Abstract
Introduction:
Alzheimer’s disease and related dementias (ADRD) cause a high burden of morbidity and mortality in the United States. Age, race, and ethnicity are important risk factors for ADRD.
Methods:
We estimated the future US burden of ADRD by age, sex, and race and ethnicity by applying subgroup-specific prevalence among Medicare Fee-for-Service beneficiaries aged ≥65 years in 2014 to subgroup-specific population estimates for 2014 and population projection data from the US Census Bureau for 2015 to 2060.
Results:
The burden of ADRD in 2014 was an estimated 5.0 million adults aged ≥65 years or 1.6% of the population, and there are significant disparities in ADRD prevalence among population subgroups defined by race and ethnicity. ADRD burden will double to 3.3% by 2060 when 13.9 million Americans are projected to have the disease.
Discussion:
These estimates can be used to guide planning and interventions related to caring for the ADRD population and supporting caregivers.
Keywords: Dementia, Alzheimer’s disease, Estimates, Prevalence, Projections, Race and ethnicity
1. Introduction
Alzheimer’s disease and related dementias (ADRD) are characterized by a decline in memory leading to loss of independence. These illnesses have wide-ranging impacts on patients, families, communities, and health-care systems. Alzheimer’s disease (AD) is the sixth leading cause of death in the United States (US) population and the fifth leading cause of death among adults aged ≥65years [1]. Although the primary risk factor for ADRD is age, race and ethnicity is also an important demographic risk factor; estimates of ADRD among these subgroups do not exist. We addressed this gap by estimating ADRD in the population aged ≥65 years by age, sex, and race and ethnicity from 2015 to 2060 using Medicare Fee-for-Service (FFS) beneficiary claims with a clinical diagnosis of ADRD in 2014.
Our estimates complement other estimates of the future burden of dementia [2–5]. The most commonly cited estimates for AD show that 4.7 million Americans had AD in 2010, and 13.8 million will have the disease by 2050 [3]. The most recent estimates in the US show that 6.1 million people had clinical AD or mild cognitive impairment in 2017, which is expected to grow to 15.0 million by 2060 [5]. We estimate that 5.0 million adults aged ≥65 years in the United States were diagnosed with ADRD in 2014, and 13.9 million Americans aged ≥65 years will be diagnosed with ADRD by 2060.
These findings support surveillance activities designed to assess cognitive decline in the population at risk for ADRD as recommended in The Healthy Brain Initiative: A National Public Health Road Map to Maintaining Cognitive Health [6]. These findings will help the public, health-care community, public health professionals, and policy-makers anticipate how disease burden among population subgroups defined by race and ethnicity is expected to increase over time.
2. Background
In the United States, the Centers for Medicare and Medicaid Services (CMS) administers the public insurance program commonly called Medicare. People aged ≥65 years become eligible for benefits on the first day of the month they turn 65, if they paid Medicare taxes for at least ten years. Most beneficiaries are enrolled in either Fee-for-Service (FFS) plans or Medicare Advantage (MA) plans. MA plans are offered by private companies approved by CMS, and claims for enrollees in MA plans are not reported to CMS. Health status differs by MA versus FFS enrollment [7], and enrollment varies by subgroup [8,9] and geography [10].
Medicare claims data are useful for epidemiological surveillance of dementia because beneficiaries are thought to be representative of population aged 65 years and older [11,12]. After determining a diagnosis of any type of dementia during a clinical encounter, the billing medical care provider is responsible for assigning the diagnosis code. This information is entered into the clinical record and used for billing to receive reimbursement from CMS for health-care services. These diagnosis codes are available in resulting claims data. The sensitivity and specificity of Medicare claims for identifying ADRD was 0.85/0.89 and 0.64/0.95, respectively, for AD when linked to a sample of persons at risk for dementia [11]. Although the gold standard for obtaining reliable estimates of the prevalence of dementia would be to clinically evaluate a nationally representative sample of older Americans [12], such studies can be prohibitively expensive, and it is unclear whether the geographically restricted cohort used to estimate the prevalence of dementia in previous studies [3] is representative of the US population.
The prevalence of ADRD is highest among the racial and ethnic groups who are expected to experience the highest rates of population growth. The US population of persons aged ≥65 years is expected to double from 46.5 million in 2014 to 83.7 million by 2060, but some groups will increase much faster than others [13]. Minority populations, those classified by the US Census Bureau as racial and ethnic groups other than non-Hispanic white, are expected to outpace the growth of the nonminority population in the next few decades. By 2060, minority populations aged ≥65 years will represent 45% of the US population for that subgroup, which is up from 22% in 2014. The percentage increase in total population by race and ethnicity by 2060 is estimated to be 75% for non-Hispanic whites, 172% for African Americans, 270% for Asian and Pacific Islanders, 274% for American Indian and Alaska Natives, and 391% for Hispanics [14,15].
