Abstract
IMPORTANCE
The results of the American College of Surgeons Oncology Group Z0011 (ACOSOG Z0011) trial were first reported in 2005 with a median follow-up of 6.3 years. Longer follow-up was necessary because the majority of the patients had estrogen receptor–positive tumors that may recur later in the disease course (the ACOSOG is now part of the Alliance for Clinical Trials in Oncology).
OBJECTIVE
To determine whether the 10-year overall survival of patients with sentinel lymph node metastases treated with breast-conserving therapy and sentinel lymph node dissection (SLND) alone without axillary lymph node dissection (ALND) is noninferior to that of women treated with axillary dissection.
DESIGN, SETTING, AND PARTICIPANTS
The ACOSOG Z0011 phase 3 randomized clinical trial enrolled patients from May 1999 to December 2004 at 115 sites (both academic and community medical centers). The last date of follow-up was September 29, 2015, in the ACOSOG Z0011 (Alliance) trial. Eligible patients were women with clinical T1 or T2 invasive breast cancer, no palpable axillary adenopathy, and 1 or 2 sentinel lymph nodes containing metastases.
INTERVENTIONS
All patients had planned lumpectomy, planned tangential whole-breast irradiation, and adjuvant systemic therapy. Third-field radiation was prohibited.
MAIN OUTCOMES AND MEASURES
The primary outcome was overall survival with a noninferiority hazard ratio (HR) margin of 1.3. The secondary outcome was disease-free survival.
RESULTS
Among 891 women who were randomized (median age, 55 years), 856 (96%) completed the trial (446 in the SLND alone group and 445 in the ALND group). At a median follow-up of 9.3 years (interquartile range, 6.93–10.34 years), the 10-year overall survival was 86.3% in the SLND alone group and 83.6% in the ALND group (HR, 0.85 [1-sided 95%CI, 0–1.16]; noninferiority P = .02). The 10-year disease-free survival was 80.2% in the SLND alone group and 78.2% in the ALND group (HR, 0.85 [95%CI, 0.62–1.17]; P = .32). Between year 5 and year 10, 1 regional recurrence was seen in the SLND alone group vs none in the ALND group. Ten-year regional recurrence did not differ significantly between the 2 groups.
CONCLUSIONS AND RELEVANCE
Among women with T1 or T2 invasive primary breast cancer, no palpable axillary adenopathy, and 1 or 2 sentinel lymph nodes containing metastases, 10-year overall survival for patients treated with sentinel lymph node dissection alone was noninferior to overall survival for those treated with axillary lymph node dissection. These findings do not support routine use of axillary lymph node dissection in this patient population based on 10-year outcomes.
TRIAL REGISTRATION
clinicaltrials.gov Identifier: NCT00003855
For more than 100 years, the extent of breast cancer surgery was based on the Halstedian concept of breast cancer as a locoregional disease that spread via the lymphatic system and was cured by resection.1,2 Since then, it has been recognized that breast cancer biology, rather than the extent of surgery, is a major risk determinant of both systemic and locoregional recurrence,3,4 opening the door to new surgical approaches to management.
Axillary lymph node dissection (ALND), long used to identify women with axillary nodal metastases, was replaced as a staging procedure by the less morbid sentinel lymph node dissection (SLND).5–7 Between 1998 and 2004, the use of ALND declined from 94% to 36% in women with no axillary nodal metastases, whereas 68% of patients with sentinel node metastases underwent ALND in 2004.8 Axillary lymph node dissection is an effective method of maintaining regional control but it is associated with a significant risk of complications such as lymphedema, numbness, axillary web syndrome, and decreased upper-extremity range of motion.6 Changes in the presentation and management of breast cancer and the selection of systemic therapy based on tumor biology raised questions regarding the necessity of ALND for some patients with sentinel lymph node metastases.
The American College of Surgeons Oncology Group Z0011 (ACOSOG Z0011) randomized clinical trial was designed to determine whether SLND alone yielded survival outcomes that were noninferior to that obtained with ALND in women with a limited number of sentinel node metastases undergoing breast-conserving surgery and receiving adjuvant whole-breast irradiation with adjuvant systemic therapy. The ACOSOG is now part of the Alliance for Clinical Trials in Oncology (Alliance). The trial protocol appears in the Supplement.
The initial study results, reported after a median follow-up of 6.3 years,9,10 demonstrated that the overall survival in patients randomized to SLND alone was no worse than patients randomized to ALND with a noninferiority hazard ratio (HR) margin of 1.3. It also showed no statistically significant difference in disease-free survival between patients randomized to SLND alone or ALND, and nodal recurrence occurred in fewer than 1% of patients in either study group.
A serious criticism of the study was the relatively short follow-up that may have not detected late death. Breast cancer, particularly hormone receptor–positive breast cancer, is a disease with a long natural history11,12 and a substantial risk of locoregional and systemic relapses occurring after 5 years. Patients enrolled in ACOSOG Z0011 reflected the demographics of patients with breast cancer in the United States. The majority of patients were postmenopausal with hormone receptor–positive breast cancer, raising concern that additional follow-up beyond 6 years was needed to document noninferiority of overall survival with SLND alone in this node-positive cohort.
