Abstract
Background
Heightened immune activation and exhaustion drive HIV disease progression and co-morbidities. Vitamin D has pleiotropic immunomodulatory effects, but little is known about the effects of supplementation in HIV. Our study investigates changes in immune activation and exhaustion markers after 12 months of supplementation in virologically-suppressed HIV-infected youth with vitamin D insufficiency.
Methods
This is a randomized, active-control, double-blind trial investigating with 3 different vitamin D3 doses [18,000 (standard/active-control dose), 60,000 (moderate dose) and 120,000 IU/monthly (high dose)] in 8–26 year old HIV-infected youth on combination antiretroviral therapy with baseline serum 25-hydroxyvitamin D (25(OH)D) concentrations ≤30 ng/mL. Only subjects (N=51) who maintained an undetectable HIV-1 RNA over the 12-month study period were included in this analysis.
Results
Baseline serum 25(OH)D concentrations and immune activation/exhaustion markers were not different between groups. By 12 months, 25(OH)D increased significantly within each dosing group with the greatest increase and most sustained concentrations ≥30 ng/mL in the high-dose group. Overall, all measured markers decreased with CD4 activation (CD4+CD38+HLA-DR+), CD8 activation (CD8+CD38+HLA-DR+), CD4 exhaustion (CD4+CD38+HLA-DR+PD1+), and inflammatory monocytes (CD14+CD16+) reaching statistical significance. When analyzed separately, there were no significant decreases in the moderate- or standard-dose groups, but CD4 and CD8 activation and inflammatory monocytes decreased significantly in the high-dose group.
Conclusions
Vitamin D supplementation decreased markers of T-cell activation/exhaustion and monocyte activation in HIV-infected youth, with subjects given the highest dose (120,000 IU/month) showing the greatest decreases. These data suggest that high-dose vitamin D supplementation may attenuate immune activation and exhaustion and serve as adjuvant therapy to antiretroviral therapy in HIV.
Keywords: HIV, immune activation, immune exhaustion, vitamin D, randomized-controlled trial, pediatrics and adolescents
INTRODUCTION
While viral suppression with combination antiretroviral therapy (cART) dramatically restores health, HIV-1-infected individuals have more age-related co-morbidities such as cardiovascular disease, neurocognitive impairment, renal disease, non-AIDS-defining malignancies, and osteoporosis than individuals in the general population [1]. While the etiology of these co-morbidities is multi-factorial, heightened immune activation and immune dysfunction have been shown to play critical roles in their development [2]. Moreover, immune activation and immune exhaustion are associated with serious HIV complications like poorer CD4+ T-cell reconstitution after cART initiation, more rapid disease progression, and higher risk of all-cause mortality [3].
Similar to their adult counterparts, data show that HIV-1-infected youth are also at an increased risk of development of these HIV-related co-morbidities later in life, despite few clinical manifestations at their younger age [4–6]. Previous data, including our own, have shown that this population exhibits a comparable pattern of increased immune activation and exhaustion as HIV-1-infected adults [7]. Given that they will live for many decades with exposure to this state of chronic immune dysfunction, the implications could be profound as the population ages. Developing complementary strategies to cART aimed at decreasing residual immune activation and immune exhaustion before clinical manifestations develop may greatly reduce future HIV-associated co-morbidities in youth.
Vitamin D supplementation is arguably an under-utilized potential adjuvant to cART based on the currently available data in both the HIV and general populations. Vitamin D, a naturally-synthesized hormone, plays a significant role in numerous immunomodulatory functions, including extensive effects on both the innate and adaptive immune systems [8–10]. Notably, a number of observational and cross-sectional studies have shown that vitamin D deficiency, as measured by the concentration of circulating 25(OH)D, an established marker of overall vitamin D status [11], impairs immune restoration after cART initiation [12, 13] and hastens HIV disease progression and mortality [14–17].
Few studies, however, have investigated the changes in immune activation and exhaustion markers that are known to be altered in HIV [18–21]. For example, Fabre-Mersseman, et al [20] showed an increase in the CD4/CD8 ratio and a decrease in the % of CD8+CD38+ cells, both indications of a reduction in immune activation, after 6–12 months of 100,000 IU of vitamin D supplementation given every 14 days for 3 months, followed by 100,000 IU/month in 17 subjects on cART. Similarly, Stallings, et al [19] showed that in 50 subjects who were given 7,000 IU/daily for 12 months had a significant increase in % of naïve T-helper cells. Interestingly, in this latter study, vitamin D supplementation appeared effective only in the presence of cART.
Expanding upon these aforementioned studies, we present the results of a randomized-controlled trial exploring the effects of three different vitamin D supplementation doses given monthly over 12 months on immune activation and exhaustion markers. To minimize potential confounders, we chose to limit our analyses to HIV-infected subjects on cART with an undetectable HIV-1 RNA level at baseline that was maintained throughout the study period. Our focus on HIV-1-infected youth represents an innovative approach to potentially identify efficacious strategies to prevent the development of HIV-related co-morbidities before the onset of established disease.
METHODS
Study Design/Population
This is a randomized, active-control, double-blinded trial designed to measure the effects of vitamin D supplementation in HIV-1-infected youth. Subjects were recruited from the HIV clinics of University Hospitals Case Medical Center, Cleveland, OH and Grady Health System, Atlanta, GA via electronic medical record system queries and case manager/provider referrals. Subjects were eligible if they were between 8–25 years of age with documented HIV-1 infection on a stable cART regimen for ≥12 weeks, with ≥6 months cumulative cART duration, HIV-1 RNA level <1,000 copies/mL, with no intent to change cART regimen, diet, sun exposure or exercise routine during study period, and a baseline serum 25(OH)D concentration ≤30 ng/mL (the Endocrine Society’s current definition of vitamin D sufficiency is ≥30 ng/mL [22]). Exclusion criteria included routine vitamin D supplementation >400 IU/day, pregnancy or lactation, acute illness or inflammatory condition, malignancy, parathyroid or calcium disorder, diabetes, creatinine clearance <50 mL/min, liver enzymes ≥2.5 times the upper limit of normal, hemoglobin ≤9.0 g/dL, medication use (e.g., chemotherapy agents, systemic steroids) which could affect results, or unwillingness/inability to comply with study procedures.
