Abstract
Acute myeloid leukemia (AML) is a hematopoietic disorder characterized by numerous cytogenetic and molecular aberrations that accounts for ~25% of childhood leukemia diagnoses. The outcome of children with AML has increased remarkably over the past 30 years, with current survival rates up to 70%, mainly due to intensification of standard chemotherapy and improvements in risk classification, supportive care, and minimal residual disease monitoring. However, childhood AML prognosis remains unfavorable and relapse rates are still around 30%. Therefore, novel therapeutic approaches are needed to increase the cure rate. In AML, the presence of gene mutations and rearrangements prompted the identification of effective targeted molecular strategies, including kinase inhibitors, cell pathway inhibitors, and epigenetic modulators. This review will discuss several new drugs that recently received US Food and Drug Administration approval for AML treatment and promising strategies to treat childhood AML, including FLT3 inhibitors, epigenetic modulators, and Hedgehog pathway inhibitors.
Keywords: Pediatric AML, targeted therapy, FLT-3 inhibitors, Hedgehog pathway inhibitors, DOT1L inhibitors
Introduction
Acute myeloid leukemia (AML) is a hematological malignancy characterized by the clonal expansion of myeloid precursors, which acquire genetic abnormalities in cellular components involved in self-renewal, proliferation, and differentiation. Pediatric AML accounts for ~25% of pediatric leukemias and although relative low frequent, it represents a clinical challenge, due to its poor prognosis. Over the last 20 years, considerable improvements in overall survival (OS) were achieved, mainly due to intensified treatment strategy, enhancements in supportive care and progresses in risk-adapted patient stratification. Despite that, OS does not exceed 70%, and relapse rates range between 25 and 35% (1), highlighting an urgent need for novel treatments. AML has an age-related profile, with regard to either incidence rate or genetic features. Indeed, the incidence of pediatric AML peaks in infants (children aged 0–1 years), and it is rare in children up to 18 years and further increases in persons between 18 and 60 years of age (2). In addition, genetic abnormalities that occur in infants distinguish a peculiar subgroup of patients (3).
In recent years, with advances in technology, there have been tremendous progresses in defining the molecular landscape of AML, and novel AML entities were included in the most recent World Health Organization (WHO) classifications (4), including AML with NPM1 or CEBPA mutations. However, the occurrence of cytogenetic abnormalities as well as genetic mutations identifying specific WHO entities (e.g., NPM1, FLT3, CEBPA mutations) is lower in pediatric than in adult AML, and a high percentage of pediatric patients (>40%) fall in the “AML not otherwise specified” (AML-NOS) category, thus limiting the applicability of WHO classification in children with AML (5). Furthermore, thanks to the recent sequencing approaches, major insights into pediatric AML genetic alterations, distinct from those of adult AML, were achieved. Importantly, these findings greatly affected patient risk stratification and provided new therapeutic targets (6). In this regard, in 2018, Bolouri et al. published the results of the TARGET project, reporting a comprehensive analysis of the molecular aberrations occurring in a large cohort of pediatric AML (7). The main features of pediatric AML emerged from this study were a low overall mutation rate, likewise adult AML, but a landscape of somatic aberrations different from that observed in adult patients, and including structural changes, aberrant DNA methylation, and novel pediatric-specific mutations in genes characteristically mutated in AML. More specifically, the most common mutated genes in pediatric AML included RAS, KIT, and FLT3, and novel pediatric-specific FLT3 mutations were identified. Conversely, DNMT3A, IDH1, and IDH2 gene mutations were nearly absent in pediatric AML. Novel focal deletions were identified in IL9R, MBNL1, and ZEB2 genes, and further deletions affected ELF1 expression. A variety of fusion genes were detected, many of which were primarily or exclusively associated to pediatric AML, for instance, CBFA2T3-GLIS2 and NUP98-NSD1. Also, multiple epigenetic regulators, particularly KMT2A and WT1, were affected by both structural and mutational anomalies. Interestingly, the associated epigenetic changes induced transcriptional silencing of activating ligands for natural killer (NK) cells or genes that converge on Wnt–β-catenin signaling, both representing potential therapeutic targets. The most remarkable information emerged from the TARGET study were the age-related distribution of genomic anomalies and the interactions among mutations that have clinical consequences, thus demonstrating the importance to improve the identification of genomic alterations to better stratify pediatric AML patients as well as to develop novel targeted therapies. Indeed, at present, all the information regarding the molecular landscape of AML marginally resulted in novel therapeutic strategies, and in the last decades, with a few exceptions, there was a general stagnation in standard chemotherapeutic approaches. Fortunately, this scenario is gradually changing (8). In this review, we provided an overview of several therapeutic approaches to target specific genetic lesion of pediatric AML, with special attention on drugs that recently received US Food and Drug Administration (FDA) approval for AML treatment together with promising strategies to treat definite subgroup of pediatric AML. A summary of selected inhibitors discussed in the present review and currently investigated in pediatric AML is provided in Table 1.
Table 1.
Targeted inhibitors in clinical trials for pediatric AML.
| Target | Drug | Intervention | Condition | Phase | Age group | Clinical trial identifier | Status |
|---|---|---|---|---|---|---|---|
| FLT3 | Sorafenib | Sorafenib in combination with chemotherapy | De novo AML | III | Up to 29 years (child, adult) | NCT01371981 | Completed |
| Sorafenib in combination with idarubicin and Ara-C | Diagnosis AML and high-risk MDS | I–II | 15–60 years (child, adult) | NCT00542971 | Completed | ||
| BTK inhibitor with chemotherapy with/without Sorafenib | Refractory/relapsed FLT3 mutant AML | II–III | 14–60 years (child, adult) | NCT03642236 | Recruiting | ||
| Sorafenib in combination with cytarabine and clofarabine | Refractory/relapsed hematologic malignancies | I | Up to 31 years (Child, Adult) | NCT00908167 | Completed | ||
| Palbociclib and Sorafenib, Decitabine, or Dexamethasone | Recurrent or refractory leukemia | I | 15 years and older (child, adult) | NCT03132454 | Recruiting | ||
| Sorafenib | Refractory/relapsed solid tumors or leukemia | I–II | 2–21 years (child, adult) | NCT01445080 | Completed | ||
| Lestaurtinib | Lestaurtinib in combination with cytarabine and idarubicin | Refractory/relapsed FLT3 mutant AML | I–II | 1–30 years (child, adult) | NCT00469859 | Completed | |
| Midostaurin | Midostaurin in combination with standard chemotherapy | De novo FLT3 mutant AML | II | 3 months to 17 years (child) | NCT03591510 | Recruiting | |
| Midostaurin | Relapsed/refractory acute leukemias (MLL-rearranged ALL ad FLT3 mutated AML) | I–II | 3 months to 18 years (child, adult) | NCT00866281 | Completed | ||
| Quizartinib | Quizartinib in combination with re-induction chemotherapy and as a single-agent maintenance | Refractory/relapsed FLT3 mutant AML | I–II | 1 month to 21 years (Child, Adult) | NCT03793478 | Recruiting | |
| Crenolanib | Crenolanib in combination with Sorafenib | Refractory/relapsed FLT3 mutant AML | I | 1 year to 39 years (Child, Adult) | NCT02270788 | Completed | |
| Gilteritinib | Gilteritinib in sequential combination with chemotherapy | Refractory/relapsed FLT3 mutant AML | I–II | 6 months to <18 years of age (and young adults) | 2215-CL-0603 | Planned | |
| Gilteritinib in sequential combination with chemotherapy | Newly diagnosed FLT3 mutant AML | II | 6 months to <18 years of age (and young adults) | 2215-CL-0604 | Planned | ||
| DOT1L | Pinometostat | Pinometostat | Relapsed/refractory leukemias with MLL rearrangements | I | 3 months to 18 years (child, adult) | NCT02141828 | Completed |
| Pinometostat with standard chemotherapy | Newly diagnosed AML with MLL Rearrangement | I–II | 14 years and older (child, adult) | NCT03724084 | Recruiting | ||
| KIT | Dasatinib | Dasatinib in consolidation therapy in CBF-AML | De novo AML | N.A. | 6 months to 16 years (child) | NCT03173612 | Recruiting |
| Dasatinib in combination with chemotherapy | Relapsed t(8;21) AML With KITD816 mutation | I | Child, adult, older adult | NCT03560908 | Recruiting | ||
| CD33 | Lintuzumab | Lintuzumab | Relapsed/refractory AML | I | 16 years and older (child, adult, older adult) | NCT00002890 | Completed |
| Actinium-225 labeled to lintuzumab | Relapsed/refractory AML | I | Child, adult, older adult | NCT00672165 | Completed |
BTK, Bruton's tyrosine kinase; MLL, mixed-lineage leukemia; FLT3, fms related tyrosine kinase 3; DOT1L, disrupter of telomeric silencing 1-like histone methyltransferase; AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia.
