arthritis, and the search for biomarkers. Nat Rev Rheumatol 12, 731–742. [DOI] [PubMed] [Google Scholar]
- Bussolino F, Camussi G, and Baglioni C (1988). Synthesis and release of platelet-activating factor by human vascular endothelial cells treated with tumor necrosis factor or interleukin 1 alpha. J Biol Chem 263, 11856–11861. [PubMed] [Google Scholar]
- Chapman CM, Beilby JP, McQuillan BM, Thompson PL, and Hung J (2004). Monocyte count, but not C-reactive protein or interleukin-6, is an independent risk marker for subclinical carotid atherosclerosis. Stroke 35, 1619–1624. [DOI] [PubMed] [Google Scholar]
- Cherepanova OA, Gomez D, Shankman LS, Swiatlowska P, Williams J, Sarmento OF, Alencar GF, Hess DL, Bevard MH, Greene ES, et al. (2016). Activation of the pluripotency factor OCT4 in smooth muscle cells is atheroprotective. Nat Med 22, 657–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH, Cheong C, Dandamudi DB, Park CG, Rodriguez A, Mehandru S, Velinzon K, Jung IH, Yoo JY, Oh GT, and Steinman RM (2011). Flt3 signaling-dependent dendritic cells protect against atherosclerosis. Immunity 35, 819–831. [DOI] [PubMed] [Google Scholar]
- Choi JH, Do Y, Cheong C, Koh H, Boscardin SB, Oh YS, Bozzacco L, Trumpfheller C, Park CG, and Steinman RM (2009). Identification of antigen-presenting dendritic cells in mouse aorta and cardiac valves. J Exp Med 206, 497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller BS (2005). Leukocytosis and ischemic vascular disease morbidity and mortality: is it time to intervene? Arterioscler Thromb Vasc Biol 25, 658–670. [DOI] [PubMed] [Google Scholar]
- Cybulsky MI, and Jongstra-Bilen J (2010). Resident intimal dendritic cells and the initiation of atherosclerosis. Curr Opin Lipidol 21, 397–403. [DOI] [PubMed] [Google Scholar]
- Cyster JG, Dang EV, Reboldi A, and Yi T (2014). 25-Hydroxycholesterols in innate and adaptive immunity. Nat Rev Immunol 14, 731–743. [DOI] [PubMed] [Google Scholar]
- Danesh J, Collins R, Appleby P, and Peto R (1998). Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA 279, 1477–1482. [DOI] [PubMed] [Google Scholar]
- Dinarello CA (2018). Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol Rev 281, 8–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA, Renfer L, and Wolff SM (1977). Human leukocytic pyrogen: purification and development of a radioimmunoassay. Proc Natl Acad Sci U S A 74, 4624–4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, and Leigh JP (2015). Effects of wages on smoking decisions of current and past smokers. Ann Epidemiol 25, 575–582 e571. [DOI] [PubMed] [Google Scholar]
- Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, et al. (2010). NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464, 1357–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, Iwamoto Y, Thompson B, Carlson AL, Heidt T, et al. (2012). Myocardial infarction accelerates atherosclerosis. Nature 487, 325–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elnabawi YA, Dey AK, Goyal A, Groenendyk JW, Chung JH, Belur AD, Rodante J, Harrington CL, Teague HL, Baumer Y, et al. (2019). Coronary artery plaque characteristics and treatment with biologic therapy in severe psoriasis: results from a prospective observational study. Cardiovasc Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensan S, Li A, Besla R, Degousee N, Cosme J, Roufaiel M, Shikatani EA, El-Maklizi M, Williams JW, Robins L, et al. (2016). Self-renewing resident arterial macrophages arise from embryonic CX3CR1(+) precursors and circulating monocytes immediately after birth. Nat Immunol 17, 159–168. [DOI] [PubMed] [Google Scholar]
- Erbel C, Akhavanpoor M, Okuyucu D, Wangler S, Dietz A, Zhao L, Stellos K, Little KM, Lasitschka F, Doesch A, et al. (2014). IL-17A influences essential functions of the monocyte/macrophage lineage and is involved in advanced murine and human atherosclerosis. J Immunol 193, 4344–4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbel C, Chen L, Bea F, Wangler S, Celik S, Lasitschka F, Wang Y, Bockler D, Katus HA, and Dengler TJ (2009). Inhibition of IL-17A attenuates atherosclerotic lesion development in apoE-deficient mice. J Immunol 183, 8167–8175. [DOI] [PubMed] [Google Scholar]
