Abstract
Here we report the synthesis, materials characterization, antimicrobial capacity, and cytocompatibility of novel antibiotic-containing scaffolds. Metronidazole (MET) or Ciprofloxacin/(CIP) was mixed with a polydioxanone (PDS)polymer solution at 5 and 25 wt% and processed into fibers. PDS fibers served as a control. Scanning electron microscopy (SEM), Fourier-transform infrared spectroscopy (FTIR), tensile testing, and high-performance liquid chromatography (HPLC) were used to assess fiber morphology, chemical structure, mechanical properties, and drug release, respectively. Antimicrobial properties were evaluated against those of Porphyromonas gingivalis/Pg and Enterococcus faecalis/Ef. Cytotoxicity was assessed in human dental pulp stem cells (hDPSCs). Statistics were performed, and significance was set at the 5% level. SEM imaging revealed a submicron fiber diameter. FTIR confirmed antibiotic incorporation. The tensile values of hydrated 25 wt% CIP scaffold were significantly lower than those of all other groups. Analysis of HPLC data confirmed gradual, sustained drug release from the scaffolds over 48 hrs. CIP-containing scaffolds significantly (p < .00001) inhibited biofilm growth of both bacteria. Conversely, MET-containing scaffolds inhibited only Pg growth. Agar diffusion confirmed the antimicrobial properties against specific bacteria for the antibiotic-containing scaffolds. Only the 25 wt% CIP-containing scaffolds were cytotoxic. Collectively, this study suggests that polymer-based antibiotic-containing electrospun scaffolds could function as a biologically safe antimicrobial drug delivery system for regenerative endodontics.
Keywords: electrospinning, nanofibers, disinfection, regeneration, double antibiotic, triple antibiotic
Introduction
In recent years, regenerative endodontics has changed the therapeutics of immature permanent teeth with pulpal necrosis (Banchs and Trope, 2004; Murray et al., 2007; Petrino et al., 2010; Ruparel et al., 2012). Root canal disinfection, a crucial step of the most current and clinically acceptable regenerative/revascularization procedure (Murray et al., 2007; Petrino et al., 2010; Ruparel et al., 2012; Yassen et al., 2013), is typically accomplished with either a triple (ciprofloxacin, metronidazole, and minocycline) or a double highly concentrated (Ruparel et al., 2012) antibiotic paste (minocycline-free) for approximately 1 mo (Murray et al., 2007; Petrino et al., 2010). Subsequently, the tooth is re-entered and irrigated, and the periapical tissues are lacerated with an endodontic file to induce bleeding. With bleeding, growth factors and stem cells from the apical area populate the natural fibrin-based scaffold, inducing pulp-dentin complex regeneration (Murray et al., 2007; Petrino et al., 2010; Ruparel et al., 2012). Regrettably, even though the antimicrobial capacities of these antibiotics are well-known (T Sato et al., 1993; Hoshino et al., 1996; I Sato et al., 1996), recent findings (Ruparel et al., 2012; Chuensombat et al., 2013) have warned of the potentially toxic effects of these highly concentrated antibiotic pastes on dental pulp stem cells and dental pulp fibroblasts.
Electrospinning has been extensively used to synthesize natural (e.g., collagen) or synthetic polymer scaffolds and/or drug delivery systems (Kenawy et al., 2002, 2009; Bottino et al., 2011, 2012; Gupte and Ma, 2012). Indeed, antibiotic-containing scaffolds have been proven to control/reduce infection by the controlled release of a wide variety of antibiotics (Kim et al., 2004; Ruckh et al., 2012). Ultimately, the ability of nano/microfibrous scaffolds to deliver intracanal, uniform, and greatly controlled amounts of antibiotics may have positive treatment implications by providing a bacteria-free environment conducive to tissue regeneration, while minimizing the adverse effects of toxicity currently associated with the use of the antibiotic paste. Here, we report for the first time on the synthesis, materials characterization, antimicrobial capacity, and cytocompatibility of novel antibiotic-containing nanofibrous scaffolds for regenerative endodontics.
