Abstract
The overall structure and architecture of the extracellular matrix undergo dramatic alterations in composition, form, and functionality over time. The stochasticity begins during development, essential for maintaining organismal homeostasis and is heavily implicated in many pathobiological states including fibrosis and cancer. Modeling and remodeling of the matrix is driven by the local cellular milieu and secreted and cell-associated components in a framework of dynamic reciprocity. This collection of expertly-written reviews aims to relay state-of-the-art information concerning the mechanisms of matrix modeling and remodeling in physiological development and disease.
Keywords: Proteoglycans, Collagens, Proteases, Senescence, Cancer
Concept of matrix modeling and remodeling
Proteoglycans are rapidly emerging as key effectors active in modeling and remodeling of both vascular and avascular tissues the stroma of most organs. Interacting with cell surface receptors [1–4], growth factors such as TGF-β [5,6], matrix remodeling enzymes [7–17], and matrix effectors imbue proteoglycans with unique and combinatorial biological properties and signaling cues [18–22], In this Special Issue on Matrix Modeling and Remodeling, novel roles of proteoglycans in orchestrating diverse biological activities are reviewed and summarized by internationally-recognized experts in the field. Possessing an array of functions, proteoglycans regulate not only structural aspects of the extracellular matrix (ECM) and tumor angiogenesis [23–28], but also cell signaling networks and behaviors governing normal and pathobiological states [29–34].
Reflecting the many biologically diverse systems in which proteoglycans operate [35,36], we will introduce the major overarching themes of this Special Issue such as senescence-driven ECM remodeling, proteolysis-mediated matrix remodeling, emerging roles in controlling tumorigenesis, and the mechanics of basement membranes in guiding development, tissue remodeling, wound re-epithelization, heart disease and myocardial infarction.
Cellular senescence drives ECM remodeling
The ECM is subjected to dynamic remodeling during development, inflammation, wound healing and diseases including cancer, atherosclerosis and osteoarthritis. Governed by cellular senescence, aging tissues display changes in both ECM function and organization. After undergoing a limited number of mitotic divisions, they enter into senescence and are unable to proliferate. This is caused by replicative senescence which is a gradual telomeric shortening that occurs every mitotic cycle. In addition, cells also experience “stress-induced premature senescence” upon exposure to certain stress factors including radiation and oxidative agents [37].
The ability of senescent cells to markedly modulate their phenotype and biosynthesis of matrix components affects tissue organization and functions as summarized by Mavrogonatou et al. found in this Special Issue. A key determinant of cellular senescence is the adaptation of senescence-associated secretory phenotype (SASP) by senescent cells that drives ECM remodeling and tissue behavior [38,39] (Fig. 1). They secrete numerous pro-inflammatory mediators including cytokines, growth factors and a plethora of proteolytic enzymes such as MMPs, plasminogen activators, and members from a class of intracellular cysteine proteases, the cathepsins [38–40], Further, senescent cells exhibit altered secretion of matrix molecules thereby establishing a provisional matrix that cooperates with the pro-inflammatory and catabolic milieu. This generates a favorable microenvironment that impairs tissue physiology, eventually leading to age-related disorders [37,39], Similarly, augmented expressions of cytokines and growth factors are implicated in wound healing [41] and in the progression of various age-related diseases including articular cartilage degeneration [42], atherosclerosis [43], and cancer development [39,44], Notably, cancer cells secrete various bioactive stimuli that induce stromal cell senescence, creating a provisional ECM to facilitate cancer cell growth and metastasis [39], Malignant cells benefit by senescent cells residing within the tumor stroma through the aberrant activation of multiple pro-growth and pro-survival pathways, driven primarily by SASP [45].
Fig. 1.
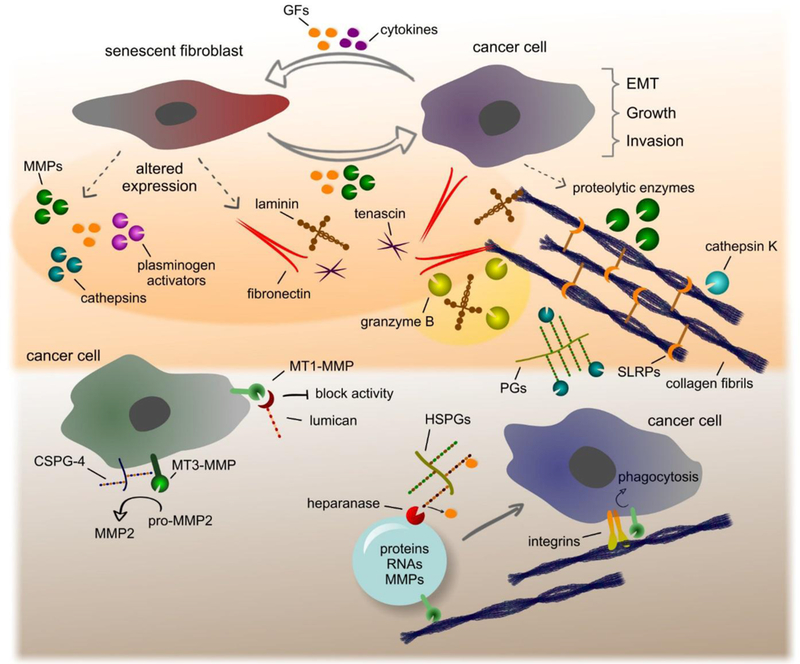
Schematic depiction of matrix modeling and remodeling driven by SASP and various proteolytic effectors including MMPs and cathepsins. Please consult the text for additional information.
As reviewed by Mavrogonatou et al., senescence-induced ECM remodeling is characterized by a reduced expression of important structural molecules including collagen, aggrecan, versican, decorin, and elastin—all essential matrix components for maintaining the proper biomechanical properties of tissues such as cartilage, skin, lungs and the vascular wall. The continuous presence of numerous proteolytic enzymes, inflammatory mediators and the expression of cross-linking enzymes such as lysyl oxidases (LOXs) increase matrix stiffness and subsequently establishes a vicious feedback cycle of ECM remodeling that gradually diminishes the physiological and homeostatic properties of the afflicted tissue (Fig. 1).
Matrix remodeling by proteolysis
Compounding the effects of SASP, various growth factors and cytokines induce epithelial to mesenchymal transition (EMT) as an important step towards local invasion and eventual systemic metastases. The composition of this “transformative” matrix is significantly enriched in adhesive proteins including fibronectin, laminin and matricellular proteins and catabolic enzymes such as matrix metalloproteinases (MMPs) that facilitate cancer cell migration and invasion. MMPs exhibit a variety of functions promoting EMT, signaling, migration and invasion [46], For example, MMP-1 and MMP-2 secreted by senescent fibroblasts trigger early EMT in keratinocytes, promoting the development of skin cancer [47], In another in vivo pre-clinical model, it was demonstrated that the beneficial effect of senescent fibroblasts on tumor development was alleviated by inhibiting the enzymatic activity of MMPs [48].
MMPs are key enzymes involved in ECM remodeling due to their ability to degrade various matrix components, cell surface receptors, cytokines and growth factors (Fig. 1). MMPs are zinc-dependent endopeptidases belonging to the metzincin superfamily of metalloproteinases that also includes their related families ADAMs and ADAMTs. MMPs are classified as collagenases, gelatinases, stromelysins, matrilysins, membrane type MMPs (MT-MMPs) and other MMPs according to their substrate specificity, homology, and domain organization [49].