Recent estimates of ADRD prevalence by race and ethnicity in the Medicare FFS population did not provide estimates of ADRD in the overall population [16]. A recent meta-analysis of six population-based studies observed that the prevalence of ADRD was 64% higher for African Americans than for Caucasians [17]. These findings are consistent with other literature about ADRD prevalence differences among racial subgroups [18]. Hispanics are one and a half times as likely to have ADRD when compared with non-Hispanic whites [19,20]. African Americans have the highest prevalence of ADRD [21–23].
Although AD is the most common type of ADRD, the prevalence of vascular dementia is higher in African Americans; this is thought to be secondary to known disparities in risk factors for vascular dementia such as cardiovascular disease. Age, family history, and heredity are unchangeable risk factors related to ADRD, but there is growing evidence proving the importance of preventive interventions to decrease prevalence. Proper management of health, lifestyle, and wellness choices can reduce the risk of ADRD. Increasing healthy behaviors can decrease the chance of developing chronic diseases that are risk factors for developing ADRD. Risk of developing ADRD can also be reduced through maintaining strong social connections, physical exercise, healthy eating, and keeping oneself mentally active [24]. Genetic testing and counseling for genetically linked dementias can also heighten awareness and be informative to the patient and provider for precautionary and preventive action.
3. Methods
The future burden of ADRD is the estimated number of persons diagnosed with ADRD aged ≥65 years among the population of all ages in a given year. The ADRD estimates were calculated by applying the prevalence of ADRD among Medicare FFS beneficiaries aged ≥65 years in 2014 to subgroup-specific population projection data from the US Census Bureau. In 2014, of the 46.5 million adults aged ≥65 years in the United States, nearly 43.1 million were enrolled in Medicare part A or B programs, of which 28.0 million (65%) were Medicare FFS beneficiaries and 15.1 million (35%) were enrolled in MA programs [25]. We used claims data for 100% of the Medicare FFS population, but data for the MA enrollees were not available as these claims are not reported to CMS. Subgroup-specific counts of FFS beneficiaries residing within the 50 states and the District of Columbia who were diagnosed with ADRD were provided by the CMS [26].
The study population consisted of 28,027,071 Medicare FFS beneficiaries aged ≥65 years in 2014, which accounts for nearly 60.2% of the adults aged ≥65 years in the United States. International Classification of Diseases 9th Revision Clinical Modification (ICD-9-CM) diagnoses codes for ADRD are shown in Table 1 [11]. A beneficiary was defined as being diagnosed with ADRD in 2014 if they had at least one inpatient, skilled nursing facility, home health agency, hospital outpatient, or carrier claim with any of the ICD-9-CM diagnosis codes that indicated a service or treatment for ADRD in 2012, 2013, or 2014. Despite the potential etiologic differences, we included the related dementias in addition to AD because of potential diagnostic misclassification [27,28] and because the burden of diseases is similar for a patient, family, community, and health system [29].
Table 1.
ICD-9-CM codes used to define Alzheimer’s disease and related dementias
| ICD-9-CM code | Descriptor |
|---|---|
| 331.0 | Alzheimer’s disease |
| 331.11 | Pick’s disease |
| 331.19 | Other frontotemporal dementia |
| 331.2 | Senile degeneration of the brain |
| 331.7 | Cerebral degeneration in diseases classified elsewhere |
| 797 | Senility without mention of psychosis |
| 290.0 | Senile dementia, uncomplicated |
| 290.10 | Presenile dementia, uncomplicated |
| 290.11 | Presenile dementia with delirium |
| 290.12 | Presenile dementia with delusional features |
| 290.13 | Presenile dementia with depressive features |
| 290.20 | Senile dementia with delusional features |
| 290.21 | Senile dementia with depressive features |
| 290.3 | Senile dementia with delirium |
| 290.40 | Vascular dementia, uncomplicated |
| 290.41 | Vascular dementia with delirium |
| 290.42 | Vascular dementia with delusions |
| 290.43 | Vascular dementia with depressed mood |
| 294.0 | Amnestic syndrome (Korsakoff’s psychosis or syndrome, nonalcoholic) |
| 294.10 | Dementia in conditions classified elsewhere without behavioral disturbance |
| 294.11 | Dementia in conditions classified elsewhere with behavioral disturbance |
| 294.2 | Dementia, unspecified, without behavioral disturbance |
| 294.21 | Dementia, unspecified, with behavioral disturbance |
| 294.8 | Other persistent mental disorders due to conditions |
Abbreviation: ICD-9-CM, International Classification of Diseases 9th Revision Clinical Modification.
We obtained data from the US Census Bureau population projections program that publishes annual population projections for all 50 states and the District of Columbia by five-year age groups, sex, race, and Hispanic origin from 2015 to 2060 [14]. We calculated the prevalence of ADRD among Medicare FFS beneficiaries for 70 subgroups defined by sex, five age groups, and seven racial and ethnic groups. Because CMS and the US Census Bureau classifies race and ethnicity differently, we created a new race classification system by matching the name of the race category in the CMS data to the same (or similarly) named racial and ethnic category in the Census data (Supplementary Appendix 1).
