Abstract
Expansion of the human brain, and specifically the neocortex, is among the most remarkable evolutionary processes that correlates with cognitive, emotional, and social abilities. Cortical expansion is determined through a tightly orchestrated process of neural stem cell proliferation, migration, and ongoing organization, synaptogenesis, and apoptosis. Perturbations of each of these intricate steps can lead to abnormalities of brain size in humans, whether small (microcephaly) or large (megalencephaly). Abnormalities of brain growth can be clinically isolated or occur as part of complex syndromes associated with other neurodevelopmental problems (eg, epilepsy, autism, intellectual disability), brain malformations, and body growth abnormalities. Thorough review of the genetic literature reveals that human microcephaly and megalencephaly are caused by mutations of a rapidly growing number of genes linked within critical cellular pathways that impact early brain development, with important pathomechanistic links to cancer, body growth, and epilepsy. Given the rapid rate of causal gene identification for microcephaly and megalencephaly understanding the roles and interplay of these important signaling pathways is crucial to further unravel the mechanisms underlying brain growth disorders and, more fundamentally, normal brain growth and development in humans. In this review, we will (a) overview the definitions of microcephaly and megalencephaly, highlighting their classifications in clinical practice; (b) overview the most common genes and pathways underlying microcephaly and megalencephaly based on the fundamental cellular processes that are perturbed during cortical development; and (c) outline general clinical molecular diagnostic workflows for children and adults presenting with microcephaly and megalencephaly.
Keywords: brain size, macrocephaly, megalencephaly, microcephaly
Abstract
El crecimiento del cerebro humano, específicamente del neocórtex, está entre los procesos evolutivos más remarcables que se correlacionan con habilidades cognitivas, emocionales y sociales. El crecimiento cortical está determinado por un proceso estrictamente coordinado de la proliferación, migración, organización, sinaptogénesis y apoptosis de las células madre neurales. La alteración de cada una de estas intrincadas etapas puede llevar a anormalidades del tamaño cerebral en los humanos, sea pequeño (microcefalia) o grande (megalencefalia). Las anormalidades del crecimiento cerebral puedan estar clínicamente aisladas o constituir parte de síndromes complejos asociados con otros problemas del neurodesarrollo (como epilepsia, autismo, incapacidad intelectual), malformaciones cerebrales y anormalidades del crecimiento corporal. La revisión de la literatura genética revela que la microcefalia y la megalencefalia son causadas por mutaciones de un número rápidamente creciente de genes relacionados con las vías celulares esenciales que influyen sobre el desarrollo precoz del cerebro, con importantes mecanismos patológicos vinculados con cáncer, crecimiento corporal y epilepsia. Dada la rápida tasa de identificación de genes que causan microcefalia y megalencefalia, la comprensión del papel y de la interacción de estas importantes vías de señalización es crucial para desentrañar los mecanismos subyacentes a los trastornos del crecimiento cerebral y, más fundamentalmente, al crecimiento y desarrollo normal del cerebro en los seres humanos. En esta revisión se presenta: a) una panorámica de las definiciones de microcefalia y megalencefalia, destacando sus clasificaciones en la práctica clínica, b) una panorámica de los genes y vías más comunes que subyacen a la microcefalia y la megalencefalia basada en los procesos celulares fundamentales que están alterados durante el desarrollo cerebral y c) un resumen del plan de trabajo general para el diagnóstico clínico molecular de niños y adultos con microcefalia y megalencefalia.
Abstract
L'expansion du cerveau humain, et surtout celle du néocortex, est l'un des processus les plus remarquables de l'évolution, corrélé avec les capacités cognitives, émotionnelles et sociales. L'expansion corticale est déterminée au travers d'un processus étroitement orchestré de prolifération, de migration, d'organisation, de synaptogenèse et d'apoptose des cellules souches neurales. Toute perturbation de chacune de ces étapes intriquées peut générer des anomalies de la taille du cerveau humain, qu'elle soit petite (microcéphalie) ou grande (macrocéphalie). Les anomalies de la croissance du cerveau peuvent être isolées cliniquement ou participer à des syndromes complexes associés à d'autres problèmes du neurodéveloppement (par exemple, épilepsie, autisme, déficit intellectuel), à des malformations cérébrales et à des anomalies de la croissance corporelle. Une revue de la littérature génétique montre que la microcéphalie et la macrocéphalie humaines sont dues à des mutations d'un nombre de gènes augmentant rapidement, liées à des voies cellulaires essentielles qui influent sur le développement précoce du cerveau avec des liens mécanistiques pathologiques importants aux cancers, à la croissance corporelle et a l'épilepsie. Compte tenu du taux d'identification rapide de gène causal pour la micro et la macrocéphalie, il est essentiel de comprendre les rôles et l'interaction de ces importantes voies de signalisation pour mieux découvrir les mécanismes sous-tendant les troubles de la croissance cérébrale et, plus fondamentalement, ceux de la croissance cérébrale et du développement normaux chez l'homme. Nous présentons dans cet article 1) une vue d'ensemble des définitions de la micro et de la macrocéphalie, en soulignant leurs classifications en pratique clinique 2) une vue d'ensemble des voies et des gènes les plus courants sous-tendant la micro- et la macrocéphalie d'après les processus cellulaires fondamentaux perturbés au cours du développement cortical et 3) un aperçu des plans de travail généraux du diagnostic clinique moléculaire pour les enfants et les adultes micro- et macrocéphales.
Introduction
The adult human brain weighs between 1200 to 1400 g, constituting of approximately 2% of the total body mass, a strikingly larger fraction than other primates.1-3 The expansion of the human brain, particularly the forebrain and total cortical surface area, is among the most remarkable evolutionary processes and correlates with a wide range of cognitive, social, and emotional abilities in humans.4-6 The increase in cortical volume and, consequently, brain size that occurred in the genus Homo is believed to explain the emergence of human-specific cognitive and social abilities that arose with this genus, although the underlying morphology-to-function relationships that arose during evolution are not fully understood.7 Nonetheless, this hypothesis is supported by evidence that different neuroanatomical regions followed different developmental patterns, possibly due to the relative cognitive and social functions that developed at any given time; with the visual cortex and the premotor area (associated to sight and motor control) developing at a slower rate compared to temporal lobe and prefrontal cortex (associated with memory, emotions and conceptual understanding).7,8
Human brain size is determined through a tightly orchestrated and intricate process of neural stem cell proliferation, expansion, migration, followed by ongoing organization, synaptogenesis, and apoptosis. The molecular mechanisms underlying the positive and negative regulation of these processes—and the extreme cases of microcephaly and megalencephaly seen in humans—have provided scientists with important evolutionary insights about the brain. Identification of mutations of key genes associated with microcephaly (eg, primary microcephaly genes such as ASPM, CDK5RAP2, MCPH1, CENPJ) for example, has led evolutionary biologists and anthropologists to establish the presence of selective pressure during human evolution.9,10 Interestingly, the development of the mammalian neocortex appears to be a species-specific process with notable differences even in species that are genetically similar.11,12 The uniqueness of the human neocortical development and evolution seems to be due to the presence of increased heterogeneity in the neural precursors population, in particular the presence of outer radial glia cells in the outer subventricular zone, which are absent or present in very low numbers in other mammals.12-15 This highlights a decades-long challenge for scientists trying to model neurodevelopmental disorders associated with brain growth dysregulation, as animal models often do not recapitulate all of the features of neurological disorders in humans primarily due to intrinsic differences in embryonic neocortical development.16,17
Neurodevelopmental disorders associated with abnormal brain growth with or without cortical malformations are important causes of morbidity and mortality with multiple neurodevelopmental consequences including intellectual disability (ID), autism spectrum disorders (ASD), and epilepsy typically manifesting in infancy or early childhood. Further, expert review of large cohorts of children with malformations of cortical development (MCDs) has identified brain size to be an important predictor of the degree of neurological impairment.18 The occipitofrontal circumference (OFC) of full term infants ranges from 32 to 37 cm at birth, increasing by 0.4 cm per week during the first several months and by ~1 cm per month during the first year of life, with the first 2 years of life characterized as the most rapid period of brain growth during development, to ultimately reach the adult human OFC of 52 to 58 cm and 52.5 to 58.5 cm in females and males, respectively.1 Assuming head size is normally distributed in populations, 0.1% of children have a head size more than 3 SD above or below the mean. This suggests that more than 220 000 of the 74 million children living in the USA have severe megalencephaly (MEG) or microcephaly (MIC), and many more have mild MEG or MIC19 The prevalence of microcephaly (OFC ≤3 SD) in Europe was found to be 1.53 per 10 000 individuals between 2003 and 2012 (95% confidence interval 1.16 to 1.96) in a recent population based study.20 Brain growth abnormalities can be isolated or occur in a wide range of neurodevelopmental syndromes. Microcephaly has been reported in more than 700 genetic syndromes, and macrocephaly in more than 200 genetic syndromes in the Online Mendelian Inheritance in Man (OMIM) database (the more specific clinical term for brain overgrowth, megalencephaly or MEG, is less used in this database).
