Abstract
Approximately half of patients with signs and symptoms of heart failure have a left ventricular ejection fraction that is not markedly abnormal. Despite the historically initial surprise, heightened risks for HF specific major adverse events occur across the broad range of ejection fraction, including normal. The recognition of the magnitude of the problem of HFpEF in the past 20 years has spurred an explosion of clinical investigation and growing intensity of informative outcome trials. This manuscript addresses the historic development of this component of the heart failure syndrome, including the epidemiology, pathophysiology, and existing and planned therapeutic studies. Looking forward, more specific phenotyping and even genotyping of subpopulations should lead to improvements in outcomes from future trials.
Keywords: heart failure, ejection fraction, cardiovascular epidemiology, pathophysiology, clinical trials
Introduction
Heart failure (HF) was undoubtedly a major contributor to the centuries old edematous condition, dropsy. As HF was recognized as a leading cause of edema and dyspnea, the pathophysiologic role of the heart also emerged as is evident from the 1933 Lewis textbook definition of HF “a condition in which the heart fails to discharge its contents adequately.”1 With the advent of hemodynamic measurements, it became clear that congestion, the core of the HF syndrome regardless of the etiology, was due to elevated cardiac filling pressures. The more current HF definition “an inability of the heart to pump blood to the body at a rate commensurate with its needs, or to do so only at the cost of high filling pressures,” prominently adds this crucial aspect.2
While this definition provides a reference standard against which other definitions can be compared, it is much less transferable to clinical bedside practice where left ventricular end diastolic pressure is not directly measured. Multiple approaches have been utilized to clinically define the syndrome of HF based on an integration of the patient’s history, presentation, physical examination and laboratory supportive findings to assess whether HF is present, each with its own advantages and disadvantages (Table 1).3, 4 As in many aspects of medicine, there is a range of diagnostic certainty regarding the time-honored constellation of signs and symptoms attributed to HF. Dyspnea, for example, a critical component of HF can also be a central manifestation of pulmonary disease rather than an aspect of impaired cardiac performance.5 The judgment and experience needed to integrate the information incorporated in the term HF challenges its diagnostic precision.
Table 1:
Controversies in the Definitions of HFpEF
| Definition | Advantages | Disadvantages |
|---|---|---|
| Braunwald Definition* | • Fits the a priori definition of cardiac failure • Objective, relies upon measurable quantities |
• Definitive measurements require invasive assessment with exercise |
| Framingham Criteria | • Well-validated and widely accepted • High specificity |
• Poorly sensitive • Requires multiple findings of right heart failure that are often restricted to patients with advanced HF |
| Hospitalization for HF | • Unequivocal event of interest • Prognostic |
• Many HFpEF patients are never hospitalized • May be confused with symptoms due to non-cardiac etiologies |
| Natriuretic Peptides | • Widely available • Easy to measure from blood samples • Prognostic |
• Many patients with proven HFpEF have normal levels |
| ICD Coding | • Pragmatic definition of primary caregiver | • Misdiagnosis or lack of diagnosis compromise sensitivity and specificity |
| Echocardiography | • Widely available • EF and diastolic function readily measurable |
• Measurement variability may be high • Normal EF does not mean normal function • Diastolic dysfunction common without HF |
| Consensus Guidelines | • Based upon expert opinion • Generally incorporate components from the definitions above |
• Lack of validation against gold standard • Poor sensitivity • Often difficult to apply |
Defined as “An inability of the heart to pump blood to the body at a rate commensurate with its needs, or to do so only at the cost of high filling pressures.”
Despite the ambiguities and overlaps with other chronic conditions, the unquestionable multifold higher risk for cardiovascular death as well as subsequent repeat exacerbations of symptoms requiring hospitalizations for HF management of those with this clinically determined diagnosis, offers firm validation that the term has important specificity and meaning. Indeed, the linkage between this longstanding clinical usage and heightened risks for HF specific major adverse events underscores that despite the seemingly inexact diagnosis, HF denotes a most grave and identifiable medical disorder.6
The adverse prognostic impact of a diagnosis of HF on an individual level is greatly amplified on a population basis since its associated risk is matched by the relatively high prevalence of HF in older individuals.7 In the Medicare population, HF is among the most frequent reason to prompt an urgent hospitalization. The mortality occurring during a hospitalization for HF is approximately 4%.8, 9 Despite major advances in HF management and care, mortality within 1 month after discharge is 10%.10 Since 1 in 5 men and women over 40 years of age will develop HF during their lifetime, the public health burden and cost to society are staggering.11 These sobering statistics utilize the bedrock clinical definition of the treating physicians for these assessments of the morbidity mortality and economic impact of a diagnosis of HF.3, 4 It is important to note, that neither the etiology nor any measure of the extent of cardiac dysfunction are incorporated into the broad overall definition or adverse impact of a diagnosis of HF.
Left Ventricular Ejection Fraction (LVEF):
The assessment of the fraction of the left ventricular volume ejected per beat (LVEF) was introduced as a measurement to “permit a more complete understanding of the activity of the left ventricle in normal subjects and in patients with a variety of cardiovascular abnormalities.”12 Even in this early quantitative study, those with overt cardiac disease ejected a lower proportion of their left ventricular volume with each contraction. LVEF was shown to both pre and afterload dependent and as such, there were other invasive measurements that better reflected ventricular contractility.13 Perhaps because of convenience and ease of noninvasive assessment, despite this load dependent shortcoming, LVEF became the leading term clinicians used to characterize the left ventricular function of their patients.
Diastolic HF:
The repeated observations that despite the same signs and symptoms, a proportion of those with HF did not have major overt reductions in systolic function led to the recognition that HF could also be the consequences of abnormalities in diastole. Initially termed diastolic HF, supporting hemodynamic studies demonstrated high filling pressures without marked increases in ventricular chamber size.14 The hallmark leftward shifted ventricular pressure-volume relationship indicated that some intrinsic or extrinsic constraining force was impeding ventricular filling rather than emptying.15 Pericardial disorders, by limiting ventricular filling, also shift both right and left ventricular pressure volume curves upward or to the left producing a similar congestive presentation. However, when clearly attributed to the pericardium it is not mechanistically generally considered HF. Rather, diastolic HF implies that there is a myocardial process impairing filling. This can be either passively from increased ventricular stiffness and/or actively from slower relaxation. A variety of abnormalities in cardiac structure and/or function, including an increase in intrinsic myocardial stiffness of the myocytes and extracellular collagen matrix, infiltrative disorders, or abnormalities in the cardiac and systemic microvasculature are associated with abnormalities in filling. Ventricular relaxation was shown to be an active process with ischemia adversely impacting filling.16 As recognition increased about the occurrence of diastolic HF, the markedly concentrically hypertrophied left ventricle with small to normal cavity volumes (increased mass to volume ratio) and maintained systolic function became the expected trademark feature of diastolic HF.17 However, the magnitude of the problem was yet to be generally appreciated.
It was only with the more widespread use of non-invasive assessments of ventricular function by the 1980s that it become better appreciated that congestive HF was not synonymous with reduced ejection fraction. In an early report of echocardiograms from patients with HF, the investigators were clear to state “neither history, physical examination or even chest radiographic findings were able to discern patients with normal versus the abnormal reduced ejection fraction.”18 Despite this emerging recognition that the constellation of signs and symptoms justifying the diagnosis of HF could be present in patients with an apparently normal ejection fraction, the magnitude of the problem was not initially adequately appreciated. In the mid-1980s, two independent though similar reports from respected nuclear cardiology laboratories raised the profile of diastolic HF from the occasional case report to the justified not to be ignored important component of the HF population.19, 20 Both evaluated consecutively referred patients with a clinical diagnosis of HF. Of 188 patients reported by Dougherty, 67 (36%) had an ejection fraction <45 % and even this early study specifically showed that there was no relationship between the ejection fraction and functional class (NYHA).19 Soufer identified 58 patients with a clinical diagnosis of HF referred to their nuclear laboratory for a quantitation of ejection fraction and found a value greater than 45% in 42% of the referrals during a 3-month sampling period.20
There were however still lingering doubts that those found to have signs and symptoms of HF with an apparently normal ejection fraction might have had transient systolic dysfunction, which was responsible for the clinical presentation. This concept was relegated to unlikely by an important study of paired measurements of ejection fraction, the first during the acute presentation in decompensated HF and the second following treatment and recovery from the pulmonary edema.21 The similar measurements of ejection fraction despite the marked difference in clinical status refocused attention to mechanisms producing elevated filling pressures with apparently adequate systolic function. With this acceptance of diastolic HF as an important entity, there was a greater emphasis on finding mechanisms and noninvasive indices of diastolic dysfunction.
Taxonomy Diastolic HF to HF with preserved Ejection Fraction (HFpEF)
In the 1980’s and 90’s, the term congestive HF was widely used to encompass all those with the clinical signs and symptoms of HF. The epidemiologic studies had yet to reveal the magnitude of the extent of those with no or minimal apparent abnormalities in ventricular ejection. Diastolic HF aptly identified this understudied, poorly understood cohort of patients.15 In this same era, major randomized clinical trials testing therapies with all-cause mortality as a primary outcome were just beginning to address patients with congestive HF. In fact, neither of the first two of these trials, the Veterans Administration Cooperative Study (V-HeFT) and Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS) specifically excluded those with what would be considered diastolic HF.22, 23 The V-HeFT investigators subsequently compared the 13% of their patients with an ejection fraction (EF) over 45% (mean 54%), to the majority with low EF (mean 26%) and reported only a minor difference in peak exercise oxygen consumption but a clearly lower rate of death in those with the more normal EF.24 This observation had important repercussions on the subsequent early mortality HF trials, which then used an arbitrary upper limit of EF above 35 or 40% as an exclusion to attempt to make sample size projections more practical.25–27 Around that time based on encouraging surrogate outcome studies, oral positive inotropic agents appeared to be a promising therapeutic breakthrough. With the exception of a subgroup in the DIG trial,28 those with diastolic HF were also understandably excluded from these mortality outcome trials.29–31 As clinical trial experience accumulated, the rates of death from the completed trials with an upper EF exclusion served as the foundation for sample size calculations of future randomized controlled clinical trials (RCTs).
The introduction of angiotensin receptor blockers (ARB), as an approved antihypertensive therapy with theoretical additional properties considered beneficial for patients with HF spurred investments in major outcome trials.32, 33 Evaluation of Losartan in the Elderly (ELITE II) directly tested whether the ARB losartan would have a superior influence on survival compared to the angiotensin converting enzyme inhibitor (ACEi) captopril on all-cause mortality in HFrEF.34 Despite the anticipated lower withdrawal rate in the ARB group, there was no advantage on rates of death.34 The Candesartan in HF Assessment of Reduction in Mortality and morbidity (CHARM) was a broader program of three distinct but complementary populations of patients based on ACEi use and LVEF, each independently assessing the potential role of candesartan on the composite outcome of cardiovascular death and/or hospitalization for HF in its unique population.35 In those with an EF ≤40%, CHARM ADDED addressed whether there was an additional benefit of adding the ARB to those on an ACEi (“ADDED), while CHARM ALTERNATIVE tested whether the ARB would improve prognosis in patients that had previously been considered intolerant of an ACEi.36, 37 The third component of this concomitant trial program selected those with the same clinical diagnosis of HF but with an EF >40%.38 This level was specifically chosen because it identified a large group of patients with HF without prior outcome trial directed evidence for treatment. As such, placebo was the appropriate comparator to the ARB.
From a historic perspective, this 1997 designed trial reflected the HF clinical climate at the end of the last century.39 Although by this time there was a general appreciation that a substantial proportion of patients with symptomatic HF did not necessarily have a low EF, except for a subgroup in the DIG trial, this higher EF group was not represented in prior outcome RCTs. Accordingly, international guidelines lacked specific evidence to base therapeutic recommendations.40, 41 Moreover, neither event rates nor extensive experiences in identifying sufficient number of these subjects were available from prior international trials. These key issues which had to be addressed in the CHARM protocol, engendered many lively pre-trial discussions amongst the study leadership.
The presumed structural and functional cardiac properties of those with diastolic HF were considered for eligibility criteria. However, aside from the impractical direct measure of ventricular filling pressure, the non-invasive indices of diastolic function did not offer adequate discrimination for identifying patients for this large outcome trial.42 The current trial’s objective was to determine whether the ARB would reduce rates of cardiovascular and hospitalization for HF in a population where there were no current evidenced-based recommendations. Therefore, the pragmatic approach of using the EF >40% for entry was adopted since this was measured in all patients, and was in fact the discriminating criteria between the well characterized patients in prior trials.38 Adopting the specific cut point of EF >40% was based on addressing this therapeutic void rather than a mechanistic distinction. This was particularly practical in this specific investigative program since it addressed the unmet need and allowed the concomitant randomization of the broadest population of patients with HF regardless of EF. Based on how these patients were being identified for the trial, the investigators designated this component trial of those with EF >40% as CHARM-Preserved. The term was to distinguish from the well-studied lower EF groups and in no way meant to imply normal left ventricular structure or function.
Although this was clearly not the first use of the term HF with preserved ejection fraction,43 the publication of a major outcome trial using that terminology was associated with an upsurge in its usage (Figure 1). As greater attention was focused on this segment of patients with HF, the acronym HFpEF was rapidly adopted.44 Although there are many demographic and cardiac structural and functional differences between those considered to have HF based on predominantly diastolic or systolic disorders, EF continues to be used as the distinguishing feature.
Figure 1.
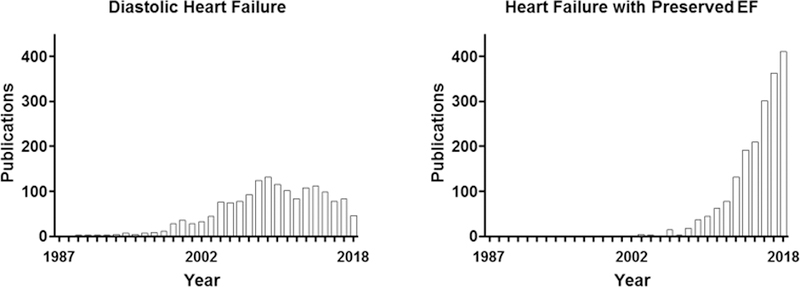
PubMed citations for heart failure with preserved ejection fraction from 1986 to 2018.
There has been a recent expansion of the EF derived categories of patient with the clinical diagnosis of HF. HF with midrange EF 40 to 49% (HFmrEF) describes a group with sub normal EF who were for the most part not included in the in the prior outcome trials.4, 45 Others have proposed recovered EF (HFrecEF) to designate a group that now has EF ≤ 50% but was known to have a lower value previously.46 The EF names continue with the term improved (HFiEF) for those that were previously <40% and have any apparently meaningful increase in EF even if still below the arbitrary 50% level for HFrecEF.47 Although clinically ascertained, EF has an expected measurement error, these additions to nomenclature highlight that the clinical presentation of HF occurs across a spectrum of current and prior EF levels. Although diastolic HF remains an important term, the pragmatic use of EF is based on linkage to clinical outcome trial data. The importance of any of these multiple EF designations of patients with the clinical diagnosis of HF will await coupling of a benefit or even risk of a specific therapeutic intervention.
Epidemiology of HFpEF
Assessment of HFpEF in Epidemiologic Studies
As a clinical syndrome, HF ascertainment in epidemiologic studies is challenging (Table 1). Ascertainment has typically relied on physician diagnostic codes with or without additional medical record abstraction and adjudication (Table 2). Diagnostic codes are readily and consistently available, but may be influenced by non-medical factors such a re-imbursement incentives. Additional adjudication helps mitigate such effects, improves consistency of the HF designation, and can more accurately assess whether HF is the cause of hospitalization or a co-existing condition. Several criteria exist for HF adjudication, including – but not limited to – the Framingham, Boston, Gothenburg, and ESC criteria.4, 48–51 All rely on some combination of symptoms, physical exam findings, radiographic findings, medical history, and response to therapy. Importantly, none include LVEF as a criteria. The most commonly employed criteria in epidemiologic studies of HFpEF has been the Framingham criteria, or a variant thereof.48 While the Framingham criteria demonstrates good specificity for HF diagnosis, its sensitivity appears particularly limited in elderly persons and HF patients without acute decompensation52 – the pattern of HF patients frequently encountered in epidemiologic cohort studies (Table 1).