Intervention consisted of 3 different monthly vitamin D3 (cholecalciferol) doses [18,000 IU/month (standard dose/active control), 60,000 IU/month (moderate dose) or 120,000 IU/month (high dose) (Tischon Corp., Salisbury, MD)]. Doses were chosen to represent an approximate monthly equivalent to 600 IU/daily (standard dose), 2,000 IU/ daily (moderate dose), and 4,000 IU/ daily (high dose), respectively. Six hundred IU/daily is the current Institute of Medicine’s (IOM) recommended dietary allowance (RDA) of vitamin D across our study population. This amount is considered sufficient by the IOM Food and Nutrition Board to meet the requirements of 97.5% of healthy individuals in each life-stage and sex group. The IOM considers a 25(OH)D concentration of ≥20 ng/mL to be sufficient. Likewise, 4,000 IU/daily is the IOM’s current tolerable upper intake level [23].
The randomization scheme was computer-generated and stratified by efavirenz (EFV) use at entry, an antiretroviral drug that has been shown to affect 25(OH)D concentrations in some studies [24]. Regardless of randomization, subjects took two capsules of vitamin D3 orally at baseline and then monthly after being prompted by a reminder phone call from study staff; capsules looked identical regardless of dose. Subjects returned for study visits every 3 months. Representative capsules were sent to an independent laboratory (Analytical Research Laboratories, Oklahoma City, OK) at regular intervals during the study period to ensure continued potency of each dose.
The study was reviewed and approved by the Institutional Review Boards of University Hospital Case Medical Center, Emory University and Grady Health System. All parents or legal guardians and subjects ≥18 years of age gave written informed consent to participate in the study. Subjects aged 17 years of age signed a written consent along with their parent or legal guardian. Subjects between the ages of 8–10 years gave verbal assent and those 11–16 years gave written assent. The study was registered on clinicaltrials.gov (NCT01523496).
Here, we present the pre-planned analysis that assessed changes in immune activation and exhaustion markers from baseline to month 12. By design and because of the confounding effect of viral replication on the markers of interest, the primary analysis focused on subjects who maintained an HIV-1 RNA level <80 copies/mL throughout the 12-month study period. Less than 80 copies/mL was chosen to define virologic suppression because there were several different assays used in the clinical laboratories with varying lower limits of detection. Less than 80 copies/mL was the highest cut-off among these assays to define undetectable HIV-1 RNA.
Study Assessments
Clinical evaluations
Relevant data were obtained by questionnaire, including demographics, current and past medical history, and tobacco use. Further information was also collected from the subjects’ medical records including past and current medical diagnoses, CD4 nadir, detailed past and current antiretroviral (ARV) and non-ARV medication use, HIV diagnosis date, and acquisition method (perinatal or horizontal). Targeted physical examination and weight and height measurements were obtained in all subjects.
Laboratory evaluations
Blood was collected from all subjects after at least an 8-hour fast. Whole blood was collected in EDTA tubes for immediate plasma and peripheral blood mononuclear cells (PBMCs) isolation and cryopreservation without prior thawing until analysis. For all laboratory assessments, laboratory personnel were blinded to clinical information and HIV status.
Serum concentrations of 25(OH)D were measured as the best measure of overall vitamin D status [11]. Samples were analyzed at the local site of the respective participant. Serum 25(OH)D concentrations were measured using either an automated chemiluminescent technique (IDS-iSYS automated machine, Immunodiagnostic Systems, Inc., Fountain Hills, AZ) or a competitive immunoassay (ADVIA Centaur XP System, Siemens Healthcare Diagnostics, Inc., Tarrytown, NY).
CD4+ and CD8+ T-cell and their respective levels of activation and exhaustion, as well as monocyte subsets, were assessed via flow cytometry. All flow cytometry staining was performed on the cryopreserved PBMCs that were thawed in a 37°C water bath and used immediately. Multi-parametric flow cytometric analysis was performed on these cells according to standard procedures, and the following panel of fluorochrome-labeled antibodies was used: anti-CD3-Alexa 700 (clone SP34-2), anti-CD8-allophycocyanin (APC)-Cy7 (clone SK1), anti-CD16-APC (clone 3G8), anti-CD38-phycoerythrin (PE) (clone HIT2), anti-CD86-fluroescein isothiocyanate (FITC) (clone FUN-1), and anti-HLA-DR-peridinin chlorophyll protein (PerCP)-Cy5.5 (clone G46-6) (BD Bioscience, San Jose, CA), anti-CD4-Pacific Blue (clone OKT4), and anti-CD20-Brilliant Violet 650 (clone 2H7) (Biolegend, San Diego, CA), anti-CD279 (PD-1)-PE-Cy7 (clone J105) (eBioscience, San Diego, CA), anti-CD14-ECD (RMO52) (Beckman Coulter, Brea, CA), and Live/Dead Fixable Aqua (Invitrogen, Carlsbad, CA).
Monocytes were identified by size and granularity, then as CD3−, CD20−, CD8−, CD4dim before being categorized into subsets by CD14 and CD16 expression: CD14+CD16− (classical), CD14+CD16+ (pro-inflammatory), and CD14dimCD16+ (non-classical/patrolling). T-cells were identified by size and granularity, as well as CD3+ and CD4+ or CD8+. T-cell activation was assessed by CD38 and HLA-DR expression, and T-cell exhaustion was assessed by CD38, HLA-DR, and programmed cell death-1 (PD-1) expression. Flow cytometric acquisition was performed on an LSRII cytometer driven by FACS DiVa software and analyzed using FlowJo software (Treestar, Ashland, OR).
