Abstract
miRNAs are potent tools that in principle can be used to control the replication of infectious agents. The objectives of the studies reported here were to design miRNAs that can block the replication of herpes simplex virus 1 and which could be delivered to infected cells via exosomes. We report the following: (1) We designed three miRNAs targeting the mRNA encoding ICP4, an essential viral regulatory protein. Of the three miRNAs, one miRNA401 effectively blocked ICP4 accumulation and viral replication on transfection into susceptible cells. (2) To facilitate packaging of the miRNA into exosomes, we incorporated into the sequence of miRNA401 an exosome-packaging motif. miRNA401 was shown to be packaged into exosomes and successfully delivered by exosomes to susceptible cells, where it remained stable for at least 72 hr. Finally, the results show that miRNA401 delivered to cells via exosomes effectively reduced virus yields in a miRNA401 dose-dependent fashion. The protocol described in this report can be applied to study viral gene functions without actually deleting or mutagenizing the gene.
Keywords: target miRNA, exosome, HSV-1, viral replication
In this issue of Molecular Therapy, Wang et al. offer important insights into the principles to design miRNAs packaged into exosomes that target viral essential genes in order to block viral replication. It may obviate the cumbersome process of deleting the targeted gene to define its function.
Introduction
In principle, controlled diminution of virus production in susceptible cells or accumulation of a specific viral protein can be done in only three ways, i.e., by the use of inhibitors of viral or cellular functions, by mutagenesis of the promoter regulating the expression of a key gene, or by delivering to the susceptible cells a microRNA (miRNA) targeting the mRNA encoding the gene product. In this report, we show that HSV-1 replication can be reduced in a dose-dependent manner by delivering to the cells via exosomes a miRNA designed to target mRNA encoding ICP4, the major regulatory protein of HSV-1. Relevant to this report are the following:
Exosomes are small, relatively uniform-sized vesicles derived from cellular membranes. They contain several key proteins (e.g., CD9, CD63, CD81, CD82, Annexin, Flotillin, etc.),1, 2, 3, 4, 5 and in addition they package proteins, mRNAs, long non-coding RNAs and miRNAs.1, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 Exosomes transport the payload from cell to cell. On entry into recipient cells, the exosome payload is released into cytoplasm.
In the studies described in this report, the desired exosome payload was a miRNA. Incorporation of RNAs into exosomes is sequence dependent and facilitated by hnRNPA2B1, a component of exosomes. hnRNPA2B1 sorts RNAs containing one of two known exosome packaging-associated motifs (EXO-motifs) into exosomes.15 A key function of hnRNPA2B1 is to regulate mRNA trafficking to axons in neural cells that is mediated by binding a 21-nt RNA sequence called RNA trafficking sequence (RTS).21 This sequence contains both of the EXO-motifs.
HSV-1 encodes more than 100 proteins, miRNAs and long non-coding RNAs.13, 22, 23, 24 We selected as the target of the miRNA the mRNA encoding ICP4. The selection was based on two properties of ICP4. Foremost ICP4 is an essential regulatory protein expressed immediately after infection. A less-cogent reason rests on evidence that ICP4 controls its accumulation in infected cells. Thus, as previously reported ICP4 binds as a dimer25, 26 with high affinity to the consensus DNA sequence ATCGTCNNNNYCGRC and with lower affinities to a number of other sequences that are not represented by a unique consensus.26, 27, 28, 29, 30, 31, 32 DNA binding sites have been thoroughly mapped in the domains of several genes. Relevant to this report is a site that conforms to the consensus and spans the transcription initiation site of the mRNA encoding ICP4.26, 29, 31, 33, 34 Binding of ICP4 to that sight inhibits the transcription of its own mRNA. In essence, ICP4 tightly regulates the synthesis of its own mRNA most likely to block the synthesis of excessive amounts of ICP4. In the absence of a surplus, the impact of decreased accumulation of ICP4 mRNA should be readily apparent.
Results
Design and Construction of a miRNA Capable of Suppressing the Replication of HSV-1
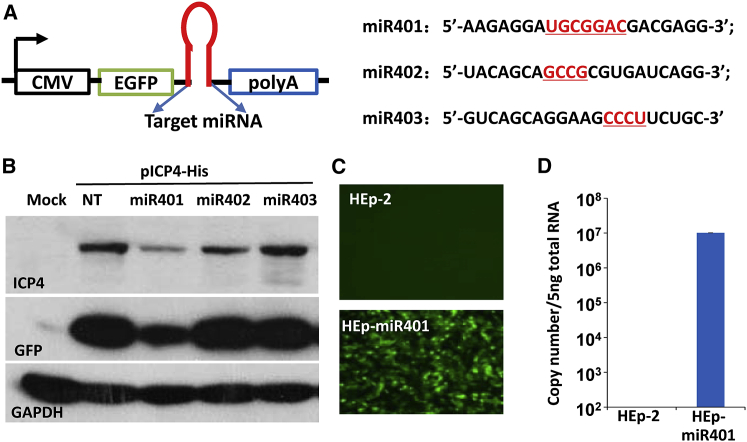
The objective of the first series of experiments was to design a miRNA targeting ICP4, the major regulatory protein of HSV-1. To this end, we have constructed three miRNAs designated miR401, miR402, and miR403. The sequence of each of the miRNAs shown in Figure 1A contains additional sequences embodying EXO-motifs downstream of miRNA seed sequence. As illustrated in Figure 1A, the miRNAs were cloned downstream of an open reading frame encoding EGFP into a miRNA expression vector named “pcDNA6.2-GW/EmGFP-miR-neg control plasmid” as described in the Materials and Methods.
Figure 1.