To calculate the future burden of ADRD in 2060, we first calculated the subgroup-specific prevalence of ADRD using the number of ADRD cases in a group as the numerator and the FFS population of the group as the denominator. The estimated number of people with ADRD in each subgroup and in a given year (2015–2060) was calculated by applying the 2014 national-level prevalence of diagnosed ADRD among FFS beneficiaries to the subgroup-specific population in the given year.
4. Results
More than 3.2 million Medicare FFS beneficiaries aged ≥65 years (11.5%) had an ADRD diagnosis in 2014 (Table 2). Prevalence was higher for women (13.3%) than men (9.2%) and increased by age from 3.6% among those aged 65–74 years to 13.6% among those aged 75–84 years and 34.6% among those aged ≥85 years. Blacks had the highest prevalence of ADRD (14.7%), followed by Hispanics (12.9%), non-Hispanic whites (11.3%), American Indian and Alaska Natives (10.5%), and Asian and Pacific Islanders (10.1%).
Table 2.
Number and percent of Medicare Fee-for-Service (FFS) beneficiaries aged ≥65 years with Alzheimer’s disease or related dementias (ADRD) and the percentage of Medicare beneficiary study population as a share of the US population aged ≥65 years, by selected characteristics (United States, 2014)
| Characteristic | FFS beneficiaries | Observed number of ADRD in FFS | Percentage of FFS beneficiaries with ADRD (%) | US population aged ≥65 years | Percentage of study population as a share of US population (%) |
|---|---|---|---|---|---|
| Total | 28,027,071 | 3,230,895 | 11.5 | 46,542,503 | 60.2 |
| Men | 12,278,223 | 1,132,634 | 9.2 | 20,485,304 | 59.9 |
| Women | 15,748,848 | 2,098,261 | 13.3 | 26,057,199 | 60.4 |
| Age group | |||||
| 65–74 | 14,906,921 | 523,522 | 3.5 | 26,597,270 | 56.0 |
| 75–84 | 859,4212 | 1,150,062 | 13.4 | 13,752,903 | 62.5 |
| ≥85 | 4,525,938 | 1,557,311 | 34.4 | 6,192,330 | 73.1 |
| Race and ethnicity | |||||
| Non-Hispanic white | 23,149,644 | 2,625,631 | 11.3 | 36,280,540 | 63.8 |
| Black | 2,117,115 | 310,924 | 14.7 | 4,147,471 | 51.0 |
| Asian and Pacific Islander | 694,419 | 69,994 | 10.1 | 1,904,886 | 36.5 |
| Hispanic | 1,460,586 | 188,211 | 12.9 | 3,534,268 | 41.3 |
| American Indian and Alaska Native | 125,087 | 13,077 | 10.5 | 300,701 | 41.6 |
| Two or more races (Census) | * | * | * | 374,637 | * |
| Other or race missing (CMS) | 480,220 | 23,058 | 4.8 | † | † |
The Centers for Medicare and Medicaid Services (CMS) does not collect data for beneficiaries with two or more races. To arrive at these estimates, we applied the age-sex–specific prevalence for all races to the total population of two or more races.
There is no comparable population group in the US Census population projections data.
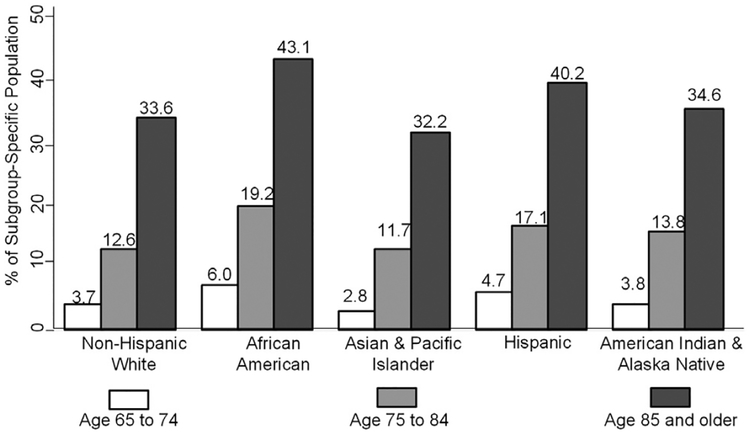
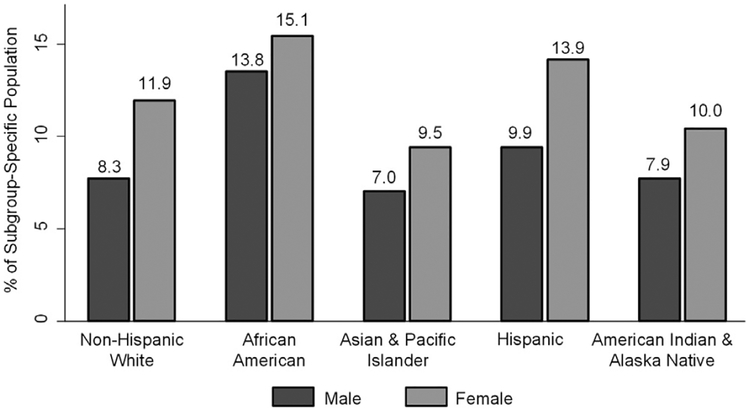
Nearly 5.0 million people in the United States aged ≥65 years (10.9% of the subgroup population) had an ADRD diagnosis in 2014 (Table 3). ADRD was more prevalent in women (12.2%) than in men (8.6%). Nearly 3.6% of those aged 65 to 74 years, 13.6% of those aged 75 to 84 years, and 34.6% of those aged ≥85 years had ADRD. Asian and Pacific Islanders had the lowest prevalence of ADRD (8.4%), followed by American Indian and Alaska Natives (9.1%), non-Hispanic whites (10.3%), population with two or more races (11.5%), Hispanics (12.2%), and blacks (13.8%). At age 85 years, over 43% of blacks and 40% of Hispanics have the greatest estimated burden of disease due to ADRD (Fig. 1). In all races and ethnicities, the prevalence of ADRD was higher for women than for men (Fig. 2).