Absolute CD4+ T-cell count, CD8+ T-cell count and plasma HIV-1 RNA level were concomitantly measured as markers of HIV disease activity.
Statistical Considerations
Analyses were performed using intent-to-treat principles based on randomized treatment assignment which used all available data, and missing values were ignored. Variables are described for the HIV-1-infected group combined (N = 51) and by dosing group (standard, moderate, and high). Continuous measures are described by medians and interquartile ranges, and nominal variables are described with frequencies and percents.
Nominal variables were compared using χ2 analysis or Fisher’s exact test. Continuous measures were tested for normality. For between-group comparisons (baseline and changes from baseline to 12 months), normally-distributed variables were compared using the t-test, and non-normally-distributed variables were compared using Wilcoxon rank sum test. For within-group changes from baseline to 12 months, normally-distributed variables were compared with the paired t-test, and non-normally-distributed variables were compared with Wilcoxon signed rank test.
Appropriate two-sample tests were used to assess marker differences in sub-groups for dichotomous variables (e.g. baseline 25(OH)D concentration <20 vs. ≥20 ng/mL). Correlations between variables of interest were assessed using Spearman correlation coefficients for continuous variables.
All statistical tests were two-sided with a 0.05 significance level. Analyses were performed with SAS version 9.2 (SAS Institute, Cary, NC).
RESULTS
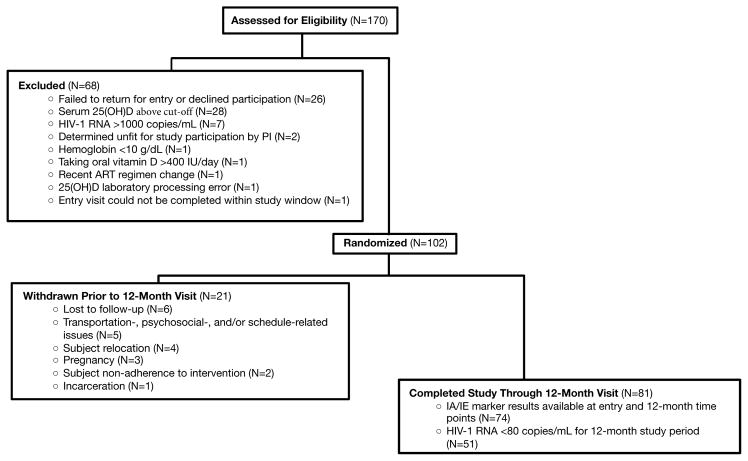
Subjects were recruited from January 2012 – July 2014. One hundred and two subjects were enrolled into the study; 81 subjects completed their 12-month visit (Figure 1). Of these 81 subjects, only those who maintained an undetectable HIV-1 RNA over the 12-month study period were included in this analysis (N=51).
Figure 1.
The baseline characteristics of the 51 subjects are described in Table 1. The median age was 19.8 years with 63% male and 86% black. Baseline median 25(OH)D concentration was 17.4 ng/mL. When these 51 subjects were compared to the other 51 of the enrolled study subjects, % of male and black subjects, as well as age, current CD4+ T-cell count and baseline 25(OH)D were similar between the two groups [male sex: 64% (P=0.32); black race: 94% (P=0.90); age: 21 years (P=0.08); CD4+ T-cell count: 583 cells/mm3 (P=0.27); 25(OH)D: 17.0 ng/mL (P=0.34)].
Table 1.
Baseline Characteristics
| Median (quartile 1, quartile 3) or no. (%) | All Subjects (N=51) | Standard Dose† (N=21) | Moderate Dose‡ (N=18) | High Dose¥ (N=12) | P* |
|---|---|---|---|---|---|
|
| |||||
| Age, years | 19.8 (15.0, 21.6) | 19.9 (15.6, 21.4) | 19.56 (15.0, 23.7) | 18.8 (16.0, 22.8) | 0.97 |
| Male sex | 32 (63%) | 13 (62%) | 12 (67%) | 7 (58%) | 0.89 |
| Black race | 44 (86%) | 19 (91%) | 15 (83%) | 10 (83%) | 0.77 |
| Body mass index, kg/m2 | 21.3 (18.2, 24.8) | 20.3 (17.0, 22.5) | 22.8 (19.2, 27.3) | 21.7 (20.9, 24.2) | 0.09 |
| Tanner stage 5 | 39 (77%) | 15 (71%) | 14 (78%) | 10 (83%) | 0.73 |
| Current smoking | 8 (16%) | 1 (5%) | 4 (22%) | 3 (25%) | 0.20 |
| 25(OH)D, ng/mL | 17.4 (13.2, 24.7) | 14.7 (12.0, 26.0) | 16.4 (14.0, 20.7) | 19.3 (17.0, 20.8) | 0.31 |
| Current CD4, cells/mm3 | 654 (451, 888) | 654 (412, 824) | 724 (548, 929) | 656 (458, 894) | 0.70 |
| Nadir CD4, cells/mm3 | 287 (178, 450) | 221 (27, 312) | 352 (274, 574) | 319 (154, 475) | 0.03 |
| HIV duration, years | 10.8 (2.8, 18.2) | 9.9 (2.7, 15.6) | 13.0 (4.5, 18.5) | 11.9 (4.6, 17.4) | 0.84 |
| ARV duration, years | 6.9 (1.7, 11.4) | 5.8 (1.3, 10.3) | 7.3 (2.5, 11.2) | 9.6 (4.2, 12.8) | 0.59 |
