Abstract
Objective
To determine if migraine with aura is associated with neuroinflammation, which has been suggested by preclinical models of cortical spreading depression (CSD) as well as imaging of human pain conditions.
Methods
Thirteen migraineurs with aura and 16 healthy controls received integrated PET/MRI brain scans with [11C]PBR28, a radioligand that binds to the 18 kDa translocator protein, a marker of glial activation. Standardized uptake value ratio (SUVR) was compared between groups, and regressed against clinical variables, using region of interest and whole-brain voxelwise analyses.
Results
Compared to healthy controls, migraineurs demonstrated SUVR elevations in nociceptive processing areas (e.g., thalamus and primary/secondary somatosensory and insular cortices) as well as in areas previously shown to be involved in CSD generation (visual cortex). SUVR levels in frontoinsular cortex, primary/secondary somatosensory cortices, and basal ganglia were correlated with frequency of migraine attacks.
Conclusions
These findings demonstrate that migraine with aura is associated with neuroimmune activation/neuroinflammation, and support a possible link between CSD and glial activation, previously observed in animals.
Despite being the third most prevalent medical disorder worldwide,1 the pathophysiology of migraine remains incompletely understood. Cortical spreading depression (CSD) is now well-accepted as the pathophysiologic mechanism underlying migraine aura,2 but the mechanisms leading to headache pain in migraine following an episode of aura are still not fully understood in humans (for review, see reference 3). Recent data from rodent models demonstrated that CSD results in a cascade of events leading to the release of proinflammatory mediators, activation of trigeminal nerve fibers, and sterile inflammation.4 However, direct evidence of neuroinflammation in migraineurs is lacking.
While the role of neuroinflammation in human pain disorders remains uncertain, our group previously demonstrated that patients with chronic low back pain exhibit elevated levels of the 18 kDa translocator protein (TSPO),5 a marker of glial activation.6 In the healthy CNS, TSPO is constitutively expressed at low levels by multiple cell types, including glia and neurons.7 However, during neuroinflammatory states, TSPO is substantially upregulated, particularly in astrocytes and microglia,8 which can be quantified by the PET ligand [11C]PBR28.9 In turn, CSD has been shown to induce elevated TSPO-ligand uptake in a rodent model.10 Despite these results, no study has ever assessed TSPO levels in human migraineurs. Here, we report findings from our investigation in human migraineurs, using combined [11C]PBR28 PET/MRI. We hypothesized that individuals who experience migraine with aura would exhibit increased glial activation, as indicated by elevated TSPO PET signal, in brain regions contributing to aura presentation and in regions involved in nociceptive processing, compared to healthy controls.
Methods
Standard protocol approvals, registrations, and patient consents
This cross-sectional study was conducted at the Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital. The institutional review board and the Radioactive Drug Research Committee approved the protocol, and all participants gave written informed consent.
Participants
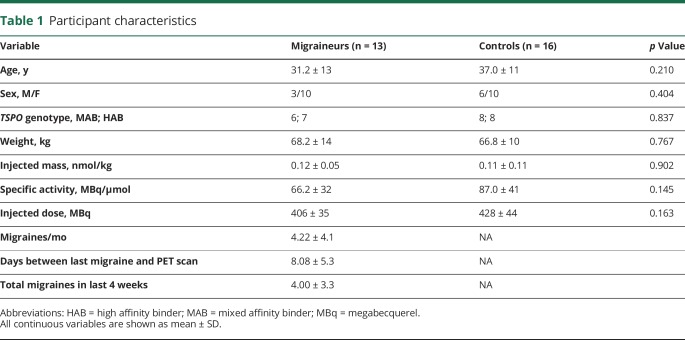
Demographic information for study participants is displayed in table 1. Thirteen patients experiencing migraine with aura completed study procedures, and were compared with 16 healthy controls recruited for other studies at our center, selected for group matching in regard to age, sex, and TPSO polymorphism. Demographic and imaging data for a portion of the healthy controls has been reported previously.5,11,12 Migraineurs were recruited via neurologists and the Partners clinical trials website from June 2012 to May 2016, and controls were recruited from the general population. Inclusion criteria for migraineurs were diagnosis meeting the 2004 International Headache Society (IHS) criteria for migraine with aura (IHS 1.1), with a minimum of 1 and a maximum of 15 attacks a month, experiencing migraine attacks for at least a year, and willingness to refrain from nonsteroidal anti-inflammatory drug (NSAID) exposure in the 2 weeks prior to scanning. Participants were excluded for recent hospitalization for a major psychiatric disorder, endorsing or testing positive for illicit drug use, any known inflammatory disease (e.g., inflammatory bowel disease), or any contraindications for PET or MRI scanning (e.g., pregnancy, claustrophobia, ferromagnetic implants). During an initial screening visit, a blood sample was obtained to genotype participants for the Ala147Thr TSPO polymorphism, which is known to affect binding affinity for [11C]PBR28.13 Low-affinity binders (Thr/Thr) were excluded from participation; high-affinity binders (Ala/Ala) and mixed-affinity binders (Ala/Thr) were included and genotype was modeled in the statistical design. Participants were scanned in the interictal period (with the exception of one patient who experienced a migraine attack during the scan session), and required to have at least one migraine attack with aura at least 15 days before the imaging session. This was selected as a threshold based on a previous animal study showing increasing TSPO levels up to 15 days after induction of CSD (no further point was examined).10 When the data were analyzed excluding the patient who experienced a migraine attack during scanning, the results were unchanged. Therefore, all analyses included this patient.
Table 1.
