Abstract
Background
The BAG3 (BLC2-associated athanogene 3) gene codes for an antiapoptotic protein located on the sarcomere Z-disc. Mutations in BAG3 are associated with dilated cardiomyopathy (DCM), but only a small number of cases have been reported to date, and the natural history of BAG3 cardiomyopathy is poorly understood.
Objectives
This study sought to describe the phenotype and prognosis of BAG3 mutations in a large multicenter DCM cohort.
Methods
The study cohort comprised 129 individuals with a BAG3 mutation (62% males, 35.1 ± 15.0 years of age) followed at 18 European centers. Localization of BAG3 in cardiac tissue was analyzed in patients with truncating BAG3 mutations using immunohistochemistry.
Results
At first evaluation, 57.4% of patients had DCM. After a median follow-up of 38 months (interquartile range: 7 to 95 months), 68.4% of patients had DCM and 26.1% who were initially phenotype-negative developed DCM. Disease penetrance in individuals >40 years of age was 80% at last evaluation, and there was a trend towards an earlier onset of DCM in men (age 34.6 ± 13.2 years vs. 40.7 ± 12.2 years; p = 0.053). The incidence of adverse cardiac events (death, left ventricular assist device, heart transplantation, and sustained ventricular arrhythmia) was 5.1% per year among individuals with DCM. Male sex, decreased left ventricular ejection fraction. and increased left ventricular end-diastolic diameter were associated with adverse cardiac events. Myocardial tissue from patients with a BAG3 mutation showed myofibril disarray and a relocation of BAG3 protein in the sarcomeric Z-disc.
Conclusions
DCM caused by mutations in BAG3 is characterized by high penetrance in carriers >40 years of age and a high risk of progressive heart failure. Male sex, decreased left ventricular ejection fraction, and enlarged left ventricular end-diastolic diameter are associated with adverse outcomes in patients with BAG3 mutations.
Key Words: BAG3, dilated cardiomyopathy, genetics, prognosis
Abbreviations and Acronyms: BAG3, BCL2-associated athanogene 3; DCM, dilated cardiomyopathy; ECG, electrocardiogram; HF, heart failure; HTx, heart transplantation; ICD, implantable cardioverter-defibrillator; IQR, interquartile range; LVAD, left ventricular assist device; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; SCD, sudden cardiac death; SVT, sustained ventricular tachycardia; VF, ventricular fibrillation
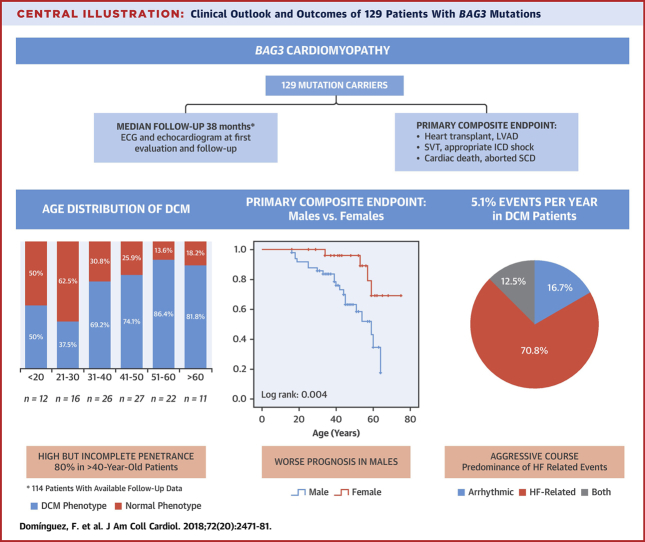
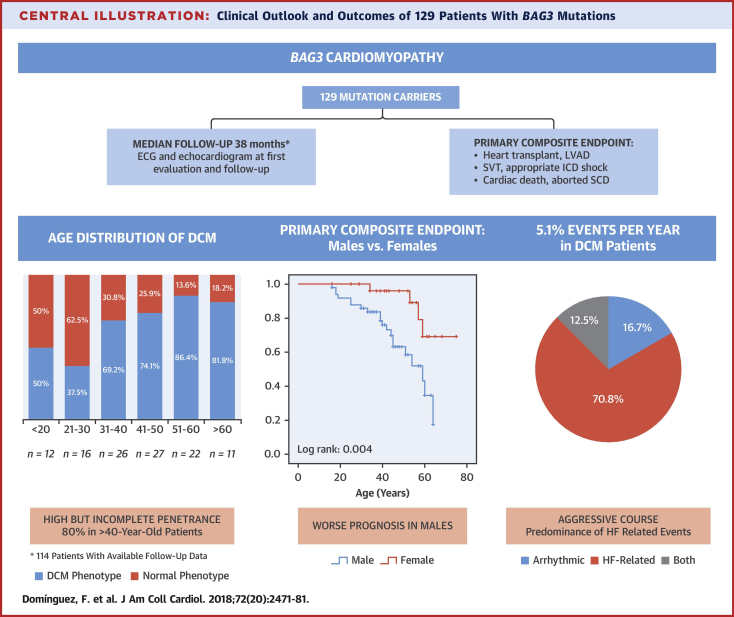
Central Illustration
Dilated cardiomyopathy (DCM) is defined as dilation and systolic impairment of the left ventricle that is not attributable to abnormal loading conditions or coronary artery disease. It is the most common cause of heart failure (HF) in the young and the most frequent indication for heart transplantation (HTx) (1). Recent studies suggest that ≥50% of patients with DCM have a genetic predisposition to the disease caused by mutations in >60 genes 2, 3, many described very recently 4, 5, 6.
One of the recent genes of interest is BAG3 7, 8, which encodes for BLC2-associated athanogene 3 (BAG3) 7, 8, a cochaperone that interacts with members of the heat shock protein (HSP) family (9). These highly conserved proteins are released by the cell in response to stress, and their role is to stimulate the repair and degradation of protein aggregates that accumulate under stress situations such as induced tension in striated muscle cells (10). Moreover, it has been shown that BAG3 is essential for the normal production and clearance of filamin (10) and modulates myocyte contraction through interaction with the β1-adrenegic receptor and the L-type calcium channel (11). Heterozygous BAG3 mutations in human induced pluripotent stem cell–derived cardiomyocytes have been shown to disrupt the myofibril structure and compromise contractile function (12).