| NRTI duration, years | 6.1 (1.7, 11.2) | 2.6 (1.3, 10.3) | 7.3 (2.5, 10.9) | 8.1 (3.0, 12.8) | 0.44 |
| Current EFV use | 14 (28%) | 5 (24%) | 6 (33%) | 3 (25%) | 0.78 |
| Current TDF use | 37 (73%) | 14 (67%) | 13 (72%) | 10 (83%) | 0.59 |
| Perinatal Transmission | 31 (61%) | 12 (57%) | 12 (67%) | 7 (58%) | 0.82 |
| % CD4+CD38+HLA-DR+a, b | 1.6 (1.2, 2.5) | 1.6 (1.2, 2.2) | 1.7 (1.2, 2.3) | 1.8 (1.3, 2.5) | 0.91 |
| % CD8+CD38+HLA-DR+a | 3.7 (2.4, 6.3) | 3.5 (2.4, 5.6) | 3.5 (2.5, 5.1) | 4.3 (2.3, 6.4) | 0.87 |
| % CD4+CD38+HLA-DR+PD1+a,b | 0.7 (0.5, 1.1) | 0.7 (0.5, 1.1) | 0.7 (0.5, 0.9) | 0.8 (0.5, 1.1) | 0.72 |
| % CD8+CD38+HLA-DR+PD1+a | 1.1 (0.7, 2.0) | 1.4 (0.6, 2.1) | 1.1 (0.7, 1.5) | 1.2 (0.9, 1.8) | 0.66 |
| % CD14+CD16+a,c | 12.2 (8.8, 20.1) | 10.9 (7.8, 18.9) | 11.8 (11.0, 20.2) | 19.5 (8.7, 29.4) | 0.65 |
| % CD14dimCD16+a,c | 9.5 (5.7, 22.1) | 11.8 (5.7, 25.1) | 7.4 (5.3, 20.3) | 8.4 (6.1, 23.3) | 0.69 |
N.B. P-values with bolded font designate those <0.05;
Standard dose = 18,000 IU given monthly for 12 months (equivalent to 600 IU/daily);
Moderate dose = 60,000 IU given monthly for 12 months (equivalent to 2,000 IU/daily);
High dose = 120,000 IU given monthly for 12 months (equivalent to 4,000 IU/daily);
P value among the three dosing groups;
Data represent the % of that cell type expressing the given phenotype;
1 subject with no data due to cell viability issues;
3 subjects with no data due to cell viability issues.
25(OH)D, 25-hydroxyvitamin D; ARV, antiretroviral; NRTI, nucleoside reverse transcriptase inhibitor; EFV, efavirenz; TDF, tenofovir
Overall smoking prevalence was low within the study population of 51 subjects (N=8). Thirty-three subjects were on a ritonavir-boosted protease inhibitor (PI), 4 subjects were on the integrase inhibitor raltegravir, and 20 subjects were on a non-nucleoside reverse transcriptase inhibitor (efavirenz = 14, rilpivirine = 4, etravirine = 1, nevirapine = 1). Nucleoside reverse transcriptase inhibitor (NRTI) backbones included emtricitabine/tenofovir (N=37), lamivudine/abacavir (N=12), lamivudine/zidovudine (N=1), and stavudine/abacavir (N=1).
The randomized groups were well-matched. There were no statistically significant differences for any of the immune activation or exhaustion markers among the three dosing groups at baseline. The only variable that was statistically different between the three groups was nadir CD4+ T-cell count [221 (27, 312), 352 (274, 574), and 319 (154, 475) cells/mm3 in the standard-, moderate-, and high-dose groups, respectively; P=0.03).
Over the 12-month study period, 9 subjects changed cART regimens, reflecting in part updates in the Guidelines for the Use of Antiretroviral Agents in HIV-1-infected Adults and Adolescents [25]. Six subjects stopped a boosted PI and switched to rilpivirine (N=1), elvitegravir (N=4), or dolutegravir (N=1). Of the 5 subjects who started an integrase inhibitor, 3 subjects were in the standard-dose group and 2 subjects were in the high-dose group. Three subjects stopped EFV (one from each dosing group) and switched to rilpivirine (N=1) or elvitegravir (N=2). No subjects changed NRTIs.
After 12 months of monthly vitamin D supplementation, subjects combined and within each dosing group had a statistically significant within-group rise in their serum 25(OH)D concentrations (Table 2). There were also significant differences in the 25(OH)D increases among the three dosing groups, where the high-dose group had the greatest increase in 25(OH)D (+31.0 ng/mL), followed by the moderate-dose group (+19.2 ng/mL) and then the standard-dose group (+14.7 ng/mL). At the 12-month time point, there were also significant differences in the 25(OH)D concentrations among the three dosing groups.
Table 2.