Derivation of Stable Cell Lines Expressing miRNAs Targeting ICP4
(A) Schematic diagram of the plasmid-encoding miRNAs targeting ICP4. The figure shows the nucleotide sequences of miR401, miR402, and miR403. The nucleotides highlighted in red indicate exosome-packaging-associated motifs (EXO-motifs). (B) Downregulation of ICP4 by designed miRNA. 12-well plates of HEp-2 were co-transfected with 0.5 μg of plasmids expressing miR401, miR402, miR403, or non-target miRNA (NT) and 0.2 μg of plasmid encoding a his-tagged ICP4 (pICP4-his). The cells were harvested after 48 hr. Accumulations of ICP4, GFP, and GAPDH were measured as described in the Materials and Methods. (C) Accumulation of GFP in stably transformed miR401 cells. The fluorescence associated with accumulation of GFP in HEp-2 cells stably transformed with miR401 (HEp-miR401) were captured with the aid of a Leica-inverted fluorescence microscope by a computer-based imaging system as described in the Materials and Methods. (D) Accumulation of miR401 in HEp-miR401 and parental HEp-2 cells. miR401 were quantified and normalized with respect to 18 s rRNA. The data are presented as mean ± SD of duplicate samples.
To test the miRNAs, HEp-2 cells were co-transfected with the miRNA expression vectors (pmiR401, pmiR402, pmiR403) described above and a plasmid encoding ICP4 tagged at the C terminus with His (pICP4-His). As shown in Figure 1B, miR401 was the most effective of the three constructs in suppressing the accumulation of ICP4. The results show that the accumulation of ICP4 is repressed by miR401 at higher efficiency. miR402 showed moderate effect, whereas the non-targeting (NT) and miR403 plasmids had no effect on accumulation of ICP4 (Figure 1B). Figure 1B shows the expression of EGFP in cells transfected with the construct containing miR401, suggesting that that the plasmid is efficiently transfected into HEp-2 cells. Therefore, miR401 was selected for further studies.
The first step in this process was the construction of a stable cell line by transfection of the plasmid into HEp-2 cell under selection of antibiotic of Blasticidin. Single-cell clone from EGPF-positive cells was selected, amplified, and designated HEp-miR401 (Figure 1C). Figure 1D shows expression of mature miR401 by qPCR analysis. As expected, the parental HEp-2 cells failed to express detectable expression of EGFP and miR401 (Figures 1C and 1D, respectively).
Accumulation of Viral Proteins Is Reduced in HEp-miR401 Cells
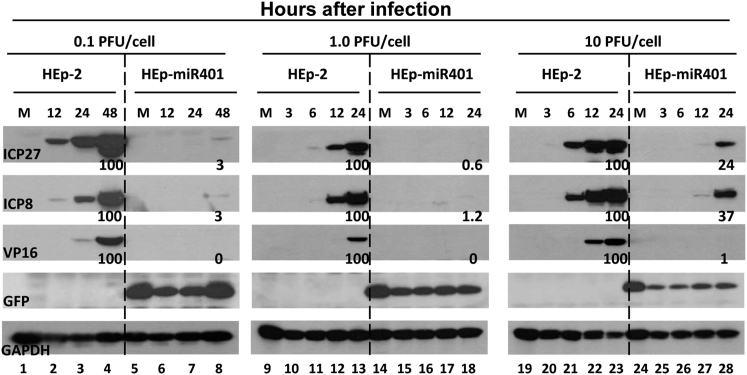
In this series of experiments, replicate cultures of HEp-2 or HEp-miR401 cells were exposed to 0.1, 1, or 10 plaque-forming units (PFU) of HSV-1(F) per cell. The cultures were harvested at indicated times after infection and then solubilized, subjected to electrophoresis in denaturing gels, reacted with antibodies to ICP27, ICP8, or VP16 representing different kinetic classes of virus replication. GFP is a positive indicator of HEp-miR401 stable cell lines, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) served as a loading control (Figure 2). The protein bands were scanned with the aid of an ImageJ scanner. The optical density of the bands was normalized with respect to the optical density of corresponding bands generated from HEp-2 cells at 24 hr (1.0, 10 PFU/cell) or 48 hr (0.1 PFU/cell) after infection. The results show that in cells exposed to 0.1 PFU or 1.0 PFU/cell, the amounts of the viral proteins decreased to undetected level. Accumulation of viral proteins in cells exposed to 10 PFU/cell was significantly lower than those detected in the HEp-2 cells (Figure 2).
Figure 2.
Accumulation of Viral Proteins in Stably Transformed HEp-miR401 Cells Infected with HSV-1(F)
12-well-plates of HEp-miR401 or HEp-2 cells were mock-infected or exposed to 0.1, 1, or 10 PFU of HSV-1(F) per cell. The cells were harvested at indicated hours post-infection. The proteins were electrophoretically separated in 10% denaturing gels and reacted with antibodies against ICP27, ICP8, VP16, GFP, or GAPDH. The protein bands were scanned with the aid of ImageJ scanner. The optical densities of the bands were normalized with respect to the optical density of corresponding bands generated from HEp-2 cells at 24 hr (1.0, 10 PFU/cell) or 48 hr (0.1 PFU/cell) after infection.
miR401 Inhibits the Replication of HSV-1(F) Virus
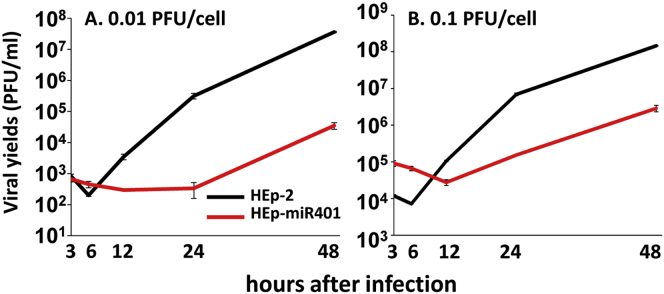
In this series of experiments, replicate cultures of HEp-2 or HEp-miR401 cells were exposed for 0.1 or 0.01 PFU of HSV-1(F) per cell. The cells harvested at the times shown in Figure 3 and viral progeny were titrated on Vero cells. The results shown indicate that the accumulations of virus in HEp-miR401 cells were significantly lower than those obtained from infected HEp-2 cells.
Figure 3.
Virus Yields Recovered from Infected HEp-miR401 Cells
HEp-miR401 or parental HEp-2 cells were exposed to 0.01 or 0.1 PFU of HSV-1(F) per cell. After 2 hr, the inoculum was replaced with fresh medium. The virus progeny was harvested at times shown and titered in Vero cells. The data are presented as mean ± SD of duplicate samples.