Since its initial description as a DCM-causing gene, several cases of DCM caused by mutations in BAG3 have been described, and the prevalence of BAG3 DCM has been reported to be between 2.3% and 3.6% in DCM patient cohorts from the United States, Europe, and Japan 7, 8, 13. Nevertheless, thus far, all published reports are mostly unicentric, include a limited number of individuals, and provide limited information on cardiac outcomes 14, 15. Furthermore, and despite the fact that BAG3 mutations have been associated predominantly with DCM, a missense mutation in BAG3 (p.Pro209Leu) has been associated with hypertrophic and restrictive cardiomyopathy, and also myofibrillary myopathy (16).
In this multicenter retrospective study, we sought to determine the mode of presentation and long-term outcomes of DCM patients and asymptomatic relatives with pathogenic BAG3 mutations. Furthermore, in an effort to gain insight into the consequences of BAG3 mutations at the cardiomyocyte level, we examined by immunohistochemistry the localization of BAG3 in cardiac tissue obtained from DCM patients with truncating BAG3 mutations.
Methods
A chart review was performed in consecutive probands and evaluated relatives with pathogenic or likely pathogenic BAG3 mutations followed at 18 European centers (Online Appendix). Clinical, electrocardiographic (resting 12-lead and ambulatory electrocardiogram [ECG] monitoring) and echocardiographic data from the first and last medical contact at each center were recorded. Left ventricular dysfunction was defined as a left ventricular ejection fraction (LVEF) ≤50% (17).
Details of clinical events that had occurred before first clinical contact and during follow-up (including the timing of events) were collected. Events were characterized as follows: left ventricular assist device (LVAD) implantation, HTx, sustained ventricular tachycardia (SVT), successfully resuscitated ventricular fibrillation (VF), appropriate implantable cardioverter-defibrillator (ICD) shock, sudden cardiac death (SCD), and cardiac and all-cause mortality. SCD was defined as an unexpected death due to cardiac causes that occur within 1 h of the onset of symptoms.
The composite of SVT, VF, ICD shock, and SCD was categorized as a serious arrhythmic event, whereas the composite of HTx, LVAD implantation, and HF death was categorized as an HF-related event. The primary endpoint of adverse cardiac events was defined as a composite of serious arrhythmic events and HF-related events.
Genetic analysis and classification of variants
Deoxyribonucleic acid (DNA) sequence analysis was performed at the participating institutions. Variants were categorized as truncating (nonsense, frameshift, copy number variations, and splice site variants) or nontruncating (missense, small insertion/deletion). Pathogenicity of variants was established according to the current American College of Medical Genetics and Genomics guidelines (18). Novel sequence variants (not found in controls) that predicted a premature truncation were also considered pathogenic.
Immunohistochemistry
Paraffin-embedded sections from explanted heart tissue samples were stained with BAG3 (ab 47124; Abcam, Cambridge, United Kingdom) and sarcomeric α-actinin (ab 9465; Abcam) antibodies, allowing visualization of the sarcomeric architecture and the localization of BAG3 in the cell. A detailed description of the tissue processing protocol can be found in the Online Appendix.
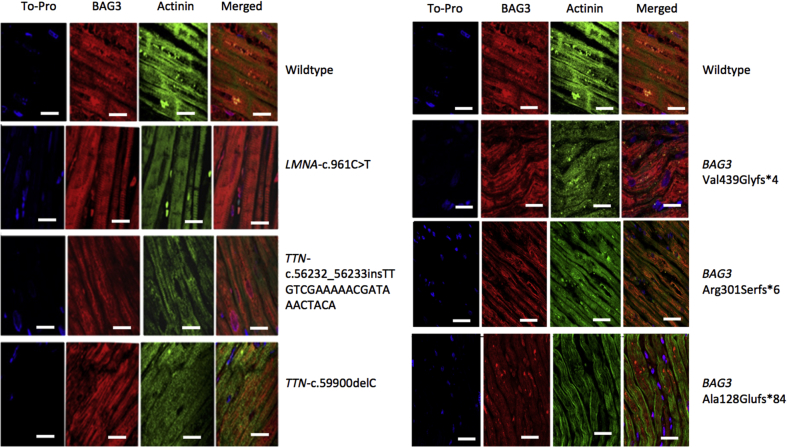
Heart tissue was studied in 3 DCM patients with pathogenic mutations in BAG3 (p.Ala128Glufs*84, p.Val439Glyfs*4, and p.Arg301Serfs*6), 2 DCM patients with truncation mutations in the titin gene (TTN) (Pro19967Leufs*8, Arg18745Leufs*69), 1 DCM patient with a pathogenic mutation in the lamin gene (LMNA) (p.Arg321*), and 2 DCM patients without identified mutations in DCM-causing genes.
Statistical analysis
Results are presented as mean ± SD for continuous variables with normal distribution, as median (interquartile range [IQR]) for continuous variables without normal distribution, and as number (percentage) for categorical data. For statistical analysis, Student's t-test and the Mann-Whitney nonparametric test were used in 2-group comparisons, whereas analysis of variance and Tukey test for multiple group comparisons were applied for 3 groups. Chi-square test or Fisher exact test were used for categorical variables. The cumulative probability of the occurrence of serious adverse cardiac events was estimated using the Kaplan-Meier method, and factors were compared using the log-rank (Mantel-Cox) method. Survival was calculated from first evaluation. A 2-sided p value <0.05 was considered statistically significant. Statistical analyses were performed using SPSS Statistics version 21.0 (IBM, Armonk, New York).
Results
The study cohort included 129 individuals (62% males, 35.1 ± 15.0 years of age at first evaluation) from 38 families (median 2 subjects per family). All individuals were Caucasians of European ancestry with the exception of 1 male Asian subject from Afghanistan with the p.Arg309* variant. Most subjects carried truncating mutations (86%). The complete list of mutations is available in Online Table 1.
