Abstract
The discovery that repeat expansions in the C9orf72 gene are a frequent cause of amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD) has revolutionized our understanding of these diseases. Substantial headway has been made in characterizing C9orf72-mediated disease and unravelling its underlying aetiopathogenesis. Three main disease mechanisms have been proposed: loss of function of the C9orf72 protein, toxic gain of function from C9orf72 repeat RNA or from dipeptide repeat proteins produced by repeat-associated non-ATG translation. Several downstream processes across a range of cellular functions have also been implicated. In this article, we review the pathological and mechanistic features of C9orf72-associated FTD and ALS (collectively termed C9FTD/ALS), the model systems used to study these conditions, and the probable initiators of downstream disease mechanisms. We suggest that a combination of upstream mechanisms involving both loss and gain of function, and downstream cellular pathways involving both cell-autonomous and non-cell-autonomous effects, contribute to disease progression.
Introduction
Amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD) are devastating and fatal neurodegenerative diseases. In common with other neurodegenerative diseases, progress towards finding disease-modifying therapies in ALS and FTD has been slow, in large part owing to an incomplete understanding of disease aetiopathogenesis. In 2011, ground-breaking progress was made with the discovery that a hexanucleotide GGGGCC repeat expansion in the C9orf72 gene (Fig. 1) is the most frequent genetic cause of both diseases in Europe and North America1,2. Interestingly, C9FTD/ALS — the collective term for C9orf72-associated diseases with clinical features of FTD, ALS or both —is extremely rare in Asia and the Middle East3,4, indicating a different genetic architecture underlying FTD and ALS in these populations. The age of onset of C9FTD/ALS ranges from 27–83 years of age3,4, and the disease duration ranges from 1–22 years3,4. C9orf72 repeat expansions have also been identified as a rare cause of other neurodegenerative diseases4, including Parkinson disease, progressive supranuclear palsy, ataxia, corticobasal syndrome, Huntington disease-like syndrome, Creutzfeldt–Jakob disease and Alzheimer disease.
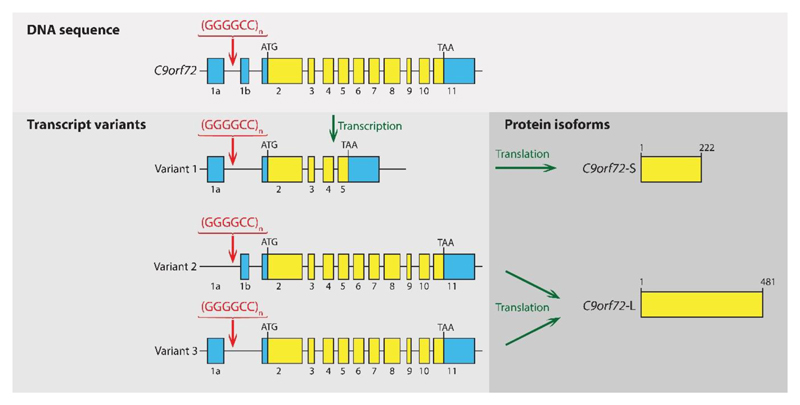
Figure 1. C9orf72 structure, transcript variants and protein isoforms.
The C9orf72 gene consists of 11 exons, has three main alternatively spliced transcript variants and produces two protein isoforms. In the figure, coding exons are indicated in yellow and noncoding exons in blue (not to scale). The GGGGCC hexanucleotide repeat expansion mutation is located in the first intron of variants 1 and 3 and within the promoter region of variant 2. Variant 1 encodes C9orf72-S (short), a 222-amino-acid protein of 24 kDa, and variants 2 and 3 encode C9orf72-L (long), a 481-amino-acid protein of 54 kDa
The vast majority (>95%) of neurologically healthy individuals have ≤11 hexanucleotide repeats in the C9orf72 gene5. The pathological repeat-length threshold has not been clearly defined; an arbitrary cut-off of 30 repeats is used in most studies, but larger expansions ranging from hundreds to thousands of repeats are most commonly observed in patients with C9FTD/ALS5–10. Importantly, the discovery of C9FTD/ALS has heightened the realization that ALS and FTD are intimately linked on a clinical, genetic, pathological and mechanistic spectrum.
The expanded GGGGCC repeats are bidirectionally transcribed into repetitive RNA, which forms sense and antisense RNA foci11–15. Remarkably, despite being within a non-coding region of C9orf72, these repetitive RNAs can be translated in every reading frame to form five different dipeptide repeat proteins (DPRs) — poly-GA, poly-GP poly-GR, poly-PA and poly-PR — via a non-canonical mechanism known as repeat-associated non-ATG (RAN) translation (Fig. 2)14–18. Although C9orf72 mutations are a relatively recent discovery, progress in understanding their pathogenenic effects has been rapid. Three competing but non-exclusive mechanisms have arisen: loss of function of C9orf72 protein, and toxic gain of function from sense and antisense C9orf72 repeat RNA or from DPRs. These mechanisms are all likely to contribute to disease to some extent, but it is crucial to determine their relative importance at various disease stages, so as to inform therapeutic strategies. The differential involvement of these mechanisms might also explain clinical, pathological and prognostic heterogeneity that is observed in patients with C9FTD/ALS.
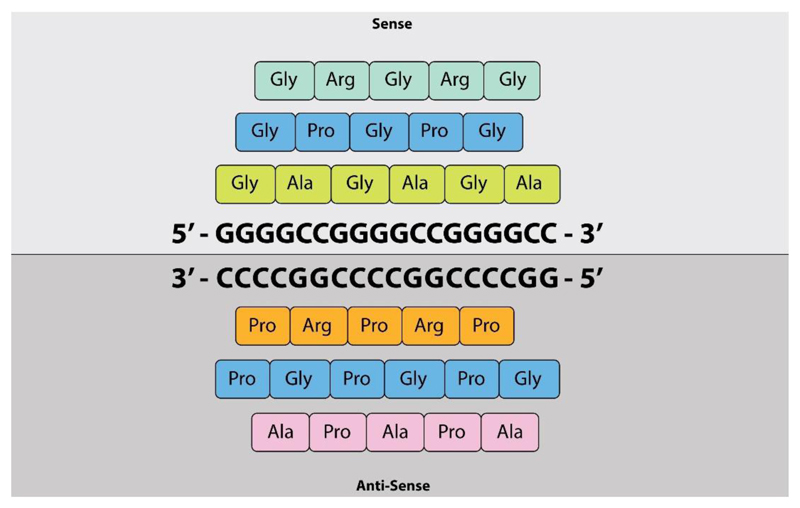
Figure 2. Dipeptide repeat proteins.
The figure shows the dipeptide repeat proteins that are generated by GGGGCC repeat-associated non-ATG (RAN) translation. The sense strand generates poly-GA, poly-GP and poly-GR and the antisense strand generates poly-GP, poly-PA and poly-PR.
In this article, we review the current understanding of the mechanisms underlying C9FTD/ALS, and the questions that remain unanswered. The clinical and genetic aspects of C9FTD/ALS have been extensively reviewed elsewhere19,20 and will not be discussed in detail here.
Repeat size and somatic mosaicism
Defining the minimum number of hexanucleotide repeats in C9orf72 that cause disease would be invaluable for genetic counselling and to guide disease modelling, but this issue is currently unresolved. Neurologically healthy individuals and patients with ALS or FTD can all have 20–30 repeats, so whether repeat lengths within this range can drive disease is unclear. For example, a screen of control post-mortem brains identified an individual with 30 repeats, sparse RNA foci and DPR inclusions, but no neurological symptoms up until death at 84 years of age21.
One confounding factor for accurate repeat sizing is somatic instability of the mutation. Individuals have been identified with large expansions within the CNS but an intermediate repeat length in DNA extracted from blood22–24. These studies show that >50 repeats in blood-derived DNA can be associated with large CNS expansions, suggesting that 50 repeats is a useful cut-off when analysing blood DNA. However, the current data do not support a single precise cut-off; for instance, 70 repeats were insufficient to cause disease in an 89-year-old whose children inherited much larger expansions and went on to develop C9FTD/ALS25. Furthermore, defining a cut-off for blood-derived DNA does not help answer the key question of the minimum repeat size required in the CNS to cause disease.
A further confounder is that repeat size varies between different brain regions6,10. This so-called somatic mosaicism within the CNS might explain some of the clinical heterogeneity that is observed between patients, but is currently not well understood.
Pathological features of C9FTD/ALS
TDP-43 inclusions
The overwhelming majority of ALS cases and approximately 50% of FTD cases are characterized by inclusions consisting of the RNA-binding protein TDP-43 (TAR DNA-binding protein 43) in neurons and glia26,27. The fact that C9orf72 mutations can lead to TDP-43 inclusions (Fig. 3) in both ALS and FTD implies a final common pathway in these diseases28. This pathology is evident in various brain regions, including the frontal, temporal and primary motor cortices, hippocampus, basal ganglia, amygdala, thalamus and midbrain29–33. Patients with C9orf72 mutations who have a predominant ALS syndrome can still exhibit extramotor pathological features consistent with FTD and, conversely, those with a predominant FTD syndrome can show pathology in the motor system31.
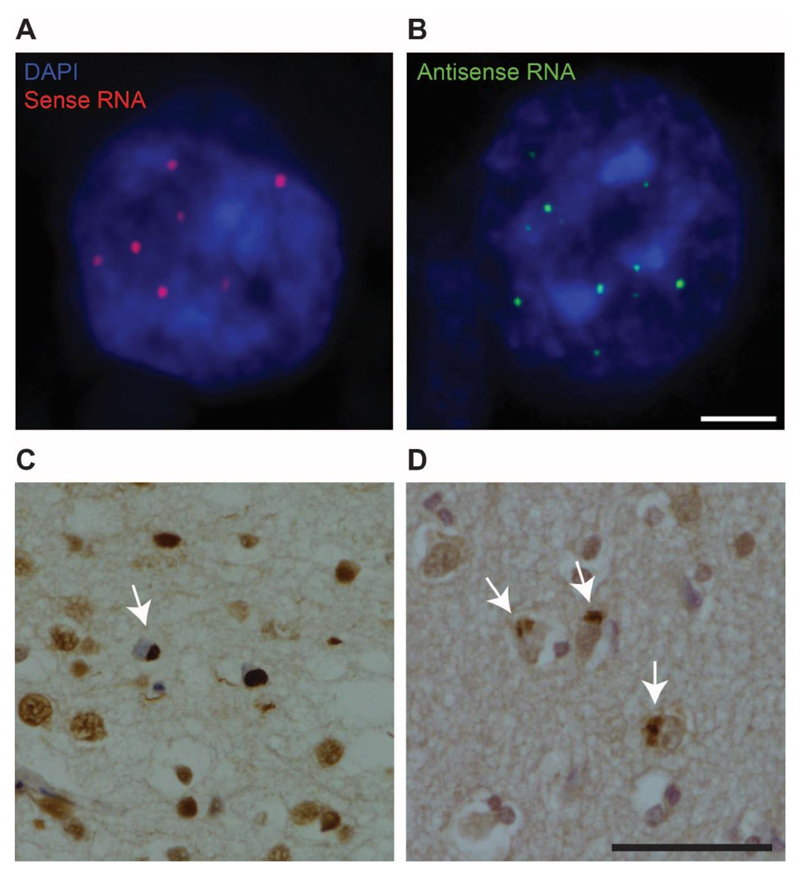
Figure 3. C9FTD/ALS neuropathology.
Sense and antisense RNA foci are a common feature in the brains of patients with C9orf72-associated frontotemporal dementia and/or amyotrophic lateral sclerosis (C9FTD/ALS). a,b |Representative images show neurons from the frontal cortex of a patient with C9FTD/ALS, containing multiple sense (red; part a) and antisense (green; part b) foci in nuclei (stained blue with DAPI). Scale bar: 2.5 μm. c | TAR DNA-binding protein 43 (TDP-43) pathology in a patient with C9FTD/ALS. Arrow indicates a neuronal cytoplasmic TDP-43 inclusion in the frontal cortex, with concomitant depletion of nuclear TDP-43. Scale bar: 50 μm. d | Dipeptide repeat protein (DPR) pathology in a patient with C9FTD/ALS. Inclusions consisting of sense and antisense DPRs are produced by repeat-associated non-ATG (RAN) translation. Arrows indicate neuronal cytoplasmic inclusions of poly-GA protein. Scale bar: 50 μm.
RNA foci
Sense and antisense RNA foci comprising C9orf72 repeat RNA are widely distributed across the CNS in patients with C9FTD/ALS (Fig. 3). These foci are found predominantly within neuronal nuclei in the frontal and motor cortices, hippocampus, cerebellum and in the spinal cord, in motor neurons and occasionally in interneurons, and sporadically in the cytoplasm11,13–15,34. Less frequently, foci are detected in glia (astrocytes, microglia, astrocytes and oligodendrocytes 11,13,15. Sense RNA foci occur in ~37% and antisense foci in ~26% of neurons in the frontal cortex, respectively11,34, and they co-occur in ~14% of frontal cortex neurons11.
DPR pathology
DPR inclusions are p62-positive and TDP-43-negative and can consist of more than one DPR (Fig. 3)14,15,17,18,35. Several studies have examined DPRs in patients with C9orf72 repeat expansions, using immunohistochemistry14–18,29,35–42, immunoblotting14,16,17,38,41 or immunoassays43, and have reached similar conclusions. DPRs most commonly form neuronal cytoplasmic inclusions15–17,29, but can also exist as neuritic inclusions29,44 or as ‘pre-inclusions’15,29, which appear as diffuse cytoplasmic staining. Neuronal intranuclear inclusions are sometimes observed15–17,29, and are occasionally paranucleolar36. Sense-derived poly-GA is the most frequent form of DPR 16,18,38–41, followed by poly-GP then poly-GR. The antisense-derived DPRs poly-PA and poly-PR are the least frequent forms15,18,35,36,38–40. Although sense-derived DPRs seem to be more prevalent than antisense-derived DPRs in patients with C9orf72 repeat expansions, both sense-derived and antisense-derived poly-GP are detected in hippocampal neurons in these individuals, with strand-specific antibodies suggesting a preponderance of antisense-derived poly-GP14. Staining of cortical tissue with antibodies specific to the carboxy-terminal region of the translated DPRs revealed that translation occurs beyond the 3′ end of the repeats in the cortex in C9orf72 repeat expansion carriers14,18.
DPR pathology is most prominent in the cerebellum, hippocampus and neocortex, is less frequent in subcortical regions, and is rarely observed in the brainstem and spinal cord. In addition, all DPRs can be detected in insoluble fractions from patient frontal cortex or cerebellum, as high-molecular-weight species, indicating they are aggregation prone 14,16,17,38. A Meso Scale Discovery immunoassay has quantitatively detected poly-GP and poly-GA — but not, to date, any of the other DPRs — in both CNS tissue43 and cerebrospinal fluid (CSF)45. CSF poly-GP levels might have utility as a biomarker for both diagnosis and pharmacodynamic response46–48.
