Abstract
Nociception is an important physiological process that detects harmful signals and results in pain perception. In this review, we discuss important experimental evidence involving some TRP ion channels as molecular sensors of chemical, thermal, and mechanical noxious stimuli to evoke the pain and itch sensations. Among them are the TRPA1 channel, members of the vanilloid subfamily (TRPV1, TRPV3, and TRPV4), and finally members of the melastatin group (TRPM2, TRPM3, and TRPM8). Given that pain and itch are pro-survival, evolutionarily-honed protective mechanisms, care has to be exercised when developing inhibitory/modulatory compounds targeting specific pain/itch-TRPs so that physiological protective mechanisms are not disabled to a degree that stimulus-mediated injury can occur. Such events have impeded the development of safe and effective TRPV1-modulating compounds and have diverted substantial resources. A beneficial outcome can be readily accomplished via simple dosing strategies, and also by incorporating medicinal chemistry design features during compound design and synthesis. Beyond clinical use, where compounds that target more than one channel might have a place and possibly have advantageous features, highly specific and high-potency compounds will be helpful in mechanistic discovery at the structure-function level.
Keywords: TRP channels, Pain, Itch, Nociceptors, Inflammation, Lipids, Temperature, Hyperalgesia, Nerve damage, Neuropathic pain, Mechanotransduction, Allodynia
Introduction
Pain and itch are vital, survival-enhancing mechanisms that protect from harm that potentially threatens organismal integrity, survival, and the ability to generate offspring. Pain and itch have unique behavioral responses. Pain-related behavior and activation of the neural pathways dedicated to sensing harm involve acute withdrawal or other protective behaviors. Conversely, itch is an irritating sensation that leads to a scratch reflex, bringing attention to the affected area so as to remove puritogens and bring temporary relief [1]. Itch and pain are distinct sensations that both rely on a nervous system with dedicated sensory subdivisions that interact.
Itch can be classified as either acute or chronic. Acute itch serves an important protective function as a sentinel against potentially harmful external agents such as insects, toxic plants, and other irritants, while at the same time, via the scratching component, removing the offending agent. On the other hand, chronic itch accompanies a number of skin diseases such as atopic dermatitis and dermatitis herpetiformis, as well as systemic conditions, including hepatic cholestasis, diabetic neuropathy, kidney failure, and lymphomas. Itch-sensitive neurons are divided into mechano-insensitive and mechano-sensitive C-fibers activated by pruritogens [2].
Pain is thought to be dominant to itch as painful stimuli relieve itch [1]. Pain with a unique conscious sensation that can be described is, by definition, exclusively human. In humans, there are numerous pathological conditions, such as diabetes, viral infections, metabolic, toxic, and traumatic nerve damage, and inflammation of neural structures, which can produce unrelenting pain that can be viewed as one form of pathological pain. Of note, pathological pain can also manifest as purely episodic, e.g. migraine or cluster headaches, trigeminal neuralgia, and peripheral or visceral neuralgic pain, in which inter-episode pain is minimal or absent, interrupted by subjectively disruptive to destructive episodes/ attacks of pain.
A gene family involved in pain sensory function is the transient receptor potential (TRP) channel super-family. This family is made up of ion channel proteins that function as non-selective cation-permeable channels, virtually all of which (28 in mammals) conduct Ca2+ [3]. In general, TRP channels have fascinated researchers, clinicians, drug developers, and generally-interested scholars because they function as molecular sensors of multiple physical and chemical stimuli, including changes in pH, chemical irritants including pungent peppers, wasabi, mustard, and menthol, as well as thermal, mechanical, osmotic, and actinic (radiation) cues. The TRP super-family is composed of 28 members divided into six subfamilies, classified as canonical (TRPC), vanilloid (TRPV), ankyrin (TRPA), melastatin (TRPM), polycystin (TRPP), and mucolipin (TRPML) [4]. Reflecting on the acronym, it has also been proposed that TRP could refer to “targeted relief of pain”. We propose here the “P” to be ambiguous and to stand for pain or pruritus.
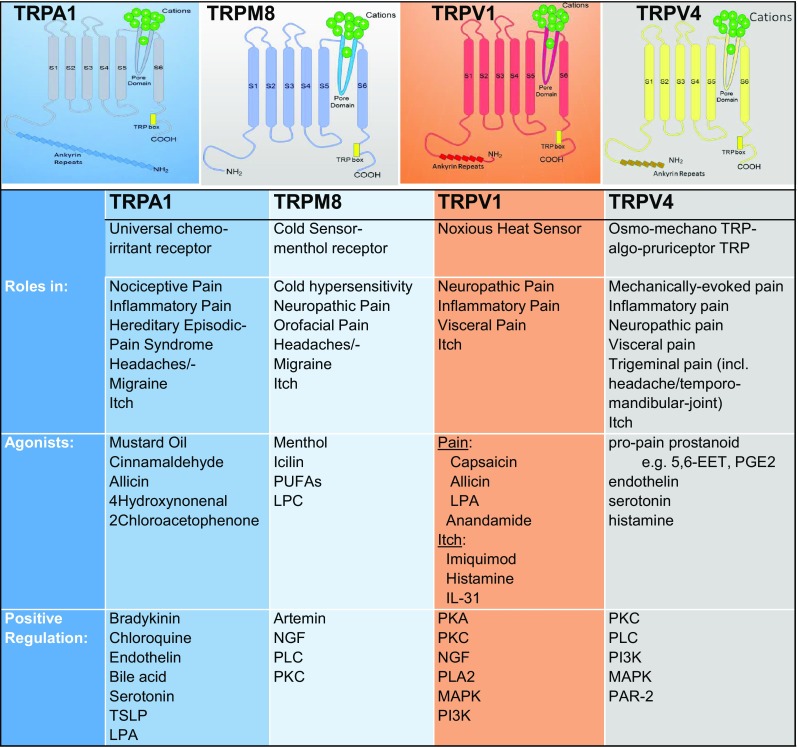
TRP channels, now elucidated as several high-resolution cryo-EM structures, consist of four subunits, each containing six transmembrane segments (S1–S6). A domain between S5 and S6, containing a hydrophilic loop, forms the ion-conducting pore and the selectivity filter [5, 6] (Fig. 1). The most highly variable regions within TRP channels are the carboxyl and amino terminal ends. The ankyrin repeat domain is located within the amino terminus of TRP channels. However, the TRP box, which is a conserved six amino-acid sequence in the TRPC, TRPM, TRPA, and TRPV subfamilies, is located at the carboxyl terminus. Several studies have shown that this region is important for ligand binding. In addition to the ankyrin repeat and TRP box domains, TRP family members contain other domains, including the Ca2+-binding EF-hand domain, PDZ domains that anchor the receptor proteins in the membrane to cytoskeletal components, or a NUDIX (hydrolase of a nucleoside diphosphate linked to some other moiety, X) domain. These domains are found in various TRP family members [7]. Diversity in their domain structure indicates that TRP channels may form complexes with multiple proteins involved in different cellular processes, facilitating their participation in many signaling processes.
Fig. 1.
TRP channels involved in the regulation of pain and itch.
Twenty years after the first description of TRPV1 as the first family member with a postulated and later verified link to pain (followed by TRPV4, 3 years thereafter), the evidence has become increasingly robust that TRP channels function in physiological and pathological pain. In this review, we elaborate on relevant highlights related to the TRPA, TRPM, and TRPV subfamilies and the roles of their members in both physiological and pathological pain as well as in itch. In addition, we propose to refer to the TRP ion channels involved in pain transduction and transmission as a functional subfamily of “pain-TRPs”, following the previously-claimed functional subfamilies of “thermo-TRPs”, and “mechano-TRPs” previously coined by one of us [8], and likewise refer to TRP ion channels involved in itch as “itch-TRPs”.
TRP Ankyrin Subfamily
The TRPA subfamily is named for the large number of ankyrin repeats in the cytosolic N-termini of its members. The mammalian genome contains a single TRPA gene, TRPA1 [9]. The TRPA1 protein has 14 ankyrin repeats and forms a nonselective cation channel permeable to Ca2+ [10, 11]. TRPA1 channels are highly expressed in peripheral nociceptors, some studies reporting additional sites of expression. TRPA1 is functionally involved in a variety of physiological or cellular processes in humans [12–14] including nociception, especially in response to chemical irritants, itch, neurogenic inflammation, and potentially thermosensation [13, 15–19], and may affect the immune system and vascular function [11].
TRPA1 is a Universal Chemo-Irritant Receptor Involved in Neuropathic Cold-Pain
TRPA1 is a polymodal nocisensor gated by a wide range of chemical irritants. It is present in a subpopulation of Aδ- and C-fiber nociceptive neurons in dorsal root, trigeminal, and vagal (nodose or jugular) ganglia. TRPA1 is activated by pain-inducing natural products, including mustard oil (allyl isothiocyanate), the pungent ingredient in mustard, wasabi, and horseradish, cinnamaldehyde from cinnamon, and pungent compounds in garlic (allicin) and onions (diallyl disulfide) [17, 20–22]. These natural products evoke pain-related behaviors in animals, and pain and irritation in humans (Fig. 1). The chemical reactivity and structural diversity of these compounds suggest that TRPA1 does not fit into the mold of the traditional definition of a pharmacological receptor, but may in fact be a chemical reactivity detector, acting as a chemical warning sensor that translates chemical reactivity into a pain signal. Indeed, reactive cysteine residues in the intracellular N-terminal domain of TRPA1 are essential for the majority of electrophilic agonists to gate the ion channel [23, 24]. More recent studies have discovered additional sites within the ion channel protein, including lysine residues, that also contribute to reactivity detection [24–26]. TRPA1 is also sensitive to non-reactive irritating natural products, including carvacrol (from clove), thymol (from thyme), gingerol (from ginger), and menthol (mint) [27–29].
Chemosensory nerve endings in the cornea, nose, and larynx constantly monitor the environment for airborne chemical threats, which initiate defensive reflexes such as lachrymation, sneezing, and coughing. The upper and lower respiratory tract are innervated by primary afferent C-fibers and Aδ-fibers. The airway-innervating trigeminal and vagal ganglia show appreciable TRPA1 expression [20, 30–32]. A significant number of airborne chemicals including industrial pollutants, but also natural substances, induce airway irritation by activating sensory neurons innervating the lung/airway in a TRPA1-dependent manner [28, 33–36]. TRPA1 expressed in airway-innervating neurons mediates acute irritation responses to tobacco smoke and smoke from fires, activated by the smoke irritants acrolein and croton aldehyde [28, 32, 36–38].
The most potent TRPA1 agonists identified so far are tear gas agents such as 2-chloroacetophenone and 2-chlorobenzalmalononitrile that activate TRPA1 in the low nanomolar or picomolar range [39, 40]. TRPA1-deficient mice lack acute nocifensive responses to these extremely painful agents [40]. TRPA1 is also sensitive to oxidizing chemical exposure, including chlorine gas, hydrogen peroxide, and ozone [32, 34]. Respiratory reflex responses to such exposure are absent in TRPA1-deficient mice [32, 34]. TRPA1 activity also contributes to the toxicological effects of particulates and metals such as zinc, cadmium, and copper, underscoring the essential role of TRPA1 in the detection of toxic environmental agents [41–43]. Airborne environmental exposure to contaminants such as ozone, particulates, and acrolein affect cardiovascular function even at very low levels, initiating changes in blood pressure and heart rate. TRPA1 activation by inhaled diesel exhaust and acrolein has been demonstrated to initiate cardiac arrhythmia in mice and rats, likely through changes in vagal sensory-autonomic control of cardiac function [44, 45].
While the function of TRPA1 as a chemosensor is firmly established, its role in thermal sensing remains a matter of debate. In mammals, exposure to temperatures below ~ 15 °C can elicit pain. TRPA1 was initially identified as a sensor for noxious cold; however, this study under-reported the prevalence of TRPA1-expressing sensory neurons that turned out to be much larger than the population responding to noxious cold [20, 46]. Studies in TRPA1-deficient mice have reported contradictory data, some finding no difference in noxious cold-induced nocifensive behavior [36, 47–49], and also when TRPA1 expression is specifically ablated in sensory neurons or inhibited by a selective antagonist [50, 51]. The complete ablation of TRPA1-expressing sensory neurons does not change the sensitivity of trigeminal nerves to cold [52]. Other studies have detected diminished responses to cold stimuli in TRPA1-deficient mice; however, the mice had been exposed to freezing temperatures that may have damaged tissue and induced inflammation [13, 53]. The thermal sensitivity of TRPA1 is clearly species-specific, some reports finding that human and other primate orthologs lack cold sensitivity, while others finding bi-modal (cold and heat) sensitivity [12, 54–56].
TRPA1 channels are highly sensitive to Ca2+, which has bimodal effects on channel function. Ca2+ potentiates and activates TRPA1, but is also essential for TRPA1 desensitization and can be indirectly regulated by G protein-coupled receptors (GPCRs), such as bradykinin receptors and proteinase-activated receptors (PAR2) or CGRP-signaling [57].
Less controversial is the role of TRPA1 in the cold allodynia associated with inflammatory and neuropathic conditions, including chemotherapy-induced neuropathies after treatment with platinum or taxol therapeutics [25, 58–61] In this case, TRPA1 may serve as an amplifier, increasing the excitability of cold-sensitive neurons [58, 62]. A proposed mechanism involves reactive oxygen species (ROS) signaling induced by cooling [63]. Inhibition of hydroxylation of a proline residue in a human TRPA1 N-terminal ankyrin repeat either by mutation or using a prolyl hydroxylase inhibitor potentiates the cold sensitivity of TRPA1 in the presence of hydrogen peroxide. The same study showed that inhibiting prolyl-hydroxylase in mice triggers TRPA1 sensitization which is sufficient to sense cold-evoked ROS, hence causing cold hypersensitivity. Furthermore, this mechanism is thought to underlie the acute cold hypersensitivity induced by the chemotherapeutic agent oxaliplatin or its metabolite oxalate [63].
TRPA1 has also been implicated in diabetic neuropathic pain, caused by chronic reactive chemical stress due to metabolic imbalance. In rodent models, TRPA1 inhibitors have been shown to protect nerves from peripheral degeneration and reduce nocifensive responses [64, 65]. Methyl glyoxal, a reactive metabolic product that is increased in diabetics, is a TRPA1 agonist that may contribute to chronic heightened channel activity, leading to increased pain sensation, Ca2+ overload, and subsequent peripheral degeneration [66, 67].
TRPA1 in Inflammatory Pain
TRPA1 sensitization leads to hyperalgesia in response to various stimuli. Its pathophysiological role in inflammation is based on its ability to activate in response to various mediators and metabolites produced under inflammatory conditions. Pro-inflammatory mediators released from non-excitable cells include ATP, bradykinin (BK), prostaglandins, leukotrienes, histamine, tumor necrosis factor-α, interleukin-1β (IL-1β), proteases, and glutamate.
BK indirectly activates TRPA1 channels to mediate pain and the inflammatory response. This requires BK to interact with its receptor (B2R), which further activates the phospholipase C (PLC)-dependent signaling pathway and promotes phosphatidylinositol 4,5-bisphosphate (PIP2) hydrolysis to produce inositol triphosphate (IP3) and diacylglycerol (DAG). Both lipids IP3 and DAG can activate TRPA1 [17]. Trpa1−/− mice have reduced thermal and mechanical pain responses to intraplantar injection of BK or allyl isothiocyanate [36].
Agents such as Complete Freund’s Adjuvant (CFA) are widely used to evoke inflammatory pain equivalents in preclinical models. This leads to increased expression of TRPA1 in dorsal root ganglion (DRG) neurons [30]. Furthermore, the TRPA1 antagonist HC-030031 reduces nocifensive behaviors induced by paw injection of formalin and suppresses mechanical hyperalgesia in the CFA model of inflammatory pain [68].
Human TRPA1 is also activated by acidosis which occurs in tissue ischemia such as myocardial infarction or peripheral vascular occlusive disease [69]. However, initial attempts to combat acidosis-evoked pain by targeting TRPA1 have not been met with success in humans [70]. Ischemia leads to an increase in ROS generation which results in the formation of reactive carbonyl species like 4-hydroxynonenal and 4-oxononenal, which act directly on TRPA1 [71, 72].
Recent studies suggest that TRPA1 may also be expressed in non-neuronal tissues. The strongest evidence for non-neuronal expression was provided by studies on intestinal enterochromaffin cells that respond to a TRPA1 agonist with the release of serotonin that, in turn, activates underlying sensory nerve endings, a mechanism regulating gastrointestinal motility [73, 74]. TRPA1 expression has also been reported in some permanent cell lines, including mast cells, fibroblasts, and epithelial lines. Quantitative PCR studies have shown that neuronal transcript levels are at least several orders of magnitude higher than those in extra-neuronal tissues, making validation of extra-neuronal expression in other organs difficult [75]. Tissue detection of TRPA1 is also hampered by the poor specificity and validation of the available antibodies, and the non-specific effects of the chemically-reactive TRPA1 agonists. Recent studies using strategies to specifically ablate TRPA1 expression in sensory neurons have recapitulated most of the findings from global TRPA1-deficient mice, including the absence of acute and inflammatory pain modalities and hypersensitivity responses, suggesting that neuronal TRPA1 accounts for most of the TRPA1-dependent physiological mechanisms [51, 52, 76]. However, as in the case of enterochromaffin cells, the expression of TRPA1 in small cell populations may be difficult to detect. Additional sites with reported TRPA1 expression are melanocytes, odontoblasts, insulin-producing β-cells of the islets of Langerhans in the pancreas, and vascular endothelial cells. Via its endothelial expression, TRPA1 might regulate vascular tone, and might also play a role in the development of atherosclerotic disease [77].