Table 2.
Definitions of HFpEF employed in key epidemiologic studies of HFpEF prevalence and prognosis
| Study | Cohort | Date | LVEF cutpoint | HFpEF diagnosis |
|---|---|---|---|---|
| Kupari et al. | Helsinki Ageing Study | 1997 | FS ≥0.25 | • At least 3 of the following: (1) history of breathlessness on ordinary effort; (2) audible ventricular gallop sound or HR >90 bpm at rest; (3) pulmonary venous congestion on CXR (consensus of 2 observers) or abnormal neck vein distention or palpable hepatomegaly; (4) cardiothoracic ratio >0.55 on CVR • FS from protocol echocardiogram at study visit |
| Senni et al. | Olmsted County | 1998 | ≥50% | • Modified Framingham criteria • LVEF from abstraction of medical record from within 3 weeks of HF diagnosis |
| Vasan et al. | FHS | 1999 | ≥50% | • Framingham criteria • LVEF from protocol echocardiogram at FHS study visit |
| Devereux et al. | SHS | 2000 | >54% | • Modified Framingham Criteria • LVEF from protocol echocardiogram at SHS study visit |
| Kitzman et al. | CHS | 2001 | Qualitative as ‘normal’; | • Expert panel adjudication based on review of pertinent data on hospitalization or outpatients visits for CHF, including history, physical examination, chest x-ray reports, and medication administration. Self-report of a physician diagnosis of CHF was confirmed by documentation in the participant’s medical records of a constellation of symptoms (dyspnea, fatigue, orthopnea, paroxysmal nocturnal dyspnea), and physical signs (edema, pulmonary rales, gallop rhythm, displaced LV apical impulse), and by supporting clinical findings such as those of the chest x-ray, and medical therapy including diuretic and digitalis or a vasodilator. • Qualitative assessment of systolic function from protocol echocardiogram at CHD study visit |
| Gottdiener et al. | CHS | 2002 | ||
| Bursi et al. | Olmsted County | 2006 | ≥50% | • Framingham criteria • Prospective study-specifc echocardiogram |
| Owan et al. | Mayo Clinic | 2006 | ≥50% | • ICD/DRG discharge codes (validated against Framingham criteria in a subset) • LVEF from clinical echocardiogram performed within 30 days of HF hospitalization |
| Bhatia et al. | EFFECT study | 2006 | >50% | • Framingham criteria • LVEF abstracted from HF hospitalization |
| Lam et al. | FHS | 2011 | >45% | • Framingham criteria • LVEF assessed within 1 year of HF hospitalization (without intervening event) |
| Gerber et al. | Olmsted County | 2015 | >50% | • ICD codes from hospital discharges or outpatient visits (validated against Framingham criteria in a subset); • LVEF assessed at Mayo clinical by echocardiography w/in 90 days of HF diagnosis |
The designation of HF subgroups by LVEF is epidemiologic studies is relatively recent (Table 2). HFpEF is typically defined by first making the clinical assessment of HF as above, which is then coupled with an LVEF assessed as ≥45 or 50% (Table 2). I In some studies, LVEF information is based on abstracted cardiac imaging results (e.g. echocardiography, nuclear imaging, angiography, MRI) from the time of HF diagnosis. The close temporal linkage between HF event and LVEF assessment is an important strength of this approach, while missing LVEF data is a limitation as LVEF is often not assessed at the time of HF diagnosis (e.g. 11 to 37% of incident cases).53–56 Another approach commonly employed in studies of HFpEF prevalence in longitudinal cohorts has been to use an LVEF assessed uniformly as part of the study protocol but performed a variable amount of time after that actual HF diagnosis.42, 57, 58 While the ability to classify all HF cases with respect to LVEF is a strength of this approach, the non-uniform lag between HF diagnosis and LVEF assessment is a limitation. Although there are concerns about longitudinal changes, LVEF is generally stable over the short to medium term unless there was an interval myocardial infarction.21, 59
Population-Based Studies of HFpEF Prevalence
For approximately a decade following the initial descriptions of HFpEF in the early to mid-1980s, published studies on HFpEF were either case series or comparative clinical studies among prevalent HF patients.60 These studies resulted in significant variability in estimates of HFpEF prevalence and prognostic relevance. In 1997, a report from the Helsinki Ageing Study of a random sample of 501 persons born in 1904, 1909, and 1914 demonstrated preserved LV systolic function – based on a fractional shorting ≥0.25 – in 51% of prevalent cases.61 Over the next 5 years, multiple diverse community based cohorts reported on HFpEF prevalence. These included the Framingham Heart Study (largely white general population),57 the Strong Heart Study (Native Americans),42 and the Cardiovascular Health Study (persons in late life),58, 62 and each confirmed that HFpEF accounts for a large proportion (51 to 63%) of HF cases in the community. These findings are also concordant with data from consecutive hospitalized patients,63 and from several large HF registries.64–66 Importantly, HFpEF ascertainment in all of these studies was based solely on the combination of HF – assessed based largely on clinical signs and symptoms – and an LVEF threshold, without additional data regarding diastolic function or atrial or ventricular structure.
Population-Based Studies of HFpEF Incidence
Studies of incident HF in community-based cohorts suggest that HFpEF accounts for approximately 40–50% of incident HF overall. In a study of all incident HF cases in Olmsted County, MN in 1991, 43% of cases with LVEF assessed had an LVEF ≥50%.53 Similarly, longitudinal follow-up of HF-free persons in the PREVEND cohort, the Framingham Heart Study, and the Cardiovascular Health Study demonstrated HFpEF accounts for 37–53% of incident HF.67 Higher proportions of incident HFpEF were observed in studies with older participants. The proportion of incident HF due to HFpEF also appears to be increasing. An analysis of HF cases evaluated at the Mayo Clinic between 1987–2001 demonstrated an increasing proportion of HFpEF patients over time, particularly among HF patients evaluated from the community.54 These findings are consistent with a broader analysis of all incident inpatient and outpatient HF cases in Olmsted County, MN occurring over the 10 year period of 2000–2010.68 Of 2,762 incident HF cases, HFpEF accounted for 53% of incident HF cases overall. While a modest increase in the proportion of HFpEF cases was observed over time, overall incidence of HF decreased during the study period by 37%. However, the magnitude of this decline was less for HFpEF (28% decrease) compared to HFrEF (45% decrease), and women in particular demonstrated a much greater reduction in incident HFrEF compared to incident HFpEF. In contrast to these data on all HF, a recent analysis of hospitalizations for acute decompensated HF in 4 U.S. communities between 2005–2014 through the ARIC Community Surveillance study demonstrated rising rates of hospitalization for acute decompensated HF, driven primarily by increasing rates of hospitalization for HFpEF.69
Demographics and Clinical Co-Morbidities in HFpEF
The clinical syndrome of HFpEF develops from a complex interaction of a number of risk factors that cause organ dysfunction and ultimately clinical symptoms (Figure 2). Since its initial description, older age and higher prevalence of women have been recognized as more frequent in HFpEF compared to HFrEF, and have been confirmed repeatedly in epidemiologic studies of prevalent and incident HFpEF. Recent data from Olmsted County suggest that persons developing HFpEF to be ~6 years older on average than those developing HFrEF,68 and HFpEF accounts for a higher proportion of incident HF cases in older persons. Similarly, the prevalence of female gender is approximately 30–40% higher in prevalent HFpEF compared to HFrEF in epidemiologic cohort studies.42, 57, 62 Not only does HFpEF account for a higher proportion of incident HF in women compared to men, but the incidence of HFrEF appears particularly low in women and has fallen more rapidly over time.68 There is less robust epidemiologic data regarding racial and ethnic differences in HFpEF epidemiology. Compared to whites, African Americans (AAs) have a 50% higher prevalence70 and ~80% higher incidence of HF,71–73 and worse outcomes once HF develops regardless of LVEF.74, 75 Over the past 20 years, the rates of HF hospitalization in AAs have remained more than twice that of whites and declines in age-standardized HF rates have been especially modest in AA women.76 HFpEF accounts for up to 70% of prevalent HF in AAs.77 However, important sex-based differences exist in HFpEF epidemiology among AAs. The incidence of HFrEF is particularly high among AA men while black women demonstrate higher rates of incident HFpEF compared to other race- and sex-based groups.69
Figure 2.
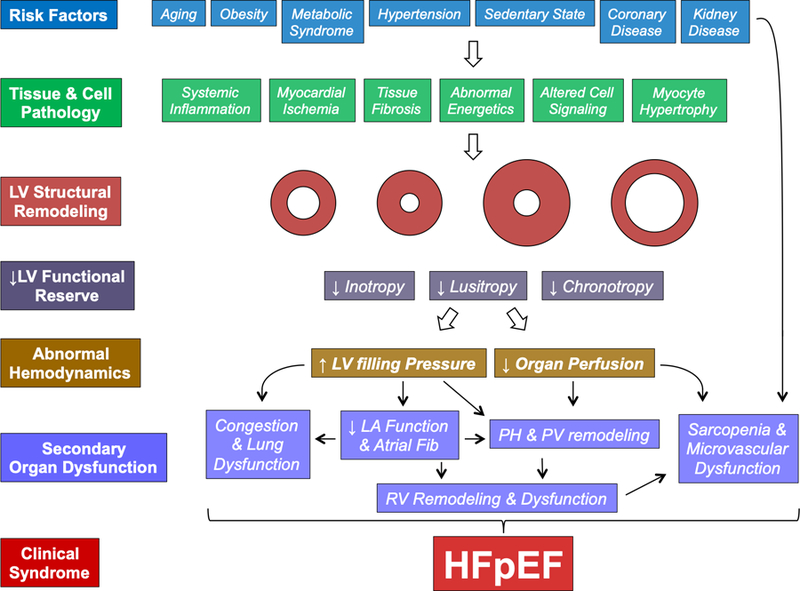
The Pathophysiologic Progression of HFpEF. By cellular mechanisms that are as yet not completely understood, established risk factors alter cell and tissue function, impairing cardiac diastolic and systolic reserve, leading to abnormal hemodynamics and secondary organ dysfunction. Note that this constellation may be seen with any pattern of cardiac geometry and remodeling, contrast to historical preconceptions that concentric left ventricular (LV) hypertrophy was requisite. See text for further details.
The high prevalence of co-morbid cardiovascular disease and cardiovascular risk factors in HFpEF is well recognized (Figure 2). The most prevalent cardiovascular disease in HFpEF is hypertension, which is present in the large majority of HFpEF cases across epidemiologic and registry studies. That HFpEF has often been considered an expression of advanced hypertensive heart disease speaks to its close association with hypertension. Several additional co-morbidities, in addition to being common in HFpEF, may also play a role in syndrome pathogenesis. Although most strongly associated with HFrEF,78 co-morbid coronary artery disease is also common in HFpEF, with prevalence in epidemiologic and registry studies of 35–60%.79 When systematically evaluated, the prevalence of epicardial CAD in HFpEF is even higher. A recent study prospectively performing coronary angiography in all HFpEF patients admitted with acute decompensation at a single center identified epicardial coronary disease in 64%. 80 When present, CAD is associated with greater risk of progressive LVEF decline and mortality in HFpEF.81
Common HFpEF co-morbidities that may also influence the pathophysiology of the syndrome include atrial fibrillation (discussed in detail below), diabetes (prevalence of 20–40%), chronic kidney disease (varying prevalence depending on definition; ~20–30%), and obesity (prevalence of up to 50% ).79 The prevalence of obesity may be even higher in HFpEF, because excess adiposity both lowers natriuretic peptide levels and makes physical examination findings of congestion less appreciable.82 For example, in a recent clinical trial that did not restrict enrollment to patients with elevated natriuretic peptide levels the prevalence of obesity was 75% and the prevalence of overweight or obesity was 95%.83 The presence of these comorbid conditions is believed to lead to the structural and functional changes that culminate in the clinical syndrome of HFpEF (Figure 2), though the cellular mechanisms and mediators remain unclear.
While these co-morbidities are common among patients with HFpEF and identify those at higher risk for adverse outcomes, it remains unclear if the burden of co-morbidities is quantitatively higher in HFpEF compared to HFrEF. Multiple studies have suggested that certain co-morbid conditions tend to be more common in HFrEF (e.g. CAD) or HFpEF (e.g. hypertension, obesity) compared to the other, and at least some studies have demonstrated that the number of comorbid conditions is higher – on average – in HFpEF compared to HFrEF.84 However, none of these co-morbidities discriminate patients with HFpEF from HFrEF. Hypertension and diabetes similarly do not appear to differentially predict incident HFpEF versus HFrEF,67 although recent studies suggest that obesity may be associated with a greater risk of incident HFpEF.85 Furthermore, the prevalence of obesity, diabetes, and hypertension also increase in late life more generally, and the extent to which they discriminate older persons with HFpEF from those without HF is unclear.86 In contrast, the reported prevalence of cardiovascular co-morbidities, such as CAD and atrial fibrillation, are much more similar in HFpEF and HFrEF compared to HF-free persons.
Clinical Outcomes in HFpEF
Regardless of LVEF, HF is associated with greater mortality compared to persons free of HF. Epidemiologic studies have consistently demonstrated a heightened risk of all-cause and CV mortality in HFpEF compared to HF-free individuals.57, 61, 62 While several studies also observed similar mortality rates between individuals with HFpEF and HFrEF,54, 55, 87 this has not been a consistent finding and several other studies have reported lower rates in HFpEF compared to HFrEF.57, 61, 62 Epidemiologic studies suggest that incidence of CV mortality is lower, and non-CV mortality higher, in HFpEF compared to HFrEF,68 findings that are concordant with observations from clinical trial samples.88 Importantly, rates of hospitalization, hospitalization duration, and impairments in patient-reported outcomes such as quality of life appear similar between HFpEF and HFrEF.89, 90 Additionally, while clinical trials impose necessary exclusions to enrollment that may limit generalizability, HFpEF patients enrolled in clinical trials clearly demonstrate heightened risk for HF and all-cause mortality compared to patients in trials of hypertension and/or diabetes but without a diagnosis of HF.6
Cardiac Structure in HFpEF
Echocardiography is the most commonly employed imaging modality in epidemiologic, registry, and clinical trial studies of HFpEF, and many have employed core laboratories to mitigate acquisition and measurement variability as recommended by professional societies.91 Still, inter-laboratory measurement differences remain a potential source of between-study differences in reports of cardiac structure and function. While studies in select patients with HFpEF have led to the view of stiff, hypertrophied walls with increased fibrosis and a relatively small chamber,14, 92 investigations in broader HFpEF samples have established a much more heterogeneous cardiac phenotype (Figure 2).93
Epidemiology
Across epidemiologic studies, perhaps the least bias study type, LV structure in HFpEF is characterized by normal mean LV cavity size with variable degrees of LV wall thickening. The Olmsted County cohort, one of the largest and most comprehensive epidemiologic evaluations of cardiac structure and function in HFpEF, demonstrated a prevalence of LVH of 42% among 244 HFpEF cases.94 Concentric hypertrophy or remodeling was present in 53%, while eccentric hypertrophy was also noted in 16%. LV geometry was normal in nearly one-third of HFpEF cases (31%). Similar average LV mass index was observed among participants in the Cardiovascular Health Study with HFpEF who were more uniformly older, but ventricular size was somewhat larger with a lesser degree of concentric remodeling.95 Perhaps the most comprehensive assessment to date of cardiac structure and function in a referral registry setting is the Northwestern HFpEF Registry, which demonstrated greater concentric remodeling and higher LVH prevalence than most epidemiologic studies.96 The relatively higher proportion of African Americans in this study may contribute to these differences. Similar to the Olmsted County cohort, 12% of patients had an eccentric pattern of ventricular hypertrophy, highlighting the heterogeneity of ventricular remodeling characterizing the HFpEF syndrome.