- Essler M, Retzer M, Bauer M, Heemskerk JW, Aepfelbacher M, and Siess W (1999). Mildly oxidized low density lipoprotein induces contraction of human endothelial cells through activation of Rho/Rho kinase and inhibition of myosin light chain phosphatase. J Biol Chem 274, 30361–30364. [DOI] [PubMed] [Google Scholar]
- Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Aros F, Gomez-Gracia E, Ruiz-Gutierrez V, Fiol M, Lapetra J, et al. (2018). Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N Engl J Med 378, e34. [DOI] [PubMed] [Google Scholar]
- Evavold CL, and Kagan JC (2019). Defying Death: The (W)hole Truth about the Fate of GSDMD Pores. Immunity 50, 15–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B, Yao PM, Li Y, Devlin CM, Zhang D, Harding HP, Sweeney M, Rong JX, Kuriakose G, Fisher EA, et al. (2003). The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat Cell Biol 5, 781–792. [DOI] [PubMed] [Google Scholar]
- Folco EJ, Sukhova GK, Quillard T, and Libby P (2014). Moderate hypoxia potentiates interleukin-1beta production in activated human macrophages. Circ Res 115, 875–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forlow SB, Schurr JR, Kolls JK, Bagby GJ, Schwarzenberger PO, and Ley K (2001). Increased granulopoiesis through interleukin-17 and granulocyte colony-stimulating factor in leukocyte adhesion molecule-deficient mice. Blood 98, 3309–3314. [DOI] [PubMed] [Google Scholar]
- Franchi L, Eigenbrod T, and Nunez G (2009). Cutting edge: TNF-alpha mediates sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation. J Immunol 183, 792–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frostegard J, Ulfgren AK, Nyberg P, Hedin U, Swedenborg J, Andersson U, and Hansson GK (1999). Cytokine expression in advanced human atherosclerotic plaques: dominance of proinflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis 145, 33–43. [DOI] [PubMed] [Google Scholar]
- Furie MB, and McHugh DD (1989). Migration of neutrophils across endothelial monolayers is stimulated by treatment of the monolayers with interleukin-1 or tumor necrosis factor-alpha. J Immunol 143, 3309–3317. [PubMed] [Google Scholar]
- Garshick MS, Barrett T, Wechter T, Azarchi S, Scher J, Neimann A, Katz S, Fuentes-Duculan J, Cannizzaro MV, Jelic S, et al. (2019). Inflammasome Signaling and Impaired Vascular Health in Psoriasis. Arterioscler Thromb Vasc Biol, ATVBAHA118312246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Hertel B, Koltsova EK, Sorensen-Zender I, Kielstein JT, Ley K, Haller H, and von Vietinghoff S (2013). Increased atherosclerotic lesion formation and vascular leukocyte accumulation in renal impairment are mediated by interleukin-17A. Circ Res 113, 965–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaffari S, Naderi Nabi F, Sugiyama MG, and Lee WL (2018). Estrogen Inhibits LDL (Low-Density Lipoprotein) Transcytosis by Human Coronary Artery Endothelial Cells via GPER (G-Protein-Coupled Estrogen Receptor) and SR-BI (Scavenger Receptor Class B Type 1). Arterioscler Thromb Vasc Biol 38, 2283–2294. [DOI] [PubMed] [Google Scholar]
- Gistera A, Robertson AK, Andersson J, Ketelhuth DF, Ovchinnikova O, Nilsson SK, Lundberg AM, Li MO, Flavell RA, and Hansson GK (2013). Transforming growth factor-beta signaling in T cells promotes stabilization of atherosclerotic plaques through an interleukin-17-dependent pathway. Sci Transl Med 5, 196ra100. [DOI] [PubMed] [Google Scholar]
- Goldstein JL, and Brown MS (1977). Atherosclerosis: the low-density lipoprotein receptor hypothesis. Metabolism 26, 1257–1275. [DOI] [PubMed] [Google Scholar]
- Gomez D, Baylis RA, Durgin BG, Newman AAC, Alencar GF, Mahan S, St Hilaire C, Muller W, Waisman A, Francis SE, et al. (2018). Interleukin-1beta has atheroprotective effects in advanced atherosclerotic lesions of mice. Nat Med 24, 1418–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabner R, Lotzer K, Dopping S, Hildner M, Radke D, Beer M, Spanbroek R, Lippert B, Reardon CA, Getz GS, et al. (2009). Lymphotoxin beta receptor signaling promotes tertiary lymphoid organogenesis in the aorta adventitia of aged ApoE−/− mice. J Exp Med 206, 233–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebe A, Hoss F, and Latz E (2018). NLRP3 Inflammasome and the IL-1 Pathway in Atherosclerosis. Circ Res 122, 1722–1740. [DOI] [PubMed] [Google Scholar]