Trigeminally-mediated pain involving TRPA1 channels could be relevant to the pathogenesis of headaches, especially migraine pain. TRPA1 channels have been shown to signal in response to the plant-derived headache- and migraine-inducing volatile compound umbellone, derived from the “headache tree”, Umbellaria californica [78, 79]. Since migraine is thought to occur when meningeal trigeminal nerves are activated, intraganglionic signaling between nasal airway-innervating and meningeal trigeminal branches would be a prerequisite [80]. Such a mechanism has been proposed for other environmental irritants that trigger headaches, including acrolein, the TRPA1 agonist in smoke and air pollution. Extended inhalational exposure of mice to acrolein has been shown to increase meningeal vascular blood flow, supporting such a mechanism [81, 82]. A common pro-migraine pathogenic mechanism is the release of calcitonin gene-related peptide (CGRP) from primary trigeminal sensory neurons, known to be triggered after TRPA1 activation [83]. CGRP is a strong meningeal vasodilator, increasing blood flow, and is a target of a novel antimigraine treatment successfully tested in clinical trials. Several clinically-effective antimigraine medicines, including herbal compounds such as petasin and parthenolides, have been shown to modulate TRPA1 channels, implicating TRPA1 as an important contributor to migraine and headaches, and as an additional target for innovative migraine drug development [83–86].
The pro-inflammatory actions of TRPA1 extend beyond pain. Recent studies suggest that TRPA1 is involved in inflammatory pathology of the respiratory system, including allergic and chemically-induced asthma and smoking-induced inflammation [37, 87, 88]. TRPA1 inhibitors, or deletion of the TRPA1 gene, strongly reduce asthmatic airway contractions and pulmonary inflammation in mice and rats allergic to the experimental allergen, ovalbumin [87].
Animal studies have revealed that the combined role of TRPA1 as a pain transducer and regulator of inflammation extends to many other inflammatory pain conditions, including arthritis and gout [89–91]. The gastrointestinal tract is also innervated by TRPA1-expressing nerve fibers. Here, TRPA1 plays a prominent role in acute mechanical distension pain of the colon and in gastrointestinal inflammatory pain and hypersensitivity during pancreatitis as well as in esophagitis due to chemical injury or allergic sensitization [92–103]. A recent study of colonic pain mechanisms reported that capsazepine desensitizes colonic afferents after the induction of colonic inflammation, yet this process is surprisingly independent of Trpv1, and apparently functions via the desensitization of TRPA1 expression in sensory neurons innervating the colon. In visceral pain of the colon and pancreas, there appears to be a particular synergy between TRPV4 and TRPA1 (see also the section on TRPV4 below) [104].
TRPA1 in an Inherited Episodic Pain Syndrome and Other Forms of Pain
The discovery of a human gain-of-function mutation in TRPA1 has greatly validated the role of TRPA1 as a “pain-TRP”. This missense mutation is associated with a familial episodic pain syndrome [105] which is characterized by the presence of severe pain in the upper body accompanied by breathing difficulty, tachycardia, sweating, generalized pallor, and stiffness of the abdominal wall [105]. These painful episodes are more likely if a carrier of the mutation has experienced a challenge through fasting, fatigue, and exposure to cold and/or physical stress. Up to this point, among the entire TRP superfamily of channels, this hereditary pain syndrome caused by a gain-of-function point mutation in TRPA1 is the only TRP-channelopathy in humans associated exclusively with pain.
This mutation is located in the linker region that connects transmembrane segments 4 and 5, thus intracellularly, where substitution of the asparagine 855 by a serine (N855S) changes the voltage-dependence to more negative potentials, thus increasing TRPA1 activity without modifying its affinity for some activators [105, 106].
Interestingly, the N855 position in TRPA1 has been shown to be involved in the inhibitory action of the TRPA1 antagonist HC-030031 in species-selectivity studies of human versus frog TRPA1 [107]. This raises the question of the correlation between the mutated amino-acid associated with human disease and the amino-acid changes found in frog TRPA1 and zebrafish TRPA1b, both of which are insensitive to HC-030031. Evolutionary comparison and these structure-activity-pharmacology studies suggest that N855 in human TRPA1 is a potential high-priority target for developing effective TRPA1-modulating compounds.
The study reporting the N855 mutation in inherited episodic pain syndrome also noted that patients experience breathing difficulties during painful episodes [105]. While no further details were provided, this finding suggests that TRPA1 may be involved in respiratory control and, potentially, in asthmatic responses. This function has been further supported by a large longitudinal study that found several polymorphisms in the TRPA1 gene to be associated with childhood asthma [108].
Adding further weight to the concept that TRPA1 is a pain-TRP in humans, the human TRPA1 gene was found to have methylation differences in identical twins who had different levels of pain sensitivity [109]. Increased expression of TRPA1 was found in the skin. This is consistent with an attenuating effect of DNA methylation on the TRPA1 promoter affecting TRPA1 gene expression.
We are optimistic that more widespread application of human DNA sequencing, especially in patients suffering from various forms of chronic pain affecting multiple generations will uncover additional polymorphisms and mutations in pain-TRPs, such as TRPA1, that co-contribute to pathological pain [109].
Two non-hereditary forms of specific pain stand out in which TRPA1 is involved, the challenging-to-manage pain [89, 90, 110–117] of sickle-cell disease [118, 119], and the largely unmet medical need of arthritis pain.
TRPA1 in Itch
Itch is defined as an unpleasant sensation that evokes the desire/reflex to scratch. TRPA1 has been suggested to mediate histamine-independent itch in response to exogenous pruritogens such as chloroquine and cowhage spicules, and to endogenous pruritic mediators produced in the skin and other organs, including bile acid, serotonin, ROS, leukotriene-B4, thymic stromal lymphopoietin, and IL-13, IL-22, IL-31, and IL-33 [3, 120–125].
Endothelin (ET-1), also known as a vasoconstricting peptide, can evoke itch by stimulating nociceptors and pruriceptors and sensitizing them to noxious and pruritic stimuli. ET-1 has been implicated in both pain and itch via TRPA1, TRPV1, and TRPV4 [94, 126–131]. Serotonin-induced itch has been shown to be mediated via activation of the serotonergic receptor HTR7 which signals to TRPA1 [132] (but see subsection “TRPV4 in itch”). Aberrant serotonin signaling has long been linked to a variety of human chronic itch conditions, including atopic dermatitis. In a mouse model of this highly prevalent condition (10% in the USA and Europe), mice lacking HTR7 or TRPA1 display reduced scratching and skin lesion severity [132]. These data highlight a role for HTR7 and TRPA1 in acute and chronic itch, and suggest that their antagonists may be useful for treating a variety of pathological itch conditions. Recent studies have also shown that TRPA1 contributes to pruritus due to allergic contact dermatitis, including that elicited by the poison ivy allergen, urushiol [75, 123].
Similarly, itch associated with severe liver disease, known as cholestatic itch, appears to be TRPA1-dependent. Bile acids induce itch in mice by activating the bile-acid receptor TGR5 and TRPA1. TRPA1 acts downstream of G-protein-coupled bile receptors such as TGR5. Antagonists of TGR5 and TRPA1, or inhibitors of the signaling mechanism by which TGR5 activates TRPA1, might be developed for the treatment of cholestatic itch [133, 134]. However, the serum levels of bile acids do not correlate with the presence and intensity of cholestatic itch in liver patients, whereas the bioreactive phospholipid lysophosphatidic acid (LPA) does. Of note, LPA also signals to pruriceptor neurons via TRPA1 [120].
Loss- and gain-of-function studies in mice have demonstrated that the G-protein-coupled receptor MrgprA3 is required for chloroquine-responsiveness in mice [135]. TRPA1 has been found to signal downstream of MrgprA3 in cultured sensory neurons and heterologous cells [122].
Again, TRPA1 not only serves as a sensor for pruritogens, but is also essential for maintaining skin inflammation, as shown in models of contact dermatitis and atopic dermatitis in which treatment with TRPA1 inhibitors reduces skin swelling, epidermal water loss, and leukocyte infiltration [75, 136, 137].
In terms of the development of TRPA1 inhibitory/modulatory compounds for human clinical use, as of today, TRPA1 inhibitors have a shared feature in that they can all be improved in terms of better efficacy and fewer side effects [138, 139]. At the basic science level, on the other hand, elegant work has been conducted. TRPA1 has been found to be evolutionarily conserved, with the involvement of TRPA1 orthologues in pain-related behavior and inflammation in invertebrate model organisms [56, 140–157].
TRP Melastatin Subfamily
The TRPM subfamily consists of eight members, three of which are associated with pain (TRPM2, TRPM3, and TRPM8). These ion channels have an overall molecular topology resembling the other TRP family members, although a major difference is the lack of ankyrin repeats in their amino end. We focus on TRPM8 here and highlight key references for TRPM2 and TRPM3.
TRPM2 Pro-pain Role
The thermo-TRP TRPM2 plays a role in pain signaling as recently discovered [54, 158–166].
TRPM3 Pro-pain Role
This is also true for the pregnenolone receptor, TRPM3 [167–170].
TRPM8 in Cold Hypersensitivity and Neuropathic Pain
Among the TRPM members in the pain pathway, TRPM8 ion channels have been researched most in-depth. TRPM8 expression is restricted to a subset of small-diameter sensory neurons of the trigeminal and dorsal root ganglia [171–173]. This channel is activated by cool and noxious cold temperatures between 10 °C and 23 °C, and by natural compounds that evoke a cold sensation such as menthol and eucalyptol (Fig. 1) [171, 172, 174]. Similar to TRPV1 and TRPA1, TRPM8 activation evokes an influx of Ca2+ into the primary sensory neuron, leading to its activation and the propagation of action potentials. The role of TRPM8 in cold thermosensation has been widely explored in vivo in Trpm8−/− mice. These mice lack a response to cold stimuli and chemical compounds that cause a cold sensation such as icilin, as well as lacking a response to acetone-evoked evaporative cooling [174–176]. TRPM8 is also involved in orofacial and visceral (colonic) pain [47, 177–180].
Nerve injury can result in cold allodynia, and it has been suggested that TRPM8 channels are a key substrate thereof [177, 181]. Rats subjected to constriction nerve injury show enhanced withdrawal reflexes in response to evaporative cooling with acetone. They also have an increase in the number of neurons immunoreactive to the TRPM8 channel and more neurons that are stimulated by menthol and cold [177].
TRPM8 has been mechanistically linked to cold hypersensitivity [181]. Unlike heat hyperalgesia which is produced by several algogenic agents, TRPM8 cold sensitization appears to be evoked by neurotrophic factors such as artemin and nerve growth factor which produce their effects through specific receptors co-expressed with TRPM8 in a subset of sensory neurons [182, 183]. This concept was convincingly demonstrated using the evaporative cooling assay, where neurotrophic factors were injected into the foot-pad with subsequent application of acetone-evoked evaporative cooling to the paw. Wild-type mice showed an increase in cold-response with growth factors, while Trpm8−/− mice did not [182, 183].
The sensitizing effects of TRPM8 ion channels on cold pain can be modulated by endogenous lipids. For example, TRPM8 activation by cold and menthol requires the presence of PIP2 (a membrane phospholipid), which interacts directly with positively-charged amino-acids located in the TRP box of the channel [184]. Furthermore, PIP2 by itself can activate the channel [184]. Therefore, TRPM8-mediated cold allodynia and hypersensitivity could be attenuated by regulating PIP2 levels such as via the activation of Ca2+-dependent PLC which then hydrolyzes PIP2 to produce DAG and IP3 [184]. Moreover, DAG can activate the PKC pathway which then attenuates TRPM8 gating. This effect is mediated by specific TRPM8 serine-phosphorylation events [185].
In addition, TRPM8 activity is endogenously regulated by the iPLA2 pathway, a calcium-insensitive phospholipase which produces lysophospholipids and free polyunsaturated fatty acids (PUFA) from the hydrolysis of sn-2-glycerophospholipids [186]. Interestingly, inhibition of iPLA2 attenuates the TRPM8 currents evoked by cold, menthol, and icilin [186]. Moreover, lysophosphatidylcholine (LPC) released from the iPLA2 pathway can activate TRPM8 even at 37 °C [186]. Furthermore, LPC evokes cold hypersensitivity in a TRPM8-dependent manner since this behavior is absent in Trpm8−/− mice [187].
Pointing to endogenous regulatory mechanisms, PUFAs such as arachidonic, eicosapentaenoic, and docosahexaenoic acids, which also are released by iPLA2, are endogenous inhibitors of TRPM8 [186].
Therefore, the iPLA2 pathway can have contrasting effects on TRPM8 activation: an activating effect by lysophospholipids and a modulating inhibitory effect by PUFAs. Is one pathway “dominant” over the other? Equimolar application of LPC and arachidonic acid favors the activation of TRPM8 by LPC, possibly implicating this mechanism in cold pain and cold hypersensitivity phenomena [186].
Selective TRPM8 inhibitors are effective in reducing cold sensing and also cold pain, suggesting that TRPM8 contributes to both innocuous and noxious thermal sensing [47, 188]. Another study using a TRPM8-specific inhibitor reported no significant effects on neuropathic mechanical allodynia, but the effects on cold allodynia were not investigated [189].
While TRPM8 inhibition may be effective in reducing neuropathic cold pain, TRPM8 agonists have long been known to have analgesic properties. Menthol is widely used as a topical analgesic and inhaled antitussive with respiratory counterirritant properties. Menthol and TRPM8-selective menthol analogs have been shown to suppress nocifensive responses to several acute pain stimuli, including capsaicin, acrolein, and acid [178]. The analgesic effects of menthol are absent in TRPM8-deficient mice [178]. Menthol also suppresses inflammatory pain in a TRPM8-dependent manner [178]. Icilin has been demonstrated to diminish colitis-associated pain in a TRPM8-dependent manner [190]. Eucalyptol, the TRPM8 agonist in eucalyptus oil, also suppresses pain and respiratory inflammation and irritation [180, 191]. TRPM8-selective agonists may have improved analgesic properties compared to menthol that can cause irritation in some patients [178].
Possibly indicative of a role for TRPM8 in controlling itch, menthol has been reported to function as an anti-pruritic in therapy-resistant pruritus in lichen amyloidosis [192] and hydroxyethyl starch-induced itch [193]. However, these clinical findings leave open the possibility of menthol also acting on TRPA1 that is also sensitive to menthol. A more recent study has demonstrated that the anti-pruritic actions of menthol depend on inhibitory interneurons in the spinal cord that receive input from menthol-sensitive fibers and dampen input from pruriceptors [194].
TRP Vanilloid Subfamily
The TRPV subfamily is named after its founding member, the TRPV1 channel, which is activated by the classic natural pungent vanilloid, capsaicin. The mammalian TRPV1 channel in this subfamily is sensitive to vanilloid compounds. The other members share amino-acid sequence homology. This subfamily consists of six members (TRPV1–TRPV6), all of which are non-selective cation channels where TRPV1/3/4 show a preference for Ca2+ and they are overall linked to pain and itch, thus they are considered in this section in more detail.
TRPV1 is an Extensively Studied Pain-TRP
The TRPV1 channel is a representative member of the TRPV subfamily and is the most well-characterized TRP channel. TRPV1 can be activated by various stimuli, including temperature (~ 42 °C), pH, and a wide range of both endogenous and exogenous compounds (Fig. 1). Its main exogenous ligand is capsaicin, a compound from pungent peppers that activates the channel. The beneficial effects of capsaicin on nociception were identified before the TRPV1 channel was discovered [195]. TRPV1 was cloned in 1997 from a cDNA library isolated from capsaicin and temperature-stimulated nociceptor neurons [196]. Subsequent characterization of TRPV1 has revealed its key role in nociception. In addition, with the use of Trpv1−/− mice, TRPV1 has been shown to be important in pain sensation [197]. Due to its importance in these physiological processes, several compounds have been developed to modulate the activity of TRPV1 to eliminate or reduce pain.
Expression of TRPV1 in the Nervous System
TRPV1 is preferentially expressed in sensory neurons of the peripheral nervous system (PNS), where it is primarily expressed by the small and medium nociceptor neurons of the DRG, trigeminal ganglion (TG), nodal ganglion, and sympathetic ganglion, in peptidergic and non-peptidergic C fibers, and in some Aδ fibers [198–200]. In addition, it is expressed at lower levels in nerve fibers that innervate the bladder [201], lungs [202, 203], and cochlea [204], as well as in the upper respiratory tract, where its function is to sense irritant compounds [205, 206].
TRPV1 is expressed in several regions of the central nervous system (CNS), specifically in laminae I and II of the dorsal horn of the spinal cord, where it modulates the synaptic transmission of nociceptive signals from the periphery [207]. TRPV1 is primarily expressed in the presynaptic terminal, though studies have demonstrated its postsynaptic expression. However, its postsynaptic expression has not been shown to be related to nociception [208]. The use of transgenic mice expressing reporter proteins driven by the TRPV1 promoter has revealed the areas in which TRPV1 is expressed [209]. Expression has been observed in the fibers of small- and medium-diameter nociceptor neurons in the cornea, bladder, and skin; in the DRG and TG; in the dorsal horn of the spinal cord; and in some regions of the brain, such as the brainstem, the nucleus caudalis, the nucleus ambiguus, the olfactory bulb, and the nucleus parabrachialis [210]. Interestingly, TRPV1—non-capsaicin receptor channels, thus TRPV1 splice-variant transcripts—has been cloned from supra-optic microdissection. These channels appear to function in the organismal response to systemic hypertonic osmolality and to organismal body temperature [211].
TRPV1 in Inflammatory Pain
When a skin lesion is produced, a wide variety of pro-inflammatory molecules are released, such as bradykinin (BK), prostaglandins, leukotrienes, serotonin, histamine, substance P, thromboxanes, platelet-activating factor, adenosine and ATP, protons, and free radicals. During inflammation, cytokines such as interleukins, tumor necrosis factor, and neurotrophins, particularly nerve growth factor, are also generated. In general, these mediators sensitize TRPV1, increasing the probability of activation by a stimulus. The first evidence of a role of TRPV1 in peripheral pain-processing was generated by observations made in mice injected with the TRPV1 antagonist capsazepine. Capsazepine injection attenuates inflammation-induced thermal hyperalgesia [197, 212] (however, note the above comments on the selectivity of capsazepine—possible TRPA1 desensitizing effects). Trpv1−/− mice do not respond to noxious temperatures and present with an attenuation of inflammation-induced thermal hyperalgesia. In addition, Trpv1−/− mice do not experience thermal hyperalgesia after CFA administration.