Table 3.
Estimated (2014) and projected (2020 to 2060) prevalence of Alzheimer’s disease and related dementias (ADRD) in the US population aged 65 years, by selected characteristics
| Estimated ADRD in 2014 population (in 1000s) | Projected estimated ADRD in population (in 1000s) | |||||
|---|---|---|---|---|---|---|
| Characteristics | N (% of subgroup population) | 2020 | 2030 | 2040 | 2050 | 2060 |
| Total | 4957 (10.9) | 5814 | 8280 | 11,088 | 12,748 | 13,894 |
| Men | 1769 (8.6) | 2163 | 3166 | 4196 | 4814 | 5377 |
| Women | 3188 (12.2) | 3651 | 5114 | 6892 | 7934 | 8517 |
| Age group | ||||||
| 65–74 | 949 (3.6) | 1232 | 1541 | 1501 | 1622 | 1930 |
| 75–84 | 1864 (13.6) | 2238 | 3554 | 4461 | 4403 | 4867 |
| ≥85 | 2144 (34.6) | 2344 | 3185 | 5126 | 6723 | 7097 |
| Race and ethnicity | ||||||
| Non-Hispanic White | 3723 (10.3) | 4186 | 5638 | 7083 | 7365 | 7061 |
| Black | 573 (13.8) | 726 | 1118 | 1575 | 1893 | 2173 |
| Asian and Pacific Islander | 161 (8.4) | 212 | 356 | 554 | 761 | 999 |
| Hispanic | 430 (12.2) | 594 | 1003 | 1620 | 2383 | 3200 |
| American Indian and Alaska Native | 27 (9.1) | 38 | 65 | 100 | 130 | 156 |
| Two or more (Census)* | 43 (11.5) | 58 | 100 | 156 | 216 | 305 |
| Other or unknown (CMS) | 23 | † | † | † | † | † |
The Centers for Medicare and Medicaid Services (CMS) does not collect data for beneficiaries with two or more races. To arrive at these estimates, we applied the age-sex–specific prevalence for all races to the total census population of two or more races.
There is no comparable population group in the US Census population projections data. Rather than excluding these groups, we used their observed ADRD cases for the 2014 estimate, but we excluded these cases for the ADRD projections. To maintain consistency of reporting over time, these ADRD cases are not included in the total number of ADRD cases in 2014.
Fig. 1.
Estimated prevalence of Alzheimer’s disease and related dementias in the US Population aged ≥65 years, by sex and race and ethnicity; United States, 2014.
Fig. 2.
Estimated prevalence of Alzheimer’s disease and related dementias in the US Population aged ≥65 years, by age and race and ethnicity; United States, 2014.
Table 3 shows that all subgroups will see an increase in the total number of ADRD cases from 2014 through 2060, which reflects the growth of the aging population. Among the 319 million persons of all ages in the United States, the number of people with ADRD in 2014 is an estimated 5.0 million adults aged ≥65 years or 1.6% of the US population in 2014. This burden will double to 3.3% by 2060 when 13.9 million Americans among a population of 417 million are projected to have the disease; this is a 178% increase from 2014.
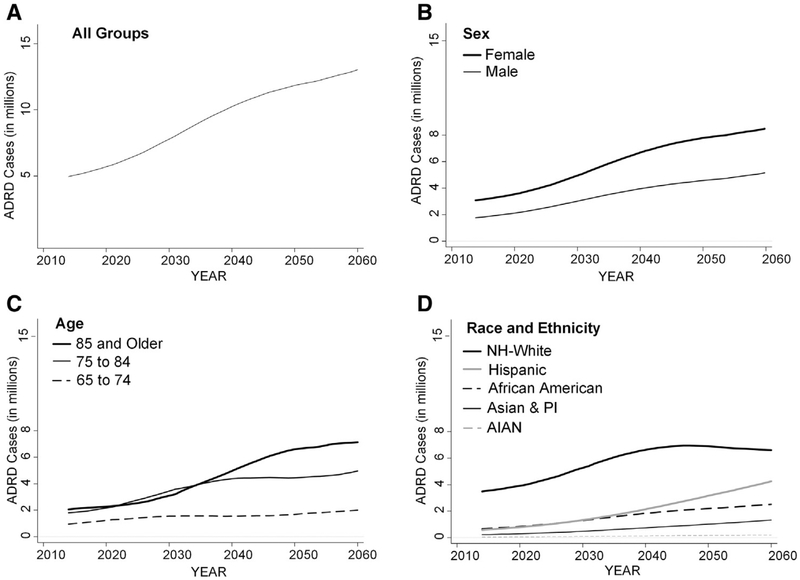
Fig. 3 depicts the projected number of individuals with ADRD for the various subgroups. In 2014, there were approximately 2 million ADRD cases estimated in each of the two oldest age groups. Beginning around 2030, the growth in the number of cases among those persons aged ≥85 years will begin to outpace those in the 75 to 84 years age group. The Hispanic population will have the largest projected increase in ADRD cases over the projection period. Given the population size relative to the other subgroups, the non-Hispanic white population will have the largest total number of ADRD cases in all years. As the United States becomes a majority-minority nation by 2050, increases in the number of non-Hispanic whites with ADRD will begin to plateau around 2030 while the number in minority populations will continue to grow, particularly among Hispanics.
