Abstract
The Japanese Society of Gastroenterological Surgery (JSGS) and the American College of Surgeons National Surgical Quality Improvement Program (ACS‐NSQIP) have collaboratively developed several clinical projects since 2011 using two nationwide clinical registries with the goal of achieving further improvement of surgical quality in both countries. In this review, the historical viewpoints and the collaboration between JSGS and ACS and their use of nationwide registries [National Clinical Database (NCD) and NSQIP] for research are reviewed. We have carried out a joint project, the 30‐day Mortality Risk Model Study and, currently, we are working on several joint projects such as the Morbidity‐Mortality Study, Japan‐USA Calibration Study, Geriatric Study, and Safety Culture Study as well as Auditing in JSGS/NCD with reference to the NSQIP method. These joint projects will continue to provide us with important information and data to drive improvements in surgical care in both countries. This will also help us to identify any unknown weaknesses in the health‐care systems of the USA and Japan.
Keywords: big data, gastroenterological surgery, surgical outcome
1. INTRODUCTION
Recent progress and advancement in information and communication technology (ICT) has allowed for nationwide data to be centralized in a data center, allowing us to use large databases for various purposes.1, 2 Within health care, use of a clinical registry to collect national data into a large database and analyze it can lead to significant improvements in health‐care quality.3, 4, 5 A nationwide multicenter registry exceeds the abilities of a small or single institution registry and allows for the collection of information necessary to contribute to improving health‐care quality on a national level.
The National Clinical Database (NCD) in Japan and the American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP) based in the USA were both established and developed to serve as nationwide clinical registries for surgery. Collaboration between the two countries has been established to support efforts within each country to improve surgical care. In this review, collaboration between the Japanese Society of Gastroenterological Surgery (JSGS) and ACS is described, and their use of nationwide registries (NCD and NSQIP) for research is reviewed by summarizing successful past collaborative studies as well as ongoing projects to improve surgical care.
2. ESTABLISHMENT AND DEVELOPMENT OF ACS‐NSQIP
National Surgical Quality Improvement Program was first established in 1994 in 132 Veterans Affairs (VA) hospitals following the success of the National VA Surgical Risk study (Table 1). The National VA Surgical Risk study developed and successfully implemented a system for the prospective collection of postoperative outcomes and assessment of risk‐adjusted hospital performance. This has grown to include more than 700 hospitals participating in NSQIP in 2018, and it continues to grow and expand (Table 2). ACS has developed NSQIP and other quality improvement programs following the same model, which comprise four important processes: (i) establish standards; (ii) build infrastructure to support the standards; (iii) develop databases to measure performance against those standards; and (iv) provide external peer‐reviewed verification.6
Table 1.
Chronological table of NCD and NSQIP development
| NCD | NSQIP | |
|---|---|---|
| 1991‐1993 | National VA Surgical Risk Study (NVASRS) | |
| 1994 | Established | |
| 1999 | Extended to Non‐VA hospitals | |
| 2000 | Japan Cardiovascular Surgery Database (JACVSD) | |
| 2001 | Pilot program in private sector hospitals | |
| 2006 | JSGS DB committee | |
| 2010 | Established | |
| 2011 | JSGS and NSQIP collaborative partnership agreement | |
| 2012 | Cancer DB (Breast, Pancreas) | |
| 2015 | Cancer DB (Liver) | |
| 2018 | Cancer DB (Stomach) | |
DB, database; JSGS, Japanese Society of Gastroenterological Surgery; NCD, National Clinical Database; NSQIP, National Surgical Quality Improvement Program.
Table 2.
Overview of NCD and ACS‐NSQIP
| NCD | ACS‐NSQIP | |
|---|---|---|
| Established year | 2011 | 1994 |
| Participating hospitals (n) | 5138a | 708b |
| % participating hospitals | ~60 | ~40 |
| No. of cases | >1.2 million cases/y | >1 million cases/y |
| Region | All 47 prefectures in Japan | 49 states and 15 countries |
| Data covering fields | General surgery | Surgery |
| Gastroenterological surgery | Pediatric surgery | |
| Cardiovascular surgery | Metabolic and Bariatric surgery | |
| Thoracic surgery | Breast surgery | |
| Breast surgery | Trauma surgery | |
| Pediatric surgery | ||
| Metabolic surgery | ||
| Thyroid surgery | ||
| Neurosurgery | ||
| Urology | ||
| Plastic surgery | ||
| Data input | Surgeon, data manager (no certification needed) | Trained surgical clinical reviewers |
| Cost of participation | 20 000‐150 000 JPY/y (according to no. of cases) | ~$30 000 USD/y |
| Education for data manager | NCD seminar biennially | SCR initial training session, ongoing education, and quality and safety conference |
| Audit | 5% hospitals, 20 cases/y | 5% hospitals, 20 cases/y |
| Management support | JSGS (Gastroenterological surgery) | ACS |
ACS‐NSQIP, American College of Surgeons National Surgical Quality Improvement Program; NCD, National Clinical Database; SCR, surgical clinical reviewers.