Pathogenesis of C9FTD/ALS
The complementary use of human tissue and in vitro and in vivo models, including illuminating mouse models examining both loss-of-function and gain-of-function mechanisms, has informed our current understanding of the contribution of C9orf72 loss of function, C9orf72 repeat RNA and DPRs to pathogenesis. These studies also have implicated several downstream mechanisms resulting from C9orf72 expansions (Fig. 4).
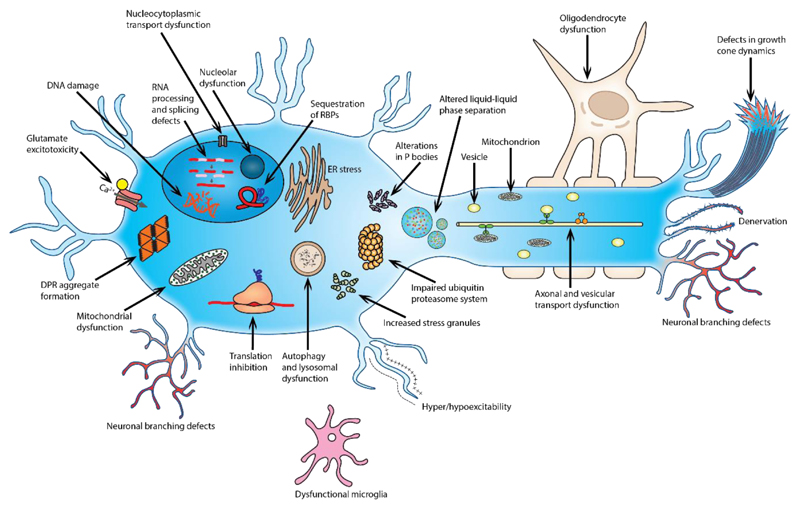
Figure 4. Cellular processes implicated in C9orf72-associated FTD and ALS.
A wide range of cellular pathways have been implicated in C9orf72-mediated disease, several of which have previously been linked to amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). C9orf72 loss-of-function and toxic gain-of-function mechanisms can both alter RNA processing and metabolism pathways, with alterations in stress granules and P-bodies, and C9orf72 gain-of-function mechanisms can lead to nucleolar dysfunction, affect RNA splicing and transcription and cause DNA damage. Proteostasis pathways have also been implicated, with impairments in autophagy and lysosomal function, the unfolded protein response and the endoplasmic reticulum, and the ubiquitin–proteasome system. Other cellular processes including nucleocytoplasmic transport, vesicular trafficking and transport granule function, and mitochondrial function, can also be impaired. In addition, neuron-specific processes, including hyperexcitabiliy and hypoexcitability, glutamate excitotoxicity, axonal transport and neuronal branching defects, have been implicated in C9FTD/ALS. Finally, loss of C9orf72 function alters immune system and microglial function (Table 3).
Loss-of-function mechanisms
C9orf72 transcription and splicing
In C9orf72 transcript variant 2, the repeats are located within the promoter region (Fig. 1), so are not incorporated into variant 2 pre-mRNA but have the potential to affect the expression of this variant. By contrast, in variants 1 and 3, the repeats are within intron 1, so are included in the respective pre-mRNAs. Variant 2 is expressed at higher levels than variants 1 and 3 in CNS tissue138,139.
Studies have demonstrated reduced levels of one or more of the C9orf72 transcript variants in blood lymphocytes1,132,140, induced pluripotent stem cell (iPSC)-derived neurons12,90,94,138, frontal cortex1,132,141–145, cerebellum12,16,143–145, motor cortex12 and cervical spinal cord12 from C9orf72 expansion carriers compared with controls. The findings are particularly robust for variants 1 and 2145. C9orf72 protein levels might be correspondingly reduced in the frontal cortex110,144. Transcripts upstream of the repeat are increased relative to downstream transcripts in blood lymphocytes and brain and spinal cord tissue66 from C9orf72 expansion carriers, possibly owing to abortive transcription in the presence of the repeat expansion66. Raised levels of variant 1 in the frontal cortex and cerebellum are associated with increased survival145 — an important consideration when developing therapies that affect transcript levels.
The intronic location of the repeats in variants 1 and 3 means they should be spliced out of the transcript, but the fact that they are translated into DPRs implies that they are either retained in the transcript or that the spliced intron is translated. Although levels of mature spliced C9orf72 mRNA are reduced in the brains of individuals with C9FTD/ALS, levels of sense and antisense transcripts containing intron 1, where the repeats are located, are increased14,16, suggesting stabilization of repeat RNAs. Mature C9orf72 transcripts with correct splicing or with retention of intron 1 are both detected in C9FTD/ALS lymphoblasts and brain tissue138,146, indicating that both species contribute to RAN translation.
The expression of C9orf72 is also modified by epigenetic effects (Box 1).
Box 1. Epigenetic modification of C9orf72.
Analysis of blood, frontal cortex and cervical spinal cord DNA from patients with C9orf72-associated frontotemporal dementia and/or amyotrophic lateral sclerosis (C9FTD/ALS) has revealed that in 20–40% of cases, the CpG island in the C9orf72 promoter region upstream of the pathogenic repeats is hypermethylated22,140,147,148. This hypermethylation is associated with increased repeat length and reduced transcription of C9orf72. Moreover, analysis of blood DNA from patients with C9FTD/ALS showed that in 97% of cases, the expanded hexanucleotide repeat itself was methylated148. A subset of C9orf72 bacterial artificial chromosome transgenic mice also demonstrate hypermethylation of the C9orf72 promoter and increasing hexanucleotide repeat methylation with age149. Treatment of C9FTD/ALS patient fibroblasts with a DNA and histone demethylating agent led to an increase in C9orf72 transcript levels143. Bromodomain-containing proteins are involved in epigenetic regulation, and bromodomain inhibitors increase C9orf72 expression in cells from patients with C9orf72-associated ALS and control individuals150.
Histone trimethylation is another epigenetic modification that can reduce gene expression. Chromatin immunoprecipitation experiments in frontal cortex and cerebellar tissue showed that the C9orf72 promoter region is bound to trimethylated histones in C9orf72 repeat expansion carriers143.
Considerable evidence points to amelioration of disease phenotypes by C9orf72 hypermethylation. Hypermethylation is associated with reductions in RNA foci and dipeptide repeat proteins (DPRs) in patients with C9FTD/ALS41 and C9orf72 cell models151, as well as reduced neuronal and grey matter loss152. In addition, hypermethylation has been linked to longer survival in patients with C9orf72-associated FTD153 and a later age of onset in ALS and FTD22. However hypermethylation also correlates with reduced disease duration before death in patients with C9orf72-associated ALS140. One possibility is that some phenotypes are dependent on gain of function and others on loss of function, so hypermethylation could have pleiotropic effects.
One might predict that reducing the levels of transcript variants 1 and 3 (Fig. 1) which are responsible for producing RNA foci and DPRs, would be protective. However, it is unclear whether reduction of variant 2 would be beneficial — this is the most highly expressed variant so is likely to be the main contributor to the functional pool of C9orf72 protein.
Autophagy and lysosomal function
Bioinformatic analysis shows that C9orf72 is structurally related to the differentially expressed in normal and neoplastic cells (DENN) guanine nucleotide exchange factor (GEF) proteins, which activate Rab proteins. Rabs are crucial for a wide range of vesicular trafficking events, and multiple lines of evidence from several independent groups point to a role for C9orf72 in autophagy and endolysosomal trafficking and function.
Knockdown of C9orf72 in human cell lines and primary neurons specifically inhibits autophagy induction, but not later stages of the autophagy pathway91,96, leading to accumulation of p6291,96 and cytoplasmic aggregation of TDP-4396. Consistent with these findings, accumulation of autophagy substrates, including p62, is observed in the spleens of C9orf72-knockout mice54,56. Conversely, overexpression of C9orf72 can activate autophagy, leading to an increase in autophagosomes in cell lines91. The role in autophagy seems to be mediated by the long C9orf72 protein isoform (C9orf72-L) rather than the short isoform (C9orf72-S)96. The mechanism underlying these changes involves Rab proteins, although no consensus has been reached on which are the most important, with Rab1a, Rab8a and Rab39b all being implicated56,78,91,96,154. C9orf72 interacts with guanine nucleotide exchange protein SMCR8 and WD repeat-containing protein 41 (WDR41)56,58,96,101,122,154, and the effect on autophagy is generally agreed to be mediated through an interaction with the serine/threonine-protein kinase ULK1 complex56,91,96,100,154, a key initiator of autophagy. Potentially, this interaction can also occur via TBK196, another serine/threonine-protein kinase implicated in autophagy. This finding is particularly intriguing, as loss-of-function mutations in TBK1 cause ALS and FTD155.
Another link to known ALS-associated genes is through ataxin-2 (ATXN2), in which intermediate expansions of polyglutamine increase the risk of ALS156. C9orf72 knockdown specifically increases the aggregation and toxicity of ataxin-2 protein with intermediate polyglutamine repeats96.
The relevance of the role of C9orf72 role in autophagy for disease pathogenesis is unclear, but neurons from patients with C9FTD/ALS have impaired basal autophagy91,93 and increased sensitivity to autophagy inhibition90, suggesting that reductions in C9orf72 levels contribute to cellular distress. Furthermore, a Src–c-Abl pathway inhibitor, which increases autophagic processes, rescues survival defects in neurons derived from patients with ALS92.
In addition to effects on autophagy, reduced endocytosis was reported in C9orf72 knockdown cell lines78, and impaired endosomal and lysosomal trafficking were observed in bone marrow-derived macrophages and microglia from homozygous C9orf72 knockout mice54, as well as in patient-derived fibroblasts and neurons93. Moreover, C9orf72 has been shown to reside on lysosomes and can directly affect lysosomal function, which might also explain the effects of C9orf72 loss of function on both endolysosomal trafficking and autophagy101. In iPSC-derived motor neurons, C9orf72 primarily localizes to early endosomes, and iPSC-derived motor neurons from patients with C9FTD/ALS have fewer lysosomes94. Both patient neurons and CRISPR–Cas9 C9orf72-knockout iPSC-derived neurons have reduced vesicular trafficking, which can be rescued by C9orf72 overexpression94. These cells also have elevated glutamate receptor levels and increased sensitivity to excitotoxicity94,127. Consistent with this observation, increased glutamate receptor levels were found in spinal cord tissue in a C9orf72-knockout mice94, and in spinal cord94,127 and cortical tissue94 from patients with C9FTD/ALS.
Taken together, these data suggest that C9orf72 is involved in multiple cellular trafficking events, and that loss of C9orf72 in both microglia and neurons can sensitize cells to other insults, thereby contributing to neurodegeneration in C9FTD/ALS.
Further insights from loss-of-function mouse models
The mouse C9orf72 orthologue shares 98% homology with human C9orf72 and is expressed in embryonic and early postnatal neurons, various regions of the adult brain and spinal cord, glia, and non-neuronal tissues, including muscle, spleen, kidney and testes50–54,157. Several C9orf72 knockout or knockdown models have now been reported (Table 1)51–58.
Table 1. Mouse models of C9orf72 loss of function.
| Study | Method(s) | Motor phenotypes in homozygotes | Cognitive and behavioural phenotypes in homozygotes | Other phenotypes in homozygotes | Survival in homozygotes |
|---|---|---|---|---|---|
| Clotilde Lagier-Tourenne et al. (2013)13 | Somatic brain transgenesis with antisense oligonucleotide | Normal function | Normal function | None reported | Not reported |
| Panda et al. (2013)49 | Non-conditional TALEN-mediated knockout | Not reported | Not reported | None reported | Not reported |
| Suzuki et al. (2013)50 | Non-conditional knockout of exons 2–6 | Not reported | Not reported | None reported | Not reported |
| Koppers et al. (2015)51 | Conditional Cre–loxP-mediated knockout in neurons and glia | Normal function | Not reported | 6% reduction in body weight in homozgotes compared with controls | Normal: oldest mice lived >24 months |
| Jiang et al. (2016)52 | Non-conditional knockout of exons 2–6 | Mild motor deficits on rotarod test | Mild social interaction and social recognition abnormalities | Reduced body weight, splenomegaly and cervical lymphadenopathy | Normal until 11 months, 7% of mice survived to 20 months |
| Atanasio et al. (2016)53 | Non-conditional knockout of the full gene | Mild motor deficits at 40 weeks | Not reported | Lymphadenopathy at 12 months | 9 of 17 mice survived to the end of the neurological assay period |
| O'Rourke et al. (2016)54 | 1. Non-conditional knockout of exons 2–6 2. Non-conditional knockout with zinc finger deletion |
Normal function | Not reported | Cervical lymphadenopathy and splenomegaly | Normal lifespan |
| Sudria-Lopez et al. (2016)55 | Not reported — full knockout in all tissues | Normal function | Not reported | Reduced body weight, lymphadenopathy and splenomegaly | Reduced survival: median lifespan ~500 days |
| Sullivan et al. (2016)56 | CRISPR–Cas9-mediated non-conditional knockout | Not reported | Not reported | Lymphadenopathy and splenomegaly | Not reported |
| Burberry et al. (2016)57 | 1. Non-conditional knockout of exons 2–6 in a C57BL/6 inbred background (model 1) 2. Same as model 1, but on an outbred background (model 2) 3. CRISPR–Cas9-mediated non-conditional knockout (model 3) |
Not reported | Not reported | Reduced body weight, splenomegaly, cervical lymphadenopathy and hepatomegaly | Model 1: 7% alive by 400 days Model 2: 64% alive by 300 days Model 3: reduced survival |
| Ugolino et al. (2017)58 | Non-conditional knockout of exons 2–6 | Not reported | Not reported | Splenomegaly and lethargy | Homozygotes: >50% dead in 600 days Heterozygotes: 20% dead in 600 days |
A range of techniques to reduce expression of or knock out the mouse C9orf72 orthologue have been used to investigate the normal function of C9orf72 protein. Homozygous C9orf72 knockouts suggest roles for the protein in immune system function, autophagy and endosomal processes. TALEN, transcription activator-like effector nuclease.
Transient reduction of C9orf72 expression in the CNS by antisense oligonucleotides (ASOs)13 and conditional homozygous knockouts of C9orf72 in neurons and glia51 do not lead to motor or behavioural phenotypes. By contrast, ubiquitous knockouts of C9orf7252–55,57,58 or CRISPR–Cas9-mediated knockouts of C9orf72 isoforms56,57 throughout development led to immune system dysregulation in homozygous mice. The phenotypes included changes in myeloid and/or lymphoid cell populations in the spleen and lymph nodes, increased levels of inflammatory cytokines, and cervical or systemic lymphadenopathy and splenomegaly, sometimes with reduced body weight52,53,55,57, neoplasia55 or increased autoimmune antibody titres53,57. In comparison, haploinsufficiency of C9orf72 does not lead to severe phenotypes.
