Abstract
Rationale:
SERCA2a, sarco-endoplasmic reticulum Ca2+-ATPase, is a critical determinant of cardiac function. Reduced level and activity of SERCA2a are major features of heart failure (HF). Accordingly, intensive efforts have been made to develop efficient modalities for SERCA2a activation. We showed that the activity of SERCA2a is enhanced by post-translational modification (PTM) with small ubiquitin-like modifier 1 (SUMO1). However, the roles of other PTMs on SERCA2a are still unknown.
Objective:
In this study, we aim to assess the role of lysine acetylation on SERCA2a function and determine whether inhibition of lysine acetylation can improve cardiac function in the setting of HF.
Methods and Results:
The acetylation of SERCA2a was significantly increased in failing hearts of humans, mice, and pigs, which is associated with the reduced level of SIRT1, a class III histone deacetylase. Down-regulation of SIRT1 increased the SERCA2a acetylation, which in turn led to SERCA2a dysfunction and cardiac defects at baseline. In contrast, pharmacological activation of SIRT1 reduced the SERCA2a acetylation, which was accompanied by recovery of SERCA2a function and cardiac defects in failing hearts. Lysine 492 (K492) was of critical importance for the regulation of SERCA2a activity via acetylation. Acetylation at K492 significantly reduced the SERCA2a activity, presumably through interfering with the binding of ATP to SERCA2a. In failing hearts, acetylation at K492 appeared to be mediated by p300, a histone acetyltransferase.
Conclusions:
These results indicate that acetylation/deacetylation at K492, which is regulated by SIRT1 and p300, is critical for the regulation of SERCA2a activity in hearts. Pharmacological activation of SIRT1 can restore SERCA2a activity through deacetylation at K492. These findings might provide a novel strategy for the treatment of HF.
Keywords: SERCA2a, heart failure, post-translational modifications, acetylation, protein, acetylation
Keywords: Basic Science Research, Contractile Function
INTRODUCTION
One of the major features of heart failure (HF) is diminished sarcoplasmic reticulum (SR) Ca2+ uptake, associated with a decrease in the expression and enzymatic activity of the SR Ca2+-ATPase 2a (SERCA2a)1, 2. In animal models of HF, increasing SERCA2a expression in cardiomyocytes normalizes intracellular Ca2+ handling, restores lusitropic and inotropic functions, and significantly improves cardiac function, energetics, and survival3–6. Phase 1 and 2a human trials, in which the SERCA2a gene was delivered to the myocardium of patients with advanced HF, have also confirmed SERCA2a as an effective therapeutic target for HF7–10.
During HF, besides changes in SERCA2a expression, there are changes in its activity, which are partially resulted from post-translational modifications (PTMs) to the protein. For example, we have previously shown that small ubiquitin-like modifier 1 (SUMO1) is conjugated to SERCA2a, and that SUMO1 levels and SERCA2a SUMOylation were simultaneously reduced in failing hearts11. Our data demonstrated that SUMOylation is essential for preserving SERCA2a ATPase activity and stability, such that reduced SUMOylation is thought to significantly contribute to SERCA2a dysfunction in failing hearts. Conversely, by increasing SUMOylation of SERCA2a, either through SUMO1 overexpression or activation of SUMOylation machinery, cardiac function in mice and porcine model with HF is markedly improved11–14. These data show that SUMOylation is a critical PTM that regulates SERCA2a activity. In addition, many other studies have demonstrated that SERCA is redox-regulated by thiol modifications15, 16. For example, glutathionylation of SERCA at cysteine 674 resulted in increased activity, however, sulfonation of this residue caused a decrease in Ca2+ uptake. Nevertheless, the precise physiological implications of these modifications have not been proven and roles of other PTMs for the regulation of SERCA2a activity are largely unknown.
Sirtuins are related to the yeast protein Silence information regulator 2 (Sir2), which is an NAD+-dependent class III histone deacetylase and mono-ADP-ribosyltransferase that plays a critical role in a variety of cellular processes, including gene silencing, DNA damage repair, and longevity17. SIRT1 activity has been implicated in the prevention against aging and oxidative stress18, and also shown to be cardio-protective in animal models of HF by regulating oxidative stress and antioxidant enzymes19. Inhibition of SIRT1 activity induces cardiomyocyte apoptosis, accompanied by caspase-3 activation, whereas adenovirus-mediated overexpression of SIRT1 prevents apoptosis in response to serum starvation20. In transgenic mice, modest overexpression of SIRT1 protects the heart from oxidative stress through Forkhead box O (FOXO)-dependent mechanisms, while at higher levels (12.5 fold overexpression), SIRT1 increases oxidative stress in the heart18. SIRT1 was also shown to regulate autophagy by deacetylating several essential autophagy molecules, including Atg5, Atg7, and Atg821. Since constitutive autophagy is essential for maintaining cardiac structure and function, it is possible that SIRT1 exerts its cardioprotective roles at least partially through the activation of autophagy.
In this study, we show that acetylation is another essential PTM that modulates the activity of SERCA2a. Acetylation of SERCA2a was significantly elevated in human and animal failing hearts and correlated with the reduced SERCA2a activity. Moreover, our data provide clear evidence that SERCA2a is a direct substrate of SIRT1 and p300. SIRT1 activation by β-lapachone (β-lap), a metabolic activator of SIRT1, significantly reduced acetylation and restored the SERCA2a function, resulting in beneficial outcomes under cardiac insults. This study provides a novel strategy for the restoration of contractile dysfunction of failing hearts.
METHODS
All data have been made publicly available at figshare and can be accessed at https://doi.org/10.6084/m9.figshare.7739828 or from the corresponding author on request. Detailed Methods section is available in the Online Data Supplement.
Animal models.
All mice were housed and treated in accordance with NIH and Institutional Animal Care and Use Committee (IACUC) guidelines, and used protocols approved by the Icahn School of Medicine at Mount Sinai or the Gwangju Institute of Science and Technology Animal Care and Use Committees. Studies were conducted in male C57BL/6J mice aged 8–10 weeks (weight, 25 ~ 30 g) purchased from Jackson Laboratories. Cardiac-specific Sirt1 knockout (SIRT1−/−) mice were generated by crossing Sirt1 flox/flox mice (Jackson Laboratory) with α-MHC-MerCreMer mice (αMHC-MerCreMer, Jackson Laboratory)19. Conditional cardiomyocyte-specific Serca2 knockout mouse model has been previously described14. All animal experiments were described in the Supplemental material.
Adult cardiomyocyte isolation and physiology.
Ventricular myocytes were isolated from mouse hearts using the method previously described11. The isolation process, analysis of mechanical property, and molecular analysis were described in the Supplemental material.
Production, purification, and administration of the adenoviruses and adeno-associated viruses.
The production and purification, and gene transfer of adenoviruses and adeno-associated viruses were described in the Supplemental material.
In vitro acetylation and deacetylation of SERCA2a.
For in vitro analysis of acetylation and deacetylation of SERCA2a, we performed cell-based and purified protein-based assays. Methods were described in detail in the Supplemental material.
SERCA2a activity assays.
For analysis of in vitro activity of SERCA2a, we performed Ca2+ uptake assay and ATPase activity assay. Methods were described in detail in the Supplemental material.
Expression plasmids.
For expression in HEK293 cells, cDNAs encoding wild type SERCA2a, K492Q SERCA2a, K492R SERCA2a, SIRT1, SIRT2, and p300 were cloned into a pcDNA vector. For expression in primary cardiomyocytes and mice, cDNAs encoding wild type SERCA2a, K492Q SERCA2a, and SIRT1 shRNA were cloned into adenoviral and adeno-associated viral vectors. Methods were described in detail in the Supplemental material.
Generation of the anti-acetylated (Ac)-K492 of SERCA2a antibody.
A peptide encompassing K492 of SERCA2a, F488SRDKSMSVYC498, was synthesized, and the lysine residue was chemically acetylated (Anygen, Korea). Antibody for the acetylated peptide was generated in mice and purified by Abfrontier (Korea).
Statistical analysis.
Statistical analysis was described in the Supplemental material.
RESULTS
SERCA2a acetylation is elevated in HF and SERCA2a interacts with SIRT1.
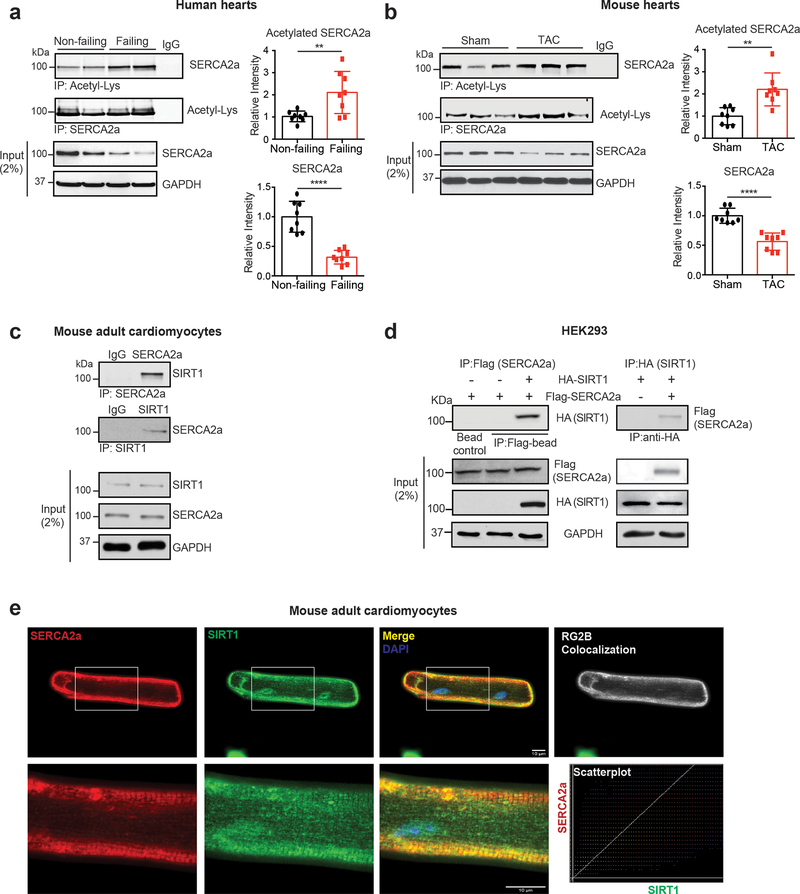
A large-scale analysis of the acetylome in human cancer cell lines has revealed that SERCA2 undergoes acetylation22. Heart samples obtained from the left ventricles (LV) of patients with HF and normal human controls were analyzed by immunoblotting. In line with previous observations, SERCA2a levels were lower in failing hearts than in normal controls. Interestingly, the acetylation of SERCA2a was markedly higher in failing hearts than the normal controls (Fig. 1a). In addition, this increase in SERCA2a acetylation was also observed in a murine model of HF induced by pressure overload (Fig. 1b) and a porcine model of HF induced by myocardial infarction (MI) (Online Fig. I), implying that an increase in acetylation is a general mechanism underlying the impaired function of SERCA2a in failing hearts.
Figure 1. SERCA2a acetylation is increased in failing hearts.