As of May 14, 2018.
As of July 2018.
The next development for ACS is to complete the ACS Data Platform Project, which aims to combine multiple ACS clinical registries including NSQIP, NSQIP‐peds, National Cancer Database (NCDB), Trauma Quality Improvement Program (TQIP), Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP), and Surgeon Specific Registry (SSR) into one integrated platform.
3. ESTABLISHMENT AND DEVELOPMENT OF NCD
No nationwide clinical data on surgical outcomes existed in Japan until JSGS formed a database committee in order to establish a nationwide surgical registry linked to the board certification system in 2006 (Table 1). NCD was established with support of the Japan Surgical Society (JSS) and JSGS and partially with support of a grant from the Ministry of Health, Labour and Welfare in 2010. The purpose of NCD was to systematically gather clinical information, analyze these data for quality improvement, follow the best medical practices, and maintain a high standard of care for all people in Japan. Prior to the establishment of NCD, the Japan Cardiovascular Surgery Database (JACVSD) was launched in 2000 to develop a nationwide database on cardiovascular surgery,7 which served as a large influence for the development of NCD. At that time, NSQIP was already established and operating, and the development of NCD was influenced by the surgical registry already established by NSQIP. In order to promote the ability for future collaboration between the USA and Japan, the NCD implemented the variables already being abstracted within NSQIP.2
National Clinical Database is now recognized as a fundamental registry linked to the board certification system in JSS and JSGS since 2016. Data registration is also one of the requirements for insurance coverage of new surgical techniques, such as laparoscopic advanced hepatobiliary pancreatic (HBP) surgery or robotics surgeries, which started in 2018. There are currently over 5000 institutions participating in the NCD and over 1.2 million cases per year have been registered between 2011 and 2017. NCD also began to gather additional information outside of the variables captured in NSQIP, including in‐hospital death, tumor node metastasis (TNM) classification for cancer, and other postoperative morbidities. Organ‐specific cancer registries have been established since 1952, but they were separately managed until recently where many of them were brought together on the same platform using the NCD system.8 This includes breast cancer registration of the Japanese Breast Cancer Society (2012‐), the Japan Pancreatic Cancer Registry (2012‐), and liver cancer registry by the Liver Cancer Study Group of Japan (2015‐) that were all integrated onto the same platform of web‐based registration system of the NCD. Other cancer registries such as the Japanese Lung Cancer Registry are now preparing to use the NCD system.
4. RELATIONSHIP OF ACS AND JSGS: FORMATION OF NCD WITH SUPPORT OF NSQIP
Collaboration between ACS and JSGS began when members of JSGS visited ACS in 2010 (H.M., M.G.) to learn about the NSQIP system in preparation for the creation of the national clinical registry for gastroenterological surgery. NCD was started using a three‐level platform for surgical registry (Figure 1). The first level includes fundamental data (date of birth, gender, procedure code, date of surgery, operator/assistants, simultaneous surgery, involvement of anesthesiologist, postoperative diagnosis, emergency/elective) from all surgical cases. The second level includes detailed information for the board certification system of the JSGS. Moreover, additional detailed data are entered for eight major gastroenterological procedures (esophagectomy, total and distal gastrectomy, right hemicolectomy, low anterior resection, hepatectomy, pancreaticoduodenectomy, and surgery for acute diffuse peritonitis), in which the data that are abstracted align with the NSQIP variables. Finally, the third level consists of more specific variables for advanced surgery, such as for hepatobiliary (HPB) surgery. In 2011, the JSGS and ACS collaboration was solidified and collaborative projects began using NCD and NSQIP with the unified goal of improving surgical quality in both countries. This successful collaboration was maintained with annual meetings and web conferences.
Figure 1.