When BK is released, it acts on β2 receptors, which in turn activate PKC to facilitate PIP2 hydrolysis by PLC to produce IP3 and DAG, two compounds that affect TRPV1 function [213]. DAG is a TRPV1 agonist that interacts with tyrosine 511 of its S3 domain [214]. PIP2 binds to the C-terminal end to positively regulate TRPV1 [215]. Prostaglandins increase the capsaicin-induced currents in DRG neurons and reduce the temperature activation threshold of the channel. These effects have been corroborated in Trpv1−/− mice [216]. TRPV1 integrates multiple pro-inflammatory stimuli, and for this reason, being able to modulate this pathway appears a desirable goal. For example, a natural compound, oleic acid, has recently been shown to inhibit TRPV1 activation, thus modulating pain and itch [217].
TRPV1 in Neuropathic Pain
Neuropathic pain can occur when there is an injury or dysfunction of the CNS or PNS and is a difficult condition to treat. The TRPV1 channel is a polymodal nociceptor, and it is modulated by pro-inflammatory mediators. The process of sensitization increases excitability, causing hyperalgesia and allodynia. However, there is contradictory evidence regarding the role of TRPV1 in neuropathic pain. It has been found that in Trpv1−/− mice, there is no change in pain-related behavior after nerve damage [197, 218]. In line with this finding, pharmacological TRPV1 inhibition decreases pain in several animal models of neuropathic pain [219, 220]. Interestingly, an increase in TRPV1 expression has been found in several models of neuropathic pain, such as the spinal nerve ligation model. Increased TRPV1 expression correlates with the development and maintenance of thermal hyperalgesia [221]. Furthermore, TRPV1 function has been shown to be enhanced in inflamed (local or cytokine-induced) DRGs [222]. Diabetic neuropathy models have difficulties in mimicking the clinical reality of this dreaded complication, and implications of TRPV1 and TRPA1 in diabetic neuropathic pain have been postulated here, but await an improvement of these models or translational findings in humans so that the validity of the results can be increased [223–225].
Finally, it has been found that TRPV1 plays an important role in cancer-induced chronic pain. In bone cancer, TRPV1 is overexpressed in DRG neurons, and this increased expression correlates with neuropathic pain [201]. In rat models of bone cancer, endogenous tissue-derived formaldehyde upregulates TRPV1 expression via mitogen-activated protein kinase (MAPK) and phosphoinositide kinase signaling [226] supporting the role of TRPV1 in cancer pain.
TRPV1 in Visceral Pain
TRPV1 is expressed in DRG neurons that innervate the colon, pancreas, stomach, duodenum, and bladder and is responsible for the pain produced by chronic gastrointestinal inflammation [227]. In tissue biopsies from patients with inflammatory bowel disease, there is an increased TRPV1 channel expression which correlates with the severity of hypersensitivity [228]. The administration of acetic acid and capsaicin increases the activity of afferent fibers in the pelvis in control mice and in mice with dextran sulfate sodium (DSS)-generated colitis. However, the response to capsaicin in DSS-treated mice is greater than in controls [229]. Interestingly, Trpv1−/− mice are less sensitive to these treatments, highlighting the role of TRPV1 in this type of pain [230]. In rat models of irritable bowel syndrome (IBS), treatment with TRPV1 antagonists in neonates prevents the development of sensitization in adults. TRPV1 antagonists have also been shown to be effective in reducing the sensitization after it develops in adults, further supporting a role of TRPV1 in IBS and colonic hypersensitivity [231]. In chronic pancreatitis, TRPV1 has been shown to be upregulated in both expression and function in DRG neurons, contributing to hyperalgesia, and suggesting TRPV1 as a target for treatment [232].
TRPV1 in Itch
TRPV1 is involved in both acute and chronic itch conditions [233]. For example, TPRV1 has been suggested to be involved in many chronic itch conditions like rosacea [234], atopic dermatitis [235], and prurigo nodularis, a skin condition characterized by itchy nodules on the arms and legs [236]. However, as an initial cautionary note, in humans, treatment with a TRPV1 inhibitor did not improve chronic itch in patients [237].
Histamine is released from mast cells when tissues are inflamed or stimulated by allergens. Histamine excites sensory neurons by activating TRPV1 and this activation is mediated by the production of 12-hydroxyeicosatetraenoic acid, a downstream metabolite of PLA2 and lipoxygenase (LOX) [238]. In cultured sensory neurons, histamine evokes inward currents that are reduced by capsazepine, a TRPV1 blocker, or when histamine receptor subtype 1 (H1R) and TRPV1 are expressed heterologously, but not when they are expressed separately. In addition, histamine causes Ca2+ influx into sensory neurons in wild-type mice but not in Trpv1−/− mice. When injected subcutaneously into the necks of mice, histamine causes bouts of scratching, which are greatly reduced by pretreatment with capsazepine, and by inhibitors of PLA2, LO, and H1R as well as in Trpv1−/− mice. These results suggest that TRPV1 might play a role in mediating the pruritogenic action of histamine via the PLA2/LO pathway [239].
IL-31RA (interleukin-31 receptor A) is a functional receptor expressed by a small subpopulation of neurons positive for IL-31RA, TRPV1, and TRPA1 and is a critical neuroimmune link between T-helper-2 cells and sensory neurons for the generation of T cell-mediated itch [121]. Cutaneous and intrathecal injection of IL-31 evoke intense itch, and its concentration increases significantly in the skin of mice with dermatitis. Both human and mouse DRG neurons express IL-31RA, largely in neurons that co-express TRPV1. IL-31-induced itch is significantly reduced in Trpv1−/− and Trpa1−/− mice [121].
Toll-like receptors (TLRs) are expressed in immune cells to regulate innate immunity. TLR 3, 4, and 7 are expressed in DRG neurons that co-express TRPV1, gastrin-releasing peptide (GRP), and MrgprA3 [240] [241]. Topical application of imiquimod, a TLR7 activator and an anti-viral and anti-tumor drug used to treat genital warts, often causes itch as a side-effect. TLR7 activation by imiquimod induces scratching and also generates inward currents and action potentials in DRG neurons [240]. Signaling mechanisms that link TLRs/TLR7 with TRPV1 need to be elucidated.
TRPV3 in Pain and Itch
A member of the TRP vanilloid subfamily important for temperature detection is the TRPV3 channel. This non-selective cation channel is abundantly expressed in skin (specifically in keratinocytes) [242] and has also been detected in neurons from the DRG and TG [243]. TRPV3 protein has 43% identity to TRPV1 and is a warm-sensitive but capsaicin- and low pH-insensitive ion channel [242, 243].
Among the ligands for TRPV3 activation are plant-derived compounds such as camphor, carvacrol, and thymol [244]; furthermore, TRPV3 activity is enhanced by endogenously produced compounds such as arachidonic acid which is an unsaturated fatty acid released during inflammation [245]. Unlike TRPV1 activation which requires the metabolism of this fatty acid to evoke currents, the enhancement of TRPV3 function is independent of arachidonic acid oxidation [245]. It is possible that in skin pathologies such as psoriasis where arachidonic acid levels are elevated [246], TRPV3 activation could contribute to chronic dermatitis.
Another TRPV3 agonist is farnesyl pyrophosphate (FPP), an endogenously produced intermediate metabolite of the mevalonate pathway [247]; it produces isoprenoids which can also activate TRPV3. FPP has been identified as a novel pain-producing compound through specific TRPV3 activation [248].
TRPV3 in Nociceptive and Inflammatory Pain
Although the physiological role of TRPV3 is still unclear, some studies suggest that this ion channel can contribute to pain/nociception. For example, mice overexpressing TRPV3 in keratinocytes release prostaglandin E2 and induce thermal nociception and hyperalgesia [249]. On the contrary, Trpv3−/− mice have an impaired response to warm and also noxious-warm temperatures [250].
Finally, endogenous antagonists to TRPV3 activation have been isolated. For example, compounds synthetized from the omega-3 fatty acids such as the resolvins are linked to anti-inflammatory and anti-nociceptive effects. One of them, 17(R)-resolvin D1 is a specific inhibitor of TRPV3 [26]. This resolvin affects the voltage-dependence of TRPV3, thus decreasing the channel activation [26]. The inhibitory effects of this resolvin are specifically through TRPV3 inhibition since the pain behavior induced by capsaicin or cinnamaldehyde is unaffected. However, nociceptive behavior induced by intradermal injection of FPP, a selective activator of TRPV3, is attenuated by co-injection with resolvin D1 [26].
TRPV3 in Itch
A missense mutation in TRPV3, Gly573Ser, has been found in individuals with Olmsted syndrome, a rare congenital disorder characterized by severe itching, peripheral pain, and palmo-plantar hyperkeratosis among other symptoms [251]. In mice, TRPV3Gly573Ser leads to increased ion channel activity in keratinocytes and is associated with hairlessness and a liability to develop severe dermatitis in DS-Nh mice [252]. Also, TRPV3Gly573Ser induces a higher nerve growth factor response to heat and increased scratching behavior. In transfected HEK293 cells expressing TRPV3Gly573Ser, increased inward currents have been recorded, defining TRPV3Gly573Ser as a gain-of-function mutation. This might lead to an increased likelihood of apoptosis in keratinocytes and keratinocyte hyperproliferation via Ca2+ signaling, explaining the hyperkeratosis phenotype in affected individuals [253]. Taken together, these observations in a human TRPV3 gain-of-function channelopathy suggest that TRPV3 channels regulate the activity and function of skin keratinocytes. Furthermore, TRPV3 over-activity in these cells can evoke not only over-proliferation and hyperkeratosis, but possibly also sensory phenomena, namely pathological itching and irritation/pain. However, more in-depth reverse-translation is needed to tease apart the contributions of keratinocyte TRPV3 and sensory neuron TRPV3 to Olmsted syndrome, and in general.
TRPV4 Functions as a Pain-TRP and an Itch-TRP
As a member of the TRPV subfamily, TRPV4 is a multi-modally activated, nonselective cation channel. Functional expression of TRPV4 has been detected in nerve cells and non-neuronal cells [254]. Importantly for this review, one of the initial discovery papers lays out a rationale of how TRPV4 can function in pain by demonstrating its expression in TG sensory neurons, in small-diameter neurons, and reasoning about a role for TRPV4 in pain signaling [254]. This initial conjecture has now been confirmed, e.g. a search for “TRPV4 pain” generated 185 references out of a total of 1085 references for “TRPV4”. Primary sensory neurons with TRPV4 expression include pain-sensing neurons in the DRG [8, 255, 256] and TG [257, 258] [94, 259], most recently also satellite cells in sensory ganglia [260], in the CNS for pain transmission in astrocytes [261], microglial cells [262], and neurons [263, 264, 265]. Outside the nervous system, TRPV4 has been found in innervated cells such as prominently in chondrocytes [266], vascular endothelia [267], and innervated epithelia such as skin keratinocytes [129, 268], airway epithelial cells [26, 269, 270], colonic epithelia [271], and odontoblasts [272]. Co-labeling experiments with neuronal markers and size determination have revealed that TRPV4-expressing sensory neurons are rather nociceptive [129, 273, 274], a finding in keeping with evidence that suggests that TRPV4 is involved in nociception both physiologically and in sensitized states such as inflammation and nerve injury [8, 273]. Of note, TRPV4 expression in TGs appears to be higher than that in DRGs; this is still not fully explained and how this difference correlates with sensory functions is not yet known [275, 276].
TRPV4 in Transduction of Mechanically-Evoked Pain
Trpv4−/− animals, generated after the initial description, have been found to have defective mechanosensation in the physiological, non-sensitized state [257, 275, 277], indicating a role for TRPV4 in non-sensitized mechano-transduction. This finding has been supported and extended by assessing the function of mammalian TRPV4 in the ASH head nociceptor neurons of Caenorhabditis elegans in a mutant line of animals lacking the proto-ancestral osmo-mechano-TRPV channel, OSM-9 [275] (see also [5]).
TRPV4 in Inflammatory Pain
TRPV4 in sensory neurons can be sensitized by pro-inflammatory mediators, such as prostaglandin E2 (activator of PAR2), an integrator of proteolytic signaling in inflammation, especially allergic inflammation, histamine, and/or serotonin, leading to increased nociception to hypotonic and mild hypertonic stimuli or mechanical stimuli [273, 278, 279]. Grant et al. found that TRPV4 is co-expressed with PAR2 in rat DRG neurons, so intraplantar injection of a PAR2 agonist causes mechanical hyperalgesia in mice and sensitizes pain responses in a TRPV4-dependent manner to, for example, the TRPV4 activator 4α-PDD (4α-phorbol 12,13-didecanoate) and hypotonic solutions. Deletion of Trpv4 prevents PAR2 agonist-induced mechanical hyperalgesia and sensitization [8]. Further studies have demonstrated that cathepsin-S [134] or elastase-mediated activation of PAR2 [280] activates TRPV4 and sensitizes nociceptors to function in a hypersensitive manner, resulting in inflammation and pain. In the lab of the senior author, we established a key role for TRPV4 ion channels in the pain response to temporomandibular joint (TMJ) inflammation. In Trpv4−/− mice with TMJ inflammation, attenuation of bite force, a surrogate of TMJ injury-mediated pain in humans and validly reverse-translated into mice, is significantly and robustly reduced versus WT mice. Furthermore, TRPV4 protein expression in the TG is dramatically upregulated after TMJ inflammation, in synchrony with the clinical severity of TMJ inflammatory injury, suggesting that TRPV4 expression in the TG is a critical locale for behavioral sensitization [94]. In favor of this concept, TRPV4 is expressed in sensory neurons innervating the TMJ, and TG sensory neurons expressing TRPV4 co-express CGRP and phosphorylated ERK, a signaling activation marker in response to injury. TG sensory neurons co-expressing pERK-TRPV4 become more numerous in response to TMJ inflammatory injury, suggesting that TRPV4-mediated Ca2+ influx into TG sensory neurons evokes MAPK activation in these neurons, and this functions as a molecular substrate of the sensitization response after inflammatory injury. Interestingly, we also found that peripheral injury to the joint after inflammatory injury is independent of the genotype, whereas the pain response is strikingly dependent on TRPV4 or the ability of the animal to generate phosphorylated ERK in neurons. In another study by Denadai-Souza et al., TRPV4 expression in the TG was demonstrated, as well as in TMJ synovial cells [117, 281]. Taken together, it has emerged that TRPV4 plays an important role in inflammatory pain of the TMJ and at the level of sensory neurons innervating a peripheral joint and TRPV4-expressing cells. TMJ disorder is a prevalent craniofacial pain disorder, and there is an increase in the prevalence of age- and obesity-associated osteoarthritis and post-traumatic osteoarthritis [282] which suggests an increasing unmet medical need. Hence, TRPV4 has become an attractive therapeutic target to address TMJ disorder, and possibly additional forms of joint pain.
The role of TRPV4 in facilitating and promoting inflammation and pain has also been supported by observations in a mouse model of skin inflammation in which UVB radiation generates sunburn tissue damage and the associated pathological pain. Following UVB over-exposure of the hindpaws, an area of mouse skin with a greater resemblance to human skin, mice with induced Trpv4 deletion in keratinocytes, as well as mice treated with topical administration of TRPV4 inhibitors to the hindpaws, become virtually resistant to noxious thermal and mechanical stimuli versus control animals, and further showed dramatically reduced skin inflammation. These findings indicate that TRPV4-expressing keratinocytes of the hindpaw can “moonlight” as non-neural sensing cells and pain-generating cells. In other words, activation of TRPV4 channels in skin keratinocytes, not in innervating sensory neurons, suffices to switch on neural pathological pain circuits and response mechanisms. Importantly, we demonstrated a form of non-neural phototransduction in response to UVB radiation in skin keratinocytes, completely dependent on TRPV4. Exploring a possible underlying mechanism, we found that epidermal keratinocyte TRPV4 is essential for UVB-evoked skin tissue damage and increased expression of the pro-pain (also pro-itch) mediator ET-1 [283]. Importantly, we also recorded an important negative finding which will become the starting point for relevant future studies, namely that keratinocyte-derived ET-1, dependent on TRPV4 function and UVB-mediated activation in these cells, is not the algogenic signal to innervating peripheral nerve projections.
TRPV4 in Neuropathic Pain
TRPV4 has been implicated in nerve pain in several preclinical rodent pain models, such as paclitaxel-induced neural injury leading to painful peripheral neuropathy (CIPN) and chronic mechanical compression-injury of the DRG [270, 284, 285]. Cerebrospinal administration of antisense oligodeoxynucleotides specific for TRPV4, which reduce the expression of TRPV4 in sensory neurons, attenuates paclitaxel-induced mechanical hyperalgesia as well as hyperalgesia caused by hypotonicity [284]. TRPV4 appears to be co-expressed with TRPC1 and TRPC6 in DRG neurons, and it has been proposed that TRPC1 and TRPC6 may act in concert with TRPV4 to mediate the mechanical hyperalgesia induced by paclitaxel and cis-platin [270], possibly representing a more general mechanism of pain in CIPN. In addition, paclitaxel can stimulate the release of mast cell tryptase, which activates PAR2 and subsequently PKA and PKC, resulting in mechanical and thermal hypersensitivity through TRPV4 sensitization [285]. These results suggest that TRPV4 plays an important role in painful peripheral neuropathies caused by paclitaxel and related taxanes, affecting more than half of taxane-treated cancer patients. This is a large unmet medical need which not only translates to patients’ quality-of-life, but indeed to their ability to successfully undergo adjuvant chemotherapy and thus emerge with increased years of survival. We reach the same conclusion as for joint- or skin-mediated forms of pain and inflammation, as discussed above, namely that TRPV4 is a promising target for anti-pain therapy. To that end, our group has recently developed novel TRPV4-inhibiting compounds [286]. Two of our compounds also potently co-inhibit TRPA1 ion channels, which could be a highly beneficial combination for CIPN pain, as well as for other forms of pain reviewed in detail below, namely visceral pain of the colon and pancreas and headaches [286].
