Abstract
Pancreatic cancer is projected to become the leading cause of cancer deaths by 2050. The risk for pancreatic cancer may be reduced by up to 27% by modifying lifestyle risk factors, most notably tobacco smoking. Based on analysis of more than 2 million unselected individuals from general population, this article quantified the risk of pancreatic cancer in relation to lifelong tobacco smoking and alcohol consumption status, both alone and in combination. It also provided a state-of-the-art review of animal studies on the effect of tobacco smoke and alcohol on genetically engineered mouse models of pancreatic precursor lesions, as well as the role of pancreatic stellate cells and immune cells in pancreatic carcinogenesis activated by tobacco and alcohol.
Keywords: pancreatic cancer, tobacco, alcohol, population risk, pancreatic stellate cells
Introduction
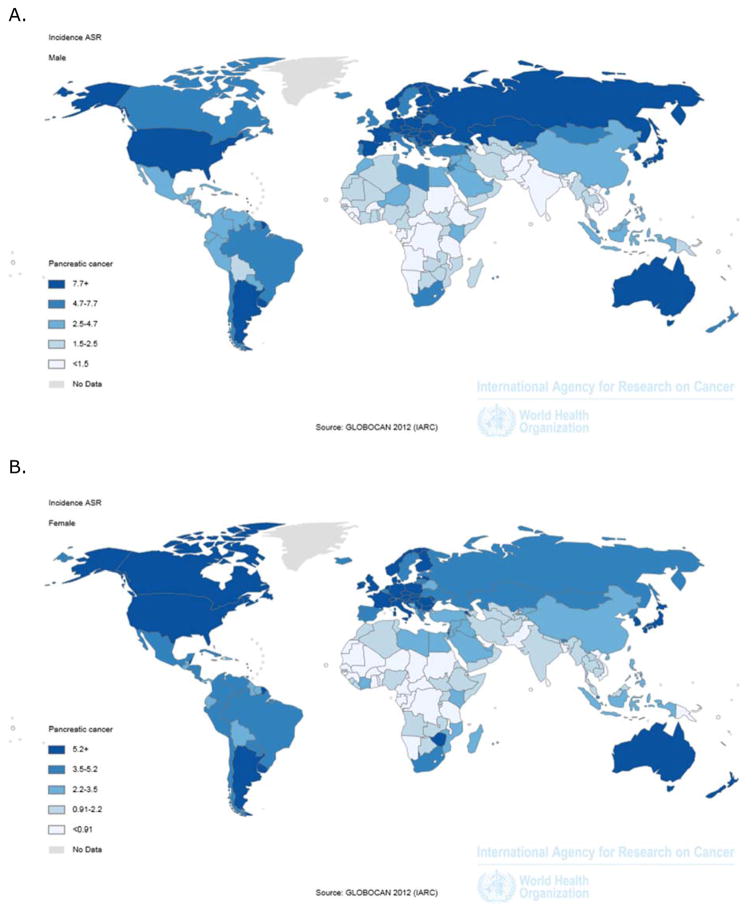
Pancreatic cancer is the second most common gastrointestinal cancer (after colorectal cancer) in North America [1] and in Europe [2]. An estimated 426,000 persons were newly diagnosed with pancreatic cancer globally in year 2015 alone [3]. The worldwide incidence of pancreatic cancer in the general population is estimated at 8.1 per 100,000 person-years [4], and is significantly higher among men than women (Figure 1). Further, pancreatic cancer incidence is significantly higher in North America than the rest of the world [4]. Given that early detection of pancreatic cancer in the general population is virtually impossible and taking into account the absence of effective therapies, prevention is currently the only way to meaningfully reduce the burden of this disease. Lifestyle factors markedly affect the risk of pancreatic cancer and it was estimated that more than a quarter of pancreatic cancer cases may have been prevented if all people in the general population were non-smokers and had limited alcohol consumption, healthy diet, normal weight, and regular physical activity [5].
Figure 1.
Incidence of pancreatic cancer worldwide in 2012 among men (A) and women (B) according to data from the International Agency for Research on Cancer
Tobacco smoking and alcohol consumption are the second and third leading risk factors of death globally, according to a study estimating the disease burden attributable to 43 risk factors in 2010 [6]. The global prevalence of current tobacco smoking was estimated to be 36% in males and 7% in females in 2015 [7]. Although prevalence of smoking has decreased considerably across the world in both low-to-middle income countries and in high-income countries (except in African and Eastern Mediterranean regions) [8], absolute number of smokers have increased with nearly one billion daily smokers worldwide in 2012. Global prevalence of heavy episodic drinking among persons 15 years and older is estimated at 7.5% (16% of drinkers), while prevalence of abstinence (no alcoholic drink within 12 months) was estimated at 62% [9]. Further, estimates for heavy episodic drinking were higher in Europe (17%) and the Americas (14%) as compared with less than 2% in the Eastern Mediterranean and South East Asian regions. Unlike smoking trends, alcohol consumption has not changed drastically in recent decades. Globally alcohol consumption has increased on average from 2005 to 2010, although decreases in consumption in certain Asian and African countries have been noted [9].
In the context of pancreatic cancer, tobacco smoking is considered the most important risk factor [10], with population attributable risk of 14%, and alcohol consumption is the fourth most important risk factor, with population attributable risk of 3% [5]. Further, given that smoking behaviour is often associated with alcohol consumption, it is plausible that there is effect modification between smoking and alcohol in affecting the risk of pancreatic cancer [10].