(a) SERCA2a acetylation was increased in human failing hearts. The human heart homogenates were immunoprecipitated with anti-acetyl-lysine antibody (reverse IP with anti-SERCA2a) and probed with anti-SERCA2a antibody (reverse blot with anti-acetyl-lysine). IgG was used as a negative control and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. Graphs show means ± SD, with each data point representing one heart sample. Donors, n = 8; failing patients n = 8. **p < 0.01; ****p < 0.0001 vs non-failing by unpaired t-test. (b) SERCA2a acetylation was increased in TAC-induced failing mouse hearts. Graphs show means ± SD, with each data point representing one heart sample; n = 8 of Sham and n = 8 of TAC mice; **p < 0.01; ****p < 0.0001 vs Sham by unpaired t-test. (c) SERCA2a interacts with SIRT1 in adult mouse cardiomyocytes (ACMs). ACM lysates were immunoprecipitated with anti-SERCA2a or anti-SIRT1 antibodies and probed with anti-SERCA2a or anti-SIRT1 antibodies. (d) SERCA2a directly interacts with SIRT1 in HEK293 cells. Flag agarose beads or HA agarose beads were incubated with lysates of HEK293 cells transfected with either Flag-tagged SERCA2a or together with HA-tagged SIRT1. Immunoprecipitates were probed with anti-Flag or anti-HA antibodies. (e) Immunofluorescence images showing co-localization of SERCA2a and SIRT1 (merge panel, yellow) in ACMs. SERCA2a staining is shown in red, SIRT1 staining in green, and DAPI staining in blue. Pixel colocalization analyzed using the JACop plugin for Image J is shown in white. Scatter plots correspond to the colocalization between SERCA2a and SIRT1 (scale bar, 10 μm). All data shown are representative of three independent experiments.
Through proteomic analysis of the SERCA2a interactome, we identified and confirmed that SIRT1 interacts with SERCA2a in porcine hearts (Online Fig. IIa-c). To verify this interaction, lysates of mouse adult cardiomyocytes (ACMs) were immunoprecipitated with an anti-SERCA2a antibody and probed with an anti-SIRT1 antibody. Inversely, the ACM lysates were also immunoprecipitated with an anti-SIRT1 antibody and probed with an anit-SERCA2a antibody. This reciprocal immunoprecipitaiton experiment showed that SERCA2a and SIRT1 are complexed in ACMs (Fig. 1c). SIRT2 was not co-immunoprecipitated with SERCA2a (Online Fig. IId) suggesting the specificity of the interaction between SERCA2a and SIRT1. Furthermore, HEK293 cells were transfected with expression plasmids for HA-SIRT1 and Flag-SERCA2a, and then cell lysates were subjected to reciprocal immunoprecipitation experiments. The results indicated that SERCA2a and SIRT1 constitute a complex in HEK293 cells (Fig. 1d). In a similar experiment, SIRT2 was not co-immunoprecipitated with SERCA2a in HEK293 cells (Online Fig. IIe). Confocal microscopy also revealed co-localization of SERCA2a and SIRT1 in ACMs (Fig. 1e).
Taken together, these data showed that SIRT1 directly interacts with SERCA2a, and raised a possibility that SIRT1 regulates the acetylation/deacetylation of SERCA2a.
SIRT1 deacetylates SERCA2a.
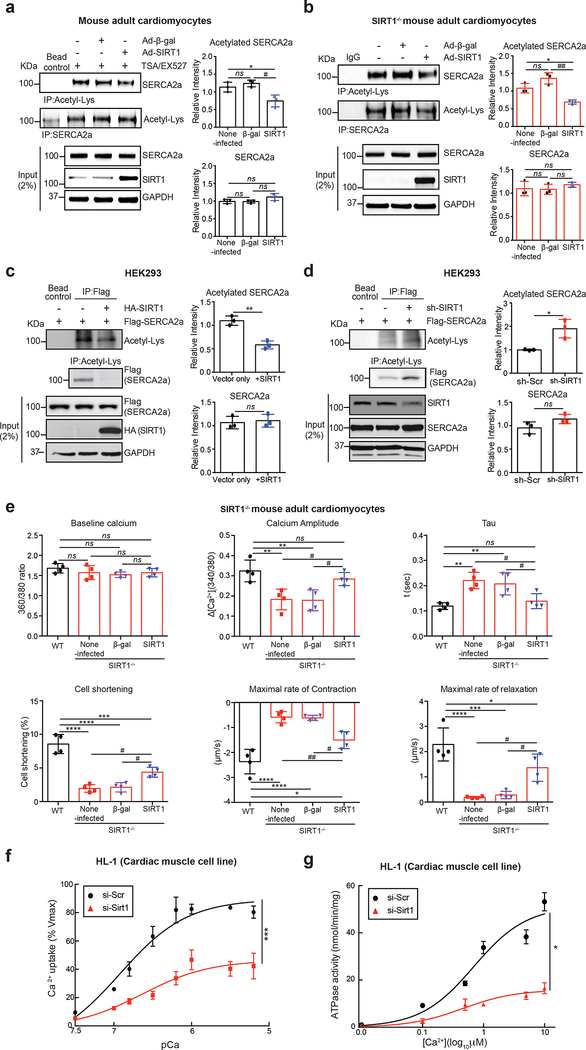
We next sought to determine the role of SIRT1 in acetylation/deacetylation of SERCA2a. In ACMs isolated from normal mice, acetylation of SERCA2a was significantly enhanced when treated simultaneously with EX-527 (SIRT1 inhibitor) and Trichostatin A (TSA, class I and II HDAC inhibitor)(data not shown), and the elevated acetylation of SERCA2a was significantly reduced by adenovirus-mediated overexpression of SIRT1 but not a control β-galactosidase (β-gal) (Fig. 2a). ACMs isolated from mice harboring cardiomyocyte-specific deletion of Sirt1 (SIRT1−/−) exhibited significantly elevated acetylation of SERCA2a compared to normal ACMs (Online Fig. III), and the elevated acetylation of SERCA2a was normalized by re-introduction of SIRT1 but not by overexpression of β-gal (Fig. 2b).
Figure 2. SIRT1 deacetylates SERCA2a and regulates its activity.
(a) SIRT1 overexpression reduced acetylation of SERCA2a in normal ACMs. ACMs were isolated from 8 week old male C57BL/6J mice and infected with the indicated adenoviruses (50 MOI). 24 hours after adenovirus infection, ACMs were treated with trichostatin A (TSA) and nicotinamide (NAM, as SIRT1 inhibitor) for 4 hours to inhibit de-acetylation of SERCA2a. ACM lysates were used for immunoprecipitation with anti-acetyl-lysine or anti-SERCA2a antibodies. Graphs show means ± SD, with each data point representing one sample. ns, not significant; *p < 0.05 vs non-infected ACMs and #p < 0.05 vs Ad-SIRT1 by one-way ANOVA. (b) Knockout of SIRT1 elevated acetylation of SERCA2a in ACMs. ACMs were isolated from 8 weeks old male SIRT1−/− mice. SIRT1−/− ACMs were infected with the indicated adenoviruses (50 MOI) for 24 hours. Anti-acetyl-lysine or anti-SERCA2a was incubated with ACM lysates. Immunoprecipitates were probed with anti-SERCA2a or anti-acetyl-lysine antibodies. Graphs show means ± SD, with each data point representing one sample. ns, not significant; *p < 0.05 vs non-infected ACMs and ##p < 0.01 vs Ad-SIRT1 by one-way ANOVA. (c) SIRT1 overexpression reduces acetylation of SERCA2a in HEK293 cells. Anti-acetyl-lysine or anti-Flag agarose beads were incubated with lysates of HEK293 cells transfected with either Flag-tagged SERCA2a alone or together with HA-tagged SIRT1. Acetylation of SERCA2a was trigged by treatment with TSA and nicotinamide (NAM, as SIRT1 inhibitor). Immunoprecipitates were probed with anti-Flag or anti-acetyl-lysine antibody. Graphs show means ± SD, with each data point representing one sample. ns, not significant; **p < 0.01 vs Vector only by unpaired t-test. (d) Knockdown of SIRT1 elevates acetylation of SERCA2a in HEK293 cells. Lysates of HEK293 cells expressing either Flag-SERCA2a alone or together with a SIRT1-specific shRNA were immunoprecipitated with anti-acetyl-lysine agarose beads or anti-Flag beads. Immunoprecipitates were probed with anti-Flag or anti-acetyl-lysine antibody. Graphs show means ± SD, with each data point representing one sample. ns, not significant; *p < 0.05 vs sh-scrambled control by unpaired t-test. (e) SIRT1 knockout showed decreased cardiomyocyte function. ACMs were isolated from SIRT1−/− mice and infected with Ad-SIRT1 or Ad-β-gal (as a negative control). ACMs isolated from wild-type (WT) mice served as control. 24 hours after infection with adenovirus (50 MOI of each virus), the contractile response of SIRT1−/− ACMs was assessed by calcium amplitude, decay time constant (tau), peak shortening, maximal rate of contraction, and maximal rate of relaxation using a video-based edge-detection system (IonOptix, Inc. Milton, MA). 15 cardiomyocytes were measured per mouse, n = 4. Graphs show means ± SD, with each data point was represented a mean average of 15 cardiomyocytes isolated from one heart sample. ns, not significant; *p < 0.05; ***p < 0.001; ****p < 0.0001 vs WT and #p < 0.05; ##p < 0.01 vs SIRT1−/− + Ad-SIRT1 by one-way ANOVA.
Flag-SERCA2a expressed in HEK293 cells was acetylated as determined by immnoprecipitation with an anti-Flag antibody followed by probing with anti-acetyl-lysine antibody. Acetylation of Flag-SERCA2a was significantly reduced when HA-SIRT1 was co-expressed (Fig. 2c), whereas it was significantly increased in cells where SIRT1 expression was knocked-down by transfection of a short hairpin RNA (shRNA) directed against SIRT1 (sh-SIRT1) (Fig. 2d). The level of SERCA2a was unaltered. The reciprocal immunoprecipitation experiments consistently showed that acetylation of SERCA2a was regulated by SIRT1.
To further verify the role of SIRT1, acetylated Flag-SERCA2a was partially purified from the transfected HEK293 cells treated with nicotinamide (NAM, SIRT1 inhibitor) and TSA. Purified acetylated Flag-SERCA2a was incubated with recombinant human SIRT1 (rhSIRT1) in the presence or absence of nicotinamide adenine dinucleotide (NAD+, SIRT1 cofactor) and/or NAM. Immunoblotting with an anti-acetyl-lysine antibody revealed that rhSIRT1 deacetylated Flag-SERCA2a in the presence of NAD+, and that this modification was inhibited completely by NAM (Online Fig. IV).
Taken together, these data demonstrated that SIRT1 directly deacetylates SERCA2a.
Acetylation suppresses SERCA2a activity.
SIRT1 supports and promotes a wide variety of cellular processes such as apoptosis/cell survival, endocrine signaling, and gene transcription. In addition, SIRT1 regulates the function of many transcription factors and cofactors through the mechanism of deacetylation17. In failing hearts, SIRT1 levels are significantly reduced, and the reduction appears to correlate with the increased SERCA2a acetylation. Thus, the increase in SERCA2a acetylation caused by the reduced SIRT1 level may contribute to SERCA2a dysfunction in failing hearts.
To test this hypothesis, we measured Ca2+ transient profiles and mechanical properties of ACMs isolated from SIRT1−/− mice using video-based edge-detection system. The SIRT1−/− ACMs exhibited significantly reduced Ca2+ handling as demonstrated by decreased Ca2+ amplitude and increased tau, and reduced contractility as demonstrated by decreased cell shortening and maximal rates of contraction and relaxation in comparison to wild type (WT) ACMs (Fig. 2e). However, the dysregulated Ca2+ handling and contractility in SIRT1−/− ACMs were significantly normalized by re-introduction of SIRT1 (Fig. 2e). These data suggest that increased acetylation of SERCA2a caused by knockdown of SIRT1 may impair its functions.