Fig. 3.
Projected number of adults aged ≥65 years with Alzheimer’s disease and related dementias for all groups (A), and by sex (B), age (C), and race and ethnicity (D); 2015 to 2060. Abbreviations: ADRD, Alzheimer’s disease and related dementia; AIAN, American Indian or Alaska Native; NH-White, Non-Hispanic white; PI, Pacific Islander.
5. Discussion
We estimated 5.0 million adults aged ≥65 years with ADRD in the US population in 2014 and 13.9 million Americans aged ≥65 years with ADRD by 2060. The burden of ADRD in 2014 was 1.6% of the US population in 2014 (n = 319 million), but this burden is expected to more than double to nearly 3.3% of the US population in 2060 (n = 417 million). These findings provide additional compelling reasons for the United States to achieve the goals outlined in the 2016 National Plan to Address ADRD [30]. This plan describes the development of potential preventive interventions or treatment options that may help to ensure these projections are never reached, to delay the onset of the disease, and to improve the quality of life for those individuals afflicted by these diseases.
The Healthy Brain Initiative: A National Public Health Road Map to Maintaining Cognitive Health [6] describes how public health agencies can promote cognitive functioning and early diagnosis in older adults, help local communities provide resources to people as they cope with cognitive impairment, and provide assistance to formal and informal caregivers. The Road Map is divided into recommendations designed to support state and local actions for monitoring and evaluation (M), education and empowerment (E), development of policy and partnerships (P), and assuring a competent workforce (W). Given the expected growth in the burden of disease, particularly among minority populations, culturally competent care for these groups will be of paramount importance. Thus, our findings highlight the need to monitor and evaluate minority populations to ensure materials and evaluations are culturally sensitive (M-06) and to define the needs of a diverse group of caregivers and persons with dementia (M-08). These findings can also support action items related to educating and empowering the nation by promoting culturally appropriate strategies designed to increase public awareness about dementia (E-01) and to promote strategies about effective communication with persons with dementia and their families (E-06). Given that some states have higher proportions of minority populations, this report can help support policy development and mobilize partnerships that specifically account for the disease burden in minority populations and disparities in ADRD burdens among racial and ethnic subgroups when developing or revising state-level AD plans (P-01) and to incorporate such information when developing state and local public health reports (P-03). These findings support efforts to develop a culturally competent workforce of health-care providers of all types. Such training would help to improve the recognition of early signs of dementia despite cultural differences (W-03) and to identify ways health-care workers can assist people with dementia in navigating the health-care system (W-04).
Given the projected growth of the US population of persons aged ≥65 years, and the estimated increase in the population living with ADRD in the United States, it is critical that older adults who are exhibiting symptoms of memory loss or cognitive decline seek an assessment and potential diagnosis from a health-care provider. However, in a recent nationally representative survey, more than 56% of Behavioral Risk Factor Surveillance System respondents who reported subjective cognitive decline had not discussed the decline with their health-care provider [1]. The benefits of early assessment, diagnosis, and disclosure of that diagnosis are well known, so efforts focused on care navigation and early adoption of the Annual Wellness Visit among minority populations could contribute to early diagnosis of ADRD in this population and facilitate timely linkages to care for patients and their caregivers [31].
Providing support for caregivers is a growing public health issue given that caregivers face financial, emotional, and social challenges and are at increased risk for negative economic, mental health, and physical health outcomes. In the United States, the proportion of younger adults [32] is declining, but members of this population are likely to become caregivers as the population of adults aged ≥65 years will double to 72 million and account for 20% of the population by 2030 [33]. Currently, there are seven potential caregivers to one adult in the high-risk age group, but this caregiver support ratio is expected to decline to four to one by 2030 [34]. Caregivers experience work disruption, reduction in household income, depletion of personal savings, increased food insecurity, and increased forgoing of personal medical care [1]. Caregivers experience physical health problems [35] and higher rates of depression and psychological stress [36], and they have a higher risk of mortality [37]. One way to support caregivers is to promote a newly available care planning and coordination mechanism under Medicare called the Cognitive Impairment Care Planning billing code. Beginning in 2017, Medicare began reimbursing services related to care planning and coordination for patients with cognitive impairment and their caregivers using code G0505 [38].