Although some studies reported mild motor or cognitive phenotypes52,53 or reduced lifespan52,53,55,57,58 in homozygous C9orf72-knockout mice, none reported neuronal loss. Transcriptomic analysis confirmed changes in immune pathways53,54, similar to those observed in CNS tissue from patients with C9orf72 repeat expansions54. Transcriptomic analysis in human tissue has shown that C9orf72 transcripts are particularly prevalent in CD14+ myeloid cells, which are involved in innate and adaptive immunity139. Overall, in line with cellular studies of C9orf72, these findings suggest an important role for C9orf72 in immune regulation, possibly through its effects on autophagosome and lysosome function and/or microglial activity, or through alteration of autoimmune responses. However, in contrast to C9orf72 loss of function, knockout of other autophagy-related genes, including ATG7158 and ATG5159, in neurons in mice leads to neurodegeneration. These findings suggest that C9orf72 is not an essential component of the autophagy pathway that mediates neuronal survival.
Crucially, none of the mouse C9orf72 knockouts recapitulate ALS or FTD, suggesting that C9orf72 loss of function is insufficient to precipitate disease. However, given the role of C9orf72 in pathways previously implicated in FTD and ALS160, haploinsufficiency might contribute to the disease process in combination with gain-of-function mechanisms, and an interesting approach will be to breed loss-of-function mouse models with gain-of-function models.
Gain-of-function mechanisms
The question of whether C9orf72 repeat RNA or DPRs produced by RAN translation are the toxic species in aetiopathogenesis is hotly debated in the field. Various approaches, each of which has limitations, have been used to address this issue. Post-mortem studies generally do not capture the earliest pathogenic events. In vitro and in vivo models often do not feature the long repeats that are found in patients, owing to methodological difficulties in cloning GC-rich repeats of this length. Therefore, these models might not fully recapitulate the disease mechanisms. Overexpression models do not necessarily reflect endogenous expression levels in patients. Many models express expanded repeats, but as these repeats can go on to produce both C9orf72 repeat RNA and DPRs, attribution of downstream mechanisms to either entity is challenging. Despite these uncertainties, however, clear mechanistic pathways have emerged (Table 3).
Table 3. Downstream mechanisms implicated in C9FTD/ALS.
| C9orf72 human tissue/model | Other C9orf72 in vitro models | C9orf72 in vivo models | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient-derived neurons/glia | Post-mortem tissue | Other system/tissue/in patients | Cell lines | Primary neurons | Yeast | Worm and zebrafish | Fly | Mouse | |
| RNA metabolism | |||||||||
| Nucleolar function/LLPS | Yes66 | Yes42,66,67 | Yes42,66 | Yes68–73 | Yes36 | – | – | Yes67,72 | – |
| Processing bodies | Yes66 | – | – | Yes74 | Yes42,74 | – | – | – | – |
| Stress granules/LLPS | Yes75 | Yes76 | Yes77 | Yes69,72–74,78–83 | Yes42,74 | – | – | Yes72 | – |
| RNA processing, splicing, transcription and transport | Yes84,85 | Yes84–86 | – | Yes68,84,85,87,88 | Yes84 | Yes89 | – | Yes84 | – |
| Nuclear speckles/LLPS | – | – | – | Yes72 | – | – | – | Yes72 | – |
| Cajal bodies/LLPS | – | – | – | Yes72 | – | – | – | Yes72 | – |
| Proteostasis | |||||||||
| Autophagy and lysosomal function | Yes75,90–94 | Yes54 | Yes93,95 | Yes54,58,78,88,91,96–101 | Yes91,96,102 | – | – | – | Yes54,56,58 |
| Endoplasmic reticulum/UPR | Yes66,75 | Yes86,103,104 | – | Yes80,83,88 | Yes80,104 | – | – | – | – |
| Translational inhibition | – | – | – | Yes79,80,87 | – | – | – | – | – |
| Ubiquitin-proteasome system | – | – | – | Yes88,99,105,106 | Yes63,102,104,107 | – | – | – | Yes63 |
| Other cellular processes | |||||||||
| Nucleocytoplasmic transport | Yes82,84,89,108,109 | Yes86,109–111 | – | Yes68,79,82,84,88,108,110–114 | Yes84,89 | Yes89 | – | Yes82,84,108,109,115 | Yes63 |
| Vesicle trafficking | Yes93 | Yes86 | Yes93 | Yes78,96 | – | – | – | Yes116 | – |
| DNA damage | Yes117 | Yes118,119 | – | Yes118,119 | Yes118 | – | – | Yes117 | – |
| Transport granule function | Yes120 | Yes120 | – | Yes121 | Yes120 | – | – | Yes120 | – |
| Mitochondrial function | Yes75,117 | – | Yes95 | Yes122 | – | – | – | – | – |
| Notch signalling | Yes123 | Yes123 | – | – | – | – | – | Yes123 | – |
| Arginine methylation | – | – | – | Yes115 | – | – | – | Yes115 | – |
| Nervous system-specific processes | |||||||||
| Neuronal excitability and glutamate toxicity | Yes12,94,124–127 | Yes12,124–126 | Yes128–131 | Yes71 | – | – | – | – | Yes94 |
| Neuronal branching and growth | – | Yes105 | – | – | Yes104,105,120 | – | Yes132–134 | Yes120,123 | – |
| Axonal transport | – | – | – | – | – | – | – | Yes135 | – |
| Actin/growth cone dynamics | Yes136 | – | – | – | Yes136 | – | – | – | – |
| Glial dysfunction | Yes137 | – | – | – | – | – | – | – | – |
| Systemic functions | |||||||||
| Immune system function | – | Yes54 | – | Yes54 | – | – | – | – | Yes (Table 1) |
Several studies (referenced within the relevant cell of the table) have identified a spectrum of downstream processes involved in C9orf72-mediated disease, using patient-derived tissue, cells or induced pluripotent stem cell-derived neurons, and in vitro or in vivo disease models. Globally, these processes can be classified into those involved in RNA metabolism and processing, proteostasis, other cellular processes, nervous system-specific functions or systemic functions. LLPS, liquid–liquid phase separation; UPR, unfolded protein response.
C9orf72 repeat RNA
In vitro, GGGGCC repeat RNA forms secondary structures, including hairpins45,66 and highly stable G-quadruplexes45,66,161–163. Other secondary structures, including DNA–RNA heteroduplexes, RNA duplexes and i-motifs164–167, might arise from the sense and antisense repeat RNA and DNA sequences. In vivo, such secondary structures are likely to mediate the sequestration — and, as a consequence, depletion — of RNA-binding proteins (RBPs)66,161 (Box 2), thus providing a clear potential route to RNA toxicity (reviewed extensively elsewhere164,168).
Box 2. RNA-binding protein sequestration.
RNA-binding proteins (RBPs) have diverse roles in splicing, translational regulation and RNA transport and degradation. In repeat-mediated diseases, possibly including C9orf72-associated disease, RBPs can become sequestered by RNA foci, leading to downstream consequences.
A number of RBPs have been shown to interact with C9orf72 repeat RNA in human tissue12,66,84,169–174, in vitro assays66,84,162,170,172,174, cell models66,70,79,169,170,172,174,175, induced pluripotent stem cell-derived neurons12,66,126 and in vivo models79,90,169,172. The most frequently identified RBPs that interact with C9orf72 repeat RNA are the heterogeneous nuclear ribonucleoprotein (hnRNPs), in particular, hnRNP H, although hnRNP A1 and hnRNP A3 are also detected. Other RBPs include ALYREF, ASF/SF2, ADARB2, nucleolin, Pur-α and SRSF2. These RBPs are not found consistently across different studies, possibly reflecting the diversity of models and methodologies. However, several of these proteins have been identified in multiple independent studies, indicating that specific RBPs can be sequestered by GGGGCC repeat RNA. Only a small subset of RNA foci seem to colocalize with RBPs in human tissue170 and cells79, indicating that sequestration of these proteins is a dynamic process, or that diffuse C9orf72 repeat RNA that is not contained within foci can sequester RBPs.
Overexpression of Pur-α, an RBP that is involved in transcription regulation176,177, mRNA localization178,179 and stress granule formation77 and was shown to interact with C9orf72 repeat RNA79,172, can ameliorate neurodegeneration in C9orf72 repeat-expressing Drosophila and neuronal cell lines172. Overexpression of Zfp106 also supresses toxiciy in C9orf72 repeat Drosophila180. To date, direct evidence regarding the effects of RBP sequestration in tissue from patients with C9FTD/ALS is limited, although some hnRNP H targets are altered86,174. A crucial next step is to establish how the implicated RBPs and their targets are mechanistically linked to pathogenesis.
DPRs: insights from post-mortem studies
The results of post-mortem studies have raised suspicions that DPR inclusions are not the primary culprit in C9FTD/ALS pathogenesis29,35–40,43,181–183. TDP-43 pathology and neurodegeneration co-occur in affected regions of the CNS in ALS and FTD29,35,37–39,43. By contrast, DPR pathology does not coincide neuroanatomically with TDP-43 pathology43 and is generally not found in the same neurons as TDP-43 inclusions16,29,35,40. Furthermore, DPR inclusions do not differ in neuroanatomical distribution between FTD and ALS cases29,37,38 and are rare in the spinal cord in C9orf72-associated ALS, whereas TDP-43 pathology is common14,17,18,29,36–40. DPR inclusions are frequent across several brain regions17,35–37,39, including structures that are thought to be minimally affected in ALS and FTD, such as the cerebellum and occipital and parietal lobes. However, one study showed that poly-GR inclusions — but not the other DPRs — correlated with areas of neurodegeneration in C9orf72-associated ALS, and also, of all the DPRs, uniquely colocalized with TDP-43 pathology in a small sample of brains that were obtained shortly after death44. These data suggest that further investigation in larger, deep-phenotyped post-mortem cohorts will provide important insights.
Despite these observations, strong counterarguments to support a pathogenic role for DPRs in C9FTD/ALS have been put forward. Post-mortem studies tend to represent the final stages of the disease process and might not reflect the early pathogenicity of DPRs. Aggregates observed at post-mortem could represent protective species, and correlations with inclusions might be misleading if soluble species mediate neurotoxicity. CNS regions that have extensive DPR pathology but are unaffected by neurodegeneration might contain protective factors; indeed, selective vulnerability is a frequent observation in neurodegenerative diseases184.
TDP-43 pathology is likely to be downstream of DPR pathology, probably explaining why it correlates more closely with neurodegeneration. This idea is consistent with downstream effects on TDP-43 in some experimental models expressing expanded repeats109,185 or pure DPRs106,185, suggesting that one or both of these gain-of-function mechanisms are linked to TDP-43. Affected individuals with DPR pathology but relatively mild or absent TDP-43 pathology have been reported16,21,43,141,182,186,187. These individuals include a young patient with evidence of intellectual disability186, a patient with C9orf72-associated ALS who had little extramotor TDP-43 pathology but showed evidence of cognitive impairment and high cerebellar DPR levels43, and patients with pathological or clinical diagnoses of FTLD or FTD16,141,182,186,187, some of whom died prematurely from other causes182. Therefore, DPR pathology without substantial TDP-43 pathology seems to be sufficient for disease to develop in some cases. Furthermore, as discussed below, considerable evidence from model systems indicates that DPRs can cause neurodegeneration.
Insights from gain-of-function models
Evidence for C9orf72 repeat RNA toxicity
The effects of C9orf72 repeat RNA were modelled in primary cortical and motor neurons transfected with expanded GGGGCC repeats within an artificial intronic region of the green fluorescent protein gene, reflecting the intronic human genomic context of C9orf72 expansions42. These neurons demonstrated nuclear RNA foci and reduced survival. Dot blots and immunocytochemistry revealed no DPRs in these cells, suggesting that the reduced survival was attributable to repeat RNA. Interestingly, however, co-expression of poly-PR and the intronic expanded GGGGCC repeats had a synergistic detrimental effect on neuronal survival.
RNA toxicity has also been implicated in eye and motor neuron degeneration in a Drosophila model that expresses 30 GGGGCC repeats with a 6 bp (CTCGAG) interruption in the middle of the repeats 109,172. DPRs were not detected in the eyes or neurons, and were only detected when the repeats were strongly induced in all tissues109.
One caveat for the interpretation of both studies is that the inability to detect DPRs is not sufficient to exclude a role for these proteins. In our experience, poly-GR can be difficult to detect even in flies that express this protein at high levels and show overt toxicity. Therefore, more sensitive detection assays for DPRs will be required to unpick the relative contributions of RNA and DPRs in these models.
A study in developing zebrafish found that both sense and antisense RNA repeats could mediate toxicity, leading to a motor axonopathy phenotype134. No DPRs were detected in this model. In a Drosophila model, however, neither sense nor antisense repeats with similar lengths to those found in patients with C9FTD/ALS led to degeneration of adult neurons189. One potential explanation for this difference is that developing neurons are more susceptible to RNA toxicity than adult neurons. Another possibility is that RNA foci in humans sequester RBPs that are not present in Drosophila, thereby limiting the utility of Drosophila as a model for RNA toxicity.
Evidence for DPR toxicity
Two studies in Drosophila models have provided evidence that neurotoxicity is attributable to DPRs rather than repeat RNA138,188. In the first study, overexpression of expanded GGGGCC repeats in Drosophila eyes or adult neurons led to neurodegeneration188. This effect was inhibited when the repeats were interrupted by stop codons in each reading frame that prevented translation of the repeats into DPRs. The second study involved ubiquitous overexpression of 160 GGGGCC repeats in an intronic context, flanked by human C9orf72 sequence138. The intron containing the repeat was spliced out and formed large numbers of sense RNA foci in neurons and glia, without production of DPRs. This model showed no evidence of neurodegeneration, reduced survival or widespread transcriptomic changes. Increasing transgene expression in this model led to DPR production and lifespan reduction, although the frequency of sense foci remained the same138, supporting the idea that DPRs rather than sense foci mediate neurodegeneration.
It should be noted that although the RNA sequence interrupted by stop codons in the first study forms the same G-quadruplex secondary structure as uninterrupted C9orf72 repeat RNA188, the tertiary and quaternary structures are not necessarily identical, which might affect the dynamics of RBP sequestration. However, the intronic model did not have an interrupted repeat sequence and exhibited multiple sense foci, yet still showed no evidence of toxicity138.
Toxicity of individual DPRs
In numerous studies in cell models14,42,69,87,104–106,190, Drosophila 42,72,108,115,123, zebrafish133,134,191 and mice63,64, the repetitive GGGGCC sequence was altered to generate coding sequences that expressed each DPR in isolation, which was often sufficient to produce neurotoxicity and implicate several downstream mechanisms. The main limitation of these models is that DPR overexpression might not reflect the endogenous mechanisms that are seen in patients.
Studies in cultured neuronal or non-neuronal cell lines or primary neuronal cultures indicate that poly-GR and poly-PR are the most toxic of the DPRs42,69,87,106. These arginine-rich DPRs — in particular, poly-PR — are toxic to yeast.89 Poly-GA and poly-PA are also toxic, but to a lesser extent89. Synthetic poly-PR and poly-GR are highly toxic when exogenously applied to cultured human astrocytes. Poly-PR has a longer half-life than poly-GR, and is especially potent in this context68. Synthetic poly-GR and poly-GA are also toxic to primary neurons192. Furthermore, overexpression of poly-PR was found to be toxic to control iPSC-derived neurons42.