Materials & Methods
Synthesis and Characterization of Electrospun Drug Delivery Systems
Food and Drug Administration (FDA)-approved polydioxanone monofilament suture material (PDS II®, Ethicon, Somerville, NJ, USA) was cut into pieces and placed into glass vials containing dichloromethane (Sigma-Aldrich, St. Louis, MO, USA) to remove the violet dye (Boland et al., 2005; Bottino et al., 2013). Then, clear PDS was dissolved in 1,1,1,3,3,3-hexafluoro-2-propanol (HFP, Sigma-Aldrich) at a 10 wt% concentration with stirring (Bottino et al., 2013). Metronidazole (MET, Sigma-Aldrich) or ciprofloxacin (CIP, Sigma-Aldrich) was directly added in 2 distinct concentrations (5 and 25 wt%, respective to the total polydioxanone/PDS weight) and mixed together under vigorous stirring. A custom-made electrospinning apparatus was used to synthesize the different drug delivery systems following standard electrospinning practices (Bottino et al., 2011, 2012, 2013). Briefly, pure PDS (control) or antibiotic-containing PDS solutions were individually loaded into 5-mL plastic syringes (Becton-Dickinson, Franklin Lakes, NJ, USA) fitted with a metallic blunt-tip needle (27G) and electrospun under optimized parameters (i.e., 2 mL/hr, 18-cm distance, and 15 kV). The fibers were collected at room temperature (RT) on the aluminum-foil-covered rotating mandrel. The electrospun scaffolds (hereafter referred to as mats) were dried in a desiccator at RT for at least 48 hrs for removal of any remaining solvent (Bottino et al., 2011, 2013).
Electrospun mats were characterized in terms of fiber morphology via scanning electron microscopy (SEM, JSM-5310LV, JEOL, Tokyo, Japan). The average fiber diameter and fiber distribution were determined from SEM images (Bottino et al., 2011). Fourier transform infrared spectroscopy (FT-IR, Jasco 4100, Easton, MD, USA) with attenuated total reflection (ATR) was done to characterize the chemical structure and investigate the possibility of interaction of chemical bonds between drug and polymer (Bottino et al., 2011; Sahoo et al., 2012). The mechanical properties of the electrospun mats (n = 8) were evaluated by uni-axial tensile testing (expert 5601, ADMET) under dry and hydrated conditions (24 hrs in PBS at 37oC) (Bottino et al., 2011, 2013).
Drug Release
Drug release from the antibiotic-containing mats was determined with a high-pressure liquid chromatograph (HPLC) equipped with a UV-Vis detector (Cui et al., 2006) (Appendix).
Antimicrobial Testing
As previously mentioned, the antimicrobial properties of the electrospun mats were quantitatively evaluated against P. gingivalis (Pg) and E. faecalis (Ef) in both biofilm and agar diffusion assays. Electrospun samples (15 × 15 mm2) were cut from the mats, mounted in plastic CellCrownTM (Scaffdex, Tampere, Finland), and placed in 24-well plates. Wells containing electrospun samples were disinfected by the addition of 2 mL of 70% ethanol for 30 min and rinsed twice with 2 mL of sterile 0.9% saline. Tested samples included 4 experimental groups [PDS+5 wt%MET, PDS+25 wt%MET, PDS+5 wt%CIP, and PDS+25 wt%CIP] and the control (pure PDS).
Ef (ATCC 29212) was cultured for 24 hrs aerobically in 5% CO2 at 37°C, and Pg (ATCC 33277) was cultured for 48 hrs anaerobically at 37°C in brain heart infusion broth (BHI) containing 5 g/L yeast extract (BHI-YE) (Difco Laboratories, Detroit, MI, USA) with 5% v/v Vitamin K/hemin solution (Thermo Scientific, Pittsburgh, PA, USA) in an anaerobic GasPak jar. A 50-µL quantity of the bacterial suspensions (ca. 106 bacteria) was inoculated into each well in quadruplicate with 2 mL of appropriate growth media. Following aerobic (24 hrs) for Ef and anaerobic (48 hrs) conditions for Pg, the electrospun samples were carefully removed from the wells with sterile forceps, washed gently twice with 1 mL of 0.9% saline, and suspended in 2 mL of 0.9% saline, followed by sonication for 10 sec and vortexing for 30 sec. For each sample, 2 dilutions were plated, one undiluted and one diluted (100×). The dislodged biofilm bacteria were plated onto anaerobic blood agar plates (Fisher Scientific, Pittsburgh, PA, USA) by means of a conventional spiral plater system. The Ef plates were incubated aerobically for 24 hrs, and the Pg plates were incubated in anaerobic conditions for 48 hrs. Subsequently, by means of an automated colony counter (Synbiosis Inc., Frederick, MD, USA), colony-forming units (CFU/mL) were quantified and compared with the PDS control. Two additional mats per group were included for qualitative evaluation of biofilm inhibition via SEM. Briefly, these mats were harvested, washed with PBS to remove unbound bacteria, and fixed in buffered 4% formaldehyde (Sigma). After dehydration, immersion in ascending ethanol gradients, and soaking in ethanol/hexamethyldisilazane gradients (Sigma), samples were mounted on Al stubs, sputter-coated with gold, and imaged by SEM (Bottino et al., 2013).