Given that minority populations tend to cluster geographically, future research about ADRD burden in local areas will provide valuable information about defining communities that will potentially bear disproportionate burdens of ADRD. Such research is important given that the prevalence of ADRD in one area may be driven by specific health conditions that may differ from those driving a similar prevalence in a different area. The disproportionate prevalence of ADRD in minority populations could further magnify existing neighborhood socioecologic disparities and potentially lead to worsening of health outcomes in these groups. Furthermore, understanding the reasons why ADRD varies by place may lead to the development of services and resources for formal and informal caregivers that are consistent with the local needs and circumstances.
This study fills a critical research gap because estimates of the future burden of ADRD in the US population by age, sex, and race and ethnicity did not exist previously. Our estimates are based on the observed prevalence of ADRD among 28.0 million Medicare FFS beneficiaries with ADRD diagnosed by a physician in a clinical setting. We included related dementias in addition to AD because of potential diagnostic misclassification [11,27] and because the burden of diseases is similar for a patient, family, community, and health system [28]. These estimates provide more detailed information about the demographic characteristics of the future population of patients with ADRD, which is important given the differences in prevalence among the subgroups coupled with the increasing diversity among persons aged ≥65 years. Another strength of this work is the use of Census population projections, which are based on a valid and robust methodology.
However, these estimates are subject to several limitations. They rely on the assumption that the ADRD prevalence among the FFS beneficiaries is not significantly different from ADRD prevalence of the US population aged ≥65 years or of those enrolled in MA programs. This is a potentially problematic assumption given that health status differs by MA versus FFS [7] and that enrollment varies by subgroup [8,9] and geography [10]. We were unable to account for these differences because CMS did not provide state-level prevalence estimates and the Census program does not publish state-level population projections by age, sex, or race and ethnicity.
Another limitation is related to potential problems with claims data, which relies on the accurate capture of the proper demographic information and diagnosis during the patient encounter [39]. Racial, ethnic, and cultural identification is not documented in the patient clinical record and subsequent claims data. More granular racial and ethnic data are needed to properly identify subgroups, which have unique risks and needs related to ADRD and other chronic diseases. Federal policy requires that vendors of electronic health record software include the capability for capturing expanded demographic information within the patient record. Although CMS claims data provide the best representation of the clinical encounter that is available today, assignment of nonspecific dementia codes, miscoding, or inaccurate coding secondary to misdiagnosis can result in underestimation or overestimation of the prevalence of a disease.
Education has a protective effect against dementia [40,41], but these estimates do not account for educational attainment because individual characteristics linked to socioeconomic status such as educational attainment and income are not reported in medical claims. The impact of excluding educational attainment when calculating these estimates is that it may lead to overestimated group-specific prevalence in 2014, which will lead to overestimated numbers of people with ADRD in the future years.
A final limitation is that we have assumed that the prevalence estimates in 2014 will be constant over time. Given the heterogeneity in research results, some have argued that the evidence is not strong enough to deduce a trend different than a stable age-specific prevalence [4,42–45]. These findings may also be complicated by the temporal changes in dementia diagnosis thresholds and recording practices and shifts in survey methods, among other factors.
Despite these limitations, the estimates of future burden of ADRD are driven more by the rapid growth in the population of those aged >65 years than by the ADRD prevalence estimates in 2014. The public health message remains the same that, given the differences in the growth rates of different population subgroups in the United States, it is critical that culturally sensitive information be provided to obtain a diagnosis as early as possible and to improve the uptake of preventive health behaviors in all racial and ethnic subgroups in the United States.
6. Conclusion
These estimates can be used for public health planning related to providing culturally competent care for the ADRD population and supporting caregivers from diverse backgrounds. Such estimates may provide a basis for planning and interventions, especially for regions that will face a disproportionately high increase of dementia cases due to their current demographic composition. The role of race and ethnicity is part of the novelty of this study, of which the implications were discussed in relation with the national Healthy Brain Initiative public health road map to maintaining cognitive health and to provide guidance for workforce development, public health planning, and caregiving.
Supplementary Material
RESEARCH IN CONTEXT.
Systematic review: We estimated that 5.0 million adults were diagnosed with Alzheimer’s disease and related dementias (ADRD) in the United States, and 12.7 million will be diagnosed by 2050. These are the first estimates of ADRD by age, sex, race and ethnicity, and decade and are based on a nationally representative sample of 28 million Medicare Fee-for-Service beneficiaries. Previous estimates were based on a small, geographically restricted set of survey respondents. Our estimates were based on physician-diagnosed ADRD in a clinical setting; other estimates were based on cognitive impairment evaluated in a research setting.
Interpretation: These results highlight the need for development of culturally sensitive public health–planning efforts related to the future burden of ADRD.
Future directions: Future research about ADRD burden in local areas and by subpopulations will help identify communities expected to bear disproportionate burdens of ADRD.