Methods
Patient Characteristics
This multicenter randomized phase 3 trial was registered with the National Cancer Institute and approved by the institutional review boards at participating centers. All patients provided written informed consent. Adult women with histologically confirmed invasive breast carcinoma clinically 5 cm or less in size, no palpable adenopathy, and with sentinel nodes containing metastatic breast cancer detected without immunohistochemical stains were eligible for participation. The eligibility criteria have been reported.9,10
Study and Design End Points
The study and design end points have been described else-where.9,10 Briefly, after stratification based on age, hormone receptor status, and tumor size, patients with 1 or 2 sentinel nodes with metastases detected by hematoxylin and eosin stain were randomized to no further axillary-specific treatment including no axillary third-field irradiation (SLND alone group) or completion ALND (ALND group). Patients were assessed for disease recurrence with a history and physical examination every 6 months for the first 36 months and yearly thereafter. Annual mammography was required; other testing was based on individual symptoms or by investigator preference.
Follow-up was planned for 10 years. The primary study end point was overall survival, which was defined as the time from randomization until death from any cause. Disease-free survival, which was defined as the time from randomization to death or first breast cancer recurrence, was a secondary end point along with morbidity and locoregional recurrence. Locoregional recurrence was defined as a tumor in the breast or in ipsilateral axillary, internal mammary, subclavicular, or supraclavicular nodes. All other disease sites were defined as distant metastases. Secondary end points have been reported.6,13
Statistical Analysis
The primary end point was overall survival as a measure of noninferiority of no further axillary-specified interventions (SLND alone group) compared with the ALND group. The study design hypothesized that overall survival would be 80% at 5 years for optimally treated women in this node-positive cohort. The SLND alone group would be considered clinically noninferior to the ALND group if the 5-year survival rate was 75% or greater and the HR was 1.3 or less. A 1-sided 95% CI for the HR from a Cox regression model was used to confirm noninferiority of SLND alone compared with ALND. An estimated 500 deaths were needed for the study to have 90% power.14
Enrollment of 1900 patients in 4 years with a minimum follow-up period of 5 years was initially planned. The trial closed early because of low accrual rates and fewer than anticipated events. The protocol specified that patients were to be followed up for a minimum of 10 years. However, the analysis of overall survival after the completion of study follow-up was not prespecified.
Analyses were performed on the intent-to-treat sample (436 patients in the SLND alone group and 420 patients in the ALND group) as well as on the patients who actually received treatment. Both analyses yielded similar results so only the intent-to-treat results are reported. Kaplan-Meier survival curves for overall survival were compared using the log-rank test for noninferiority. The unadjusted HR (95% CI) was calculated using a Cox regression analysis. Patients who were lost to follow-up (ie, missing data) were censored at the time of their last follow-up in the time-to-event analyses (disease-free survival and overall survival). Most patients were lost to follow-up because the site investigator left an institution and the institution stopped active follow-up on the patients. Hence, the missing data were not associated with outcome.
Patients who withdrew consent for use of their information were omitted from the analyses. All secondary analyses were tested for differences. As a secondary analysis, known prognostic factors (including adjuvant treatment) were included in the Cox regression model to generate an adjusted HR for overall survival. Disease-free survival was analyzed with a log-rank analysis using Kaplan-Meier curves and unadjusted and multivariable Cox regression analyses. The proportional hazards assumptions for the Cox models were evaluated using Schoenfeld residual plots, and none of the reported models appeared to violate the proportional hazards assumption.
An exploratory analysis was conducted to determine the effect of treatment (SLND alone vs ALND) on overall survival for patients with hormone receptor–positive tumors. A comparison was done among 4 groups of patients (hormone receptor–positive treated with ALND, hormone receptor–positive treated with SLND alone, hormone receptor–negative treated with ALND, hormone receptor–negative treated with SLND alone) using a log-rank test. In addition, a log-rank test was used to determine whether hormone receptor status was associated with overall survival in the ALND group.
Data collection and statistical analyses in the ACOSOG Z0011 (Alliance) trial were conducted by the Alliance Statistics and Data Center. Data quality was ensured by review of data by the Alliance Statistics and Data Center and by the study chairperson following Alliance policies. The study database was frozen on September 29, 2015. Except for the primary overall survival analysis, each analysis was performed with a 2-sided, .05 significance level, and 95% CIs. SAS version 9.4 (SAS Institute Inc) was used for all analyses.
Results
Patient Characteristics
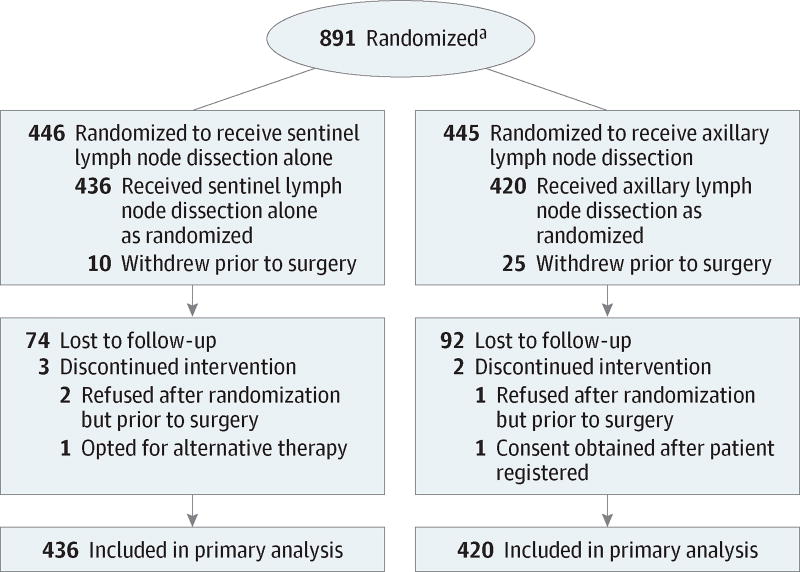
Enrollment began in May 1999 with a planned accrual of 1900 patients and closed in December 2004 due to a lower than expected event rate.9 There were 891 patients randomized from 115 institutions (both academic and community medical centers); 35 withdrew consent (Figure 1). Demographic and disease characteristics at baseline were well balanced between the SLND alone group and the ALND group (Table 1).9
Figure 1. Flow of Patients Through Treatment and Follow-up in the ACOSOG Z0011 (Alliance) Trial.
