Acute GVHD - clinical presentation and risk factors
Allogeneic hematopoietic cell transplantation (allo-HCT) is a well-established treatment for hematological diseases incurable by conventional treatments1. More than one million hematopoietic cell transplants have been performed, of which 40% were allogeneic2. The most common life-threatening complication is graft-versus-host disease (GVHD). GVHD occurs when immune competent T cells in the donated tissue (the graft) recognize the recipient (the host) as foreign (nonself). The resulting immune response activates donor T cells to gain cytolytic capacity then attack the recipient to eliminate foreign antigen(s)-bearing cells. The two main clinical presentations are acute GVHD and chronic GVHD. Typical acute GVHD signs include a maculopapular rash (skin), hyperbilirubinemia with jaundice due to small bile duct damage leading to cholestasis (liver), nausea, vomiting and anorexia [upper gastrointestinal tract (GI)], and watery or bloody diarrhea and crampy abdominal pain (lower GI) (Suppl. text, Suppl. Table 1 in Suppl. Appendix; Figure 1). Acute GVHD diagnosis relies on clinical, laboratory, and biopsy assessment of target organs. Acute GVHD severity is graded clinically by tabulating the extent of involvement of the three main target organs: skin (the most frequent and often the earliest clinical manifestation of acute GVHD), gastrointestinal tract (second most common), and liver3,4. Overall grades are grade I (mild), II (moderate), III (severe), and IV (very severe). Amongst all allogeneic hematopoietic cell transplant patients, 30-50% develop acute GVHD (grade I-IV) and 14% experience severe acute GVHD (grade III-IV)5. Risk factors are summarized in Table 2 (Suppl. Appendix) and include degree of HLA mismatch, unrelated donors, female donors for male recipients, peripheral blood stem cell grafts and conditioning regimen intensity6–8.
Figure 1. Clinical features of aGVHD.

Representative pictures for clinical aGVHD in early and advanced stages of the skin and intestinal tract are shown (A-D). Histologic aspects of skin (E), hepatic (F, G) and intestinal (H, I) lesions in GVHD. E. AGVHD of the skin, the black arrows indicate apoptotic cells in the basal layer of the epidermis. F. Damage to small interlobular bile ducts characterized by epithelial irregularities and rare apoptosis. Moderate inflammation of the adjacent portal area (hematoxylin & eosin, medium magnification). G. Infiltration of bile duct epithelium by CD3+ lymphocytes (anti-CD3 immunolabeling, high magnification). H. Mucosal surface denudation and partial crypt destruction in intestinal GVHD (hematoxylin & eosin, low magnification). I. Scattered apoptotic bodies in regenerating crypts in close association with exploding crypts containing karyorrhectic nuclear debris (hematoxylin & eosin, high magnification). Histological images were provided by Dr. Technau, Dept of Dermatology, Univ of Freiburg (skin), and Prof Schmitt-Gräff, Dept of Pathology, Univ. of Freiburg (liver, intestines).
Donor T-cell recognition that induces acute GVHD can be directed against host MHC and/or minor histocompatibility antigen (miH) disparities. HLA class I molecules (A, B, and C) are expressed at variable levels by all cells whereas MHC class II molecules (DR, DQ, and DP) are mainly expressed by hematopoietic cells, especially antigen-presenting cells (APCs) such as host B-cells, dendritic cells (DC), macrophages and monocytes. Recent data indicate that recognition of a mismatch in the polymorphic MHC class I chain-related gene A or MICA is connected to higher GVHD risk9. The role for miH is supported by genome-wide analysis of single nucleotide polymorphisms (SNPs) resulting in amino acid coding differences between recipients and donors10; each 1% increase in genome-wide recipient mismatching is associated with a 20% increase in the hazard of severe acute GVHD10. MiH mismatches and allo-hematopoietic cell transplant are connected to GVHD11. Although high-resolution HLA typing with next generation sequencing will likely detect more HLA gene mismatches, it is unclear whether such information will lead to improved allo- hematopoietic cell transplant outcomes, since not all mismatches may be recognized by the donor T cells. Additionally, SNPs for chemokines, cytokines, costimulatory molecules and micro-RNAs (miRs) are also associated with acute GVHD risk (Suppl text). Although acute GVHD risk can be increased by mismatches and closer matching may reduce the risk, mismatched antigens present in normal tissue may be shared with malignant cells; thus, some mismatches may be important for graft-versus-leukemia (GVL) responses and greater matching could increase relapse risk.
Tissue damage and early events of acute GVHD investigated in the mouse model
Our understanding of acute GVHD biology is mostly based on murine studies, albeit limited by the physiological and immune system differences of mice and humans, transplant procedures12, and microbiome13. Nonetheless, GVHD mouse models have been the basis for much of our understanding of GVHD biology. The earliest acute GVHD pathophysiological events are neoangiogenesis14,15 and intestinal tract infiltration by innate myeloid cells such as neutrophil granulocytes (neutrophils)16–19 and monocytes20,21, first-wave immune responders to tissue injury and foreign pathogens. Recipient neutrophils impact GVHD through their activation and reactive oxygen species (ROS) production in the GI tract17. The impact of neutrophils on acute GVHD is further supported by observations that a high density of neutrophil infiltration correlated with an unfavorable outcomes18, increased intestinal permeability after allo-hematopoietic cell transplant19 and conversely, defective neutrophil ROS production in chronic granulomatous disease patients resulted in low acute GVHD rates22. However, tissue protective effects can be conferred by neutrophils and monocytes dependent upon the specific cell subset involved, time point related to allo-hematopoietic cell transplant and the tissue and environment context (Suppl. text, Suppl. Appendix).
In the early acute GVHD phase, inflammatory triggers can drive both the innate and adaptive immune responses. These triggers can be divided into sterile damage associated molecular patterns (DAMPS) and pathogen-associated molecular patterns (PAMPS). The DAMPS comprise molecules released into the extracellular space only when tissue damage occurs that cause immune activation23. GVHD can be enhanced by extracellular adenosine triphosphate (ATP) activating the purinergic P2X7 receptor24 and P2Y2 receptor20. ATP is metabolized by the ectonucleotidase CD39, expressed by endothelial and immune cells, into adenosine monophosphate and then into anti-inflammatory adenosine (by CD73, expressed in acute GVHD organs such as colon, liver, and lung; endothelial cells; leukocytes)25. Consequently, the lack of CD73 enhances GVHD but also GVL effects26,27. ATP and uric acid can cause activation of the Nlrp3 inflammasome, a myeloid expressed multiprotein oligomer containing caspase-1 or -11, leading to pro-IL-1β cleavage into its bioactive form, enhancing GVHD28. Other DAMPs include heparan sulfate, high mobility group box 1 protein (HMGB1), sialic acid-binding immunoglobulin-type lectins (siglecs), mitochondrial components, IL-33 or the small leucine-rich repeat proteoglycan, biglycan29–36 (Figure 2). Like myeloid cells, tissue and inflammatory context are important for the net DAMP effects. This was particularly evident for IL-33 that has anti-inflammatory properties when given before tissue damage due to expansion of IL-33 receptor [suppressor of tumorigenicity (ST2)] expressing suppressor CD4+CD25+FoxP3+ regulatory T cells (Treg)34. Conversely, IL-33 administration during evolving GVHD promotes interferon (IFN)-γ producing T cell expansion and acute GVHD34, while blockade of the IL-33/ST2-axis can reduce acute GVHD34,35.
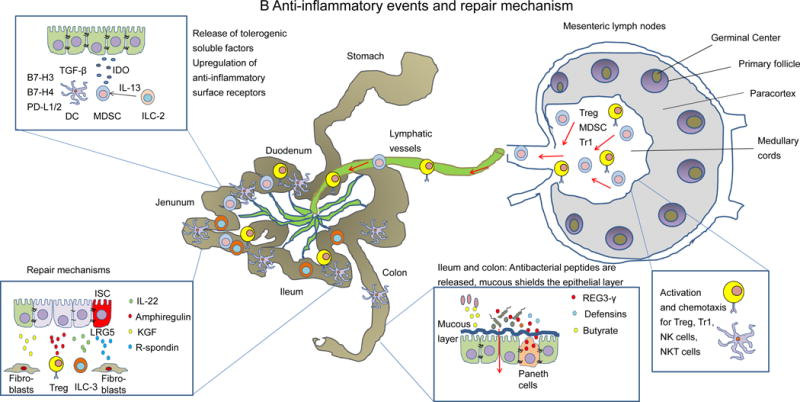
Figure 2. Schematic overview of the early events of GVHD.
