Abstract
The tubular endolysosomal network is a quality control system that ensures the proper delivery of internalized receptors to specific subcellular destinations in order to maintain cellular homeostasis. Although retromer was originally described in yeast as a regulator of endosome-to-Golgi receptor recycling, mammalian retromer has emerged as a central player in endosome-to-plasma membrane recycling of a variety of receptors. Over the past decade, information regarding the mechanism by which retromer facilitates receptor trafficking has emerged, as has the identification of numerous retromer-associated molecules including the WASH complex, sorting nexins and TBC1d5. Moreover, the recent demonstration that several sorting nexins can directly interact with retromer cargo to facilitate endosome-to-Golgi retrieval has provided new insight into how these receptors are trafficked in cells. The mechanism by which sorting nexin 17 cargoes are recycled out of the endosomal system was demonstrated to involve a retromer-like complex termed the retriever, which is recruited to WASH positive endosomes through an interaction with the COMMD/CCDC22/CCDC93 (CCC) complex. Lastly, the mechanisms by which bacterial and viral pathogens highjack this complex sorting machinery in order to escape the endolysosomal system or remain hidden within the cells are beginning to emerge. In this review, we will highlight recent studies that have begun to unravel the intricacies by which the retromer and associated molecules contribute to receptor trafficking and how deregulation at this sorting domain can contribute to disease or facilitate pathogen infection.
Keywords: Retromer, WASH, Retriever, Endosome, Receptor Trafficking, Sorting Nexin
Graphical Abstract
INTRODUCTION
Maintaining cellular homeostasis is necessary for every aspect of cellular life including cell growth, cell death, various signal transduction pathways, and immune response 1,2,3. Receptor endocytosis and subsequent sorting in endosomes are major pathways to preserve cellular homeostasis. Integral membrane proteins and their associated macromolecules are internalized via endocytosis. They can be further delivered to lysosomes for degradation, or targeted to the trans-Golgi network (TGN) or the plasma membrane for reuse (Figure 1). These processes, known as endosomal protein sorting, are essential for a wide array of physiological functions, including nutrient uptake, developmental and neural signaling, as such, genetic defects in these processes have been linked with pathologies such as neurological disorders and diabetes 1,4,5.
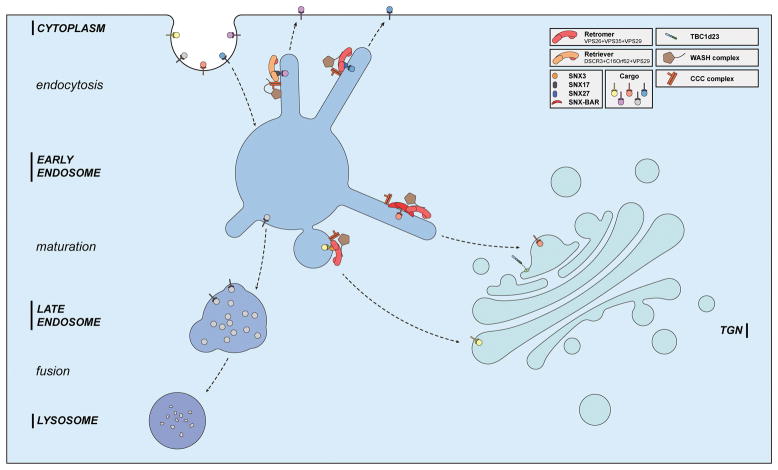
Figure 1.
Representative trafficking pathways of transmembrane receptors. Transmembrane proteins are internalized into early endosomes via endocytosis. Maturation of early endosomes into late endosomes leads to protein degradation via lysosome. Some proteins are delivered to the plasma membrane or to the TGN with the assistance of retromer, retriever, WASH, CCC, and a variety of SNX proteins, thus escaping lysosomal degradation. At the TGN, TBC1d23 and associated proteins are responsible for receiving endosomal vesicles.
One of the best-characterized protein complexes regulating endosomal sorting is the evolutionarily conserved retromer complex, which was first identified in the yeast Saccharomyces cerevisiae two decades ago 6. Retromer is a coat complex that assembles on endosomes, and mediates the transport of receptors that traverse the endosomal compartment on their way to an ultimate physiologic destination such as the plasma membrane or the TGN, and are thus referred to as ‘cargo’ proteins. The core of retromer is the VPS35–VPS26–VPS29 heterotrimer, which is conserved from yeast to human (Table 1). Since the core complex is able to recognize certain cargo, it was termed the cargo-selective complex (CSC). Retromer functions together with a large number of accessory proteins to package cargo into tubular or vesicular structures for transport to the TGN or plasma membrane.
Table 1.
Conservation of genes encoding endosomal protein sorting machinery in model organisms.
| Homo sapiens | Mus musculus | Danio rerio | D.melanogaster | C.elegans | A.thaliana | Dictyostelium | S.cerevisiae | |
|---|---|---|---|---|---|---|---|---|
| Retromer | √ | √ | √ | √ | √ | √ | √ | √ |
| TBC1d5 | √ | √ | √ | √ | √ | √ | √ | |
| SNX-BAR | √ | √ | √ | √ | √ | √ | √ | √ |
| SNX3 | √ | √ | √ | √ | √ | √ | √ | √ |
| SNX27 | √ | √ | √ | √ | √ | |||
| SNX17 | √ | √ | √ | √ | √ | |||
| WASH | √ | √ | √ | √ | √a | √ | ||
| CCC | √ | √ | √ | √ | √b | √ | √ | |
| Retriever | √ | √ | √ | √ | √c | √ | √ | |
| TBC1d23 | √ | √ | √ | √ | √ |
Four homologues of WASH complex components have been reported in C. elegans (WASH, Strumpellin, SWIP and CCDC53). A possible FAM21 C. elegans homolog (C05G5.2), which is also conserved in other nematode species, could also be identified. However, it remains to be determined whether these proteins associate with each other to form a functional WASH complex and regulate endosomal receptor trafficking in nematodes.
C. elegans has CCC subunits: 3 homologs of CCDC93 (C16A11.2, C31E10.6, C31E10.5), no homologs of CCDC22 and one COMMD gene homolog (T28F2.2). It remains to be determined whether these proteins associate with each other and function in endosomal receptor trafficking.
C elegans has a possible retriever subunit, F26G1.1, which according to blast is more similar to VPS35L than to VPS35; interestingly, no VPS26C homolog is apparently present.
Although the role of retromer in endosomal sorting is well established, recent work has identified additional retromer-dependent and retromer-independent trafficking pathways, and revealed their functions in development and human disease (Figure 1). Several novel regulators, including TBC1d5, TBC1d23, the WASH actin regulatory complex, and the recently described COMMD/CCDC22/CCDC93 (CCC) and retriever complexes have been identified and characterized (Table 1). Accumulating evidence suggests that sorting nexin (SNX) proteins, in addition to binding to membranes play a critical role in cargo selection. In this review, we focus on these newly identified endosomal sorting machineries, and summarize the current understanding of their roles in endosomal trafficking and retrograde vesicular transport. We also discuss how viral and bacterial pathogens exploit endosomal trafficking pathways to promote their replication during infection. We do not intend to discuss every aspect of endosomal sorting due to space limitations, and interested readers are referred to excellent reviews published elsewhere 1,4,5,7.
TBC1d5 REGULATION OF RECEPTOR TRAFFICKING
Study of classic coat proteins including the clathrin/adaptor protein, COPI, and COPII, has revealed that small GTPases are essential for the formation of coated vesicles 8–11. Similarly, the endosomal-localized Rab7a GTPase is required for the membrane recruitment of the retromer CSC, and this role is conserved in yeast, plants and mammals 12–19. TBC1d5 is a member of the Tre2–Bub2–Cdc16 (TBC) family, and was first identified through its association with retromer in yeast-2-hybrid and immunoprecipitation experiments 19,20. Both human and worm TBC1d5 function as GTPase-activating proteins (GAP) for Rab7a, catalyzing GTP hydrolysis and thus, inactivating Rab7a 21,22. Over-expression of TBC1d5 was shown to decrease the amount of Rab7a-GTP and reduce the amount of VPS35 on endosomes 19. Therefore, TBC1d5 was initially proposed to inhibit retromer trafficking; recent evidence, however, suggests that TBC1d5 is more than an inhibitory factor for retromer.
Among all the known endogenous regulators of retromer, TBC1d5 displays the highest affinity toward retromer 21. TBC1d5 forms a stable complex with retromer, with a dissociation constant of 220~450 nM 21,23. Such an affinity is comparable to that measured between VPS29 and VPS35 (~200 nM), and is at least one order of magnitude higher than the affinity between retromer and its other binding partners, such as SNX3, VARP, Rab7a, or the WASH complex subunit FAM21 16,21,23–26. TBC1d5 harbors a TBC domain on its N-terminus, and a largely disordered C-terminus. The TBC domain mediates the interaction with retromer, through contacting both VPS35 and VPS29 21. A loop from TBC1d5 binds to a conserved hydrophobic pocket on VPS29, and a second loop may interact with the N-terminus of VPS35. Interestingly, a VPS35 mutant unable to associate with TBC1d5 did not localize properly to endosomes, suggesting that TBC1d5 may play a role in recruiting VPS35 to endosomal membrane 14,21.
The tight association between TBC1d5 and retromer is akin to the Sec23-Sec24 complex in the COPII coat. Similar to TBC1d5, Sec23 is a GAP for the Sar1 GTPase. The interaction between Sec23 and Sar1 is needed for the recruitment of the COPII coat to ER membranes, at least in an in vitro reconstitution system10,11. GTP hydrolysis of Sar1, promoted by Sec23, is believed to be essential for the maturation of COPII vesicles and coat disassembly. Past studies have provided mechanistic insights into the formation process of the COPII coat, and recognition of the analogy between the retromer and COPII systems may help to understand how retromer-coated vesicles are formed.