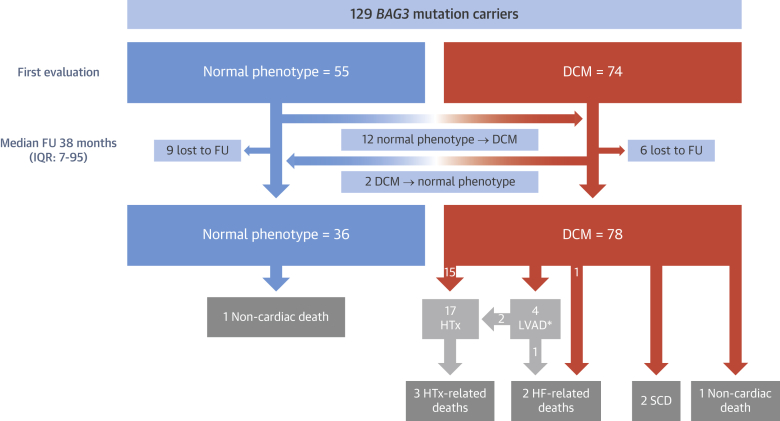
At first evaluation, 74 patients had DCM, whereas 55 subjects had normal left ventricular size and function (Table 1, Figure 1). After a median follow-up of 38 months (IQR: 7 to 95 months), 78 patients had DCM (78 of 114; 68.4%), 36 (36 of 114; 32.6%) showed normal phenotype, and 15 (6 with DCM) were lost to follow-up (15 of 129; 11.6%). A total of 12 patients without DCM at initial evaluation developed DCM (12 of 46 with available follow-up; 26.1%), 2 patients with DCM at initial evaluation normalized LVEF (2 of 68 with available follow-up; 2.9%), and 80% (48 of 60) of individuals older than 40 years at last evaluation exhibited DCM (Figure 1). A total of 18 DCM patients had received transplants (n = 17) or underwent LVAD implantation (n = 1) at last evaluation (18 of 78; 23%) (Figure 1). Overall and cardiac mortality during follow-up in patients with DCM was 10.3% (8 of 78) and 8.9% (7 of 78), respectively.
Table 1.
Baseline Characteristics of Individuals With BAG3 Mutation
| Total Cohort (N = 129) | Phenotype-Negative at First Evaluation (n = 55) | DCM at First Evaluation (n = 74) | |
|---|---|---|---|
| Male | 80 (62.0) | 30 (54.5) | 50 (67.6) |
| Age at first evaluation, yrs | 35.1 ± 15.0 | 28.9 ± 15.0 | 40.1 ± 13.2 |
| Type of mutation | |||
| Truncating | 111 (86.0) | 51 (92.7) | 60 (81.1) |
| Non-truncating | 18 (14.0) | 4 (7.3) | 14 (18.9) |
| Creatine kinase levels, UI/l | 129.3 ± 208.9 | 148.4 ± 323.7 | 109.5 ± 68.3 |
| ECG | |||
| Sinus rhythm | 118/121 (97.5) | 52/52 (100.0) | 66/69 (95.6) |
| QRS duration, ms | 94.7 ± 17.2 | 88.5 ± 10.7 | 99.4 ± 20.3 |
| Negative T-wave all locations | 21/109 (19.3) | 8/50 (16.0) | 13/59 (22.0) |
| Echocardiogram | |||
| LVEF, % | 44.4 ± 17.0 | 59.7 ± 5.7 | 32.9 ± 13.1 |
| LVEDD, mm | 58.0 ± 9.8 | 50.3 ± 6.0 | 64.3 ± 7.7 |
| TAPSE, mm | 21.6 ± 4.5 | 22.1 ± 3.1 | 19.1 ± 4.9 |
| NYHA functional class | |||
| I | 76/128 (59.3) | 52/54 (96.4) | 24/74 (32.4) |
| II | 20/128 (15.6) | 2/54 (3.6) | 18/74 (24.4) |
| III | 17/128 (13.3) | 0/54 (0.0) | 17/74 (22.9) |
| IV | 15/128 (11.7) | 0/54 (0.0) | 15/74 (20.3) |
Values are n (%), n/N (%), or mean ± SD.
ECG = electrocardiogram; DCM = dilated cardiomyopathy; LVEDD = left ventricular end-diastolic diameter; LVEF = left ventricular ejection fraction; NYHA = New York Heart Association; TAPSE = tricuspid annular plane systolic excursion.
Figure 1.
Flowchart of Patients Included in the Study
*1 patient was still on LVAD at last evaluation. BAG3 = BLC2-associated athanogene 3; DCM = dilated cardiomyopathy; FU = follow-up; HF = heart failure; HTx = heart transplantation; IQR = interquartile range; LVAD = left ventricular assist device; SCD = sudden cardiac death.
Natural history of BAG3 mutations
The diagnosis of DCM was made at a mean age of 36.9 ± 13.1 years, with a trend toward an earlier onset in men (34.6 ± 13.2 years vs. 40.7 ± 12.2 years; p = 0.053). The type of mutation (truncating vs. nontruncating) had no impact on the mean age at DCM diagnosis (37.3 ± 12.7 years in truncating vs. 35.5 ± 14.5 years in nontruncating; p = 0.62). A total of 12 individuals (83.3% male) were diagnosed with DCM under 20 years of age (12 of 71 patients with available information; 17%); a 14-year-old male with a nonsense mutation (p.Gln353Argfs*10) was the youngest patient diagnosed. Seven percent of patients were diagnosed with DCM over 55 years.
Among patients with DCM at first evaluation (n = 74), 24 (32.4%) were in New York Heart Association (NYHA) functional class I, 18 (24.4%) were in NYHA functional class II, and 32 (43.2%) were in NYHA functional class III to IV. ECG and echocardiographic findings at first evaluation are shown in Table 1. Patients with DCM at first evaluation were in sinus rhythm in 95.6% of cases, 22% showed negative T waves, and the mean QRS duration was 99.4 ± 20.3 ms. Mean ejection fraction was 32.9 ± 13.1%, and the mean left ventricular end-diastolic diameter (LVEDD) was 64.3 ± 7.7 mm. Mutation carriers without the DCM phenotype were all in sinus rhythm, and 16% exhibited negative T waves on the ECG (Table 1).
Of the 12 patients who developed DCM during follow-up (38.5% males, age at DCM diagnosis 37.6 ± 16.0 years), mean LVEF and LVEDD at last follow-up was 45.5 ± 6.6% and 58.5 ± 3.6 mm, respectively (Table 2). One-quarter of these patients (3 of 12) had nontruncating variants. Taking into account that only 4 subjects without DCM at first evaluation had a nontruncating variant (Table 1), 75% of them developed DCM, as compared with 21.4% in subjects with truncating variants (p = 0.02) (Table 2). ECG features at first evaluation were comparable between subjects who developed or did not develop DCM (Table 2).
Table 2.
