Abstract
The development of post-transplant antibodies against non-HLA autoantigens is associated with rejection and decreased long-term graft survival. Although our knowledge of non-HLA antibodies is incomplete, recent studies provide compelling experimental and clinical findings demonstrating that antibodies directed against autoantigens contribute to the process of antibody-mediated acute and chronic rejection. Important areas for investigation remain understanding the mechanisms underlying the production of autoantibodies in the setting of organ transplantation. Ischaemia-reperfusion injury, surgical trauma and/or alloimmune responses can result in the release of organ-derived autoantigens in the form of soluble antigens, extracellular vesicles or apoptotic bodies that are presented in context of the transplant recipient’s antigen presenting cells to stimulate autoantibody production. Th17 cells are essential in the orchestrating autoantibody production by supporting the proliferation and maturation of autoreactive B cells within ectopic tertiary lymphoid tissue. Conversely, autoantibody-mediated graft damage can trigger alloimmunity and the development of donor-specific HLA antibodies that can act in synergy to promoteallograft rejection. Identification of the immunologic phenotypes of transplant recipients at risk of non-HLA antibody-mediated rejection and the development of targeted therapies to treat these rejections are sorely needed to improve both transplant and patient survival.
Introduction
Antibody-mediated rejection (AMR) contributes to both acute and chronic allograft rejection and impedes long-term renal transplant survival1–7. The principal targets of the humoral immune response to the renal allograft are the highly polymorphic HLA antigens, but studies have also implicated antibodies directed against non-HLA antigens in the process of AMR. The most convincing evidence of this mechanism comes from reports of accelerated AMR in recipients of renal transplants from HLA-identical siblings8–10. Similarly, immunity to non-HLA antigens also portends poorer long-term allograft outcome. Two large multicenter studies using independent registry data unexpectedly showed reduced long-term survival of renal transplants performed between HLA-haplotype-matched sibling donors, underscoring the importance of non-HLA immunity to the allograft in chronic rejection11, 12.
Non-HLA antibodies are classified into two main categories: alloantibodies directed against polymorphic antigens that differ between the recipient and donor, and antibodies that recognize self-antigens — autoantibodies13, 14. As the vasculature is at the interface of the recipient immune system and the transplanted organ, a substantial proportion of the non-HLA antibodies reported to mediate renal rejection recognize autoantigens expressed by endothelial cells. Brasile et al. were the first to report a patient with pretransplant anti-endothelial cell antibodies (AECA) that caused hyperacute rejection of a maternal renal allograft15. This patient subsequently received a second transplant from a deceased donor that was also rejected in a hyperacute manner. AECA directed against both donors were detected in pretransplant sera from the recipient, implicating these antibodies in the pathogenesis of accelerated AMR. This early case report was followed by several analogous accounts of transplant recipients who experienced multiple successive hyperacute renal rejections and/or accelerated AMR due to pretransplant AECA16–20. Notably, these recipients displayed negative lymphocyte crossmatches and tested negative for donor-specific HLA antibodies (DSAs), implicating AECA as the mediator of graft injury.
At the population level, the frequency of AECA has been reported to be higher in renal recipients with failed transplants than in those with functioning grafts at 1 year post-transplant21. AECA were also found at a significantly higher frequency in pretransplant sera from HLA-sensitized renal transplant candidates than in non-sensitized candidates22 and have been reported to correlate with AMR in heart transplantation23. In a prospective multicentre trial that used peripheral blood endothelial cell precursors for antibody detection, kidney transplant recipients with pretransplant AECA had increased episodes of early acute rejection and higher creatinine levels than those without AECA24. However, a comparable study of 71 living donor renal transplants showed that pretransplant AECA were not associated with acute rejection or with inferior renal graft function, suggesting that organs from deceased donors might exhibit higher levels of non-HLA antibody ligands than those from living donors25. Interestingly, many of the renal rejections that were reported to be associated with AECA were negative for the complement degradation product C4d and graded as cellular rejection. Although AECA have been shown to activate complement resulting in C4d or C3d deposition in the graft in patients26 and in animal models 27, 28, others have reported the absence of C4d staining in biopsy samples from patients with AECA17, 19, 29, 30. This discrepancy could result from the fact that endothelial-cell-reactive antibodies are enriched for noncomplement-fixing subclasses IgG2 and IgG431. Even in the absence of complement deposition, however, AECA+ biopsy samples exhibit histologic findings consistent with AMR, including glomerulitis, transplant glomerulopathy, focal interstitial haemorrhage, focal capillary thrombosis, and margination of neutrophils, monocytes and macrophages in the peritubular capillaries29, 30, 32, 33.
The specificity of the autoantibodies developed during the immune response to the allograft is diverse. Using a proteomics discovery approach, Sarwal and colleagues provided insights into the broad array of autoantibodies developed following renal allograft rejection34, 35. Some of these autoantibodies were capable of activating endothelial cells in vitro, causing up-regulation of HLA class I, E-selectin and ICAM1 expression36. Notably, despite generating autoantibodies following transplantation, the majority of patients did not experience rejection or graft dysfunction. This finding suggests that the pathogenicity of the autoantibodies is conditional upon other factors such as ligand expression, ischaemic injury and/or the state of inflammation within the microenvironment of the allograft. The expression of autoantigens on the endothelium can vary widely depending upon their anatomical location, vessel type and inflammatory milieu, which might pose challenges to ascribe clinical relevance to non-HLA autoantibodies34. This finding underscores the importance of identifying the nature of the autoantibody ligands on the cells of the allograft to gain mechanistic insight into their pathogenesis. In this Review, we focus on the clinical significance of a selected group of well-characterized autoantibodies and discuss current theories concerning their pathogens and production in renal transplantation.
Angiotensin type 1 receptor (AT1R)
Clinical studies in renal transplantation
Angiotensin type 1 receptor (AT1R) is a G-protein coupled receptor that is expressed at the endothelial cell surface, binds to angiotensin II and regulates water–salt balance and blood pressure37. Hyperactivity of AT1R causes hypertension, vasoconstriction and vascular smooth muscle migration and proliferation38. Antibodies to AT1R were first implicated in pre-eclampsia leading to maternal and fetal mortality and morbidity39. In renal transplantation, elevated levels of AT1R antibodies were first reported in recipients with severe steroid-refractory vascular rejection and malignant hypertension in the absence of HLA-DSA (Table 1)33. In this study, 13 recipients had DSA whereas the remaining 20 were HLA-DSA negative. Among those without DSA, 16 recipients tested positive for AT1R antibodies and presented with vascular injury and malignant hypertension. Even though the AT1R antibodies were IgG1 and IgG3, graft biopsy samples from anti-AT1R positive patients with vascular rejection did not show evidence of complement deposition. Instead the samples displayed increased expression of tissue factor, which was reduced following treatment with the angiotensin II receptor antagonist losartan. Treatment of AT1R antibody positive patients with a combination of plasmapheresis, intravenous immunoglobulin (IVIG), and losartan resulted in significantly improved allograft survival compared to patients receiving standard anti-rejection therapy. These results indicate that agonistic antibodies targeting AT1R can mediate vascular injury.
Table 1.
AT1R antibodies in human organ transplantation
| Cohort | Time course | Key findings | Synergy with HLA-DSA | Ref. |
|---|---|---|---|---|
| Kidney recipients | ||||
| 33 patients with steroid-refractory rejection: 16 with AT1R Ab+, HLA-DSA− 13 with AT1R Ab−, HLA-DSA+ |
Post-TX | Biopsy samples from 11 of 16 AT1R Ab+ patients did not show C4d deposition AT1R+, HLA DSA- patients had much rapid allograft loss as compared to AT1R-, HLA DSA+ recipients Treatment with plasmapheresis, IVIG and losartan improved allograft survival in AT1R ab+ recipients |
NA | - 33 |
| 63 patients with no HLA-DSA or MICA-DSA, including 16 patients with AR (7 AMR, 9 ACR) | Pre-TX & post-TX | AT1R Ab associated with increased AMR but not ACR 6 of 7 patients with AMR did not have C4d deposition |
NA | - 30 |
| 134 patients with abnormal biopsies* 217 control patients |
Pre-TX & post-TX | AT1R Ab associated with abnormal biopsy samples De novo AT1R Ab associated with increased graft failure as compared to recipients without AT1R and HLA DSA |
Yes | - 44 |
| 283 AT1R Ab+ patients 316 AT1R Ab- patients |
Pre-TX | Pre-formed AT1R Ab associated with increased AR within 4 months and increased graft failure >3-year post-TX | NA | 40 |
| 7 AT1R Ab+ patients 72 AT1R Ab− patients |
Pre-TX | Pre-formed AT1R Ab associated with increased AMR but not ACR | NA | 41 |
| 7 AT1R Ab+ patients 58 AT1R Ab− patients |
Post-TX | Post-Tx AT1R Ab associated with Increased graft injury and graft failure | NA | - 46 |
| 11 patients with AR and no HLA-DSA | Pre-TX & post-TX | 10 of 11 patients had pre-TX AT1R Ab 10 of 11 patients had no C4d deposition |
NA | - 29 |
| 12 patients with AMR and no HLA-DSA | Pre-TX & post-TX | 9 of 10 patients had AT1R Ab+ sera before transplantation 10 of 12 patients were AT1R Ab+ at the time of biopsy AT1R Ab increased the risk of AMR |
NA | - 43 |
| 98 AT1R Ab+ patients 64 AT1R Ab− patients |
Pre-TX | Pre-Tx AT1R Ab associated with increased AR | NA | - 42 |
| Paediatric recipients: 10 autoAb+ 10 autoAb− |
Pre & post Tx | 50% of patients developed de novo Abs to AT1R, FN, or collagen IV in the first year after TX; development of these Ab did not correlate with graft function | NA | 45 |
| Heart recipients | ||||
| 14 AT1R Ab+ patients 16 AT1R Ab− patients |
Pre-TX Post-TX |
Pre and post-transplant AT1R ab is associated with increased AMR, ACR and microvasculopathy | NA | 55 |
| 76 AT1R Ab+ patients 124 AT1R Ab− patients |
Pre-TX & post-TX | Pre-Tx and post-Tx AT1R Ab did not correlate with AMR, CMR and CAV AMR and CMR increased with HLA-DSA and AT1R |
Yes | - 56 |
| 69 patients with LVAD: 8 with AT1R Ab pre-LVAD 44 with de novo AT1R Ab post-LVAD 17 without AT1R Ab |
Pre-TX | 3 of 5 patients with primary graft dysfunction had AT1R Ab No difference in 1 year and 5 year survival No difference on ACR and AMR between AT1R Ab+ and AT1R Ab− groups |
NA | -57 |
AT1R, angiotensin II type 1 receptor; GPCR, G protein coupled receptors; ab, antibody; TX, transplant; AR, acute rejection; AMR, antibody mediated rejection; ACR, acute cellular rejection; CMR, cellular mediated rejection; CAV, coronary artery vasculopathy; LVAD, left ventricular assist device. NA, not assessed.