Recently, Steinberg and colleagues have revealed a novel role for both retromer and TBC1d5 in regulating the activation and subcellular localization of Rab7a 27. They found that Rab7a resides on multiple subcellular organelles in addition to the interface between EEA1+ sorting endosomes and LAMP1/2+ late endosomes/lysosomes. These include the TGN, endoplasmic reticulum, and mitochondrial membranes. In the absence of either retromer or TBC1d5, Rab7a-GTP levels substantially increase and Rab7a accumulates over the lysosomal domains, which results in decreased Rab7a mobilization, depletion of inactive Rab7a from other subcellular organelles and defective membrane turnover. Intriguingly, whereas we previously showed that TBC1d5 was critical for the recycling of integrin alpha 5 and CI-MPR, Steinberg et al showed that loss of TBC1d5 did not impact the recycling of the retromer cargoes CI-MPR or GLUT1. The exact reason for the discrepancy remains unclear, but could involve either the way in which the knockout cells were generated or how the trafficking assays were performed.
TBC1d5 also plays a role in autophagy, a process in which cellular components are selectively targeted and degraded 27–30. Studies by Dikic and colleagues showed that TBC1d5 switches between endosomes and autophagosomes 28,29. More recently, the Debnath group extended this study and showed that this switch could modulate retromer function under different physiological conditions such that metabolic stress leads to the association TBC1d5 with autophagosomes, promoting the recycling of GLUT1 and glucose uptake 30.
SORTING NEXIN PROTEINS
SNX proteins are characterized by the presence of a particular and highly conserved phox-homology (PX) domain and participate in variety of cellular activities 31,32. S. cerevisiae and human genomes encode 10 and 33 SNXs, respectively31. Although PX domains are capable of contacting PtdIns(3)P, recent studies reveal that certain PX domains can also interact with phosphoinositides other than PtdIns(3)P, and can also interact with a variety of proteins 31. With endosomes being the major organelle enriched with PtdIns(3)P, the functions of SNXs have been most extensively investigated in this subcellular compartment.
In addition to the PX domain, SNXs often possess other domains, including BAR (Bin/Amphiphysin/Rvs), FERM (protein 4.1/ezrin/radixin/moesin), and SH3 domains 31. The presence of these additional domains allow SNX proteins to be divided into five subfamilies: SNX-PX, SNX-BAR, SNX-FERM, SNX-PXA-RGS-PXC and SNX-MIT 31. Several SNXs from three different subfamilies have been the focus of recent studies: SNX3 of the SNX-PX subfamily, SNX1/2/5/6 of the SNX-BAR subfamily, and SNX27 and SNX17 of the SNX-FERM subfamily (Figure 2A). These SNXs associate with retromer or the recently identified retriever complex, and mediate distinct endosomal trafficking pathways. Remarkably, recent studies have revealed that these SNXs play a central role in cargo recognition, in addition to binding phosphatidylinositides (PtdIns) (Figure 2B).
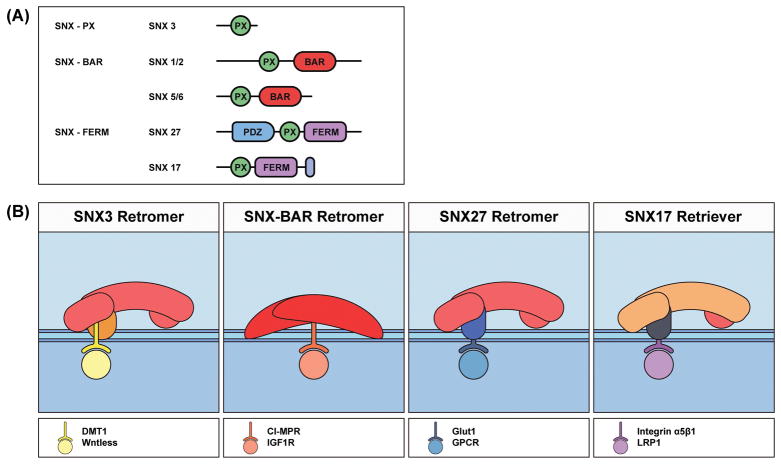
Figure 2.
Domain organization and functional modes of select SNXs.
(A) Domain structures of retromer and retriever-associated SNXs. All SNXs share a conserved PX domain. SNX-BAR and SNX-FERM subfamilies possess a BAR or FERM domain, respectively, C-terminal to the PX domain. Within the SNX-FERM subfamily, SNX27 features a unique N-terminal PDZ domain whereas SNX17 has a unique C-terminal tail.
(B) Distinct cargo recognition modes of SNXs. Representative cargo proteins are listed under the cartoon. SNX3 interacts with both VPS35 and VPS26 subunits of retromer, and cargo recognition is achieved through cooperative action of SNX3 and VPS26. SNX-BAR may directly bind to cargo such as CI-MPR and IGF1R. The PDZ domain of SNX27 recognizes a subset of cargo proteins independent of retromer; however, interaction with VPS26 enhances the affinity of SNX27 for cargo. SNX17 may bridge cargo with retriever through simultaneous interaction with cargo and DSCR3.
SNX3 (SNX-PX subfamily) contains only one structural domain, the PX domain, which binds to PtdIns(3)P (Figure 2). SNX3 is required for endosome-to-TGN retrieval of Wntless in human, worm and fly 33,34. In S. cerevisiae, Grd19p/SNX3 is also needed for cargo trafficking from endosomes to the TGN 35. Burd and colleagues found that retromer is recruited to the endosomal membrane through both Rab7a and SNX3, and in vitro the recruitment depends on the presence of a transmembrane cargo, DMT1, on the liposome 17. More recently, a crystal structure of the quaternary VPS35-VPS26-SNX3-DMT1 tail complex revealed that SNX3 binds at the interface of VPS35 and VPS26 36. Upon binding to SNX3, VPS26 undergoes a conformational change in its cargo-binding motif, which allows recognition of the DMT1 tail by both VPS26 and SNX3. Thus, the SNX3/retromer complex integrates two different activities: membrane binding and cargo recognition.
Unlike SNX3, the SNX-BAR proteins possess an additional BAR domain, which can sense membrane curvature and induce membrane tubulation 31,32. The SNX-BAR proteins can target to endosomes through coincidence detection of membrane curvature and PtdIns. Members of the SNX-BAR subfamily, SNX1 or SNX2, form a heterodimer with SNX5 or SNX6 in cells (Figure 2B). Intriguingly, although the PX domains of SNX1 and SNX2 bind to PtdIns(3)P, the PX domain of SNX6 interacts with PtdIns(4)P 37. Such an interaction may facilitate the dissociation of SNX-BAR-coated vesicles from motors at the TGN 37. Interestingly, whereas a wealth of earlier studies38–40 suggested that the retromer CSC associates with CI-MPR for its endosome-to-TGN transport, two recent studies indicate that SNX5 and SNX6 interact with the cytoplasmic tails of CI-MPR (and IGF1R), independent of retromer (Figure 2B) 41,42. Even more surprisingly, these studies also showed that deletion of SNX1/2/5/6, but not VPS35, leads to a pronounced defect in CI-MPR trafficking 41,42. The exact reasons for these discrepancies remain unclear, but given that both SNX3 and retromer bind to DMT1, SNX-BAR and retromer may recognize the CI-MPR tail through an analogous mechanism36. Future studies will be necessary to address how CI-MPR and IGF1R are recognized and transported.
Lastly, two members of the SNX-FERM subfamily, SNX27 and SNX17, have been shown to be critical for recycling of numerous cell surface proteins (Figure 2). In addition to the PX domain, both SNX27 and SNX17 contain a FERM domain, which recognizes NPxY/NxxY motifs found in a variety of proteins including growth factor receptors, solute carriers and integrins 43. Although SNX27 contains a unique PDZ domain at its N-terminus, SNX17 does not harbor this domain, but instead possesses a unique C-terminal polypeptide sequence. Intriguingly, it is these two unique regions that determine the cargo selectivity of these two SNXs. The PDZ domain of SNX27 binds directly to VPS26 and cargo proteins with PDZ-binding motifs including glucose transporter GLUT1 and the β2-adrenergic receptor (β2AR) 44–48. Thus, SNX27 mediates cargo recycling through a retromer-dependent manner. In contrast, SNX17 utilizes its unique C-terminal tail to interact with DSCR3 (VPS26C) of the retriever complex (see below), whereas its FERM domain binds to NPxY/NxxY motif-containing cargo proteins such as β1 integrin 49. As a result, SNX17 mediates the recycling of α5β1 integrin in a retromer-independent, retriever-dependent manner. Collectively, through association with retromer or retriever, SNXs provide cargo specificity and help to establish diverse endosomal trafficking pathways.
WASH COMPLEX
The WASP family promotes actin nucleation via the Arp2/3 complex, and function in cell migration/invasion, cell-cell adhesion, endocytosis/phagocytosis, cytokinesis, and intracellular membrane transport 50,51. WASH1 shares the conserved VCA domain (also known as the WCA domain) with other members of the WASP family, including WASP/N-WASP and WAVE 50–52. In cells, WASH1 tightly associates with four other proteins, including FAM21 (WASHC2), Strumpellin (WASHC5), Strumpellin and WASH1-interacting protein (SWIP or WASHC4), and CCDC53 (WASHC3) (Figure 3A) 53–55. The WASH complex is conserved in many eukaryotic taxa, including some unicellular organisms, but is not found in the yeast S. cerevisiae (Table 1). Although some controversy exists, in general, depletion of individual subunits impacts the stability of the other subunits. For example, mouse embryonic fibroblasts lacking WASH show a dramatic effect on CCDC53 stability and incorporation with the remaining complex members, whose levels are also diminished 56. FAM21 is comprised of a head domain (~220 amino acids), which is necessary to interact with other members of the WASH complex, and an extended C-terminal tail containing 21 repeats of a novel acidic motif (L-F-[D/E]3-10-L-F), termed the LFa motif 25,55. The FAM21 tail has been suggested to function as an endosomal signaling hub recruiting numerous proteins, including the actin-capping protein CapZ, ANKRD50, FKBP15, TBC1d23, RME-8, and the COMMD/CCDC22/CCDC93 (CCC) complex (Figure 3A) 25,55,57–63.