The agar diffusion test has been considered the standard method to test for antibiotic susceptibility (Siqueira and de Uzeda, 1997; Siqueira et al., 1998; Kim et al., 2004). Thus, to further confirm the inhibitory effects of the antibiotic-containing electrospun mats on the growth of bacterial strains, we carried out an agar diffusion assay. Four sterile 6-mm disk-shaped samples from each of the 5 experimental groups were placed on cultured blood agar plates containing bacterial lawns of Ef and Pg. After 5 days of incubation, the inhibition zones (in mm) were determined.
Cell Culture and Cytotoxicity Test
We followed the guidelines provided by the International Organization for Standardization (1993) to detect toxicity levels of the electrospun scaffolds on human dental pulp stem cells (Appendix Fig.).
Statistical Analysis
Results are shown as the mean value ± the standard deviation (SD) of the mean. One-way analyses of variance (ANOVAs) followed by Tukey’s multiple comparisons were used to compare the groups for differences in fiber diameter. Two-way ANOVAs followed by Tukey’s multiple comparisons were used to compare the groups for differences in tensile strength and modulus. All analyses were performed on the natural logarithm of the data, to satisfy the ANOVA assumptions. The comparisons for biofilm were analyzed by one-way ANOVA, on the natural-log-transformed data. The comparisons for inhibition zones were performed by Wilcoxon rank-sum tests. A 5% significance level was used for all tests.
Results
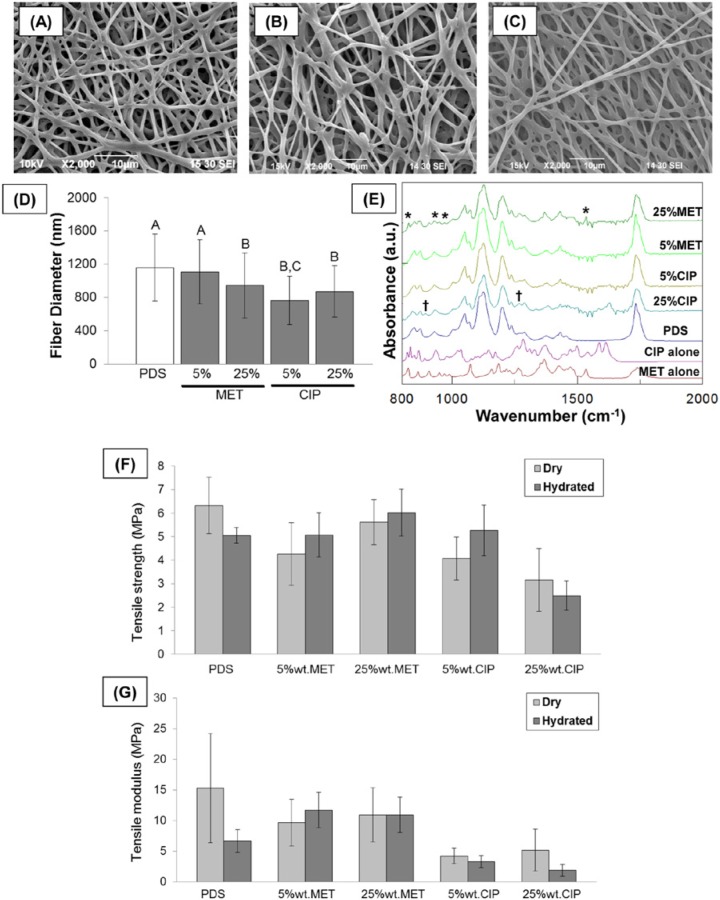
Representative SEM images of the processed PDS and antibiotic-containing mats are shown in Figs. 1A-1C. A three-dimensional micro/nanofibrous network with interconnected micron-sized pores was seen for all groups. Fig. 1D displays the mean fiber diameter for the processed mats. MET at 25 wt% and CIP (at both 5 and 25 wt%) led to significant (p < .05) fiber diameter decrease when compared with the control (PDS). The IR spectra of pure PDS and antibiotic-containing mats are given in Fig 1E. Characteristic absorption peaks for PDS—namely, an ester carbonyl group at around 1736 cm-1, C–O–C of ester at 1124 cm-1 with shoulder broadening at 1100 cm-1 due to ether group, and C–O bands of ester at 1066 cm-1 and 1055 cm1—were evident. Antibiotic-containing scaffolds displayed (Fig. 1E) peaks comparable with pure PDS in addition to MET- (e.g., 823 cm-1 1534 cm-1) and CIP- (e.g., 867 cm-1 and 1282 cm-1) related peaks.
Figure 1.