Participant characteristics
Twenty-nine participants with migraine were enrolled in the study; out of these, 13 completed scan visits. Causes for attrition were low-affinity binding (n = 2), cancelling after first screening for reasons concerning issues with injection of a radioactive tracer (n = 8), not wanting to stop taking anti-inflammatories (n = 1), lost to follow-up (n = 1), and failing to be able to schedule a scanning session following at least 2 episodes of migraine in the last 15 days (n = 4).
No migraineurs were taking antimigraine preventive medications. Because there are no previously published studies using TSPO PET to image migraineurs, we had no prior effect sizes on which to base power analyses for sample size determination.
Image acquisition
Dynamic [11C]PBR28 scans were performed with an integrated MRI/PET scanner consisting of a dedicated brain avalanche photodiode-based PET scanner in the bore of a Siemens (Munich, Germany) 3T Tim Trio MRI. Structural T1 images were acquired prior to tracer injection for anatomical localization, spatial normalization of PET data, and generation of attenuation correction maps.14 Dynamic PET acquisition began with an IV bolus injection of [11C]PBR28. PET data were stored in listmode format.
Data analysis
Standardized uptake value (SUV) images (60–90 minutes post-administration of [11C]PBR28) were generated as described previously.5,12 Magnetization-prepared rapid gradient echo (MPRAGE)–based attenuation correction was performed according to published methods.14 SUV maps were coregistered to the MPRAGE, transformed to Montreal Neurological Institute (MNI) space, and smoothed with an 8 mm (full width at half maximum) Gaussian kernel using FSL tools (FMRIB's Software Library, version 4.1.9, fmrib.ox.ac.uk/fsl/). Reconstruction of cortical surface from the T1 images was performed using Freesurfer 5.3.0 (surfer.nmr.mgh.harvard.edu/). Previously, TSPO PET signal has been shown to exhibit considerable interindividual variability, which may not be linked to neuroinflammation.15 In order to account for this variability, several groups have reduced variability by normalizing PET signal to uptake in whole brain or whole gray matter.5,12,16,17 While this approach may improve the detection of focal effects by robustly reducing between-participant variability, it may be not be appropriate when the condition investigated is characterized by widespread, rather than regional, inflammation, or when the extent of suspected inflammatory response is unknown. For some pathologies, a viable option is the identification of an a priori, anatomically defined pseudo-reference region, based on knowledge that this region is relatively unaffected in the diseases studied. For instance, our group has recently validated the use of the occipital cortex in patients with chronic low back pain and amyotrophic lateral sclerosis.6 However, because the occipital cortex is known to be affected in migraine with aura, for this study we devised a strategy aimed at identifying a data-driven pseudoreference region, based on the absence of statistically significant SUV differences between groups. To this end, whole-brain voxelwise group SUV comparisons were conducted independently for high-affinity and mixed-affinity binders, covarying for age and injected dose (although these variables were not different across groups; ps > 0.16; table 1). The resulting Z-map was thresholded to include only voxels exhibiting values between −0.2 and +0.2 (i.e., p > 0.84), and the thresholded Z-maps for each genotype were combined to create a conjunction mask. The resulting mask was further intersected with an eroded MNI brain mask to ensure that no CSF or extra-brain voxels were included (figure 1). Voxel-wise SUV ratio (SUVR) images were obtained by dividing SUV images by average SUV from this minimal group-difference region. Mean SUVR was extracted from several a priori selected regions of interest (ROIs): thalamus, primary somatosensory (S1) representation of the face, insula, trigeminal nuclei, and primary visual cortex (V1). Thalamus and S1 were chosen because they previously demonstrated elevated [11C]PBR28 SUVR in another pain disorder: chronic back pain.5 Specifically, thalamic ROI corresponded to the left thalamic cluster that exhibited statistically significant differences across groups in our previous study.5 For the S1 ROI, we selected the somatotopic representation of the face, as our previous study suggested that [11C]PBR28 S1 elevations in pain patients may occur within the somatotopic representations of the body site affected by their pain symptoms (i.e., the representation of the lumbar spine in chronic low back pain patients5). Since the face representation is immediately ventral to the representation of the hand, the somatosensory face area ROI was created by having an expert neuroscientist (N.H.) manually draw the portions of the postcentral gyrus and central sulcus ventral to the hand knob18 on the postcentral gyrus label from the Harvard-Oxford Cortical Structural Atlas, which is distributed with FSL (fmrib.ox.ac.uk/fsl). The insula, trigeminal nuclei, and V1 were included as these regions have been highly implicated in migraine pathology,19,20 including aura presentation. Insula and V1 ROIs were obtained using the “insular cortex” and “intracalcarine cortex” labels from the Harvard-Oxford atlas, respectively. All ROI data were extracted from images in standard MNI space. All probabilistic labels were thresholded at the arbitrary threshold of 30. Trigeminal nucleus ROIs were obtained by creating a 3 mm3 sphere around the peak voxel from a previous study employing an air-puff challenge in migraineurs to functionally localize the spinal trigeminal nuclei.21
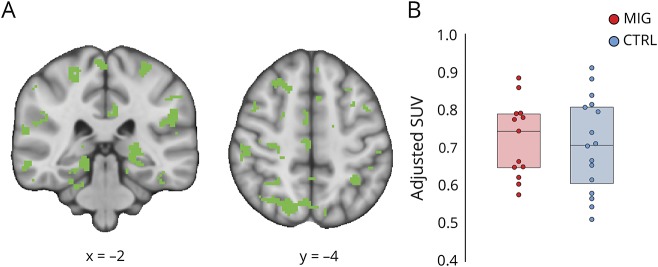
Figure 1. No group difference in pseudoreference uptake.