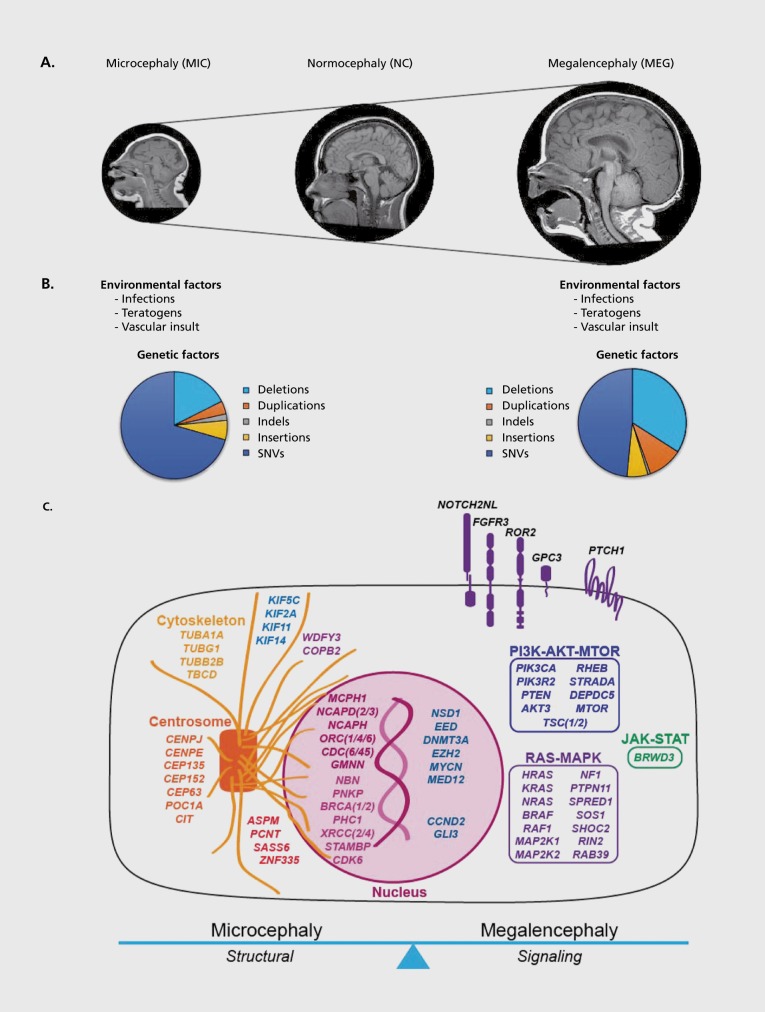
In this review, we will (a) overview the definitions of microcephaly and megalencephaly, highlighting their classifications in clinical practice; (b) overview the most common genes and pathways underlying microcephaly and megalencephaly based on the fundamental cellular processes that are perturbed during cortical development; and (c) outline general clinical molecular diagnostic workflows for children and adults presenting with microcephaly and megalencephaly. Table I lists the most common genes, pathways, and syndromes known to be associated with brain growth abnormalities in humans, to date. Figure 1 presents a schematic overview of the genetic and non-genetic causes of microcephaly and megalencephaly broadly, highlighting the known cellular mechanisms and/or pathways. Importantly, this review will not cover metabolic etiologies of microcephaly and megalencephaly, as the underlying mechanisms of neurometabolic brain growth abnormalities are quite distinct and beyond the scope of this review.
Figure 1. (Opposite) Overview of causes of MIC and MEG. A, Representative brain MR images of microcephaly (MEG), normocephaly (NC) and megalencephaly (MEG). B, Pie graphs demonstrating the types of genetic abnormalities identified in MIC and MEG, along with other (environmental) causes listed as well. C, Schematic representation of the most common MIC—and MEG—associated gene in relationship to their cell function and/or pathway. * The contributions of these genetic abnormalities are derived from the numbers of pathogenic and likely pathogenic mutations deposited in the ClinVar Database for microcephaly and megalencephaly (last accessed July 2018). ** These environmental factors are specifically associated with macrocephaly (large head size) most often due to hydrocephalus or ventriculomegaly, not true brain overgrowth (aka. true MEG), as the latter is most often, if not always, caused by genetic factors. Excluded from this figure are causes of neurometabolic MEG.
TABLE I. Pathways and genes underlying microcephaly (MIC) and megalencephaly (MEG).
| Pathway/cell function | Genes | Inheritance | Neuronal phenotype | Brain MRI findings | Syndromic association | Body growth abnormalities | Cancer predisposition* | Epilepsy |
| MICROCEPHALY | ||||||||
| Cell cycle: Centro-some formation, spindle orientation, microtubule organization, cytokinesis | MCPH1 | AR | Premature chromosome condensation, decreased neuronal proliferation, premature differentiation | SIMP | Congenital MIC | + | - | - |
| ASPM | AR | Decreased neuronal proliferation | Decreased brain volume, SIMP | Congenital MIC | - | - | +/- | |
| WDR62 | AR | Decreased neuronal proliferation | MCDs, complex | Congenital MIC, cortical dysplasia | - | - | - | |
| CD-K5RAP2 | AR | Premature differentiation | Decreased brain volume, SIMP | Congenital MIC | - | - | - | |
| CASC5 | AR | Decreased neuronal proliferation | Decreased brain volume, SIMP | Congenital MIC | - | - | - | |
| CENPJ | AR | Increased apoptosis | Decreased brain volume, SIMP | Congenital MIC, Seckel syndrome | + | - | + | |
| SASS6** | AR | Decreased neuronal proliferation, exact neuronal phenotype not well-understood | Decreased brain volume, SIMP | Congenital MIC | - | - | + | |
| STIL | AR | Neural tube defects, increased sensitivity to neurotoxic insult | Holoprosencephaly, SIMP | Congenital MIC | - | - | - | |
| CEP152 | AR | Abnormal centrosome structure and function, decreased neuronal proliferation | Decreased brain volume, SIMP | Congenital MIC, Seckel syndrome | + | - | - | |
| CEP63** | AR | Increased cell death due to increased centrosome-based mitotic errors. | Decreased brain volume, SIMP | Seckel syndrome | + | - | - | |
| NDE1 | AR | Decreased neuronal proliferation | MCDs, complex | Microhydranencephaly, lissencephaly | + | - | - | |
| NIN | AR | Defects of the anterior neuroectoderm | Decreased brain volume, SIMP | Seckel syndrome | + | - | - | |
| PCNT | AR | Disorganized mitotic pindles and misaggregation of chromosomes, decreased neuronal proliferation | Decreased brain volume, SIMP, vascular abnormalities | MOPD type II | + | - | - | |
| BUB1B | AR | Decreased neuronal proliferation, increased apoptosis | Complex | Mosaic variegated aneuploidy | + | + | - | |
| CENPE | AR | Potential defect of neuronal proliferation | Decreased brain volume, SIMP | Congenital MIC, MOPD | + | - | - | |
| Centro-some formation, spindle orientation, micro-tubule organization, cytokinesis | KIF5C | AD/ de novo | Abnormal microtubule function | Complex | Cortical dysplasia | - | - | + |
| KIF2A | AD/ de novo | Abnormal axonal branching with consequently reduced neuronal volume | Complex | Cortical dysplasia | + | - | + | |
| KIF11 | AD/ de novo | Abnormal mitotic spindles, decreased neuronal proliferation | Decreased brain volume, SIMP | Microcephaly-chorioretinopathy-lymphedema | - | - | - | |
| KIF14 | AR | Increased neuronal apoptosis, impaired cell migration and motility, decreased myelination | Decreased brain volume, large basal cisterns, optic nerve atrophy | Congenital MIC, Meckel syndrome | + | - | - | |
| TUBA1A | AD/ de novo | Abnormal neuronal migration | Complex | Tubulinopathy | - | - | + | |
| TUBG1 | AD/ de novo | Abnormal neuronal migration | Complex | Tubulinopathy | - | - | - | |
| TUBB2B | AD/ de novo | Abnormal neuronal migration | Complex | Tubulinopathy | - | - | - | |
| TBCD | AR | Abnormal microtubule structure and function with likely effects on neuronal proliferation and migration | Complex | Encephalopathy, atrophy, thin corpus callosum | + | - | + | |
| POC1A | AR | Abnormal mitotic spindles and centrioles with likely effects on neuronal proliferation and migration | - | Short stature, on-ychodysplasia, facial dysmorphism, hypotrichosis | + | - | - | |
| ZNF335 | AR | Reduced proliferation, defects of neuronal differentiation and migration | SIMP | Congenital MIC | + | - | - | |
| CIT | AR | Impaired neuronal cytokinesis, delayed mitosis, cellular blebbing, multipolar spindles, genome instability, increased apoptosis | Complex | Congenital MIC | - | - | - | |
| NCAPD2 | AR | Impaired chromosome segregation, reduced neuronal proliferation, reduced cell survival | Decreased brain volume, SIMP | Congenital MIC | + | - | - | |
| NCAPD3 | AR | Impaired chromosome segregation, reduced neuronal proliferation, reduced cell survival | - | - | - | |||
| NCAPH** | AR | Impaired chromosome segregation, reduced neuronal proliferation, reduced cell survival | - | - | - | |||
| KATNB1 | AR | Supernumerary centrosomes and spindle abnormalities | Decreased brain volume, SIMP, cortical dysplasia | Congenital MIC, cortical dysplasia | - | - | + | |
| Cell cycle: kinetochore | CEP135 | AR | Fragmented or lack of centrosomes with disorganized microtubules, decreased neuronal proliferation | Decreased brain volume, SIMP | Congenital MIC | - | - | - |
| CENPF | AR | Abnormal spindle orientation and ciliogenesis | Decreased brain volume, SIMP | Stromme syndrome | - | - | - | |
| CHAMP1 | AD/ de novo | Abnormal kinetochore-microtubule attachment | Decreased brain volume, SIMP | Intellectual disability, microcephaly | - | - | - | |