Targeting Gene Mutations: Focus on FLT3 and KIT
AML development is a multistep process that requires the cooperation of at least two genetic abnormalities, classified as type I (that confer a proliferation advantage on hematopoietic cells) and type II alterations (that impair hematopoietic differentiation) (9). These anomalies include both karyotypic alterations and gene mutations, with the latter frequently occurring in cytogenetically normal AML. Although AML is a cancer with a very low rate of somatic alterations, because of the constant identification of novel recurrent gene mutations, nowadays more than 90% of pediatric AML are identified to have at least one genomic alteration (10), among which those affecting FLT3 and KIT genes are very common in children, with more than 20% and 10% frequency, respectively, according to the TARGET study (7).
FLT3 is a transmembrane type III receptor tyrosine kinase that is activated by the specific FLT3 ligand and, subsequently, regulates hematopoiesis through phosphorylation of downstream targets, including STAT5, and activation of critical oncogenic pathways such as Ras/Raf/MAPK and PI3K/Akt/mTOR (11). Activating mutations of FLT3 include both internal tandem duplication (FLT3-ITD) and point mutations of the activation loop domain (FLT3-TKD), with a prevalence of ~15 and 7%, respectively, in pediatric AML (12). Ligand-independent FLT3 activation leads to a decreased maturation and an increased proliferation of myeloid progenitors. Importantly, FLT3 mutations are prognostically relevant in pediatric AML, and the presence of ITD particularly with an high allelic ratio (AR) of ≥0.5 have a prognostic impact and are significant predictive factors for an adverse outcome (12–14). Therefore, FLT3 mutated pediatric AML patients are considered high risk and, nowadays, they are offered allogenic hematopoietic stem cell transplantation (HSCT) in first complete remission (15). The use of HSCT can override the negative prognostic impact of FLT3 mutations, as demonstrated by similar probability of 8-year event free survival (EFS) in both FLT3-ITD and wild-type subgroups (15). However, there are potentially severe side effects correlated to this procedure, and there is still a consistent proportion of patients not eligible for HSCT, thus supporting the relevance to improve current treatments for FLT3 mutated patients. In addition, FLT3 mutations, even if not detectable at diagnosis, can subsequently appear at relapse because of clonal selection, and may further affect prognosis (16). Given the high number of both adult and pediatric AML patients harboring FLT3 mutations (7, 17) and their poor outcome, many efforts have been made to develop FLT3 targeted inhibitors, and a variety of compounds have entered clinical trials for both adult and pediatric patients (Table 1). The first generation of FLT3 inhibitors, which entered clinical trials since the early 2000s, were not FLT3 specific but targeted multiple kinases. In pediatric AML, the most extensively studied first-generation FLT3 inhibitor is Sorafenib, which was investigated as single agent or in combination with other drugs in several formal clinical trials enrolling both de novo or refractory/relapsed AML. In pediatric AML, the MTD of Sorafenib was defined as 150 mg/m2 (18, 19). Sorafenib showed a significant antileukemic activity in relapsed or refractory pediatric AML, inducing a reduction by more than 50% of bone marrow blasts and, in combination with clofarabine and cytarabine, it achieved a complete remission in 8 out 12 patients, including both wild type and mutated FLT3 (18). As a single agent, the activity of Sorafenib was observed in 2/8 pediatric refractory AML, both with FLT3-ITD (19). Importantly, in these trials, remission achievement allowed to proceed with allergenic HSCT. These findings resulted in further investigation of Sorafenib in newly diagnosed or FLT3 mutant AML (NCT01371981, NCT03642236). Other first-generation FLT3 inhibitors evaluated for pediatric AML treatment include Sunitinib (20), Lestaurtinib (NCT00469859), and Midostaurin (NCT00866281, NCT03591510). The latter is extremely important because in 2017, the FDA approved Midostaurin in combination with chemotherapy for newly diagnosed FLT3-mutated adult AML based on data of a multi-institutional, randomized phase 3 trial (RATIFY, NCT00651261). This trial showed that addition of Midostaurin to standard chemotherapy significantly prolonged overall and event-free survival among adult patients with AML and an FLT3 mutation (21). In children, Midostaurin is being evaluated as a single agent or in combination with chemotherapy in refractory/relapsed or newly diagnosed AML, respectively (NCT00866281, NCT03591510), and preliminary data indicated that single-agent Midostaurin, although adequately tolerated, has only limited clinical activity (22). However, since Midostaurin was the first drug approved in an AML genetic subgroup characterized by a specific gene mutation, this represents a starting point for novel therapy employing additional targeted agents, including second- and third-generation FLT3 inhibitors in ongoing clinical trials. Indeed, newer generation FLT3 inhibitors have a great specificity against FLT3, and consequently, they are more potent in inhibiting FLT3 with hopefully fewer off-target side effects. Among such compounds, Quizartinib, evaluated in relapsed childhood AML in combination with salvage chemotherapy, demonstrated a favorable toxicity profile and an encouraging response, consisting in complete FLT3 inhibition in all patients, and 4/17 and 10/17 complete remissions or stable disease, respectively (23). Currently, Quizartinib is being evaluated in a phase 1/2 study both in combination with re-induction chemotherapy and as a single-agent maintenance therapy in relapsed/refractory pediatric AML with FLT3-ITD mutations (NCT03793478). Crenolanib, a tyrosine kinase inhibitor developed as a selective and potent PDGFRα/β inhibitor, has also high affinity for FLT3, including both FLT3-ITD and FLT3-TKD mutations (24), and a phase 1 pilot study is currently assessing its toxicity profile in combination with Sorafenib in relapsed or refractory pediatric AML with mutated FLT3 (NCT02270788). Gilteritinib is a potent and selective FLT3 inhibitor with activity against both FLT3-ITD and FLT3-TKD mutations that demonstrated clinical efficacy in subjects with both wild-type or mutated FLT3 in phase 1/2 clinical trials enrolling adult patients with relapsed or refractory AML (25). In 2018, the FDA approved Gilteritinib as monotherapy to treat adult patients with relapsed/refractory AML and FLT3 mutations based on interim analysis of the ADMIRAL phase 3 trial (NCT02421939), which proved the superiority of Gilteritinib as compared to salvage chemotherapy in adults with relapsed and/or refractory FLT3 mutated AML. Indeed, the initial results of this trial reported a median OS significantly longer in the Gilteritinib arm than in the salvage chemotherapy arm (9.3 vs. 5.6 months), with 21% and 11% of complete remissions (CR) achieved in the two arms, respectively (26). Noteworthy, Gilteritinib is the first FLT3 inhibitor to be approved as monotherapy for AML patients. In 2016, the pediatric development program for Gilteritinib started, and in 2018, EMA approved several modifications to the pediatric investigation plan that now include two clinical studies evaluating Gilteritinib used in sequential combination with chemotherapy in pediatric patients from 6 months to <18 years of age with FLT3-ITD positive relapse/refractory AML (2215-CL-0603) or newly diagnosed AML (2215-CL-0604). Collectively, these trials provided important data regarding the efficacy of FLT3 inhibitors for AML treatment, and their application, particularly in combination with traditional chemotherapeutic agents as well as novel agents, would represent an important shift in the outcome of pediatric AML patients.