Clinical Trials
Imaging sub-studies of large clinical trials provide the opportunity for detailed characterization of large numbers of HFpEF cases, although trial inclusion and exclusion criteria likely impact the observed phenotypes. For example, normal LV geometry was observed in only 14% of 875 HFpEF patients in the echocardiographic sub-study of the TOPCAT trial,97 but was seen in nearly half (46%) of 745 HFpEF patients in the I-PRESERVE echocardiographic sub-study.98 Despite this variability, LVH is consistently predictive of worse prognosis in HFpEF, including higher risk of death or HF hospitalization independent of clinical predictors and measures of diastolic function.98, 99
HFpEF in Non-Western Regions of the World
Information is more limited in other regions of the world. Data from the multiregional cross-sectional Identification of patients with heart failure and PREserved systolic Function: an Epidemiological Regional (I-PREFER) study demonstrated that HFpEF also accounts for a significant proportion of HF in non-Western countries.100 Notably, important regional variation was observed, with HFpEF accounting for a higher prevalence of HF in Latin American (69%) and North Africa (75%) compared to the Middle East (41%). HFpEF similarly accounted for 51% of HF in the Japanese Chronic Heart failure Analysis and Registry in the Tohoku district (CHART-1) study.101 Notably, while CHART-1 recruited patients in 2000, in the follow-up CHART-2 study (2006–2010) HFpEF accounted for 69% of HF,102 highlighting the increasing burden of HFpEF in that region. A recent report from the Asian Sudden Cardiac Death in Heart Failure (ASIAN-HF) registry highlighted the diversity of the HFpEF syndrome between, and within, regions.103 For example, HFpEF patients in that study were nearly a decade younger with lower BMI than compared to reports from Western samples. Furthermore, appreciable differences existed in co-morbidity prevalence, cardiac remodeling, and outcomes between regions within Asia.
Integrated Pathophysiology of HFpEF
From a clinical perspective patients with HFpEF generally manifest effort intolerance and symptoms of dyspnea and fatigue on activity. As HFpEF progresses, these symptoms are noted by patients with progressively lower levels of activity, and frank evidence of venous congestion becomes evident, which may then lead to hospitalization. Fundamentally, the signs and symptoms of HFpEF are largely related to abnormal hemodynamics, and these are ultimately caused by abnormalities in cardiovascular structure and function (Figures 2 and 3).104–110 In addition to cardiac derangements, recent data have pointed to an important role in the periphery in HFpEF, including abnormalities in the systemic vasculature, endothelium, adipocytes, and skeletal muscle.82, 111–117 One important overarching theme common to all aspects of pathophysiology in HFpEF is the concept of abnormal reserve. Even when baseline or resting function appears normal, cardiac, vascular and peripheral reserve to cope with stressors is significantly diminished.111
Figure 3.
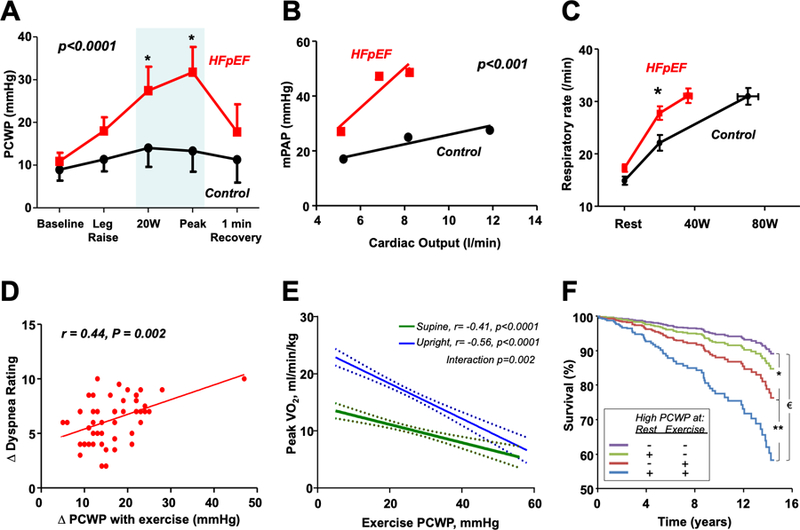
[A] As compared to controls (black), patients with HFpEF (red) display greater elevation in pulmonary capillary wedge pressure (PCWP) during exercise. [B] In tandem with abnormalities in pulmonary vasodilation and vascular recruitment, this promotes greater increases in mean pulmonary artery pressure (mPAP) as cardiac output increases during exercise. These hemodynamic perturbations are correlated with abnormalities in ventilatory drive [C] and the perception of dyspnea [D], reductions in peak aerobic capacity (peak oxygen consumption, VO2) [E], and increased mortality [F]. *Hazard ratio (HR) 2.37, 95% confidence interval (CI) 1.09–5.17, p=0.029; **HR 3.14, 95% CI 1.22–8.01; € HR 4.75, 95% CI 1.90–11.84, p<0.001. Adapted with permission from references 100, 104, 105, 106, and 118.
LV Diastolic Dysfunction
The most conspicuous and unifying hemodynamic finding of HFpEF is an elevation in LV filling pressures (LVFP). In advanced stages of HFpEF, LVFP are elevated at rest, but in patients with earlier stages of disease, LVFP become markedly elevated only during the stress of exercise.108, 111 High LVFP during exertion in HFpEF are directly correlated with heightened inspiratory drive, symptoms of dyspnea, alterations in gas exchange and pulmonary ventilation, and reductions in aerobic capacity (Figure 3).109, 110 Elevation in LVFP alter the Starling forces across the pulmonary capillaries, favoring filtration of water out of the vascular space and into the interstitium.118, 119 Over time, high LVFP may promote capillary stress failure120 and vascular remodeling, particularly in the pulmonary veins.121 High LVFP, even when normal at rest and restricted to exercise, identifies patients at increased risk for both HF hospitalization and death.122, 123
Elevation in LVFP in HFpEF is caused predominantly by diastolic dysfunction. As compared to controls, patients with HFpEF display an LV diastolic pressure-volume relationship that is shifted up and to the left, indicating an increase in passive chamber stiffness.106 During dynamic exercise, LV diastolic stiffness acutely increases to an even greater extent in HFpEF.107 Patients with HFpEF display prolonged relaxation,106 and an inability to hasten relaxation as heart rate increases, as during exercise.107 This, together with an impairment in systolic reserve (discussed below), impairs LV diastolic “suction”, so that left atrial hypertension becomes necessary to drive LV filling, in striking contrast to the normal heart where blood is “pulled” into the LV due to vigorous suction.124 While prolonged relaxation does not usually affect LVFP when heart rate is normal at rest, this can significantly impact LVFP during exertion as cycle length shortens.107 Indeed, the inability to enhance early LV diastolic velocities during exertion is directly correlated with the magnitude of LVFP increase in patients with HFpEF.108
While these mechanisms underlying elevated LVFP in HFpEF have been described in select patients undergoing invasive hemodynamic assessment, the extent to which they generalize to the broader group of persons with the HFpEF syndrome identified in epidemiologic studies and therapeutic clinical trials is less clear. Diastolic dysfunction is most commonly assessed noninvasively in clinical practice, in clinical trials, and in population-based studies, and multiple potential indices of diastolic performance exist.125
One important challenge in determining the prevalence of diastolic dysfunction in HFpEF is that non-invasive measures of diastolic function change with age. However, the extent to which these prominent age-related changes are pathologic versus benign is unclear. Indeed, using existing guideline-based cutpoints, at least 1 abnormal diastolic measure is noted in >90% of persons >65 years of age.126 By using reference limits for abnormal diastolic measures derived from low risk elderly persons (>65 years old) without CV disease or risk factors, fewer elderly persons were classified as having diastolic abnormalities compared to guideline cutpoints, while risk prediction for incident HF or death was improved. Notably, even using these age-appropriate cutpoints, diastolic abnormalities were identified in 54% of elderly persons. These finding argue that age-appropriate reference limits need to be used in interpreting diastolic indices.
Detailed data on diastolic function in epidemiologic studies have been limited as assessment in most existing studies relied primarily on transmitral diastolic flow patterns, and specifically the ratio of early (E wave) to atrial (A wave) inflow velocity (E/A ratio).42, 58 The most comprehensive assessment to date has been performed by the Olmsted County investigators. Among 556 HF cases in Olmsted County, LVEF was ≥50% in 55% among whom Doppler evidence of diastolic dysfunction was present in 78% (mild 7%, moderate 63% severe 8%). Diastolic assessment was normal in 10% and indeterminate in the remainder.87
More comprehensive data on LV diastolic measures is available from HFpEF clinical trials compared to epidemiology studies. Prevalence estimates in these studies for abnormal diastolic measures varies appreciably, possibly related to trial inclusion criteria, and are unlikely generalizable to persons in the community with HFpEF.97 However, both community-based and clinical trial studies have consistently demonstrated an association between diastolic dysfunction severity, and worse individual diastolic measures, and adverse outcomes in HFpEF including mortality and HF hospitalization.99
Extrinsic Restraint and LV Filling Pressures
Elevation in LVFP may also be driven in part by increases in extrinsic restraint on the heart, mediated by the right heart and pericardium.127, 128 This effect is amplified when venous return to the heart increases, as during exercise.107 Patients with the obese phenotype of HFpEF display greater cardiac hypertrophy and plasma volume expansion, which increase total heart volume from within, and an increase in epicardial fat thickness, which increases external restraint from without.82 The net effect is an increase in diastolic ventricular interaction in obese HFpEF patients, whereby a greater proportion of the increase in intracavitary pressures is mediated by external restraint.82 In addition to mechanical effects,82 the increase in epicardial fat in patients with HFpEF may promote cardiac injury, inflammation, fibrosis, and coronary microvascular dysfunction.129, 130
LV Systolic Dysfunction
The LVEF is, by definition, preserved in HFpEF, but LV systolic function is not necessarily normal. Impairments in myocardial and chamber-level function are present at rest, and these become much more dramatic during exercise.108, 131–133 This limits the ability of the heart to augment stroke volume, which substantially impairs the cardiac output response to exercise.105, 108–112, 116, 132, 134 In addition, LV systolic reserve deficits importantly compromise diastolic reserve, because the ability to enhance contractility plays a key role in determining the restoring forces that enhance early diastolic annular motion or recoil.108
Impairments in LV systolic performance at rest can also be detected among broader samples of HFpEF patients based on transthoracic echocardiography. Recently, significant attention has focused on strain imaging, which quantitatively assesses myocardial deformation in multiple planes (longitudinal, circumferential), and appears to be a less load-dependent index of systolic function than LVEF.135 Worse longitudinal strain has been associated with several common HF risk factors, including hypertension, diabetes, obesity, and CAD, and is predictive of incident HF in community-based studies.136 Importantly, worse longitudinal strain despite preserved LVEF is robustly prognostic of worse outcomes in HFpEF. In the echocardiographic substudy of the TOPCAT trial, longitudinal strain was abnormal in over half of patients, and was associated with a heightened risk of CV death or HF hospitalization beyond clinical and other echocardiographic predictors.137
Left Atrial Dysfunction
As LV diastolic filling pressures are elevated intermittently over time, there is secondary remodeling and dysfunction that develops in the left atrium (LA) in HFpEF. It has been hypothesized that preservation of LA function may be an important adaptation in HFpEF that “protects” the lungs and right heart,138 because development of LA dysfunction is associated with worse exercise capacity, more profound pulmonary vascular disease, and increased risk of death.138–140 Atrial fibrillation is very common in HFpEF and is further associated with LA remodeling and dysfunction.141 As discussed below HFpEF patients with LA dysfunction and atrial fibrillation are even more likely to also develop pulmonary hypertension and right HF (Figure 2).142, 143
Pulmonary Hypertension
The prevalence of pulmonary hypertension (PH) in patients with HFpEF varies widely based on the sample being studied144 and method of assessment, but its presence uniformly identifies patients at increased risk of death.145 In addition to PH from “passive” LA hypertension, a substantial number of patients go on to develop pulmonary vascular disease, with elevations in pulmonary vascular resistance (PVR).120, 146, 147 As compared to patients with pure passive PH (i.e. caused by isolated LA hypertension), this cohort displays further increased risk of death.146 Elevated PVR in HFpEF is related in part to vasoconstriction, since acute vasodilators can reduce PVR,148 but recent data also indicate that there is structural remodeling in the lung vasculature that importantly contributes, though its mechanisms remain unresolved.121
Patients with HFpEF and pulmonary vascular disease display a unique pathophysiology whereby the inability to enhance RV ejection leads to augmented right heart distention and LV underfilling, despite the fact that LVFP are elevated.147 Even among HFpEF patients that display normal PVR at rest, there is inadequate pulmonary vasodilation during exercise in HFpEF.108, 149 This is associated with adverse clinical outcomes149 and impairment in right ventricular (RV) functional reserve.108 Patients with HFpEF and greater lung congestion display more pulmonary vascular disease,118 possibly due to effects of vessel wall edema itself150 or chronic structural remodeling related to increased hydrostatic pressures.121
Right Ventricular Dysfunction
Longstanding PH in HFpEF eventually causes RV dysfunction, which develops in roughly one-third of patients.142, 143 However, this is not mediated purely by afterload mismatch, as RV myocardial dysfunction is demonstrable even after accounting for the severity of PH and non-hemodynamic risk factors have been identified including ischemic heart disease, obesity and atrial fibrillation.142, 143, 151 The development of RV dysfunction identifies patients with HFpEF with markedly increased risk of death, and might be preventable through treatment of PH and risk factors.142, 143,151
Coronary Microvascular Dysfunction
Given the combined systolic and diastolic myocardial reserve limitations in HFpEF, which are demonstrable in both ventricles, it is logical to consider abnormalities in cardiomyocyte energy availability or utilization as potential contributors. Indeed, one human study did reveal deficits in myocardial oxygen utilization in HFpEF which was correlated with hemodynamic severity.152 Numerous recent studies suggest that coronary microvascular dysfunction may plan an important role in the pathophysiology of HFpEF. Myocardial ischemia and injury are common in HFpEF, and are correlated with the ventricular functional abnormalities noted in human physiologic studies.153 Ischemia may be caused by supply-demand mismatch due to high LVFP,153 macrovascular (epicardial) disease154 as well as coronary microvascular dysfunction.153, 155, 156
Redfield and colleagues first demonstrated in an autopsy study that microvascular density is reduced in patients with HFpEF, and the severity of microvascular rarefaction is correlated with the magnitude of myocardial fibrosis.157 In addition to anatomic causes, there is evidence for impaired coronary flow reserve in HFpEF, with is associated with markers of more advanced disease.156 These data have led to the emerging hypothesis that much of HFpEF is related to coronary microvascular dysfunction.158, 159
Large Vessel and Microvascular Dysfunction
In addition to cardiac and pulmonary vascular abnormalities, there is abnormal systemic vascular function in HFpEF.111, 116 Aortic and conduit vessel stiffness is increased in HFpEF, leading to excessive blood pressure variability and greater arterial afterload mismatch, particularly during exercise.111, 116, 160 This appears to be mediated in part by endothelial dysfunction, since abnormal flow-mediated dilation is present in HFpEF111 and because acute provision of NO improves vascular function and central hemodynamics in tandem.111, 116
Microvascular function is impaired in the periphery, evidenced by impaired flow mediated dilation during reactive hyperemia in HFpEF.161 The degree of impairment in systemic microvascular function is associated with abnormal regional vasodilation during exercise, pulmonary vasoconstriction, greater dyspnea and fatigue severity, reduced aerobic capacity, and increased risk for HF hospitalization.161–164
Peripheral Abnormalities
Recent studies have also identified important roles peripheral to the heart and large arteries. Skeletal muscle composition is altered in HFpEF, with increased fatty infiltration and frank sarcopenia, even when total body weight is increased (sarcopenic obesity).111–113, 115 The ability to enhance oxygen extraction in skeletal muscle is impaired in HFpEF due to an inability to augment diffusional conductance for gas transfer within skeletal muscle, and an inability to improve oxygen utilization, likely due at least in part to mitochondrial dysfunction.114, 115, 117
Cardiac Amyloidosis
Patients with infiltrative cardiomyopathies such as cardiac amyloid have pathophysiologically distinct causes for HF are generally not considered to be part of the “garden variety” HFpEF syndrome. While cardiac amyloidosis is generally considered rare, recent data suggest that the prevalence of wild type ATTR cardiac amyloidosis, characterized by deposition of misfolded transthyretin protein, may be present in 13–19% of persons with prevalent HFpEF. This has become particularly important to recognize as novel therapeutic agents such as Inotersen, Tafamidis, and Patisiran have demonstrated efficacy in treating TTR amyloidosis. Furthermore, the high sensitivity and specificity of 99mtechnetium pyrophosphate SPECT imaging to detect cardiac TTR deposition now provides a non-invasive approach to screen appropriate patients.