(A) Pseudoreference region used to create standardized uptake value (SUV) ratio images, computed by identifying regions showing no differences (p > 0.84) in SUV between groups, regressing out effects of TSPO polymorphism, age, and injected dose. (B) For visualization purposes, average adjusted SUV for each group is displayed in box plots, where boxes represent the 25th to 75th interquartile range, and the horizontal line represents the median.
Statistical analysis
Group differences in demographics were assessed with t tests for continuous variables and Fisher exact test for categorical variables. ROI SUVR was compared across groups using general linear models, employing Bonferroni correction for multiple comparisons. Associations between ROI SUVR and clinical variables (i.e., number of migraine attacks per month, days between the scanning visit and last attack, and total number of attacks in the last 4 weeks) were assessed with linear regression analyses, with TSPO polymorphism as a regressor of no interest. In addition, we assessed group differences and relationships between SUVR and clinical variables on a whole-brain voxelwise level with a mixed-effect analysis using FSL's FEAT tool, a cluster-forming threshold of Z = 2.3, and a cluster size significance threshold of p = 0.05 to correct for multiple comparisons. Age and injected dose were included as regressors of no interest in statistical models assessing between-group differences. TSPO polymorphism was included as a regressor of no interest in all statistical models.
Data availability statement
Anonymized data will be shared by request from any qualified investigator for purposes of replicating procedures and results.
Results
Participant characteristics
Participant information is displayed in table 1. There were no group differences in distribution of sex or TSPO polymorphism, or in average age, weight, or any tracer injection measure (ps > 0.145). Clinical details are given in table 2.
Table 2.
Clinical characteristics
A priori ROI group differences
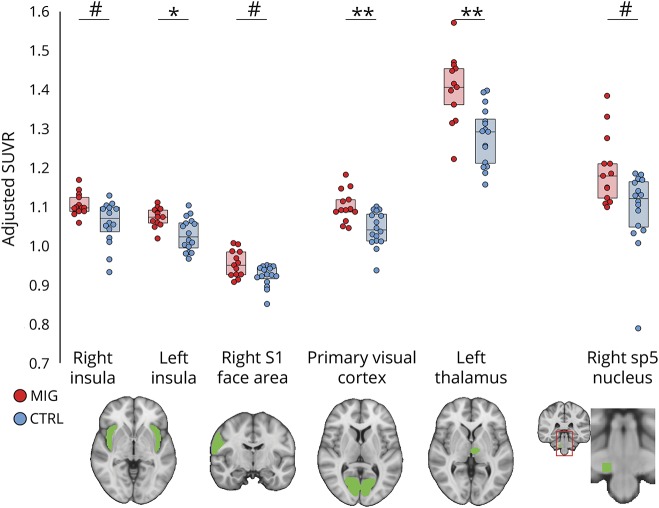
Group comparisons (figure 2) indicated that SUVR in patients was elevated in the left thalamus (p = 0.002; corrected), left insula (p = 0.016; corrected), and V1 (p = 0.008; corrected). SUVR was higher on average for right spinal trigeminal nucleus (p = 0.056; corrected), right S1 face area (p = 0.08; corrected), and right insula (p = 0.096, corrected), though these differences were not statistically significant. Additional brain regions (left S1 face area and left spinal trigeminal nucleus) did not yield group differences (p > 0.384, corrected).
Figure 2. Region of interest (ROI) [11C]PBR28 standardized uptake value ratio (SUVR) is elevated in migraineurs with aura.
Group comparison of ROI SUVR. SUVR adjusted for age, TSPO polymorphism, and injected dose is displayed. Boxes represent the 25th to 75th interquartile range, and the horizontal line represents the median. **Significant group differences at p < 0.01, corrected; *significant group differences at p < 0.05, corrected; #group differences at p < 0.10, corrected.
Voxelwise group differences
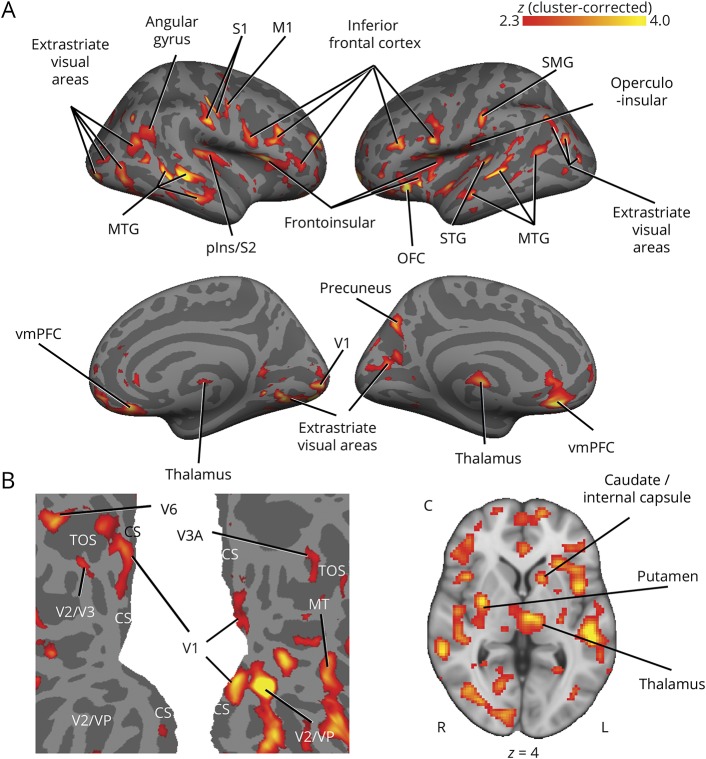
A voxelwise group comparison revealed statistically significant elevations in [11C]PBR28 signal in several regions throughout the brain in migraineurs compared to controls, including posterior insula, frontoinsular cortices, primary and secondary somatosensory cortex, primary motor, visual, and auditory cortices, dorsolateral, ventrolateral, and ventromedial prefrontal cortex, orbitofrontal cortex, and putamen (figure 3, A and C; see table 3 for a complete list of regions). Notably, in addition to primary visual cortex, increased PET signal was observed in motion-processing areas MT and V3A (figure 3B). There were no regions that showed an increase in controls compared to migraineurs.