It should be noted that pancreatic cancer is an umbrella term that includes several malignancies. About 90% of these cancers are pancreatic ductal adenocarcinomas (PDACs). The most common precursor lesions to PDAC are pancreatic intra-epithelial neoplasia (PanIN) that progress from stage IA to stage III and ultimately to PDAC [11]. PanIN arise from acinar to ductal metaplasia (ADM) lesions [11], and stage I–II PanIN may occur in patients who never develop PDAC. By contrast, stage III PanIN lesions have a high propensity to become PDAC. Importantly, 90 to 95% of PDAC harbor a mutated KRAS, and this mutation is the major contributor to PDAC initiation [12], progression and metastasis [13,14].
Critique of study designs of tobacco, alcohol and risk for pancreatic cancer
Multiple studies have reported on associations between tobacco smoking and risk of pancreatic cancer as well as alcohol consumption and risk of pancreatic cancer. However, their findings must be interpreted with caution because study designs were often suboptimal to investigate the associations between exposure to smoking and/or alcohol and subsequent risk of pancreatic cancer [10]. In particular, case-control studies should be interpreted with caution. They are frequently used because this study design requires considerably less time and funding to achieve adequate statistical power than a prospective cohort study. However, given late diagnosis and dismal prognosis of pancreatic cancer, majority of individuals become physically unable to participate in epidemiological studies or pass away before they can be interviewed regarding exposure factors. This violates the fundamental assumption of the case-control design, i.e. that the selected cases and controls must unbiasedly represent the underlying cohort. The other common problem with case-control studies is survivor bias, which occurs when the exposure (specifically, tobacco smoking and/or alcohol consumption) and the outcome (specifically, incidence of pancreatic cancer) are conditionally associated with survival time. Case losses in pancreatic cancer case-control studies could therefore introduce survivor bias and lead to a significant deviation in statistical estimates from the true associations in the general population [15]. For example, given that smoking is associated with shorter mortality in general, survivor bias could potentially underestimate the prevalence of smoking in pancreatic cancer cases. Also, recall bias is another common problem with investigations on smoking and alcohol, in particular retrospective studies, because cases tend to recall past exposures differently than control do [16]. Epidemiologic research on risk factors for pancreatic cancer that is conducted on special cohorts (e.g., veterans only, nurses only, insurance claims) may introduce a selection bias and, hence, may not be generalisable [17]. Cohort studies in general population is arguably the best available study design but also have limitations as they require extremely long follow-up before enough pancreatic cancer cases have accrued, during which time alcohol consumption and tobacco smoking habits may have changed.
Exposure to tobacco and alcohol and risk for pancreatic cancer in general population
To provide the most robust quantifiable data on the effect of smoking and alcohol on risk of pancreatic cancer, we conducted a literature search in PubMed for articles published between January 1, 2000, and July 1, 2017. The inclusion criteria were all of the following:
study design: prospective, population-based, cohort studies;
study population: adult individuals of both sexes in a given geographic area in a given period of time;
exposure: tobacco smoking and/or alcohol consumption
outcome: incident case of pancreatic cancer
Studies were excluded if:
they were retrospective cohort, (nested) case-control, cross-sectional, or interventional studies; or
study population was not representative of the general population (e.g., cohorts constrained to 1 gender only or a particular ethnicity; insurance claims, secondary or tertiary settings); or
small sample size, arbitrarily defined as the total size of the cohort of less than 25,000 or
they were conducted in animals; or
risk of developing pancreatic cancer was not reported, and/or it was not possible to reconstruct a 2×2 contingency table and meta-analyze data.
If tobacco smoking or alcohol consumption was investigated in more than 1 study derived from the same cohort, the study with the most complete information on either of the exposures was selected. StatsDirect was used to generate forest plots and pooled relative risks (RR) with corresponding 95% confidence intervals (CI). Data were statistically aggregated using the DerSimonian-Laird random-effects model to account for potential random variation of associations between study populations.
A total of 12 prospective, population-based, cohort studies met all the eligibility criteria [18–29]. Of them, 11 investigated smoking as risk factor for pancreatic cancer, 5 – alcohol as risk factor for pancreatic cancer, and 3 - interaction of smoking with alcohol as risk for pancreatic cancer (Table 1).
Table 1.