Characterization of miR401 Packaged in Exosomes
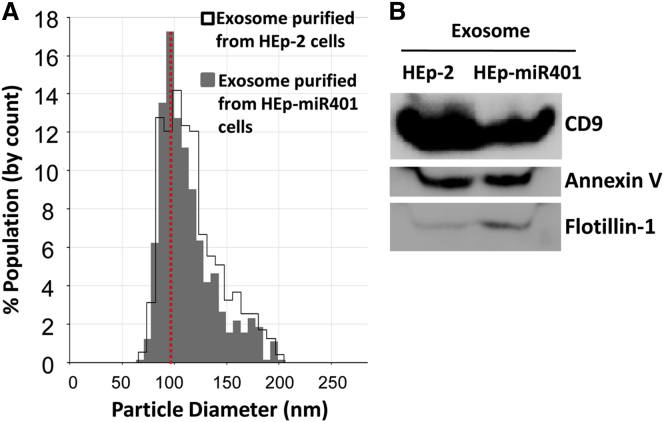
Several experiments were carried out to test the hypothesis that miR401 produced in HEp-miR401 cells is packaged into exosomes. First, exosomes produced in HEp-2 or HEp-miR401 were purified as described in the Materials and Methods. Next, 200 μL of purified exosomes obtained from cultures grown in T150 cells were then measured with respect to size by nanoparticle tracking analysis using Izon’s qNano technology. The results (Figure 4A) show that the exosomes purified from HEp-2 cells or HEp-miR401 cells overlapped in size and formed a single band ranging from 75–150 nm in size.
Figure 4.
Characterization of Exosomes Purified from Extracellular Medium Harvested from HEp-2 or HEp-miR401 Cell Cultures
(A) Exosomes purified from HEp-miR401 or parental HEp-2 cells conform with known size of exosomal particles. Cultured HEp-2 or HEp-miR401 cells each containing 1 × 107 cells were rinsed with PBS and incubated in serum-free medium. After 18 hr, exosomes were isolated from collected cells by using Total Exosome Isolation Reagent (Thermo Fisher, cat. no. 4478359). Particle size distribution and number of isolated exosomes were determined by Izon’s qNano technology as described in the Materials and Methods. (B) Characterization of purified exosomes with respect to the presence of exosome-associated proteins. Purified exosomes were lysed with RIPA lysis buffer, and 45 μg of exosome proteins were subjected to electrophoresis in denaturing gels and reacted with antibodies to exosome marker proteins CD9, Annexin V, or Flotillin-1, respectively.
Next, the purified exosomes were tested for the presence of proteins associated with exosomes that is CD9, Annexin V, and Flotillin-1. In brief, purified exosomes were lysed by RIPA lysis buffer, 45 μg of solubilized exosome protein subjected to electrophoresis in denaturing gels, and reacted with antibodies to CD9, Annexin V, or Flotillin-1 (Figure 4B). The results indicate that the exosome proteins were present in purified exosomes derived from HEp-2 or HEp-miR401 cell lines.
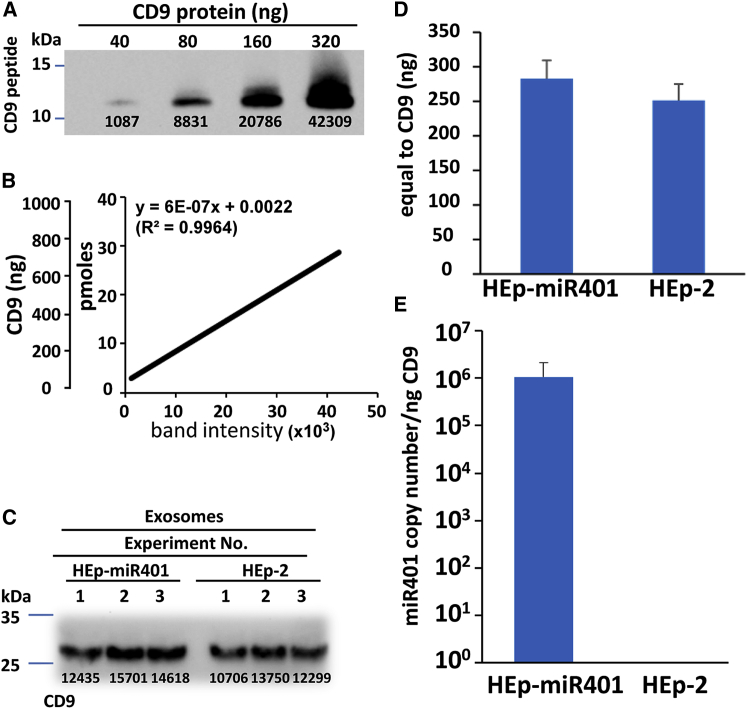
To quantify the amounts of miRNA packaged in exosomes, we selected CD9 as an indicator of exosome concentration. To quantify CD9 protein, we used an 11-kDa CD9 fragment purchased from Sino Biological as a substitute for the full-size CD9 protein. In brief, serial dilutions of CD9 protein fragment were subjected to electrophoresis in denaturing gels and reacted with antibodies to CD9 (Figure 5A), and the bands intensities were quantified using ImageJ to construct a standard curve. Equivalence of full-length of CD9 protein (28 kDa) was calculated accordingly (Figure 5B). Next, 200 μL of exosome preparations was purified from either T150 of HEp-miR401 or T150 of parental HEp-2 cells in three independent experiments. Fifty microliters of preparation from each was subjected to electrophoresis in denaturing gels and reacted with antibodies to CD9 (Figure 5C), and the band intensities were quantified using ImageJ. The results showed that on average, 50 μL of purified exosomes equaled 283 ng of CD9 obtained from HEp-miR401 and 251 ng of CD9 obtained from HEp-2 parental cells (Figure 5D). We measured the amounts of miR401 packaged in exosomes produced in the two cell lines. The results were that in exosomes produced in HEp-miR401 cells, 1 ng of CD9 corresponded to 1 × 106 miR401. miR401 was not detected in exosomes produced in HEp-2 cells (Figure 5E).