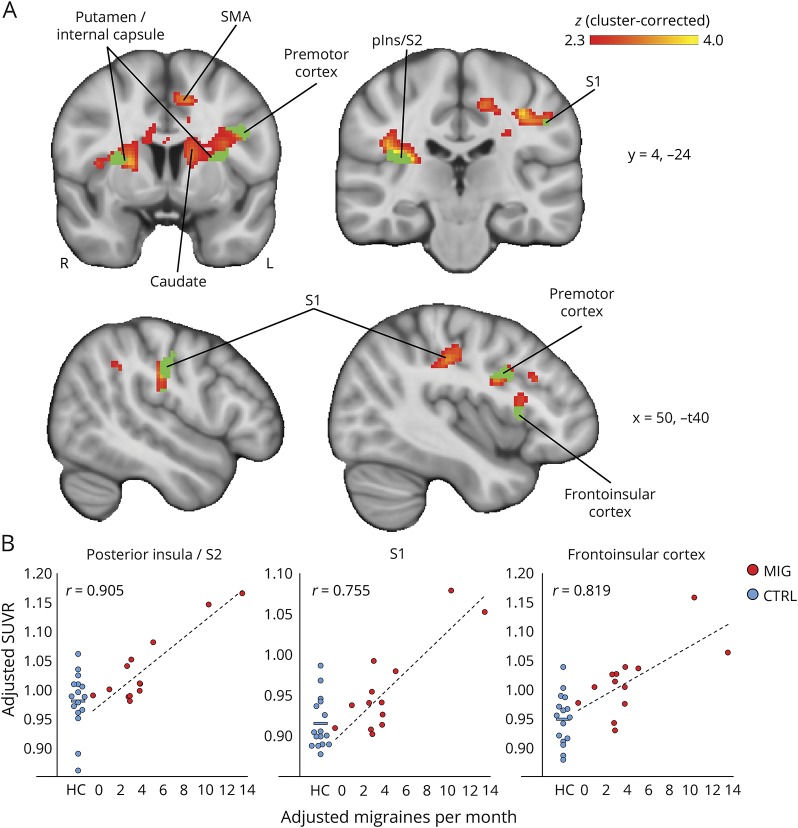
Figure 3. Voxelwise [11C]PBR28 standardized uptake value ratio (SUVR) is elevated in migraineurs with aura.
Regions of elevated PET signal in migraineurs with aura compared to controls are shown in red–yellow color scale. (A) Group differences in cortical [11C]PBR28 SUVR are displayed on surface projections. There were no significant regions for the controls > migraineurs contrast. (B) Flat map of occipital cortex. Left hemisphere is shown on the left, right hemisphere on the right. Lighter gray = gyrus, darker gray = sulcus. (C) An axial slice is shown to display subcortical SUVR differences. CS = calcarine sulcus; M1 = primary motor cortex; MTG = middle temporal gyrus; pIns = posterior insula; OFC = orbitofrontal cortex; S1/S2 = primary/secondary somatosensory cortex; SMG = supramarginal gyrus; STG = superior temporal gyrus; TOS = transverse occipital sulcus; V1 = primary visual cortex; vmPFC = ventromedial prefrontal cortex.
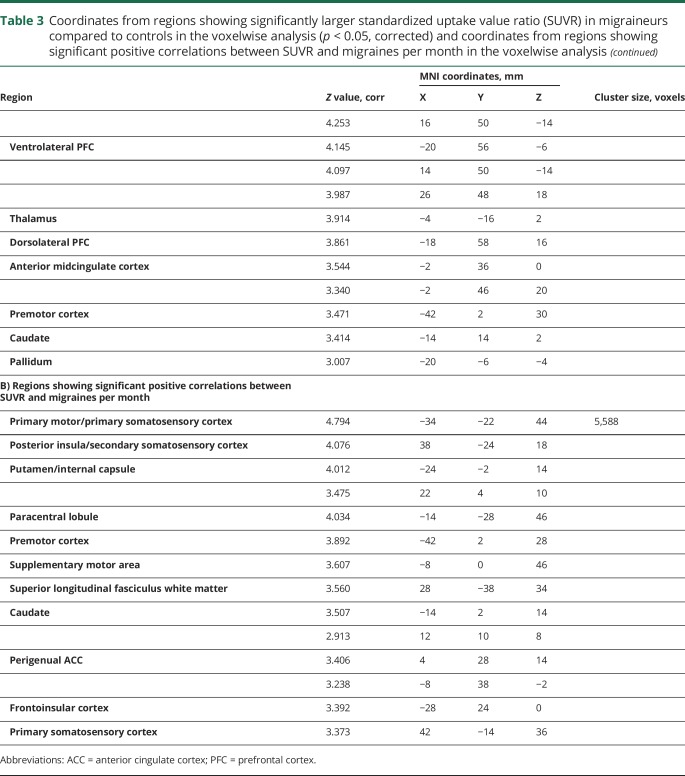
Table 3.
