Abstract
Gene therapy for X-linked severe combined immunodeficiency (SCID-X1) has proven highly effective for long-term restoration of immunity in human subjects. However, the development of lymphoproliferative complications due to dysregulated proto-oncogene expression has underlined the necessity for developing safer vector systems. To reduce the potential for insertional mutagenesis, we have evaluated the efficacy of self-inactivating (SIN) gammaretroviral vectors in cellular and in vivo models of SCID-X1. Vectors incorporating an internal human elongation factor-1α regulatory element were capable of fully restoring the lymphoid differentiation potential of γc-deficient lineage negative cells. Multilineage lymphoid reconstitution of a murine model was achieved at a similar level to that achieved by a conventional long-terminal repeat (LTR)-regulated vector used in previous clinical trials. Functional proliferative responses to mitogenic stimuli were also restored, and serum immunoglobulin levels were normalized. The reduced mutagenic potential conferred by SIN vector configurations and alternative non-LTR-based regulatory elements, together with proven efficacy in correction of cellular defects provides an important platform for development of the next phase of clinical trials for SCID-X1.
Introduction
X-linked severe combined immunodeficiency (SCID-X1) is caused by mutations in the gene encoding the common cytokine receptor gamma chain (γc) which forms an essential component of the interleukin (IL) receptors for IL-2, 4, 7, 9, 15, and 21.1,2 The absence of signaling through these cytokine receptors results in severe defects in both cellular and humoral immunity, characterized typically by an absence of T and natural killer cells and intrinsic defects of B-cell function.3 Allogeneic bone marrow transplantation is highly successful for this disorder; however, in cases where a genotypically matched donor is unavailable, the survival rates for human leukocyte antigen–mismatched transplantations are lower and associated with significant morbidity and mortality.4–6
The strong growth and survival advantage conferred upon gene-corrected hematopoietic progenitors in the SCID-X1 setting means that this disorder is a good candidate for gene therapy and substantial immunological reconstitution has been observed in 19 infants in two similar clinical trials using a long-terminal repeat (LTR)-regulated gammaretroviral vector.7–9 However, consistent changes in the profile of integration sites in vivo after infusion of transduced progenitor cells, and the development of unexpected clonal proliferation in some patients, has highlighted the potential for side effects of gene therapy using integrating vectors in several disease settings.10–15
To reduce the potential for insertional mutagenesis, we have developed a series of self-inactivating (SIN) gammaretroviral vectors for the treatment of SCID-X1. These vectors incorporate a deletion within the U3 region of the 3′ viral LTR resulting in inactivation of the viral promoter/enhancer elements, including the CAAT and TATA boxes, following reverse transcription.16,17 Expression of the γc transgene is therefore achieved via a single internal regulatory element. In this study, transgene expression was directed by either the spleen focus forming virus (SFFV) U3 LTR, or a short intron-less form of the human elongation factor 1-α (EFS) promoter, which were selected for evaluation based on their activity in hematopoietic cells.18–21 Here we have demonstrated the capacity of both these constructs to effectively regulate human γc expression both in vitro and in vivo. The SIN design together with a less potent, endogenous promoter will potentially decrease the likelihood of insertional mutagenesis following transplantation of gene-corrected cells in vivo whilst maintaining the therapeutic efficacy thus far seen in clinical trials.
Results
Gammaretroviral SIN vectors for gene therapy of SCID-X1
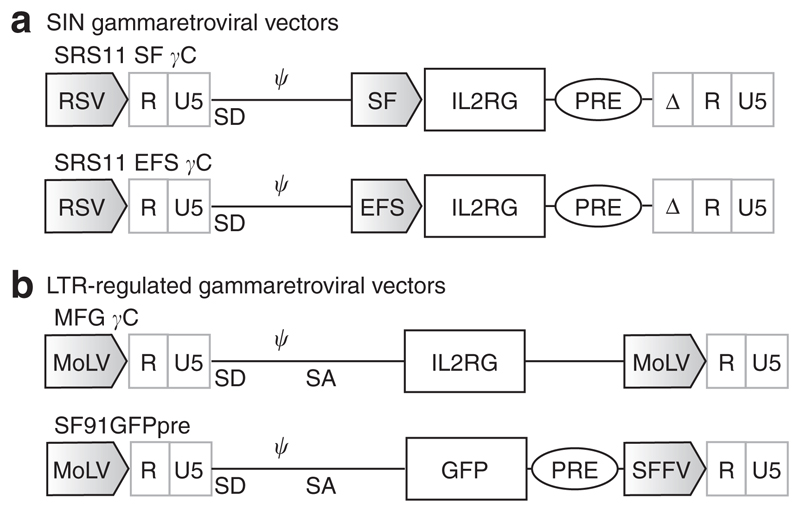
SIN gammaretroviral vectors incorporating the human IL2RG complementary DNA (cDNA) transgene were constructed using the previously described SRS11 vector backbone (Figure 1a).17 These vectors contain a Rous sarcoma virus promoter within the 5′ LTR enabling the production of high-titre gammaretro-viral supernatants. After an initial evaluation of several regulatory elements including the endogenous Wiskott–Aldrich syndrome and human phosphoglycerate kinase gene promoters (data not shown), two were chosen for further study. These were the U3 region of the SFFV LTR (SFFV promoter) and the short form (intron-deleted) of the human elongation factor 1-α promoter (EFS), both of which have been demonstrated to regulate high levels of transgene expression in hematopoietic lineages, including human CD34+ cells.18,19,22 In addition, a safety-modified post-transcriptional regulatory element (devoid of the X-protein open-reading frame) was introduced to enhance titres and gene expression levels. The IL2RG cDNA (equipped with a Kozak consensus sequence) and post-transcriptional regulatory element were verified by sequencing.
Figure 1. Self-inactivating (SIN) gammaretroviral vectors for X-linked severe combined immunodeficiency (SCID-X1).
(a) Schematic of the SRS11 series of SIN gammaretroviral vectors for SCID-X1 incorporating internal spleen focus forming virus (SFFV) and EFS promoters. (b)Long-terminal repeat (LTR)-regulated vectors used in this study for comparison. Ψ, packaging signal; MoLV, Moloney murine leukemia virus; MPSV, Myeloproliferative sarcoma virus U3; RSV, Rous sarcoma virus U3; WPRE, mutated (devoid of X-protein ORF) Woodchuck hepatitis virus post-transcriptional regulatory element.
Vector characterization in cell lines and wild-type lineage negative cells
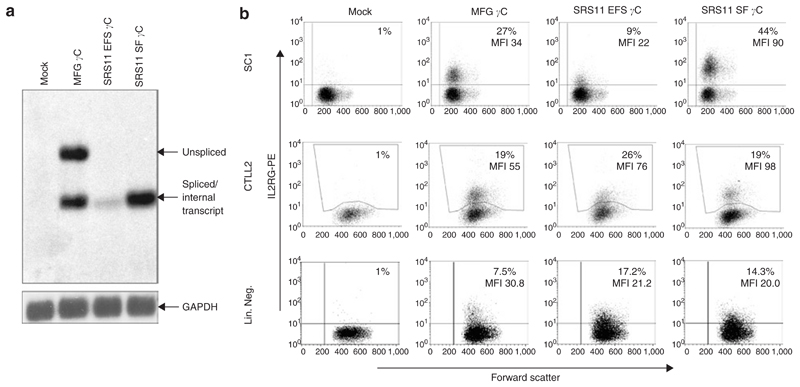
First, the performance of the new SIN vectors was compared to MFG γc, the LTR-regulated gammaretroviral vector used in the SCID-X1 clinical trials. Figure 2a shows a Northern blot analysis of transduced SC1 cells (murine fibroblast cell line) indicating the expected RNA processing. MFG γc, which harbors an intron within the leader region, shows spliced and unspliced transcripts while the SIN vectors (SRS11 derivatives on right) show only the transcript initiated by the internal promoter. To assess expression levels, murine fibroblasts (SC1), cytotoxic T cells (CTLL-2, constitutively expressing IL-2 receptors) and murine lin– cells (C57/Bl6 wild-type background) were transduced. To ensure that the majority of cells contained only single integration events, transduction efficiencies <35% were used (Figure 2b). Whilst the SRS11 SF γc vector clearly mediated the highest expression in SC1 cells, differences in expression strength were less prominent between vectors in transduced CTLL-2 cells. Following transduction of murine lin– cells, the level of γc expression in MFG γc-transduced cells was slightly superior to the levels observed following transduction with either of the SIN vectors. Taken together, expression differences between vectors were minor and dependent on cell type.
