The advent of the tragic congenital Zika virus epidemic in 2016 focused considerable and well-deserved attention on the recognition and prevention of this infection. However, even as this evolving human tragedy continues to unfold, the ongoing problem of congenital cytomegalovirus (cCMV) infection deserves similar scrutiny. In this article, we provide an overview of the impact of cCMV on pediatric practice, with an emphasis on evolving concepts in maternal and newborn screening, counseling and education, diagnosis in the newborn, and medical management of children with CMV infection.
How Common is Congenital CMV Infection?
Congenital infections with CMV are common and appear to be under-recognized. In the US, the Centers for Disease Control (CDC) has estimated an overall birth prevalence of 0.65%.1 Congenital infections are even more prevalent in the developing world, with estimates of rates as high as 6.5% in some populations.2 In the US, this corresponds to over 25,000 newborns every year with cCMV. There appears to be considerable variation in birth prevalence based on maternal age, race, socioeconomic status and the entire spectrum of the social determinants of heatlh.3 Rates of cCMV are highest in black infants.4, 5 This fact makes cCMV an example of a disease reflecting health disparities in the US.6 To put this number in perspective, as of June 2016 there had been 7830 suspected cases of congenital Zika syndrome reported to the Brazilian Ministry of Health.7 The largest total of cases of congenital infection with another teratogenic virus in the US, rubella, was in 1969, when 57,686 cases were reported.8 Thus, the total number of cases of cCMV in the US (and globally) far exceeds the cases of congenital Zika syndrome, and is similar in scope to the magnitude of congenital rubella syndrome observed in the pre-vaccine era.
Given the high prevalence of cCMV, why isn’t it more commonly recognized by clinicians? One important issue is the lack of knowledge and awareness – not only in the lay public in general, but among health care providers, including obstetricians and pediatricians. Women of childbearing age in particular lack knowledge about the risks associated with cCMV. Transmission of CMV requires exposure to infectious body fluids, including urine, saliva, blood, and breast milk. Women who have children in group daycare are at particular risk, since CMV shedding rates are high in infants and toddlers attending daycare centers. Surveys have shown that women are less well informed about cCMV than they are about neural tube defects, fetal alcohol syndrome, Down syndrome, and toxoplasmosis – even though all of these threats to healthy pregnancy are less common that CMV!9 Thus, there is a great unmet need for programs that can increase the public’s familiarity with cCMV (discussed in more detail below).
Another major challenge that diminishes overall awareness of cCMV is the recognition that the majority of infants with cCMV (85-90%) are asymptomatic at birth.10 In the 10-15% of infants who have signs or symptoms at birth, clinical manifestations may include growth retardation, petechiae, hepatosplenomegaly, microcephaly, jaundice, seizures, rashes, and jaundice (Table 1). Symptomatic infants are more likely to have long-term neurodevelopmental sequelae, including mental retardation, seizure disorders, cerebral palsy, sensorineural hearing loss (SNHL), microcephaly, and learning disabilities.11 Of these sequelae, SNHL is the most common. It is important to keep in perspective asymptomatic cCMV is not innocuous. Asymptomatic infections can portend long-term risk, particularly as it relates to SNHL. Approximately 22-65% of children with symptomatic disease at birth, and 6-23% of children with asymptomatic cCMV infection, will have SNHL following congenital CMV infection.12 It is also of note that SNHL caused by cCMV infection may not be present at birth, and won’t be noticeable until later in childhood. An infant with cCMV may hear normally at birth, only to have progression to severe SNHL in early childhood. The fluctuating and (in many cases) delayed nature of cCMV-associated SNHL means that the majority of cases will be missed by routine newborn hearing screening13, providing a compelling rationale for universal CMV screening for all newborn infants – base on the rationale that any infant (even asymptomatic babies) with known cCMV could then be provided with serial, regular audiological assessment to facilitate early intervention for infants demonstrating evidence of SNHL.
