Abstract
Objectives
– Routine histopathological grading for salivary gland mucoepidermoid carcinoma (MEC) have failed to prognosticate these tumors, resulting in poor post-surgical outcomes. In developing countries, the lack of technologically advanced infrastructure curtails, efficient treatment modalities. This study aimed at determining if MUC4β can characterize salivary gland MEC and serve as a practical and inexpensive method to prognosticate salivary gland MEC.
Materials and methods
– Fifteen cases of archived paraffin embedded tissue blocks of mucoepidermoid carcinomas were reassessed for histopathological grading using Healey's system, modified by Batsakis and Luna and immunohistochemically evaluated for expression of MUC4β. Statistical analysis (Kappa statistics and Spearman's rho correlation coefficient) was performed to assess inter-observer reproducibility and to correlate the expression of MUC4β with the histopathological grade of the tumor.
Results
MUC4β expression is related to tumor differentiation in an inverse relationship. Two cases of high grade MEC were the exception to this rule.
Conclusion
Our study revealed that MUC4β alone cannot serve as a reliable prognostic marker due to its divergent tumor suppressor and oncogenic pathway. The role of MUC4β needs further evaluation and research so as to potentiate therapeutics depending upon its context dependent function, as a cancer marker or an oncogenic factor.
Keywords: Cancer research, Dentistry, Oncology, Salivary gland cancer, Mucoepidermoid carcinoma, MUC4, Prognosis
Cancer Research; Dentistry; Oncology; Salivary gland cancer; Mucoepidermoid carcinoma; MUC4; Prognosis
1. Introduction
Salivary glands are diffusely distributed in the oral and para-oral tissues. Salivary gland neoplasms are rare, accounting for just 3–10% of all head and neck neoplasms (Ansari, 2007). The global incidence of malignant salivary gland neoplasms is 0.5–2 per 100,000 (Parkin et al., 2010). Mucoepidermoid carcinomas (MECs) account for 30%–40% of all salivary gland neoplasms and are known for their clinical, histopathological and genetic diversity (Coca-Pelaz et al., 2015; Honjo et al., 2018).
The aggressive behavior of MEC dictates a grade dependent treatment strategy (To et al., 2012). However, an efficient prognostic histopathological grading system is yet to be established (Qannam and Bello, 2016). Qannam in 2016 compared the commonly used grading systems for Mucoepidermoid carcinomas and reported a very low percentage of agreement across all the grading systems, especially in case of minor salivary gland MECs. Thus, research into molecular markers that can be used as an adjunct to routine histopathology becomes important for prognostication of MECs. MUC4 is known for its divergent, tumor suppressor and oncogenic potential (Khiavi et al., 2012; Honjo et al., 2018). Hence, this study, aimed to evaluate MUC4, as a prognostic marker for salivary gland MECs. The review of literature includes a comprehensive list of prognostic markers and molecular cascades that delineates aggressiveness in MEC.
2. Materials and methods
2.1. Collection of samples and data
Fifteen diagnosed cases of MECs were selected at the department of Oral and Maxillofacial Pathology, Government Dental College and Hospital, Goa, India. The demographic records were retrieved from the department archives. All the patients had undergone surgical excision of the tumors as standard treatment. Radiotherapy and chemotherapy were added as adjunctive modalities in advanced cases. The haematoxylin and eosin stained sections were reassessed to determine the histopathological grade by three blinded investigators using the Healey's system.
2.2. Immunohistochemistry of MUC4β
Representative paraffin wax blocks were selected from each of the fifteen cases for immunohistochemistry. The Abcam [ab150381] Rabbit monoclonal MUC4β (targets the β subunit of MUC4) antibody was used in 1:100 dilution. Standard immunohistochemistry procedure was followed. Briefly, 4 μm sections were floated from the water bath onto bar coded (Dako Seymour SystemTM) silanized slides. Antigen retrieval was performed using the Heat Induced Epitope Retrieval (HIER) system (DAKO PTLinkTM) and Dako target retrieval solution (Ethylene diamine tetra-acetic acid, pH 9). The Dako AutoStainer and Dako reagents were used to carry out the immunohistochemical staining procedure. The MUC4β antibody was applied to the tissue sections for 20 min and the diaminobenzidine substrate chromogen solution was applied for 10 min. The sections were then counterstained with haematoxylin and washed with phosphate buffer solution, to remove the excess stain. Lastly, the slides were dehydrated in 100% alcohol (30 s), cleared in xylene (two dips) and mounted using DPX (Dibutyl Phthlate Xylene) mounting media. The colonic mucosa was used as the positive control.
The immunohistochemical results were evaluated by three independent observers by counting the percentage of positive neoplastic cells at 400X magnification in 5 different fields. MUC4β was considered positive, when the tissue section showed more than 5% positively stained neoplastic cells (Jeon et al., 2010). The proportion of tumor cells which stained positive with the MUC4β marker were graded as follows: <5% (score 0), <33% (score 1), 33–66% (score 2) and >66% (score 3). When the opinions of the investigators differed, a median of the three scores was taken as the final score and a consensus decision was made. The final score of MUC4β staining was compared to the histopathological grade.
2.3. Statistical analysis
Statistical analysis was performed with SPSS (Statistical Package for Social Sciences) version 20.0 for windows (Microsoft, Armonk, NY, United States of America). Kappa statistical analysis was applied to assess the inter-observer reproducibility. Spearman's rho correlation coefficient was used for comparison between the expression of the MUC4β marker and the histopathological grade of the tumor. A p-value ≤ 0.05 was defined as a statistically significant difference.
3. Results
3.1. Clinicopathological characteristics of the patients
The clinicopathological characteristics of the patients are summarized in Table 1. MECs showed approximately equal gender distribution. The palate was the most common site followed by the parotid gland. There was unequal distribution of grades with the majority of cases being high grade MEC.
Table 1.
Clinicopathological characteristics of the patients at diagnosis.