Clinical Characteristics of Initially Unaffected BAG3 Genotype-Positive Relatives Who Did or Did Not Develop DCM at Last Follow-Up
| Normal Phenotype After Follow-Up (n = 34) | DCM After Follow-Up (n = 12) | p Value | |
|---|---|---|---|
| Male | 20/34 (58.8) | 5/12 (41.7) | 0.31 |
| Age at first evaluation, yrs | 26.2 ± 13.7 | 27.9 ± 11.5 | 0.70 |
| Age at last evaluation, yrs | 32.0 ± 13.6 | 37.3 ± 12.5 | 0.25 |
| Follow-up, months | 28 (5–67.3) | 59 (25–190) | 0.13 |
| Creatine kinase levels, UI/l | 80.3 ± 26.4 | 105.7 ± 51.3 | 0.12 |
| Nontruncating variants | 1/34 (2.9) | 3/12 (25.0) | 0.02 |
| ECG | |||
| Sinus rhythm first ECG | 33/33 (100.0) | 10/10 (100.0) | 0.58 |
| Sinus rhythm follow-up ECG | 32/33 (97.0)∗ | 11/11 (100.0) | 0.70 |
| QRS duration first ECG, ms | 88.6 ± 10.4 | 85.1 ± 15.3 | 0.56 |
| QRS duration follow-up ECG, ms | 92.0 ± 11.7 | 105.8 ± 24.2 | 0.025 |
| Negative T waves first ECG | 4/32 (12.5) | 2/11 (18.2) | 0.55 |
| Negative T waves follow-up ECG | 4/32 (12.5) | 2/10 (20.0) | 0.76 |
| Echocardiogram | |||
| LVEF first evaluation, % | 59.9 ± 5.2 | 57.9 ± 4.4 | 0.26 |
| LVEF follow-up, % | 56.6 ± 5.6 | 45.5 ± 6.6 | <0.001 |
| LVEDD first evaluation, mm | 49.5 ± 4.9 | 52.6 ± 8.7 | 0.30 |
| LVEDD follow-up, mm | 50.1 ± 4.8 | 58.5 ± 3.6 | <0.001 |
| TAPSE first evaluation, mm | 21.4 ± 2.6 | 25.3 ± 1.9 | 0.01 |
| TAPSE follow-up, mm | 22.3 ± 2.5 | 22.6 ± 5.0 | 0.87 |
Values are n/N (%), mean ± SD, or median (interquartile range).
Abbreviations as in Table 1.
1 patient showed junctional rhythm. Bold indicates statistical significance.
Regarding patients with DCM at first evaluation (n = 74, 67.6% males, 40.1 ± 13.2 years), no significant changes in LVEF (34.1 ± 12.4% vs. 34.1 ± 13.0%; p = 0.81) and LVEDD (64.0 ± 9.3 mm vs. 64.5 ± 7.9 mm; p = 0.66) were found after a median follow-up of 42.5 months (IQR: 6 to 94 months) (Table 3). Considering only DCM patients with available ECG at first evaluation and follow-up (n = 53, 71.6%), 1 subject exhibited new atrial fibrillation (1.8%), 8 subjects showed new negative T waves (17%), and mean QRS duration was 103.1 ± 24.4 ms as compared with 98.6 ± 21.5 ms at initial evaluation (p = 0.10) (Table 3).
Table 3.
ECG and Echocardiographic Characteristics of BAG3 Mutation Carriers With DCM at First Evaluation
| First Evaluation (n = 74) | Last Evaluation (n = 68)∗ | p Value | |
|---|---|---|---|
| Male | 67.6 | 66.1 | |
| Age, yrs | 40.1 ± 13.2 | 45.3 ± 13.5 | |
| ECG | |||
| Sinus rhythm | 51/53 (96.2) | 50/53 (94.3) | 0.50 |
| QRS duration, ms | 98.6 ± 21.5 | 103.1 ± 24.4 | 0.10 |
| Negative T waves, % | 8/47 (17.0) | 16/47 (34.0) | 0.04 |
| Echocardiogram | |||
| LVEF, % | 34.1 ± 13.0 | 34.1 ± 12.4 | 0.99 |
| LVEDD, mm | 64.5 ± 7.9 | 64.0 ± 9.3 | 0.66 |
| TAPSE, mm | 19.1 ± 4.9 | 20.2 ± 5.3 | 0.25 |
Values are n, mean ± SD, or n/N (%).
Abbreviations as in Table 1.
6 patients were lost to follow-up, 2 normalized phenotype, and 66 maintained DCM phenotype. Last available echocardiogram and ECG was before heart transplantation in the 17 patients that received transplants.
Clinical events
A total of 114 subjects were followed for a median of 38 months (IQR: 7 to 95 months). During this period, 9 patients died (7.9%; 7 cardiac deaths and 2 noncardiac). Among the cardiac deaths, 2 were SCD (28.6%). Three additional patients presented an aborted SCD, whereas 4 patients received a LVAD and 17 underwent HTx (14.3%), including 1 of the patients who had an aborted SCD. Only 1 patient had an LVAD at last evaluation (Figure 1). The remaining cardiac deaths were due to HF (n = 2) or to cardiac complications after HTx (n = 3) (Figures 1 and 2).
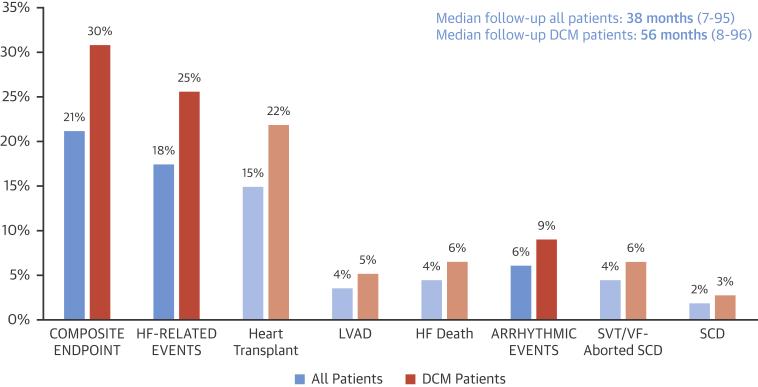
Figure 2.
Clinical Events During Follow-Up of Individuals With BAG3 Mutation
SVT/FV = sustained ventricular tachycardia/ventricular fibrillation; other abbreviations as in Figure 1.