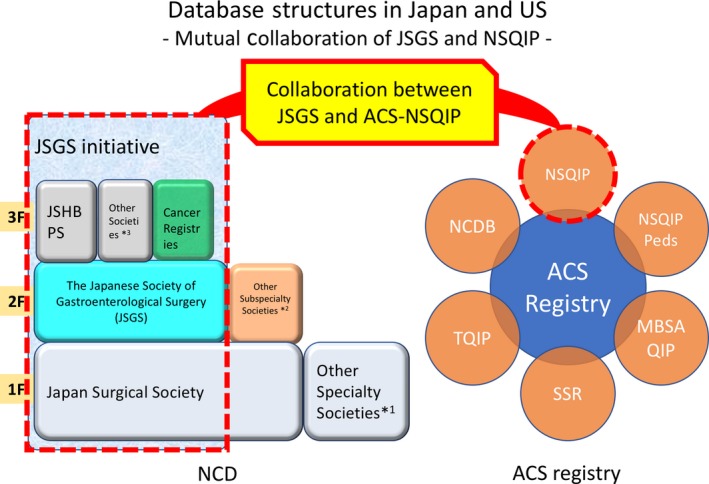
Database structures in Japan and the USA: Mutual Collaboration of Japanese Society of Gastroenterological Surgery (JSGS) and National Surgical Quality Improvement Program (NSQIP). A database structure in the National Clinical Database (NCD) has been built as stair‐like expert‐related complexity of each surgical society. NSQIP is part of the American College of Surgeons (ACS) registry. Collaboration projects between JSGS and ACS‐NSQIP are currently undertaken to further improve surgical quality in both countries. JSHBPS, Japanese Society of Hepato‐Biliary‐Pancreatic Surgery; NSQIP peds, National Surgical Quality Improvement Program pediatric; NCDB, National Cancer Database; TQIP, Trauma Quality Improvement Program; SSR, Surgeon Specific Registry; MBSAQIP, Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program. Other specialty societies*1 include The Japan Neurosurgical Society, The Japanese Urological Association, and Japan Society of Plastic and Reconstructive Surgery. Other subspecialty societies*2 include The Japanese Society for Cardiovascular Surgery, The Japanese Society for Vascular Surgery, The Japanese Society of Pediatric Surgeons, The Japanese Association for Thoracic Surgery, The Japanese Association for Chest Surgery, Japanese Breast Cancer Society, and Japanese Society of Thyroid Surgery. Other societies*3 include Japanese Gastric Cancer Association, The Japan Esophageal Society, and The Japan Society for Endoscopic Surgery
5. COLLABORATIVE STUDIES BETWEEN USA AND JAPAN
There are various aspects of health care between the USA and Japan that differ greatly between the two countries. There are system‐based differences such as insurance coverage, cost of treatment, and hospital volume, as well as patient population differences such as average age and body mass index (BMI).1, 9 Nevertheless, it is still possible to compare the outcomes as the same variables are used in gathering clinical data for each clinical registry and can be accounted for when creating models.
5.1. Thirty‐day Mortality Risk Model Study
The first collaborative study between NCD and NSQIP was a comparison of the risk models created for postoperative 30‐day mortality in major gastroenterological surgery.10 Risk calculators were created from both NCD and NSQIP individually, modeling 30‐day mortality in right hemicolectomy (RH), low anterior resection (LAR), and pancreaticoduodenectomy (PD).11, 12, 13, 14, 15 These calculators provided risk‐adjusted estimations for mortality in each case, which is essential for understanding factors leading to 30‐day mortality from each country.
This study involved two steps to accurately evaluate the predictive value of each risk calculator. The first step set out to establish the model, using the same predictive variables from NCD and NSQIP data to develop risk models for each procedure (RH, LAR, and PD). The second step involved determining the applicability of one country's model to the other country by exchanging the risk calculators developed using NCD and NSQIP and applying them to the other data set (NCD calculator in NSQIP dataset and vice versa). We found significant differences in the patient populations captured by NCD and NSQIP, especially in age, BMI class, and chronic kidney disease (CKD). Patients in Japan were older, had lower BMI, and had higher stages of renal dysfunction when compared to patients in the USA. There were also differences in length of hospital stay (LOS) with LOS being shorter in the USA in all three procedures studied (procedure, mean (IQR): RH 5 (4‐7) vs 14 (10‐20) days; LAR 6 (4‐8) vs 16 (12‐25) days; PD 9 (7‐14) vs 31 (22‐43) days), respectively.
Odds ratios for each of the risk factors in the NCD and NSQIP models were compared and appeared to be similar; however, further analysis with a Hosmer‐Lemeshow test demonstrated that the risk calculators created in Japan were not accurate in predicting mortality using the NSQIP data and the risk calculators created in the USA were not accurate in predicting mortality using the NCD data, thus demonstrating that local risk models were not accurate in predicting mortality using data from other countries. This was the first study that showed the feasibility and utility of international collaboration using nationwide clinical registries, but showed that local risk models remain important at this time.