| Characteristics | Cases (%) |
|---|---|
| Total number of cases | 15 |
| Gender | |
| Female | 8 (53.33%) |
| Male | 7 (46.66%) |
| Tumor location | |
| Parotid | 4 (26.66%) |
| Palate | 8 (53.33%) |
| Floor of mouth | 1 (6.66%) |
| Buccal mucosa | 1 (6.66%) |
| Retro molar region | 1 (6.66%) |
| Tumor grade | |
| Grade I | 4 (26.66%) |
| Grade II | 3 (20.00%) |
| Grade III | 8 (53.33%) |
MUC4 expression in controls and in MECs:
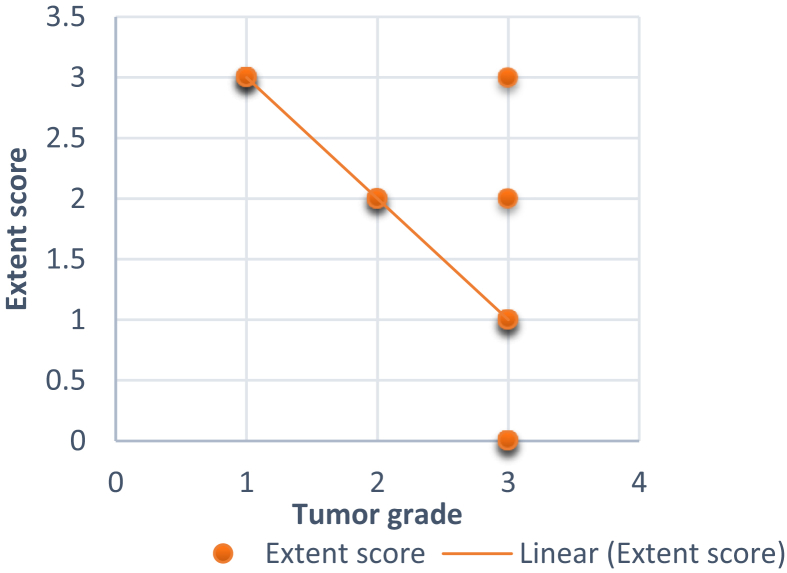
The colon mucosa and the luminal surface of the excretory ducts of normal salivary glands stained positive. Salivary acini were negative for MUC4β. In general, MUC4β showed cytoplasmic and membranous expression in the neoplastic cells. A variation was noted in the staining pattern of MUC4β in different grades of MECs (Table 2). All the grade I (4/4 i.e. 100%) MECs showed high expression of MUC4β, grade II MECs showed moderate (66.6%) to high expression (33.3%) of MUC4β, whereas the grade III MECs also showed variable expression of MUC4β. Six cases (75%) of grade III MECs showed low expression of MUC4β (Score 0–1) and two cases (25%) of MEC showed Moderate to high expression of MUC4β (Score 2–3).The three grades of MEC showed a statistically significant difference in the expression of MUC4β (p = 0.001). In general, an inverse relationship was noted between the grade of MEC and the expression of MUC4β (13/15 cases i.e. 86.66% cases) (Figs. 1 and 2). Two cases (13.33%) of high grade MEC showed moderate to high expression of MUC4β (Fig. 3).
Table 2.
Tabulation of the scores given by the three observers following evaluation of the MUC4 immunohistochemically (IHC) stained slides of Mucoepidermoid carcinoma.
| No. of MECs | Tumor Grade | Extent Score |
Final Score | ||
|---|---|---|---|---|---|
| Observer 1 | Observer 2 | Observer 3 | |||
| I | 1 | 3 | 3 | 3 | 3 |
| II | 1 | 3 | 3 | 3 | 3 |
| III | 1 | 3 | 3 | 3 | 3 |
| IV | 1 | 3 | 2 | 3 | 3 |
| V | 2 | 1 | 2 | 2 | 2 |
| VI | 2 | 2 | 3 | 2 | 2 |
| VII | 2 | 2 | 3 | 3 | 3 |
| VIII | 3 | 0 | 0 | 0 | 0 |
| IX | 3 | 0 | 0 | 0 | 0 |
| X | 3 | 0 | 0 | 0 | 0 |
| XI | 3 | 1 | 1 | 1 | 1 |
| XII | 3 | 1 | 2 | 1 | 1 |
| XIII | 3 | 0 | 1 | 1 | 1 |
| XIV | 3 | 2 | 2 | 2 | 2 |
| XV | 3 | 3 | 3 | 3 | 3 |
Fig. 1.
Graphical representation of negative correlation between, grade of MECs and extent of MUC4β positive neoplastic cells.
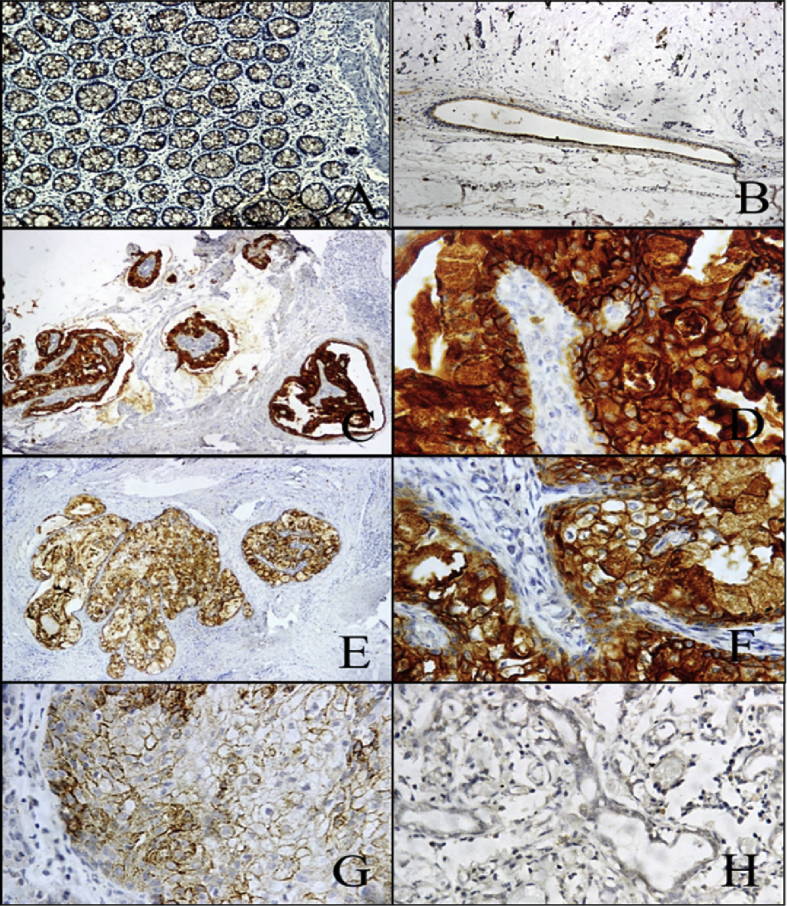
Fig. 2.
Photomicrographs of MUC4β immunohistochemistry in different grades of MECs: (A) and (B) (100X magnification) - Positive control – Colon mucosa and excretory duct of normal salivary gland tissue respectively. (C) (100X magnification) and (D) (400X magnification) - Low grade (Grade 1) MEC, showing strong (Score 3) membranous and cytoplasmic staining of neoplastic cells. (E) (100X magnification) and (F) (400X magnification) – Intermediate grade (Grade II) MEC, showing moderate (Score 2) membranous and cytoplasmic staining of neoplastic cells. (G) and (H) (400X magnification) – High grade (Grade III) MEC, showing weak (Score 1) or negative (Score 0) staining for MUC4β in neoplastic cells, respectively.
Fig. 3.
Photomicrographs from the two cases of Grade III MEC that revealed high expression of MUC4β. (A) (100X magnification) and (B) (400X magnification), are the haematoxylin and eosin stained sections of CASE 1, showing histomorphology of tumor cells and perineural, perivascular invasion, respectively. (C) (400X magnification), CASE 1, immunohistochemistry revealed high expression (Score 3) of MUC4β in neoplastic cells. (D) (100X magnification) and (E) (400X magnification), are the haematoxylin and eosin stained sections of CASE 2, showing histomorphology of tumor cells and intravascular spread, respectively. (F) (400X magnification), CASE 2, immunohistochemistry revealed moderate (Score 2) to high expression (Score 3) of MUC4β in neoplastic cells.