To explore the effects of SIRT1-mediated deacetylation on the enzyme activity of SERCA2a, HL-1 cardiac muscle cells were transfected with a siRNA directed against SIRT1 (si-SIRT1). Immunoblotting showed that SIRT1 levels were markedly reduced in si-SIRT1-transfected cells, but those of SERCA2a and its endogenous regulator phospholamban (PLN) were not affected. Acetylation of p53, a well-studied substrate of SIRT117, was significantly increased in si-SIRT1-transfected cells compared to si-Scramble-transfected cells (si-Scr), indicating successful knockdown of SIRT1 in si-SIRT1-transfected cells. As expected, SIRT1 knockdown significantly elevated levels of acetylated SERCA2a, indicating that SIRT1 deacetylates SERCA2a in HL-1 cells (Online Fig. V). Microsomal fractions were isolated and their Ca2+ uptake and ATPase activities, which are contributed mainly by SERCA2a, were determined. Knockdown of SIRT1 significantly reduced Ca2+ uptake activity (Vmax= 89.53±3.81 and Km=6.92±0.14 of si-Scr vs Vmax= 46.41±4.09 and Km=6.63±0.24 of si-Sirt1, Fig. 2f) and ATPase activity (Vmax= 51.33±3.16 and Km=0.69±0.16 of si-Scr vs Vmax= 15.89±1.34 and Km=0.46±0.16 of si-Sirt1, Fig. 2g). These data showed that the enzymatic activity of SERCA2a is regulated by its acetylation.
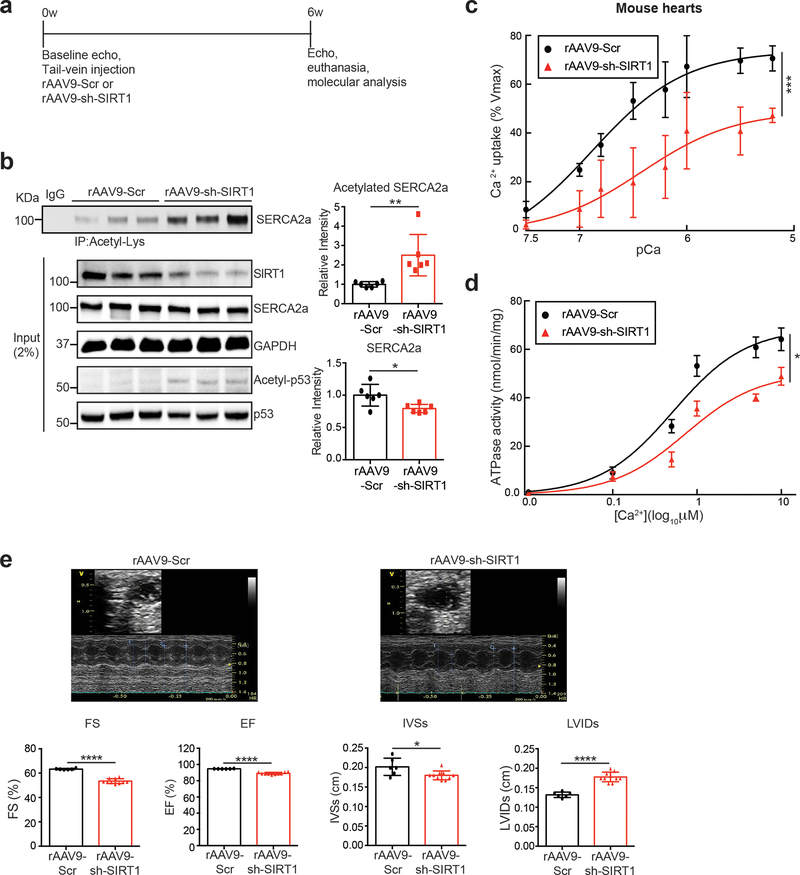
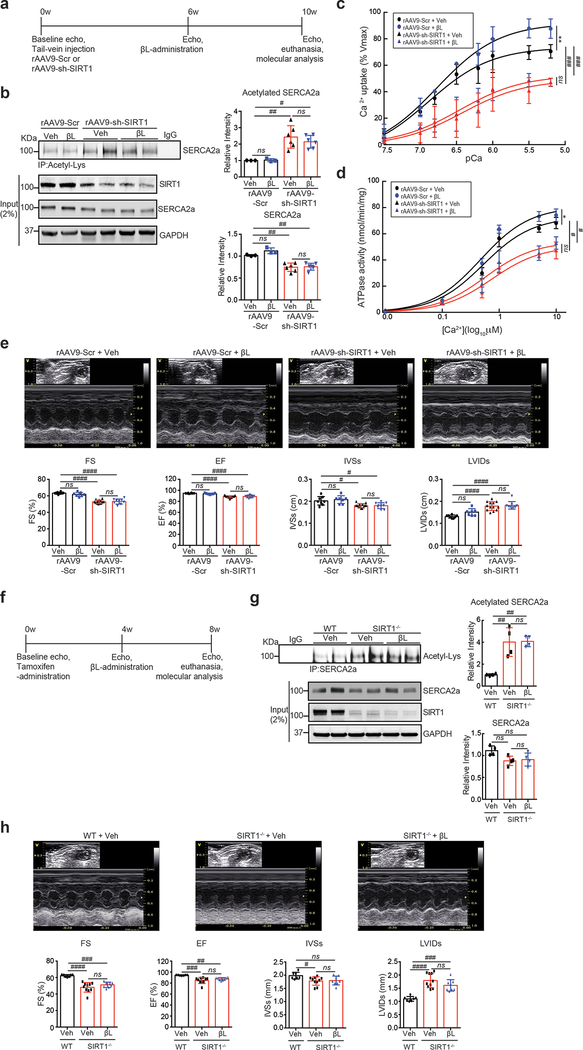
To further evaluate the role of SIRT1-mediated deacetylation of SERCA2a in vivo, mice were injected with a recombinant adeno-associated virus serotype 9 designed to express either a SIRT1-specific shRNA (rAAV9-sh-SIRT1) or a scrambled sequence (rAAV9-Scr) (Fig. 3a). A rAAV9-sh-SIRT1-mediated reduction in SIRT1 level was evident as early as 4 weeks after viral injection, and persisted for up to several months (data not shown). Immunoblotting and immunoprecipitation analyses were performed 6 weeks after the injection of the viruses. Knockdown of SIRT1 significantly increased acetylation of SERCA2a and slightly decreased the SERCA2a level (Fig. 3b). The increased acetylation of SERCA2a was associated reduced Ca2+ uptake (Vmax= 73.67±2.86 and Km=6.88±0.13 of sh-Scr vs Vmax= 48.95±5.51 and Km=6.42±0.25 of sh-Sirt1, Fig. 3c) and ATPase activities (Vmax= 68.68±2.78 and Km=0.52±0.09 of sh-Scr vs Vmax= 50.35±2.78 and Km=0.73±0.15 of sh-Sirt1, Fig. 3d) in the microsomal fractions. In addition, systolic function was significantly decreased in rAAV9-sh-SIRT1-injected mice as demonstrated by decreased left ventricular (LV) fractional shortening (FS) and ejection fraction (EF). Adverse LV remodeling was also evident in mice that received rAAV9-sh-SIRT1 compared to mice that received rAAV9-Scr, as demonstrated by reduced interventricular septal thickness at systole (IVSs) and increased LV internal dimensions at systole (LVIDs) (Fig. 3e and Online Table I). These data showed that SIRT1-mediated deacetylation plays an important role on SERCA2a activity in vivo.
Figure 3. Knockdown of SIRT1 elevates SERCA2a acetylation in vivo.
(a) Protocol for AAV9-sh-SIRT1-mediated SIRT1 knockdown in normal mice. (b) Knockdown of SIRT1 elevated levels of SERCA2a acetylation in normal mouse hearts. 8 week old male mice were injected with 11011 copies of rAAV9-scrambled (Scr) or rAAV9-shRNA-SIRT1 (sh-SIRT1). 6 weeks later, hearts were harvested and homogenized. The heart homogenates were immunoprecipitated with anti-acetyl-lysine agarose beads and probed with anti-SERCA2a antibody. The representative immnoublots were presented. Graphs show means ± SD, with each data point representing one heart sample. n = 6 per each group. *p < 0.05; **p < 0.01 vs sh-scrambled control by unpaired t-test. (c-d) Knockdown of SIRT1 reduced SERCA2a activity. (c) Calcium uptake and (d) ATPase activity were measured using microsomal fractions isolated from mouse hearts injected with rAAV9- Scr or rAAV9- sh-SIRT1 (normalized to expression levels of SERCA2a). Data are represented as the mean ± SD of n = 3 hearts. * p < 0.05; *** p < 0.001 vs scrambled control by paired t-test. (e) Knockdown of SIRT1 induced cardiac dysfunction. Echocardiographic M-mode images show that mice injected with rAAV9-shRNA-SIRT1 underwent functional deterioration. Ejection fraction (EF), fractional shortening (FS), LV internal diameter during systole (LVIDs), and interventricular septal thickness at end-systole (IVSs) were determined in mice injected with rAAV9-sh-scrambled control (n = 6) and mice injected with rAAV9-shRNA-SIRT1 (n = 10). Graphs show means ± SD, with each data point representing one mouse. * p < 0.05; *** p < 0.001 vs sh-scrambled control by unpaired t-test.
SIRT1 activation reduces SERCA2a acetylation and restores its activity in HF.
We have previously shown that β-lapachone (β-lap), a natural quinine compound, indirectly activates SIRT1 by elevating intracellular levels of NAD+, a critical regulator of SIRT1 activity23, 24. In mice, transverse aortic constriction (TAC) caused a significant reduction in the intracellular NAD+/NADH ratio and SIRT1 protein expression levels (Online Fig. VIa and Fig. 4b), which was consistent with an increase in SERCA2a acetylation (Fig. 1b and 4b). In parallel, increased acetylation of known SIRT1 substrates (p53, PARP-1, and Hif-2α) in TAC mouse hearts were observed25, further indicating a reduction in the levels of SIRT1 expression and activity (Online Fig. VIb).
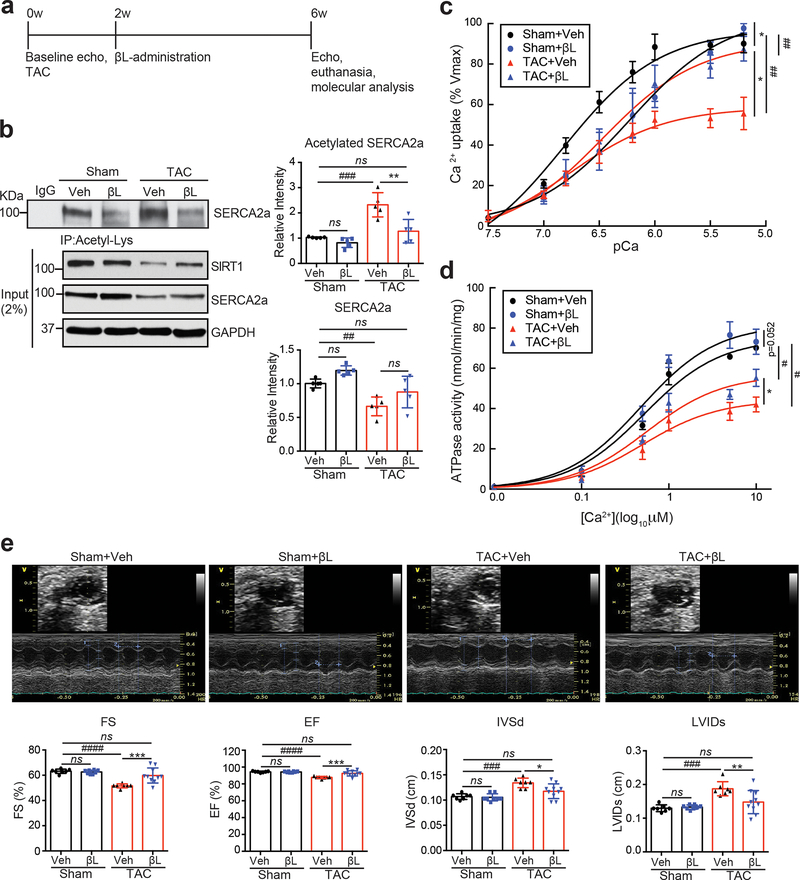
Figure 4. β-lap treatment diminishes acetylation and restores activity of SERCA2a in a pressure-overload mouse model.