Morphological, chemical and mechanical characterizations of the electrospun scaffolds. (A-C) Representative SEM micrographs of the PDS-based electrospun mats (magnification 2000×): (A) pure PDS, (B) 25 wt% MET, and (C) 25 w.% CIP. (D) Mean fiber diameter (nm). Different letters indicate statistically significant differences (p < .05). (E) FTIR spectra of different electrospun mats. Note the presence of characteristic peaks for both MET and CIP (* and † denote metronidazole- and ciprofloxacin-related peaks, respectively). (F-G) Effect of hydration on (F) the tensile strength and (G) tensile modulus of the electrospun mats.
Figs. 1F and 1G show tensile strength and modulus data (mean ± SD) for the different groups. Analysis of the mechanical testing data demonstrated no significance in tensile strength between dry and wet samples (p = .64). In detail, PDS (control) presented significantly higher dry strength than 5 wt% CIP (p = .0167), 25 wt% CIP (p < .0001), and 5 wt% MET (p = .0281). Twenty-five wt% MET demonstrated significantly higher strength (dry) than its CIP counterpart (p < .0001). Twenty-five wt% CIP revealed significantly (p < .0001) lower strength (wet) than all other groups, but the strengths (wet) of the other groups did not differ significantly from each other (p > .05). Both 5 and 25 wt% CIP had significantly lower modulus, regardless of the test conditions, when compared with the other groups (i.e., 5 and 25 wt% MET and control).
Fig. 2A shows the in vitro cumulative release for CIP- and MET-containing electrospun mats as a function of time. Within the first 48 hrs, while CIP-containing mats released between 22.4% (5 wt% CIP) and 27.52% (25 wt% CIP) of their total drug amount theoretically present within the mats, the MET-containing counterparts released 51.4% (5 wt% MET) and 44.6% (25 wt% MET). Apart from the 25 wt% CIP-containing mats, all other electrospun mats exhibited an initial burst followed by an overall linear sustained drug release after 8 hrs up to the assessed time period (Fig. 2B). The mean drug amounts released of the 5 wt% CIP, 25 wt% CIP, 5 wt% MET, and 25 wt% MET mats after 48 hrs were 8.6 ± 0.78 µg, 57.5 ± 6.3 µg, 28.68 ± 2.3 µg, and 88.6 µg, respectively (Fig. 2B).
Figure 2.
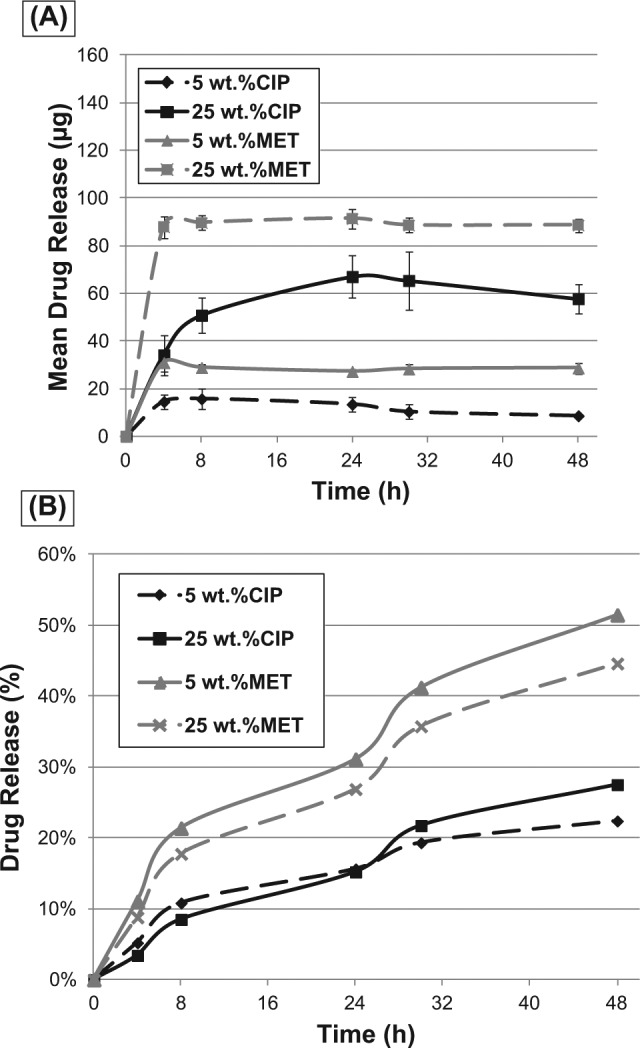
In vitro cumulative release for antibiotic-containing electrospun mats as a function of time. (A) Mean drug release (in µg). (B) Percentage of drug release from the antibiotic-containing PDS-based electrospun mats.