Characteristics of the included studies
| Study ID | Country | Total cohort size | No of pancreatic cancer cases | Risk factor | Suitable for meta-analysis | ||||
|---|---|---|---|---|---|---|---|---|---|
| Smoking | Alcohol | Both | Smoking | Alcohol | Both | ||||
| Gapstur et al., 2000 [18] | USA | 35,658 | 139 | Yes | No | No | Yes | No | No |
| Lin et al., 2002 [19] | Japan | 110,792 | 225 | Yes | No | No | Yes | No | No |
| Larsson et al., 2005 [20] | Sweden | 83,506 | 131 | Yes | No | No | Yes | No | No |
| Gallicchio et al., 2006 [21] | USA | 48,172 | 92 | Yes | No | No | Yes | No | No |
| Luo et al., 2007 [22] | Japan | 99,670 | 224 | Yes | Yes | No | Yes | No | No |
| Arnold et al., 2009 [23] | USA | 1,060,389 | 6,243 | Yes | No | No | Yes | No | No |
| Jiao et al., 2009 [24] | USA | 450,416 | 1,057 | Yes | Yes | Yes | No | Yes | No |
| Johansen et al., 2009 [25] | Sweden | 33,346 | 183 | Yes | Yes | Yes | Yes | No | No |
| Gapstur et al., 2011 [26] | USA | 1,030,467 | 6,847 | No | Yes | Yes | No | Yes | No |
| Nakamura et al., 2011 [27] | Japan | 30,826 | 52 | Yes | Yes | No | Yes | No | No |
| Meyer et al, 2015 [28] | Switzerland | 35,784 | 127 | Yes | No | No | Yes | No | No |
| Andersson et al., 2016 [29] | Sweden | 28,098 | 163 | Yes | No | No | Yes | No | No |
Smoking as risk factor for pancreatic cancer
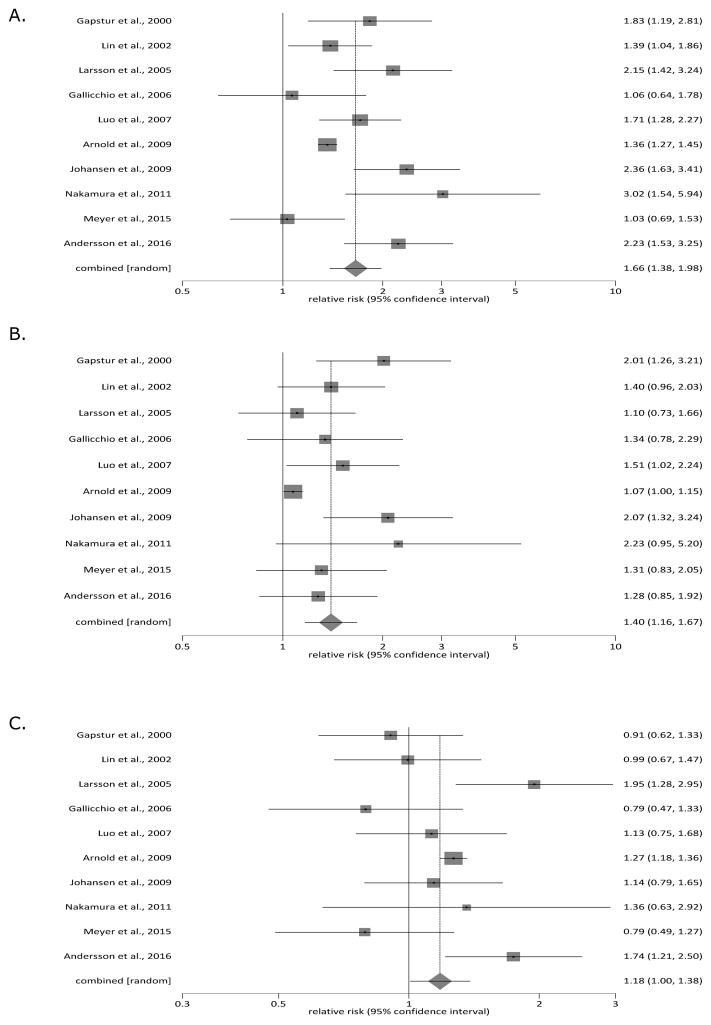
Ten of the eligible studies were suitable for meta-analysis of smoking as risk factor for pancreatic cancer. In the pooled cohort, a total of 366,653 individuals were current smokers, 361,944 – former smokers, and 675,400 – never smokers. Current smokers had the pooled RR of 1.66 (95% CI 1.38 to 1.98) when compared with never smokers (p < 0.05), and the pooled RR of 1.18 (95% CI 1.00 to 1.38) when compared with former smokers (p < 0.05). Former smokers had the pooled RR of 1.40 (95% CI 1.16 to 1.67) when compared with never smokers (p < 0.05). Figure 2 details the pair-wise comparisons between the 3 studied subgroups. Statistical heterogeneity (as eveidenced by I2 metric) was 70%, 47%, and 63%, correspondingly.
Figure 2.
Tobacco smoking status and risk factor for pancreatic cancer: (A) current smokers versus never smokers, (B) former smokers versus never smokers, and (C) current smokers versus former smokers
Alcohol as risk factor for pancreatic cancer
Two of the eligible studies were suitable for meta-analysis of smoking as risk factor for pancreatic cancer. In the pooled cohort, a total of 133,213 individuals were heavy drinkers, 685,428 – light drinkers, and 682,497 – never drinkers. Heavy drinkers had the pooled RR of 1.29 (95% CI 1.20 to 1.38) when compared with never drinkers (p < 0.05), and the pooled RR of 1.36 (95% CI 1.02 to 1.80) when compared with light drinkers (p < 0.05). Light drinkers had the pooled RR of 0.96 (95% CI 0.75 to 1.22) when compared with never drinkers. Figure 3 details the pair-wise comparisons between the 3 studied subgroups. Statistical heterogeneity (as eveidenced by I2 metric) was 28%, 87%, and 91%, correspondingly.
Figure 3.