5.2. Morbidity‐Mortality Study
Based on the results of the 30‐day Mortality Risk Model Study, future research is being directed towards studying the differences in patient demographics between the two countries, especially the differences in preoperative comorbidities and their association with postoperative outcomes. A current ongoing project includes comparing preoperative comorbidities between NCD and NSQIP and their association with differences in postoperative outcomes such as 30‐day mortality and morbidities in major gastroenterological surgeries (RH, LAR, hepatectomy and PD; Figure 2). We are now summarizing the results and interpretation of them.
Figure 2.
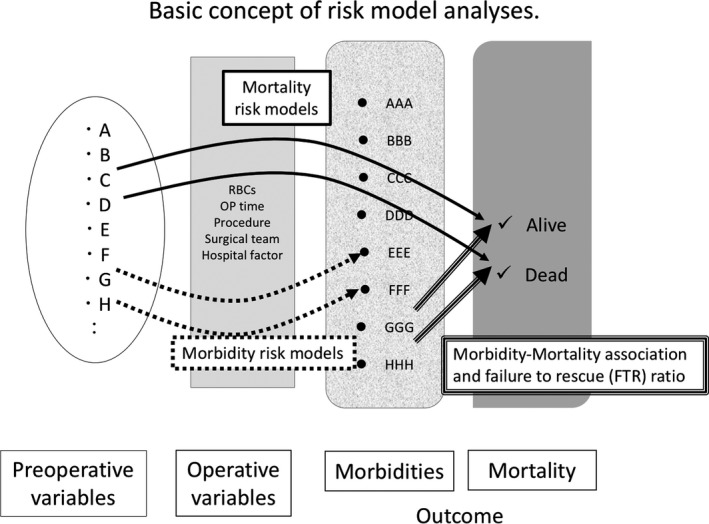
Basic concept of our risk model analyses. Association between preoperative and operative variables and postoperative morbidities and mortality. The risk models in each country will be compared
5.3. Japan‐USA Calibration Study
Another ongoing project within the JSGS and ACS collaboration involves validating a global model of the NSQIP risk calculator. NSQIP recently launched a universal risk calculator using a global model, in which all current procedural terminology (CPT) codes are included.16 We are planning to conduct a large‐scale validation study using the NSQIP risk calculator on NCD data, which will provide insight into the differences in expectations and outcomes between the USA and Japan. As we would have a very large sample from nearly all Japanese hospitals, this would be a country‐wide validation exercise. Analyses are being scheduled between NCD and NSQIP.
5.4. Geriatric Study
Surgery for the geriatric population is rapidly growing in both countries. In Japan, it is projected that people aged over 65 years will make up 33.3% of the population by 2036.17 The age distribution of patients who undergo major gastroenterological surgery is also changing rapidly. From 2011 to 2016, the rate of octogenarian or older individuals receiving surgery increased by as little as 4.3% in those undergoing LAR to 27.6% in those undergoing major hepatectomy.5 Given these data, it was important to conduct further research into the field of geriatric surgery using prospectively collected national data, which had not been previously done in Japan. The geriatric pilot study at the ACS began in 2015 with 24 hospitals enrolled, and data of more than 20 000 cases collected.18, 19, 20, 21, 22 NSQIP and a panel of experts in geriatrics established a set of standards for geriatric surgery and developed these standards into data variables for collection into the pilot study at participating hospitals. Additionally, a verification process was established that involved site visits to participating hospitals to check that these geriatric standards have been upheld. So far, several studies have been carried out and published in a geriatric pilot study in NSQIP.21, 23, 24 Similarly, the JSGS geriatric pilot prospective study was started with support from a Health and Labour Sciences Research Grant, and will gather data from 22 academic and community hospitals in 2018, and will be using similar variables to the geriatric pilot in NSQIP (Table 3). Data analyses and comparison between Japan and the USA will be conducted in 2019.
Table 3.