- Guo H, Callaway JB, and Ting JP (2015). Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med 21, 677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, and Harrison DG (2007). Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204, 2449–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajra L, Evans AI, Chen M, Hyduk SJ, Collins T, and Cybulsky MI (2000). The NF-kappa B signal transduction pathway in aortic endothelial cells is primed for activation in regions predisposed to atherosclerotic lesion formation. Proc Natl Acad Sci U S A 97, 9052–9057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson GK, and Hermansson A (2011). The immune system in atherosclerosis. Nat Immunol 12, 204–212. [DOI] [PubMed] [Google Scholar]
- He Y, Hara H, and Nunez G (2016). Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem Sci 41, 1012–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Mohanta SK, Yin C, Peng L, Ma Z, Srikakulapu P, Grassia G, MacRitchie N, Dever G, Gordon P, et al. (2015). Artery Tertiary Lymphoid Organs Control Aorta Immunity and Protect against Atherosclerosis via Vascular Smooth Muscle Cell Lymphotoxin beta Receptors. Immunity 42, 1100–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu SC, and Lan CE (2017). Psoriasis and Cardiovascular Comorbidities: Focusing on Severe Vascular Events, Cardiovascular Risk Factors and Implications for Treatment. Int J Mol Sci 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LH, Zinselmeyer BH, Chang CH, Saunders BT, Elvington A, Baba O, Broekelmann TJ, Qi L, Rueve JS, Swartz MA, et al. (2019). Interleukin-17 Drives Interstitial Entrapment of Tissue Lipoproteins in Experimental Psoriasis. Cell Metab 29, 475–487 e477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber AR, Kunkel SL, Todd RF 3rd, and Weiss SJ (1991). Regulation of transendothelial neutrophil migration by endogenous interleukin-8. Science 254, 99–102. [DOI] [PubMed] [Google Scholar]
- Ishibashi S, Brown MS, Goldstein JL, Gerard RD, Hammer RE, and Herz J (1993). Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J Clin Invest 92, 883–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, Lindsley RC, Mermel CH, Burtt N, Chavez A, et al. (2014). Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med 371, 2488–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongstra-Bilen J, Haidari M, Zhu SN, Chen M, Guha D, and Cybulsky MI (2006). Low-grade chronic inflammation in regions of the normal mouse arterial intima predisposed to atherosclerosis. J Exp Med 203, 2073–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz SS, Shipley GG, and Small DM (1976). Physical chemistry of the lipids of human atherosclerotic lesions. Demonstration of a lesion intermediate between fatty streaks and advanced plaques. J Clin Invest 58, 200–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, et al. (2015). Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526, 666–671. [DOI] [PubMed] [Google Scholar]
- Kellner-Weibel G, Yancey PG, Jerome WG, Walser T, Mason RP, Phillips MC, and Rothblat GH (1999). Crystallization of free cholesterol in model macrophage foam cells. Arterioscler Thromb Vasc Biol 19, 1891–1898. [DOI] [PubMed] [Google Scholar]
- Kim K, Shim D, Lee JS, Zaitsev K, Williams JW, Kim KW, Jang MY, Seok Jang H, Yun TJ, Lee SH, et al. (2018). Transcriptome Analysis Reveals Nonfoamy Rather Than Foamy Plaque Macrophages Are Proinflammatory in Atherosclerotic Murine Models. Circ Res 123, 1127–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T, Kobiyama K, Winkels H, Tse K, Miller J, Vassallo M, Wolf D, Ryden C, Orecchioni M, Dileepan T, et al. (2018). Regulatory CD4(+) T Cells Recognize Major Histocompatibility Complex Class II Molecule-Restricted Peptide Epitopes of Apolipoprotein B. Circulation 138, 1130–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T, Tse K, Sette A, and Ley K (2015). Vaccination to modulate atherosclerosis. Autoimmunity 48, 152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirii H, Niwa T, Yamada Y, Wada H, Saito K, Iwakura Y, Asano M, Moriwaki H, and Seishima M (2003). Lack of interleukin-1beta decreases the severity of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol 23, 656–660. [DOI] [PubMed] [Google Scholar]