Biopsy-proven rejection and/or lesions.
Subsequent studies confirmed these initial findings and showed that AT1R antibody correlated with an increased incidence of AMR and inferior graft survival (Table 1)29, 30, 32, 40–46. For example, a strong correlation between AT1R antibody and diagnosis of AMR was identified in a cohort of 97 kidney transplant recipients who did not have HLA or MHC class I -related chain A (MICA)-DSA30. The researchers observed a relationship between antibody strength and AMR as six of the seven patients diagnosed with AMR had high levels of AT1R antibody (>17 U/ml) both pretransplant and at the time of rejection, whereas none of the nine patients diagnosed with acute cellular rejection (ACR) had AT1R antibodies at these time points. Notably, only one of 6 AT1R+ recipients with AMR had C4d staining in their biopsy sample, again suggesting that complement-independent mechanisms contributed to graft injury.
Giral et al. showed that >45% of 599 kidney transplant recipients had pretransplant AT1R antibodies >10 U/ml40. AT1R autoantibody positivity was associated with a higher risk of acute rejection within the first 4 months after transplantation and a 2.6-fold higher risk of graft failure beyond 3 years post-transplantation, suggesting that AT1R autoantibodies might impact long-term graft survival40. Pre-transplant and post-transplant AT1R antibodies were studied in 351 consecutive kidney transplant recipients using a cut-off value of 15 U/ml44. In this study 35 patients were positive for AT1R antibodies before transplantation but became negative for these antibodies after transplantation with no impact on graft survival. By contrast, 12 of 25 patients (48%) with persistent pretransplant and post-transplant AT1R antibodies, and 7 of 11 patients (64%) who developed de novo AT1R antibodies following transplantation lost their grafts. Furthermore, patients who were positive for both AT1R antibody and HLA-DSA had lower graft survival than those with HLA-DSA alone, suggesting a synergistic effect of these autoantibodies44. The prevalence of pretransplant AT1R antibody reported across these studies was high, but varied widely from 17% to 59%. A possible explanation for this variation is the use of differing methods for AT1R antibody detection or different cut-off values for determining positive or negative results.
Recurrence of focal segmental glomerulosclerosis (FSGS) after kidney transplantation causes accelerated allograft loss in approximately 30% of recipients47, 48,49, 50. The hallmark of FSGS is podocyte damage resulting in proteinuria and loss of graft function. Causes of FSGS can be hereditary or idiopathic, but the underlying pathophysiology of FSGS recurrence is not well understood. Studies in an experimental rat model suggest a role for AT1R in FSGS; overexpression of human AT1R in rat podocytes was sufficient to cause podocyte damage and foot process effacement consistent with a FSGS phenotype51. Autoantibodies to AT1R were reported in a transplant recipient diagnosed with AMR and FSGS with severe podocyte effacement52. Depletion of AT1R antibodies by plasma exchange combined with losartan therapy ameliorated AMR and reduced podocyte injury in this patient. The presence of pretransplant AT1R antibodies was also assessed in a cohort of 28 renal transplant recipients with a history of idiopathic FSGS. Although no differences were found in pretransplant AT1R antibody levels between patients with FSGS and a control group of patients with polycystic kidney disease, significantly higher levels of AT1R antibodies were observed in recipients with FSGS recurrence53. Using an integrative bioinformatics approach on high density protoarrays, a panel of autoantibodies were identified as clinically relevant predictors of FSGS recurrence after transplantation54. Mechanistic studies suggest that the patient-derived autoantibody to CD40 synergizes with the soluble urokinase receptor to elicit podocyte injury. These studies underscore the clinical importance of AT1R autoantibodies in renal transplant outcome and support the feasibility of developing an autoantibody panel that might serve as a biomarker for risk of FSGS recurrence.
Clinical studies in heart transplantation
Increased pretransplant levels of AT1R antibodies are associated with heart transplant ACR and AMR as well as early onset of microvasculopathy55. A study showed that elevated pretransplant AT1R antibody levels alone were not associated with increased risk of rejection, but when both HLA-DSA and AT1R antibodies were present, the risk of AMR and ACR increased significantly, suggesting a synergism between AT1R and HLA antibodies in promoting rejection56.
Elevated AT1R antibody levels were also reported in patients with a left ventricular assist device (LVAD) as a bridge to transplantation. Over 60% of heart transplant candidates who were AT1R negative prior to LVAD implantation developed de novo AT1R antibodies after the procedure independent of blood product usage57. Development of AT1R antibodies post-LVAD did not impact incidence of rejection or transplant survival. The mechanism by which LVADs promote autoantibody production is unknown and research is needed to determine if these devices have unique immunologic properties that lead to the formation of de novo autoantibodies, or if they elicit inflammation that leads to stimulation of existing memory B cells.
Mechanisms of injury
Although AT1R antibodies implicated in renal rejection were identified as IgG1 and IgG3 isotypes, C4d deposition was only detected in a subset of patients, suggesting the involvement of complement-independent pathogenesis of these antibodies19, 29, 30, 33. Indeed, Dragun et al. showed that binding of antibodies to AT1R mimicked the action of angiotensin II and triggered phosphorylation of ERK kinase and activation of the transcription factors AP-1 and NF-κB in endothelial and smooth muscle cells33. Renal biopsy specimens from transplant recipients with AT1R-mediated rejection also had increased tissue factor expression and thrombotic occlusions33. These occlusions were lessened following treatment with losartan and this effect was accompanied by improved renal function and graft outcome. The effects of AT1R antibodies were recapitulated in a rat transplant model in which passive transfer of agonistic AT1R antibodies caused hypertension and induced vascular changes in the renal transplant including endarteritis and intravascular infiltrates33. Together, these clinical and experimental data are consistent with the hypothesis that binding of autoantibody binding to AT1R contributes to rejection by mimicking the action of angiotensin II.
Although angiotensin II binds to several sites in the extracellular loops and transmembrane helices of AT1R58, AT1R antibodies implicated in renal allograft rejection have been mapped to the AFJYESQ and ENTNIT epitopes present in the second extracellular loop (ECL2) of AT1R33. Molecular dynamics simulations in rat models have shown that the ECL2 has an important role in the conformation of AT1R and consequently different functional states of the receptor59, 60. ECL2 conformation differs between the angiotensin II-bound and losartan-bound states that affect AT1R activation 61, 62, AT1R antibodies directed toward ECL2 can stabilize the receptor in a permanently activated state63. In line with this hypothesis, the time course of vasoconstriction caused by AT1R antibodies in a human fetoplacental pre-eclamptic model was significantly longer in duration than that induced by angiotensin II64.
As mentioned above, the presence of AT1R autoantibodies prior to renal transplantation is a risk factor for vascular rejection and malignant hypertension. Not all renal recipients with AT1R antibodies reject their grafts, however, indicating that additional co-factors or environmental conditions might be needed to promote rejection. For example, in a rat model of ischaemia reperfusion injury (IRI), AT1R antibodies mediate renal vascular contraction only under ischaemic conditions65. Similarly, AT1R antibodies isolated in sera from patients with HLA-DSA negative vascular rejection induced severe vasoconstriction in arteries of the renal allograft, but not in native arteries65 These results support the concept that IRI-mediated damage of the endothelium modulates AT1R expression and/or its conformation to facilitate interactions with AT1R antibodies. These findings were corroborated by studies showing that IRI can lead to the unmasking of neo-epitopes that, once exposed, can serve as a target of natural antibodies66, 67.
Perlecan
Clinical studies in renal transplantation
Perlecan — a large heparan sulfate proteoglycan — is a major component of the vessel wall. The C-terminal domain of perlecan, endorepellin, is best known for its anti-angiogenic properties68 and contains three laminin-like globular (LG) domains separated by two sets of epidermal growth factor (EGF)-like repeats. Most of the antiangiogenic activity of endorepellin resides in the LG3 subdomain. Apoptotic endothelial cells liberate cathepsin-L, which cleaves the LG3 domain from the C-terminal fragment of perlecan69. Studies have shown increased serum LG3 levels in renal transplant recipients with immune-mediated vascular injury and renal dysfunction70–72 (Table 2). In a retrospective case-control study, serum LG3 levels were significantly higher in renal transplant recipients diagnosed with Banff grade II or III acute vascular rejection than in those with Banff grade I acute tubulo-interstitial rejection or normal graft function72. Furthermore, vascular rejection and elevated LG3 levels were associated with heightened neointima formation. These data identify LG3 as a regulator of vascular remodelling.
Table 2.
Perlecan antibodies in kidney transplant recipients
| Cohort | Time | Key findings | Ref. |
|---|---|---|---|
| 16 patients with AVR 16 patients with ATIR 32 control patients |
Post-TX | Post-transplant LG3 Ab are associated with increased AVR and reduced survival | 72 |
| 15 patients with AVR 15 patients with ATIR 30 controls |
Pre-TX & post-TX | Pre-transplant LG3 Ab and HLA-DSA were both independently associated with AVR | - 70 |
AVR, acute vascular rejection; ATIR; acute tubulointerstitial rejection; TX, transplantation.
An important question is whether or not LG3 also behaves as a neoantigen capable of driving the production of LG3 antibodies that can enhance the process of chronic rejection. Cardinal et al. reported that pre-transplant and post-transplant levels of LG3 antibodies of the IgG1 and IgG3 isotypes were significantly higher in renal transplant recipients with acute vascular rejection than in those without evidence of rejection70. Levels of circulating LG3 sampled at the same time as the LG3 antibodies showed a concomitant increase. Moreover, higher pretransplant levels of LG3 antibodies predicted recipients at risk of vascular rejection. Neither pretransplant HLA-DSA alone or post-transplant LG3 antibodies alone reduced renal allograft survival in patients diagnosed with vascular rejection, but patients with pretransplant DSA and strong post-transplant LG3 antibodies had significantly reduced 1-year graft survival. These results suggest that similar to AT1R antibodies, HLA-DSA and LG3 autoantibodies can synergize to elicit endothelial cell and graft damage.
Mechanisms of injury
The current paradigm of perlecan-mediated graft injury considers two different, but interdependent mechanisms whereby bioactive forms of perlecan cause vascular injury and neointimal formation directly by promoting migration of donor vascular smooth muscle and/or recipient-derived mesenchymal cells, and/or elicit humoral immune responses that accelerate immune-mediated vascular injury and remodelling. Studies by the Hebert group have provided supporting evidence for both models. First, they showed that passive transfer of anti-LG3 IgG in a murine model of vascular rejection significantly increased infiltration of T cells and natural killer cells, C4d deposition and obliterative vascular remodelling in recipients of ischaemic aortic allografts, but not in adjacent non-ischaemic native aortas70. Second, they showed that escalating serum LG3 levels in murine aortic allograft recipients significantly increased neointima formation70. To address the question of how LG3 contributes directly to vascular remodelling, recombinant LG3 was administered to mice transplanted with aortic allografts. Injection of LG3 enhanced smooth muscle cell migration and neointima formation via an ERK dependent pathway72. Moreover, in addition to cells of donor origin, injection of LG3 furthered the accumulation of recipient-derived mesenchymal stem cells (MSCs) and smooth muscle cells into the sites of neointima formation, which was dependent upon MSC expression of α2β1 integrin73. Together these findings suggest that LG3 causes vascular injury and neointimal formation by stimulating autoantibody production and/or by promoting the migration of vascular smooth muscle cells and/or MSCs.