Geriatric variables for Japanese Society of Gastroenterological Surgery pilot study
| Preoperative |
| 1. Origin status from home |
| 2. Use of mobility aid |
| 3. Fall history |
| 4. History of dementia (cognitive status on admission) |
| 5. Not competent on admission |
| 6. From hospice upon admission |
| 7. Evidence of advanced care planning |
| Postoperative |
| 8. Postoperative pressure ulcer(s) |
| 9. Postoperative delirium |
| 10. New do‐not‐resuscitate (DNR) order during hospitalization |
| 11. Transition to postoperative hospice or comfort care |
| 12. Functional health status on day of discharge following surgery |
| 13. Fall risk on discharge |
| 14. Postoperative new use of mobility aid |
| 15. Hospital discharge (to home) with or without services |
| 30‐day postoperative |
| 16. Functional health status 30 days postoperatively |
| 17. Living location 30 days postoperatively |
| 18. Physical function comparing preoperative baseline to 30 days postoperatively |
5.5. Safety Culture Study
Patient safety culture is one of the key factors for quality improvement in surgery and within a health‐care system. A study by Sheetz et al25 showed that hospital safety characteristics influenced the rate of failure to rescue following major surgery. The Agency for Healthcare Research and Quality (AHRQ) released the Hospital Survey on Patient Safety Culture for providers and other staff to assess the patient safety culture in their hospitals. Since then, hundreds of hospitals across the USA and internationally have implemented the survey. In Japan, hospital safety culture has not been assessed or provided to health‐care workers in the field of surgery.
Through the ongoing collaboration between JSGS and ACS, another ongoing collaborative project involves comparison of safety culture and its impact on surgical outcomes. The safety culture survey questionnaires used by the ACS were translated into Japanese and turned into a web‐based survey that was sent to 2972 institutions and hospitals and answered by 1696 hospitals (57.1%) in 2016. The list of questions in the survey is provided in Table 4, and these data will be interpreted and analyzed in our ongoing collaboration.
Table 4.
List of questionnaires in safety culture in Japan and USA
| ACS‐NSQIP | JSGS | ||
|---|---|---|---|
| 2 | What is your role in the hospital? | JQ1 | What is your role in the hospital? |
| Surgeon Champion | Chief Surgeon | ||
| SCR | Surgeon in charge | ||
| Other | Data manager | ||
| Other (If other, specify: ____) | |||
| 3 | In what surgical quality programs does your hospital participate? | ||
| Perioperative quality and safety practices | |||
| 6 | If your hospital employs a surgical safety checklist (e.g. the WHO Safe Surgery Checklist or the Joint Commission Universal Protocol), how often is the checklist performed in a meaningful way (e.g. all intended participants cease other activities and are engaged)? | JQ39 | Do you employ a surgical safety checklist prior to surgery in the operating room (i.e. the WHO Safe Surgery Checklist)? |
| Always | Over 90% (most of the time) | ||
| Most of the time | Some of the time | ||
| Some of the time | We do not use a surgical checklist | ||
| Rarely | Unsure | ||
| We do not use a surgical checklist | |||
| Unsure | |||
| 8 | If your hospital has a protocol regarding prophylactic antibiotic redosing during surgery, how often is this protocol followed? | JQ18 | Does your hospital have a policy regarding routine preoperative administration of prophylactic antibiotics to prevent SSI? |
| Always | Yes | ||
| Most of the time | No | ||
| Some of the time | Unsure | ||
| Rarely | |||
| We do not use a surgical checklist | |||
| Unsure | |||
| 9 | If your hospital has a protocol regarding early urinary bladder (Foley) catheter removal after surgery, how often is this protocol followed? | JQ19 | Does your hospital have a policy in place regarding timely removal of urinary catheters after surgery to prevent UTI? |
| Always | Yes | ||
| Most of the time | No | ||
| Some of the time | Unsure | ||
| Rarely | |||
| We do not use a surgical checklist | |||
| Unsure | |||
| 10 | If your hospital has a protocol regarding routine administration of VTE prophylaxis, how often is the protocol followed? | JQ20 | Does your hospital have a policy regarding routine administration of VTE prophylaxis, including acceptable contraindications to VTE prophylaxis? |
| Always | Yes | ||
| Most of the time | No | ||
| Some of the time | Unsure | ||
| Rarely | |||
| We do not use a surgical checklist | |||
| Unsure | |||
| 11 | If your hospital has electronic order sets, how often are they used (vs bypassed or not used)? | JQ21 | “Smart order sets” are bundles of orders in an electronic medical record that can be standardized. They often include best‐practice measures such as VTE prophylaxis, timely Foley discontinuation post‐op, or early ambulation. Does your hospital use “smart order sets”? |
| Always used | Yes | ||
| Used most of the time | No | ||