- Kojima Y, Volkmer JP, McKenna K, Civelek M, Lusis AJ, Miller CL, Direnzo D, Nanda V, Ye J, Connolly AJ, et al. (2016). CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature 536, 86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka M, and Kuchroo VK (2009). IL-17 and Th17 Cells. Annu Rev Immunol 27, 485–517. [DOI] [PubMed] [Google Scholar]
- Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S, Saito S, Inoue K, Kamatani N, Gillespie MT, et al. (1999). IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest 103, 1345–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacic JC, Mercader N, Torres M, Boehm M, and Fuster V (2012). Epithelial-to-mesenchymal and endothelial-to-mesenchymal transition: from cardiovascular development to disease. Circulation 125, 1795–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachmann HJ, Kone-Paut I, Kuemmerle-Deschner JB, Leslie KS, Hachulla E, Quartier P, Gitton X, Widmer A, Patel N, Hawkins PN, and Canakinumab in CSG (2009). Use of canakinumab in the cryopyrin-associated periodic syndrome. N Engl J Med 360, 2416–2425. [DOI] [PubMed] [Google Scholar]
- Lee SE, Chang HJ, Sung JM, Park HB, Heo R, Rizvi A, Lin FY, Kumar A, Hadamitzky M, Kim YJ, et al. (2018). Effects of Statins on Coronary Atherosclerotic Plaques: The PARADIGM Study. JACC Cardiovasc Imaging 11, 1475–1484. [DOI] [PubMed] [Google Scholar]
- Lee SI, Patel M, Jones CM, and Narendran P (2015). Cardiovascular disease and type 1 diabetes: prevalence, prediction and management in an ageing population. Ther Adv Chronic Dis 6, 347–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy E, Rizwan Y, Thibault L, Lepage G, Brunet S, Bouthillier L, and Seidman E (2000). Altered lipid profile, lipoprotein composition, and oxidant and antioxidant status in pediatric Crohn disease. Am J Clin Nutr 71, 807–815. [DOI] [PubMed] [Google Scholar]
- Libby P, Ordovas JM, Auger KR, Robbins AH, Birinyi LK, and Dinarello CA (1986). Endotoxin and tumor necrosis factor induce interleukin-1 gene expression in adult human vascular endothelial cells. Am J Pathol 124, 179–185. [PMC free article] [PubMed] [Google Scholar]
- Libby P, and Pasterkamp G (2015). Requiem for the ‘vulnerable plaque’. Eur Heart J 36, 2984–2987. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Clinton SK, Iiyama K, Connelly PW, Libby P, and Cybulsky MI (1999). Hyperlipidemia and atherosclerotic lesion development in LDL receptor-deficient mice fed defined semipurified diets with and without cholate. Arterioscler Thromb Vasc Biol 19, 1938–1944. [DOI] [PubMed] [Google Scholar]
- Lim HY, Lim SY, Tan CK, Thiam CH, Goh CC, Carbajo D, Chew SHS, See P, Chakarov S, Wang XN, et al. (2018). Hyaluronan Receptor LYVE-1-Expressing Macrophages Maintain Arterial Tone through Hyaluronan-Mediated Regulation of Smooth Muscle Cell Collagen. Immunity 49, 1191. [DOI] [PubMed] [Google Scholar]
- Lin J, Li H, Yang M, Ren J, Huang Z, Han F, Huang J, Ma J, Zhang D, Zhang Z, et al. (2013). A role of RIP3-mediated macrophage necrosis in atherosclerosis development. Cell Rep 3, 200–210. [DOI] [PubMed] [Google Scholar]
- Liu B, Tan W, Barsoum A, Gu X, Chen K, Huang W, Ramsay A, Kolls JK, and Schwarzenberger P (2010). IL-17 is a potent synergistic factor with GM-CSF in mice in stimulating myelopoiesis, dendritic cell expansion, proliferation, and functional enhancement. Exp Hematol 38, 877–884 e871. [DOI] [PubMed] [Google Scholar]
- Liu P, Yu YR, Spencer JA, Johnson AE, Vallanat CT, Fong AM, Patterson C, and Patel DD (2008). CX3CR1 deficiency impairs dendritic cell accumulation in arterial intima and reduces atherosclerotic burden. Arterioscler Thromb Vasc Biol 28, 243–250. [DOI] [PubMed] [Google Scholar]
- Macritchie N, Grassia G, Sabir SR, Maddaluno M, Welsh P, Sattar N, Ialenti A, Kurowska-Stolarska M, McInnes IB, Brewer JM, et al. (2012). Plasmacytoid dendritic cells play a key role in promoting atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 32, 2569–2579. [DOI] [PubMed] [Google Scholar]
- Madhur MS, Funt SA, Li L, Vinh A, Chen W, Lob HE, Iwakura Y, Blinder Y, Rahman A, Quyyumi AA, and Harrison DG (2011). Role of interleukin 17 in inflammation, atherosclerosis, and vascular function in apolipoprotein e-deficient mice. Arterioscler Thromb Vasc Biol 31, 1565–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, Gibson H, Gordon D, Copeland T, D’Agostino D, et al. (2019). Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N Engl J Med 380, 33–44.30415629 [Google Scholar]