Of all the DPRs, poly-GR had the greatest detrimental effect on development, locomotor activity and survival in a zebrafish model133. In Drosophila models, poly-GR and poly-PR were neurotoxic when expressed in the eyes42,72,108,115,123,188. In addition, flies expressing these proteins exhibited reduced survival42,108,123,188 and locomotor phenotypes42,123,135. Most of these studies found that poly-GA42,115,123, poly-PA42,188 and poly-GP108 were not toxic in Drosophila, although one study reported a mild reduced survival phenotype when poly-GA was expressed in adult neurons188. Consistent with this finding, poly-GA overexpression in cultured cells104,105, primary neurons104, zebrafish133,191 or mouse brain63 leads to toxicity, and synthetic poly-GA exogenously applied to human cells190 or primary neurons192 is also toxic. In addition, cryo-electron tomography revealed that poly-GA forms twisted ribbon structures that sequester the 26S proteasome107.
Overall, these studies show that poly-GR and poly-PR are potently neurotoxic and poly-GA also exerts toxicity. The remaining DPRs, poly-PA and poly-GP, are unlikely to be toxic species. As poly-GR, poly-PR and poly-GA can all be damaging when overexpressed, a key aim is to establish the levels of each of these species in patient tissue, particularly at early disease stages. New techniques to extract and measure DPRs from patient tissue and assess their toxicity will be essential for this endeavour.
Mouse gain-of-function models
Several mouse models of C9orf72 gain of function have been characterized. Adeno-associated virus-mediated CNS overexpression of 66 GGGGCC repeats leads to motor and behavioural phenotypes by 6 months, with RNA foci, DPRs, phospho-TDP-43 inclusions and neuronal loss being observed59. The same approach was used to generate mice specifically overexpressing poly-GA in the CNS63. These mice developed neuronal cytoplasmic inclusions of fibrillar poly-GA, as well as neurodegeneration and motor, cognitive and behavioural phenotypes. However, these effects were not observed when the poly-GA sequence was mutated to a sequence that was unable to aggregate. Phospho-TDP-43 inclusions were rarely found; therefore, this model does not fully recapitulate the (GGGGCC)66 repeat mouse phenotype, indicating that species other than poly-GA are the main drivers of phospho-TDP-43 accumulation. A further poly-GA model, with expression levels more comparable to those in the cortex in patients with C9FTD/ALS, demonstrated motor deficits, but overall a less severe phenotype than the viral poly-GA model 64.
Bacterial artificial chromosome transgenic mouse models
Bacterial artificial chromosome (BAC) transgenic mice have the advantages of expressing the human C9orf72 gene, with surrounding regulatory regions and flanking sequences, at more physiological levels. Three BAC transgenic models have produced similar findings. Two of these models used BAC constructs52,60, containing exons 1–5 of the gene and 300–500 repeats (Table 2). The third BAC model expressed the full gene and protein with 100–1000 repeats61. All three models developed RNA foci and DPRs in the CNS, but none demonstrated TDP-43 inclusion pathology, neuronal loss or reduced survival. One model developed memory impairment and loss of hippocampal neurons, with an increase in levels of phosphorylated TDP-43, but was not fully reflective of ALS or FTD52. In this model, a single injection of an ASO targeting C9orf72 RNA led to sustained reductions in RNA foci and DPR pathology, and improved cognition. Repeat length had a strong influence on the formation of RNA foci, with 100-repeat mice developing no foci and 400-repeat mice developing many foci, despite considerably higher transgene expression in the 100-repeat mice52.
Table 2. Mouse models of C9orf72 gain of function.
| Study | Mouse strain and methodology | RNA foci | DPRs detected by immunocytochemistry | DPRs detected by Immunoassay | TDP-43 pathology | Motor and cognitive phenotypes | Survival |
|---|---|---|---|---|---|---|---|
| Chew et al. (2015)59 | C57BL/6J mice Somatic brain transgenesis: AAV-mediated expression of (GGGGCC)66 repeats and 119 bp upstream and 100 bp downstream C9orf72 sequence | Sense foci throughout CNS | Poly-GA and poly-GP inclusions | Poly-GP expression in (GGGGCC)66 mice | Nuclear and occasionally cytoplasmic pTDP-43 inclusions | Rotarod impairments from day 2 of testing onwards, plus anxiety-like behaviour and hyperactivity | Not reported |
| Peters et al. (2015)60 | SJL/B6 mice BAC transgenic, 140.5 kb upstream C9orf72 sequence, exons 1–5, and 300 or 500 repeats | Abundant sense foci throughout CNS, antisense foci more sparse | Poly-GP inclusions, increase with age | Poly-GP throughout brain, lower than in patients with C9FTD/ALS | No TDP-43 pathology | Normal rotarod and grip strength testing, normal social behaviour | Normal survival |
| O'Rourke et al. (2015)61 | C57BL/6J mice BAC transgenic, 110 kb upstream C9orf72 sequence, full gene and 100–1000 repeats, 20 kb downstream sequence | Sense and antisense RNA foci throughout CNS | Poly-GP inclusions, increase with age | Soluble and insoluble poly-GP, similar levels to patients with C9orf72-associatedFTLD | No TDP-43 pathology | Normal grip strength, rotarod and open field testing; normal behaviour | Not reported |
| Liu et al. (2016)62 | FVB/NJ mice BAC transgenic, 52 kb upstream C9orf72 sequence, full gene and different lines with repeat lengths and copy numbers up to 500 repeats, 19.4 kb downstream sequence | Sense and antisense RNA foci | Poly-GA aggregates throughout CNS, increase with age | Not reported | Nuclear and cytoplasmic TDP-43 aggregates | Acute rapidly progressive disease with motor phenotype; normal open field behaviour test | Mice with acute rapidly progressive disease have decreased survival |
| Jiang et al. (2016)52 | C57BL/6 mice BAC transgenic, 140 kb upstream C9orf72 sequence, exons 1–5 and ~110 repeats or ~450 repeats | Sense and antisense foci | Poly-GA, poly-GP and poly-GR inclusions | 2% SDS-soluble poly-GP detected | Increased levels of pTDP-43, no mislocalization or aggregates | No motor deficits; spatial and working memory deficits and anxiety found | Not reported |
| Zhang et al. (2016)63 | C57BL/6J mice Somatic brain transgenesis: AAV-mediated, GFP-(GA)50 or GFP-(GA)50-mutated | Not applicable | GFP-(GA)50 inclusions | Poly-GA levels twofold higher than in (GGGGCC)66 mice from the Chew et al. study59 | Rare pTDP-43 inclusions | Motor phenotype, hyperactivity and anxiety | Not reported |
| Schludi et al. (2017)64 | C57BL/6 mice Germline transgenesis: neuronal expression, (GA)149-CFP + 31 carboxy-terminal amino acids from endogenous human locus | Not applicable | (GA)149-CFP inclusions in brainstem, cerebellar nuclei and spinal cord, increase with age | Similar poly-GA levels in mouse spinal cord and motor neurons from patients with C9FTD/ALS | pTDP-43 levels higher in the urea-soluble fraction, as determined by ELISA; no TDP-43 inclusions or mislocalization. | Progressive gait and balance deficits; muscle strength and spatial memory normal | Not reported |
| Herranz-Martin et al. (2017)65 | C57BL/6J mice Somatic brain transgenesis: AAV-mediated expression of 10 pure or 102 interrupted (by TCGAG linker) GGGGCC repeats No C9orf72 flanking sequence | Sense foci throughout CNS, less frequent in spinal cord | Poly-GA, primarily in cerebellum and brainstem | Not reported | Infrequent TDP-43 aggregates found equally in 10 pure or 102 interrupted repeats | Progressive gait and behavioural deficits on open field and novel object recognition | Not reported |
Several mouse gain-of-function models are described. The approaches used have included somatic brain or germline transgenesis and expression of BAC constructs containing the full-length or partial human C9orf72 gene and repeat expansion. AAV, adeno-associated virus; BAC, bacterial artificial chromosome; C9FTD/ALS, C9orf72-associated frontotemporal dementia and/or amyotrophic lateral sclerosis; CFP, cyan fluorescent protein; DPRs, dipeptide repeat proteins; ELISA, enzyme-linked immunosorbent assay; FTLD, frontotemporal lobar degeneration; GFP, green fluorescent protein; pTDP-43, phospho-TDP-43; SDS, sodium dodecyl sulphate; TDP-43, TAR DNA-binding protein 43.
Only one study has reported BAC transgenic mice with a striking neurodegenerative phenotype62. The mice expressed the full gene and different repeat lengths in different lines. Three independent lines, two with ~500 repeats and one with high expression levels of 36 repeats, developed RNA foci, DPR aggregates, TDP-43 pathology and neurodegeneration. A subset of female mice developed an acute motor phenotype, with paralysis, weight loss and decreased survival, with other female and male mice showing slower progressive neurodegeneration. Antisense transcripts and foci were observed at especially high levels in some CNS regions that showed neurodegeneration, whereas sense foci were more evenly distributed. These data show that high expression of short repeats can be toxic, indicating that large repeats are not the critical factor for toxicity in this mouse model. The reason why female mice are particularly susceptible and only a subset develops acute disease is currently unclear, although factors such as methylation might be important.
In-depth molecular and phenotypic comparisons between all the BAC models should further our understanding of C9orf72-associated pathogenic mechanisms. One model that expresses the full-length human gene with the repeat expansion recapitulates disease62 whereas the other does not61. These mice feature different genetic backgrounds and flanking C9orf72 sequences (Table 2), which might be contributory factors. Backcrossing the models that did not show a phenotype into different genetic backgrounds, so as to determine whether certain backgrounds facilitate disease, would provide new insight.
Downstream mechanisms
A range of downstream mechanisms in C9FTD/ALS have been validated across multiple human and non-human model systems and different laboratories (Table 3). Dysfunctional nucleocytoplasmic transport (NCT) is a prominent mechanism that has been identified in genome-wide screens in yeast89 and Drosophila108,109,115, and in CRISPR–Cas9 screens in human cells and primary neurons88 (reviewed elsewhere168,193).
Poly-PR and poly-GR have been shown to interact with proteins that contain low complexity domains (LCDs), which include many RBPs72,194. LCD proteins can undergo liquid–liquid phase separation (LLPS) to form droplets. Through this process, the proteins become compartmentalized in the cell, forming membrane-less organelles such as nucleoli and stress granules. These organelles facilitate the assembly of RNA and RBPs into ribonucleoproteins, and also aid subsequent RNA metabolism. Furthermore, these LCDs can form reversible hydrogels, which have the propensity to fibrillize into irreversible hydrogel-like structures. Mutations frequently occur in the LCD domains in several RBPs that are involved in ALS, including FUS and TDP-43195. These mutations can alter the LLPS dynamics of RBPs, leading to their fibrillization and aggregation196–198. Poly-GR and poly-PR interact with LCD proteins in nucleoli and stress granules, thereby impairing LLPS, disrupting the dynamics of assembly of these organelles, and affecting mRNA translation and NCT72,73,82. Aliphatic alcohols, which disrupt phase separation and hydrogel formation, can reduce poly-PR’s protein interactions194 and disrupt the nucleolar localization of poly-PR and poly-GR72. Knockdown of several of these LCD proteins modifies the eye degeneration phenotype in a Drosophila poly-GR model72. Poly-PR also interacts with LCDs in intermediate filament proteins194. Interestingly, arginine-rich DPRs at high concentrations can undergo LLPS73. In addition, GGGGCC repeat RNA can undergo gel transition81,199 and induce phase transition of RNA granule proteins81 in the absence of other LCD proteins. These data further implicate altered LLPS in C9FTD/ALS pathogenesis.
Effects on membrane-less organelles have been observed in several other studies. Primary cortical neurons that overexpress poly-PR, leading to the formation of nuclear poly-PR aggregates, show a reduction in cytoplasmic P-bodies and an increase in stress granule formation42. In cultured cells treated with arsenite, a stress granule inducer, overexpression of poly-PR and poly-GR reduced stress granule formation, whereas poly-GA, poly-GP and poly-PA induced stress granule formation69.
Poly-PR interacts with translation initiation and elongation factors, and overexpression of poly-GR and poly-PR, but not poly-GA, inhibited translation in vitro and in cell lines87. Translational inhibition was attributed to direct binding of poly-GR and poly-PR to mRNA, thereby blocking access to the translation machinery87. A second study also observed translational inhibition when (GGGGCC)31 was expressed in cell lines79. This inhibition was accompanied by an increase in stress granule formation (a marker of translational arrest) and nuclear accumulation of poly-A mRNAs and PABPc, a protein that facilitates mRNA translation in the cytoplasm79. GGGGCC repeat-induced stress granule formation was also observed in subsequent studies80,81. The translational inhibition might be explained by sequestration of PABPc by GGGGCC repeat RNA, but could also be due to the DPR-mediated mechanisms described above. Interestingly, two studies that identified translation inhibition suggested that this process was independent of eukaryotic translation initiation factor 2 subunit 1 (eIF2α) phosphorylation, an important master regulator of translation that has been implicated in other neurodegenerative diseases200. However, stress granule induction secondary to poly-PR has been shown to require eIF2α phosphorylation73.
A study published in 2018 showed that poly-GR and poly-PR induced stress granule assembly and localization of NCT factors into these stress granules, thereby mediating NCT dysfunction82. Inhibition of stress granule assembly abrogated NCT dysfunction and neurodegeneration in patient-derived neurons and in vivo82. Stress granules and translation inhibition have been implicated in FTD and ALS associated with TDP-43 and FUS inclusions201, and they provide an interesting potential common theme across the disease spectrum.
In three elegant studies, GGGGCC repeat overexpression constructs were used to investigate the mechanisms underlying RAN translation. Two of these studies showed that the integrated stress response, via eIF2α phosphorylation, selectively increased RAN translation80,83. Therefore, repeat-induced cellular stress could lead to both impaired translation and enhanced RAN translation, causing a negative feedback loop. In addition, two of the studies showed that RAN translation occurred on unspliced, capped mRNA (in which the repeat-containing intron is retained), and was initiated by an upstream CUG acting as a start codon80,202. By contrast, the third study found that RAN translation occurred on the spliced intronic RNA and was cap-independent, although cap-dependent RAN translation was more efficient83. These mechanisms now need to be investigated in the context of endogenous RAN translation.