ACOSOG indicates American College of Surgeons Oncology Group; Alliance, Alliance for Clinical Trials in Oncology.
a Data are not available for the number of patients screened for eligibility.
Table 1.
Baseline Participant Characteristics
| Lymph Node Dissection | ||
|---|---|---|
| Sentinel Alone (n = 436) |
Axillary (n = 420) |
|
| Age, median (range), y | 54 (25–90) | 56 (24–92) |
| Age group, No. (%) | ||
| ≤50 y | 160 (36.7) | 135 (32.1) |
| >50 y | 266 (61.0) | 278 (66.2) |
| Missing | 10 (2.3) | 7 (1.7) |
| Clinical stage, No. (%) | ||
| T1 | 303 (69.5) | 284 (67.6) |
| T2 | 126 (28.9) | 134 (31.9) |
| Missing | 7 (1.6) | 2 (0.5) |
| Tumor size, median (range), cma | 1.6 (0–5.0) | 1.7 (0.4–7.0) |
| Receptor status, No. (%) | ||
| ER and PR positive | 270 (61.9) | 256 (61.0) |
| ER positive and PR negative | 54 (12.4) | 61 (14.5) |
| ER negative and PR positive | 4 (0.9) | 3 (0.7) |
| ER and PR negative | 64 (14.7) | 63 (15.0) |
| Missing | 44 (10.1) | 37 (8.8) |
| Lymphovascular invasion, No. (%) | ||
| Present | 113 (25.9) | 129 (30.7) |
| Absent | 208 (47.7) | 189 (45.0) |
| Missing | 115 (26.4) | 102 (24.3) |
| Grade, No. (%)b | ||
| 1 | 81 (18.6) | 71 (16.9) |
| 2 | 148 (33.9) | 158 (37.6) |
| 3 | 87 (20.0) | 94 (22.4) |
| Missing | 120 (27.5) | 97 (23.1) |
| Histological type, No. (%) | ||
| Ductal | 356 (81.7) | 344 (81.9) |
| Lobular | 36 (8.3) | 27 (6.4) |
| Mixed ductal and lobular | 10 (2.3) | 23 (5.5) |
| Other | 22 (5.0) | 22 (5.2) |
| Missing | 12 (2.7) | 4 (1.0) |
Abbreviations: ER, estrogen receptor; PR, progesterone receptor.
There were missing data for 14 patients in the sentinel lymph node dissection alone group and for 6 patients in the axillary lymph node dissection group.
Defined using the modified Bloom-Richardson system. Patients with lower grades have a better prognosis.
Treatment Results
Fewer lymph nodes were removed in the SLND alone group (median, 2; interquartile range [IQR], 1–4) than in the ALND group (median, 17; IQR, 13–22) (P < .001), and the total number of involved nodes was greater in the ALND group. The median total number of nodes containing metastases in both groups was 1 (IQR, 1–2).
Micrometastases (≤2 mm) were identified in the sentinel nodes of 164 patients (44.8%) in the SLND alone group compared with 137 patients (37.5%) in the ALND group (P = .046). In addition, 27.3% of patients in the ALND group had macrometastases (>2 mm) in nonsentinel nodes removed during ALND, including 10% of patients with micrometastases in a sentinel node.
Adjuvant systemic therapy was delivered to 423 women (97.0%) in the SLND alone group and 403 women (96.0%) in the ALND group, with no between-group difference in the type of chemotherapy or the proportion receiving endocrine therapy, chemotherapy, or both. The majority of women received radiation therapy (277 women [89.6%] in the SLND alone group vs 263 women [88.9%] in the ALND group). An independent analysis of radiation fields in a subset of participants demonstrated no between-group difference in the use of high tangents, nodal irradiation, or no irradiation; 18.9% received protocol-prohibited nodal-field irradiation.15 Eleven percent received no irradiation.
