Abstract
The ability of normally soluble proteins to convert into amyloid fibrils is now recognized to be a generic phenomenon. The overall cross-β architecture of the core elements of such structures is closely similar for different amino acid sequences, as this architecture is dominated by interactions associated with the common polypeptide main chain. In contrast, the multiplicity of complex and intricate structures of the functional states of proteins is dictated by specific interactions involving the variable side chains, the sequence of which is unique to a given protein. Nevertheless, the side chains dictate important aspects of the amyloid structure, including the regions of the sequence that form the core elements of the fibrils and the kinetics and mechanism of the conversion process. The formation of the amyloid state of proteins is of particular importance in the context of a range of medical disorders that include Alzheimer’s and Parkinson’s diseases and type 2 diabetes. These disorders are becoming increasingly common in the modern world, primarily as a consequence of increasing life spans and changing lifestyles, and now affect some 500 million people worldwide. This review describes recent progress in our understanding of the molecular origins of these conditions and discusses emerging ideas for new and rational therapeutic strategies by which to combat their onset and progression.
The ability of soluble proteins to convert to amyloid fibrils is now recognized to be a generic phenomenon. About 50 human disorders, including type 2 diabetes and Alzheimer’s disease, are associated with such amyloid fibrils.
Until about a century ago, the major causes of death in the world were infectious diseases caused by bacteria, viruses, and parasites (Dobson 2015b). The spread of such diseases was dramatically enhanced by changes in human behavior associated with the advance of civilization. Population densities increased, which hastened disease transmission within specific locations, and interactions between peoples from different regions of the world increased, which quickened the global spread of infectious agents, often to populations with low immunity as a result of the lack of previous exposure to such agents (Haines and Steckel 2014). Diseases such as measles, smallpox, bubonic plague, and cholera wrought havoc both as endemic conditions and recurring pandemics; the “Black Death” in the 14th century, for example, killed between a third and a half of the population of Europe.
The changes in human behavior that resulted in such outbreaks of infectious disease were, however, accompanied by an increasing realization of their causes and by more effective means of their prevention and treatment. Improvements in sanitation and hygiene and the development of effective vaccines and antibiotics have been dramatic, such that in much of the world the chances of dying from one of the classic infectious diseases that plagued humanity until modern times have been hugely reduced (Dobson and Dobson 2017). The risk of pandemics has not been completely abolished, however, as resistant strains of many diseases that were thought to have been largely suppressed, such as tuberculosis, have emerged, and “new” types of disease have appeared, such as HIV/AIDS and Ebola (Peacock 2014; Dobson 2015a). Even in such cases, however, modern science has so far managed to limit the severity and spread of these more recent “plagues.”
The decline in the number of cases of infectious diseases has itself led to major changes in the human condition, not least as a result of greatly increased life spans in most parts of the modern world; life expectancy in the most highly developed countries is now ∼80 years compared with ∼50 years a century ago (Riley 2005). The result, however, has been a large increase in cases of noninfectious conditions—notably, cancer and cardiovascular disease—which are now the greatest causes of death in much of the world other than sub-Saharan Africa. In the last few decades, however, there has been a dramatic increase in another group of medical conditions, collectively called “protein misfolding disorders,” that include Alzheimer’s disease (AD) and type 2 diabetes (Knowles et al. 2014). AD is the most common form of dementia and has recently been called a “21st century plague,” while type 2 diabetes is now endemic across much of the world. These disorders (and many other related conditions) are directly attributable to changes in the human condition; in the case of AD, the key factor is age, while for type 2 diabetes, it is obesity. In this article, we will illustrate some of the fundamental principles underlying protein-misfolding disorders and their links with aggregation and amyloid formation.
THE INCREASING PREVALENCE OF PROTEIN-MISFOLDING DISORDERS
Alois Alzheimer first described the disease that bears his name in a lecture in 1906 after studying the case of a 51-year-old woman, Auguste Deter, who was admitted to the Frankfurt Asylum suffering from severe dementia (Möller and Graeber 1998). At the time, her case was a curiosity, and it is now evident that she was suffering from a rare early-onset form of AD. It was only some 40 years ago that AD began to be recognized as an increasingly common condition among the elderly, and since then the number of cases has increased dramatically within our aging population (Alzheimer’s Association 2015). The number of cases in the United States, for example, now exceeds five million, and it is estimated that there are some 40 million sufferers worldwide. This number is anticipated to rise to about 135 million by 2050, and >70% of patients will be in low- or middle-income nations (Alzheimer’s Association 2015). AD can, therefore, no longer be considered as a phenomenon confined to the wealthy nations of the world but is a global issue. The increase in the number of cases is attributable to the increasing number of people over the age of 65, where AD affects between 1% and 2% of the population, the incidence rising to between one-third and one-half of those living to the age of 85 (Alzheimer’s Association 2015).