- McAlpine CS, Kiss MG, Rattik S, He S, Vassalli A, Valet C, Anzai A, Chan CT, Mindur JE, Kahles F, et al. (2019). Sleep modulates haematopoiesis and protects against atherosclerosis. Nature 566, 383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer Zu Horste G, Wu C, Wang C, Cong L, Pawlak M, Lee Y, Elyaman W, Xiao S, Regev A, and Kuchroo VK (2016). RBPJ Controls Development of Pathogenic Th17 Cells by Regulating IL-23 Receptor Expression. Cell Rep 16, 392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, Akira S, Rajavashisth TB, and Arditi M (2004). Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proceedings of the National Academy of Sciences of the United States of America 101, 10679–10684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millonig G, Niederegger H, Rabl W, Hochleitner BW, Hoefer D, Romani N, and Wick G (2001). Network of vascular-associated dendritic cells in intima of healthy young individuals. Arterioscler Thromb Vasc Biol 21, 503–508. [DOI] [PubMed] [Google Scholar]
- Misharin AV, Morales-Nebreda L, Reyfman PA, Cuda CM, Walter JM, McQuattie-Pimentel AC, Chen CI, Anekalla KR, Joshi N, Williams KJN, et al. (2017). Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J Exp Med 214, 2387–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser R, Schleiffenbaum B, Groscurth P, and Fehr J (1989). Interleukin 1 and tumor necrosis factor stimulate human vascular endothelial cells to promote transendothelial neutrophil passage. J Clin Invest 83, 444–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundi S, Massaro M, Scoditti E, Carluccio MA, van Hinsbergh VWM, Iruela-Arispe ML, and De Caterina R (2018). Endothelial permeability, LDL deposition, and cardiovascular risk factors-a review. Cardiovasc Res 114, 35–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasir K, Guallar E, Navas-Acien A, Criqui MH, and Lima JA (2005). Relationship of monocyte count and peripheral arterial disease: results from the National Health and Nutrition Examination Survey 1999–2002. Arterioscler Thromb Vasc Biol 25, 1966–1971. [DOI] [PubMed] [Google Scholar]
- Nestle FO, Di Meglio P, Qin JZ, and Nickoloff BJ (2009). Skin immune sentinels in health and disease. Nat Rev Immunol 9, 679–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordlohne J, Helmke A, Ge S, Rong S, Chen R, Waisman A, Haller H, and von Vietinghoff S (2018). Aggravated Atherosclerosis and Vascular Inflammation With Reduced Kidney Function Depend on Interleukin-17 Receptor A and Are Normalized by Inhibition of Interleukin-17A. JACC Basic Transl Sci 3, 54–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermoser G, and Pascual V (2010). The interferon-alpha signature of systemic lupus erythematosus. Lupus 19, 1012–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paramel Varghese G, Folkersen L, Strawbridge RJ, Halvorsen B, Yndestad A, Ranheim T, Krohg-Sorensen K, Skjelland M, Espevik T, Aukrust P, et al. (2016). NLRP3 Inflammasome Expression and Activation in Human Atherosclerosis. J Am Heart Assoc 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsa R, Lund H, Georgoudaki AM, Zhang XM, Ortlieb Guerreiro-Cacais A, Grommisch D, Warnecke A, Croxford AL, Jagodic M, Becher B, et al. (2016). BAFF-secreting neutrophils drive plasma cell responses during emergency granulopoiesis. J Exp Med 213, 1537–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson KE, Zhu SN, Chen M, Nurmohamed S, Jongstra-Bilen J, and Cybulsky MI (2010). Resident intimal dendritic cells accumulate lipid and contribute to the initiation of atherosclerosis. Circ Res 106, 383–390. [DOI] [PubMed] [Google Scholar]
- Pober JS, Gimbrone MA Jr., Lapierre LA, Mendrick DL, Fiers W, Rothlein R, and Springer TA (1986). Overlapping patterns of activation of human endothelial cells by interleukin 1, tumor necrosis factor, and immune interferon. J Immunol 137, 1893–1896. [PubMed] [Google Scholar]