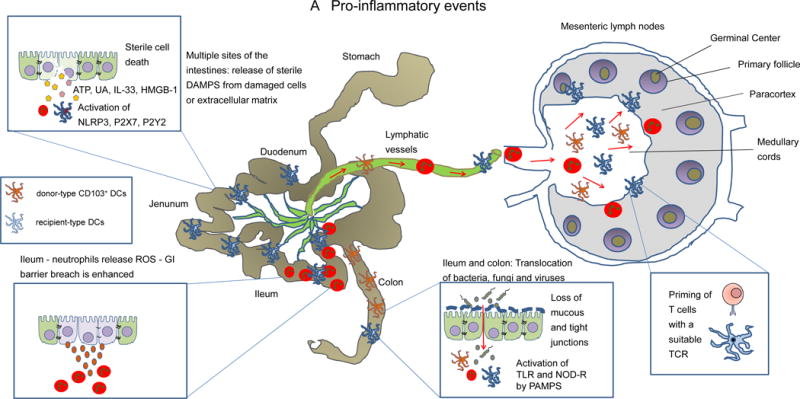
A: DAMPs (e.g. uric acid, ATP, heparan sulfate, HMGB-1 or IL-33) that are released from the dying cells or disrupted extracellular matrix and activate the respective receptors, e.g. ATP activates P2X7 and P2Y2, uric acid activates the Nlrp3 inflammasome. PAMPS derived from invading bacteria activate innate immune cells including donor derived CD103+ dendritic cells, inflammatory monocytes and neutrophils. A fraction of these cells migrates from the damaged intestinal epithelium towards the draining mesenteric lymph nodes where donor T-cells are activated. ATP: Adenosine triphosphate, HMGB-1: High mobility group box 1 protein, ROS: reactive oxygen species, DAMPs: danger associated molecular patterns, PAMPs: pathogen associated molecular patterns.
B: Anti-inflammatory events and repair mechanism. Cells in the GVHD target organs attempt to counterbalance inflammation via the release of tolerogenic soluble factors, upregulation of anti-inflammatory surface receptors and repair mechanisms. Activation signals and chemotactic signals for Treg and Tr1 cells are provided in the lymph nodes. KGF: keratinocyte growth factor.
Although the role of sterile DAMPS in GVHD was discovered in the last decade, the role of bacteria and the resulting PAMPS such as lipopolysaccharide (LPS) in GVHD has been studied since the 1970s with the first seminal studies in mice37. Direct effects of bacterial components that engage immune system pattern recognition receptors (PRRs) such as Toll-like receptors (TLR) and nucleotide-binding oligomerization domain-like receptors (NOD-like receptors; NLRs) can activate APCs, promoting acute GVHD. Novel technologies such as bacterial genome sequencing permit better understanding of which bacteria in the GI tract decline with antibiotics diminishing acute GVHD and conversely, which bacteria have a protective role for acute GVHD38,39 as well as relapse- and progression-free survival40. GVHD itself induces dysbiosis in mice41. Increased GVHD-related mortality that can occur in mice and in patients due to antibiotic treatment39 is likely due to the loss of GI homeostasis that heavily depends on microbiota-derived metabolites. Intestinal bacteria-secreted butyrate functions as a histone deacetylase (HDAC) inhibitor, and potently reduces GVHD by inhibiting indoleamine-2,3-dioxygenase (IDO)-dependent innate immune and allo-stimulating APC functions in a STAT-3-dependent manner42. IDO expession itself is upregulated on intestinal parenchymal cells and APCs in mice43 as a result of IFN-γ produced by alloreactive T- cells44. IDO causes local depletion of the essential amino acid tryptophan through a stress response mechanism, resulting in T-cell metabolic starvation and apoptosis at sites of high IDO expression (e.g. colon)43. Fungi and viruses also are connected to GVHD severity, e.g. α-Mannan derived from fungi induces Th17-mediated pulmonary GVHD in mice45. Cumulatively, DAMPS and PAMPS lead to rapid intracellular biochemical cascades that induce caspase-1 cleavage of inactive cytokines stored intracellularly (e.g. IL-1β; IL-18) and the transcription of genes that encode for cytokines, chemokines and their receptors. Overall, the early events of acute GVHD set the stage for later T-cell priming and expansion.
T-cell activation, co-stimulation, survival and metabolism in mouse models of aGVHD
A key event for the development of acute GVHD is the interaction of T cells expressing a suitable T-cell receptor (TCR) with APCs that express host MHC or miH peptides. Recent reports from mouse acute GVHD models also point to the role of non-hematopoietic cells in the antigen presentation process46,47. T cells are the main effectors causing target tissue cell death that can be mediated by the expression of the TNF family member, FAS-ligand, and release of intracellular granule contents including the serine protease, granzyme B, and pore-forming cytolytic protein, perforin48,49. Where MHC class I only is mismatched, donor CD8+ T cells alone are sufficient to induce GVHD. For miH disparities, CD8+ T cells require cognate (direct) interactions with GVHD target tissues miH50, a situation particularly relevant in human allo-hematopoietic cell transplant recipients due to frequent miH mismatches. CD4+ T cells can cause GVHD by cognate interactions with MHC class II alone or with miH peptides or can damage tissues without cognate interactions by releasing cytotoxic cytokines, such as TNF-α, that induce apoptosis in epithelial cells51. In contrast to T cells, NK cells reduce GVHD via the elimination of recipient-type APC based on killer-cell immunoglobulin-like receptor (KIR)-mismatches52 and elaboration of TGFβ that can suppress T-cell activation53.
Besides the TCR activation, T cells need co-stimulation, a second T-cell signal required to lower the TCR activation threshold, amplify and sustain cytokine production, inhibit apoptosis, and support T-effector metabolism. The role of multiple costimulatory pathways has been studied in acute GVHD including positive regulatory axes [CD2854,55, ICOS (CD278)56], TNFR-superfamily receptors [CD40L(CD154), OX40(CD134), 4-1BB(CD137)] and negative regulatory pathways [CTLA-4(CD152)55, PD-1(CD279)/PD-L1(CD274)57,58 and B7-H359(CD276)], amongst others (Suppl. text, Suppl. Appendix). The third signal for the T-cell activation and survival is cytokine-mediated. Multiple cytokines (Suppl. text, Suppl. Appendix) were found to play a role in the pathogenesis of GVHD such as IL-1β and T-helper 1 (Th1) cytokines (IFN-γ, IL-2, and TNF). The differentiation stage (naive, effector/memory) of T cells is decisive for their ability to cause acute GVHD. In acute GVHD mouse models, memory CD4+ T cells cause less or almost no acute GVHD but mediate GVL effects60,61. Naïve T cells migrate to lymph nodes, via L-selectin (CD62L) and the chemokine CCR7, where priming takes place, e.g. via interaction with donor-derived CD11b−CD103+ DCs that have migrated to mesenteric lymph nodes from the colon, imprinting donor T cells to express gut-homing integrin receptors62.
T-cell differentiation and proliferation during GVHD require multiple energy sources to keep pace with the high metabolic demands. Aerobic glycolysis is essential for optimal GVHD Teff responses63–65. In some studies, Teffs also have been shown to utilize oxidative phosphorylation and fatty acid oxidation63,65,66, supplying vital energy needs not accomplished by glycolysis alone. Targeting metabolic pathways and subverting T-cell energy utilization by inhibition of glycolysis, fatty acid oxidation and oxidative phosphorylation, and/or glutaminolysis or via essential or conditionally essential amino acid deprivation (e.g. tryptophan or L-arginine, respectively) may reduce the frequency of rapidly proliferating T cells responsible for acute GVHD. This field of investigation builds upon early studies demonstrating that blockade of the nutrient sensor, mammalian target of rapamycin (mTOR), ameliorates acute GVHD67 and CD28 costimulation regulates glycolysis68 along with more recent studies showing that the negative regulator, PD-1, suppresses glycolysis and promotes lipolysis and fatty acid oxidation69. The specific nutrients and magnitude of energy needed during GVHD likely depends upon the T-cell subset, proliferation rate, intensity of inflammatory response, and local tissue environment. As metabolism drives cell cycle and proliferation, other novel targets are cyclin-dependent kinases (CDK) that affect cell cycle regulation. For instance the CDK-inhibitor roscovitine prevents alloreactive T-cell expansion and protects against acute GVHD in mice70. The role of the three signals for T-cell activation and differentiation, key cytokines, and metabolic demands derived from murine acute GVHD studies provide pathophysiology-based targets for exploration in humans.
Biomarkers for aGVHD severity - studies in patients
To predict the risk of GVHD and the response to immunosuppressive therapy, multiple biomarkers have been investigated in allo-hematopoietic cell transplant patients (Suppl. text, Table 3, Suppl. Appendix). The serum level of the soluble form of ST2 was reported to be an important biomarker for therapy-resistance in patients developing acute GVHD71. Based on this observation an early-biomarker algorithm was studied for its predictive value for lethal acute GVHD72. A 4-biomarker panel [ST2, TNFR1, IL-2Rα chain(CD25), regenerating islet-derived protein-3 alpha(REG3α)], released from injured tissue or activated Teffs, was used to predict increased acute GVHD-related death. By modeling 6-month non-relapse mortality in an independent test set and validation set, a 2-biomarker model using ST2 and REG3α concentrations identified patients with a cumulative incidence of 6-month nonrelapse mortality of 28% in the high-risk and 7% in the low-risk group72.
Micro-RNAs (miRs) are potent regulators of multiple pro-inflammatory target genes and are readily measurable in patient serum. Multiple miRs in sera were strongly connected to acute GVHD risk73, in particular miR-155 and miR146a74. In initial studies, the presence of a miR-146a polymorphism (rs2910164) in the donor or the allo- hematopoietic cell transplant recipient was connected to higher rates of grade III and IV acute GVHD75,76, a finding requiring confirmation in larger patient cohorts. These and possibly other yet to be discovered biomarker panels hold promise to better predict the risk of acute GVHD and acute GVHD-related mortality, which could lead to a more individualized GVHD-prophylaxis approach.