Figure 2. Comparison of vector performance in cell lines and wild-type (wt) primary cells.
(a) Northern blot analysis of total RNA preparations from transduced SC1 cells. The IL2RG transcript was detected by a specific probe. The expected RNA bands (unspliced versus spliced/internal transcript) are indicated by arrows on the right. Re-hybridization with probes to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was performed as an internal control. (b) Flow cytometry of SC1, CTLL-2 and wt lineage negative cells transduced with the indicated MFG γc and self-inactivating (SIN) vectors (SRS11 EFS γc and SRS11 SF γc). Cells were analyzed 5 days post-transduction using phycoerythrin-conjugated anti-human CD132 antibody (y-axis). Representative examples are shown. The percentage of positive cells and corresponding mean fluorescent intensity (MFI) are indicated.
In vitro differentiation of T and B lymphocytes from transduced murine Il2rg−/− lin– cells
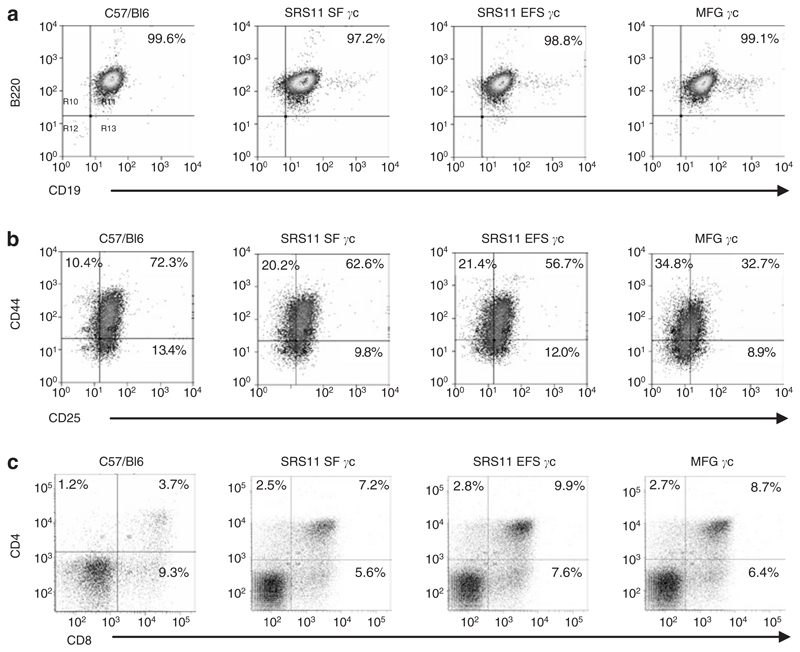
To assess the ability of the SIN gammaretroviral vectors to restore the lymphoid differentiation potential of γc-deficient hematopoietic progenitors, lin– cells isolated from the bone marrow of Il2rg−/− mice were transduced with the SIN vector series, MFG γc or a control vector encoding enhanced green fluorescent protein (eGFP) (SF91GFPpre) and seeded onto OP9-eGFP or OP9-DL1 stromal layers, which provide a convenient system for the in vitro development of B or T lymphocytes, respectively.23,24 Untransduced C57/Bl6 wild-type lin– cells were also seeded as controls. The γc-deficient lin- cells transduced with either of the SIN gammaretroviral vectors or MFG γc were found to have restored lymphoid differentiation potential and to have undergone in vitro B- or T-cell development following 13 days of co-culture on OP9 stromal layers at a similar rate as observed for C57/Bl6 wild-type cells (Figure 3a and b). Furthermore, the transduced γc-deficient lin– cells were able to develop into mature CD4+ and CD8+ T lymphocytes following 31 days of co-culture on OP9-DL1 stromal layers (Figure 3c). γc-Deficient lin– cells transduced with the control SF91GFPpre vector however, failed to undergo lymphoid differentiation under identical culture conditions and were no longer viable at the time of analysis. These results demonstrate that both the SFFV and EFS promoters in the context of gammaretroviral SIN vectors are able to regulate expression of the human γc transgene in murine γc-deficient hematopoietic progenitors to sufficient levels that restore lymphoid development in vitro.
Figure 3. Transduced murine Il2rg−/− lin– cells have restored T and B lymphoid differentiation potential in vitro.
(a) Murine γc–deficient lin– cells transduced with SRS11 SF γc or SRS11 EFS γc had restored B-cell differentiation potential following co-culture on OP9-eGFP stromal layers. (b) The Il2rg−/− progenitors transduced with the self-inactivating vectors also exhibited T-cell development in vitro at comparable levels to MFG γc-transduced cells or wild-type C57/Bl6 progenitors. (c) Development of CD4 and CD8 single positive cells 31 days post-transduction (control C57/Bl6 cells were analyzed 15 days post-seeding on OP9 stromal layers). In addition to CD4+/CD8+ double positive cells (upper right quadrant), mature CD4+ and CD8+ lymphocytes are indicated in upper left and lower right quadrants, respectively. eGFP, enhanced green fluorescent protein.
Reconstitution of T, B and natural killer cells following ex vivo gene therapy of the SCID-X1 murine model
The SIN gammaretroviral vectors were subsequently tested in vivo using a murine model of SCID-X1. In contrast to SCID-X1 patients, Il2rg−/− mice develop a proportion of T lymphocytes and are susceptible to inflammatory bowel disease. A second strain, Il2rg−/−Rag2−/−c5−/− mice, were therefore used as transplant recipients as these animals have a stable, alymphoid phenotype and are a convenient model for reconstitution studies.25 γc-Deficient lin– cells transduced twice with ecotropic retroviral particles at a defined multiplicity of infection (MOI) were engrafted into sub-lethally irradiated, Il2rg−/−Rag2−/−c5−/− recipient mice. To assess lin– cell transduction efficiency, a proportion of transduced progenitors were seeded in semi-solid medium supplemented with murine cytokines and cultured for ~2 weeks, after which colonies were picked and assessed for the presence of integrated provirus by polymerase chain reaction (PCR) using primers specific to the IL2RG transgene. High transduction levels were achieved for both the SIN gammaretroviral vectors and the LTR-regulated vector, with between 79 and 91% of colonies found to harbor integrated provirus (Figure 4a).