Cellular Mechanisms
While many studies have elucidated the organ-level pathophysiology of HFpEF, the cellular mechanisms that drive these changes remain unresolved (Figures 2 and 3). This is related in large part to the paucity of data from human tissue, which is difficult to procure.
Systemic Inflammation
One major paradigm relates cardiac and extracardiac reserve limitations to abnormalities in NO-cGMP signaling, which are believed to be ultimately related to low grade systemic inflammation.165 Elevation in inflammatory biomarkers predict development of incident HFpEF,166 and have been shown to be higher in patients with HFpEF as compared to HFrEF.167, 168
Comorbidities such as obesity, hypertension and metabolic syndrome are extremely common in HFpEF are accordingly believed to cause low-grade systemic inflammation, which impairs endothelial formation of NO in the heart and elsewhere (Figure 2).165 This paradigm is supported by data from human HFpEF LV biopsy specimens demonstrating reduced PKG activity and cGMP concentrations that were correlated with increased passive myocyte stiffness.169 In myocardium of HFpEF patients there is microvascular endothelial activation, presumably related to underlying systemic inflammation, that is demonstrated by upregulation of E-selectin and intercellular adhesion molecule-1 expression levels and uncoupling of endothelial NO synthase associated with reduced myocardial nitrite/nitrate concentration, cGMP content, and PKG activity.170
Other Cellular Mechanisms
Intracellular calcium also plays a key role in relaxation. A recent study of isolated myocardium from patients with HFpEF has revealed that contraction and relaxation are prolonged compared to controls, in tandem with elevated sarcomere calcium levels, even as intracellular sodium and calcium handling protein expression appear unaffected.171 In myocardial strip preparations from patients with LV hypertrophy undergoing cardiac surgery, relaxation reserve was inversely related to LV mass and associated with increased calcium load at high heart rates, with increased resting tone due to diastolic cross-bridge cycling.172
Increases in diastolic LV stiffness in HFpEF have also been related to alterations in titin content and phosphorylation as well as fibrillar collagen.173 Titin in particular appears to be a major player since it is a dominant cellular determinant of stiffness that can be dynamically modulated, either by altering phosphorylation status, or protein expression.174, 175 Other possible unifying cellular mechanisms include exaggerated cardiomyocyte senescence, lipotoxicity, or deranged autophagy.176–178
Barriers to Improved Mechanistic Understanding
There are no well-established animal models of HFpEF, though a number recapitulate certain features of human HFpEF. Models based upon combinations of accelerated senescence, metabolic stress (western diet), obesity, pressure overload, neurohormonal stimulation, and even radiation exposure have been developed in recent years.170, 179–184 These models have demonstrated increases in mitochondrial ROS180 and energy deficiency with decreased ATP production.179 While each of these model systems generally produce hemodynamic and even systemic alterations that are relatively typical of “garden variety” HFpEF, the relevance of new discoveries from these models to the human condition will continue to be questioned until more data from direct human tissue can be obtained and analyzed.
Existing Therapeutic Clinical Trials in HFpEF
The findings of therapeutic clinical trials in HFpEF have been recently thoroughly reviewed.185 While the categorization of patients with the HF syndrome by LVEF in therapeutic trials was initially motivated by the practical need to enhance for risk, notable differences in the efficacy of therapeutic agents have been observed between patients with HFpEF and HFrEF. Inhibitors of the renin-angiotensin-aldosterone system have proven efficacious at reducing morbidity and mortality in HFrEF, and are part of the foundation of evidence-based therapies for this condition. To-date, four large phase III randomized clinical trials have tested their safety and efficacy in HFpEF.
As previously mentioned, the first of these was CHARM-Preserved, which randomized 3,023 patients with NYHA class II-IV HF, a prior cardiac hospitalization, and an LVEF >40% (by site report) to candesartan versus placebo as part of the larger CHARM program.38 The primary endpoint, a composite of CV death or HF hospitalization, occurred in 22% and 24% of participants in the candesartan and placebo arms respectively at a median follow-up of 36.6 months (hazard ratio 0.89 [95% CI 0.77–1.03], p=0.12). Similar results, but of borderline statistical significance, were observed in prespecified analyses either adjusting for several baseline characteristics (HR 0.86 [0.74–1.00], p=0.051) or using investigator-reported events as opposed to end-point committee adjudicated events (HR 0.85 [0.73–0.98], p=0.03). Randomization to candesartan did not impact CV death, but a signal was observed for reduction in incident of HF hospitalization (unadjusted HR 0.85 [0.72–1.01], p=0.07; adjusted HR 0.84 [0.70–1.00], p=0.051) and for total number of HF hospitalizations. Therefore, no definitive benefit with candesartan was observed in CHARM-Preserved, although several secondary analyses suggested potential benefit particularly for reduction in HF hospitalization. These data led to a IIb recommendation for consideration of the use of ARBs to decrease hospitalizations in HFpEF.186
The Perindopril in Elderly People with Chronic Heart Failure (PEP-CHF) trial randomized 850 HF patients aged ≥70 years with an LVEF >40% and 2D or Doppler echocardiographic findings suggestive of diastolic dysfunction to perindopril versus placebo.187 The primary endpoint, a composite of all-cause mortality and HF hospitalization, was not significantly reduced with perindopril compared to placebo (HR 0.92 [0.70–1.21], p=0.55) at a mean follow-up of 26.2 months, nor was the secondary endpoint of HF hospitalization (HR 0.86 [0.61–1.20], p=0.38). It is notable that study drug discontinuation was substantial subsequent to the 1-year visit.
The Irbesartan in patients with Heart Failure with Preserved Ejection Fraction (I-PRESERVE) trial tested the angiotensin receptor blocker irbesartan against placebo in 4,128 persons ≥60 years of age, with NYHA class II-IV HF and an LVEF ≥45%, for the primary endpoint of all-cause mortality or cardiovascular hospitalization.188 Irbesartan was not associated with improvements in the primary or any prespecified secondary endpoints. The signal for possible benefit of ARB therapy for HF hospitalization observed in CHARM-Preserved was not replicated in I-PRESERVE.
The most recent outcomes trial in HFpEF was the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial, which tested the impact of spironolactone compared to placebo on the composite endpoint of CV death, HF hospitalization, or aborted cardiac arrest in patients with signs and symptoms of HF, an LVEF ≥45%, and either a prior HF hospitalization or elevated natriuretic peptide level.189 TOPCAT enrolled 3,445 patients, 51% from North or South America and 49% from Russia or Georgia. Spironolactone was not associated with a reduction in the primary endpoint at a mean follow-up of 3.3 years (HR 0.89 [0.77–1.04], p=0.14), although a reduction in the secondary endpoint of HF hospitalization was observed (HR 0.83 [0.69–0.99], p=0.04). Spironolactone was associated with an increase in serum creatinine and higher rate of hyperkalemia. However, surprising differences were noted between patients enrolled in the Americas compared to Russia or Georgia that raised concerns regarding the appropriateness of the patients enrolled and treatment administration in Russia and Georgia.190 The incidence of the primary outcome was greater than 4-fold lower in Russia or Georgia (11.5 versus 2.4 per 100 patient-years). Furthermore, expected treatment associated changes in blood pressure, serum potassium, and serum creatinine were significantly less in Russia and Georgia despite a higher reported treatment adherence in Russia and Georgia. Finally, in the Americas, but not in Russia or Georgia, spironolactone was associated with reductions in the primary outcome (HR 0.82 [069–0.98], p=0.03) and its components. These findings led the AHA/ACC HF Guideline Committee, in their 2017 update, to include a IIb recommendation for the use of an aldosterone receptor antagonist to reduce hospitalizations in HFpEF patients meeting TOPCAT inclusion criteria.186
Numerous going phase 2 and 3 trials are currently evaluating novel therapeutics in HFpEF, and have been thoroughly reviewed elsewhere.185 Given the clear efficacy of combination neprolysin inhibition and ARB in HFrEF and the robust data for HF prevention with SGLT2 inhibitors in high risk patients with diabetes, perhaps the two most eagerly anticipated include the PARAGON-HF trial and EMPEROR-Preserved trial. Both are phase III trials with a endpoint of CV death or HF hospitalization. PARAGON-HF is investigating the efficacy of sacubitril-valsartan compared to valsartan alone among 4,822 patients with NYHA II-IV HF, LVEF ≥45%, evidence of cardiac structural remodeling (increased LV wall thickness or LA enlargement), and either a prior HF hospitalization or elevated natriuretic peptide level.191 EMPEROR-Preserved will test the efficacy of empagliflozin compared to placebo, and is aiming to enroll 4,126 patients with NYHA II-IV HF, LVEF >40%, elevated natriuretic peptide levels, and either a prior HF hospitalization or evidence of structural heart disease. Similarly, the efficacy of dapagliflozin in reducing the composite of CV death and HF events in ~4,700 patients with NYHA II-IV HF, LVEF >40%, and evidence of structural heart disease is being tested in the DELIVER trial.
Treatments Targeted to High Filling Pressures
As described above, tissue congestion caused by high cardiac filling pressures plays a central role in the pathophysiology of HFpEF (Figure 3), and as would be expected, interventions to reduce filling pressures have been shown to improve outcomes in this population. In CHAMPION trial, clinical management guided by physician knowledge of central hemodynamics significantly reduced HF hospitalizations.192 This finding was confirmed in an ancillary analysis restricted to HFpEF,193 and in more recent analyses of Medicare beneficiaries.194 In addition to direct hemodynamic monitoring, other methods to detect congestion allowing for intervention have shown promise including measurement of blood and plasma volume by radiolabeled indicator-dilution techniques.195
Lifestyle Interventions in HFpEF
In 2010, Kitzman and colleagues reported the first randomized controlled trial evaluating exercise training as a treatment for HFpEF, showing substantial improvement in cardiorespiratory fitness with training.196 Since that time a number of studies have corroborated this benefit, and demonstrated favorable effects on quality of life.197 While most studies suggest that exercise training causes favorable effects predominantly in the periphery,198 there is one study that also suggested a potential cardiac benefit as well.199 More recently, Kitzman et al. demonstrated in the SECRET trial that exercise training and weight loss induced by caloric restriction significantly improved aerobic capacity, and the combination of both interventions was additive.200 Sodium restriction is less well studied in HFpEF, but one study did demonstrate improvements in ventricular-arterial coupling with sodium restriction as part of the DASH diet.201
Prevention of HFpEF
Hypertension Treatment
The Framingham Heart Study established hypertension as one of the earliest ‘factors of risk’ for coronary heart disease202, and subsequently also for incident HF 203. Several randomized clinical trials of antihypertensive agents have subsequently established the reduction in incidence of HF resulting from effective blood pressure control.204 The Systolic Hypertension in the Elderly (SHEP) trial specifically expanded evidence for this benefit to persons >60 years of age,205 while the Hypertension in the Very Elderly Trial (HYVET) expanded it to persons ≥80 years of age who are highest risk of HF development.206 More recently, the Systolic Blood Pressure Intervention Trial (SPRINT) demonstrated the further reduction in incident HF associated with targeting a systolic blood pressure of <120 mmHg compared to <140 mmHg among 9,361 non-diabetic persons with elevated cardiovascular risk.207 In that study, intensive compared to conventional BP treatment targets reduced incident HF by 37%, with separation of HF event curves between groups evident after 6 months. Importantly, among the 2,636 participants who were ≥ 75 years old at enrollment 208, intensive compared to conventional treatment targets was also associated with significant reduction in risk of incident HF (HR 0.62; 95% CI 0.40–0.95). The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) results also demonstrated the importance of the antihypertensive class employed. The doxazosin arm of that trial was terminated prematurely due to a 2-fold increase in risk of incident HF compared to chlorthalidone 209. Furthermore, risk of incident HF was lower among those randomized to chlorthalidone compared to lisinopril or amlodipine.210 Among randomized trials addressing cardiovascular outcomes with BP lowering, a decrease in HF risk is one of the most prominent effects.
SGLT2 Inhibitors
In contrast to SPRINT, a more aggressive blood pressure treatment target in ACCORD was not associated with lower risk of incident HF among diabetic patients.211 In addition, limited data exist linking degree of glycemic control to HF risk, with several oral hypoglycemic agents associated with heightened risk of HF. However, SGLT2 inhibitors have demonstrated convincing reductions in incidence of HF among diabetic patients with varying degrees of baseline cardiovascular risk.212–214
Lifestyle Modification
The beneficial impacts of lifestyle modification, including weight reduction, dietary consumption, and physical activity and cardiorespiratory fitness on HF risk have recently been thoroughly reviewed.215 While there is limited randomized controlled trial data, robust epidemiologic data support the conclusion that optimization of these lifestyle factors is associated with reductions in important HF risk factors (e.g. hypertension, diabetes, atherosclerotic disease), and suggest that reductions in incident HF independent of these risk factors.
Conclusions
Heart failure is a devastating disease encompassing multiple etiologies. It is diagnosed by a constellation of clinical signs and symptoms, most of which relate to the fact that the ventricle operates at a higher filling pressure. Since this congestive pathophysiology occurs across the spectrum of LVEF, this aspect of cardiac function is not discriminating and is not part of the diagnostic criteria for HF. Regardless of LVEF, the clinical recognition the signs and symptoms of HF are associated with major adverse impacts on both the quality as well as quantity of life. The relatively recent appreciation that approximately half of those with this disorder have a normal or near normal LVEF takes on even greater importance with the results of major randomized trials demonstrating differential responses to HF therapies based on LVEF.
Although not needed for the diagnosis of HF, major differences in current international guideline recommendations for therapies and devices based on LVEF elevates its ascertainment to central for management decisions in HF.3, 4 The initially arbitrary categorization of patients with HF based on LVEF has, as a result of clinical trials, acquired more pragmatic importance. Design concerns, regarding event rates and sample size considerations, led most of the prior therapeutic outcome trials in HF to focus on those with more reduced LVEF. As a consequence, the randomized trial evidence upon which to base therapeutic decisions for those with preserved LVEF is much more limited and less definitive.
This pattern is now changing. HFpEF patients are currently considered the larger “unmet need” in cardiology. In addition, the appropriateness of testing new therapies against placebo in this population, rather than on top of known multiple proven agents and devices, has enhanced the appeal to conduct major RCTs in these patients, and many are currently underway.
Frustration over the paucity of beneficial outcome results in HFpEF trials has led some to suggest that more restrictive inclusion criteria, such as increased natriuretic peptide levels, are needed for future RCTs, predominantly to enhance risk.216 However, in so doing, many patients with different pathophysiologies will likely be excluded. For example, such natriuretic peptide thresholds would be expected to differentially exclude individuals with obesity, with genetically low natriuretic peptide levels82, 217 and persons of African American race/ethnicity who are known to be at elevated risk.218 Trialists are responding by more selectively defining higher risk subpopulations based on additional biomarker and/or cardiac structural criteria. However enhancing for risk is not the same as matching mechanism of therapy to the patient population.
Patients with cardiac amyloid were formerly “lumped” into the broader category of patients with HFpEF, but recent data of the efficacy of Tafamidis in reducing mortality and total CV-related hospitalizations among patients with TTR amyloid cardiomyopathy, in addition to a secondary analysis of a small surrogate outcome trial showing improvement in LV wall thickness, strain, and NT-proBNP associated with Patisiran in highly selected patients with hereditary TTR amyloid, points to a future of better matching (precision) between the presumed drug mechanism and the patient population.219, 220 If (Big if) this line of research leads to improved outcomes in subsequent major trials for specific HFpEF phenotypes outside of amyloid, the potential benefits (as well as risks) would only apply to that subpopulation of HFpEF. This type of progress would lead to better matching patients with their effective therapy and reducing unnecessary treatments. As trialists continue to operationally define narrower subgroups of HFpEF for therapeutic trials, it remains important to recognize that not being eligible for a trial is not tantamount to not having HFpEF.