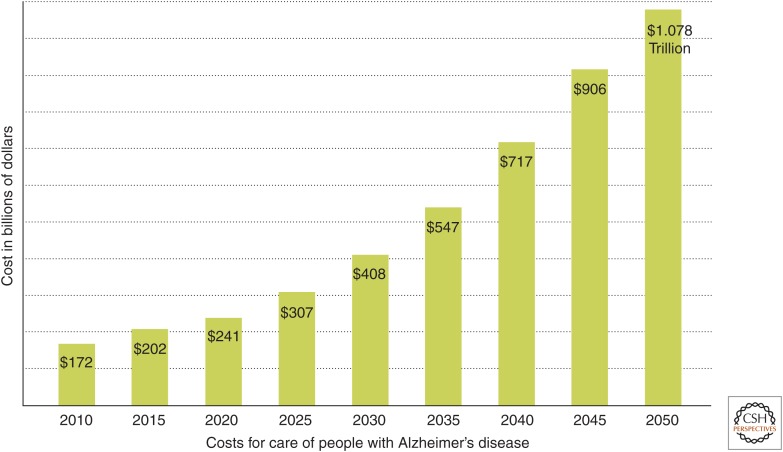
It is rapidly becoming evident that the extremely debilitating and uniquely distressing nature of AD has huge implications not only for individuals and their relatives but also for society as a whole; indeed, the flood of stories in the media is such that the fear of this condition is becoming comparable to that associated with the pandemics of infectious diseases in past centuries. Moreover, the financial burdens of this condition (because of the need for continuous care of patients, often for years) are reaching levels that no health care system can ignore. In the United States, for example, the costs of the disease are estimated to be ∼$200 billion in 2015 and are predicted to exceed $1 trillion by 2050 (Fig. 1); in the next 35 years, therefore, the cost of care for patients with AD is anticipated to be ∼$20 trillion in the United States alone (Alzheimer’s Association 2015). It is for these reasons that dementia in general, and AD in particular, has recently become recognized as an emerging international catastrophe, resulting in the first G8 Dementia Summit in London in 2013, where the need to increase spending on research into the nature of the disease and into new approaches to the development of therapeutic strategies for its prevention and treatment was clearly recognized (G8 Dementia Summit 2013). In addition, the number of sufferers of type 2 diabetes throughout the world is even today estimated to be a staggering 400 million, with a global price tag of $600 billion, and, again, is anticipated to increase rapidly as developing countries become more affluent. Indeed, the rise of type 2 diabetes has been suggested to be a major factor that could slow down, and ultimately bring to a halt, the present steady increase in predicted human life expectancy (Olshansky et al. 2005).
Figure 1.
Predicted costs of AD in the United States (see also Alzheimer’s Association 2015). (From Alzheimer’s Disease Facts and Figures 2015, Alzheimer’s Association 2015; reprinted, with permission, from Elsevier © 2015.)
AMYLOID FORMATION AND ITS LINKS WITH HUMAN DISEASE
It was evident from the earliest autopsies of patients who had died with AD that a characteristic feature of this condition is the presence of proteinaceous deposits within the brain. Indeed, these deposits were named “amyloid” because they were found to stain with dyes that also stain for starch (the Latin word for which is amylum). Moreover, it is now known that approximately 50 human diseases in addition to AD are associated with similar deposits (Chiti and Dobson 2006), including other neurodegenerative conditions such as Parkinson's disease (PD), Huntington’s disease (HD), motor neuron disease (MND), also known as amyotrophic lateral sclerosis (ALS), and the transmissible prion disorders such as Creutzfeldt–Jakob disease (CJD). This group of diseases also includes systemic amyloidoses, where proteinaceous deposits are widespread in organs such as the liver, kidney, and spleen; type 2 diabetes, where the deposits are found in the pancreas; and dialysis-related amyloidosis (DRA), where the deposits are formed in joints and skeletal tissue and are a consequence of long-term hemodialysis procedures used to treat patients with kidney failure. In each of these conditions, the deposits are predominantly composed of one specific protein, which differs from one disease to another, and are often made up of masses of highly intractable thread-like fibrils that are just a few nanometers in diameter but often microns in length (Chiti and Dobson 2006).
Our interest in this class of diseases began in the mid-1990s by chance, as we were focused at that time on the study of protein folding (Dobson 1992). One of the proteins that we were studying in the greatest detail was lysozyme, an antibacterial enzyme that we were using as a primary experimental system for exploring the key determinants of the process through which a globular protein reaches its functional state (Radford et al. 1992). It then emerged that several familial mutations had been identified in the lysozyme gene, and that some give rise to a fatal form of systemic amyloidosis, bringing this enzyme into the group of proteins associated with protein-deposition diseases (Pepys et al. 1993). Further research on this system resulted in the discovery that these mutations greatly increased the probability of the protein misfolding, and that the misfolding process resulted in the conversion of the protein into pathogenic amyloid fibrils (Booth et al. 1997). One of the fascinating questions that then arose concerned the nature of these fibrils and the determinants of their structures, X-ray fiber diffraction studies of which had shown to contain a similar “cross-β” motif consisting of β-strands oriented perpendicular to the fibril axis that then form β-sheets oriented parallel with this axis (Sunde and Blake 1997). Of particular significance was the fact that this apparently common motif for proteins in amyloid fibrils contrasts so dramatically with the well-established observation that the native states of proteins are extremely varied, usually forming intricate and complex folds that are determined by the sequence of the amino acid building blocks in the polypeptide chains of which they are composed (Dobson 1999).