Alcohol consumption and risk factor for pancreatic cancer: (A) heavy drinkers versus never drinkers, (B) light drinkers versus never drinkers, and (C) heavy drinkers versus light drinkers
Interaction of smoking with alcohol as risk for pancreatic cancer
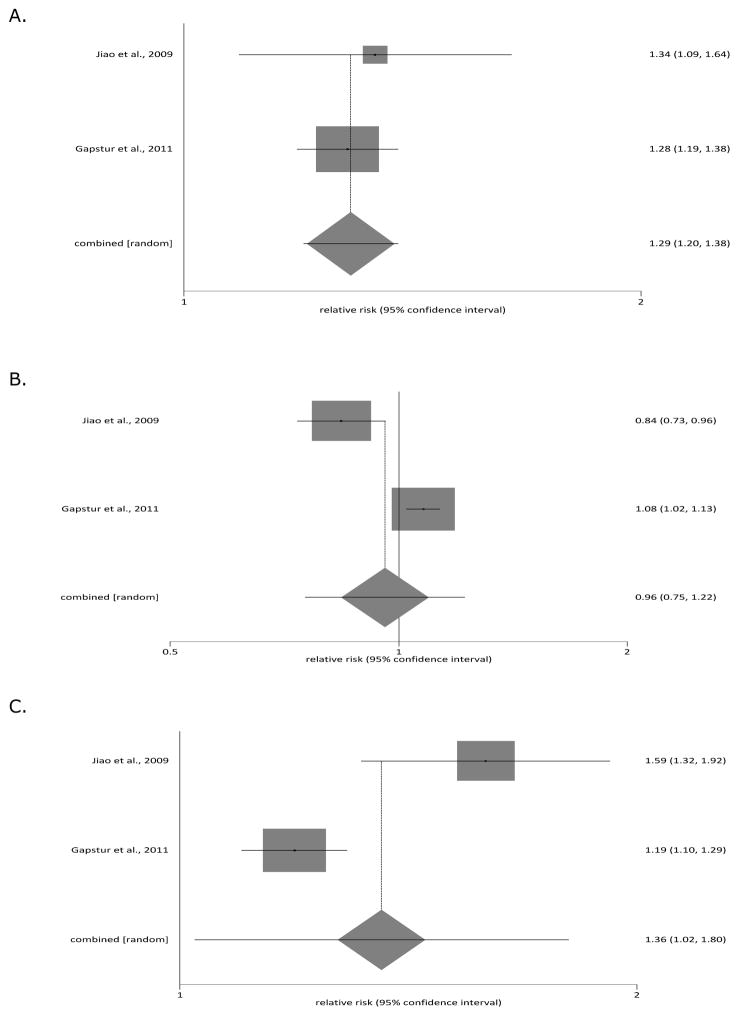
Three prospective, population-based, cohort studies investigated interaction between smoking and alcohol as risk factor for pancreatic cancer but none was suitable for meta-analysis. The study by Jiao et al. [24] followed up a total of 450,416 individuals in the USA (who originally resided in 1 of 6 states - California, Florida, Louisiana, New Jersey, North Carolina, and Pennsylvania) who reported servings of alcohol use per day for total beverages and stratified their analysis by smoking status. One serving (1 drink) was defined on the basis of the US Department of Agriculture’s Food Guide Pyramid as 12 fluid ounces of regular beer (12.96 g of alcohol), 5 fluid ounces of wine (13.72 g of alcohol), or 1.5 ounces of 80 proof distilled spirits liquor (13.93 g of alcohol). In multivariate analysis (adjusted for sex, total energy intake, energy-adjusted saturated fat, red meat, and total folate intake, body mass index, physical activity, and history of diabetes), the authors found a statistically significant increase in the risk of pancreatic cancer among ever smokers, but not never smokers. Compared with that for light use (i.e. less than 1 drink per day), the relative risk for heavy total alcohol use (i.e. at least 3 drinks per day) was 1.50 (95% CI 1.18 to 1.90) in ever smokers. Specifically, the relative risk was 1.54 (95% CI: 1.11 to 2.13) in current smokers and recent quitters and 1.41 (95% CI 1.01 to 2.00) in those who quit at least 10 years ago. Compared with that for light use, the relative risk for heavy total alcohol use was 1.35 (95% CI 0.79 to 2.30) in never smokers. There were no statistically significant interactions of alcohol use on risk of pancreatic cancer by smoking status (p interaction = 0.25) or the number of years since quit smoking (p interaction = 0.61).
The study by Johansen et al. [25] followed up a total of 33,346 individuals in Sweden who reported a modified version of the Michigan Alcoholism Screening Test (which contained 7 questions regarding drinking habits but no questions on absolute amounts of alcohol intake). In multivariate analysis (adjusted for age, sex, body mass index), the authors found a statistically significant increase in the risk of pancreatic cancer among former smokers, but not never smokers or current smokers. Compared with that for low alcohol consumption (i.e. score 0–1), the relative risk for intermediate or heavy alcohol consumption (i.e. score 2 or above) was 2.13 (95% CI 1.05 to 4.32) in former smokers; 1.39 (95% CI 0.91 to 2.12) in current smokers; and 1.31 (95% CI 0.63 to 2.72) in never smokers. There was no statistically significant interaction of alcohol use on risk of pancreatic cancer by smoking status (p interaction = 0.29).
The study by Gapstur et al. [26] followed up a total of 1,030,467 individuals in the USA (who came from all the US states, the District of Columbia, and Puerto Rico) who completed a questionnaire detailing absolute amounts of alcohol intake. In multivariate analysis (adjusted for age, sex, ethnicity, education, marital status, body mass index, family history of pancreatic cancer, and personal history of gallstones and diabetes mellitus), the authors found a statistically significant increase in the risk of pancreatic cancer among both ever smokers and never smokers. Compared with that for non-drinkers, the relative risk for heavy alcohol consumption (i.e. at least 3 drinks per day) was 1.36 (95% CI 1.13 to 1.62) in never smokers and 1.22 (95% CI 1.12 to 1.34) in ever smokers. In further subgroup analyses, the combined effect of smoking and alcohol on risk of pancreatic cancer remained statistically significant only in those ever smokers who reported heavy consumption of liquor (relative risk 1.18; 95% CI 1.03 to 1.35) but not beer (relative risk 1.08; 95% CI 0.92 to 1.26) or wine (relative risk 0.98; 95% CI 0.76 to 1.26). There was no statistically significant interaction of alcohol use on risk of pancreatic cancer by smoking status (p interaction = 0.58).