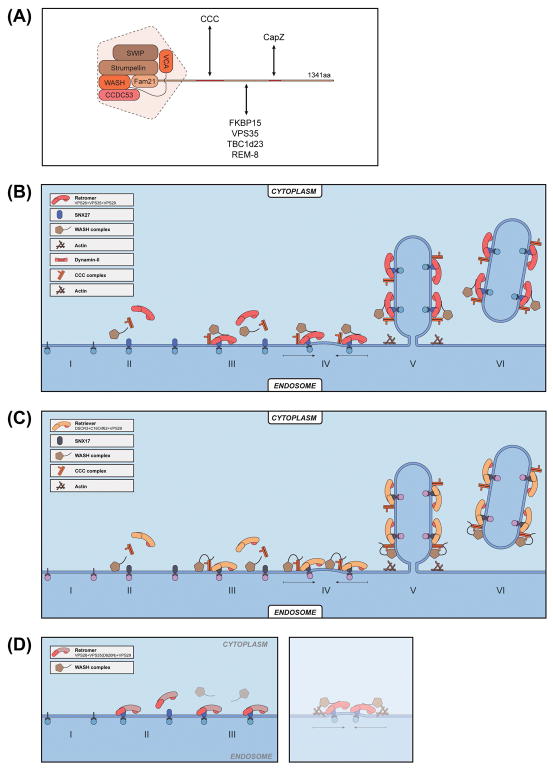
Figure 3.
Function of the WASH complex in retromer- and retriever-mediated trafficking.
(A) Organization of the WASH complex and some of its associated proteins. Whereas CCC and CapZ interact with FAM21 fragments containing amino acids 448-631 and 1010-1067 (as indicated), respectively, other proteins either bind to multiple regions of FAM21 or the exact interacting regions are not fully defined. For instance, VPS35 binds to many LFa repeats of FAM21, with highest affinity toward those at the C-terminal end of the tail.
(B) A model depicting the role of WASH complex in regulating retromer-mediating trafficking. Increasing concentration of cargo (step I) leads to membrane recruitment of the retromer-SNX27 complex (step II), which, in turn, recruits the WASH complex (step III). WASH-mediated actin polymerization could promote the organization of retromer endosomal subdomains (step IV), and formation of retromer tubules (step V). WASH could also function to assist the scission of tubular structures together with other proteins, such as Dynamin II (step VI).
(C) A model depicting the role of WASH complex in regulating retriever-mediating trafficking. SNX17 binds to cargo protein on the membrane, and WASH is recruited to membrane in a retromer-dependent manner (steps I and II). WASH and SNX17 in turn recruit CCC (step III), and retriever complexes (step IV). Similar to its role in retromer trafficking, WASH-mediated actin polymerization could promote the formation of SNX17/cargo/CCC/retriever endosomal subdomains (step V), vesicle budding (step VI), and vesicle scission (step VII).
(D) A mutation in VPS35 (D620N) diminishes the interaction of the WASH1 complex with the retromer, and impairs retromer-mediated cargo trafficking.
WASH1 predominantly localizes to endosomes, but can be also found associated with other organelles and even within the nucleus 64. In the cytosol, WASH complex-mediated actin polymerization has been observed to function in several distinct endosomal recycling pathways: (1) endosome-to-Golgi retrieval of CI-MPR 54, which also requires retromer, SNX3 and SNX-BAR proteins. (2) endosome-to-cell surface recycling of the transferrin receptor (TfnR), the β2-adrenoceptor (β2AR), the glucose transporter GLUT1, the copper transporter ATP7A, and α5β1 integrin 53,56,60,65–67. Interestingly whereas recycling of TfnR, β2AR, ATP7A, and GLUT1 requires retromer activity, recycling of α5β1 is retriever-dependent (see below). (3) WASH complex is also involved in epidermal growth factor receptor (EGFR) delivery to lysosomes 68. It should be noted that currently no evidence supports that retromer or retriever is directly required for the delivery of cargo to the degradation pathway. SNX6 appears to be needed for EGFR degradation, which may or may not be related to retromer activity 69. (4) Finally, the WASH complex can also associate with BLOC-1 (biogenesis of lysosomal organelles complex-1), but the significance of this interaction remains to be determined 70,71.
How does the WASH complex function to promote trafficking in such diverse pathways? Most of our understanding is derived from the better-characterized retromer-WASH pathway. In conjunction with SNX proteins, retromer mediates both endosome-to-TGN retrieval, and endosome-to-plasma membrane recycling. The WASH complex directly associates with retromer through an interaction between the LFa motif of FAM21 and VPS35 25,57. Knockdown of VPS35 in cells decreases the amount of endosomal-localized WASH complex; however, a significant amount of the WASH complex is still present on endosomes in VPS35-KO cells, suggesting that the WASH complex can localize to endosomes in both a retromer-dependent and -independent manner 25,49,57. Current data suggests a model by which WASH-mediated actin polymerization promotes retromer trafficking: (1) Endosome-bound proteins are recognized by specific combinations of retromer and SNXs; (2) Retromer recruits the WASH complex through a direct interaction; (3) The WASH complex is activated through ubiquitination or other yet to be characterized mechanisms, promoting actin polymerization via Arp2/3 complex recruitment and activation. A combination of the action of BAR domains, motor proteins, and actin polymerization leads to the formation of tubular structures; (4) Subsequently, actin polymerization, together with the activity of the dynein–dynactin complex, kinesin microtubular motors, and/or Dynamin II, promotes the fission of tubular structures; (5) Ultimately, tubular vesicles carrying various cargo proteins are delivered to their final destinations (Figure 3B). In addition to functioning in retromer-dependent receptor trafficking, the WASH complex may function in retromer-independent pathways through similar mechanisms (Figure 3C). For instance, the SNX17-retriever complex recognizes a different subset of endosomal cargo (see below). These proteins associate with the WASH complex, which may help to form discrete endomembrane subdomains and to facilitate vesicular scission through actin polymerization.
The following evidence supports the role of the WASH complex in both the formation of retromer tubules, and subsequent scission. First, in mouse embryonic fibroblasts devoid of WASH1, a collapse of the endolysosomal network to the perinuclear region was observed 56. There was no F-actin accumulation on the collapsed structures and only re-expression of wild-type WASH1, but not a VCA-deleted mutant, could restore the normal architecture of the endolysosomal system, F-actin accumulation and receptor trafficking 56. Second, in WASH1-knockdown cells, cargo-laden tubules have been observed suggestive of a defect in actin-dependent tubule scission 54. Third, recycling β2AR is localized in a subset of tubular endosome microdomains, which do not include degrading receptors and bulk recycling proteins 66. Recycling of β2AR requires both SNX27 and retromer 44,45. WASH, but not other WASP family members, including WASP, N-WASP, and WAVE, is concentrated on β2AR tubules 66. Finally, a mutation in VPS35 (D620N), that is associated with early onset Parkinson’s Disease (PD) was shown to diminish the interaction of the WASH1 complex with retromer and impact vesicular trafficking from the late endosome as well as impair autophagy 72–77 (Figure 3D). Interestingly, gain-of-function mutations in the FAM21-associated molecule, RME-8, which is involved in coordinating the activity of the WASH complex with the tabulating activities of SNXs 63, were identified in patients with PD and Lewy body pathology 78. Thus perturbation of receptor trafficking from the retromer subdomain seems to be a common mechanism contributing to the development of PD.
Due to the importance of WASH-mediated actin polymerization in endosomal trafficking, its activity must be tightly regulated, as is the case for other WASP proteins. Indeed, although recombinant WASH1 is active toward Arp2/3 in vitro, the assembly of WASH1 into the pentameric complex inhibits this activity 55. Interestingly, the WASH complex has structural similarity to the WAVE complex, which promotes actin polymerization at the leading edge of migrating cells 55,57. Several distinct mechanisms, including small GTPase Rac, phospholipids, and phosphorylation are known to activate the intrinsically inactive WAVE complex 79. Similarly, mammalian WASH complex can be activated through TRIM27-mediated poly-ubiquitination (K63 linked) on WASH1 at lysine 22080. Of note, this lysine residue lies within what would be the meander region of WAVE, and thus, ubiquitination at this residue might activate WASH1 by disrupting an inhibitory fold surrounding the VCA. More recently, USP7, a deubiquitinase that interacts with TRIM27 and the MAGE-L2 cargo adaptor protein, was shown to be required to not only prevent TRIM27 from ubiquitin-mediated degradation, but also regulates WASH1 activity by controlling the level of ubiquitination 81. Interestingly, the gene encoding USP7 is mutated in Autism-spectrum disorders, and the same has been noted for MAGE-L2, suggesting that aberrant WASH1 regulation may be involved in the pathology of these neurological diseases 81. Lastly, it should be pointed out that TRIM27 and MAGE-L2 are not ubiquitously expressed, thus the identification of signaling mechanisms contributing to the activation of WASH in cells devoid of these molecules remains to be determined.