Changes in Variables after 12 Months of Vitamin D Supplementation
| A. Absolute Changes over 12 Months | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Median (quartile 1, quartile 3) | All Subjects (N=51) | P∘ | Standard Dose† (N=21) | P∘ | Moderate Dose‡ (N=18) | P∘ | High Dose¥ (N=12) | P∘ | P* |
| 25(OH)D, ng/mL | +15.6 (+9.2, +30.1) | <0.0001 | +14.7 (+12.0, +18.1) | <0.0001 | +19.2 (+10.0, +32.0) | <0.0001 | +31.0 (+16.2, +41.1) | <0.0001 | 0.001 |
| Body mass index, kg/m2 | +0.67 (−0.49, +1.38) | 0.02 | +0.56 (−0.4, +0.9) | 0.18 | +1.4 (−0.37, +1.9) | 0.01 | +0.3 (−1.1, +3.1) | 0.94 | 0.23 |
| Current CD4, cells/mm3 | +24 (−108, +154) | 0.40 | −1 (−118, +127) | 0.72 | +52 (−167, +137) | 0.87 | +62 (−50, +228) | 0.34 | 0.66 |
| Current CD8, cells/mm3 | −23 (−147, +123) | 0.84 | −59 (−168, +129) | 0.86 | −56 (−205, +93) | 0.61 | +36 (−112, +234) | 0.67 | 0.67 |
| CD4/CD8 ratio | +0.05 (−0.05, +0.18) | 0.03 | +0.03 (−0.7, +0.13) | 0.47 | +0.06 (−0.06, +0.18) | 0.076 | +0.05 (−0.01, +2.7) | 0.13 | 0.65 |
|
| |||||||||
| B. Variables at 12 Months | |||||||||
|
| |||||||||
| Median (quartile 1, quartile 3) | All Subjects (N=51) | Standard Dose† (N=21) | Moderate Dose‡ (N=18) | High Dose¥ (N=12) | P* | ||||
|
| |||||||||
| 25(OH)D, ng/mL | 36.5 (30.2, 43.0) | 31.5 (23.8, 36.5) | 37.8 (30.6, 42.0) | 51.5 (37.6 (27.0, 58.5) | 0.001 | ||||
| Body mass index, kg/m2 | 21.7 (10.0, 25.4) | 20.7 (17.8, 23.2) | 22.2 (20.0, 28.1) | 22.6 (20.4, 24.7) | 0.12 | ||||
| Current CD4, cells/mm3 | 703 (515, 948) | 665 (554, 812) | 689 (498, 961) | 783 (456, 1122) | 0.63 | ||||
| Current CD8, cells/mm3 | 650 (505, 908) | 713 (508, 937) | 630 (477, 761) | 684 (506, 1015) | 0.75 | ||||
| CD4/CD8 ratio | 1.0 (0.8, 1,5) | 0.9 (0.6, 1.3) | 1.2 (0.8, 1.5) | 1.0 (0.9, 1.6) | 0.39 | ||||
N.B. P-values with bolded font designate those <0.05;
P value within group;
P value among the three dosing groups;
Standard dose = 18,000 IU given monthly for 12 months (equivalent to 600 IU/daily);
Moderate dose = 60,000 IU given monthly for 12 months (equivalent to 2,000 IU/daily);
High dose = 120,000 IU given monthly for 12 months (equivalent to 4,000 IU/daily).
25(OH)D, 25-hydroxyvitamin D
In the high-dose group, 11 out of 12 subjects (92%) achieved a 25(OH)D concentration ≥30 ng/mL as early as the 3-month time point and maintained it throughout the 12-month time point. One subject had a 25(OH)D concentration of 27 ng/mL at the 12-month time point, but had a concentration of ≥32 ng/mL at the earlier time points. Among the moderate-dose group, 14 out of 18 subjects (78%) achieved a 25(OH)D concentration ≥30 ng/mL at the 12-month time point (range 19–60 ng/mL), Among the standard-dose group, 15 out of 21 subjects (71%) achieved a 25(OH)D concentration ≥30 ng/mL at the 12-month time point (range 14–43 ng/mL). No subject ever achieved a 25(OH)D ≥100 ng/mL, the concentration above which one may experience toxicity. There were no study-related adverse events, interruptions in study drug administration, or known non-adherence for any subject in any dosing group.
When all 51 subjects were analyzed together, there was a significant increase in body mass index (BMI) and CD4/CD8 ratio but no significant changes in CD4+ or CD8+ T-cell counts over the 12-month time period. Other than an increase in BMI in the moderate-dose group, there were no other significant changes in BMI, CD4/CD8 ratio, or CD4+ and CD8+ T-cell counts for any of the groups when analyzed individually.
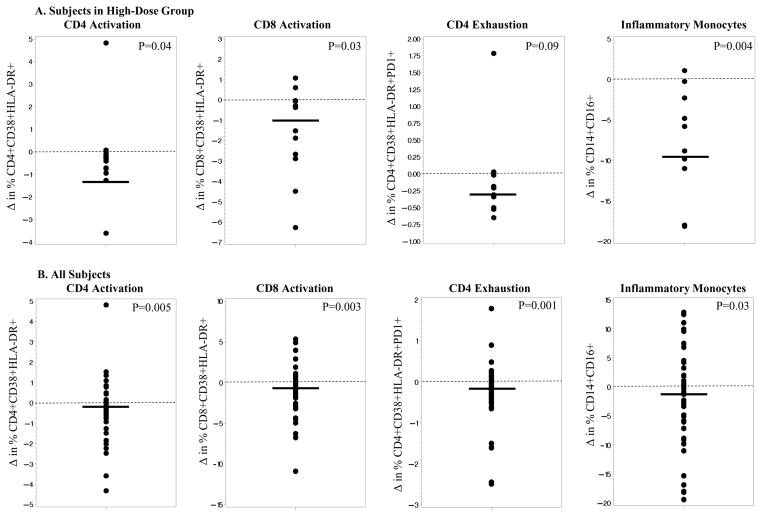
Changes in immune activation and exhaustion markers are shown in Table 3 and Figure 2. With all groups combined, there was a significant decrease in % CD4+CD38+HLA-DR+ (CD4 activation), % CD8+CD38+HLA-DR+ (CD8 activation), % CD4+CD38+HLA-DR+PD1+ (CD4 exhaustion), and % of proinflammatory monocytes (CD14+CD16+). Although the median decreased over time for all markers, changes were not statistically significant within either the standard-dose or moderate-dose groups. However, within the high-dose group, there were significant decreases in % CD4+CD38+HLA-DR+, % CD8+CD38+HLA-DR+, and % CD14+CD16+, and decreases in % CD4+CD38+HLA-DR+PD1+ approached significance. Differences between the treatment groups did not reach significance.
Table 3.