- Rajamaki K, Lappalainen J, Oorni K, Valimaki E, Matikainen S, Kovanen PT, and Eklund KK (2010). Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS One 5, e11765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM, Everett BM, Pradhan A, MacFadyen JG, Solomon DH, Zaharris E, Mam V, Hasan A, Rosenberg Y, Iturriaga E, et al. (2019). Low-Dose Methotrexate for the Prevention of Atherosclerotic Events. N Engl J Med 380, 752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, et al. (2017). Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med 377, 1119–1131. [DOI] [PubMed] [Google Scholar]
- Robbins CS, Hilgendorf I, Weber GF, Theurl I, Iwamoto Y, Figueiredo JL, Gorbatov R, Sukhova GK, Gerhardt LM, Smyth D, et al. (2013). Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med 19, 1166–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanato G, Scarpa M, Angriman I, Faggian D, Ruffolo C, Marin R, Zambon S, Basato S, Zanoni S, Filosa T, et al. (2009). Plasma lipids and inflammation in active inflammatory bowel diseases. Aliment Pharmacol Ther 29, 298–307. [DOI] [PubMed] [Google Scholar]
- Rong JX, Shapiro M, Trogan E, and Fisher EA (2003). Transdifferentiation of mouse aortic smooth muscle cells to a macrophage-like state after cholesterol loading. Proc Natl Acad Sci U S A 100, 13531–13536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R, Glomset J, and Harker L (1977). Response to injury and atherogenesis. Am J Pathol 86, 675–684. [PMC free article] [PubMed] [Google Scholar]
- Roubille C, Richer V, Starnino T, McCourt C, McFarlane A, Fleming P, Siu S, Kraft J, Lynde C, Pope J, et al. (2015). The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal antiinflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Ann Rheum Dis 74, 480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roufaiel M, Gracey E, Siu A, Zhu SN, Lau A, Ibrahim H, Althagafi M, Tai K, Hyduk SJ, Cybulsky KO, et al. (2016). CCL19-CCR7-dependent reverse transendothelial migration of myeloid cells clears Chlamydia muridarum from the arterial intima. Nat Immunol 17, 1263–1272. [DOI] [PubMed] [Google Scholar]
- Ruhl S, Shkarina K, Demarco B, Heilig R, Santos JC, and Broz P (2018). ESCRT-dependent membrane repair negatively regulates pyroptosis downstream of GSDMD activation. Science 362, 956–960. [DOI] [PubMed] [Google Scholar]
- Ruperto N, Brunner HI, Quartier P, Constantin T, Wulffraat N, Horneff G, Brik R, McCann L, Kasapcopur O, Rutkowska-Sak L, et al. (2012). Two randomized trials of canakinumab in systemic juvenile idiopathic arthritis. N Engl J Med 367, 2396–2406. [DOI] [PubMed] [Google Scholar]
- Sage AP, Murphy D, Maffia P, Masters LM, Sabir SR, Baker LL, Cambrook H, Finigan AJ, Ait-Oufella H, Grassia G, et al. (2014). MHC Class II-restricted antigen presentation by plasmacytoid dendritic cells drives proatherogenic T cell immunity. Circulation 130, 1363–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage AP, Tsiantoulas D, Binder CJ, and Mallat Z (2018). The role of B cells in atherosclerosis. Nat Rev Cardiol. [DOI] [PubMed] [Google Scholar]
- Schett G, Dayer JM, and Manger B (2016). Interleukin-1 function and role in rheumatic disease. Nat Rev Rheumatol 12, 14–24. [DOI] [PubMed] [Google Scholar]
- Schwartz SM, Campbell GR, and Campbell JH (1986). Replication of smooth muscle cells in vascular disease. Circ Res 58, 427–444. [DOI] [PubMed] [Google Scholar]
- Schwartz SM, Virmani R, and Majesky MW (2018). An update on clonality: what smooth muscle cell type makes up the atherosclerotic plaque? F1000Res 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessa WC (2018). Estrogen Reduces LDL (Low-Density Lipoprotein) Transcytosis. Arterioscler Thromb Vasc Biol 38, 2276–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman LS, Gomez D, Cherepanova OA, Salmon M, Alencar GF, Haskins RM, Swiatlowska P, Newman AA, Greene ES, Straub AC, et al. (2015). KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med 21, 628–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, and Shao F (2015a). Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665. [DOI] [PubMed] [Google Scholar]
- Shi X, Xie WL, Kong WW, Chen D, and Qu P (2015b). Expression of the NLRP3 Inflammasome in Carotid Atherosclerosis. J Stroke Cerebrovasc Dis 24, 2455–2466. [DOI] [PubMed] [Google Scholar]