Emphasizing the importance of the WASH complex in development and human diseases is the observation that mutations in the complex lead to multiple neurological disorders. A mutation in the gene encoding SWIP (P1019R) has been observed in patients with non-syndromic autosomal recessive intellectual disability (ID) and results in diminished levels of Strumpellin and other members of the WASH complex 82,83. In addition, mutations in Strumpellin have been found in patients with autosomal-dominant hereditary spastic paraplegia (HSP), but these mutations do not impact WASH complex assembly or endosomal localization, and thus might either be impacting WASH1 activity directly or could result from other unappreciated cellular functions of Strumpellin 55,84,85. Lastly, a splice-site mutation in Strumpellin was recently observed in Ritscher-Schinzel/3C syndrome, which among its clinical features includes intellectual disability 86. Although other WASH complex components were not assessed, this splice-site mutation leads to an eight-fold reduction in Strumpellin transcript and a ~60% loss of Strumpellin protein. The mechanism by which WASH deregulation contributes to the pathology of intellectual disability syndromes remains to be determined, but likely involves the regulation of vesicle recycling of proteins involved in neuronal maturation, survival or function during brain development.
RETRIEVER COMPLEX
It is of interest that WASH also regulates the recycling of SNX17 cargoes including the integrin α5β1, EGFR, LDLR, and Notch ligand Jag1 87–92. However, how SNX17 couples trafficking of its cargos to WASH-regulated subdomains remained a mystery until very recently 49,93. Through a series of proteomic and functional studies it was uncovered that SNX17 interacts with C16orf62, DSCR3 and VPS29, among other proteins (Figure 2B). C16orf62 is a distant homolog of VPS35, and has thus been named VPS35L 49. DSCR3, on the other hand, is predicted to have a fold analogous to VPS26, and has been named VPS26C 49,94. VPS35L, VPS26C and VPS29 form a stable trimeric complex, and co-elute following size-exclusion chromatography 49. Due to the similarity with retromer, this new complex has been named ‘retriever’. Knockdown or deletion of VPS35 results in a substantial loss of VPS26A, while VPS35L deletion results in depletion of VPS26C; interestingly, VPS29 is depleted primarily upon loss of VPS35 and not VPS35L 49. Importantly, despite the similarities between the retromer and retriever complexes, it is not known whether Rab proteins might also participate in the endosomal recruitment of the retriever complex like they do for the retromer complex. However, it is tempting to speculate that mechanisms regulating retromer recruitment might also operate to control retriever recruitment since both retromer and retriever share the VPS29 subunit that interacts with the Rab7a GAP TBC1d5.
Significantly, loss of retriever components (VPS35L and VPS26C) resulted in defective retrieval of SNX17 cargo from the endosome 49,60. The mechanism by which SNX17 coupled to the retriever was revealed when a highly conserved four amino acid sequence in the C-terminus of SNX17 was found to be necessary and sufficient to interact with VPS26C and recruit SNX17 to endosomes where WASH and the CCC complexes reside 49. Thus, WASH sits at the nexus of two major recycling pathways coordinating both SNX17 and SNX27 cargo retrieval (Figure 3).
CCC COMPLEX
Although a role for COMMD1 in regulating copper homeostasis in dogs and mice had been known for some time, the cellular mechanism was unclear 95. The explanation for this phenotype emerged when COMMD1 was found to associate with CCDC22 and CCDC93, two uncharacterized proteins found to interact with a specific region of the FAM21 C-terminal tail. This interaction is responsible for the recruitment of the CCC complex to endosomes 57,60. Loss of CCC complex components, as well as loss of the retromer subunit VPS35 or SNX27, lead to impaired endosome to plasma membrane recycling of the copper transporter ATP7A, indicating that CCC is required for recycling of at least certain retromer/SNX27 cargoes 60. Interestingly, a splice-site mutation in CCDC22 was identified as the causative mutation for X-linked intellectual disability in two families, a phenotype that resembles defects of WASH function in humans 96–98. Patients with this mutation showed a substantial loss of CCDC22 protein, which also impacted the levels of CCDC93 and resulted in the loss of COMMD1 from endosomes. Consistent with the idea that the CCC complex is involved in regulating copper transport, patients harboring CCDC22 mutations showed disturbances in copper handling that are similarly seen in animal models of COMMD1 loss.
Another prototypical SNX17 cargo is the LDL receptor and other members of this receptor family 99. Interestingly, mice lacking COMMD1 in hepatocytes showed high levels of LDL and cholesterol, which was also observed in patients with CCDC22 deficiency and as well as in patients with Strumpellin mutations associated with ID 99. Significantly, in mice and cells lacking COMMD1, the surface levels of LDLR were diminished, suggestive of a recycling defect. In addition, WASH deficiency also resulted in LDLR trafficking defects resulting in LDLR accumulation in lysosomes. In aggregate, studies of both CCC and WASH deficient states suggest that defective LDLR trafficking resulting in reduced cell surface expression likely accounts for the increased cholesterol and LDL levels seen in patients. Consistent with a broader role for the CCC in SNX17-dependent recycling, integrins were also found to be dependent on the CCC complex for proper trafficking 49. Thus, the studies to date indicate that the CCC complex is recruited to endosomes by the FAM21 subunit of WASH and is required for recycling events mediated by retriever/SNX17as well as retromer/SNX27. However, the exact mechanism by which it participates in these sorting events remains to be determined.
TBC1d23
In addition to the generation of endosomal vesicles, recent work also provides insights into the mechanisms by which endosomal vesicles are captured by the TGN 61,100. Interestingly, another member of the TBC family, TBC1d23, plays a critical role in the process. Unlike TBC1d5, TBC1d23 is apparently catalytically inactive due to mutations in key residues important for its GAP activity (Figure 4A). TBC1d23 also harbors a Rhodanese-like domain, which can be found in eukaryotic proteins such as protein phosphatases and ubiquitin ligases, and a C-terminal domain that bears little similarity with other proteins (Figure 4A).
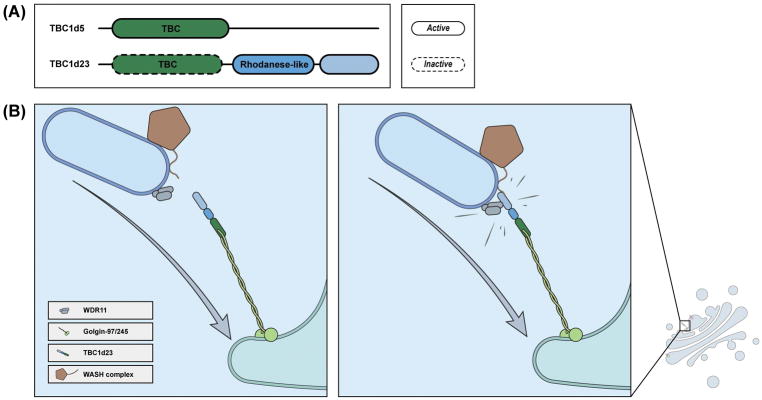
Figure 4.
Function of TBC1d23 in endosomal sorting.
(A) Domain organization of TBC1d23 and TBC1d5. Although both proteins possess a TBC domain, TBC1d5, but not TBC1d5, is catalytically active.
(B) A model depicting the function of TBC1d23 in tethering endosomal vesicles with TGN. Left: The WASH and WDR11 complexes decorate endosome-derived vesicles, and TBC1d23 is localized at the TGN through its interaction with Goglin-97/245. Right: Interaction between TBC1d23 and FAM21 or the WDR11 complex allows tethering and subsequent fusion of endosomal vesicles with the TGN.
The Golgi apparatus has a specific set of proteins, called golgins, which are able to capture vesicles from endosomes, endoplasmic reticulum, or the Golgi itself 101,102. Among them, golgin-97 and golgin-245 are responsible for receiving endosome-derived vesicles. Shin et al found that TBC1d23 bridges the interaction between golgin-97, golgin-245, and endosomal vesicles 61. The TBC domain of TBC1d23 binds to a conserved region of golgin-97 and golgin-245, and the C terminus binds to FAM21 (Figure 4B). Critically, deletion of TBC1d23, or deletion of both golgin-97 and golgin-245 leads to defective endosome-to-Golgi trafficking of CI-MPR and TGN46 61. Thus, TBC1d23 links WASH-coated endosomal vesicles to the TGN during endosome-to-TGN retrieval.
In addition to golgin-97, golgin-245 and the WASH complex, TBC1d23 also interacts with a trimeric complex consisting of WDR11, FAM91A1, and C7orf75 (Figure 4B) 61,100. Similar to the WASH complex, the WDR11 complex associates with vesicles derived from endosomes, but not with the TGN 100. The interaction between the WDR11 complex and TBC1d23 is proposed to promote the tethering of vesicles to the TGN; however, the exact mechanism remains to be elucidated. Intriguingly, homozygous mutation in TBC1d23 has been recently linked with pontocerebellar hypoplasia, a developmental disorder characterized by impaired growth of the pons and cerebellum, furthering emphasizing the importance of vesicular trafficking in neuronal development and function 103,104.