Classical acute GVHD preventive and therapeutic strategies that have been tested in the clinic
Acute GVHD prophylaxis with the calcineurin inhibitor cyclosporin A and methotrexate, a folate antagonist, is used in the majority of allo-hematopoietic cell transplant recipients based on a sequential, prospective randomized trial showing cyclosporine A and methotrexate was superior to cyclosporine A alone77 (Suppl. text, Suppl. Appendix). Randomized, multicenter phase-III trials of the pan-T-cell-depleting reagent, rabbit anti-thymocyte globulin (ATG-F), showed decreased acute GVHD and chronic GVHD incidence without increased relapse or non-relapse mortality when added to standard prophylaxis78,79. The mode of action of the most frequently applied classical immunosuppressive medications is summarized in Figure 3A. The cyclosporine A and FK506, inhibit GVHD by preventing nuclear factor of activated T cells (NFAT) activation thereby reducing IL-2 transcription and Teff activation, albeit with a concurrent reduction in anti-inflammatory Treg that are IL-2-dependent. In contrast to calcineurin inhibitors, rapamycin is more potent in suppressing expansion of conventional T cells compared to Treg, most likely due to their greater dependence on the mTOR/protein kinase-B-pathway compared to Tregs80. More recently, post-transplant cyclophosphamide has been used to deplete conventional T cells while relatively preserving Tregs81, resulting in a relatively low incidence of GVHD even in haploidentical transplant recipients82.
Figure 3. Classical and novel approaches to target T-cell and dendritic cell activation.
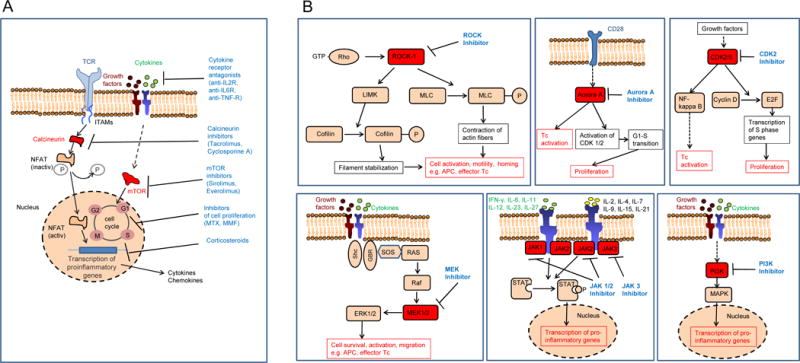
A: Sketch showing the mode of action of multiple immunosuppressive strategies that are currently applied in the clinic for prevention and therapy of aGVHD. mTOR: mammalian target of rapamycin, MTX: methotrexate, MMF: mycophenolate mofetil
B: Kinases that have been subject to targeted therapy approaches in aGVHD are shown. Blockade of the kinases ROCK-1, Aurora A, CDK2, MEK-1/2, JAK1-3 and PI3K was shown to reduce aGVHD in mouse models. The different signaling pathways in which these kinases have a non-redundant function are displayed. Tc: T cell, TCR: T-cell receptor
Cell-based approaches to prevent acute GVHD include the manipulation of the donor graft. Ex vivo T-cell depletion accomplished by positive selection of CD34+ cells or negative selection against T cells and B cells was reported83. A promising strategy is the enrichment of T-cell receptor gamma/delta T cells to prevent relapse84. T-cell-depletion specifically for naive T cells alone showed a reduced chronic GVHD incidence85. Adoptive transfer of Treg or donor lymphocytes cultured with IL-10-treated host APCs that enriches for IL-10 and TGFβ producing Tregulatory type 1 (Tr1) has been investigated. Clinically, prophylactic Treg transfer was associated with low acute GVHD rates and adequate immune reconstitution86,87. Tr1 enhanced immune reconstitution in 5/12 patients when given at 1-2 months after allo-hematopoietic cell transplant88. Besides Treg and Tr1, mesenchymal stroma cells were tested in the clinic for their ability to reduce GVHD severity. Different clinical studies showed either impressive responses or, in a randomized clinical trial, failed to show improvement of GVHD-related mortality89,90 which may be due to mesenchymal stem cell preparation, transfer time point, GVHD severity or organ involvement.
Despite prophylaxis acute GVHD still evolves and is treated first with glucocorticoids based on randomized controlled trials91. Acute GVHD patients that are glucocorticoid refractory have a dismal long-term prognosis with only 5-30% overall survival. A summary of acute GVHD drug therapies is listed in the supplementary appendix. Currently only two randomized phase-III trials in glucocorticoid-refractory acute GVHD have been reported using gavilimomab (murine anti-CD147; ABX-CBL)92 or inolimomab (murine anti-CD25)93. The first trial reported an 18-month overall survival after treatment initiation that was less favorable in the gavilimomab arm compared to the ATG arm (35% versus 45%)92. In the second trial, inolimomab was compared to ATG with the primary objective to evaluate 1-year overall survival93. The primary end point was not reached and there was no significant difference in overall survival93. As both antibodies had been promising in early phase clinical studies but failed to show improvement in phase-III trials, it is important that promising agents used to treat glucocorticoid-resistant acute GVHD identified in phase I/II or phase II trials are tested in randomized phase-III trials in the future. As such, no proven second-line therapy has been uniformly adopted or approved for glucocorticoid-resistant acute GVHD.
Novel acute GVHD preventive and therapeutic strategies being tested
Since T-cell migration to GVHD organs is needed to cause acute GVHD and is initiated by chemokine gradients produced within these target organs as a result of tissue injury or innate cell infiltration, chemotaxis should be considered a central event in acute GVHD pathogenesis. In support of this contention, a clinical phase-II trial reported that CCR5 inhibition prevents GVHD of liver and gut before day 10094. The phase-II trial used reduced-intensity conditioning which may be relevant to the successful outcome because the CCR5 migratory signals appear less important in the context of myeloablative radiation in mouse acute GVHD models. For example, CCR5 inhibition was protective against GVHD in a non-irradiated GVHD mouse model but GVHD onset was earlier and severity worsened when CCR5-deficient T cells were transferred into heavily irradiated GVHD model95. In a different approach to inhibit migration, the sphingosphine-1-phosphate receptor antagonist, FTY720, has been shown to reduce murine acute GVHD by trapping T cells in lymphoid organs or reducing DC migration96 and is currently in clinical trials to prevent acute GVHD.
The fundamental role of IL-22 has been explored. IL-22 is produced by innate lymphoid cells type-3 (ILC3) that are depleted by GVHD, resulting in cypt apoptosis, ILC3 depletion and epithelial integrity loss97–99. Exogenous IL-22 can enhance the regeneration of intestinal stem cells (ISC) that express IL-22 receptors97–99. In a clinical trial (NCT02406651,ClinicalTrials.gov), IL-22 IgG2-Fc(F-652) is being given to patients with grade II-IV acute GVHD of the lower intestinal tract. Another recent approach to protect the intestinal tract involves the direct transfer of microbial species as acute GVHD therapy. In a small pioneer study, the first successful and safe application of related fecal microbiota transplants via nasoduodenal tubes in patients suffering from glucocorticoid-resistant acute GVHD was reported100. Three of four patients responded by 28 days after the first fecal transplant, allowing reduction of the glucocorticoid dose by 69%100. While encouraging, fecal transplantation is still to be considered a highly experimental treatment approach and needs validation in carefully designed prospective clinical trials. Other approaches currently being explored to prevent or treat GVHD in patients are blockade of T-cell co-stimulation101, α-GalCer, a glycolipid that expands and activates natural killer T cells and subsequently expands Treg in patients102, anti-inflammatory antibodies, proteins or drugs targeting signaling by IL-6103, IL-23104, or multiple cytokine signaling pathways using HDAC inhibitors105, proteosomal inhibition106, or the anti-inflammatory protease inhibitor, alpha-1-antitrypsin107. Additionally, Janus-activated kinase (JAK)-1/2 inhibitors have shown promising results in preclinical studies108,109 as well as in retrospective clinical analyses110 and are currently being tested in prospective randomized studies.
Promising novel strategies against acute GVHD tested in preclinical models but not yet in GVHD trials
In contrast to the approaches that have already reached clinical application, preclinical strategies being tested have continued to focus on targeting the signalling of multiple cytokine receptors, pro-inflammatory pathways and intestinal stem cells. Novel targets include kinase inhibitors that block the protein serine/threonine kinase ROCK1111, Aurora kinase A112, MEK113, and others (Supplemental appendix and Figure 3B). Strategies being investigated to improve Treg efficacy by in vivo Treg expansion in mouse models include TNFRSF25 (DR3) stimulation using a fusion protein to ligate the receptor114, agonistic DR3 antibody115 and agonistic TNFR2 antibody116.
Administration of R-spondin-1(R-Spo1), a WNT agonist, to mice reduced GVHD by protecting intestinal stem cells from conditioning injury117 and stimulating them to differentiate into secretory cells thereby inhibiting GVHD-associated microbiome changes. The protective effect of R-Spo1 was connected to the microbial microflora in the intestinal tract and partly abrogated when mice received broad-spectrum antibiotics. While bacterial components can activate innate immune cells like neutrophils and monocytes, bacteria are also critical for intestinal tissue homeostasis. Indeed, transfer of selected strains of Clostridia known to produce the short chain fatty acid butyrate results in increased Treg frequencies in the GI tract118. Butyrate given via the GI tract may be proven to be effective in the absence of Clostridia transfer, albeit repetitive administration would likely be needed during the period of acute GVHD risk. Since Treg can potently suppress acute GVHD,119,120 these data link intestinal metabolism to GVHD. Transfer of innate lymphoid cells type 2 (ILC-2) into mice both prevented and treated acute GVHD by stimulating the expansion of anti-inflammatory regulatory cell populations121. While still in pre-clinical testing, these approaches may be more effective than classical broadly immunosuppressive strategies compared to targeting of individual cytokine or chemokine signals or preferentially targeting the GI tract.