Table 1:
Clinical, laboratory and imaging findings suggesting cCMV infection.
| Potential Diagnostic Clues in Congenital CMV Infection |
|---|
| Clinical Findings |
| Small for gestational age/intrauterine growth retardation |
| Neurologic findings |
| • Microcephaly • Hypotonia • Poor suck/feeding • Seizures |
| Jaundice |
| Hepatosplenomegaly |
| Rash: petechiae and/or purpura |
| Failed newborn hearing screen |
| Laboratory/Imaging Abnormalities |
| Thrombocytopenia |
| Transaminitis (elevated alanine aminotransferase) |
| Conjugated hyperbilirubinemia |
| Anemia with hemolysis |
| CSF pleiocytosis, elevated CSF protein |
| Chorioretinitis |
| Ventriculomegaly, intracerebral (particularly periventricular) calcifications, periventricular echogenicity, leukostriate vasculopathy |
When and How to Test for cCMV Infection
The cornerstone of diagnosis of cCMV is based on virology, not serology. Although the term “TORCH titers” is unfortunately still used in clinical practice, this nomenclature should be abandoned, since antibody studies are rarely useful in the work-up and management of congenital viral infections. The traditional “gold standard” of diagnosis of cCMV has been demonstration of virus by culture in specimens of saliva, urine, or blood in the infected newborn. However, few diagnostic laboratories offer culture today, and virological diagnosis is predicated on identification of viral DNA by PCR assay. PCR of urine or saliva are equally definitive in making the diagnosis of cCMV.14 Most experts recommend obtaining blood PCR for CMV DNA in addition to urine and saliva samples. The finding of CMV IgG antibodies is not informative, since it neither confirms that an infant has congenital CMV (since transplacental transfer of IgG can occur without bona fide infection), nor does it exclude the possibility of congenital CMV infection (since late gestation transmission can occur from mother to fetus prior to the appearance of IgG antibodies). Evaluation of neonatal serum for IgM antibodies can be useful, but the test is relatively insensitive and should not be relied upon to confirm or exclude the diagnosis.
One critically important element to consider in the diagnostic evaluation is timing of obtaining specimens for definitive testing. It is imperative that diagnostic specimens be obtained in the first 21 days of life – preferably, in the first 14 days of life. This is because shedding of CMV beyond 21 days of age may reflect perinatal transmission, most commonly from breast-feeding.15 Although breast milk-acquired infections are generally of no clinical importance in term babies, this mode of acquisition can complicate the evaluation for cCMV infection. This is a particular concern for infants that fail the newborn hearing screen and require audiological referral to investigate for possible etiologies of SNHL. In this setting, infants are often beyond three weeks of age when they present to an audiologist for diagnostic evaluation. Under these circumstances a positive urine or saliva PCR for CMV DNA must be interpreted cautiously, since a positive result could reflect post-natal acquisition and may have nothing to do with an infant’s hearing loss.
Once diagnostic virology has confirmed the diagnosis, other ancillary studies are important in the evaluation of cCMV. These are summarized in Table 2. The pattern of laboratory abnormalities, if present, is valuable in defining whether an infant has symptomatic or asymptomatic congenital infection. A complete blood count and differential leukocyte count is important, since thrombocytopenia in the neonate stands out as a predictive biomarker for an increased risk of neurodevelopmental sequelae.16 Liver function tests, including assessment for cholestasis, are useful. Imaging studies are a key component of the evaluation. Head ultrasound is recommended in the neonatal period, and has excellent sensitivity for demonstrating periventricular calcifications, structural lesions, and ventriculomegaly.11 Ophthalmological evaluation is warranted in all proven cases of cCMV. Serial audiological assessment is essential, including brainstem auditory evoked responses in the sett
Table 2:
Suggested diagnostic studies in the evaluation and work-up of cCMV infection.