MMPs are associated with disease development and progression including cancer, atherosclerosis, osteoarthritis and inflammation by affecting multiple signaling pathways. As reviewed by Hannocks et al. in this Special Issue, gelatinases MMP-2 and MMP-9 are key regulatory enzymes operative during neuroinflammation. It has been shown by in vivo and in vitro studies that both enzymes drive experimental autoimmune encephalopathy and subsume complementary functions in a murine model of the disease [50], Gelatinases MMP-2 and MMP-9 are secreted by immune and resident cells at the blood-brain barrier under the control of pro-inflammatory cytokines produced by recruited T-effector cells. Together, MMP-2 and MMP-9 promote the penetration of the outer parenchymal barrier by invading leukocytes. The elevated expression of MMP-2 and MMP-9 coordinates with pro-inflammatory cytokines to evoke the expression and modulate the activity of chemokines, thereby establishing a potent chemotactic gradient [51,52], MMPs also degrade ECM components and cell surface receptors such as β-dystroglycan from the astrocyte cell membrane, thus compromising parenchymal basement membrane attachment and reducing both their survival and the integrity of brain-blood barrier [50,51,53], Cleavage of cytokines and their receptors on the T-cell surface is postulated as a critical step for the prevalence of T-effector over T- ory cells for the inflammatory cascade.
The activity of MMPs is finely regulated by various mechanisms involving transcriptional regulation, activation by certain enzymes, inactivation by tissue inhibitors of MMPs (TIMPs) and α2-macroglobulin in serum and interactions with allosteric modulators [49], Initially, MMPs are synthesized as inactive proenzymes that are activated by proteolytic removal of their N-terminal pro-peptide. The activation of MMPs is mediated by plasmin, other MMPs, ADAMs, ADAMTs as well as an autocatalytic activation extracellularly and furin intracellularly [49], This process is critical for the function of MMPs and is thereby tightly regulated by several matrix constituents.
Proteoglycans (PGs) and glycosaminoglycans (GAGs) are among these regulatory molecules that interact with MMPs to modify their activity [54] (Fig. 1). A prime example is the interaction of MMP-2 with syndecan-2 on the cell surface that ultimately inhibits activation of pro-MMP-2 [55], On the other hand, MMP-2 forms a complex with CSPG-4 and MT3-MMP on the melanoma cell surface for pro-MMP-2 activation via MT3-MMP to evoke tumor cell invasion in collagen l-enriched matrices. This mode of activation requires the CS chains on CSPG-4 [56], Similarly, MMP-9 interacts with glypicans on the cell surface promoting colon cancer cell motility [57], Intriguingly, MMP-9 further interacts with matrix- localized serglycin and versican forming large, multimeric complexes that modulate pro-MMP-9 activation and target substrate binding [58], Lumican, a small leucine rich proteoglycan (SLRP), binds directly to the catalytic domain of MMP-14 (MT1-MMP) to inhibit its enzymatic activity. This binding is mediated by specific fragments of the lumican core protein [59,60], Lumican affects endothelial cell migration by regulating the expression and activity of MMP-9 and MT1-MMP by integrins [61]. The delicate interplay between MT1-MMP and lumican and their ensuing biological effects on tumorigenesis are elegantly presented by Pietraszek-Gremplewicz et al. in this Special Issue.
Independent of regulating collagen fibrillogenesis and mediating ECM organization, lumican exhibits potent anti-tumor effects by interfering with integrins and MT1-MMP at the cell membrane, inhibiting angiogenesis and tumor cell migration [59–62]. MT1-MMP localizes at lamellipodia forming complexes with CD44 and promoting tumor cell migration, invasion, and metastasis [63]. Co-localization of CD44 and MT1-MMP links both molecules to the actin cytoskeleton via the cytoplasmic tail of CD44, directing MT1-MMP to the migration front during cell migration [63]. MT1-MMP efficiently degrades a wide range of matrix components including collagens, fibronectin, laminins, proteoglycans, elastin and various cell surface receptors such as integrins, CD44, syndecan-1 and ICAM-1 [14]. Degradation of these targets are essential for pericellular remodeling and modulation of cell-matrix interactions [14].
MMPs play a central role in processing essential matrix components such as fibrillar collagens (Fig. 1). Collagen fibrils are widely distributed in all tissues providing unique mechanical properties and tensile strength. Their balanced synthesis and degradation is required for tissue homeostasis and imbalanced remodeling is recurrent in fibrosis, arthritis and cancer [64,65]. The tightly packed helical structure of collagen fibrils provide resistance against proteolytic degradation. The molecular pathways and enzymes involved in fibrillar collagen degradation are discussed by Sprangers and Everts in the corresponding review of this Special Issue. The ability of MMPs to degrade either intact or denatured collagen fibrils such as collagenases MMP-1, MMP-8, MMP-13, MT1-MMP, MT3-MMP and gelatinases MMP-2 and MMP-9 is presented. Extracellular degradation of collagen fibrils by MMPs is complemented with the action of other proteases including cathepsin K [66]. Apart from extracellular degradation, collagen fibrils are internalized by phagocytosis, a process dependent on integrins and MT1-MMP, and stored in phagosomes [67,68]. Soluble collagen fragments processed in the ECM are internalized by macropinocytosis and endocytosis that mechanistically requires urokinase plasminogen activator receptor associated protein (uPARAP/Endo180) and clathrin-coated vesicles [69]. Internalized collagen fibrils and fragments within vesicles are directed to the endosomal/lysosomal compartment and subsequently degraded by cysteine proteases.
Cysteine cathepsins are the fundamental proteases of the endolysosomal system and are increasingly implicated as extracellular matrix remodeling enzymes involved in numerous diseases [70] (Fig. 1). For instance, there are 11 papain-like cysteine cathepsins in humans that show specific and differential tissue expressions to accomplish their requisite biological functions. They act as acid proteases and possess biological activity within the slightly acidic microenvironment prevalent in diseases such as cancer, osteoarthritis, and osteoporosis [71]. In addition, the interaction of cathepsins with GAGs evokes stabilization and autoactivation at a neutral pH [70,72], Cathepsin-mediated ECM remodeling is reviewed by Vizovisek et al. in this Special Issue. The authors describe the structural and functional roles of cathepsins with emphasis on ECM remodeling and its relevance to disease progression. Indeed, dysregulated cathepsin expression and extracellular activity has been implicated in several cancer types, inflammatory diseases, arthritis, cardiovascular, and skeletal disorders.
The implication of other non-classical matrix proteases such as granzyme B in pathologic matrix remodeling with a focus on skin inflammation and related diseases is discussed by Turner and colleagues. Granzyme B belongs to a family of five serine proteases called granzymes (granule-secreted enzymes) in humans. It is synthesized by immune cells and non-immune cells alike (chondrocytes and keratinocytes) and secreted in the presence or absence of perforin. It can be internalized by target cells for apoptotic initiation following the cleavage of various substrates. A portion of granzyme B that is not internalized, is biologically active in the extracellular milieu [73].
Together, MMPs, cathepsins, and granzyme B are capable of degrading a wide variety of ECM components, junctional proteins, and cell surface receptors culminating in authoritarian control of cell-cell, cell-matrix interactions and downstream signaling [46,49,73–75] (Fig. 1). The degradation of ECM components upon remodeling also results in the release of bioactive fragments [76], These matrikines [9] exert their activity by interacting with specific cell surface receptors in multiple cell types to guide matrix re-organization, tissue architecture, and function. The concept of producing bioactive fragments via certain proteases, their functional roles, and their potential applications as drugs and biomarkers is elegantly highlighted by Ricard-Blum and Vallet. For example, bioactive fragments such as endorepellin and endostatin produced by cleavage of perlecan and collagen XVIII, respectively, bind to integrins and VEGFR1, thereby inhibiting angiogenesis and evoking autophagy [77,78], In contrast, lumikine, a proteolytically-liberated peptide derived from lumican, binds to ALK5/TGFβR1 to activate signaling and promote wound healing [79].