MANIPULATION OF ENDOSOMAL PROTEIN SORTING BY PATHOGENS
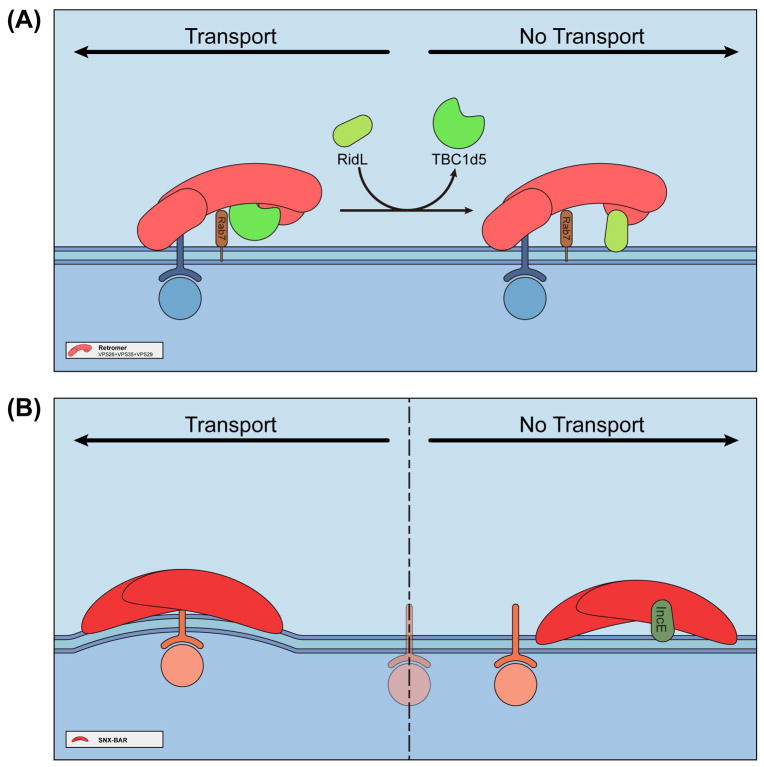
Given the importance of endosomal sorting pathways for cellular homeostasis and other cellular functions, these pathways emerge as opportune targets by a variety of viral and bacterial pathogens 105. Pathogens target key sorting protein machineries, such as retromer, SNX proteins, and the WASH complex, to promote cellular entry and replication during infection, and to evade degradation. For instance, the envelope glycoprotein of the HIV type-1 and the tyrosine kinase-interacting (Tip) protein of the herpesvirus saimiri bind directly to retromer during viral infection 106,107. Human papillomavirus type 16, and the effector IncE of Chlamydia trachomatis, target to SNX27, and SNX5/SNX6, respectively 108–110. The WASH-FAM21 complex is required by Vaccinia Virus for cellular entry and subsequent intracellular transport 111,112. Among all cases, two bacterial proteins, RidL and IncE, have been most extensively studied. Biochemical, structural, and cellular studies have collectively revealed not only important bacteria-host interactions, but have also uncovered important, but previously less appreciated host regulatory mechanisms.
Legionella pneumophila is the causative agent of Legionnaires’ disease. During infection, these bacteria reside in a special membrane compartment known as the Legionella-containing vacuole (LCV) 113, which delivers nearly 300 different proteins, known as effectors, to exploit various host functions including vesicle transport. The effector RidL appears to target to retromer and interfere with retromer function, which has been shown to restrict intracellular growth of L. pneumophila 114. RidL is a large protein with over 1100 amino acids, but lacks sequence homology to known proteins. The interaction between RidL and retromer is mediated by the N-terminal 200 amino acids of RidL and the VPS29 subunit of retromer 23,115,116. RidL contacts VPS29 with a dissociation constant of ~200 nM, similar to the affinity between TBC1d5 and VPS35/VPS29 23,116. Structural and biochemical studies reveal that both RidL and TBC1d5 bind to a highly conserved hydrophobic surface of VPS29, opposite to the VPS35 binding surface 20,23,115,116. Remarkably, both RidL and TBC1d5, and likely VARP, interact with VPS29 through a conserved P-L/I motif from a hairpin loop 23,24,116. Outside of this motif, these three proteins bear little sequence similarity. In fact, RidL and TBC1d5 have opposite main chain directions in their aligned regions 116. Consistent with structural analysis, RidL competes with TBC1d5 and VARP in vitro and in vivo 23,116. Thus, RidL interferes with retromer trafficking by outcompeting critical endogenous regulators (Figure 5A). In addition to retromer, the N-terminus of RidL also associates with the endosomal lipid PtdIns(3)P, which aids in the recruitment of RidL together with retromer116. Despite these studies, functions of the C-terminus of RidL remain unclear 117. Since many multiple-domain Legionella effectors have related functions in each of their domains, RidL likely regulates endosomal trafficking through other mechanisms, such as post-translational modifications of retromer or related proteins.
Figure 5.
Mechanisms of interference of endosomal trafficking by bacterial effector proteins RidL and IncE.
(A) Model showing how RidL subverts retromer-dependent transport. Left: TBC1d5 is required for retromer-dependent endosomal trafficking. TBC1d5 interacts with both VPS35 and VPS29. Right: During L. pneumophila infection, RidL replaces TBC1d5, and likely VARP (not shown), to block retromer-mediated trafficking. In contrast with TBC1d5, RidL interacts only with VPS29.
(B) Model showing how IncE inhibits SNX-BAR-dependent transport. Left: SNX-BAR complex mediates endosome-to-TGN transport of certain proteins, such as CI-MPR. Right: Bacterial effector protein IncE interacts with the PX domain of SNX5/SNX6, and may inhibit their association with cargo proteins.
Another pathogenic protein that has been extensively characterized is the Chlamydial effector protein IncE 110,118–121. Chlamydiae infection leads to human blindness, respiratory, and genital tract diseases. Chlamydiae replicates within a unique membrane-bound compartment, termed the inclusion. IncE is one of the effector proteins localized on the inclusion membrane, which specifically interacts with SNX5/SNX6 and inhibits retromer- and SNX5/SNX6-mediated endosomal trafficking 110. Structural studies from three different groups revealed that IncE binds to a conserved hydrophobic groove in the PX domain of SNX5 118–120. Although the exact inhibitory mechanisms exerted by IncE remain to be determined, two observations indicate that IncE may function through interfering with the interaction between SNX5 and its cargo, such as IGFR1 and CI-MPR (Figure 5B) 118–120. First, a SNX5 mutant unable to bind to IncE shows decreased binding to IGFR1 and CI-MPR 120. Second, overexpression of IncE reduces co-localization of CI-M6PR with VPS35, and inhibits the delivery of CI-MPR to the TGN 118. Therefore, two distinct types of bacteria utilize diverse mechanisms to interfere with endosomal sorting.
CONCLUSIONS
The last several years have seen remarkable progress in the field of endosomal sorting. In addition to the well-established retromer-dependent trafficking pathway, retriever is found to mediate retromer-independent endosomal trafficking. Remarkably, retriever shows a certain degree of homology to retromer, including complex organization, protein sequences, and interaction with SNXs. Additionally, several novel protein machineries have been identified and characterized to regulate endosomal sorting including the WASH complex, TBC1d5, TBC1d23, the CCC complex, and WDR11. The identification of these complexes has allowed the dissection of the mechanistic details of retromer membrane recruitment, cargo recognition, vesicular transport, and vesicle tethering with target membranes. Despite this progress, many key questions remain to be answered in the field including but not limited to: (1) Are there other complexes that function similar to retromer and retriever? (2) What is the exact role of the WASH and CCC complexes in retromer and retriever-mediated trafficking? (3) Since endosomal proteins can be targeted to both the plasma membrane and the TGN, how are specific trafficking routes selected? (4) Why do viral and bacterial pathogens target endosomal trafficking pathways? Undoubtedly, answers to these questions will lead us to a better understanding of endosomal receptor trafficking and their functions in organismal development and human disease.
Synopsis.
The proper delivery of internalized receptors to specific subcellular destinations is critical to normal cellular physiology. Several endosomal-associated molecules including sorting nexins, the retromer and WASH complexes, as well as the recently identified retriever and CCC complexes regulate receptor recycling through the tubular endolysosomal network. Herein, we highlight the intricacies by which the retromer and associated molecules contribute to receptor trafficking and how deregulation at this sorting domain can contribute to disease or facilitate pathogen infection.
Acknowledgments
We thank members of our laboratories for critical discussions, Mr. Chengxin Weng for help with figures and apologize for our colleagues whose work could not be cited here due to space constraints. This research is supported by Natural Science Foundation of China (NSFC) grants #31671477 (D.J.), NIH grants R01DK073639 (E.B.) and R01DK107733 (D.D.B. and E.B.). D.J. is a “One Thousand Talents” program scholar. The authors declare no financial conflicts of interest.