| Suggested Evaluation of Infant with Congenital CMV Infection |
|---|
| Laboratory and Radiographic Evaluation |
| Complete blood count, platelet count, differential leukocyte count |
| Hepatic panel (transaminases, bilirubin) |
| CMV DNA PCR (blood, urine, saliva) |
| Placental histopathology (if available) |
| Head ultrasound (screening test; consider follow-up MRI) |
| Subspecialty Consultants |
| Audiology (auditory evoked response studies) |
| Otolaryngology |
| Child neurology |
| Developmental specialist |
| Ophthalmology |
| Physical therapy/occupational therapy |
| Pediatric infectious diseases |
Which infants require diagnostic evaluation for cCMV infection? Certainly, any infant with signs and symptoms (Table 1) suggestive of cCMV warrants virological evaluation, with addition studies as outlined above if CMV is demonstrated. However, the notion that cCMV is asymptomatic in 85-90% of cases may substantially underestimate the frequency of subtle clinical manifestations of infection. Infants born to women with ultrasonographic abnormalities, particularly echogenic bowel, should be tested for cCMV in the newborn period. Infants with unexplained intrauterine growth retardation or a small-for-gestational-age presentation at birth should be tested for cCMV. The diagnosis of cCMV should be considered in infants with unexplained premature birth, since there appears to be a higher birth prevalence in premature infants.
Finally, in any newborn who is “refer” on the newborn hearing screen, consideration should be given to performing CMV testing prior to hospital discharge. Although only a small percentage of infants who do not pass the newborn hearing screen actually have hearing loss, there is an enrichment for cCMV in this group of infants.17, 18 Moreover, as noted above, obtaining a diagnostic specimen for CMV in the immediate newborn period eliminates the diagnostic uncertainty intrinsic to the finding of viral shedding in an infant older than 21 days of age. Therefore, we recommend that if an infant does not pass the newborn hearing screen, that testing for congenital CMV should be performed immediately in the newborn nursery.
When and How to Treat cCMV Infections
Which infants require treatment for cCMV infection? Currently, treatment is reserved for infants with symptomatic congenital infection – infants with obviously signs of neurological involvement at birth. This includes microcephaly, radiographic abnormalities consistent with cCMV central nervous system disease (ventriculomegaly, intracerebral calcifications, periventricular echogenicity, cortical or cerebellar malformations), abnormal cerebrospinal fluid indices for age, chorioretinitis, sensorineural hearing loss, or the detection of CMV DNA in cerebrospinal fluid.11, 17 Infants with clinical evidence of cCMV who have clear-cut symptomatic disease, even without CNS involvement, should also be offered therapy, since the risk of long-term neurodevelopment sequelae is high. This includes infants with thrombocytopenia, petechiae, hepatomegaly, splenomegaly, intrauterine growth restriction, hepatitis (raised transaminases or bilirubin), or other signs of infection. Those infants who have isolated sensorineural hearing loss with no other clinical manifestation of infection, and those with asymptomatic congenital infection, are not currently considered candidates for antiviral therapy, although this potential benefits of treatment are being evaluated in a number of active clinical trials, and consultation with an expert is recommended.
Treatment, when indicated, should consist of oral valganciclovir suspension. The suggested dose is 16 mg/kg/dose orally, twice daily. Treatment should be commenced in the first month of life. The finding of CMV by PCR or culture in urine, saliva or blood in an infant beyond 21 days of age cannot be presumed to be diagnostic of cCMV infection, since breast-fed babies may acquire infection post-natally15, confounding the interpretation of diagnostic studies in infants who have clinical features of congenital infection. My laboratory (https://www.cmvscreening.org/) will perform CMV DNA PCR on saved, archived newborn dried blood spots, if available (these are routinely obtained in the course of normal newborn care, and retained in most states) with permission of the infant’s family and the respective state health department; clinicians interested in this can contact our lab for further discussion. In some cases, this test helps resolve the question of whether an infant was born with congenital CMV infection.18, 19 There are also active clinical studies examining whether delayed initiation of antiviral therapy (i.e., beyond one month of age) is beneficial. In infants unable to tolerate oral therapy, intravenous therapy with ganciclovir can be considered: again, consultation with a pediatric infectious diseases specialist is recommended in this circumstance. Evidence of benefit conferred by therapy with oral valganciclovir was demonstrated in a randomized placebo-controlled trial that showed a statistically significant benefit of treatment in symptomatic neonates.20 All symptomatic cytomegalovirus-infected neonates received valganciclovir for six weeks, and were then randomized to receive either placebo or additional valganciclovir treatment to complete a six-month course. Neonates receiving six months of valganciclovir had increased likelihood of improved hearing at 24 months than those who received only six weeks of valganciclovir treatment (followed by placebo). Importantly, neurodevelopmental outcomes were also improved with therapy.20 Based on these data, antiviral therapy with valganciclovir should be considered in all infants with symptomatic cCMV infection.