Remodeling on the fly: the rise of exosomes in ECM modeling
Recently, extracellular vesicles (EVs) have emerged as integral components of ECM that play central roles in matrix remodeling. This concept is thoroughly reviewed by Rilla et al. and Sanderson et al. in this Special Issue. EVs are released by all cell types and found ubiquitously in body fluids. They are classified into three different categories according their size and mode of biogenesis. The smaller in diameter, named exosomes (30–150 nm), arise from the endocytic pathway with the subsequent formation of multivesicular bodies [80], They are transported to plasma membrane and fused to release exosomes to the ECM. Other larger extracellular vesicles such as microvesicles (100–1000 nm) and apoptotic bodies (1–5 μm) are derived from the direct budding of plasma membrane [80], EVs, especially exosomes, play fundamental roles in cell-cell communication as they transfer a plethora of chemical signals to neighboring cells that directly modulate their behavior [80], For example, nucleic acids (mRNAs, miRNAs, and non-coding RNAs), matrix proteins, growth factors, cytokines, enzymes and lipids are components of exosomes that affect cell differentiation, proliferation, migration, and matrix synthesis. The surface of exosomes contains numerous cell surface receptors including cell surface proteoglycans, integrins, and the specific enzymes that mediate their interaction with ECM molecules and cellular uptake [81], Exosomes are involved in ECM organization/re-organization that occurs under normal and pathological circumstances. A plethora of miRNAs influences the expression of matrix components involved in wound healing and matrix remodeling in various diseases [82], Exosomes derived from different cellular sources also regulate wound healing by promoting fibroblasts proliferation, migration, matrix biogenesis, and the release of inflammatory mediators. EVs transfer several matrix-degrading enzymes including MMPs and cathepsins that remodel the ECM [83,84], In addition, they induce the expression and release of proteolytic enzymes in target cells to induce matrix degradation. For instance, microvesicles enriched in EMMPRIN/CD147 are released by tumor cells to stimulate the expression of MMP-2 in stromal fibroblasts [85], Enzymes which are involved in matrix remodeling are also located on EV surfaces and are able to act directly on their matrix substrates. Lysyl oxidase-like 2 is enzymatically active on the vesicular surface and catalyzes the crosslink of collagen fibrils in the ECM [86], Increased tissue stiffness occurs in remodeled tissues in various diseases including cancer and is associated with disease progression [64], Also on vesicular surfaces, MT1-MMP degrades fibrillar collagens among other matrix components and is a potent regulator of cell migration and cancer cell invasion and metastasis [87,88], Glycosidases such as sialidase and heparanase are components of the exosomal surface and are involved in the removal of sialic acid from cell surface components and degradation of HS chains from HSPGs [89,90], Heparanase acts on various matrix and cell surface HSPGs to promote cell migration either via liberation of HS-sequestered growth factors and cytokines within the matrix or by modulating cell-matrix interactions [91], Notably, heparanase trimming of HS chains on syndecan stimulates exosome biogenesis by modulating the function of the syndecan-syntenin-ALIX complex [92], Heparanase up-regulation induced by chemotherapy treatment in multiple myeloma patients evokes exosome secretion, further supporting the role of the heparanase-syndecan axis on exosome biogenesis [89].
Proteoglycans as interior designers for the matrix
Basement membranes are chiefly composed of four requisite components: laminin [93], collagen IV [94], nidogen, and the heparan sulfate proteoglycan (HSPG) perlecan [95,96], The biology and contributions of collagen XVIII, a related basement HSPG implicated in tissue homeostasis and dysfunction, has been recently reviewed [97], Unbiased quantitative proteomic definitions of basement membrane compositions [98] and extracellular matrices [99,100] are leading an “-omics” revolution in understanding the functional contributions of basement membrane in associated diseases [101–103], development [104,105] and potentially as viable cell-based therapeutic modalities [106], Perlecan and related components [107] are critical for maintaining the structural integrity of cartilage and assembly of basement membrane [108], while maintaining sufficient plasticity for remodeling as the organism grows and develops over time. Indeed, embryo implantation triggers dynamic expression of the basement membrane toolkit [109].
The core regulatory mechanism is found in generating biomechanical forces that is sensed by and ultimately influences cell function, and vice-versa as discussed by Mrkonjic and colleagues in this Special Issue. The aggregate effect of basement membrane protein deposition (modeling) and degradation (remodeling) controls cellular and tissue stiffness as determined by atomic force microscopy, tissue plasticity [110], organ shape, and ligand retention by Ramos-Lewis et al. in this Special Issue. Reciprocal signaling, such as that mediated by DDR1 for collagen IV synthesis, is also necessary to maintain properties of the basement membranes [111,112], This functional paradigm for basement membrane synthesis, deposition, and remodeling is aptly and thoroughly demonstrated in the model organism Drosophila melanogaster and its step-wise assembly process is elegantly presented in the review of Ramos-Lewis and colleagues (Fig. 2). The subsequent examination of the wing disc basement membrane provides a microcosm for discerning the roles of cellular compression [113] processes and ligand retention for developing biological systems, which is especially applicable for stem and progenitor cell biology. The mechanisms gleaned from this invertebrate model organism is significantly advancing our knowledge regarding the subtle nuances of basement membrane intricacies and architecture and the roles that proteoglycans subsume.
Fig. 2.
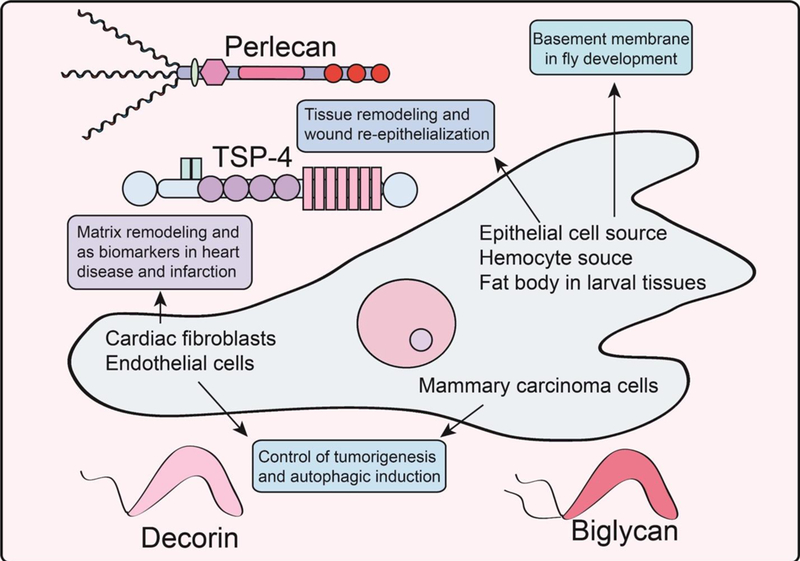
Schematic representation of various roles discovered for proteoglycans in regulating development, as biomarkers in adverse cardiac events, and in controlling tumorigenesis and catabolism. Please refer to the text for additional information.
The next major portion of articles deals with cutaneous wound healing which comprises three distinct phases of inflammation, re-epithelization, and tissue remodeling. The recurring theme of matrix remodeling is prevalent within all four continuous processes: hemostasis, inflammation, proliferation, and maturation [114], This thene is highlighted, largely in sequence, by recent advances in the role of CD44 in modulating inflammation, re-epithelialization, and thrombospondin-4 activity in tissue remodeling, all found within reviews by Govindaraju et al., Rousselle et al., and Stenina-Adognravi et al., respectively (Fig. 2).
During wound healing, deposition of abundant of fibrillar cartilage maintains tissue and tendon biomechanics [23,25,26,115,116] while forming scar tissue, impairing normal tissue function and elastoplasticity. Dissecting the molecular and cellular processes governing this form of ECM deposition and remodeling are critical towards efforts to prevent fibrotic scarring. It has recently been demonstrated that CD44, the hyaluronan receptor, functions in a heretofore unknown pathway for wound healing. As reviewed by Govindaraju et al., mice lacking CD44 have increased levels of inflammatory markers with ablated fibrogenic responses during the initial injury response stage of wound healing. At later stages, as the wound is closing, proteolytic degradation of the deposited collagen is significantly diminished, resulting in increased retention times of the fibrillar collagen post closure, ultimately manifesting in a more severe scarring phenotype.