Although pharmacological strategies to overcome acute GVHD inflammation are typically short-lived unless a state of deep tolerance is acquired during drug therapy, the transfer of a tolerogenic cell population that persists in the body, could ideally lead to the achievement of long-term tolerance. First steps towards this strategy were made when Treg adoptively transferred into mice reduced acute GVHD119,120.
Summary and outlook
Acute GVHD remains a major life-threatening allo-hematopoietic cell transplant complication leading to high mortality and rendering patients that survive often profoundly immune deficient for several years. Acute GVHD clinical diagnosis, pathophysiology, standard as well as experimental prevention and treatment procedures and novel biomarkers to tailor GVHD-treatment are important developments that hold promise to lead to reduced acute GVHD rates. Major pathophysiologic pathways that drive acute GVHD include tissue damage due to the conditioning regimen or infection, recognition of non-self-MHC/miH and altered repair/tissue protective mechanisms including microbiome changes that cause a decline in protective microbial-derived metabolites.
Therapeutic directions that are particularly promising to pursue based on early clinical trial data include costimulatory pathway blockade, anti-IL-6R mAb103, HDAC-inhibitors105, kinase108,109- and proteasome-inhibitors106, the anti-inflammatory protease-inhibitor alpha-1-antitrypsin107, CTLA-4 antagonism101, CCR5 blockade94 and adoptive Treg transfer86,87. These and other novel strategies being developed have to be tested in prospective phase-III trials to become standard therapy for acute GVHD.
Supplementary Material
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Forman SJ, Negrin RS, Antin JH, Appelbaum FR. Thomas’ Hematopoietic Cell Transplantation. 5 Wiley-Blackwell; 2016. [Google Scholar]
- 2.Gratwohl A, et al. One million haemopoietic stem-cell transplants: a retrospective observational study. Lancet Haematol. 2015;3:e91–e100. doi: 10.1016/S2352-3026(15)00028-9. [DOI] [PubMed] [Google Scholar]
- 3.Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, Lerner KG, Thomas ED. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Harris AC, Young R, Devine S, Hogan WJ, Ayuk F, Bunworasate U, Chanswangphuwana C, Efebera YA, Holler E, Litzow M, Ordemann R, Qayed M, Renteria AS, Reshef R, Wölfl M, Chen YB, Goldstein S, Jagasia M, Locatelli F, Mielke S, Porter D, Schechter T, Shekhovtsova Z, Ferrara JL, Levine JE. International, Multicenter Standardization of Acute Graft-versus-Host Disease Clinical Data Collection: A Report from the Mount Sinai Acute GVHD International Consortium. Biol Blood Marrow Transplant. 2016;22:4–10. doi: 10.1016/j.bbmt.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pasquini MC, Zhu X. Current use and outcome of hematopoietic stem cell transplantation. 2015 CIBMTR Summary Slides 2014. http://www.cibmtr.org.
- 6.Loiseau P, Busson M, Balere ML, Dormoy A, Bignon JD, Gagne K, Gebuhrer L, Dubois V, Jollet I, Bois M, Perrier P, Masson D, Moine A, Absi L, Reviron D, Lepage V, Tamouza R, Toubert A, Marry E, Chir Z, Jouet JP, Blaise D, Charron D, Raffoux C. HLA Association with hematopoietic stem cell transplantation outcome: the number of mismatches at HLA-A, -B, -C, -DRB1, or -DQB1 is strongly associated with overall survival. Biol Blood Marrow Transplant. 2007;13:965–74. doi: 10.1016/j.bbmt.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Flowers ME, Inamoto Y, Carpenter PA, Lee SJ, Kiem HP, Petersdorf EW, Pereira SE, Nash RA, Mielcarek M, Fero ML, Warren EH, Sanders JE, Storb RF, Appelbaum FR, Storer BE, Martin PJ. Comparative analysis of risk factors for acute graft-versus-host disease and for chronic graft-versus-host disease according to National Institutes of Health consensus criteria. Blood. 2011;117:3214–9. doi: 10.1182/blood-2010-08-302109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jagasia M, Arora M, Flowers ME, Chao NJ, McCarthy PL, Cutler CS, Urbano-Ispizua A, Pavletic SZ, Haagenson MD, Zhang MJ, Antin JH, Bolwell BJ, Bredeson C, Cahn JY, Cairo M, Gale RP, Gupta V, Lee SJ, Litzow M, Weisdorf DJ, Horowitz MM, Hahn T. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood. 2012;119:296–307. doi: 10.1182/blood-2011-06-364265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carapito R, Jung N, Kwemou M, Untrau M, Michel S, Pichot A, Giacometti G, Macquin C, Ilias W, Morlon A, Kotova I, Apostolova P, Schmitt-Graeff A, Cesbron A, Gagne K, Oudshoorn M, van der Holt B, Labalette M, Spierings E, Picard C, Loiseau P, Tamouza R, Toubert A, Parissiadis A, Dubois V, Lafarge X, Maumy-Bertrand M, Bertrand F, Vago L, Ciceri F, Paillard C, Querol S, Sierra J, Fleischhauer K, Nagler A, Labopin M, Inoko H, von dem Borne PA, Kuball J, Ota M, Katsuyama Y, Michallet M, Lioure B, Peffault de Latour R, Blaise D, Cornelissen JJ, Yakoub-Agha I, Claas F, Moreau P, Milpied N, Charron D, Mohty M, Zeiser R, Socié G, Bahram S. Matching for the nonconventional MHC-I MICA gene significantly reduces the incidence of acute and chronic GVHD. Blood. 2016;128:1979–86. doi: 10.1182/blood-2016-05-719070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin PJ, Levine DM, Storer BE, Warren EH, Zheng X, Nelson SC, Smith AG, Mortensen BK, Hansen JA. Genome-wide minor histocompatibility matching as related to the risk of graft-versus-host disease. Blood. 2017;129:791–98. doi: 10.1182/blood-2016-09-737700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santos N, Rodríguez-Romanos R, Nieto JB, Buño I, Vallejo C, Jiménez-Velasco A, Brunet S, Buces E, López-Jiménez J, González M, Ferrá C, Sampol A, de la Cámara R, Martínez C, Gallardo D, GvHD/Immunotherapy Working Party of the Spanish Group of Hematopoietic Transplant (GETH) UGT2B17 minor histocompatibility mismatch and clinical outcome after HLA-identical sibling donor stem cell transplantation. Bone Marrow Transplant. 2016;51:79–82. doi: 10.1038/bmt.2015.207. [DOI] [PubMed] [Google Scholar]
- 12.Zeiser R, Blazar BR. Preclinical models of acute and chronic graft-versus-host disease: how predictive are they for a successful clinical translation? Blood. 2016;127:3117–26. doi: 10.1182/blood-2016-02-699082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beura LK, Hamilton SE, Bi K, Schenkel JM, Odumade OA, Casey KA, Thompson EA, Fraser KA, Rosato PC, Filali-Mouhim A, Sekaly RP, Jenkins MK, Vezys V, Haining WN, Jameson SC, Masopust D. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature. 2016;532:512–6. doi: 10.1038/nature17655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Penack O, Henke E, Suh D, et al. Inhibition of neovascularization to simultaneously ameliorate graft-vs-host disease and decrease tumor growth. J Natl Cancer Inst. 2010;102:894–908. doi: 10.1093/jnci/djq172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riesner K, Shi Y, Jacobi A, Kräter M, Kalupa M, McGearey A, Mertlitz S, Cordes S, Schrezenmeier JF, Mengwasser J, Westphal S, Perez-Hernandez D, Schmitt C, Dittmar G, Guck J, Penack O. Initiation of acute graft-versus-host disease by angiogenesis. Blood. 2017;129:2021–32. doi: 10.1182/blood-2016-08-736314. [DOI] [PubMed] [Google Scholar]
- 16.Giroux M, Delisle JS, Gauthier SD, Heinonen KM, Hinsinger J, Houde B, et al. SMAD3 prevents graft-versus-host disease by restraining Th1 differentiation and granulocyte-mediated tissue damage. Blood. 2011;117:1734–44. doi: 10.1182/blood-2010-05-287649. [DOI] [PubMed] [Google Scholar]
- 17.Schwab L, Goroncy L, Palaniyandi S, Gautam S, Triantafyllopoulou A, Mocsai A, Reichardt W, Karlsson FJ, Radhakrishnan SV, Hanke K, Schmitt-Graeff A, Freudenberg M, von Loewenich FD, Wolf P, Leonhardt F, Baxan N, Pfeifer D, Schmah O, Schönle A, Martin SF, Mertelsmann R, Duyster J, Finke J, Prinz M, Henneke P, Häcker H, Hildebrandt GC, Häcker G, Zeiser R. Neutrophil granulocytes recruited upon translocation of intestinal bacteria enhance GvHD via tissue damage. Nat Med. 2014;20:648–54. doi: 10.1038/nm.3517. [DOI] [PubMed] [Google Scholar]
- 18.Socié G, Mary JY, Lemann M, Daneshpouy M, Guardiola P, Meignin V. Prognostic value of apoptotic cells and infiltrating neutrophils in graft-versus-host disease of the gastrointestinal tract in humans: TNF and Fas expression. Blood. 2004;103:50–7. doi: 10.1182/blood-2003-03-0909. [DOI] [PubMed] [Google Scholar]