THE GENERIC NATURE OF THE AMYLOID STATE
Important clues to the intrinsic nature of amyloid fibrils came from the unexpected, and again initially chance, observation that in the laboratory it is possible to convert many proteins not associated with disease from their normal functional states into amyloid fibrils (Fig. 2) (Guijarro et al. 1998; Chiti and Dobson 2006). Moreover, in a crucial series of experiments with polypeptide sequences in which all of the amino acid residues are the same (such as polylysine polythreonine), it became evident that, unlike their functional forms, the architecture of the amyloid state of a protein is not encoded in its sequence (Fändrich and Dobson 2002). This observation led to the realization that there are two alternative highly organized forms of proteins: the unique functional native states and the generic amyloid state (Fig. 3) (Guijarro et al. 1998; Dobson 1999, 2008; Fändrich and Dobson 2002). The varied and complex structures of native proteins, including those where all or some of the polypeptide chain is disordered, result from the dominance of specific interactions between the side chains in the unique sequences of those proteins selected by evolution for functional purposes. In contrast, the structure of the amyloid state is dominated by the interactions of the polypeptide main chain that is common to all proteins; the amyloid structures of different proteins therefore possess a common core structure, albeit with variations in the spacing of the β-sheets and other details, such as the way the various component protofilament structures assemble together to form the mature fibrils (Fändrich and Dobson 2002; Chiti and Dobson 2006).
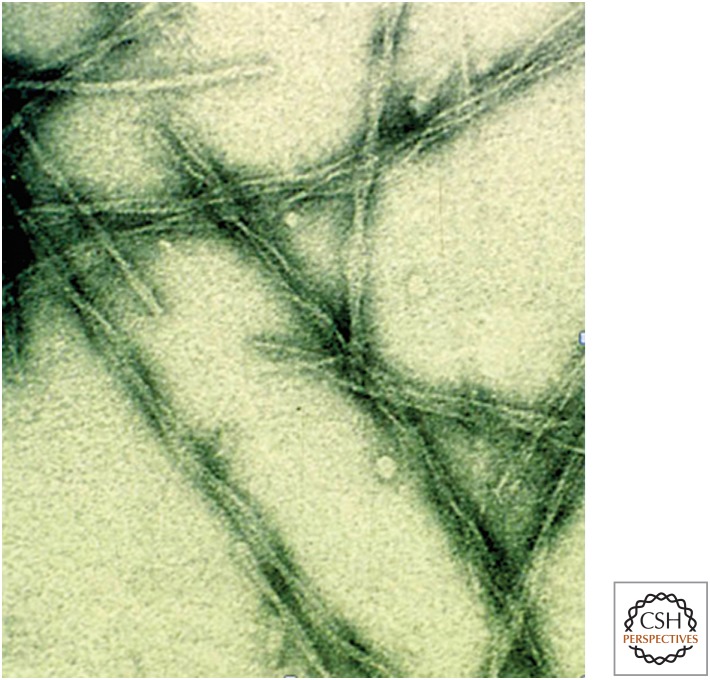
Figure 2.
Images of amyloid fibrils formed from the SH3 domain of PI3 kinase. The fibrils were formed in vitro by incubation of the protein (whose native structure is represented in Fig. 3) at low pH (Guijarro et al. 1998). Although the SH3 domain has no links with disease, the fibrils (which have diameters of just a few nanometers) have all the characteristics of the fibrillar aggregates isolated from the tissue of patients suffering from amyloid diseases such as Alzheimer’s and Parkinson’s (Chiti and Dobson 2006). This observation gave rise to the concept that the amyloid structure is a common or “generic” form of protein architecture, albeit one that is generally observed in nature only in pathological states (Dobson 1999).
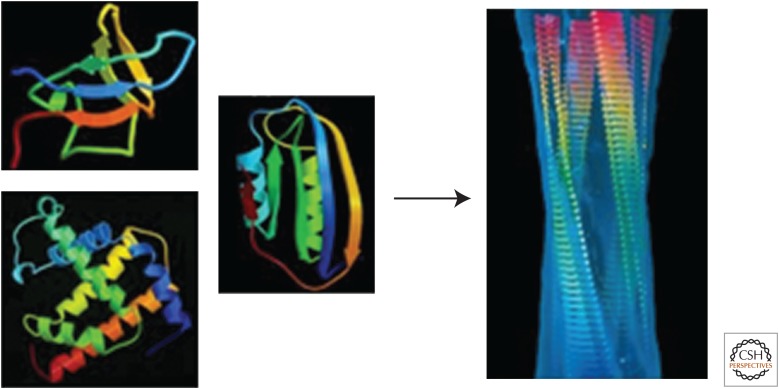
Figure 3.