Coordinates from regions showing significantly larger standardized uptake value ratio (SUVR) in migraineurs compared to controls in the voxelwise analysis (p < 0.05, corrected) and coordinates from regions showing significant positive correlations between SUVR and migraines per month in the voxelwise analysis
Associations between imaging and clinical characteristics
In migraineurs, number of migraines per month preceding the scan demonstrated a positive correlation with SUVR in the left S1 face area ROI (r = 0.737; p = 0.006, uncorrected). Monthly migraine frequency also showed a positive association with SUVR in V1 (r = 0.549; p = 0.065, uncorrected), though it did not meet statistical significance. There were no other significant associations in ROI analyses. Voxelwise regression analyses showed several brain regions where SUVR was positively correlated with number of migraine attacks per month (figure 4, table 3), including the right posterior insula/S2, right S1, left frontoinsular cortex, and pregenual anterior cingulate cortex (ACC). There were no other significant associations between SUVR and clinical variables, whether in ROI or voxelwise analyses.
Figure 4. Migraine frequency is positively associated with [11C]PBR28 standardized uptake value ratio (SUVR).
(A) Regions showing a significant positive correlation between [11C]PBR28 SUVR and migraine attacks per month preceding the scan in migraineurs are shown in red–yellow color scale (p < 0.05, cluster-corrected). Regions in green showed significant effects in both voxel-wise analyses, that is, SUVR in these regions was significantly higher in migraineurs compared to controls (see figure 3), and also significantly positively correlated with migraine frequency. No regions displayed significant negative associations between SUVR and migraine frequency. pIns = posterior insula; S1/S2 = primary/secondary somatosensory cortex; SMA = supplementary motor area. (B) Average SUVR from several regions showing a positive association between migraine attacks per month and SUVR. SUVR in migraineurs is plotted against migraine frequency; healthy control SUVR is displayed to the left for comparison. Group differences were significant for right posterior insula/S2 (p = 0.012), S1 (p = 0.016), and frontoinsular cortex (p = 0.001). All data are adjusted for age, TSPO polymorphism, and injected dose.
Discussion
We provide in vivo evidence that migraineurs with one or several attacks of migraine with aura in the preceding 2 weeks exhibit brain elevations in [11C]PBR28 signal, indicative of glial activation. Furthermore, we provide preliminary evidence that neuroinflammation in migraineurs is positively associated with the frequency of migraine attacks.
Previous studies using PET in migraine have examined function/metabolism with H2O15 (e.g., references22–26) or evaluated receptor availability (for example, 5HT1A receptor with [18F]MPFF),27 whereas the present study addressed the question of neuroinflammation with glial uptake of [11C]PBR28 as a proxy for neuroinflammation produced by CSD.
Among the regions displaying elevated [11C]PBR28 signal in the migraineurs were structures involved in pain processing, including basal ganglia, thalamus, insula, primary and secondary somatosensory cortices, and ACC,28 that our previous work has directly implicated in migraine pathophysiology, by demonstrating increased intrinsic connectivity with the periaqueductal gray matter.29 Interestingly, several of these regions had been shown to have lower 5-HT1B binding in migraineurs, including ACC, sensorimotor cortex, and insula,30 further implicating them in migraine pathology. In addition to pain-relevant regions, we observed elevation in [11C]PBR28 signal in one area previously directly shown to be involved in CSD generation (extrastriate area V3A), as well as in areas affected during episodes of aura (e.g., Broca area).
The present work furthers the interpretation of brain alterations demonstrated in previous studies, particularly in the thalamus and in the basal ganglia. For instance, our group previously showed increased thalamic T1 relaxation time in migraineurs with aura, compared to migraineurs without aura and healthy controls, which could reflect increased glial content, or iron deposition.31 In addition, a recent study in migraineurs showed that the thalamus (dorsomedial) demonstrated abnormal oscillatory activity compared to controls.32 The observed oscillations were at a similar frequency to astrocytic Ca2+ waves, which could indicate increased astrocytic activation in this region, as has been previously suggested in a study of orofacial pain.33 By demonstrating that the thalamus is associated with increases in [11C]PBR28 signal, this work suggests that glial activation may underlie the alterations reported in those studies. Interestingly, elevation in dorsomedial thalamic [11C]PBR28 signal was observed in our previous study in low-back pain patients.5 Basal ganglia disorders have also been associated with migraine,34 and one recent study reported anatomical and functional changes in the basal ganglia of migraineurs that they associated with repeated episodes of pain or the usage of triptans.35 In this article, the authors also hypothesized that differences in the basal ganglia may be related to altered cortico-thalamic inputs and increased excitability state. Interestingly, we found a significant positive correlation between migraine frequency and [11C]PBR28 uptake in the caudate and the putamen, but not in the thalamus. The precise relation between the basal ganglia, the cortico-thalamic activity, and the excitation/inhibition imbalance in migraine should be further examined in future studies.