4. Discussion
The World Health Organization defines MEC as ‘a malignant glandular epithelial neoplasm characterized by mucous, intermediate and epidermoid cells, with columnar, clear cell and oncocytoid features’ (Coca-Pelaz et al., 2015). MECs can show diverse histologic morphologies, depending on the predominant cell type and pattern. The clinical behavior of this tumor is usually predicted by its histologic grade. Histologically, a predominantly cystic architecture with numerous mucous cells, minimal cytological atypia and scant mitoses qualifies the tumor as a low-grade (Grade I) MECs. On the contrary, the high grade (Grade III) MECs are predominantly cellular, mainly consisting of intermediate and epidermoid cells, interspersed by few mucous cells. High grade MECs are highly anaplastic with large number of mitotic figures, evidence of tumor necrosis, neural, vascular and osseous invasion with infiltrative margins. However, the histopathological criteria used for grading MECs are controversial. Some histopathologically low-grade MECs have shown an aggressive clinical behavior (Auclair et al., 1992; Goode et al., 1998; Brandwein et al., 2001; Bai et al., 2013).
Mucins are high molecular weight glycoproteins normally expressed by various epithelial cell types, including salivary glands (Hollingsworth and Swanson, 2004). Till date, twenty-one mucin genes have been identified in humans, which are further classified into secretory mucins and membrane bound (transmembrane) mucins (Dhanisha et al., 2018). Mucins are usually perceived as the biomolecules implicated in the protection and lubrication of epithelial surfaces. However, current research indicates that mucins, particularly MUC4, can also function as signaling modulators and affect tumor cell phenotype (Singh et al., 2007).
MUC4 is a high molecular weight heterodimeric transmembrane glycoprotein located on chromosome 3 locus 3q29. This gene was first identified in 1991, from a tracheobronchial cDNA library (Dhanisha et al., 2018). The MUC4 glycoprotein complex consists of MUC4 alpha (MUC4α) subunit tightly bound to a transmembrane subunit, MUC4 beta (MUC4β). The MUC4 beta subunit has two epidermal growth factor (EGF)–like domains that act as a ligand for human epidermal growth receptor 2 (HER2) (also known as ErbB2), suggesting that MUC4 acts as an intramembranous autocrine activator of HER2 receptor (Dhanisha et al., 2018). The anti-MUC4β antibody is a more tumor-specific antibody than anti-MUC4α (Weed et al., 2004). In our study, anti-MUC4β antibody was used for immunostaining of MECs.
During embryogenesis, MUC4 is first expressed during the canalicular stage of salivary gland morphogenesis, at the apical surface of well-developed excretory ducts. The expression of MUC4 precedes cytodifferentiation of salivary gland, thus emphasizing its role in cell differentiation. Fully developed salivary glands show weak or no expression of MUC4 in acinar lobules and marked expression of MUC4 on the luminal surface of the ductal cells, particularly the excretory ducts (Liu et al., 2002; Alos et al., 2005; Teshima et al., 2011).
Aberrant expression of MUC4 has been reported in various inflammatory diseases and carcinomas, highlighting the diverse roles of MUC4 in biological processes, such as, epithelial cell renewal and differentiation, cell signaling, cell adhesion and malignancies (Chaturvedi et al., 2008). MUC4 has been studied as a potential biomarker in the diagnosis and prognosis of various neoplasms. Current research has revealed that MUC4 can serve diverse functions in a context-dependent manner. It has been noted that, high expression of MUC4 in oral squamous cell carcinoma, breast cancer, extra hepatic bile duct cancer, pancreatic cancer, colorectal cancer, cholangiocarcinoma and ovarian cancer, is associated with aggressive behavior and high chances of metastasis of these malignancies (Tamada et al., 2006; Chauhan et al., 2006; Shanmugam et al., 2010; Hamada et al., 2012; Mukhopadhyay et al., 2013; Huang et al., 2015; Li et al., 2016; Gautam et al., 2017). On the contrary, improved patient survival is associated with high MUC4 expression in, squamous cell carcinoma of the lung and mucoepidermoid carcinoma of the salivary glands (Weed et al., 2004; Alos et al., 2005; Handra-Luca et al., 2005; Majhi et al., 2013). Thus, the role of MUC4 as a sole marker of prognostication remains inconclusive.
The demographic characteristics of the patients included in this study were consistent with the results of similar previous studies (Weed et al., 2004; Bai et al., 2013). The MUC4β expression was seen in the cell membrane and the cytoplasm of all the neoplastic cells, indicating that during the process of carcinogenesis, there are post-transcriptional alterations that produce aberrantly glycosylated mucins (Remmers et al., 2013; Padler-Karavani, 2014). Although limited by a small sample size, our study demonstrated some important findings. As reported by Weed et al. (2004); Alos et al. (2005) and Handra-Luca et al. (2005), a statistically significant negative correlation between MUC4β expression and the histopathological grade of MEC was observed in the present study. However, among the eight cases of high grade MEC, two cases revealed high MUC4β expression, which is a novel, hitherto unreported finding. Till date, very few studies have evaluated MUC4 as a marker of prognostication in salivary gland MECs. Literature research suggests that MUC4β can serve context dependent diverse functions in cell signaling (Singh et al., 2007).
MUC4β may help identify a subset of patients with favorable prognosis, in cases where it plays a role in tumor differentiation. On the other hand, loss of MUC4-associated antigens, as seen with increase in the grade of MEC, may represent a later event in the dedifferentiation of these tumors. As seen in two of our cases, high-grade MECs showing high expression of MUC4β may either exhibit clinical behavior more consistent with lower-grade tumors or may follow the alternate pathway of MUC4β mediated phosphorylation of ErbB2, ErbB3 and neuregulin, resulting in tumor progression (Weed et al., 2004; Jepson et al., 2002).
Under physiologic conditions, MUC4 is localized to the apical surface of normal epithelial cells, while the receptor tyrosine kinases (RTKs) are restricted to the basolateral membranes. The ErbB family of transmembrane receptor tyrosine kinases consisting of ErbB1/epidermal growth factor receptor, ErbB2, ErbB3 and ErbB4, have been implicated in both cell differentiation and neoplasias (Alroy and Yarden, 1997). Physiological expression of ErbB2 and MUC4, functions as a regulator of cell differentiation via the MUC4β -ErbB2 complex. In normal epithelial cells, the MUC4β-ErbB2 interaction sequesters ErbB2, thereby preventing it from heterodimerizing with the other ErbB family members and activating ErbB2 signaling cascade.During oncogenic transformation, there is loss of polarity of the cells resulting in repositioning of the MUC4 over the entire cell membrane, thus potentiating the interaction between MUC4β and RTKs (Singh et al., 2004). On the contrary, high expression and activation of the ErbB2 receptor is more likely to be mediated by the activated MUC4β-ErbB2-ErbB3-neuregulin complex, potentiating tumor progression. MUC4 expression could thus be an indicator of tumor cell differentiation or a mediator of tumor growth and progression (Cho et al., 1997; Weed et al., 2004; Carraway et al., 2009) (Fig. 4). In neoplastic cells, MUC4 has been reported to cause an increase in the cell-surface populations of ErbB2 and ErbB3 by inducing relocalization of the ErbB2 and ErbB3 receptors from the intracellular compartments to the plasma membrane, thus potentiating ErbB2/ErbB3 signaling and tumor progression (Funes et al., 2006).