References
- 1.Burd C, Cullen PJ. Retromer: A Master Conductor of Endosome Sorting. Csh Perspect Biol. 2014;6(2) doi: 10.1101/cshperspect.a016774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo Y, Sirkis DW, Schekman R. Protein sorting at the trans-Golgi network. Annu Rev Cell Dev Biol. 2014;30:169–206. doi: 10.1146/annurev-cellbio-100913-013012. [DOI] [PubMed] [Google Scholar]
- 3.Bonifacino JS, Hurley JH. Retromer. Curr Opin Cell Biol. 2008;20(4):427–436. doi: 10.1016/j.ceb.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McMillan KJ, Korswagen HC, Cullen PJ. The emerging role of retromer in neuroprotection. Curr Opin Cell Biol. 2017;47:72–82. doi: 10.1016/j.ceb.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lucas M, Hierro A. Retromer. Curr Biol. 2017;27(14):R687–R689. doi: 10.1016/j.cub.2017.05.072. [DOI] [PubMed] [Google Scholar]
- 6.Seaman MN, McCaffery JM, Emr SD. A membrane coat complex essential for endosome-to-Golgi retrograde transport in yeast. J Cell Biol. 1998;142(3):665–681. doi: 10.1083/jcb.142.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu JJ. Retromer-Mediated Protein Sorting and Vesicular Trafficking. J Genet Genomics. 2016;43(4):165–177. doi: 10.1016/j.jgg.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Palmer DJ, Helms JB, Beckers CJ, Orci L, Rothman JE. Binding of coatomer to Golgi membranes requires ADP-ribosylation factor. J Biol Chem. 1993;268(16):12083–12089. [PubMed] [Google Scholar]
- 9.Orcl L, Palmer DJ, Amherdt M, Rothman JE. Coated vesicle assembly in the Golgi requires only coatomer and ARF proteins from the cytosol. Nature. 1993;364(6439):732–734. doi: 10.1038/364732a0. [DOI] [PubMed] [Google Scholar]
- 10.Yoshihisa T, Barlowe C, Schekman R. Requirement for a GTPase-activating protein in vesicle budding from the endoplasmic reticulum. Science. 1993;259(5100):1466–1468. doi: 10.1126/science.8451644. [DOI] [PubMed] [Google Scholar]
- 11.Barlowe C, Orci L, Yeung T, et al. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994;77(6):895–907. doi: 10.1016/0092-8674(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 12.Nakada-Tsukui K, Saito-Nakano Y, Ali V, Nozaki T. A retromerlike complex is a novel Rab7 effector that is involved in the transport of the virulence factor cysteine protease in the enteric protozoan parasite Entamoeba histolytica. Mol Biol Cell. 2005;16(11):5294–5303. doi: 10.1091/mbc.E05-04-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balderhaar HJ, Arlt H, Ostrowicz C, et al. The Rab GTPase Ypt7 is linked to retromer-mediated receptor recycling and fusion at the yeast late endosome. J Cell Sci. 2010;123(Pt 23):4085–4094. doi: 10.1242/jcs.071977. [DOI] [PubMed] [Google Scholar]
- 14.Liu TT, Gomez TS, Sackey BK, Billadeau DD, Burd CG. Rab GTPase regulation of retromer-mediated cargo export during endosome maturation. Mol Biol Cell. 2012;23(13):2505–2515. doi: 10.1091/mbc.E11-11-0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zelazny E, Santambrogio M, Pourcher M, et al. Mechanisms Governing the Endosomal Membrane Recruitment of the Core Retromer in Arabidopsis. J Biol Chem. 2013;288(13):8815–8825. doi: 10.1074/jbc.M112.440503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Priya A, Kalaidzidis IV, Kalaidzidis Y, Lambright D, Datta S. Molecular insights into Rab7-mediated endosomal recruitment of core retromer: deciphering the role of Vps26 and Vps35. Traffic. 2015;16(1):68–84. doi: 10.1111/tra.12237. [DOI] [PubMed] [Google Scholar]
- 17.Harrison MS, Hung CS, Liu TT, Christiano R, Walther TC, Burd CG. A mechanism for retromer endosomal coat complex assembly with cargo. Proc Natl Acad Sci U S A. 2014;111(1):267–272. doi: 10.1073/pnas.1316482111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rojas R, van Vlijmen T, Mardones GA, et al. Regulation of retromer recruitment to endosomes by sequential action of Rab5 and Rab7. J Cell Biol. 2008;183(3):513–526. doi: 10.1083/jcb.200804048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seaman MN, Harbour ME, Tattersall D, Read E, Bright N. Membrane recruitment of the cargo-selective retromer subcomplex is catalysed by the small GTPase Rab7 and inhibited by the Rab-GAP TBC1D5. J Cell Sci. 2009;122(Pt 14):2371–2382. doi: 10.1242/jcs.048686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harbour ME, Breusegem SY, Antrobus R, Freeman C, Reid E, Seaman MN. The cargo-selective retromer complex is a recruiting hub for protein complexes that regulate endosomal tubule dynamics. J Cell Sci. 2010;123(Pt 21):3703–3717. doi: 10.1242/jcs.071472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jia D, Zhang JS, Li F, et al. Structural and mechanistic insights into regulation of the retromer coat by TBC1d5. Nat Commun. 2016;7:13305. doi: 10.1038/ncomms13305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukhopadhyay A, Pan X, Lambright DG, Tissenbaum HA. An endocytic pathway as a target of tubby for regulation of fat storage. EMBO Rep. 2007;8(10):931–938. doi: 10.1038/sj.embor.7401055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romano-Moreno M, Rojas AL, Williamson CD, et al. Molecular mechanism for the subversion of the retromer coat by the Legionella effector RidL. Proc Natl Acad Sci U S A. 2017;114(52):E11151–E11160. doi: 10.1073/pnas.1715361115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hesketh GG, Perez-Dorado I, Jackson LR, et al. VARP Is Recruited on to Endosomes by Direct Interaction with Retromer, Where Together They Function in Export to the Cell Surface. Developmental Cell. 2014;29(5):591–606. doi: 10.1016/j.devcel.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jia D, Gomez TS, Billadeau DD, Rosen MK. Multiple repeat elements within the FAM21 tail link the WASH actin regulatory complex to the retromer. Mol Biol Cell. 2012;23(12):2352–2361. doi: 10.1091/mbc.E11-12-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Norwood SJ, Shaw DJ, Cowieson NP, Owen DJ, Teasdale RD, Collins BM. Assembly and solution structure of the core retromer protein complex. Traffic. 2011;12(1):56–71. doi: 10.1111/j.1600-0854.2010.01124.x. [DOI] [PubMed] [Google Scholar]
- 27.Jimenez-Orgaz A, Kvainickas A, Nagele H, et al. Control of RAB7 activity and localization through the retromer-TBC1D5 complex enables RAB7-dependent mitophagy. EMBO J. 2018;37(2):235–254. doi: 10.15252/embj.201797128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Popovic D, Akutsu M, Novak I, Harper JW, Behrends C, Dikic I. Rab GTPase-activating proteins in autophagy: regulation of endocytic and autophagy pathways by direct binding to human ATG8 modifiers. Mol Cell Biol. 2012;32(9):1733–1744. doi: 10.1128/MCB.06717-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Popovic D, Dikic I. TBC1D5 and the AP2 complex regulate ATG9 trafficking and initiation of autophagy. EMBO Rep. 2014;15(4):392–401. doi: 10.1002/embr.201337995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roy S, Leidal AM, Ye J, Ronen SM, Debnath J. Autophagy-Dependent Shuttling of TBC1D5 Controls Plasma Membrane Translocation of GLUT1 and Glucose Uptake. Mol Cell. 2017;67(1):84–95. e85. doi: 10.1016/j.molcel.2017.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teasdale Rohan D, Collins Brett M. Insights into the PX (phox-homology) domain and SNX (sorting nexin) protein families: structures, functions and roles in disease. Biochemical Journal. 2012;441(1):39–59. doi: 10.1042/BJ20111226. [DOI] [PubMed] [Google Scholar]
- 32.van Weering JR, Cullen PJ. Membrane-associated cargo recycling by tubule-based endosomal sorting. Semin Cell Dev Biol. 2014;31:40–47. doi: 10.1016/j.semcdb.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 33.Harterink M, Port F, Lorenowicz MJ, et al. A SNX3-dependent retromer pathway mediates retrograde transport of the Wnt sorting receptor Wntless and is required for Wnt secretion. Nat Cell Biol. 2011;13(8):914–923. doi: 10.1038/ncb2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang P, Wu Y, Belenkaya TY, Lin X. SNX3 controls Wingless/Wnt secretion through regulating retromer-dependent recycling of Wntless. Cell Res. 2011;21(12):1677–1690. doi: 10.1038/cr.2011.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strochlic TI, Setty TG, Sitaram A, Burd CG. Grd19/Snx3p functions as a cargo-specific adapter for retromer-dependent endocytic recycling. J Cell Biol. 2007;177(1):115–125. doi: 10.1083/jcb.200609161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lucas M, Gershlick DC, Vidaurrazaga A, Rojas AL, Bonifacino JS, Hierro A. Structural Mechanism for Cargo Recognition by the Retromer Complex. Cell. 2016 doi: 10.1016/j.cell.2016.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niu Y, Zhang C, Sun Z, et al. PtdIns(4)P regulates retromer-motor interaction to facilitate dynein-cargo dissociation at the trans-Golgi network. Nat Cell Biol. 2013;15(4):417–429. doi: 10.1038/ncb2710. [DOI] [PubMed] [Google Scholar]
- 38.Arighi CN, Hartnell LM, Aguilar RC, Haft CR, Bonifacino JS. Role of the mammalian retromer in sorting of the cation-independent mannose 6-phosphate receptor. J Cell Biol. 2004;165(1):123–133. doi: 10.1083/jcb.200312055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seaman MN. Cargo-selective endosomal sorting for retrieval to the Golgi requires retromer. J Cell Biol. 2004;165(1):111–122. doi: 10.1083/jcb.200312034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seaman MN. Identification of a novel conserved sorting motif required for retromer-mediated endosome-to-TGN retrieval. J Cell Sci. 2007;120(Pt 14):2378–2389. doi: 10.1242/jcs.009654. [DOI] [PubMed] [Google Scholar]