Numerous preclinical studies provide evidence for a key role of glial cells (such as microglia and astrocytes) in the establishment or maintenance of persistent pain (e.g., reference 36). In the presence of an injury, for instance, these cells respond by undergoing a series of cellular and molecular changes collectively known as glial activation, which include proliferation (e.g., reference 37), upregulation of surface markers and receptors (e.g., P2RX4 and CX3CR138), and production of cytokines and mediators and factors that activate or sensitize nociceptive neurons, including interleukin-6, interleukin-1-β, tumor necrosis factor–α, and the brain-derived neurotrophic factor.36,39 Since pharmacologic inhibition of microglial40,41 or astrocytic activation39 prevents, reverts, or delays the establishment of persistent pain, animal studies provide evidence in support of a key role for glial cells in persistent pain, and may therefore be a promising therapeutic target for human pain as well. While the clinical significance of our observations remains to be evaluated, our data suggest that glial activation does occur in migraine, extending to this disorder our previous observation on a separate group of chronic pain patients.5
CSD is now well-established as the substrate of migraine aura2 (for review, see reference 3). CSD is a slow propagating depolarization of neurons and glia that translates into neurologic symptoms depending on location. The most commonly reported auras in migraine are visual, but other types of aura exist, indicating involvement of nonvisual cortical regions, for example, somatosensory cortex with the sensation of tingling, or of inferior frontal cortex and Broca area with transitory aphasia.42 However, it is possible that some auras originate in brain areas that do not express any specific externalizing symptoms, as could happen in prefrontal cortex, for example. Here, in addition to the primary visual cortex activation, we also observed elevated uptake in 2 visual extrastriate motion processing areas that have been demonstrated to be implicated in migraine pathophysiology, namely V3A and MT,2,43 in which we previously reported the originating focus of a visual aura2 as well as increased cortical thickness.43 In addition, many patients in the present report also described other aura symptoms, such as difficulty finding words, speech difficulties, ringing in one ear, or mental confusion. Interestingly, we observed elevated glial activation in the inferior frontal cortex, frontal pole, orbitofrontal cortex, and the primary auditory cortex. The elevation in [11C]PBR28 uptake in these areas could be interpreted as evidence of astrocytic activation following CSD in these cortical areas, which is in line with preclinical data showing a proinflammatory role for CSD-induced astrocytic activation.44 However, there is also evidence of microglial activation following CSD,45 and the specific contributions of these cellular populations to the TSPO PET signal cannot be resolved with our current methods.
In addition to the observed group differences in [11C]PBR28 PET signal, we also report a significant positive correlation between SUVR and migraine attack frequency, in the posterior insula, basal ganglia, frontoinsular cortex, S1, and perigenual ACC. Several studies reported associations between the frequency of migraine attacks and functional activity in the insula, both in anterior and posterior subdivisions,29,46,47 regions that have been associated with different aspects of migraine pathology,20 emphasizing the importance of insular activity in moderating the probability of migraine attacks. In fact, our recent fMRI study with episodic migraine patients found cortical amplification and reduced habituation in the insula for trigeminal somatosensory afference.21 In addition, one of the aforementioned studies also found increased pain-related activity in S1 of migraineurs experiencing frequent attacks compared to those with less frequent attacks.46 This area lies in close proximity to the one in which we found a positive correlation between [11C]PBR28 SUVR and migraine frequency (figure 4), and both are situated in the S1 somatotopic representation of the face. Moreover, migraineurs exhibited significantly elevated TSPO PET signal in this area compared to controls, indicating that glial activation in S1 may be increased as a result of more frequent attacks. Interestingly, while few previous studies have reported differential basal ganglia function in migraineurs, one recent study found that noxious stimuli-induced brain activation of the basal ganglia was significantly different between migraineurs dichotomized by the frequency of migraine attacks.35 This suggests that caudate and putamen may play a role in the transition from episodic to chronic migraine states. Our data indicate that neuroinflammation in several regions, including insula, S1, and basal ganglia, is also associated with the prevalence of migraine attacks. Further research is needed to investigate the putative role of glial activation in both migraine frequency and the transition from episodic to chronic migraine.
There are limitations to the present study. First, the sample size of the current cohort was relatively small, due in part to the stringent criteria requiring a recent migraine attack with aura. Despite this, we were able to detect statistically significant elevations in [11C]PBR28 signal in migraineurs and associations between TSPO PET and the frequency of attacks. These results will need to be replicated in larger samples. Second, in the present study we did not perform kinetic modeling with radiometabolite-corrected arterial input function, which is considered by many to be the gold standard for quantification of TSPO binding. While previous work suggests that semiquantitative ratio metrics may be at least similarly sensitive to detect pathology-related group differences in [11C]PBR28 signal as classic kinetic modeling techniques,6,11 future studies will need to validate the use of these metrics in migraine datasets.
Our data demonstrate important in vivo evidence that migraine with aura is accompanied by prolonged signs of neuroinflammation in humans. Our observation may open relatively unexplored therapeutic strategies in migraine, aimed at attenuating glial activation, which have already demonstrated neuroprotective effects in rodent studies.48,49 Although a recent study suggested that this approach may be ineffective in chronic migraine,50 the lack of therapeutic efficacy for this condition may be due to the fact that chronic migraine pathophysiology is different than that underlying episodic migraine with aura. Future research is needed to better understand the role of neuroinflammation in migraine, and to evaluate whether the patterns of glial activation observed here are specific to migraine with aura, or can also be observed in migraine without aura.
Acknowledgment
The authors thank Judit Sore, Shirley Hsu, Regan Butterfield, and Grae Arabasz for technical support; Minhae Kim, Courtney Bergan, Anisha Bhanot, Elena Herranz, Constantina A. Treaba, and Russell Ouellette for assistance with data collection; and Shahin Nasr for comments on the manuscript.