Looking back, the recognition of the magnitude of the problem of HFpEF in the past 20 years has spurred an explosion of clinical investigation and growing intensity of informative outcome trials. Looking forward, more specific phenotying and even genotyping of subpopulations should lead to improvements in outcomes from future trials, further diminish the burden of HFpEF in their specifically targeted population, and identify others within the broad term HFpEF for future studies.
Acknowledgments
Disclosures:
Dr. Pfeffer receives research support from Novartis. He serves as a consultant for AstraZeneca, Corvidia, DalCor, GlaxoSmithKline, Novartis, Novo Nordisk, Pfizer, Roche, Sanofi, Servier and Takeda; and has equity in DalCor.
Dr. Shah is supported by NIH/NHLBI grants K08HL116792, R01HL135008, and R01HL143224. He receives research support through Brigham and Women’s Hospital from Novartis. He serves as a consultant from Bellerophon Therapeutics and Philips Ultrasound.
Dr. Borlaug is supported by the National Institutes of Health (R01 HL128526). No other relevant disclosures.
Financial support: None
Abbreviations
- HF
heart failure
- HFpEF
heart failure with preserved ejection fraction
- LVEF
left ventricular ejection fraction
- NYHA
New York heart association functional class
- RCT
randomized clinical trial
- ESC
European Society of Cardiology
- AA
African American
- HFrEF
heart failure with reduced ejection fraction
- LVH
left ventricular hypertrophy
- LVFP
left ventricular filling pressure
- LA
left atrium
- PH
pulmonary hypertension
- PVR
pulmonary vascular resistance
- RV
right ventricle
- NO
nitric oxide
- cGMP
cyclic guanosine monophosphate
- PKG
protein kinase G
References
- 1.Lewis T Diseases of the Heart 1st ed. London: MacMillian; 1933. [Google Scholar]
- 2.Braunwald E Heart Disease: A textbook of cardiovascular medicine 4th ed. Philadelphia: W.B. Saunders; 1992. [Google Scholar]
- 3.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ and Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013;128:1810–52. [DOI] [PubMed] [Google Scholar]
- 4.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P and Authors/Task Force M. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–200. [DOI] [PubMed] [Google Scholar]
- 5.Miner B, Tinetti ME, Van Ness PH, Han L, Leo-Summers L, Newman AB, Lee PJ and Vaz Fragoso CA. Dyspnea in Community-Dwelling Older Persons: A Multifactorial Geriatric Health Condition. J Am Geriatr Soc 2016;64:2042–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell RT, Jhund PS, Castagno D, Hawkins NM, Petrie MC and McMurray JJ. What have we learned about patients with heart failure and preserved ejection fraction from DIG-PEF, CHARM-preserved, and I-PRESERVE? J Am Coll Cardiol 2012;60:2349–56. [DOI] [PubMed] [Google Scholar]
- 7.Curtis LH, Whellan DJ, Hammill BG, Hernandez AF, Anstrom KJ, Shea AM and Schulman KA. Incidence and prevalence of heart failure in elderly persons, 1994–2003. Arch Intern Med 2008;168:418–24. [DOI] [PubMed] [Google Scholar]
- 8.Abraham WT, Fonarow GC, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, O’Connor CM, Sun JL, Yancy CW, Young JB, Investigators O-H and Coordinators. Predictors of in-hospital mortality in patients hospitalized for heart failure: insights from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF). J Am Coll Cardiol 2008;52:347–56. [DOI] [PubMed] [Google Scholar]
- 9.Adams KF Jr., Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, Berkowitz RL, Galvao M, Horton DP, Committee ASA and Investigators. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J 2005;149:209–16. [DOI] [PubMed] [Google Scholar]
- 10.Loehr LR, Rosamond WD, Chang PP, Folsom AR and Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study). Am J Cardiol 2008;101:1016–22. [DOI] [PubMed] [Google Scholar]
- 11.Lloyd-Jones DM, Larson MG, Leip EP, Beiser A, D’Agostino RB, Kannel WB, Murabito JM, Vasan RS, Benjamin EJ, Levy D and Framingham Heart S. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation 2002;106:3068–72. [DOI] [PubMed] [Google Scholar]
- 12.Folse R and Braunwald E. Determination of fraction of left ventricular volume ejected per beat and of ventricular end-diastolic and residual volumes. Experimental and clinical observations with a precordial dilution technic. Circulation 1962;25:674–85. [DOI] [PubMed] [Google Scholar]
- 13.Cikes M and Solomon SD. Beyond ejection fraction: an integrative approach for assessment of cardiac structure and function in heart failure. Eur Heart J 2016;37:1642–50. [DOI] [PubMed] [Google Scholar]
- 14.Zile MR, Baicu CF and Gaasch WH. Diastolic heart failure--abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med 2004;350:1953–9. [DOI] [PubMed] [Google Scholar]
- 15.Gaasch WH and Zile MR. Left ventricular diastolic dysfunction and diastolic heart failure. Annu Rev Med 2004;55:373–94. [DOI] [PubMed] [Google Scholar]
- 16.Mann T, Goldberg S, Mudge GH Jr. and Grossman W. Factors contributing to altered left ventricular diastolic properties during angina pectoris. Circulation 1979;59:14–20. [DOI] [PubMed] [Google Scholar]
- 17.Aurigemma GP and Gaasch WH. Clinical practice. Diastolic heart failure. The New England journal of medicine 2004;351:1097–105. [DOI] [PubMed] [Google Scholar]
- 18.Echeverria HH, Bilsker MS, Myerburg RJ and Kessler KM. Congestive heart failure: echocardiographic insights. Am J Med 1983;75:750–5. [DOI] [PubMed] [Google Scholar]
- 19.Dougherty AH, Naccarelli GV, Gray EL, Hicks CH and Goldstein RA. Congestive heart failure with normal systolic function. The American journal of cardiology 1984;54:778–82. [DOI] [PubMed] [Google Scholar]
- 20.Soufer R, Wohlgelernter D, Vita NA, Amuchestegui M, Sostman HD, Berger HJ and Zaret BL. Intact systolic left ventricular function in clinical congestive heart failure. Am J Cardiol 1985;55:1032–6. [DOI] [PubMed] [Google Scholar]
- 21.Gandhi SK, Powers JC, Nomeir AM, Fowle K, Kitzman DW, Rankin KM and Little WC. The pathogenesis of acute pulmonary edema associated with hypertension. N Engl J Med 2001;344:17–22. [DOI] [PubMed] [Google Scholar]
- 22.Cohn JN, Archibald DG, Ziesche S, Franciosa JA, Harston WE, Tristani FE, Dunkman WB, Jacobs W, Francis GS, Flohr KH and et al. Effect of vasodilator therapy on mortality in chronic congestive heart failure. Results of a Veterans Administration Cooperative Study. N Engl J Med 1986;314:1547–52. [DOI] [PubMed] [Google Scholar]
- 23.Group CTS. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med 1987;316:1429–35. [DOI] [PubMed] [Google Scholar]
- 24.Cohn JN and Johnson G. Heart failure with normal ejection fraction. The V-HeFT Study. Veterans Administration Cooperative Study Group. Circulation 1990;81:III48–53. [PubMed] [Google Scholar]
- 25.Cohn JN, Johnson G, Ziesche S, Cobb F, Francis G, Tristani F, Smith R, Dunkman WB, Loeb H, Wong M and et al. A comparison of enalapril with hydralazine-isosorbide dinitrate in the treatment of chronic congestive heart failure. N Engl J Med 1991;325:303–10. [DOI] [PubMed] [Google Scholar]
- 26.Investigators S, Yusuf S, Pitt B, Davis CE, Hood WB and Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991;325:293–302. [DOI] [PubMed] [Google Scholar]
- 27.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J and Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999;341:709–17. [DOI] [PubMed] [Google Scholar]
- 28.Digitalis Investigation G. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med 1997;336:525–33. [DOI] [PubMed] [Google Scholar]
- 29.Packer M, Carver JR, Rodeheffer RJ, Ivanhoe RJ, DiBianco R, Zeldis SM, Hendrix GH, Bommer WJ, Elkayam U, Kukin ML and et al. Effect of oral milrinone on mortality in severe chronic heart failure. The PROMISE Study Research Group. N Engl J Med 1991;325:1468–75. [DOI] [PubMed] [Google Scholar]
- 30.Ferguson JJ and Fox R. Meeting highlights. The 69th Scientific Sessions of the American Heart Association in New Orleans, La, November 10–13, 1996. Circulation 1997;95:761–4. [DOI] [PubMed] [Google Scholar]
- 31.Packer M, Pitt B, Rouleau JL, Swedberg K, DeMets DL and Fisher L. Long-Term Effects of Flosequinan on the Morbidity and Mortality of Patients With Severe Chronic Heart Failure: Primary Results of the PROFILE Trial After 24 Years. JACC Heart Fail 2017;5:399–407. [DOI] [PubMed] [Google Scholar]
- 32.Goodfriend TL, Elliott ME and Catt KJ. Angiotensin receptors and their antagonists. N Engl J Med 1996;334:1649–54. [DOI] [PubMed] [Google Scholar]
- 33.Pitt B, Segal R, Martinez FA, Meurers G, Cowley AJ, Thomas I, Deedwania PC, Ney DE, Snavely DB and Chang PI. Randomised trial of losartan versus captopril in patients over 65 with heart failure (Evaluation of Losartan in the Elderly Study, ELITE). Lancet 1997;349:747–52. [DOI] [PubMed] [Google Scholar]
- 34.Pitt B, Poole-Wilson PA, Segal R, Martinez FA, Dickstein K, Camm AJ, Konstam MA, Riegger G, Klinger GH, Neaton J, Sharma D and Thiyagarajan B. Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: randomised trial--the Losartan Heart Failure Survival Study ELITE II. Lancet 2000;355:1582–7. [DOI] [PubMed] [Google Scholar]
- 35.Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J, Yusuf S, Pocock S, Investigators C and Committees. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet 2003;362:759–66. [DOI] [PubMed] [Google Scholar]
- 36.McMurray JJ, Ostergren J, Swedberg K, Granger CB, Held P, Michelson EL, Olofsson B, Yusuf S, Pfeffer MA, Investigators C and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet 2003;362:767–71. [DOI] [PubMed] [Google Scholar]
- 37.Granger CB, McMurray JJ, Yusuf S, Held P, Michelson EL, Olofsson B, Ostergren J, Pfeffer MA, Swedberg K, Investigators C and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet 2003;362:772–6. [DOI] [PubMed] [Google Scholar]
- 38.Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J, Investigators C and Committees. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet 2003;362:777–81. [DOI] [PubMed] [Google Scholar]
- 39.Swedberg K, Pfeffer M, Granger C, Held P, McMurray J, Ohlin G, Olofsson B, Ostergren J and Yusuf S. Candesartan in heart failure--assessment of reduction in mortality and morbidity (CHARM): rationale and design. Charm-Programme Investigators. J Card Fail 1999;5:276–82. [DOI] [PubMed] [Google Scholar]
- 40.Cronin L, Mehta SR, Zhao F, Pogue J, Budaj A, Hunt D and Yusuf S. Stroke in relation to cardiac procedures in patients with non-ST-elevation acute coronary syndrome: a study involving >18 000 patients. Circulation 2001;104:269–74. [DOI] [PubMed] [Google Scholar]
- 41.Remme WJ, Swedberg K, Task Force for the D and Treatment of Chronic Heart Failure ESoC. Guidelines for the diagnosis and treatment of chronic heart failure. Eur Heart J 2001;22:1527–60. [DOI] [PubMed] [Google Scholar]
- 42.Devereux RB, Roman MJ, Liu JE, Welty TK, Lee ET, Rodeheffer R, Fabsitz RR and Howard BV. Congestive heart failure despite normal left ventricular systolic function in a population-based sample: the Strong Heart Study. Am J Cardiol 2000;86:1090–6. [DOI] [PubMed] [Google Scholar]
- 43.Given BD, Lee TH, Stone PH and Dzau VJ. Nifedipine in severely hypertensive patients with congestive heart failure and preserved ventricular systolic function. Arch Intern Med 1985;145:281–5. [PubMed] [Google Scholar]
- 44.Borlaug BA, Melenovsky V, Russell SD, Kessler K, Pacak K, Becker LC and Kass DA. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation 2006;114:2138–47. [DOI] [PubMed] [Google Scholar]
- 45.Lam CS and Solomon SD. The middle child in heart failure: heart failure with mid-range ejection fraction (40–50%). Eur J Heart Fail 2014;16:1049–55. [DOI] [PubMed] [Google Scholar]
- 46.Florea VG, Rector TS, Anand IS and Cohn JN. Heart Failure With Improved Ejection Fraction: Clinical Characteristics, Correlates of Recovery, and Survival: Results From the Valsartan Heart Failure Trial. Circ Heart Fail 2016;9. [DOI] [PubMed] [Google Scholar]
- 47.Gulati G and Udelson JE. Heart Failure With Improved Ejection Fraction: Is it Possible to Escape One’s Past? JACC Heart Fail 2018;6:725–733. [DOI] [PubMed] [Google Scholar]
- 48.McKee PA, Castelli WP, McNamara PM and Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med 1971;285:1441–6. [DOI] [PubMed] [Google Scholar]
- 49.Carlson KJ, Lee DC, Goroll AH, Leahy M and Johnson RA. An analysis of physicians’ reasons for prescribing long-term digitalis therapy in outpatients. J Chronic Dis 1985;38:733–9. [DOI] [PubMed] [Google Scholar]
- 50.Eriksson H, Caidahl K, Larsson B, Ohlson LO, Welin L, Wilhelmsen L and Svardsudd K. Cardiac and pulmonary causes of dyspnoea--validation of a scoring test for clinical-epidemiological use: the Study of Men Born in 1913. Eur Heart J 1987;8:1007–14. [DOI] [PubMed] [Google Scholar]
- 51.Rosamond WD, Chang PP, Baggett C, Johnson A, Bertoni AG, Shahar E, Deswal A, Heiss G and Chambless LE. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail 2012;5:152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Di Bari M, Pozzi C, Cavallini MC, Innocenti F, Baldereschi G, De Alfieri W, Antonini E, Pini R, Masotti G and Marchionni N. The diagnosis of heart failure in the community. Comparative validation of four sets of criteria in unselected older adults: the ICARe Dicomano Study. J Am Coll Cardiol 2004;44:1601–8. [DOI] [PubMed] [Google Scholar]
- 53.Senni M, Tribouilloy CM, Rodeheffer RJ, Jacobsen SJ, Evans JM, Bailey KR and Redfield MM. Congestive heart failure in the community: a study of all incident cases in Olmsted County, Minnesota, in 1991. Circulation 1998;98:2282–9. [DOI] [PubMed] [Google Scholar]
- 54.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL and Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006;355:251–9. [DOI] [PubMed] [Google Scholar]
- 55.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y and Liu PP. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med 2006;355:260–9. [DOI] [PubMed] [Google Scholar]
- 56.Lam CS, Lyass A, Kraigher-Krainer E, Massaro JM, Lee DS, Ho JE, Levy D, Redfield MM, Pieske BM, Benjamin EJ and Vasan RS. Cardiac dysfunction and noncardiac dysfunction as precursors of heart failure with reduced and preserved ejection fraction in the community. Circulation 2011;124:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vasan RS, Larson MG, Benjamin EJ, Evans JC, Reiss CK and Levy D. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population-based cohort. Journal of the American College of Cardiology 1999;33:1948–55. [DOI] [PubMed] [Google Scholar]
- 58.Kitzman DW, Gardin JM, Gottdiener JS, Arnold A, Boineau R, Aurigemma G, Marino EK, Lyles M, Cushman M, Enright PL and Cardiovascular Health Study Research G. Importance of heart failure with preserved systolic function in patients > or = 65 years of age. CHS Research Group. Cardiovascular Health Study. Am J Cardiol 2001;87:413–9. [DOI] [PubMed] [Google Scholar]
- 59.Dunlay SM, Roger VL, Weston SA, Jiang R and Redfield MM. Longitudinal changes in ejection fraction in heart failure patients with preserved and reduced ejection fraction. Circ Heart Fail 2012;5:720–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vasan RS, Benjamin EJ and Levy D. Prevalence, clinical features and prognosis of diastolic heart failure: an epidemiologic perspective. J Am Coll Cardiol 1995;26:1565–74. [DOI] [PubMed] [Google Scholar]