Collagen
Clinical studies in lung transplantation
The clinical significance of collagen autoantibodies has mainly been assessed in the setting of lung transplantation (Table 3). Collagen V is present on airway epithelial cells and its expression increases following immune-mediated injury and/or IRI74, 75. Graft injury is thought to induce the expression of matrix metalloproteases that modify collagen and release collagen fragments that serve as a major target for autoantibody production76. Post-transplant development of collagen V antibody is associated with the development of bronchiolitis obliterans syndrome (BOS) in lung transplant recipients76–79. A trans vivo delayed type hypersensitivity assay showed that a collagen-V-specific cellular response in these patients was dependent on both CD4 T cells and monocytes that required IL-17, TNF-α and IL-1β77. Patients with idiopathic pulmonary fibrosis and cystic fibrosis reportedly have the highest prevalence of antibodies to collagen V and Kα-tubulin and are at increased risk of developing primary graft dysfunction, HLA-DSA and BOS79.
Table 3.
Collagen antibodies in human organ transplantation
| Cohort | Time of Ab detection | Key findings | Ref. |
|---|---|---|---|
| Lung recipients | |||
| 54 patients 11 controls (5 renal TX recipients and 6 healthy individuals) |
Post-TX | Post-transplant development of collagen V Ab was associated with increased incidence of BOS caused by TH17 cells, monocytes and macrophages | 77 |
| 5 patients with PGD 5 patients without PGD |
Within hours post-TX | Pre-formed collagen V Ab was strongly associated with PGD development | 74 |
| 12 patients with BOS and collagen V Ab | Post-TX | A shift in the immunodominant epitopes of collagen V antibody from α1 and α2 to α1 only, occurred at the time of BOS diagnosis The shift in immunodominant epitopes correlated with a decrease in the expression of IL-10 and increased expression of IFN-γ and IL-17 |
76 |
| 72 collagen V and/or Kα-tubulin Ab+ patients 36 collagen V and Kα-tubulin Ab− patients |
Post-TX | Correlation between the development of Ab to self-antigens and HLA-DSA Ab to self-antigens are an important risk factor for BOS |
78 |
| 317 patients | Pre-TX | Patients with idiopathic pulmonary fibrosis and cystic fibrosis have the highest prevalence of Ab to collagen V and Kα-tubulin Patients with pre-existing Ab to collagen V and Kα-tubulin are at increased risk of development of PGD, DSA and BOS |
79 |
| 30 collagen V Ab+ patients 69 Ab− patients |
Pre-TX & post-TX | Patient transplanted with DR15 donor were at significant risk of developing anti-collagen V responses and BOS. This may result from presentation of self-peptides by donor HLA DR15 to recipient T cells | 112 |
| Heart recipients | |||
| 60 patients with early TX* (9 AMR+, 51 AMR−) 77 patients with late TX‡ (14 CAV+, 63 CAV−) |
Post-TX | Development of collagen V and Kα-tubulin Ab correlated with the development of DSA, AMR and DSA+ CAV CAV+ patients had increased IL-17 and decreased IL-10 secreting collagen V specific CD4+ T cells |
81 |
| Kidney recipients | |||
| 26 patients with transplant glomerulopathy 10 stable controls |
Pre-TX & post-TX | Patients with Ab to collagen IV and fibronectin had increased risk for transplant glomerulopathy (OR of 22) Patients with transplant glomerulopathy had increased collagen-IV-specific and fibronectin-specific CD4+ T cells secreting IFN-γ and IL-17, but reduced levels of IL-10 |
80 |
BOS, bronchiolitis obliterans syndrome; TX, transplant; PGD, primary graft dysfunction; TG, transplant glomerulopathy; ab, antibody; DSA, donor specific antibody; AMR, antibody mediated rejection; CAV, coronary artery vasculopathy.
Transplant duration ≤12 months.
Transplant duration >12 months.
Clinical studies in renal and heart transplantation
Late renal allograft failure owing to chronic allograft nephropathy is one of the major challenges to the long-term success of renal transplantation. Transplant glomerulopathy is characterized by duplication of the glomerular basement membrane and is associated with chronic renal allograft rejection. Development of autoantibodies to collagen IV and fibronectin has been reported in renal transplant recipients diagnosed with transplant glomerulopathy80. These patients displayed increased collagen IV-specific and fibronectin-specific CD4+ T cells that secreted IFN-γ and IL-17, as well as a reduction in IL-10 levels, implicating collagen IV in the pathogenesis of chronic rejection80.
In heart transplantation, development of HLA DSA in recipients diagnosed with AMR correlated with the development of autoantibodies to collagen V81. Production of DSA to HLA and autoantibodies to collagen V was accompanied by increased frequencies of collagen V specific CD4+Th cells secreting IL-17 and higher risk of CAV.
Mechanisms of injury
Administration of collagen-V-specific CD4+ T cells or antibodies to collagen V can induce BOS in experimental models of lung transplantation77. The role of IL-17-dependent cell-mediated immunity to collagen V in the development of BOS was first demonstrated in a study of lung transplant recipients who were monitored over a period of 7 years following transplantation. Strong collagen V responses correlated with both the frequency and severity of BOS77.
An orthotopic rat lung isograft model and adoptive transfer experiments were utilized to demonstrate that type 17 T helper (TH17)-mediated immunity to collagen V can induce transplant vasculopathy in the absence of alloimmunity. Immunization of rats with collagen V induced upregulation of IL17 and IL-23 mRNA expression in T cells and adoptive transfer of these TH17 cells caused development of BOS lesions in lung isografts77. The causal role of TH17 cells in acute allograft rejection was further confirmed by adoptive transfer of CD4+TH17+ collagen-V-specific T cells into rat lung isografts82. Treatment with neutralizing IL-17 prevented BOS in orthotopic lung transplantation and chronic corneal allograft rejection in experimental mouse models83, 84. Furthermore, adoptive transfer of CD4+ collagen-V -specific regulatory T cells successfully blocked TH17-mediated acute lung transplant rejection in a rat model82. The mechanism for this TH17 suppression is consistent with induction of tolerance to collagen V, suggesting that targeting TH17 development might lead to improved long-term outcomes in lung transplantation.
Transfer of anti-collagen V antibodies to rat recipients of lung isografts induced primary graft dysfunction, a leading cause of early mortality in lung transplantation and a major risk factor for BOS74. The adoptively transferred anti-collagen V antibodies were capable of inducing airway epithelial cell cytotoxicity in the lung isografts, implicating complement as a mechanism of graft injury. Intrabronchial administration of anti-MHC class I or control antibodies to wild type and IL-17a knockout C57BL/6 mice induced a marked reduction in cellular infiltration, decreased fibrosis, and a substantial (2.17-fold) decrease in collagen V antibody levels, when compared with wild-type mice 85. These findings indicate that IL-17A and IL-17F secreted by CD4+ TH17 cells specific to lung self-antigens are critical mediators of autoimmunity leading to the pathogenesis of BOS.
Autoantibody production in transplantation
Autoantibody production in the transplant setting depends on multiple factors. Graft damage mediated through IRI, alloimmunity and chronic inflammation can cause intracellular proteins to be expressed on the surface of apoptotic cells, raising the possibility that these cells act as a autoantigen reservoirs86. The autoantigens can be subsequently presented to autoreactive T cells and B cells either by recipient or donor antigen presenting cells (APCs), in the context of extracellular vesicles (EV) or in tertiary lymphoid tissue (TLT). Improved understanding of the mechanisms that underlie autoantibody production could lead to the identification of new therapeutic strategies to prevent or control transplant rejection and aberrant vascular remodelling.
Ischaemia reperfusion injury
Organ transplantation inevitably involves varying levels of surgical trauma, tissue damage and IRI, all of which can elicit innate inflammatory responses contributing to the generation of alloimmune and autoimmune responses to the graft. Although the immune system has a number of checkpoints to preserve tolerance to self-antigens, including central and peripheral tolerance, defects in these checkpoints coupled with the constant presence of autoantigen leads to chronic inflammation87. IRI is a complex pathophysiological process that involves the generation of reactive oxygen species, complement activation, coagulation, endothelial activation and leukocyte recruitment (FIG. 1). The tissue damage caused by IRI can lead to acute kidney injury and delayed graft function, which can impair graft survival88. Apoptosis and necrosis caused by IRI results in the release of damage associated molecular patterns (DAMPs) including self-nucleic acids, histones, and high mobility group protein B1. DAMPs can interact with pattern recognition receptors including Toll-like receptor (TLR) 2 and TLR4 expressed on myeloid cells89, 90, dendritic cells89, vascular endothelial cells91, 92, and tubular epithelial cells93. Ligation of TLRs results in the recruitment of MYD88 and the production of NF-κB. This process in turn causes downstream production of proinflammatory cytokines, including IL-1β, IL-6, and TNF, which promote the activation of adaptive immune responses that can exacerbate allograft damage and exposure to autoantigens93.
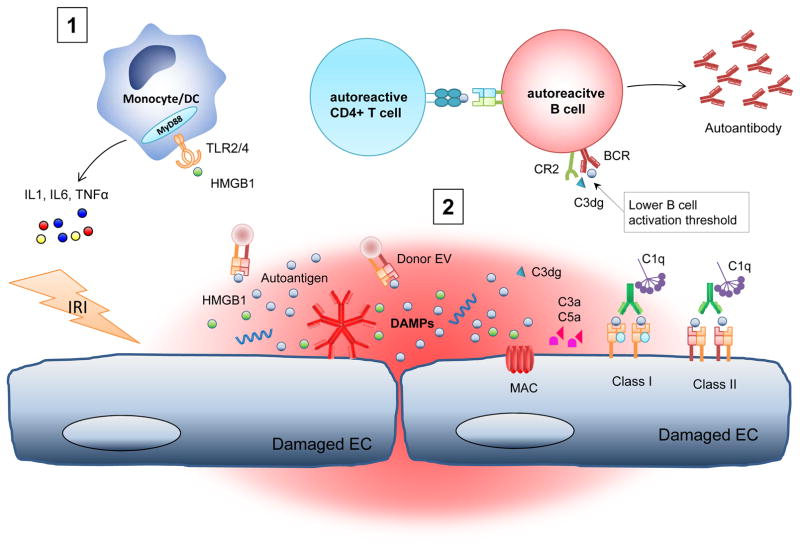
Figure 1. Allograft injury and potential mechanisms of autoantibody production.