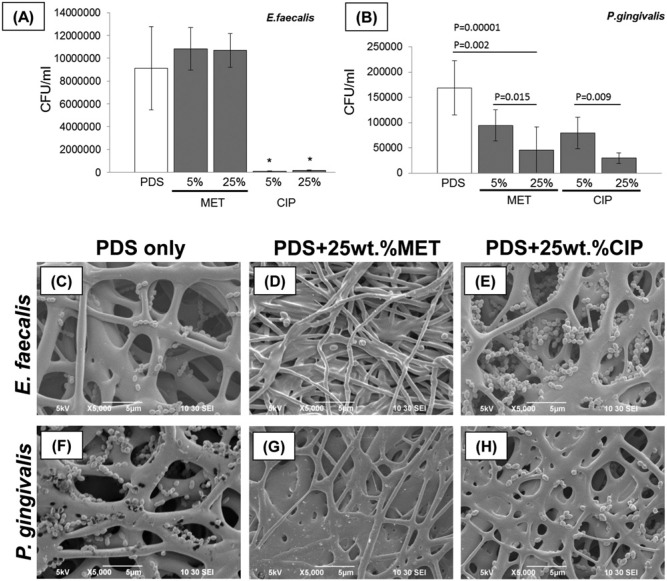
Spiral plating revealed clear trends in biofilm reduction: All CIP-containing mats significantly inhibited growth of both Ef and Pg (p < .00001) (Figs. 3A, 3B, respectively). MET-containing mats significantly (p = .002) inhibited the growth of Pg at 25 wt% MET (Fig. 3B). Moreover, compared with PDS-only mats (Figs. 3C, 3F), SEM analysis provided further qualitative evidence that antibiotic-containing PDS-based electrospun mats inhibited growth of sensitive microbials (Figs. 3D, 3E, 3G, 3H). Data from the agar diffusion tests were in agreement with the results from spiral plating (Figs. 4A, 4B). CIP-containing mats inhibited growth of both bacteria tested, and there was no difference in growth-inhibitory effect on both micro-organisms between 2 CIP concentrations (Figs. 4A, 4B, 4D, 4F). Both MET-containing mats demonstrated an inhibitory effect only on Pg, with the largest inhibition zone seen at 25 wt%MET (Figs. 4B, 4E).
Figure 3.
Effects of antibiotic-containing PDS-based electrospun mats on the growth of bacteria. (A-B) Spiral plating was used to calculate CFU/mL of samples of dislodged bacteria: (A) Ef and (B) Pg from biofilms containing 5 wt% CIP, 25 wt% CIP, 5 wt% MET, and 25 wt% MET, and PDS (control). Significant difference is denoted with an asterisk (*p < .05) when compared with the control. (C-H) Representative SEM micrographs showing growth inhibition of Ef and Pg on the antibiotic-containing electrospun mats.
Figure 4.
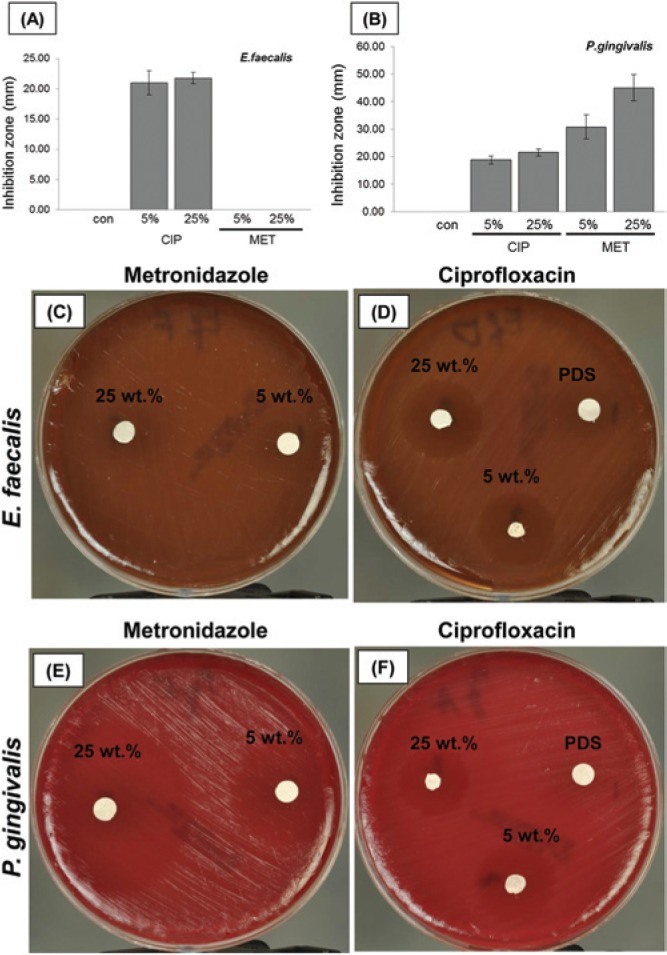
Growth inhibition of (A) Ef and (B) Pg at day 5 by antibiotic-containing electrospun mats with 5 or 25 wt% of CIP or MET. PDS used as a negative control. Significant difference is denoted with an asterisk (*p < .05) when compared with the control. (C-F) Representative macrophotographs showing growth inhibition of Ef and Pg.