(a) Protocol for β-lap administration in TAC mice. (b) β-lap treatment reduced levels of SERCA2a acetylation in mouse hearts. 8 week old male C57BL/6J mice were subjected TAC operation. Vehicle (Veh) or 150 mg/kg/day of β-lap (βL) was administered to mice 2 weeks post sham or TAC surgery. Four weeks later, hearts were harvested, and heart homogenates were immunoprecipitated with anti-acetyl-lysine agarose beads and probed with anti-SERCA2a antibody. The representative immnoublots were presented. Graphs show means ± SD, with each data point representing one heart sample. n = 5 sham mice treated with vehicle (Sham+ Veh), n = 5 sham mice treated with β-lap (Sham+ β L), n = 5 TAC mice treated with vehicle (TAC+ Veh), and n = 5 TAC mice treated with β-lap (TAC+ βL). ns, not significant; *p < 0.05; **p < 0.01; ***p < 0.001 vs β-lap treatment and ##p < 0.01; ###p < 0.001 vs Sham+Veh by one-way ANOVA. (c-d) β-lap administration restored SERCA2a activities in TAC mouse hearts. (c) Calcium uptake and (d) ATPase activity were measured in mouse hearts (normalized to expression levels of SERCA2a). Data are represented as the mean ± SD of n = 3 experiments. *p < 0.05 vs β-lap treatment and #p < 0.05; ##p < 0.01 vs Sham+Veh by paired t-test. (e) β-lap administration restored cardiac function in TAC-operated mice. Echocardiographic M-mode images show that β-lap administration restored cardiac function in TAC-operated mice. LV chamber dimensions and LV systolic function were measured 6 weeks post sham or TAC surgery. Data are represented as the mean ± SD, with each data point representing one heart sample. Sham mice treated with vehicle (Sham+ Veh, n = 7), sham mice treated with β-lap (Sham+ βL, n = 8), TAC mice treated with vehicle (TAC+ Veh, n = 7), and TAC mice treated with β-lap (TAC+ βL, n = 10). ns, not significant; *p < 0.05; **p < 0.01; ***p < 0.001 vs β-lap treatment and ###p < 0.001; ####p < 0.0001 vs Sham+ Veh by one-way ANOVA.
At two weeks post TAC surgery, β-lap administration was initiated to induce SIRT1 activation (Fig. 4a). As expected, daily oral administration of β-lap for 4 consecutive weeks corrected these metabolic abnormalities and inhibited acetylation of known SIRT1 substrates in failing mouse hearts (Online Fig. VI). TAC-induced acetylation of SERCA2a was also reduced by β-lap treatment (Fig. 4b). As expected, the TAC-induced acetylation of SERCA2a caused a significant decrease in SERCA2a activity compared to Sham mice. The maximum rate of Ca2+ uptake (Vmax= 96.95±2.38 and Km=6.82±0.07 of Sham+Veh vs Vmax= 58.59±2.03 and Km=6.77±0.10 of TAC+Veh) was reduced by approximately 40% in TAC heart tissues, as compared to Sham hearts. However, this SERCA2a dysfunction was almost completely restored in β-lap-treated TAC-operated mice (Vmax= 104.30±3.65 and Km=6.23±0.07 of Sham+ βL and Vmax= 91.14±3.42 and Km=6.48±0.08 of TAC+ βL) (Fig. 4c). The ATPase activity of SERCA2a was also reduced in TAC-operated mice (Vmax= 74.75±2.61 and Km=0.52±0.08 of Sham+Veh vs Vmax= 44.26±2.13 and Km=0.50±0.10 of TAC+Veh), and this defect was significantly normalized by β-lap (Vmax= 81.66±3.29 and Km=0.48±0.08 of Sham+ βL and Vmax= 56.76±2.51 and Km=0.55±0.10 of TAC+ βL) (Fig. 4d). The levels of PLN phosphorylation and expression of SIRT1 were not altered by β-lap (Online Fig. VII), suggesting that the observed changes in the microsomal Ca2+ uptake and ATPase activities were the result of decreased SERCA2a acetylation due to activation of SIRT1. β-lap also restored TAC-induced contractile dysfunction and adverse cardiac remodeling, as determined by echocardiography (Fig. 4e and Online Table II).
Taken together, these results indicate that pharmacological activation of SIRT1 protects cardiac function by reducing SERCA2a acetylation during pressure overload.
The effects of β-lap on SERCA2a are SIRT1-dependent.
To confirm that the observed beneficial effect of β-lap on SERCA2a function is SIRT1-dependent, we utilized two independent SIRT1 deficient mouse models. First, WT mice were intravenously injected with rAAV9-sh-SIRT1 or rAAV9-Scr for 6 weeks and then exposed to β-lap for 4 weeks (Fig. 5a). In rAAV9-sh-SIRT1-injected mice, SERCA2a acetylation was significantly elevated, which was not restored by β-lap (Fig. 5b). In these mice, Ca2+ uptake and ATPase activities of SERCA2a were also significantly impaired, and these defects were not normalized by β-lap (Fig. 5c, d, and Online Table VII). The contractile dysfunction and adverse cardiac remodeling in rAAV9-sh-SIRT1-treated mice were not rescued by β-lap (Fig. 5e and Online Table III).
Figure 5. β-lap treatment does not affect SERCA2a function in SIRT1 knock-down hearts.
(a) Protocol for β-lap administration in AAV9-sh-SIRT1-mediated SIRT1 knock-down mice. (b) β-lap (βL) did not restore levels of SERCA2a acetylation in SIRT1 knockdown mice. 8 week old male C57BL/6J mice were injected with 11011 copies of rAAV9-Scr or rAAV9-sh-SIRT1. Six weeks later, vehicle or 150 mg/kg/day of β-lap was administered orally for 4 weeks. Heart homogenates were immunoprecipitated with anti-acetyl-lysine agarose beads and probed with anti-SERCA2a antibody. The representative immnoublots were presented. Graphs show means ± SD, with each data point representing one heart sample. n = 3 rAAV9-Scr injected mice treated with vehicle (rAAV9-Scr + Veh), n = 3 rAAV9-Scr injected mice treated with β-lap (rAAV9-Scr + β L), n = 6 rAAV9-sh-SIRT1 injected mice treated with vehicle (rAAV9-sh-SIRT1+ Veh), and n = 6 rAAV9-sh-SIRT1 injected mice treated with β-lap (rAAV9-sh-SIRT1+ βL). ns, not significant vs β-lap treatment and #p < 0.05; ##p < 0.01 vs rAAV9-Scr +Veh by one-way ANOVA. (c) β-lap administration did not restore SERCA2a activity in the hearts of mice treated with rAAV9-sh-SIRT1. (c) Calcium uptake and (d) ATPase activity were measured in mouse hearts. Data are represented as the mean ± SD of n = 3 experiments. ns, not significant; *p < 0.05; **p < 0.01 vs β-lap treatment and #p < 0.05; ###p < 0.001 vs rAAV9-Scr + Veh by paired t-test. (e) β-lap administration did not recover cardiac function in mice treated with rAAV9-sh-SIRT1. Echocardiographic M-mode images show that β-lap administration did not restore normal cardiac function in SIRT1 knockdown mice. LV chamber dimensions and LV systolic function were measured. Data are represented as the mean ± SD, with each data point representing one heart sample. rAAV9-Scr + Veh (n = 8), rAAV9-Scr + βL (n = 7), rAAV9-sh-SIRT1+ Veh (n = 13) and rAAV9-sh-SIRT1+ βL (n = 9). ns, not significant vs β-lap treatment and #p < 0.05; ####p < 0.0001 vs rAAV9-Scr +Veh by one-way ANOVA. (f) Protocol for β-lap administration in cardiac-specific SIRT1 knockout (SIRT1−/−) mice. (g) β-lap administration did not affect levels of SERCA2a acetylation in SIRT1−/− mice. 8 week old male SIRT1−/− mice were injected intraperitoneally with tamoxifen to induce Sirt1 gene disruption. Four weeks after tamoxifen administration vehicle or 1 mg/g/day of β-lap was administered orally for 4 weeks. Heart homogenates were immunoprecipitated with anti-SERCA2a antibody and probed with anti-acetyl-lysine antibody. The representative immnoublots were presented. Graphs show means ± SD, with each data point representing one heart sample. n=4 per each group. ns, not significant vs β-lap treatment and ##p < 0.01 vs WT+Veh by one-way ANOVA. (h) β-lap administration did not recover cardiac function in SIRT1−/− mice. Echocardiographic M-mode, LV chamber dimensions, and LV systolic function were measured. Data are represented as the mean ± SD, with each data point representing one heart sample. WT + Veh (n = 7), SIRT1−/− + Veh (n = 10) and SIRT1−/− + βL (n = 8). ns, not significant vs β-lap treatment and #p < 0.05; ##p < 0.01; ###p < 0.001; ####p < 0.0001 vs WT + Veh by one-way ANOVA.
Second, SIRT1−/− mice19 were fed with β-lap for four weeks (Fig. 5f). While SIRT1 level was prominently reduced, the acetylation of p53, a well-known substrate of SIRT117, was significantly elevated (Online Fig. VIIIa). SIRT1 deficiency did not alter the expression levels of SIRT2 and SIRT3 nor the acetylation of tubulin, a well-known target of SIRT2. However, overall lysine acetylation levels were significantly elevated in these mice (Online Fig. VIIIb). In SIRT1−/− mice, elevated SERCA2a acetylation was not restored by β-lap (Fig. 5g). The contractile dysfunction and adverse cardiac remodeling in SIRT1−/− mice were not rescued by β-lap (Fig. 5h and Online Table IV). It is of note that both Serca2a mRNA and protein levels were not significantly altered by β-lap in SIRT1−/− mice (Fig. 5g and Online Fig. IX).
In order to further address the critical link between SIRT1 activity and SERCA2a function, we set out to determine the effects of β-lap on cardiac function of mice harboring cardiac-specific deletion of Serca2 (SERCA2−/−). Two weeks after induction of Serca2 gene excision, the SERCA2−/− mice were subjected to β-lap administration for 4 consecutive weeks (Online Fig. Xa). These mice displayed significantly decreased cardiac function in terms of EF, FS, IVSs, and LVIDs. β-lap-mediated activation of SIRT1 did not rescue the cardiac dysfunction in SERCA2−/− mice (Online Fig. Xb). The endogenous SERCA2a level was barely detectable and the SERCA2a acetylation was not significantly affected by β-lap in SERCA2−/− mice (Online Fig. Xc). ACMs isolated from SERCA2−/− mice exhibited defective Ca2+ handling and contractility. The cardioprotective effects of β-lap were not observed in these ACMs (Online Fig. Xd-h). These data suggest that β-lap exerts its beneficial effects through SIRT1-mediated deacetyaltion of SERCA2a.
Taken together, these results demonstrate that SIRT1 play a critical role in modulating SERCA2a activity through deacetylation in the heart.
Identification and characterization of acetylated lysine residues in SERCA2a.