One of the most critical processes of mammalian wound healing is re-epithelization, which is considered the second step under proliferation, with defects resulting in chronic wounds and pressure ulcers [65]. Several ECM-derived factors are deposited, and the precise composition of this milieu is influenced by the local wound microenvironment, involving cytokines, growth factors, and MMPs [117]. Following wound injury, provisional matrix is deposited for proficient tissue repair at approximately 3 days to 1 week by granular fibroblasts and is characterized by the temporal synthesis of key effectors including fibronectin isoforms that incorporate the EDA and EDB domains, fibronectin species lacking these domains, and fibrillar and non-fibrillar collagens (I, V, VI, VII, XVIII) [118,119]. Recent studies, as discussed by Rousselle et al., have determined precise functions for each of the EDA and EDB domains as critical for the recruitment of collagen I and an assortment of matricellular proteins, such thrombospondin (TSP), within the wound ECM (see below). As described above, a major constituent of basement membranes is the HSPG perlecan [120]. Structurally, perlecan augments cellular adhesion and co-localizes with laminins, specifically laminin 322, to further stabilize keratinocyte attachment. Primarily, perlecan functions as a repository for HS-binding growth factors whereupon degradation of the its HS chains or perlecan itself releases the pleiotropic growth factor progranulin [121,122] as well as other mitogens and trophic factors necessary for the reparative process. Supporting this concept, transgenic mice lacking these HS chains have delayed wound healing and decreased vascular density, despite maintaining wound closure rates [123].
Intriguingly, both collagen XVIII and perlecan harbor proteolytically sensitive C-termini, [124] in the form of endostatin [125] and endorepellin [126–133], respectively, that acts to fine-tune angiogenesis [77]. Recently, both anti-angiogenic fragments have been implicated in coordinating the evolutionarily-conversed catabolic process of autophagy [78,134,135]. Intriguingly, there appears to be a fundamental antithetical correlation between autophagic activation and angiogenesis insofar as pro-autophagic molecules possess anti-angiogenic properties [95,136,137] and vice versa. This concept is further underscored by transgenic mice expressing HS-less perlecan; perlecan no longer exhibits angiogenic bivalency (pro- and anti-angiogenic cues embedded within the same molecule) and only contains anti- angiogenic signals via C-terminal endorepellin. The form of perlecan sans the HS chains results in a less favorable angiogenic wound healing environment characterized by decreased granularity and vascularization. Therefore, proteoglycans, such as collagen XVIII and perlecan, may exert their roles in wound healing by controlling autophagy as an under-appreciated contributor to wound healing.
In contrast to the anti-angiogenic modalities inherent to the basement membrane HSPGs, collagen XVIII and perlecan, as well as to other thrombospondin family members, TSP-4 promotes angiogenesis. Expressed throughout embryonic development and within the adult organism in the osteogenic mesenchyme, tendons, skeletal and nervous tissues as well as the eye and cardiovascular tissue, TSP-4 performs several other key roles in opposition to TSP-1 and TSP-2. Specifically, TSP-4 reduces fibrosis and collagen production. As an evolutionarily conserved multi-modular molecule, TSP4 subsumes a critical role as an organizational factor for the ECM and provides structural support. Being a proangiogenic factor, TSP-4 has been implicated in cancer due to high levels of expression and its subsequent association to cancer progression, particularly facilitating progression and invasion as described by Stenina-Adognravi et al. However, the precise role of TSP-4 is uncertain as studies have demonstrated both tumor-promoting and tumor-inhibiting properties, depending on the tissue of origin.
The role of proteoglycans in remodeling the cardiovascular system has long been underappreciated; however, this emerging field is spotlighted within this Special Issue in two elegantly written reviews by Nielson et al. and Christensen et al. in this Special Issue. Cardiovascular diseases are primarily characterized by extensive (and aberrant) ECM remodeling over decades of life, leading to fibrotic scarring and inevitable heart failure. Importantly, SLRPs, including decorin, biglycan, and lumican, have surfaced as vital regulators of this process as well as membrane-localized proteoglycans including glypican and syndecans. In this context, they function as receptors to regulate cardiac fibroblast signaling and may represent therapeutic targets or biomarkers. In the same vein, ECM components are considered as biomarkers for myocardial infarction of the left ventricle to predict patient outcome (Fig. 2).
Proteoglycans also exert major roles as key molecular determinants throughout tumorigenesis and angiogenesis as comprehensively reviewed by Theocharis and Karamanos in this Special Issue. Proteoglycans are able to remodel the tumor stroma in a cell- and context-specific manner that can generate a provisional matrix for tumorigenic growth and drug resistance. Recently, it was discovered that decorin, the prototypical member of the SLRP family, evokes endothelial autophagy and tumor cell mitophagy, as reviewed by Buraschi et al. in this Special Issue (Fig. 2). We found that decorin evokes excessive flux through this conserved catabolic process under nutrient-rich conditions downstream of receptor tyrosine kinase activity, chiefly VEGFR2 and Met for endothelial cells and breast carcinoma, respectively. Under these specific circumstances, autophagic regulation is considered “non-canonical”. Engagement of the autophagic or mitophagic machinery relied on two novel effectors, Peg3 or mitostatin, respectively. These proteins represent a novel, matrix-regulated nexus for augmenting cellular catabolism and organelle clearance. The resultant effects of pro-autophagic/pro-mitophagic process underlies the molecular versatility of decorin as an anti-tumorigenic and angiostatic factor.
Highlights.
During development and organ pathology, the extracellular matrix undergoes a dramatic restructuring in composition, form and functionality.
Modeling and remodeling of the matrix are driven by the local cellular milieu and secreted components via a framework of dynamic reciprocity.
We will critically address key concepts related to the role of extracellular matrix modeling and remodeling in various disease processes.
Acknowledgments
This work was supported, in part, by National Institutes of Health Grants, CA39481, and CA47282 (R.V.I.). We wish to thank all past and present members of our laboratories and Carolyn Chen for critically reading of our manuscript.