- Shimokawa H, Ito A, Fukumoto Y, Kadokami T, Nakaike R, Sakata M, Takayanagi T, Egashira K, and Takeshita A (1996). Chronic treatment with interleukin-1 beta induces coronary intimal lesions and vasospastic responses in pigs in vivo. The role of platelet-derived growth factor. J Clin Invest 97, 769–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DM, Bond MG, Waugh D, Prack M, and Sawyer JK (1984). Physicochemical and histological changes in the arterial wall of nonhuman primates during progression and regression of atherosclerosis. The Journal of clinical investigation 73, 1590–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E, Prasad KM, Butcher M, Dobrian A, Kolls JK, Ley K, and Galkina E (2010). Blockade of interleukin-17A results in reduced atherosclerosis in apolipoprotein E-deficient mice. Circulation 121, 1746–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spann NJ, Garmire LX, McDonald JG, Myers DS, Milne SB, Shibata N, Reichart D, Fox JN, Shaked I, Heudobler D, et al. (2012). Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell 151, 138–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speeckaert R, Lambert J, Grine L, Van Gele M, De Schepper S, and van Geel N (2016). The many faces of interleukin-17 in inflammatory skin diseases. Br J Dermatol 175, 892–901. [DOI] [PubMed] [Google Scholar]
- Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, and Ley K (2005). Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity 22, 285–294. [DOI] [PubMed] [Google Scholar]
- Stender S, and Hjelms E (1984). In vivo influx of free and esterified plasma cholesterol into human aortic tissue without atherosclerotic lesions. J Clin Invest 74, 1871–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian M, Thorp E, Hansson GK, and Tabas I (2013). Treg-mediated suppression of atherosclerosis requires MYD88 signaling in DCs. J Clin Invest 123, 179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian S, and Hirsch IB (2018). Intensive Diabetes Treatment and Cardiovascular Outcomes in Type 1 Diabetes Mellitus: Implications of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study 30-Year Follow-up. Endocrinol Metab Clin North Am 47, 65–79. [DOI] [PubMed] [Google Scholar]
- Suntsova M, Garazha A, Ivanova A, Kaminsky D, Zhavoronkov A, and Buzdin A (2015). Molecular functions of human endogenous retroviruses in health and disease. Cell Mol Life Sci 72, 3653–3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swirski FK, Pittet MJ, Kircher MF, Aikawa E, Jaffer FA, Libby P, and Weissleder R (2006). Monocyte accumulation in mouse atherogenesis is progressive and proportional to extent of disease. Proc Natl Acad Sci U S A 103, 10340–10345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang WH, and Hazen SL (2014). The contributory role of gut microbiota in cardiovascular disease. J Clin Invest 124, 4204–4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theofilopoulos AN, Kono DH, and Baccala R (2017). The multiple pathways to autoimmunity. Nat Immunol 18, 716–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorp E, Cui D, Schrijvers DM, Kuriakose G, and Tabas I (2008). Mertk receptor mutation reduces efferocytosis efficiency and promotes apoptotic cell accumulation and plaque necrosis in atherosclerotic lesions of apoe−/− mice. Arterioscler Thromb Vasc Biol 28, 1421–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorp E, and Tabas I (2009). Mechanisms and consequences of efferocytosis in advanced atherosclerosis. J Leukoc Biol 86, 1089–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting JP, Lovering RC, Alnemri ES, Bertin J, Boss JM, Davis BK, Flavell RA, Girardin SE, Godzik A, Harton JA, et al. (2008). The NLR gene family: a standard nomenclature. Immunity 28, 285–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyama M, Kong Y, Song E, Jayewickreme T, Kang I, and Iwasaki A (2018). ERVmap analysis reveals genome-wide transcription of human endogenous retroviruses. Proc Natl Acad Sci U S A 115, 12565–12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treger RS, Pope SD, Kong Y, Tokuyama M, Taura M, and Iwasaki A (2019). The Lupus Susceptibility Locus Sgp3 Encodes the Suppressor of Endogenous Retrovirus Expression SNERV. Immunity 50, 334–347 e339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lammeren GW, den Ruijter HM, Vrijenhoek JE, van der Laan SW, Velema E, de Vries JP, de Kleijn DP, Vink A, de Borst GJ, Moll FL, et al. (2014). Time-dependent changes in atherosclerotic plaque composition in patients undergoing carotid surgery. Circulation 129, 2269–2276. [DOI] [PubMed] [Google Scholar]