- 19.Fischer JC, Wintges A, Haas T, Poeck H. Assessment of mucosal integrity by quantifying neutrophil granulocyte influx in murine models of acute intestinal injury. Cellular immunology. 2017;316:70–6. doi: 10.1016/j.cellimm.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Klämbt V, Wohlfeil SA, Schwab L, Hülsdünker J, Ayata K, Apostolova P, Schmitt-Graeff A, Dierbach H, Prinz G, Follo K, Prinz M, Idzko M, Zeiser R. A novel function for P2Y2 in myeloid recipient-derived cells during GvHD. J Immunol. 2015;195:5795–804. doi: 10.4049/jimmunol.1501357. [DOI] [PubMed] [Google Scholar]
- 21.Reinhardt K, Foell D, Vogl T, Mezger M, Wittkowski H, Fend F, et al. Monocyte-induced development of Th17 cells and the release of S100 proteins are involved in the pathogenesis of graft-versus-host disease. J Immunol. 2014;193:3355–65. doi: 10.4049/jimmunol.1400983. [DOI] [PubMed] [Google Scholar]
- 22.Güngör T, Teira P, Slatter M, Stussi G, Stepensky P, Moshous D, et al. Reduced-intensity conditioning and HLA-matched haemopoietic stem-cell transplantation in patients with chronic granulomatous disease: a prospective multicentre study. Lancet. 2014;383:436–48. doi: 10.1016/S0140-6736(13)62069-3. [DOI] [PubMed] [Google Scholar]
- 23.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–5. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 24.Wilhelm K, Ganesan J, Müller T, Dürr C, Grimm M, Beilhack A, Krempl CD, Sorichter S, Gerlach UV, Jüttner E, Zerweck A, Gärtner F, Pellegatti P, Di Virgilio F, Ferrari D, Kambham N, Fisch P, Finke J, Idzko M, Zeiser R. Graft-versus-host disease enhanced by extracellular adenosine triphosphate activating P2X7R. Nature medicine. 2010;12:1434–8. doi: 10.1038/nm.2242. [DOI] [PubMed] [Google Scholar]
- 25.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–65. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsukamoto H, Chernogorova P, Ayata K, Gerlach UV, Rughani A, Ritchey JW, Ganesan J, Follo M, Zeiser R, Thompson LF, Idzko M. Deficiency of CD73/ecto-5′-nucleotidase in mice enhances acute graft-versus-host disease. Blood. 2012;119:4554–64. doi: 10.1182/blood-2011-09-375899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L, Fan J, Chen S, Zhang Y, Curiel TJ, Zhang B. Graft-versus-host disease is enhanced by selective CD73 blockade in mice. PLoS One. 2013;8:e58397. doi: 10.1371/journal.pone.0058397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jankovic D, Ganesan J, Bscheider M, Stickel N, Weber F, Guarda G, Follo M, Pfeifer D, Tardivel A, Ludigs K, Bouazzaoui A, Kerl K, Fischer J, Haas T, Schmitt-Gräff A, Manoharan A, Müller L, Finke J, Martin S, Gorka O, Peschel C, Ruland J, Idzko M, Duyster J, Holler E, French LE, Poeck H, Contassot E, Zeiser R. The Nlrp3-inflammasome regulates acute graft-versus-host disease. J Exp Med. 2013;210:1899–910. doi: 10.1084/jem.20130084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toubai T, Hou G, Mathewson N, Liu C, Wang Y, Oravecz-Wilson K, Cummings E, Rossi C, Evers R, Sun Y, Wu J, Choi SW, Fang D, Zheng P, Liu Y, Reddy P. Siglec-G-CD24 axis controls the severity of graft-versus-host disease in mice. Blood. 2014 doi: 10.1182/blood-2013-12-545335. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matta BM, Reichenbach DK, Zhang X, Mathews L, Koehn BH, Dwyer GK, Lott JM, Uhl FM, Pfeifer D, Feser CJ, Smith MJ, Liu Q, Zeiser R, Blazar BR, Turnquist HR. Peri-alloHCT IL-33 administration expands recipient T regulatory cells that protect mice against acute GVHD. Blood. 2016 doi: 10.1182/blood-2015-12-684142. blood-201512-684142. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Babelova A, Moreth K, Tsalastra-Greul W, et al. Biglycan, a danger signal that activates the NLRP3 inflammasome via toll-like and P2X receptors. J Biol Chem. 2009;284:24035–48. doi: 10.1074/jbc.M109.014266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brennan TV, Lin L, Huang X, Cardona DM, Li Z, Dredge K, et al. Heparan sulfate, an endogenous TLR4 agonist, promotes acute GVHD after allogeneic stem cell transplantation. Blood. 2012;120:2899–908. doi: 10.1182/blood-2011-07-368720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, Parroche P, Drabic S, Golenbock D, Sirois C, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8:487–96. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 34.Reichenbach DK, Schwarze V, Matta BM, Tkachev V, Lieberknecht E, Liu Q, Koehn BH, Pfeifer D, Taylor PA, Prinz G, Dierbach H, Stickel N, Beck Y, Warncke M, Junt T, Schmitt-Graeff A, Nakae S, Follo M, Wertheimer T, Schwab L, Devlin J, Watkins SC, Duyster J, Ferrara JL, Turnquist HR, Zeiser R, Blazar BR. The IL-33/ST2 axis augments effector T cell responses during acute GVHD. Blood. 2015;125:3183–92. doi: 10.1182/blood-2014-10-606830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang J, Ramadan AM, Griesenauer B, Li W, Turner MJ, Liu C, Kapur R, Hanenberg H, Blazar BR, Tawara I, Paczesny S. ST2 blockade reduces sST2-producing T cells while maintaining protective mST2-expressing T cells during graft-versus-host disease. Sci Transl Med. 2015;308:308ra160. doi: 10.1126/scitranslmed.aab0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–7. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Bekkum DW, Roodenburg J, Heidt PJ, van der Waaij D. Mitigation of secondary disease of allogeneic mouse radiation chimeras by modification of the intestinal microflora. J Nat Cancer Inst. 1974;52:401–4. doi: 10.1093/jnci/52.2.401. [DOI] [PubMed] [Google Scholar]
- 38.Jenq RR, Ubeda C, Taur Y, Menezes CC, Khanin R, Dudakov JA, et al. Regulation of intestinal inflammation by microbiota following allogeneic bone marrow transplantation. J Exp Med. 2012;209:903–11. doi: 10.1084/jem.20112408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shono Y, Docampo MD, Peled JU, Perobelli SM, Velardi E, Tsai JJ, Slingerland AE, Smith OM, Young LF, Gupta J, Lieberman SR, Jay HV, Ahr KF, Porosnicu Rodriguez KA, Xu K, Calarfiore M, Poeck H, Caballero S, Devlin SM, Rapaport F, Dudakov JA, Hanash AM, Gyurkocza B, Murphy GF, Gomes C, Liu C, Moss EL, Falconer SB, Bhatt AS, Taur Y, Pamer EG, van den Brink MR, Jenq RR. Increased GVHD-related mortality with broad-spectrum antibiotic use after allogeneic hematopoietic stem cell transplantation in human patients and mice. Sci Transl Med. 2016;339:339ra71. doi: 10.1126/scitranslmed.aaf2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peled JU, Devlin SM, Staffas A, et al. Intestinal Microbiota and Relapse After Hematopoietic-Cell Transplantation. J Clin Oncol. 2017 Mar 15;:JCO2016703348. doi: 10.1200/JCO.2016.70.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eriguchi Y, Takashima S, Oka H, Shimoji S, Nakamura K, Uryu H, Shimoda S, Iwasaki H, Shimono N, Ayabe T, Akashi K, Teshima T. Graft-versus-host disease disrupts intestinal microbial ecology by inhibiting Paneth cell production of alpha-defensins. Blood. 2012;120:223–31. doi: 10.1182/blood-2011-12-401166. [DOI] [PubMed] [Google Scholar]
- 42.Mathewson ND, Jenq R, Mathew AV, Koenigsknecht M, Hanash A, Toubai T, Oravecz-Wilson K, Wu SR, Sun Y, Rossi C, Fujiwara H, Byun J, Shono Y, Lindemans C, Calafiore M, Schmidt TC, Honda K, Young VB, Pennathur S, van den Brink M, Reddy P. Gut microbiome-derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease. Nat Immunol. 2016;17:505–13. doi: 10.1038/ni.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jasperson LK, Bucher C, Panoskaltsis-Mortari A, Taylor PA, Mellor AL, Munn DH, Blazar BR. Indoleamine 2,3-dioxygenase is a critical regulator of acute graft-versus-host disease lethality. Blood. 2008;111:3257–65. doi: 10.1182/blood-2007-06-096081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jasperson LK, Bucher C, Panoskaltsis-Mortari A, Mellor AL, Munn DH, Blazar BR. Inducing the tryptophan catabolic pathway, indoleamine 2,3-dioxygenase (IDO), for suppression of graft-versus-host disease (GVHD) lethality. Blood. 2009;114:5062–70. doi: 10.1182/blood-2009-06-227587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uryu H, Hashimoto D, Kato K, Hayase E, Matsuoka S, Ogasawara R, Takahashi S, Maeda Y, Iwasaki H, Miyamoto T, Saijo S, Iwakura Y, Hill GR, Akashi K, Teshima T. α-Mannan induces Th17-mediated pulmonary graft-versus-host disease in mice. Blood. 2015;125:3014–23. doi: 10.1182/blood-2014-12-615781. [DOI] [PubMed] [Google Scholar]