The Appendix Fig. shows the cell viability data. Only the 25 wt% CIP-containing scaffold extract resulted in significant adverse effects at the cellular level [IC(50%) = 21].
Discussion
The most current regenerative endodontics strategy (Banchs and Trope, 2004; Petrino et al., 2010; Yassen et al., 2013) uses either the triple- or, as an alternative, the double-antibiotic paste, due to minocycline-associated dentin staining (Reynolds et al., 2009; Kim et al., 2010; Ruparel et al., 2012). Even though MET is known to be effective against obligate anaerobes, it has been shown that it cannot eliminate all the bacteria in a necrotic root canal (Sato et al., 1996). Nonetheless, the combination of MET/CIP, such as in the double-antibiotic paste, has been reported to sterilize a root canal (T Sato et al., 1993; Hoshino et al., 1996; I Sato et al., 1996). Thus, both MET and CIP were investigated to lay foundational knowledge that could lead to the development of a bi-mix antibiotic electrospun scaffold. To the best of our knowledge, this is the first report on the synthesis of a mechanically strong biodegradable polymer-based electrospun antibiotic-containing nano/microfibrous scaffold that could potentially be used as a drug delivery system for root canal disinfection prior to regenerative endodontics.
In the present study, the mechanical properties of the synthesized antibiotic-containing electrospun scaffolds were evaluated. Generally, the synthesized materials seemed to present adequate mechanical properties not only to sustain handling but more importantly to maintain its structure in the root canal space during regenerative endodontics procedures. Worth mentioning, 25 wt% MET demonstrated significantly higher strength than its CIP counterpart. At present, one could suggest that CIP incorporation might play a detrimental role in polymer crystallinity, which possibly contributed to lower mechanical strength.
We observed an important difference in terms of drug release. After 48 hrs, MET-containing mats released ca. 50% of the drug incorporated within the mats (Fig. 2B). One plausible reason for this observation might be related to the differences in molecular weight (Mw) of the antibiotics, since no major changes in fiber diameter (except for the 5 wt% CIP when compared with the other antibiotics-containing groups) and fiber surface morphology were seen. The Mw of CIP (331.346 g/mol) is almost 2 times higher than that of MET (171.15 g/mol). Analysis of our data corroborates results of previous studies (Taepaiboon et al., 2006; Tungprapa et al., 2007) in which the total amount of the drugs released from the mats decreased with increasing drug Mw.
Analysis of the collective microbiological data (i.e., biofilm and agar diffusion assays) revealed a significant inhibition and/or reduction of growth of sensitive bacteria. Analysis of our antibacterial data clearly showed that the electrospinning process did not affect antimicrobial properties. Similar studies on the incorporation of a wide range of drugs (e.g., sodium salicylate, diclofenac sodium, naproxen, and indomethacin) (Taepaiboon et al., 2006) and biomolecules (e.g., fibroblast growth factor) (Sahoo et al., 2010) have proven that electrospinning does not jeopardize therapeutic properties. Herein, the incorporation of CIP, a gyrase inhibitor (Strahilevitz and Hooper, 2005), into the electrospun mats led to a significant inhibition of growth of both Ef and Pg. Analysis of data from the agar diffusion test further confirmed the efficacy of CIP-containing mats against both bacteria. As expected, MET-containing mats did not display any antimicrobial effects against Ef. In contrast, MET at both concentrations tested significantly inhibited the growth of Pg, which was also confirmed by the agar diffusion assay. Analysis of our cell viability data revealed that only the 25 wt% CIP scaffolds, which released approximately 440 µg over a 48-hour period, resulted in a significant reduction in hDPSCs cell survival. When the clinical use of the obtained scaffolds is considered, it is worth mentioning that the total amounts of MET and CIP entrapped within these systems, as 15 × 15 mm2 mats which can be rolled up and inserted intracanal, are significantly lower (5 wt% MET = 386 µg, 25 wt% MET = 1.38 mg, 5 wt% CIP = 280 µg, and 25 wt% CIP = 1 mg) than that currently used clinically in the form of a paste (1 g/mL). Indeed, a recent study (Ruparel et al., 2012) demonstrated that, at clinically used concentrations, the antibiotics found in the triple- and double-antibiotic pastes have deleterious effects on the survival of stem cells from the apical papillae. More importantly, the study pointed out increased survival rates as the antibiotic concentrations decreased to 1 mg/mL or below for either of the antibiotics tested (Ruparel et al., 2012). Our antimicrobial results indicated that there was no significant difference in Ef inhibition between the 5 and 25 wt% CIP. Therefore, the lowest concentration could be clinically more beneficial not only to reduce the overuse of antibiotics but also, more importantly, to avoid deleterious effects on viable stem cells.