When applied exogenously to astrocyte cultures, poly-GR and poly-PR 20mers accumulate in the nucleolus, leading to splicing changes and impaired ribosomal RNA (rRNA) maturation68. This finding led to the suggestion that these DPRs bind to and impair nuclear RBP complexes that are involved in mRNA splicing, which could in turn affect ribosome biogenesis and other important genes68. Interestingly, given the effect of poly-PR and poly-GR on NCT, splicing of RanGAP, an essential regulator of NCT, was altered68. Poly-PR and poly-GR also colocalize with nucleoli in cultured cells, primary neurons, iPSC-derived neurons and Drosophila, leading to abnormal nucleolar morphology36,42,67,69,71. Importantly, the brains of individuals with C9orf79-associated FTLD exhibit bidirectional nucleolar volume changes, with smaller neuronal nucleoli overall but enlarged nucleoli in neurons containing poly-GR inclusions67. Other interactome studies suggest that poly-PR and poly-GR associate with the spliceosome component U2 snRNP in cell nuclear extracts, block spliceosome assembly, and disrupt splicing when applied to cell nuclear extracts85. These proteins also interact with mitochondrial ribosomal proteins and other ribosomal proteins117, and evidence of impaired mitochondrial function has been observed in iPSC-derived neurons from patients with C9FTD/ALS75,117. Ribosomal proteins, hnRNPs, nucleolar proteins and RBPs associated with RNA granules have been shown to interact with poly-GR, and overexpression of poly-GR and poly-PR led to decreased levels of rRNA in human cell lines69. In agreement with these data, poly-PR was shown to interact with proteins involved in mRNA splicing and ribosome assembly, and with ribosomal proteins87. Three studies have shown that in addition to effects on RNA, C9orf72 repeats can induce DNA damage, probably mediated by DPRs117–119.
Overall, many cellular pathways have been implicated in gain-of-function toxicity, with the majority of studies focused on poly-GR and poly-PR. Given the highly toxic nature of these DPRs, they are likely to influence multiple pathways. Links between the downstream mechanisms and specific DPRs or repeat RNA have not yet been established, and sensitive techniques to measure both repeat RNA and DPRs in models expressing pure GGGGCC repeats will be required to enable the effects of individual molecular species to be distinguished.
Another important issue that remains to be addressed is whether different DPRs or repeat RNA and DPRs act synergistically, potentially in conjunction with loss of function of C9orf72, to elicit downstream effects. Crossing of models that express different DPR species should help to address this question. A further priority is to develop physiologically relevant models to reflect endogenous levels of C9orf72 repeat RNA and DPRs, and human iPSC-derived neuronal models will be a key tool in this regard. Furthermore, C9orf72 protein depletion, repeat RNA and DPRs might have non-cell-autonomous effects, and iPSC models of neuronal, glial and muscle co-culture should increase our understanding of these complex interactions.
Therapeutic strategies
Targeting C9orf72 RNA or DNA
ASOs that target C9orf72 RNA can rescue C9orf72-specific pathologies12,13,46,126, downstream gene expression changes12,126, NCT defects109 and TDP-43 mislocalization109 in C9orf72 fibroblasts or iPSC-derived neurons. These ASOs also reduce NCT defects and neurodegeneration in Drosophila109 and diminish sense RNA foci and DPRs in mice46,52. ASO trials in humans are planned, and the feasibility of this strategy has a precedent in studies of superoxide dismutase 1 (SOD1)-targeting ASOs in patients with ALS203.
An alternative strategy is to use compounds that target the secondary structure of C9orf72 repeat RNA45,109,204–206. These compounds could prevent sequestration of RBPs and/or interfere with the RNA structure to prevent RAN translation. Such molecules have been shown to affect the secondary structure of C9orf72 repeat RNA in vitro45,204,206, to reduce the production of RNA foci and DPRs when applied to patient neurons45,205 and Drosophila205, and to rescue nuclear import defects and neurodegeneration in a Drosophila model109.
Targeting of the transcription elongation factor SPT4 reduces levels of sense and antisense C9orf72 repeat transcripts and ameliorates disease phenotypes in vitro and in vivo, including in C9orf72 iPSC-derived neurons207. Single-stranded small inhibitory RNAs have also been proposed as a strategy to silence the C9orf72 repeat RNA208. In addition, two studies have shown that use of the CRISPR–Cas9 system to target either GGGGCC repeat DNA209 — thus reducing repeat transcription — or repeat RNA210 can reduce RNA foci and DPR levels in cell lines. On the basis of these studies, targeting of C9orf72 RNA or DNA are promising strategies, and ASOs are currently the most advanced in terms of clinical development.
Targeting DPRs and TDP-43
In other neurodegenerative diseases, active and passive immunological approaches have been used to target toxic proteins such as amyloid-β211, tau212,213 and α-synuclein214,215, leading to improved pathology and phenotypes in model systems. With the ongoing development of specific DPR antibodies, passive immunization to the DPRs could present a novel therapeutic approach for C9FTD/ALS, and poly-GA-specific antibodies have been shown to reduce intracellular poly-GA aggregation and seeding activity of C9FTD/ALS brain extracts216. In addition, given that C9FTD/ALS pathogenesis converges on TDP-43, anti-TDP-43 immunotherapy would be a compelling strategy.
Current strategies in other neurodegenerative diseases are based on targeting of the extracellular pool of protein, which is presumably involved in cell-to-cell transmission. The success of such an approach in the case of DPRs and TDP-43 would depend on whether there is a disease-relevant extracellular pool to target. If not, methods to target antibodies intracellularly would be required. Other important factors to consider are the ideal timing of these treatments, which DPRs and conformations to target, the specificity and safety of the treatment, and the risks of precipitating an autoimmune response. As short repetitive sequences similar to DPRs are present across the proteome, specificity might present a barrier. Increasing clearance of DPRs by other mechanisms could also be effective; for example, the small heat shock protein HSPB8 was shown to reduce DPR levels, probably via the autophagy pathway98.
Targeting downstream mechanisms
Targeting of downstream mechanisms might represent a useful therapeutic strategy for C9FTD/ALS. Reducing nuclear export by targeting the nuclear export factors SRSF1 or exportin 1 ameliorates toxicity in C9orf72-repeat Drosophila84,109; this was suggested to be due to either by reducing the levels of cytoplasmic repeat RNA (and, thus, DPRs) or by a more general mechanism to alterations in NCT. Importantly, in addition to the proof of concept genetic knockdown approaches that were used to reduce SRSF1 and exportin 1, small molecule exportin 1 inhibitors were also used, indicating a potential route to the clinic. It will now be important to address whether there is a large enough therapeutic window when targeting nuclear export, which is a fundamental cellular process. Inhibition of stress granule formation using either ASOs targeting Ataxin-2 or small molecules, also prevents NCT defects in C9-ALS iPSC-neurons and neurodegeneration in a C9orf72 repeat fly model82. Given the success of Ataxin-2-targeting ASOs in extending lifespan in an ALS mouse model overexpressing TDP-43, this strategy and the mechanisms underlying it, are of clear interest. A Src–c-Abl pathway inhibitor that augments autophagy, improved the survival of iPSC-derived neurons from patients with ALS, including those with C9FTD/ALS, suggesting this approach could be beneficial for several forms of FTD/ALS92. Knockdown of TMX2, an endoplasmic reticulum protein that was identified in CRISPR–Cas9 screens in human cells and primary neurons, modulated endoplasmic reticulum stress in primary neurons overexpressing DPRs and increased survival of neurons derived from patients with C9orf72-associated ALS 88. Whether TMX2 is druggable awaits further study. High-throughput drug screens — for example, using iPSC-derived neuronal models92,94,217 — will be of great importance in establishing treatments to reduce levels of repeat RNA and DPRs, repair dysfunctional cellular mechanisms and rescue disease phenotypes88.
Conclusions
Our knowledge of C9FTD/ALS has increased exponentially within a relatively short time period. Dissection of the disease mechanisms has not been straightforward, and the emerging picture is one of a combination of a diverse range of factors that lead to neurodegeneration. Given the relative importance of C9orf72 repeat expansions as a causative factor for ALS and FTD, rapid translation of the accumulated knowledge into therapeutic strategies would have a substantial impact on patients with these devastating neurodegenerative diseases.
Key points.
Rapid progress has been made in the understanding how repeat expansions in C9orf72 cause C9FTD/ALS
Both loss of function of C9orf72 and gain of toxic function of the repeats are implicated
A range of new models including mice, Drosophila and patient neurons have provided new insights
Several cellular pathways are affected and could provide new options for treatment
Targeted therapeutic strategies against the repeats themselves are most advanced and progressing towards clinical trials
Glossary.
Repeat-associated non-ATG (RAN) translation
Translation is canonically dependent on an ATG start codon for initiation. RAN translation is a non-canonical form of translation that in the presence of repetitive sequences can start without the need for an ATG codon.
Hairpins
A secondary structure in which an RNA or DNA molecule folds back onto itself to resemble a hairpin.
G-quadruplexes
A secondary structure formed by guanine rich RNA or DNA molecules consisting of a stack of G-quartets (four guanine residues aligned in a square planar configuration).
i-motifs
A four-stranded secondary structure formed by cytosine-rich DNA or RNA molecules.
Frontotemporal lobar degeneration
Frontotemporal lobar degeneration (FTLD) describes the pathological findings observed in patients with frontotemporal dementia (FTD), however FTLD and FTD are also often used interchangeably to describe the clinical syndrome.
Bacterial artificial chromosome (BAC)
A vector for maintaining large pieces of DNA, often 50-200 kilobases in size.
Cryo-electron tomography
A high-resolution technique that involves collecting a series of tilted images of frozen hydrated samples using an electron microscope to produce a £D reconstruction of the sample
P-bodies
Processing bodies – membraneless organelles within the cytoplasm that are involved in translational repression of mRNAs and mRNA silencing and degradation
Acknowledgements
R.B. is a Leonard Wolfson Clinical Research Training Fellow and is funded by a Wellcome Trust Research Training Fellowship (107196/Z/14/Z). A.M.I. is funded by the Motor Neuron Disease Association, Alzheimer’s Research UK, the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (648716 — C9ND) and the UK Dementia Research Institute. We thank Dr Sarah Mizielinska for reviewing the manuscript and assistance with figures, and Mr Martino Guadalupi and Dr Rachele Saccon for assistance with and design of figures.
Footnotes
Author contributions
Both authors researched data for the article, discussed the content, wrote the article, and reviewed and edited the manuscript before submission.
Competing interests statement
The authors declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.DeJesus-Hernandez M, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72:245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Renton AE, et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS–FTD. Neuron. 2011;72:257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Majounie E, et al. Frequency of the C9orf72 hexanucleotide repeat expansion in patients with amyotrophic lateral sclerosis and frontotemporal dementia: a cross-sectional study. Lancet Neurol. 2012;11:323–330. doi: 10.1016/S1474-4422(12)70043-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woollacott IO, Mead S. The C9ORF72 expansion mutation: gene structure, phenotypic and diagnostic issues. Acta Neuropathol. 2014;127:319–332. doi: 10.1007/s00401-014-1253-7. [DOI] [PubMed] [Google Scholar]
- 5.Rutherford NJ, et al. Length of normal alleles of C9ORF72 GGGGCC repeat do not influence disease phenotype. Neurobiol Aging. 2012;33:2950.e5–2950.e7. doi: 10.1016/j.neurobiolaging.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beck J, et al. Large C9orf72 hexanucleotide repeat expansions are seen in multiple neurodegenerative syndromes and are more frequent than expected in the UK population. Am J Hum Genet. 2013;92:345–353. doi: 10.1016/j.ajhg.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simón-Sánchez J, et al. The clinical and pathological phenotype of C9ORF72 hexanucleotide repeat expansions. Brain. 2012;135:723–735. doi: 10.1093/brain/awr353. [DOI] [PubMed] [Google Scholar]
- 8.Harms MB, et al. Lack of C9ORF72 coding mutations supports a gain of function for repeat expansions in amyotrophic lateral sclerosis. Neurobiol Aging. 2013;34:2234.e13–2234.e19. doi: 10.1016/j.neurobiolaging.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Zee J, et al. A pan-European study of the C9orf72 repeat associated with FTLD: geographic prevalence, genomic instability, and intermediate repeats. Hum Mutat. 2013;34:363–373. doi: 10.1002/humu.22244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Blitterswijk M, et al. Association between repeat sizes and clinical and pathological characteristics in carriers of C9ORF72 repeat expansions (Xpansize-72): a cross-sectional cohort study. Lancet Neurol. 2013;12:978–988. doi: 10.1016/S1474-4422(13)70210-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mizielinska S, et al. C9orf72 frontotemporal lobar degeneration is characterised by frequent neuronal sense and antisense RNA foci. Acta Neuropathol. 2013;126:845–857. doi: 10.1007/s00401-013-1200-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donnelly CJ, et al. RNA toxicity from the ALS/FTD C9ORF72 expansion is mitigated by antisense intervention. Neuron. 2013;80:415–428. doi: 10.1016/j.neuron.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lagier-Tourenne C, et al. Targeted degradation of sense and antisense C9orf72 RNA foci as therapy for ALS and frontotemporal degeneration. Proc Natl Acad Sci USA. 2013;110:E4530–E4539. doi: 10.1073/pnas.1318835110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zu T, et al. RAN proteins and RNA foci from antisense transcripts in C9ORF72 ALS and frontotemporal dementia. Proc Natl Acad Sci USA. 2013;110:E4968–E4977. doi: 10.1073/pnas.1315438110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gendron TF, et al. Antisense transcripts of the expanded C9ORF72 hexanucleotide repeat form nuclear RNA foci and undergo repeat-associated non-ATG translation in c9FTD/ALS. Acta Neuropathol. 2013;126:829–844. doi: 10.1007/s00401-013-1192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mori K, et al. The C9orf72 GGGGCC repeat is translated into aggregating dipeptide-repeat proteins in FTLD/ALS. Science. 2013;339:1335–1338. doi: 10.1126/science.1232927. [DOI] [PubMed] [Google Scholar]