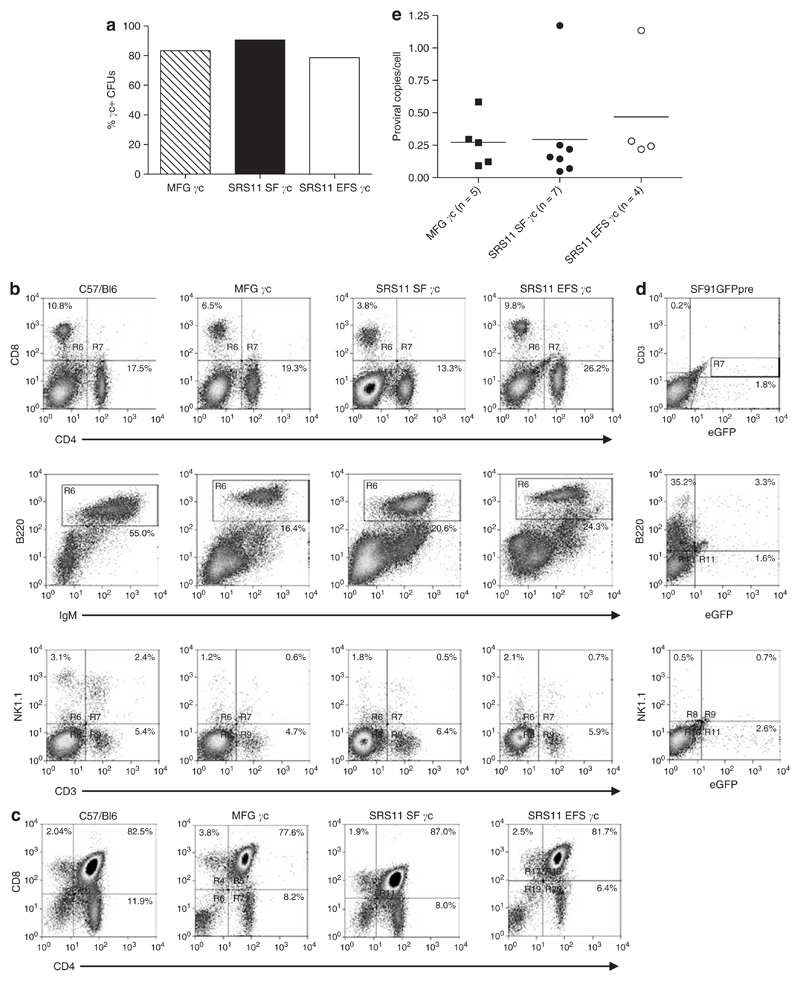
Figure 4. Efficient immunological reconstitution of a X-linked severe combined immunodeficiency (SCID-X1) murine model following ex vivo gene therapy using self-inactivating (SIN) gammaretroviral vectors.
(a) Equivalent high levels of transgene-positive Il2rg−/− lin– cells are observed for the SIN gammaretroviral vectors and MFG γc following two rounds of ex vivo transduction. (b) Il2rg−/−Rag2−/−c5−/− mice transplanted with SRS11 SF γc–, SRS11 EFS γc– or MFG γc–transduced γc-deficient progenitors were assessed for lymphoid reconstitution ~3 months post-transplant (see Materials and Methods). Comparable levels of CD4+ and CD8+ T cells and mature B220+IgM+ B lymphocytes in the spleens and NK1.1+ NK cells in the bone marrow were detected for the SIN vectors and long terminal repeat (LTR)-regulated vector. (c) Thymopoiesis is successfully restored in transplant recipients, with equivalent proportions of single-positive and double-positive thymocytes detected for transplant recipients and wild-type C57/Bl6 mice. (d) Il2rg−/−Rag2−/−c5−/− mice transplanted with SFFV-eGFP-transduced Il2rg−/− progenitors failed to develop T or mature B lymphocytes or NK cells in the spleen and bone marrow. (e) Proviral copy number in splenocytes from reconstituted Il2rg−/−Rag2−/−c5−/− mice was determined by quantitative polymerase chain reaction (see Materials and Methods). Equivalent low copy number was observed for SRS11 SF γc, SRS11 EFS γc and MFG γc transplant recipients indicating that immunological reconstitution was achieved with only a single proviral integration for the majority of animals. Bars represent the mean values for each group. CFU, colony forming units; eGFP, enhanced green fluorescent protein; Ig, immunoglobulin; NK cells, natural killer cells; SFFV, spleen focus forming virus.
Lymphoid reconstitution of transplanted Il2rg−/−Rag2−/−c5−/− mice was confirmed by the detection of lymphocytes in tail-bleed samples 15–19 weeks post-engraftment, with similar levels of T and B lymphocytes detected for animals transplanted with SIN gammaretrovirally transduced (n = 14) or MFG γc–transduced (n = 6) γc-deficient lin– cells (data not shown). Recipient mice that had been injected with Il2rg−/− lin– cells transduced with the control SF91GFPpre vector had no detectable circulating B or T lymphocytes (n = 4); eGFP+ cells were however detected in the periphery of two out of four animals (data not shown).
Engrafted mice were killed 23 weeks post-transplantation and the spleens, bone marrow, and thymi assessed for lymphoid reconstitution. Analysis by flow cytometry revealed the presence of T, B, and natural killer cell populations in transplanted animals, with comparable levels of lymphoid reconstitution observed for the SIN vectors (SRS11 SF γc and SRS11 EFS γc) and the LTR-regulated vector (MFG γc) (Figure 4b and c and Table 1). By contrast, Il2rg−/−Rag2−/−c5−/− mice transplanted with SF91GFPpre-transduced Il2rg−/− progenitors failed to reconstitute (Figure 4d). The proviral copy number in the spleens of transplant recipients was determined by quantitative PCR. A comparable mean vector copy number was observed for mice transplanted with SRS11 SF γc–transduced (0.29 per cell), SRS11 EFS γc–transduced (0.47 per cell) or MFG γc–transduced (0.27 per cell) γc-deficient progenitors, indicating that lymphoid reconstitution following ex vivo gene therapy with either of the SIN gammaretroviral vectors is achievable at relatively low vector copy number (Figure 4e).
Table 1. Lymphoid reconstitution in the spleens of transplanted mice.
| CD4% | CD8% | B220 IgM% | NK1.1% | |
|---|---|---|---|---|
| C57/Bl6 | 15.96 ± 0.70 | 11.46 ± 1.31 | 58.52 ± 1.25 | 2.61 ± 0.61 |
| MFG (n = 6) | 18.55 ± 2.69 | 5.47 ± 0.58 | 17.28 ± 3.58 | 0.36 ± 0.09 |
| SRS11 SF γc (n = 7)a | 26.9 ± 5.92 | 9.66 ± 3.27 | 18.99 ± 3.90 | 0.65 ± 0.17 |
| SRS11 EFS γc (n = 7)b | 16.83 ± 1.87 | 6.92 ± 1.19 | 20.30 ± 3.21 | 1.36 ± 1.12 |
Abbreviations: IgM, immunoglobulin M; NK cells, natural killer cells.
Results are presented as the mean % ± SEM.
n = 6 for NK1.1
n = 6 for B220 IgM.