Changes over 12 Months in Immune Activation and Exhaustion Markers
| Median (quartile 1, quartile 3) | All Subjects (N=51) |
P∘ | Standard Dose (N=21) |
P∘ | Moderate Dose (N=18) |
P∘ | High Dose (N=12) |
P∘ | Moderate+High Dose (N=30) |
P∘ | P* | P** | P*** |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % CD4+CD38+HLA-DR+a, b | −0.2 (−0.7, +0.1) | 0.005 | −0.1 (−0.6, +0.2) | 0.31 | −0.2 (−0.8, +0.03) | 0.07 | −0.4 (−0.9, −0.1) | 0.04 | −0.3 (−0.8, −0.1) | 0.002 | 0.35 | 0.20 | 0.20 |
| % CD8+CD38+HLA-DR+a | −0.7 (−1.9, +0.2) | 0.003 | −0.1 (−1.6, +0.4) | 0.30 | −1.0 (−2.6, +0.2) | 0.55 | −1.0 (−2.8, −0.1) | 0.03 | −1.0 (−2.7, 0.0) | 0.004 | 0.54 | 0.76 | 0.33 |
| % CD4+CD38+HLA-DR+PD1+a,b | −0.2 (−0.4, +0.03) | 0.001 | −0.2 (−0.4, +0.1) | 0.10 | −0.06 (−0.3, −0.02) | 0.37 | −0.3 (−0.4, +0.001) | 0.09 | −0.2 (−0.3, −0.02) | 0.004 | 0.98 | 0.27 | 0.77 |
| % CD8+CD38+HLA-DR+PD1+a | −0.2 (−0.7, +0.2) | 0.12 | −0.02 (−0.7, +0.3) | 0.74 | −0.2 (−0.7, +0.2) | 1.00 | −0.3 (−0.6, −0.01) | 0.18 | −0.3 (−0.7, +0.1) | 0.08 | 0.68 | 0.54 | 0.45 |
| % CD14+CD16+a,c | −2.7 (−10.4, +2.6) | 0.03 | −3.2 (12.2, +5.1) | 0.25 | 0.5 (−2.9, +4.5) | 0.70 | −8.9 (−18.0, +2.3) | 0.004 | −2.5 (−10.4, +15) | 0.04 | 0.27 | 0.36 | 0.86 |
| % CD14dimCD16+a,c | −2.6 (−5.8, +4.9) | 0.32 | −0.2 (−9.3, +7.3) | 0.70 | −1.9 (−4.3, +3.3) | 0.43 | −3.5 (−5.7, +3.8) | 0.46 | −3.3 (−5.7, +3.5) | 0.25 | 0.99 | 0.26 | 0.83 |
N.B. P-values with bolded font designate those <0.05;
P value within group;
P value between standard vs. moderate groups;
P value between standard vs. high groups;
P value between standard vs. moderate+high groups;
Standard dose = 18,000 IU given monthly for 12 months (equivalent to 600 IU/daily);
Moderate dose = 60,000 IU given monthly for 12 months (equivalent to 2,000 IU/daily);
High dose = 120,000 IU given monthly for 12 months (equivalent to 4,000 IU/daily).
Data represent the % of that cell type expressing the given phenotype;
1 subject with no data due to cell viability issues;
3 subjects with no data due to cell viability issues
Figure 2.
In pre-planned analyses, we combined the moderate-dose and high-dose groups and compared to the standard-dose group. There were significant changes for the same markers as for the high-dose group alone, and in addition, the changes in % CD4+CD38+HLA-DR+PD1+ became significant, and decreases in % CD8+CD38+HLA-DR+PD1+ (CD8+ exhaustion) approached significance. Again, there were no significant between-group changes when high- plus moderate-dose groups were compared with the standard-dose group. Also, changes in activation/exhaustion markers did not differ by baseline 25(OH)D (e.g., <10 vs. ≥10 ng/ml; <20 vs. ≥20) or by degree of change in 25(OH)D (e.g., subjects above vs. below the median 25(OH)D concentration at the 12-month time point; subjects in the lowest vs. highest quartile for change in 25(OH)D; half of subjects with the greatest increase in 25(OH)D regardless of baseline); results not shown.
Next, correlations between variables of interest were considered. There were no significant correlations between changes in serum 25(OH)D concentrations and changes in immune activation/exhaustion markers, CD4+ T-cell count, CD8+ T-cell count, or CD4/CD8 ratio. Likewise, there were no significant correlations between 25(OH)D concentrations and immune activation/exhaustion markers at the 12-month time point. There were also no significant correlations between changes in activation/exhaustion markers and changes in BMI or ARV duration.
DISCUSSION
To our knowledge, this is the first RCT of vitamin D supplementation to comprehensively investigate changes in immune activation and exhaustion markers among HIV-1-infected subjects viralogically suppressed on cART. Our results suggest that 120,000 IU of vitamin D3 given monthly over 12 months decreases immune activation and exhaustion markers in this population. In addition, this dose safely raised 25(OH)D to a serum concentration ≥30 ng/mL, the current definition of vitamin D sufficiency as defined by the Endocrine Society [22].
Although the significant decreases in immune activation and exhaustion markers in the high-dose group (with statistical significance lacking in the moderate- and standard-dose groups) suggest a potential benefit of high-dose supplementation, there were no statistically significant between-group differences. While this perhaps diminishes the strength of our proposed conclusion, it is likely merely a reflection of small sample size, especially in the high-dose group. This is supported by the fact that the number of markers with statistically significant decreases increased when the high- and moderate-dose groups were combined and when all groups were considered together. Our study design using an active-control arm instead of a true placebo also limits our ability to detect differences between groups. However, due to ethical reasons about failing to treat subjects with vitamin D insufficiency/deficiency, we were unable to have a true control arm.
Another reason that we did not observe between-group differences may be due to our monthly dosing strategy which was designed to minimize additional pill burden, given the risk of poor adherence to medication among adolescents and young adults. Serum 25(OH)D, the major circulating form of vitamin D and the metabolite most commonly used to assess overall vitamin D status, has a long half-life of ~15 days [26], theoretically allowing a longer interval between vitamin D doses, which has been supported by the results from other RCTs among HIV-1-infected individuals [18, 20]. On the other hand, a systematic review evaluating 7 RCTs of vitamin D supplementation for the prevention of childhood acute respiratory infections suggested that a daily dosing schedule exerts superior therapeutic effects compared to large bolus doses, which can result in both a steep and rapid increase in circulating 25(OH)D concentrations, followed by a slow decline [27]. However, there was considerable heterogeneity among the small number of available trials, and two studies utilized bolus dosing that included a single dose of 100,000 IU with a follow-up of 3 months, and 3 monthly bolus doses of 100,000 IU with a follow-up of 18 months.