- 41.Kvainickas A, Jimenez-Orgaz A, Nagele H, Hu Z, Dengjel J, Steinberg F. Cargo-selective SNX-BAR proteins mediate retromer trimer independent retrograde transport. J Cell Biol. 2017;216(11):3677–3693. doi: 10.1083/jcb.201702137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simonetti B, Danson CM, Heesom KJ, Cullen PJ. Sequence-dependent cargo recognition by SNX-BARs mediates retromer-independent transport of CI-MPR. J Cell Biol. 2017;216(11):3695–3712. doi: 10.1083/jcb.201703015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghai R, Bugarcic A, Liu H, et al. Structural basis for endosomal trafficking of diverse transmembrane cargos by PX-FERM proteins. Proc Natl Acad Sci U S A. 2013;110(8):E643–652. doi: 10.1073/pnas.1216229110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steinberg F, Gallon M, Winfield M, et al. A global analysis of SNX27-retromer assembly and cargo specificity reveals a function in glucose and metal ion transport. Nat Cell Biol. 2013;15(5):461–471. doi: 10.1038/ncb2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Temkin P, Lauffer B, Jager S, Cimermancic P, Krogan NJ, von Zastrow M. SNX27 mediates retromer tubule entry and endosome-to-plasma membrane trafficking of signalling receptors. Nat Cell Biol. 2011;13(6):715–721. doi: 10.1038/ncb2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gallon M, Clairfeuille T, Steinberg F, et al. A unique PDZ domain and arrestin-like fold interaction reveals mechanistic details of endocytic recycling by SNX27-retromer. Proc Natl Acad Sci U S A. 2014;111(35):E3604–3613. doi: 10.1073/pnas.1410552111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clairfeuille T, Mas C, Chan AS, et al. A molecular code for endosomal recycling of phosphorylated cargos by the SNX27-retromer complex. Nat Struct Mol Biol. 2016;23(10):921–932. doi: 10.1038/nsmb.3290. [DOI] [PubMed] [Google Scholar]
- 48.Lee S, Chang J, Blackstone C. FAM21 directs SNX27-retromer cargoes to the plasma membrane by preventing transport to the Golgi apparatus. Nat Commun. 2016;7:10939. doi: 10.1038/ncomms10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McNally KE, Faulkner R, Steinberg F, et al. Retriever is a multiprotein complex for retromer-independent endosomal cargo recycling. Nat Cell Biol. 2017;19(10):1214–1225. doi: 10.1038/ncb3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Padrick SB, Rosen MK. Physical mechanisms of signal integration by WASP family proteins. Annu Rev Biochem. 2010;79:707–735. doi: 10.1146/annurev.biochem.77.060407.135452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burianek LE, Soderling SH. Under lock and key: spatiotemporal regulation of WASP family proteins coordinates separate dynamic cellular processes. Semin Cell Dev Biol. 2013;24(4):258–266. doi: 10.1016/j.semcdb.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Linardopoulou EV, Parghi SS, Friedman C, Osborn GE, Parkhurst SM, Trask BJ. Human subtelomeric WASH genes encode a new subclass of the WASP family. PLoS Genet. 2007;3(12):e237. doi: 10.1371/journal.pgen.0030237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Derivery E, Sousa C, Gautier JJ, Lombard B, Loew D, Gautreau A. The Arp2/3 activator WASH controls the fission of endosomes through a large multiprotein complex. Dev Cell. 2009;17(5):712–723. doi: 10.1016/j.devcel.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 54.Gomez TS, Billadeau DD. A FAM21-containing WASH complex regulates retromer-dependent sorting. Dev Cell. 2009;17(5):699–711. doi: 10.1016/j.devcel.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jia D, Gomez TS, Metlagel Z, et al. WASH and WAVE actin regulators of the Wiskott-Aldrich syndrome protein (WASP) family are controlled by analogous structurally related complexes. Proc Natl Acad Sci U S A. 2010;107(23):10442–10447. doi: 10.1073/pnas.0913293107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gomez TS, Gorman JA, de Narvajas AA, Koenig AO, Billadeau DD. Trafficking defects in WASH-knockout fibroblasts originate from collapsed endosomal and lysosomal networks. Mol Biol Cell. 2012;23(16):3215–3228. doi: 10.1091/mbc.E12-02-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harbour ME, Breusegem SY, Seaman MN. Recruitment of the endosomal WASH complex is mediated by the extended “tail” of Fam21 binding to the retromer protein VPS35. Biochem J. 2011;(442):209–220. doi: 10.1042/BJ20111761. [DOI] [PubMed] [Google Scholar]
- 58.Takeda S, Minakata S, Koike R, et al. Two distinct mechanisms for actin capping protein regulation--steric and allosteric inhibition. PLoS Biol. 2010;8(7):e1000416. doi: 10.1371/journal.pbio.1000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hernandez-Valladares M, Kim T, Kannan B, et al. Structural characterization of a capping protein interaction motif defines a family of actin filament regulators. Nat Struct Mol Biol. 2010;17(4):497–503. doi: 10.1038/nsmb.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Phillips-Krawczak CA, Singla A, Starokadomskyy P, et al. COMMD1 is linked to the WASH complex and regulates endosomal trafficking of the copper transporter ATP7A. Mol Biol Cell. 2015;26(1):91–103. doi: 10.1091/mbc.E14-06-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shin JJH, Gillingham AK, Begum F, Chadwick J, Munro S. TBC1D23 is a bridging factor for endosomal vesicle capture by golgins at the trans-Golgi. Nat Cell Biol. 2017;19(12):1424–1432. doi: 10.1038/ncb3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kvainickas A, Orgaz AJ, Nagele H, et al. Retromer- and WASH-dependent sorting of nutrient transporters requires a multivalent interaction network with ANKRD50. J Cell Sci. 2017;130(2):382–395. doi: 10.1242/jcs.196758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Freeman CL, Hesketh G, Seaman MN. RME-8 coordinates the activity of the WASH complex with the function of the retromer SNX dimer to control endosomal tubulation. J Cell Sci. 2014;127(Pt 9):2053–2070. doi: 10.1242/jcs.144659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Verboon JM, Rincon-Arano H, Werwie TR, et al. Wash interacts with lamin and affects global nuclear organization. Curr Biol. 2015;25(6):804–810. doi: 10.1016/j.cub.2015.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zech T, Calaminus SD, Caswell P, et al. The Arp2/3 activator WASH regulates alpha5beta1-integrin-mediated invasive migration. J Cell Sci. 2011;124(Pt 22):3753–3759. doi: 10.1242/jcs.080986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Puthenveedu MA, Lauffer B, Temkin P, et al. Sequence-dependent sorting of recycling proteins by actin-stabilized endosomal microdomains. Cell. 2010;143(5):761–773. doi: 10.1016/j.cell.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Piotrowski JT, Gomez TS, Schoon RA, Mangalam AK, Billadeau DD. WASH knockout T cells demonstrate defective receptor trafficking, proliferation, and effector function. Mol Cell Biol. 2013;33(5):958–973. doi: 10.1128/MCB.01288-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Duleh SN, Welch MD. WASH and the Arp2/3 complex regulate endosome shape and trafficking. Cytoskeleton (Hoboken) 2010;67(3):193–206. doi: 10.1002/cm.20437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cavet ME, Pang J, Yin G, Berk BC. An epidermal growth factor (EGF) -dependent interaction between GIT1 and sorting nexin 6 promotes degradation of the EGF receptor. FASEB J. 2008;22(10):3607–3616. doi: 10.1096/fj.07-094086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Monfregola J, Napolitano G, D’Urso M, Lappalainen P, Ursini MV. Functional characterization of Wiskott-Aldrich syndrome protein and scar homolog (WASH), a bi-modular nucleation-promoting factor able to interact with biogenesis of lysosome-related organelle subunit 2 (BLOS2) and gamma-tubulin. J Biol Chem. 2010;285(22):16951–16957. doi: 10.1074/jbc.M109.078501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ryder PV, Vistein R, Gokhale A, Seaman MN, Puthenveedu MA, Faundez V. The WASH complex, an endosomal Arp2/3 activator, interacts with the Hermansky-Pudlak syndrome complex BLOC-1 and its cargo phosphatidylinositol-4-kinase type IIalpha. Mol Biol Cell. 2013;24(14):2269–2284. doi: 10.1091/mbc.E13-02-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McGough IJ, Steinberg F, Jia D, et al. Retromer binding to FAM21 and the WASH complex is perturbed by the Parkinson disease-linked VPS35(D620N) mutation. Curr Biol. 2014;24(14):1670–1676. doi: 10.1016/j.cub.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vilarino-Guell C, Wider C, Ross OA, et al. VPS35 mutations in Parkinson disease. Am J Hum Genet. 2011;89(1):162–167. doi: 10.1016/j.ajhg.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zimprich A, Benet-Pages A, Struhal W, et al. A mutation in VPS35, encoding a subunit of the retromer complex, causes late-onset Parkinson disease. Am J Hum Genet. 2011;89(1):168–175. doi: 10.1016/j.ajhg.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zavodszky E, Seaman MN, Moreau K, et al. Mutation in VPS35 associated with Parkinson’s disease impairs WASH complex association and inhibits autophagy. Nat Commun. 2014;5:3828. doi: 10.1038/ncomms4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Follett J, Norwood SJ, Hamilton NA, et al. The Vps35 D620N mutation linked to Parkinson’s disease disrupts the cargo sorting function of retromer. Traffic. 2014;15(2):230–244. doi: 10.1111/tra.12136. [DOI] [PubMed] [Google Scholar]
- 77.Miura E, Hasegawa T, Konno M, et al. VPS35 dysfunction impairs lysosomal degradation of alpha-synuclein and exacerbates neurotoxicity in a Drosophila model of Parkinson’s disease. Neurobiol Dis. 2014;71:1–13. doi: 10.1016/j.nbd.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 78.Vilarino-Guell C, Rajput A, Milnerwood AJ, et al. DNAJC13 mutations in Parkinson disease. Hum Mol Genet. 2014;23(7):1794–1801. doi: 10.1093/hmg/ddt570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen Z, Borek D, Padrick SB, et al. Structure and control of the actin regulatory WAVE complex. Nature. 2010;468(7323):533–538. doi: 10.1038/nature09623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hao YH, Doyle JM, Ramanathan S, et al. Regulation of WASH-dependent actin polymerization and protein trafficking by ubiquitination. Cell. 2013;152(5):1051–1064. doi: 10.1016/j.cell.2013.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hao YH, Fountain MD, Jr, Fon Tacer K, et al. USP7 Acts as a Molecular Rheostat to Promote WASH-Dependent Endosomal Protein Recycling and Is Mutated in a Human Neurodevelopmental Disorder. Mol Cell. 2015;59(6):956–969. doi: 10.1016/j.molcel.2015.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ropers F, Derivery E, Hu H, et al. Identification of a novel candidate gene for non-syndromic autosomal recessive intellectual disability: the WASH complex member SWIP. Hum Mol Genet. 2011;20(13):2585–2590. doi: 10.1093/hmg/ddr158. [DOI] [PubMed] [Google Scholar]