Ischaemia-reperfusion injury (IRI)-induced damage to vascular endothelial and tubular epithelial cells triggers the release of damage associated molecular patterns (DAMPs), including nucleic acids, histones and high mobility group protein B1 (HMGB1). Ligation of Toll-like receptor (TLR) 2 and TLR4 on myeloid, dendritic, and vascular endothelial cells activates the MYD88 and NF-κB pathways, resulting in production of proinflammatory cytokines. Autoantibody-mediated inflammation activates complement and promotes further activation of autoreactive B cells and leukocyte recruitment through the production of C3a and C5a. Tissue damage caused by IRI, natural antibodies or HLA-DSA can also lead to the activation of autoreactive T and B cells and the subsequent production of autoantibodies. Complement can potentiate autoantibody production by lowering the threshold of B-cell activation through the binding of C3dg-coated antigen to complement receptor 2 (CR2) and the B cell receptor (BCR). MAC, membrane attack complex; TNF, tumour necrosis factor.
Several studies have demonstrated that IRI is sufficient to induce an autoimmune response in the absence of alloimmunity70, 75. For example, adoptive transfer of collagen-V-reactive lymphocytes to Wistar Kyoto rats induced grade 2 rejection in fresh isografts and collagen V expression (detected using immunhistochemistry) in fresh and well-healed isografts, but not in native lungs75. These data indicate that IRI predisposes to anti-collagen-V-mediated pathology. TLR4 expression was also elevated in deceased donor kidney transplants, and loss-of-function mutations in TLR4 were associated with lower intragraft expression of proinflammatory genes such as TNFα and monocyte chemoattractant protein-1 (MCP-1)93. IRI, natural antibodies, HLA-DSA and complement can damage the vascular endothelium leading to release of autoantigens from necrotic and apoptotic cells (FIG. 1). Autoantigens such as vimentin, perlecan, and collagen V released from the injured allograft are processed and presented to autoreactive T cells by APC recruited during IRI70, 94, 95. Circulating B cells bind these autoantigens and become activated by autoreactive T cells, resulting in the secretion of autoantibodies. In addition, complement activation can potentiate autoantibody production in the transplant setting.
In a murine chronic lung transplant model, C3a increased IL-17 production by collagen-V-reactive T cells, suggesting an important role of complement in the development of autoantibody responses96. In addition, C3 can bind directly to self-antigens leading to a more efficient retention of antigens in the B cell areas of lymphoid tissue and a reduced threshold for B-cell stimulation by these autoantigens97, 98. Mice lacking complement fail to initiate high-affinity IgG production against MHC in skin grafts99. The mechanism involves antigen bound C3dg, an iC3b cleavage product, binding to CR2 (CD21) receptor on B cells and lowering the threshold for B-cell activation98. Work from several groups has uncovered a novel role for complement spilt products on T cell and APC activation, resulting in signals that induce T cell proliferation and APC upregulation of costimulatory molecules and cytokines100.
Interplay between alloimmunity and autoimmunity
Alloimmune responses to the transplanted organ or tissue occur through the direct, indirect and semi-direct allorecognition pathways101. Once initiated, the indirect alloimmune response can spread to additional determinants within the primary target antigen, this process is termed intramolecular epitope spreading102, 103. This expansion of alloreactive T cells to donor-derived MHC antigen via the indirect allorecognition pathway is associated with chronic rejection and linked to the generation of DSAs103–106.
Similarly, the indirect recognition pathway can also promote the development of autoimmune T and B cells that contribute to the rejection process107. The indirect alloresponse triggers autoimmunity after transplantation presumably via antigen mimicry between autoantigen peptides and donor MHC peptides108. Several studies have shown that autoreactive proinflammatory T cells specific for collagen V and cardiac myosin are detected after lung and heart transplantation, respectively. Clonal expansion of these T cells occurs only after an alloresponse, and once stimulated they can induce rejection of allogeneic transplants77, 81, 109, 110. Alternatively the tissue damage caused by the alloresponse to donor HLA antigens can cause the release of sequestered autoantigens resulting in the presentation of cryptic self-determinants and thereby triggering an autoimmune process at the site of the graft.
Once autoreactive T cells are generated, chronic stimulation of these cells can lead to epitope spreading, which has been reported to contribute to the generation of autoantibodies111. For example, sequential analysis of antibody development against collagen V following lung transplantation demonstrated that patients initially develop antibodies to both a1(V) and a2(V) of collagen V, yet at the time of BOS diagnosis, the antibodies shifted entirely to a1(V) demonstrating the importance of epitope spreading in the development of this disease76. A more refined epitope analysis of collagen-V-specific CD4+ TH17 cells in patients with BOS showed HLA-restricted epitope binding of a1(V) in association with the HLA-DR15 genotype112. Interestingly, an HLA-DR15 negative recipient who received a transplant from a HLA-DR15 positive donor also responded to DR15-restricted collagen V epitopes after lung transplantation, suggesting determinant spreading from recipient DR-restricted epitopes to donor HLA-DR restricted epitopes112, 113.
During progression of chronic rejection in heart and kidney transplant recipients, ‘inter-molecular epitope spreading’ has been described for indirect pathway responses to donor alloantigen114. It is plausible, therefore, that acquisition of a donor class II-restricted response to self-antigens is yet another mechanism contributing to chronic rejection. This hypothesis has important clinical implications for the development of autoimmunity post-transplantation and underscores the importance of identifying the specific autoantigens and their HLA-restricted epitopes. Ultimately this information might guide the allocation of lung transplants and the development of therapeutic strategies to prevent chronic rejection.
Extracellular vesicles
Cell-to-cell communication through extracellular vesicles is increasingly recognized as a mechanism that elicits autoimmune and alloimmune responses115, 116. Depending on their cellular source, extracellular vesicles contain mRNAs, miRNAs, DNA, proteins, lipids and carbohydrates, and can positively or negatively modulate immune responses116. Based on their biogenesis, extracellular vesicles can be categorized into three classes — exosomes, microparticles and apoptotic bodies — all of which contain numerous autoantigens. Extracellular vesicles released by APCs also carry surface MHC class I and II molecules plus bound peptides that can activate T cells117–122. Although high concentrations of exosomes can directly activate T cells118, 119, the activatory effect of this type of antigen presentation is much weaker than that of antigen presentation by professional APCs owing to the lack of co-stimulatory molecules (FIG. 2). By contrast, exosomes efficiently activate T cells when they interact with dendritic cells117, 120.
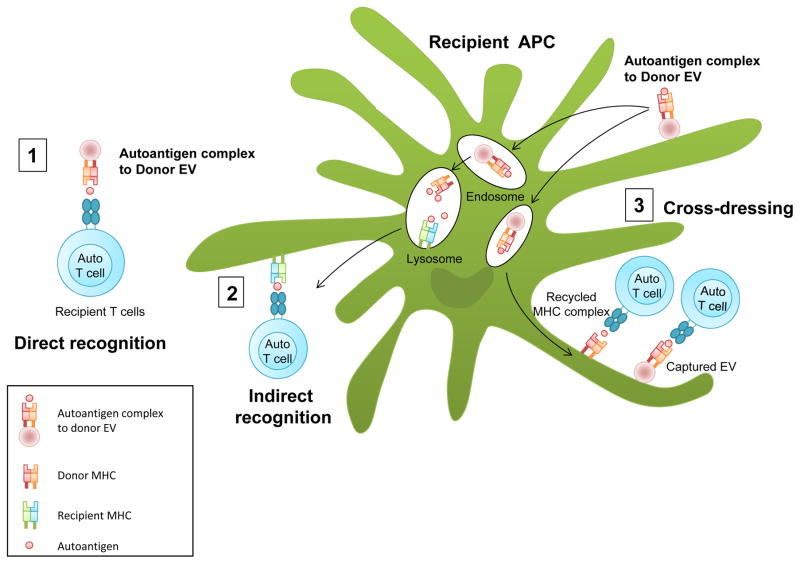
Figure 2. Autoantigen presentation by extracelluar vesicles.
(1) Donor-derived extracellular vesicles containing major histocompatibility complex (MHC) class I and/or MHC class II molecules complexed to autoantigens released from the allograft can directly activate recipient autoreactive CD4+ and CD8+ T cells. Antigen presentation by free extracellular vesicles is much less efficient than presentation by professional antigen presenting cells (APCs). (2) Donor-derived extracellular vesicles can be internalized by recipient APCs and the processed donor antigens presented by these cells through the indirect recognition pathway to recipient autoreactive CD4+ and CD8+ T cells. (3) Antigen presentation through ‘cross-dressing’ of recipient APCs with donor MHC molecules activates recipient autoreactive CD4+ and CD8+ T cells. Cross-dressing occurs when donor-derived extracellular vesicles are internalized and recycled to the surface of recipient APCs without peptide–MHC reprocessing. Alternatively, donor derived extracellular vesicles EV can be captured and fused to the APC surface.
In mouse models of cardiac and skin transplantation, internalization of host-derived exosomes by recipient splenic dendritic cells led to activation of anti-donor CD4 T cells by the indirect pathway of allorecognition117. In addition, donor autoantigen–MHC complexes from internalized extracellular vesicles can be recycled and presented as intact molecules on the surface of recipient APCs to recipient T cells through the process of cross-dressing (FIG. 2)116. The role of apoptotic exosome-like vesicles in regulating the production of autoantibodies was recently demonstrated using large scale proteomics123. The study showed high levels of the immunogenic LG3 fragment of perlecan was present in apoptotic exosome-like vesicles, but not in apoptotic bodies 123 Injection of apoptotic exosome-like vesicles in an in vivo murine aorta transplant model not only induced the production of LG3 antibodies, but also increased the severity of rejection.
TH17 cells and tertiary lymphoid tissue
TH17 cells are the major producers of IL-17 and have been implicated in many autoimmune diseases124, 125. TH17 cells activated by TGF-β and IL-6 are potent inducers of leukocyte recruitment and mediate protective responses to foreign pathogens, whereas Th17 cells activated by IL-23 promote chronic tissue inflammation and autoimmunity. A large body of data demonstrates the importance of IL-17 in solid organ transplant rejection and generation of autoimmune responses, particularly in lung allograft rejection55, 76, 77, 79, 81, 112. Early lung allograft rejection caused by TH17 cells can be attributed to recruitment of neutrophils into the graft (FIG. 3). For example, intradermal injection of IL-17 into human skin grafts transplanted on mice with severe combined immunodeficiency induced neutrophil infiltration126. In addition, IL-17-knockout mice showed delayed neutrophil infiltration after cardiac allograft transplantation127.