| Cell cycle: mitotic chromosome structure | NCAPD2 | Abnormal chromosome condensation, and sister chromatid disentanglement | Decreased brain volume, SIMP | AR microcephaly | + | - | - | |
| NCAPH | AR | Decreased brain volume, SIMP | AR microcephaly | - | - | - | ||
| NCAPD3 | AR | Decreased brain volume, SIMP | AR microcephaly | + | - | |||
| Origin Recognition Complex | ORC1 | AR | Abnormal DNA replication and likely abnormal neuronal proliferation | SIMP | MGORS | + | - | - |
| ORC4 | AR | SIMP | MGORS | + | - | - | ||
| ORC6 | AR | SIMP | MGORS | + | - | - | ||
| CDT1 | AR | SIMP | MGORS | + | - | - | ||
| CDC6 | AR | SIMP | MGORS | + | - | |||
| GMNN | AR | SIMP | MGORS | + | - | - | ||
| CDC45 | AR | SIMP | MGORS | + | - | - | ||
| DDR and chromosome stability and cell cycle regulation | ATR | AR/AD | Exact neuronal phenotype not well-understood | Complex | Seckel syndrome, Familial cancer | + | - | - |
| ATRIP | AR | Exact neuronal phenotype not well-understood | SIMP | Seckel syndrome | + | - | - | |
| RBBP8 | AR | Exact neuronal phenotype not well-understood | Complex | Seckel syndrome, Jawad syndrome | + | - | - | |
| NBN | AR | Aberrant regulation of early brain development | SIMP | Nijmegen breakage syndrome | + | + | - | |
| RAD50 | AR | Exact neuronal phenotype not well-understood | SIMP | Nijmegen breakage syndrome-like disorder | + | + | - | |
| MRE11A | AR | Exact neuronal phenotype not well-understood | Complex | Ataxia-telan-giectasia-like disorder | - | + | + | |
| PNKP | AR | Increased neurogenesis, defects in neuronal differentiation | Complex | Microcephaly with seizures and developmental delay, ataxia-oculomotor apraxia | - | - | + | |
| BRCA1 | AR | Neuroepithelial defects, reduced proliferation | SIMP | Fanconi anemia, complementation group S | - | + | - | |
| DDR and chromosome stability and cell cycle regulation | BRCA2 | AR | Reduced neuronal proliferation | SIMP | Fanconi anemia, complementation group D1 | - | + | - |
| LIG4 | AR | Exact neuronal phenotype not well-understood | SIMP | LIG4 syndrome | + | + | - | |
| NHEJ1 | AR | Exact neuronal phenotype not well-understood | SIMP | SCID with microcephaly | + | + | - | |
| DDX11 | AR | Exact neuronal phenotype not well-understood | SIMP | Warsaw breakage syndrome | + | + | - | |
| PHC1 | AR | Reduced proliferation, defects of differentiation | SIMP | Congenital MIC | + | - | - | |
| DNA2 | AR | Increased senescence, needs research | SIMP | Seckel syndrome | + | - | - | |
| XRCC2 | AR | Increased apoptosis of postmitotic neurons | SIMP | Fanconi anemia, complementation group U | + | + | - | |
| XRCC4 | AR | Increased apoptosis, reduced proliferation | Complex | Short stature, microcephaly, and endocrine dysfunction | + | + | - | |
| RECQL3 | AR | Exact neuronal phenotype not well-understood | SIMP | Bloom syndrome | + | + | - | |
| DONSON | AR | Decreased neuronal proliferation | SIMP, abnormal WM, and MCDs | Microcephaly-micromelia syndrome | + | - | - | |
| STAMBP | AR | Increased apoptosis | Complex | Microcephaly-capillary malformation | + | - | + | |
| CDK6 | AR | Exact neuronal phenotype not well-understood | SIMP | Congenital MIC | - | + | - | |
| ANKLE2 | AR | Decreased cell proliferation and increased apoptosis of neuroblasts | SIMP, MCDs, ventricular abnormalities, ACC | Congenital MIC | + | - | + | |
| MFSD2A | AR | BBB defects, neuronal cell loss, loss of transport activity | SIMP | Congenital MIC | + | - | + | |
| Cellular trafficking, fatty acid metabolism, lipid binding proteins | WDFY3** | AD | Abnormal WNT activation, increased proliferation of apical progenitor cells, lack of neuronal differentiation, impaired cortical development (dominant-negative effect) | MIC | Congenital MIC | - | - | - |
| COPB2** | AR | Increased apoptosis, reduction of upper layer neurons | SIMP, thin CC, enlarged XAX, delayed myelination, progressive atrophy | Congenital MIC | + | - | + | |
| MEGALENCEPHALY | ||||||||
| PI3K-AKT-MTOR | PIK3 CA | AD/de novo (mosaic) | Exact neuronal phenotype understudy, likely cell hypertrophy, abnormal neuronal organization | PMG (BPP) | PIK3CA-related overgrowth disorders | + | +/-(tentative, Wilms tumor) | + |
| PTEN | AD/ de novo | Cell hypertrophy | PMG, FCD | PTEN-hamarto-ma tumor syndrome | + | + | - | |
| PIK3R2 | AD/de novo | Exact neuronal phenotype understudy, likely cell hypertrophy, abnormal neuronal organization | PMG (BPP), mega CC | Megalencephaly-polymicrogyria-polydactyly-hydrocephalus (MPPH) syndrome | - | - | + | |
| MTOR | AD/de novo (mosaic) | Cell hypertrophy, abnormal neuronal migration | PMG/HMEG/FCD (depending on level of mosaicism) | MTOR-related disorders | - | - | + | |
| CCND2 | AD/de novo | Increased neuronal proliferation | PMG (BPP), mega CC | MPPH syndrome | - | - | + | |
| RHEB | AD/de novo | Cell hypertrophy, abnormal migration | - | MEG-ID | - | - | + | |
| STRADA (LYK5) | AR | Abnormal neuronal lamination, mTORC1 hyperactivation | VMEG, subependymal dysplasia, WM abnormalities | Polyhydramnios-MEG-symptomatic epilepsy (PMSE) | - | - | + | |
| DEPDC5 | AD/de novo | Neuronal hypertrophy and abnormal neuronal organization | FCD, MCDs | Familial epilepsy | - | - | + | |
| AKT3*** | AD/de novo (mosaic) | Exact neuronal phenotype under study, likely cell hypertrophy, abnormal neuronal organization | PMG/HMEG/FCD (depending on level of mosaicism) | MPPH syndrome | - | - | + | |
| TSC1/TSC2 | AD/de novo | Neuronal hypertrophy | Cortical tubers, subependymal nodules, HMEG/FCD | Tuberous Sclerosis | - | + | + | |
| DNA methyltrans-ferases, transcription initiation, and regulators | NSD1*** | AD/de novo | Exact neuronal phenotype not well-understood | VMEG,XAX | Sotos syndrome | + | - | - |
| EED | AD/de novo | Abnormal EZH2 function, exact neuronal phenotype not well understood | - | Cohen-Gibson syndrome | + | - | - | |
| DNMT3A | AD/de novo | Exact neuronal phenotype not well-understood | Tatton-Brown-Rahman syndrome | + | - | + | ||
| EZH2 | AD/de novo | Exact neuronal phenotype not well-understood | MCDs, VMEG | Weaver syndrome | + | - | + | |
| DNA methyl-trans-ferases, transcription initiation, and regulators | MYCN*** | AD/de novo | Decreased apoptosis | - | 2p24.3 duplication syndrome | - | + (w/ amplifications) | - |
| MED12 | X-linked | Exact neuronal phenotype not well-understood | Callosal abnormalities, VMEG, HET | Opitz-Kaveggia syndrome Lujan (Lujan-Fryns) syndrome | + | - | + | |
| NFIX | AD/de novo | Exact neuronal phenotype not well-understood | - | Malan syndrome | + | - | + | |
| SETD2 | AD/ de novo | Exact neuronal phenotype not well-understood | Likely complex | Luscan-Lumish syndrome | +/- | - | + | |
| RAS-MAPK | NF1 | AD/ de novo | RAS-MAPK mediated effects on brain development (pleitropic) | VMEG, UBOs on T2 imaging, CC abnormalities | Neurofibromatosis type I | - | + (Optic gliomas) | - |
| SPRED1 | AD/ de novo | - | Legius syndrome | - | - | - | ||
| HRAS | AD/ de novo | CBTH, VMEG/ HYD | Costello syndrome | + Short stature | + | - | ||
| BRAF, MAP2K1, MAP2K2, KRAS | AD/ de novo | CBTH, VMEG/HYD, cortical atrophy, MCDs | Cardiofaciocutaneous syndrome | + Short stature | - | - | ||
| PTPN11, SOS1, RAF1, KRAS, BRAF, SHOC2, NRAS, MAP2K1 | AD/ de novo | CBTH, VMEG/HYD | Noonan syndrome | + Short stature | - | - | ||