- 17.Ash PE, et al. Unconventional translation of C9ORF72 GGGGCC expansion generates insoluble polypeptides specific to c9FTD/ALS. Neuron. 2013;77:639–646. doi: 10.1016/j.neuron.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mori K, et al. Bidirectional transcripts of the expanded C9orf72 hexanucleotide repeat are translated into aggregating dipeptide repeat proteins. Acta Neuropathol. 2013;126:881–893. doi: 10.1007/s00401-013-1189-3. [DOI] [PubMed] [Google Scholar]
- 19.Rohrer JD, et al. C9orf72 expansions in frontotemporal dementia and amyotrophic lateral sclerosis. Lancet Neurol. 2015;14:291–301. doi: 10.1016/S1474-4422(14)70233-9. [DOI] [PubMed] [Google Scholar]
- 20.Renton AE, Chio A, Traynor BJ. State of play in amyotrophic lateral sclerosis genetics. Nat Neurosci. 2014;17:17–23. doi: 10.1038/nn.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gami P, et al. A 30-unit hexanucleotide repeat expansion in C9orf72 induces pathological lesions with dipeptide-repeat proteins and RNA foci, but not TDP-43 inclusions and clinical disease. Acta Neuropathol. 2015;130:599–601. doi: 10.1007/s00401-015-1473-5. [DOI] [PubMed] [Google Scholar]
- 22.Gijselinck I, et al. The C9orf72 repeat size correlates with onset age of disease, DNA methylation and transcriptional downregulation of the promoter. Mol Psychiatry. 2016;21:1112–1124. doi: 10.1038/mp.2015.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nordin A, et al. Extensive size variability of the GGGGCC expansion in C9orf72 in both neuronal and non-neuronal tissues in 18 patients with ALS or FTD. Hum Mol Genet. 2015;24:3133–3142. doi: 10.1093/hmg/ddv064. [DOI] [PubMed] [Google Scholar]
- 24.Fratta P, et al. Screening a UK amyotrophic lateral sclerosis cohort provides evidence of multiple origins of the C9orf72 expansion. Neurobiol Aging. 2015;36:546.e1–546.e7. doi: 10.1016/j.neurobiolaging.2014.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xi Z, et al. Jump from pre-mutation to pathologic expansion in C9orf72. Am J Hum Genet. 2015;96:962–970. doi: 10.1016/j.ajhg.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neumann M, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 27.Arai T, et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351:602–611. doi: 10.1016/j.bbrc.2006.10.093. [DOI] [PubMed] [Google Scholar]
- 28.Ling SC, Polymenidou M, Cleveland DW. Converging mechanisms in ALS and FTD: disrupted RNA and protein homeostasis. Neuron. 2013;79:416–438. doi: 10.1016/j.neuron.2013.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mackenzie IR, et al. Dipeptide repeat protein pathology in C9ORF72 mutation cases: clinico-pathological correlations. Acta Neuropathol. 2013;126:859–879. doi: 10.1007/s00401-013-1181-y. [DOI] [PubMed] [Google Scholar]
- 30.Murray ME, et al. Clinical and neuropathologic heterogeneity of c9FTD/ALS associated with hexanucleotide repeat expansion in C9ORF72. Acta Neuropathol. 2011;122:673–690. doi: 10.1007/s00401-011-0907-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsiung GY, et al. Clinical and pathological features of familial frontotemporal dementia caused by C9ORF72 mutation on chromosome 9p. Brain. 2012;135:709–722. doi: 10.1093/brain/awr354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahoney CJ, et al. Frontotemporal dementia with the C9ORF72 hexanucleotide repeat expansion: clinical, neuroanatomical and neuropathological features. Brain. 2012;135:736–750. doi: 10.1093/brain/awr361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Irwin DJ, et al. Cognitive decline and reduced survival in C9orf72 expansion frontotemporal degeneration and amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2013;84:163–169. doi: 10.1136/jnnp-2012-303507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeJesus-Hernandez M, et al. In-depth clinico-pathological examination of RNA foci in a large cohort of C9ORF72 expansion carriers. Acta Neuropathol. 2017;134:255–269. doi: 10.1007/s00401-017-1725-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mann DM, et al. Dipeptide repeat proteins are present in the p62 positive inclusions in patients with frontotemporal lobar degeneration and motor neurone disease associated with expansions in C9ORF72. Acta Neuropathol Commun. 2013;1:68. doi: 10.1186/2051-5960-1-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schludi MH, et al. Distribution of dipeptide repeat proteins in cellular models and C9orf72 mutation cases suggests link to transcriptional silencing. Acta Neuropathol. 2015;130:537–555. doi: 10.1007/s00401-015-1450-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davidson YS, et al. Brain distribution of dipeptide repeat proteins in frontotemporal lobar degeneration and motor neurone disease associated with expansions in C9ORF72. Acta Neuropathol Commun. 2014;2:70. doi: 10.1186/2051-5960-2-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mackenzie IR, et al. Quantitative analysis and clinico-pathological correlations of different dipeptide repeat protein pathologies in C9ORF72 mutation carriers. Acta Neuropathol. 2015;130:845–861. doi: 10.1007/s00401-015-1476-2. [DOI] [PubMed] [Google Scholar]
- 39.Davidson Y, et al. Neurodegeneration in frontotemporal lobar degeneration and motor neurone disease associated with expansions in C9orf72 is linked to TDP-43 pathology and not associated with aggregated forms of dipeptide repeat proteins. Neuropathol Appl Neurobiol. 2016;42:242–254. doi: 10.1111/nan.12292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gomez-Deza J, et al. Dipeptide repeat protein inclusions are rare in the spinal cord and almost absent from motor neurons in C9ORF72 mutant amyotrophic lateral sclerosis and are unlikely to cause their degeneration. Acta Neuropathol Commun. 2015;3:38. doi: 10.1186/s40478-015-0218-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu EY, et al. C9orf72 hypermethylation protects against repeat expansion-associated pathology in ALS/FTD. Acta Neuropathol. 2014;128:525–541. doi: 10.1007/s00401-014-1286-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wen X, et al. Antisense proline-arginine RAN dipeptides linked to C9ORF72-ALS/FTD form toxic nuclear aggregates that initiate in vitro and in vivo neuronal death. Neuron. 2014;84:1213–1225. doi: 10.1016/j.neuron.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gendron TF, et al. Cerebellar c9RAN proteins associate with clinical and neuropathological characteristics of C9ORF72 repeat expansion carriers. Acta Neuropathol. 2015;130:559–573. doi: 10.1007/s00401-015-1474-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saberi S, et al. Sense-encoded poly-GR dipeptide repeat proteins correlate to neurodegeneration and uniquely co-localize with TDP-43 in dendrites of repeat-expanded C9orf72 amyotrophic lateral sclerosis. Acta Neuropathol. 2018;135:459–474. doi: 10.1007/s00401-017-1793-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Su Z, et al. Discovery of a biomarker and lead small molecules to target r(GGGGCC)-associated defects in c9FTD/ALS. Neuron. 2014;83:1043–1050. doi: 10.1016/j.neuron.2014.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gendron TF, et al. Poly(GP) proteins are a useful pharmacodynamic marker for C9ORF72-associated amyotrophic lateral sclerosis. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aai7866. eaai7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lehmer C, et al. Poly-GP in cerebrospinal fluid links C9orf72-associated dipeptide repeat expression to the asymptomatic phase of ALS/FTD. EMBO Mol Med. 2017;9:859–868. doi: 10.15252/emmm.201607486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Balendra R, Moens TG, Isaacs AM. Specific biomarkers for C9orf72 FTD/ALS could expedite the journey towards effective therapies. EMBO Mol Med. 2017;9:853–855. doi: 10.15252/emmm.201707848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Panda SK, et al. Highly efficient targeted mutagenesis in mice using TALENs. Genetics. 2013;195:703–713. doi: 10.1534/genetics.113.156570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suzuki N, et al. The mouse C9ORF72 ortholog is enriched in neurons known to degenerate in ALS and FTD. Nat Neurosci. 2013;16:1725–1727. doi: 10.1038/nn.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koppers M, et al. C9orf72 ablation in mice does not cause motor neuron degeneration or motor deficits. Ann Neurol. 2015;78:426–438. doi: 10.1002/ana.24453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang J, et al. Gain of toxicity from ALS/FTD-linked repeat expansions in C9ORF72 Is alleviated by antisense oligonucleotides targeting GGGGCC-containing RNAs. Neuron. 2016;90:535–550. doi: 10.1016/j.neuron.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Atanasio A, et al. C9orf72 ablation causes immune dysregulation characterized by leukocyte expansion, autoantibody production, and glomerulonephropathy in mice. Sci Rep. 2016;6 doi: 10.1038/srep23204. 23204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O'Rourke JG, et al. C9orf72 is required for proper macrophage and microglial function in mice. Science. 2016;351:1324–1329. doi: 10.1126/science.aaf1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sudria-Lopez E, et al. Full ablation of C9orf72 in mice causes immune system-related pathology and neoplastic events but no motor neuron defects. Acta Neuropathol. 2016;132:145–147. doi: 10.1007/s00401-016-1581-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sullivan PM, et al. The ALS/FTLD associated protein C9orf72 associates with SMCR8 and WDR41 to regulate the autophagy-lysosome pathway. Acta Neuropathol Commun. 2016;4:51. doi: 10.1186/s40478-016-0324-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Burberry A, et al. Loss-of-function mutations in the C9ORF72 mouse ortholog cause fatal autoimmune disease. Sci Transl Med. 2016;8:347ra93. doi: 10.1126/scitranslmed.aaf6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ugolino J, et al. Loss of C9orf72 enhances autophagic activity via deregulated mTOR and TFEB signaling. PLoS Genet. 2016;12:e1006443. doi: 10.1371/journal.pgen.1006443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chew J, et al. C9ORF72 repeat expansions in mice cause TDP-43 pathology, neuronal loss, and behavioral deficits. Science. 2015;348:1151–1154. doi: 10.1126/science.aaa9344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peters OM, et al. Human C9ORF72 hexanucleotide expansion reproduces RNA foci and dipeptide repeat proteins but not neurodegeneration in BAC transgenic mice. Neuron. 2015;88:902–909. doi: 10.1016/j.neuron.2015.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.O'Rourke JG, et al. C9orf72 BAC transgenic mice display typical pathologic features of ALS/FTD. Neuron. 2015;88:892–901. doi: 10.1016/j.neuron.2015.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu Y, et al. C9orf72 BAC mouse model with motor deficits and neurodegenerative features of ALS/FTD. Neuron. 2016;90:521–534. doi: 10.1016/j.neuron.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 63.Zhang YJ, et al. C9ORF72 poly(GA) aggregates sequester and impair HR23 and nucleocytoplasmic transport proteins. Nat Neurosci. 2016;19:668–677. doi: 10.1038/nn.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schludi MH, et al. Spinal poly-GA inclusions in a C9orf72 mouse model trigger motor deficits and inflammation without neuron loss. Acta Neuropathol. 2017;134:241–254. doi: 10.1007/s00401-017-1711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Herranz-Martin S, et al. Viral delivery of C9orf72 hexanucleotide repeat expansions in mice leads to repeat-length-dependent neuropathology and behavioural deficits. Dis Model Mech. 2017;10:859–868. doi: 10.1242/dmm.029892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haeusler AR, et al. C9orf72 nucleotide repeat structures initiate molecular cascades of disease. Nature. 2014;507:195–200. doi: 10.1038/nature13124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mizielinska S, et al. Bidirectional nucleolar dysfunction in C9orf72 frontotemporal lobar degeneration. Acta Neuropathol Commun. 2017;5:29. doi: 10.1186/s40478-017-0432-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kwon I, et al. Poly-dipeptides encoded by the C9orf72 repeats bind nucleoli, impede RNA biogenesis, and kill cells. Science. 2014;345:1139–1145. doi: 10.1126/science.1254917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tao Z, et al. Nucleolar stress and impaired stress granule formation contribute to C9orf72 RAN translation-induced cytotoxicity. Hum Mol Genet. 2015;24:2426–2441. doi: 10.1093/hmg/ddv005. [DOI] [PubMed] [Google Scholar]
- 70.Stopford MJ, et al. C9ORF72 hexanucleotide repeat exerts toxicity in a stable, inducible motor neuronal cell model, which is rescued by partial depletion of Pten. Hum Mol Genet. 2017;26:1133–1145. doi: 10.1093/hmg/ddx022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bennion Callister J, Ryan S, Sim J, Rollinson S, Pickering-Brown SM. Modelling C9orf72 dipeptide repeat proteins of a physiologically relevant size. Hum Mol Genet. 2016;25:5069–5082. doi: 10.1093/hmg/ddw327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee KH, et al. C9orf72 dipeptide repeats impair the assembly, dynamics, and function of membrane-less organelles. Cell. 2016;167:774–788.e17. doi: 10.1016/j.cell.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boeynaems S, et al. Phase separation of C9orf72 dipeptide repeats perturbs stress granule dynamics. Mol Cell. 2017;65:1044–1055.e5. doi: 10.1016/j.molcel.2017.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maharjan N, Kunzli C, Buthey K, Saxena S. C9ORF72 regulates stress granule formation and its deficiency impairs stress granule assembly, hypersensitizing cells to stress. Mol Neurobiol. 2017;54:3062–3077. doi: 10.1007/s12035-016-9850-1. [DOI] [PubMed] [Google Scholar]
- 75.Dafinca R, et al. C9orf72 hexanucleotide expansions are associated with altered ER calcium homeostasis and stress granule formation in iPSC-derived neurons from patients with amyotrophic lateral sclerosis and frontotemporal dementia. Stem Cells. 2016;34:2063–2078. doi: 10.1002/stem.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McGurk L, et al. Poly-A binding protein-1 localization to a subset of TDP-43 inclusions in amyotrophic lateral sclerosis occurs more frequently in patients harboring an expansion in C9orf72. J Neuropathol Exp Neurol. 2014;73:837–845. doi: 10.1097/NEN.0000000000000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Daigle JG, et al. Pur-alpha regulates cytoplasmic stress granule dynamics and ameliorates FUS toxicity. Acta Neuropathol. 2016;131:605–620. doi: 10.1007/s00401-015-1530-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Farg MA, et al. C9ORF72, implicated in amytrophic lateral sclerosis and frontotemporal dementia, regulates endosomal trafficking. Hum Mol Genet. 2014;23:3579–3595. doi: 10.1093/hmg/ddu068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rossi S, et al. Nuclear accumulation of mRNAs underlies G4C2-repeat-induced translational repression in a cellular model of C9orf72 ALS. J Cell Sci. 2015;128:1787–1799. doi: 10.1242/jcs.165332. [DOI] [PubMed] [Google Scholar]