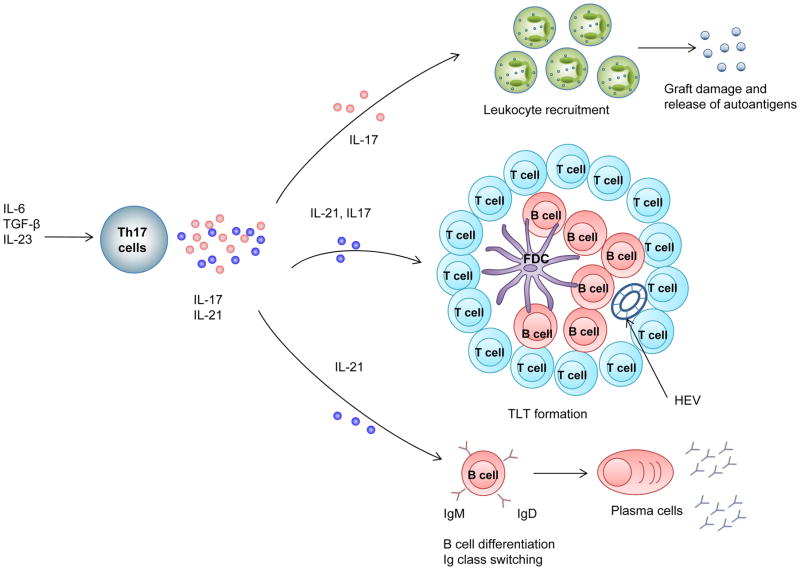
Figure 3. Effector mechanisms of TH17-cell-mediated autoantibody production.
Type 17 T helper (TH17) cells secrete several effector molecules including IL-21, IL-22 and IL-17. Production of IL-17 in the allograft leads to leukocyte recruitment, resulting in graft damage and release of autoantigens. Production of IL-21 by TH17 cells can trigger formation of tertiary lymphoid tissue (TLT) and mediate B-cell differentiation and antibody class switch which is important for B cell maturation and autoantibody production.
The development of TLT has been reported in chronic allograft rejection of kidney128–131, lung132 and heart transplants131, 133. TLT is an ectopic accumulation of lymphoid cells, with characteristics similar to that of a germinal center within a secondary lymphoid organ that arise in the setting of chronic inflammation through a process called lymphoid neogenesis (FIG. 3). TLT form in certain autoimmune diseases and support the proliferation and maturation of autoreactive B cells134. Hsu and colleagues demonstrated that TH17 cells secreting IL-17 orchestrated the spontaneous formation of autoreactive germinal centers in a mouse model of autoimmunity135. Consistent with these findings, transfer of TH17 cells into T-cell deficient mice induced a pronounced autoantibody response with preferential isotype class switching as well as the formation of germinal centers124. Isotype class switching was dependent upon the production of IL-21 by TH17 cells124. TH17 cells have a greater capacity to provide cognate B cell help than do TH1 cell populations by higher clonal expansion, persistence in the B cell follicle and/or expression of inducible T cell costimulator (ICOS). ICOS is highly expressed by T follicular helper cells which is important for T cell dependent antibody production. IL-17 has also been demonstrated to promote TLT formation in mouse lung by up-regulating the expression of CXCL13 and CCL19 that is central for T and B lymphocyte recruitment and TLT organization136,137. Autoreactive B cells might also escape apoptosis and negative selection in TLT due to sustained local production of BAFF and IL-21 that promote B-cell survival138. Furthermore, under pro-inflammatory conditions, TLT is constantly fed neo-antigens released by tissue injury and trapped by defective lymphatic drainage leading to the production of pathogenic autoantibodies139. Collectively, these data underscore the important role of TH17 cells in B-cell differentiation and antibody production.
Conclusions and future perspectives
Mounting evidence supports the importance of immunity to self-antigens in both acute and chronic rejection of solid-organ transplants. Autoantibody production might occur via several mechanisms, including failure of efficient clearance of extracellular vesicles, particularly apoptotic bodies, cross-reactivity between self and foreign antigens, and interplay between innate and adaptive immunity.
The patterns of clinical presentation and response to desensitization therapy of non-HLA antibodies have not been clearly established. Current treatment strategies for AMR due to autoantibodies are similar to that reported for HLA antibodies, including antibody depletion140, B-cell depletion141, IVIG142 proteosome inhibitors143, 144 and complement inhibitors145, 146. However, it is not clear whether these regimens are effective either alone or in combination. This issue was highlighted in a case report of renal artery thrombosis, accelerated rejection and graft loss associated with AT1R antibody that was refractory to AT1R blockade, plasmapheresis, IVIG, corticosteroids and eculizumab 32. A need clearly exists for further investigation into the pathogenic mechanisms of autoantibodies and the identification of more effective therapies. A potential candidate is the IL-6 inhibitor tocilizumab, which reduced the production of HLA-DSAs in desensitization-resistant kidney transplant recipients in a small pilot study147. Therapeutic strategies targeting IL-17 pathways, miRNA regulation148–150 and exosomes might also become viable options to treat autoantibody-mediated AMR and improve graft survival.
Incomplete knowledge of the autoantigens that elicit immune responses during transplantation hampers the diagnosis and treatment of AMR. Modern genomics and proteomics platforms provide extraordinary new tools to examine antibody repertoires34, 35, 151, 152, and continued efforts to define their targets is warranted to understand the mechanism and pathogenesis of autoantibodies and to develop strategies to improve long-term transplant outcomes.
Key points.
Humoral immunity to self-antigens expressed on the allograft is associated with rejection and reduced long-term renal allograft survival
Autoantibodies can mediate graft damage via complement-dependent and complement-independent pathways.
Compelling experimental data suggest that autoantibodies are generated through multiple mechanisms, including presentation of donor-derived extracellular vesicles and apoptotic bodies by host antigen presenting cells
IL-17 and type 17 T helper (TH17) cell pathways might have a central role in autoantibody production in the setting of allotransplantation through development of tertiary lymphoid tissue (TLT)
TLT promotes B-cell survival and immunoglobulin isotype class switching
More investigation is required to elucidate the pathogenesis of autoantibodies and to develop targeted therapeutic strategies to prevent acute and chronic antibody-mediated rejection
Acknowledgments
This work was supported by the following grants: NIH RO1AI42819 to EFR.
Biographies
Elaine F. Reed, Ph.D. is Professor of Pathology, David Geffen School of Medicine at UCLA and Director of the UCLA Immunogenetics Center. Her research interests over the last 29 years have focused on mechanisms of antibody-mediated acute and chronic allograft rejection. Dr. Reed has published extensively in the field of Immunogenetics and Transplant Immunology, and she has trained over 50 graduate students, post-doctoral research scientists, fellows and junior faculty.
Dr. Qiuheng Jennifer Zhang, Ph.D. is Associate Professor of Pathology, David Geffen School of Medicine at UCLA and Associate Director of the UCLA Immunogenetics Center. Dr. Zhang’s research interests are focused on understanding the role of both HLA and non-HLA antibodies in allograft rejection and developing new technologies to define/measure the patient’s immune responses and identify transplant recipients at risk of rejection to improve graft outcome.
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
Both authors discussed the content, researcher the data, wrote the article and reviewed or edited the manuscript before submission.
References
- 1.Stegall MD, et al. Terminal complement inhibition decreases antibody-mediated rejection in sensitized renal transplant recipients. Am J Transplant. 2011;11:2405–13. doi: 10.1111/j.1600-6143.2011.03757.x. [DOI] [PubMed] [Google Scholar]
- 2.Amico P, et al. Clinical relevance of pretransplant donor-specific HLA antibodies detected by single-antigen flow-beads. Transplantation. 2009;87:1681–8. doi: 10.1097/TP.0b013e3181a5e034. [DOI] [PubMed] [Google Scholar]
- 3.Gloor JM, et al. Baseline donor-specific antibody levels and outcomes in positive crossmatch kidney transplantation. Am J Transplant. 2010;10:582–9. doi: 10.1111/j.1600-6143.2009.02985.x. [DOI] [PubMed] [Google Scholar]
- 4.Everly MJ. Incidence and hazards of alloantibodies in renal transplantation. Clin Transpl. 2013:313–7. [PubMed] [Google Scholar]
- 5.Wiebe C, et al. Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. Am J Transplant. 2012;12:1157–67. doi: 10.1111/j.1600-6143.2012.04013.x. [DOI] [PubMed] [Google Scholar]
- 6.Lefaucheur C, et al. Preexisting donor-specific HLA antibodies predict outcome in kidney transplantation. J Am Soc Nephrol. 2010;21:1398–406. doi: 10.1681/ASN.2009101065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gourishankar S, et al. Pathological and clinical characterization of the ‘troubled transplant’: data from the DeKAF study. Am J Transplant. 2010;10:324–30. doi: 10.1111/j.1600-6143.2009.02954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grafft CA, et al. Antibody-mediated rejection following transplantation from an HLA-identical sibling. Nephrol Dial Transplant. 2010;25:307–10. doi: 10.1093/ndt/gfp526. [DOI] [PubMed] [Google Scholar]
- 9.Kalil J, et al. Humoral rejection in two HLA identical living related donor kidney transplants. Transplant Proc. 1989;21:711–3. [PubMed] [Google Scholar]
- 10.Montoliu J, et al. Delayed hyperacute rejection in recipients of kidney transplants from HLA identical sibling donors. Am J Med. 1979;67:590–6. doi: 10.1016/0002-9343(79)90239-0. [DOI] [PubMed] [Google Scholar]
- 11.Terasaki PI. Deduction of the fraction of immunologic and non-immunologic failure in cadaver donor transplants. Clin Transpl. 2003:449–52. [PubMed] [Google Scholar]
- 12.Opelz G Collaborative Transplant S. Non-HLA transplantation immunity revealed by lymphocytotoxic antibodies. Lancet. 2005;365:1570–6. doi: 10.1016/S0140-6736(05)66458-6. [DOI] [PubMed] [Google Scholar]
- 13.Bordron A, et al. Functional heterogeneity of anti-endothelial cell antibodies. Clin Exp Immunol. 2001;124:492–501. doi: 10.1046/j.1365-2249.2001.01528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zou Y, Stastny P, Susal C, Dohler B, Opelz G. Antibodies against MICA antigens and kidney-transplant rejection. N Engl J Med. 2007;357:1293–300. doi: 10.1056/NEJMoa067160. [DOI] [PubMed] [Google Scholar]
- 15.Brasile L, Rodman E, Shield CF, 3rd, Clarke J, Cerilli J. The association of antivascular endothelial cell antibody with hyperacute rejection: a case report. Surgery. 1986;99:637–40. [PubMed] [Google Scholar]
- 16.Harmer AW, Haskard D, Koffman CG, Welsh KI. Novel antibodies associated with unexplained loss of renal allografts. Transpl Int. 1990;3:66–9. doi: 10.1007/BF00336205. [DOI] [PubMed] [Google Scholar]
- 17.Jackson AM, Kuperman MB, Montgomery RA. Multiple hyperacute rejections in the absence of detectable complement activation in a patient with endothelial cell reactive antibody. Am J Transplant. 2012;12:1643–9. doi: 10.1111/j.1600-6143.2011.03955.x. [DOI] [PubMed] [Google Scholar]