Regarding arrhythmic events, SVT occurred in 3 patients, and VF was documented in the 3 patients with aborted SCD (Figure 2). At last follow-up, 12 of the 61 DCM patients without transplants with available follow-up data had an ICD implanted (19.7%), 91.7% for primary prevention. Only 1 patient had received an appropriate ICD shock.
Composite endpoint of cardiac death, heart transplant, LVAD, aborted SCD, or serious ventricular arrhythmia occurred in 24 of the patients with DCM (30.1%) (Figure 2), and the incidence of the composite endpoint was 5.1% per year. No events were found in individuals with BAG3 mutation without a DCM phenotype.
Predictors of adverse events
DCM patients (n = 78) who reached the composite endpoint of cardiac events (n = 24, 83.3% males, 40.4 ± 15.0 years at last follow-up) exhibited lower LVEF at first evaluation (24.1 ± 9.9% vs. 42.4 ± 14.0%; p < 0.001) and higher LVEDD (68.7 ± 8.1 vs. 60.9 ± 8.5 mm; p = 0.002) than DCM patients who did not have adverse events (Table 4). Non-SVT on ECG Holter was more frequent in DCM patients with adverse events (57.1% vs. 33.3%), but this difference did not reach statistical significance (p = 0.26). Male sex was also more prevalent in patients who had events (Figure 3, Central Illustration). However, electrocardiographic features and the type of mutation did not differ between DCM patients with or without cardiac events (Table 4). The subgroup of patients with HF-related events (HTx, LVAD, or HF death, n = 20) were more frequently male and exhibited a lower LVEF and a larger LVEDD at first evaluation. No other clinical predictors were observed (Online Table 2). Regarding the subgroup of DCM patients with serious arrhythmic events (n = 8), male sex was not significantly associated with these complications, but LVEF at first evaluation was still lower as compared with DCM patients without arrhythmic events (Online Table 3).
Table 4.
Clinical Parameters in DCM Patients With BAG3 Mutations Classified According to the Presence of Cardiac Events During Follow-Up
| DCM Patients Without Cardiac Events (n = 54) | DCM Patients With Cardiac Events (n = 24) | p Value | |
|---|---|---|---|
| Male | 55.6 | 83.3 | 0.02 |
| Truncating mutation | 77.8 | 83.3 | 0.58 |
| Non-truncating mutation | 22.2 | 16.7 | 0.58 |
| Age at DCM onset, yrs | 38.0 ± 12.3 | 33.4 ± 14.4 | 0.18 |
| QRS width on 1st ECG, ms | 98.4 ± 21.8 | 96.7 ± 19.2 | 0.79 |
| Negative T waves on 1st ECG | 17.0 | 33.3 | 0.18 |
| LVEDD on 1st echo, mm | 60.9 ± 8.5 | 68.7 ± 8.1 | 0.002 |
| LVEF on 1st echo, % | 42.4 ± 14.0 | 24.1 ± 9.9 | <0.001 |
| TAPSE on 1st echo, mm | 20.5 ± 5.1 | 15.5 ± 2.1 | 0.21 |
| NSVT on Holter monitor | 33.3 | 57.1 | 0.26 |
| CK, UI/l | 116.2 ± 67.9 | 89.7 ± 58.8 | 0.22 |
Values are % or mean ± SD. Bold indicates statistical significance.
CK = creatine kinase; NSVT = non-sustained ventricular tachycardia; other abbreviations as in Table 1.
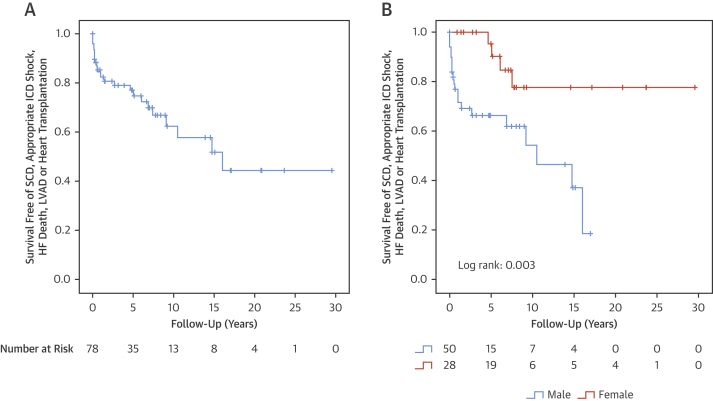
Figure 3.
Survival Analysis in BAG3 DCM
(A) Global survival free of the primary composite endpoint (heart transplantation, left ventricular assist device, cardiac death, aborted SCD and appropriate ICD shock). Analysis from date of first evaluation until last follow-up or event. (B) Male sex as risk factor for clinical events. ICD = implantable cardioverter-defibrillator; other abbreviations as in Figure 1.
Central Illustration.
Clinical Outlook and Outcomes of 129 Patients With BAG3 Mutations
BAG3 = BLC2-associated athanogene 3; DCM = dilated cardiomyopathy; ECG = electrocardiogram; HF = heart failure; ICD = implantable cardioverter defibrillator; LVAD = left ventricular assist device; SCD = sudden cardiac death; SVT = sustained ventricular tachycardia.
Immunohistochemistry
Immunofluorescence confocal microscopy of myocardial tissue from 3 patients with different truncating mutations in BAG3 showed both myofibrillar disarray and a decrease and a relocation of BAG3 protein in the sarcomeric Z-disc, exhibiting a disorganized pattern (Figure 4, Online Figures 1 and 2). By contrast, appropriate BAG3 localization was evident in the Z-disc region in DCM cases caused by TTN and LMNA mutations or without identifiable genetic cause (Figure 4).
Figure 4.
BAG3 Localization in Explanted Hearts
Samples fluorescently labeled for actinin (green) and BAG3 (red). Scale bar: 50 μm. (Left column) The first row belongs to a patient without mutations. The second row to a patient with a mutation in Lamin, and the third and fourth rows to patients with truncating mutations in TTN. Samples labeled with actinin and BAG3 show normal localization of the proteins on Z-discs on all the patients. (Right column) The first row belongs to a patient without mutations. Subsequent rows belong to patients with BAG3 truncating mutations. In patients with BAG3 mutations, BAG3 is present at Z-discs but appears disorganized. Myofibrillar disarray can also be observed. BAG3 = BLC2-associated athanogene 3.
Discussion
This study represents the largest cohort of DCM caused by BAG3 mutations reported to date. Our findings show that DCM caused by mutations in BAG3 is characterized by early-onset disease in the majority of patients, with a high risk of progression to end-stage heart failure and a worse prognosis in men.