Laboratory monitoring is essential in infants treated with valganciclovir. Treatment is associated with neutropenia, and absolute neutrophil counts should be followed weekly for 6-8 weeks, then monthly for the duration of therapy. Transaminases should be followed monthly throughout therapy. For infants with drug-induced neutropenia, although there are no consensus management guidelines on this issue, I believe therapy with G-CSF should be offered, as needed. This allows many infants to complete a full six-month course of treatment.
Many parents and clinicians become invested in their commitment to finish a six-month course of therapy, and G-CSF can safely enable this. I also recommend that audiological testing should be done at 3-month intervals for the first 3 years of life in all cases of cCMV, irrespective of whether symptoms are present at birth or whether the infant is treated with valganciclovir, and annually thereafter through adolescence (ages 10–19).
Serial developmental assessments, beginning at the first year of life, are helpful in some children with symptomatic congenital cytomegalovirus disease, as is neuroimaging. Since some infants with cCMV with evolving sensorineural hearing loss are or become candidates for cochlear implantation, brain MRI can be considered at the same time that temporal bone MRI is performed prior to implant placement.
Treatment and monitoring of cCMV involves much more than just antiviral therapy and monitoring for drug toxicity; it requires a coordinated, team-based approach including, in many instances, specialists in ophthalmology, audiology, otolaryngology, neurology, developmental pediatrics, occupational and physical therapy, orthopedic surgeons, physiatrists, and pediatric infectious diseases specialists. The pediatrician can play a central role in enabling the multi-disciplinary evaluations required by many of these infants.
Finally, the infant with cCMV can and should receive routine childhood immunizations, including infants on antiviral therapy, since there is no evidence that such infants have over-arching immune deficiencies or problems handling live-virus vaccines.
The Case for Newborn CMV Screening
Laboratory monitoring is essential in infants treated with valganciclovir. Treatment is associated with neutropenia, there has been considerable recent interest in the question of whether congenital CMV should be added to the Recommended Uniform Screening Panel, or RUSP (https://www.hrsa.gov/advisory-committees/heritable-disorders/rusp/index.html), recommended for all newborns. There are two major issues that have precluded adding cCMV to the RUSP panel. First, it is not yet clear what constitutes the optimal specimen for newborn screening for CMV infection. Performing PCR for CMV DNA on the dried newborn blood spot would, in principle, represent an ideal strategy, since it is obtained routinely in the nursery and therefore would obviate the need for procuring additional samples for CMV testing. However, a multi-center cCMV screening study of blood spot PCR demonstrated sub-optimal sensitivity.21 Alternative approaches could include PCR testing of saliva or urine samples, but the cost associated with obtaining such samples in all newborns may be prohibitive. A second issue is that, in contrast to most newborn screening tests – which are typically performed to identify uniformly serious and even life-threatening conditions – cCMV screen will, in most cases, identify infants who are destined to have a normal clinical outcome. Advocates for universal screening point out that even asymptomatically congenitally infected infants are at risk for developmental of sensorineural hearing loss, even if they pass the newborn hearing screen, and that identification of such infants is not only capable of improving their clinical outcomes, but is also cost-effective.22, 23
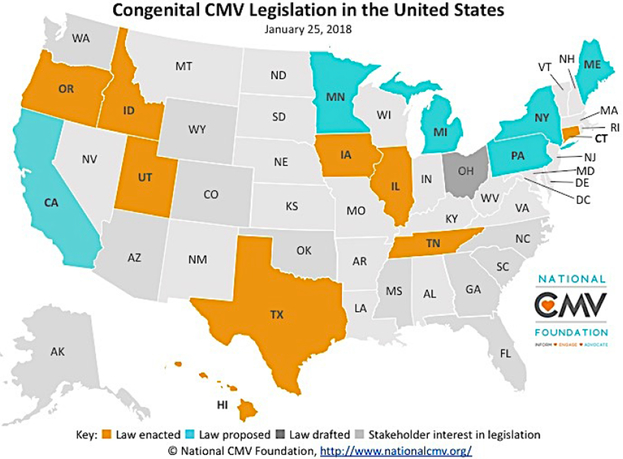