| Used some of the time | Unsure | ||
| Rarely used | |||
| We do not use electronic order sets | |||
| Unsure | |||
| Surgical Quality Officer (SQO) | |||
| 17 | My hospital has a Surgical Quality Officer (SQO) or designated quality leader in the department of surgery. | JQ40‐1 | My hospital has a Surgical Quality Officer (SQO) or designated quality leader in the department of surgery |
| Yes | Yes | ||
| No | No | ||
| Unsure | Unsure | ||
| If you responded “No” or “Unsure,” please skip to question 22 | |||
| 18 | The SQO has had formal training in quality improvement method(s) (i.e. DMAIC, PDSA, Six Sigma). | JQ40‐2 | The SQO or designated quality leader has had formal training in quality improvement methods (i.e. DMAIC, PSDA, Six Sigma) |
| Yes, more than one of the above | Yes | ||
| Yes, one of the above | No | ||
| No | Unsure | ||
| Unsure | |||
| If you answered “yes,” please specify which quality improvement method(s) (DMAIC, PDSA, Six Sigma etc.) | |||
| 27 | Does your hospital have a formal overarching committee dedicated to overall surgical quality and safety? | JQ41‐1 | My hospital has one or more formal quality and/or safety committees. |
| Yes | Yes | ||
| No | No | ||
| Unsure | Unsure | ||
| If you responded “No” or “Unsure,” continue to question 30. | |||
| 28 | If yes, what providers are members of this committee? | JQ41‐2 | Across all quality/safety committees at your hospital, who participates? Please check all that apply. |
| Surgeons | Surgeons | ||
| RNs | RNs | ||
| Anesthesiologists | Anesthesiologists | ||
| Hospital administrators (CMO etc.) | Hospital administrators (CMO etc.) | ||
| Other | Other | ||
| Unsure | |||
| If other, please specify: | |||
| 33 | How frequently does your division conduct a morbidity & mortality conference? | JQ11 | Does your division conduct a morbidity & mortality conference? |
| Weekly | Yes | ||
| Less than weekly, more than monthly | No | ||
| Monthly | |||
| Less than monthly | |||
| 38 | Is there a formal process for ad hoc individual surgeon review when concerns are raised regarding a surgeon's professionalism or outcomes? | JQ13 | Is there a formal process for ad hoc individual surgeon review when concerns are raised regarding a surgeon's professionalism or outcomes? |
| Yes | Yes | ||
| No | No | ||
| Unsure | |||
ACS‐NSQIP, American College of Surgeons National Surgical Quality Improvement Program; CMO, chief medical officer; DMAIC, Define, Measure, Analyze, Improve and Control; JSGS, Japanese Society of Gastroenterological Surgery; PDSA, Plan‐Do‐Study‐Act; RN, registered nurse; SCR, surgical clinical reviewer; SSI, surgical site infection; UTI, urinary tract infection; VTE, venous thromboembolism; WHO, World Health Organization.
5.6. Audit for data verification
National Clinical Database and NSQIP are both web‐based clinical registries that are very similar in many aspects. In both NCD and NSQIP, data are entered online in a Health Insurance Portability and Accountability Act (HIPAA)‐compliant, secure, web‐based platform that can be accessed 24 hours a day. Blinded, risk‐adjusted information is then shared with all hospitals, allowing them to nationally benchmark their complication rates and surgical outcomes. NSQIP provides auditing to ensure data reliability and, in the NCD, only the first level of clinical data was initially targeted for auditing in the field of gastroenterological surgery. Audits for second‐level clinical data in NCD were then implemented in 2015 for 2014 data, following the same strategy used by NSQIP, where NCD selects 5% of participating institutions with JSGS accreditation (from n = 853) and checks 45 items including 27 items for evaluation of discrepancy. A rate of <5% discrepancy is accepted, and audit results were provided to each institution. In 2015 data, all 45 hospitals audited passed the audit with <5% discrepancy.26
6. FUTURE PERSPECTIVES AND SUMMARY
Establishment and development of the NCD was largely influenced by American College of Surgeons National Surgical Quality Improvement Program (ACS‐NSQIP), sharing the same goal of providing the best medical care and maintaining a high standard of care for all patients. Although there are substantial differences in the health‐care systems between Japan and the USA, the level of surgical quality and safety within each country is among the best in the world and would serve as a good model for other countries to emulate.
In Europe, there are surgical data registries such as Eurostat (https://ec.europa.eu/eurostat/web/main/home) or the European Society of Thoracic Surgery Database (ESTSD),27 but they use restricted variables or different formats compared with NCD/NSQIP. Although European counterparts of NCD/NSQIP are not present, it might be possible to compare basic characteristics among international registries such as demography, length of hospital stay, and mortality after specific procedures, which would be very interesting and important as a future project.