Furthermore, this is the first study to provide data on the impact of BAG3 mutations on cardiomyocyte architecture in patients with DCM. Previous cardiac histological data of BAG3 mutations were limited to postmortem tissue from a patient with DCM and the p.His243Thrfr*64 mutation that showed mild fibrosis and nuclear pleomorphism (19). However, BAG3 localization or further histological features were not provided. Additionally, histological data are available for 2 patients with the p.Pro209Leu mutation, which does not cause DCM, but rather restrictive cardiomyopathy and myofibrillary myopathy 20, 21. In the present study, confocal microscopy of heart samples revealed that BAG3 protein was diminished and disorganized in the sarcomeric Z-disc in patients with truncating mutations in the BAG3 gene, which is similar to what has been observed in patients with the p.Pro209Leu mutation (21).
BAG3 protein interacts with the actin capping protein CapZ, stabilizing the myofibril structure in response to mechanical stress (22), and has been shown to be essential for the stability of the cardiac sarcomere (10). Accordingly, alteration in BAG3 location in the Z-disc could directly interfere with its function by preventing interaction with CapZ.
BAG3 is expressed mainly in skeletal muscle cells and cardiomyocytes, and mutations in the gene were initially reported to be associated with myofibrillar myopathy (16). Studies in knockout mice showed striated muscle fiber degeneration and apoptotic nuclei, leading to fulminant skeletal myopathy and cardiomyopathy (9). However, to date, Pro209Leu is the only genetic variant that has been associated with a neuromuscular phenotype in humans (16). This mutation was not present in our cohort, and there was no evidence of relevant neuromuscular involvement in any of our patients. Only 1 patient with the p.Gln353Argfs*10 mutation and without a DCM phenotype had a single significantly increased creatine kinase value (1,822 UI/l), from a sample taken soon after heavy training.
Natural history of BAG3 mutations
Our study provides detailed longitudinal data of a large cohort with BAG3 mutations, allowing specific important information for physicians handling patients with this genetic diagnosis. Our data show that 26.1% of genotype-positive individuals who were phenotype-negative at first evaluation developed LVEF impairment and LV dilatation during a median follow-up of 33 months. The mean age at DCM onset was 36.9 years, and penetrance was 80% in subjects >40 years of age (Central Illustration). Although this penetrance is high, it is lower than that of other genetic forms of DCM such as the recently described truncating filamin C form (FLNC), in which penetrance is almost complete at >40 years of age (23). Previous studies including DCM patients with BAG3 mutations did not include detailed clinical information, but reported a mean age at DCM diagnosis of 44 and 37 years in the 2 largest cohorts reported to date from the United States (n = 22) and Canada (n = 21), respectively 7, 24.
Data on other factors that could have accelerated the onset of DCM phenotype, such as alcohol, pregnancy, or infections 25, 26, were unavailable in ours and in previous cohorts. Nevertheless, the observed rate of progression into DCM phenotype is substantially higher than in other genetic DCM forms. For instance, LMNA genotype-positive phenotype-negative individuals were reported to develop left ventricular dilation in 24% of cases after a median follow-up of 7 years (27). Furthermore, the response to treatment in DCM patients with BAG3 mutations seemed less prominent than in other genetic forms of DCM, as only 2.9% of the patients with DCM at first evaluation normalized LVEF during follow-up (Figure 1). Although medical treatment data were not available in the present study, our results reveal a very low left ventricular reverse remodeling rate. In line with these results, a recent study has reported that rare genetic variants in genes that code for proteins of the cytoskeleton or Z-disc are independently associated with a lower rate of left ventricular reverse remodeling in DCM (28).
Prognosis of DCM caused by BAG3 mutations
Our study shows that patients with DCM caused by BAG3 mutations have an adverse prognosis. The incidence of adverse cardiac events was 5.1% per year in patients with overt DCM phenotype (Figure 3). More men than women with DCM developed cardiac events during follow-up, and the survival analysis showed a better prognosis in females. Male sex has also been found to be a predictor of worse outcome in other forms of genetic DCM such as those caused by mutations in TTN (29) or LMNA (27). However, male sex was not associated with serious arrhythmic events in our cohort. In fact, serious arrhythmias were infrequent, with a rate of only 1% per year in all individuals with a BAG3 mutation and 1.5% in those patients with a DCM phenotype. This phenotype contrasts with other genetic causes of DCM with a heavy burden of arrhythmia, such as mutations in LMNA (27) or FLNC truncating variants (23) and is more similar to DCM caused by TTN truncating variants, in which arrhythmic events are less common (30).
By contrast, the number of patients who developed HF adverse events was high. Up to 27% of DCM patients required HTx, LVAD, or had an HF-related death (4.3% per year), which depicts a more “HF”-oriented phenotype in DCM caused by BAG3 mutations. Along this line, Norton et al. (7) reported that 8 of 17 patients with DCM caused by BAG3 mutations underwent HTx or presented HF-related death in a non-European cohort. Overall, these data support a phenotype dominated by HF-related complications irrespective of patients' geographical origin.
Although data on the prevalence of BAG3 mutations at all participant centers were not available, the prevalence found at 2 of the participating centers (Hospital Universitario Puerta de Hierro, Madrid, Spain, and the Cardinal Stefan Wyszyński Institute of Cardiology, Warsaw, Poland) was 6.7% (14 of 208 DCM probands tested for mutations in BAG3), whereas it ranged from 2.3% to 3.6% in previous reports 6, 7, 13. Interestingly, the Hospital Universitario Puerta de Hierro and the Cardinal Stefan Wyszyński Institute of Cardiology are centers with important HTx/LVAD programs that could have enriched the BAG3 mutation uptake and potentially reflect again an “HF”-oriented phenotype.
Other factors associated with adverse outcomes in our study were initial LVEF and LVEDD. However, there was a trend toward more arrhythmic events in subjects with a later DCM onset (p = 0.10) (Online Table 3). Because late debuts were not very frequent, these results again underline the fact that prognosis is determined by an HF-related phenotype, especially in young patients.
In recent years, advances in pharmacological and nonpharmacological treatments have substantially improved the prognosis of DCM, and the estimated survival free from death or HTx in DCM have been reported to be 85% at 10 years (31). Thus, the 4.3% yearly rate of HF-related events found in our study highlights the aggressive course of BAG3 DCM. This information and the factors associated with adverse events identified in this study could help physicians in referring BAG3 DCM patients promptly for HTx evaluation and to promote aggressive pharmacological treatment from early stages of the disease.