Comparison of examples of native and amyloid structures of protein molecules. On the left are ribbon diagrams of the native structures of three small proteins: an SH3 domain (top), myoglobin (bottom), and acylphosphatase (middle). The native structures differ in their topologies and contents of α-helices and β-sheets resulting from the dominance of side-chain interactions within their highly evolved sequences. On the right is a molecular model of an amyloid fibril. (Image kindly provided by Helen Saibil, Birkbeck College, London, from data reported in Jiménez et al. 1999.) The fibril was produced from the SH3 domain whose native structure is shown on the left and consists of four “protofilaments” that twist around one another to form a hollow tube with a diameter of ∼6 nm. The β-strands (flat arrows) are oriented perpendicularly to the fibril axis and are linked together by hydrogen bonds involving main-chain amide and carbonyl groups, many of which are intermolecular, to form a continuous structure in each protofilament. The protofilaments are held together by much weaker interactions involving primarily side-chain contacts. As the main chain is common to all polypeptides, the core protofilament structures of fibrils from different sequences have common features, differing only in detail as a result of differences in the nondominant effects of side-chain packing. The arrow indicates that when the native states of globular proteins are destabilized, they tend to convert into the generic amyloid structure, as described in the text. (Reprinted from Dobson 2008.)
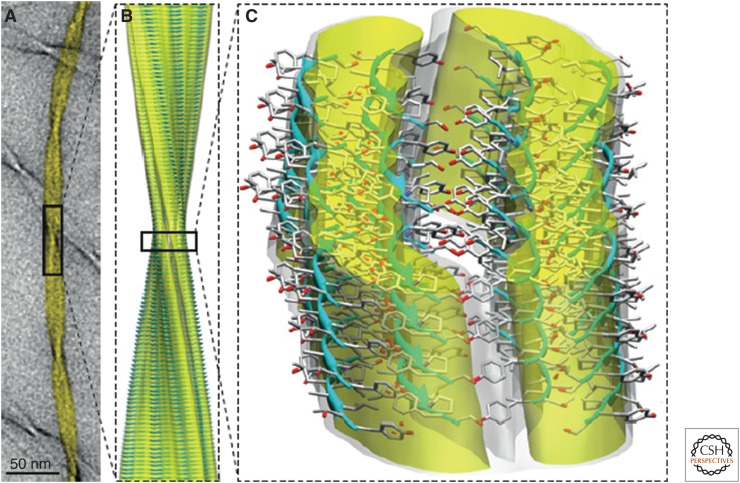
The atomic-level structure of an amyloid fibril, an 11-residue peptide from the protein transthyretin, has recently been determined by a combination of biophysical techniques, particularly solid-state nuclear magnetic resonance spectroscopy and cryo-electron microscopy (cryo-EM) (Fitzpatrick et al. 2013). The structure (Fig. 4) shows that the core of the protofilaments is made up of a pair of nearly flat β-sheets, a feature proposed earlier on the basis of earlier cryo-EM studies of an SH3 protein (Jiménez et al. 1999), and suggests the presence of water-filled cavities of the type observed in the X-ray structures of amyloid-like peptide microcrystals (Nelson et al. 2005). The fact that the cross-β structure is a characteristic arrangement of polypeptide chains is supported by the results of computer simulations (Auer et al. 2008), as well as by theoretical analysis, and by the results of investigations of the role of the arrays of hydrogen bonds that are features of this molecular architecture (Knowles et al. 2007). Although the specific protein sequence does not itself define this overall architecture, it does play a major role in defining the relative propensity of a given protein to convert from its normal functional state to the polymeric amyloid state, a feature that turns out to be crucial in enabling proteins in living systems to avoid rapid conversion into their amyloid states, which, in many cases, may be thermodynamically the most stable state of a protein molecule (Baldwin et al. 2011).
Figure 4.
Structure of an amyloid fibril at atomic resolution. The structure shown is one of several polymorphs of the amyloid fibrils that are formed from an 11-residue fragment of transthyretin. The combination of cryo-electron microscopy imaging (A) with solid-state NMR analysis has enabled the determination of an atomic-level structure (B). A more detailed view (C) shows the hierarchical organization of the amyloid fibril in which the three filaments that form the mature fibril illustrated here are in turn formed by pairs of cross-β protofilaments, which are each composed of pairs of β-sheets. The fibril surfaces are shown as electron density maps, and the constituent β-sheets are shown in a ribbon representation; oxygen, carbon, and nitrogen atoms are shown in red, gray, and blue, respectively. (From Knowles et al. 2014; reprinted, with permission, from The American Association for the Advancement of Science © 2009 and Fitzpatrick et al. 2013 with permission from National Academy of Sciences © 2013.)