- 18.Jordan SC, Yap HK, Sakai RS, Alfonso P, Fitchman M. Hyperacute allograft rejection mediated by anti-vascular endothelial cell antibodies with a negative monocyte crossmatch. Transplantation. 1988;46:585–7. doi: 10.1097/00007890-198810000-00024. [DOI] [PubMed] [Google Scholar]
- 19.Niikura T, et al. Probable C4d-negative accelerated acute antibody-mediated rejection due to non-HLA antibodies. Nephrology (Carlton) 2015;20(Suppl 2):75–8. doi: 10.1111/nep.12467. [DOI] [PubMed] [Google Scholar]
- 20.Sumitran-Karuppan S, Tyden G, Reinholt F, Berg U, Moller E. Hyperacute rejections of two consecutive renal allografts and early loss of the third transplant caused by non-HLA antibodies specific for endothelial cells. Transpl Immunol. 1997;5:321–7. doi: 10.1016/s0966-3274(97)80016-0. [DOI] [PubMed] [Google Scholar]
- 21.Perrey C, Brenchley PE, Johnson RW, Martin S. An association between antibodies specific for endothelial cells and renal transplant failure. Transpl Immunol. 1998;6:101–6. doi: 10.1016/s0966-3274(98)80024-5. [DOI] [PubMed] [Google Scholar]
- 22.Le Bas-Bernardet S, et al. Non-HLA-type endothelial cell reactive alloantibodies in pre-transplant sera of kidney recipients trigger apoptosis. Am J Transplant. 2003;3:167–77. doi: 10.1034/j.1600-6143.2003.00021.x. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Q, et al. HLA and MICA: targets of antibody-mediated rejection in heart transplantation. Transplantation. 2011;91:1153–8. doi: 10.1097/TP.0b013e3182157d60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Breimer ME, et al. Multicenter evaluation of a novel endothelial cell crossmatch test in kidney transplantation. Transplantation. 2009;87:549–56. doi: 10.1097/TP.0b013e3181949d4e. [DOI] [PubMed] [Google Scholar]
- 25.Daniel V, et al. Clinical Relevance of Preformed IgG and IgM Antibodies Against Donor Endothelial Progenitor Cells in Recipients of Living Donor Kidney Grafts. Clin Transplant. 2015 doi: 10.1111/ctr.12665. [DOI] [PubMed] [Google Scholar]
- 26.Sun Q, et al. Detectable circulating antiendothelial cell antibodies in renal allograft recipients with C4d-positive acute rejection: a report of three cases. Transplantation. 2005;79:1759–62. doi: 10.1097/01.tp.0000163290.19788.e7. [DOI] [PubMed] [Google Scholar]
- 27.Mahesh B, et al. Autoantibodies to vimentin cause accelerated rejection of cardiac allografts. Am J Pathol. 2007;170:1415–27. doi: 10.2353/ajpath.2007.060728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahesh B, et al. Autoimmunity to vimentin potentiates graft vasculopathy in murine cardiac allografts. Transplantation. 2010;90:4–13. doi: 10.1097/TP.0b013e3181dfa694. [DOI] [PubMed] [Google Scholar]
- 29.Fuss A, et al. C4d-negative antibody-mediated rejection with high anti-angiotensin II type I receptor antibodies in absence of donor-specific antibodies. Nephrology (Carlton) 2015;20:467–73. doi: 10.1111/nep.12441. [DOI] [PubMed] [Google Scholar]
- 30.Reinsmoen NL, et al. Anti-angiotensin type 1 receptor antibodies associated with antibody mediated rejection in donor HLA antibody negative patients. Transplantation. 2010;90:1473–7. doi: 10.1097/TP.0b013e3181fd97f1. [DOI] [PubMed] [Google Scholar]
- 31.Jackson AM, Lucas DP, Melancon JK, Desai NM. Clinical relevance and IgG subclass determination of non-HLA antibodies identified using endothelial cell precursors isolated from donor blood. Transplantation. 2011;92:54–60. doi: 10.1097/TP.0b013e31821b60e9. [DOI] [PubMed] [Google Scholar]
- 32.Pearl MH, et al. Accelerated rejection, thrombosis, and graft failure with angiotensin II type 1 receptor antibodies. Pediatr Nephrol. 2015;30:1371–4. doi: 10.1007/s00467-015-3123-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dragun D, et al. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N Engl J Med. 2005;352:558–69. doi: 10.1056/NEJMoa035717. [DOI] [PubMed] [Google Scholar]
- 34.Li L, et al. Identifying compartment-specific non-HLA targets after renal transplantation by integrating transcriptome and “antibodyome” measures. Proc Natl Acad Sci U S A. 2009;106:4148–53. doi: 10.1073/pnas.0900563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sigdel TK, et al. Non-HLA antibodies to immunogenic epitopes predict the evolution of chronic renal allograft injury. J Am Soc Nephrol. 2012;23:750–63. doi: 10.1681/ASN.2011060596. [DOI] [PubMed] [Google Scholar]
- 36.Jackson AM, et al. Endothelial cell antibodies associated with novel targets and increased rejection. J Am Soc Nephrol. 2015;26:1161–71. doi: 10.1681/ASN.2013121277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seva Pessoa B, et al. Key developments in renin-angiotensin-aldosterone system inhibition. Nat Rev Nephrol. 2013;9:26–36. doi: 10.1038/nrneph.2012.249. [DOI] [PubMed] [Google Scholar]
- 38.Xia Y, Kellems RE. Angiotensin receptor agonistic autoantibodies and hypertension: preeclampsia and beyond. Circ Res. 2013;113:78–87. doi: 10.1161/CIRCRESAHA.113.300752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wallukat G, et al. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest. 1999;103:945–52. doi: 10.1172/JCI4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giral M, et al. Pretransplant sensitization against angiotensin II type 1 receptor is a risk factor for acute rejection and graft loss. Am J Transplant. 2013;13:2567–76. doi: 10.1111/ajt.12397. [DOI] [PubMed] [Google Scholar]
- 41.In JW, et al. Anti-angiotensin type 1 receptor antibodies associated with antibody-mediated rejection in patients without preformed HLA-donor-specific antibody. Transplant Proc. 2014;46:3371–4. doi: 10.1016/j.transproceed.2014.09.096. [DOI] [PubMed] [Google Scholar]
- 42.Lee J, et al. The clinicopathological relevance of pretransplant anti-angiotensin II type 1 receptor antibodies in renal transplantation. Nephrol Dial Transplant. 2015 doi: 10.1093/ndt/gfv375. [DOI] [PubMed] [Google Scholar]
- 43.Lee J, et al. Clinical implications of angiotensin II type 1 receptor antibodies in antibody-mediated rejection without detectable donor-specific HLA antibodies after renal transplantation. Transplant Proc. 2015;47:649–52. doi: 10.1016/j.transproceed.2014.11.055. [DOI] [PubMed] [Google Scholar]
- 44.Taniguchi M, et al. Higher risk of kidney graft failure in the presence of anti-angiotensin II type-1 receptor antibodies. Am J Transplant. 2013;13:2577–89. doi: 10.1111/ajt.12395. [DOI] [PubMed] [Google Scholar]
- 45.Hesemann LE, Subramanian V, Mohanakumar T, Dharnidharka VR. De novo development of antibodies to kidney-associated self-antigens angiotensin II receptor type I, collagen IV, and fibronectin occurs at early time points after kidney transplantation in children. Pediatr Transplant. 2015;19:499–503. doi: 10.1111/petr.12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Banasik M, et al. Non-HLA antibodies: angiotensin II type 1 receptor (anti-AT1R) and endothelin-1 type A receptor (anti-ETAR) are associated with renal allograft injury and graft loss. Transplant Proc. 2014;46:2618–21. doi: 10.1016/j.transproceed.2014.09.029. [DOI] [PubMed] [Google Scholar]
- 47.Cravedi P, Kopp JB, Remuzzi G. Recent progress in the pathophysiology and treatment of FSGS recurrence. Am J Transplant. 2013;13:266–74. doi: 10.1111/ajt.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abbott KC, et al. Graft loss due to recurrent focal segmental glomerulosclerosis in renal transplant recipients in the United States. Am J Kidney Dis. 2001;37:366–73. doi: 10.1053/ajkd.2001.21311. [DOI] [PubMed] [Google Scholar]
- 49.Vincenti F, Ghiggeri GM. New insights into the pathogenesis and the therapy of recurrent focal glomerulosclerosis. Am J Transplant. 2005;5:1179–85. doi: 10.1111/j.1600-6143.2005.00968.x. [DOI] [PubMed] [Google Scholar]
- 50.Choy BY, Chan TM, Lai KN. Recurrent glomerulonephritis after kidney transplantation. Am J Transplant. 2006;6:2535–42. doi: 10.1111/j.1600-6143.2006.01502.x. [DOI] [PubMed] [Google Scholar]
- 51.Hoffmann S, Podlich D, Hahnel B, Kriz W, Gretz N. Angiotensin II type 1 receptor overexpression in podocytes induces glomerulosclerosis in transgenic rats. J Am Soc Nephrol. 2004;15:1475–87. doi: 10.1097/01.asn.0000127988.42710.a7. [DOI] [PubMed] [Google Scholar]
- 52.Alachkar N, Gupta G, Montgomery RA. Angiotensin antibodies and focal segmental glomerulosclerosis. N Engl J Med. 2013;368:971–3. doi: 10.1056/NEJMc1207233. [DOI] [PubMed] [Google Scholar]
- 53.Mujtaba MA, et al. Pre-transplant angiotensin receptor II type 1 antibodies and risk of post-transplant focal segmental glomerulosclerosis recurrence. Clin Transplant. 2015;29:606–11. doi: 10.1111/ctr.12562. [DOI] [PubMed] [Google Scholar]
- 54.Delville M, et al. A circulating antibody panel for pretransplant prediction of FSGS recurrence after kidney transplantation. Sci Transl Med. 2014;6:256ra136. doi: 10.1126/scitranslmed.3008538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hiemann NE, et al. Non-HLA antibodies targeting vascular receptors enhance alloimmune response and microvasculopathy after heart transplantation. Transplantation. 2012;94:919–24. doi: 10.1097/TP.0b013e3182692ad2. [DOI] [PubMed] [Google Scholar]
- 56.Reinsmoen NL, et al. Increased negative impact of donor HLA-specific together with non-HLA-specific antibodies on graft outcome. Transplantation. 2014;97:595–601. doi: 10.1097/01.TP.0000436927.08026.a8. [DOI] [PubMed] [Google Scholar]
- 57.Urban M, et al. The impact of angiotensin II type 1 receptor antibodies on post-heart transplantation outcome in Heart Mate II bridged recipients. Interact Cardiovasc Thorac Surg. 2015 doi: 10.1093/icvts/ivv344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fillion D, et al. Structure of the human angiotensin II type 1 (AT1) receptor bound to angiotensin II from multiple chemoselective photoprobe contacts reveals a unique peptide binding mode. J Biol Chem. 2013;288:8187–97. doi: 10.1074/jbc.M112.442053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Unal H, Jagannathan R, Bhat MB, Karnik SS. Ligand-specific conformation of extracellular loop-2 in the angiotensin II type 1 receptor. J Biol Chem. 2010;285:16341–50. doi: 10.1074/jbc.M109.094870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Unal H, et al. Long range effect of mutations on specific conformational changes in the extracellular loop 2 of angiotensin II type 1 receptor. J Biol Chem. 2013;288:540–51. doi: 10.1074/jbc.M112.392514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang W, et al. Stimulatory activity of anti-peptide antibodies against the second extracellular loop of human M2 muscarinic receptors. Chin Med J (Engl) 2000;113:867–71. [PubMed] [Google Scholar]