| RIN2 | AR | - | Macrocephaly-alopecia-cutis laxa-scoliosis (MACS) syndrome | + Short stature | - | - | ||
| RAB39 | XL | - | XLID, ASD, epilepsy | - | - | + | ||
| RTKs | FGFR3 | AR | Abnormal neuronal proliferation and apoptosis of cortical progenitors | HYD, cervico-medullary compression, MCDs, other | Achondroplasia Thanatophoric dysplasia | + Multiple skeletal anomalies | - | - |
| ROR2 | AR | Abnormal neuronal sternness | MCDs | Robinow syndrome | + Short stature | - | - | |
| NOTCH | GPC3 | X-linked | Abnormal cell growth and proliferation, exact neuronal phenotype not well-understood | HYD, cerebellar tonsillar herniation, CC abnormalities | Simpson Golabi Behmel syndrome I | + | + (Embryonal tumors, Wilms tumor) | |
| NOTCH2NL*** | AD/ de novo | Delayed neuronal differentiation | - | 1q21.1 microduplication syndrome | - | - | - | |
| SHH | PTCH1 | AD/ de novo | Abnormal neuronal proliferation and organization | Calcifications (>90%) | Nevoid basal cell carcinoma syndrome | - | +(PNET) | - |
| KIF7 | AR | Abnormal neuronal proliferation and organization | CC abnormalities (ACC) | Acrocallosal syndrome | + Growth retardation | - | + | |
| GLI3 | AD/ de novo | Abnormal neuronal proliferation | CC abnormalities (ACC) | Greig cephalosyndactyly | - | - | - | |
| Cilia structure and function | OFD1 | X-linked | Abnormal ciliary function in proliferating sells | VMEG | Simpson Golabi Behmel syndrome II | + | - | - |
| RTTN | AR | Abnormal centriole formation | Decreased brain volume, SIMP, cortical dysplasia, callosal, cerebellar abnormalities | Microcephaly, short stature, and poly-microgyria with seizures | + | - | + | |
| JAK-STAT | BRWD3 | X-linked | Abnormal cell morphology and cytoskeletal organization | - | XLID | - | - | - |
| Abbreviations: | ||||||||
| ACC, agenesis of the corpus callosum; AD, autosomal dominant; AR, autosomal recessive; ASD, autism spectrum disorders; BPP, bilateral frontoparietal polymicrogyria; CBTH, cerebellar tonsillar herniation; CC, corpus callosum; FCD, focal cortical dysplasia; HET, heterotopia; HMEG, hemimegalencephaly; HYD, hydrocephalus; MCDs, malformations of cortical development; MCPH, primary microcephaly genes 1-23; MEG, megalencephaly; MGORS, Meier-Gorlin syndrome 1-7; MIC, microcephaly; MOPD, microcephalic osteodysplastic primordial dwarfism; PMG, polymicrogyria; RTKs, receptor tyrosine kinases; SCID; Severe Combined Immunodeficiency; SIMP, simplified gyral pattern; UBOs, unidentified bright objects on brain MRI; VMEG, ventriculomegaly; WM, white matter; XAX, extra-axial space; XLID, X-linked intellectual disability. | ||||||||
| Notes: | ||||||||
| * Cancer predisposition specifically refers to germline (constitutional) cancer risk | ||||||||
| ** MIC-/MEG-associated mutations in these genes have been reported in one or few families, to date. | ||||||||
| *** Reciprocal deletions/duplications of these gene loci are associated with MIC or MEG and include the following genes: AKT3 (1q43.q44; del MIC, dup MEG); NOTCH2NL (1q21.1; del MIC, dup MEG), MYCN (2p.24.3; del MIC, dup MEG); NSD1 (5q35.3; del MEG, dup MIC). Other MIC-MEG loci include 10q22q23 (del MEG, dup MIC) and 16p11.2 (dup MIC, dup MEG) with no MIC/MEG candidate genes identified, to our knowledge. |
Microcephaly
Definition, clinical classifications, and clinical features
Microcephaly (MIC) is classically defined as a head circumference more than two standard deviations (SD) below the mean for age and sex. However, most experts define clinically significant (ie, diagnostically useful) MIC as 3 SD or more below the mean, as many children with a head OFC between 2-3 SD are developmentally normal.19,21,22 About 15% of children referred to child neurologists for evaluation of developmental disabilities have MIC, which is often associated with comorbidities such as epilepsy, autism, and other birth defects.19 Genetic and non-genetic factors can cause an abnormally small brain size. Genetic causes including a rapidly growing list of single gene disorders, copy number abnormalities (ie, deletions/duplications), and large chromosomal rearrangements (eg, triosomies) (Figure 1, Table I). Non-genetic or environmental insults include in utero or congenital infections (eg, congenital Zika virus, cytomegalovirus, and herpes simple virus, among others), teratogenic exposures (eg, alcohol, drugs such as hydantoin or aminopterin) and vascular insults (eg, placental insufficiency).19 However, knowledge regarding the underlying causes of MIC remains limited in scope, and the diagnostic and therapeutic approaches for children with MIC are not uniform, as highlighted by the Zika virus epidemic in 2015-2016.1,2,5,6
A confusing number of terms have been used to clinically classify MIC including “primary microcephaly” which refers to early-onset (ie, typically congenital) MIC with no other syndromic features including (classically) no additional brain abnormalities besides a simplified cortical gyral pattern.22 “Autosomal recessive primary MIC” (or MCPH) was a clinical diagnostic term used for the earliest familial reports and loci of MIC, later defined by several key features including (a) severe congenital MIC (OFC ≤4 SD below the mean at birth), (b) ID in the absence of more severe neurological problems including spasticity, seizures or developmental regression and (c) absence of other syndromic features including body growth abnormalities or additional brain malformations.23-25 To date, mutations in 23 genes have been identified as causative of primary microcephaly (Table I). However, the remarkable emerging genetic and clinical heterogeneity of MIC syndromes has challenged traditional classifications and uncovered a wide range of syndromes in which individuals can have overlapping features including brain size abnormalities, body growth abnormalities and complex malformations of cortical development.26,27 Indeed, mutations of the same genes have now been identified in expanding spectra involving these features. Examples include MIC and cancer phenotypes due to mutations in DNA Damage Response genes, such as Nijmegan Breakage syndrome, and isolated MIC and Microcephalic Osteodysplastic Primordial Dwarfism (MOPD) phenotypes due to centrosomal defects.26
When the brain is small, the most common abnormality identified on brain imaging (typically magnetic resonance imaging, MRI) is a simplified cortical gyral pattern that is typically diffuse but can disproportionately affect the frontal lobes (ie, microcephaly with a simplified gyral pattern).28 By definition, this is the most common brain MRI abnormality identified in children with most forms of MIC. However, additional brain abnormalities can co-occur with MIC, partly depending on the underlying etiology, including cortical malformations such pachygyria, polymicrogyria, dysgyria (eg, due to mutations of the tubulin genes), callosal abnormalities (including most notably a small or thin corpus callosum), as well as abnormalities of the cerebellum, brainstem, basal ganglia, white matter among others.28 Therefore, thorough examination of neuroimaging features associated with MIC is often diagnostically very helpful, as shown in Table I.