Overall Survival
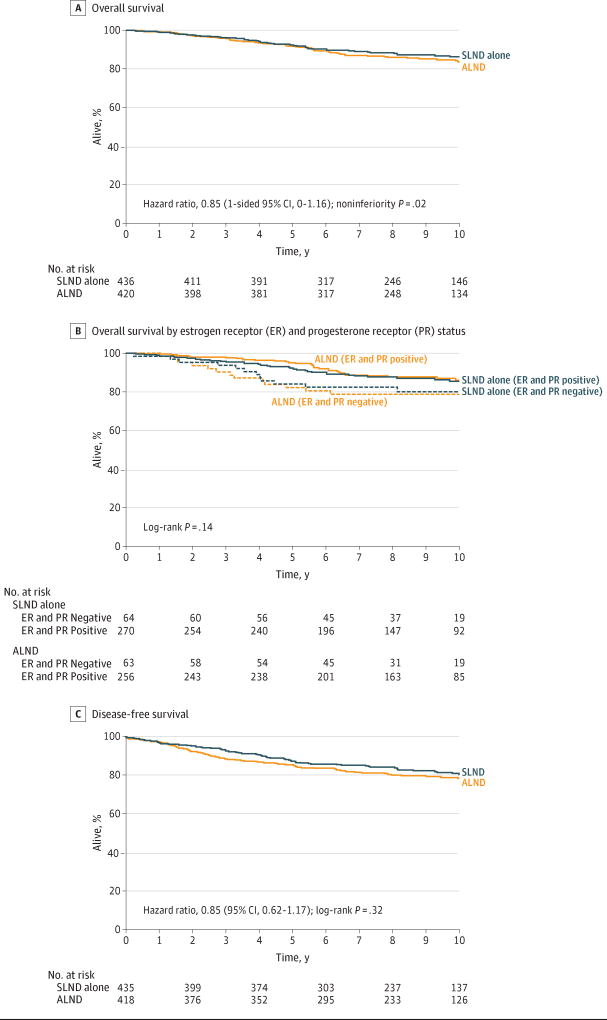
At a median follow-up of 9.3 years (IQR, 6.93–10.34 years), there were 110 deaths (51 in the SLND alone group and 59 in the ALND group). Compared with ALND, SLND alone was found to be noninferior for overall survival (log-rank P = .02; Figure 2A). The 10-year overall survival rate was 86.3% (95% CI, 82.2%–89.5%) in the SLND alone group and 83.6% (95% CI, 79.1%–87.1%) in the ALND group. The unadjusted HR comparing overall survival between the SLND alone group and the ALND group was 0.85 (1-sided 95% CI, 0–1.16), which did not cross the prespecified noninferiority HR margin of 1.3. The HR for overall survival adjusting for adjuvant therapy (chemotherapy, endocrine therapy, radiation, or a combination of these 3) and age for the SLND alone group compared with the ALND group was 0.93 (1-sided 95% CI, 0–1.28) (Table 2).
Figure 2. Overall and Disease-Free Survival in the ACOSOG Z0011 (Alliance) Trial.
ACOSOG indicates American College of Surgeons Oncology Group; Alliance, Alliance for Clinical Trials in Oncology. In part A, there were 51 events in the sentinel lymph node dissection (SLND) alone group (n = 436) during a median follow-up of 9.3 years (interquartile range [IQR], 6.8–10.3 years) vs 59 events in the axillary lymph node dissection (ALND) group (n = 420) during a median follow-up of 9.4 years (IQR, 7.2–10.3 years). In part B, there were 12 events in the ER- and PR-negative SLND alone group (n = 64) during a median follow-up of 9.3 years (IQR, 7.7–10.7 years) with a hazard ratio of 1.71 (95% CI, 0.88–3.34); 33 events in the ER- and PR-positive SLND alone group (n = 270) during a median follow-up of 9.3 years (IQR, 6.7–10.4 years) with a hazard ratio of 1.10 (95% CI, 0.67–1.80); 13 events in the ER- and PR-negative ALND group (n = 63) during a median follow-up of 9.3 years (IQR, 7.1–10.4 years) with a hazard ratio of 1.91 (95% CI, 1.00–3.66); and 30 events in the ER- and PR-positive ALND group (n = 256) during a median follow-up of 9.5 years (IQR, 7.7–10.3 years) with a hazard ratio of 1 [Reference]. In part C, there were 73 events in the SLND alone group (n = 435) during a median follow-up of 9.3 years (IQR, 6.8–10.3 years) vs 82 events in the ALND group (n = 418) during a median follow-up of 9.4 years (IQR, 7.2–10.3 years).
Table 2.
Survival Outcomes by Study Group
| Lymph Node Dissection | P Value | ||
|---|---|---|---|
| Sentinel Alone | Axillary | ||
| Primary End Point: Overall Survival | |||
| 10-y Disease-free survival Kaplan-Meier estimate (95% CI) | 86.3 (82.2–89.5) | 83.6 (79.1–87.1) | |
| No. of events/No. of patients | 51/436 | 59/420 | |
| Unadjusted HR (1-sided 95% CI) | 0.85 (0–1.16) | 1 [Reference] | .02a |
| Adjusted HR (1-sided 95% CI)b | 0.93 (0–1.28) | 1 [Reference] | .72 |
| Disease-Free Survival | |||
| 10-y Kaplan-Meier estimate (95% CI) | 80.2 (75.6–84.1) | 78.2 (73.5–82.2) | |
| No. of events/No. of patients | 73/435 | 82/418 | |
| Unadjusted HR (95% CI) | 0.85 (0.62–1.17) | 1 [Reference] | .32 |
| Adjusted HR (95% CI)b | 0.90 (0.68–1.18) | 1 [Reference] | .51 |
| Locoregional Relapse-Free Survival | |||
| 10-y Kaplan-Meier estimate (95% CI) | 83.0 (78.6–86.6) | 81.2 (76.7–84.9) | |
| No. of events/No. of patients | 64/436 | 71/418 | |
| Unadjusted HR (95% CI) | 0.87 (0.62–1.22) | 1 [Reference] | .41 |
| Adjusted HR (95% CI)b | 0.93 (0.66–1.31) | 1 [Reference] | .66 |
Abbreviation: HR, hazard ratio.