- 46.Koyama M, Kuns RD, Olver SD, Raffelt NC, Wilson YA, Don AL, et al. Recipient nonhematopoietic antigen-presenting cells are sufficient to induce lethal acute graft-versus-host disease. Nature medicine. 2012;18:135–42. doi: 10.1038/nm.2597. [DOI] [PubMed] [Google Scholar]
- 47.Li H, Demetris AJ, McNiff J, Matte-Martone C, Tan HS, Rothstein DM, Lakkis FG, Shlomchik WD. Profound depletion of host conventional dendritic cells, plasmacytoid dendritic cells, and B cells does not prevent graft-versus-host disease induction. J Immunol. 2012;188:3804–11. doi: 10.4049/jimmunol.1102795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baker MB, Altman NH, Podack ER, Levy RB. The role of cell-mediated cytotoxicity in acute GVHD after MHC-matched allogeneic bone marrow transplantation in mice. J Exp Med. 1996;183:2645–56. doi: 10.1084/jem.183.6.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Graubert TA, DiPersio JF, Russell JH, Ley TJ. Perforin/granyme-dependent and independent mechanisms are both important for the development of graft-versus-host disease after murine bone marrow transplantation. J Clin Invest. 1997;100:9-4-11. doi: 10.1172/JCI119606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matte-Martone C, Liu J, Jain D, McNiff J, Shlomchik WD. CD8+ but not CD4+ T cells require cognate interactions with target tissues to mediate GVHD across only minor H antigens, whereas both CD4+ and CD8+ T cells require direct leukemic contact to mediate GVL. Blood. 2008;111:3884–92. doi: 10.1182/blood-2007-11-125294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Borsotti C, Franklin AR, Lu SX, Kim TD, Smith OM, Suh D, et al. Absence of donor T-cell-derived soluble TNF decreases graft-versus-host disease without impairing graft-versus-tumor activity. Blood. 2007;110:783–6. doi: 10.1182/blood-2006-10-054510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, Posati S, Rogaia D, Frassoni F, Aversa F, Martelli MF, Velardi A. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 53.Asai O, Longo DL, Tian ZG, Hornung RL, Taub DD, Ruscetti FW, Murphy WJ. Suppression of graft-versus-host disease and amplification of graft-versus-tumor effects by activated natural killer cells after allogeneic bone marrow transplantation. J Clin Invest. 1998:1835–42. doi: 10.1172/JCI1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blazar B, Taylor PA, Boyer MW, Panoskaltsis-Mortari A, Allison JP, Vallera DA. CD28/B7 interactions are required for sustaining the graft-versus-leukemia effect of delayed post-bone marrow transplantation splenocyte infusion in murine recipients of myeloid or lymphoid leukemia cells. J Immunol. 1997;159:3460–73. [PubMed] [Google Scholar]
- 55.Blazar BR, Taylor PA, Panoskaltsis-Mortari A, Sharpe AH, Vallera DA. Opposing roles of CD28:B7 and CTLA-4:B7 pathways in regulating in vivo alloresponses in murine recipients of MHC disparate T cells. J Immunol. 1999;162:6368–77. [PubMed] [Google Scholar]
- 56.Taylor PA, Panoskaltsis-Mortari A, Freeman GJ, Sharpe AH, Noelle RJ, Rudensky AY, Mak TW, Serody JS, Blazar BR. Targeting of inducible costimulator (ICOS) expressed on alloreactive T cells down-regulates graft-versus-host disease (GVHD) and facilitates engraftment of allogeneic bone marrow (BM) Blood. 2005;105:3372–80. doi: 10.1182/blood-2004-10-3869. [DOI] [PubMed] [Google Scholar]
- 57.Li X, Deng R, He W, Liu C, Wang M, Young J, Meng Z, Du C, Huang W, Chen L, Chen Y, Martin P, Forman S, Zeng D. Loss of B7-H1 expression by recipient parenchymal cells leads to expansion of infiltrating donor CD8+ T cells and persistence of graft-versus-host disease. J Immunol. 2012;188:724–34. doi: 10.4049/jimmunol.1102630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saha A, O’Connor RS, Thangavelu G, Lovitch SB, Dandamudi DB, Wilson CB, Vincent BG, Tkachev V, Pawlicki JM, Furlan SN, Kean LS, Aoyama K, Taylor PA, Panoskaltsis-Mortari A, Foncea R, Ranganathan P, Devine SM, Burrill JS, Guo L, Sacristan C, Snyder NW, Blair IA, Milone MC, Dustin ML, Riley JL, Bernlohr DA, Murphy WJ, Fife BT, Munn DH, Miller JS, Serody JS, Freeman GJ, Sharpe AH, Turka LA, Blazar BR. Programmed death ligand-1 expression on donor T cells drives graft-versus-host disease lethality. The Journal of clinical investigation. 2016;126:2642–60. doi: 10.1172/JCI85796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Veenstra RG, Flynn R, Kreymborg K, McDonald-Hyman C, Saha A, Taylor PA, Osborn MJ, Panoskaltsis-Mortari A, Schmitt-Graeff A, Lieberknect E, Murphy WJ, Serody JS, Munn DH, Freeman GJ, Allison JP, Mak TW, van den Brink M, Zeiser R, Blazar BR. B7-H3 expression in donor T cells and host cells negatively regulates acute graft-versus-host disease lethality. Blood. 2015;125:3335–46. doi: 10.1182/blood-2014-09-603357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen BJ, Deoliveira D, Cui X, Le NT, Son J, Whitesides JF, Chao NJ. Inability of memory T cells to induce graft-versus-host disease is a result of an abortive alloresponse. Blood. 2007;109:3115–23. doi: 10.1182/blood-2006-04-016410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Anderson BE, McNiff J, Matte C, Ananasiadis I, Shlomchik WD, Shlomchik MJ. Recipient CD4+ T Cells That Survive Irradiation Regulate Chronic Graft-vs-Host Disease. Blood. 2004;104:1565–73. doi: 10.1182/blood-2004-01-0328. [DOI] [PubMed] [Google Scholar]
- 62.Koyama M, Cheong M, Markey KA, Gartlan KH, Kuns RD, Locke KR, et al. Donor colonic CD103+ dendritic cells determine the severity of acute graft-versus-host disease. J Exp Med. 2015;212:1303–21. doi: 10.1084/jem.20150329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gatza E, Wahl DR, Opipari AW, Sundberg TB, Reddy P, Liu C, Glick GD, Ferrara JL. Manipulating the bioenergetics of alloreactive T cells causes their selective apoptosis and arrests graft-versus-host disease. Sci Transl Med. 2011;67:67ra8. doi: 10.1126/scitranslmed.3001975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nguyen HD, Chatterjee S, Haarberg KM, Wu Y, Bastian D, Heinrichs J, Fu J, Daenthanasanmak A, Schutt S, Shrestha S, Liu C, Wang H, Chi H, Mehrotra S, Yu XZ. Metabolic reprogramming of alloantigen-activated T cells after hematopoietic cell transplantation. J Clin Invest. 2016;126:1337–52. doi: 10.1172/JCI82587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saha A, Aoyama K, Taylor PA, et al. Host programmed death ligand 1 is dominant over programmed death ligand 2 expression in regulating graft-versus-host disease lethality. Blood. 2013;122:3062–73. doi: 10.1182/blood-2013-05-500801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Byersdorfer CA, Tkachev V, Opipari AW, Goodell S, Swanson J, Sandquist S, Glick GD, Ferrara JL. Effector T cells require fatty acid metabolism during murine graft-versus-host disease. Blood. 2013;122:3230–7. doi: 10.1182/blood-2013-04-495515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blazar B, Taylor PA, Snover DC, Sehgal SN, Vallera DA. Murine recipients of fully mismatched donor marrow are protected from lethal graft-versus-host disease by the in vivo administration of rapamycin but develop an autoimmune-like syndrome. J Immunol. 1993;151:5726–41. [PubMed] [Google Scholar]