THE PATHOGENIC CONSEQUENCES OF AMYLOID FORMATION
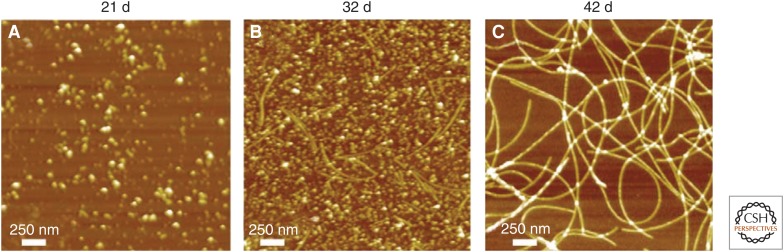
The conversion of soluble protein molecules into amyloid fibrils is accompanied by a loss of biological activity, as the latter is generated by the proximity of the functional groups of specific side chains that are brought together in the unique fold of the native state. The conversion of a protein into the amyloid state, however, is associated with more than a loss of biological activity. Biological systems have evolved in such a way that the correctly folded structures of protein molecules (and, indeed, other biological molecules) can be packed together at high densities within cells and in regions of intercellular space (Minton 2000). The amyloid state of a protein molecule (except in the rare cases where this form of proteins has a functional role) is intrinsically misfolded and so is inherently prone to interact inappropriately within the crowded and complex environment of a living system, thereby generating toxicity (Bucciantini et al. 2002). The assembly into amyloid fibrils is a stepwise process that begins with the formation of small clusters of misfolded protein molecules that ultimately grow into mature fibrils (Fig. 5) (Apetri et al. 2006; Knowles et al. 2009, 2014; Cohen et al. 2012). Moreover, these oligomeric species have been found generally to be much more toxic than the fibrils, a phenomenon that can be attributed to the greater exposure of “sticky” hydrophobic side chains that is associated with these prefibrillar species, which have much higher surface-to-volume ratios than do the larger and more structured fibrils (Knowles et al. 2014; Bucciantini et al. 2002; Cremades et al. 2012).
Figure 5.
Different types of aggregates. AFM images of aggregates formed during the conversion of α-synuclein from the soluble monomeric form into amyloid fibrils. The series of images shows the spectrum of approximately spherical oligomers prior to more numerous aggregates that include thin protofibrils and mature fibrils. (From Apetri et al. 2006; reprinted, with permission, from the Journal of Molecular Biology © 2006.)
A key issue that arises in this context is why such species form and give rise to disease. Protein biosynthesis in living systems involves the generation of polypeptide chains having specific sequences of amino acids, the information for which is encoded in the genomic DNA, following which the folding process takes place in complex cellular environments. There is a finite possibility that any given chain will not fold correctly, but there are sophisticated and highly efficient systems within cells that can detect that a given polypeptide chain is misfolded and then target it for degradation (Rubinsztein 2006); such a mechanism avoids the accumulation of potentially toxic aggregates, enables the component amino acids to be recycled and incorporated into new protein molecules, and, in addition, plays a key role in the functioning of the immune system. Indeed, both cells and extracellular spaces have a wide range of specialized proteins, many of which are called “molecular chaperones,” as they discourage inappropriate interactions during protein folding and whose purpose is the prevention of misfolding and its consequences (Hartl and Hayer-Hartl 2009). It is now clear that misfolding disorders such as AD result at the most fundamental level from the failure of such protective mechanisms, with the result that pathogenic forms of proteins can accumulate and progressively damage or destroy those cells in their vicinity (Chiti et al. 1999; Dobson 2003).
AMYLOID FORMATION AND THE LOSS OF PROTEIN HOMEOSTASIS
The actions of molecular chaperones and other systems that prevent misfolding play a key role in the maintenance of protein homeostasis, the mechanism by which every living system maintains the appropriate balance of the generation and degradation of proteins that enables all the normal functions of the organism to be carried out. Once such protective systems fail to prevent the progressive aggregation of one or more proteins, such homeostasis can be lost and pathology results (Balch et al. 2008). It is clear that the ability to avoid protein aggregation is an important factor in biological evolution, such that proteins are able to remain in their functional states under normal physiological conditions. Nevertheless, recent evidence indicates that most proteins within their biological environments are at the highest concentrations at which they can avoid aggregation (Tartaglia et al. 2007). The reason for this situation is likely to result from the fact that the sequences and structures of protein molecules have evolved to enable them to be stable at the concentrations that are required for their optimal function. As random mutations will in general reduce their stability and solubility, proteins in living systems will tend to be just capable of remaining in their functional states under normal conditions (Tartaglia et al. 2007).
This conclusion enables us, at least qualitatively, to be able to rationalize the reasons that protein-misfolding diseases—virtually unknown a century ago—are now becoming frighteningly common (Dobson 2002). Many of these “modern diseases” can be attributed to the fact that we are now living to ages unprecedented in all of human history, and as we get older, our protective mechanisms have a greater risk of failing—for example, because proteins have an increasing tendency to be damaged by processes such as oxidative stress, and as the production of energy required for protein mechanisms to function starts to decline, a loss of protein homeostasis results. Indeed, it appears that certain proteins are particularly vulnerable, many of which are found in the pathways associated with neurodegenerative diseases, as they are “supersaturated”—that is, they accumulate to levels that make them particularly vulnerable to aggregation (Cyriam et al. 2015). Other misfolding disorders are associated with different features of modern life—for example, increasing obesity can change the levels of crucial protein hormones in the pancreas and result in type 2 diabetes. Another example is dialysis-related amyloidosis, as prolonged treatment by dialysis for kidney failure changes the levels of some serum proteins, notably β2-microglobulin, which can be increased by well over an order of magnitude, resulting in its slow deposition in joints and skeletal tissue (Chiti and Dobson 2006). And, indeed, the infectious forms of protein-misfolding and aggregation disorders (Prusiner 1998) (those associated with prions, such as CJD) result from the ingestion of aggregated forms of the protein from an external source, such as contaminated growth hormone or brain tissue. Such species then spread by the recruitment into the aggregates of normally soluble protein molecules in the host organism (Prusiner 1998, 2012; Dobson 1999; Collins et al. 2004; Knowles et al. 2011; Cohen et al. 2014).