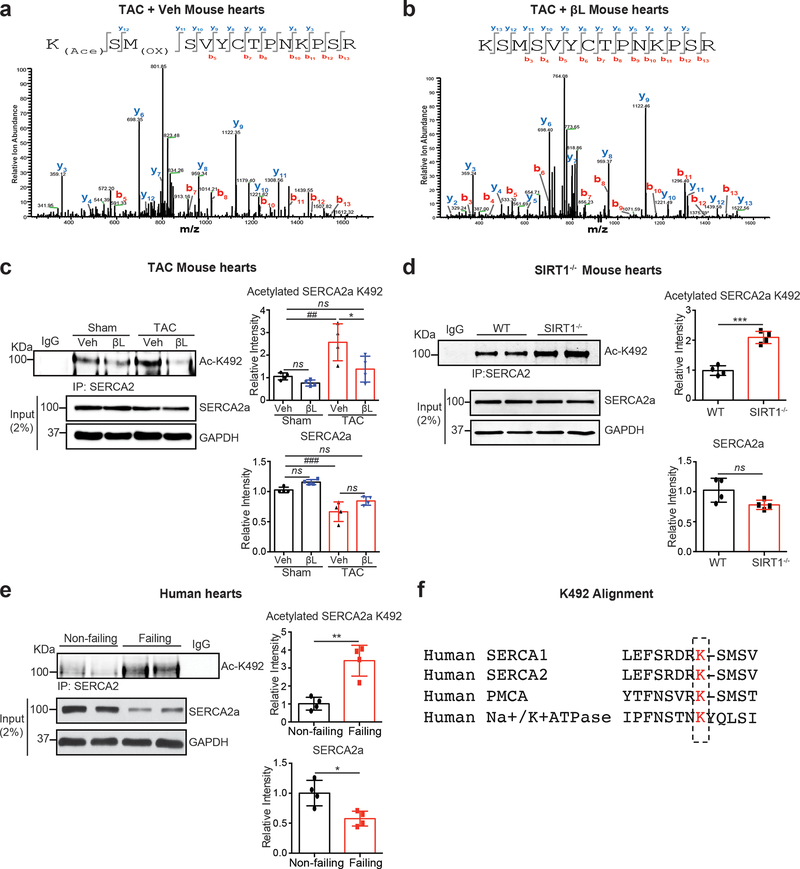
To identify critical acetylation sites on SERCA2a, a series of one-dimensional SDS-PAGE and LC-MS/MS analyses were performed on SERCA2a acetylated in vitro and in vivo. Using this approach, a total of 12 acetylation sites on SERCA2a were identified (data not shown). Among these sites, we recognized a previously identified acetylation site on SERCA, Lys46422. To identify SIRT1-specific deacetylation sites in SERCA2a, semi-quantitative LC-MS/MS analyses were performed on SERCA2a isolated from mouse hearts in which SIRT1 was either inhibited or activated. Among the 12 putative lysine residues, K492 was highly acetylated in TAC-operated mice, which was normalized by β-lap (Fig. 6a, b). In addition, a peptide containing acetylated K492 was identified in hearts of SIRT1−/− mice (Online Fig. XI).
Figure 6. Acetylation of K492 on SERCA2a is increased in failing and SIRT1 knockout hearts.
(a-b) Representative MS/MS spectra of tryptic peptides of SERCA2a at K492 identified in TAC mouse hearts treated with vehicle or β-lap. (a) Acetyl-Lys peptide KACSMOXSVYCTPNKPSR (residues 492–505) was detected in heart extracts from TAC mice. (b) Lys peptide KSMSVYCTPNKPSR (residues 492–505) was detected in heart extracts from TAC mice treated with β-lap (TAC + βL). KAc: acetyl-lysine; MOx: oxidized methionine. (c) Levels of K492 acetylation of SERCA2a were elevated in TAC-induced failing mouse hearts, as determined by a specific anti-acetyl-K492 antibody. The representative immnoublots were presented. Graphs show means ± SD, with each data point representing one heart sample. n = 4 per each group. ns, not significant; *p < 0.05 vs β-lap treatment and ##p < 0.01; ###p < 0.001 vs Sham+Veh by one-way ANOVA. (d) Acetylation levels of SERCA2a at K492 were elevated in SIRT1−/− mouse hearts. The representative immnoublots were presented. Graphs show means ± SD, with each data point representing one heart sample. n = 4 per each group. ns, not significant; ***p < 0.001 vs WT by unpaired t-test. (e) Acetylation levels of SERCA2a at K492 were increased in human failing hearts. The representative immnoublots were presented. Graphs show means ± SD, with each data point representing one heart sample. n = 4 per each group. *p < 0.05; **p < 0.01 vs non-failing by unpaired t-test. (f) Amino acid sequence alignment of different members of the human P-ATPase family of proteins showing high degree of conservation of K492 (K492 and homologous lysine residues are highlighted in red).
To confirm these results, an antibody specific for acetyl-K492 of SERCA2a (Ac-K492) was generated. To test the specificity of Ac-K492 antibody against acetyl-K492 of SERCA2a, Flag-tagged WT SERCA2a (Flag-SERCA2a) or K492R SERCA2a (non-acetylated K492) was expressed in HEK293 cells. To induce SERCA2a acetylation, the transfected cells were treated simultaneously with Ex-527 and TSA. Immunoblotting with Ac-K492 antibody detected a strong SERCA2a band in WT SERCA2a-transfected cells, but not in cells transfected with the K492R mutant of SERCA2a (Online Fig. XII). Next, immunoblotting with this antibody revealed that the level of acetylation at K492 was signifcantly elevated in the TAC-induced HF mice and was normalized upon SIRT1 activation by β-lap (Fig. 6c). Up-regulation of K492 acetylation was also found in SIRT1−/− mice (Fig. 6d) and failing human hearts (Fig. 6e). K492 is highly conserved among the family of human P-type ATPases supporting its essential role in the regulation of ATPase activity (Fig. 6f).
Taken together, these results suggest that acetylation at K492 may be associated with the reduced SERCA2a activity in failing hearts and its deacetyaltion may be mediated by SIRT1.
Effects of acetylated K492 on SERCA2a activity.
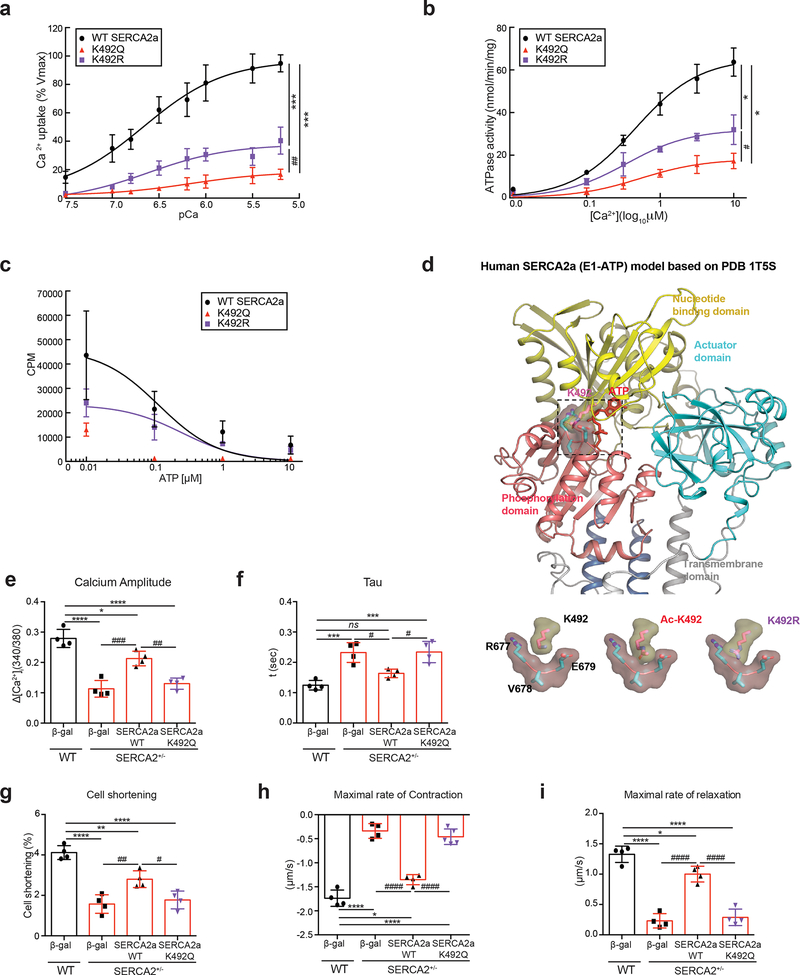
To explore the functional significance of acetylation of SERCA2a at K492, we generated Flag-tagged acetylation mimicking (K492Q) and non-acetylated (K492R) mutants of SERCA2a. HEK293 cells were transfected with plasmids expressing these recombinant proteins. The protein levels of both K492Q and K492R SERCA2a were comparable to WT SERCA2a level. Total lysine acetylation levels in these mutants were slightly lower than WT (Online Fig. XIIIa). Ca2+ uptake, ATPase activity, and ATP-binding affinity were measured and the values were normalized by the protein levels of SERCA2a. K492Q SERCA2a exhibited significantly decreased Ca2+ uptake and ATPase activities compared to WT SERCA2a, which is consistent with our view that acetylation at 492 significantly reduces the SERCA2a activity (Fig. 7a, b). However, K492R SERCA2a also exhibited significantly reduced Ca2+ uptake and ATPase activities, although the reduction was lesser than K492Q SERCA2a (Fig. 7a, b). ATP binding affinity was completely abolished in K492Q and significantly reduced in K492R SERCA2a, which suggest that K492 might play a key role in ATP-binding activity of SERCA2a (Fig. 7c). We carried out molecular modeling of SERCA2a based on the crystal structure of the rabbit SERCA1a complexed with ATP26. The deduced model suggested that acetylation at K492 might alter the ATP-binding pocket to render the ATP-binding of SERCA2a less favorable (Fig. 7d). Notably, previous mutagenesis studies of SERCA1a, the major SERCA isoform in skeletal muscles, showed that K492 plays a critical role in ATP binding26, 27. In addition, our modeling revealed that the K492R mutant might exert steric hindrance to the ATP-binding pocket that is composed of R677, V678, and E679 in the phosphorylation domain of the SERCA2a. Thus, K492R, like K492Q, appears to interfere with the binding of ATP to SERCA2a. Therefore, it is likely that K492 is highly vulnerable to modification (i.e. acetylation) or substitution so that any alteration at K492 can negatively influence the ATP-binding properties of ultimately leading to decreased activity of SERCA2a.
Figure 7. Acetylation at K492 plays a critical role in regulating SERCA2a activity.
(a) The calcium uptake, (b) ATPase activity, and (c) ATP binding assays of SERCA2a in microsomal fractions isolated from HEK293 cells transfected with wild-type (WT) or mutant (K492Q or K492R) forms of SERCA2a (normalized to expression levels of SERCA2a). Data are represented as the mean ± SD of n = 3 experiments. CPM, counts per minute. *p < 0.05; ***p < 0.001 vs WT SERCA2a and #p < 0.05; ##p < 0.01 vs K492R SERCA2a by paired t-test. (d) K492 acetylation site is located in the ATP binding pocket of SERCA2a. Cartoon representation of SERCA2a based on the crystal structure of SERCA1a (PDB: 1T5S). The phosphorylation domain, nucleotide-binding domain, and actuator domain are shown in salmon, yellow, and cyan, respectively. The transmembrane domain is shown in grey. K492 (pink) is located within the nucleotide-binding domain adjacent to the ATP molecule (red). Close-up views of K492, acetylated K492 (Ac-K492), and non-acetylated K492 mutant (K492R) relative to the phosphorylation domain are shown on the bottom. (e-i) Overexpression of the acetyl-mimicking mutant (K492Q) of SERCA2a showed contractile dysfunction in adult cardiomyocytes isolated from SERCA2+/− mice. Adult ventricular cardiomyocytes were isolated from SERCA2+/− mice and infected with Ad-WT SERCA2a, Ad- K492Q SERCA2a, or Ad-β-gal (as a negative control). ACMs isolated from wild-type (WT) mice infected with Ad-β-gal served as control. The contractile response of SERCA2a restoration in SERCA2+/− ACMs was assessed by calcium amplitude (e), decay time constant (tau) (f), peak shortening (g), maximal rate of contraction (h), and maximal rate of relaxation (i) using a video-based edge-detection system (IonOptix, Inc. Milton, MA) 24 hour after infection of ACMs with adenovirus (50 MOI of each virus). n = 4. Graphs show means ± SD, with each data point was represented a mean average of 15 ACMs isolated from one heart sample. ns, not significant; *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001 vs WT + β-gal and #p < 0.05; ###p < 0.001; ####p < 0.0001 vs SERCA2+/− + SERCA2a-WT by one-way ANOVA.