Restoration of lymphocyte function following ex vivo gene therapy of the SCID-X1 murine model using SIN gammaretroviral vectors
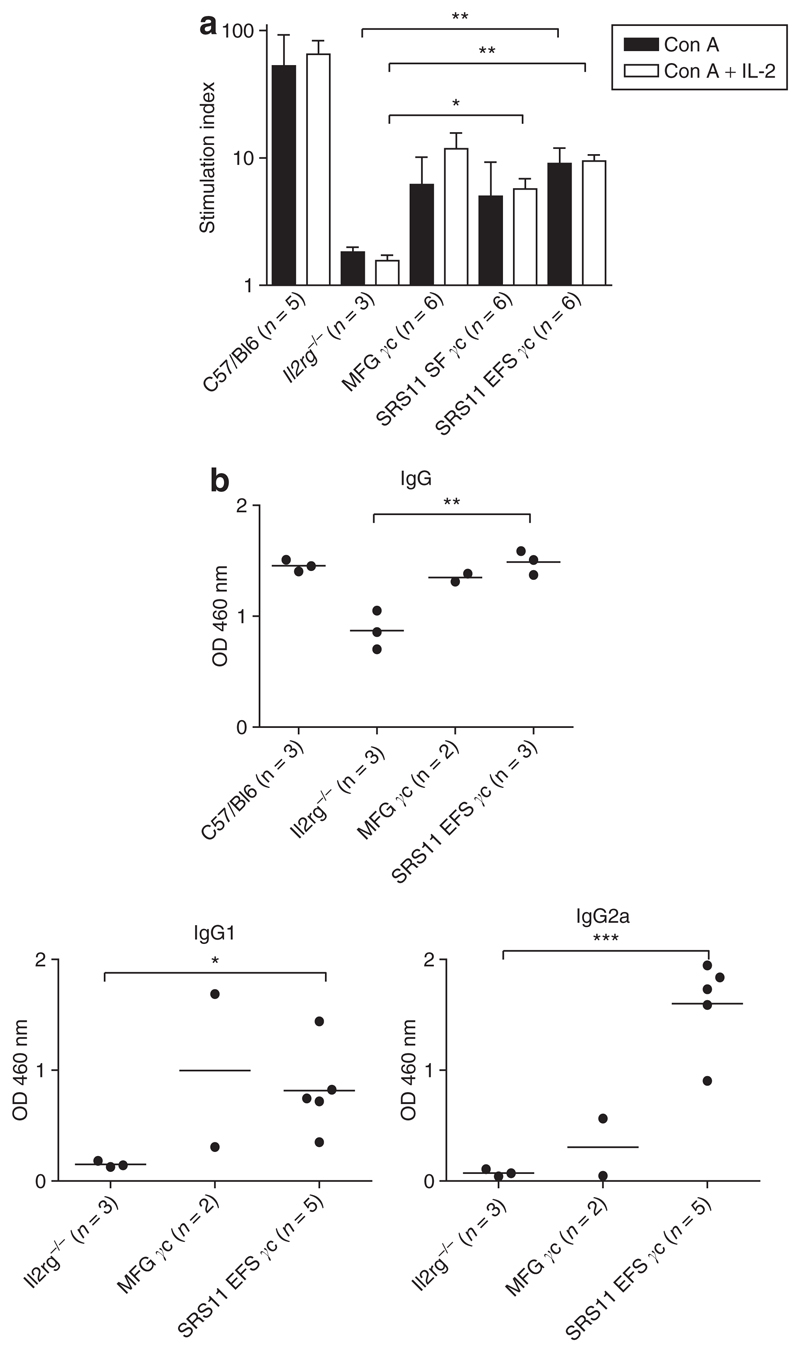
To assess functionality of the restored T lymphocytes in the SIN vector transplanted mice, splenocytes were stimulated in vitro with the mitogen concanavalin A alone or in the presence of the γc-dependent cytokine, IL-2. In reconstituted Il2rg−/−Rag2−/−c5−/− mice, splenocytes were capable of proliferation in response to concanavalin A at levels higher than those observed for splenocytes from Il2rg−/− donor mice, with significantly higher levels of proliferation observed for the SRS11 EFS γc group (Figure 5a). Furthermore, in all but two of the transplanted mice tested, IL-2 further stimulated the concanavalin A mitogenic response, with significantly increased levels of proliferation observed for animals reconstituted with lin– cells transduced with either SIN vector. It is worth noting, however, that the basal proliferation levels for Il2rg−/− mice and transplant recipients were significantly higher than for wild-type C57/Bl6 mice; consequently, even though the absolute increase in proliferation was equivalent to that seen in C57/Bl6 animals for several experimental mice, the relative increase as expressed here is lower.26 These data demonstrate that SIN gammaretroviral human γc gene transfer in this model is able to restore expression of functional IL-2 receptor complexes on murine T cells.
Figure 5. Lymphocyte function is restored following ex vivo gene therapy using self-inactivating (SIN) gammaretroviral vectors.
(a) Splenocytes from transplanted Il2rg−/−Rag2−/−c5−/− or control mice were stimulated with concanavalin A (Con A) alone or in the presence of interleukin-2 (IL-2). Proliferating cells were assessed by incorporation of 3H-thymidine and expressed as a proliferation index (the ratio of the stimulated cells to unstimulated cells). Splenocytes from mice transplanted with SRS11 SF γc–, SRS11 EFS γc– or MFG γc–transduced Il2rg−/− lin– cells exhibited increased levels of proliferation as compared to untreated γc-deficient animals. The mean + SD stimulation indices for each experimental group are shown. (*P < 0.05; **P < 0.01). (b) Serum immunoglobulin (Ig) levels in SRS11 EFS γc– and MFG γc–transplanted mice and control animals were determined by enzyme-linked immunosorbent assay. Levels of IgG detected in the sera of transplanted mice were equivalent to those detected in three wild-type C57/Bl6 controls, with levels of IgG1 and IgG2a for SRS11 EFS γc mice significantly increased as compared to untreated Il2rg−/− animals. Bars represent the mean values for each group. (*P < 0.05; **P < 0.01; ***P < 0.001).