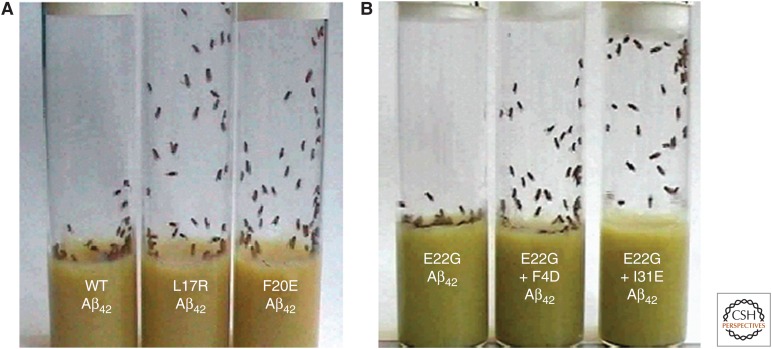
In addition to the “sporadic” events associated with misfolding diseases, the familial amyloid diseases, such as the systemic amyloidosis associated with lysozyme or the small fraction of cases of AD with early onset, can be attributed to the effects of mutations that reduce the solubility and stability of the proteins involved. A particularly dramatic example of these principles has come from the study of transgenic organisms such as fruit flies that express the 42-residue protein fragment (Aβ42) that is the major component of the aggregates that form in AD. By using the knowledge of the effects of amino acid substitutions on the propensity of a given protein to aggregate, it has been possible to design mutations within the sequence of Aβ42 that increase or decrease its propensity to aggregate and form amyloid deposits (Luheshi et al. 2007). Insertion of these mutational variants into transgenic flies (Fig. 6) shows a remarkable correlation between aggregation propensity and neuronal damage (as revealed by locomotor ability and life span), a finding that both reveals the role of the aggregation process in neurodegeneration and the impact that the aggregation of a single (in this case nonessential) protein can have on the viability of a complex living system.
Figure 6.
The effect of mutations in the sequence of the 42-residue human Alzheimer Aβ-peptide on neuronal dysfunction in transgenic fruit flies (Luheshi et al. 2007). (A) A climbing assay of flies expressing the wild-type sequence (left) and two mutational variants (right) predicted to reduce the peptide's aggregation propensity; the healthier the flies, the higher up the tube they can climb. (B) A similar experiment with flies expressing the Aβ-peptide containing the E22G “Arctic mutation” (left-hand tube). The two right-hand tubes are of peptides that contain additional mutations that decrease the propensity to form oligomeric prefibrillar aggregates. (Adapted from Dobson 2008.)
THE MECHANISM OF THE ONSET AND PROGRESSION OF AMYLOID DISEASES
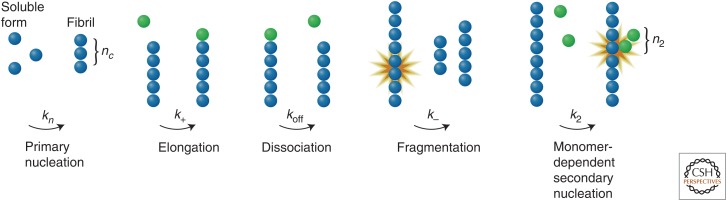
One of the key advances in our understanding of protein-misfolding disorders has been the realization that for many proteins, particularly those that are relatively small, are intrinsically disordered, or have been fragmented by proteolytic enzymes, the amyloid state can be even more stable thermodynamically than the native state even under physiological conditions (Baldwin et al. 2011). The reason for the ability of such species to resist the conversion from their functional forms into the amyloid state is, therefore, strongly dependent on the rate of this transition, the details of which are now beginning to emerge as a result of a transformation in our ability to analyze the complex kinetics of protein aggregation reactions. It is now clear, for example, that the macroscopic process of amyloid fibril formation involves a variety of distinct microscopic steps (Fig. 7) (Knowles et al. 2009; Cohen et al. 2012). The first of these steps involves a primary nucleation event in which two or more monomeric species interact with each other. The resulting oligomers can then grow through sequestration of further soluble species, and, in addition, proliferation can result from secondary processes including fragmentation and surface-induced nucleation (Cohen et al. 2013). For filamentous aggregates such as amyloid fibrils, fragmentation increases the number of sites from which growth can occur, and, in addition, further nucleation can occur on the surfaces of the growing aggregates. The aggregation reaction therefore includes a feedback loop such that the growth of aggregates catalyzes the formation of more aggregates, leading to their rapidly increasing proliferation and spread (Cohen et al. 2013; Buell et al. 2014).
Figure 7.