Acknowledgment
Kimberly A. Lochner, Centers for Medicare & Medicaid Services, Office of Enterprise Data and Analytics. The findings and conclusions in this report are those of the authors and do not necessarily represent the official positions of the Centers for Disease Control and Prevention or the Centers for Medicare and Medicaid Services.
Footnotes
Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jalz.2018.06.3063.
References
- [1].2016 Alzheimer’s disease facts and figures. Alzheimers Dement 2016; 12:459–509. [DOI] [PubMed] [Google Scholar]
- [2].Brookmeyer R, Evans DA, Hebert L, Langa KM, Heeringa SG, Plassman BL, et al. National estimates of the prevalence of Alzheimer’s disease in the United States. Alzheimers Dement 2011; 7:61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 2013;80:1778–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rocca WA, Petersen RC, Knopman DS, Hebert LE, Evans DA, Hall KS, et al. Trends in the incidence and prevalence of Alzheimer’s disease, dementia, and cognitive impairment in the United States. Alzheimers Dement 2011;7:80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Brookmeyer R, Abdalla N, Kawas CH, Corrada MM. Forecasting the prevalence of preclinical and clinical Alzheimer’s disease in the United States. Alzheimer’s Demen 2018;14:121–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].The Healthy Brain Initiative: The Public Health Road Map for State and National Partnerships, 2013–2018 2013 Chicago, IL: Alzheimer’s Association; 2013. [Google Scholar]
- [7].Mirel LB, Wheatcroft G, Parker JD, Makuc D. Health Characteristics of Medicare Traditional Fee-for-Service and Medicare Advantage enrollees: 1999–2004 National Health and Nutrition Examination Survey linked to 2007 Medicare Data (US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics, 2012). [PubMed] [Google Scholar]
- [8].Byhoff E, Harris JA, Ayanian JZ. Characteristics of decedents in Medicare Advantage and traditional Medicare. JAMA Intern Med 2016; 176:1020–3. [DOI] [PubMed] [Google Scholar]
- [9].Miller EA, Decker SL, Parker JD. Characteristics of Medicare advantage and Fee-for-Service beneficiaries upon enrollment in Medicare at Age 65. J Ambul Care Manag 2016;39:231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jacobson G, Damico A, Neuman T, Gold M. Medicare Advantage 2015 Spotlight: Enrollment Market Update. Available at: http://kff.org/medicare/issue-brief/medicare-advantage-2015-spotlight-enrollment-market-update/; 2015. Accessed November 2017.
- [11].Taylor DH Jr, Østbye T, Langa KM, Weir D, Plassman BL. The accuracy of Medicare claims as an epidemiological tool: The case of dementia revisited. J Alzheimers Dis 2009;17:807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, et al. Prevalence of dementia in the United States: The aging, demographics, and memory study. Neuroepidemiology 2007; 29:125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ortman JM, Velkoff VA, Hogan H, An Aging Nation: The Older Population in the United States (United States Census Bureau, Economics and Statistics Administration, US Department of Commerce, 2014). [Google Scholar]
- [14].Colby S, Ortman J. Projections of the size and composition of the US population: 2014 to 2060 (Current Population Reports, P25–1143). Washington, DC: US Census Bureau; 2017. Accessed November 2017. [Google Scholar]
- [15].Bernstein R Census Bureau projections show a slower growing, older, more diverse nation a half century from now (Press Release, CB12–243). Washington, DC: US Census Bureau; 2015. Accessed Mar 2016. [Google Scholar]
- [16].Goodman RA, Lochner KA, Thambisetty M, Wingo TS, Posner SF, Ling SM. Prevalence of dementia subtypes in United States Medicare Fee-for-Service beneficiaries, 2011–2013. Alzheimers Dement 2017; 13:28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Steenland K, Goldstein FC, Levey A, Wharton W. A meta-analysis of Alzheimer’s disease incidence and prevalence comparing African-Americans and Caucasians. J Alzheimer’s Dis 2015;50:71–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimer’s Demen 2016;12:216–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Samper-Ternent R, Kuo YF, Ray LA, Ottenbacher KJ, Markides KS, Al Snih S. Prevalence of health conditions and predictors of mortality in oldest old Mexican Americans and non-Hispanic whites. J Am Med Directors Assoc 2012;13:254–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Haan MN, Mungas DM, Gonzalez HM, Ortiz TA, Acharya A, Jagust WJ. Prevalence of dementia in older Latinos: The influence of type 2 diabetes mellitus, stroke and genetic factors. J Am Geriatr Soc 2003;51:169–77. [DOI] [PubMed] [Google Scholar]
- [21].Gurland BJ, Wilder DE, Lantigua R, Stern Y, Chen J, Killeffer EH, et al. Rates of dementia in three ethnoracial groups. Int J Geriatr Psychiatry 1999;14:481–93. [PubMed] [Google Scholar]