- 61.Kupari M, Lindroos M, Iivanainen AM, Heikkila J and Tilvis R. Congestive heart failure in old age: prevalence, mechanisms and 4-year prognosis in the Helsinki Ageing Study. J Intern Med 1997;241:387–94. [DOI] [PubMed] [Google Scholar]
- 62.Gottdiener JS, McClelland RL, Marshall R, Shemanski L, Furberg CD, Kitzman DW, Cushman M, Polak J, Gardin JM, Gersh BJ, Aurigemma GP and Manolio TA. Outcome of congestive heart failure in elderly persons: influence of left ventricular systolic function. The Cardiovascular Health Study. Ann Intern Med 2002;137:631–9. [DOI] [PubMed] [Google Scholar]
- 63.Tribouilloy C, Rusinaru D, Mahjoub H, Souliere V, Levy F, Peltier M, Slama M and Massy Z. Prognosis of heart failure with preserved ejection fraction: a 5 year prospective population-based study. Eur Heart J 2008;29:339–47. [DOI] [PubMed] [Google Scholar]
- 64.Lenzen MJ, Scholte op Reimer WJ, Boersma E, Vantrimpont PJ, Follath F, Swedberg K, Cleland J and Komajda M. Differences between patients with a preserved and a depressed left ventricular function: a report from the EuroHeart Failure Survey. Eur Heart J 2004;25:1214–20. [DOI] [PubMed] [Google Scholar]
- 65.Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow GC, Committee ASA and Investigators. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol 2006;47:76–84. [DOI] [PubMed] [Google Scholar]
- 66.Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade M, Greenberg BH, O’Connor CM, Sun JL, Yancy CW, Young JB, Investigators O-H and Hospitals. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol 2007;50:768–77. [DOI] [PubMed] [Google Scholar]
- 67.Ho JE, Enserro D, Brouwers FP, Kizer JR, Shah SJ, Psaty BM, Bartz TM, Santhanakrishnan R, Lee DS, Chan C, Liu K, Blaha MJ, Hillege HL, van der Harst P, van Gilst WH, Kop WJ, Gansevoort RT, Vasan RS, Gardin JM, Levy D, Gottdiener JS, de Boer RA and Larson MG. Predicting Heart Failure With Preserved and Reduced Ejection Fraction: The International Collaboration on Heart Failure Subtypes. Circ Heart Fail 2016;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gerber Y, Weston SA, Redfield MM, Chamberlain AM, Manemann SM, Jiang R, Killian JM and Roger VL. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern Med 2015;175:996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chang PP, Wruck LM, Shahar E, Rossi JS, Loehr LR, Russell SD, Agarwal SK, Konety SH, Rodriguez CJ and Rosamond WD. Trends in Hospitalizations and Survival of Acute Decompensated Heart Failure in Four US Communities (2005–2014): ARIC Study Community Surveillance. Circulation 2018;138:12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brown DW, Haldeman GA, Croft JB, Giles WH and Mensah GA. Racial or ethnic differences in hospitalization for heart failure among elderly adults: Medicare, 1990 to 2000. Am Heart J 2005;150:448–54. [DOI] [PubMed] [Google Scholar]
- 71.Bahrami H, Kronmal R, Bluemke DA, Olson J, Shea S, Liu K, Burke GL and Lima JA. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Arch Intern Med 2008;168:2138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Okin PM, Kjeldsen SE, Dahlof B and Devereux RB. Racial differences in incident heart failure during antihypertensive therapy. Circ Cardiovasc Qual Outcomes 2011;4:157–64. [DOI] [PubMed] [Google Scholar]
- 73.Eaton CB, Abdulbaki AM, Margolis KL, Manson JE, Limacher M, Klein L, Allison MA, Robinson JG, Curb JD, Martin LA, Liu S and Howard BV. Racial and ethnic differences in incident hospitalized heart failure in postmenopausal women: the Women’s Health Initiative. Circulation 2012;126:688–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dries DL, Exner DV, Gersh BJ, Cooper HA, Carson PE and Domanski MJ. Racial differences in the outcome of left ventricular dysfunction. N Engl J Med 1999;340:609–16. [DOI] [PubMed] [Google Scholar]
- 75.East MA, Peterson ED, Shaw LK, Gattis WA and O’Connor CM. Racial differences in the outcomes of patients with diastolic heart failure. Am Heart J 2004;148:151–6. [DOI] [PubMed] [Google Scholar]
- 76.Ziaeian B, Kominski GF, Ong MK, Mays VM, Brook RH and Fonarow GC. National Differences in Trends for Heart Failure Hospitalizations by Sex and Race/Ethnicity. Circ Cardiovasc Qual Outcomes 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gupta DK, Shah AM, Castagno D, Takeuchi M, Loehr LR, Fox ER, Butler KR, Mosley TH, Kitzman DW and Solomon SD. Heart failure with preserved ejection fraction in African Americans: The ARIC (Atherosclerosis Risk In Communities) study. JACC Heart Fail 2013;1:156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ho JE, Gona P, Pencina MJ, Tu JV, Austin PC, Vasan RS, Kannel WB, D’Agostino RB, Lee DS and Levy D. Discriminating clinical features of heart failure with preserved vs. reduced ejection fraction in the community. Eur Heart J 2012;33:1734–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lam CS, Donal E, Kraigher-Krainer E and Vasan RS. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail 2011;13:18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Trevisan L, Cautela J, Resseguier N, Laine M, Arques S, Pinto J, Orabona M, Barraud J, Peyrol M, Paganelli F, Bonello L and Thuny F. Prevalence and characteristics of coronary artery disease in heart failure with preserved and mid-range ejection fractions: A systematic angiography approach. Arch Cardiovasc Dis 2018;111:109–118. [DOI] [PubMed] [Google Scholar]
- 81.Hwang SJ, Melenovsky V and Borlaug BA. Implications of coronary artery disease in heart failure with preserved ejection fraction. J Am Coll Cardiol 2014;63:2817–27. [DOI] [PubMed] [Google Scholar]
- 82.Obokata M, Reddy YN, Pislaru SV, Melenovsky V and Borlaug BA. Evidence Supporting the Existence of a Distinct Obese Phenotype of Heart Failure with Preserved Ejection Fraction. Circulation 2017;136:6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Borlaug BA, Anstrom KJ, Lewis GD, Shah SJ, Levine JA, Koepp GA, Givertz MM, Felker GM, LeWinter MM, Mann DL, Margulies KB, Smith AL, Tang WHW, Whellan DJ, Chen HH, Davila-Roman VG, McNulty S, Desvigne-Nickens P, Hernandez AF, Braunwald E, Redfield MM, National Heart L and Blood Institute Heart Failure Clinical Research N. Effect of Inorganic Nitrite vs Placebo on Exercise Capacity Among Patients With Heart Failure With Preserved Ejection Fraction: The INDIE-HFpEF Randomized Clinical Trial. JAMA 2018;320:1764–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ather S, Chan W, Bozkurt B, Aguilar D, Ramasubbu K, Zachariah AA, Wehrens XH and Deswal A. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol 2012;59:998–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Savji N, Meijers WC, Bartz TM, Bhambhani V, Cushman M, Nayor M, Kizer JR, Sarma A, Blaha MJ, Gansevoort RT, Gardin JM, Hillege HL, Ji F, Kop WJ, Lau ES, Lee DS, Sadreyev R, van Gilst WH, Wang TJ, Zanni MV, Vasan RS, Allen NB, Psaty BM, van der Harst P, Levy D, Larson M, Shah SJ, de Boer RA, Gottdiener JS and Ho JE. The Association of Obesity and Cardiometabolic Traits With Incident HFpEF and HFrEF. JACC Heart Fail 2018;6:701–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shah AM, Claggett B, Loehr LR, Chang PP, Matsushita K, Kitzman D, Konety S, Kucharska-Newton A, Sueta CA, Mosley TH, Wright JD, Coresh J, Heiss G, Folsom AR and Solomon SD. Heart Failure Stages Among Older Adults in the Community: The Atherosclerosis Risk in Communities Study. Circulation 2017;135:224–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, Meverden RA and Roger VL. Systolic and diastolic heart failure in the community. JAMA 2006;296:2209–16. [DOI] [PubMed] [Google Scholar]
- 88.Solomon SD, Anavekar N, Skali H, McMurray JJ, Swedberg K, Yusuf S, Granger CB, Michelson EL, Wang D, Pocock S, Pfeffer MA and Candesartan in Heart Failure Reduction in Mortality I. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation 2005;112:3738–44. [DOI] [PubMed] [Google Scholar]
- 89.Lewis EF, Lamas GA, O’Meara E, Granger CB, Dunlap ME, McKelvie RS, Probstfield JL, Young JB, Michelson EL, Halling K, Carlsson J, Olofsson B, McMurray JJ, Yusuf S, Swedberg K, Pfeffer MA and Investigators C. Characterization of health-related quality of life in heart failure patients with preserved versus low ejection fraction in CHARM. Eur J Heart Fail 2007;9:83–91. [DOI] [PubMed] [Google Scholar]
- 90.Loop MS, Van Dyke MK, Chen L, Brown TM, Durant RW, Safford MM and Levitan EB. Comparison of Length of Stay, 30-Day Mortality, and 30-Day Readmission Rates in Medicare Patients With Heart Failure and With Reduced Versus Preserved Ejection Fraction. Am J Cardiol 2016;118:79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Douglas PS, DeCara JM, Devereux RB, Duckworth S, Gardin JM, Jaber WA, Morehead AJ, Oh JK, Picard MH, Solomon SD, Wei K, Weissman NJ, American Society of Echocardiography S and American College of Cardiology F. Echocardiographic imaging in clinical trials: American Society of Echocardiography Standards for echocardiography core laboratories: endorsed by the American College of Cardiology Foundation. J Am Soc Echocardiogr 2009;22:755–65. [DOI] [PubMed] [Google Scholar]
- 92.Kitzman DW, Little WC, Brubaker PH, Anderson RT, Hundley WG, Marburger CT, Brosnihan B, Morgan TM and Stewart KP. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA 2002;288:2144–50. [DOI] [PubMed] [Google Scholar]
- 93.Shah AM and Pfeffer MA. The many faces of heart failure with preserved ejection fraction. Nat Rev Cardiol 2012;9:555–6. [DOI] [PubMed] [Google Scholar]
- 94.Lam CS, Roger VL, Rodeheffer RJ, Bursi F, Borlaug BA, Ommen SR, Kass DA and Redfield MM. Cardiac structure and ventricular-vascular function in persons with heart failure and preserved ejection fraction from Olmsted County, Minnesota. Circulation 2007;115:1982–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Maurer MS, Burkhoff D, Fried LP, Gottdiener J, King DL and Kitzman DW. Ventricular structure and function in hypertensive participants with heart failure and a normal ejection fraction: the Cardiovascular Health Study. J Am Coll Cardiol 2007;49:972–81. [DOI] [PubMed] [Google Scholar]
- 96.Burke MA, Katz DH, Beussink L, Selvaraj S, Gupta DK, Fox J, Chakrabarti S, Sauer AJ, Rich JD, Freed BH and Shah SJ. Prognostic importance of pathophysiologic markers in patients with heart failure and preserved ejection fraction. Circ Heart Fail 2014;7:288–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shah AM, Shah SJ, Anand IS, Sweitzer NK, O’Meara E, Heitner JF, Sopko G, Li G, Assmann SF, McKinlay SM, Pitt B, Pfeffer MA, Solomon SD and Investigators T. Cardiac structure and function in heart failure with preserved ejection fraction: baseline findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist trial. Circ Heart Fail 2014;7:104–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zile MR, Gottdiener JS, Hetzel SJ, McMurray JJ, Komajda M, McKelvie R, Baicu CF, Massie BM, Carson PE and Investigators IP. Prevalence and significance of alterations in cardiac structure and function in patients with heart failure and a preserved ejection fraction. Circulation 2011;124:2491–501. [DOI] [PubMed] [Google Scholar]
- 99.Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, O’Meara E, Desai AS, Heitner JF, Li G, Fang J, Rouleau J, Zile MR, Markov V, Ryabov V, Reis G, Assmann SF, McKinlay SM, Pitt B, Pfeffer MA and Solomon SD. Cardiac structure and function and prognosis in heart failure with preserved ejection fraction: findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) Trial. Circ Heart Fail 2014;7:740–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Magana-Serrano JA, Almahmeed W, Gomez E, Al-Shamiri M, Adgar D, Sosner P, Herpin D and Investigators IP. Prevalence of heart failure with preserved ejection fraction in Latin American, Middle Eastern, and North African Regions in the I PREFER study (Identification of Patients With Heart Failure and PREserved Systolic Function: an epidemiological regional study). Am J Cardiol 2011;108:1289–96. [DOI] [PubMed] [Google Scholar]
- 101.Shiba N, Watanabe J, Shinozaki T, Koseki Y, Sakuma M, Kagaya Y, Shirato K and Investigators C. Analysis of chronic heart failure registry in the Tohoku district: third year follow-up. Circ J 2004;68:427–34. [DOI] [PubMed] [Google Scholar]
- 102.Shiba N, Nochioka K, Miura M, Kohno H, Shimokawa H and Investigators C-. Trend of westernization of etiology and clinical characteristics of heart failure patients in Japan--first report from the CHART-2 study. Circ J 2011;75:823–33. [DOI] [PubMed] [Google Scholar]
- 103.Tromp J, Teng TH, Tay WT, Hung CL, Narasimhan C, Shimizu W, Park SW, Liew HB, Ngarmukos T, Reyes EB, Siswanto BB, Yu CM, Zhang S, Yap J, MacDonald M, Ling LH, Leineweber K, Richards AM, Zile MR, Anand IS, Lam CSP and Investigators A-H. Heart failure with preserved ejection fraction in Asia. Eur J Heart Fail 2019;21:23–36. [DOI] [PubMed] [Google Scholar]
- 104.Borlaug BA, Nishimura RA, Sorajja P, Lam CS and Redfield MM. Exercise Hemodynamics Enhance Diagnosis of Early Heart Failure with Preserved Ejection Fraction. Circ Heart Fail 2010;3:588–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Maeder MT, Thompson BR, Brunner-La Rocca HP and Kaye DM. Hemodynamic basis of exercise limitation in patients with heart failure and normal ejection fraction. J Am Coll Cardiol 2010;56:855–63. [DOI] [PubMed] [Google Scholar]
- 106.Westermann D, Kasner M, Steendijk P, Spillmann F, Riad A, Weitmann K, Hoffmann W, Poller W, Pauschinger M, Schultheiss HP and Tschope C. Role of left ventricular stiffness in heart failure with normal ejection fraction. Circulation 2008;117:2051–60. [DOI] [PubMed] [Google Scholar]
- 107.Borlaug BA, Jaber WA, Ommen SR, Lam CS, Redfield MM and Nishimura RA. Diastolic relaxation and compliance reserve during dynamic exercise in heart failure with preserved ejection fraction. Heart 2011;97:964–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Borlaug BA, Kane GC, Melenovsky V and Olson TP. Abnormal right ventricular-pulmonary artery coupling with exercise in heart failure with preserved ejection fraction. Eur Heart J 2016;37:3293–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Obokata M, Olson TP, Reddy YN, Melenovsky V, Kane GC and Borlaug BA. Hemodynamics, Dyspnea, and Pulmonary Reserve in Heart Failure with Preserved Ejection Fraction. Eur Heart J 2018;39:2810–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Reddy YNV, Olson TP, Obokata M, Melenovsky V and Borlaug BA. Hemodynamic Correlates and Diagnostic Role of Cardiopulmonary Exercise Testing in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail 2018;6:665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Borlaug BA, Olson TP, Lam CS, Flood KS, Lerman A, Johnson BD and Redfield MM. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol 2010;56:845–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM and Kitzman DW. Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. J Am Coll Cardiol 2011;58:265–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Haykowsky MJ, Kouba EJ, Brubaker PH, Nicklas BJ, Eggebeen J and Kitzman DW. Skeletal muscle composition and its relation to exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Cardiol 2014;113:1211–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dhakal BP, Malhotra R, Murphy RM, Pappagianopoulos PP, Baggish AL, Weiner RB, Houstis NE, Eisman AS, Hough SS and Lewis GD. Mechanisms of Exercise Intolerance in Heart Failure with Preserved Ejection Fraction: The Role of Abnormal Peripheral Oxygen Extraction. Circ Heart Fail 2015;8:286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Molina AJ, Bharadwaj MS, Van Horn C, Nicklas BJ, Lyles MF, Eggebeen J, Haykowsky MJ, Brubaker PH and Kitzman DW. Skeletal Muscle Mitochondrial Content, Oxidative Capacity, and Mfn2 Expression Are Reduced in Older Patients With Heart Failure and Preserved Ejection Fraction and Are Related to Exercise Intolerance. JACC Heart Fail 2016;4:636–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Reddy YNV, Andersen MJ, Obokata M, Koepp KE, Kane GC, Melenovsky V, Olson TP and Borlaug BA. Arterial Stiffening With Exercise in Patients With Heart Failure and Preserved Ejection Fraction. J Am Coll Cardiol 2017;70:136–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Houstis NE, Eisman AS, Pappagianopoulos PP, Wooster L, Bailey CS, Wagner PD and Lewis GD. Exercise Intolerance in Heart Failure With Preserved Ejection Fraction: Diagnosing and Ranking Its Causes Using Personalized O2 Pathway Analysis. Circulation 2018;137:148–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Melenovsky V, Andersen MJ, Andress K, Reddy YN and Borlaug BA. Lung congestion in chronic heart failure: haemodynamic, clinical, and prognostic implications. Eur J Heart Fail 2015;17:1161–71. [DOI] [PubMed] [Google Scholar]