- 83.Vardarajan BN, Bruesegem SY, Harbour ME, et al. Identification of Alzheimer disease-associated variants in genes that regulate retromer function. Neurobiol Aging. 2012;33(9):2231.e2215–2231.e2230. doi: 10.1016/j.neurobiolaging.2012.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Valdmanis PN, Meijer IA, Reynolds A, et al. Mutations in the KIAA0196 gene at the SPG8 locus cause hereditary spastic paraplegia. Am J Hum Genet. 2007;80(1):152–161. doi: 10.1086/510782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.de Bot ST, Vermeer S, Buijsman W, et al. Pure adult-onset spastic paraplegia caused by a novel mutation in the KIAA0196 (SPG8) gene. J Neurol. 2013;260(7):1765–1769. doi: 10.1007/s00415-013-6870-x. [DOI] [PubMed] [Google Scholar]
- 86.Elliott AM, Simard LR, Coghlan G, et al. A novel mutation in KIAA0196: identification of a gene involved in Ritscher-Schinzel/3C syndrome in a First Nations cohort. J Med Genet. 2013;50(12):819–822. doi: 10.1136/jmedgenet-2013-101715. [DOI] [PubMed] [Google Scholar]
- 87.Osborne DG, Piotrowski JT, Dick CJ, Zhang JS, Billadeau DD. SNX17 affects T cell activation by regulating TCR and integrin recycling. J Immunol. 2015;194(9):4555–4566. doi: 10.4049/jimmunol.1402734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Steinberg F, Heesom KJ, Bass MD, Cullen PJ. SNX17 protects integrins from degradation by sorting between lysosomal and recycling pathways. J Cell Biol. 2012;197(2):219–230. doi: 10.1083/jcb.201111121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stockinger W, Sailler B, Strasser V, et al. The PX-domain protein SNX17 interacts with members of the LDL receptor family and modulates endocytosis of the LDL receptor. EMBO J. 2002;21(16):4259–4267. doi: 10.1093/emboj/cdf435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yin W, Liu D, Liu N, et al. SNX17 regulates Notch pathway and pancreas development through the retromer-dependent recycling of Jag1. Cell Regen (Lond) 2012;1(1):4. doi: 10.1186/2045-9769-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bottcher RT, Stremmel C, Meves A, et al. Sorting nexin 17 prevents lysosomal degradation of beta1 integrins by binding to the beta1-integrin tail. Nat Cell Biol. 2012;14(6):584–592. doi: 10.1038/ncb2501. [DOI] [PubMed] [Google Scholar]
- 92.Li H, Koo Y, Mao X, et al. Endosomal sorting of Notch receptors through COMMD9-dependent pathways modulates Notch signaling. J Cell Biol. 2015;211(3):605–617. doi: 10.1083/jcb.201505108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mallam AL, Marcotte EM. Systems-wide Studies Uncover Commander, a Multiprotein Complex Essential to Human Development. Cell Syst. 2017;4(5):483–494. doi: 10.1016/j.cels.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Aubry L, Klein G. True arrestins and arrestin-fold proteins: a structure-based appraisal. Prog Mol Biol Transl Sci. 2013;118:21–56. doi: 10.1016/B978-0-12-394440-5.00002-4. [DOI] [PubMed] [Google Scholar]
- 95.van De Sluis B, Rothuizen J, Pearson PL, van Oost BA, Wijmenga C. Identification of a new copper metabolism gene by positional cloning in a purebred dog population. Hum Mol Genet. 2002;11(2):165–173. doi: 10.1093/hmg/11.2.165. [DOI] [PubMed] [Google Scholar]
- 96.Kolanczyk M, Krawitz P, Hecht J, et al. Missense variant in CCDC22 causes X-linked recessive intellectual disability with features of Ritscher-Schinzel/3C syndrome. Eur J Hum Genet. 2015;23(5):633–638. doi: 10.1038/ejhg.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Voineagu I, Huang L, Winden K, et al. CCDC22: a novel candidate gene for syndromic X-linked intellectual disability. Mol Psychiatry. 2012;17(1):4–7. doi: 10.1038/mp.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Starokadomskyy P, Gluck N, Li H, et al. CCDC22 deficiency in humans blunts activation of proinflammatory NF-kappaB signaling. J Clin Invest. 2013;123(5):2244–2256. doi: 10.1172/JCI66466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bartuzi P, Billadeau DD, Favier R, et al. CCC- and WASH-mediated endosomal sorting of LDLR is required for normal clearance of circulating LDL. Nat Commun. 2016;7:10961. doi: 10.1038/ncomms10961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Navarro Negredo P, Edgar JR, Manna PT, Antrobus R, Robinson MS. The WDR11 complex facilitates the tethering of AP-1-derived vesicles. Nat Commun. 2018;9(1):596. doi: 10.1038/s41467-018-02919-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cheung PY, Pfeffer SR. Transport Vesicle Tethering at the Trans Golgi Network: Coiled Coil Proteins in Action. Front Cell Dev Biol. 2016;4:18. doi: 10.3389/fcell.2016.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wong M, Munro S. Membrane trafficking. The specificity of vesicle traffic to the Golgi is encoded in the golgin coiled-coil proteins. Science. 2014;346(6209):1256898. doi: 10.1126/science.1256898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ivanova EL, Mau-Them FT, Riazuddin S, et al. Homozygous Truncating Variants in TBC1D23 Cause Pontocerebellar Hypoplasia and Alter Cortical Development. Am J Hum Genet. 2017;101(3):428–440. doi: 10.1016/j.ajhg.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Marin-Valencia I, Gerondopoulos A, Zaki MS, et al. Homozygous Mutations in TBC1D23 Lead to a Non-degenerative Form of Pontocerebellar Hypoplasia. Am J Hum Genet. 2017;101(3):441–450. doi: 10.1016/j.ajhg.2017.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Personnic N, Bärlocher K, Finsel I, Hilbi H. Subversion of Retrograde Trafficking by Translocated Pathogen Effectors. Trends in Microbiology. 24(6):450–462. doi: 10.1016/j.tim.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 106.Kingston D, Chang H, Ensser A, et al. Inhibition of retromer activity by herpesvirus saimiri tip leads to CD4 downregulation and efficient T cell transformation. J Virol. 2011;85(20):10627–10638. doi: 10.1128/JVI.00757-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Groppelli E, Len AC, Granger LA, Jolly C. Retromer regulates HIV-1 envelope glycoprotein trafficking and incorporation into virions. PLoS Pathog. 2014;10(10):e1004518. doi: 10.1371/journal.ppat.1004518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pim D, Broniarczyk J, Bergant M, Playford MP, Banks L. A Novel PDZ Domain Interaction Mediates the Binding between Human Papillomavirus 16 L2 and Sorting Nexin 27 and Modulates Virion Trafficking. J Virol. 2015;89(20):10145–10155. doi: 10.1128/JVI.01499-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Popa A, Zhang W, Harrison MS, et al. Direct binding of retromer to human papillomavirus type 16 minor capsid protein L2 mediates endosome exit during viral infection. PLoS Pathog. 2015;11(2):e1004699. doi: 10.1371/journal.ppat.1004699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mirrashidi KM, Elwell CA, Verschueren E, et al. Global Mapping of the Inc-Human Interactome Reveals that Retromer Restricts Chlamydia Infection. Cell host & microbe. 2015;18(1):109–121. doi: 10.1016/j.chom.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Huang CY, Lu TY, Bair CH, Chang YS, Jwo JK, Chang W. A novel cellular protein, VPEF, facilitates vaccinia virus penetration into HeLa cells through fluid phase endocytosis. J Virol. 2008;82(16):7988–7999. doi: 10.1128/JVI.00894-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hsiao JC, Chu LW, Lo YT, et al. Intracellular Transport of Vaccinia Virus in HeLa Cells Requires WASH-VPEF/FAM21-Retromer Complexes and Recycling Molecules Rab11 and Rab22. J Virol. 2015;89(16):8365–8382. doi: 10.1128/JVI.00209-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Qiu J, Luo ZQ. Legionella and Coxiella effectors: strength in diversity and activity. Nat Rev Microbiol. 2017 doi: 10.1038/nrmicro.2017.67. [DOI] [PubMed] [Google Scholar]
- 114.Finsel I, Ragaz C, Hoffmann C, et al. The Legionella effector RidL inhibits retrograde trafficking to promote intracellular replication. Cell host & microbe. 2013;14(1):38–50. doi: 10.1016/j.chom.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 115.Barlocher K, Hutter CAJ, Swart AL, et al. Structural insights into Legionella RidL-Vps29 retromer subunit interaction reveal displacement of the regulator TBC1D5. Nat Commun. 2017;8(1):1543. doi: 10.1038/s41467-017-01512-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yao J, Yang F, Sun X, et al. Mechanism of inhibition of retromer transport by the bacterial effector RidL. Proc Natl Acad Sci U S A. 2018;115(7):E1446–E1454. doi: 10.1073/pnas.1717383115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Barlocher K, Welin A, Hilbi H. Formation of the Legionella Replicative Compartment at the Crossroads of Retrograde Trafficking. Front Cell Infect Microbiol. 2017;7:482. doi: 10.3389/fcimb.2017.00482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sun Q, Yong X, Sun X, et al. Structural and functional insights into sorting nexin 5/6 interaction with bacterial effector IncE. Signal Transduction and Targeted Therapy. 2017;2(1) doi: 10.1038/sigtrans.2017.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Paul B, Kim HS, Kerr MC, Huston WM, Teasdale RD, Collins BM. Structural basis for the hijacking of endosomal sorting nexin proteins by Chlamydia trachomatis. Elife. 2017:6. doi: 10.7554/eLife.22311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Elwell CA, Czudnochowski N, von Dollen J, et al. Elife. 2017. Chlamydia interfere with an interaction between the mannose-6-phosphate receptor and sorting nexins to counteract host restriction; p. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Luo Z-Q. Catch and arrest: exploiting the retromer by a Chlamydial effector. Signal Transduction and Targeted Therapy. 2017;2(1) doi: 10.1038/sigtrans.2017.39. [DOI] [PMC free article] [PubMed] [Google Scholar]