- 62.Lebesgue D, et al. An agonist-like monoclonal antibody against the human beta2-adrenoceptor. Eur J Pharmacol. 1998;348:123–33. doi: 10.1016/s0014-2999(98)00136-8. [DOI] [PubMed] [Google Scholar]
- 63.Unal H, Jagannathan R, Karnik SS. Mechanism of GPCR-directed autoantibodies in diseases. Adv Exp Med Biol. 2012;749:187–99. doi: 10.1007/978-1-4614-3381-1_13. [DOI] [PubMed] [Google Scholar]
- 64.Zhang S, et al. Angiotensin type 1 receptor autoantibody from preeclamptic patients induces human fetoplacental vasoconstriction. J Cell Physiol. 2013;228:142–8. doi: 10.1002/jcp.24113. [DOI] [PubMed] [Google Scholar]
- 65.Lukitsch I, et al. Renal ischemia and transplantation predispose to vascular constriction mediated by angiotensin II type 1 receptor-activating antibodies. Transplantation. 2012;94:8–13. doi: 10.1097/TP.0b013e3182529bb7. [DOI] [PubMed] [Google Scholar]
- 66.Ehrenstein MR, Notley CA. The importance of natural IgM: scavenger, protector and regulator. Nat Rev Immunol. 2010;10:778–86. doi: 10.1038/nri2849. [DOI] [PubMed] [Google Scholar]
- 67.Zhang M, et al. The role of natural IgM in myocardial ischemia-reperfusion injury. J Mol Cell Cardiol. 2006;41:62–7. doi: 10.1016/j.yjmcc.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 68.Iozzo RV, Sanderson RD. Proteoglycans in cancer biology, tumour microenvironment and angiogenesis. J Cell Mol Med. 2011;15:1013–31. doi: 10.1111/j.1582-4934.2010.01236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cailhier JF, et al. Caspase-3 activation triggers extracellular cathepsin L release and endorepellin proteolysis. J Biol Chem. 2008;283:27220–9. doi: 10.1074/jbc.M801164200. [DOI] [PubMed] [Google Scholar]
- 70.Cardinal H, et al. Antiperlecan antibodies are novel accelerators of immune-mediated vascular injury. Am J Transplant. 2013;13:861–74. doi: 10.1111/ajt.12168. [DOI] [PubMed] [Google Scholar]
- 71.O’Riordan E, Addabbo F, Goligorsky MS. Urine proteomics--prospects for future diagnostics. Acta Physiol Hung. 2007;94:133–41. doi: 10.1556/APhysiol.94.2007.1-2.12. [DOI] [PubMed] [Google Scholar]
- 72.Soulez M, et al. The perlecan fragment LG3 is a novel regulator of obliterative remodeling associated with allograft vascular rejection. Circ Res. 2012;110:94–104. doi: 10.1161/CIRCRESAHA.111.250431. [DOI] [PubMed] [Google Scholar]
- 73.Pilon EA, et al. The Perlecan Fragment LG3 Regulates Homing of Mesenchymal Stem Cells and Neointima Formation During Vascular Rejection. Am J Transplant. 2015;15:1205–18. doi: 10.1111/ajt.13119. [DOI] [PubMed] [Google Scholar]
- 74.Iwata T, et al. Anti-type V collagen humoral immunity in lung transplant primary graft dysfunction. J Immunol. 2008;181:5738–47. doi: 10.4049/jimmunol.181.8.5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yoshida S, et al. Anti-type V collagen lymphocytes that express IL-17 and IL-23 induce rejection pathology in fresh and well-healed lung transplants. Am J Transplant. 2006;6:724–35. doi: 10.1111/j.1600-6143.2006.01236.x. [DOI] [PubMed] [Google Scholar]
- 76.Tiriveedhi V, et al. A shift in the collagen V antigenic epitope leads to T helper phenotype switch and immune response to self-antigen leading to chronic lung allograft rejection. Clin Exp Immunol. 2012;167:158–68. doi: 10.1111/j.1365-2249.2011.04486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Burlingham WJ, et al. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest. 2007;117:3498–506. doi: 10.1172/JCI28031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hachem RR, et al. Antibodies to K-alpha 1 tubulin and collagen V are associated with chronic rejection after lung transplantation. Am J Transplant. 2012;12:2164–71. doi: 10.1111/j.1600-6143.2012.04079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tiriveedhi V, et al. Pre-transplant antibodies to Kalpha1 tubulin and collagen-V in lung transplantation: clinical correlations. J Heart Lung Transplant. 2013;32:807–14. doi: 10.1016/j.healun.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Angaswamy N, et al. Immune responses to collagen-IV and fibronectin in renal transplant recipients with transplant glomerulopathy. Am J Transplant. 2014;14:685–93. doi: 10.1111/ajt.12592. [DOI] [PubMed] [Google Scholar]
- 81.Nath DS, et al. A role for antibodies to human leukocyte antigens, collagen-V, and K-alpha1-Tubulin in antibody-mediated rejection and cardiac allograft vasculopathy. Transplantation. 2011;91:1036–43. doi: 10.1097/TP.0b013e318211d2f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Braun RK, et al. Transfer of tolerance to collagen type V suppresses T-helper-cell-17 lymphocyte-mediated acute lung transplant rejection. Transplantation. 2009;88:1341–8. doi: 10.1097/TP.0b013e3181bcde7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fan L, et al. Neutralizing IL-17 prevents obliterative bronchiolitis in murine orthotopic lung transplantation. Am J Transplant. 2011;11:911–22. doi: 10.1111/j.1600-6143.2011.03482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yin XT, Zobell S, Jarosz JG, Stuart PM. Anti-IL-17 therapy restricts and reverses late-term corneal allorejection. J Immunol. 2015;194:4029–38. doi: 10.4049/jimmunol.1401922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Basha HI, et al. Critical role for IL-17A/F in the immunopathogenesis of obliterative airway disease induced by Anti-MHC I antibodies. Transplantation. 2013;95:293–300. doi: 10.1097/TP.0b013e3182772244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rosen A, Casciola-Rosen L. Autoantigens as substrates for apoptotic proteases: implications for the pathogenesis of systemic autoimmune disease. Cell Death Differ. 1999;6:6–12. doi: 10.1038/sj.cdd.4400460. [DOI] [PubMed] [Google Scholar]
- 87.Bluestone JA, Bour-Jordan H, Cheng M, Anderson M. T cells in the control of organ-specific autoimmunity. J Clin Invest. 2015;125:2250–60. doi: 10.1172/JCI78089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jang HR, Rabb H. Immune cells in experimental acute kidney injury. Nat Rev Nephrol. 2015;11:88–101. doi: 10.1038/nrneph.2014.180. [DOI] [PubMed] [Google Scholar]
- 89.Zhai Y, Petrowsky H, Hong JC, Busuttil RW, Kupiec-Weglinski JW. Ischaemia-reperfusion injury in liver transplantation--from bench to bedside. Nat Rev Gastroenterol Hepatol. 2013;10:79–89. doi: 10.1038/nrgastro.2012.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Methe H, Zimmer E, Grimm C, Nabauer M, Koglin J. Evidence for a role of toll-like receptor 4 in development of chronic allograft rejection after cardiac transplantation. Transplantation. 2004;78:1324–31. doi: 10.1097/01.tp.0000137930.40597.03. [DOI] [PubMed] [Google Scholar]
- 91.Stribos EG, et al. Renal expression of Toll-like receptor 2 and 4: dynamics in human allograft injury and comparison to rodents. Mol Immunol. 2015;64:82–9. doi: 10.1016/j.molimm.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 92.Chen J, et al. Toll-like receptor 4 regulates early endothelial activation during ischemic acute kidney injury. Kidney Int. 2011;79:288–99. doi: 10.1038/ki.2010.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kruger B, et al. Donor Toll-like receptor 4 contributes to ischemia and reperfusion injury following human kidney transplantation. Proc Natl Acad Sci U S A. 2009;106:3390–5. doi: 10.1073/pnas.0810169106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tanaka M, et al. Progression of alloresponse and tissue-specific immunity during graft coronary artery disease. Am J Transplant. 2005;5:1286–96. doi: 10.1111/j.1600-6143.2005.00880.x. [DOI] [PubMed] [Google Scholar]
- 95.Rose ML. Role of anti-vimentin antibodies in allograft rejection. Hum Immunol. 2013;74:1459–62. doi: 10.1016/j.humimm.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Suzuki H, et al. Role of complement activation in obliterative bronchiolitis post-lung transplantation. J Immunol. 2013;191:4431–9. doi: 10.4049/jimmunol.1202242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Carroll MC. The complement system in regulation of adaptive immunity. Nat Immunol. 2004;5:981–6. doi: 10.1038/ni1113. [DOI] [PubMed] [Google Scholar]
- 98.Dempsey PW, Allison ME, Akkaraju S, Goodnow CC, Fearon DT. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science. 1996;271:348–50. doi: 10.1126/science.271.5247.348. [DOI] [PubMed] [Google Scholar]
- 99.Marsh JE, et al. The allogeneic T and B cell response is strongly dependent on complement components C3 and C4. Transplantation. 2001;72:1310–8. doi: 10.1097/00007890-200110150-00022. [DOI] [PubMed] [Google Scholar]
- 100.Sheen JH, Heeger PS. Effects of complement activation on allograft injury. Curr Opin Organ Transplant. 2015;20:468–75. doi: 10.1097/MOT.0000000000000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sayegh MH, Turka LA. The role of T-cell costimulatory activation pathways in transplant rejection. N Engl J Med. 1998;338:1813–21. doi: 10.1056/NEJM199806183382506. [DOI] [PubMed] [Google Scholar]
- 102.Valujskikh A, Lantz O, Celli S, Matzinger P, Heeger PS. Cross-primed CD8(+) T cells mediate graft rejection via a distinct effector pathway. Nat Immunol. 2002;3:844–51. doi: 10.1038/ni831. [DOI] [PubMed] [Google Scholar]
- 103.Reed EF, Tugulea SL, Suciu-Foca N. Influence of HLA class I and class II antigens on the peripheral T-cell receptor repertoire. Hum Immunol. 1994;40:111–22. doi: 10.1016/0198-8859(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 104.Fangmann J, Dalchau R, Fabre JW. Rejection of skin allografts by indirect allorecognition of donor class I major histocompatibility complex peptides. J Exp Med. 1992;175:1521–9. doi: 10.1084/jem.175.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hanvesakul R, et al. Indirect recognition of T-cell epitopes derived from the alpha 3 and transmembrane domain of HLA-A2. Am J Transplant. 2007;7:1148–57. doi: 10.1111/j.1600-6143.2007.01743.x. [DOI] [PubMed] [Google Scholar]
- 106.Vella JP, et al. Cellular and humoral mechanisms of vascularized allograft rejection induced by indirect recognition of donor MHC allopeptides. Transplantation. 1999;67:1523–32. doi: 10.1097/00007890-199906270-00005. [DOI] [PubMed] [Google Scholar]