An additional gene frequently mutated in pediatric AML is KIT (7), a proto-oncogene that encodes a transmembrane glycoprotein type III receptor tyrosine kinase (RTK). The stem cell factor (SCF) promotes KIT dimerization and auto-phosphorylation that in turn lead to activation of complex downstream signaling pathways, including Ras/Erk, PI3K/Akt/mTOR, PLC-γ, Src kinase, and JAK/STAT signaling pathways, all essential to proliferation, differentiation, and survival of hematopoietic stem cells (27). In pediatric AML patients, KIT mutations occur in the extracellular portion of the receptor (exon 8), in the transmembrane domain (exons 10), in the juxtamembrane domain (exon 11), and in the activation loop of the tyrosine kinase domain (exon 17). These mutations affect RTK activity, due to ligand-independent activation of KIT, and tyrosine kinase inhibitors with activity against mutated KIT may represent effective therapeutic approaches. KIT mutations frequently associate with specific AML subtype, including core binding factor (CBF) AML. CBF-AML is characterized by the presence of aberrancies at CBF genes, and comprises t(8;21) and inv (16)/t(16;16), resulting in the RUNX1-RUNX1T1 and CBFB-MYH11 gene fusions, respectively. Both alterations affect CBF transcriptional complex, that is involved in the regulation of normal hematopoiesis, thus inducing leukemic transformation by blocking differentiation and promoting the self-renewal of stem cells and early progenitors (28). Collectively, CBF AML accounts about 20% of pediatric AML and is considered favorable (10). Accordingly, these patients receive a regimen of treatment based on four courses of chemotherapy (usually at lower dosages compared to other risk groups) not comprising HSCT (29). However, in some recent studies, the subgroup of t(8;21) AML patients showed a cumulative incidence of relapse of ~30%, similarly to high-risk patients (29–31). Although most of these patients have been subsequently rescued by an allograft, this resulting in an 8-year OS approaching 83%, the event free survival (EFS) remains unsatisfactory (29). These observation prompted to better investigate the impact of KIT mutation on prognosis of t(8;21) AML. Indeed, according to the multistep model of leukemogenesis, RUNX1-RUNX1T1 alone is not sufficient for leukemogenesis and requires co-operation with additional genetic hits, such as KIT mutations (32). In an interesting retrospective analysis of children with CBF-AML, Manara et al. found several differences between t(8;21) and inv(16)/t(16;16) AML, with a higher occurrence of KIT mutation in RUNX1-RUNX1T1- compared to CBFB-MYH11-rearranged patients (33). More importantly, t(8;21) AML with KIT mutations had a significantly worse prognosis than patients harboring only the translocation, suggesting that KIT mutations might contribute to the outcome and might be considered for risk stratification and therapeutically targetable markers in this subgroup of CBF-AML patients (33). Given the high frequency of KIT mutations and consequent elevated expression of this gene in AML with t(8;21), the addition to chemotherapy of the multikinase inhibitor Dasatinib has been evaluated in adult patients, and the results showed a favorable outcome (34). A recent phase 1 study is evaluating the clinical efficacy and tolerability of combination therapy of Dasatinib with multi-agent chemotherapy in relapsed child and adult AML patients with t(8;21) translocation and KITD816 mutation (NCT03560908) (Table 1).
Targeting Deregulated Signaling Pathways
In AML, uncontrolled proliferation and increased survival of leukemic cells can be sustained by deregulation of signal transduction pathways, whose components represent potential actionable targets.
Signaling regulated by Ras proteins are among the best characterized but most intricate signal transduction pathways in cell biology. Indeed, there are three members belonging to the Ras family (HRAS, KRAS, and NRAS), all found to be activated by mutations in human tumors, that play essential roles in controlling cellular functions involved in tumorigenesis including cell growth, division, differentiation, cell cycle regulation, cell migration, and angiogenesis (35). In addition, Ras proteins operate through two distinct pathways, the mitogen-activated protein kinases (MAPK) and phosphoinositide-3 kinase (PI3K) pathways (35). Collectively, mutations in both NRAS and KRAS genes account for more than 30% of pediatric AML patients, with a prominent age-related profile, and further RAS-related mutations, that affect RAS downstream components, may occur (7). On the other hand, PI3K/Akt/mTOR pathway deregulation occurs in a large proportion of AML patients. Its constitutive activation results from a variety of mechanisms besides Ras activating-mutations, including activating mutation in RTK (e.g., FLT3 and KIT mutations), mutations and/or over-expression in key signaling components (PI3K subunits, Akt, mTOR), alterations in the activity of the negative regulators PTEN and SHIP phosphatases, and deregulation in molecules that interact with this pathway (36). A plethora of compounds targeting Ras/MAPK and PI3K/Akt/mTOR signaling components were developed and tested in adult AML patients (37, 38); however, at present, only few of those inhibitors are investigated in pediatric AML patients (39). Since this review focuses on novel and promising targeted therapies potentially available for study in pediatric AML in the near future, an exhaustive review of all the Ras/MAPK and PI3K/Akt/mTOR signaling inhibitors is beyond our scope, and only few examples will be reported. Among the Ras/MAPK pathway inhibitors, Trametinib, a highly specific and potent MEK1/2 inhibitor, is currently investigated in children with Juvenile myelomonocytic leukemia (JMML) in a phase 2 trial (NCT03190915). Various clinical trials investigated FLT3 and KIT inhibitors, as above discussed, which in turn may down-modulate PI3K/Akt/mTOR signaling, and showed encouraging results. The PI3K/Akt/mTOR pathway inhibition can be achieved by targeting the key signaling components. In pediatric AML, both Akt (MK2206) and mTOR (RAD001) inhibitors were investigated in phase 1 clinical trials (NCT01231919 and NCT00081874, respectively). However, PI3K/Akt/mTOR inhibitors may induce significant toxicities, particularly in association with chemotherapy (39), without objective responses (40), thus limiting the clinical development of therapeutic approaches based on their application.
An interesting pathway deregulated in AML and recently investigated in adult patients is the Hedgehog (Hh) pathway, an evolutionary conserved process that regulates embryonic development and organ morphogenesis (41). Hh pathway has also been implicated in hematopoiesis, although its requirements depend on developmental stage (primitive or definitive hematopoiesis), cell maturation stage, and cell physiologic state, and a wide range of in vitro and in vivo studies demonstrated that targeting specific pathway components severely impairs proper hematopoiesis (42). Classical Hh pathway can be activated by one of three different ligands (Sonic Hedgehog, SHH; Indian Hedgehog, IHH; Desert Hedgehog, DHH) that bind the transmembrane receptor Patched (PTCH) that functions as a negative regulator of the pathway through inhibition of Smoothened (Smo). As a result of ligand binding, Smo is activated and induces a signaling cascade that culminate in the activation and nucleus translocation of GLI transcription factors that in turn regulate target gene expression (41). Due to its physiological role, it is not surprising that aberrant activation of Hh pathway is commonly observed in human cancers, including myeloid malignancies. Currently, the Smo inhibitors Sonidegib, Vismodegib, and Glasdegib are evaluated with multiple intervention approaches in phase 1, 2, and 3 clinical trials, enrolling adult patients with myeloid malignancies at different stages, including AML (43). In a phase 1 study, Glasdegib, administered as a single agent, demonstrated a biological activity in 16/28 adult AML patients by inducing one CR, four partial remissions, four minor responses, and seven stable diseases (44). A more recent randomized phase 2 clinical trial evaluated low-dose cytarabine plus/minus Glasdegib in newly diagnosed AML or high-risk myelodysplastic syndrome adult patients. Addition of Glasdegib increased median OS from 4.9 to 8.8 months, with complete remission rates of 17% for the Glasdegib arm vs. 2% for the standard arm, and a general favorable benefit–risk profile (45). Based on these results, in 2018, FDA approved Glasdegib in combination with low-dose cytarabine, for newly diagnosed AML patients who are 75 years old or older or who have comorbidities that preclude intensive induction chemotherapy. Several Smo inhibitors, including Sonidegib and Vismodegib, have been evaluated in pediatric patients affected by medulloblastoma (MB), the most common malignant brain tumor in children, demonstrating efficient HH pathway inhibition and anti-tumor activity (46). Although no evidence has still been reported in childhood AML, both results obtained in adult AML and pediatric MB patients prompted an extensive investigation of Hh inhibitors in the pediatric setting (47). With regard to Smo inhibitors, it should be taken into account the induction of permanent defects in bone growth, a toxicity profile that has been observed in both preclinical models and patients enrolled in clinical trials (46, 48). As a result, in 2016, the European Medicines Agency (EMA) modified the pediatric investigation plan of Glasdegib in pediatric patients with MB, with a waiver applied to the pediatric population from birth to <4 months of age. However, this drug-related toxicity was observed only with several compounds, including Glasdegib and Sonidegib (46, 48), thus supporting further investigation of additional Smo inhibitors especially in combinatorial drug regimens.