Similarly, Arpadi, et al [28] evaluated the bone mass accrual in 64 perinatally HIV-1-infected individuals, aged 6–16 years, after 2 years of 100,000 IU of vitamin D3 every other month plus daily calcium compared to placebo. They did not find any difference in bone mass parameters between the two groups after adjusting for confounding variables. However, while the intervention group increased their mean 25(OH)D concentrations after two years compared to the placebo group, 75% in the treatment group had at least 1 month with 25(OH)D <30 ng/mL. In contrast, our study used monthly dosing that continued for the entire 12-month study period, and in the high-dose group, subjects maintained a serum concentration ≥30 ng/mL except for one subject who dipped to 27 ng/mL at one single time point. Thus, we believe our results suggest that utilizing monthly doses that are sufficient enough to induce a sustained 25(OH)D elevation should produce results similar to daily dosing. This finding should be confirmed in additional RCTs, as minimizing pill burden while producing adequate therapeutic effects is particularly important in HIV-1-infected youth who often struggle with adherence to daily medications.
One limitation of many of the previous studies investigating the association between vitamin D and HIV parameters, such as CD4+ T-cell count and HIV-1 RNA level, is the inclusion of very heterogeneous individuals with different states of HIV disease. With uncontrolled viremia, immune activation is higher than in individuals with cART-induced viral suppression [29]. Our study focused on subjects with sustained virologic suppression, and, as such, the significant decreases in the measured immune activation/exhaustion markers strengthen the argument that vitamin D supplementation played a direct role in these changes.
A limitation to our current analysis includes a lack of adherence measurements to study drug, such as pill counts. However, we limited our study population to those who were clearly adherent to their cART regimen as evidenced by their sustained viral suppression so these subjects were arguably more likely to be adherent to the study drug. We also investigated the relationships between changes in 25(OH)D and marker changes regardless of randomized treatment group, as another indicator of adherence to the study drug. However, this latter approach assumes that serum 25(OH)D concentration is the appropriate measure to assess vitamin D’s effect on changes in immune markers that result from vitamin D supplementation. Because the active form of vitamin D acts at the cellular level/vitamin D receptor, it is possible that one needs to look, for example, at changes in gene expression, as recently demonstrated [30]. In addition, uncertainty still remains at to the what 25(OH)D concentration (or change in 25(OH)D) is needed to demonstrate immunological effects; however, some recent data suggest that reaching a threshold of ~40 ng/mL may be relevant [30, 31]. As previously discussed, our relatively small sample size, especially among those receiving the high dose, and a lack of a true placebo arm due to ethical concerns may have diminished our ability to detect effects.
Nevertheless, our results are novel and suggest a potential benefit of high-dose monthly vitamin D supplementation in HIV-1-infected individuals with vitamin D insufficiency by decreasing immune activation and exhaustion markers that are associated with HIV disease progression, mortality, and HIV-related co-morbidities. Larger studies should be done to validate and expand upon these findings.
Acknowledgments
Sources of Support: This work was made possible by the National Institute of Child Health and Development at the National Institutes of Health [K23 HD069199 to ARE; R01 HD070490 to GAM; K12 HD072245 to AC], Case Western Reserve University’s Center for AIDS Research (P30 AI36219), Emory University’s Center for AIDS Research (P30 AI050409), Emory+Children’s Pediatric Research Center (Immunology and Flow Cytometry Cores), Clinical and Translational Science Award and the Clinical and Translational Science Collaborative of Cleveland (UL1TR000439) from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
ClinicalTrials.gov Identifier: NCT01523496
Conflicts of Interest: ARE has received research funding from Bristol-Myers Squibb, Cubist Pharmaceuticals, and GlaxoSmithKline and has served as an advisor and speaker for Gilead. ARE has received research funding from Bristol-Myers Squibb, Cubist Pharmaceuticals, and GlaxoSmithKline and has served as an advisor and speaker for Gilead. GAM serves as a consultant for Bristol-Myers Squibb, ViiV/GlaxoSmithKline, Gilead, Pfizer, and ICON, and has received grant funding from Bristol-Myers Squibb, ViiV/GlaxoSmithKline, Merck, AstraZeneca, and Gilead. All other declare no conflicts of interest.