Indicates noninferiority for primary end point.
Model includes study group, age, and adjuvant therapy.
In a multivariable analysis of overall survival, type of treatment was not significantly associated with overall survival (Table 3). An exploratory analysis of the effect of treatment and hormone receptor status revealed no statistically significant difference in overall survival among the 4 groups (log-rank P = .14; Figure 2B). Operation had no significant effect on overall survival with respect to estrogen receptor and progesterone receptor status.
Table 3.
Multivariable Analysis of the Association of Treatment and Prognostic Variables With Overall Survival
| No. of Patients |
No. of Deaths |
Adjusted HR (95% CI)a | P Value | |
|---|---|---|---|---|
| Lymph node dissection group | ||||
| Sentinel alone | 426 | 51 | 0.93 (0.64–1.36) | .72 |
| Axillary | 413 | 56 | 1 [Reference] | |
| Age group, y | ||||
| ≤50 | 295 | 23 | 1 [Reference] | .002 |
| >50 | 544 | 84 | 2.08 (1.31–3.30) | |
| Estrogen receptor and progesterone receptor status | ||||
| Both negative | 101 | 25 | 1 [Reference] | .02 |
| ≥1 Positive | 514 | 61 | 0.57 (0.36–0.91) | |
| Lymphovascular invasion | ||||
| Absent | 238 | 31 | 1 [Reference] | .74 |
| Present | 387 | 48 | 0.92 (0.59–1.46) | |
| Sentinel lymph node met size | ||||
| Micrometastases (≤2 mm) | 296 | 37 | 1 [Reference] | .97 |
| Macrometastases (>2 mm) | 418 | 53 | 1.01 (0.66–1.54) | |
| Pathological tumor size, cm (continuous) | 1.19 (1.07–1.32) | .001 | ||
| Histological type | ||||
| Ductal | 687 | 86 | 1 [Reference] | .25 |
| Lobular | 63 | 9 | 1.04 (0.52–2.07) | |
| Mixed ductal and lobular | 32 | 8 | 2.06 (0.99–4.27) | |
| Other | 41 | 4 | 0.79 (0.29–2.16) | |
| Gradeb | ||||
| 1 | 150 | 20 | 1 [Reference] | .46 |
| 2 | 300 | 31 | 0.74 (0.42–1.30) | |
| 3 | 178 | 26 | 1.07 (0.60–1.92) | |
| Unknown or missing | 144 | 21 | 1.06 (0.58–1.96) |
Abbreviation: HR, hazard ratio.
Model includes study group, age, and adjuvant therapy.
Defined using the modified Bloom-Richardson system. Patients with lower grades have a better prognosis.
Disease-Free Survival
Disease-free survival and locoregional recurrence have been reported.13 The 10-year disease-free survival was 80.2%(95% CI, 75.6%–84.1%) for the SLND alone group and 78.2% (95% CI, 73.5%–82.2%) for the ALND group (log-rank P = .32; Figure 2C). The unadjusted HR comparing the SLND alone group with the ALND group was 0.85 (95% CI, 0.62–1.17) (Table 2). Only 1 nodal recurrence was observed in a patient in the SLND alone group after 5 years and none in the ALND group. In an unplanned analysis of the subset of the 228 patients with detailed radiation records available, those treated with nodal-field irradiation experienced no difference in disease-free survival, overall survival, or locoregional recurrence compared with those who did not receive irradiation.
Discussion
Even with follow-up extended to a median of 9.3 years, the ACOSOG Z0011 (Alliance) randomized clinical trial demonstrated that SLND alone did not result in inferior overall survival outcomes compared with ALND for patients with clinical T1 or T2 node-negative (by palpation) breast cancer and 1 or 2 positive sentinel nodes treated with breast-conserving therapy and adjuvant systemic therapy. Before publication of the initial ACOSOG Z0011 trial results,9 there was a general consensus that axillary dissection was necessary for better cancer control when metastases were identified in sentinel lymph nodes.
Axillary dissections are associated with considerable morbidity, and the results of this trial demonstrated that this morbidity can be avoided without decreasing cancer control. The long-term outcome of this study provides additional support that axillary dissection is not necessary for long-term disease control and survival for patients with positive sentinel nodes, even for those with generally late-recurring hormone receptor–positive tumors.
In addition, there was no significant difference in disease-free survival between patients treated with SLND alone and ALND. This confirms that although distant recurrence among hormone receptor–positive tumors is a later event, nodal recurrence among these patients is primarily an early event. The stability of these results over time is important because patients with hormone receptor–positive breast cancer, who comprise the majority of study participants and the majority of breast cancer patients in the United States, are known to be at prolonged risk for disease recurrence.