TRPV4 is involved in mediating mechanical allodynia in preclinical models of chronic compression of the DRG (CCD model) [256]. After CCD, Trpv4 mRNA and protein expression increase compared to the sham group, with the highest level at 7 days post-CCD. Knockdown of TRPV4 partly reverses the CCD-induced mechanical allodynia. CCD rats show thermal hyperalgesia and increased nitrite production [287]. The thermal hyperalgesia is reduced by intrathecal blockers targeted at any site along the TRPV4-NO-cGMP-PKG pathway. Inhibition of TRPV4 and/or nitric oxide synthase decreases the nitrite production in the DRGs of CCD rats. Changes in the level of nitrite are positively associated with the changes in thermal hyperalgesia. Identified signaling pathways involved in the CCD neuropathic pain model include NF-kappa-B, which mediates the TRPV4-NO pathway [288] and the p38 pathway [289].
TRPV4 in Visceral Pain
Trpv4 mRNA is enriched in sensory neurons innervating the colon and TRPV4 protein is co-localized in a subset of fibers with CGRP, the substrate of neurogenic inflammation in mice. Mechanosensory responses of colonic serosal and mesenteric afferents are enhanced by a TRPV4 agonist and are dramatically reduced in Trpv4−/− mice. The behavioral responses to noxious colonic distention are also substantially reduced in mice lacking Trpv4 [290]. Similarly, another study found that the TRPV4 agonist 4α-phorbol 12,13-didecanoate (4α-PDD) specifically activates a cationic current and Ca2+ influx in DRG neurons innervating the colon and causes dose-dependent visceral hypersensitivity. Intrathecal administration of TRPV4-targeted but not mismatched siRNA is effective in reducing basal visceral nociception, and the TRPV4-activator 4α-PDD or PAR2 agonists induce hypersensitivity [255]. PAR2 exacerbates visceromotor responses, an indicator of mechanical hyperalgesia, and this is absent in Trpv4−/− mice, indicating that TRPV4 is required for PAR2-induced mechanical hyperalgesia in the colon, and the excitation of sensory neurons innervating the colon [291]. A more recent study has shown that both TRPV4 and TRPA1 mediate colonic distension pain and CGRP release. Unlike TRPV4 and TRPA1, the role of TRPM8 seems to be confined to signaling more extreme noxious distension [179]. Interestingly in this context, levels of the TRPV4 agonist 5′,6′-epoxyeicosatrienoic acid are increased in IBS colon biopsies. The increases correlate with pain and bloating scores. Small interfering RNA knockdown of TRPV4 in mouse primary afferent neurons inhibits the hypersensitivity caused by supernatant from IBS biopsies in mice. Polyunsaturated fatty acid metabolites extracted from IBS biopsies or the colons of mice with visceral hypersensitivity activate mouse sensory neurons in culture via TRPV4 [292]. These data indicate that TRPV4 contributes to visceral pain, and is relevant to human disease.
Of note, TRPV4 also plays an important role in pancreatitis pain, a large unmet clinical need in visceral pain. Immunoreactive TRPV4 has been detected in pancreatic nerve fibers and in DRG neurons innervating the pancreas, as identified by retrograde tracing. Activation of TRPV4 with 4α-PDD increases the intracellular Ca2+ in these neurons in culture. The secretagogue cerulein induces chemical pancreatitis, c-Fos expression in spinal cord dorsal horn neurons in segments innervating the pancreas, and pancreatitis pain behavior. All of these phenomena are suppressed in Trpv4−/− mice [293]. Using a chronic pancreatitis model induced by a high-fat and alcohol diet (HFA), Zhang et al. demonstrated that TRPV4 expression is increased in pancreatic stellate cells (PSCs). The Ca2+ signal in PSCs from HFA-fed rats in response to 4α-PDD is dramatically higher than that of cells from control rats. Tumor necrosis factor-α increases responses to the TRPV4-activator 4α-PDD in control PSCs [294]. Furthermore, inhibition of TRPV4 by systemic injection of the selective blocker HC067047 effectively alleviates the mechanical and thermal hypersensitivity of rats with HFA-induced chronic pancreatitis in a dose-dependent manner [295]. In the laboratory of the senior author, we recently found that blockade of both TRPV4 and TRPA1 by intraperitoneal injection of the dual inhibitor compound 16-8 dramatically attenuates the pancreatic edema and inflammation induced by caerulein. Furthermore, serum amylase, a marker of inflammatory injury of the pancreas is also significantly reduced by 16-8 treatment. Perhaps most importantly, pancreatitis pain behavior is virtually eliminated in response to compound 16-8 [286]. Using this well-established pre-clinical model, these studies provide evidence that dual channel inhibitors targeting TRPV4 and TRPA1 might be a rewarding new concept to meet the grave unmet clinical need of pancreatitis pain.
TRPV4 in Craniofacial Pain and Headache
Using in vitro patch-clamp electrophysiology of trigeminal neurons retrogradely labeled from the dura, around half of identified dural afferents generated currents in response to hypotonic solutions and 4α-PDD, indicating robust dural innervation via TRPV4. This is relevant because of the known involvement of TRPV4 in mechanotransduction, and its sensitization to mechanical cues under inflammatory conditions. Activation of meningeal TPRV4 using hypotonic solution or 4α-PDD in vivo results in facial allodynia that is blocked by the TRPV4 antagonist RN1734 [296]. These data indicate that activation of TRPV4 within the meninges produces afferent nociceptive signaling from the head that may contribute to migraine headache. In the laboratory of the senior author, we recently found TRPV4 to be important for the trigeminal nocifensive behavior evoked by formalin whisker-pad injection. This conclusion is supported by studies with Trpv4−/− mice and the TRPV4-specific antagonists GSK205 and HC067047 [268]. Our results suggest that TRPV4 acts as a relevant signaling molecule in irritation-evoked trigeminal pain, at the level of a functional formalin receptor in TG sensory neurons. TRPV4-antagonistic therapies can therefore be envisioned as novel analgesics, possibly for specific targeting of trigeminal pain disorders, including headaches, with a mandate to further elucidate the pathophysiology but also to continue translational medical development.
Taken together, based on the role of TRPV4 in pain as currently understood, future studies are needed to address many important questions, a few of which are: (1) how sensory-neuronal TRPV4 signaling in a pain-relevant manner, particularly pathological pain, can be readily addressed via appropriate genetic targeting strategies; (2) what is the co-contributory role of glial TRPV4 [261] in pain; (3) how TRPV4 functionally interacts and mutually modulates pain with other pain-TRP channels, in particular TRPA1. Of note, there is an overlap in TRPV4-TRPA1 function in several important and relevant forms of pain, such as visceral pain of the colon and pancreas, chemotherapy-induced peripheral neuropathic pain (especially that mediated by taxane), headache, formalin-evoked pain as an important model, mechanically-evoked pain in general, and chronic cough as an upper airway equivalent of chronic pain [57, 78, 179, 255, 285, 286, 290–292, 297].
TRPV4 in Itch
The finding of TRPV4-dependent secretion of the pruritogen ET-1 by keratinocytes led the senior author’s group to question whether TRPV4 plays a role in itch, in particular whether TRPV4 in keratinocytes of the epidermis can drive scratching behavior [283]. To address this question, the focus was first on acute itch, and specifically to examine prototypic examples of histaminergic itch, including ET-1-evoked itch plus chloroquine-induced non-histaminergic itch as control. In this study, an exciting new function of TRPV4 in forefront itch transduction from the integument was reported, namely that TRPV4 in epidermal keratinocytes functions as a pruriceptor-TRP channel in acute histaminergic itch, including itch evoked by ET-1, but not in non-histaminergic itch evoked by chloroquine. Direct activation of TRPV4 channels also evokes scratching behavior which appears to be completely dependent on TRPV4 expression in keratinocytes, thus underscoring the role of this cell and its expression of TRPV4 in itch [131]. Complementary findings in Trpv4 keratinocyte-specific inducible knockout mice have shown that Ca2+ transients in response to histaminergic pruritogens in cultured primary keratinocytes depend on TRPV4. Ca2+ influx via TRPV4 then up-regulates phosphorylation of the MAP-kinase ERK in keratinocytes. Consequently, topical transdermal treatment with a selective inhibitor of TRPV4 is effective as an anti-pruritogen. Moreover, we found similar in vivo anti-pruritic effects when topically targeting MEK, upstream of ERK, with a selective inhibitor.
Serotonin (5-hydroxytryptamine [74])-induced itch has been shown to be associated with TRPV4 [166]. Approximately 90% of 5-HT-sensitive DRG neurons assessed by Ca2+ imaging are immunoreactive for TRPV4. TRPV4-knockout mice exhibit significantly fewer 5-HT-evoked scratching bouts than wild-type mice. Pretreatment with a TRPV4 antagonist significantly attenuates 5-HT-evoked scratching in vivo. In cultured primary DRG neurons from Trpv4−/− mice, the response to 5-HT application is attenuated. A TRPV4 antagonist suppresses the 5-HT-evoked responses in DRG cells from wild-type mice. These results indicate that 5-HT-induced itch is linked to TRPV4. Extending on the role of TRPV4 in itch, TRPV4 signaling in subgroups of DRG neurons has been suggested to be facilitated by TRPV1 in itch [298]. Both of these studies, on the role of serotonin in itch, and the suggested TRPV4/TRPA1 co-signaling, are interesting and push back the knowledge barrier, yet there are clear limitations on the use of Trpv1−/− and Trpv4−/− pan-null animals as key tools, in contrast to cell-type specific and inducible genetically-engineered animals.
Concluding Remarks
Nociception is an important physiological process for detecting harmful signals that results in pain and itch perception. Here, we have reviewed important experimental evidence involving some TRP ion channels as molecular sensors of chemical, thermal, and mechanical noxious stimuli to evoke pain and itch sensation. Among them are the TRPA1 channel, members of the vanilloid subfamily (TRPV1, TRPV3, and TRPV4), and finally members of the melastatin group (TRPM2, TRPM3, and TRPM8).
Given that pain is a pro-survival, evolutionarily-honed protective mechanism, care has to be exercised when developing inhibitory/modulatory compounds targeting specific pain/itch-TRPs so that protective physiological mechanisms are not disabled to a degree that stimulus-mediated injury can occur. Such events have impeded the development of safe and effective TRPV1-modulating compounds and diverted substantial resources. A beneficial outcome can be readily accomplished via simple dosing strategies, and also by the incorporation of medicinal chemistry design features during compound design and synthesis. Beyond clinical use, where compounds that target more than one channel might have a place, possibly having advantageous features, highly specific and high-potency compounds will be helpful in mechanistic discovery at the structure-function level.
Acknowledgements
All the research works cited in this review were supported by the National Institutes of Health, USA (DE018549, UL1TR001117, P30AR066527, and AR48182 to WL, AR48182-S1 to WL as co-investigator; F33DE024668 and K12DE022793 to YC), the US Department of Defense (W81XWH-13-1-0299 to WL), and the Harrington Discovery Institute, Cleveland OH (to WL).
Author Contributions
All authors wrote the paper and approved the final version.
Contributor Information
Yong Chen, Email: yong.chen@duke.edu.
Wolfgang B. Liedtke, Email: liedt001@duke.edu
References
- 1.Dhand A, Aminoff MJ. The neurology of itch. Brain. 2014;137:313–322. doi: 10.1093/brain/awt158. [DOI] [PubMed] [Google Scholar]
- 2.Nilius B, Flockerzi V, editors. Mammalian Transient Receptor Potential (TRP) Cation Channels. Berlin Heidelberg: Springer-Verlag; 2014. [PubMed] [Google Scholar]
- 3.Julius D. TRP channels and pain. Annu Rev Cell Dev Biol. 2013;29:355–384. doi: 10.1146/annurev-cellbio-101011-155833. [DOI] [PubMed] [Google Scholar]
- 4.Wu LJ, Sweet TB, Clapham DE. International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol Rev. 2010;62:381–404. doi: 10.1124/pr.110.002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindy AS, Parekh PK, Zhu R, Kanju P, Chintapalli SV, Tsvilovskyy V, et al. TRPV channel-mediated calcium transients in nociceptor neurons are dispensable for avoidance behaviour. Nat Commun. 2014;5:4734. doi: 10.1038/ncomms5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Owsianik G, Talavera K, Voets T, Nilius B. Permeation and selectivity of TRP channels. Annu Rev Physiol. 2006;68:685–717. doi: 10.1146/annurev.physiol.68.040204.101406. [DOI] [PubMed] [Google Scholar]
- 7.Owsianik G, D’Hoedt D, Voets T, Nilius B. Structure-function relationship of the TRP channel superfamily. Rev Physiol Biochem Pharmacol. 2006;156:61–90. [PubMed] [Google Scholar]
- 8.Grant AD, Cottrell GS, Amadesi S, Trevisani M, Nicoletti P, Materazzi S, et al. Protease-activated receptor 2 sensitizes the transient receptor potential vanilloid 4 ion channel to cause mechanical hyperalgesia in mice. J Physiol. 2007;578:715–733. doi: 10.1113/jphysiol.2006.121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaquemar D, Schenker T, Trueb B. An ankyrin-like protein with transmembrane domains is specifically lost after oncogenic transformation of human fibroblasts. J Biol Chem. 1999;274:7325–7333. doi: 10.1074/jbc.274.11.7325. [DOI] [PubMed] [Google Scholar]
- 10.Latorre R, Zaelzer C, Brauchi S. Structure-functional intimacies of transient receptor potential channels. Q Rev Biophys. 2009;42:201–246. doi: 10.1017/S0033583509990072. [DOI] [PubMed] [Google Scholar]
- 11.Gees M, Colsoul B, Nilius B. The role of transient receptor potential cation channels in Ca2+ signaling. Cold Spring Harb Perspect Biol. 2010;2:a003962. doi: 10.1101/cshperspect.a003962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J, Kang D, Xu J, Lake M, Hogan JO, Sun C, et al. Species differences and molecular determinant of TRPA1 cold sensitivity. Nat Commun. 2013;4:2501. doi: 10.1038/ncomms3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karashima Y, Talavera K, Everaerts W, Janssens A, Kwan KY, Vennekens R, et al. TRPA1 acts as a cold sensor in vitro and in vivo. Proc Natl Acad Sci U S A. 2009;106:1273–1278. doi: 10.1073/pnas.0808487106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKemy DD. How cold is it? TRPM8 and TRPA1 in the molecular logic of cold sensation. Mol Pain. 2005;1:16. doi: 10.1186/1744-8069-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moldenhauer H, Latorre R, Grandl J. The pore-domain of TRPA1 mediates the inhibitory effect of the antagonist 6-methyl-5-(2-(trifluoromethyl)phenyl)-1H-indazole. PLoS One. 2014;9:e106776. doi: 10.1371/journal.pone.0106776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jabba S, Goyal R, Sosa-Pagan JO, Moldenhauer H, Wu J, Kalmeta B, et al. Directionality of temperature activation in mouse TRPA1 ion channel can be inverted by single-point mutations in ankyrin repeat six. Neuron. 2014;82:1017–1031. doi: 10.1016/j.neuron.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, et al. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- 18.Caspani O, Zurborg S, Labuz D, Heppenstall PA. The contribution of TRPM8 and TRPA1 channels to cold allodynia and neuropathic pain. PLoS One. 2009;4:e7383. doi: 10.1371/journal.pone.0007383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nozadze I, Tsiklauri N, Gurtskaia G, Tsagareli MG. Role of thermo TRPA1 and TRPV1 channels in heat, cold, and mechanical nociception of rats. Behav Pharmacol. 2016;27:29–36. doi: 10.1097/FBP.0000000000000176. [DOI] [PubMed] [Google Scholar]
- 20.Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 21.Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Hogestatt ED, et al. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci U S A. 2005;102:12248–12252. doi: 10.1073/pnas.0505356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Macpherson LJ, Geierstanger BH, Viswanath V, Bandell M, Eid SR, Hwang S, et al. The pungency of garlic: activation of TRPA1 and TRPV1 in response to allicin. Curr Biol. 2005;15:929–934. doi: 10.1016/j.cub.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 23.Hinman A, Chuang HH, Bautista DM, Julius D. TRP channel activation by reversible covalent modification. Proc Natl Acad Sci U S A. 2006;103:19564–19568. doi: 10.1073/pnas.0609598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, et al. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445:541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- 25.Materazzi S, Fusi C, Benemei S, Pedretti P, Patacchini R, Nilius B, et al. TRPA1 and TRPV4 mediate paclitaxel-induced peripheral neuropathy in mice via a glutathione-sensitive mechanism. Pflugers Arch. 2012;463:561–569. doi: 10.1007/s00424-011-1071-x. [DOI] [PubMed] [Google Scholar]