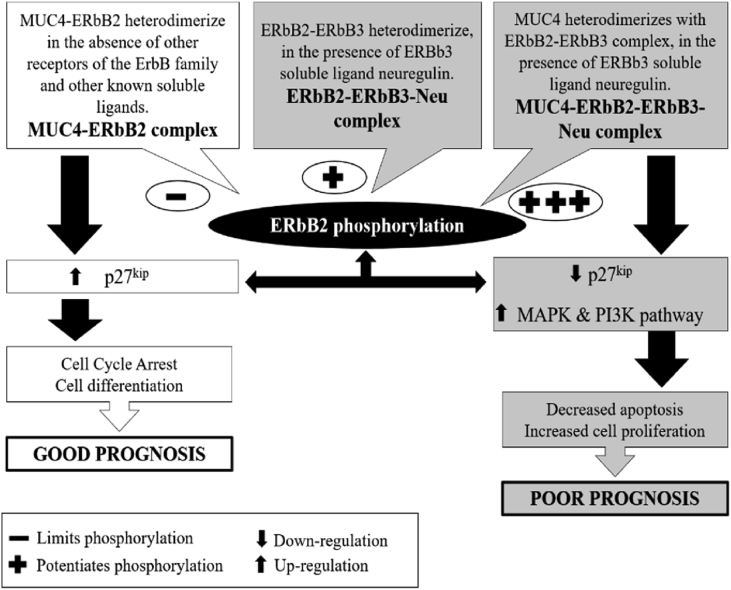
Fig. 4.
Pathways for MUC4β mediated ErbB2 phosphorylation: Phosphorylation and activation of ERbB2 can be mediated by three pathways, subsequent to formation of either MUC4β-ERbB2 complex, ERbB2-ERbB3-Neu complex or MUC4β -ERbB2-ERbB3-Neu complex. The latter being characterized by high degree of phosphorylation (indicated by the plus sign) resulting in down-regulation of p27kip (cyclin-dependent kinase inhibitor) and activation of the Mitogen-activated protein kinase and phosphoinositide 3-kinase pathway, thus inhibiting cell differentiation and apoptosis and promoting cell proliferation associated with tumor progression.
In a study by El-Attar RH (El-Attar and Deraz, 2014) it was concluded that normal salivary glands do not express ErbB2. Hence, aberrant expression of ErbB2 in salivary gland neoplasms, like MEC, indicates a distinctly aggressive behavior of these neoplasms. Immunohistochemical studies on expression of ErbB2 in MEC have shown conflicting data, with reports of negative (0%) to as high as 38% ErbB2 expression. However, all the studies have linked expression of ErbB2 with tumor progression and unfavorable prognosis (Kernohan et al., 1991; Sugano et al., 1992; Press et al., 1994). High expression of EGFR has been reported in 73% of high grade tumors and is associated with a poor prognosis (Ettl et al., 2008; Lujan et al., 2010).
A review of literature has shown that some low grade MECs can show an unpredictable aggressive course, while certain high grade MECs may show different clinical outcomes (better/worse) (Coca-Pelez et al., 2015; Aro et al., 2011; Herd et al., 2012). Therefore, it is crucial to predict which MEC patients are prone to an aggressive behavior i.e. recurrence and metastasis, irrespective of the histopathological grade of the tumor. Till date, several diagnostic and prognostic biomarkers for MEC have been evaluated (Table 3). Earlier studies had proposed that the CRTC1-MAML2 or CRTC3-MAML2 translocation positive MECs showed a better prognosis, irrespective of their histopathological grade (Okabe et al., 2006; Behboudi et al., 2006). However, subsequent studies revealed that the CRTC1-MAML2 or CRTC3-MAML2 translocation had a questionable prognostic significance and can only be used as a diagnostic marker (Anzick et al., 2010; Schwarz et al., 2011; Saade et al., 2016). Interestingly, AREG (ligand for ErbB2) expression is positively correlated with CRTC1-MAML2 positive MECs and could be linked to the ErbB2 signaling in MEC (Shinomiya et al., 2016). Studies on p53 expression in MEC revealed its expression in intermediate and high grade MECs and suggested that p53 mutations might represent a genetic switch from a low-grade to a high grade phenotype (Faur et al., 2015). Therefore, irrespective of whether MECs are fusion positive or not, their prognosis is governed by other molecular markers (summarized in Fig. 5).
Table 3.
Review of the prognostic and diagnostic markers in MEC (Alos et al., 2005; Anzick et al., 2010; Aro et al., 2011; Coca-Pelaz et al., 2015; Dillard et al., 2001; Handra-Luca A. et al., 2005; Honjo et al., 2018; Luna, 2006; Saade et al., 2016; Weed et al., 2004).