As a measure of restoration of humoral immunity in reconstituted MFG γc and SRS11 EFS γc mice, circulating plasma immunoglobulin (Ig) levels were analyzed by enzyme-linked immunosorbent assay (ELISA). γc-Deficient mice exhibit abnormal serum Ig levels reflecting the defective B-cell development in these animals, with levels of IgG1 and IgG2a in particular diminished in this model.27 Following ex vivo gene therapy however, levels of IgG and IgG1 in the plasma of mice reconstituted with MFG γc or SRS11 EFS γc retrovirally transduced cells had increased as compared to levels in Il2rg−/− donor mice, with reconstitution of serum IgG2a levels appearing more efficient for the SRS11 EFS γc mice (Figure 5b). IgG1 class-switching is dependent on IL-4 secreted by TH2 CD4+ T-helper cells, hence these data in particular indicate restoration of both T-lymphocyte function and γc-containing cytokine receptors on B lymphocytes in the reconstituted mice.28
Restoration of γc-mediated signaling in a SCID-X1 T-cell line following transduction with GALV-pseudotyped SRS11 EFS γc
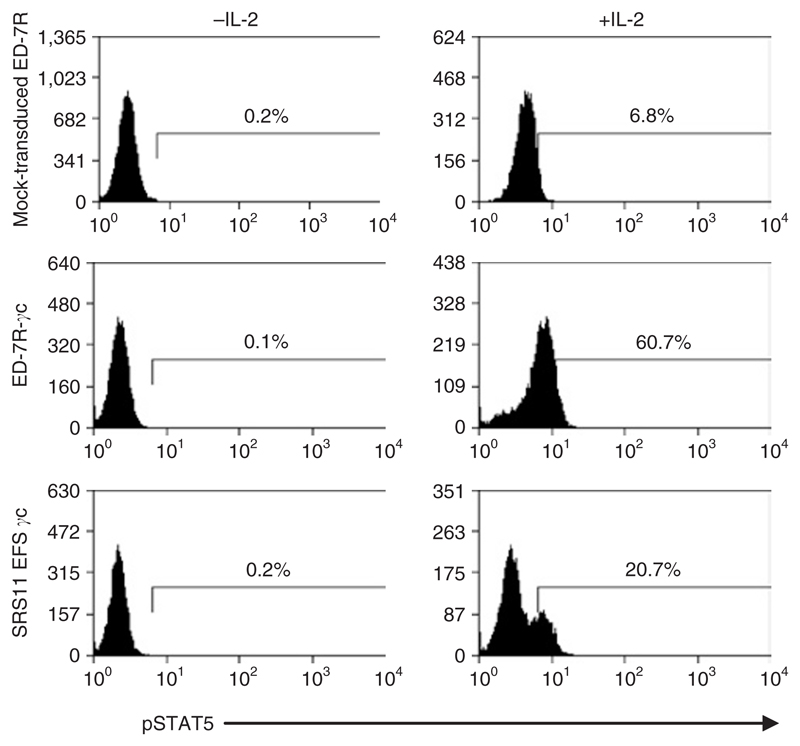
The common cytokine receptor γc is shared by the receptors for IL-2, 4, 7, 9, 15, and 21, which signal through association with the JAK family of tyrosine kinases. In particular γc associates with JAK3 following ligand binding, which causes the subsequent phosphorylation of the transcription factor, STAT5. To assess whether the SIN vector containing the internal EFS promoter (SRS11 EFS γc) can express the γc transgene to therapeutic levels in human cells, the γc-deficient T-cell line, ED-7R, was transduced with GALV-pseudotyped gammaretrovirus. The transduced cells were analyzed for the presence of restored functional IL-2 receptor complexes by stimulation with IL-2 and subsequent analysis of downstream signaling by staining for phosphorylated STAT5. Following two rounds of transduction with SRS11 EFS γc at low MOI, ~20% of cells had restored IL-2-mediated signaling as assessed by the presence of phosphorylated STAT5, demonstrating that the SRS11 EFS γc vector is capable of directing γc expression in human cells to levels that enable the rescue of γc-dependent cytokine signaling (Figure 6).
Figure 6. Interleukin-2 (IL-2)-mediated signaling is restored in SRS11 EFS γc–transduced ED-7R cells.
The γc-deficient T-cell line, ED-7R, was transduced with SRS11 EFS γc using two rounds of transduction at a multiplicity of infection of 3, yielding 90% of γc+ cells as assessed by flow cytometry (data not shown). The transduced ED-7R and control cells (mock-transduced ED-7R and IL2RG-expressing ED-7R-γc cells) were stimulated with IL-2. γc-mediated signaling in response to IL-2 was partially restored in transduced cells as assessed by the presence of phosphorylated STAT5 (pSTAT5) by flow cytometry.
Discussion
Gene therapy for SCID-X1 has proved to be an effective treatment in two clinical trials, with the restoration of cellular and humoral immunity in the majority of patients enabling them to return to normal social environments and cope with environmental pathogens.9,29,30 Given the morbidity and mortality associated with haploidentical stem cell transplantation and the frequent failure to develop functional humoral immunity following these transplants, gene therapy for this condition offers an effective alternative treatment.4–6 The recent development of lymphoproliferative diseases in four patients, however, has underlined the potential risks of such a treatment and reinforced the necessity for continued research into vector design and safety (Dr. M Cavazzana-Calvo, Hopital Necker-Enfants Malades, Paris, personal communication, 28 October 2007).14,15 Furthermore recent studies of integration sites in T lymphocytes from patients receiving gene therapy for SCID-X1 has revealed an unexpected skewing suggesting that there are also more widespread subtle vector-mediated influences on gene expression that alter survival and growth of cells in vivo.10,11
To begin to address this issue we have developed a series of SIN gammaretroviral vectors for the treatment of SCID-X1 incorporating SFFV and EFS internal promoters, selected due to their high activity levels in hematopoietic cells, including human CD34+ progenitors (Figure 2a).18–22,31 The complete elimination of the potent dual LTR enhancer–promoter sequences in these vectors should reduce the risk of insertional activation of endogenous genes and this is supported by observations that SIN gammaretroviral vectors encoding reporter genes regulated by an internal SFFV promoter demonstrate a significantly reduced capacity to immortalize murine hematopoietic cells in vitro as compared to their LTR-regulated counterparts.32 In this study, we have assessed the SIN gammaretroviral vectors both in vitro and in vivo and compared their performance to MFG γc, the LTR-regulated vector used in the SCID-X1 clinical trials. In vitro, γc-deficient hematopoietic progenitors transduced with the SIN vectors had restored lymphoid differentiation potential and were able to undergo B- and T-cell development at similar levels to wild-type cells (Figure 3). Furthermore, in vivo, the transduced Il2rg−/− progenitors reconstituted functional humoral and cellular immunity in the SCID-X1 murine model to comparable, if not superior, levels observed for MFG γc at relatively low vector copy number (Figures 4 and 5). Limited evaluation in secondary transplant recipients has also demonstrated the long-term expression of human γc in hematopoietic progenitors from both promoters in the context of SIN gammaretroviral vectors (data not shown).
Whilst the SIN design confers a degree of safety over classical LTR-regulated vectors, the choice of internal promoter remains important with regard to the potential for inducing insertional mutagenesis. As discussed above, SIN gammaretroviral vectors incorporating internal SFFV promoters were shown to be significantly less likely than their LTR-regulated counter-parts to immortalize murine hematopoietic progenitors in vitro, although they retained a residual propensity to induce immortalization under the experimental conditions that were used. More recently, a SIN gammaretroviral vector incorporating the EFS promoter was found to be incapable of inducing immortilization in this in vitro assay and to be significantly less mutagenic than the corresponding SIN vector incorporating the internal SFFV promoter. This provides good evidence for the decreased trans-activation potential of gammaretroviral vectors incorporating the SIN design together with an EFS promoter and to further address this issue we have initiated long-term in vivo studies (data not shown).
The CpG-rich upstream region of the human house-keeping gene, elongation factor 1α, together with the first intron have been used to regulate the expression of various transgenes and at high levels in hematopoietic cells.21,31 The 242-base pair EFS promoter incorporated into the SIN gammaretroviral vector used in this study is the intron-deleted (short) form, and our data indicate that regulation of human γc by this promoter in a SIN gammaretroviral vector is sufficient to correct the SCID-X1 phenotype both in vitro and in vivo to comparable levels as observed for MFG γc and SRS11 SF γc vectors containing potent viral promoter elements (Figures 3 and 4 and Table 1). Furthermore, reconstitution of serum Ig levels and isotype-switching appear more efficient for SRS11 EFS γc as compared to the LTR-regulated vector (Figure 5). Finally, SRS11 EFS γc pseudotyped with the GALV envelope was demonstrated to be capable of restoring transgene expression to functional levels in a human γc-deficient T-cell line that enabled the rescue of IL-2-mediated signaling (Figure 6).