- 26.Bang S, Yoo S, Yang TJ, Cho H, Hwang SW. 17(R)-resolvin D1 specifically inhibits transient receptor potential ion channel vanilloid 3 leading to peripheral antinociception. Br J Pharmacol. 2012;165:683–692. doi: 10.1111/j.1476-5381.2011.01568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu H, Delling M, Jun JC, Clapham DE. Oregano, thyme and clove–derived flavors and skin sensitizers activate specific TRP channels. Nat Neurosci. 2006;9:628–635. doi: 10.1038/nn1692. [DOI] [PubMed] [Google Scholar]
- 28.Escalera J, von Hehn CA, Bessac BF, Sivula M, Jordt SE. TRPA1 mediates the noxious effects of natural sesquiterpene deterrents. J Biol Chem. 2008;283:24136–24144. doi: 10.1074/jbc.M710280200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang MQ, Ye LL, Liu XL, Qi XM, Lv JD, Wang G, et al. Gingerol activates noxious cold ion channel TRPA1 in gastrointestinal tract. Chin J Nat Med. 2016;14:434–440. doi: 10.1016/S1875-5364(16)30040-1. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, et al. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J Comp Neurol. 2005;493:596–606. doi: 10.1002/cne.20794. [DOI] [PubMed] [Google Scholar]
- 31.Nassenstein C, Kwong K, Taylor-Clark T, Kollarik M, Macglashan DM, Braun A, et al. Expression and function of the ion channel TRPA1 in vagal afferent nerves innervating mouse lungs. J Physiol. 2008;586:1595–1604. doi: 10.1113/jphysiol.2007.148379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor-Clark TE, Undem BJ. Ozone activates airway nerves via the selective stimulation of TRPA1 ion channels. J Physiol. 2010;588:423–433. doi: 10.1113/jphysiol.2009.183301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guimaraes MZP, Jordt SE. TRPA1: A sensory channel of many talents. In: Liedtke WB, Heller S, editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton (FL): CRC Press/Taylor & Francis; 2007. [Google Scholar]
- 34.Bessac BF, Jordt SE. Breathtaking TRP channels: TRPA1 and TRPV1 in airway chemosensation and reflex control. Physiology (Bethesda) 2008;23:360–370. doi: 10.1152/physiol.00026.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bessac BF, Sivula M, von Hehn CA, Escalera J, Cohn L, Jordt SE. TRPA1 is a major oxidant sensor in murine airway sensory neurons. J Clin Invest. 2008;118:1899–1910. doi: 10.1172/JCI34192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 37.Andre E, Campi B, Materazzi S, Trevisani M, Amadesi S, Massi D, et al. Cigarette smoke-induced neurogenic inflammation is mediated by alpha, beta-unsaturated aldehydes and the TRPA1 receptor in rodents. J Clin Invest. 2008;118:2574–2582. doi: 10.1172/JCI34886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kichko TI, Kobal G, Reeh PW. Cigarette smoke has sensory effects through nicotinic and TRPA1 but not TRPV1 receptors on the isolated mouse trachea and larynx. Am J Physiol Lung Cell Mol Physiol. 2015;309:L812–L820. doi: 10.1152/ajplung.00164.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brone B, Peeters PJ, Marrannes R, Mercken M, Nuydens R, Meert T, et al. Tear gasses CN, CR, and CS are potent activators of the human TRPA1 receptor. Toxicol Appl Pharmacol. 2008;231:150–156. doi: 10.1016/j.taap.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 40.Bessac BF, Sivula M, von Hehn CA, Caceres AI, Escalera J, Jordt SE. Transient receptor potential ankyrin 1 antagonists block the noxious effects of toxic industrial isocyanates and tear gases. FASEB J. 2009;23:1102–1114. doi: 10.1096/fj.08-117812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gu Q, Lin RL. Heavy metals zinc, cadmium, and copper stimulate pulmonary sensory neurons via direct activation of TRPA1. J Appl Physiol. 1985;2010(108):891–897. doi: 10.1152/japplphysiol.01371.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andersson DA, Gentry C, Moss S, Bevan S. Clioquinol and pyrithione activate TRPA1 by increasing intracellular Zn2+ Proc Natl Acad Sci U S A. 2009;106:8374–8379. doi: 10.1073/pnas.0812675106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu H, Bandell M, Petrus MJ, Zhu MX, Patapoutian A. Zinc activates damage-sensing TRPA1 ion channels. Nat Chem Biol. 2009;5:183–190. doi: 10.1038/nchembio.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hazari MS, Haykal-Coates N, Winsett DW, Krantz QT, King C, Costa DL, et al. TRPA1 and sympathetic activation contribute to increased risk of triggered cardiac arrhythmias in hypertensive rats exposed to diesel exhaust. Environ Health Perspect. 2011;119:951–957. doi: 10.1289/ehp.1003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kurhanewicz N, McIntosh-Kastrinsky R, Tong H, Ledbetter A, Walsh L, Farraj A, et al. TRPA1 mediates changes in heart rate variability and cardiac mechanical function in mice exposed to acrolein. Toxicol Appl Pharmacol. 2017;324:51–60. doi: 10.1016/j.taap.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 47.Knowlton WM, Bifolck-Fisher A, Bautista DM, McKemy DD. TRPM8, but not TRPA1, is required for neural and behavioral responses to acute noxious cold temperatures and cold-mimetics in vivo. Pain. 2010;150:340–350. doi: 10.1016/j.pain.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Oliveira C, Garami A, Lehto SG, Pakai E, Tekus V, Pohoczky K, et al. Transient receptor potential channel ankyrin-1 is not a cold sensor for autonomic thermoregulation in rodents. J Neurosci. 2014;34:4445–4452. doi: 10.1523/JNEUROSCI.5387-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Touska F, Winter Z, Mueller A, Vlachova V, Larsen J, Zimmermann K. Comprehensive thermal preference phenotyping in mice using a novel automated circular gradient assay. Temperature (Austin) 2016;3:77–91. doi: 10.1080/23328940.2015.1135689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen J, Joshi SK, DiDomenico S, Perner RJ, Mikusa JP, Gauvin DM, et al. Selective blockade of TRPA1 channel attenuates pathological pain without altering noxious cold sensation or body temperature regulation. Pain. 2011;152:1165–1172. doi: 10.1016/j.pain.2011.01.049. [DOI] [PubMed] [Google Scholar]
- 51.Zappia KJ, O’Hara CL, Moehring F, Kwan KY, Stucky CL. Sensory neuron-specific deletion of TRPA1 results in mechanical cutaneous sensory deficits. eNeuro 2017, 4. [DOI] [PMC free article] [PubMed]
- 52.Yarmolinsky DA, Peng Y, Pogorzala LA, Rutlin M, Hoon MA, Zuker CS. Coding and plasticity in the mammalian thermosensory system. Neuron. 2016;92:1079–1092. doi: 10.1016/j.neuron.2016.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, et al. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 54.Babes A, Sauer SK, Moparthi L, Kichko TI, Neacsu C, Namer B, et al. Photosensitization in porphyrias and photodynamic therapy involves TRPA1 and TRPV1. J Neurosci. 2016;36:5264–5278. doi: 10.1523/JNEUROSCI.4268-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moparthi L, Survery S, Kreir M, Simonsen C, Kjellbom P, Hogestatt ED, et al. Human TRPA1 is intrinsically cold- and chemosensitive with and without its N-terminal ankyrin repeat domain. Proc Natl Acad Sci U S A. 2014;111:16901–16906. doi: 10.1073/pnas.1412689111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Laursen WJ, Anderson EO, Hoffstaetter LJ, Bagriantsev SN, Gracheva EO. Species-specific temperature sensitivity of TRPA1. Temperature (Austin) 2015;2:214–226. doi: 10.1080/23328940.2014.1000702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cattaruzza F, Spreadbury I, Miranda-Morales M, Grady EF, Vanner S, Bunnett NW. Transient receptor potential ankyrin-1 has a major role in mediating visceral pain in mice. Am J Physiol Gastrointest Liver Physiol. 2010;298:G81–G91. doi: 10.1152/ajpgi.00221.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vetter I, Touska F, Hess A, Hinsbey R, Sattler S, Lampert A, et al. Ciguatoxins activate specific cold pain pathways to elicit burning pain from cooling. EMBO J. 2012;31:3795–3808. doi: 10.1038/emboj.2012.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Obata K, Katsura H, Mizushima T, Yamanaka H, Kobayashi K, Dai Y, et al. TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J Clin Invest. 2005;115:2393–2401. doi: 10.1172/JCI25437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nassini R, Gees M, Harrison S, De Siena G, Materazzi S, Moretto N, et al. Oxaliplatin elicits mechanical and cold allodynia in rodents via TRPA1 receptor stimulation. Pain. 2011;152:1621–1631. doi: 10.1016/j.pain.2011.02.051. [DOI] [PubMed] [Google Scholar]
- 61.Zhao M, Isami K, Nakamura S, Shirakawa H, Nakagawa T, Kaneko S. Acute cold hypersensitivity characteristically induced by oxaliplatin is caused by the enhanced responsiveness of TRPA1 in mice. Mol Pain. 2012;8:55. doi: 10.1186/1744-8069-8-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.del Camino D, Murphy S, Heiry M, Barrett LB, Earley TJ, Cook CA, et al. TRPA1 contributes to cold hypersensitivity. J Neurosci. 2010;30:15165–15174. doi: 10.1523/JNEUROSCI.2580-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miyake T, Nakamura S, Zhao M, So K, Inoue K, Numata T, et al. Cold sensitivity of TRPA1 is unveiled by the prolyl hydroxylation blockade-induced sensitization to ROS. Nat Commun. 2016;7:12840. doi: 10.1038/ncomms12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wei H, Hamalainen MM, Saarnilehto M, Koivisto A, Pertovaara A. Attenuation of mechanical hypersensitivity by an antagonist of the TRPA1 ion channel in diabetic animals. Anesthesiology. 2009;111:147–154. doi: 10.1097/ALN.0b013e3181a1642b. [DOI] [PubMed] [Google Scholar]
- 65.Koivisto A, Hukkanen M, Saarnilehto M, Chapman H, Kuokkanen K, Wei H, et al. Inhibiting TRPA1 ion channel reduces loss of cutaneous nerve fiber function in diabetic animals: sustained activation of the TRPA1 channel contributes to the pathogenesis of peripheral diabetic neuropathy. Pharmacol Res. 2012;65:149–158. doi: 10.1016/j.phrs.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 66.Ohkawara S, Tanaka-Kagawa T, Furukawa Y, Jinno H. Methylglyoxal activates the human transient receptor potential ankyrin 1 channel. J Toxicol Sci. 2012;37:831–835. doi: 10.2131/jts.37.831. [DOI] [PubMed] [Google Scholar]
- 67.Andersson DA, Gentry C, Light E, Vastani N, Vallortigara J, Bierhaus A, et al. Methylglyoxal evokes pain by stimulating TRPA1. PLoS One. 2013;8:e77986. doi: 10.1371/journal.pone.0077986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Petrus M, Peier AM, Bandell M, Hwang SW, Huynh T, Olney N, et al. A role of TRPA1 in mechanical hyperalgesia is revealed by pharmacological inhibition. Mol Pain. 2007;3:40. doi: 10.1186/1744-8069-3-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.de la Roche J, Eberhardt MJ, Klinger AB, Stanslowsky N, Wegner F, Koppert W, et al. The molecular basis for species-specific activation of human TRPA1 protein by protons involves poorly conserved residues within transmembrane domains 5 and 6. J Biol Chem. 2013;288:20280–20292. doi: 10.1074/jbc.M113.479337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schwarz MG, Namer B, Reeh PW, Fischer MJM. TRPA1 and TRPV1 antagonists do not inhibit human acidosis-induced pain. J Pain. 2017;18:526–534. doi: 10.1016/j.jpain.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 71.Trevisani M, Siemens J, Materazzi S, Bautista DM, Nassini R, Campi B, et al. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci U S A. 2007;104:13519–13524. doi: 10.1073/pnas.0705923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Andersson DA, Gentry C, Moss S, Bevan S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J Neurosci. 2008;28:2485–2494. doi: 10.1523/JNEUROSCI.5369-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nozawa K, Kawabata-Shoda E, Doihara H, Kojima R, Okada H, Mochizuki S, et al. TRPA1 regulates gastrointestinal motility through serotonin release from enterochromaffin cells. Proc Natl Acad Sci U S A. 2009;106:3408–3413. doi: 10.1073/pnas.0805323106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bellono NW, Bayrer JR, Leitch DB, Castro J, Zhang C, O’Donnell TA, et al. Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways. Cell. 2017;170(185–198):e116. doi: 10.1016/j.cell.2017.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu B, Escalera J, Balakrishna S, Fan L, Caceres AI, Robinson E, et al. TRPA1 controls inflammation and pruritogen responses in allergic contact dermatitis. FASEB J. 2013;27:3549–3563. doi: 10.1096/fj.13-229948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Trankner D, Hahne N, Sugino K, Hoon MA, Zuker C. Population of sensory neurons essential for asthmatic hyperreactivity of inflamed airways. Proc Natl Acad Sci U S A. 2014;111:11515–11520. doi: 10.1073/pnas.1411032111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yanaga A, Goto H, Nakagawa T, Hikiami H, Shibahara N, Shimada Y. Cinnamaldehyde induces endothelium-dependent and -independent vasorelaxant action on isolated rat aorta. Biol Pharm Bull. 2006;29:2415–2418. doi: 10.1248/bpb.29.2415. [DOI] [PubMed] [Google Scholar]
- 78.Nassini R, Materazzi S, Vriens J, Prenen J, Benemei S, De Siena G, et al. The ‘headache tree’ via umbellulone and TRPA1 activates the trigeminovascular system. Brain. 2012;135:376–390. doi: 10.1093/brain/awr272. [DOI] [PubMed] [Google Scholar]
- 79.Edelmayer RM, Le LN, Yan J, Wei X, Nassini R, Materazzi S, et al. Activation of TRPA1 on dural afferents: a potential mechanism of headache pain. Pain. 2012;153:1949–1958. doi: 10.1016/j.pain.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kunkler PE, Ballard CJ, Pellman JJ, Zhang L, Oxford GS, Hurley JH. Intraganglionic signaling as a novel nasal-meningeal pathway for TRPA1-dependent trigeminovascular activation by inhaled environmental irritants. PLoS One. 2014;9:e103086. doi: 10.1371/journal.pone.0103086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kunkler PE, Zhang L, Pellman JJ, Oxford GS, Hurley JH. Sensitization of the trigeminovascular system following environmental irritant exposure. Cephalalgia. 2015;35:1192–1201. doi: 10.1177/0333102415574845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kunkler PE, Ballard CJ, Oxford GS, Hurley JH. TRPA1 receptors mediate environmental irritant-induced meningeal vasodilatation. Pain. 2011;152:38–44. doi: 10.1016/j.pain.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Benemei S, Fusi C, Trevisan G, Geppetti P. The TRPA1 channel in migraine mechanism and treatment. Br J Pharmacol. 2014;171:2552–2567. doi: 10.1111/bph.12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Benemei S, De Logu F, Li Puma S, Marone IM, Coppi E, Ugolini F, et al. The anti-migraine component of butterbur extracts, isopetasin, desensitizes peptidergic nociceptors by acting on TRPA1 cation channel. Br J Pharmacol 2017. [DOI] [PMC free article] [PubMed]
- 85.Materazzi S, Benemei S, Fusi C, Gualdani R, De Siena G, Vastani N, et al. Parthenolide inhibits nociception and neurogenic vasodilatation in the trigeminovascular system by targeting the TRPA1 channel. Pain. 2013;154:2750–2758. doi: 10.1016/j.pain.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nassini R, Materazzi S, Benemei S, Geppetti P. The TRPA1 channel in inflammatory and neuropathic pain and migraine. Rev Physiol Biochem Pharmacol. 2014;167:1–43. doi: 10.1007/112_2014_18. [DOI] [PubMed] [Google Scholar]
- 87.Caceres AI, Brackmann M, Elia MD, Bessac BF, del Camino D, D’Amours M, et al. A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma. Proc Natl Acad Sci U S A. 2009;106:9099–9104. doi: 10.1073/pnas.0900591106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hox V, Vanoirbeek JA, Alpizar YA, Voedisch S, Callebaut I, Bobic S, et al. Crucial role of transient receptor potential ankyrin 1 and mast cells in induction of nonallergic airway hyperreactivity in mice. Am J Respir Crit Care Med. 2013;187:486–493. doi: 10.1164/rccm.201208-1358OC. [DOI] [PubMed] [Google Scholar]
- 89.Trevisan G, Hoffmeister C, Rossato MF, Oliveira SM, Silva MA, Ineu RP, et al. Transient receptor potential ankyrin 1 receptor stimulation by hydrogen peroxide is critical to trigger pain during monosodium urate-induced inflammation in rodents. Arthritis Rheum. 2013;65:2984–2995. doi: 10.1002/art.38112. [DOI] [PubMed] [Google Scholar]
- 90.Horvath A, Tekus V, Boros M, Pozsgai G, Botz B, Borbely E, et al. Transient receptor potential ankyrin 1 (TRPA1) receptor is involved in chronic arthritis: in vivo study using TRPA1-deficient mice. Arthritis Res Ther. 2016;18:6. doi: 10.1186/s13075-015-0904-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Moilanen LJ, Hamalainen M, Lehtimaki L, Nieminen RM, Moilanen E. Urate crystal induced inflammation and joint pain are reduced in transient receptor potential ankyrin 1 deficient mice–potential role for transient receptor potential ankyrin 1 in gout. PLoS One. 2015;10:e0117770. doi: 10.1371/journal.pone.0117770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu Z, Hu Y, Yu X, Xi J, Fan X, Tse CM, et al. Allergen challenge sensitizes TRPA1 in vagal sensory neurons and afferent C-fiber subtypes in guinea pig esophagus. Am J Physiol Gastrointest Liver Physiol. 2015;308:G482–G488. doi: 10.1152/ajpgi.00374.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Terada Y, Fujimura M, Nishimura S, Tsubota M, Sekiguchi F, Nishikawa H, et al. Contribution of TRPA1 as a downstream signal of proteinase-activated receptor-2 to pancreatic pain. J Pharmacol Sci. 2013;123:284–287. doi: 10.1254/jphs.13128sc. [DOI] [PubMed] [Google Scholar]