- Virmani R, Burke AP, Farb A, and Kolodgie FD (2006). Pathology of the vulnerable plaque. J Am Coll Cardiol 47, C13–18. [DOI] [PubMed] [Google Scholar]
- von Vietinghoff S, and Ley K (2009). IL-17A controls IL-17F production and maintains blood neutrophil counts in mice. J Immunol 183, 865–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltner-Romen M, Falkensammer G, Rabl W, and Wick G (1998). A previously unrecognized site of local accumulation of mononuclear cells. The vascular-associated lymphoid tissue. J Histochem Cytochem 46, 1347–1350. [DOI] [PubMed] [Google Scholar]
- Wan X, Zinselmeyer BH, Zakharov PN, Vomund AN, Taniguchi R, Santambrogio L, Anderson MS, Lichti CF, and Unanue ER (2018). Pancreatic islets communicate with lymphoid tissues via exocytosis of insulin peptides. Nature 560, 107–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Subramanian M, Abramowicz S, Murphy AJ, Gonen A, Witztum J, Welch C, Tabas I, Westerterp M, and Tall AR (2014). Interleukin-3/granulocyte macrophage colony-stimulating factor receptor promotes stem cell expansion, monocytosis, and atheroma macrophage burden in mice with hematopoietic ApoE deficiency. Arterioscler Thromb Vasc Biol 34, 976–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JW, Elvington A, Ivanov S, Kessler S, Luehmann H, Baba O, Saunders BT, Kim KW, Johnson MW, Craft CS, et al. (2017). Thermoneutrality but Not UCP1 Deficiency Suppresses Monocyte Mobilization Into Blood. Circ Res 121, 662–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JW, Martel C, Potteaux S, Esaulova E, Ingersoll MA, Elvington A, Saunders BT, Huang LH, Habenicht AJ, Zinselmeyer BH, and Randolph GJ (2018). Limited Macrophage Positional Dynamics in Progressing or Regressing Murine Atherosclerotic Plaques. Arterioscler Thromb Vasc Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KJ, and Tabas I (1995). The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol 15, 551–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf D, and Ley K (2019). Immunity and Inflammation in Atherosclerosis. Circ Res 124, 315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Thabet SR, Kirabo A, Trott DW, Saleh MA, Xiao L, Madhur MS, Chen W, and Harrison DG (2014). Inflammation and mechanical stretch promote aortic stiffening in hypertension through activation of p38 mitogen-activated protein kinase. Circ Res 114, 616–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P, Jia F, Zhang B, and Zhang P (2017). Risk of cardiovascular disease in inflammatory bowel disease. Exp Ther Med 13, 395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H, Lu M, Lin TY, Chen Z, Chen G, Wang WC, Marin T, Shentu TP, Wen L, Gongol B, et al. (2013). Sterol regulatory element binding protein 2 activation of NLRP3 inflammasome in endothelium mediates hemodynamic-induced atherosclerosis susceptibility. Circulation 128, 632–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young A, Koduri G, Batley M, Kulinskaya E, Gough A, Norton S, and Dixey J (2006). Mortality in rheumatoid arthritis. Increased in the early course of disease, in ischaemic heart disease and in pulmonary fibrosis. Rheumatology 46, 350–357. [DOI] [PubMed] [Google Scholar]
- Yun TJ, Lee JS, Machmach K, Shim D, Choi J, Wi YJ, Jang HS, Jung IH, Kim K, Yoon WK, et al. (2016). Indoleamine 2,3-Dioxygenase-Expressing Aortic Plasmacytoid Dendritic Cells Protect against Atherosclerosis by Induction of Regulatory T Cells. Cell Metab 23, 852–866. [DOI] [PubMed] [Google Scholar]
- Zhang SH, Reddick RL, Piedrahita JA, and Maeda N (1992). Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science 258, 468–471. [DOI] [PubMed] [Google Scholar]
- Zhang X, Sessa WC, and Fernandez-Hernando C (2018). Endothelial Transcytosis of Lipoproteins in Atherosclerosis. Front Cardiovasc Med 5, 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng F, Xing S, Gong Z, and Xing Q (2013). NLRP3 inflammasomes show high expression in aorta of patients with atherosclerosis. Heart Lung Circ 22, 746–750. [DOI] [PubMed] [Google Scholar]