Abbreviations
- ECM
extracellular matrix
- EMT
epithelial to mesenchymal transition
- EV
extracellular vesicle
- HSPG
heparan sulfate proteoglycan
- LOX
lysyl oxidase
- MMP
matrix metalloprotease
- MT-MMP
membrane type MMP
- SASP
senescence-associated secretory phenotype
- SLRP
small leucine rich proteoglycan
- TGF-β
transforming growth factor β
- TSP
thrombospondin
Footnotes
Conflicts of interest
The author declares no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Iozzo RV, Moscatello D, McQuillan DJ, Eichstetter I, Decorin is a biological ligand for the epidermal growth factor receptor, J. Biol. Chem. 274 (1999) 4489–4492. [DOI] [PubMed] [Google Scholar]
- [2].Goldoni S, Humphries A, Nyström A, Sattar S, Owens RT, McQuillan DJ et al. , Decorin is a novel antagonistic ligand of the Met receptor, J. Cell Biol. 185 (2009) 743–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Goldoni S, Owens RT, McQuillan DJ, Shriver Z, Sasisekharan R, Birk DE et al. , Biologically active decorin is a monomer in solution, J. Biol. Chem. 279 (2004) 6606–6612. [DOI] [PubMed] [Google Scholar]
- [4].Ferdous Z, Wei VM, Iozzo RV, Höök M, Grande-Allen KJ, Decorin-transforming growth factor-β interaction regulates matrix organization and mechanical characteristics of three-dimensional collagen matrices, J. Biol. Chem. 282 (2007) 35887–35898. [DOI] [PubMed] [Google Scholar]
- [5].Robertson IB, Horiguchi M, Zilberberg L, Dabovic B, Hadjiolova K, Rifkin DB, Latent TGF-β-binding proteins, Matrix Biol. 47 (2015) 44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gubbiotti MA, Vallet SD, Ricard-Blum S, Iozzo RV, Decorin interacting network: A comprehensive analysis of decorin-binding partners and their versatile functions, Matrix Biol 55 (2016) 7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kessenbrock K, Wang C-Y, Werb Z, Matrix metalloproteinases in stem cell mobilization, Matrix Biol. 44–46 (2015) 184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Arpino V, Brock M, Gill SE, The role of TIMPs in regulation of extracellular matrix proteolysis, Matrix Biol. 44–46 (2015) 247–254. [DOI] [PubMed] [Google Scholar]
- [9].Wells JM, Gaggar A, Blalock JE, MMP generated matrikines, Matrix Biol. 44–46 (2015) 122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Vadon-Le Goff S, Hulmes DJ, Moali C, BMP-1/tolloid-like proteinases synchronize matrix assembly with growth factor activation to promote morphogenesis and tissue remodeling, Matrix Biol. 44–46 (2015) 14–23. [DOI] [PubMed] [Google Scholar]
- [11].Deryugina EI, Quigley JP, Tumor angiogenesis: MMP-mediated induction of intravasation- and metastasis-sustaining neovasculature, Matrix Biol. 44–46 (2015) 94–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Apte SS, Parks WC, Metalloproteinases: A parade of functions in matrix biology and an outlook for the future, Matrix Biol. 44–46 (2015) 1–6. [DOI] [PubMed] [Google Scholar]
- [13].Rohani MG, Parks WC, Matrix remodelling by MMPs during wound repair, Matrix Biol. 44–46 (2015) 113–121. [DOI] [PubMed] [Google Scholar]
- [14].ltoh Y, Membrane-type matrix metalloproteinases: Their functions and regulations, Matrix Biol 44–46 (2015) 207–223. [DOI] [PubMed] [Google Scholar]
- [15].Hubmacher D, Apte SS, ADAMTS proteins as modulators of microfibril formation and function, Matrix Biol. 47 (2015) 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Scilabra SD, Yamamoto K, Pigoni M, Sakamoto K, Muller SA, Papadopoulou A et al. , Dissecting the interaction between tissue inhibitor of metalloproteinases-3 (TIMP-3) and low density lipoprotein receptor-related protein-1 (LRP-1): Development of a “TRAP” to increase levels of TIMP-3 in the tissue, Matrix Biol 59 (2016) 69–79. [DOI] [PubMed] [Google Scholar]
- [17].Alvarez RJ, Sun MJ, Haverty TP, Iozzo RV, Myers JC, Neilson EG, Biosynthetic and proliferative characteristics of tubulointerstitial fibroblasts probed with paracrine cytokines, Kidney Int. 41 (1992) 14–23. [DOI] [PubMed] [Google Scholar]
- [18].Bi Y, Stueltens CH, Kilts T, Wadhwa S, Iozzo RV, Robey PG et al. , Extracellular matrix proteoglycans control the fate of bone marrow stromal cells, J. Biol. Chem. 280 (2005) 30481–30489. [DOI] [PubMed] [Google Scholar]
- [19].Bi X, Tong C, Dokendorff A, Banroft L, Gallagher L, Guzman-Hartman G et al. , Genetic deficiency of decorin causes intestinal tumor formation through disruption of intestinal cell maturation, Carcinogenesis 29 (2008) 1435–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bi X, Pohl NM, Yang GR, Gou Y, Guzman G, Kajdacsy-Balla A et al. , Decorin-mediated inhibition of colorectal cancer growth and migration is associated with E-cadherin in vitro and in mice, Carcinogenesis 33 (2012) 326–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Neill T, Schaefer L, Iozzo RV, Decorin, a guardian from the matrix, Am. J. Pathol. 181 (2012) 380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Neill T, Painter H, Buraschi S, Owens RT, Lisanti MP, Schaefer L et al. , Decorin antagonizes the angiogenic network. Concurrent inhibition of Met, hypoxia inducible factor-1 α and vascular endothelial growth factor A and induction of thrombospondin-1 and TIMP3, J. Biol. Chem. 287 (2012) 5492–5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chen S, Young MF, Chakravarti S, Birk DE, Interclass small leucine-rich repeat proteoglycan interactions regulate collagen fibrillogenesis and corneal stromal assembly, Matrix Biol. 35 (2014) 103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Reese SP, Underwood CJ, Weiss JA, Effects of decorin proteoglycan on fibrillogenesis, ultrastructure, and mechanics of type I collagen gels, Matrix Biol. 32 (2013) 414–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dunkman AA, Buckley MR, Mienaltowski MJ, Adams SM, Thomas SJ, Satchell L et al. , Decorin expression is important for age-related changes in tendon structure and mechanical properties, Matrix Biol. 32 (2013) 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Dunkman AA, Buckley MR, Mienaltowski MJ, Adams SM, Thomas SJ, Kumar A et al. , The injury response of aged tendons in the absence of biglycan and decorin, Matrix Biol. 35 (2014) 232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Robinson PS, Lin TW, Reynolds PR, Derwin KA, Iozzo RV, Soslowsky LJ, Strain-rate sensitive mechanical properties of tendon fascicles from mice with genetically engineered alterations in collagen and decorin, J. Biomech. Eng 126 (2004) 252–257. [DOI] [PubMed] [Google Scholar]
- [28].Iozzo RV, Chakrani F, Perrotti D, McQuillan DJ, Skorski T, Calabretta B et al. , Cooperative action of germline mutations in decorin and p53 accelerates lymphoma tumorigenesis, Proc. Natl. Acad. Sci. USA 96 (1999) 3092–3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Iozzo RV, Schaefer L, Proteoglycan form and function: A comprehensive nomenclature of proteoglycans, Matrix Biol. 42 (2015) 11–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Neill T, Schaefer L, Iozzo RV, Decorin as a multivalent therapeutic agent against cancer, Adv. Drug Deliv. Rev. 97 (2016) 174–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Neill T, Sharpe C, Owens RT, Iozzo RV, Decorin-Evoked Paternally Expressed Gene 3 (PEG3) is an Upstream Regulator of the Transcription Factor EB (TFEB) in Endothelial Cell Autophagy, J. Biol Chem. 292 (2017) 16211–16220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Neill T, Andreuzzi E, Wang Z-X, Peiper SC, Mongiat M, Iozzo RV, Endorepellin remodels the endothelial transcriptome toward a pro-autophagic and pro-mitophagic gene signature, J. Biol. Chem. 293 (2018) 12137–12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bouris P, Manou D, Sopaki-Valalaki A, Kolokotroni A, Moustakas A, Kapoor A et al. , Serglycin promotes breast cancer cell aggressiveness: Induction of epithelial to mesenchymal transition, proteolytic activity and IL-8 signaling, Matrix Biol2018). [DOI] [PubMed] [Google Scholar]