- 94.Cattaruzza F, Johnson C, Leggit A, Grady E, Schenk AK, Cevikbas F, et al. Transient receptor potential ankyrin 1 mediates chronic pancreatitis pain in mice. Am J Physiol Gastrointest Liver Physiol. 2013;304:G1002–G1012. doi: 10.1152/ajpgi.00005.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vermeulen W, De Man JG, De Schepper HU, Bult H, Moreels TG, Pelckmans PA, et al. Role of TRPV1 and TRPA1 in visceral hypersensitivity to colorectal distension during experimental colitis in rats. Eur J Pharmacol. 2013;698:404–412. doi: 10.1016/j.ejphar.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 96.Brozmanova M, Ru F, Surdenikova L, Mazurova L, Taylor-Clark T, Kollarik M. Preferential activation of the vagal nodose nociceptive subtype by TRPA1 agonists in the guinea pig esophagus. Neurogastroenterol Motil. 2011;23:e437–e445. doi: 10.1111/j.1365-2982.2011.01768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schwartz ES, Christianson JA, Chen X, La JH, Davis BM, Albers KM, et al. Synergistic role of TRPV1 and TRPA1 in pancreatic pain and inflammation. Gastroenterology. 2011;140(1283–1291):e1281–e1282. doi: 10.1053/j.gastro.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mitrovic M, Shahbazian A, Bock E, Pabst MA, Holzer P. Chemo-nociceptive signalling from the colon is enhanced by mild colitis and blocked by inhibition of transient receptor potential ankyrin 1 channels. Br J Pharmacol. 2010;160:1430–1442. doi: 10.1111/j.1476-5381.2010.00794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kondo T, Obata K, Miyoshi K, Sakurai J, Tanaka J, Miwa H, et al. Transient receptor potential A1 mediates gastric distention-induced visceral pain in rats. Gut. 2009;58:1342–1352. doi: 10.1136/gut.2008.175901. [DOI] [PubMed] [Google Scholar]
- 100.Yu S, Gao G, Peterson BZ, Ouyang A. TRPA1 in mast cell activation-induced long-lasting mechanical hypersensitivity of vagal afferent C-fibers in guinea pig esophagus. Am J Physiol Gastrointest Liver Physiol. 2009;297:G34–G42. doi: 10.1152/ajpgi.00068.2009. [DOI] [PubMed] [Google Scholar]
- 101.Yu S, Ouyang A. TRPA1 in bradykinin-induced mechanical hypersensitivity of vagal C fibers in guinea pig esophagus. Am J Physiol Gastrointest Liver Physiol. 2009;296:G255–G265. doi: 10.1152/ajpgi.90530.2008. [DOI] [PubMed] [Google Scholar]
- 102.Yang J, Li Y, Zuo X, Zhen Y, Yu Y, Gao L. Transient receptor potential ankyrin-1 participates in visceral hyperalgesia following experimental colitis. Neurosci Lett. 2008;440:237–241. doi: 10.1016/j.neulet.2008.05.093. [DOI] [PubMed] [Google Scholar]
- 103.Kimball ES, Prouty SP, Pavlick KP, Wallace NH, Schneider CR, Hornby PJ. Stimulation of neuronal receptors, neuropeptides and cytokines during experimental oil of mustard colitis. Neurogastroenterol Motil. 2007;19:390–400. doi: 10.1111/j.1365-2982.2007.00939.x. [DOI] [PubMed] [Google Scholar]
- 104.Kistner K, Siklosi N, Babes A, Khalil M, Selescu T, Zimmermann K, et al. Systemic desensitization through TRPA1 channels by capsazepine and mustard oil—a novel strategy against inflammation and pain. Sci Rep. 2016;6:28621. doi: 10.1038/srep28621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kremeyer B, Lopera F, Cox JJ, Momin A, Rugiero F, Marsh S, et al. A gain-of-function mutation in TRPA1 causes familial episodic pain syndrome. Neuron. 2010;66:671–680. doi: 10.1016/j.neuron.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zima V, Witschas K, Hynkova A, Zimova L, Barvik I, Vlachova V. Structural modeling and patch-clamp analysis of pain-related mutation TRPA1-N855S reveal inter-subunit salt bridges stabilizing the channel open state. Neuropharmacology. 2015;93:294–307. doi: 10.1016/j.neuropharm.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 107.Gupta R, Saito S, Mori Y, Itoh SG, Okumura H, Tominaga M. Structural basis of TRPA1 inhibition by HC-030031 utilizing species-specific differences. Sci Rep. 2016;6:37460. doi: 10.1038/srep37460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gallo V, Dijk FN, Holloway JW, Ring SM, Koppelman GH, Postma DS, et al. TRPA1 gene polymorphisms and childhood asthma. Pediatr Allergy Immunol. 2017;28:191–198. doi: 10.1111/pai.12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bell JT, Loomis AK, Butcher LM, Gao F, Zhang B, Hyde CL, et al. Differential methylation of the TRPA1 promoter in pain sensitivity. Nat Commun. 2014;5:2978. doi: 10.1038/ncomms3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Oehler B, Kistner K, Martin C, Schiller J, Mayer R, Mohammadi M, et al. Inflammatory pain control by blocking oxidized phospholipid-mediated TRP channel activation. Sci Rep. 2017;7:5447. doi: 10.1038/s41598-017-05348-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pereira I, Mendes SJ, Pereira DM, Muniz TF, Colares VL, Monteiro CR, et al. Transient receptor potential ankyrin 1 channel expression on peripheral blood leukocytes from rheumatoid arthritic patients and correlation with pain and disability. Front Pharmacol. 2017;8:53. doi: 10.3389/fphar.2017.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fernandes ES, Russell FA, Alawi KM, Sand C, Liang L, Salamon R, et al. Environmental cold exposure increases blood flow and affects pain sensitivity in the knee joints of CFA-induced arthritic mice in a TRPA1-dependent manner. Arthritis Res Ther. 2016;18:7. doi: 10.1186/s13075-015-0905-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Garrison SR, Stucky CL. Contribution of transient receptor potential ankyrin 1 to chronic pain in aged mice with complete Freund’s adjuvant-induced arthritis. Arthritis Rheumatol. 2014;66:2380–2390. doi: 10.1002/art.38724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bautista DM, Pellegrino M, Tsunozaki M. TRPA1: A gatekeeper for inflammation. Annu Rev Physiol. 2013;75:181–200. doi: 10.1146/annurev-physiol-030212-183811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Brain SD. TRPV1 and TRPA1 channels in inflammatory pain: elucidating mechanisms. Ann N Y Acad Sci. 2011;1245:36–37. doi: 10.1111/j.1749-6632.2011.06326.x. [DOI] [PubMed] [Google Scholar]
- 116.Fernandes ES, Russell FA, Spina D, McDougall JJ, Graepel R, Gentry C, et al. A distinct role for transient receptor potential ankyrin 1, in addition to transient receptor potential vanilloid 1, in tumor necrosis factor alpha-induced inflammatory hyperalgesia and Freund’s complete adjuvant-induced monarthritis. Arthritis Rheum. 2011;63:819–829. doi: 10.1002/art.30150. [DOI] [PubMed] [Google Scholar]
- 117.Kochukov MY, McNearney TA, Fu Y, Westlund KN. Thermosensitive TRP ion channels mediate cytosolic calcium response in human synoviocytes. Am J Physiol Cell Physiol. 2006;291:C424–C432. doi: 10.1152/ajpcell.00553.2005. [DOI] [PubMed] [Google Scholar]
- 118.Zappia KJ, Garrison SR, Hillery CA, Stucky CL. Cold hypersensitivity increases with age in mice with sickle cell disease. Pain. 2014;155:2476–2485. doi: 10.1016/j.pain.2014.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hillery CA, Kerstein PC, Vilceanu D, Barabas ME, Retherford D, Brandow AM, et al. Transient receptor potential vanilloid 1 mediates pain in mice with severe sickle cell disease. Blood. 2011;118:3376–3383. doi: 10.1182/blood-2010-12-327429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kittaka H, Uchida K, Fukuta N, Tominaga M. Lysophosphatidic acid-induced itch is mediated by signalling of LPA5 receptor, phospholipase D and TRPA1/TRPV1. J Physiol. 2017;595:2681–2698. doi: 10.1113/JP273961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cevikbas F, Wang X, Akiyama T, Kempkes C, Savinko T, Antal A, et al. A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: Involvement of TRPV1 and TRPA1. J Allergy Clin Immunol. 2014;133:448–460. doi: 10.1016/j.jaci.2013.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wilson SR, Gerhold KA, Bifolck-Fisher A, Liu Q, Patel KN, Dong X, et al. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat Neurosci. 2011;14:595–602. doi: 10.1038/nn.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu B, Tai Y, Achanta S, Kaelberer MM, Caceres AI, Shao X, et al. IL-33/ST2 signaling excites sensory neurons and mediates itch response in a mouse model of poison ivy contact allergy. Proc Natl Acad Sci U S A. 2016;113:E7572–E7579. doi: 10.1073/pnas.1606608113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wilson SR, The L, Batia LM, Beattie K, Katibah GE, McClain SP, et al. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell. 2013;155:285–295. doi: 10.1016/j.cell.2013.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu T, Ji RR. Oxidative stress induces itch via activation of transient receptor potential subtype ankyrin 1 in mice. Neurosci Bull. 2012;28:145–154. doi: 10.1007/s12264-012-1207-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liang J, Bi H, Ji W. Involvement of TRPA1 in ET-1-induced pain-like behavior in mice. Neuroreport. 2010;21:201–205. doi: 10.1097/WNR.0b013e328335b3c5. [DOI] [PubMed] [Google Scholar]
- 127.Liang J, Ji Q, Ji W. Role of transient receptor potential ankyrin subfamily member 1 in pruritus induced by endothelin-1. Neurosci Lett. 2011;492:175–178. doi: 10.1016/j.neulet.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 128.Kido-Nakahara M, Buddenkotte J, Kempkes C, Ikoma A, Cevikbas F, Akiyama T, et al. Neural peptidase endothelin-converting enzyme 1 regulates endothelin 1-induced pruritus. J Clin Invest. 2014;124:2683–2695. doi: 10.1172/JCI67323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen Y, Williams SH, McNulty AL, Hong JH, Lee SH, Rothfusz NE, et al. Temporomandibular joint pain: a critical role for Trpv4 in the trigeminal ganglion. Pain. 2013;154:1295–1304. doi: 10.1016/j.pain.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bressan E, Touska F, Vetter I, Kistner K, Kichko TI, Teixeira NB, et al. Crotalphine desensitizes TRPA1 ion channels to alleviate inflammatory hyperalgesia. Pain. 2016;157:2504–2516. doi: 10.1097/j.pain.0000000000000669. [DOI] [PubMed] [Google Scholar]
- 131.Chen Y, Fang Q, Wang Z, Zhang JY, MacLeod AS, Hall RP, et al. Transient receptor potential vanilloid 4 ion channel functions as a pruriceptor in epidermal keratinocytes to evoke histaminergic itch. J Biol Chem. 2016;291:10252–10262. doi: 10.1074/jbc.M116.716464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Morita T, McClain SP, Batia LM, Pellegrino M, Wilson SR, Kienzler MA, et al. HTR7 mediates serotonergic acute and chronic itch. Neuron. 2015;87:124–138. doi: 10.1016/j.neuron.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Alemi F, Kwon E, Poole DP, Lieu T, Lyo V, Cattaruzza F, et al. The TGR5 receptor mediates bile acid-induced itch and analgesia. J Clin Invest. 2013;123:1513–1530. doi: 10.1172/JCI64551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhao P, Lieu T, Barlow N, Metcalf M, Veldhuis NA, Jensen DD, et al. Cathepsin S causes inflammatory pain via biased agonism of PAR2 and TRPV4. J Biol Chem. 2014;289:27215–27234. doi: 10.1074/jbc.M114.599712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chen L, Liu C, Liu L. Osmolality-induced tuning of action potentials in trigeminal ganglion neurons. Neurosci Lett. 2009;452:79–83. doi: 10.1016/j.neulet.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wilson SR, Nelson AM, Batia L, Morita T, Estandian D, Owens DM, et al. The ion channel TRPA1 is required for chronic itch. J Neurosci. 2013;33:9283–9294. doi: 10.1523/JNEUROSCI.5318-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Oh MH, Oh SY, Lu J, Lou H, Myers AC, Zhu Z, et al. TRPA1-dependent pruritus in IL-13-induced chronic atopic dermatitis. J Immunol. 2013;191:5371–5382. doi: 10.4049/jimmunol.1300300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Andrade EL, Meotti FC, Calixto JB. TRPA1 antagonists as potential analgesic drugs. Pharmacol Ther. 2012;133:189–204. doi: 10.1016/j.pharmthera.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 139.Baraldi PG, Romagnoli R, Saponaro G, Aghazadeh Tabrizi M, Baraldi S, Pedretti P, et al. 7-Substituted-pyrrolo[3,2-d]pyrimidine-2,4-dione derivatives as antagonists of the transient receptor potential ankyrin 1 (TRPA1) channel: a promising approach for treating pain and inflammation. Bioorg Med Chem. 2012;20:1690–1698. doi: 10.1016/j.bmc.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 140.Birkholz TR, Beane WS. The planarian TRPA1 homolog mediates extraocular behavioral responses to near-ultraviolet light. J Exp Biol. 2017;220:2616–2625. doi: 10.1242/jeb.152298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chaudhuri J, Bose N, Gong J, Hall D, Rifkind A, Bhaumik D, et al. A Caenorhabditis elegans model elucidates a conserved role for TRPA1-Nrf signaling in reactive alpha-dicarbonyl detoxification. Curr Biol. 2016;26:3014–3025. doi: 10.1016/j.cub.2016.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhang X. Targeting TRP ion channels for itch relief. Naunyn Schmiedebergs Arch Pharmacol. 2015;388:389–399. doi: 10.1007/s00210-014-1068-z. [DOI] [PubMed] [Google Scholar]
- 143.Xiao R, Zhang B, Dong Y, Gong J, Xu T, Liu J, et al. A genetic program promotes C. elegans longevity at cold temperatures via a thermosensitive TRP channel. Cell. 2013;152:806–817. doi: 10.1016/j.cell.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Garrity PA. Neuroscience: Feel the light. Nature. 2010;468:900–901. doi: 10.1038/468900a. [DOI] [PubMed] [Google Scholar]
- 145.Yin J, Kuebler WM. Mechanotransduction by TRP channels: general concepts and specific role in the vasculature. Cell Biochem Biophys. 2010;56:1–18. doi: 10.1007/s12013-009-9067-2. [DOI] [PubMed] [Google Scholar]
- 146.Prober DA, Zimmerman S, Myers BR, McDermott BM, Jr, Kim SH, Caron S, et al. Zebrafish TRPA1 channels are required for chemosensation but not for thermosensation or mechanosensory hair cell function. J Neurosci. 2008;28:10102–10110. doi: 10.1523/JNEUROSCI.2740-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kindt KS, Viswanath V, Macpherson L, Quast K, Hu H, Patapoutian A, et al. Caenorhabditis elegans TRPA-1 functions in mechanosensation. Nat Neurosci. 2007;10:568–577. doi: 10.1038/nn1886. [DOI] [PubMed] [Google Scholar]
- 148.Montell C. The TRP superfamily of cation channels. Sci STKE 2005, 2005: re3. [DOI] [PubMed]
- 149.Viswanath V, Story GM, Peier AM, Petrus MJ, Lee VM, Hwang SW, et al. Opposite thermosensor in fruitfly and mouse. Nature. 2003;423:822–823. doi: 10.1038/423822a. [DOI] [PubMed] [Google Scholar]
- 150.Lee JE, Kim Y, Kim KH, Lee DY, Lee Y. Contribution of Drosophila TRPA1 to metabolism. PLoS One. 2016;11:e0152935. doi: 10.1371/journal.pone.0152935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Guntur AR, Gu P, Takle K, Chen J, Xiang Y, Yang CH. Drosophila TRPA1 isoforms detect UV light via photochemical production of H2O2. Proc Natl Acad Sci U S A. 2015;112:E5753–E5761. doi: 10.1073/pnas.1514862112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Braun AP. Structural remodeling of the N-terminus tunes TRPA1 channel activation and regulates behavioral responses in Drosophila. Channels (Austin) 2012;6:50–51. doi: 10.4161/chan.19351. [DOI] [PubMed] [Google Scholar]
- 153.Neely GG, Keene AC, Duchek P, Chang EC, Wang QP, Aksoy YA, et al. TrpA1 regulates thermal nociception in Drosophila. PLoS One. 2011;6:e24343. doi: 10.1371/journal.pone.0024343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kwon Y, Kim SH, Ronderos DS, Lee Y, Akitake B, Woodward OM, et al. Drosophila TRPA1 channel is required to avoid the naturally occurring insect repellent citronellal. Curr Biol. 2010;20:1672–1678. doi: 10.1016/j.cub.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kim SH, Lee Y, Akitake B, Woodward OM, Guggino WB, Montell C. Drosophila TRPA1 channel mediates chemical avoidance in gustatory receptor neurons. Proc Natl Acad Sci U S A. 2010;107:8440–8445. doi: 10.1073/pnas.1001425107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kang K, Pulver SR, Panzano VC, Chang EC, Griffith LC, Theobald DL, et al. Analysis of Drosophila TRPA1 reveals an ancient origin for human chemical nociception. Nature. 2010;464:597–600. doi: 10.1038/nature08848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rosenzweig M, Brennan KM, Tayler TD, Phelps PO, Patapoutian A, Garrity PA. The Drosophila ortholog of vertebrate TRPA1 regulates thermotaxis. Genes Dev. 2005;19:419–424. doi: 10.1101/gad.1278205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Perraud AL, Fleig A, Dunn CA, Bagley LA, Launay P, Schmitz C, et al. ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature. 2001;411:595–599. doi: 10.1038/35079100. [DOI] [PubMed] [Google Scholar]
- 159.Koh WU, Choi SS, Kim JH, Yoon HJ, Ahn HS, Lee SK, et al. The preventive effect of resiniferatoxin on the development of cold hypersensitivity induced by spinal nerve ligation: involvement of TRPM8. BMC Neurosci. 2016;17:38. doi: 10.1186/s12868-016-0273-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Liedtke WB. Deconstructing mammalian thermoregulation. Proc Natl Acad Sci U S A. 2017;114:1765–1767. doi: 10.1073/pnas.1620579114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kraft R, Grimm C, Grosse K, Hoffmann A, Sauerbruch S, Kettenmann H, et al. Hydrogen peroxide and ADP-ribose induce TRPM2-mediated calcium influx and cation currents in microglia. Am J Physiol Cell Physiol. 2004;286:C129–C137. doi: 10.1152/ajpcell.00331.2003. [DOI] [PubMed] [Google Scholar]