Although the annual rate of distant recurrence after completion of 5 years of endocrine therapy has been reported to range from 0.9% to 1.5% through year 15 after diagnosis,12 regional recurrence in the ACOSOG Z0011 (Alliance) trial was rare after either SLND alone or ALND between years 5 and 10 even though more than 80% of patients had hormone receptor–positive tumors.13 These findings are compatible with those of the International Breast Cancer Study Group in which rates of regional recurrence in patients with estrogen receptor–positive tumors were seen to increase minimally from 5% to 6.2% between years 5 and 10, with substantially greater increases in the rates of late local (8.8%–11.2%) and distant recurrence (23.4%–31.9%) observed.16
Because the patient characteristics were well balanced, any decrease in disease-free survival or overall survival in the SLND alone group would have been anticipated to occur due to an increase in regional recurrences; however, only a single regional recurrence was observed in the SLND alone group with additional follow-up in the ACOSOG Z0011 (Alliance) trial. Consistent with this finding, the incremental decreases in disease-free survival (3.7% for the SLND alone group and 4.0% for the ALND group) and overall survival (6.2% for the SLND alone group and 8.2% for the ALND group) between years 5 and 10 among patients undergoing either SLND alone or ALND were not meaningfully different. In an adjusted analysis, well documented prognostic factors such as age, hormone receptor status, tumor size, and the use of adjuvant therapy (but not elimination of ALND) were associated with overall survival.
Although the initial results of the ACOSOG Z0011 study generated controversy,17,18 management of women in the United States with sentinel node metastases changed substantially as a result of the study. Among 701 consecutive patients with node-positive tumors at Memorial Sloan Kettering Cancer Center who met ACOSOG Z0011 eligibility criteria, 83% did not have to undergo ALND.19 In a 12-hospital network, use of ALND decreased from 71% to 17% after development of a guideline using ACOSOG Z0011 eligibility criteria and age of 50 years or older as indications for SLND alone.20
In a National Cancer Database study of 74 309 patients, Yao et al21 observed that use of SLND in patients meeting ACOSOG Z0011 eligibility criteria increased from 23% to 56% between 2009 and 2011. In that study, age younger than 50 years and a triple-negative subtype predicted a greater use of ALND, a practice neither supported by the reported 5-year outcomes of ACOSOG Z0011,9 nor by the findings of the current report. In ACOSOG Z0011, age was not significantly associated with locoregional recurrence after controlling for other factors.13 Studies examining the application of findings from ACOSOG Z0011 among young women or among those with triple-negative breast cancer have found neither a greater need for ALND in these groups, nor heavier axillary tumor burdens in those undergoing ALND.19,22
The role of nodal irradiation, specifically in ACOSOG Z0011 and in the management of patients with node-positive breast cancer, is controversial. Although 19% of patients received prohibited third-field irradiation, nodal irradiation was distributed similarly by treatment group, as was omission of irradiation and the use of high–tangent-field irradiation,15 indicating that choice of radiotherapy fields was unlikely to have affected the study outcome. In addition, the unplanned analysis showed that no survival differences were observed among patients treated with conventional tangent-field irradiation or nodal-field irradiation.
Since the initial publication of ACOSOG Z0011,9 2 studies (the MA.2023 and the European Organization for Research and Treatment of Cancer24 [EORTC] 22922/10925) examined the role of regional nodal irradiation in patients with similar characteristics (T1 or T2 and 1, 2, or 3 axillary nodal metastases) and their findings have caused some25 to question whether comprehensive nodal irradiation should be routine. In the MA.20 study,23 patients with node-positive tumors were randomized to axillary dissection or axillary dissection plus extensive postoperative nodal irradiation, including supraclavicular and internal mammary nodal basins. The EORTC 22922/10925 study24 randomized high-risk women postoperatively to whole-breast or chest-wall irradiation alone or with regional nodal irradiation. These studies, with 10 years of follow-up and 5836 enrolled patients, demonstrate a very modest 1% to 1.5% decrease in regional recurrence with nodal irradiation, and no significant difference in overall survival.
The 10-year rates of overall survival in the SLND alone and ALND groups of the ACOSOG Z0011 (Alliance) trial were 86.3% and 83.6%, respectively, compared with 82.8% in the nodal irradiation group in MA.20 and 82.3% in EORTC 22922/10925, suggesting that the ACOSOG Z0011 eligibility criteria identified a population that may not benefit from comprehensive nodal irradiation. Thus, although nodal irradiation may be added to the management of some patients with node-positive tumors based on an evaluation of their overall risk profile, the routine use of nodal irradiation for all patients with 1 or 2 sentinel node metastases managed with SLND alone may not be justified.
Ten years of follow-up confirm that women with 1 or 2 positive sentinel nodes and clinical T1 or T2 tumors undergoing lumpectomy with whole-breast irradiation and systemic therapy experience no worse local control, disease-free survival, or overall survival with elimination of ALND. Application of these findings in clinical practice has the potential to avoid the morbidity of ALND without diminution of survival outcomes in 61% to 83% of women with these characteristics.19,26 The routine use of ALND for all patients with positive sentinel nodes is no longer justified based on these 10-year overall survival results.