Mechanisms of effects of tobacco and alcohol
Given that pancreas is one of the least accessible organs and taking into account that pancreatic tissues are rarely availble for studies, mechanisms of effects of tobacco and alcohol has been maiinly investigated in animal studies [30–34]. Administration of tobacco to healthy rodents does not cause pancreatic cancer in rats. Nonetheless, rats exposed for four months to daily cigarette smoke exhibit acinar cell loss, increased pancreatic matrix deposition and infiltration of the pancreas with CD45-positive immune cells [35]. Importantly, CD45 is a receptor-like tyrosine phosphatase that is crucial for proper B and T cell function [36], highlighting the ability of tobacco smoke tobacco smoke to alter the immune microenvironment within the pancreas.
The effects of cigarette smoke have also been examined in genetically engineered mouse models (GEMMs) of PDAC. The most studied model in this regard consists of mice designed to express the oncogenic Kas protein in the pancreas (termed KC mice) [30]. These studies revealed that that tobacco exerts dramatic effects in the pancreas. Thus, exposure of KC mice to cigarette smoke (for 20 weeks) enhances ADM formation and accelerates PanIN development in conjunction with decreased number of myeloid-derived suppressor cells (MDSCs) and an increase in the number of M2 macrophages and dendritic cells in the pancreas [37], underscoring the deleterious effects of cigarette smoke on the immune system in the pancreatic microenvironment that has already been compromised by the expression of oncogenic Kras.
The trans-differentiation of acinar cells to ADM precursor lesions requires the loss of acinar cell identity, and the latter is dictated by expression of several transcription factors including Gata6, Mist1, and PTf1 [38,39]. When administered prior to tumor formation, nicotine accelerates PDAC formation in several types of GEMMs due to the activation of an AKT-ERK-MYC signaling cascade that promotes ADM formation by decreasing Gata6 expression and inducing acinar cell de-differentiation [40]. In these GEMMs, when nicotine is administered once PDAC is established, there is enhanced epithelial–mesenchymal transition (EMT) and a greater propensity to form hepatic metastases. Moreover, in KC mice, exposure to cigarette smoke promotes PanIN and stroma formation, EMT, and appearance of M2 macrophages, and these effects can be reversed by the HDAC inhibitor suberoylanilide hydroxamic acid (SAHA), in agreement with the ability of cigarette smoke to decrease histone acetylation [41]. Taken together, the above studies support the concept that prolonged exposure to tobacco smoke over many years, as seen in humans, can lead to pancreatic malignant transformation.
As in the case of tobacco, alcohol per se has not been shown to cause neoplastic transformation in the rodent pancreas. However, alcohol was shown to promote an increase in the number of M2 macrophages within PDAC in the KC GEMM, and to slightly enhance fibrosis [42]. This is an important observation since M2 macrophages promote cancer progression by suppressing cancer directed immune mechanisms and impeding T cell recruitment into the tumor [43]. Moreover, caerulein causes stellate cell activation and contributes to PDAC fibrosis, but also induces histone deacetylation, and this latter effect is enhanced by alcohol [42]. Histone deacetylation represses gene transcription and may promote loss of tumor suppressor gene functions.
Present challenges and future directions
Cessation of smoking for 10 or more years returns the relative risk of pancreatic cancer to levels equivalent to non-smoker [44,45]. However, communication to the public of the harmful effect of tobacco smoking and the beneficial effect of quitting is poor. In this regard, rate advancement periods is a useful statistic for lay people. A recent study showed that being a current smoker (compared with never smoking) increases the risk of developing pancreatic cancer by more than 7 years [46]. Quitting smoking within 10 years (compared with not quitting) delays the risk of development of pancreatic cancer by more than 3 years; within 20 years – by more than 5 years; and quitting for more than 20 years – by more than 10 years [46].
Studies on associations between alcohol consumption and risk for pancreatic cancer have largely focused on average drinking over lifetime or drinking at baseline or recent drinking [47], but it is unknown how changes in alcohol consumption during the follow-up period (in particular, cessation of drinking and drinking during adolescence/young adulthood) is associated with risk for pancreatic cancer. Given that alcohol cessation leads to reductions in risk of recurrent acute pancreatitis [48] and taking into account the recurrent acute pancreatitis-chronic pancreatitis-pancreatic cancer continuum [49,50], future studies should investigate whether alcohol cessation also reduces the risk of pancreatic cancer.
Little is known about the direct effects of tobacco products and their metabolites on pancreatic stellate cells activation and secretion. Considering the evidence presented above, it would be very useful to determine how these compounds could regulate pancreatic stellate cells and contribute to the microenvironment within the pancreas [32,34].
Most studies published to date assumed additivity between average effects of tobacco and alcohol, and hence oversimplified their impact on burden of pancreatic cancer. Carefully designed modelling studies on hypothetical changes in lifestyle risk factors at the population-level are warranted to examine the interactions between smoking, alcohol (and metabolic conditions such adiposity and diabetes [51,52]) and their impact on burden of pancreatic cancer. Such studies will need to be large enough to detect multiplicative interaction between tobacco, alcohol (and metabolic factors) and have sufficient number of cases for pancreatic cancer in each permutation risk factors.
To date, high quality population-based studies on tobacco and alcohol as risk factors for pancreatic cancer have almost invariably come from only 3 countries – USA, Japan, and Sweden. Studies from other part of the world would greatly enrich the knowledge on these relationships and should be viewed favorably by funding agencies [4,10].