Genes and pathways underlying human MIC
In examining the large body of literature on genes and pathways underlying human—particularly congenital—MIC, several important functional classes of defects can be clearly discerned.26 The earliest identified MIC-associated genes and loci were critical centrosomal and cell cycle defects that continue to account for the largest fraction of MIC molecular diagnoses worldwide. These include centrosome-specific proteins (eg, CEP135, CENPJ, PCNT, MCHP1), spindle-associated proteins (eg, ASPM, WDR62) and, less commonly, kinetochore associated defects (eg, CENPE). By far, mutations in the majority of these genes are inherited in an autosomal recessive fashion and are, therefore, more widely seen in in-bred or consanguineous populations.29 Mutations of the ASPM (Abnormal Spindle Microtubule Assembly) gene alone are estimated to account for 10%-40% of the causes of autosomal recessive congenital MIC (or MCPH).30 Mutations of this broad class of genes have been well-documented to cause a wide range of cell cycle defects including abnormal centrosome structure and function, abnormal spindle-kinetochore assembly, and abnormal microtubule structure and function.26 More recently, mutations of a critical class of microtubule-associated genes including those belonging to the tubulin family (eg, TUBA1A, TUBB2B, TUBG1, TBCD, among many others) and the kinesin protein (eg, KIF11, KIF14) have been identified to cause complex MIC syndromes characterized by a wide range of brain malformations including cortical malformations, callosal abnormalities, and dysgenesis of the brainstem, basal ganglia, and thaiami, with wide variability among affected individuals.31,32 In contrast to the most common centrosomal defects underlying MIC, mutations of these genes are typically de novo, with consequently a low-recurrence risk.32
The second-largest group of human functional defects associated with MIC is defects in the DNA Damage Response (DDR) pathway. The DDR is a signaling cascade critical for repairing DNA strand repairs due to endogenous and exogenous insults. The large number of associated mutations, genes, and syndromes known to be associated with congenital MIC that are related to DDR and centriole/spindle organization support the fundamental roles these pathways play in neuronal development and genomic stability.33,34 The intimate relationship between these pathways and cancer is further supported by the association of many of these MIC-associated genes with cancer phenotypes in humans, as occurs with mutations in NBN, NHEJ1, XRCC2, XRCC4 among many others, for example.35 Collectively, the overwhelming majority of genes associated with congenital MIC are critical nodes in very early stages of neuronal development and have yielded substantial insights into their fundamental roles.36 In contrast, acquired causes of MIC, such as Zika virus (ZIKV) related MIC, for example, appear to typically affect later stages of neuronal progenitors, inducing apoptosis and defects of differentiation rather than severe neuroproliferative defects, although the mechanisms of ZIKV-related MIC continues to be under intense study.37-39
Megalencephaly
Definition, clinical classifications and clinical features
From DeMyer's work, MEG or “large brain” has been defined as an oversized and overweight brain that exceeds the mean by two SD for age and gender, consistent with standard medical practice which uses a normal range of ±2 SD for growth parameters.40 Similar to MIC, clinically significant MEG is now defined as 3 or more SD above the mean, as most individuals with mildly large head size (+2-3 SD) have been found to have normal development.40 The largest documented brain sizes in human reach more than 10 SD above the mean, reported more recently in individuals with mutations of the phosphatidyl inositol 3-kinase (PI3K)-AKT-MTOR pathway including AKT3.41 Abnormalities of brain size can occur early on during fetal development and manifest at or shortly after birth (ie, congenital MEG), or evolve more slowly during infancy and early childhood (ie, postnatal MEG).26 Clinically significant MEG has been classified into anatomic and metabolic subtypes, a classification scheme first proposed by DeMyer that continues to be of clinical use as these two large MEG groups differ substantially in their underlying genetic causes, pathomechanisms, clinical features and medical management.40 Metabolic (or neurometabolic) syndromes associated with MEG include a wide range of disorders characterized by abnormal accumulation of metabolic substrates, typically associated with consequent neuronal hypertrophy with ballooning of the cytoplasm, and fundamentally less cellular hyperplasia.40,42 Anatomic MEG includes a wide range of disorders characterized by a multitude of cellular defects including increased cell size and/or number.
From the early literature, brain overgrowth has been proposed to occur in several distinct “non-syndromic” clinical forms. The earliest is familial MEG (also termed “idiopathic MEG”) that has been reported and proposed to be the most common form of MEG seen in children.40 Individuals with this clinical “entity” classically had mild MEG (OFC +2-3 SD above the mean) with familial recurrence reported in up to 50% of children in large series.43-46 Importantly, a subset of these children with familial MEG had neurodevelopmental problems including epilepsy and tone abnormalities, suggesting that brain overgrowth, even if presenting as an isolated feature, can be associated with neurological consequences.45,47-49 Further, neuroimaging on many children with this reported MEG subtype identified ventriculomegaly, enlarged extra-axial spaces, or hydrocephalus requiring shunting, overlapping with syndromic forms of MEG (such as the PI3K-AKT-MTOR related MEG syndromes) and suggesting a more complex process underlying head overgrowth that may involve both brain overgrowth and cerebrospinal fluid expansion causing hydrocephalus.47,49-51
MEG has also been reported as a very common clinical feature in children with autism spectrum disorders (ASD), with several studies suggesting that it is the most common physical manifestation seen in ASD.52 Mutations of the PTEN gene have been identified in ~10% to 20% of megalencephalic children with ASD, which has led to the delineation of the “macrocephaly-autism syndrome” as a subtype of the PTEN-hamartoma tumor related disorders.53-56 However, wider scale genomic testing has now identified mutations of many other genes associated with MEG-ASD as well, including most notably MTOR, PPP2R5D, CHD8, among others, strongly suggesting that the etiologies of MEG and ASD are much more genetically heterogeneous but likely linked to some of the same critical cellular pathways.57
Genes and pathways underlying human MEG
Disorders causing an abnormally large brain size (ie, true MEG) most often occur as a result of a genetic insult that may cause isolated brain overgrowth (eg, PTEN related hamartoma tumor syndrome) or combined brain and body overgrowth as part of a generalized overgrowth disorder (eg, Sotos syndrome, PIK3CA related overgrowth disorders).58,59 Most of these disorders are caused by defects of single genes, although a growing number of copy number abnormalities have also been identified in overgrowth syndromes (Table I). Mutations of key nodes in several signaling pathways regulating cellular growth and proliferation cause a wide range of human brain overgrowth disorders including the PI3K-AKT-MTOR, RAS-MAPK-ERK pathways, among others.60-63 Importantly, a growing number of human overgrowth disorders are caused by mutations in epigenetic regulators such as NSD1, EZH2, DNTM3A, among others.64 A broad overview of the involved genes and pathways highlights that most of these are within critical intermediary signaling pathways that are highly pleiotropic in function and, unlike MIC, the cellular effects of these pathway perturbations on neuronal development are not as well-understood (Figure 1).
The most common genetic defects causing human MEG localize to the PI3K-AKT-MTOR pathway. Proper MTOR signaling is critical in key aspects of brain development including neuronal progenitor maintenance, differentiation, migration, synaptogenesis, and regulating protein translation.65-68 Therefore, mutations of different nodes causing functional upregulation of this pathway are associated with a wide range of neurodevelopmental phenotypes including MEG, ID, ASD, and epilepsy in humans and animal models. These nodes include a growing number of genes located upstream (PIK3CA, PIK3R2, PTEN), midstream (AKT3) and downstream (TSC1, TSC2, MTOR, CCND2, TBC1D7, RHEB, and STRADA) within the pathway.69 Besides diffuse brain overgrowth (MEG), a growing number of mosaic (postzygotic) mutations of this pathway are associated with focal malformations of cortical development with intractable epilepsy, including focal cortical dysplasia (FCD), hemimegalencephaly (HMEG) and dysplastic megalencephaly (DMEG).70-72 The functional endpoint of all mutations associated with diffuse or focal MEG phenotypes is pathway hyperactivation. The proposed mechanisms by which PI3K-AKT-MTOR pathway mutations are believed to cause brain overgrowth include neuronal hypertrophy (eg, PTEN- and MTOR- related brain overgrowth), and increased neuronal proliferation (eg, CCND2- related brain overgrowth).73-76 Specifically, mutations of the AKT3 gene have been associated with most severe MEG, and further shown to have very strong associations with increased intracranial volume (ICV) in a large meta-analysis of reactome gene sets in relationship to brain size, along with other MTOR pathway genes.41,69
Recently, mutations of a new family of genes—the PP2A phosphatase family including specifically PPP2R5D—have been identified in children with congenital onset MEG, autism, and hypotonia. This class of phosphatases is believed to have a negative regulatory effect on the PI3K-AKT-MTOR pathway. Therefore, PI3K-AKT-MTOR dysregulation is believed to underlie the brain overgrowth of PPP2R5D-related disorders as well.77-79 Finally, abnormalities of the evolutionarily-conserved partitioning defective protein complex, including PARD3, PARD6, and atypical protein kinase C (aPKC), that regulate asymmetric cell division of Radial Glial Progenitors (RGPs) have been proposed as a mechanism underlying MEG and heterotopia in humans.80,81 However, no mutations of these genes have been identified in human MEG, to date. A comprehensive review of additional genes associated with human MEG are further provided in Table I.