However, these conclusions apply only to patients meeting ACOSOG Z0011 eligibility criteria and should not be extrapolated to the management of patients with positive palpable nodes, those with metastases in more than 2 sentinel nodes, patients forgoing whole-breast irradiation, those treated with mastectomy without radiation, or patients receiving neoadjuvant therapy because all of these are circumstances in which the elimination of ALND is not known to be safe. The ongoing Positive Sentinel Node-Adjuvant Therapy Alone vs Adjuvant Therapy Plus Clearance or Axillary Radiotherapy trial for women with metastases in 1 or 2 sentinel nodes treated with breast-conserving surgery or mastectomy will provide important information about the safety of omitting ALND after mastectomy, but this study is not expected to complete accrual until 2018.17
Limitations
This study has several limitations. Like most large randomized trials in breast cancer management, not all biological subtypes are represented in large numbers. Differences in outcomes may be seen for patients with different individual circumstances. However, not all biological subtypes can be analyzed for small variations in locoregional treatment. Furthermore, due to low accrual and the low event rate, the study did not reach the prespecified sample size of 1900 participants or 500 deaths. In addition, some patients had irradiation protocol variations that could have resulted in a small alteration of outcomes; however, these patients were distributed similarly in both study groups.
Conclusions
Among women with T1 or T2 invasive primary breast cancer, no palpable axillary adenopathy, and 1 or 2 sentinel lymph nodes containing metastases, 10-year overall survival for patients treated with sentinel lymph node dissection alone was noninferior to overall survival for those treated with axillary lymph node dissection. These findings do not support routine use of axillary lymph node dissection in this patient population based on 10-year outcomes.
Supplementary Material
Key Points.
Question
Is there any diminution in 10-year overall survival for women with cT1-2N0 breast cancer and metastases to 1 or 2 sentinel lymph nodes undergoing breast-conserving surgery, whole-breast irradiation, and adjuvant systemic therapy treated with sentinel node dissection alone compared with that of patients treated with axillary dissection?
Findings
In this randomized clinical trial including 856 women, after median follow-up of 9.3 years, overall survival for patients treated with sentinel lymph node dissection alone was not inferior to those treated with completion axillary lymph node dissection (86.3% vs 83.6%, respectively; noninferiority hazard ratio margin of 1.3).
Meaning
These findings do not support the use of axillary lymph node dissection when metastases are found with sentinel lymph node sampling in women with cT1-2M0 breast cancer.
Acknowledgments
Dr Giuliano reported receiving travel support from the American College of Surgeons Oncology Group (ACOSOG) to attend Alliance for Clinical Trials in Oncology (Alliance) meetings. Dr Ballman and Ms McCall received compensation for their statistics work for ACOSOG (Alliance). Dr Ballman also reported receiving grant support from the National Cancer Institute during the conduct of the study. Drs Giuliano, Beitsch, Ollila, Hansen, Whitworth, Blumencranz, Leitch, Saha, Hunt, and Morrow reported their respective institutions received per capita compensation for patient accrual from ACOSOG (Alliance). Dr Hansen also reported receiving speakers fees from Genentech and Genomic Health. Dr Leitch also reported receiving grant support from ACOSOG.
Funding/Support: Supported by grants U10CA180821 and U10CA180882 (awarded to the Alliance), U10CA047559, U10CA077651, U10CA180791, U10CA180838, U10CA180858, and U10CA180870 from the National Cancer Institute.
Role of the Funder/Sponsor: The National Cancer Institute had a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. Neither the Alliance nor the National Cancer Institute had the right to veto the submission of the manuscript.
Footnotes
Author Contributions: Drs Giuliano and Ballman had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Giuliano, Ballman, Brennan, Kelemen, Ollila, Whitworth, Leitch, Saha, Hunt.
Acquisition, analysis, or interpretation of data: Giuliano, Ballman, McCall, Beitsch, Brennan, Kelemen, Ollila, Hansen, Whitworth, Blumencranz, Leitch, Saha, Hunt, Morrow.
Drafting of the manuscript: Giuliano, Ballman, McCall, Brennan, Morrow.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Ballman, McCall.
Obtained funding: Giuliano.
Administrative, technical, or material support: Giuliano.
Supervision: Giuliano.
Conflict of Interest Disclosures: The authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. No other disclosures were reported.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Meeting Presentation: This study was presented in part at the American Society of Clinical Oncology annual meeting; June 3–7, 2016; Chicago, Illinois.