A summary of the general classes of mechanisms that create protein aggregates. Primary nucleation pathways result in the formation of new aggregates from interactions solely between soluble monomers. Such aggregates can grow (and in the case of fibrillar species, elongate) by the addition of further monomeric species. But the number of aggregates can increase by additional “secondary” processes including monomer-independent events, such as fibril fragmentation, which generate new aggregates at a rate that depends only on the level of the aggregates already present in the solution, and monomer-dependent events such as surface-catalyzed secondary nucleation; this latter process creates new aggregates at a rate that depends on the concentrations of both monomeric protein and existing aggregates. The rate constants of the various processes are labeled. Further details are given in Knowles et al. (2009). (From Knowles et al. 2009; reprinted, with permission, from The American Association for the Advancement of Science © 2009.)
The development of a multitude of experimental and theoretical techniques, ranging from single molecule measurements to mathematical modeling, has now made it possible to extract not only the rates of the individual microscopic steps from experimental measurements of the time-dependent kinetics of amyloid formation under defined conditions, but also the populations of the variety of intermediate species, including those that are known to be highly toxic to cells (Knowles et al. 2009; Cohen et al. 2012). Studies of the different peptides and proteins whose aggregation is linked to different disorders (e.g., the Aβ42 peptide associated with AD and α-synuclein associated with Parkinson's disease) show that there can be profound differences in the relative importance of individual microscopic processes in determining both rates and populations. In the case of the Aβ42 peptide, for example, analysis of experimental data has revealed the importance of the catalysis of the aggregation reaction by secondary nucleation on the surfaces of those aggregates that have already formed (Cohen et al. 2013), while for α-synuclein, a vital step appears to be primary nucleation and its catalysis by surfaces such as membranes (Buell et al. 2014; Galvagnion et al. 2015). Interestingly, a key factor in the infectivity of the prion diseases may well be that primary nucleation is extremely slow under all conditions but that secondary processes can be relatively rapid; under these circumstances, however, the process of aggregation can be triggered by the introduction to the system of preformed aggregates (e.g., by injection or ingestion) (Prusiner 2012).
MOLECULAR CHAPERONES AND INHIBITION OF AMYLOID FORMATION
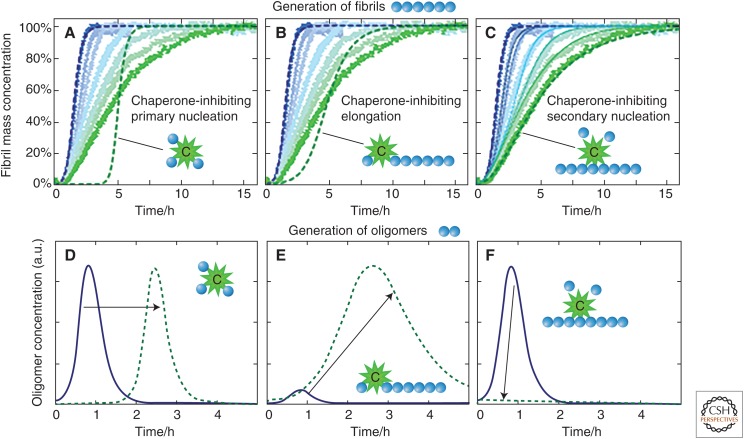
Kinetic analysis of the type discussed above provides strong evidence that the process of amyloid formation by different polypeptides is generic, but that the rates of different steps in the process are dependent on the nature of the polypeptide involved and the conditions under which they occur. The generic nature of the aggregation process provides vital clues as to the means by which biological systems can prevent such processes occurring under normal physiological conditions. Living systems have a host of processes by which protein homeostasis is maintained (including molecular chaperones, quality-control systems, and degradation mechanisms) that we now know can act very specifically on different microscopic steps in the aggregation process of given proteins. Thus, for example, some chaperones target soluble misfolded proteins for degradation while others, however, interact with such species and inhibit their involvement in the initial primary nucleation process (Cohen et al. 2015; Wright et al. 2015; Arosio et al. 2016). Both such mechanisms, if completely effective, could prevent the aggregation of misfolded proteins completely unless, of course, preformed aggregates are introduced from external sources, as in the prion disorders. But if such “primary defenders” fail to prevent completely the formation of nuclei by this initial step, such that aggregates begin to form, other protective species exist that can interact with such aggregates and act as second- or even later-stage defensive mechanisms. Indeed, it is clear, for example, that specific chaperones are able to switch off the catalytic process that drives the proliferation of the aggregation of Aβ42 very selectively, and hence suppress the production of the most highly toxic oligomeric species associated with AD (Fig. 8) (Cohen et al. 2015). It is therefore evident that biological systems have developed a multiplicity of mechanics by which prevention of the formation and, indeed, the subsequent spreading (by secondary processes including those often described as “prion-like” [Prusiner 2012; Knowles et al. 2014]) of the species that are most damaging to cells can be achieved.
Figure 8.