| Marker | Diagnostic significance | Prognostic significance |
|---|---|---|
| Mucins/Glycoproteins | ||
| MUC1 | Expressed in cytoplasm and cell membranes of all neoplastic cells | Expression is directly proportional to histologic grade, lymph node metastases & disease progression (>50% positive cells correlated to a shorter disease free interval) |
| MUC2 | Rarely expressed (5%–21% MECs) in cytoplasm of mucous cells | No prognostic significance |
| MUC4β (used in present study) | Expressed in the cytoplasm and cell membrane of all neoplastic cells | Inverse statistical relationship with the histologic grade of the MECs. Indicator of good prognosis. |
| MUC5AC | Expressed in 72% MEC in cytoplasm of mucous cells. No correlation with grade | No prognostic significance |
| MUC5B | Expressed in 82% MEC in cytoplasm of mucous cells | No prognostic significance |
| MUC6 | Rarely expressed (32% MECs) in cytoplasm of neoplastic cells | No prognostic significance |
| MUC7 | Rarely expressed (5% MECs) in cytoplasm of mucous cells | No prognostic significance |
| MUC16 (CA-125) & Sialyl Lewis antigen (CA19-9) |
Expressed apically in neoplastic luminal cells | Not elucidated |
| CD63 | Expressed in microliths | Not elucidated |
| CD68 | Expressed in high grade MEC | Hypothesized to play a role in tumour progression via the release of pro-angiogenic growth factors. |
| Proteins | ||
| Intermediate filament proteins: | ||
| - Cytokeratin (CK): CK 7, CK 19, CK 14, CK 5, CK 6 |
Expressed in the squamoid and intermediate cells. Secretory material from neoplastic cells is thought to cause displacement of the cytoskeleton & affect immunoreactivity of the CKs. |
No association with the grade |
| - Vimentin & Glial fibrillary acidic protein (GFAP) | Expression is seen in 30% of MECs. | Not elucidated |
| Oncofetal antigens: | ||
| - α-fetoprotein | Expressed in neoplastic parenchyma | Not elucidated |
| - Carcino-embryonic antigen (CEA) | Low specificity for neoplastic parenchyma | Not elucidated |
| Tumor suppressor proteins: | ||
| - p53 | Expressed in the nuclei of intermediate and squamoid cells in 60% of MECs | Correlates with the grade of tumour. Associated with poor prognosis. |
| - Deletion/Inactivation of CDKN2A (p16) | Not elucidated | Regarded as an adverse prognosticator |
| Growth factors: | ||
| - TGF-β1-TGF-β RII | Expressed in neoplastic cells independent of histological grade | Not elucidated |
| - FGF 1 & FGF 2 | Expressed in neoplastic parenchyma | Thought to play a role in facilitating neoplastic progression. |
| Transmembrane proteins: | ||
| - E-cadherin (HECD1) | Focal loss of expression seen in neoplastic parenchyma | No prognostic significance |
| - Claudins 1 & Claudin 3 | Expressed in low grade MEC (89.7%). Expressed in intermediate & high grade MEC (71.8%) |
Auxiliary prognostic marker |
| - Caveolin-1 | Expressed in the cytoplasm & membrane of non-mucous cells & is inversely proportional to histopathological grade. | Negative expression may indicate poor prognosis |
| Enzymes: | ||
| - Endoribonuclease Dicer | Expressed in squamoid and intermediate cells, particularly in high grade MEC | Predictor of poor disease-specific survival |
| - Activated protein kinase (phosphorylated ERK1 & ERK2) | Expressed in neoplastic cells | Expression is correlated with aggressive tumour behaviour and high Ki67 index. Does not correspond with histological grade & HER-2/Neu or p16 expression |
| - COX2 | Expressed in neoplastic cells & is inversely proportional to the grade | High expression in node positive MECs |
| - MMP-2 & MMP-9 | Expressed in the cytoplasm of intermediate cells | Not elucidated |
| Viral antigens: | ||
| - E6 (Human Papilloma Virus 16/18) | Expressed in the mucous & squamoid cells | Not elucidated |
| - Cytomegalovirus (CMV) (IE1, pt65) | Expressed in the squamoid & intermediate cells | Not elucidated |
| Miscellaneous: | ||
| - S100 | ||
| - Actin | Expressed in dendritic cells in 23% MECs | Not elucidated |
| - Monoclonal antibody B 72.3 | Expressed in neoplastic parenchyma | Not elucidated |
| - IgG4 | Expressed in glandular neoplastic cells | Not elucidated |
| - p63 | Expressed in plasma cells of the sclerosing variant of MEC | Not elucidated |
| - p27 | Expressed in oncocytic MEC | Not elucidated |
| - Involucrin | Expression was detected in the intracystic component | Not elucidated |
| - SMA, Caldesmon | Expressed in squamoid cells | Inversely correlated with histologic grade & prognosis |
| - HSP 27 | Not expressed in MEC | Not elucidated |
| - BCL2 | Expressed in squamoid & intermediate cells | Not elucidated |
| - Amphiregulin (AREG) | Expressed in neoplastic cells & is inversely proportional to the grade. | BCL2 negative tumours had a poor prognosis. |
| Expressed in low & intermediate grade MEC, in association with the fusion transcript, hence known as the surrogate marker for fusion transcript in MEC. | High expression is associated with good prognosis. | |
| Proliferation markers: | ||
| - Proliferating cell nuclear antigen (PCNA) | Nuclear expression in neoplastic parenchyma | >7% (positively stained nuclei divided by total number of neoplastic cells) was associated with a poor prognosis. |
| - Ki 67/MIB1 | Expressed in squamoid & intermediate cells | An index >10 % correlated with an unfavourable prognosis. |
| Receptors: | ||
| - HER1/ErbB/EGFR | Expressed in squamoid tumour cells & mucous cells | Not sensitive or specific to the grade. Prognostic significance unknown. |
| - HER2(Neu)/ErbB2/EGFR2 | Expressed in the squamoid tumour cells in <20 % of MECs | Indicator of poor prognosis, independent of histological grade, and T or N status. |
| Genetic abberations: | ||
| - t(11:19) (q21; p13.1) | Seen mostly seen in low & intermediate grade MEC. | Usually associated with favourable prognosis. |
| - 9p21 | Mutations seen in high-grade MECs in limited studies | No prognostic significance |
| - 8q | ||
| - 5p | ||
| - 16q | ||
| - 12p | ||
| - H-ras gene mutation | ||
Fig. 5.
Prognosticators of Mucoepidermoid carcinomas (MECs): MECs can broadly be classified based on the presence or absence of CRTC1-MAML2/CRTC3-MAML2 translocation. MECs positive for CRTC1-MAML2 translocation and associated with deletion and hypermethylation of cyclin-dependent kinase Inhibitor 2A (CDKN2A), show poor prognosis. CDKN2A gene codes for p16INK4a and p14arf, tumor suppressor proteins that act by regulating the cell cycle by inhibiting CDK4 and CDK6; and by preventing degradation of p53 (Kozloski et al., 2010; Weber et al., 2002). Irrespective of MEC being fusion positive or not, high expression of ErbB2 and/or evidence of p53 mutations indicate poor prognosis. Independent of the histopathological grade of MEC, aberrant co-expression of ErbB2 and MUC4β, potentiates phosphorylation of ErbB2 causing activation of downstream signaling pathways responsible for tumor progression and poor prognosis. (MAPK-Mitogen-activated protein kinase; PI3K-phosphoinositide 3-kinase; CDK- cyclin-dependent kinase; (CREB)-regulated transcriptional coactivator 1 (CRTC1) and mastermind-like 2 (MAML2).
We would like to hypothesize that, over expression of MUC4β (as seen in two cases of our study) in high grade (grade III) MECs, if associated with a simultaneous high expression of ErBb2/Neu, can synergistically activate the downstream signaling pathways associated with the MUC4β-ERbB2-ERbB3-Neu complex. Thus contributing to the aggressive behavior of the neoplasm (Lujan et al., 2010; Funes et al., 2006; Kang et al., 2017).
In our opinion, MUC4β alone cannot be considered a reliable prognostic marker. The co-expression of ErbB2 and MUC4β needs to be evaluated further as it can serve as a potential and economical marker of prognostication, irrespective of the histological grade of the tumor.
5. Conclusions
In conclusion, the present study demonstrates that MUC4 expression does show a negative correlation with the histopathological grade of MECs. However, there may be some peculiar cases that do not follow this pattern, as seen in two cases of high grade MECs in our study,a hitherto unreported finding. Hence, although previous studies have supported that MUC4 is an indicator of good prognosis; further research is needed to determine whether the role of MUC4 as a marker of prognostication is governed by its association with ErbB2 in view of its divergent tumor suppressor and oncogenic pathway.