Targeting Fusion Proteins
One of the most recurrent translocation in pediatric AML is t(15, 17), which results in the fusion transcript PML-RARA and identifies a specific AML subtype, acute promyelocytic leukemia (APL), accounting for ~12% of pediatric AML (10). Among pediatric leukemias, APL is the first case where an effective targeted therapy was used, consisting of all-trans retinoic acid (ATRA) and arsenic trioxide (ATO), and this successful treatment radically changed APL from a detrimental to a curable malignancy, with complete remission rate over 90% (49, 50). These successful results constantly encourage the efforts in identifying effective targeted therapies against leukemia driver mutations.
The most common genetic events occurring in pediatric AML are the rearrangements of the MLL gene, accounting for 18% of patients (10), and in infants, the frequency is much higher, reaching 50% in several studies (3). MLL is a nuclear protein critical for hematopoietic development that normally regulates gene expression by catalyzing methylation of lysine 4 on histone 3 (H3K4). MLL rearranges with more than 80 different partner genes, and the resulting fusion proteins deregulate expression of MLL target genes. The most frequently overexpressed genes in MLL-rearranged leukemias are HOX cluster genes and the HOX cofactor MEIS1, which are normally expressed at highest levels only in the stem cells and early lineage progenitor cells, whereas they are down-regulated during differentiation (51). Although with several differences depending on fusion partner, the overall prognosis of MLL-rearranged AML is unfavorable (51). Great advancements in our knowledge of relevant mechanisms that determine the oncogenicity of MLL fusions have been made in recent years. The histone methyltransferase disrupter of telomeric silencing 1-like (DOT1L) is a methyltransferase that catalyzes methylation of lysine 79 on histone H3 (H3K79) and this epigenetic mark associates with active gene transcription (52). DOT1L acts in a multiprotein complex that also includes partners of MLL in the formation of MLL-fusion proteins (53). Therefore, in MLL-rearranged leukemias, DOT1L is recruited on MLL target sites, and its mislocated enzymatic activity promotes the leukemic gene expression program (54–56). It is not surprising that considerable efforts are being made to identify effective inhibitors to target DOT1L. Impressive results have been obtained in preclinical studies investigating pharmacologic inhibition of DOT1L (57), and the DOT1L inhibitor Pinometostat entered a phase 1 clinical trial to treat pediatric patients with relapsed/refractory leukemias bearing a rearrangement of the MLL gene (NCT02141828) (Table 1). However, despite Pinometostat biological activity and acceptable safety profile, no objective responses were observed (58). Currently, a phase 1/2 clinical trial evaluating Pinometostat in combination with standard chemotherapy to treat both child and adult patients with newly diagnosed MLL-rearranged leukemia is ongoing (NCT03724084) (Table 1).
An attractive therapeutic target for MLL-rearranged leukemias is represented by the menin–MLL interaction. Menin is a tumor suppressor protein that interacts with both wild type and rearranged MLL proteins and is required for the proper recruitment of MLL to the target genes. Because MLL-fusion proteins are difficult to target directly, pharmacological inhibition of the menin–MLL interaction is a promising therapeutic approach, since the leukemogenic activity of MLL fusion proteins is dependent on this interaction. In in vivo preclinical studies, the first orally bioavailable small-molecule inhibitors of the menin–MLL interaction, MI-463 and MI-503, resulted in growth inhibition and survival benefit in mouse models of MLL leukemia (59). In addition, further preclinical studies demonstrated that combining DOT1L and menin inhibition enhances the treatment efficacy in MLL-rearranged leukemia models (60). Interestingly, the synergistic effect of DOT1L and menin inhibitors has also been observed in AML models harboring NPM1 gene mutations (61). Currently, two phase 1/2 clinical trials are evaluating the menin inhibitors KO-539 (NCT04067336) and SNDX-5613 (NCT04065399) in adult patients with relapsed/refractory AML or MLL-rearranged/NPM1-mutated AML, respectively.
CBFA2T3-GLIS2 is a recently identified fusion transcript resulting from a cryptic inversion of chromosome 16 and specific to pediatric AML (62, 63). This chimeric oncogene identifies a peculiar subgroup of extremely aggressive pediatric AML with an incidence ranging between 9 and 30% among the whole cytogenetically normal (CN) AML (64) or the non-Down syndrome acute megakaryoblastic leukemia (non-DS-AMKL) subgroups (62), respectively. CBFA2T3 is a CBFA2T-family member that belongs to the RUNX1T1 complex and acts as a transcriptional co-repressor via its association with DNA-binding transcription factors, other corepressors, and histone-modifying enzymes, including the chimeric protein RUNX1-RUNX1T1 resulting from t(8;21) (64). GLIS2 (GLI-similar 2) is a member of the Kruppel-like zinc finger transcription factor group, which is closely related to the GLI family proteins, the transcription factors activated by the Hedgehog signal transduction cascade that regulate cell proliferation and self-renewal (64). Based on the homology between GLIS2 and GLI proteins, our group explored the possibility to target CBFA2T3-GLIS2 employing GANT61, a GLI1, and GLI2 inhibitor. Remarkably, in vitro treatment with GANT61 induced apoptosis in CBFA2T3-GLIS2-positive AML cells and reduced the expression of GLIS2-specific signature genes (65). Even if preliminary, these results are encouraging and prompt to extend the investigation of GLI inhibitors as a promising strategy to treat CBFA2T3-GLIS2 AML. Due to the discovery that induction of polyploidization and differentiation mediated by Aurora kinase A (AURKA) provides a therapeutic strategy for AMKL (66), Thiollier et al. investigated the efficacy of the AURKA inhibitor Alisertib (MLN8237) in a xenograft model of human AMKL expressing the CBFA2T3-GLIS2 fusion, demonstrating that Alisertib efficiently inhibits leukemic blast proliferation and increases survival, because of induction of terminal differentiation and apoptosis (67). However, a phase 2 clinical trial documented a poor response rate in children and adolescents affected by solid tumors or leukemia and receiving Alisertib as a single agent (68).
Targeting Epigenetic Regulators
A common feature of AML is an altered epigenetic pattern resulting from both somatic mutations in epigenetic regulators or specific translocations that interfere with the normal epigenetic program (69). As a whole, mutations in epigenetic regulators are frequent in adult AML while they are uncommon in pediatric AML. For example, mutations in the NADP+-dependent isocitrate dehydrogenase genes 1 and 2 (IDH1 and IDH2) were found in up to 33% adult AML and only in 3–4% of pediatric AML (70–72). Other epigenetic regulators mutated in pediatric AML, albeit rarely, are TET2, DNMT3A, and ASXL1 (73). A relevant finding that emerged from these studies is the frequent co-occurrence of mutations in epigenetic regulators with other genetic anomalies in signaling transduction pathways and hematopoietic transcription factor genes, suggesting that mutations in epigenetic regulators cooperate in leukemogenesis. The last point also implies the possibility to combine treatments that target different class of mutations to eradicate the leukemic clone. In this regard, targeting the methyltransferase DOT1L is particularly intriguing for several reasons. Firstly, DOT1L is the only known methyltrasferase that catalyze mono-, di-, and tri-methylation of H3K79, and only recently a histone demethylase that can catalyze the removal of di- and tri-methyl groups from the H3K79 lysine residue has been identified (74); therefore, most likely DOT1L plays an essential role in AML cells. Next, DOT1L has diverse functions in mammalian cells, since methylation of H3K79 is primarily linked to active gene transcription and transcription elongation, but DOT1L also acts in DNA damage response and cell cycle regulation (53). Then, DOT1L associates with several complexes, including the elongation complexes EAP (75), SEC (76), and AEP (77), which, in turn, contain other proteins with oncogenic/leukemogenic activity. Finally, although the involvement of DOT1L in leukemia was initially linked to its mislocation due to MLL fusion proteins, some of the most recent works demonstrated a role of DOT1L also in non MLL-rearranged leukemias. For example, in preclinical studies, pharmacologic inhibition of DOT1L has been reported to impair proliferation, to induce cell differentiation, or to impact gene expression of primary AML cells harboring IDH1/2 mutations (78) as well as AML cell lines and a xenograft model with partial tandem duplication (PTD) of MLL (MLL-PTD) (79). DOT1L inhibition has been explored as a therapeutic target for the treatment of DNMT3A mutant AML, wherein reversed DNMT3Amut induced gene activation and resulted in apoptosis and cell differentiation induction in both in vitro and in vivo AML models (80, 81). Finally, DOT1L inhibition has also been assessed in NPM1 mutant AML and resulted synergistic with menin–MLL inhibitors in suppressing HOX, MEIS1, and FLT3 gene expression and inducing AML cell differentiation (61). Overall, these studies demonstrated a remarkable role of DOT1L in AML cells irrespective of MLL fusion proteins, and based on these preclinical results, further investigation of combination treatments employing DOT1L inhibitors is warranted.