Figure 5.
Analysis of miR401 from Purified Exosomes
(A and B) Quantification of full-length CD9 protein (28 kDa) from band intensities of a CD9 protein fragment (11 kDa). (A) Immunoblot of standard dilution of CD9 protein fragment purchased from Sino Biological. Band intensities were quantified using ImageJ. (B) Standard curve relating band intensities of the 11-kDa CD9 fragment in (A) to full-size 28-kDa CD9 protein. (C and D) Quantitative analysis of purified exosomes with respect to CD9 content. Exosomes in 50 μL amounts purified from either extracellular medium of HEp-miR401 or HEp-2 cell cultures were lysed with RIPA lysis buffer and loaded in triplicate onto a 12% denaturing gel. The electrophoretically separated bands were reacted with CD9 antibody, and band intensities were quantified using ImageJ (C). (D) Calculated amounts of CD9 protein in 50 μL of purified exosomes on the basis of data presented in (B). The amounts of recovered CD9 were 283 ng from HEp-miR401 versus 251 ng from HEp-2 cells. (E) Quantitative analysis of miR401 from purified exosomes. miR401 were extracted from exosomes containing 1 ng of equivalent of CD9. The data are presented as mean ± SD of triplicate samples.
Delivery of miR401 by Exosomes into HEp-2 Cells
The experiments described below were designed to measure the entry and stability of miR401 delivered by exosomes to recipient HEp-2 cells. Replicate cultures of HEp-2 cells containing 2.5 × 105 cells were each exposed to 10 μg of exosomes purified from extracellular medium of HEp-miR401 cell cultures. The HEp-2 cells were harvested at 2, 8, 12, 24, 48, or 72 hr after exposure, extensively rinsed with PBS, and analyzed for the miR401 content by qPCR as described in Materials and Methods. The results (Figure 6) suggest that miR401 was efficiently delivered by exosomes into recipient cells by 2 hr after exposure and that it remained stable for at least 24 hr then decreased approximately 10-fold by 72 hr.
Figure 6.
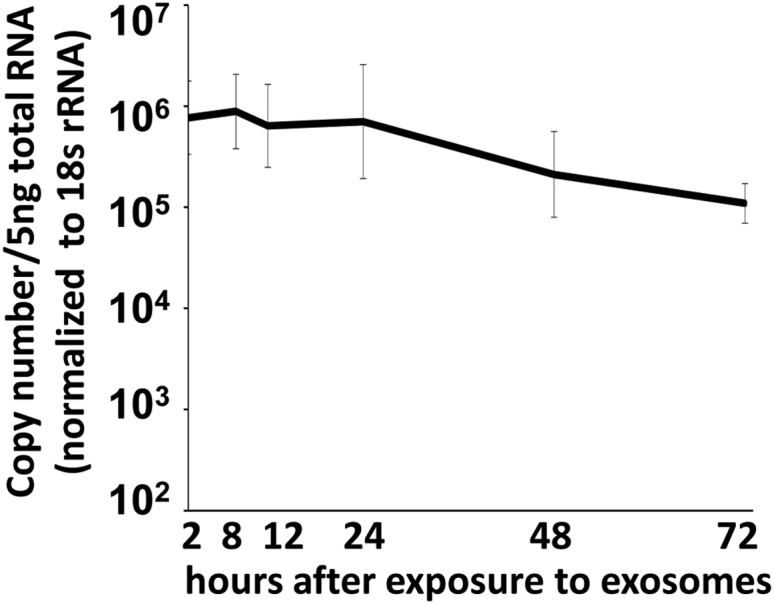
Penetration of miR401 Contained in Exosomes into HEp-2 Recipient Cells
Replicate HEp-2 cultures containing 2.5 × 105 cells were exposed to 10 μg of exosomes purified from HEp-miR401 cells for 2, 8, 12, 24, 48, or 72 hr. The cells were harvested and extensively rinsed with PBS, miR401 was extracted, quantified, and normalized with respect to 18 s rRNA. The data are presented as mean ± SD of duplicate samples.
Exosome-Delivered miR401 Inhibits HSV-1 Replication in a Dose-Dependent Manner
In this series of experiments, we examined whether miR401 delivered via exosome is effective in diminishing the replication of HSV-1. We report two series of experiments.
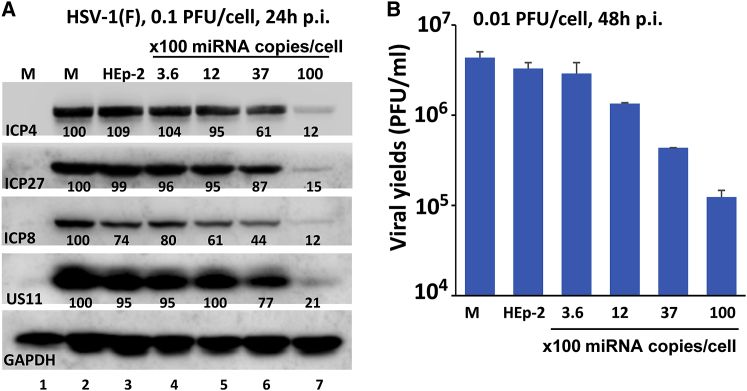
In the first replicate cultures each containing 2.5 × 105 HEp-2 cells were exposed for 12 hr to purified exosomes produced in HEp-miR401 cells or HEp-2 cells. The amounts of miR401 contained in the exosomes ranged from 360 to 10,000 copies per cell. The culture exposed to exosomes produced in HEp-2 cell was equivalent in amount to the highest concentration of exosomes containing miRNA401. After 12 hr of incubation, the cells were rinsed, exposed to 0.1 PFU of HSV-1(F) per cell, and harvested at 24 hr. The cell lysate was subjected to an electrophoresis in denaturing gels and reacted with antibodies to ICP4, ICP27, ICP8, or US11. Figure 7A shows that the accumulations of viral proteins ICP27, ICP8, and US11 decreased in HEp-2 exposed to exosomes containing miR401 in a dose-dependent manner.