Large prospective cohort studies of individuals at high risk of pancreatic cancer, such as patients with pancreatitis or diabetes of the exocrine pancreas [53–55], will provide opportunities to accurately measure exposure to smoking and alcohol in a standardized manner and allow for an analysis of the synergistic impact of alcohol and smoking, as well as metabolic disorders.
Summary
The last two decades have witnessed the appearance of large population-based studies that established tobacco smoking as the strongest modifiable risk factor for pancreatic cancer. This opens up real opportunities for primary prevention. Progress has been slow and difficult for understanding the molecular pathogenesis of pancreatic cancer. However, GEMMs including lifestyle risk factors such as tobacco and alcohol are positioned well to provide deeper insights into the complex interplay between the microenvironment and the cancer cells, which may lead to important discoveries of drugs that target molecular pathways that are activated by tobacco and alcohol.
Practice Points.
A large body of evidence consistently demonstrates that tobacco smoking status is significantly associated with risk of developing pancreatic cancer in general population
Current smokers have a 66% higher risk of pancreatic cancer and former smokers have a 40% higher risk of pancreatic cancer in comparison with never smokers
A limited body of evidence shows that alcohol consumption status may be associated with risk of developing pancreatic cancer but findings of population-based studies are inconsistent
The combined effect of smoking and total alcohol on risk of pancreatic cancer is likely to be non-additive; as an example only heavy consumption of liquor, but not wine or beer, increases the risk of pancreatic cancer in ever smokers
Research Agenda.
The recurrent acute pancreatitis-chronic pancreatitis-pancreatic cancer continuum is a valuable framework to adopt in future studies on pathogenesis of pancreatic cancer
Time since smoking cessation, smoking intensity, and duration need to be investigated as comprehensively as lifelong smoking status
Adequately powered epidemiological studies are warranted to examine the association between alcohol consumption and risk for pancreatic cancer by tobacco smoking status, most pressingly in lifelong never smokers
Given that incidence of pancreatic cancer in general population is low, (inter)national consortiums will help achieve the sample size needed
Footnotes
Conflict of interest & financial disclosure
Research reported in this publication was supported by National Cancer Institute and National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award numbers U01DK108323 and U01DK108314. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Cancer Society. Cancer Facts & Figures 2017. Atlanta, GA: American Cancer Society; 2017. [Google Scholar]
- 2.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 3.Global Burden of Disease Cancer C. Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3:524–48. doi: 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiao AY, Tan ML, Wu LM, Asrani VM, Windsor JA, Yadav D, et al. Global incidence and mortality of pancreatic diseases: a systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol Hepatol. 2016;1:45–55. doi: 10.1016/S2468-1253(16)30004-8. [DOI] [PubMed] [Google Scholar]
- 5.Jiao L, Mitrou PN, Reedy J, Graubard BI, Hollenbeck AR, Schatzkin A, Stolzenberg-Solomon R. A combined healthy lifestyle score and risk of pancreatic cancer in a large cohort study. Arch Intern Med. 2009;169:764–70. doi: 10.1001/archinternmed.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim SS, Vos T, Flaxman AD, Danaei G, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–60. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. WHO report on the global tobacco epidemic, 2015: raising taxes on tobacco. Geneva: World Health Organization; 2015. p. 198. [Google Scholar]
- 8.Bilano V, Gilmour S, Moffiet T, d’Espaignet ET, Stevens GA, Commar A, et al. Global trends and projections for tobacco use, 1990–2025: an analysis of smoking indicators from the WHO Comprehensive Information Systems for Tobacco Control. Lancet. 2015;385:966–76. doi: 10.1016/S0140-6736(15)60264-1. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. Global status report on alcohol and health 2014. Geneva: World Health Organization; 2014. p. xiv.p. 376. [Google Scholar]
- 10.Alsamarrai A, Das SL, Windsor JA, Petrov MS. Factors that affect risk for pancreatic disease in the general population: a systematic review and meta-analysis of prospective cohort studies. Clin Gastroenterol Hepatol. 2014;12:1635–44. doi: 10.1016/j.cgh.2014.01.038. [DOI] [PubMed] [Google Scholar]
- 11.Maitra A, Fukushima N, Takaori K, et al. Precursors to invasive pancreatic cancer. Adv Anat Pathol. 2005;12:81–91. doi: 10.1097/01.pap.0000155055.14238.25. [DOI] [PubMed] [Google Scholar]
- 12.Kleeff J, Korc M, Apte M, et al. Pancreatic cancer. Nat Rev Dis Primers. 2016;21:16022. doi: 10.1038/nrdp.2016.22. [DOI] [PubMed] [Google Scholar]