Proposed diagnostic workflow for microcephaly to megalencephaly
Given the causal heterogeneity of MIC and MEG, and based on our broad review of the literature, we propose the following clinical diagnostic workflow for pediatric-onset MIC and MEG. For any individual presenting with an abnormally small or large brain size, a careful assessment of the individual's growth (including stature and weight) are crucial as this will help delineate whether brain growth abnormalities are isolated or due to more generalized growth disorders (eg, MOPD disorders with MIC, somatic overgrowth disorders with MEG). Further, assessment of the brain magnetic resonance imaging is extremely valuable as many MIC-MEG syndromes are associated with other developmental brain disorders that can be clinically delineated by good quality imaging, such as mutations of the tubulin genes (TUBA1A, TUBB2B, TUBG1, among many others, for example).
Microcephaly
Among the most clinically useful distinguishing features of MIC is its' age of onset (ie, congenital vs postnatal). Congenital MIC, if isolated (ie, not associated with any other major structural anomalies) is typically autosomal recessive in nature (also known as Primary MIC or MCPH) caused by mutations in cell cycle genes such as ASPM most notably, among others. Congenital MIC in association with other structural defects is most often syndromic and likely extremely genetically heterogeneous. Therefore, etiologies falling under this category include microdeletion or microduplication syndromes that can be identified by chromosomal microarrays, or a growing number of single gene disorders, many of which can be delineated based on their associated key clinical features. If clinical delineation is not possible, then consideration for wide-scale genomic testing (eg, exome sequencing) is recommended. It is important to remember the non-genetic causes of congenital MIC including congenital viral infections, vascular insults or injuries and environmental exposures (eg, fetal alcohol syndrome). Postnatal MIC, on the other hand, is extremely genetically heterogeneous, as MIC occurs as a secondary features in numerous neurodevelopmental syndromes characterized by epilepsy, ID and ASD; many of which are caused by de novo mutations. Therefore, clinical delineation of the specific syndrome is recommended. If a specific syndrome is not identified based on the history and physical examination, then consideration of wide scale genomic testing (eg, exome sequencing) is similarly recommended. Detailed recommendations for clinical evaluations of children with MIC have been previously published.19
Megalencephaly
Similar to MIC, careful review of overall body growth measurements, in conjunction with a detailed neurological examination and/or brain imaging, are very useful in evaluating a child with MEG. Importantly, when a child presents with a large head size, it is imperative to rule out other important causes of macrocephaly such as hydrocephalus or progressive ventriculomegaly. True and isolated brain overgrowth — MEG — is seen most commonly with single gene disorders, most notably PI3K-AKT-MTOR pathway related disorders.59,72 Given the likelihood of low-frequency postzygotic (mosaic) mutations in MEG disorders, careful selection of molecular diagnostic approaches needs to be made using a method that generates good quality, high depth data (eg, ultra deep targeted capture). Syndromic forms of MEG require a consideration of microdeletion-microduplication syndromes (using chromosomal arrays), followed by careful and thorough clinical assessments to delineate specific overgrowth syndromes. In the presence of diffuse MEG with multiple other congenital anomalies, exome sequencing may be utilized to efficiently and rapidly identify the underlying genetic etiology, given the emerging genetic heterogeneity of MEG.58 In spite of wider integration of high-quality and high-depth NGS methods in clinical practice, the genetic causes of a substantial fraction of MEG in children remain unidentified, suggesting the involvement of other genes/loci and/or low-level mosaicism challenging detection using standard molecular methods.69
Summary
A review of the microcephaly and megalencephaly disorders in humans highlights the remarkable genetic heterogeneity of these disorders, mutations of which perturb key cellular processes with disruption of various neurodevelopmental stages. There is a strong need for updated classifications of these disorders based on molecular pathways or underlying mechanisms rather than descriptive clinical parameters that may be outdated and in need of refinement, particularly in light of rapidly emerging molecular data. Most MIC—and MEG—associated pathways are critical cell signaling pathways that regulate cellular growth, proliferation, with strong overlap with cancer pathways, the aberrations of which cause complex syndromes where neurodevelopmental features and cancer phenotypes overlap (eg, DDR-associated disorders with MIC, and PI3K-AKT-MTOR related disorders with MEG). With the ever-increasing use of NGS methods in clinical molecular practice, the genetic heterogeneity of these disorders is only expected to increase, with the very strong likelihood of uncovering low frequency mosaic mutations in MEG and related disorders including focal malformations of cortical development. These genetic insights will likely shed light on more fundamental mechanisms underlying MIC and MEG that may require validation ideally using high throughput in in vivo and in vitro systems. Ultimately, MIC-MEG disorders are collectively not that rare and have substantially informed biological mechanisms underlying normal brain growth and development in humans.
Acknowledgments
We thank our patients, their families, and providers for their contribution and support of our ongoing research on developmental brain disorders. We also thank Joshua Scheck (Center for Integrative Brain Research, Seattle Children's Research Institute, Seattle, WA) for his assistance with this manuscript. Research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke (NINDS) under award number K08NS092898 and Jordan's Guardian Angels (to G.M.M), and NIH grants R21 OD023838 and R24 HD000836, the Keck Foundation, and the Seattle Children's Foundation (to B.R.N). The content is solely the responsibility of the authors, and does not necessarily represent the official views of the National Institutes of Health. The funding sources had no role in the design and conduct of the study, collection, management, analysis and interpretation of the data, preparation, review or approval of the manuscript, or decision to submit the manuscript for publication. The authors report no conflict of interest.
Contributor Information
Filomena Pirozzi, Center for Integrative Brain Research, Seattle Children's Research Institute, Seattle, Washington, USA.
Branden Nelson, Center for Integrative Brain Research, Seattle Children's Research Institute, Seattle, Washington, USA.
Ghayda Mirzaa, Center for Integrative Brain Research, Seattle Children's Research Institute, Seattle, Washington, USA; Division of Genetic Medicine, Department of Pediatrics, University of Washington, Seattle, Washington, USA.