Declarations
Author contribution statement
P. Sawant: conceived and designed the experiments; performed the experiments; wrote the paper.
A. Spadigam: conceived and designed the experiments.
A. Dhupar: performed the experiments.
S. Syed: contributed reagents, materials, analysis tools or data.
K. Carvalho: analyzed and interpreted the data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We thank the Departments of Oral and Maxillofacial Pathology, Goa Dental College and Hospital, Bambolim-Goa, India; the Department of General Pathology, Goa Medical College and Hospital, Bambolim-Goa, India; Maratha Mandal's Institute of Dental Sciences and Research Centre, Belgavi-Karnataka, India and KLE VK Institute of Dental Sciences, Belgavi-Karnataka, India; for assistance in data collection.
References
- Alos L., Lujan B., Castillo M., Nadal A., Carreras M., Caballero M., de, Bolos C., Cardesa A. Expression of membrane-bound mucins (MUC1 and MUC4) and secreted mucins (MUC2, MUC5AC, MUC5B, MUC6 and MUC7) in mucoepidermoid carcinomas of salivary glands. Am. J. Surg. Pathol. 2005;29:806–813. doi: 10.1097/01.pas.0000155856.84553.c9. [DOI] [PubMed] [Google Scholar]
- Alroy I., Yarden Y. The ErbB signaling network in embryogenesis and oncogenesis: signal diversification through combinatorial ligand-receptor interactions. FEBS let. 1997;410:83–86. doi: 10.1016/s0014-5793(97)00412-2. PMID: 9247128. [DOI] [PubMed] [Google Scholar]
- Ansari M.H. Salivary gland tumors in an Iranian population: a retrospective study of 130 cases. J. Oral Maxillofac. Surg. 2007;65:2187–2194. doi: 10.1016/j.joms.2006.11.025. [DOI] [PubMed] [Google Scholar]
- Anzick S.L., Chen W.D., Park Y., Meltzer P., Bell D., El-Naggar A.K., Kaye F.J. Unfavorable prognosis of CRTC1-MAML2 positive mucoepidermoid tumors with CDKN2A deletions. Genes Chromosomes Cancer. 2010;49:59–69. doi: 10.1002/gcc.20719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aro K., Rosa L.E., Bello I.O., Soini Y., Mäkitie A.A., Salo T., Leivo I. Expression pattern of claudins 1 and 3-an auxiliary tool in predicting behavior of mucoepidermoid carcinoma of salivary gland origin. Virchows Arch. 2011;458:341–348. doi: 10.1007/s00428-010-1026-1. [DOI] [PubMed] [Google Scholar]
- Auclair P.L., Goode R.K., Ellis G.L. Mucoepidermoid carcinoma of intraoral salivary glands evaluation and application of grading criteria in 143 cases. Cancer. 1992;69:2021–2030. doi: 10.1002/1097-0142(19920415)69:8<2021::aid-cncr2820690803>3.0.co;2-7. 2-7. [DOI] [PubMed] [Google Scholar]
- Bai S., Clubwala R., Adler E., Sarta C., Schiff B., Smith R.V., Gnepp D.R., Brandwein-Gensler M. Salivary mucoepidermoid carcinoma: a multi-institutional review of 76 patients. Head and Neck Pathol. 2013;7:105–112. doi: 10.1007/s12105-012-0405-0. PMID: 23080318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behboudi A., Enlund F., Winnes M., Andrén Y., Nordkvist A., Leivo I., Flaberg E., Szekely L., Mäkitie A., Grenman R., Mark J., Stenman G. Molecular classification of mucoepidermoid carcinomas—prognostic significance of the MECT1–MAML2 fusion oncogene. Genes Chromosomes Cancer. 2006;45:470–481. doi: 10.1002/gcc.20306. [DOI] [PubMed] [Google Scholar]
- Brandwein M.S., Ivanov K., Wallace D.I., Hille J.J., Wang B., Fahmy A., Bodian C., Urken M.L., Gnepp D.R., Huvos A., Lumerman H., Mills S.E. Mucoepidermoid carcinoma: a clinicopathologic study of 80 patients with special reference to histological grading. Am. J. Surg. Pathol. 2001;25:835–845. doi: 10.1097/00000478-200107000-00001. PMID: 11420454. [DOI] [PubMed] [Google Scholar]
- Carraway K.L., Theodoropoulos G., Kozloski G.A., Carothers, Carraway C.A. Muc4/MUC4 functions and regulation in cancer. Future Oncol. 2009;5:1631–1640. doi: 10.2217/fon.09.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi P., Singh A.P., Batra S.K. Structure, evolution, and biology of the MUC4 mucin. FASEB J. 2008;22:966–981. doi: 10.1096/fj.07-9673rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan S.C., Singh A.P., Ruiz F., Johansson S.L., Jain M., Smith L.M., Moniaux N., Batra S.K. Aberrant expression of MUC4 in ovarian carcinoma: diagnostic significance alone and in combination with MUC1 and MUC16 (CA125) Mod. Pathol. 2006;19:1386–1394. doi: 10.1038/modpathol.3800646. PMID: 16880776. [DOI] [PubMed] [Google Scholar]
- Cho K., Kim J., Lee S., Oh K. Mucoepidermoid carcinoma of the salivary gland—a clinicopathologic and immunohistochemical study for c-erbB2 oncoprotein. J. Korean Med. Sci. 1997;12:499–504. doi: 10.3346/jkms.1997.12.6.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coca-Pelaz A., Rodrigo J.P., Triantafyllou A., Hunt J.L., Rinaldo A., Strojan P., Haigentz M., Mendenhall W.M., Takes R.P., Vander Poorten V., Ferlito A. Salivary mucoepidermoid carcinoma revisited. Eur. Arch. Oto-Rhino-Laryngol. 2015;272:799–819. doi: 10.1007/s00405-014-3053-z. [DOI] [PubMed] [Google Scholar]
- Dhanisha S.S., Guruvayoorappan C., Drishya S., Abeesh P. Mucins:structural diversity, biosynthesis, its role in pathogenesis and as possible therapeutic targets. Crit. Rev. Oncol. Hematol. 2018;122:98–122. doi: 10.1016/j.critrevonc.2017.12.006. [DOI] [PubMed] [Google Scholar]
- Dillard G.D., Muller S., Cohen C., Bloch D., Del Gaudio J.M., Gal A.A. High tumor grade in salivary gland mucoepidermoid carcinomas and loss of expression of transforming growth factor β receptor type II. Arch. Otolaryngol. Head Neck Surg. 2001;127:683–686. doi: 10.1001/archotol.127.6.683. [DOI] [PubMed] [Google Scholar]
- El-Attar R.H., Deraz E.M. Expression of estrogen receptors (ER) and human epidermal growth factor receptor 2 (HER2) in mucoepidermoid carcinoma: relationship to its grading. Tanta Dent J. 2014;11:194–198. [Google Scholar]