Glossary
- ACC
anterior cingulate cortex
- CSD
cortical spreading depression
- IHS
International Headache Society
- MNI
Montreal Neurological Institute
- MPRAGE
magnetization-prepared rapid gradient echo
- NSAID
nonsteroidal anti-inflammatory drug
- ROI
region of interest
- SUV
standardized uptake value
- SUVR
standardized uptake value ratio
- TSPO
translocator protein
Appendix. Authors
Study funding
This work was supported by 5R21NS082926-02 (N.H.), National MS Society RG 4729A2/1, Department of Defense US Army W81XWH-13-1-0112 Award (C.M.), 5T32EB13180 (T32 supporting DSA), NIH R01NS07832201 A1 (C.M.), 1R01NS094306-01A1 (M.L.L.), 1R01NS095937-01A1 (M.L.L.), 1R21NS087472-01A1 (M.L.L.), R61AT009306 (V.N.), R01AR064367 (V.N.), R01AT007550 (V.N.), 1UL1TR001102-01, 8UL1TR000170-05, from the National Center for Advancing Translational Science, and 1UL1RR025758-04, from the National Center for Research Resources, Harvard Catalyst Advanced Imaging Pilot Grant (J.M.H.), Harvard Clinical and Translational Science Center, and financial contributions from Harvard University and its affiliated academic health care centers.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2197–2223. [DOI] [PubMed] [Google Scholar]
- 2.Hadjikhani N, Sanchez Del Rio M, Wu O, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci USA 2001;98:4687–4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pietrobon D, Moskowitz MA. Pathophysiology of migraine. Annu Rev Physiol 2013;75:365–391. [DOI] [PubMed] [Google Scholar]
- 4.Karatas H, Erdener SE, Gursoy-Ozdemir Y, et al. Spreading depression triggers headache by activating neuronal Panx1 channels. Science 2013;339:1092–1095. [DOI] [PubMed] [Google Scholar]
- 5.Loggia ML, Chonde DB, Akeju O, et al. Evidence for brain glial activation in chronic pain patients. Brain 2015;138:604–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albrecht D, Shcherbinin S, Wooten D, et al. Occipital lobe as a pseudo-reference region for [11C] PBR28 PET imaging: validation in chronic pain and amyotrophic lateral sclerosis cohorts. J Nucl Med 2016;57:1814. [Google Scholar]
- 7.Cosenza-Nashat M, Zhao ML, Suh HS, et al. Expression of the translocator protein of 18 kDa by microglia, macrophages and astrocytes based on immunohistochemical localization in abnormal human brain. Neuropathol Appl Neurobiol 2009;35:306–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen MK, Guilarte TR. Translocator protein 18 kDa (TSPO): molecular sensor of brain injury and repair. Pharmacol Ther 2008;118:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hannestad J, Gallezot JD, Schafbauer T, et al. Endotoxin-induced systemic inflammation activates microglia: [(1)(1)C]PBR28 positron emission tomography in nonhuman primates. NeuroImage 2012;63:232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui Y, Takashima T, Takashima-Hirano M, et al. 11C-PK11195 PET for the in vivo evaluation of neuroinflammation in the rat brain after cortical spreading depression. J Nucl Med 2009;50:1904–1911. [DOI] [PubMed] [Google Scholar]
- 11.Herranz E, Gianni C, Louapre C, et al. Neuroinflammatory component of gray matter pathology in multiple sclerosis. Ann Neurol 2016;80:776–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zurcher NR, Loggia ML, Lawson R, et al. Increased in vivo glial activation in patients with amyotrophic lateral sclerosis: assessed with [(11)C]-PBR28. Neuroimage Clin 2015;7:409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Owen DR, Yeo AJ, Gunn RN, et al. An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J Cereb Blood flow Metab 2012;32:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Izquierdo-Garcia D, Hansen AE, Forster S, et al. An SPM8-based approach for attenuation correction combining segmentation and nonrigid template formation: application to simultaneous PET/MR brain imaging. J Nucl Med 2014;55:1825–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Owen DR, Guo Q, Rabiner EA, Gunn RN. The impact of the rs6971 polymorphism in TSPO for quantification and study design. Clin Transl Imaging 2015;3:1–6. [Google Scholar]
- 16.Bloomfield PS, Selvaraj S, Veronese M, et al. Microglial activity in people at ultra high risk of psychosis and in schizophrenia: an [(11)C]PBR28 PET brain imaging study. Am J Psychiatry 2016;173:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turkheimer FE, Rizzo G, Bloomfield PS, et al. The methodology of TSPO imaging with positron emission tomography. Biochem Soc Trans 2015;43:586–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yousry TA, Schmid UD, Alkadhi H, et al. Localization of the motor hand area to a knob on the precentral gyrus: a new landmark. Brain 1997;120:141–157. [DOI] [PubMed] [Google Scholar]
- 19.Goadsby PJ, Holland PR, Martins-Oliveira M, Hoffmann J, Schankin C, Akerman S. Pathophysiology of migraine: a disorder of sensory processing. Physiol Rev 2017;97:553–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borsook D, Veggeberg R, Erpelding N, et al. The insula: a "Hub of activity" in migraine. Neuroscientist 2016;22:632–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee J, Lin RL, Garcia RG, et al. Reduced insula habituation associated with amplification of trigeminal brainstem input in migraine. Cephalalgia 2017;37:1026–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woods RP, Iacoboni M, Mazziotta JC. Brief report: bilateral spreading cerebral hypoperfusion during spontaneous migraine headache [see comments]. N Engl J Med 1994;331:1689–1692. [DOI] [PubMed] [Google Scholar]