REFERENCES
- 1.Nellhaus G. Head circumference from birth to eighteen years. Practical composite international and interracial graphs. Pediatrics. 1968;41(1):106–114. [PubMed] [Google Scholar]
- 2.Roth G., Dicke U. Evolution of the brain and intelligence. Trends Cogn Sci. 2005;9(5):250–257. doi: 10.1016/j.tics.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Wilson SA. Megalencephaly. J Neurol Psychopathol. 1934;14(55):193–216. doi: 10.1136/jnnp.s1-14.55.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reardon PK., Seidlitz J., Vandekar S., et al. Normative brain size variation and brain shape diversity in humans. Science. 2018;360(6394):1222–1227. doi: 10.1126/science.aar2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rilling JK. Comparative primate neuroimaging: insights into human brain evolution. Trends Cogn Sci. 2014;18(1):46–55. doi: 10.1016/j.tics.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Van Essen DC. Scaling of human brain size. Science. 2018;360(6394):1184–1185. doi: 10.1126/science.aat8948. [DOI] [PubMed] [Google Scholar]
- 7.Balzeau A., Gilissen E., Holloway RL., Prima S., Grimaud-Herve D. Variations in size, shape and asymmetries of the third frontal convolution in hominids: paleoneurological implications for hominin evolution and the origin of language. J Hum Evol. 2014;76:116–128. doi: 10.1016/j.jhevol.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Conroy GC., Smith RJ. The size of scalable brain components in the human evolutionary lineage: with a comment on the paradox of Homo floresiensis. Homo. 2007;58(1):1–12. doi: 10.1016/j.jchb.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Montgomery SH., Capellini I., Venditti C., Barton RA., Mundy Nl. Adaptive evolution of four microcephaly genes and the evolution of brain size in anthropoid primates. Mol Biol Evol. 2011;28(1):625–638. doi: 10.1093/molbev/msq237. [DOI] [PubMed] [Google Scholar]
- 10.Murray JE., Jackson AP. Exploring microcephaly and human brain evolution. Dev Med Child Neurol. 2012;54(7):580–581. doi: 10.1111/j.1469-8749.2012.04330.x. [DOI] [PubMed] [Google Scholar]
- 11.Northcutt RG., Kaas JH. The emergence and evolution of mammalian neocortex. Trends Neurosci. 1995;18(9):373–379. doi: 10.1016/0166-2236(95)93932-n. [DOI] [PubMed] [Google Scholar]
- 12.Florio M., Huttner WB. Neural progenitors, neurogenesis and the evolution of the neocortex. Development. 2014;141(11):2182–2194. doi: 10.1242/dev.090571. [DOI] [PubMed] [Google Scholar]
- 13.Borrell V., Gotz M. Role of radial glial cells in cerebral cortex folding. Curr Opin Neurobiol. 2014;27:39–46. doi: 10.1016/j.conb.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Gertz CC., Lui JH., La Monica BE., Wang X., Kriegstein AR. Diverse behaviors of outer radial glia in developing ferret and human cortex. J Neurosci. 2014;34(7):2559–2570. doi: 10.1523/JNEUROSCI.2645-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansen DV., Lui JH., Parker PR., Kriegstein AR. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature. 2010;464(7288):554–561. doi: 10.1038/nature08845. [DOI] [PubMed] [Google Scholar]
- 16.Rakic P. A small step for the cell, a giant leap for mankind: a hypothesis of neocortical expansion during evolution. Trends Neurosci. 1995;18(9):383–388. doi: 10.1016/0166-2236(95)93934-p. [DOI] [PubMed] [Google Scholar]
- 17.Molnar Z., Clowry G. Cerebral cortical development in rodents and primates. Prog Brain Res. 2012;195:45–70. doi: 10.1016/B978-0-444-53860-4.00003-9. [DOI] [PubMed] [Google Scholar]
- 18.Guerrini R., Dobyns WB. Malformations of cortical development: clinical features and genetic causes. Lancet Neurol. 2014;13(7):710–726. doi: 10.1016/S1474-4422(14)70040-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ashwal S., Michelson D., Plawner L., Dobyns WB. Practice parameter: Evaluation of the child with microcephaly (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2009;73(11):887–897. doi: 10.1212/WNL.0b013e3181b783f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris JK., Rankin J., Game E., et al. Prevalence of microcephaly in Europe: population based study. BMJ. 2016;354:i4721. doi: 10.1136/bmj.i4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dobyns WB. Primary microcephaly: new approaches for an old disorder. Am J Med Genet. 2002;112(4):315–317. doi: 10.1002/ajmg.10580. [DOI] [PubMed] [Google Scholar]
- 22.Woods CG., Parker A. Investigating microcephaly. Arch Dis Child. 2013;98(9):707–713. doi: 10.1136/archdischild-2012-302882. [DOI] [PubMed] [Google Scholar]
- 23.Jackson AP., McHale DP., Campbell DA., et al. Primary autosomal recessive microcephaly (MCPH1) maps to chromosome 8p22-pter. Am J Hum Genet. 1998;63(2):541–546. doi: 10.1086/301966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roberts E., Jackson AP., Carradice AC., et al. The second locus for autosomal recessive primary microcephaly (MCPH2) maps to chromosome 19q13.1-13.2. Eur J Hum Genet. 1999;7(7):815–820. doi: 10.1038/sj.ejhg.5200385. [DOI] [PubMed] [Google Scholar]
- 25.Woods CG., Bond J., Enard W. Autosomal recessive primary microcephaly (MCPH): a review of clinical, molecular, and evolutionary findings. Am J Hum Genet. 2005;76(5):717–728. doi: 10.1086/429930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alcantara D., O'Driscoll M. Congenital microcephaly. Am J Med Genet C Semin Med Genet. 2014;166c(2):124–139. doi: 10.1002/ajmg.c.31397. [DOI] [PubMed] [Google Scholar]
- 27.Seltzer LE., Paciorkowski AR. Genetic disorders associated with postnatal microcephaly. Am J Med Genet C Semin Med Genet. 2014;166c(2):140–155. doi: 10.1002/ajmg.c.31400. [DOI] [PubMed] [Google Scholar]
- 28.Basel-Vanagaite L., Dobyns WB. Clinical and brain imaging heterogeneity of severe microcephaly. Pediatr Neurol. 2010;43(1):7–16. doi: 10.1016/j.pediatrneurol.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 29.Roberts E., Hampshire DJ., Pattison L., et al. Autosomal recessive primary microcephaly: an analysis of locus heterogeneity and phenotypic variation. J Med Genet. 2002;39(10):718–721. doi: 10.1136/jmg.39.10.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicholas AK., Swanson EA., Cox JJ., et al. The molecular landscape of ASPM mutations in primary microcephaly. J Med Genet. 2009;46(4):249–253. doi: 10.1136/jmg.2008.062380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fallet-Bianco C., Laquerriere A., Poirier K., et al. Mutations in tubulin genes are frequent causes of various foetal malformations of cortical development including microlissencephaly. Acta Neuropathol Commun. 2014;2:69. doi: 10.1186/2051-5960-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poirier K., Lebrun N., Broix L., et al. Mutations in TUBG1, DYNC1H1, KIF5C and KIF2A cause malformations of cortical development and microcephaly. Nat Genet. 2013;45(6):639–647. doi: 10.1038/ng.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lancaster MA., Knoblich JA. Spindle orientation in mammalian cerebral cortical development. Curr Opin Neurobiol. 2012;22(5):737–746. doi: 10.1016/j.conb.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Driscoll M., Jeggo PA. The role of the DNA damage response pathways in brain development and microcephaly: insight from human disorders. DNA Repair (Amst). 2008;7(7):1039–1050. doi: 10.1016/j.dnarep.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 35.Venkatesh T., Suresh PS. Emerging roles of MCPH1: expedition from primary microcephaly to cancer. Eur J Cell Biol. 2014;93(3):98–105. doi: 10.1016/j.ejcb.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 36.Gilmore EC., Walsh CA. Genetic causes of microcephaly and lessons for neuronal development. Wiley Interdiscip Rev Dev Biol. 2013;2(4):461–478. doi: 10.1002/wdev.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cugola FR., Fernandes IR., Russo FB., et al. The Brazilian Zika virus strain causes birth defects in experimental models. Nature. 2016;534(7606):267–271. doi: 10.1038/nature18296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dang J., Tiwari SK., Lichinchi G., et al. Zika virus depletes neural progenitors in human cerebral organoids through activation of the innate immune receptor TLR3. Cell Stem Cell. 2016;19(2):258–265. doi: 10.1016/j.stem.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qian X., Nguyen HN., Song MM., et al. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell. 2016;165(5):1238–1254. doi: 10.1016/j.cell.2016.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DeMyer W. Megalencephaly: types, clinical syndromes, and management. Pediatr Neurol. 1986;2(6):321–328. doi: 10.1016/0887-8994(86)90072-x. [DOI] [PubMed] [Google Scholar]