- 107.Valujskikh A, Fedoseyeva E, Benichou G, Heeger PS. Development of autoimmunity after skin graft rejection via an indirect alloresponse. Transplantation. 2002;73:1130–7. doi: 10.1097/00007890-200204150-00021. [DOI] [PubMed] [Google Scholar]
- 108.Macdonald WA, et al. T cell allorecognition via molecular mimicry. Immunity. 2009;31:897–908. doi: 10.1016/j.immuni.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 109.Fukami N, et al. Antibodies to MHC class I induce autoimmunity: role in the pathogenesis of chronic rejection. J Immunol. 2009;182:309–18. doi: 10.4049/jimmunol.182.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Saini D, et al. Alloimmunity-induced autoimmunity as a potential mechanism in the pathogenesis of chronic rejection of human lung allografts. J Heart Lung Transplant. 2011;30:624–31. doi: 10.1016/j.healun.2011.01.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Subramanian V, et al. Immune response to tissue-restricted self-antigens induces airway inflammation and fibrosis following murine lung transplantation. Am J Transplant. 2014;14:2359–66. doi: 10.1111/ajt.12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Keller MR, et al. Epitope analysis of the collagen type V-specific T cell response in lung transplantation reveals an HLA-DRB1*15 bias in both recipient and donor. PLoS One. 2013;8:e79601. doi: 10.1371/journal.pone.0079601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lehmann PV, Forsthuber T, Miller A, Sercarz EE. Spreading of T-cell autoimmunity to cryptic determinants of an autoantigen. Nature. 1992;358:155–7. doi: 10.1038/358155a0. [DOI] [PubMed] [Google Scholar]
- 114.Liu Z, et al. Indirect recognition of donor HLA-DR peptides in organ allograft rejection. J Clin Invest. 1996;98:1150–7. doi: 10.1172/JCI118898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pallet N, Dieude M, Cailhier J, Hebert M. The molecular legacy of apoptosis in transplantation. Am J Transplant. 2012;12:1378–84. doi: 10.1111/j.1600-6143.2012.04015.x. [DOI] [PubMed] [Google Scholar]
- 116.Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14:195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Montecalvo A, et al. Exosomes as a short-range mechanism to spread alloantigen between dendritic cells during T cell allorecognition. J Immunol. 2008;180:3081–90. doi: 10.4049/jimmunol.180.5.3081. [DOI] [PubMed] [Google Scholar]
- 118.Peche H, Heslan M, Usal C, Amigorena S, Cuturi MC. Presentation of donor major histocompatibility complex antigens by bone marrow dendritic cell-derived exosomes modulates allograft rejection. Transplantation. 2003;76:1503–10. doi: 10.1097/01.TP.0000092494.75313.38. [DOI] [PubMed] [Google Scholar]
- 119.Segura E, et al. ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming. Blood. 2005;106:216–23. doi: 10.1182/blood-2005-01-0220. [DOI] [PubMed] [Google Scholar]
- 120.Thery C, et al. Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol. 2002;3:1156–62. doi: 10.1038/ni854. [DOI] [PubMed] [Google Scholar]
- 121.Muntasell A, Berger AC, Roche PA. T cell-induced secretion of MHC class II-peptide complexes on B cell exosomes. EMBO J. 2007;26:4263–72. doi: 10.1038/sj.emboj.7601842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nolte-’t Hoen EN, Buschow SI, Anderton SM, Stoorvogel W, Wauben MH. Activated T cells recruit exosomes secreted by dendritic cells via LFA-1. Blood. 2009;113:1977–81. doi: 10.1182/blood-2008-08-174094. [DOI] [PubMed] [Google Scholar]
- 123.Dieude M, et al. The 20S proteasome core, active within apoptotic exosome-like vesicles, induces autoantibody production and accelerates rejection. Sci Transl Med. 2015;7:318ra200. doi: 10.1126/scitranslmed.aac9816. [DOI] [PubMed] [Google Scholar]
- 124.Mitsdoerffer M, et al. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc Natl Acad Sci U S A. 2010;107:14292–7. doi: 10.1073/pnas.1009234107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yao Z, et al. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity. 1995;3:811–21. doi: 10.1016/1074-7613(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 126.Rao DA, et al. Interleukin (IL)-1 promotes allogeneic T cell intimal infiltration and IL-17 production in a model of human artery rejection. J Exp Med. 2008;205:3145–58. doi: 10.1084/jem.20081661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gorbacheva V, Fan R, Li X, Valujskikh A. Interleukin-17 promotes early allograft inflammation. Am J Pathol. 2010;177:1265–73. doi: 10.2353/ajpath.2010.091106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cheng J, et al. Ectopic B-cell clusters that infiltrate transplanted human kidneys are clonal. Proc Natl Acad Sci U S A. 2011;108:5560–5. doi: 10.1073/pnas.1101148108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kerjaschki D, et al. Lymphatic neoangiogenesis in human kidney transplants is associated with immunologically active lymphocytic infiltrates. J Am Soc Nephrol. 2004;15:603–12. doi: 10.1097/01.asn.0000113316.52371.2e. [DOI] [PubMed] [Google Scholar]
- 130.Thaunat O, et al. Chronic rejection triggers the development of an aggressive intragraft immune response through recapitulation of lymphoid organogenesis. J Immunol. 2010;185:717–28. doi: 10.4049/jimmunol.0903589. [DOI] [PubMed] [Google Scholar]
- 131.Thaunat O, et al. Lymphoid neogenesis in chronic rejection: evidence for a local humoral alloimmune response. Proc Natl Acad Sci U S A. 2005;102:14723–8. doi: 10.1073/pnas.0507223102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sato M, et al. The role of intrapulmonary de novo lymphoid tissue in obliterative bronchiolitis after lung transplantation. J Immunol. 2009;182:7307–16. doi: 10.4049/jimmunol.0803606. [DOI] [PubMed] [Google Scholar]
- 133.Huibers MM, et al. The composition of ectopic lymphoid structures suggests involvement of a local immune response in cardiac allograft vasculopathy. J Heart Lung Transplant. 2015;34:734–45. doi: 10.1016/j.healun.2014.11.022. [DOI] [PubMed] [Google Scholar]
- 134.Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol. 2006;6:205–17. doi: 10.1038/nri1786. [DOI] [PubMed] [Google Scholar]
- 135.Hsu HC, et al. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat Immunol. 2008;9:166–75. doi: 10.1038/ni1552. [DOI] [PubMed] [Google Scholar]
- 136.Rangel-Moreno J, et al. The development of inducible bronchus-associated lymphoid tissue depends on IL-17. Nat Immunol. 2011;12:639–46. doi: 10.1038/ni.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Patakas A, et al. Th17 effector cells support B cell responses outside of germinal centres. PLoS One. 2012;7:e49715. doi: 10.1371/journal.pone.0049715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Szodoray P, Jellestad S, Teague MO, Jonsson R. Attenuated apoptosis of B cell activating factor-expressing cells in primary Sjogren’s syndrome. Lab Invest. 2003;83:357–65. [PubMed] [Google Scholar]
- 139.Thaunat O, et al. A stepwise breakdown of B-cell tolerance occurs within renal allografts during chronic rejection. Kidney Int. 2012;81:207–19. doi: 10.1038/ki.2011.317. [DOI] [PubMed] [Google Scholar]
- 140.Iyer HS, Jackson AM, Zachary AA, Montgomery RA. Transplanting the highly sensitized patient: trials and tribulations. Curr Opin Nephrol Hypertens. 2013;22:681–8. doi: 10.1097/MNH.0b013e328365b3b9. [DOI] [PubMed] [Google Scholar]
- 141.Lefaucheur C, et al. Comparison of combination Plasmapheresis/IVIg/anti-CD20 versus high-dose IVIg in the treatment of antibody-mediated rejection. Am J Transplant. 2009;9:1099–107. doi: 10.1111/j.1600-6143.2009.02591.x. [DOI] [PubMed] [Google Scholar]
- 142.Jordan SC, Toyoda M, Kahwaji J, Vo AA. Clinical aspects of intravenous immunoglobulin use in solid organ transplant recipients. Am J Transplant. 2011;11:196–202. doi: 10.1111/j.1600-6143.2010.03400.x. [DOI] [PubMed] [Google Scholar]
- 143.Morrow WR, et al. Rapid reduction in donor-specific anti-human leukocyte antigen antibodies and reversal of antibody-mediated rejection with bortezomib in pediatric heart transplant patients. Transplantation. 2012;93:319–24. doi: 10.1097/TP.0b013e31823f7eea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Woodle ES, et al. Prospective iterative trial of proteasome inhibitor-based desensitization. Am J Transplant. 2015;15:101–18. doi: 10.1111/ajt.13050. [DOI] [PubMed] [Google Scholar]
- 145.Fan J, et al. Eculizumab Salvage Therapy for Antibody-Mediated Rejection in a Desensitization-Resistant Intestinal Re-Transplant Patient. Am J Transplant. 2015;15:1995–2000. doi: 10.1111/ajt.13183. [DOI] [PubMed] [Google Scholar]
- 146.Cornell LD, Schinstock CA, Gandhi MJ, Kremers WK, Stegall MD. Positive crossmatch kidney transplant recipients treated with eculizumab: outcomes beyond 1 year. Am J Transplant. 2015;15:1293–302. doi: 10.1111/ajt.13168. [DOI] [PubMed] [Google Scholar]
- 147.Vo AA, et al. A Phase I/II Trial of the Interleukin-6 Receptor Specific Humanized Monoclonal (Tocilizumab) + Intravenous Immunoglobulin in Difficult to Desensitize Patients. Transplantation. 2015 doi: 10.1097/TP.0000000000000741. [DOI] [PubMed] [Google Scholar]
- 148.Sarma NJ, et al. Modulation of immune responses following solid organ transplantation by microRNA. Exp Mol Pathol. 2012;93:378–85. doi: 10.1016/j.yexmp.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Xu Z, et al. De novo-developed antibodies to donor MHC antigens lead to dysregulation of microRNAs and induction of MHC class II. J Immunol. 2015;194:6133–43. doi: 10.4049/jimmunol.1401848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Xu Z, et al. Dysregulated MicroRNA Expression and Chronic Lung Allograft Rejection in Recipients With Antibodies to Donor HLA. Am J Transplant. 2015;15:1933–47. doi: 10.1111/ajt.13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sutherland SM, et al. Protein microarrays identify antibodies to protein kinase Czeta that are associated with a greater risk of allograft loss in pediatric renal transplant recipients. Kidney Int. 2009;76:1277–83. doi: 10.1038/ki.2009.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hagedorn PH, et al. Integrative analysis correlates donor transcripts to recipient autoantibodies in primary graft dysfunction after lung transplantation. Immunology. 2011;132:394–400. doi: 10.1111/j.1365-2567.2010.03373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]