- 23.Boulloche N, Denuelle M, Payoux P, Fabre N, Trotter Y, Géraud G. Photophobia in migraine: an interictal PET study of cortical hyperexcitability and its modulation by pain. J Neurol Neurosurg Psychiatry 2010;81:978–984. [DOI] [PubMed] [Google Scholar]
- 24.Denuelle M, Boulloche N, Payoux P, Fabre N, Trotter Y, Geraud G. A PET study of photophobia during spontaneous migraine attacks. Neurology 2011;76:213–218. [DOI] [PubMed] [Google Scholar]
- 25.Maniyar FH, Sprenger T, Schankin C, Goadsby PJ. The origin of nausea in migraine: a PET study. J Headache Pain 2014;15:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim JH, Kim S, Suh SI, Koh SB, Park KW, Oh K. Interictal metabolic changes in episodic migraine: a voxel-based FDG-PET study. Cephalalgia 2010;30:53–61. [DOI] [PubMed] [Google Scholar]
- 27.Demarquay G, Lothe A, Royet JP, et al. Brainstem changes in 5-HT1A receptor availability during migraine attack. Cephalalgia 2011;31:84–94. [DOI] [PubMed] [Google Scholar]
- 28.Jensen KB, Regenbogen C, Ohse MC, Frasnelli J, Freiherr J, Lundström JN. Brain activations during pain: a neuroimaging meta-analysis of pain patients and healthy controls. Pain 2016;157:1279–1286. [DOI] [PubMed] [Google Scholar]
- 29.Mainero C, Boshyan J, Hadjikhani N. Altered functional magnetic resonance imaging resting-state connectivity in periaqueductal gray networks in migraine. Ann Neurol 2011;70:838–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deen M, Hansen HD, Hougaard A, et al. Low 5-HT1B receptor binding in the migraine brain: a PET study. Cephalalgia 2018;38:519–527. [DOI] [PubMed] [Google Scholar]
- 31.Granziera C, Daducci A, Romascano D, et al. Structural abnormalities in the thalamus of migraineurs with aura: a multiparametric study at 3 T. Hum Brain Mapp 2014;35:1461–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hodkinson DJ, Wilcox SL, Veggeberg R, et al. Increased amplitude of thalamocortical low-frequency oscillations in patients with migraine. J Neurosci 2016;36:8026–8036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alshelh Z, Di Pietro F, Youssef AM, et al. Chronic neuropathic pain: it's about the rhythm. J Neurosci 2016;36:1008–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teixeira AL, Jr., Meira FC, Maia DP, Cunningham MC, Cardoso F. Migraine headache in patients with Sydenham's chorea. Cephalalgia 2005;25:542–544. [DOI] [PubMed] [Google Scholar]
- 35.Maleki N, Becerra L, Nutile L, et al. Migraine attacks the basal ganglia. Mol Pain 2011;7:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ji RR, Berta T, Nedergaard M. Glia and pain: is chronic pain a gliopathy? Pain 2013;154(suppl 1):S10–S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calvo M, Bennett DL. The mechanisms of microgliosis and pain following peripheral nerve injury. Exp Neurol 2012;234:271–282. [DOI] [PubMed] [Google Scholar]
- 38.Tsuda M, Shigemoto-Mogami Y, Koizumi S, et al. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature 2003;424:778–783. [DOI] [PubMed] [Google Scholar]
- 39.Guo W, Wang H, Watanabe M, et al. Glial-cytokine-neuronal interactions underlying the mechanisms of persistent pain. J Neurosci 2007;27:6006–6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ledeboer A, Sloane EM, Milligan ED, et al. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain 2005;115:71–83. [DOI] [PubMed] [Google Scholar]
- 41.Raghavendra V, Tanga F, Rutkowski MD, DeLeo JA. Anti-hyperalgesic and morphine-sparing actions of propentofylline following peripheral nerve injury in rats: mechanistic implications of spinal glia and proinflammatory cytokines. Pain 2003;104:655–664. [DOI] [PubMed] [Google Scholar]
- 42.Vincent MB, Hadjikhani N. Migraine aura and related phenomena: beyond scotomata and scintillations. Cephalalgia 2007;27:1368–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Granziera C, DaSilva AF, Snyder J, Tuch DS, Hadjikhani N. Anatomical alterations of the visual motion processing network in migraine with and without aura. PLos Med 2006;3:e402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghaemi A, Alizadeh L, Babaei S, et al. Astrocyte-mediated inflammation in cortical spreading depression. Cephalalgia 2017:333102417702132. [DOI] [PubMed] [Google Scholar]
- 45.Shibata M, Suzuki N. Exploring the role of microglia in cortical spreading depression in neurological disease. J Cereb Blood Flow Metab 2017;37:1182–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maleki N, Becerra L, Brawn J, Bigal M, Burstein R, Borsook D. Concurrent functional and structural cortical alterations in migraine. Cephalalgia 2012;32:607–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mathur VA, Moayedi M, Keaser ML, et al. High frequency migraine is associated with lower acute pain sensitivity and abnormal insula activity related to migraine pain intensity, attack frequency, and pain catastrophizing. Front Hum Neurosci 2016;10:489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jin WJ, Feng SW, Feng Z, Lu SM, Qi T, Qian YN. Minocycline improves postoperative cognitive impairment in aged mice by inhibiting astrocytic activation. Neuroreport 2014;25:1–6. [DOI] [PubMed] [Google Scholar]
- 49.Ortega FJ, Vukovic J, Rodriguez MJ, Bartlett PF. Blockade of microglial KATP-channel abrogates suppression of inflammatory-mediated inhibition of neural precursor cells. Glia 2014;62:247–258. [DOI] [PubMed] [Google Scholar]
- 50.Kwok YH, Swift JE, Gazerani P, Rolan P. A double-blind, randomized, placebo-controlled pilot trial to determine the efficacy and safety of ibudilast, a potential glial attenuator, in chronic migraine. J Pain Res 2016;9:899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared by request from any qualified investigator for purposes of replicating procedures and results.