A compromise that has emerged in some states is “targeted screening” – that is, the testing for cCMV in all infants that fail the newborn hearing screen. Such programs will miss the majority of cases of cCMV, but will facilitate timely diagnosis and early intervention for infants who could benefit from intervention.24 An exciting development has been the engagement of state legislative bodies across the USA where several bills have been passed in recent years that require state health departments to provide educational resources, targeting in particular healthcare providers and young women of child-bearing age, about the problem of cCMV infection (Figure 1). Such legislation – in many instances, driven by parents of children with cCMV – is, in some instances, linked to mandates for targeted screening of newborn referred for evaluation of failed hearing screens. For example, a CMV knowledge and awareness bill, the “Vivian Act”, is currently under consideration by the Minnesota House of Representatives (http://www.house.leg.state.mn.us/members/pressrelease.asp?pressid=28204&party=2&memid=15434). It is hoped that these measures can address the substantial and disconcerting knowledge deficit that exists – both in the lay public and among physicians – regarding the risks of acquiring CMV infections during pregnancy.9, 25 Since education about simple hygienic precautions that women can take to avoid infection has been shown in other studies to be effective in preventing acquisition of CMV during pregnancy26, such legislation could have a significant impact on future cCMV infections.
Figure 1: States that have passed or are considering legislation to enhance knowledge and awareness of congenital CMV.
Three states, Illinois, Iowa, and Utah require both education and screening. Eight states require the state to educate the public and professionals about cCMV: Hawaii, Idaho, Illinois, Iowa, Oregon, Texas, Utah. Tennessee requires healthcare providers to educate women of childbearing age. Connecticut, Iowa, and Utah require each newborn that fails the newborn hearing screening to be tested for cCMV, and Illinois requires that a CMV test be offered to the parents of every child who fails the newborn hearing screening.
Source: https://www.nationalcmv.org/cmv-research/legislation.aspx
The Future: CMV Vaccines
Ultimately, prevention of cCMV will most likely require development and implementation of an effective vaccine. Several CMV vaccine platforms have been developed and assessed in pre-clinical models and phase I and II human studies.27 The best-studied candidate to date, a purified and adjuvanted recombinant vaccine against the immunodominant glycoprotein B present in the CMV viral envelope, has demonstrated efficacy ranging from 43%-50% in preventing primary CMV infection in young women.28, 29 Many questions remain about how a CMV vaccine would be used in clinical practice. Should a CMV vaccine be given universally to young children, toward the goal of universal coverage, using a paradigm that was successful for vaccine-mediated protection against congenital rubella syndrome? Or, should a vaccine selectively target young women (and young men!) of children-bearing age, to enhance protection during the child-bearing years? Should serological screening be performed before administration of vaccine to young women, knowing that the greatest risk for disability in infants occurs in the context of primary maternal infection during pregnancy – or should all women be vaccinated prior to pregnancy, irrespective of CMV serology, since it is also clear that CMV “immune” women can become re-infected with new strains during pregnancy, and that this scenario can result in congenital transmission (although probably less commonly, and with fewer sequelae, than in primary maternal infection)?30 Regardless of how these questions play out, these are exciting times with encouraging prospects ahead for solving the problem of cCMV. The combination effects of increased awareness, evolution of newborn screening programs, and development and deployment of an effective vaccine should synergize to give us solutions on the near horizon for this common, under-recognized and disabling infection in newborns.