To further verify the functional consequences of acetylation of SERCA2a at K492 on contractile function in vitro, adenoviruses expressing WT or K492Q SERCA2a were infected to ACMs isolated from SERCA2+/− mice. In these mice, the SERCA2a level was reduced to ~ 30% of the normal level, and infection with the viruses restored the expression level of SERCA2a to ~70% (Online Fig. XIIIb). WT SERCA2a significantly restored the defective Ca2+ handling and contractility of SERCA2+/− ACMs. However, K492Q SERCA2a had no effects on these properties, supporting the importance of K492 for the function of SERCA2a (Fig. 7e-i). We further extended these findings to in vivo studies. WT or K492Q SERCA2a was re-introduced via AAV-mediated gene delivery to SERCA2+/− mice (Online Fig. XIVa). SERCA2+/− mice showed significantly decreased cardiac function compared to WT mice (Online Fig. XIVb and Online Table VI). Re-introduction of WT SERCA2a restored the contractile function, whereas re-introduction of K492Q SERCA2a did not rescue the impaired cardiac function (Online Fig. XIVb and Online Table VI). AAV-mediated re-introduction of both WT and K492Q SERCA2a in SERCA2+/− mice restored the total SERCA2a protein level to the range of WT mice (Online Fig. XIVc). ACMs were isolated from SERCA2+/− mice injected with rAAV9-WT or K492Q SERCA2a. The defective Ca2+ handling and contractility was completely restored by WT but not by K492Q SERCA2a (Online Fig. XIVd-h).
Taken together, these results suggest that acetylation of SERCA2a at K492 profoundly impairs the SERCA2a activities.
p300 acetyltransferase acetylates SERCA2a.
We finally pursued to identify the acetyltransferase that is involved in the SERCA2a acetylation in failing hearts. Expression levels of acetyltransferases, p300, GCN5, and PCAF, were elevated in human failing hearts compared to normal donors (Online Fig. XV).
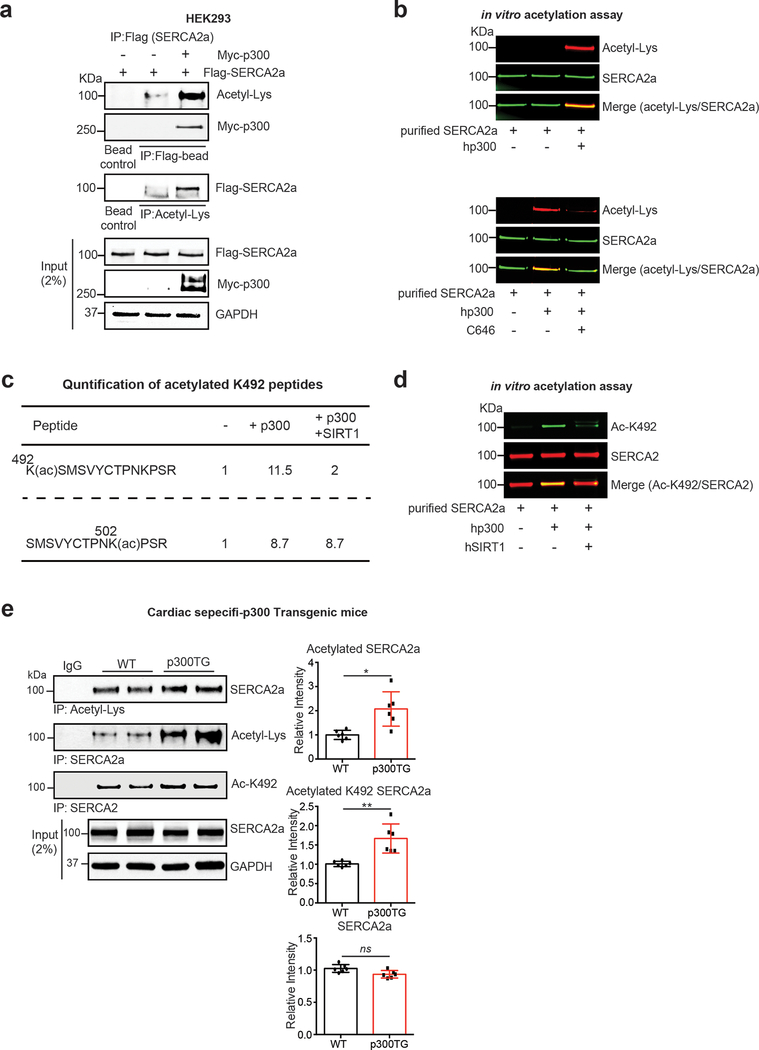
Flag-tagged SERCA2a was co-expressed either with or without Myc-tagged p300. Cell lysates were immunoprecipitated with an anti-Flag antibody and the resulting precipitates were probed with anti-acetyl-lysine antibody (Fig. 8a). Reverse immunoprecipitation experiment was also performed where cell lysates were immunoprecipitated with an anti-acetyl-lysine antibody and probed with anti-Flag antibody. Results from both experiments showed that p300 directly interacts with SERCA2a and that p300 acetylates SERCA2a.
Figure 8. SERCA2a is acetylated by p300 acetyltransferase.
(a) p300 directly acetylates and interacts with SERCA2a in HEK293 cells. HEK293 cells were co-transfected with Flag-SERCA2a alone or with Myc-p300. Interaction between p300 and SERCA2a as well as acetylation levels of SERCA2a were determined by immunoprecipitation followed by probing with appropriate antibodies. The experiments shown are representative of three independent experiments. (b) SERCA2a was acetylated by p300 in vitro. Purified porcine SERCA2a protein was incubated with 10 μM acetyl-CoA in the presence or absence of human recombinant p300 (hp300) (upper panel) protein. p300-mediated acetylation of SERCA2a was prevented by treatment with C646, a selective inhibitor of p300 (lower panel). (c) K492 acetylation on SERCA2a was regulated by p300 and SIRT1. Purified porcine SERCA2a protein was incubated with 10 μM acetyl-CoA and hp300 followed by incubation in the presence or absence of recombinant SIRT1. Following incubation, the samples were subjected to analysis by mass spectrometry which showed that p300-mediated acetylation at K492 was deacetylation by SIRT1. Comparison of acetylated lysine 492 levels using MRM (multiple reaction monitoring) anaylsis. Acetyl-Lys 492 peptide KACSMSVYCTPNKPSR (residues 492–505) was acetylated by p300 and deacetylated by SIRT1. Acetyl-Lys 502 peptide SMSVYCTPNKACPSR (residues 493–505) was not perturbed in the presence of SIRT1. (d) Acetylation status of K492 in purified porcine SERCA2a protein was confirmed by immnobloting with anti-acetyl-K492 antibody. Graphs show means ± SD, with each data point representing one heart sample. n = 6 per each group. ns, not significant; *p < 0.05 vs wild-type (WT) mice by unpaired t-test.
To further confirm acetylation of SERCA2a by p300 in vitro, SERCA2a purified from normal porcine hearts was incubated in the presence or absence of recombinant human p300 (hp300). Co-immunoblotting with an anti-acetyl-lysine and anti-SERCA2a antibodies revealed that SERCA2a is acetylated when p300 is co-incubated (Fig. 8b upper). This SERCA2a acetylation was inhibited by C646 (selective p300 inhibitor) (Fig. 8b bottom). MS-based analysis and co-immunoblotting experiment revealed that the acetylation at K492 was prominently elevated by p300 treatment, which is prevented by co-incubation with rhSIRT1 (Fig. 8c, d). Acetylation at several other lysine residues were also increased by p300, but not prevented by co-incubation with rhSIRT1 (data not shown). Acetylation at K502 was shown as such an example (Fig. 8c).
It was previously shown that the transgenic (TG) mice harboring cardiac-specific overexpression of p300 displayed severe contractile dysfunction51, 52. Total levels of SERCA2a acetylation and acetylation at K492 were significantly increased in the hearts of p300 TG mice compared to WT mice (Fig. 8e).
Taken together, our data suggest that p300 directly acetylates SERCA2a and that acetylation/deacetylation of SERCA2a at K492 is determined by SIRT1 and p300.
DISCUSSION
SERCA2a is SUMOylated by SUMO1, and this modification is essential for preserving SERCA2a activity and stability11. Elevation of SERCA2a expression or SUMOylation has been suggested as an effective therapeutic strategy for the treatment of HF11–14. The results presented here show that SERCA2a also undergoes acetylation, which is increased in failing human hearts and animal models of HF. Moreover, acetylation of SERCA2a is mediated by p300 and this modification is reversed by SIRT1. SIRT1 deficiency elevated acetylation of SERCA2a and promoted its enzymatic dysfunction. In contrast, deacetylating SERCA2a by activating SIRT1 with SIRT1 activating compound, β-lap, effectively restored SERCA2a function. However, knock-down of SERCA2a abolished the inotropic effects of SIRT1 activation. Mass spectrometry analysis revealed a SIRT1-regulated SERCA2a deacetylation at K492. The level of acetylation of SERCA2a at K492 was shown to be significantly increased in HF and K492 is critical in modulating SERCA2a activity, suggesting that acetylation of SERCA2a at K492 may be a marker of pathophysiological condition in HF.
Over the years, several PTMs of SERCA2a, such as glutathionylation and nitration, have been directly implicated in modulating its activity under normal and stress conditions15, 16. In our previous work, we found that covalent attachment of SUMO1 to K480 and K585 of SERCA2a enhances its stability and enzymatic activity11. Cardiac-specific overexpression of SUMO1 by AAV-mediated gene delivery significantly improved cardiac contractility and protected SERCA2a from oxidative stress in mouse and porcine models of HF11–13. Recently, we found that SUMO1 is negatively regulated by microRNA-146a, which results in the inhibition of SERCA2a SUMOylation28. In addition, we have shown that SUMOylation of SERCA2a can be up-regulated by enhancing the activity of a SUMO-activating enzyme (E1) using specific pharmacological molecules, resulting in improved contractile function and further supporting the cardioprotective properties of SUMOylation in the heart14.
Since lysine residues can undergo multiple PTMs, such as ubiquitination, SUMOylation, acetylation, methylation, and glycation, it is likely that cross-talk between different PTMs of SERCA2a exists and modulates its function in the heart. Cross-talk between different PTMs is likely to be important for signal-dependent regulation of protein activity29. At the organism level, acetylation plays an important role in immunity, circadian rhythmicity, and memory formation30. Protein acetylation is enzymatically mediated by histone acetyltransferases (HATs) and can be reversed by histone deacetylases (HDACs) and is becoming an important target in drug design for numerous disease conditions31, 32. Many HDACs have been identified with impacts on cardioprotection. Specifically, Sirtuins (silent mating type information regulation 2 homolog, HDAC class III) are important in ischemia/reperfusion studies due to their impact on aging, apoptosis, metabolic homeostasis, and stress responses19, 33, 34. In addition, both class I and class II HDACs have been closely linked to cardiac hypertrophy35. Mitochondrial acetylation has been shown to be important in the pathophysiology of HF36. Functional interplay between acetylation and SUMOylation have been reported to regulate the transcriptional activity of myocyte enhancer factor-2, histones, p53, and tumor suppressor HIC1 in multiple cancers37–40. Furthermore, several HATs and HDACs undergo SUMOylation, and its SUMOylation regulates localization and/or activity of some of these enzymes41, 42. Aberrant acetylation or deacetylation has been implicated in human diseases such as cancer and neurodegenerative diseases43. Despite the increasing number of studies related to protein acetylation, our understanding of the exact role of acetyltransferases and deacetylases in cardiac function is controversial as these enzymes have numerous protein targets. A large-scale analysis of human acetylome in cancer cell lines suggested that acetylation of SERCA2 plays a role in changes in the cell phenotype22. A recent study has identified three acetylated lysine residues (K464, K510, K533) in SERCA2a from guinea pig hearts, raising the possibility that acetylation of SERCA2a may also influence intracellular Ca2+ cycling in the heart44.