- [22].Clark PC, Kutner NG, Goldstein FC, Peterson-Hazen S, Garner V, Zhang R, et al. Impediments to timely diagnosis of Alzheimer’s disease in African Americans. J Am Geriatr Soc 2005;53:2012–7. [DOI] [PubMed] [Google Scholar]
- [23].Fitten LJ, Ortiz F, Pont on M. Frequency of Alzheimer’s disease and other dementias in a community outreach sample of Hispanics. J Am Geriatr Soc 2001;49:1301–8. [DOI] [PubMed] [Google Scholar]
- [24].Solomon A, Mangialasche F, Richard E, Andrieu S, Bennett DA, Breteler M, et al. Advances in the prevention of Alzheimer’s disease and dementia. J Intern Med 2014;275:229–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Medicare Beneficiary Characteristics. Baltimore, MD: Centers for Medicare and Medicaid Services; 2014. [Google Scholar]
- [26].Unpublished tabular data from the Office of Enterprise Data and Analytics. Baltimore, MD: Centers for Medicare and Medicaid Services; 2016. [Google Scholar]
- [27].Newcomer R, Clay T, Luxenberg JS, Miller RH. Misclassification and selection bias when identifying Alzheimer’s disease solely from Medicare claims records. J Am Geriatr Soc 1999;47:215–9. [DOI] [PubMed] [Google Scholar]
- [28].Erkinjuntti T, Østbye T, Steenhuis R, Hachinski V. The effect of different diagnostic criteria on the prevalence of dementia. N Engl J Med 1997;337:1667–74. [DOI] [PubMed] [Google Scholar]
- [29].Sosa-Ortiz AL, Acosta-Castillo I, Prince MJ. Epidemiology of dementias and Alzheimer’s disease. Arch Med Res 2012;43:600–8. [DOI] [PubMed] [Google Scholar]
- [30].US Department of Health and Human Services. National Plan to Address Alzheimer’s Disease. Available at: https://aspe.hhs.gov/national-plan-address-alzheimer%E2%80%99s-disease-2015-update; 2016. Accessed July 2018.
- [31].Cordell CB, Borson S, Boustani M, Chodosh J, Reuben D, Verghese J, et al. Alzheimer’s Association recommendations for operationalizing the detection of cognitive impairment during the Medicare Annual Wellness Visit in a primary care setting. Alzheimer’s Demen 2013; 9:141–50. [DOI] [PubMed] [Google Scholar]
- [32].Hamilton BE, Martin JA, Osterman MJK, Driscoll AK, Roseen LM. Births: Provisional Data for 2016 (Vital Statistics Rapid Release; No 2). Bethesda, MD: National Center for Health Statistics; 2017. [Google Scholar]
- [33].Older American 2012: Key Indicators of Well-Being. Washington, DC: Federal Interagency Forum on Aging-Related Statistics. US Government Printing Office; 2012. [Google Scholar]
- [34].Redfoot D, Feinberg L, Houser A. The Aging of the Baby Boom and the Growing Care Gap: A Look at Future Declines in the Availability of Family Caregivers. Washington, DC: AARP Public Policy Institute; 2013. [Google Scholar]
- [35].Vitaliano PP, Zhang J, Scanlan JM. Is caregiving hazardous to one’s physical health? A meta-analysis. Psychol Bull 2003;129:946. [DOI] [PubMed] [Google Scholar]
- [36].Sörensen S, Duberstein P, Gill D, Pinquart M. Dementia care: Mental health effects, intervention strategies, and clinical implications. Lancet Neurol 2006;5:961–73. [DOI] [PubMed] [Google Scholar]
- [37].Schulz R, Beach SR. Caregiving as a risk factor for mortality: The Caregiver Health Effects Study. JAMA 1999;282:2215–9. [DOI] [PubMed] [Google Scholar]
- [38].Revisions to payment policies under the physician fee schedule and other revisions to Part B for CY 2017. Fed Regist 2016; 2016:80170. [PubMed] [Google Scholar]
- [39].Douglas MD, Dawes DE, Holden KB, Mack D. Missed policy opportunities to advance health equity: Missed policy opportunities to advance health equity by recording demographic data in electronic health records. Am J Public Health 2015;105:S380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Meng X, D’Arcy C. Education and dementia in the context of the cognitive reserve hypothesis: A systematic review with meta-analyses and qualitative analyses. PLoS One 2012;7:e38268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimers DemenT 2015;11:718–26. [DOI] [PubMed] [Google Scholar]
- [42].Manton K, Gu X, Ukraintseva S. Declining prevalence of dementia in the US elderly population. Adv Gerontol 2005;16:30–7. [PubMed] [Google Scholar]
- [43].Prince M, Ali GC, Guerchet M, Prina AM, Albanese E, Wu YT. Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimer’s Res Ther 2016;8:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Langa KM, Larson EB, Karlawish JH, Cutler DM, Kabeto MU, Kim SY, et al. Trends in the prevalence and mortality of cognitive impairment in the United States: Is there evidence of a compression of cognitive morbidity? Alzheimer’s Demen 2008;4:134–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wu YT, Beiser AS, Breteler MM, Fratiglioni L, Helmer C, Hendrie HC, et al. The changing prevalence and incidence of dementia over time–current evidence. Nature Rev Neurol 2017;13:327. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.