- 162.Yamamoto S, Shimizu S, Kiyonaka S, Takahashi N, Wajima T, Hara Y, et al. TRPM2-mediated Ca2+influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat Med. 2008;14:738–747. doi: 10.1038/nm1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Matsumoto K, Takagi K, Kato A, Ishibashi T, Mori Y, Tashima K, et al. Role of transient receptor potential melastatin 2 (TRPM2) channels in visceral nociception and hypersensitivity. Exp Neurol. 2016;285:41–50. doi: 10.1016/j.expneurol.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 164.Haraguchi K, Kawamoto A, Isami K, Maeda S, Kusano A, Asakura K, et al. TRPM2 contributes to inflammatory and neuropathic pain through the aggravation of pronociceptive inflammatory responses in mice. J Neurosci. 2012;32:3931–3941. doi: 10.1523/JNEUROSCI.4703-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chung MK, Asgar J, Lee J, Shim MS, Dumler C, Ro JY. The role of TRPM2 in hydrogen peroxide-induced expression of inflammatory cytokine and chemokine in rat trigeminal ganglia. Neuroscience. 2015;297:160–169. doi: 10.1016/j.neuroscience.2015.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Akiyama T, Ivanov M, Nagamine M, Davoodi A, Carstens MI, Ikoma A, et al. Involvement of TRPV4 in serotonin-evoked scratching. J Invest Dermatol. 2016;136:154–160. doi: 10.1038/JID.2015.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wagner TF, Loch S, Lambert S, Straub I, Mannebach S, Mathar I, et al. Transient receptor potential M3 channels are ionotropic steroid receptors in pancreatic beta cells. Nat Cell Biol. 2008;10:1421–1430. doi: 10.1038/ncb1801. [DOI] [PubMed] [Google Scholar]
- 168.Vriens J, Owsianik G, Hofmann T, Philipp SE, Stab J, Chen X, et al. TRPM3 is a nociceptor channel involved in the detection of noxious heat. Neuron. 2011;70:482–494. doi: 10.1016/j.neuron.2011.02.051. [DOI] [PubMed] [Google Scholar]
- 169.Held K, Kichko T, De Clercq K, Klaassen H, Van Bree R, Vanherck JC, et al. Activation of TRPM3 by a potent synthetic ligand reveals a role in peptide release. Proc Natl Acad Sci U S A. 2015;112:E1363–E1372. doi: 10.1073/pnas.1419845112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Krugel U, Straub I, Beckmann H, Schaefer M. Primidone inhibits TRPM3 and attenuates thermal nociception in vivo. Pain. 2017;158:856–867. doi: 10.1097/j.pain.0000000000000846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 172.Peier AM. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 173.Knowlton WM, Palkar R, Lippoldt EK, McCoy DD, Baluch F, Chen J, et al. A sensory-labeled line for cold: TRPM8-expressing sensory neurons define the cellular basis for cold, cold pain, and cooling-mediated analgesia. J Neurosci. 2013;33:2837–2848. doi: 10.1523/JNEUROSCI.1943-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, et al. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- 175.Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. TRPM8 is required for cold sensation in mice. Neuron. 2007;54:371–378. doi: 10.1016/j.neuron.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 176.Colburn RW, Lubin ML, Stone DJ, Jr, Wang Y, Lawrence D, D’Andrea MR, et al. Attenuated cold sensitivity in TRPM8 null mice. Neuron. 2007;54:379–386. doi: 10.1016/j.neuron.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 177.Xing H, Chen M, Ling J, Tan W, Gu JG. TRPM8 mechanism of cold allodynia after chronic nerve injury. J Neurosci. 2007;27:13680–13690. doi: 10.1523/JNEUROSCI.2203-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Liu B, Fan L, Balakrishna S, Sui A, Morris JB, Jordt SE. TRPM8 is the principal mediator of menthol-induced analgesia of acute and inflammatory pain. Pain. 2013;154:2169–2177. doi: 10.1016/j.pain.2013.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mueller-Tribbensee SM, Karna M, Khalil M, Neurath MF, Reeh PW, Engel MA. Differential contribution of TRPA1, TRPV4 and TRPM8 to colonic nociception in mice. PLoS One. 2015;10:e0128242. doi: 10.1371/journal.pone.0128242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Caceres AI, Liu B, Jabba SV, Achanta S, Morris JB, Jordt SE. Transient Receptor Potential Cation Channel Subfamily M Member 8 channels mediate the anti-inflammatory effects of eucalyptol. Br J Pharmacol. 2017;174:867–879. doi: 10.1111/bph.13760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Allchorne AJ, Broom DC, Woolf CJ. Detection of cold pain, cold allodynia and cold hyperalgesia in freely behaving rats. Mol Pain. 2005;1:36. doi: 10.1186/1744-8069-1-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lippoldt EK, Elmes RR, McCoy DD, Knowlton WM, McKemy DD. Artemin, a glial cell line-derived neurotrophic factor family member, induces TRPM8-dependent cold pain. J Neurosci. 2013;33:12543–12552. doi: 10.1523/JNEUROSCI.5765-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lippoldt EK, Ongun S, Kusaka GK, McKemy DD. Inflammatory and neuropathic cold allodynia are selectively mediated by the neurotrophic factor receptor GFRalpha3. Proc Natl Acad Sci U S A. 2016;113:4506–4511. doi: 10.1073/pnas.1603294113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Rohacs T, Lopes CM, Michailidis I, Logothetis DE. PI(4,5)P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat Neurosci. 2005;8:626–634. doi: 10.1038/nn1451. [DOI] [PubMed] [Google Scholar]
- 185.Premkumar LS, Raisinghani M, Pingle SC, Long C, Pimentel F. Downregulation of transient receptor potential melastatin 8 by protein kinase C-mediated dephosphorylation. J Neurosci. 2005;25:11322–11329. doi: 10.1523/JNEUROSCI.3006-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Andersson DA, Nash M, Bevan S. Modulation of the cold-activated channel TRPM8 by lysophospholipids and polyunsaturated fatty acids. J Neurosci. 2007;27:3347–3355. doi: 10.1523/JNEUROSCI.4846-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Gentry C, Stoakley N, Andersson DA, Bevan S. The roles of iPLA2, TRPM8 and TRPA1 in chemically induced cold hypersensitivity. Mol Pain. 2010;6:4. doi: 10.1186/1744-8069-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Knowlton WM, Daniels RL, Palkar R, McCoy DD, McKemy DD. Pharmacological blockade of TRPM8 ion channels alters cold and cold pain responses in mice. PLoS One. 2011;6:e25894. doi: 10.1371/journal.pone.0025894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lehto SG, Weyer AD, Zhang M, Youngblood BD, Wang J, Wang W, et al. AMG2850, a potent and selective TRPM8 antagonist, is not effective in rat models of inflammatory mechanical hypersensitivity and neuropathic tactile allodynia. Naunyn Schmiedebergs Arch Pharmacol. 2015;388:465–476. doi: 10.1007/s00210-015-1090-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ramachandran R, Hyun E, Zhao L, Lapointe TK, Chapman K, Hirota CL, et al. TRPM8 activation attenuates inflammatory responses in mouse models of colitis. Proc Natl Acad Sci U S A. 2013;110:7476–7481. doi: 10.1073/pnas.1217431110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Willis DN, Liu B, Ha MA, Jordt SE, Morris JB. Menthol attenuates respiratory irritation responses to multiple cigarette smoke irritants. FASEB J. 2011;25:4434–4444. doi: 10.1096/fj.11-188383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Frolich M, Enk A, Diepgen TL, Weisshaar E. Successful treatment of therapy-resistant pruritus in lichen amyloidosis with menthol. Acta Derm Venereol. 2009;89:524–526. doi: 10.2340/00015555-0725. [DOI] [PubMed] [Google Scholar]
- 193.Haught JM, Jukic DM, English JC., 3rd Hydroxyethyl starch-induced pruritus relieved by a combination of menthol and camphor. J Am Acad Dermatol. 2008;59:151–153. doi: 10.1016/j.jaad.2008.03.034. [DOI] [PubMed] [Google Scholar]
- 194.Kardon AP, Polgar E, Hachisuka J, Snyder LM, Cameron D, Savage S, et al. Dynorphin acts as a neuromodulator to inhibit itch in the dorsal horn of the spinal cord. Neuron. 2014;82:573–586. doi: 10.1016/j.neuron.2014.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Frias B, Merighi A. Capsaicin, nociception and pain. Molecules 2016, 21. [DOI] [PMC free article] [PubMed]
- 196.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 197.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 198.Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI, et al. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci U S A. 2009;106:9075–9080. doi: 10.1073/pnas.0901507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Huang D, Li S, Dhaka A, Story GM, Cao YQ. Expression of the transient receptor potential channels TRPV1, TRPA1 and TRPM8 in mouse trigeminal primary afferent neurons innervating the dura. Mol Pain. 2012;8:66. doi: 10.1186/1744-8069-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hwang SJ, Oh JM, Valtschanoff JG. Expression of the vanilloid receptor TRPV1 in rat dorsal root ganglion neurons supports different roles of the receptor in visceral and cutaneous afferents. Brain Res. 2005;1047:261–266. doi: 10.1016/j.brainres.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 201.Li Y, Cai J, Han Y, Xiao X, Meng XL, Su L, et al. Enhanced function of TRPV1 via up-regulation by insulin-like growth factor-1 in a rat model of bone cancer pain. Eur J Pain. 2014;18:774–784. doi: 10.1002/j.1532-2149.2013.00420.x. [DOI] [PubMed] [Google Scholar]
- 202.Zhao Q, Wang W, Wang R, Cheng Y. TRPV1 and neuropeptide receptor immunoreactivity and expression in the rat lung and brainstem after lung ischemia-reperfusion injury. J Surg Res. 2016;203:183–192. doi: 10.1016/j.jss.2016.03.050. [DOI] [PubMed] [Google Scholar]
- 203.Dinh QT, Groneberg DA, Peiser C, Mingomataj E, Joachim RA, Witt C, et al. Substance P expression in TRPV1 and trkA-positive dorsal root ganglion neurons innervating the mouse lung. Respir Physiol Neurobiol. 2004;144:15–24. doi: 10.1016/j.resp.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 204.Vass Z, Dai CF, Steyger PS, Jancso G, Trune DR, Nuttall AL. Co-localization of the vanilloid capsaicin receptor and substance P in sensory nerve fibers innervating cochlear and vertebro-basilar arteries. Neuroscience. 2004;124:919–927. doi: 10.1016/j.neuroscience.2003.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.De Logu F, Patacchini R, Fontana G, Geppetti P. TRP functions in the broncho-pulmonary system. Semin Immunopathol. 2016;38:321–329. doi: 10.1007/s00281-016-0557-1. [DOI] [PubMed] [Google Scholar]
- 206.Lehmann R, Schobel N, Hatt H, van Thriel C. The involvement of TRP channels in sensory irritation: a mechanistic approach toward a better understanding of the biological effects of local irritants. Arch Toxicol. 2016;90:1399–1413. doi: 10.1007/s00204-016-1703-1. [DOI] [PubMed] [Google Scholar]
- 207.Spicarova D, Palecek J. The role of the TRPV1 endogenous agonist N-Oleoyldopamine in modulation of nociceptive signaling at the spinal cord level. J Neurophysiol. 2009;102:234–243. doi: 10.1152/jn.00024.2009. [DOI] [PubMed] [Google Scholar]
- 208.Spicarova D, Palecek J. The role of spinal cord vanilloid (TRPV1) receptors in pain modulation. Physiol Res. 2008;57(Suppl 3):S69–S77. doi: 10.33549/physiolres.931601. [DOI] [PubMed] [Google Scholar]
- 209.Cavanaugh DJ, Chesler AT, Braz JM, Shah NM, Julius D, Basbaum AI. Restriction of transient receptor potential vanilloid-1 to the peptidergic subset of primary afferent neurons follows its developmental downregulation in nonpeptidergic neurons. J Neurosci. 2011;31:10119–10127. doi: 10.1523/JNEUROSCI.1299-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Cavanaugh DJ, Chesler AT, Jackson AC, Sigal YM, Yamanaka H, Grant R, et al. Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. J Neurosci. 2011;31:5067–5077. doi: 10.1523/JNEUROSCI.6451-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zaelzer C, Hua P, Prager-Khoutorsky M, Ciura S, Voisin DL, Liedtke W, et al. DeltaN-TRPV1: A molecular co-detector of body temperature and osmotic stress. Cell Rep. 2015;13:23–30. doi: 10.1016/j.celrep.2015.08.061. [DOI] [PubMed] [Google Scholar]
- 212.Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- 213.Morales-Lazaro SL, Simon SA, Rosenbaum T. The role of endogenous molecules in modulating pain through transient receptor potential vanilloid 1 (TRPV1) J Physiol. 2013;591:3109–3121. doi: 10.1113/jphysiol.2013.251751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Woo DH, Jung SJ, Zhu MH, Park CK, Kim YH, Oh SB, et al. Direct activation of transient receptor potential vanilloid 1(TRPV1) by diacylglycerol (DAG) Mol Pain. 2008;4:42. doi: 10.1186/1744-8069-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Brauchi S, Orta G, Mascayano C, Salazar M, Raddatz N, Urbina H, et al. Dissection of the components for PIP2 activation and thermosensation in TRP channels. Proc Natl Acad Sci U S A. 2007;104:10246–10251. doi: 10.1073/pnas.0703420104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Moriyama T, Higashi T, Togashi K, Iida T, Segi E, Sugimoto Y, et al. Sensitization of TRPV1 by EP1 and IP reveals peripheral nociceptive mechanism of prostaglandins. Mol Pain. 2005;1:3. doi: 10.1186/1744-8069-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Morales-Lazaro SL, Llorente I, Sierra-Ramirez F, Lopez-Romero AE, Ortiz-Renteria M, Serrano-Flores B, et al. Inhibition of TRPV1 channels by a naturally occurring omega-9 fatty acid reduces pain and itch. Nat Commun. 2016;7:13092. doi: 10.1038/ncomms13092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Bolcskei K, Helyes Z, Szabo A, Sandor K, Elekes K, Nemeth J, et al. Investigation of the role of TRPV1 receptors in acute and chronic nociceptive processes using gene-deficient mice. Pain. 2005;117:368–376. doi: 10.1016/j.pain.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 219.Kanai Y, Nakazato E, Fujiuchi A, Hara T, Imai A. Involvement of an increased spinal TRPV1 sensitization through its up-regulation in mechanical allodynia of CCI rats. Neuropharmacology. 2005;49:977–984. doi: 10.1016/j.neuropharm.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 220.Yamamoto W, Sugiura A, Nakazato-Imasato E, Kita Y. Characterization of primary sensory neurons mediating static and dynamic allodynia in rat chronic constriction injury model. J Pharm Pharmacol. 2008;60:717–722. doi: 10.1211/jpp.60.6.0006. [DOI] [PubMed] [Google Scholar]
- 221.Fukuoka T, Tokunaga A, Tachibana T, Dai Y, Yamanaka H, Noguchi K. VR1, but not P2X(3), increases in the spared L4 DRG in rats with L5 spinal nerve ligation. Pain. 2002;99:111–120. doi: 10.1016/s0304-3959(02)00067-2. [DOI] [PubMed] [Google Scholar]
- 222.Dong F, Du YR, Xie W, Strong JA, He XJ, Zhang JM. Increased function of the TRPV1 channel in small sensory neurons after local inflammation or in vitro exposure to the pro-inflammatory cytokine GRO/KC. Neurosci Bull. 2012;28:155–164. doi: 10.1007/s12264-012-1208-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Pabbidi RM, Yu SQ, Peng S, Khardori R, Pauza ME, Premkumar LS. Influence of TRPV1 on diabetes-induced alterations in thermal pain sensitivity. Mol Pain. 2008;4:9. doi: 10.1186/1744-8069-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Hong S, Wiley JW. Early painful diabetic neuropathy is associated with differential changes in the expression and function of vanilloid receptor 1. J Biol Chem. 2005;280:618–627. doi: 10.1074/jbc.M408500200. [DOI] [PubMed] [Google Scholar]
- 225.Cui YY, Xu H, Wu HH, Qi J, Shi J, Li YQ. Spatio-temporal expression and functional involvement of transient receptor potential vanilloid 1 in diabetic mechanical allodynia in rats. PLoS One. 2014;9:e102052. doi: 10.1371/journal.pone.0102052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Han Y, Li Y, Xiao X, Liu J, Meng XL, Liu FY, et al. Formaldehyde up-regulates TRPV1 through MAPK and PI3K signaling pathways in a rat model of bone cancer pain. Neurosci Bull. 2012;28:165–172. doi: 10.1007/s12264-012-1211-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Beyak MJ, Vanner S. Inflammation-induced hyperexcitability of nociceptive gastrointestinal DRG neurones: the role of voltage-gated ion channels. Neurogastroenterol Motil. 2005;17:175–186. doi: 10.1111/j.1365-2982.2004.00596.x. [DOI] [PubMed] [Google Scholar]
- 228.Hicks GA. TRP channels as therapeutic targets: hot property, or time to cool down? Neurogastroenterol Motil. 2006;18:590–594. doi: 10.1111/j.1365-2982.2006.00823.x. [DOI] [PubMed] [Google Scholar]
- 229.Makimura Y, Ito K, Kuwahara M, Tsubone H. Augmented activity of the pelvic nerve afferent mediated by TRP channels in dextran sulfate sodium (DSS)-induced colitis of rats. J Vet Med Sci. 2012;74:1007–1013. doi: 10.1292/jvms.11-0547. [DOI] [PubMed] [Google Scholar]