A further recently identified epigenetic target is the Bromodomain and Extra-Terminal Domain (BET) family of proteins, which includes BRD2, BRD3, BRD4, and BRDT proteins. This is the most prominent group of epigenetic reader proteins that regulates gene transcription by interacting with acetylated histones, thus facilitating transcriptional activation. Different small-molecule BRD inhibitors were developed and tested in cancers characterized by altered histone acetylation and aberrant gene transcription, including AML wherein epigenetic alterations are common (82). BET inhibitors demonstrated an anti-leukemic activity in preclinical models as both single agents or in combination with other drugs. In addition, their application is currently investigated in several clinical trials enrolling adult AML patients (82, 83), encouraging their application for pediatric AML treatment.
Immunotherapy
A promising treatment to fight cancer is immunotherapy, an approach that exploits components of the immune system. There are several types of immunotherapy, including monoclonal antibodies, T-cell therapy, cancer vaccines, and other non-specific immunotherapies (84). All these therapeutic strategies have been employed to treat different types of cancer, including AML (85). In pediatric AML, the most relevant immunotherapy-based approach is represented by targeted therapy directed against surface antigens, in particular CD33 (sialic acid-binding immunoglobulin lectin, SIGLEC) and CD123 (IL3Rα), that are highly expressed, albeit not exclusively, on AML cells. A wide range of clinical trials were conducted to investigate the efficacy of gentuzumab ozogamycin (GO, Mylotarg) treatment in pediatric AML. GO is a monoclonal antibody to CD33 conjugated with the cytotoxic antibiotic calicheamicin that specifically induces cell death in CD33-expressing cells. GO originally received accelerated approval in 2000 as a stand-alone treatment for older patients with CD33-positive AML who had experienced a relapse, but it was withdrawn from the market after subsequent confirmatory trials failed to verify clinical benefit and demonstrated safety concerns, including a high number of early deaths. Nevertheless, in 2017, FDA approved GO for the treatment of adults with newly CD33-positive AML and patients aged 2 years and older with CD33-positive AML who have experienced a relapse or who are refractory (86). The newest FDA approval includes a lower dosing regimen, which induces less adverse events and is active for induction of remission, without curative intent (86). GO as monotherapy demonstrated a limited efficacy in children with relapsed/refractory AML (87) and did not delay the time to relapse when administered in postconsolidation therapy (88). However, GO was effective in reducing MRD levels in pediatric AML patients, when administered in combination with chemotherapy (89), and this is particularly beneficial in conditioning regimen prior to HSCT (90). The use of GO was also explored in consolidation targeted immunotherapy following HSCT (91). CD33-expressing AML cells may also be targeted by the unconjugated antibody Lintuzumab that was investigated in relapsed/refractory pediatric AML in phase 1 clinical trials (NCT00002890, NCT00672165). An exhaustive review describing all the other immunotherapies investigated in pediatric AML was recently published (92).
Conclusions
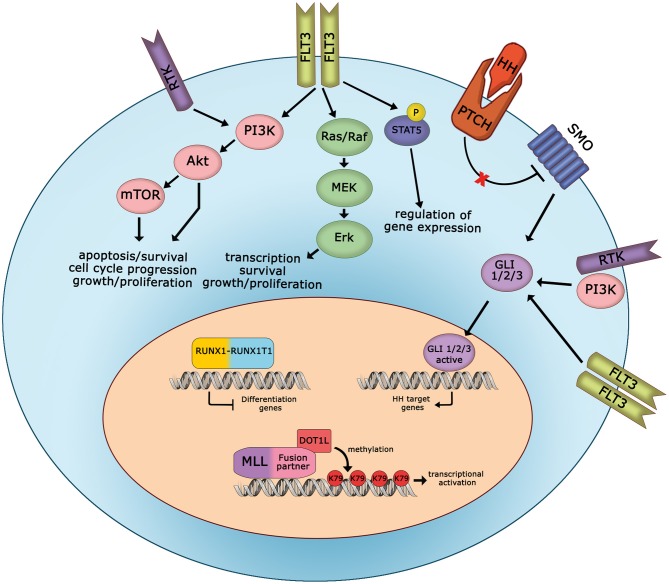
After decades of therapeutic advances for AML based almost exclusively on optimizing older drugs, since 2017, the field of novel therapies for AML has been rapidly developing. A major point to take into account is that lots of novel target inhibitors might be associated with each other or with conventional chemotherapy to increase treatment efficacy. Indeed, in AML, the genomic lesions often cooperate (Figure 1). For example, one of the most significantly up-regulated gene in MLL-rearranged leukemias is FLT3. Further, NPM1-mutated AML has aberrant HOX expression and frequently concomitant FLT3 mutations. Also, the presence of fusion proteins interferes with normal cellular functions, and the efficacy of fusion protein targeting might be increased with additional drugs. In conclusion, such progresses in drug development, together with continuous efforts in our understanding of the molecular landscape of AML, provide great hope that more effective treatments may be offered to pediatric AML patients in the near future.
Figure 1.
Cooperation between oncogenic signaling pathways in pediatric AML. Schematic representation of deregulated signaling and mutated proteins, involved in AML cell proliferation and survival, that can be targeted in pediatric patients. HH, hedgehog; RTK, receptor tyrosine kinase.
Author Contributions
All authors contributed to the conception and editing of this review and approved the final manuscript. AL conducted literature review and wrote the manuscript in consultation with RM. AP critically revised the work. RM provided final approval of the version to publish.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by Associazione Italiana per la Ricerca sul Cancro (grant number MFAG2016, Id.19117) to RM.