- [34].Karalis TT, Heldin P, Vynios DH, Neill T, Buraschi S, Iozzo RV et al. , Tumor-suppressive functions of 4-MU on breast cancer cells of different ER status: Regulation of hyaluronan/HAS2/CD44 and specific matrix effectors, Matrix Biol2018). [DOI] [PubMed] [Google Scholar]
- [35].Frey T, Schroeder N, Manon-Jensen T, Iozzo RV, Schaefer L, Biological interplay between proteoglycans and their innate immune receptors in inflammation, FEBS J. 280 (2013) 2165–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Iozzo RV, Gubbiotti MA, Extracellular matrix: The driving force of mammalian diseases, Matrix Biol2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Campisi J, d’Adda di FF, Cellular senescence: when bad things happen to good cells, Nat. Rev. Mol. Cell Biol 8 (2007) 729–740. [DOI] [PubMed] [Google Scholar]
- [38].Davalos AR, Coppe JP, Campisi J, Desprez PY, Senescent cells as a source of inflammatory factors for tumor progression, Cancer Metastasis Rev. 29 (2010) 273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Herranz N, Gil J, Mechanisms and functions of cellular senescence, J.Clin. Invest 128 (2018) 1238–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Funk WD, Wang CK, Shelton DN, Harley CB, Pagon GD, Hoeffler WK, Telomerase expression restores dermal integrity to in vitro-aged fibroblasts in a reconstituted skin model, Exp. Cell Res. 258 (2000) 270–278. [DOI] [PubMed] [Google Scholar]
- [41].Patel S, Maheshwari A, Chandra A, Biomarkers for wound healing and their evaluation, J.Wound. Care 25 (2016) 46–55. [DOI] [PubMed] [Google Scholar]
- [42].Ashraf S, Cha BH, Kim JS, Ahn J, Han I, Park H et al. , Regulation of senescence associated signaling mechanisms in chondrocytes for cartilage tissue regeneration, Osteoarthritis. Cartilage. 24 (2016) 196–205. [DOI] [PubMed] [Google Scholar]
- [43].Huet F, Akodad M, Fauconnier J, Lacampagne A, Roubille F, Anti-inflammatory drugs as promising cardiovascular treatments, Expert. Rev. Cardiovasc. Ther. 15 (2017) 109–125. [DOI] [PubMed] [Google Scholar]
- [44].Setrerrahmane S, Xu H, Tumor-related interleukins: old validated targets for new anti-cancer drui development, Mol. Cancer 16 (2017) 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Schosserer M, Grillari J, Breitenbach M, The Dual Role of Cellular Senescence in Developing Tumors and Their Response to Cancer Therapy, Front Oncol. 7 (2017) 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Piperigkou Z, Manou D, Karamanou K, Theocharis AD, Strategies to Target Matrix Metalloproteinases as Therapeutic Approach in Cancer, Methods Mol. Biol 1731 (2018) 325–348. [DOI] [PubMed] [Google Scholar]
- [47].Malaquin N, Vercamer C, Bouali F, Martien S, Deruy E, Wernert N et al. , Senescent fibroblasts enhance early skin carcinogenic events via a paracrine MMP-PAR-1 axis, PLoS. One. 8 (2013) e63607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Liu D, Hornsby PJ, Senescent human fibroblasts increase the early growth of xenograft tumors via matrix metalloproteinase secretion, Cancer Res. 67 (2007) 3117–3126. [DOI] [PubMed] [Google Scholar]
- [49].Gialeli C, Theocharis AD, Karamanos NK, Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting, FEBS J. 278 (2011) 16–27. [DOI] [PubMed] [Google Scholar]
- [50].Agrawal S, Anderson P, Durbeej M, van Rooijen N, Ivars F, Opdenakker G et al. , Dystroglycan is selectively cleaved at the parenchymal basement membrane at sites of leukocyte extravasation i experimental autoimmune encephalomyelitis, J. Exp. Med. 203 (2006) 1007–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Song J, Wu C, Korpos E, Zhang X, Agrawal SM, Wang Y et al. , Focal MMP-2 and MMP-9 activity at the blood-brain barrier promotes chemokine-induced leukocyte migration, Cell Rep. 10 (2015) 1040–1054. [DOI] [PubMed] [Google Scholar]
- [52].Sorokin L, The impact of the extracellular matrix on inflammation, Nat. Rev. Immunol. 10 (2010) 712–723. [DOI] [PubMed] [Google Scholar]
- [53].Wu C, Ivars F, Anderson P, Hallmann R, Vestweber D, Nilsson P et al. , Endothelial basement membrane laminin alpha5 selectively inhibits T lymphocyte extravasation into the brain, Nat. Me 15 (2009) 519–527. [DOI] [PubMed] [Google Scholar]
- [54].Theocharis AD, Gialeli C, Bouris P, Giannopoulou E, Skandalis SS, Aletras AJ et al. , Cell-matrix interactions: focus on proteoglycan-proteinase interplay and pharmacological targeting in cancer, FEBS J. 281 (2014) 5023–5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Munesue S, Yoshitomi Y, Kusano Y, Koyama Y, Nishiyama A, Nakanishi H et al. , A novel function of syndecan-2, suppression of matrix metalloprotease-2 activation, which causes suppression of metastasis, J. Biol. Chem. 282 (2007) 28164–28174. [DOI] [PubMed] [Google Scholar]
- [56].Iida J, Wilhelmson KL, Ng J, Lee P, Morrison C, Tam E et al. , Cell surface chondroitin sulfate glycosaminoglycan in melanoma: role in the activation of pro-MMP-2 (pro-gelatinase A), Biochem. J. 403 (2007) 553–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Koyama Y, Naruo H, Yoshitomi Y, Munesue S, Kiyono S, Kusano Y et al. , Matrix metalloproteinase-9 associated with heparan sulphate chains of GPI-anchored cell surface proteoglycans mediates motility of murine colon adenocarcinoma cells, J. Biochem. 143 (2008) 581–592. [DOI] [PubMed] [Google Scholar]
- [58].Malla N, Berg E, Theocharis AD, Svineng G, Uhlin-Hansen L, Winberg JO, In vitro reconstitution of complexes between pro-matrix metalloproteinase-9 and the proteoglycans serglycin and versican, FEBS J. 280 (2013) 2870–2887. [DOI] [PubMed] [Google Scholar]
- [59].Pietraszek K, Chatron-Colliet A, Brezillon S, Perreau C, Jakubiak-Augustyn A, Krotkiewski H et al. , Lumican: A new inhibitor of matrix metalloproteinase-14 activity, FEBS Lett. 588 (2014) 4319–4324. [DOI] [PubMed] [Google Scholar]
- [60].Stasiak M, Boncela J, Perreau C, Karamanou K, Chatron-Colliet A, Proult I et al. , Lumican Inhibits SNAIL-Induced Melanoma Cell Migration Specifically by Blocking MMP-14 Activity, PLoS. One. 11 (2016) e0150226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Niewiarowska J, Brezillon S, Sacewicz-Hofman I, Bednarek R, Maquart FX, Malinowski M et al. , Lumican inhibits angiogenesis by interfering with alpha2beta1 receptor activity and downregulating MMP-14 expression, Thromb. Res. 128 (2011) 452–457. [DOI] [PubMed] [Google Scholar]
- [62].Zeltz C, Brezillon S, Kapyla J, Eble JA, Bobichon H, Terryn C et al. , Lumican inhibits cell migration through α2β1 integrin, Exp. Cell Res. 316 (2010) 2922–2931. [DOI] [PubMed] [Google Scholar]
- [63].Mori H, Tomari T, Koshikawa N, Kajita M, ltoh Y, Sato H et al. , CD44 directs membrane-type 1 matrix metalloproteinase to lamellipodia by associating with its hemopexin-like domain, EMBO J. 21 (2002) 3949–3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Theocharis AD, Skandalis SS, Gialeli C, Karamanos NK, Extracellular matrix structure, Adv. Drug Deliv. Rev. 97 (2016) 4–27. [DOI] [PubMed] [Google Scholar]
- [65].Wells A, Nuschke A, Yates CC, Skin tissue repair: Matrix microenvironmental influences, Matrix Biol 49 (2016) 25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Aguda AH, Panwar P, Du X, Nguyen NT, Brayer GD, Bromme D, Structural basis of collagen fiber degradation by cathepsin K, Proc. Natl. Acad. Sci. U. S. A 111 (2014) 17474–17479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Woltersdorf C, Bonk M, Leitinger B, Huhtala M, Kapyla J, Heino J et al. , The binding capacity of alphalbetal-, alpha2beta1- and alpha10beta1-integrins depends on non-collagenous surface macromolecules rather than the collagens in cartilage fibrils, Matrix Biol 63 (2017) 91–105. [DOI] [PubMed] [Google Scholar]