References
- 1.Halsted WS. I: a clinical and histological study of certain adenocarcinomata of the breast: and a brief consideration of the supraclavicular operation and of the results of operations for cancer of the breast from 1889 to 1898 at the Johns Hopkins Hospital. Ann Surg. 1898;28(5):557–576. [PMC free article] [PubMed] [Google Scholar]
- 2.Halsted WS. I: the results of radical operations for the cure of carcinoma of the breast. Ann Surg. 1907;46(1):1–19. doi: 10.1097/00000658-190707000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lowery AJ, Kell MR, Glynn RW, Kerin MJ, Sweeney KJ. Locoregional recurrence after breast cancer surgery: a systematic review by receptor phenotype. Breast Cancer Res Treat. 2012;133(3):831–841. doi: 10.1007/s10549-011-1891-6. [DOI] [PubMed] [Google Scholar]
- 4.Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98(19):10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giuliano AE, Jones RC, Brennan M, Statman R. Sentinel lymphadenectomy in breast cancer. J Clin Oncol. 1997;15(6):2345–2350. doi: 10.1200/JCO.1997.15.6.2345. [DOI] [PubMed] [Google Scholar]
- 6.Lucci A, McCall LM, Beitsch PD, et al. American College of Surgeons Oncology Group. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol. 2007;25(24):3657–3663. doi: 10.1200/JCO.2006.07.4062. [DOI] [PubMed] [Google Scholar]
- 7.Veronesi U, Paganelli G, Viale G, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med. 2003;349(6):546–553. doi: 10.1056/NEJMoa012782. [DOI] [PubMed] [Google Scholar]
- 8.Rescigno J, Zampell JC, Axelrod D. Patterns of axillary surgical care for breast cancer in the era of sentinel lymph node biopsy. Ann Surg Oncol. 2009;16(3):687–696. doi: 10.1245/s10434-008-0195-5. [DOI] [PubMed] [Google Scholar]
- 9.Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305(6):569–575. doi: 10.1001/jama.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giuliano AE, McCall L, Beitsch P, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg. 2010;252(3):426–432. doi: 10.1097/SLA.0b013e3181f08f32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colleoni M, Sun Z, Price KN, et al. Annual hazard rates of recurrence for breast cancer during 24 years of follow-up: results from the International Breast Cancer Study Group Trials I to V. J Clin Oncol. 2016;34(9):927–935. doi: 10.1200/JCO.2015.62.3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan H, Gray RG, Davies C, et al. Predictors of recurrence during years 5–14 in 46,138 women with ER+ breast cancer allocated 5 years only of endocrine therapy (ET) J Clin Oncol. 2016;34(15 suppl):505–505. [Google Scholar]
- 13.Giuliano AE, Ballman K, McCall L, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: long-term follow-up from the American College of Surgeons Oncology Group (Alliance) ACOSOG Z0011 randomized trial. Ann Surg. 2016;264(3):413–420. doi: 10.1097/SLA.0000000000001863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gail MH, Lubin JH, Rubinstein LV. Likelihood calculations for matched case-control studies and survival studies with tied death times. Biometrika. 1981;68(3):703–707. [Google Scholar]
- 15.Jagsi R, Chadha M, Moni J, et al. Radiation field design in the ACOSOG Z0011 (Alliance) trial. J Clin Oncol. 2014;32(32):3600–3606. doi: 10.1200/JCO.2014.56.5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colleoni M, Gray KP, Gelber S, et al. Low-dose oral cyclophosphamide and methotrexate maintenance for hormone receptor-negative early breast cancer: International Breast Cancer Study Group Trial 22-00. J Clin Oncol. 2016;34(28):3400–3408. doi: 10.1200/JCO.2015.65.6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goyal A, Dodwell D. POSNOC: a randomised trial looking at axillary treatment in women with one or two sentinel nodes with macrometastases. Clin Oncol (R Coll Radiol) 2015;27(12):692–695. doi: 10.1016/j.clon.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Güth U, Myrick ME, Viehl CT, Schmid SM, Obermann EC, Weber WP. The post ACOSOG Z0011 era: does our new understanding of breast cancer really change clinical practice? Eur J Surg Oncol. 2012;38(8):645–650. doi: 10.1016/j.ejso.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 19.Mamtani A, Patil S, Van Zee KJ, et al. Age and receptor status do not indicate the need for axillary dissection in patients with sentinel lymph node metastases. Ann Surg Oncol. 2016;23(11):3481–3486. doi: 10.1245/s10434-016-5259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsao MW, Cornacchi SD, Hodgson N, et al. A population-based study of the effects of a regional guideline for completion axillary lymph node dissection on axillary surgery in patients with breast cancer. Ann Surg Oncol. 2016;23(10):3354–3364. doi: 10.1245/s10434-016-5310-4. [DOI] [PubMed] [Google Scholar]
- 21.Yao K, Liederbach E, Pesce C, Wang CH, Winchester DJ. Impact of the American College of Surgeons Oncology Group Z0011 randomized trial on the number of axillary nodes removed for patients with early-stage breast cancer. J Am Coll Surg. 2015;221(1):71–81. doi: 10.1016/j.jamcollsurg.2015.02.035. [DOI] [PubMed] [Google Scholar]
- 22.Chung A, Gangi A, Mirocha J, Giuliano A. Applicability of the ACOSOG Z0011 criteria in women with high-risk node-positive breast cancer undergoing breast conserving surgery. Ann Surg Oncol. 2015;22(4):1128–1132. doi: 10.1245/s10434-014-4090-y. [DOI] [PubMed] [Google Scholar]
- 23.Whelan TJ, Olivotto IA, Parulekar WR, et al. MA. 20 Study Investigators. Regional nodal irradiation in early-stage breast cancer. N Engl JMed. 2015;373(4):307–316. doi: 10.1056/NEJMoa1415340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poortmans PM, Collette S, Kirkove C, et al. EORTC Radiation Oncology and Breast Cancer Groups. Internal mammary and medial supraclavicular irradiation in breast cancer. N Engl J Med. 2015;373(4):317–327. doi: 10.1056/NEJMoa1415369. [DOI] [PubMed] [Google Scholar]
- 25.Poortmans PM, Coles C, Bernier J. Treatment of regional lymph nodes in breast cancer-evidence in favor of radiation therapy. JAMA Oncol. 2016;2(8):989–990. doi: 10.1001/jamaoncol.2016.0183. [DOI] [PubMed] [Google Scholar]
- 26.Verheuvel NC, Voogd AC, Tjan-Heijnen VC, Roumen RM. Potential impact of application of Z0011 derived criteria to omit axillary lymph node dissection in node positive breast cancer patients. Eur J Surg Oncol. 2016;42(8):1162–1168. doi: 10.1016/j.ejso.2016.05.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.