References
- 1.Zwaan CM, Kolb EA, Reinhardt D, Abrahamsson J, Adachi S, Aplenc R, et al. Collaborative efforts driving progress in pediatric acute myeloid leukemia. J Clin Oncol. (2015) 33:2949–62. 10.1200/JCO.2015.62.8289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dores GM, Devesa SS, Curtis RE, Linet MS, Morton LM. Acute leukemia incidence and patient survival among children and adults in the United States, 2001-2007. Blood. (2012) 119:34–43. 10.1182/blood-2011-04-347872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masetti R, Vendemini F, Zama D, Biagi C, Pession A, Locatelli F. Acute myeloid leukemia in infants: biology and treatment. Front Pediatr. (2015) 3:37. 10.3389/fped.2015.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. (2016) 127:2391–405. 10.1182/blood-2016-03-643544 [DOI] [PubMed] [Google Scholar]
- 5.Sandahl JD, Kjeldsen E, Abrahamsson J, Ha SY, Heldrup J, Jahnukainen K, et al. The applicability of the WHO classification in paediatric AML. A NOPHO-AML study Br J Haematol. (2015) 169:859–67. 10.1111/bjh.13366 [DOI] [PubMed] [Google Scholar]
- 6.Rubnitz JE. Current management of childhood acute myeloid leukemia. Paediatr Drugs. (2017) 19:1–10. 10.1007/s40272-016-0200-6 [DOI] [PubMed] [Google Scholar]
- 7.Bolouri H, Farrar JE, Triche T, Jr, Ries RE, Lim EL, Alonzo TA, et al. The molecular landscape of pediatric acute myeloid leukemia reveals recurrent structural alterations and age-specific mutational interactions. Nat Med. (2018) 24:103–12. 10.1038/nm.4439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walasek A. The new perspectives of targeted therapy in acute myeloid leukemia. Adv Clin Exp Med. (2019) 28:271–6. 10.17219/acem/81610 [DOI] [PubMed] [Google Scholar]
- 9.Balgobind BV, Hollink IH, Arentsen-Peters ST, Zimmermann M, Harbott J, Beverloo HB, et al. Integrative analysis of type-I and type-II aberrations underscores the genetic heterogeneity of pediatric acute myeloid leukemia. Haematologica. (2011) 96:1478–87. 10.3324/haematol.2010.038976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pui CH, Carroll WL, Meshinchi S, Arceci RJ. Biology, risk stratification, and therapy of pediatric acute leukemias: an update. J Clin Oncol. (2011) 29:551–65. 10.1200/JCO.2010.30.7405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sexauer AN, Tasian SK. Targeting FLT3 signaling in childhood acute myeloid leukemia. Front Pediatr. (2017) 5:248. 10.3389/fped.2017.00248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meshinchi S, Alonzo TA, Stirewalt DL, Zwaan M, Zimmerman M, Reinhardt D, et al. Clinical implications of FLT3 mutations in pediatric AML. Blood. (2006) 108:3654–61. 10.1182/blood-2006-03-009233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Staffas A, Kanduri M, Hovland R, Rosenquist R, Ommen HB, Abrahamsson J, et al. Presence of FLT3-ITD and high BAALC expression are independent prognostic markers in childhood acute myeloid leukemia. Blood. (2011) 118:5905–13. 10.1182/blood-2011-05-353185 [DOI] [PubMed] [Google Scholar]
- 14.Manara E, Basso G, Zampini M, Buldini B, Tregnago C, Rondelli R, et al. Characterization of children with FLT3-ITD acute myeloid leukemia: a report from the AIEOP AML-2002 study group. Leukemia. (2017) 31:18–25. 10.1038/leu.2016.177 [DOI] [PubMed] [Google Scholar]
- 15.Locatelli F, Masetti R, Rondelli R, Zecca M, Fagioli F, Rovelli A, et al. Outcome of children with high-risk acute myeloid leukemia given autologous or allogeneic hematopoietic cell transplantation in the aieop AML-2002/01 study. Bone Marrow Transplant. (2015) 50:181–8. 10.1038/bmt.2014.246 [DOI] [PubMed] [Google Scholar]
- 16.Cloos J, Goemans BF, Hess CJ, van Oostveen JW, Waisfisz Q, Corthals S, et al. Stability and prognostic influence of FLT3 mutations in paired initial and relapsed AML samples. Leukemia. (2006) 20:1217–20. 10.1038/sj.leu.2404246 [DOI] [PubMed] [Google Scholar]
- 17.Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. (2016) 374:2209–21. 10.1056/NEJMoa1516192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inaba H, Rubnitz JE, Coustan-Smith E, Li L, Furmanski BD, Mascara GP, et al. Phase I pharmacokinetic and pharmacodynamic study of the multikinase inhibitor sorafenib in combination with clofarabine and cytarabine in pediatric relapsed/refractory leukemia. J Clin Oncol. (2011) 29:3293–300. 10.1200/JCO.2011.34.7427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Widemann BC, Kim A, Fox E, Baruchel S, Adamson PC, Ingle AM, et al. A phase I trial and pharmacokinetic study of sorafenib in children with refractory solid tumors or leukemias: a Children's Oncology Group Phase I Consortium report. Clin Cancer Res. (2012) 18:6011–22. 10.1158/1078-0432.CCR-11-3284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baker SD, Zimmerman EI, Wang YD, Orwick S, Zatechka DS, Buaboonnam J, et al. Emergence of polyclonal FLT3 tyrosine kinase domain mutations during sequential therapy with sorafenib and sunitinib in FLT3-ITD-positive acute myeloid leukemia. Clin Cancer Res. (2013) 19:5758–68. 10.1158/1078-0432.CCR-13-1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. (2017) 377:454–64. 10.1056/NEJMoa1614359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zwaan CM, Soderhall S, Brethon B, Luciani M, Rizzari C, Stam RW, et al. A phase 1/2, open-label, dose-escalation study of midostaurin in children with relapsed or refractory acute leukaemia. Br J Haematol. (2019) 185:623–7. 10.1111/bjh.15593 [DOI] [PubMed] [Google Scholar]
- 23.Cooper TM, Cassar J, Eckroth E, Malvar J, Sposto R, Gaynon P, et al. A phase I study of quizartinib combined with chemotherapy in relapsed childhood leukemia: a therapeutic advances in childhood leukemia & lymphoma (TACL) study. Clin Cancer Res. (2016) 22:4014–22. 10.1158/1078-0432.CCR-15-1998 [DOI] [PubMed] [Google Scholar]
- 24.Zimmerman EI, Turner DC, Buaboonnam J, Hu S, Orwick S, Roberts MS, et al. Crenolanib is active against models of drug-resistant FLT3-ITD-positive acute myeloid leukemia. Blood. (2013) 122:3607–15. 10.1182/blood-2013-07-513044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perl AE, Altman JK, Cortes J, Smith C, Litzow M, Baer MR, et al. Selective inhibition of FLT3 by gilteritinib in relapsed or refractory acute myeloid leukaemia: a multicentre, first-in-human, open-label, phase 1-2 study. Lancet Oncol. (2017) 18:1061–75. 10.1016/S1470-2045(17)30416-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perl AE, Martinelli G, Cortes JE, Neubauer A, Berman E, Paolini S, et al. Gilteritinib significantly prolongs overall survival in patients with FLT3-mutated relapsed/refractory acute myeloid leukemia: results from the phase III ADMIRAL trial. Cancer Res. (2019) 79(13 Suppl):Abstract#CT184. 10.1158/1538-7445.AM2019-CT184 [DOI] [Google Scholar]
- 27.Liang J, Wu YL, Chen BJ, Zhang W, Tanaka Y, Sugiyama H. The C-kit receptor-mediated signal transduction and tumor-related diseases. Int J Biol Sci. (2013) 9:435–43. 10.7150/ijbs.6087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin S, Mulloy JC, Goyama S. RUNX1-ETO leukemia. Adv Exp Med Biol. (2017) 962:151–73. 10.1007/978-981-10-3233-2_11 [DOI] [PubMed] [Google Scholar]
- 29.Pession A, Masetti R, Rizzari C, Putti MC, Casale F, Fagioli F, et al. Results of the AIEOP AML 2002/01 multicenter prospective trial for the treatment of children with acute myeloid leukemia. Blood. (2013) 122:170–8. 10.1182/blood-2013-03-491621 [DOI] [PubMed] [Google Scholar]
- 30.Gibson BE, Webb DK, Howman AJ, De Graaf SS, Harrison CJ, Wheatley K. Results of a randomized trial in children with Acute Myeloid Leukaemia: medical research council AML12 trial. Br J Haematol. (2011) 155:366–76. 10.1111/j.1365-2141.2011.08851.x [DOI] [PubMed] [Google Scholar]