These findings provide an encouraging platform on which to base new clinical trials for SCID-X1. The SIN design of this vector, together with the endogenous EFS promoter, will likely decrease the potential for dangerous insertional mutagenesis following transplantation of gene-corrected cells in vivo whilst maintaining the therapeutic efficacy thus far seen in clinical trials.32
Materials and Methods
Vector construction
The coding sequence for the human common γc (IL2RG) was amplified via PCR using primers 5′ IL2RG age (5′-CTGAACCGGTC GCCACCATGTTGAAGCCATCATTACCAT-3′; including a Kozak consensus sequence, restriction sites underlined) and 3′ IL2RG γ sal bam (5′-GCTAGGATCCGTCGACTCAGT TATCTAGGTTTCAGG CTTTAGGGTGT-3′), and verified by sequencing. To construct the gammaretroviral vector SRS11 SF γc, the γc cDNA was cloned into pSRS11 SF GFPpre,17 a SIN vector optimized for high-titre virus production and harboring a safety-modified post-transcriptional regulatory element (devoid of the X-protein) of the woodchuck hepatitis virus.33 To design SRS11 EFS γc, the EF1-α short (EFS) promoter (242 base pairs)22 was cloned into SRS11 SF γc. As control vectors, we used pSF91GFPpre,34,35 a retroviral vector harboring the eGFP transgene under control of the SFFV U3 promoter, and MFG γc, the conventionally used Moloney murine leukemia virus LTR-driven retroviral vector used in the clinical trial in Paris and London (kindly provided by Dr. M Cavazzana-Calvo, Hopital Necker-Enfants Malades, Paris, France).
Production of vector supernatants
Retroviral supernatant production was performed on 293T cells using Moloney murine leukemia virus gag/pol (M57-DAW) and GALV or ecotropic Moloney murine leukemia virus envelope helper plasmids and titrated on HT1080 or SC1 cells, respectively, as previously described.16,36 For titration of γc vectors, transduced cells were stained with an anti-human CD132 antibody (BD Pharmingen, Oxford, UK) prior to analysis by flow cytometry. 293T, HT1080, and SC1 (murine fibroblast) cells were maintained in Dulbecco’s modified Eagle’s medium (Biochrom, Berlin, Germany) supplemented with 10% fetal calf serum (FCS), 100 U/ml penicillin/streptomycin, and 2 mmol/l glutamine.
Transduction of cell lines and primary cells
CTLL-2 (murine lymphoblast cell line, American Type Culture Collection TIB-214) and ED-7R cells (derived from an adult T-cell leukaemia line deficient in IL2RG gene expression37) were cultured in Roswell Park Memorial Institute-1640 medium (Biochrom, Berlin, Germany), SC1 cells as described above. Both media were supplemented with 10% FCS, 100 U/ml penicillin/streptomycin, and 2 mmol/l glutamine. 1 × 105 cells were transduced in 12-well plates in the presence of protamine sulfate using centrifugation at 2,000 r.p.m. for 1 hour. Transduction of CTLL-2 and SC-1 cells aimed for <35% positive cells (MOI of 1.5 and 0.3, respectively) to exclude multiple integrants in the majority of transduced cells. ED-7R cells were transduced using two rounds of transduction at an MOI of 3 over 2 days.
Bone marrow cells were harvested from femurs and tibias of C57/Bl6 mice (Charles River Laboratories, Wilmington, MA) and further purified by magnetic sorting using the Lineage Cell depletion kit (Miltenyi, Bergisch Gladbach, Germany) to obtain lin– cells. Lin– cells were cultured in StemSpan (Cell Systems, St. Katharinen, Germany) supplemented with 2 mmol/l glutamine, 200 U/ml penicillin/streptomycin, and cytokines [50 ng/ml murine stem cell factor, 100 ng/ml hFlt3L, 100 ng/ml hIL-11, 20 ng/ml mIL-3; all from PeproTech, London, UK], and pre-stimulated for 2 days. Transduction at an MOI of 3 was performed on RetroNectin-coated (TaKaRa, Otsu, Japan) dishes by spinoculation for 30 minutes at 4 °C. Cells were analyzed by flow cytometry (FACSCalibur, Becton-Dickinson, Heidelberg, Germany) 5 days after transduction.
Northern blot
Standard procedures were performed as previously described.22 Specific probes were directed against the coding sequence of IL2RG and GAPDH.
Co-culture of transduced lin– cells on OP9 stromal layers
Early passage OP9-eGFP and OP9-DL1 stromal cells were grown to confluency in 10-cm tissue culture dishes in minimum essential media alpha (Gibco BRL, Invitrogen, Paisley, UK) supplemented with 20% FCS and 5% penicillin/streptomycin. 2 × 104 stromal cells per well were seeded in 24-well tissue culture plates and left to adhere overnight. The following day the media was removed from the plates and 6,500–10,000 lin– cells (transduced or untransduced) were seeded on top of the stromal layers in 2 ml of minimum essential media alpha supplemented with 12% FCS, 1 mmol/l HEPES, 1 mmol/l sodium pyruvate, 50 μg/ml gentamicin, 1% penicillin/streptomycin, 2 mmol/l Glutamax, 0.5 mmol/l 2-mercaptoethanol, and cytokines (0.5 ng/ml mIL-7, 5 ng/ml mFlt3L for OP9-DL1 co-cultures or 5 ng/ml mIL-7, 5 ng/ml mFlt3L for OP9-eGFP co-cultures). Every 4 days 1 ml of media was removed from the top of the plates so as not to disturb the differentiating lin– cells and 1 ml of fresh co-culture media added. On days 13 and 31 (the final analysis of control C57/Bl6 cells was performed on day 15) the cells were removed from the plates by vigorous pipetting, strained through a 70-μm nylon cell strainer to remove the OP9 stromal layers, stained with antibodies to lymphocyte cell surface markers (BD Pharmingen, Oxford, UK) and analyzed by flow cytometry (CyAn ADP, DakoCytomation, Cambridgeshire, UK) for lymphoid differentiation.
Animals
Il2rg−/− mice38 were a kind gift from Dr. J DiSanto (Hopital Necker-Enfants Malades, Paris, France). Il2rg−/−Rag2−/−c5−/− mice were generated by crossing Il2rg−/−Rag2−/−c5+/+ mice39 with A/J mice (Il2rg+/+ Rag+/+c5−/−; Harlan UK, Bicester, UK). All experimental procedures were approved by the Institutional Research Ethics Committee (Institute of Child Health, University College London, UK) and performed according to UK Home Office Animal Welfare Legislation.
Ex vivo gene therapy of the SCID-X1 murine model
Bone marrow cells were harvested from the femurs and tibias of Il2rg−/− or C57/Bl6 mice and the lin– progenitors isolated using a StemSep mouse progenitor enrichment cocktail and negative selection columns as per the manufacturer’s instructions (StemCell Technologies, London, UK). Lin– cells were pre-stimulated in StemSpan SFEM serum-free medium (StemCell Technologies) supplemented with 1% penicillin/streptomycin and cytokines (100 ng/ml murine stem cell factor, 100 ng/ml mFlt3L, 100 ng/ml hIL-11, 20 ng/ml mIL-3) for 2 days. Two rounds of transduction at an MOI of 1–2 were performed on RetroNectin-coated (TaKaRa, Otsu, Japan) dishes by spinoculation for 30 minutes at 950g at 4 °C. The transduced cells were harvested on day 5 and between 3 and 10 × 105 transduced lin– cells, resuspended in 300 μl Roswell Park Memorial Institute medium, were intravenously injected into the tail veins of sub-lethally irradiated Il2rg−/−Rag2−/−c5−/− mice (6 Gy).