Study limitations
Follow-up data were not available in all the patients. The database was not designed to assess response to pharmacological treatments, and data regarding this issue are lacking. Finally, the immunohistochemical analysis was only performed in heart tissue from individuals with truncating mutations, and the effect of missense variants could not be evaluated.
Conclusions
DCM caused by mutations in BAG3 is characterized by an aggressive clinical course dominated by HF complications and a high, but incomplete, penetrance in carriers >40 years of age. Male sex, low LVEF and increased LVEDD at first evaluation were associated with an adverse prognosis during follow-up.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Patients with dilated cardiomyopathy caused by BAG3 mutations exhibit a high rate of heart failure–related events.
TRANSLATIONAL OUTLOOK: Because penetrance of BAG3 mutations is incomplete, further studies are needed to identify the factors leading some affected individuals to develop DCM.
Footnotes
This work was supported by grants from the following institutions: Instituto de Salud Carlos III (ISCIII) (PI14/0967, PI15/01551, AC16/0014), CIBERCV (CB16/11/00403), Progreso and Salud Foundation (Junta de Andalucia) (PI-0011/201), Ministry of Economy, Industry and Competitiveness (IJCI-2016-29393), Mutual Medical (Research Award 2017), ERA-CVD Joint Transnational Call 2016 (Genprovic), British Heart Foundation (SP/10/10/28431), Wellcome Trust (107469/Z/15/), NIHR Royal Brompton Cardiovascular Biomedical Research Unit, NIHR Imperial Biomedical Research Center, Health Innovation Challenge Fund award from the Wellcome Trust and Department of Health, U.K. (HICF-R6-373), Danish Heart Foundation (16-R107-A6617), University of Southern Denmark, the Region of Southern Denmark, Odense University Hospital, German Competence Network Heart Failure, (TP9, FKZ 01GI0205), DETECTIN-HF project (ERA-CVD framework), and PROMEX Charitable Foundation. UCL Hospitals NIHR Biomedical Research Center and Netherlands Cardiovascular Research Initiative, an initiative with support of the Dutch Heart Foundation, CVON2015-12 eDETECT and CVON2014-40 DOSIS. The CNIC is supported by the Ministry of Economy, Industry and Competitiveness and the Pro-CNIC Foundation, and is a Severo Ochoa Center of Excellence (SEV-2015-0505). Grants from ISCIII and the Spanish Ministry of Economy and Competitiveness are supported by the Plan Estatal de I+D+I 2013-2016 – European Regional Development Fund (FEDER) “A way of making Europe.” The Hospital Universitario Puerta de Hierro Majadahonda, Hôpital Pitié-Salpêtrière, and Saint Bartholomews' Hospital are members of the European Reference Network for rare, low-prevalence, and complex diseases of the heart (ERN GUARD-Heart). Drs. Ochoa, Cicerchia, and Salazar-Mendiguchia are employees of Health In Code. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Contributor Information
Pablo Garcia-Pavia, Email: pablogpavia@yahoo.es, @cardiopdh.
European Genetic Cardiomyopathies Initiative Investigators:
Hans Eiskjær, Roberto Barriales, Xusto Fernández Fernández, Marcos Cicerchia, Lorenzo Monserrat, Juan Pablo Ochoa, Joel Salazar-Mendiguchia, Maria Victoria Mogollón, Tomás Ripoll, Philippe Charron, Pascale Richard, Eric Villard, Julian Palomino Doza, Ana Fontalba, Luis Alonso-Pulpón, Marta Cobo-Marcos, Fernando Domínguez, Pablo Garcia-Pavia, Manuel Gómez-Bueno, Esther González-López, Aitor Hernández-Hernández, Francisco José Hernández-Pérez, Ángela López-Sainz, Alejandra Restrepo-Córdoba, Javier Segovia-Cubero, Rocio Toro, David de Gonzalo-Calvo, Félix Rosa Longobardo, Javier Limeres, Jose F. Rodriguez-Palomares, Jose Manuel Garcia-Pinilla, Miguel A. López-Garrido, Juan Jiménez-Jaimez, Dolores Garcia-Medina, Diego Rangel Sousa, Maria Luisa Peña, Jens Mogensen, Thomas Morris-Hey, Paul J. Barton, Stuart A. Cook, William Midwinter, Angharad M. Roberts, James S. Ware, Roddy Walsh, Mohammed Akhtar, Perry M. Elliott, Luis Rocha-Lopes, Konstantinos Savvatis, Petros Syrris, Ewa Michalak, Rafal Ploski, Malgorzata Sobieszczanska-Malek, Zofia Bilińska, Sabine Pankuweit, Folkert Asselbergs, Annette Baas, Dennis Dooijes, and Arjan Sammani
Appendix
References
- 1.Japp A.G., Gulati A., Cook S.A., Cowie M.R., Prasad S.K. The diagnosis and evaluation of dilated cardiomyopathy. J Am Coll Cardiol. 2016;67:2996–3010. doi: 10.1016/j.jacc.2016.03.590. [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Pavia P., Cobo-Marcos M., Guzzo-Merello G. Genetics in dilated cardiomyopathy. Biomark Med. 2013;7:517–533. doi: 10.2217/bmm.13.77. [DOI] [PubMed] [Google Scholar]
- 3.Cuenca S., Ruiz-Cano M.J., Gimeno-Blanes J.R. Genetic basis of familial dilated cardiomyopathy patients undergoing heart transplantation. J Heart Lung Transplant. 2016;35:625–635. doi: 10.1016/j.healun.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 4.Michels V.V., Moll P.P., Miller F.A. The frequency of familial dilated cardiomyopathy in a series of patients with idiopathic dilated cardiomyopathy. N Engl J Med. 1992;326:77–82. doi: 10.1056/NEJM199201093260201. [DOI] [PubMed] [Google Scholar]
- 5.Burkett E.L., Hershberger R.E. Clinical and genetic issues in familial dilated cardiomyopathy. J Am Coll Cardiol. 2005;45:969–981. doi: 10.1016/j.jacc.2004.11.066. [DOI] [PubMed] [Google Scholar]