In sum, our antibiotic-containing scaffolds hold promise for the improvement of current regenerative strategies by providing a drug delivery system to disinfect necrotic immature permanent teeth through a controllable release of low, yet effective, antibiotic doses when compared with conventional treatments, while serving as a matrix for the growth and differentiation of stem cells from the apical papilla after bleeding is induced.
Supplementary Material
Acknowledgments
The authors are grateful to Mr. Timothy Kamp for his assistance with mechanical testing. We are also grateful to Mr. Aaron Waeiss (IUSD) and Dr. Rodolfo Pinal (Industrial Pharmacy, Purdue University, West Lafayette, IN, USA) as well as to Dr. Miriam Fussae Suzuki (Institute for Energy and Nuclear Research, IPEN, São Paulo, Brazil) for their helpful discussions on HPLC analyses and cytocompatibility experiments, respectively.
Footnotes
This work was supported by a Research Grant from the American Association of Endodontists Foundation (AAE) to M.C. Bottino.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Banchs F, Trope M. (2004). Revascularization of immature permanent teeth with apical periodontitis: New treatment protocol? J Endod 30:196-200. [DOI] [PubMed] [Google Scholar]
- Boland ED, Coleman BD, Barnes CP, Simpson DG, Wnek GE, Bowlin GL. (2005). Electrospinning polydioxanone for biomedical applications. Acta Biomater 1:115-123. [DOI] [PubMed] [Google Scholar]
- Bottino MC, Thomas V, Janowski GM. (2011). A novel spatially designed and functionally graded electrospun membrane for periodontal regeneration. Acta Biomater 7:216-224. [DOI] [PubMed] [Google Scholar]
- Bottino MC, Thomas V, Schmidt G, Vohra YK, Chu TM, Kowolik MJ, et al. (2012). Recent advances in the development of GTR/GBR membranes for periodontal regeneration—A materials perspective. Dent Mater 28:703-721. [DOI] [PubMed] [Google Scholar]
- Bottino MC, Yassen G, Platt J, Labban N, Windsor L, Spolnik K, et al. (2013). A novel three-dimensional scaffold for regenerative endodontics:materials and biological characterizations. J Tissue Eng Regen Med [Epub ahead of print 3/August/2013] (in press). [DOI] [PubMed] [Google Scholar]
- Chuensombat S, Khemaleelakul S, Chattipakorn S, Srisuwan T. (2013). Cytotoxic effects and antibacterial efficacy of a 3-antibiotic combination: an in vitro study. J Endod 39:813-819. [DOI] [PubMed] [Google Scholar]
- Cui W, Li X, Zhu X, Yu G, Zhou S, Weng J. (2006). Investigation of drug release and matrix degradation of electrospun poly(DL-lactide) fibers with paracetanol inoculation. Biomacromolecules 7:1623-1629. [DOI] [PubMed] [Google Scholar]
- Gupte MJ, Ma PX. (2012). Nanofibrous scaffolds for dental and craniofacial applications. J Dent Res 91:227-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino E, Kurihara-Ando N, Sato I, Uematsu H, Sato M, Kota K, et al. (1996) In-vitro antibacterial susceptibility of bacteria taken from infected root dentine to a mixture of ciprofloxacin, metronidazole and minocycline. Int Endod J 29:125-130. [DOI] [PubMed] [Google Scholar]
- International Organization for Standardization (1993). ISO 10993-5. Biological evaluation of medical devices. Part 5: Tests for cytotoxicity: in vitro methods. URL accessed on 8/28/2013 at: http://www.iso.org/iso/catalogue_detail.htm?csnumber=36406.