- 80.Green KM, et al. RAN translation at C9orf72-associated repeat expansions is selectively enhanced by the integrated stress response. Nat Commun. 2017;8:2005. doi: 10.1038/s41467-017-02200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fay MM, Anderson PJ, Ivanov P. ALS/FTD-associated C9ORF72 repeat RNA promotes phase transitions in vitro and in cells. Cell Rep. 2017;21:3573–3584. doi: 10.1016/j.celrep.2017.11.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang K, et al. Stress granule assembly disrupts nucleocytoplasmic transport. Cell. 2018;173:958–971.e17. doi: 10.1016/j.cell.2018.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cheng W, et al. C9ORF72 GGGGCC repeat-associated non-AUG translation is upregulated by stress through eIF2α phosphorylation. Nat Commun. 2018;9:51. doi: 10.1038/s41467-017-02495-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hautbergue GM, et al. SRSF1-dependent nuclear export inhibition of C9ORF72 repeat transcripts prevents neurodegeneration and associated motor deficits. Nat Commun. 2017;8 doi: 10.1038/ncomms16063. 16063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yin S, et al. Evidence that C9ORF72 dipeptide repeat proteins associate with U2 snRNP to cause mis-splicing in ALS/FTD patients. Cell Rep. 2017;19:2244–2256. doi: 10.1016/j.celrep.2017.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Prudencio M, et al. Distinct brain transcriptome profiles in C9orf72-associated and sporadic ALS. Nat Neurosci. 2015;18:1175–1182. doi: 10.1038/nn.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kanekura K, et al. Poly-dipeptides encoded by the C9ORF72 repeats block global protein translation. Hum Mol Genet. 2016;25:1803–1813. doi: 10.1093/hmg/ddw052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kramer NJ, et al. CRISPR–Cas9 screens in human cells and primary neurons identify modifiers of C9ORF72 dipeptide-repeat-protein toxicity. Nat Genet. 2018;50:603–612. doi: 10.1038/s41588-018-0070-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jovicic A, et al. Modifiers of C9orf72 dipeptide repeat toxicity connect nucleocytoplasmic transport defects to FTD/ALS. Nat Neurosci. 2015;18:1226–1229. doi: 10.1038/nn.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Almeida S, et al. Modeling key pathological features of frontotemporal dementia with C9ORF72 repeat expansion in iPSC-derived human neurons. Acta Neuropathol. 2013;126:385–399. doi: 10.1007/s00401-013-1149-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Webster CP, et al. The C9orf72 protein interacts with Rab1a and the ULK1 complex to regulate initiation of autophagy. EMBO J. 2016;35:1656–1676. doi: 10.15252/embj.201694401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Imamura K, et al. The Src/c–Abl pathway is a potential therapeutic target in amyotrophic lateral sclerosis. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aaf3962. eaaf3962. [DOI] [PubMed] [Google Scholar]
- 93.Aoki Y, et al. C9orf72 and RAB7L1 regulate vesicle trafficking in amyotrophic lateral sclerosis and frontotemporal dementia. Brain. 2017;140:887–897. doi: 10.1093/brain/awx024. [DOI] [PubMed] [Google Scholar]
- 94.Shi Y, et al. Haploinsufficiency leads to neurodegeneration in C9ORF72 ALS/FTD human induced motor neurons. Nat Med. 2018;24:313–325. doi: 10.1038/nm.4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Onesto E, et al. Gene-specific mitochondria dysfunctions in human TARDBP and C9ORF72 fibroblasts. Acta Neuropathol Commun. 2016;4:47. doi: 10.1186/s40478-016-0316-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sellier C, et al. Loss of C9ORF72 impairs autophagy and synergizes with polyQ Ataxin-2 to induce motor neuron dysfunction and cell death. EMBO J. 2016 doi: 10.15252/embj.201593350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Busch JI, et al. Increased expression of the frontotemporal dementia risk factor TMEM106B causes C9orf72-dependent alterations in lysosomes. Hum Mol Genet. 2016;35:1276–1297. doi: 10.1093/hmg/ddw127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cristofani R, et al. The small heat shock protein B8 (HSPB8) efficiently removes aggregating species of dipeptides produced in C9ORF72-related neurodegenerative diseases. Cell Stress Chaperones. 2018;23:1–12. doi: 10.1007/s12192-017-0806-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cristofani R, et al. Inhibition of retrograde transport modulates misfolded protein accumulation and clearance in motoneuron diseases. Autophagy. 2017;13:1280–1303. doi: 10.1080/15548627.2017.1308985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jung J, et al. Multiplex image-based autophagy RNAi screening identifies SMCR8 as ULK1 kinase activity and gene expression regulator. Elife. 2017;6 doi: 10.7554/eLife.23063. e23063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Amick J, Roczniak-Ferguson A, Ferguson SM. C9orf72 binds SMCR8, localizes to lysosomes, and regulates mTORC1 signaling. Mol Biol Cell. 2016;27:3040–3051. doi: 10.1091/mbc.E16-01-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gupta R, et al. The proline/arginine dipeptide from hexanucleotide repeat expanded C9ORF72 inhibits the proteasome. eNeuro. 2017;4 doi: 10.1523/ENEURO.0249-16.2017. ENEURO.0249-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee S, et al. Activation of HIPK2 promotes ER stress-mediated neurodegeneration in amyotrophic lateral sclerosis. Neuron. 2016;91:41–55. doi: 10.1016/j.neuron.2016.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang YJ, et al. Aggregation-prone c9FTD/ALS poly(GA) RAN-translated proteins cause neurotoxicity by inducing ER stress. Acta Neuropathol. 2014;128:505–524. doi: 10.1007/s00401-014-1336-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.May S, et al. C9orf72 FTLD/ALS-associated Gly–Ala dipeptide repeat proteins cause neuronal toxicity and Unc119 sequestration. Acta Neuropathol. 2014;128:485–503. doi: 10.1007/s00401-014-1329-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yamakawa M, et al. Characterization of the dipeptide repeat protein in the molecular pathogenesis of c9FTD/ALS. Hum Mol Genet. 2015;24:1630–1645. doi: 10.1093/hmg/ddu576. [DOI] [PubMed] [Google Scholar]
- 107.Guo Q, et al. In situ structure of neuronal C9orf72 poly-GA aggregates reveals proteasome recruitment. Cell. 2018;172:696–705.e12. doi: 10.1016/j.cell.2017.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Freibaum BD, et al. GGGGCC repeat expansion in C9orf72 compromises nucleocytoplasmic transport. Nature. 2015;525:129–133. doi: 10.1038/nature14974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang K, et al. The C9orf72 repeat expansion disrupts nucleocytoplasmic transport. Nature. 2015;525:56–61. doi: 10.1038/nature14973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xiao S, et al. Isoform-specific antibodies reveal distinct subcellular localizations of C9orf72 in amyotrophic lateral sclerosis. Ann Neurol. 2015;78:568–583. doi: 10.1002/ana.24469. [DOI] [PubMed] [Google Scholar]
- 111.Chou CC, et al. TDP-43 pathology disrupts nuclear pore complexes and nucleocytoplasmic transport in ALS/FTD. Nat Neurosci. 2018;21:228–239. doi: 10.1038/s41593-017-0047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shani T, Levy M, Israelson A. Assay to measure nucleocytoplasmic transport in real time within motor neuron-like NSC-34 cells. J Vis Exp. 2017 doi: 10.3791/55676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shi KY, et al. Toxic PRn poly-dipeptides encoded by the C9orf72 repeat expansion block nuclear import and export. Proc Natl Acad Sci USA. 2017;114:E1111–E1117. doi: 10.1073/pnas.1620293114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Khosravi B, et al. Cytoplasmic poly-GA aggregates impair nuclear import of TDP-43 in C9orf72 ALS/FTLD. Hum Mol Genet. 2017;26:790–800. doi: 10.1093/hmg/ddw432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Boeynaems S, et al. Drosophila screen connects nuclear transport genes to DPR pathology in c9ALS/FTD. Sci Rep. 2016;6 doi: 10.1038/srep20877. 20877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Coyne AN, et al. Post-transcriptional inhibition of Hsc70-4/HSPA8 expression leads to synaptic vesicle cycling defects in multiple models of ALS. Cell Rep. 2017;21:110–125. doi: 10.1016/j.celrep.2017.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lopez-Gonzalez R, et al. Poly(GR) in C9ORF72-related ALS/FTD compromises mitochondrial function and increases oxidative stress and DNA damage in iPSC-derived motor neurons. Neuron. 2016;92:383–391. doi: 10.1016/j.neuron.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Walker C, et al. C9orf72 expansion disrupts ATM-mediated chromosomal break repair. Nat Neurosci. 2017;20:1225–1235. doi: 10.1038/nn.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Farg MA, Konopka A, Ying Soo K, Ito D, Atkin JD. The DNA damage response (DDR) is induced by the C9orf72 repeat expansion in amyotrophic lateral sclerosis. Hum Mol Genet. 2017;26:2882–2896. doi: 10.1093/hmg/ddx170. [DOI] [PubMed] [Google Scholar]
- 120.Schweizer Burguete A, et al. GGGGCC microsatellite RNA is neuritically localized, induces branching defects, and perturbs transport granule function. Elife. 2015;4 doi: 10.7554/eLife.08881. e08881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ishiguro A, Kimura N, Watanabe Y, Watanabe S, Ishihama A. TDP-43 binds and transports G-quadruplex-containing mRNAs into neurites for local translation. Genes Cells. 2016;21:466–481. doi: 10.1111/gtc.12352. [DOI] [PubMed] [Google Scholar]
- 122.Blokhuis AM, et al. Comparative interactomics analysis of different ALS-associated proteins identifies converging molecular pathways. Acta Neuropathol. 2016;132:175–196. doi: 10.1007/s00401-016-1575-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yang D, et al. FTD/ALS-associated poly(GR) protein impairs the Notch pathway and is recruited by poly(GA) into cytoplasmic inclusions. Acta Neuropathol. 2015;130:525–535. doi: 10.1007/s00401-015-1448-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Devlin AC, et al. Human iPSC-derived motoneurons harbouring TARDBP or C9ORF72 ALS mutations are dysfunctional despite maintaining viability. Nat Commun. 2015;6 doi: 10.1038/ncomms6999. 5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wainger BJ, et al. Intrinsic membrane hyperexcitability of amyotrophic lateral sclerosis patient-derived motor neurons. Cell Rep. 2014;7:1–11. doi: 10.1016/j.celrep.2014.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sareen D, et al. Targeting RNA foci in iPSC-derived motor neurons from ALS patients with a C9ORF72 repeat expansion. Sci Transl Med. 2013;5:208ra149. doi: 10.1126/scitranslmed.3007529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Selvaraj BT, et al. C9ORF72 repeat expansion causes vulnerability of motor neurons to Ca2+-permeable AMPA receptor-mediated excitotoxicity. Nat Commun. 2018;9:347. doi: 10.1038/s41467-017-02729-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schanz O, et al. Cortical hyperexcitability in patients with C9orf72 mutations: relationship to phenotype. Muscle Nerve. 2016;54:264–269. doi: 10.1002/mus.25047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Geevasinga N, et al. Cortical function in asymptomatic carriers and patients with C9orf72 amyotrophic lateral sclerosis. JAMA Neurol. 2015;72:1268–1274. doi: 10.1001/jamaneurol.2015.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Williams KL, et al. Pathophysiological insights into ALS with C9ORF72 expansions. J Neurol Neurosurg Psychiatry. 2013;84:931–935. doi: 10.1136/jnnp-2012-304529. [DOI] [PubMed] [Google Scholar]
- 131.Benussi A, et al. Impaired long-term potentiation-like cortical plasticity in presymptomatic genetic frontotemporal dementia. Ann Neurol. 2016;80:472–476. doi: 10.1002/ana.24731. [DOI] [PubMed] [Google Scholar]
- 132.Ciura S, et al. Loss of function of C9orf72 causes motor deficits in a zebrafish model of amyotrophic lateral sclerosis. Ann Neurol. 2013;74:180–187. doi: 10.1002/ana.23946. [DOI] [PubMed] [Google Scholar]
- 133.Swaminathan A, et al. Expression of C9orf72-related dipeptides impairs motor function in a vertebrate model. Hum Mol Genet. 2018;27:1754–1762. doi: 10.1093/hmg/ddy083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Swinnen B, et al. A zebrafish model for C9orf72 ALS reveals RNA toxicity as a pathogenic mechanism. Acta Neuropathol. 2018;135:427–443. doi: 10.1007/s00401-017-1796-5. [DOI] [PubMed] [Google Scholar]
- 135.Baldwin KR, Godena VK, Hewitt VL, Whitworth AJ. Axonal transport defects are a common phenotype in Drosophila models of ALS. Hum Mol Genet. 2016;25:2378–2392. doi: 10.1093/hmg/ddw105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sivadasan R, et al. C9ORF72 interaction with cofilin modulates actin dynamics in motor neurons. Nat Neurosci. 2016;19:1610–1618. doi: 10.1038/nn.4407. [DOI] [PubMed] [Google Scholar]
- 137.Ferraiuolo L, et al. Oligodendrocytes contribute to motor neuron death in ALS via SOD1-dependent mechanism. Proc Natl Acad Sci USA. 2016;113:E6496–E6505. doi: 10.1073/pnas.1607496113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tran H, et al. Differential toxicity of nuclear RNA foci versus dipeptide repeat proteins in a Drosophila model of C9ORF72 FTD/ALS. Neuron. 2015;87:1207–1214. doi: 10.1016/j.neuron.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rizzu P, et al. C9orf72 is differentially expressed in the central nervous system and myeloid cells and consistently reduced in C9orf72, MAPT and GRN mutation carriers. Acta Neuropathol Commun. 2016;4:37. doi: 10.1186/s40478-016-0306-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Xi Z, et al. Hypermethylation of the CpG island near the G4C2 repeat in ALS with a C9orf72 expansion. Am J Hum Genet. 2013;92:981–989. doi: 10.1016/j.ajhg.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gijselinck I, et al. A C9orf72 promoter repeat expansion in a Flanders-Belgian cohort with disorders of the frontotemporal lobar degeneration–amyotrophic lateral sclerosis spectrum: a gene identification study. Lancet Neurol. 2012;11:54–65. doi: 10.1016/S1474-4422(11)70261-7. [DOI] [PubMed] [Google Scholar]
- 142.Fratta P, et al. Homozygosity for the C9orf72 GGGGCC repeat expansion in frontotemporal dementia. Acta Neuropathol. 2013;126:401–409. doi: 10.1007/s00401-013-1147-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Belzil VV, et al. Reduced C9orf72 gene expression in c9FTD/ALS is caused by histone trimethylation, an epigenetic event detectable in blood. Acta Neuropathol. 2013;126:895–905. doi: 10.1007/s00401-013-1199-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Waite AJ, et al. Reduced C9orf72 protein levels in frontal cortex of amyotrophic lateral sclerosis and frontotemporal degeneration brain with the C9ORF72 hexanucleotide repeat expansion. Neurobiol Aging. 2014;35:1779.e5–1779.e13. doi: 10.1016/j.neurobiolaging.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.van Blitterswijk M, et al. Novel clinical associations with specific C9ORF72 transcripts in patients with repeat expansions in C9ORF72. Acta Neuropathol. 2015;130:863–876. doi: 10.1007/s00401-015-1480-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Niblock M, et al. Retention of hexanucleotide repeat-containing intron in C9orf72 mRNA: implications for the pathogenesis of ALS/FTD. Acta Neuropathol Commun. 2016;4:18. doi: 10.1186/s40478-016-0289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Belzil VV, et al. Characterization of DNA hypermethylation in the cerebellum of c9FTD/ALS patients. Brain Res. 2014;1584:15–21. doi: 10.1016/j.brainres.2014.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xi Z, et al. The C9orf72 repeat expansion itself is methylated in ALS and FTLD patients. Acta Neuropathol. 2015;129:715–727. doi: 10.1007/s00401-015-1401-8. [DOI] [PubMed] [Google Scholar]