Lin– cell transduction efficiency
To assess lin– cell transduction efficiency, 1 × 104 transduced lin– cells were treated with 25 U of Benzonase nuclease to remove any contaminating plasmid DNA and seeded in 3 ml of methocult (StemCell Technologies) into two 10-mm tissue culture dishes and incubated at 37 °C for 14–21 days. Approximately 28 colonies per condition were picked using a P20 Gilson pipette, lyzed immediately in 20 μl DNA lysis buffer (10 mmol/l Tris 1 mmol/l EDTA pH 8.0, 0.5% NP40, 0.5% Tween 20, 1.25 mg/ml Proteinase K) and genomic DNA isolated. To confirm the presence of DNA, PCR was performed using primers to the murine house-keeping gene, Hprt, using primers HPRT-F: 5′-TCCCCAGACTTTTGATTTGC-3′ and HPRT-R: 5′-GGAAAATACAGCCAACACTGC-3′. To assess for the presence of integrated provirus, PCR was performed using primers γc-F (exon 3): 5′-CTGGCTGTCAGTTGCAAAAA-3′ and gammac-R (exon 8): 5′-GAGATAACCACGGCTTCCAA-3′ specific for the human IL2RG cDNA.
Analysis of immune reconstitution
Analysis of immune reconstitution by flow cytometry and T-cell proliferation assay was performed 3–5 months post-transplantation as previously described.25
Ig ELISA
Serum isolated from the peripheral blood of transplanted mice was diluted appropriately and added to NUNC-Immuno ELISA plates (NUNC, Fischer Scientific UK, Loughborough, UK) pre-coated with capture antibody—IgG (AbD Serotec, Oxford, UK), IgG1 (BD Pharmingen), or IgG2a (BD Pharmingen). Plates were incubated for 1–2 hours at room temperature, washed three times with phosphate-buffered saline/0.05% Tween 20 and incubated with a horseradish peroxidase–conjugated detection antibody at room temperature for 1 hour. For the IgG ELISAs a biotinylated detection antibody was used followed by 30 minute incubation with a Strepavidin–horseradish peroxidase tertiary antibody. Plates were washed a further three times in phosphate-buffered saline/0.05% Tween 20 after which substrates A and B from an ELISA Duo-set substrate reagent pack (R&D Systems, Abingdon, UK) were mixed in equal quantities and 100 μl of the mixed solutions added to each well, plates were incubated at room temperature in the dark for ~10 minutes to allow color change, at which time the reaction was stopped by adding 50 μl stop solution/well (2 N H2SO4). The absorbance of each plate was then read at 405 nm using a FLUOstar Optima plate reader (BMG LABTECH).
Determination of gammaretroviral copy number by quantitative real-time PCR
To harvest genomic DNA, 1 × 105 splenocytes were lyzed in 20 μl DNA lysis buffer (10 mmol/l Tris, 1 mmol/l EDTA pH 8.0, 0.5% NP40, 0.5% Tween 20, 1.25 mg/ml Proteinase K) and genomic DNA isolated. Quantitative real-time PCR for copy number was performed using primers and probes to the IL2RG cDNA and murine Titin gene as previously described.25
STAT5 phosphorylation assay
This assay was performed as previously described.25 The control ED-7R-γc cells have been described previously37 and were maintained in Roswell Park Memorial Institute-1640 medium supplemented with 10% FCS, 100 U/ml penicillin/streptomycin, and 2 mmol/l glutamine.
Acknowledgments
We thank Ian Alexander, for the gift of ED-7R and ED-7R-γc cell lines and Juan Carlos Zúñiga-Pflücker for the provision of the OP9-DL1 and OP9-eGFP stromal cells. We also thank the Translational Trials and Development Support Laboratory and the Viral Vector Core at Cincinnati Children’s Hospital Medical Center for technical support. We acknowledge the support of the following funding sources; The Wellcome Trust (A.J.T.), Medical Research Council (A.J.T., S.I.T., H.B.G., C.B.), The Department of Health (M.U.), German Ministry for Research and Education (Consortium TreatID) and European Union Framework VI programme CONSERT (LSHB-CT-2004-005242). This work was carried out as part of the Transatlantic Gene Therapy Consortium.
References
- 1.Fischer A, Le Deist F, Hacein-Bey-Abina S, André-Schmutz I, Basile Gde S, de Villartay JP, et al. Severe combined immunodeficiency. A model disease for molecular immunology and therapy. Immunol Rev. 2005;203:98–109. doi: 10.1111/j.0105-2896.2005.00223.x. [DOI] [PubMed] [Google Scholar]
- 2.Buckley RH. Molecular defects in human severe combined immunodeficiency and approaches to immune reconstitution. Annu Rev Immunol. 2004;22:625–655. doi: 10.1146/annurev.immunol.22.012703.104614. [DOI] [PubMed] [Google Scholar]
- 3.White H, Thrasher A, Veys P, Kinnon C, Gaspar HB. Intrinsic defects of B cell function in X-linked severe combined immunodeficiency. Eur J Immunol. 2000;30:732–737. doi: 10.1002/1521-4141(200003)30:3<732::AID-IMMU732>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 4.Buckley RH, Schiff SE, Schiff RI, Markert L, Williams LW, Roberts JL, et al. Hematopoietic stem-cell transplantation for the treatment of severe combined immunodeficiency. N Engl J Med. 1999;340:508–516. doi: 10.1056/NEJM199902183400703. [DOI] [PubMed] [Google Scholar]
- 5.Antoine C, Muller S, Cant A, Cavazzana-Calvo M, Veys P, Vossen J, et al. Long-term survival and transplantation of haemopoietic stem cells for immunodeficiencies: report of the European experience 1968–99. Lancet. 2003;361:553–560. doi: 10.1016/s0140-6736(03)12513-5. [DOI] [PubMed] [Google Scholar]
- 6.Haddad E, Landais P, Friedrich W, Gerritsen B, Cavazzana-Calvo M, Morgan G, et al. Long-term immune reconstitution and outcome after HLA-nonidentical T-cell-depleted bone marrow transplantation for severe combined immunodeficiency: a European retrospective study of 116 patients. Blood. 1998;91:3646–3653. [PubMed] [Google Scholar]
- 7.Stephan V, Wahn V, Le Deist F, Dirksen U, Broker B, Muller-Fleckenstein I, et al. Atypical X-linked severe combined immunodeficiency due to possible spontaneous reversion of the genetic defect in T cells. N Engl J Med. 1996;335:1563–1567. doi: 10.1056/NEJM199611213352104. [DOI] [PubMed] [Google Scholar]
- 8.Cavazzana-Calvo M, Hacein-Bey S, De Saint BG, Gross F, Yvon E, Nusbaum P, et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288:669–672. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- 9.Gaspar HB, Parsley KL, Howe S, King D, Gilmour KC, Sinclair J, et al. Gene therapy of X-linked severe combined immunodeficiency by use of a pseudotyped gammaretroviral vector. Lancet. 2004;364:2181–2187. doi: 10.1016/S0140-6736(04)17590-9. [DOI] [PubMed] [Google Scholar]
- 10.Deichmann A, Hacein-Bey-Abina S, Schmidt M, Garrigue A, Brugman MH, Hu J, et al. Vector integration is nonrandom and clustered and influences the fate of lymphopoiesis in SCID-X1 gene therapy. J Clin Invest. 2007;117:2225–2232. doi: 10.1172/JCI31659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwarzwaelder K, Howe SJ, Schmidt M, Brugman MH, Deichmann A, Glimm H, et al. Gammaretrovirus-mediated correction of SCID-X1 is associated with skewed vector integration site distribution in vivo. J Clin Invest. 2007;117:2241–2249. doi: 10.1172/JCI31661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ott MG, Schmidt M, Schwarzwaelder K, Stein S, Siler U, Koehl U, et al. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat Med. 2006;12:401–409. doi: 10.1038/nm1393. [DOI] [PubMed] [Google Scholar]