- 119.Thompson RB, Pagano JJ, Chow K, Sekowski V, Ezekowitz J, Anderson TJ, Dyck JRB and Haykowsky MJ. Subclinical Pulmonary Edema is Associated with Reduced Exercise Capacity in HFpEF and HFrEF. J Am Coll Card 2017;70:1827–8. [DOI] [PubMed] [Google Scholar]
- 120.Guazzi M and Borlaug BA. Pulmonary hypertension due to left heart disease. Circulation 2012;126:975–90. [DOI] [PubMed] [Google Scholar]
- 121.Fayyaz AU, Edwards WD, Maleszewski JJ, Konik EA, DuBrock HM, Borlaug BA, Frantz RP, Jenkins SM and Redfield MM. Global Pulmonary Vascular Remodeling in Pulmonary Hypertension Associated with Heart Failure and Preserved or Reduced Ejection Fraction. Circulation 2018;137:1796–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dorfs S, Zeh W, Hochholzer W, Jander N, Kienzle RP, Pieske B and Neumann FJ. Pulmonary capillary wedge pressure during exercise and long-term mortality in patients with suspected heart failure with preserved ejection fraction. Eur Heart J 2014;35:3103–12. [DOI] [PubMed] [Google Scholar]
- 123.Eisman AS, Shah RV, Dhakal BP, Pappagianopoulos PP, Wooster L, Bailey C, Cunningham TF, Hardin KM, Baggish AL, Ho JE, Malhotra R and Lewis GD. Pulmonary Capillary Wedge Pressure Patterns During Exercise Predict Exercise Capacity and Incident Heart Failure. Circ Heart Fail 2018;11:e004750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cheng CP, Noda T, Nozawa T and Little WC. Effect of heart failure on the mechanism of exercise-induced augmentation of mitral valve flow. Circ Res 1993;72:795–806. [DOI] [PubMed] [Google Scholar]
- 125.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA and Waggoner AD. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016;29:277–314. [DOI] [PubMed] [Google Scholar]
- 126.Shah AM, Claggett B, Kitzman D, Biering-Sorensen T, Jensen JS, Cheng S, Matsushita K, Konety S, Folsom AR, Mosley TH, Wright JD, Heiss G and Solomon SD. Contemporary Assessment of Left Ventricular Diastolic Function in Older Adults: The Atherosclerosis Risk in Communities Study. Circulation 2017;135:426–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Borlaug BA, Schaff HV, Pochettino A, Pedrotty DM, Asirvatham SJ, Abel MD, Carter RE and Mauermann WJ. Pericardiotomy Enhances Left Ventricular Diastolic Reserve With Volume Loading in Humans: Implications for the Surgical Management of Heart Failure With Preserved Ejection Fraction. Circulation 2018;epub ahead of press. [DOI] [PMC free article] [PubMed]
- 128.Borlaug BA, Carter RE, Melenovsky V, DeSimone CV, Gaba P, Killu A, Naksuk N, Lerman L and Asirvatham SJ. Percutaneous Pericardial Resection: A Novel Potential Treatment for Heart Failure With Preserved Ejection Fraction. Circ Heart Fail 2017;10:e003612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.van Woerden G, Gorter TM, Westenbrink BD, Willems TP, van Veldhuisen DJ and Rienstra M. Epicardial fat in heart failure patients with mid-range and preserved ejection fraction. Eur J Heart Fail 2018. [DOI] [PMC free article] [PubMed]
- 130.Iacobellis G, Corradi D and Sharma AM. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med 2005;2:536–43. [DOI] [PubMed] [Google Scholar]
- 131.Borlaug BA, Lam CS, Roger VL, Rodeheffer RJ and Redfield MM. Contractility and ventricular systolic stiffening in hypertensive heart disease insights into the pathogenesis of heart failure with preserved ejection fraction. J Am Coll Cardiol 2009;54:410–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tan YT, Wenzelburger F, Lee E, Heatlie G, Leyva F, Patel K, Frenneaux M and Sanderson JE. The pathophysiology of heart failure with normal ejection fraction: exercise echocardiography reveals complex abnormalities of both systolic and diastolic ventricular function involving torsion, untwist, and longitudinal motion. J Am Coll Cardiol 2009;54:36–46. [DOI] [PubMed] [Google Scholar]
- 133.Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, Liu L, Pitt B, Pfeffer MA and Solomon SD. Prognostic Importance of Impaired Systolic Function in Heart Failure With Preserved Ejection Fraction and the Impact of Spironolactone. Circulation 2015;132:402–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Abudiab MM, Redfield MM, Melenovsky V, Olson TP, Kass DA, Johnson BD and Borlaug BA. Cardiac output response to exercise in relation to metabolic demand in heart failure with preserved ejection fraction. Eur J Heart Fail 2013;15:776–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shah AM and Solomon SD. Myocardial deformation imaging: current status and future directions. Circulation 2012;125:e244–8. [DOI] [PubMed] [Google Scholar]
- 136.Biering-Sorensen T, Biering-Sorensen SR, Olsen FJ, Sengelov M, Jorgensen PG, Mogelvang R, Shah AM and Jensen JS. Global Longitudinal Strain by Echocardiography Predicts Long-Term Risk of Cardiovascular Morbidity and Mortality in a Low-Risk General Population: The Copenhagen City Heart Study. Circ Cardiovasc Imaging 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, Liu L, Pitt B, Pfeffer MA and Solomon SD. Prognostic Importance of Impaired Systolic Function in Heart Failure With Preserved Ejection Fraction and the Impact of Spironolactone. Circulation 2015;132:402–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Melenovsky V, Hwang SJ, Redfield MM, Zakeri R, Lin G and Borlaug BA. Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circ Heart Fail 2015;8:295–303. [DOI] [PubMed] [Google Scholar]
- 139.Freed BH, Daruwalla V, Cheng JY, Aguilar FG, Beussink L, Choi A, Klein DA, Dixon D, Baldridge A, Rasmussen-Torvik LJ, Maganti K and Shah SJ. Prognostic Utility and Clinical Significance of Cardiac Mechanics in Heart Failure With Preserved Ejection Fraction: Importance of Left Atrial Strain. Circ Cardiovasc Imag 2016;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zakeri R, Moulay G, Chai Q, Ogut O, Hussain S, Takahama H, Lu T, Wang XL, Linke WA, Lee HC and Redfield MM. Left Atrial Remodeling and Atrioventricular Coupling in a Canine Model of Early Heart Failure With Preserved Ejection Fraction. Circ Heart Fail 2016;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zakeri R, Chamberlain AM, Roger VL and Redfield MM. Temporal Relationship and Prognostic Significance of Atrial Fibrillation in Heart Failure Patients with Preserved Ejection Fraction: A Community-Based Study. Circulation 2013;128:1085–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Melenovsky V, Hwang SJ, Lin G, Redfield MM and Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J 2014;35:3452–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mohammed SF, Hussain I, Abou Ezzeddine OF, Takahama H, Kwon SH, Forfia P, Roger VL and Redfield MM. Right ventricular function in heart failure with preserved ejection fraction: a community-based study. Circulation 2014;130:2310–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Shah AM and Pfeffer MA. Heart failure with preserved ejection fraction: a forest of a variety of trees. Eur Heart J 2014;35:3410–2. [DOI] [PubMed] [Google Scholar]
- 145.Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT and Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol 2009;53:1119–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Vanderpool RR, Saul M, Nouraie M, Gladwin MT and Simon MA. Association Between Hemodynamic Markers of Pulmonary Hypertension and Outcomes in Heart Failure With Preserved Ejection Fraction. JAMA Cardiol 2018. [DOI] [PMC free article] [PubMed]
- 147.Gorter TM, Obokata M, Reddy YNV, Melenovsky V and Borlaug BA. Exercise unmasks distinct pathophysiologic features in heart failure with preserved ejection fraction and pulmonary vascular disease. Eur Heart J 2018;39:2825–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Andersen MJ, Hwang SJ, Kane GC, Melenovsky V, Olson TP, Fetterly K and Borlaug BA. Enhanced pulmonary vasodilator reserve and abnormal right ventricular: pulmonary artery coupling in heart failure with preserved ejection fraction. Circ Heart Fail 2015;8:542–50. [DOI] [PubMed] [Google Scholar]
- 149.Huang W, Oliveira RKF, Lei H, Systrom DM and Waxman AB. Pulmonary Vascular Resistance During Exercise Predicts Long-Term Outcomes in Heart Failure With Preserved Ejection Fraction. J Card Fail 2017;24:169–176. [DOI] [PubMed] [Google Scholar]
- 150.West JB, Dollery CT and Heard BE. Increased Pulmonary Vascular Resistance in the Dependent Zone of the Isolated Dog Lung Caused by Perivascular Edema. Circ Res 1965;17:191–206. [DOI] [PubMed] [Google Scholar]
- 151.Obokata M, Reddy YNV, Melenovsky V, Pislaru S and Borlaug BA. Deterioration in right ventricular structure and function over time in patients with heart failure and preserved ejection fraction. Eur Heart J 2018. [DOI] [PMC free article] [PubMed]
- 152.van Empel VP, Mariani J, Borlaug BA and Kaye DM. Impaired myocardial oxygen availability contributes to abnormal exercise hemodynamics in heart failure with preserved ejection fraction. J Am Heart Assoc 2014;3:e001293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Obokata M, Reddy YNV, Melenovsky V, Kane GC, Olson TP, Jarolim P and Borlaug BA. Myocardial Injury and Cardiac Reserve in Patients With Heart Failure and Preserved Ejection Fraction. J Am Coll Cardiol 2018;72:29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hwang SJ, Melenovsky V and Borlaug BA. Implications of coronary artery disease in heart failure with preserved ejection fraction. J Am Coll Cardiol 2014;63:2817–27. [DOI] [PubMed] [Google Scholar]
- 155.Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ and Redfield MM. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation 2015;131:550–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Shah SJ, Lam CSP, Svedlund S, Saraste A, Hage C, Tan RS, Beussink-Nelson L, Fermer ML, Broberg MA, Gan LM and Lund LH. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur Heart J 2018. [DOI] [PMC free article] [PubMed]
- 157.Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ and Redfield MM. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation 2015;131:550–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Crea F, Bairey Merz CN, Beltrame JF, Kaski JC, Ogawa H, Ong P, Sechtem U, Shimokawa H, Camici PG and Coronary Vasomotion Disorders International Study G. The parallel tales of microvascular angina and heart failure with preserved ejection fraction: a paradigm shift. Eur Heart J 2017;38:473–477. [DOI] [PubMed] [Google Scholar]
- 159.Elgendy IY and Pepine CJ. Heart failure with preserved ejection fraction: Is ischemia due to coronary microvascular dysfunction a mechanistic factor? Am J Med 2019. [DOI] [PMC free article] [PubMed]
- 160.Tartiere-Kesri L, Tartiere JM, Logeart D, Beauvais F and Cohen Solal A. Increased proximal arterial stiffness and cardiac response with moderate exercise in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol 2012;59:455–61. [DOI] [PubMed] [Google Scholar]
- 161.Borlaug BA, Olson TP, Lam CS, Flood KS, Lerman A, Johnson BD and Redfield MM. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol 2010;56:845–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Akiyama E, Sugiyama S, Matsuzawa Y, Konishi M, Suzuki H, Nozaki T, Ohba K, Matsubara J, Maeda H, Horibata Y, Sakamoto K, Sugamura K, Yamamuro M, Sumida H, Kaikita K, Iwashita S, Matsui K, Kimura K, Umemura S and Ogawa H. Incremental prognostic significance of peripheral endothelial dysfunction in patients with heart failure with normal left ventricular ejection fraction. J Am Coll Cardiol 2012;60:1778–86. [DOI] [PubMed] [Google Scholar]
- 163.Lee JF, Barrett-O’Keefe Z, Nelson AD, Garten RS, Ryan JJ, Nativi-Nicolau JN, Richardson RS and Wray DW. Impaired skeletal muscle vasodilation during exercise in heart failure with preserved ejection fraction. Int J Cardiol 2016;211:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Farrero M, Blanco I, Batlle M, Santiago E, Cardona M, Vidal B, Castel MA, Sitges M, Barbera JA and Perez-Villa F. Pulmonary hypertension is related to peripheral endothelial dysfunction in heart failure with preserved ejection fraction. Circ Heart Fail 2014;7:791–8. [DOI] [PubMed] [Google Scholar]
- 165.Paulus WJ and Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013;62:263–71. [DOI] [PubMed] [Google Scholar]
- 166.Kalogeropoulos A, Georgiopoulou V, Psaty BM, Rodondi N, Smith AL, Harrison DG, Liu Y, Hoffmann U, Bauer DC, Newman AB, Kritchevsky SB, Harris TB, Butler J and Health ABCSI. Inflammatory markers and incident heart failure risk in older adults: the Health ABC (Health, Aging, and Body Composition) study. J Am Coll Cardiol 2010;55:2129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sanders-van Wijk S, van Empel V, Davarzani N, Maeder MT, Handschin R, Pfisterer ME, Brunner-La Rocca HP and investigators T-C. Circulating biomarkers of distinct pathophysiological pathways in heart failure with preserved vs. reduced left ventricular ejection fraction. Eur J Heart Fail 2015;17:1006–14. [DOI] [PubMed] [Google Scholar]
- 168.Putko BN, Wang Z, Lo J, Anderson T, Becher H, Dyck JR, Kassiri Z, Oudit GY and Alberta HI. Circulating levels of tumor necrosis factor-alpha receptor 2 are increased in heart failure with preserved ejection fraction relative to heart failure with reduced ejection fraction: evidence for a divergence in pathophysiology. PLoS One 2014;9:e99495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.van Heerebeek L, Hamdani N, Falcao-Pires I, Leite-Moreira AF, Begieneman MP, Bronzwaer JG, van der Velden J, Stienen GJ, Laarman GJ, Somsen A, Verheugt FW, Niessen HW and Paulus WJ. Low myocardial protein kinase g activity in heart failure with preserved ejection fraction. Circulation 2012;126:830–9. [DOI] [PubMed] [Google Scholar]