- 6.Hershberger R.E., Siegfried J.D. Update 2011: clinical and genetic issues in familial dilated cardiomyopathy. J Am Coll Cardiol. 2011;57:1641–1649. doi: 10.1016/j.jacc.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Norton N., Li D., Rieder M.J. Genome-wide studies of copy number variation and exome sequencing identify rare variants in BAG3 as a cause of dilated cardiomyopathy. Am J Hum Genet. 2011;88:273–282. doi: 10.1016/j.ajhg.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villard E., Perret C., Gary F. A genome-wide association study identifies two loci associated with heart failure due to dilated cardiomyopathy. Eur Heart J. 2011;32:1065–1076. doi: 10.1093/eurheartj/ehr105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knezevic T., Myers V.D., Gordon J. BAG3: a new player in the heart failure paradigm. Heart Fail Rev. 2015;20:423–434. doi: 10.1007/s10741-015-9487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ulbricht A., Hohfeld J. Tension-induced autophagy: may the chaperone be with you. Autophagy. 2013;9:920–922. doi: 10.4161/auto.24213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feldman A.M., Gordon J., Wang J. BAG3 regulates contractility and Ca2+ homeostasis in adult mouse ventricular myocytes. J Mol Cell Cardiol. 2016;92:10–20. doi: 10.1016/j.yjmcc.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Judge L.M., Perez-Bermejo J.A., Truong A. A BAG3 chaperone complex maintains cardiomyocyte function during proteotoxic stress. JCI Insight. 2017;2:e94623. doi: 10.1172/jci.insight.94623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arimura T., Ishikawa T., Nunoda S., Kawai S., Kimura A. Dilated cardiomyopathy-associated BAG3 mutations impair Z-disc assembly and enhance sensitivity to apoptosis in cardiomyocytes. Hum Mutat. 2011;32:1481–1491. doi: 10.1002/humu.21603. [DOI] [PubMed] [Google Scholar]
- 14.Franaszczyk M., Bilinska Z.T., Sobieszczańska-Małek M. The BAG3 gene variants in Polish patients with dilated cardiomyopathy: four novel mutations and a genotype-phenotype correlation. J Transl Med. 2014;12:192. doi: 10.1186/1479-5876-12-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feldman A.M., Begay R.L., Knezevic T. Decreased levels of BAG3 in a family with a rare variant and in idiopathic dilated cardiomyopathy. J Cell Physiol. 2014;229:1697–1702. doi: 10.1002/jcp.24615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selcen D., Muntoni F., Burton B.K. Mutation in BAG3 causes severe dominant childhood muscular dystrophy. Ann Neurol. 2009;65:83–89. doi: 10.1002/ana.21553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinto Y.M., Elliott P.M., Arbustini E. Proposal for a revised definition of dilated cardiomyopathy, hypokinetic non-dilated cardiomyopathy, and its implications for clinical practice: a position statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2016;37:1850–1858. doi: 10.1093/eurheartj/ehv727. [DOI] [PubMed] [Google Scholar]
- 18.Richards S., Aziz N., Bale S. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toro R., Pérez-Serra A., Campuzano O. Familial dilated cardiomyopathy caused by a novel frameshift in the BAG3 gene. PLoS One. 2016;11:e0158730. doi: 10.1371/journal.pone.0158730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konersman C.G., Bordini B.J., Scharer G. BAG3 myofibrillar myopathy presenting with cardiomyopathy. Neuromuscul Disord. 2015;25:418–422. doi: 10.1016/j.nmd.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 21.Schänzer A., Rupp S., Gräf S. Dysregulated autophagy in restrictive cardiomyopathy due to Pro209Leu mutation in BAG3. Mol Genet Metab. 2018;123:388–399. doi: 10.1016/j.ymgme.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Hishiya A., Kitazawa T., Takayama S. BAG3 and Hsc70 interact with actin capping protein CapZ to maintain myofibrillar integrity under mechanical stress. Circ Res. 2010;107:1220–1231. doi: 10.1161/CIRCRESAHA.110.225649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ortiz-Genga M.F., Cuenca S., Dal Ferro M. Truncating FLNC mutations are associated with high-risk dilated and arrhythmogenic cardiomyopathies. J Am Coll Cardiol. 2016;68:2440–2451. doi: 10.1016/j.jacc.2016.09.927. [DOI] [PubMed] [Google Scholar]
- 24.Chami N., Tadros R., Lemarbre F. Nonsense mutations in BAG3 are associated with early-onset dilated cardiomyopathy in French Canadians. Can J Cardiol. 2014;30:1655–1661. doi: 10.1016/j.cjca.2014.09.030. [DOI] [PubMed] [Google Scholar]
- 25.Ware J.S., Amor-Salamanca A., Tayal U. Genetic etiology for alcohol-induced cardiac toxicity. J Am Coll Cardiol. 2018;71:2293–2302. doi: 10.1016/j.jacc.2018.03.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ware J.S., Seidman J.G., Arany Z. Shared genetic predisposition in peripartum and dilated cardiomyopathies. N Engl J Med. 2016;374:2601–2602. doi: 10.1056/NEJMc1602671. [DOI] [PubMed] [Google Scholar]
- 27.Kumar S., Baldinger S.H., Gandjbakhch E. Long-term arrhythmic and nonarrhythmic outcomes of lamin A/C mutation carriers. J Am Coll Cardiol. 2016;68:2299–2307. doi: 10.1016/j.jacc.2016.08.058. [DOI] [PubMed] [Google Scholar]
- 28.Dal Ferro M., Stolfo D., Altinier A. Association between mutation status and left ventricular reverse remodelling in dilated cardiomyopathy. Heart. 2017;103:1704–1710. doi: 10.1136/heartjnl-2016-311017. [DOI] [PubMed] [Google Scholar]
- 29.Herman D.S., Lam L., Taylor M.R.G. Truncations of Titin causing dilated cardiomyopathy. N Engl J Med. 2012;366:619–628. doi: 10.1056/NEJMoa1110186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tayal U., Newsome S., Buchan R. Truncating variants in titin independently predict early arrhythmias in patients with dilated cardiomyopathy. J Am Coll Cardiol. 2017;69:2466–2468. doi: 10.1016/j.jacc.2017.03.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merlo M., Cannatà A., Gobbo M., Stolfo D., Elliott P.M., Sinagra G. Evolving concepts in dilated cardiomyopathy. Eur J Heart Fail. 2018;20:228–239. doi: 10.1002/ejhf.1103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.