- Ettl T., Schwarz S., Kleinsasser N., Hartmann A., Reichert T.E., Driemel O. Overexpression of EGFR and absence of C-KIT expression correlate with poor prognosis in salivary gland carcinomas. Histopathology. 2008;53:567–577. doi: 10.1111/j.1365-2559.2008.03159.x. [DOI] [PubMed] [Google Scholar]
- Faur A.C., Sas I., Motoc A.G., Cornianu M.Ă., Zamfir C.L., Lazăr D.C., Folescu R. Ki-67 and p53 immunostaining assessment of proliferative activity in salivary tumors. Rom. J. Morphol. Embryol. 2015;56:1429–1439. PMID: 26743291. [PubMed] [Google Scholar]
- Funes M., Miller J.K., Lai C., Carraway K.L., Sweeney C. The mucin Muc4 potentiates neuregulin signaling by increasing the cell-surface populations of ErbB2 and ErbB3. J. Biol. Chem. 2006;281:19310–19319. doi: 10.1074/jbc.M603225200. PMID: 16690615. [DOI] [PubMed] [Google Scholar]
- Gautam S.K., Kumar S., Cannon A., Hall B., Bhatia R., Nasser M.W., Mahapatra S., Batra S.K., Jain M. MUC4 mucin-a therapeutic target for pancreatic ductal adenocarcinoma. Expert Opin. Ther. Targets. 2017;21:657–669. doi: 10.1080/14728222.2017.1323880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode R.K., Auclair P.L., Ellis G.L. Mucoepidermoid carcinoma of the major salivary glands:clinical and histopathologic analysis of 234 cases with evaluation of grading criteria. Cancer. 1998;82:1217–1224. doi: 10.1002/(sici)1097-0142(19980401)82:7<1217::aid-cncr2>3.0.co;2-c. PMID: 9529011. [DOI] [PubMed] [Google Scholar]
- Hamada T., Wakamatsu T., Miyahara M., Nagata S., Nomura M., Kamikawa Y., Yamada N., Batra S.K., Yonezawa S., Sugihara K. MUC4: a novel prognostic factor of oral squamous cell carcinoma. Int. J. Cancer. 2012;130:1768–1776. doi: 10.1002/ijc.26187. PMID: 21618516. [DOI] [PubMed] [Google Scholar]
- Handra-Luca A., Lamas G., Bertrand J.C., Fouret P. MUC1, MUC2, MUC4, and MUC5AC expression in salivary gland mucoepidermoid carcinoma: diagnostic and prognostic implications. Am. J. Surg. Pathol. 2005;29:881–889. doi: 10.1097/01.pas.0000159103.95360.e8. PMID: 12742253. [DOI] [PubMed] [Google Scholar]
- Herd M.K., Murugaraj V., Ghataura S.S., Brennan P.A., Anand R. Low-grade mucoepidermoid carcinoma of the palate—a previously unreported case of metastasis to the liver. J. Oral Maxillofac. Surg. 2012;70:2343–2346. doi: 10.1016/j.joms.2011.11.019. [DOI] [PubMed] [Google Scholar]
- Hollingsworth M.A., Swanson B.J. Mucins in cancer: protection and control of the cell surface. Nat. Rev. Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- Honjo K., Hiraki T., Higashi M., Noguchi H., Nomoto M., Yoshimura T., Batra S.K., Yonezawa S., Semba I., Nakamura N., Tanimoto A. Immunohistochemical expression profiles of mucin antigens in salivary gland mucoepidermoid carcinoma: MUC4-and MUC6-negative expression predicts a shortened survival in the early postoperative phase. Histol. Histopathol. 2018;33:201–213. doi: 10.14670/HH-11-913. PMID: 28649694. [DOI] [PubMed] [Google Scholar]
- Huang X., Wang X., Lu S.M., Chen C., Wang J., Zheng Y.Y., Ren B.H., Xu L. Clinicopathological and prognostic significance of MUC4 expression in cancers: evidence from meta-analysis. Int. J. Clin. Exp. Med. 2015;8:10274–10283. [PMC free article] [PubMed] [Google Scholar]
- Jeon J.M., Lee H.W., Park J.Y., Jung H.R., Hwang I., Kwon S.Y., Choe M.S., Kang Y.N., Kim S.P., Lee S.S., Choi W.I., Kwon K.Y. Korean J Pathol. 2010;44:397–403. [Google Scholar]
- Jepson S., Komatsu M., Haq B., Arango M.E., Huang D., Carraway C.A.C., Carraway K.L. Muc4/sialomucin complex, the intramembrane ErbB2 ligand, induces specific phosphorylation of ErbB2 and enhances expression of p27 kip, but does not activate mitogen-activated kinase or protein kinaseB/Akt pathways. Oncogene. 2002;21:7524–7532. doi: 10.1038/sj.onc.1205970. [DOI] [PubMed] [Google Scholar]
- Kang H., Tan M., Bishop J.A., Jones S., Sausen M., Ha P.K., Agrawal N. Whole-exome sequencing of salivary gland mucoepidermoid carcinoma. Clin. Cancer Res. 2017;23:283–288. doi: 10.1158/1078-0432.CCR-16-0720. PMID: 27340278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernohan N.M., Blessing K., King G., Corbett I.P., Miller I.D. Expression of c-erbB-2 oncoprotein in salivary gland tumors: an immunohistochemical study. J. Pathol. 1991;163:77–80. doi: 10.1002/path.1711630113. [DOI] [PubMed] [Google Scholar]
- Khiavi M.M., Vosoughhosseini S., Saravani S., Halimi M. Immunohistochemical correlation of epidermal growth factor receptor and c-erbB-2 with histopathologic grading of mucoepidermoid carcinoma. J. Cancer Res. Ther. 2012;8:586. doi: 10.4103/0973-1482.106550. [DOI] [PubMed] [Google Scholar]
- Kozloski G.A., Carraway C.A., Carraway K.L. Mechanistic and signaling analysis of Muc4–ErbB2 signaling module: new insights into the mechanism of ligand-independent ErbB2 activity. J. Cell. Physiol. 2010;224:649–657. doi: 10.1002/jcp.22163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Tang H., Zhang A., Dong J. Prognostic role of mucin antigen MUC4 for cholangiocarcinoma: a meta-analysis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0157878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Lague J.R., Nunes D.P., Toselli P., Oppenheim F.G., Soares R.V., Troxler R.F., Offner G.D. Expression of membrane-associated mucins MUC1 and MUC4 in major human salivary glands. J. Histochem. Cytochem. 2002;50:811–820. doi: 10.1177/002215540205000607. [DOI] [PubMed] [Google Scholar]
- Lujan B., Hakim S., Moyano S., Nadal A., Caballero M., Diaz A., Valera A., Carrera M., Cardesa A., Alos L. Activation of the EGFR/ERK pathway in high-grade mucoepidermoid carcinomas of the salivary glands. Br. J. Canc. 2010;103:510–516. doi: 10.1038/sj.bjc.6605788. PMID: 20664595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna M.A. Salivary mucoepidermoid carcinoma: revisited. Adv. Anat. Pathol. 2006;13:293–307. doi: 10.1097/01.pap.0000213058.74509.d3. [DOI] [PubMed] [Google Scholar]