Figure 7.
Inhibition of Viral Gene Expression and Replication by Exosome-Mediated miR401
(A) Accumulation of selected viral proteins in cells exposed to exosomes containing miR401. Cultures containing 2.5 × 105 HEp-2 cells were incubated with purified exosomes from HEp-miR401 cells. The miR401 copy numbers were determined as described in the legend of Figure 5. Exosomes purified from HEp-2 parental cells served as controls. After 12 hr of incubation, the cells were exposed to 0.1 PFU of HSV-1(F) per cell. After 24 hr, the cells were harvested and the cells lysates were electrophoretically separated in a 10% denaturing gel and reacted with indicated antibodies. The band density was normalized with respect to GAPDH and the yields obtained in mock-infected cells. (B) Virus yield from cells treated with exosomes containing miR401. 2.5 × 105 HEp-2 cells were exposed to purified exosomes as described in (A) and then infected with 0.01 PFU per cell. After 2 hr, the inoculum was replaced with fresh culture medium. The infected cells were harvested at 48 hr post-infection (p.i.), and the virus yields were titered in Vero cells. The data are presented as mean ± SD of duplicate samples.
The second series of experiments was a replica of the first, except that the infected cells were harvested at 48 hr after infection, and the virus produced in the infected cells was titered in Vero cells. The results shown in Figure 7B indicate that the accumulation of virus in cells exposed to exosomes containing miR401 decreased significantly in a dose-dependent manner, as predicted by the effects of miR401 on the accumulation of viral proteins.
Discussion
The objectives of the studies described in this report were two-fold. Foremost, the question posed was whether a single miRNA targeting a viral mRNA delivered to susceptible cells by transfection could block the replication of HSV-1. The results presented in this report show that of the three miRNAs designed to target the miRNA encoding ICP4, the major HSV-1 regulatory protein one, miRNA401 effectively blocked the accumulation of the protein and the production of infectious virus.
The second objective of the studies was to determine whether inhibitory amounts of miRNA targeting ICP4 mRNA could be delivered to susceptible cells in exosomes. As noted in the Introduction, exosomes are small, relatively uniform-sized vesicles derived from cellular membranes that package and export to other cells proteins, mRNAs, miRNAs, and long non-coding RNAs.1, 10, 18, 20, 35, 36 Recent studies have shown that packaging of RNAs is not random, but rather it is facilitated by short nucleotide sequences designated as EXO-motifs.15 To enhance the packaging of miRNAs tested in this study, EXO-motifs were incorporated in the sequence of miRNAs designed to target ICP4 mRNA.
The result reported here show that miRNA401 is packaged in exosomes and is readily delivered to susceptible cells. We have also shown that the miRNA delivered via exosomes persists in recipient cells for at least 72 hr. We have quantified the packaged miRNA in terms of miRNA copies per ng of CD9, a key protein component of the exosomes.1, 37, 38 Last, we have shown that miRNA401 delivered to cells in exosomes reduces viral yields in a dose-dependent manner.
The results presented in this report also show that miRNAs can be used to define the targeted gene function as well as block viral replication if the targeted gene plays an essential role in viral replication. In many instances, it may obviate the cumbersome process of deleting the targeted gene to define its function.
Materials and Methods
Cell Lines and Virus
HEp-2 and Vero cells were obtained from the American Type Culture Collection and were cultured in DMEM (high glucose) supplemented with 5% (v/v) fetal bovine serum (FBS) or 5% (v/v) newborn calf serum (NBCS), respectively. HSV-1(F), the prototype HSV-1 strain used in this laboratory39 was propagated and titrated on Vero cells.
Antibodies
Antibodies against ICP27, ICP4,40 ICP8 (Rumbaugh Goodwin Institute for Cancer Research), VP16,41 and US1142 have been described elsewhere. Additional antibodies used in this study were anti-GFP monoclonal antibody (cat no. KM8009, Sungene Biotech), anti-His-tag (cat no. 66005-1-Ig, Proteintech Group), and anti-GAPDH (cat no. 2118, Cell Signaling Technology). The exosomal marker antibody kit against CD9, Annexin V,1, 43 or Flotillin-12, 3 were purchased from Cell Signaling Technology (cat no. 74220).
Proteins
CD9 protein fragment (11 kDa; cat no. 11029-H08H-10) was purchased from Sino Biological (Beijing, China).
Plasmid Construction
Three sets of distinct miRNA sequences targeting for ICP4 coding sequence were designed online by using “BLOCK-iT RNAi Designer”44, 45 (http://rnaidesigner.thermofisher.com/rnaiexpress/). The sequence of the miRNAs were further modified by the addition of at least one of the known EXO-motifs that facilitates packaging of the miRNAs into exosomes.15 The miRNAs were synthesized by IGE Biotechnology (Guangzhou, China) according to the sequence provided to them. The sequences of miRNAs are as follows: miR401, 5′-AAGAGGATGCGGACGACGAGGGTTTTGGCCACTGACTGACCCTCGTCGCGCATCCTCTT-3′; miR402, 5′-TACAGCAGCCGCGTGATCAGGGTTTTGGCCACTGACTGACCCTGATCACGGCTGCTGTA-3′; miR403, 5′-GTCAGCAGGAAGCCCTTCTGCGTTTTGGCCACTGACTGACGCAGAAGGTTCCTGCTGAC-3′. Underlines indicate the mature miRNA sequence. Synthesized miRNA fragments were digested with BamHI and XhoI restriction enzymes and cloned into the corresponding sites of pcDNA6.2-GW/EmGFP-miR-neg control plasmid (Invitrogen).
The His-tagged ICP4 was obtained by PCR using primers: forward, 5′-GAATTCATGGCGTCGGAGAACAAGCAGCGCCC-3′; reverse, 5′-CTCGAGTTATCAGTGATGGTGATGGTGATG CAGCACCCCGTCCCCCTCGAACGCGC-3′. The PCR fragment was then subcloned in the multiple cloning site of pcDNA3.1(+). The resulting plasmids were named pICP4-His.