Through participating in large nationwide registries such as NCD and NSQIP, the risk‐adjusted mortality and morbidity rate in both countries has decreased significantly after only a few years of participation, suggesting that the use of large data sets in health care is effective at improving surgical care and is important for the future. Moreover, we are facing increasing obstacles in the future of health care with an aging society and high costs of medical care, and participating in continued efforts such as these to improve surgical quality will help us overcome these challenges.
The collaboration between JSGS and ACS on these joint projects will continue to provide us with important information and data to drive improvements in surgical care. The data from the risk calculators project will provide us with an internationally validated calculator and will also help us determine specific features of surgical care that impact each country differently. This will also help us to identify any unknown weaknesses in the health‐care systems of the USA and Japan. Our collaborative partnership between JSGS and ACS remains strong, and we look forward to continuation of the important work being done with reviews of the studies as these two organizations work together to improve surgical care on an international basis.
DISCLOSURE
Funding: This work was supported with a grant from the Health Labour Sciences Research Grant (201119010B, 201221064A, 201708014A), and Grant‐in‐Aid for Scientific Research (C) (16K10437).
Conflicts of Interest: Authors declare no conflicts of interest for this article.
Marubashi S, Liu JY, Miyata H, Cohen ME, Ko CY, Gotoh M. Surgical quality improvement programs in Japan and USA: Report from the collaborative projects between Japanese Society of Gastroenterological Surgery and American College of Surgeons National Surgical Quality Improvement Program. Ann Gastroenterol Surg. 2019;3:343–351. 10.1002/ags3.12250
Funding information
This work was supported with a grant from the Health Labour Sciences Research Grant (201119010B, 201221064A, 201708014A), and Grant‐in‐Aid for Scientific Research (C) (16K10437).
REFERENCES
- 1. Miyata H, Gotoh M, Hashimoto H, Motomura N, Murakami A, Tomotaki A, et al. Challenges and prospects of a clinical database linked to the board certification system. Surg Today. 2014;44:1991–351. [DOI] [PubMed] [Google Scholar]
- 2. Gotoh M, Miyata H, Hashimoto H, Wakabayashi G, Konno H, Miyakawa S, et al. National Clinical Database feedback implementation for quality improvement of cancer treatment in Japan: from good to great through transparency. Surg Today. 2016;46:38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hall BL, Hamilton BH, Richards K, Bilimoria KY, Cohen ME, Ko CY. Does surgical quality improve in the American College of Surgeons National Surgical Quality Improvement Program: an evaluation of all participating hospitals. Ann Surg. 2009;250:363–76. [DOI] [PubMed] [Google Scholar]
- 4. Ingraham AM, Richards KE, Hall BL, Ko CY. Quality improvement in surgery: the American College of Surgeons National Surgical Quality Improvement Program approach. Adv Surg. 2010;44:251–67. [DOI] [PubMed] [Google Scholar]
- 5. Kakeji Y, Takahashi A, Udagawa H, Unno M, Endo I, Kunisaki C, et al. Surgical outcomes in gastroenterological surgery in Japan: Report of National Clinical database 2011‐2016. Ann Gastroenterol Surg. 2018;2:37–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ko CY, Paruch JL, Hoyt DB. Achieving high‐quality surgical care: observations from the American College of Surgeons Quality of Care Programs. Ann Surg. 2015;261:240. [DOI] [PubMed] [Google Scholar]
- 7. Marubashi S, Nagano H, Eguchi H, Wada H, Asaoka T, Tomimaru Y, et al. Minimum graft size calculated from preoperative recipient status in living donor liver transplantation. Liver Transpl. 2016;22:599–606. [DOI] [PubMed] [Google Scholar]
- 8. Anazawa T, Miyata H, Gotoh M. Cancer registries in Japan: National Clinical Database and site‐specific cancer registries. Int J Clin Oncol. 2015;20:5–10. [DOI] [PubMed] [Google Scholar]
- 9. Anell A, Willis M. International comparison of health care systems using resource profiles. Bull World Health Organ. 2000;78:770–8. [PMC free article] [PubMed] [Google Scholar]