- Kenawy ER, Bowlin GL, Mansfield K, Layman J, Simpson DG, Sanders EH, et al. (2002). Release of tetracycline hydrochloride from electrospun poly(ethylene-co-vinylacetate), poly(lactic acid), and a blend. J Control Release 81:57-64. [DOI] [PubMed] [Google Scholar]
- Kenawy ER, Abdel-Hay FI, El-Newehy MH, Wnek GE. (2009). Processing of polymer nanofibers through electrospinning as drug delivery systems. Mater Chemistry Physics 113:296-302. [Google Scholar]
- Kim JH, Kim Y, Shin SJ, Park JW, Jung IY. (2010). Tooth discoloration of immature permanent incisor associated with triple antibiotic therapy: a case report. J Endod 36:1086-1091. [DOI] [PubMed] [Google Scholar]
- Kim K, Luu YK, Chang C, Fang D, Hsiao BS, Chu B, et al. (2004). Incorporation and controlled release of a hydrophilic antibiotic using poly(lactide-co-glycolide)-based electrospun nanofibrous scaffolds. J Control Release 98:47-56. [DOI] [PubMed] [Google Scholar]
- Murray PE, Garcia-Godoy F, Hargreaves KM. (2007). Regenerative endodontics: a review of current status and a call for action. J Endod 33:377-390. [DOI] [PubMed] [Google Scholar]
- Petrino JA, Boda KK, Shambarger S, Bowles WR, McClanahan SB. (2010). Challenges in regenerative endodontics: a case series. J Endod 36:536-541. [DOI] [PubMed] [Google Scholar]
- Reynolds K, Johnson JD, Cohenca N. (2009). Pulp revascularization of necrotic bilateral bicuspids using a modified novel technique to eliminate potential coronal discolouration: a case report. Int Endod J 42:84-92. [DOI] [PubMed] [Google Scholar]
- Ruckh TT, Oldinski RA, Carroll DA, Mikhova K, Bryers JD, Popat KC. (2012). Antimicrobial effects of nanofiber poly(caprolactone) tissue scaffolds releasing rifampicin. J Mater Sci Mater Med 23:1411-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruparel NB, Teixeira FB, Ferraz CC, Diogenes A. (2012). Direct effect of intracanal medicaments on survival of stem cells of the apical papilla. J Endod 38:1372-1375. [DOI] [PubMed] [Google Scholar]
- Sahoo S, Ang LT, Goh JC, Toh SL. (2010). Growth factor delivery through electrospun nanofibers in scaffolds for tissue engineering applications. J Biomed Mater Res A 93:1539-1550. [DOI] [PubMed] [Google Scholar]
- Sahoo S, Chakraborti CK, Behera PK. (2012). FTIR and Raman spectroscopic investigations of a controlled release ciprofloxacin/carbopol940 mucoadhesive suspension. Asian J Pharmaceutical Clin Res 5:125-130. [Google Scholar]
- Sato I, Ando-Kurihara N, Kota K, Iwaku M, Hoshino E. (1996). Sterilization of infected root-canal dentine by topical application of a mixture of ciprofloxacin, metronidazole and minocycline in situ. Int Endod J 29:118-124. [DOI] [PubMed] [Google Scholar]
- Sato T, Hoshino E, Uematsu H, Noda T. (1993). In-vitro antimicrobial susceptibility to combinations of drugs of bacteria from carious and endodontic lesions of human decidous teeth. Oral Microbiol Immunol 8:172-176. [DOI] [PubMed] [Google Scholar]
- Siqueira JF, Jr, de Uzeda M. (1997). Intracanal medicaments: evaluation of the antibacterial effects of chlorhexidine metronidazole, and calcium hydroxide associated with three vehicles. J Endod 23:167-169. [DOI] [PubMed] [Google Scholar]
- Siqueira JF, Jr, Batista MM, Fraga RC, de Uzeda M. (1998). Antibacterial effects of endodontic irrigants on black-pigmented Gram-negative anaerobes and facultative bacteria. J Endod 24:414-416. [DOI] [PubMed] [Google Scholar]
- Strahilevitz J, Hooper DC. (2005). Dual targeting of topoisomerase IV and gyrase to reduce mutant selection: direct testing of the paradigm by using WCK-1734, a new fluoroquinolone, and ciprofloxacin. Antimicrob Agents Chemother 49:1949-1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taepaiboon P, Rungsardthong U, Supaphol P. (2006). Drug-loaded electrospun mats of poly(vinyl alcohol) fibres and their release characteristics of four model drugs. Nanotechnology 17:2317-2329. [Google Scholar]
- Tungprapa S, Jangchud I, Supaphol P. (2007). Release characteristics of four model drugs from drug-loaded electrospun cellulose acetate fiber mats. Polymer 48:5030-5041. [Google Scholar]
- Yassen GH, Chu TM, Eckert G, Platt JA. (2013). Effect of medicaments used in endodontic regeneration technique on the chemical structure of human immature radicular dentin: an in vitro study. J Endod 39:269-273. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.