- 13.Collins MA, Bednar F, Zhang Y, et al. Oncogenic Kras is required for both the initiation and maintenance of pancreatic cancer in mice. J Clin Invest. 2012;122:639–65. doi: 10.1172/JCI59227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins MA, Brisset JC, Zhang, et al. Metastatic pancreatic cancer is dependent on oncogenic Kras in mice. PLOS One. 2012;7:e49707. doi: 10.1371/journal.pone.0049707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu ZH, Connett JE, Yuan JM, Anderson KE. Role of survivor bias in pancreatic cancer case-control studies. Ann Epidemiol. 2016;26:50–6. doi: 10.1016/j.annepidem.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wacholder S, Silverman DT, McLaughlin JK, Mandel JS. Selection of controls in case-control studies. III. Design options. Am J Epidemiol. 1992;135:1042–50. doi: 10.1093/oxfordjournals.aje.a116398. [DOI] [PubMed] [Google Scholar]
- 17.Hernán MA, Hernández-Díaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15:615–25. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 18.Gapstur SM, Gann PH, Lowe W, et al. Abnormal glucose metabolism and pancreatic cancer mortality. JAMA. 2000;283:2552–8. doi: 10.1001/jama.283.19.2552. [DOI] [PubMed] [Google Scholar]
- 19.Lin Y, Tamakoshi A, Kawamura T, et al. A prospective cohort study of cigarette smoking and pancreatic cancer in Japan. Cancer Causes Control. 2002;13:249–54. doi: 10.1023/a:1015052710213. [DOI] [PubMed] [Google Scholar]
- 20.Larsson SC, Permert J, Hakansson N, et al. Overall obesity, abdominal adiposity, diabetes and cigarette smoking in relation to the risk of pancreatic cancer in two Swedish populationbased cohorts. Br J Cancer. 2005;93:1310–1315. doi: 10.1038/sj.bjc.6602868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallicchio L, Kouzis A, Genkinger JM, et al. Active cigarette smoking, household passive smoke exposure, and the risk of developing pancreatic cancer. Prev Med. 2006;42:200–5. doi: 10.1016/j.ypmed.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 22.Luo J, Iwasaki M, Inoue M, et al. Body mass index, physical activity and the risk of pancreatic cancer in relation to smoking status and history of diabetes: a large-scale population-based cohort study in Japan—the JPHC study. Cancer Causes Control. 2007;18:603–12. doi: 10.1007/s10552-007-9002-z. [DOI] [PubMed] [Google Scholar]
- 23.Arnold LD, Patel AV, Yan Y, et al. Are racial disparities in pancreatic cancer explained by smoking and overweight/obesity? Cancer Epidemiol Biomarkers Prev. 2009;18:2397–405. doi: 10.1158/1055-9965.EPI-09-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiao L, Silverman DT, Schairer C, et al. Alcohol use and risk of pancreatic cancer: the NIH-AARP Diet and Health Study. Am J Epidemiol. 2009;169:1043–51. doi: 10.1093/aje/kwp034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johansen D, Borgstrom A, Lindkvist B, et al. Different markers of alcohol consumption, smoking and body mass index in relation to risk of pancreatic cancer: a prospective cohort study within the Malmo Preventive Project. Pancreatology. 2009;9:677–686. doi: 10.1159/000212088. [DOI] [PubMed] [Google Scholar]
- 26.Gapstur SM, Jacobs EJ, Deka A, et al. Association of alcohol intake with pancreatic cancer mortality in never smokers. Arch Intern Med. 2011;171:444–51. doi: 10.1001/archinternmed.2010.536. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura K, Nagata C, Wada K, et al. Cigarette smoking and other lifestyle factors in relation to the risk of pancreatic cancer death: a prospective cohort study in Japan. Jpn J Clin Oncol. 2011;41:225–31. doi: 10.1093/jjco/hyq185. [DOI] [PubMed] [Google Scholar]
- 28.Meyer J, Rohrmann S, Bopp M, Faeh D Swiss National Cohort Study Group. Impact of Smoking and Excess Body Weight on Overall and Site-Specific Cancer Mortality Risk. Cancer Epidemiol Biomarkers Prev. 2015;24:1516–22. doi: 10.1158/1055-9965.EPI-15-0415. [DOI] [PubMed] [Google Scholar]
- 29.Andersson G, Wennersten C, Borgquist S, Jirström K. Pancreatic cancer risk in relation to sex, lifestyle factors, and pre-diagnostic anthropometry in the Malmö Diet and Cancer Study. Biol Sex Differ. 2016 Dec 9;7:66. doi: 10.1186/s13293-016-0120-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Apte MV, Wilson JS, Lugea A, Pandol SJ. A starring role for stellate cells in the pancreatic cancer microenvironment. Gastroenterology. 2013;144:1210–1219. doi: 10.1053/j.gastro.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Habtezion A, Edderkaoui M, Pandol SJ. Macrophages and pancreatic ductal adenocarcinoma. Cancer Lett. 2016;381:211–6. doi: 10.1016/j.canlet.2015.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pandol S, Gukovskaya A, Edderkaoui M, Edderkoui M, Dawson D, Eibl G, Lugea A. Epidemiology, risk factors, and the promotion of pancreatic cancer: role of the stellate cell. J Gastroenterol Hepatol. 2012;27(Suppl 2):127–134. doi: 10.1111/j.1440-1746.2011.07013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.di Magliano MP, Logsdon CD. Roles for KRAS in pancreatic tumor development and progression. Gastroenterology. 2013;144:1220–1229. doi: 10.1053/j.gastro.2013.01.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pandol S, Gukovskaya A, Edderkaoui M, Dawson D, Eibl G, Lugea A. Epidemiology, risk factors, and the promotion of pancreatic cancer: role of the stellate cell. J Gastroenterol Hepatol. 2012;27(Suppl 2):127–134. doi: 10.1111/j.1440-1746.2011.07013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wittel UA, Pandey KK, Andrianifahanana M, et al. Chronic pancreatic inflammation induced by environmental tobacco smoke inhalation in rats. Am J Gastroenterol. 2006;101:148–59. doi: 10.1111/j.1572-0241.2006.00405.x. [DOI] [PubMed] [Google Scholar]