- 170.Franssen C, Chen S, Unger A, Korkmaz HI, De Keulenaer GW, Tschope C, Leite-Moreira AF, Musters R, Niessen HW, Linke WA, Paulus WJ and Hamdani N. Myocardial Microvascular Inflammatory Endothelial Activation in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail 2016;4:312–24. [DOI] [PubMed] [Google Scholar]
- 171.Runte KE, Bell SP, Selby DE, Haussler TN, Ashikaga T, LeWinter MM, Palmer BM and Meyer M. Relaxation and the Role of Calcium in Isolated Contracting Myocardium From Patients With Hypertensive Heart Disease and Heart Failure With Preserved Ejection Fraction. Circ Heart Fail 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Selby DE, Palmer BM, Lewinter MM and Meyer M. Tachycardia-induced diastolic dysfunction and resting tone in myocardium from patients with a normal ejection fraction. Journal of the American College of Cardiology 2011;58:147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zile MR, Baicu CF, Ikonomidis JS, Stroud RE, Nietert PJ, Bradshaw AD, Slater R, Palmer BM, Van Buren P, Meyer M, Redfield MM, Bull DA, Granzier HL and LeWinter MM. Myocardial stiffness in patients with heart failure and a preserved ejection fraction: contributions of collagen and titin. Circulation 2015;131:1247–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bishu K, Hamdani N, Mohammed SF, Kruger M, Ohtani T, Ogut O, Brozovich FV, Burnett JC Jr., Linke WA and Redfield MM. Sildenafil and B-type natriuretic peptide acutely phosphorylate titin and improve diastolic distensibility in vivo. Circulation 2011;124:2882–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Methawasin M, Strom JG, Slater RE, Fernandez V, Saripalli C and Granzier H. Experimentally Increasing the Compliance of Titin Through RNA Binding Motif-20 (RBM20) Inhibition Improves Diastolic Function In a Mouse Model of Heart Failure With Preserved Ejection Fraction. Circulation 2016;134:1085–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Loffredo FS, Nikolova AP, Pancoast JR and Lee RT. Heart failure with preserved ejection fraction: molecular pathways of the aging myocardium. Circ Res 2014;115:97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lavandero S, Chiong M, Rothermel BA and Hill JA. Autophagy in cardiovascular biology. J Clin Invest 2015;125:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sarma S, Carrick-Ranson G, Fujimoto N, Adams-Huet B, Bhella PS, Hastings JL, Shafer KM, Shibata S, Boyd K, Palmer D, Szczepaniak EW, Szczepaniak LS and Levine BD. Effects of age and aerobic fitness on myocardial lipid content. Circ Cardiovasc Imaging 2013;6:1048–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Luptak I, Sverdlov AL, Panagia M, Qin F, Pimentel DR, Croteau D, Siwik DA, Ingwall JS, Bachschmid MM, Balschi JA and Colucci WS. Decreased ATP production and myocardial contractile reserve in metabolic heart disease. J Mol Cell Cardiol 2018;116:106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sverdlov AL, Elezaby A, Qin F, Behring JB, Luptak I, Calamaras TD, Siwik DA, Miller EJ, Liesa M, Shirihai OS, Pimentel DR, Cohen RA, Bachschmid MM and Colucci WS. Mitochondrial Reactive Oxygen Species Mediate Cardiac Structural, Functional, and Mitochondrial Consequences of Diet-Induced Metabolic Heart Disease. J Am Heart Assoc 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sorop O, Heinonen I, van Kranenburg M, van de Wouw J, de Beer VJ, Nguyen ITN, Octavia Y, van Duin RWB, Stam K, van Geuns RJ, Wielopolski PA, Krestin GP, van den Meiracker AH, Verjans R, van Bilsen M, Danser AHJ, Paulus WJ, Cheng C, Linke WA, Joles JA, Verhaar MC, van der Velden J, Merkus D and Duncker DJ. Multiple common comorbidities produce left ventricular diastolic dysfunction associated with coronary microvascular dysfunction, oxidative stress, and myocardial stiffening. Cardiovasc Res 2018;114:954–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Schwarzl M, Hamdani N, Seiler S, Alogna A, Manninger M, Reilly S, Zirngast B, Kirsch A, Steendijk P, Verderber J, Zweiker D, Eller P, Hofler G, Schauer S, Eller K, Maechler H, Pieske BM, Linke WA, Casadei B and Post H. A porcine model of hypertensive cardiomyopathy: implications for heart failure with preserved ejection fraction. Am J Physiol Heart Circ Physiol 2015;309:H1407–18. [DOI] [PubMed] [Google Scholar]
- 183.Gevaert AB, Shakeri H, Leloup AJ, Van Hove CE, De Meyer GRY, Vrints CJ, Lemmens K and Van Craenenbroeck EM. Endothelial Senescence Contributes to Heart Failure With Preserved Ejection Fraction in an Aging Mouse Model. Circ Heart Fail 2017;10. [DOI] [PubMed] [Google Scholar]
- 184.Saiki H, Moulay G, Guenzel AJ, Liu W, Decklever TD, Classic KL, Pham L, Chen HH, Burnett JC, Russell SJ and Redfield MM. Experimental cardiac radiation exposure induces ventricular diastolic dysfunction with preserved ejection fraction. Am J Physiol Heart Circ Physiol 2017;313:H392–H407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Parikh KS, Sharma K, Fiuzat M, Surks HK, George JT, Honarpour N, Depre C, Desvigne-Nickens P, Nkulikiyinka R, Lewis GD, Gomberg-Maitland M, O’Connor CM, Stockbridge N, Califf RM, Konstam MA, Januzzi JL Jr., Solomon SD, Borlaug BA, Shah SJ, Redfield MM and Felker GM. Heart Failure With Preserved Ejection Fraction Expert Panel Report: Current Controversies and Implications for Clinical Trials. JACC Heart Fail 2018;6:619–632. [DOI] [PubMed] [Google Scholar]
- 186.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW and Westlake C. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017;136:e137–e161. [DOI] [PubMed] [Google Scholar]
- 187.Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J and Investigators P-C. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J 2006;27:2338–45. [DOI] [PubMed] [Google Scholar]
- 188.Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A and Investigators IP. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 2008;359:2456–67. [DOI] [PubMed] [Google Scholar]
- 189.Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O’Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM and Investigators T. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014;370:1383–92. [DOI] [PubMed] [Google Scholar]
- 190.Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Heitner JF, Lewis EF, O’Meara E, Rouleau JL, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, McKinlay SM and Pitt B. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation 2015;131:34–42. [DOI] [PubMed] [Google Scholar]
- 191.Solomon SD, Rizkala AR, Lefkowitz MP, Shi VC, Gong J, Anavekar N, Anker SD, Arango JL, Arenas JL, Atar D, Ben-Gal T, Boytsov SA, Chen CH, Chopra VK, Cleland J, Comin-Colet J, Duengen HD, Echeverria Correa LE, Filippatos G, Flammer AJ, Galinier M, Godoy A, Goncalvesova E, Janssens S, Katova T, Kober L, Lelonek M, Linssen G, Lund LH, O’Meara E, Merkely B, Milicic D, Oh BH, Perrone SV, Ranjith N, Saito Y, Saraiva JF, Shah S, Seferovic PM, Senni M, Sibulo AS Jr., Sim D, Sweitzer NK, Taurio J, Vinereanu D, Vrtovec B, Widimsky J Jr., Yilmaz MB, Zhou J, Zweiker R, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, Van Veldhuisen DJ, Zannad F, Zile MR and McMurray JJV. Baseline Characteristics of Patients With Heart Failure and Preserved Ejection Fraction in the PARAGON-HF Trial. Circ Heart Fail 2018;11:e004962. [DOI] [PubMed] [Google Scholar]
- 192.Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, Strickland W, Neelagaru S, Raval N, Krueger S, Weiner S, Shavelle D, Jeffries B, Yadav JS and Group CTS. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 2011;377:658–66. [DOI] [PubMed] [Google Scholar]
- 193.Adamson PB, Abraham WT, Bourge RC, Costanzo MR, Hasan A, Yadav C, Henderson J, Cowart P and Stevenson LW. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ Heart Fail 2014;7:935–44. [DOI] [PubMed] [Google Scholar]
- 194.Desai AS, Bhimaraj A, Bharmi R, Jermyn R, Bhatt K, Shavelle D, Redfield MM, Hull R, Pelzel J, Davis K, Dalal N, Adamson PB and Heywood JT. Ambulatory Hemodynamic Monitoring Reduces Heart Failure Hospitalizations in “Real-World” Clinical Practice. J Am Coll Cardiol 2017;69:2357–2365. [DOI] [PubMed] [Google Scholar]
- 195.Strobeck JE, Feldschuh J and Miller WL. Heart Failure Outcomes With Volume-Guided Management. JACC Heart Fail 2018;6:940–948. [DOI] [PubMed] [Google Scholar]
- 196.Kitzman DW, Brubaker PH, Morgan TM, Stewart KP and Little WC. Exercise training in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. Circ Heart Fail 2010;3:659–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Pandey A, Parashar A, Kumbhani D, Agarwal S, Garg J, Kitzman D, Levine B, Drazner M and Berry J. Exercise training in patients with heart failure and preserved ejection fraction: meta-analysis of randomized control trials. Circ Heart Fail 2015;8:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Haykowsky MJ, Brubaker PH, Stewart KP, Morgan TM, Eggebeen J and Kitzman DW. Effect of endurance training on the determinants of peak exercise oxygen consumption in elderly patients with stable compensated heart failure and preserved ejection fraction. J Am Coll Cardiol 2012;60:120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Edelmann F, Gelbrich G, Dungen HD, Frohling S, Wachter R, Stahrenberg R, Binder L, Topper A, Lashki DJ, Schwarz S, Herrmann-Lingen C, Loffler M, Hasenfuss G, Halle M and Pieske B. Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: results of the Ex-DHF (Exercise training in Diastolic Heart Failure) pilot study. J Am Coll Cardiol 2011;58:1780–91. [DOI] [PubMed] [Google Scholar]
- 200.Kitzman DW, Brubaker P, Morgan T, Haykowsky M, Hundley G, Kraus WE, Eggebeen J and Nicklas BJ. Effect of Caloric Restriction or Aerobic Exercise Training on Peak Oxygen Consumption and Quality of Life in Obese Older Patients With Heart Failure With Preserved Ejection Fraction: A Randomized Clinical Trial. JAMA 2016;315:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hummel SL, Seymour EM, Brook RD, Sheth SS, Ghosh E, Zhu S, Weder AB, Kovacs SJ and Kolias TJ. Low-sodium DASH diet improves diastolic function and ventricular-arterial coupling in hypertensive heart failure with preserved ejection fraction. Circ Heart Fail 2013;6:1165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kannel WB, Dawber TR, Kagan A, Revotskie N and Stokes J 3rd. Factors of risk in the development of coronary heart disease--six year follow-up experience. The Framingham Study. Ann Intern Med 1961;55:33–50. [DOI] [PubMed] [Google Scholar]
- 203.Kannel WB, Castelli WP, McNamara PM, McKee PA and Feinleib M. Role of blood pressure in the development of congestive heart failure. The Framingham study. N Engl J Med 1972;287:781–7. [DOI] [PubMed] [Google Scholar]
- 204.Psaty BM, Smith NL, Siscovick DS, Koepsell TD, Weiss NS, Heckbert SR, Lemaitre RN, Wagner EH and Furberg CD. Health outcomes associated with antihypertensive therapies used as first-line agents. A systematic review and meta-analysis. JAMA 1997;277:739–45. [PubMed] [Google Scholar]
- 205.Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA 1991;265:3255–64. [PubMed] [Google Scholar]
- 206.Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, Stoyanovsky V, Antikainen RL, Nikitin Y, Anderson C, Belhani A, Forette F, Rajkumar C, Thijs L, Banya W, Bulpitt CJ and Group HS. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008;358:1887–98. [DOI] [PubMed] [Google Scholar]
- 207.Group SR, Wright JT Jr., Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC Jr., Fine LJ, Cutler JA, Cushman WC, Cheung AK and Ambrosius WT. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med 2015;373:2103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Williamson JD, Supiano MA, Applegate WB, Berlowitz DR, Campbell RC, Chertow GM, Fine LJ, Haley WE, Hawfield AT, Ix JH, Kitzman DW, Kostis JB, Krousel-Wood MA, Launer LJ, Oparil S, Rodriguez CJ, Roumie CL, Shorr RI, Sink KM, Wadley VG, Whelton PK, Whittle J, Woolard NF, Wright JT Jr., Pajewski NM and Group SR. Intensive vs Standard Blood Pressure Control and Cardiovascular Disease Outcomes in Adults Aged >/=75 Years: A Randomized Clinical Trial. JAMA 2016;315:2673–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Major cardiovascular events in hypertensive patients randomized to doxazosin vs chlorthalidone: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). ALLHAT Collaborative Research Group. JAMA 2000;283:1967–75. [PubMed] [Google Scholar]
- 210.Officers A, Coordinators for the ACRGTA and Lipid-Lowering Treatment to Prevent Heart Attack T. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002;288:2981–97. [DOI] [PubMed] [Google Scholar]
- 211.Group AS, Cushman WC, Evans GW, Byington RP, Goff DC Jr., Grimm RH Jr., Cutler JA, Simons-Morton DG, Basile JN, Corson MA, Probstfield JL, Katz L, Peterson KA, Friedewald WT, Buse JB, Bigger JT, Gerstein HC and Ismail-Beigi F. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010;362:1575–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE and Investigators E-RO. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med 2015;373:2117–28. [DOI] [PubMed] [Google Scholar]
- 213.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR and Group CPC. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med 2017;377:644–657. [DOI] [PubMed] [Google Scholar]
- 214.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS and Investigators D-T. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med 2018.
- 215.Aggarwal M, Bozkurt B, Panjrath G, Aggarwal B, Ostfeld RJ, Barnard ND, Gaggin H, Freeman AM, Allen K, Madan S, Massera D, Litwin SE, American College of Cardiology’s N and Lifestyle Committee of the Prevention of Cardiovascular Disease C. Lifestyle Modifications for Preventing and Treating Heart Failure. J Am Coll Cardiol 2018;72:2391–2405. [DOI] [PubMed] [Google Scholar]
- 216. Diagnosing heart failure with preserved ejection fraction: The new HFA consensus.
- 217.Newton-Cheh C, Larson MG, Vasan RS, Levy D, Bloch KD, Surti A, Guiducci C, Kathiresan S, Benjamin EJ, Struck J, Morgenthaler NG, Bergmann A, Blankenberg S, Kee F, Nilsson P, Yin X, Peltonen L, Vartiainen E, Salomaa V, Hirschhorn JN, Melander O and Wang TJ. Association of common variants in NPPA and NPPB with circulating natriuretic peptides and blood pressure. Nat Genet 2009;41:348–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Bajaj NS, Gutierrez OM, Arora G, Judd SE, Patel N, Bennett A, Prabhu SD, Howard G, Howard VJ, Cushman M and Arora P. Racial Differences in Plasma Levels of N-Terminal Pro-B-Type Natriuretic Peptide and Outcomes: The Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. JAMA Cardiol 2018;3:11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington-Cruz M, Kristen AV, Grogan M, Witteles R, Damy T, Drachman BM, Shah SJ, Hanna M, Judge DP, Barsdorf AI, Huber P, Patterson TA, Riley S, Schumacher J, Stewart M, Sultan MB, Rapezzi C and Investigators A-AS. Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy. N Engl J Med 2018;379:1007–1016. [DOI] [PubMed] [Google Scholar]
- 220.Solomon SD, Adams D, Kristen A, Grogan M, Gonzalez-Duarte A, Maurer MS, Merlini G, Damy T, Slama MS, Brannagan Iii TH, Dispenzieri A, Berk JL, Shah AM, Garg P, Vaishnaw A, Karsten V, Chen J, Gollob J, Vest J and Suhr O. Effects of Patisiran, an RNA Interference Therapeutic, on Cardiac Parameters in Patients with Hereditary Transthyretin-Mediated Amyloidosis: An Analysis of the APOLLO Study. Circulation 2018. [DOI] [PubMed]