This study clearly indicated that SERCA2a is acetylated and that this acetylation is more prominent in the setting of HF. In addition, our proteomic analysis of SERCA2a interactome indicated that there is indeed interaction between SERCA2a and Sirtuin1 (SIRT1), which has known anti-aging and stress-resistance effects17, 18. Activation of SIRT1 exerts a number of beneficial effects in failing hearts, including normalization of metabolic deficits45. SIRT1-mediated deacetylation of SERCA2a may contribute to the cardio-protective effects of SIRT1 activating compounds such as β-lap.
β-lap is a quinone-containing natural compound that is obtained from the bark of the South American Lapacho tree (Tabebuia avellandedae)46. This compound is an anti-tumor agent with strong cytotoxic activity against a variety of cancer cell lines47. A recent study showed that oral administration of β-lap prevents obesity and obesity-related metabolic phenotypes in mice23, while another study demonstrated that β-lap prevents arterial restenosis in rats by activating AMPK25. The pharmacological activity of β-lap is dependent on a FAD-containing enzyme NADH:quinone oxidoreductase (NQO1). NQO1 mediates reduction of β-lap using NADH as an electron source48. Thus, β-lap treatment promotes oxidation of NADH to NAD+ resulting in increased intracellular levels of NAD+. Since SIRT1 activity strictly requires NAD+ as a cofactor, β-lap was supposed to increase SIRT1 activity. Previous reports showed that β-lap indeed increased SIRT1 activity through elevation of intracellular NAD+/NADH ratio in neuronal cells and cardiomyocytes25. Therefore, in this study, β-lap (as a SIRT1 activating compound) exerts cardio-protective effects at least partially through the SIRT1-mediated deacetylation of SERCA2a. Further studies examining the long-term effects of targeting SERCA2a acetylation are warranted to evaluate the potential of this therapeutic strategy.
p300 is a transcriptional co-activator that functions as an integrator of numerous signaling pathways and it regulates many DNA-binding proteins to facilitate transcriptional activation42. p300 has been implicated in numerous disease processes including several forms of cancer and neurodegenerative diseases49. Several lines of evidence suggest that p300 plays an important role in the growth of cardiac myocytes during development50. p300 knockout mice presented cardiac defects such as impaired expression of cardiac genes and reduced ventricular trabeculation50. In adult mouse hearts, p300 overexpression exhibits symptoms representative of HF accompanied by acetylation of hypertrophy responsive transcription factors51. Cardiac specific p300 transgenic mice showed significantly more ventricular dilations and diminished systolic function after MI than wild-type mice52. Genetic reduction of p300 limited both hypertrophy and the attendant risk of HF53. Together, these findings imply that in some cases a therapeutic benefit may be obtained through inhibition of p300. The present study demonstrates that p300 expression is increased in failing hearts and p300 induces SERCA2a acetylation in vitro and in vivo.
AAV-mediated transfer of SERCA2a to patients with advanced HF yielded highly promising results in phase 1 and 2a clinical studies8, 9. However, the SERCA2a gene therapy failed to meet the primary goals in a recent phase 2b clinical trial that encompassed 250 HF patients10. This disappointing result can be explained by several factors such as doses and qualities of the infused AAV. In addition, we need to consider that SERCA2a activity, as well as its level, is regulated at multiple levels. Previous and present studies showed that SUMOylation and acetylation are critical PTMs which regulate SERCA2a activity. Any modalities that enhance SUMOylation and/or deacetylation of SERCA2a can be valid therapeutic strategies for the treatment of HF. We previously showed that SUMO1 gene transfer indeed led to restoration of SERCA2a levels, improved hemodynamic performance, and reduced mortality in a mouse model of pressure overload-induced HF11. We further demonstrated that gene transfer of SUMO1 in combination with SERCA2a led to reversal of HF in a porcine model of ischemic HF12. Since the two lysine residues (K480 and K585) of SERCA2a that are subject to SUMOylation are not acetylated, there should be no direct competitions between SUMOylation and acetylation. Rather these two modifications appear to be regulated through independent mechanisms but toward opposite directions. Yet, SUMOylation, due to the relatively large size of SUMO1, may sterically hinder the acetylation at near lysine residues. In the TAC-induced HF mouse hearts, SERCA2a SUMOylation was decreased, while its acetylation was increased. Increased SUMOylation of SERCA2a by SUMO1 gene transfer resulted in normalization of SERCA2a acetylation in TAC mice, while reduced SUMO1 expression by AAV9.sh-Sumo1 gene transfer increased acetylation of SERCA2a accompanied by a reduction of SERCA2a SUMOylation in vivo (data not shown). In this study, we first reported the functional and physiological effects of acetylation at K492 on SERCA2a. Acetylation at K492 directly regulates the access of ATP to its binding site thus regulates the activities of SERCA2a. K492 is one of a group of residues forming the ATP-binding pocket of SERC2a and was previously shown to have a critical role in assuring proper binding of ATP to SERCA1a26. Therefore, we conclude that K492 is highly vulnerable to modification (i.e., acetylation) or substitution, and any alteration in this site can significantly alter the ATP-binding capacity of SERCA2a in a less favorable way. However, in this study, we did not find definite evidence to support the possibility that acetylation and SUMOylation regulate each other directly in the heart.
In this study, we demonstrate a novel regulatory mechanism for cardiac function whereby lysine acetylation influences the activity of SERCA2a, a key molecule involved in the regulation of cardiac contractility. In addition, the beneficial effects of SERCA2a deacetyaltion on cardiac function via SIRT1 activation suggest that targeting SERCA2a’s PTMs may provide a novel therapeutic strategy for the treatment of HF.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
The sarco-endoplasmic reticulum Ca2+-ATPase (SERCA2a) pump is dysregulated in the setting of heart failure (HF).
SERCA2a undergoes several post-translational modifications (PTMs), such as SUMOylation, glutathionylation, and nitration
PTMs of SERCA2a are directly implicated in modulating its activity under normal and stress conditions.
The roles of other PTMs on SERCA2a functions are still unknown.
What New Information Does This Article Contribute?
SERCA2a is acetylated, particularly in the setting of HF.
Acetylation of SERCA2a is mediated by p300, which could be reversed by SIRT1.
Inhibition of SERCA2a acetylation by activating SIRT1 activity reverses contractile dysfunction in the setting of HF.
Acetylation/deacetylation of SERCA2a at lysine position 492 (K492) is critical for regulation of SERCA2a activity in normal and diseased hearts.
Regulation of SERCA2a by PTMs has emerged as an important mechanism by which Ca2+ homeostasis is maintained in health and disrupted in disease. We report a novel regulatory mechanism whereby lysine acetylation of SERCA2a directly affects its function. We show that SERCA2a is a direct substrate of SIRT1 and p300. The beneficial effects of SERCA2a deacetylation on cardiac function via SIRT1 activation suggest that targeting SERCA2a’s PTMs may provide a novel therapeutic strategy for the treatment of HF.
Acknowledgments
SOURCES OF FUNDING
During this work, C.K. was supported by NIH R00 HL116645 and AHA 18TPA34170460. R.J.H. was supported by R01 HL119046, R01 HL128099, R01 HL 117505, R01 HL129814, R01 HL131404, R42 HL132684, P50 HL112324, T32 HL007824, and a Transatlantic Leducq Foundation grant. W.J.P. was supported by a grant from Basic Science Research Program (2017R1A2B4007340), a grant from Basic Research Laboratory Program (2016R1A4A1009895), and a grant from Bio & Medical Technology Development Program (NRF-2015M3A9E6028951), funded by the NRF of Korea. Y.J.Y., D.H.K., and W.J.P. were supported by the Institute for Basic Science program (IBS-R025-D1), funded by the Ministry of Science and ICT, Korea. W.J.P. and H.K. were supported by Basic Research Laboratory Program (2016R1A4A1009895) from NRF of Korea. D.J. was supported by The assistant Secretary of Defense for Health Affairs endorsed by the Department of Defense, through the FY17, DMDRP, Career Development Award program under Award No. W81XWH-18-1-0322. A.L. was supported by R43 HL144223. P.A.G. was supported by Canadian Institutes of Health Research postdoctoral fellowship. J.G.O. was supported by the American Heart Association Postdoctoral Fellowship AHA 17POST33410877.
Nonstandard Abbreviations and Acronyms:
- AAV9
adeno-associated virus serotype 9
- ACM
adult cardiomyocyte
- AcK
acetylated lysine
- β-lap
β-lapachone
- Ca2+
calcium
- EF
ejection fraction
- FS
fractional shortening
- HF
heart failure
- Lys
lysine
- LV
left ventricle
- NAD+
nicotinamide adenine dinucleotide
- p300
histone acetyltransferase p300
- PTM
post-translational modification
- SERCA2a
sarco-endoplasmic reticulum Ca2+-ATPase
- SIRT1
sirtuin 1
- SUMO1
small ubiquitin-related modifier 1
- TAC
transverse aortic constriction
Footnotes
DISCLOSURE
None.