- 41.Alcantara D., Timms AE., Gripp K., et al. Mutations of AKT3 are associated with a wide spectrum of developmental disorders including extreme megalencephaly. Brain. 2017;140(10):2610–2622. doi: 10.1093/brain/awx203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoffmann GF., Gibson KM., Trefz FK., Nyhan WL., Bremer HJ., Rating D. Neurological manifestations of organic acid disorders. Eur J Pediatr. 1994;153(7 Suppl 1):S94–100. doi: 10.1007/BF02138786. [DOI] [PubMed] [Google Scholar]
- 43.Day RE., Schutt WH. Normal children with large heads-benign familial megalencephaly. Arch Dis Child. 1979;54(7):512–517. doi: 10.1136/adc.54.7.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gragg GW. Familial megalencephaly. Birth Defects Orig Artic Ser. 1971;7(1):228–230. [PubMed] [Google Scholar]
- 45.Hartel C., Bachmann S., Bonnemann C., Meinecke P., Sperner J. Familial megalencephaly with dilated Virchow-Robin spaces in magnetic resonance imaging: an autosomal recessive trait? Clin Dysmorphol. 2005;14(1):31–34. [PubMed] [Google Scholar]
- 46.Schreier H., Rapin I., Davis J. Familial megalencephaly or hydrocephalus? Neurology. 1974;24(3):232–236. doi: 10.1212/wnl.24.3.232. [DOI] [PubMed] [Google Scholar]
- 47.Alvarez LA., Maytal J., Shinnar S. Idiopathic external hydrocephalus: natural history and relationship to benign familial macrocephaly. Pediatrics. 1986;77(6):901–907. [PubMed] [Google Scholar]
- 48.Asch AJ., Myers GJ. Benign familial macrocephaly: report of a family and review of the literature. Pediatrics. 1976;57(4):535–539. [PubMed] [Google Scholar]
- 49.Laubscher B., Deonna T., Uske A., van Melle G. Primitive megalencephaly in children: natural history, medium term prognosis with special reference to external hydrocephalus. Eur J Pediatr. 1990;149(7):502–507. doi: 10.1007/BF01959405. [DOI] [PubMed] [Google Scholar]
- 50.Conway RL., Pressman BD., Dobyns WB., et al. Neuroimaging findings in macrocephaly-capillary malformation: a longitudinal study of 17 patients. Am J Med Genet A. 2007;143A(24):2981–3008. doi: 10.1002/ajmg.a.32040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mirzaa GM., Conway RL., Gripp KW., et al. Megalencephaly-capillary malformation (MCAP) and megalencephaly-polydactyly-polymicrogyria-hydrocephalus (MPPH) syndromes: two closely related disorders of brain overgrowth and abnormal brain and body morphogenesis. Am J Med Genet A. 2012;158a(2):269–291. doi: 10.1002/ajmg.a.34402. [DOI] [PubMed] [Google Scholar]
- 52.Bailey A., Luthert P., Bolton P., Le Couteur A., Rutter M., Harding B. Autism and megalencephaly. Lancet. 1993;341(8854):1225–1226. doi: 10.1016/0140-6736(93)91065-t. [DOI] [PubMed] [Google Scholar]
- 53.Herman GE., Butter E., Enrile B., Pastore M., Prior TW., Sommer A. Increasing knowledge of PTEN germline mutations: Two additional patients with autism and macrocephaly. Am J Med Genet A. 2007;143A(6):589–593. doi: 10.1002/ajmg.a.31619. [DOI] [PubMed] [Google Scholar]
- 54.Herman GE., Henninger N., Ratliff-Schaub K., Pastore M., Fitzgerald S., McBride KL. Genetic testing in autism: how much is enough? Genet Med. 2007;9(5):268–274. doi: 10.1097/gim.0b013e31804d683b. [DOI] [PubMed] [Google Scholar]
- 55.Orrico A., Galli L., Buoni S., Orsi A., Vonella G., Sorrentino V. Novel PTEN mutations in neurodevelopmental disorders and macrocephaly. Clin Genet. 2009;75(2):195–198. doi: 10.1111/j.1399-0004.2008.01074.x. [DOI] [PubMed] [Google Scholar]
- 56.Varga EA., Pastore M., Prior T., Herman GE., McBride KL. The prevalence of PTEN mutations in a clinical pediatric cohort with autism spectrum disorders, developmental delay, and macrocephaly. Genet Med. 2009;11(2):111–117. doi: 10.1097/GIM.0b013e31818fd762. [DOI] [PubMed] [Google Scholar]
- 57.O'Roak BJ., Deriziotis P., Lee C., et al. Exome sequencing in sporadic autism spectrum disorders identifies severe de novo mutations. Nat Genet. 2011;43(6):585–589. doi: 10.1038/ng.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tatton-Brown K., Weksberg R. Molecular mechanisms of childhood overgrowth. Am J Med Genet C Semin Med Genet. 2013;163C(2):71–75. doi: 10.1002/ajmg.c.31362. [DOI] [PubMed] [Google Scholar]
- 59.Mirzaa GM., Riviere JB., Dobyns WB. Megalencephaly syndromes and activating mutations in the PI3K-AKT pathway: MPPH and MCAP. Am J Med Genet C Semin Med Genet. 2013;163C(2):122–130. doi: 10.1002/ajmg.c.31361. [DOI] [PubMed] [Google Scholar]
- 60.Bentires-Alj M., Kontaridis Ml., Neel BG. Stops along the RAS pathway in human genetic disease. Nat Med. 2006;12(3):283–285. doi: 10.1038/nm0306-283. [DOI] [PubMed] [Google Scholar]
- 61.Rauen KA. The RASopathies. Annu Rev Genomics Hum Genet. 2013;14:355–369. doi: 10.1146/annurev-genom-091212-153523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tidyman WE., Rauen KA. The RASopathies: developmental syndromes of Ras/MAPK pathway dysregulation. Curr Opin Genet Dev. 2009;19(3):230–236. doi: 10.1016/j.gde.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Engelman JA., Luo J., Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7(8):606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 64.Tatton-Brown K., Loveday C., Yost S., et al. Mutations in epigenetic regulation genes are a major cause of overgrowth with intellectual disability. Am J Hum Genet. 2017;100(5):725–736. doi: 10.1016/j.ajhg.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Laplante M., Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122(Pt20):3589–3594. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sarbassov DD., Ali SM., Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17(6):596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 67.Saxton RA., Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;169(2):361–371. doi: 10.1016/j.cell.2017.03.035. [DOI] [PubMed] [Google Scholar]
- 68.Zoncu R., Efeyan A., Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12(1):21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reijnders MRF., Kousi M., vanWoerden GM., et al. Variation in a range of mTOR-related genes associates with intracranial volume and intellectual disability. Nature Communications. 2017;8(1):1052. doi: 10.1038/s41467-017-00933-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jansen LA., Mirzaa GM., Ishak GE., et al. PI3K/AKT pathway mutations cause a spectrum of brain malformations from megalencephaly to focal cortical dysplasia. Brain. 2015;138:1613–1628. doi: 10.1093/brain/awv045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jamuar SS., Lam AT., Kircher M., et al. Somatic mutations in cerebral cortical malformations. N Engl J Med. 2014;371(8):733–743. doi: 10.1056/NEJMoa1314432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mirzaa GM., Poduri A. Megalencephaly and hemimegalencephaly: breakthroughs in molecular etiology. Am J Med Genet C Semin Med Genet. 2014;166c(2):156–172. doi: 10.1002/ajmg.c.31401. [DOI] [PubMed] [Google Scholar]
- 73.Mirzaa GM., Parry DA., Fry AE., et al. De novo CCND2 mutations leading to stabilization of cyclin D2 cause megalencephaly-polymicrogyriapolydactyly-hydrocephalus syndrome. Nat Genet. 2014;46(5):510–515. doi: 10.1038/ng.2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kwon CH., Luikart BW., Powell CM., et al. Pten regulates neuronal arborization and social interaction in mice. Neuron. 2006;50(3):377–388. doi: 10.1016/j.neuron.2006.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kwon CH., Zhu X., Zhang J., Baker SJ. mTor is required for hypertrophy of Pten-deficient neuronal soma in vivo. Proc Natl Acad Sci U S A. 2003;100(22):12923–12928. doi: 10.1073/pnas.2132711100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kwon CH., Zhu X., Zhang J., et al. Pten regulates neuronal soma size: a mouse model of Lhermitte-Duclos disease. Nat Genet. 2001;29(4):404–411. doi: 10.1038/ng781. [DOI] [PubMed] [Google Scholar]
- 77.Loveday C., Tatton-Brown K., Clarke M., et al. Mutations in the PP2A regulatory subunit B family genes PPP2R5B, PPP2R5C and PPP2R5D cause human overgrowth. Hum Mol Genet. 2015;24(17):4775–4779. doi: 10.1093/hmg/ddv182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Houge G., Haesen D., Vissers LE., et al. B56delta-related protein phosphatase 2A dysfunction identified in patients with intellectual disability. J Clin Invest. 2015;125(8):3051–3062. doi: 10.1172/JCI79860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shang L., Henderson LB., Cho MT., et al. De novo missense variants in PPP2R5D are associated with intellectual disability, macrocephaly, hypotonia, and autism. Neurogenetics. 2016;17(1):43–49. doi: 10.1007/s10048-015-0466-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mazzeu JF., Pardono E., Vianna-Morgante AM., et al. Clinical characterization of autosomal dominant and recessive variants of Robinow syndrome. Am J Med Genet A. 2007;143(4):320–325. doi: 10.1002/ajmg.a.31592. [DOI] [PubMed] [Google Scholar]
- 81.Liu WA., Chen S., Li Z., et al. PARD3 dysfunction in conjunction with dynamic HIPPO signaling drives cortical enlargement with massive heterotopia. Genes Dev. 2018;32(11-12):763–780. doi: 10.1101/gad.313171.118. [DOI] [PMC free article] [PubMed] [Google Scholar]