Generation of Stable Cell Lines
The stable cell line expressing miRNA targeting ICP4 was generated by transfection of miR401 plasmid into HEp-2 cells. After 48 hr transfection, the cells were selected by adding antibiotic blasticidin (Solarbio Life Sciences) to a final concentration of 6 μg/mL. GFP-positive single-cell-derived colony was selected and cultured in 5% FBS DMEM complete medium with 6 μg/mL of blasticidin. The cell line was monitored for expression of GFP and miRNA401.
Exosome Isolation and Quantification
Cells seeded in T150 flask for 24 hr were extensively rinsed with PBS and then incubated in serum-free medium. After 18 hr, the cell-free extracellular medium was centrifuged at 2000 × g for 30 min. The supernatant fluid was harvested, mixed with recommended dose of Total Exosome Isolation kit reagent (Thermo Fisher, catalog no. 4478359), stored overnight at 4°C, and then centrifuged for 1 hr. The pelleted exosomes were then resuspended in 200 μL of PBS or were lysed in RIPA buffer and then quantified by a bicinchoninic acid (BCA) assay using the Enhanced BCA Protein Assay Kit (Beyotime Biotechnology, China) according to manufacturer’s instructions. Exosome protein content was determined by calibration against standard curve, which was prepared by plotting the absorbance at 562 nm versus BSA standard concentration.
Exosome Size Analysis
Exosome size distribution analysis was done using the qNano system (Izon, Christchurch, New Zealand). Izon’s qNano technology (http://izon.com) was employed to detect extracellular vesicles passing through a nanopore by way of a single-molecule electrophoresis.46 In practice, it enables accurate particle-by-particle characterization of vesicles from 75 to 150 nm in size of exosomes, without averaging the particle sizes. Purified exosomes were diluted to 1:10 in PBS with 0.05% Tween 20, vigorously shaken, and measured by using an NP150 (A45540) nanopore aperture according to the manufacturer’s instructions. Data processing and analysis were carried out on the Izon Control Suite software v3.3 (Izon Science).
qRT-PCR for miRNA
Total RNAs from cells and liquid exosomes were isolated using TRIzol reagent (Thermo Fisher Scientific) and TRIzol LS reagent (Thermo Fisher Scientific) according to the respective manufacturer’s instructions. The procedure was performed as described.22 The miRNA tested were reverse-transcribed from 50 ng total RNA in duplicate by specific stem-loop primer as described in the TaqMan miRNA reverse transcription kit (Applied Biosystems). The expression of miRNA was determined by real-time PCR using TaqMan Universal Master Mix II kit purchased from Applied Biosystems. miRNA copy number was normalized by comparison with cellular 18 s rRNA. The primers of miR401 were designed according to Chen et al.47 and synthesized by Ige Biotechnology. The sequences are as follows: miR401 stem loop primer, 5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCCTCGT-3′; forward primer, 5′-TGCTGGCGAAGAGGATGC-3′; reverse primer, 5′-CCAGTGCAGGGTCCGAGGTA-3′; probe, 5′-(6-FAM)CTGGATACGACCCTCGTC(MGB)-3′.
Immunoblot Assays
Purified exosomes were harvested and lysed with a RIPA lysis buffer (Beyotime) supplemented with 1 mM protease inhibitor PMSF (Beyotime) and phosphatase inhibitor (Beyotime). Cell lysates were heat denatured, separated by SDS-PAGE, and transferred to polyvinylidene difluoride membranes (Millipore). The proteins were detected by incubation with appropriate primary antibody followed by horseradish peroxidase-conjugated secondary antibody (Pierce) and the ECL reagent (Pierce) and exposed to a film, or images were captured using a ChemiDoc Touch Imaging System (Bio-Rad) and processed using ImageLab software. The densities of corresponding bands were quantified using ImageJ software.
Virus Titration
Cells were seeded in 6-well plates or 24-well plates at densities of 1 × 106 cells per well or 2.5 × 105 cells per well, respectively, for 24 hr and then exposed to 0.01 or 0.1 PFU of HSV-1(F) per cell. The cells were harvested at 3, 6, 12, 24, and 48 hr post-infection or at indicated time point. Viral progeny was titrated on Vero cells after three freeze-thaw cycles and brief sonication.48
Author Contributions
L.W., X.C., B.R., and G.G.Z. designed research; L.W., X.C., and X.Z. performed research; X.C., B.R., and G.Z. analyzed data and wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Acknowledgments
These studies were supported by grants from the National Nature Science Foundation of China (NSFC 81472826 and NSFC 31600137), Guangzhou Science Technology, Innovation Commission Project (201504010016 to Guangzhou Medical University), and Developmental Funding of Dapeng New District (KY20160302 to Shenzhen International Institute for Biomedical Research).
Contributor Information
Bernard Roizman, Email: bernard.roizman@bsd.uchicago.edu.
Grace Guoying Zhou, Email: zhoug@siitm.org.cn.