- 36.Zikherman J, Doan K, Parameswaran R, et al. Quantitative differences in CD45 expression unmask functions for CD45 in B-cell development, tolerance, and survival. Proc Natl Acad Sci USA. 2012;109:E3–12. doi: 10.1073/pnas.1117374108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumar S, Torres MP, Kaur S, et al. Smoking accelerates pancreatic cancer progression by promoting differentiation of MDSCs and inducing HB-EGF expression in macrophages. Oncogene. 2015;34:2052–60. doi: 10.1038/onc.2014.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martinelli P, Cañamero M, del Pozo N, et al. Gata6 is required for complete acinar differentiation and maintenance of the exocrine pancreas in adult mice. Gut. 2013;62:1481–8. doi: 10.1136/gutjnl-2012-303328. [DOI] [PubMed] [Google Scholar]
- 39.Jiang M, Azevedo-Pouly A, Deering TG, et al. MIST1 and PTF1 Collaborate in Feed-forward Regulatory Loops that Maintain the Pancreatic Acinar Phenotype in Adult Mice. Mol Cell Biol. 2016 Sep 19; doi: 10.1128/MCB.00370-16. pii: MCB.00370-16. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hermann PC, Sancho P, Cañamero M, et al. Nicotine promotes initiation and progression of KRAS-induced pancreatic cancer via Gata6-dependent dedifferentiation of acinar cells in mice. Gastroenterology. 2014;147:1119–33. doi: 10.1053/j.gastro.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 41.Edderkaoui M, Xu S, Chheda C, et al. HDAC3 mediates smoking-induced pancreatic cancer. Oncotarget. 2016;7:7747–60. doi: 10.18632/oncotarget.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu S, Chheda C, Ouhaddi Y, et al. Characterization of Mouse Models of Early Pancreatic Lesions Induced by Alcohol and Chronic Pancreatitis. Pancreas. 2015;44:882–7. doi: 10.1097/MPA.0000000000000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quail DF, Joyce JA. Molecular Pathways: Deciphering Mechanisms of Resistance to Macrophage-Targeted Therapies. Clin Cancer Res. 2017;23:876–84. doi: 10.1158/1078-0432.CCR-16-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iodice S, Gandini S, Maisonneuve P, Lowenfels AB. Tobacco and the risk of pancreatic cancer: a review and meta-analysis. Langenbecks Arch Surg. 2008;393:535–45. doi: 10.1007/s00423-007-0266-2. [DOI] [PubMed] [Google Scholar]
- 45.Vrieling A, Bueno-de-Mesquita HB, Boshuizen HC, Michaud DS, Severinsen MT, Overvad K, et al. Cigarette smoking, environmental tobacco smoke exposure and pancreatic cancer risk in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2010;126:2394–403. doi: 10.1002/ijc.24907. [DOI] [PubMed] [Google Scholar]
- 46.Ordóñez-Mena JM, Schöttker B, Mons U, et al. Quantification of the smoking-associated cancer risk with rate advancement periods: meta-analysis of individual participant data from cohorts of the CHANCES consortium. BMC Med. 2016;14:62. doi: 10.1186/s12916-016-0607-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang YT, Gou YW, Jin WW, Xiao M, Fang HY. Association between alcohol intake and the risk of pancreatic cancer: a dose-response meta-analysis of cohort studies. BMC Cancer. 2016;16:212. doi: 10.1186/s12885-016-2241-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nordback I, Pelli H, Lappalainen-Lehto R, Jarvinen S, Raty S, Sand J. The recurrence of acute alcohol-associated pancreatitis can be reduced: a randomized controlled trial. Gastroenterology. 2009;136:848–55. doi: 10.1053/j.gastro.2008.11.044. [DOI] [PubMed] [Google Scholar]
- 49.Petrov MS. Therapeutic implications of oxidative stress in acute and chronic pancreatitis. Curr Opin Clin Nutr Metab Care. 2010;13:562–568. doi: 10.1097/MCO.0b013e32833b64b9. [DOI] [PubMed] [Google Scholar]
- 50.Sankaran SJ, Xiao AY, Wu LM, Windsor JA, Forsmark CE, Petrov MS. Frequency of progression from acute to chronic pancreatitis and risk factors: a meta-analysis. Gastroenterology. 2015;149:1490–1500. doi: 10.1053/j.gastro.2015.07.066. [DOI] [PubMed] [Google Scholar]
- 51.Petrov MS. Abdominal fat: a key player in metabolic acute pancreatitis. Am J Gastroenterol. 2013;108:140–142. doi: 10.1038/ajg.2012.384. [DOI] [PubMed] [Google Scholar]
- 52.Pendharkar SA, Mathew J, Petrov MS. Age- and sex-specific prevalence of diabetes associated with diseases of the exocrine pancreas: a population-based study. Dig Liver Dis. 2017;49:540–544. doi: 10.1016/j.dld.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 53.Andersen DK. Mechanisms and emerging treatments of the metabolic complications of chronic pancreatitis. Pancreas. 2007;35:1–15. doi: 10.1097/mpa.0b013e31805d01b0. [DOI] [PubMed] [Google Scholar]
- 54.Woodmansey C, McGovern A, McCullough K, et al. Incidence, demographics and clinical characteristics of diabetes of the exocrine pancreas (type 3c): a retrospective cohort study. Diabtes Care. 2017 doi: 10.2337/dc17-0542. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 55.Petrov MS. Diabetes of the exocrine pancreas: American Diabetes Association-compliant lexicon. Pancreatology. 2017;17:523–526. doi: 10.1016/j.pan.2017.06.007. [DOI] [PubMed] [Google Scholar]