- [68].Lee H, Overall CM, McCulloch CA, Sodek J, A critical role for the membrane-type 1 matrix metalloproteinase in collagen phagocytosis, Mol. Biol Cell 17 (2006) 4812–4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Madsen DH, Jurgensen HJ, lngvarsen S, Melander MC, Vainer B, Egerod KL et al. , Endocytic collagen degradation: a novel mechanism involved in protection against liver fibrosis, J. Pathol. 227 (2012) 94–105. [DOI] [PubMed] [Google Scholar]
- [70].Fonovic M, Turk B, Cysteine cathepsins and extracellular matrix degradation, Biochim. Biophys. Acta 1840 (2014) 2560–2570. [DOI] [PubMed] [Google Scholar]
- [71].Turk V, Stoka V, Vasiljeva O, Renko M, Sun T, Turk B et al. , Cysteine cathepsins: from structure, function and regulation to new frontiers, Biochim. Biophys. Acta 1824 (2012) 68–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Almeida PC, Nantes IL, Chagas JR, Rizzi CC, Faljoni-Alario A, Carmona E et al. , Cathepsin B activity regulation. Heparin-like glycosaminogylcans protect human cathepsin B from alkaline pH- induced inactivation, J. Biol Chem. 276 (2001) 944–951. [DOI] [PubMed] [Google Scholar]
- [73].Boivin WA, Cooper DM, Hiebert PR, Granville DJ, Intracellular versus extracellular granzyme B in immunity and disease: challenging the dogma, Lab Invest 89 (2009) 1195–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Boivin WA, Shackleford M, Hoek AV, Zhao H, Hackett TL, Knight DA et al. , Granzyme B cleaves decorin, biglycan and soluble betaglycan, releasing active transforming growth factor-β1, PLoS ONE 7 (2012) e33163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Kramer L, Turk D, Turk B, The Future of Cysteine Cathepsins in Disease Management, Trends Pharmacol. Sci. 38 (2017) 873–898. [DOI] [PubMed] [Google Scholar]
- [76].Ricard-Blum S, Vallet SD, Proteases decode the extracellular matrix cryptome, Biochimie 122 (2016) 300–313. [DOI] [PubMed] [Google Scholar]
- [77].Poluzzi C, Iozzo RV, Schaefer L, Endostatin and endorepellin: A common route of action for similar angiostatic cancer avengers, Adv. Drug Deliv. Rev. 97 (2016) 156–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Poluzzi C, Casulli J, Goyal A, Mercer TJ, Neill T, Iozzo RV, Endorepellin evokes autophagy in endothelial cells, J. Biol. Chem. 289 (2014) 16114–16128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Gesteira TF, Coulson-Thomas VJ, Yuan Y, Zhang J, Nader HB, Kao WW, Lumican Peptides: Rational Design Targeting ALK5/TGFBRI, Sci. Rep. 7 (2017) 42057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Sedgwick AE, D’Souza-Schorey C, The biology of extracellular microvesicles, Traffic. 19 (2018) 319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Groth E, Pruessmeyer J, Babendreyer A, Schumacher J, Pasqualon T, Dreymueller D et al. , Stimulated release and functional activity of surface expressed metalloproteinase ADAM 17 in exosomes, Biochim. Biophys. Acta 1863 (2016) 2795–2808. [DOI] [PubMed] [Google Scholar]
- [82].Piperigkou Z, Gotte M, Theocharis AD, Karamanos NK, Insights into the key roles of epigenetics in matrix macromolecules-associated wound healing, Adv. Drug Deliv. Rev. 129 (2018) 16–36. [DOI] [PubMed] [Google Scholar]
- [83].Giusti I, D’Ascenzo S, Millimaggi D, Taraboletti G, Carta G, Franceschini N et al. , Cathepsin B mediates the pH-dependent proinvasive activity of tumor-shed microvesicles, Neoplasia. 10 (2008) 481–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Shimoda M, Khokha R, Proteolytic factors in exosomes, Proteomics. 13 (2013) 1624–1636. [DOI] [PubMed] [Google Scholar]
- [85].Milia-Argeiti E, Mourah S, Vallee B, Huet E, Karamanos NK, Theocharis AD et al. , EMMPRIN/CD147-encriched membrane vesicles released from malignant human testicular germ cells increase MMP production through tumor-stroma interaction, Biochim. Biophys. Acta 1840 (2014) 2581–2588. [DOI] [PubMed] [Google Scholar]
- [86].de Jong OG, van Balkom BW, Gremmels H, Verhaar MC, Exosomes from hypoxic endothelial cells have increased collagen crosslinking activity through up-regulation of lysyl oxidase-like 2, J. Cell Mol. Med. 20 (2016) 342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Hakulinen J, Sankkila L, Sugiyama N, Lehti K, Keski-Oja J, Secretion of active membrane type 1 matrix metalloproteinase (MMP-14) into extracellular space in microvesicular exosomes, J. Cell Biochem. 105 (2008) 1211–1218. [DOI] [PubMed] [Google Scholar]
- [88].Paterson EK, Courtneidge SA, Invadosomes are coming: new insights into function and disease relevance, FEBS J. 285 (2018) 8–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Bandari SK, Purushothaman A, Ramani VC, Brinkley GJ, Chandrashekar DS, Varambally S et al. , Chemotherapy induces secretion of exosomes loaded with heparanase that degrades extracellular matrix and impacts tumor and host cell behavior, Matrix Biol 65 (2018) 104–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Sumida M, Hane M, Yabe U, Shimoda Y, Pearce OM, Kiso M et al. , Rapid Trimming of Cell Surface Polysialic Acid (PolySia) by Exovesicular Sialidase Triggers Release of Preexisting Surface Neurotrophin, J. Biol Chem. 290 (2015) 13202–13214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Sanderson RD, Elkin M, Rapraeger AC, Ilan N, Vlodavsky I, Heparanase regulation of cancer, autophagy and inflammation: New mechanisms and targets for therapy, FEBS J. 284 (2016) 42–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Roucourt B, Meeussen S, Bao J, Zimmermann P, David G, Heparanase activates the syndecan- syntenin-ALIX exosome pathway, Cell Res. 25 (2015) 412–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Di Russo J, Hannocks MJ, Luik AL, Song J, Zhang X, Yousif L et al. , Vascular laminins in physiology and pathology, Matrix Biol 57–58 (2017) 140–148. [DOI] [PubMed] [Google Scholar]
- [94].Jeanne M, Gould DB, Genotype-phenotype correlations in pathology caused by collagen type IV alpha 1 and 2 mutations, Matrix Biol, In Press2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Gubbiotti MA, Neill T, Iozzo RV, A current view of perlecan in physiology and pathology: A mosaic of functions, Matrix Biol 57–58 (2017) 285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Iozzo RV, Zoeller JJ, Nystrom A, Basement membrane proteoglycans: Modulators par excellence of cancer growth and angiogenesis, Mol. Cells 27 (2009) 503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Heljasvaara R, Aikio M, Ruotsalainen H, Pihlajaniemi T, Collagen XVIII in tissue homeostasis and dysregulation - Lessons learned from model organisms and human patients, Matrix Biol 57–58 (2017) 55–75. [DOI] [PubMed] [Google Scholar]
- [98].Randles MJ, Humphries MJ, Lennon R, Proteomic definitions of basement membrane composition in health and disease, Matrix Biol 57–58 (2017) 12–28. [DOI] [PubMed] [Google Scholar]
- [99].Naba A, Clauser KR, Ding H, Whittaker CA, Carr SA, Hynes RO, The extracellular matrix: Tools and insights for the “omics” era, Matrix Biol. 49 (2016) 10–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Naba A, Clauser KR, Mani DR, Carr SA, Hynes RO, Quantitative proteomic profiling of the extracellular matrix of pancreatic islets during the angiogenic switch and insulinoma progression, Sci. Rep. 7 (2017) 40495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Cosgrove D, Liu S, Collagen IV diseases: A focus on the glomerular basement membrane in Alport syndrome, Matrix Biol 57–58 (2017) 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Uitto J, Has C, Vahidnezhad H, Youssefian L, Bruckner-Tuderman L, Molecular pathology of the basement membrane zone in heritable blistering diseases:: The paradigm of epidermolysis bullosa, Matrix Biol 57–58 (2017) 76–85. [DOI] [PubMed] [Google Scholar]