References
- 1.Théry C., Zitvogel L., Amigorena S. Exosomes: composition, biogenesis and function. Nat. Rev. Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 2.Kowal J., Tkach M., Théry C. Biogenesis and secretion of exosomes. Curr. Opin. Cell Biol. 2014;29:116–125. doi: 10.1016/j.ceb.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Min L., Shen J., Tu C., Hornicek F., Duan Z. The roles and implications of exosomes in sarcoma. Cancer Metastasis Rev. 2016;35:377–390. doi: 10.1007/s10555-016-9630-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Higginbotham J.N., Demory Beckler M., Gephart J.D., Franklin J.L., Bogatcheva G., Kremers G.J., Piston D.W., Ayers G.D., McConnell R.E., Tyska M.J., Coffey R.J. Amphiregulin exosomes increase cancer cell invasion. Curr. Biol. 2011;21:779–786. doi: 10.1016/j.cub.2011.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bobrie A., Colombo M., Krumeich S., Raposo G., Théry C. Diverse subpopulations of vesicles secreted by different intracellular mechanisms are present in exosome preparations obtained by differential ultracentrifugation. J. Extracell. Vesicles. 2012;1:18397. doi: 10.3402/jev.v1i0.18397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan Q., Yang L., Zhang X., Peng X., Wei S., Su D., Zhai Z., Hua X., Li H. The emerging role of exosome-derived non-coding RNAs in cancer biology. Cancer Lett. 2018;414:107–115. doi: 10.1016/j.canlet.2017.10.040. [DOI] [PubMed] [Google Scholar]
- 7.Fu Y., Zhang L., Zhang F., Tang T., Zhou Q., Feng C., Jin Y., Wu Z. Exosome-mediated miR-146a transfer suppresses type I interferon response and facilitates EV71 infection. PLoS Pathog. 2017;13:e1006611. doi: 10.1371/journal.ppat.1006611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casadei L., Calore F., Creighton C.J., Guescini M., Batte K., Iwenofu O.H., Zewdu A., Braggio D.A., Bill K.L., Fadda P. Exosome-derived miR-25-3p and miR-92a-3p stimulate liposarcoma progression. Cancer Res. 2017;77:3846–3856. doi: 10.1158/0008-5472.CAN-16-2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang J., Li S., Li L., Li M., Guo C., Yao J., Mi S. Exosome and exosomal microRNA: trafficking, sorting, and function. Genomics Proteomics Bioinformatics. 2015;13:17–24. doi: 10.1016/j.gpb.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valadi H., Ekström K., Bossios A., Sjöstrand M., Lee J.J., Lötvall J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 11.Shi J. Considering exosomal miR-21 as a biomarker for cancer. J. Clin. Med. 2016;5:42. doi: 10.3390/jcm5040042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li W., Li C., Zhou T., Liu X., Liu X., Li X., Chen D. Role of exosomal proteins in cancer diagnosis. Mol. Cancer. 2017;16:145. doi: 10.1186/s12943-017-0706-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalamvoki M., Du T., Roizman B. Cells infected with herpes simplex virus 1 export to uninfected cells exosomes containing STING, viral mRNAs, and microRNAs. Proc. Natl. Acad. Sci. USA. 2014;111:E4991–E4996. doi: 10.1073/pnas.1419338111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahadi A., Brennan S., Kennedy P.J., Hutvagner G., Tran N. Long non-coding RNAs harboring miRNA seed regions are enriched in prostate cancer exosomes. Sci. Rep. 2016;6:24922. doi: 10.1038/srep24922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Villarroya-Beltri C., Gutiérrez-Vázquez C., Sánchez-Cabo F., Pérez-Hernández D., Vázquez J., Martin-Cofreces N., Martinez-Herrera D.J., Pascual-Montano A., Mittelbrunn M., Sánchez-Madrid F. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat. Commun. 2013;4:2980. doi: 10.1038/ncomms3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y., Gu Y., Han Y., Zhang Q., Jiang Z., Zhang X., Huang B., Xu X., Zheng J., Cao X. Tumor exosomal RNAs promote lung pre-metastatic niche formation by activating alveolar epithelial TLR3 to recruit neutrophils. Cancer Cell. 2016;30:243–256. doi: 10.1016/j.ccell.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 17.Beltrami C., Besnier M., Shantikumar S., Shearn A.I., Rajakaruna C., Laftah A., Sessa F., Spinetti G., Petretto E., Angelini G.D., Emanueli C. Human pericardial fluid contains exosomes enriched with cardiovascular-expressed microRNAs and promotes therapeutic angiogenesis. Mol. Ther. 2017;25:679–693. doi: 10.1016/j.ymthe.2016.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang C., Zhang C., Liu L., A X., Chen B., Li Y., Du J. Macrophage-derived mir-155-containing exosomes suppress fibroblast proliferation and promote fibroblast inflammation during cardiac injury. Mol. Ther. 2017;25:192–204. doi: 10.1016/j.ymthe.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Squadrito M.L., Baer C., Burdet F., Maderna C., Gilfillan G.D., Lyle R., Ibberson M., De Palma M. Endogenous RNAs modulate microRNA sorting to exosomes and transfer to acceptor cells. Cell Rep. 2014;8:1432–1446. doi: 10.1016/j.celrep.2014.07.035. [DOI] [PubMed] [Google Scholar]
- 20.Qu L., Ding J., Chen C., Wu Z.J., Liu B., Gao Y., Chen W., Liu F., Sun W., Li X.F. Exosome-transmitted lncARSR promotes sunitinib resistance in renal cancer by acting as a competing endogenous RNA. Cancer Cell. 2016;29:653–668. doi: 10.1016/j.ccell.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Hoek K.S., Kidd G.J., Carson J.H., Smith R. hnRNP A2 selectively binds the cytoplasmic transport sequence of myelin basic protein mRNA. Biochemistry. 1998;37:7021–7029. doi: 10.1021/bi9800247. [DOI] [PubMed] [Google Scholar]
- 22.Han Z., Liu X., Chen X., Zhou X., Du T., Roizman B., Zhou G. miR-H28 and miR-H29 expressed late in productive infection are exported and restrict HSV-1 replication and spread in recipient cells. Proc. Natl. Acad. Sci. USA. 2016;113:E894–E901. doi: 10.1073/pnas.1525674113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Du T., Han Z., Zhou G., Roizman B. Patterns of accumulation of miRNAs encoded by herpes simplex virus during productive infection, latency, and on reactivation. Proc. Natl. Acad. Sci. USA. 2015;112:E49–E55. doi: 10.1073/pnas.1422657112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roizman B., Knipe D.M., Whitley R.J. Herpes simplex viruses. In: Knipe D.M., Howley P.M., editors. Fields’ Virology. Sixth Edition. Lippincott, Williams & Wilkins; 2013. pp. 1823–1897. [Google Scholar]