- 31.Ustun C, Morgan E, Moodie EEM, Pullarkat S, Yeung C, Broesby-Olsen S, et al. Core-binding factor acute myeloid leukemia with t(8, 21): risk factors and a novel scoring system (I-CBFit). Cancer Med. (2018) 7:4447–55. 10.1002/cam4.1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang YY, Zhao LJ, Wu CF, Liu P, Shi L, Liang Y, et al. C-KIT mutation cooperates with full-length AML1-ETO to induce acute myeloid leukemia in mice. Proc Natl Acad Sci USA. (2011) 108:2450–5. 10.1073/pnas.1019625108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manara E, Bisio V, Masetti R, Beqiri V, Rondelli R, Menna G, et al. Core-binding factor acute myeloid leukemia in pediatric patients enrolled in the AIEOP AML 2002/01 trial: screening and prognostic impact of c-KIT mutations. Leukemia. (2014) 28:1132–4. 10.1038/leu.2013.339 [DOI] [PubMed] [Google Scholar]
- 34.Paschka P, Schlenk RF, Weber D, Benner A, Bullinger L, Heuser M, et al. Adding dasatinib to intensive treatment in core-binding factor acute myeloid leukemia-results of the AMLSG 11-08 trial. Leukemia. (2018) 32:1621–30. 10.1038/s41375-018-0129-6 [DOI] [PubMed] [Google Scholar]
- 35.Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. (2003) 3:11–22. 10.1038/nrc969 [DOI] [PubMed] [Google Scholar]
- 36.Park S, Chapuis N, Tamburini J, Bardet V, Cornillet-Lefebvre P, Willems L, et al. Role of the PI3K/AKT and mTOR signaling pathways in acute myeloid leukemia. Haematologica. (2010) 95:819–28. 10.3324/haematol.2009.013797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Evangelisti C, Bressanin D, Buontempo F, Chiarini F, Lonetti A, Soncin M, et al. Targeting phosphatidylinositol 3-kinase signaling in acute myelogenous leukemia. Expert Opin Ther Targets. (2013) 17:921–36. 10.1517/14728222.2013.808333 [DOI] [PubMed] [Google Scholar]
- 38.Johnson DB, Smalley KS, Sosman JA. Molecular pathways: targeting NRAS in melanoma and acute myelogenous leukemia. Clin Cancer Res. (2014) 20:4186–92. 10.1158/1078-0432.CCR-13-3270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tasian SK, Teachey DT, Rheingold SR. Targeting the PI3K/mTOR pathway in pediatric hematologic malignancies. Front Oncol. (2014) 4:108. 10.3389/fonc.2014.00108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Becher OJ, Millard NE, Modak S, Kushner BH, Haque S, Spasojevic I, et al. A phase I study of single-agent perifosine for recurrent or refractory pediatric CNS and solid tumors. PLoS ONE. (2017) 12:e0178593 10.1371/journal.pone.0178593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Briscoe J, Therond PP. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat Rev Mol Cell Biol. (2013) 14:416–29. 10.1038/nrm3598 [DOI] [PubMed] [Google Scholar]
- 42.Lim Y, Matsui W. Hedgehog signaling in hematopoiesis. Crit Rev Eukaryot Gene Expr. (2010) 20:129–39. 10.1615/CritRevEukarGeneExpr.v20.i2.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Campbell V, Copland M. Hedgehog signaling in cancer stem cells: a focus on hematological cancers. Stem Cells Cloning. (2015) 8:27–38. 10.2147/SCCAA.S58613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martinelli G, Oehler VG, Papayannidis C, Courtney R, Shaik MN, Zhang X, et al. Treatment with PF-04449913, an oral smoothened antagonist, in patients with myeloid malignancies: a phase 1 safety and pharmacokinetics study. Lancet Haematol. (2015) 2:e339–346. 10.1016/S2352-3026(15)00096-4 [DOI] [PubMed] [Google Scholar]
- 45.Cortes JE, Heidel FH, Hellmann A, Fiedler W, Smith BD, Robak T, et al. Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia. (2019) 33:379–89. 10.1038/s41375-018-0312-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Y, Song Q, Day BW. Phase I and phase II sonidegib and vismodegib clinical trials for the treatment of paediatric and adult MB patients: a systemic review and meta-analysis. Acta Neuropathol Commun. (2019) 7:123. 10.1186/s40478-019-0773-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pession A, Lonetti A, Bertuccio S, Locatelli F, Masetti R. Targeting Hedgehog pathway in pediatric acute myeloid leukemia: challenges and opportunities. Expert Opin Ther Targets. (2019) 23:87–91. 10.1080/14728222.2019.1559822 [DOI] [PubMed] [Google Scholar]
- 48.Cortes JE, Gutzmer R, Kieran MW, Solomon JA. Hedgehog signaling inhibitors in solid and hematological cancers. Cancer Treat Rev. (2019) 76:41–50. 10.1016/j.ctrv.2019.04.005 [DOI] [PubMed] [Google Scholar]
- 49.Abla O, Kutny MA, Testi AM, Feusner JH, Creutzig U, Gregory J, Jr, et al. Management of relapsed and refractory childhood acute promyelocytic leukaemia: recommendations from an international expert panel. Br J Haematol. (2016) 175:588–601. 10.1111/bjh.14313 [DOI] [PubMed] [Google Scholar]
- 50.Kutny MA, Alonzo TA, Gerbing RB, Wang YC, Raimondi SC, Hirsch BA, et al. Arsenic trioxide consolidation allows anthracycline dose reduction for pediatric patients with acute promyelocytic leukemia: report from the children's oncology group phase III historically controlled trial AAML0631. J Clin Oncol. (2017) 35:3021–9. 10.1200/JCO.2016.71.6183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Winters AC, Bernt KM. MLL-rearranged leukemias-an update on science and clinical approaches. Front Pediatr. (2017) 5:4. 10.3389/fped.2017.00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Steger DJ, Lefterova MI, Ying L, Stonestrom AJ, Schupp M, Zhuo D, et al. DOT1L/KMT4 recruitment and H3K79 methylation are ubiquitously coupled with gene transcription in mammalian cells. Mol Cell Biol. (2008) 28:2825–39. 10.1128/MCB.02076-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nguyen AT, Zhang Y. The diverse functions of Dot1 and H3K79 methylation. Genes Dev. (2011) 25:1345–58. 10.1101/gad.2057811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bernt KM, Zhu N, Sinha AU, Vempati S, Faber J, Krivtsov AV, et al. MLL-rearranged leukemia is dependent on aberrant H3K79 methylation by DOT1L. Cancer Cell. (2011) 20:66–78. 10.1016/j.ccr.2011.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nguyen AT, Taranova O, He J, Zhang Y. DOT1L, the H3K79 methyltransferase, is required for MLL-AF9-mediated leukemogenesis. Blood. (2011) 117:6912–22. 10.1182/blood-2011-02-334359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen L, Deshpande AJ, Banka D, Bernt KM, Dias S, Buske C, et al. Abrogation of MLL-AF10 and CALM-AF10-mediated transformation through genetic inactivation or pharmacological inhibition of the H3K79 methyltransferase Dot1l. Leukemia. (2013) 27:813–22. 10.1038/leu.2012.327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Daigle SR, Olhava EJ, Therkelsen CA, Basavapathruni A, Jin L, Boriack-Sjodin PA, et al. Potent inhibition of DOT1L as treatment of MLL-fusion leukemia. Blood. (2013) 122:1017–25. 10.1182/blood-2013-04-497644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stein EM, Garcia-Manero G, Rizzieri DA, Tibes R, Berdeja JG, Savona MR, et al. The DOT1L inhibitor pinometostat reduces H3K79 methylation and has modest clinical activity in adult acute leukemia. Blood (2018) 131:2661–9. 10.1182/blood-2017-12-818948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Borkin D, He S, Miao H, Kempinska K, Pollock J, Chase J, et al. Pharmacologic inhibition of the Menin-MLL interaction blocks progression of MLL leukemia in vivo. Cancer Cell. (2015) 27:589–602. 10.1016/j.ccell.2015.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dafflon C, Craig VJ, Mereau H, Grasel J, Schacher Engstler B, Hoffman G, et al. Complementary activities of DOT1L and Menin inhibitors in MLL-rearranged leukemia. Leukemia. (2017) 31:1269–77. 10.1038/leu.2016.327 [DOI] [PubMed] [Google Scholar]
- 61.Kuhn MW, Song E, Feng Z, Sinha A, Chen CW, Deshpande AJ, et al. Targeting chromatin regulators inhibits leukemogenic gene expression in NPM1 mutant leukemia. Cancer Discov. (2016) 6:1166–81. 10.1158/2159-8290.CD-16-0237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gruber TA, Larson Gedman A, Zhang J, Koss CS, Marada S, Ta HQ, et al. An Inv(16)(p13.3q24.3)-encoded CBFA2T3-GLIS2 fusion protein defines an aggressive subtype of pediatric acute megakaryoblastic leukemia. Cancer Cell. (2012) 22:683–97. 10.1016/j.ccr.2012.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Masetti R, Pigazzi M, Togni M, Astolfi A, Indio V, Manara E, et al. CBFA2T3-GLIS2 fusion transcript is a novel common feature in pediatric, cytogenetically normal AML, not restricted to FAB M7 subtype. Blood. (2013) 121:3469–72. 10.1182/blood-2012-11-469825 [DOI] [PubMed] [Google Scholar]