- 149.Esanov R, et al. A C9ORF72 BAC mouse model recapitulates key epigenetic perturbations of ALS/FTD. Mol Neurodegener. 2017;12:46. doi: 10.1186/s13024-017-0185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zeier Z, et al. Bromodomain inhibitors regulate the C9ORF72 locus in ALS. Exp Neurol. 2015;271:241–250. doi: 10.1016/j.expneurol.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bauer PO. Methylation of C9orf72 expansion reduces RNA foci formation and dipeptide-repeat proteins expression in cells. Neurosci Lett. 2016;612:204–209. doi: 10.1016/j.neulet.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 152.McMillan CT, et al. C9orf72 promoter hypermethylation is neuroprotective: neuroimaging and neuropathologic evidence. Neurology. 2015;84:1622–1630. doi: 10.1212/WNL.0000000000001495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Russ J, et al. Hypermethylation of repeat expanded C9orf72 is a clinical and molecular disease modifier. Acta Neuropathol. 2015;129:39–52. doi: 10.1007/s00401-014-1365-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yang M, et al. A C9ORF72/SMCR8-containing complex regulates ULK1 and plays a dual role in autophagy. Sci Adv. 2016;2:e1601167. doi: 10.1126/sciadv.1601167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Freischmidt A, et al. Haploinsufficiency of TBK1 causes familial ALS and fronto-temporal dementia. Nat Neurosci. 2015;18:631–636. doi: 10.1038/nn.4000. [DOI] [PubMed] [Google Scholar]
- 156.Elden AC, et al. Ataxin-2 intermediate-length polyglutamine expansions are associated with increased risk for ALS. Nature. 2010;466:1069–1075. doi: 10.1038/nature09320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ferguson R, Serafeimidou-Pouliou E, Subramanian V. Dynamic expression of the mouse orthologue of the human amyotropic lateral sclerosis associated gene C9orf72 during central nervous system development and neuronal differentiation. J Anat. 2016;229:871–891. doi: 10.1111/joa.12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Komatsu M, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- 159.Hara T, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 160.Robberecht W, Philips T. The changing scene of amyotrophic lateral sclerosis. Nat Rev Neurosci. 2013;14:248–264. doi: 10.1038/nrn3430. [DOI] [PubMed] [Google Scholar]
- 161.Fratta P, et al. C9orf72 hexanucleotide repeat associated with amyotrophic lateral sclerosis and frontotemporal dementia forms RNA G-quadruplexes. Sci Rep. 2012;2:1016. doi: 10.1038/srep01016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Reddy K, Zamiri B, Stanley SY, Macgregor RB, Jr, Pearson CE. The disease-associated r(GGGGCC)n repeat from the C9orf72 gene forms tract length-dependent uni- and multimolecular RNA G-quadruplex structures. J Biol Chem. 2013;288:9860–9866. doi: 10.1074/jbc.C113.452532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhou B, et al. Characterizations of distinct parallel and antiparallel G-quadruplexes formed by two-repeat ALS and FTD related GGGGCC sequence. Sci Rep. 2018;8 doi: 10.1038/s41598-018-20852-w. 2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Vatovec S, Kovanda A, Rogelj B. Unconventional features of C9ORF72 expanded repeat in amyotrophic lateral sclerosis and frontotemporal lobar degeneration. Neurobiol Aging. 2014;35:2421.e1–2421.e12. doi: 10.1016/j.neurobiolaging.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 165.Kovanda A, Zalar M, Sket P, Plavec J, Rogelj B. Anti-sense DNA d(GGCCCC)n expansions in C9ORF72 form i-motifs and protonated hairpins. Sci Rep. 2015;5 doi: 10.1038/srep17944. 17944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhang Y, Roland C, Sagui C. Structure and dynamics of DNA and RNA double helices obtained from the GGGGCC and CCCCGG hexanucleotide repeats that are the hallmark of C9FTD/ALS diseases. ACS Chem Neurosci. 2017;8:578–591. doi: 10.1021/acschemneuro.6b00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zamiri B, et al. Stress-induced acidification may contribute to formation of unusual structures in C9orf72-repeats. Biochim Biophys Acta. 2018;1862:1482–1491. doi: 10.1016/j.bbagen.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 168.Haeusler AR, Donnelly CJ, Rothstein JD. The expanding biology of the C9orf72 nucleotide repeat expansion in neurodegenerative disease. Nat Rev Neurosci. 2016;17:383–395. doi: 10.1038/nrn.2016.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lee YB, et al. Hexanucleotide repeats in ALS/FTD form length-dependent RNA foci, sequester RNA binding proteins, and are neurotoxic. Cell Rep. 2013;5:1178–1186. doi: 10.1016/j.celrep.2013.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cooper-Knock J, et al. Sequestration of multiple RNA recognition motif-containing proteins by C9orf72 repeat expansions. Brain. 2014;137:2040–2051. doi: 10.1093/brain/awu120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Cooper-Knock J, et al. Antisense RNA foci in the motor neurons of C9ORF72-ALS patients are associated with TDP-43 proteinopathy. Acta Neuropathol. 2015;130:63–75. doi: 10.1007/s00401-015-1429-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Xu Z, et al. Expanded GGGGCC repeat RNA associated with amyotrophic lateral sclerosis and frontotemporal dementia causes neurodegeneration. Proc Natl Acad Sci USA. 2013;110:7778–7783. doi: 10.1073/pnas.1219643110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Davidson YS, et al. Heterogeneous ribonuclear protein A3 (hnRNP A3) is present in dipeptide repeat protein containing inclusions in frontotemporal lobar degeneration and motor neurone disease associated with expansions in C9orf72 gene. Acta Neuropathol Commun. 2017;5:31. doi: 10.1186/s40478-017-0437-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Conlon EG, et al. The C9ORF72 GGGGCC expansion forms RNA G-quadruplex inclusions and sequesters hnRNP H to disrupt splicing in ALS brains. Elife. 2016;5:e17820. doi: 10.7554/eLife.17820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mori K, et al. hnRNP A3 binds to GGGGCC repeats and is a constituent of p62-positive/TDP43-negative inclusions in the hippocampus of patients with C9orf72 mutations. Acta Neuropathol. 2013;125:413–423. doi: 10.1007/s00401-013-1088-7. [DOI] [PubMed] [Google Scholar]
- 176.Haas S, et al. A 39-kD DNA-binding protein from mouse brain stimulates transcription of myelin basic protein gene in oligodendrocytic cells. J Cell Biol. 1995;130:1171–1179. doi: 10.1083/jcb.130.5.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gallia GL, Johnson EM, Khalili K. Puralpha: a multifunctional single-stranded DNA- and RNA-binding protein. Nucleic Acids Res. 2000;28:3197–3205. doi: 10.1093/nar/28.17.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ohashi S, et al. The single-stranded DNA- and RNA-binding proteins pur alpha and pur beta link BC1 RNA to microtubules through binding to the dendrite-targeting RNA motifs. J Neurochem. 2000;75:1781–1790. doi: 10.1046/j.1471-4159.2000.0751781.x. [DOI] [PubMed] [Google Scholar]
- 179.Kanai Y, Dohmae N, Hirokawa N. Kinesin transports RNA: isolation and characterization of an RNA-transporting granule. Neuron. 2004;43:513–525. doi: 10.1016/j.neuron.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 180.Celona B, et al. Suppression of C9orf72 RNA repeat-induced neurotoxicity by the ALS-associated RNA-binding protein Zfp106. Elife. 2017;6:e19032. doi: 10.7554/eLife.19032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mackenzie IR. The role of dipeptide-repeat protein pathology in C9orf72 mutation cases. Neuropathol Appl Neurobiol. 2016;42:217–219. doi: 10.1111/nan.12296. [DOI] [PubMed] [Google Scholar]
- 182.Baborie A, et al. Accumulation of dipeptide repeat proteins predates that of TDP-43 in frontotemporal lobar degeneration associated with hexanucleotide repeat expansions in C9ORF72 gene. Neuropathol Appl Neurobiol. 2015;41:601–612. doi: 10.1111/nan.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mann DM. Dipeptide repeat protein toxicity in frontotemporal lobar degeneration and in motor neurone disease associated with expansions in C9ORF72 — a cautionary note. Neurobiol Aging. 2015;36:1224–1226. doi: 10.1016/j.neurobiolaging.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 184.Mattsson N, Schott JM, Hardy J, Turner MR, Zetterberg H. Selective vulnerability in neurodegeneration: insights from clinical variants of Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2016;87:1000–1004. doi: 10.1136/jnnp-2015-311321. [DOI] [PubMed] [Google Scholar]
- 185.Mori K, et al. Reduced hnRNPA3 increases C9orf72 repeat RNA levels and dipeptide-repeat protein deposition. EMBO Rep. 2016;17:1314–1325. doi: 10.15252/embr.201541724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Proudfoot M, et al. Early dipeptide repeat pathology in a frontotemporal dementia kindred with C9ORF72 mutation and intellectual disability. Acta Neuropathol. 2014;127:451–458. doi: 10.1007/s00401-014-1245-7. [DOI] [PubMed] [Google Scholar]
- 187.Vatsavayai SC, et al. Timing and significance of pathological features in C9orf72 expansion-associated frontotemporal dementia. Brain. 2016;139:3202–3216. doi: 10.1093/brain/aww250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mizielinska S, et al. C9orf72 repeat expansions cause neurodegeneration in Drosophila through arginine-rich proteins. Science. 2014;345:1192–1194. doi: 10.1126/science.1256800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Moens TG, et al. Sense and antisense RNA are not toxic in Drosophila models of C9orf72-associated ALS/FTD. Acta Neuropathol. 2018;135:445–457. doi: 10.1007/s00401-017-1798-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Chang YJ, Jeng US, Chiang YL, Hwang IS, Chen YR. The glycine–alanine dipeptide repeat from C9orf72 hexanucleotide expansions forms toxic amyloids possessing cell-to-cell transmission properties. J Biol Chem. 2016;291:4903–4911. doi: 10.1074/jbc.M115.694273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ohki Y, et al. Glycine–alanine dipeptide repeat protein contributes to toxicity in a zebrafish model of C9orf72 associated neurodegeneration. Mol Neurodegener. 2017;12:6. doi: 10.1186/s13024-016-0146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Flores BN, et al. Distinct C9orf72-associated dipeptide repeat structures correlate with neuronal toxicity. PLoS ONE. 2016;11:e0165084. doi: 10.1371/journal.pone.0165084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Prpar Mihevc S, et al. Nuclear trafficking in amyotrophic lateral sclerosis and frontotemporal lobar degeneration. Brain. 2017;140:13–26. doi: 10.1093/brain/aww197. [DOI] [PubMed] [Google Scholar]
- 194.Lin Y, et al. Toxic PR poly-dipeptides encoded by the C9orf72 repeat expansion target LC domain polymers. Cell. 2016;167:789–802.e12. doi: 10.1016/j.cell.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lagier-Tourenne C, Polymenidou M, Cleveland DW. TDP-43 and FUS/TLS: emerging roles in RNA processing and neurodegeneration. Hum Mol Genet. 2010;19:R46–R64. doi: 10.1093/hmg/ddq137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Molliex A, et al. Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell. 2015;163:123–133. doi: 10.1016/j.cell.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Patel A, et al. A liquid-to-solid phase transition of the ALS protein FUS accelerated by disease mutation. Cell. 2015;162:1066–1077. doi: 10.1016/j.cell.2015.07.047. [DOI] [PubMed] [Google Scholar]
- 198.Murakami T, et al. ALS/FTD mutation-induced phase transition of FUS liquid droplets and reversible hydrogels into irreversible hydrogels impairs RNP granule function. Neuron. 2015;88:678–690. doi: 10.1016/j.neuron.2015.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Jain A, Vale RD. RNA phase transitions in repeat expansion disorders. Nature. 2017;546:243–247. doi: 10.1038/nature22386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Halliday M, Mallucci GR. Review: Modulating the unfolded protein response to prevent neurodegeneration and enhance memory. Neuropathol Appl Neurobiol. 2015;41:414–427. doi: 10.1111/nan.12211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Bowden HA, Dormann D. Altered mRNP granule dynamics in FTLD pathogenesis. J Neurochem. 2016;138(Suppl 1):112–133. doi: 10.1111/jnc.13601. [DOI] [PubMed] [Google Scholar]
- 202.Tabet R, et al. CUG initiation and frameshifting enable production of dipeptide repeat proteins from ALS/FTD C9ORF72 transcripts. Nat Commun. 2018;9:152. doi: 10.1038/s41467-017-02643-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Miller TM, et al. An antisense oligonucleotide against SOD1 delivered intrathecally for patients with SOD1 familial amyotrophic lateral sclerosis: a phase 1, randomised, first-in-man study. Lancet Neurol. 2013;12:435–442. doi: 10.1016/S1474-4422(13)70061-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zamiri B, Reddy K, Macgregor RB, Jr, Pearson CE. TMPyP4 porphyrin distorts RNA G-quadruplex structures of the disease-associated r(GGGGCC)n repeat of the C9orf72 gene and blocks interaction of RNA-binding proteins. J Biol Chem. 2014;289:4653–4659. doi: 10.1074/jbc.C113.502336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Simone R, et al. G-quadruplex-binding small molecules ameliorate C9orf72 FTD/ALS pathology in vitro and in vivo. EMBO Mol Med. 2018;10:22–31. doi: 10.15252/emmm.201707850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Alniss H, Zamiri B, Khalaj M, Pearson CE, Macgregor RB., Jr Thermodynamic and spectroscopic investigations of TMPyP4 association with guanine- and cytosine-rich DNA and RNA repeats of C9orf72. Biochem Biophys Res Commun. 2018;495:2410–2417. doi: 10.1016/j.bbrc.2017.12.108. [DOI] [PubMed] [Google Scholar]
- 207.Kramer NJ, et al. Spt4 selectively regulates the expression of C9orf72 sense and antisense mutant transcripts. Science. 2016;353:708–712. doi: 10.1126/science.aaf7791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hu J, Rigo F, Prakash TP, Corey DR. Recognition of c9orf72 mutant RNA by single-stranded silencing RNAs. Nucleic Acid Ther. 2017;27:87–94. doi: 10.1089/nat.2016.0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Pinto BS, et al. Impeding transcription of expanded microsatellite repeats by deactivated Cas9. Mol Cell. 2017;68:479–490.e5. doi: 10.1016/j.molcel.2017.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Batra R, et al. Elimination of toxic microsatellite repeat expansion RNA by RNA-targeting Cas9. Cell. 2017;170:899–912.e10. doi: 10.1016/j.cell.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Barry AE, et al. Alzheimer's disease brain-derived amyloid-β-mediated inhibition of LTP in vivo is prevented by immunotargeting cellular prion protein. J Neurosci. 2011;31:7259–7263. doi: 10.1523/JNEUROSCI.6500-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Asuni AA, Boutajangout A, Quartermain D, Sigurdsson EM. Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements. J Neurosci. 2007;27:9115–9129. doi: 10.1523/JNEUROSCI.2361-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Boutajangout A, Ingadottir J, Davies P, Sigurdsson EM. Passive immunization targeting pathological phospho-tau protein in a mouse model reduces functional decline and clears tau aggregates from the brain. J Neurochem. 2011;118:658–667. doi: 10.1111/j.1471-4159.2011.07337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Masliah E, et al. Effects of α-synuclein immunization in a mouse model of Parkinson's disease. Neuron. 2005;46:857–868. doi: 10.1016/j.neuron.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 215.Masliah E, et al. Passive immunization reduces behavioral and neuropathological deficits in an alpha-synuclein transgenic model of Lewy body disease. PLoS ONE. 2011;6:e19338. doi: 10.1371/journal.pone.0019338. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 216.Zhou Q, et al. Antibodies inhibit transmission and aggregation of C9orf72 poly-GA dipeptide repeat proteins. EMBO Mol Med. 2017;9:687–702. doi: 10.15252/emmm.201607054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Yang YM, et al. A small molecule screen in stem-cell-derived motor neurons identifies a kinase inhibitor as a candidate therapeutic for ALS. Cell Stem Cell. 2013;12:713–726. doi: 10.1016/j.stem.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]