- 25.Metzler D.W., Wilcox K.W. Isolation of herpes simplex virus regulatory protein ICP4 as a homodimeric complex. J. Virol. 1985;55:329–337. doi: 10.1128/jvi.55.2.329-337.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michael N., Roizman B. Binding of the herpes simplex virus major regulatory protein to viral DNA. Proc. Natl. Acad. Sci. USA. 1989;86:9808–9812. doi: 10.1073/pnas.86.24.9808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DiDonato J.A., Muller M.T. DNA binding and gene regulation by the herpes simplex virus type 1 protein ICP4 and involvement of the TATA element. J. Virol. 1989;63:3737–3747. doi: 10.1128/jvi.63.9.3737-3747.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DiDonato J.A., Spitzner J.R., Muller M.T. A predictive model for DNA recognition by the herpes simplex virus protein ICP4. J. Mol. Biol. 1991;219:451–470. doi: 10.1016/0022-2836(91)90186-a. [DOI] [PubMed] [Google Scholar]
- 29.Faber S.W., Wilcox K.W. Association of the herpes simplex virus regulatory protein ICP4 with specific nucleotide sequences in DNA. Nucleic Acids Res. 1986;14:6067–6083. doi: 10.1093/nar/14.15.6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kristie T.M., Roizman B. DNA-binding site of major regulatory protein alpha 4 specifically associated with promoter-regulatory domains of alpha genes of herpes simplex virus type 1. Proc. Natl. Acad. Sci. USA. 1986;83:4700–4704. doi: 10.1073/pnas.83.13.4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kristie T.M., Roizman B. Alpha 4, the major regulatory protein of herpes simplex virus type 1, is stably and specifically associated with promoter-regulatory domains of alpha genes and of selected other viral genes. Proc. Natl. Acad. Sci. USA. 1986;83:3218–3222. doi: 10.1073/pnas.83.10.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michael N., Spector D., Mavromara-Nazos P., Kristie T.M., Roizman B. The DNA-binding properties of the major regulatory protein alpha 4 of herpes simplex viruses. Science. 1988;239:1531–1534. doi: 10.1126/science.2832940. [DOI] [PubMed] [Google Scholar]
- 33.Kristie T.M., Roizman B. Separation of sequences defining basal expression from those conferring alpha gene recognition within the regulatory domains of herpes simplex virus 1 alpha genes. Proc. Natl. Acad. Sci. USA. 1984;81:4065–4069. doi: 10.1073/pnas.81.13.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leopardi R., Michael N., Roizman B. Repression of the herpes simplex virus 1 alpha 4 gene by its gene product (ICP4) within the context of the viral genome is conditioned by the distance and stereoaxial alignment of the ICP4 DNA binding site relative to the TATA box. J. Virol. 1995;69:3042–3048. doi: 10.1128/jvi.69.5.3042-3048.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simons M., Raposo G. Exosomes—vesicular carriers for intercellular communication. Curr. Opin. Cell Biol. 2009;21:575–581. doi: 10.1016/j.ceb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 36.Abd Elmageed Z.Y., Yang Y., Thomas R., Ranjan M., Mondal D., Moroz K., Fang Z., Rezk B.M., Moparty K., Sikka S.C. Neoplastic reprogramming of patient-derived adipose stem cells by prostate cancer cell-associated exosomes. Stem Cells. 2014;32:983–997. doi: 10.1002/stem.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boker K.O., Lemus-Diaz N., Rinaldi Ferreira R., Schiller L., Schneider S., Gruber J. The Impact of the CD9 Tetraspanin on Lentivirus Infectivity and Exosome Secretion. Mol Ther. 2017;26:634–647. doi: 10.1016/j.ymthe.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu S., Cao H., Shen B., Feng J. Tumor-derived exosomes in cancer progression and treatment failure. Oncotarget. 2015;6:37151–37168. doi: 10.18632/oncotarget.6022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ejercito P.M., Kieff E.D., Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J. Gen. Virol. 1968;2:357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- 40.Ackermann M., Braun D.K., Pereira L., Roizman B. Characterization of herpes simplex virus 1 alpha proteins 0, 4, and 27 with monoclonal antibodies. J. Virol. 1984;52:108–118. doi: 10.1128/jvi.52.1.108-118.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McKnight J.L., Kristie T.M., Roizman B. Binding of the virion protein mediating alpha gene induction in herpes simplex virus 1-infected cells to its cis site requires cellular proteins. Proc. Natl. Acad. Sci. USA. 1987;84:7061–7065. doi: 10.1073/pnas.84.20.7061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roller R.J., Roizman B. The herpes simplex virus 1 RNA binding protein US11 is a virion component and associates with ribosomal 60S subunits. J. Virol. 1992;66:3624–3632. doi: 10.1128/jvi.66.6.3624-3632.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Conde-Vancells J., Rodriguez-Suarez E., Embade N., Gil D., Matthiesen R., Valle M., Elortza F., Lu S.C., Mato J.M., Falcon-Perez J.M. Characterization and comprehensive proteome profiling of exosomes secreted by hepatocytes. J. Proteome Res. 2008;7:5157–5166. doi: 10.1021/pr8004887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang W., Fang H., Groom L., Cheng A., Zhang W., Liu J., Wang X., Li K., Han P., Zheng M. Superoxide flashes in single mitochondria. Cell. 2008;134:279–290. doi: 10.1016/j.cell.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ibrišimović M., Kneidinger D., Lion T., Klein R. An adenoviral vector-based expression and delivery system for the inhibition of wild-type adenovirus replication by artificial microRNAs. Antiviral Res. 2013;97:10–23. doi: 10.1016/j.antiviral.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Momen-Heravi F., Balaj L., Alian S., Tigges J., Toxavidis V., Ericsson M., Distel R.J., Ivanov A.R., Skog J., Kuo W.P. Alternative methods for characterization of extracellular vesicles. Front. Physiol. 2012;3:354. doi: 10.3389/fphys.2012.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen C., Ridzon D.A., Broomer A.J., Zhou Z., Lee D.H., Nguyen J.T., Barbisin M., Xu N.L., Mahuvakar V.R., Andersen M.R. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005;33:e179. doi: 10.1093/nar/gni178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roizman B., Spear P.G. Preparation of herpes simplex virus of high titer. J. Virol. 1968;2:83–84. doi: 10.1128/jvi.2.1.83-84.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]