- 64.Masetti R, Bertuccio SN, Pession A, Locatelli F. CBFA2T3-GLIS2-positive acute myeloid leukaemia. A peculiar paediatric entity. Br J Haematol. (2019) 184:337–47. 10.1111/bjh.15725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Masetti R, Bertuccio SN, Astolfi A, Chiarini F, Lonetti A, Indio V, et al. Hh/Gli antagonist in acute myeloid leukemia with CBFA2T3-GLIS2 fusion gene. J Hematol Oncol. (2017) 10:26. 10.1186/s13045-017-0396-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wen Q, Goldenson B, Silver SJ, Schenone M, Dancik V, Huang Z, et al. Identification of regulators of polyploidization presents therapeutic targets for treatment of AMKL. Cell. (2012) 150:575–89. 10.1016/j.cell.2012.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thiollier C, Lopez CK, Gerby B, Ignacimouttou C, Poglio S, Duffourd Y, et al. Characterization of novel genomic alterations and therapeutic approaches using acute megakaryoblastic leukemia xenograft models. J Exp Med. (2012) 209:2017–31. 10.1084/jem.20121343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mosse YP, Fox E, Teachey DT, Reid JM, Safgren SL, Carol H, et al. A phase II study of Alisertib in children with recurrent/refractory solid tumors or leukemia: Children's Oncology Group phase I and Pilot Consortium (ADVL0921). Clin Cancer Res. (2019) 25:3229–38. 10.1158/1078-0432.CCR-18-2675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wouters BJ, Delwel R. Epigenetics and approaches to targeted epigenetic therapy in acute myeloid leukemia. Blood. (2016) 127:42–52. 10.1182/blood-2015-07-604512 [DOI] [PubMed] [Google Scholar]
- 70.Ho PA, Alonzo TA, Kopecky KJ, Miller KL, Kuhn J, Zeng R, et al. Molecular alterations of the IDH1 gene in AML: a Children's Oncology Group and Southwest Oncology Group study. Leukemia. (2010) 24:909–13. 10.1038/leu.2010.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Andersson AK, Miller DW, Lynch JA, Lemoff AS, Cai Z, Pounds SB, et al. IDH1 and IDH2 mutations in pediatric acute leukemia. Leukemia. (2011) 25:1570–7. 10.1038/leu.2011.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Damm F, Thol F, Hollink I, Zimmermann M, Reinhardt K, van den Heuvel-Eibrink MM, et al. Prevalence and prognostic value of IDH1 and IDH2 mutations in childhood AML: a study of the AML-BFM and DCOG study groups. Leukemia. (2011) 25:1704–10. 10.1038/leu.2011.142 [DOI] [PubMed] [Google Scholar]
- 73.Liang DC, Liu HC, Yang CP, Jaing TH, Hung IJ, Yeh TC, et al. Cooperating gene mutations in childhood acute myeloid leukemia with special reference on mutations of ASXL1, TET2, IDH1, IDH2, and DNMT3A. Blood. (2013) 121:2988–95. 10.1182/blood-2012-06-436782 [DOI] [PubMed] [Google Scholar]
- 74.Kang JY, Kim JY, Kim KB, Park JW, Cho H, Hahm JY, et al. KDM2B is a histone H3K79 demethylase and induces transcriptional repression via sirtuin-1-mediated chromatin silencing. FASEB J. (2018) 32:5737–50. 10.1096/fj.201800242R [DOI] [PubMed] [Google Scholar]
- 75.Mueller D, Bach C, Zeisig D, Garcia-Cuellar MP, Monroe S, Sreekumar A, et al. A role for the MLL fusion partner ENL in transcriptional elongation and chromatin modification. Blood. (2007) 110:4445–54. 10.1182/blood-2007-05-090514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lin C, Smith ER, Takahashi H, Lai KC, Martin-Brown S, Florens L, et al. AFF4, a component of the ELL/P-TEFb elongation complex and a shared subunit of MLL chimeras, can link transcription elongation to leukemia. Mol Cell. (2010) 37:429–37. 10.1016/j.molcel.2010.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yokoyama A, Lin M, Naresh A, Kitabayashi I, Cleary ML. A higher-order complex containing AF4 and ENL family proteins with P-TEFb facilitates oncogenic and physiologic MLL-dependent transcription. Cancer Cell. (2010) 17:198–212. 10.1016/j.ccr.2009.12.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sarkaria SM, Christopher MJ, Klco JM, Ley TJ. Primary acute myeloid leukemia cells with IDH1 or IDH2 mutations respond to a DOT1L inhibitor in vitro. Leukemia. (2014) 28:2403–6. 10.1038/leu.2014.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kuhn MW, Hadler MJ, Daigle SR, Koche RP, Krivtsov AV, Olhava EJ, et al. MLL partial tandem duplication leukemia cells are sensitive to small molecule DOT1L inhibition. Haematologica. (2015) 100:e190–3. 10.3324/haematol.2014.115337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lu R, Wang P, Parton T, Zhou Y, Chrysovergis K, Rockowitz S, et al. Epigenetic perturbations by Arg882-mutated DNMT3A potentiate Aberrant stem cell gene-expression program and acute leukemia development. Cancer Cell. (2016) 30:92–107. 10.1016/j.ccell.2016.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rau RE, Rodriguez BA, Luo M, Jeong M, Rosen A, Rogers JH, et al. DOT1L as a therapeutic target for the treatment of DNMT3A-mutant acute myeloid leukemia. Blood. (2016) 128:971–81. 10.1182/blood-2015-11-684225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alqahtani A, Choucair K, Ashraf M, Hammouda DM, Alloghbi A, Khan T, et al. Bromodomain and extra-terminal motif inhibitors: a review of preclinical and clinical advances in cancer therapy. Future Sci OA. (2019) 5:FSO372. 10.4155/fsoa-2018-0115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Berthon C, Raffoux E, Thomas X, Vey N, Gomez-Roca C, Yee K, et al. Bromodomain inhibitor OTX015 in patients with acute leukaemia: a dose-escalation, phase 1 study. Lancet Haematol. (2016) 3:e186–95. 10.1016/S2352-3026(15)00247-1 [DOI] [PubMed] [Google Scholar]
- 84.Khalil DN, Smith EL, Brentjens RJ, Wolchok JD. The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat Rev Clin Oncol. (2016) 13:273–90. 10.1038/nrclinonc.2016.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kruger S, Ilmer M, Kobold S, Cadilha BL, Endres S, Ormanns S, et al. Advances in cancer immunotherapy 2019 - latest trends. J Exp Clin Cancer Res. (2019) 38:268. 10.1186/s13046-019-1266-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Norsworthy KJ, Ko CW, Lee JE, Liu J, John CS, Przepiorka D, et al. FDA approval summary: mylotarg for treatment of patients with relapsed or refractory CD33-positive acute myeloid leukemia. Oncologist. (2018) 23:1103–8. 10.1634/theoncologist.2017-0604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Reinhardt D, Diekamp S, Fleischhack G, Corbacioglu C, Jurgens H, Dworzak M, et al. Gemtuzumab ozogamicin (Mylotarg) in children with refractory or relapsed acute myeloid leukemia. Onkologie. (2004) 27:269–72. 10.1159/000075606 [DOI] [PubMed] [Google Scholar]
- 88.Hasle H, Abrahamsson J, Forestier E, Ha SY, Heldrup J, Jahnukainen K, et al. Gemtuzumab ozogamicin as postconsolidation therapy does not prevent relapse in children with AML: results from NOPHO-AML 2004. Blood. (2012) 120:978–84. 10.1182/blood-2012-03-416701 [DOI] [PubMed] [Google Scholar]
- 89.O'Hear C, Inaba H, Pounds S, Shi L, Dahl G, Bowman WP, et al. Gemtuzumab ozogamicin can reduce minimal residual disease in patients with childhood acute myeloid leukemia. Cancer. (2013) 119:4036–43. 10.1002/cncr.28334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Satwani P, Bhatia M, Garvin JH, Jr, George D, Dela Cruz F, Le Gall J, et al. A phase I study of gemtuzumab ozogamicin (GO) in combination with busulfan and cyclophosphamide (Bu/Cy) and allogeneic stem cell transplantation in children with poor-risk CD33+ AML: a new targeted immunochemotherapy myeloablative conditioning (MAC) regimen. Biol Blood Marrow Transplant. (2012) 18:324–9. 10.1016/j.bbmt.2011.11.007 [DOI] [PubMed] [Google Scholar]
- 91.Zahler S, Bhatia M, Ricci A, Roy S, Morris E, Harrison L, et al. A phase I study of reduced-intensity conditioning and allogeneic stem cell transplantation followed by dose escalation of targeted consolidation immunotherapy with gemtuzumab ozogamicin in children and adolescents with CD33+ acute myeloid leukemia. Biol Blood Marrow Transplant. (2016) 22:698–704. 10.1016/j.bbmt.2016.01.019 [DOI] [PubMed] [Google Scholar]
- 92.Bonifant CL, Velasquez MP, Gottschalk S. Advances in immunotherapy for pediatric acute myeloid leukemia. Expert Opin Biol Ther. (2018) 18:51–63. 10.1080/14712598.2018.1384463 [DOI] [PubMed] [Google Scholar]