- 68.Frauwirth KA, et al. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16:769–77. doi: 10.1016/s1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- 69.Patsoukis N, Bardhan K, Chatterjee P, Sari D, Liu B, Bell LN, Karoly ED, Freeman GJ, Petkova V, Seth P, Li L, Boussiotis VA. PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat Commun. 2015;6:6692–7. doi: 10.1038/ncomms7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li L, Wang H, Kim J, Pihan G, Boussiotis V. The cyclin dependent kinase inhibitor (R)-roscovitine prevents alloreactive T cell clonal expansion and protects against acute GvHD. Cell Cycle. 2009;8:1794–802. doi: 10.4161/cc.8.11.8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vander Lugt MTB, B TM, Hanash S, Ritz J, Ho VT, Antin JH, Zhang Q, Wong CH, Wang H, Chin A, Gomez A, Harris AC, Levine JE, Choi SW, Couriel D, Reddy P, Ferrara JL, Paczesny S. ST2 as a marker for risk of therapy-resistant graft-versus-host disease and death. N Engl J Med. 2013;369:529–39. doi: 10.1056/NEJMoa1213299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hartwell MJ, Özbek U, Holler E, Renteria AS, Major-Monfried H, Reddy P, Aziz M, Hogan WJ, Ayuk F, Efebera YA, Hexner EO, Bunworasate U, Qayed M, Ordemann R, Wölfl M, Mielke S, Pawarode A, Chen YB, Devine S, Harris AC, Jagasia M, Kitko CL, Litzow MR, Kröger N, Locatelli F, Morales G, Nakamura R, Reshef R, Rösler W, Weber D, Wudhikarn K, Yanik GA, Levine JE, Ferrara JL. An early-biomarker algorithm predicts lethal graft-versus-host disease and survival. JCI Insight. 2017;2:e89798. doi: 10.1172/jci.insight.89798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xiao B, Wang Y, Li W, et al. Plasma microRNA signature as a noninvasive biomarker for acute graft-versus-host disease. Blood. 2013;122:3365–75. doi: 10.1182/blood-2013-06-510586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Atarod S, Ahmed MM, Lendrem C, Pearce KF, Cope W, Norden J, Wang XN, Collin M, Dickinson AM. miR-146a and miR-155 Expression Levels in Acute Graft-Versus-Host Disease Incidence. Front Immunol. 2016;7:56–61. doi: 10.3389/fimmu.2016.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stickel N, Prinz G, Pfeifer D, Hasselblatt P, Schmitt-Graeff A, Follo M, Thimme R, Finke J, Duyster J, Salzer U, Zeiser R. MiR-146a regulates the TRAF6/TNF-axis in donor T cells during GvHD. Blood. 2014;124:2586–95. doi: 10.1182/blood-2014-04-569046. [DOI] [PubMed] [Google Scholar]
- 76.Stickel N, Hanke K, Marschner D, Prinz G, Köhler M, Melchinger W, Pfeifer D, Schmitt-Graeff A, Brummer T, Heine A, Brossart P, Wolf D, von Bubnoff N, Finke J, Duyster J, Ferrara J, Salzer U, Zeiser R. MicroRNA-146a reduces MHC-II expression via targeting JAK/STAT-signaling in dendritic cells after stem cell transplantation. Leukemia. 2017 doi: 10.1038/leu.2017.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Storb R, Deeg HJ, Whitehead J, et al. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N Engl J Med. 1986;314:729–35. doi: 10.1056/NEJM198603203141201. [DOI] [PubMed] [Google Scholar]
- 78.Finke J, Bethge WA, Schmoor C, Ottinger HD, Stelljes M, Zander AR, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009;10:855–64. doi: 10.1016/S1470-2045(09)70225-6. [DOI] [PubMed] [Google Scholar]
- 79.Kröger N, Solano C, Wolschke C, Bandini G, Patriarca F, Pini M, et al. Antilymphocyte Globulin for Prevention of Chronic Graft-versus-Host Disease. N Engl J Med. 2016;374:43–53. doi: 10.1056/NEJMoa1506002. [DOI] [PubMed] [Google Scholar]
- 80.Zeiser R, Leveson-Gower DB, Zambricki EA, Kambham N, Beilhack A, Loh J, Hou JZ, Negrin RS. Differential impact of mTOR inhibition on CD4+CD25+Foxp3+ regulatory T cells as compared to conventional CD4+ T cells. Blood. 2008;111:453–62. doi: 10.1182/blood-2007-06-094482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ganguly S, Ross DB, Panoskaltsis-Mortari A, Kanakry CG, Blazar BR, Levy RB, Luznik L. Donor CD4+ Foxp3+ regulatory T cells are necessary for posttransplantation cyclophosphamide-mediated protection against GVHD in mice. Blood. 2014;124:2131–41. doi: 10.1182/blood-2013-10-525873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Luznik L, Bolaños-Meade J, Zahurak M, Chen AR, Smith BD, Brodsky R, et al. High-dose cyclophosphamide as single-agent, short-course prophylaxis of graft-versus-host disease. Blood. 2010;115:3224–30. doi: 10.1182/blood-2009-11-251595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Urbano-Ispizua A, Rozman C, Martínez C, Marín P, Briones J, Rovira M, Féliz P, Viguria MC, Merino A, Sierra J, Mazzara R, Carreras E, Montserrat E. Rapid engraftment without significant graft-versus-host disease after allogeneic transplantation of CD34+ selected cells from peripheral blood. Blood. 1997;89:3967–73. [PubMed] [Google Scholar]
- 84.Lang P, Feuchtinger T, Teltschik HM, Schwinger W, Schlegel P, Pfeiffer M, Schumm M, Lang AM, Lang B, Schwarze CP, Ebinger M, Urban C, Handgretinger R. Improved immune recovery after transplantation of TCRαβ/CD19-depleted allografts from haploidentical donors in pediatric patients. Bone Marrow Transplant. 2015;50:6–10. doi: 10.1038/bmt.2015.87. [DOI] [PubMed] [Google Scholar]
- 85.Bleakley M, Heimfeld S, Loeb KR, Jones LA, Chaney C, Seropian S, et al. Outcomes of acute leukemia patients transplanted with naive T cell-depleted stem cell grafts. J Clin Invest. 2015;125:2677–89. doi: 10.1172/JCI81229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Martelli MF, Di Ianni M, Ruggeri L, Falzetti F, Carotti A, Terenzi A, Pierini A, Massei MS, Amico L, Urbani E, Del Papa B, Zei T, Iacucci Ostini R, Cecchini D, Tognellini R, Reisner Y, Aversa F, Falini B, Velardi A. HLA-haploidentical transplantation with regulatory and conventional T-cell adoptive immunotherapy prevents acute leukemia relapse. Blood. 2014;124:638–44. doi: 10.1182/blood-2014-03-564401. [DOI] [PubMed] [Google Scholar]
- 87.Brunstein CG, Miller JS, McKenna DH, Hippen KL, DeFor TE, Sumstad D, et al. Umbilical cord blood-derived T regulatory cells to prevent GVHD: kinetics, toxicity profile and clinical effect. Blood. 2016;127:1044–51. doi: 10.1182/blood-2015-06-653667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bacchetta R, Lucarelli B, Sartirana C, et al. Immunological Outcome in Haploidentical-HSC Transplanted Patients Treated with IL-10-Anergized Donor T Cells. Front Immunol. 2014;5:16. doi: 10.3389/fimmu.2014.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kordelas L, Rebmann V, Ludwig AK, et al. MSC-derived Exosomes: A Novel Tool to Treat Therapy-Refractory Graft-versus-Host Disease. Leukemia. 2014 doi: 10.1038/leu.2014.41. in press. [DOI] [PubMed] [Google Scholar]
- 90.Le Blanc K, Rasmusson I, Sundberg B, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–41. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 91.Mielcarek M, Furlong T, Storer BE, Green ML, McDonald GB, Carpenter PA, Flowers ME, Storb R, Boeckh M, Martin PJ. Effectiveness and safety of lower dose prednisone for initial treatment of acute graft-versus-host disease: a randomized controlled trial. Haematologica. 2015;100:842–8. doi: 10.3324/haematol.2014.118471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Macmillan ML, Couriel D, Weisdorf DJ, Schwab G, Havrilla N, Fleming TR, Huang S, Roskos L, Slavin S, Shadduck RK, Dipersio J, Territo M, Pavletic S, Linker C, Heslop HE, Deeg HJ, Blazar BR. A phase 2/3 multicenter randomized clinical trial of ABX-CBL versus ATG as secondary therapy for steroid-resistant acute graft-versus-host disease. Blood. 2007;109:2657–62. doi: 10.1182/blood-2006-08-013995. [DOI] [PubMed] [Google Scholar]
- 93.Socié G, Vigouroux S, Yakoub-Agha I, Bay JO, Fürst S, Bilger K, Suarez F, Michallet M, Bron D, Gard P, Medeghri Z, Lehert P, Lai C, Corn T, Vernant JP. A phase 3 randomized trial comparing inolimomab vs usual care in steroid-resistant acute GVHD. Blood. 2017;129:643–9. doi: 10.1182/blood-2016-09-738625. [DOI] [PubMed] [Google Scholar]
- 94.Reshef R, Luger SM, Hexner EO, Loren AW, Frey NV, Nasta SD, et al. Blockade of lymphocyte chemotaxis in visceral graft-versus-host disease. N Engl J Med. 2012;367:135–45. doi: 10.1056/NEJMoa1201248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wysocki CA, Burkett SB, Panoskaltsis-Mortari A, Kirby SL, Luster AD, McKinnon K, Blazar BR, Serody JS. Differential roles for CCR5 expression on donor T cells during graft-versus-host disease based on pretransplant conditioning. J Immunol. 2004;173:845–54. doi: 10.4049/jimmunol.173.2.845. [DOI] [PubMed] [Google Scholar]