- 13.Aiuti A, Slavin S, Aker M, Ficara F, Deola S, Mortellaro A, et al. Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science. 2002;296:2410–2413. doi: 10.1126/science.1070104. [DOI] [PubMed] [Google Scholar]
- 14.Hacein-Bey-Abina S, von Kalle C, Schmidt M, Le Deist F, Wulffraat N, McIntyre E, et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl J Med. 2003;348:255–256. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- 15.Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 16.Kraunus J, Schaumann DH, Meyer J, Modlich U, Fehse B, Brandenburg G, et al. Self-inactivating retroviral vectors with improved RNA processing. Gene Ther. 2004;11:1568–1578. doi: 10.1038/sj.gt.3302309. [DOI] [PubMed] [Google Scholar]
- 17.Schambach A, Mueller D, Galla M, Verstegen MM, Wagemaker G, Loew R, et al. Overcoming promoter competition in packaging cells improves production of self-inactivating retroviral vectors. Gene Ther. 2006;13:1524–1533. doi: 10.1038/sj.gt.3302807. [DOI] [PubMed] [Google Scholar]
- 18.Demaison C, Parsley K, Brouns G, Scherr M, Battmer K, Kinnon C, et al. High-level transduction and gene expression in hematopoietic repopulating cells using a human immunodeficiency [correction of imunodeficiency] virus type 1-based lentiviral vector containing an internal spleen focus forming virus promoter. Hum Gene Ther. 2002;13:803–813. doi: 10.1089/10430340252898984. [DOI] [PubMed] [Google Scholar]
- 19.Baum C, Hegewisch-Becker S, Eckert HG, Stocking C, Ostertag W. Novel retroviral vectors for efficient expression of the multidrug resistance (mdr-1) gene in early hematopoietic cells. J Virol. 1995;69:7541–7547. doi: 10.1128/jvi.69.12.7541-7547.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tumas DB, Spangrude GJ, Brooks DM, Williams CD, Chesebro B. High-frequency cell surface expression of a foreign protein in murine hematopoietic stem cells using a new retroviral vector. Blood. 1996;87:509–517. [PubMed] [Google Scholar]
- 21.Salmon P, Kindler V, Ducrey O, Chapuis B, Zubler RH, Trono D. High-level transgene expression in human hematopoietic progenitors and differentiated blood lineages after transduction with improved lentiviral vectors. Blood. 2000;96:3392–3398. [PubMed] [Google Scholar]
- 22.Schambach A, Bohne J, Chandra S, Will E, Margison GP, Williams DA, et al. Equal potency of gammaretroviral and lentiviral SIN vectors for expression of O6-methylguanine-DNA methyltransferase in hematopoietic cells. Mol Ther. 2006;13:391–400. doi: 10.1016/j.ymthe.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 23.Kodama H, Nose M, Niida S, Nishikawa S, Nishikawa S. Involvement of the c-kit receptor in the adhesion of hematopoietic stem cells to stromal cells. Exp Hematol. 1994;22:979–984. [PubMed] [Google Scholar]
- 24.Schmitt TM, Zúñiga-Pflucker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17:749–756. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- 25.Zhang F, Thornhill SI, Howe SJ, Ulaganathan M, Schambach A, Sinclair J, et al. Lentiviral vectors containing an enhancer-less ubiquitously-acting chromatin opening element (UCOE) provide highly reproducible and stable transgene expression in haematopoietic cells. Blood. 2007;110:1448–1457. doi: 10.1182/blood-2006-12-060814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lo M, Bloom ML, Imada K, Berg M, Bollenbacher JM, Bloom ET, et al. Restoration of lymphoid populations in a murine model of X-linked severe combined immunodeficiency by a gene-therapy approach. Blood. 1999;94:3027–3036. [PubMed] [Google Scholar]
- 27.Cao X, Shores EW, Hu-Li J, Anver MR, Kelsall BL, Russell SM, et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity. 1995;2:223–238. doi: 10.1016/1074-7613(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 28.Kuhn R, Rajewsky K, Muller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991;254:707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- 29.Hacein-Bey-Abina S, De Saint Basile G, Cavazzana-Calvo M. Gene therapy of X-linked severe combined immunodeficiency. Methods Mol Biol. 2003;215:247–259. doi: 10.1385/1-59259-345-3:247. [DOI] [PubMed] [Google Scholar]
- 30.Hacein-Bey-Abina S, Le Deist F, Carlier F, Bouneaud C, Hue C, de Villartay JP, et al. Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. N Engl J Med. 2002;346:1185–1193. doi: 10.1056/NEJMoa012616. [DOI] [PubMed] [Google Scholar]
- 31.Dardalhon V, Herpers B, Noraz N, Pflumio F, Guetard D, Leveau C, et al. Lentivirus-mediated gene transfer in primary T cells is enhanced by a central DNA flap. Gene Ther. 2001;8:190–198. doi: 10.1038/sj.gt.3301378. [DOI] [PubMed] [Google Scholar]
- 32.Modlich U, Bohne J, Schmidt M, von Kalle C, Knoss S, Schambach A, et al. Cell-culture assays reveal the importance of retroviral vector design for insertional genotoxicity. Blood. 2006;108:2545–2553. doi: 10.1182/blood-2005-08-024976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schambach A, Bohne J, Baum C, Hermann FG, Egerer L, von Laer D, et al. Woodchuck hepatitis virus post-transcriptional regulatory element deleted from X protein and promoter sequences enhances retroviral vector titer and expression. Gene Ther. 2006;13:641–645. doi: 10.1038/sj.gt.3302698. [DOI] [PubMed] [Google Scholar]
- 34.Hildinger M, Abel KL, Ostertag W, Baum C. Design of 5′ untranslated sequences in retroviral vectors developed for medical use. J Virol. 1999;73:4083–4089. doi: 10.1128/jvi.73.5.4083-4089.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schambach A, Wodrich H, Hildinger M, Bohne J, Kräusslich HG, Baum C. Context dependence of different modules for posttranscriptional enhancement of gene expression from retroviral vectors. Mol Ther. 2000;2:435–445. doi: 10.1006/mthe.2000.0191. [DOI] [PubMed] [Google Scholar]
- 36.Morita S, Kojima T, Kitamura T. Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther. 2000;7:1063–1066. doi: 10.1038/sj.gt.3301206. [DOI] [PubMed] [Google Scholar]
- 37.Kumaki S, Ishii N, Minegishi M, Tsuchiya S, Cosman D, Sugamura K, et al. Functional role of interleukin-4 (IL-4) and IL-7 in the development of X-linked severe combined immunodeficiency. Blood. 1999;93:607–612. [PubMed] [Google Scholar]
- 38.DiSanto JP, Müller W, Guy-Grand D, Fischer A, Rajewsky K. Lymphoid development in mice with a targeted deletion of the interleukin 2 receptor gamma chain. Proc Natl Acad Sci USA. 1995;92:377–381. doi: 10.1073/pnas.92.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldman JP, Blundell MP, Lopes L, Kinnon C, Di Santo JP, Thrasher AJ. Enhanced human cell engraftment in mice deficient in RAG2 and the common cytokine receptor gamma chain. Br J Haematol. 1998;103:335–342. doi: 10.1046/j.1365-2141.1998.00980.x. [DOI] [PubMed] [Google Scholar]