- Majhi P.D., Lakshmanan I., Ponnusamy M.P., Jain M., Das S., Kaur S., Shimizu S.T., West W.W., Johansson S.L., Smith L.M., Yu F., Roller C.E., Sharma P., Carey G.B., Batra S.K., Ganti A.K. Pathobiological implications of MUC4 in non–small-cell lung cancer. J. Thorac. Oncol. 2013;8:398–407. doi: 10.1097/JTO.0b013e3182829e06. PMID: 23370366. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay P., Lakshmanan I., Ponnusamy M.P., Chakraborty S., Jain M., Pai P., Smith L.M., Lele S.M., Batra S.K. MUC4 overexpression augments cell migration and metastasis through EGFR family proteins in triple negative breast cancer cells. PLoS One. 2013;8 doi: 10.1371/journal.pone.0054455. PMID: 23408941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe M., Miyabe S., Nagatsuka H., Terada A., Hanai N., Yokoi M., Shimozato K., Eimoto T., Nakamura S., Nagai N., Hasegawa Y., Inagaki H. MECT1-MAML2 fusion transcript defines a favorable subset of mucoepidermoid carcinoma. Clin. Cancer Res. 2006;12:3902–3907. doi: 10.1158/1078-0432.CCR-05-2376. PMID: 16818685. [DOI] [PubMed] [Google Scholar]
- Padler-Karavani V. Aiming at the sweet side of cancer: aberrant glycosylation as possible target for personalized-medicine. Cancer Lett. 2014;352:102–112. doi: 10.1016/j.canlet.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Parkin D.M., Ferlay J., Curado M.P., Bray F., Edwards B., Shin H.R., Forman D. Fifty years of cancer incidence: CI5 I–IX. Int. J. Cancer. 2010;127:2918–2927. doi: 10.1002/ijc.25517. [DOI] [PubMed] [Google Scholar]
- Press M.F., Pike M.C., Hung G., Zhou J.Y., Ma Y., George J., Dietz-Band J., James W., Slamon D.J., Batsakis J.G., El-Naggar A.K. Amplification and overexpression of HER-2/neu in carcinomas of the salivary gland: correlation with poor prognosis. Cancer Res. 1994;54:5675–5682. [PubMed] [Google Scholar]
- Qannam A., Bello I.O. Comparison of histological grading methods in mucoepidermoid carcinoma of minor salivary glands. Indian J. Pathol. Microbiol. 2016;59:457–462. doi: 10.4103/0377-4929.191765. [DOI] [PubMed] [Google Scholar]
- Remmers N., Anderson J.M., Linde E.M., DiMaio D.J., Lazenby A.J., Wandall H.H., Mandel U., Clausen H., Yu F., Hollingsworth M.A. Aberrant expression of mucin core proteins and o-linked glycans associated with progression of pancreatic cancer. Clin. Cancer Res. 2013;19:1–24. doi: 10.1158/1078-0432.CCR-12-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saade R.E., Bell D., Garcia J., Roberts D., Weber R. Role of CRTC1/MAML2 translocation in the prognosis and clinical outcomes of mucoepidermoid carcinoma. JAMA Otolaryngol Head Neck Surg. 2016;142:234–240. doi: 10.1001/jamaoto.2015.3270. [DOI] [PubMed] [Google Scholar]
- Schwarz S., Stiegler C., Müller M., Ettl T., Brockhoff G., Zenk J., Agaimy A. Salivary gland mucoepidermoid carcinoma is a clinically, morphologically and genetically heterogeneous entity: a clinicopathological study of 40 cases with emphasis on grading, histological variants and presence of the t (11; 19) translocation. Histopathology. 2011;58:557–570. doi: 10.1111/j.1365-2559.2011.03777.x. [DOI] [PubMed] [Google Scholar]
- Shanmugam C., Jhala N.C., Katkoori V.R., Wan W., Meleth S., Grizzle W.E., Manne U. Prognostic value of mucin 4 expression in colorectal adenocarcinomas. Cancer. 2010;116:3577–3586. doi: 10.1002/cncr.25095. PMID: 20564074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinomiya H., Ito Y., Kubo M., Yonezawa K., Otsuki N., Iwae S., Inagaki H., Nibu K.I. Expression of amphiregulin in mucoepidermoid carcinoma of the major salivary glands: a molecular and clinicopathological study. Hum. Pathol. 2016;57:37–44. doi: 10.1016/j.humpath.2016.06.016. [DOI] [PubMed] [Google Scholar]
- Singh A.P., Moniaux N., Chauhan S.C., Meza J.L., Batra S.K. Inhibition of MUC4 expression suppresses pancreatic tumor cell growth and metastasis. Cancer Res. 2004;64:622–630. doi: 10.1158/0008-5472.can-03-2636. PMID: 14744777. [DOI] [PubMed] [Google Scholar]
- Singh A.P., Chaturvedi P., Batra S.K. Emerging roles of MUC4 in cancer:a novel target for diagnosis and therapy. Cancer Res. 2007;67:433–436. doi: 10.1158/0008-5472.CAN-06-3114. PMID: 17234748. [DOI] [PubMed] [Google Scholar]
- Sugano S., Mukai K., Tsuda H., Hirohashi S., Furuya S., Shimosato Y., Ebihara S., Takeyama I. Immunohistochemical study of c-erbB-2 oncoprotein overexpression in human major salivary gland carcinoma: an indicator of aggressiveness. The Laryngoscope. 1992;102:923–927. doi: 10.1288/00005537-199208000-00013. [DOI] [PubMed] [Google Scholar]
- Tamada S., Shibahara H., Higashi M., Goto M., Batra S.K., Imai K., Yonezawa S. MUC4 is a novel prognostic factor of extrahepatic bile duct carcinoma. Clin. Cancer Res. 2006;12:4257–4264. doi: 10.1158/1078-0432.CCR-05-2814. PMID: 16857800. [DOI] [PubMed] [Google Scholar]
- Teshima T.H., Ianez R.F., Coutinho-Camillo C.M., Buim M.E., Soares F.A., Lourenço S.V. Development of human minor salivary glands: expression of mucins according to stage of morphogenesis. J. Anat. 2011;219:410–417. doi: 10.1111/j.1469-7580.2011.01405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To V.S.H., Chan J.Y.W., Tsang R.K., Wei W.I. Review of salivary gland neoplasms. ISRN otolaryngology. 2012;2012 doi: 10.5402/2012/872982. PMID: 23724273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber H.O., Samuel T., Rauch P., Funk J.O. Human p14 ARF-mediated cell cycle arrest strictly depends on intact p53 signaling pathways. Oncogene. 2002;21:3207–3212. doi: 10.1038/sj.onc.1205429. [DOI] [PubMed] [Google Scholar]
- Weed D.T., Gomez-Fernandez C., Pacheco J., Ruiz J., Hamilton-Nelson K., Arnold D.J., Civantos F.J., Zhang J., Yasin M., Goodwin W.J., Carraway K.L. MUC4 and ERBB2 expression in major and minor salivary gland mucoepidermoid carcinoma. Head Neck. 2004;26:353–364. doi: 10.1002/hed.10387. [DOI] [PubMed] [Google Scholar]