References
- 1.Guaraldi G, Orlando G, Zona S, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis. 2011;53:1120–1126. doi: 10.1093/cid/cir627. [DOI] [PubMed] [Google Scholar]
- 2.Eckard AR, Meissner EG, Singh I, McComsey GA. Cardiovascular Disease, Statins, and HIV. J Infect Dis. 2016;214(Suppl 2):S83–92. doi: 10.1093/infdis/jiw288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hunt PW, Lee SA, Siedner MJ. Immunologic Biomarkers, Morbidity, and Mortality in Treated HIV Infection. J Infect Dis. 2016;214(Suppl 2):S44–50. doi: 10.1093/infdis/jiw275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eckard AR, Rosebush JC, O’Riordan MA, et al. Neurocognitive dysfunction in HIV-infected youth: investigating the relationship with immune activation. Antivir Ther. 2017 Mar 22; doi: 10.3851/IMP3157. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eckard AR, Mora S. Bone health in HIV-infected children and adolescents. Curr Opin HIV AIDS. 2016;11:294–300. doi: 10.1097/COH.0000000000000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eckard AR, Fowler SL, Haston JC, Dixon TC. Complications of Treatment in Youth with HIV. Curr HIV/AIDS Rep. 2016;13:226–233. doi: 10.1007/s11904-016-0320-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eckard AR, Rosebush JC, Lee ST, et al. Increased Immune Activation and Exhaustion in HIV-infected Youth. Pediatr Infect Dis J. 2016;35:e370–e377. doi: 10.1097/INF.0000000000001326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamen DL, Tangpricha V. Vitamin D and molecular actions on the immune system: modulation of innate and autoimmunity. J Mol Med (Berl) 2010;88:441–450. doi: 10.1007/s00109-010-0590-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coussens AK, Martineau AR, Wilkinson RJ. Anti-Inflammatory and Antimicrobial Actions of Vitamin D in Combating TB/HIV. Scientifica (Cairo) 2014;2014:903680. doi: 10.1155/2014/903680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coussens AK, Wilkinson RJ, Hanifa Y, et al. Vitamin D accelerates resolution of inflammatory responses during tuberculosis treatment. Proc Natl Acad Sci U S A. 2012;109:15449–15454. doi: 10.1073/pnas.1200072109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 12.Ross AC, Judd S, Kumari M, et al. Vitamin D is linked to carotid intima-media thickness and immune reconstitution in HIV-positive individuals. Antivir Ther. 2011;16:555–563. doi: 10.3851/IMP1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aziz M, Livak B, Burke-Miller J, et al. Vitamin D insufficiency may impair CD4 recovery among Women’s Interagency HIV Study participants with advanced disease on HAART. AIDS. 2013;27:573–578. doi: 10.1097/QAD.0b013e32835b9ba1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viard JP, Souberbielle JC, Kirk O, et al. Vitamin D and clinical disease progression in HIV infection: results from the EuroSIDA study. AIDS. 2011;25:1305–1315. doi: 10.1097/QAD.0b013e328347f6f7. [DOI] [PubMed] [Google Scholar]
- 15.Mehta S, Giovannucci E, Mugusi FM, et al. Vitamin D status of HIV-infected women and its association with HIV disease progression, anemia, and mortality. PLoS One. 2010;5:e8770. doi: 10.1371/journal.pone.0008770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vescini F, Cozzi-Lepri A, Borderi M, et al. Prevalence of hypovitaminosis D and factors associated with vitamin D deficiency and morbidity among HIV-infected patients enrolled in a large Italian cohort. J Acquir Immune Defic Syndr. 2011;58:163–172. doi: 10.1097/QAI.0b013e31822e57e9. [DOI] [PubMed] [Google Scholar]
- 17.Sudfeld CR, Wang M, Aboud S, Giovannucci EL, Mugusi FM, Fawzi WW. Vitamin D and HIV progression among Tanzanian adults initiating antiretroviral therapy. PLoS One. 2012;7:e40036. doi: 10.1371/journal.pone.0040036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giacomet V, Vigano A, Manfredini V, et al. Cholecalciferol supplementation in HIV-infected youth with vitamin D insufficiency: effects on vitamin D status and T-cell phenotype: a randomized controlled trial. HIV Clin Trials. 2013;14:51–60. doi: 10.1310/hct1402-51. [DOI] [PubMed] [Google Scholar]
- 19.Stallings VA, Schall JI, Hediger ML, et al. High-dose vitamin D3 supplementation in children and young adults with HIV: a randomized, placebo-controlled trial. Pediatr Infect Dis J. 2015;34:e32–40. doi: 10.1097/INF.0000000000000483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fabre-Mersseman V, Tubiana R, Papagno L, et al. Vitamin D supplementation is associated with reduced immune activation levels in HIV-1-infected patients on suppressive antiretroviral therapy. AIDS. 2014;28:2677–2682. doi: 10.1097/QAD.0000000000000472. [DOI] [PubMed] [Google Scholar]
- 21.Bang U, Kolte L, Hitz M, et al. Correlation of increases in 1,25-dihydroxyvitamin D during vitamin D therapy with activation of CD4+ T lymphocytes in HIV-1-infected males. HIV Clin Trials. 2012;13:162–170. doi: 10.1310/hct1303-162. [DOI] [PubMed] [Google Scholar]
- 22.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 23.Ross AC, Taylor CL, Yaktine AL, Del Valle HB. Dietary Reference Intakes for Calcium and Vitamin D. City: 2011. [PubMed] [Google Scholar]
- 24.Longenecker CT, Hileman CO, Carman TL, et al. Vitamin D supplementation and endothelial function in vitamin D deficient HIV-infected patients: a randomized placebo-controlled trial. Antivir Ther. 2012;17:613–621. doi: 10.3851/IMP1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. [Google Scholar]
- 26.Ish-Shalom S, Segal E, Salganik T, Raz B, Bromberg IL, Vieth R. Comparison of daily, weekly, and monthly vitamin D3 in ethanol dosing protocols for two months in elderly hip fracture patients. J Clin Endocrinol Metab. 2008;93:3430–3435. doi: 10.1210/jc.2008-0241. [DOI] [PubMed] [Google Scholar]
- 27.Xiao L, Xing C, Yang Z, et al. Vitamin D supplementation for the prevention of childhood acute respiratory infections: a systematic review of randomised controlled trials. Br J Nutr. 2015;114:1026–1034. doi: 10.1017/S000711451500207X. [DOI] [PubMed] [Google Scholar]
- 28.Arpadi SM, McMahon D, Abrams EJ, et al. Effect of bimonthly supplementation with oral cholecalciferol on serum 25-hydroxyvitamin D concentrations in HIV-infected children and adolescents. Pediatrics. 2009;123:e121–126. doi: 10.1542/peds.2008-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wada NI, Jacobson LP, Margolick JB, et al. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS. 2015;29:463–471. doi: 10.1097/QAD.0000000000000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hardiman G, Savage SJ, Hazard ES, et al. Systems analysis of the prostate transcriptome in African-American men compared with European-American men. Pharmacogenomics. 2016;17:1129–1143. doi: 10.2217/pgs-2016-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mirzakhani H, Litonjua AA, McElrath TF, et al. Early pregnancy vitamin D status and risk of preeclampsia. J Clin Invest. 2016;126:4702–4715. doi: 10.1172/JCI89031. [DOI] [PMC free article] [PubMed] [Google Scholar]