Acknowledgments
Grant Support
Support from the National Institutes of Health (R01HD079918) and March of Dimes Birth Defects Foundation (#6-FY17-849) is gratefully acknowledged.
Biography
Mark R. Schleiss, MD, is Professor of Pediatrics at the University of Minnesota Medical School. He holds the American Legion and Auxiliary Heart Research Foundation Endowed Chair in the Department of Pediatrics. In additional to maintaining a clinical practice in pediatric infectious diseases, his laboratory’s research focuses on congenital and neonatal cytomegalovirus infection. His work focuses both on clinical research programs in congenital CMV infection and in small animal models of CMV vaccine development. His laboratory is funded by the National Institutes of Health and the March of Dimes Birth Defects Foundation.
REFERENCES
- 1.Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol. 2007;17(4):253–76. [DOI] [PubMed] [Google Scholar]
- 2.Lanzieri TM, Dollard SC, Bialek SR, Grosse SD. Systematic review of the birth prevalence of congenital cytomegalovirus infection in developing countries. Int J Infect Dis. 2014;22:44–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.United States Department of Health and Human Service. Office of Disease and Health Promotion (2018). 2020 Topics and Objectives: Social Determinants of Health. Retrieved from: www.healthypeople.gov.
- 4.Fowler KB, Boppana SB. Congenital cytomegalovirus infection. Semin Perinatol. 2018; pii: S0146–0005(18)30008–9. doi: 10.1053/j.semperi.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Fowler KB, Ross SA, Shimamura M, et al. Racial and ethnic differences in the prevalence of congenital cytomegalovirus infection. J Pediatr. 2018; pii: S0022–3476(18)30597–3. doi: 10.1016/j.jpeds.2018.04.043. PMID: 29784513. [DOI] [PubMed] [Google Scholar]
- 6.Hotez PJ. Neglected infections of poverty in the United States of America. PLoS Negl Trop Dis. 2008;2(6):e256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.França GV, Schuler-Faccini L, Oliveira WK, et al. Congenital Zika virus syndrome in Brazil: a case series of the first 1501 livebirths with complete investigation. Lancet. 2016;388(10047):891–7. [DOI] [PubMed] [Google Scholar]
- 8.Modlin JF, Brandling-Bennett D, Witte JJ, et al. A review of five years’ experience with rubella vaccine in the United States. Pediatrics. 1975;55(1):20–29. [PubMed] [Google Scholar]
- 9.Cannon MJ, Westbrook K, Levis D, et al. Awareness of and behaviors related to child-to-mother transmission of cytomegalovirus. Prev Med. 2012;54(5):351–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boppana SB, Ross SA, Fowler KB. Congenital cytomegalovirus infection: clinical outcome. Clin Infect Dis. 2013;57(S4):S178–S181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheeran MC, Lokensgard JR, Schleiss MR. Neuropathogenesis of congenital cytomegalovirus infection: disease mechanisms and prospects for intervention. Clin Microbiol Rev. 2009;22(1):99–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fowler KB. Congenital cytomegalovirus infection: audiologic outcome. Clin Infect Dis. 2013;57(S4):S182–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fowler KB, Dahle AJ, Boppana SB, Pass RF. Newborn hearing screening: will children with hearing loss caused by congenital cytomegalovirus infection be missed? J Pediatr. 1999;135(1):60–4. [DOI] [PubMed] [Google Scholar]
- 14.Cardoso ES, Jesus BL, Gomes LG, et al. The use of saliva as a practical and feasible alternative to urine in large-scale screening for congenital cytomegalovirus infection increases inclusion and detection rates. Rev Soc Bras Med Trop. 2015;48(2):206–7. [DOI] [PubMed] [Google Scholar]