- [103].Foster MH, Basement membranes and autoimmune diseases, Matrix Biol 57–58 (2017) 149–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Pozzi A, Yurchenco PD, Iozzo RV, The nature and biology of basement membranes, Matrix Biol 57–58 (2017) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Patel VN, Pineda DL, Hoffman MP, The function of heparan sulfate during branching morphogenesis, Matrix Biol 57–58 (2017) 311–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Nystrom A, Bornert O, Kuhl T, Cell therapy for basement membrane-linked diseases, Matrix Biol 57–58 (2017) 124–139. [DOI] [PubMed] [Google Scholar]
- [107].Viquez OM, Yazlovitskaya EM, Tu T, Mernaugh G, Secades P, McKee KK et al. , Integrin α6 maintains the structural integrity of the kidney collecting system, Matrix Biol 57–58 (2017) 244–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Costell M, Gustafsson E, Aszódi A, Mörgelin M, BIoch W, Hunziker E et al. , Perlecan maintains the integrity of cartilage and some basement membranes, J. Cell Biol. 147 (1999) 1109–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Jones-Paris CR, Paria S, Berg T, Saus J, Bhave G, Paria BC et al. , Embryo implantation triggers dynamic spatiotemporal expression of the basement membrane toolkit during uterine reprogramming, Matrix Biol 57–58 (2017) 347–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Miller RT, Mechanical properties of basement membrane in health and disease, Matrix Biol 57–58 (2017) 366–373. [DOI] [PubMed] [Google Scholar]
- [111].Borza CM, Pozzi A, Discoidin domain receptors in disease, Matrix Biol. 34 (2014) 185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Borza CM, Su Y, Tran TL, Yu L, Steyns N, Temple KJ et al. , Discoidin domain receptor 1 kinase activity is required for regulating collagen IV synthesis, Matrix Biol 57–58 (2017) 258–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Kim OV, Litvinov RI, Chen J, Chen DZ, Weisel JW, AIber MS, Compression-induced structural and mechanical changes of fibrin-collagen composites, Matrix Biol 60–61 (2017) 141–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Page-McCaw A, Ewald AJ, Werb Z, Matrix metalloproteinases and the regulation of tissue remodelling, Nat. Rev. Mol. Cell Biol 8 (2007) 221–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Robinson KA, Sun M, Barnum CE, Weiss SN, Huegel J, Shetye SS et al. , Decorin and biglycan are necessary for maintaining collagen fibril structure, fiber realignment, and mechanical properties of mature tendons, Matrix Biol 64 (2017) 81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Chen S, Birk DE, The regulatory roles of small leucine-rich proteoglycans in extracellular matrix assembly, FEBS J. 280 (2013) 2120–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Muir AM, Massoudi D, Nguyen N, Keene DR, Lee SJ, Birk DE et al. , BMP1-like proteinases are essential to the structure and wound healing of skin, Matrix Biol 56 (2016) 114–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Barker TH, Engler AJ, The provisional matrix: setting the stage for tissue repair outcomes, Matrix Biol 60–61 (2017) 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Chester D, Brown AC, The role of biophysical properties of provisional matrix proteins in wound repair, Matrix Biol 60–61 (2017) 124–140. [DOI] [PubMed] [Google Scholar]
- [120].Iozzo RV, Basement membrane proteoglycans: from cellar to ceiling, Nat. Rev. Mol. Cell Biol. 6 (2005) 646–656. [DOI] [PubMed] [Google Scholar]
- [121].Neill T, Buraschi S, Goyal A, Sharpe C, Natkanski E, Schaefer L et al. , EphA2 is a functional receptor for the growth factor progranulin, J. Cell Biol 215 (2016) 687–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Tanimoto R, Palladino C, Xu SQ, Buraschi S, Neill T, Gomella LG et al. , The perlecan- interacting growth factor progranulin regulates ubiquitination, sorting, and lysosomal degradation of sortilin, Matrix Biol. 64 (2017) 27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Zhou Z, Wang J, Cao R, Morita H, Soininen R, Chan KM et al. , Impaired angiogenesis, delayed wound healing and retarded tumor growth in perlecan heparan sulfate-deficient mice, Cancer Res. 64 (2004) 4699–4702. [DOI] [PubMed] [Google Scholar]
- [124].Cailhier J-F, Sirois I, Raymond M-A, Lepage S, Laplante P, Brassard N et al. , Caspase-3 activation triggers extracellular release of cathepsin L and endorepellin proteolysis, J. Biol. Chem. 283 (2008) 27220–27229. [DOI] [PubMed] [Google Scholar]
- [125].Skovseth DK, Veuger MJ, Sorensen DR, De Angelis PM, Haraldsen G, Endostatin dramatically inhibits endothelial cell migration, vascular morphogenesis, and perivascular cell recruitment in vivo, Blood 105 (2005) 1044–1051. [DOI] [PubMed] [Google Scholar]
- [126].Mongiat M, Sweeney S, San Antonio JD, Fu J, Iozzo RV, Endorepellin, a novel inhibitor of angiogenesis derived from the C terminus of perlecan, J. Biol. Chem. 278 (2003) 4238–4249. [DOI] [PubMed] [Google Scholar]
- [127].Bix G, Fu J, Gonzalez E, Macro L, Barker A, Campbell S et al. , Endorepellin causes endothelial cell disassembly of actin cytoskeleton and focal adhesions through the α2β1 integrin, J. Cell Biol. 166 (2004) 97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Gonzalez EM, Reed CC, Bix G, Fu J, Zhang Y, Gopalakrishnan B et al. , BMP- 1/Tolloid-like metalloproteases process endorepellin, the angiostatic C-terminal fragment of perlecan, J. Biol. Chem. 280 (2005) 7080–7087. [DOI] [PubMed] [Google Scholar]
- [129].Bix G, Castello R, Burrows M, Zoeller JJ, Weech M, Iozzo RA et al. , Endorepellin in vivo: targeting the tumor vasculature and retarding cancer growth and metabolism, J. Natl. Cancer Inst. 98 (2006) 1634–1646. [DOI] [PubMed] [Google Scholar]
- [130].Bix G, Iozzo RA, Woodall B, Burrows M, McQuillan A, Campbell S et al. , Endorepellin, the C- terminal angiostatic module of perlecan, enhances collagen-platelet responses via the α2β1 integrin receptor, Blood 109 (2007) 3745–3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Woodall BP, Nystrom A, Iozzo RA, Eble JA, Niland S, Krieg T et al. , Integrin α2β1 is the required receptor for endorepellin angiostatic activity, J. Biol. Chem. 283 (2008) 2335–2343. [DOI] [PubMed] [Google Scholar]
- [132].Zoeller JJ, Iozzo RV, Proteomic profiling of endorepellin angiostatic activity on human endothelial cells, Proteome Sci. 6 (2008) 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Goyal A, Pal N, Concannon M, Paulk M, Doran M, Poluzzi C et al. , Endorepellin, the angiostatic module of perlecan, interacts with both the α2β1 integrin and vascular endothelial growth factor receptor 2 (VEGFR2), J. Biol. Chem. 286 (2011) 25947–25962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Nguyen TMB, Subramanian IV, Xiao X, Ghosh G, Nguyen P, Kelekar A et al. , Endostatin induces autophagy in endothelial cells by modulating Beclin 1 and β-catenin levels, J. Cell. Mol. Med. 13 (2009) 3687–3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Goyal A, Gubbiotti MA, Chery DR, Han L, Iozzo RV, Endorepellin-evoked autophagy contributes to angiostasis, J. Biol. Chem. 291 (2016) 19245–19256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Gubbiotti MA, Iozzo RV, Proteoglycans regulate autophagy via outside-in signaling: An emerging new concept, Matrix Biol. 48 (2015) 6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Schaefer L, Tredup C, Gubbiotti MA, Iozzo RV, Proteoglycan neofunctions: regulation of inflammation and autophagy in cancer biology, FEBS J. 284 (2017) 10–26. [DOI] [PMC free article] [PubMed] [Google Scholar]