REFERENCES
- 1.Kho C, Lee A and Hajjar RJ. Altered sarcoplasmic reticulum calcium cycling--targets for heart failure therapy. Nat Rev Cardiol. 2012;9:717–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kawase Y and Hajjar RJ. The cardiac sarcoplasmic/endoplasmic reticulum calcium ATPase: a potent target for cardiovascular diseases. Nat Clin Pract Cardiovasc Med. 2008;5:554–65. [DOI] [PubMed] [Google Scholar]
- 3.Sakata S, Lebeche D, Sakata N, Sakata Y, Chemaly ER, Liang LF, Takewa Y, Jeong D, Park WJ, Kawase Y and Hajjar RJ. Targeted gene transfer increases contractility and decreases oxygen cost of contractility in normal rat hearts. American journal of physiology Heart and circulatory physiology. 2007;292:H2356–63. [DOI] [PubMed] [Google Scholar]
- 4.del Monte F, Williams E, Lebeche D, Schmidt U, Rosenzweig A, Gwathmey JK, Lewandowski ED and Hajjar RJ. Improvement in survival and cardiac metabolism after gene transfer of sarcoplasmic reticulum Ca(2+)-ATPase in a rat model of heart failure. Circulation. 2001;104:1424–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyamoto MI, del Monte F, Schmidt U, DiSalvo TS, Kang ZB, Matsui T, Guerrero JL, Gwathmey JK, Rosenzweig A and Hajjar RJ. Adenoviral gene transfer of SERCA2a improves left-ventricular function in aortic-banded rats in transition to heart failure. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:793–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawase Y, Ly HQ, Prunier F, Lebeche D, Shi Y, Jin H, Hadri L, Yoneyama R, Hoshino K, Takewa Y, Sakata S, Peluso R, Zsebo K, Gwathmey JK, Tardif JC, Tanguay JF and Hajjar RJ. Reversal of cardiac dysfunction after long-term expression of SERCA2a by gene transfer in a pre-clinical model of heart failure. Journal of the American College of Cardiology. 2008;51:1112–9. [DOI] [PubMed] [Google Scholar]
- 7.Zsebo K, Yaroshinsky A, Rudy JJ, Wagner K, Greenberg B, Jessup M and Hajjar RJ. Long-term effects of AAV1/SERCA2a gene transfer in patients with severe heart failure: analysis of recurrent cardiovascular events and mortality. Circulation research. 2014;114:101–8. [DOI] [PubMed] [Google Scholar]
- 8.Jaski BE, Jessup ML, Mancini DM, Cappola TP, Pauly DF, Greenberg B, Borow K, Dittrich H, Zsebo KM, Hajjar RJ and Calcium Up-Regulation by Percutaneous Administration of Gene Therapy In Cardiac Disease Trial I. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase 1/2 clinical trial. Journal of cardiac failure. 2009;15:171–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jessup M, Greenberg B, Mancini D, Cappola T, Pauly DF, Jaski B, Yaroshinsky A, Zsebo KM, Dittrich H, Hajjar RJ and Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease I. Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID): a phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2+-ATPase in patients with advanced heart failure. Circulation. 2011;124:304–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenberg B, Yaroshinsky A, Zsebo KM, Butler J, Felker GM, Voors AA, Rudy JJ, Wagner K and Hajjar RJ. Design of a phase 2b trial of intracoronary administration of AAV1/SERCA2a in patients with advanced heart failure: the CUPID 2 trial (calcium up-regulation by percutaneous administration of gene therapy in cardiac disease phase 2b). JACC Heart failure. 2014;2:84–92. [DOI] [PubMed] [Google Scholar]
- 11.Kho C, Lee A, Jeong D, Oh JG, Chaanine AH, Kizana E, Park WJ and Hajjar RJ. SUMO1-dependent modulation of SERCA2a in heart failure. Nature. 2011;477:601–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tilemann L, Lee A, Ishikawa K, Aguero J, Rapti K, Santos-Gallego C, Kohlbrenner E, Fish KM, Kho C and Hajjar RJ. SUMO-1 gene transfer improves cardiac function in a large-animal model of heart failure. Science translational medicine. 2013;5:211ra159. [DOI] [PubMed] [Google Scholar]
- 13.Lee A, Jeong D, Mitsuyama S, Oh JG, Liang L, Ikeda Y, Sadoshima J, Hajjar RJ and Kho C. The role of SUMO-1 in cardiac oxidative stress and hypertrophy. Antioxidants & redox signaling. 2014;21:1986–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kho C, Lee A, Jeong D, Oh JG, Gorski PA, Fish K, Sanchez R, DeVita RJ, Christensen G, Dahl R and Hajjar RJ. Small-molecule activation of SERCA2a SUMOylation for the treatment of heart failure. Nature communications. 2015;6:7229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adachi T, Weisbrod RM, Pimentel DR, Ying J, Sharov VS, Schoneich C and Cohen RA. S-Glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nature medicine. 2004;10:1200–7. [DOI] [PubMed] [Google Scholar]
- 16.Knyushko TV, Sharov VS, Williams TD, Schoneich C and Bigelow DJ. 3-Nitrotyrosine modification of SERCA2a in the aging heart: a distinct signature of the cellular redox environment. Biochemistry. 2005;44:13071–81. [DOI] [PubMed] [Google Scholar]
- 17.Haigis MC and Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annual review of pathology. 2010;5:253–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, Tian B, Wagner T, Vatner SF and Sadoshima J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circulation research. 2007;100:1512–21. [DOI] [PubMed] [Google Scholar]
- 19.Hsu CP, Zhai P, Yamamoto T, Maejima Y, Matsushima S, Hariharan N, Shao D, Takagi H, Oka S and Sadoshima J. Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation. 2010;122:2170–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alcendor RR, Kirshenbaum LA, Imai S, Vatner SF and Sadoshima J. Silent information regulator 2alpha, a longevity factor and class III histone deacetylase, is an essential endogenous apoptosis inhibitor in cardiac myocytes. Circulation research. 2004;95:971–80. [DOI] [PubMed] [Google Scholar]
- 21.Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE, Tsokos M, Alt FW and Finkel T. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:3374–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV and Mann M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834–40. [DOI] [PubMed] [Google Scholar]
- 23.Hwang JH, Kim DW, Jo EJ, Kim YK, Jo YS, Park JH, Yoo SK, Park MK, Kwak TH, Kho YL, Han J, Choi HS, Lee SH, Kim JM, Lee I, Kyung T, Jang C, Chung J, Kweon GR and Shong M. Pharmacological stimulation of NADH oxidation ameliorates obesity and related phenotypes in mice. Diabetes. 2009;58:965–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shin BH, Lim Y, Oh HJ, Park SM, Lee SK, Ahnn J, Kim DH, Song WK, Kwak TH and Park WJ. Pharmacological activation of Sirt1 ameliorates polyglutamine-induced toxicity through the regulation of autophagy. PloS one. 2013;8:e64953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeong MH, Tran NK, Kwak TH, Park BK, Lee CS, Park TS, Lee YH, Park WJ and Yang DK. beta-Lapachone ameliorates lipotoxic cardiomyopathy in acyl CoA synthase transgenic mice. PloS one. 2014;9:e91039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McIntosh DB, Woolley DG, Vilsen B and Andersen JP. Mutagenesis of segment 487Phe-Ser-Arg-Asp-Arg-Lys492 of sarcoplasmic reticulum Ca2+-ATPase produces pumps defective in ATP binding. The Journal of biological chemistry. 1996;271:25778–89. [DOI] [PubMed] [Google Scholar]
- 27.Ma H, Lewis D, Xu C, Inesi G and Toyoshima C. Functional and structural roles of critical amino acids within the”N”, “P”, and “A” domains of the Ca2+ ATPase (SERCA) headpiece. Biochemistry. 2005;44:8090–100. [DOI] [PubMed] [Google Scholar]
- 28.Oh JG, Watanabe S, Lee A, Gorski PA, Lee P, Jeong D, Liang Y, Baccarini A, Sahoo S, Brown BD, Hajjar RJ and Kho C. miR-146a Suppresses SUMO1 Expression and induces Cardiac Dysfunction in Maladaptive Hypertrophy. Circulation research. 2018;123(6):673–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beltrao P, Bork P, Krogan NJ and van Noort V. Evolution and functional cross-talk of protein post-translational modifications. Mol Syst Biol. 2013;9:714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herskovits AZ and Guarente L. SIRT1 in neurodevelopment and brain senescence. Neuron. 2014;81:471–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marks P, Rifkind RA, Richon VM, Breslow R, Miller T and Kelly WK. Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer. 2001;1:194–202. [DOI] [PubMed] [Google Scholar]
- 32.Dekker FJ, van den Bosch T and Martin NI. Small molecule inhibitors of histone acetyltransferases and deacetylases are potential drugs for inflammatory diseases. Drug Discov Today. 2014;19:654–60. [DOI] [PubMed] [Google Scholar]
- 33.Winnik S, Auwerx J, Sinclair DA and Matter CM. Protective effects of sirtuins in cardiovascular diseases: from bench to bedside. Eur Heart J. 2015;36:3404–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poulose N and Raju R. Sirtuin regulation in aging and injury. Biochim Biophys Acta. 2015;1852:2442–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang CL, McKinsey TA, Chang SR, Antos CL, Hill JA and Olson EN. Class II histone deacetylases act as signal-responsive repressors of cardiac hypertrophy. Cell. 2002;110:479–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wagner GR and Payne RM. Mitochondrial acetylation and diseases of aging. J Aging Res. 2011;2011:234875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gregoire S and Yang XJ. Association with class IIa histone deacetylases upregulates the sumoylation of MEF2 transcription factors. Molecular and cellular biology. 2005;25:2273–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shilo Y and Eisenman RN. Histone sumoylation is associated with transcriptional repression. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:13225–13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stankovic-Valentin N, Deltour S, Seeler J, Pinte S, Vergoten G, Guerardel C, Dejean A and Leprince D. An acetylation/deacetylation-SUMOylation switch through a phylogenetically conserved psi KXEP motif in the tumor suppressor HIC1 regulates transcriptional repression activity. Molecular and cellular biology. 2007;27:2661–2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu SY and Chiang CM. Crosstalk between sumoylation and acetylation regulates p53-dependent chromatin transcription and DNA binding. Embo J. 2009;28:1246–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.David G, Neptune MA and DePinho RA. SUMO-1 modification of histone deacetylase 1 (HDAC1) modulates its biological activities. The Journal of biological chemistry. 2002;277:23658–63. [DOI] [PubMed] [Google Scholar]
- 42.Girdwood D, Bumpass D, Vaughan OA, Thain A, Anderson LA, Snowden AW, Garcia-Wilson E, Perkins ND and Hay RT. p300 transcriptional repression is mediated by SUMO modification. Mol Cell. 2003;11:1043–1054. [DOI] [PubMed] [Google Scholar]
- 43.Grunstein M Histone acetylation in chromatin structure and transcription. Nature. 1997;389:349–52. [DOI] [PubMed] [Google Scholar]
- 44.Foster DB, Liu T, Rucker J, O’Meally RN, Devine LR, Cole RN and O’Rourke B. The Cardiac Acetyl-Lysine Proteome. PloS one. 2013;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tanno M, Kuno A, Horio Y and Miura T. Emerging beneficial roles of sirtuins in heart failure. Basic research in cardiology. 2012;107:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park HJ, Choi EK, Choi J, Ahn KJ, Kim EJ, Ji IM, Kook YH, Ahn SD, Williams B, Griffin R, Boothman DA, Lee CK and Song CW. Heat-induced up-regulation of NAD(P)H:quinone oxidoreductase potentiates anticancer effects of beta-lapachone. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005;11:8866–71. [DOI] [PubMed] [Google Scholar]
- 47.Bey EA, Bentle MS, Reinicke KE, Dong Y, Yang CR, Girard L, Minna JD, Bornmann WG, Gao J and Boothman DA. An NQO1- and PARP-1-mediated cell death pathway induced in non-small-cell lung cancer cells by beta-lapachone. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:11832–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jaiswal AK. Regulation of genes encoding NAD(P)H:quinone oxidoreductases. Free radical biology & medicine. 2000;29:254–62. [DOI] [PubMed] [Google Scholar]
- 49.Valor LM, Viosca J, Lopez-Atalaya JP and Barco A. Lysine acetyltransferases CBP and p300 as therapeutic targets in cognitive and neurodegenerative disorders. Current pharmaceutical design. 2013;19:5051–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yao TP, Oh SP, Fuchs M, Zhou ND, Ch’ng LE, Newsome D, Bronson RT, Li E, Livingston DM and Eckner R. Gene dosage-dependent embryonic development and proliferation defects in mice lacking the transcriptional integrator p300. Cell. 1998;93:361–72. [DOI] [PubMed] [Google Scholar]
- 51.Yanazume T, Hasegawa K, Morimoto T, Kawamura T, Wada H, Matsumori A, Kawase Y, Hirai M and Kita T. Cardiac p300 is involved in myocyte growth with decompensated heart failure. Molecular and cellular biology. 2003;23:3593–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miyamoto S, Kawamura T, Morimoto T, Ono K, Wada H, Kawase Y, Matsumori A, Nishio R, Kita T and Hasegawa K. Histone acetyltransferase activity of p300 is required for the promotion of left ventricular remodeling after myocardial infarction in adult mice in vivo. Circulation. 2006;113:679–90. [DOI] [PubMed] [Google Scholar]
- 53.Wei JQ, Shehadeh LA, Mitrani JM, Pessanha M, Slepak TI, Webster KA and Bishopric NH. Quantitative control of adaptive cardiac hypertrophy by acetyltransferase p300. Circulation. 2008;118:934–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.