- 10. Anazawa T, Paruch JL, Miyata H, Gotoh M, Ko CY, Cohen ME, et al. Comparison of National Operative Mortality in Gastroenterological Surgery using web‐based prospective data entry systems. Medicine (Baltimore). 2015;94:e2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kobayashi H, Miyata H, Gotoh M, Baba H, Kimura W, Kitagawa Y, et al. Risk model for right hemicolectomy based on 19,070 Japanese patients in the National Clinical Database. J Gastroenterol. 2014;49:1047–55. [DOI] [PubMed] [Google Scholar]
- 12. Matsubara N, Miyata H, Gotoh M, Tomita N, Baba H, Kimura W, et al. Mortality after common rectal surgery in Japan: a study on low anterior resection from a newly established nationwide large‐scale clinical database. Dis Colon Rectum. 2014;57:1075–81. [DOI] [PubMed] [Google Scholar]
- 13. Kimura W, Miyata H, Gotoh M, Hirai I, Kenjo A, Kitagawa Y, et al. A pancreaticoduodenectomy risk model derived from 8575 cases from a national single‐race population (Japanese) using a web‐based data entry system: the 30‐day and in‐hospital mortality rates for pancreaticoduodenectomy. Ann Surg. 2014;259:773–80. [DOI] [PubMed] [Google Scholar]
- 14. Cohen ME, Bilimoria KY, Ko CY, Hall BL. Development of an American College of Surgeons National Surgery Quality Improvement Program: morbidity and mortality risk calculator for colorectal surgery. J Am Coll Surg. 2009;208:1009–16. [DOI] [PubMed] [Google Scholar]
- 15. Parikh P, Shiloach M, Cohen ME, Bilimoria KY, Ko CY, Hall BL, et al. Pancreatectomy risk calculator: an ACS‐NSQIP resource. HPB (Oxford). 2010;12:488–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bilimoria KY, Liu Y, Paruch JL, Zhou L, Kmiecik TE, Ko CY, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217:833–42. e831‐833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. National Institute of Population and Social Security Research . Population Projections for Japan: 2016 to 2065. http://www.ipss.go.jp/pp-zenkoku/e/zenkoku_e2017/pp_zenkoku2017e.asp. Accessed April 9, 2019.
- 18. Robinson TN, Rosenthal RA. The ACS NSQIP Geriatric Surgery Pilot Project: improving care for older surgical patients. Bull Am Coll Surg. 2014;99:21–3. [PubMed] [Google Scholar]
- 19. Mohanty S, Rosenthal RA, Russell MM, Neuman MD, Ko CY, Esnaola NF, et al. Optimal perioperative management of the geriatric patient: a best practices guideline from the American College of Surgeons NSQIP and the American Geriatrics Society. J Am Coll Surg. 2016;222:930–47. [DOI] [PubMed] [Google Scholar]
- 20. Berian JR, Zhou L, Russell MM, Hornor MA, Cohen ME, Finlayson E, et al. Postoperative delirium as a target for surgical quality improvement. Ann Surg. 2017;268(1):93–351. [DOI] [PubMed] [Google Scholar]
- 21. Berian JR, Zhou L, Hornor MA, Russell MM, Cohen ME, Finlayson E, et al. Optimizing surgical quality datasets to care for older adults: lessons from the American College of Surgeons NSQIP Geriatric Surgery Pilot. J Am Coll Surg. 2017;225:702–12. e701. [DOI] [PubMed] [Google Scholar]
- 22. Wang X, Hu Y, Zhao B, Su Y. Predictive validity of the ACS‐NSQIP surgical risk calculator in geriatric patients undergoing lumbar surgery. Medicine (Baltimore). 2017;96:e8416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Berian JR, Mohanty S, Ko CY, Rosenthal RA, Robinson TN. Association of loss of independence with readmission and death after discharge in older patients after surgical procedures. JAMA Surg. 2016;151:e161689. [DOI] [PubMed] [Google Scholar]
- 24. Berian JR, Zhou L, Russell MM, Hornor MA, Cohen ME, Finlayson E, et al. Postoperative delirium as a target for surgical quality improvement. Ann Surg. 2018;268:93–351. [DOI] [PubMed] [Google Scholar]
- 25. Sheetz KH, Dimick JB, Ghaferi AA. Impact of hospital characteristics on failure to rescue following major surgery. Ann Surg. 2016;263:692–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kanaji S, Takahashi A, Miyata H, Marubashi S, Kakeji Y, Konno H, et al. Initial verification of data from a clinical database of gastroenterological surgery in Japan. Surg Today. 2018;49(4):328–33. [DOI] [PubMed] [Google Scholar]
- 27. Agzarian J, Shargall Y. Beyond borders‐international database collaboration in thoracic surgery. J Thorac Dis. 2018;10:S3521–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