- 230.Jones RC, 3rd, Xu L, Gebhart GF. The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. J Neurosci. 2005;25:10981–10989. doi: 10.1523/JNEUROSCI.0703-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Winston J, Shenoy M, Medley D, Naniwadekar A, Pasricha PJ. The vanilloid receptor initiates and maintains colonic hypersensitivity induced by neonatal colon irritation in rats. Gastroenterology. 2007;132:615–627. doi: 10.1053/j.gastro.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 232.Xu GY, Winston JH, Shenoy M, Yin H, Pendyala S, Pasricha PJ. Transient receptor potential vanilloid 1 mediates hyperalgesia and is up-regulated in rats with chronic pancreatitis. Gastroenterology. 2007;133:1282–1292. doi: 10.1053/j.gastro.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 233.Sun S, Dong X. Trp channels and itch. Semin Immunopathol. 2016;38:293–307. doi: 10.1007/s00281-015-0530-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Sulk M, Seeliger S, Aubert J, Schwab VD, Cevikbas F, Rivier M, et al. Distribution and expression of non-neuronal transient receptor potential (TRPV) ion channels in rosacea. J Invest Dermatol. 2012;132:1253–1262. doi: 10.1038/jid.2011.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Li L, Liu C, Chen L, Chen L. Hypotonicity modulates tetrodotoxin-sensitive sodium current in trigeminal ganglion neurons. Mol Pain. 2011;7:27. doi: 10.1186/1744-8069-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Stander S, Moormann C, Schumacher M, Buddenkotte J, Artuc M, Shpacovitch V, et al. Expression of vanilloid receptor subtype 1 in cutaneous sensory nerve fibers, mast cells, and epithelial cells of appendage structures. Exp Dermatol. 2004;13:129–139. doi: 10.1111/j.0906-6705.2004.0178.x. [DOI] [PubMed] [Google Scholar]
- 237.Gibson RA, Robertson J, Mistry H, McCallum S, Fernando D, Wyres M, et al. A randomised trial evaluating the effects of the TRPV1 antagonist SB705498 on pruritus induced by histamine, and cowhage challenge in healthy volunteers. PLoS One. 2014;9:e100610. doi: 10.1371/journal.pone.0100610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Numata T, Shimizu T, Okada Y. Direct mechano-stress sensitivity of TRPM7 channel. Cell Physiol Biochem. 2007;19:1–8. doi: 10.1159/000099187. [DOI] [PubMed] [Google Scholar]
- 239.Hondoh A, Ishida Y, Ugawa S, Ueda T, Shibata Y, Yamada T, et al. Distinct expression of cold receptors (TRPM8 and TRPA1) in the rat nodose-petrosal ganglion complex. Brain Res. 2010;1319:60–69. doi: 10.1016/j.brainres.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 240.Liu T, Xu ZZ, Park CK, Berta T, Ji RR. Toll-like receptor 7 mediates pruritus. Nat Neurosci. 2010;13:1460–1462. doi: 10.1038/nn.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Barajon I, Serrao G, Arnaboldi F, Opizzi E, Ripamonti G, Balsari A, et al. Toll-like receptors 3, 4, and 7 are expressed in the enteric nervous system and dorsal root ganglia. J Histochem Cytochem. 2009;57:1013–1023. doi: 10.1369/jhc.2009.953539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Peier AM, Reeve AJ, Andersson DA, Moqrich A, Earley TJ, Hergarden AC, et al. A heat-sensitive TRP channel expressed in keratinocytes. Science. 2002;296:2046–2049. doi: 10.1126/science.1073140. [DOI] [PubMed] [Google Scholar]
- 243.Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, et al. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature. 2002;418:181–186. doi: 10.1038/nature00882. [DOI] [PubMed] [Google Scholar]
- 244.Vogt–Eisele AK, Weber K, Sherkheli MA, Vielhaber G, Panten J, Gisselmann G, et al. Monoterpenoid agonists of TRPV3. Br J Pharmacol 2007, 151: 530–540. [DOI] [PMC free article] [PubMed]
- 245.Hu HZ, Xiao R, Wang C, Gao N, Colton CK, Wood JD, et al. Potentiation of TRPV3 channel function by unsaturated fatty acids. J Cell Physiol. 2006;208:201–212. doi: 10.1002/jcp.20648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Brash AR. Arachidonic acid as a bioactive molecule. J Clin Invest. 2001;107:1339–1345. doi: 10.1172/JCI13210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Holstein SA, Hohl RJ. Isoprenoids: remarkable diversity of form and function. Lipids. 2004;39:293–309. doi: 10.1007/s11745-004-1233-3. [DOI] [PubMed] [Google Scholar]
- 248.Bang S, Yoo S, Yang TJ, Cho H, Hwang SW. Farnesyl pyrophosphate is a novel pain-producing molecule via specific activation of TRPV3. J Biol Chem. 2010;285:19362–19371. doi: 10.1074/jbc.M109.087742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Huang SM, Lee H, Chung MK, Park U, Yu YY, Bradshaw HB, et al. Overexpressed transient receptor potential vanilloid 3 ion channels in skin keratinocytes modulate pain sensitivity via prostaglandin E2. J Neurosci. 2008;28:13727–13737. doi: 10.1523/JNEUROSCI.5741-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Moqrich A, Hwang SW, Earley TJ, Petrus MJ, Murray AN, Spencer KS, et al. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science. 2005;307:1468–1472. doi: 10.1126/science.1108609. [DOI] [PubMed] [Google Scholar]
- 251.Andersson DA, Gentry C, Bevan S. TRPA1 has a key role in the somatic pro-nociceptive actions of hydrogen sulfide. PLoS One. 2012;7:e46917. doi: 10.1371/journal.pone.0046917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Yoshioka T, Imura K, Asakawa M, Suzuki M, Oshima I, Hirasawa T, et al. Impact of the Gly573Ser substitution in TRPV3 on the development of allergic and pruritic dermatitis in mice. J Invest Dermatol. 2009;129:714–722. doi: 10.1038/jid.2008.245. [DOI] [PubMed] [Google Scholar]
- 253.Lin Z, Chen Q, Lee M, Cao X, Zhang J, Ma D, et al. Exome sequencing reveals mutations in TRPV3 as a cause of Olmsted syndrome. Am J Hum Genet. 2012;90:558–564. doi: 10.1016/j.ajhg.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, Sali A, et al. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000;103:525–535. doi: 10.1016/s0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Cenac N, Altier C, Chapman K, Liedtke W, Zamponi G, Vergnolle N. Transient receptor potential vanilloid-4 has a major role in visceral hypersensitivity symptoms. Gastroenterology 2008, 135: 937–946, 946 e931–932. [DOI] [PubMed]
- 256.Zhang Y, Wang YH, Ge HY, Arendt-Nielsen L, Wang R, Yue SW. A transient receptor potential vanilloid 4 contributes to mechanical allodynia following chronic compression of dorsal root ganglion in rats. Neurosci Lett. 2008;432:222–227. doi: 10.1016/j.neulet.2007.12.028. [DOI] [PubMed] [Google Scholar]
- 257.Suzuki M, Mizuno A, Kodaira K, Imai M. Impaired pressure sensation in mice lacking TRPV4. J Biol Chem. 2003;278:22664–22668. doi: 10.1074/jbc.M302561200. [DOI] [PubMed] [Google Scholar]
- 258.Chen L, Liu C, Liu L. Changes in osmolality modulate voltage-gated calcium channels in trigeminal ganglion neurons. Brain Res. 2008;1208:56–66. doi: 10.1016/j.brainres.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Chen J. The evolutionary divergence of TRPA1 channel: heat-sensitive, cold-sensitive and temperature-insensitive. Temperature (Austin) 2015;2:158–159. doi: 10.1080/23328940.2014.998903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Rajasekhar P, Poole DP, Liedtke W, Bunnett NW, Veldhuis NA. P2Y1 Receptor Activation of the TRPV4 Ion Channel Enhances Purinergic Signaling in Satellite Glial Cells. J Biol Chem. 2015;290:29051–29062. doi: 10.1074/jbc.M115.689729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Benfenati V, Amiry-Moghaddam M, Caprini M, Mylonakou MN, Rapisarda C, Ottersen OP, et al. Expression and functional characterization of transient receptor potential vanilloid-related channel 4 (TRPV4) in rat cortical astrocytes. Neuroscience. 2007;148:876–892. doi: 10.1016/j.neuroscience.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 262.Konno M, Shirakawa H, Iida S, Sakimoto S, Matsutani I, Miyake T, et al. Stimulation of transient receptor potential vanilloid 4 channel suppresses abnormal activation of microglia induced by lipopolysaccharide. Glia. 2012;60:761–770. doi: 10.1002/glia.22306. [DOI] [PubMed] [Google Scholar]
- 263.Shibasaki K, Suzuki M, Mizuno A, Tominaga M. Effects of body temperature on neural activity in the hippocampus: regulation of resting membrane potentials by transient receptor potential vanilloid 4. J Neurosci. 2007;27:1566–1575. doi: 10.1523/JNEUROSCI.4284-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Shibasaki K, Sugio S, Takao K, Yamanaka A, Miyakawa T, Tominaga M, et al. TRPV4 activation at the physiological temperature is a critical determinant of neuronal excitability and behavior. Pflugers Arch. 2015;467:2495–2507. doi: 10.1007/s00424-015-1726-0. [DOI] [PubMed] [Google Scholar]
- 265.Kanju P, Liedtke W. Pleiotropic function of TRPV4 ion channels in the central nervous system. Exp Physiol. 2016;101:1472–1476. doi: 10.1113/EP085790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Phan MN, Leddy HA, Votta BJ, Kumar S, Levy DS, Lipshutz DB, et al. Functional characterization of TRPV4 as an osmotically sensitive ion channel in porcine articular chondrocytes. Arthritis Rheum. 2009;60:3028–3037. doi: 10.1002/art.24799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Watanabe H, Vriens J, Suh SH, Benham CD, Droogmans G, Nilius B. Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J Biol Chem. 2002;277:47044–47051. doi: 10.1074/jbc.M208277200. [DOI] [PubMed] [Google Scholar]
- 268.Chen Y, Kanju P, Fang Q, Lee SH, Parekh PK, Lee W, et al. TRPV4 is necessary for trigeminal irritant pain and functions as a cellular formalin receptor. Pain. 2014;155:2662–2672. doi: 10.1016/j.pain.2014.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Li J, Ghio AJ, Cho SH, Brinckerhoff CE, Simon SA, Liedtke W. Diesel exhaust particles activate the matrix-metalloproteinase-1 gene in human bronchial epithelia in a beta-arrestin-dependent manner via activation of RAS. Environ Health Perspect. 2009;117:400–409. doi: 10.1289/ehp.0800311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Alessandri-Haber N, Dina OA, Chen X, Levine JD. TRPC1 and TRPC6 channels cooperate with TRPV4 to mediate mechanical hyperalgesia and nociceptor sensitization. J Neurosci. 2009;29:6217–6228. doi: 10.1523/JNEUROSCI.0893-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.D’Aldebert E, Cenac N, Rousset P, Martin L, Rolland C, Chapman K, et al. Transient receptor potential vanilloid 4 activated inflammatory signals by intestinal epithelial cells and colitis in mice. Gastroenterology. 2011;140:275–285. doi: 10.1053/j.gastro.2010.09.045. [DOI] [PubMed] [Google Scholar]
- 272.Kwon M, Baek SH, Park CK, Chung G, Oh SB. Single-cell RT-PCR and immunocytochemical detection of mechanosensitive transient receptor potential channels in acutely isolated rat odontoblasts. Arch Oral Biol. 2014;59:1266–1271. doi: 10.1016/j.archoralbio.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 273.Alessandri-Haber N, Yeh JJ, Boyd AE, Parada CA, Chen X, Reichling DB, et al. Hypotonicity induces TRPV4-mediated nociception in rat. Neuron. 2003;39:497–511. doi: 10.1016/s0896-6273(03)00462-8. [DOI] [PubMed] [Google Scholar]
- 274.Cao DS, Yu SQ, Premkumar LS. Modulation of transient receptor potential Vanilloid 4-mediated membrane currents and synaptic transmission by protein kinase C. Mol Pain. 2009;5:5. doi: 10.1186/1744-8069-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Liedtke W, Tobin DM, Bargmann CI, Friedman JM. Mammalian TRPV4 (VR-OAC) directs behavioral responses to osmotic and mechanical stimuli in C. elegans. Proc Natl Acad Sci U S A. 2003;100:14531–14536. doi: 10.1073/pnas.2235619100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Vandewauw I, Owsianik G, Voets T. Systematic and quantitative mRNA expression analysis of TRP channel genes at the single trigeminal and dorsal root ganglion level in mouse. BMC Neurosci. 2013;14:21. doi: 10.1186/1471-2202-14-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Liedtke W, Friedman JM. Abnormal osmotic regulation in trpv4-/- mice. Proc Natl Acad Sci U S A. 2003;100:13698–13703. doi: 10.1073/pnas.1735416100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Alessandri-Haber N, Joseph E, Dina OA, Liedtke W, Levine JD. TRPV4 mediates pain-related behavior induced by mild hypertonic stimuli in the presence of inflammatory mediator. Pain. 2005;118:70–79. doi: 10.1016/j.pain.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 279.Alessandri-Haber N, Dina OA, Joseph EK, Reichling D, Levine JD. A transient receptor potential vanilloid 4-dependent mechanism of hyperalgesia is engaged by concerted action of inflammatory mediators. J Neurosci. 2006;26:3864–3874. doi: 10.1523/JNEUROSCI.5385-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Zhao P, Lieu T, Barlow N, Sostegni S, Haerteis S, Korbmacher C, et al. Neutrophil elastase activates protease-activated receptor-2 (par2) and transient receptor potential vanilloid 4 (trpv4) to cause inflammation and pain. J Biol Chem. 2015;290:13875–13887. doi: 10.1074/jbc.M115.642736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Denadai–Souza A, Martin L, de Paula MA, de Avellar MC, Muscara MN, Vergnolle N, et al. Role of transient receptor potential vanilloid 4 in rat joint inflammation. Arthritis Rheum 2012, 64: 1848–1858. [DOI] [PubMed]
- 282.O’Conor CJ, Ramalingam S, Zelenski NA, Benefield HC, Rigo I, Little D, et al. Cartilage-specific knockout of the mechanosensory ion channel TRPV4 decreases age-related osteoarthritis. Sci Rep. 2016;6:29053. doi: 10.1038/srep29053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Moore C, Cevikbas F, Pasolli HA, Chen Y, Kong W, Kempkes C, et al. UVB radiation generates sunburn pain and affects skin by activating epidermal TRPV4 ion channels and triggering endothelin-1 signaling. Proc Natl Acad Sci U S A. 2013;110:E3225–E3234. doi: 10.1073/pnas.1312933110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Alessandri-Haber N, Dina OA, Yeh JJ, Parada CA, Reichling DB, Levine JD. Transient receptor potential vanilloid 4 is essential in chemotherapy-induced neuropathic pain in the rat. J Neurosci. 2004;24:4444–4452. doi: 10.1523/JNEUROSCI.0242-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Chen Y, Yang C, Wang ZJ. Proteinase-activated receptor 2 sensitizes transient receptor potential vanilloid 1, transient receptor potential vanilloid 4, and transient receptor potential ankyrin 1 in paclitaxel-induced neuropathic pain. Neuroscience. 2011;193:440–451. doi: 10.1016/j.neuroscience.2011.06.085. [DOI] [PubMed] [Google Scholar]
- 286.Kanju P, Chen Y, Lee W, Yeo M, Lee SH, Romac J, et al. Small molecule dual-inhibitors of TRPV4 and TRPA1 for attenuation of inflammation and pain. Sci Rep. 2016;6:26894. doi: 10.1038/srep26894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Ding XL, Wang YH, Ning LP, Zhang Y, Ge HY, Jiang H, et al. Involvement of TRPV4-NO-cGMP-PKG pathways in the development of thermal hyperalgesia following chronic compression of the dorsal root ganglion in rats. Behav Brain Res. 2010;208:194–201. doi: 10.1016/j.bbr.2009.11.034. [DOI] [PubMed] [Google Scholar]
- 288.Wang C, Ning LP, Wang YH, Zhang Y, Ding XL, Ge HY, et al. Nuclear factor-kappa B mediates TRPV4-NO pathway involved in thermal hyperalgesia following chronic compression of the dorsal root ganglion in rats. Behav Brain Res. 2011;221:19–24. doi: 10.1016/j.bbr.2011.02.028. [DOI] [PubMed] [Google Scholar]
- 289.Qu YJ, Zhang X, Fan ZZ, Huai J, Teng YB, Zhang Y, et al. Effect of TRPV4-p38 MAPK pathway on neuropathic pain in rats with chronic compression of the dorsal root ganglion. Biomed Res Int. 2016;2016:6978923. doi: 10.1155/2016/6978923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Brierley SM, Page AJ, Hughes PA, Adam B, Liebregts T, Cooper NJ, et al. Selective role for TRPV4 ion channels in visceral sensory pathways. Gastroenterology. 2008;134:2059–2069. doi: 10.1053/j.gastro.2008.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Sipe WE, Brierley SM, Martin CM, Phillis BD, Cruz FB, Grady EF, et al. Transient receptor potential vanilloid 4 mediates protease activated receptor 2-induced sensitization of colonic afferent nerves and visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1288–G1298. doi: 10.1152/ajpgi.00002.2008. [DOI] [PubMed] [Google Scholar]
- 292.Cenac N, Bautzova T, Le Faouder P, Veldhuis NA, Poole DP, Rolland C, et al. Quantification and potential functions of endogenous agonists of transient receptor potential channels in patients with irritable bowel syndrome. Gastroenterology. 2015;149(433–444):e437. doi: 10.1053/j.gastro.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 293.Ceppa E, Cattaruzza F, Lyo V, Amadesi S, Pelayo JC, Poole DP, et al. Transient receptor potential ion channels V4 and A1 contribute to pancreatitis pain in mice. Am J Physiol Gastrointest Liver Physiol. 2010;299:G556–G571. doi: 10.1152/ajpgi.00433.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Zhang LP, Ma F, Abshire SM, Westlund KN. Prolonged high fat/alcohol exposure increases TRPV4 and its functional responses in pancreatic stellate cells. Am J Physiol Regul Integr Comp Physiol. 2013;304:R702–R711. doi: 10.1152/ajpregu.00296.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Zhang LP. Kline RHt, Deevska G, Ma F, Nikolova-Karakashian M, Westlund KN. Alcohol and high fat induced chronic pancreatitis: TRPV4 antagonist reduces hypersensitivity. Neuroscience. 2015;311:166–179. doi: 10.1016/j.neuroscience.2015.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Wei X, Edelmayer RM, Yan J, Dussor G. Activation of TRPV4 on dural afferents produces headache-related behavior in a preclinical rat model. Cephalalgia. 2011;31:1595–1600. doi: 10.1177/0333102411427600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, et al. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci U S A. 2007;104:13525–13530. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Kim S, Barry DM, Liu XY, Yin S, Munanairi A, Meng QT, et al. Facilitation of TRPV4 by TRPV1 is required for itch transmission in some sensory neuron populations. Sci Signal 2016, 9: ra71. [DOI] [PMC free article] [PubMed]