- 96.Taylor PA, Ehrhardt MJ, Lees CJ, Tolar J, Weigel BJ, Panoskaltsis-Mortari A, Serody JS, Brinkmann V, Blazar BR. Insights into the mechanism of FTY720 and compatibility with regulatory T cells for the inhibition of graft-versus-host disease (GVHD) Blood. 2007;110:3480–8. doi: 10.1182/blood-2007-05-087940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hanash AM, Dudakov JA, Hua G, O’Connor MH, Young LF, Singer NV, West ML, Jenq RR, Holland AM, Kappel LW, Ghosh A, Tsai JJ, Rao UK, Yim NL, Smith OM, Velardi E, Hawryluk EB, Murphy GF, Liu C, Fouser LA, Kolesnick R, Blazar BR, van den Brink MR. Interleukin-22 protects intestinal stem cells from immune-mediated tissue damage and regulates sensitivity to graft versus host disease. Immunity. 2012;37:339–50. doi: 10.1016/j.immuni.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lindemans CA, Calafiore M, Mertelsmann AM, O’Connor MH, Dudakov JA, Jenq RR, Velardi E, Young LF, Smith OM, Lawrence G, Ivanov JA, Fu YY, Takashima S, Hua G, Martin ML, O’Rourke KP, Lo YH, Mokry M, Romera-Hernandez M, Cupedo T, Dow LE, Nieuwenhuis EE, Shroyer NF, Liu C, Kolesnick R, van den Brink MR, Hanash AM. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature. 2016;528:560–4. doi: 10.1038/nature16460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dudakov JA, Hanash AM, van den Brink MR. Interleukin-22: immunobiology and pathology. Annu Rev Immunol. 2015;33:747–85. doi: 10.1146/annurev-immunol-032414-112123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kakihana K, Fujioka Y, Suda W, et al. Fecal microbiota transplantation for patients with steroid-resistant acute graft-versus-host disease of the gut. Blood. 2016;128:2083–8. doi: 10.1182/blood-2016-05-717652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Koura DT, Horan JT, Langston AA, Qayed M, Mehta A, Khoury HJ, et al. In vivo T cell costimulation blockade with abatacept for acute graft-versus-host disease prevention: a first-in-disease trial. Biol Blood Marrow Transplant. 2013;11:1638–49. doi: 10.1016/j.bbmt.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 102.Chen YB, Efebera YA, Johnston L, et al. Increased Foxp3+Helios+ Regulatory T Cells and Decreased Acute Graft-versus-Host Disease after Allogeneic Bone Marrow Transplantation in Patients Receiving Sirolimus and RGI-2001, an Activator of Invariant Natural Killer T Cells. Biol Blood Marrow Transplant. 2017;23:625–34. doi: 10.1016/j.bbmt.2017.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kennedy GA, Varelias A, Vuckovic S, Le Texier L, Gartlan KH, Zhang P, et al. Addition of interleukin-6 inhibition with tocilizumab to standard graft-versus-host disease prophylaxis after allogeneic stem-cell transplantation: a phase 1/2 trial. Lancet Oncol. 2014;13:1451–9. doi: 10.1016/S1470-2045(14)71017-4. [DOI] [PubMed] [Google Scholar]
- 104.Sofen H, Smith S, Matheson RT, et al. Guselkumab (an IL-23-specific mAb) demonstrates clinical and molecular response in patients with moderate-to-severe psoriasis. J Allergy Clin Immunol. 2014;133:1032–40. doi: 10.1016/j.jaci.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 105.Choi SW, Braun T, Chang L, Ferrara JL, Pawarode A, Magenau JM, et al. Vorinostat plus tacrolimus and mycophenolate to prevent graft-versus-host disease after related-donor reduced-intensity conditioning allogeneic haemopoietic stem-cell transplantation: a phase 1/2 trial. Lancet Oncol. 2014;1:87–95. doi: 10.1016/S1470-2045(13)70512-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Koreth J, Stevenson KE, Kim HT, Garcia M, Ho VT, Armand P, et al. Bortezomib, tacrolimus, and methotrexate for prophylaxis of graft-versus-host disease after reduced-intensity conditioning allogeneic stem cell transplantation from HLA-mismatched unrelated donors. Blood. 2009;114:3956–9. doi: 10.1182/blood-2009-07-231092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tawara I, Sun Y, Lewis EC, Toubai T, Evers R, Nieves E, Azam T, Dinarello CA, Reddy P. Alpha-1-antitrypsin monotherapy reduces graft-versus-host disease after experimental allogeneic bone marrow transplantation. Proc Natl Acad Sci U S A. 2012;109:564–9. doi: 10.1073/pnas.1117665109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Spoerl S, Mathew NR, Bscheider M, Schmitt-Graeff A, Chen S, Mueller T, et al. Activity of therapeutic JAK 1/2 blockade in graft-versus-host disease. Blood. 2014;123:3832–42. doi: 10.1182/blood-2013-12-543736. [DOI] [PubMed] [Google Scholar]
- 109.Betts BC, Abdel-Wahab O, Curran SA, St Angelo ET, Koppikar P, Heller G, Levine RL, Young JW. Janus kinase-2 inhibition induces durable tolerance to alloantigen by human dendritic cell-stimulated T cells yet preserves immunity to recall antigen. Blood. 2011;118:5330–9. doi: 10.1182/blood-2011-06-363408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zeiser R, Burchert A, Lengerke C, Verbeek M, Maas-Bauer K, Metzelder SK, et al. Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogeneic stem cell transplantation: a multi-center survey. Leukemia. 2015;29:2062–8. doi: 10.1038/leu.2015.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Iyengar S, Zhan C, Lu J, Korngold R, Schwartz DH. Treatment with a rho kinase inhibitor improves survival from graft-versus-host disease in mice after MHC-haploidentical hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2014;8:1104–11. doi: 10.1016/j.bbmt.2014.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Furlan SN, W B, Tkachev V, Flynn R, Cooley S, Ramakrishnan S, et al. Transcriptome analysis of GVHD reveals aurora kinase A as a targetable pathway for disease prevention. Sci Transl Med. 2015;315:315ra191. doi: 10.1126/scitranslmed.aad3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shindo T, Kim TK, Benjamin CL, Wieder ED, Levy RB, Komanduri KV. MEK inhibitors selectively suppress alloreactivity and graft-versus-host disease in a memory stage-dependent manner. Blood. 2013;121:4617–26. doi: 10.1182/blood-2012-12-476218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wolf D, Barreras H, Bader CS, Copsel S, Lightbourn CO, Pfeiffer BJ, Altman NH, Podack ER, Komanduri KV, Levy RB. Marked in Vivo Donor Regulatory T Cell Expansion via Interleukin-2 and TL1A-Ig Stimulation Ameliorates Graft-versus-Host Disease but Preserves Graft-versus-Leukemia in Recipients after Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2017;23:757–66. doi: 10.1016/j.bbmt.2017.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nishikii H, Kim BS, Yokoyama Y, Chen Y, Baker J, Pierini A, Alvarez M, Mavers M, Maas-Bauer K, Pan Y, Chiba S, Negrin RS. DR3 signaling modulates the function of Foxp3+ regulatory T cells and the severity of acute graft-versus-host disease. Blood. 2016;128:2846–58. doi: 10.1182/blood-2016-06-723783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chopra M, Biehl M, Steinfatt T, Brandl A, Kums J, Amich J, Vaeth M, Kuen J, Holtappels R, Podlech J, Mottok A, Kraus S, Jordán-Garrote AL, Bäuerlein CA, Brede C, Ribechini E, Fick A, Seher A, Polz J, Ottmüller KJ, Baker J, Nishikii H, Ritz M, Mattenheimer K, Schwinn S, Winter T, Schäfer V, Krappmann S, Einsele H, Müller TD, Reddehase MJ, Lutz MB, Männel DN, Berberich-Siebelt F, Wajant H, Beilhack A. Exogenous TNFR2 activation protects from acute GvHD via host T reg cell expansion. J Exp Med. 2016;213:1881–900. doi: 10.1084/jem.20151563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Takashima S, Kadowaki M, Aoyama K, Koyama M, Oshima T, Tomizuka K, Akashi K, Teshima T. () The Wnt agonist R-spondin1 regulates systemic graft-versus-host disease by protecting intestinal stem cells. J Exp Med. 2011;208:285–94. doi: 10.1084/jem.20101559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, Kim S, Fritz JV, Wilmes P, Ueha S, Matsushima K, Ohno H, Olle B, Sakaguchi S, Taniguchi T, Morita H, Hattori M, Honda K. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–6. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 119.Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002;99:3493–9. doi: 10.1182/blood.v99.10.3493. [DOI] [PubMed] [Google Scholar]
- 120.Edinger M, Hoffmann P, Ermann J, Drago K, Fathman CG, Strober S, Negrin RS. CD4+CD25+ Regulatory T Cells Preserve Graft-vs-Tumor Activity while Inhibiting Graft-vs-Host Disease After Bone Marrow Transplantation. Nature medicine. 2003;9:1144–9. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- 121.Bruce DW, Stefanski HE, Vincent BG, Dant TA, Reisdorf S, Bommiasamy H, Serody DA, Wilson JE, McKinnon KP, Shlomchik WD, Armistead PM, Ting JP, Woosley JT, Blazar BR, Zaiss DM, McKenzie AN, Coghill JM, Serody JS. Type 2 innate lymphoid cells treat and prevent acute gastrointestinal graft-versus-host disease. The Journal of clinical investigation. 2017 Apr 4; doi: 10.1172/JCI. pii: 91816. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