- 15.Schleiss MR. Acquisition of human cytomegalovirus infection in infants via breast milk: natural immunization or cause for concern? Rev Med Virol. 2006;16(2):73–82. [DOI] [PubMed] [Google Scholar]
- 16.Swanson EC, Schleiss MR. Congenital cytomegalovirus infection: new prospects for prevention and therapy. Pediatr Clin North Am.2013;60(2):335–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rawlinson WD, Boppana SB, Fowler KB, et al. Lancet Infect Dis. 2017;17(6):e177–e188. [DOI] [PubMed] [Google Scholar]
- 18.Choi KY, Schimmenti LA, Jurek AM, et al. Detection of cytomegalovirus DNA in dried blood spots of Minnesota infants who do not pass newborn hearing screening. Pediatr Infect Dis J. 2009;28(12):1095–8. [DOI] [PubMed] [Google Scholar]
- 19.Meyer L, Sharon B, Huang TC, et al. Analysis of archived newborn dried blood spots (DBS) identifies congenital cytomegalovirus as a major cause of unexplained pediatric sensorineural hearing loss. Am J Otolaryngol. 2017;38(5):565–570. [DOI] [PubMed] [Google Scholar]
- 20.Kimberlin DW, Jester PM, Sanchez PJ, et al. Valganciclovir for symptomatic congenital cytomegalovirus disease. N Engl J Med. 2015;372(10):933–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boppana SB, Ross SA, Novak Z, et al. Dried blood spot real-time polymerase chain reaction assays to screen newborns for congenital cytomegalovirus infection. JAMA. 2010;303(14):1375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gantt S, Dionne F, Kozak FK, et al. Cost-effectiveness of universal and targeted newborn screening for congenital cytomegalovirus infection. JAMA Pediatr. 2016; 170(12): 1173–1180. [DOI] [PubMed] [Google Scholar]
- 23.Ronchi A, Shimamura M, Malhotra PS, Sanchez PJ. Encouraging postnatal cytomegalovirus (CMV) screening: the time is NOW for universal screening! Expert Rev Anti Infect Ther. 2017;15(5):417–419. [DOI] [PubMed] [Google Scholar]
- 24.Diener ML, Zick CD, McVicar SB, et al. Outcomes from a hearing-targeted cytomegalovirus screening program. Pediatrics. 2017;139(2). pii: e20160789. [DOI] [PubMed] [Google Scholar]
- 25.Thackeray R, Magnusson BM. Women's attitudes toward practicing cytomegalovirus prevention behaviors. Prev Med Rep. 2016;4:517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vauloup-Fellous C, Picone O, Cordier A-G. Does hygiene counseling have an impact on the rate of CMV primary infection during pregnancy? Results of a 3-year prospective study in a French hospital. J Clin Virol. 2009;46S:S49–S53. [DOI] [PubMed] [Google Scholar]
- 27.Schleiss MR, Permar SR, Plotkin SA. Progress toward development of a vaccine against congenital cytomegalovirus infection. Clin Vaccine Immunol. 2017;24(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pass RF, Zhang C, Evans A, et al. Vaccine prevention of maternal cytomegalovirus infection. N Engl J Med. 2009;360(12):1191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernstein DI, Munoz FM, Callahan ST, et al. Safety and efficacy of a cytomegalovirus glycoprotein B (gB) vaccine in adolescent girls: A randomized clinical trial. Vaccine. 2016;34(3):313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Permar SR, Schleiss MR, Plotkin SA. Advancing our understanding of protective maternal immunity as a guide for development of vaccines to reduce congenital cytomegalovirus infections. J Virol. 2018;92(7). pii: e00030-18. [DOI] [PMC free article] [PubMed] [Google Scholar]