Identification of the inhibition of secondary nucleation and evaluation of the consequences on the generation of toxic oligomers by means of kinetic analysis. (A–C) Kinetic reaction profiles for the aggregation of Aβ42 are shown from left (blue) to right (green) for reactions in the absence of the molecular chaperone called BRICHOS, and with 10%, 20%, 50%, 75%, and 400% Aβ42 monomer equivalents of BRICHOS. The effect of BRICHOS saturates at a stoichiometry of approximately one monomer equivalent, and the blue dashed line is the integrated rate law for Aβ42 aggregation through primary and secondary nucleation using previously determined rate constants. The dashed green lines show predictions for the resulting reaction profiles when each of (A) primary nucleation, (B) fibril elongation, and (C) secondary nucleation are inhibited by the chaperone. Note the characteristic differences in the change in the shape of the reaction profile in each case. The prediction for the case where the chaperone solely and entirely suppresses secondary nucleation is matched essentially perfectly in the presence of excess BRICHOS. The thin dotted lines in C are theoretical predictions for the intermediate BRICHOS concentrations using the association and dissociation rate constants determined from separate binding experiments. (D–F) Time evolution of the concentration of low-molecular-weight oligomeric species predicted by kinetic analysis. The blue line corresponds to the situation without the presence of BRICHOS, and the dashed green lines show predictions when each of (D) primary nucleation, (E) fibril elongation, and (F) secondary nucleation are suppressed by BRICHOS. The results reveal that this particular chaperone effectively inhibits the latter process, which has been shown to be that responsible for the proliferation of toxic oligomeric species in the aggregation process in the absence of the chaperone. (From Cohen et al. 2015; adapted, with permission, from the authors.)
TOWARD THERAPEUTIC INTERVENTION IN AMYLOID DISEASE
Research into the causes of misfolding disorders (notably AD, which is now reaching the level of a “21st century plague”) has only been conducted in earnest for a few decades. In contrast, the bacterial and viral diseases that once afflicted humankind were studied for centuries in the context of progressive developments in our understanding of biological and medical science (Dobson 2015a). Moreover, huge strides have been made in the last 100 years or so in developing the means of treating and preventing noncommunicable diseases such as heart disease and cancer. Such conditions that were until recently thought to be incurable are increasingly considered to be manageable and eminently treatable. Protective compounds such as statins reduce the risk of heart disease, and drugs such as tamoxifen have proved to be effective in treating cancer. Although some advances in treatments over the ages have been fortuitous, the large majority has emerged through painstaking scientific study of the causes of the disease in question, followed by careful evaluation and development of therapeutic compounds that emerge from the testing and screening of possible lead compounds (Dobson 2013).
The progress that has been made in understanding the molecular origins of AD is now at the point where rational exploration of therapies can be undertaken (Arioso et al. 2014; Knowles et al. 2014). The pace of advances in all aspects of science and medicine has never been greater, and collaborative efforts that bring together experts in different disciplines to address major problems have never been more strongly encouraged. The funding of research into dementia is at present only a fraction of that into cancer, and it is essential that this situation changes. Initiatives that replicate the U.S. National Cancer Act of 1971, which led to a “war on cancer,” are needed to change this situation for neurodegenerative diseases. We need to understand more about the details of the underlying causes of these conditions, and we need to develop new biomarkers that enable the progression of disease to be monitored rigorously and regularly; the latter is essential to enable clinical trials to be carried out more effectively and more rapidly.
The fact that biology has managed to generate such effective protection mechanisms, as we have discussed above, means that few cases of AD develop until old age, except where pathogenic mutations act to increase the risk of misfolding and aggregation. This conclusion suggests that we might learn from the mechanisms that have evolved in living systems to develop new types of therapeutic strategies. The increasing emergence of misfolding diseases in the modern world, and indeed experiments such as those with fruit flies discussed above, show that relatively modest changes in the concentrations or properties of proteins are sufficient to cause a profound shift from health to disease. The corollary of this observation is that relatively modest changes in the other direction should be able to reduce the risk of these types of disorders—for example, making it less likely that AD will develop or progress until much more advanced ages (Arioso et al. 2014). One particularly attractive therapeutic strategy, therefore, would be to find means of enabling our natural defense systems to continue to act effectively in old age. We are therefore exploring ways of achieving this objective by searching for molecules that act (rather like the chaperones discussed above) as inhibitors of one or more of the microscopic processes that are involved in the aggregation reaction. Initial results suggest that small molecules that resemble existing types of pharmaceutical compounds may be able to act in this way (Habchi et al. 2016; Joshi et al. 2016), and we are now developing methods of screening such potential drugs that are based on the techniques that we have developed to probe the intrinsic phenomena that underlie specific disorders.
CONCLUDING REMARKS
With the progress that is being made in such directions, we are optimistic that protein-misfolding diseases, like infectious diseases and illnesses such as cancer, will become conditions that are increasingly and successfully prevented and treated (Dobson and Dobson 2017). In this manner, dementia will be like the plagues of the past in that its rapid rise in incidence will be followed by its steady decline to a condition that can be managed effectively in the future (Dobson 2013). Moreover, given the nature of misfolding diseases, there is little if any risk that methods will need to be found to prevent resistance to effective drugs, as is the case for infectious diseases and even some cancers, because the toxic agents of disorders such as AD are not themselves evolving independently of the human hosts. There is every reason to believe, therefore, that these “postevolutionary” diseases (Dobson 2002), which have become prevalent in the modern world because of our success in addressing those diseases that have plagued humankind as a result of previous changes in lifestyle, will themselves become aspects of medical history rather than medical emergencies.
ACKNOWLEDGMENTS
This article is focused particularly on studies carried out by members of my own laboratory and our many collaborators. There are too many people for me to mention them all in this section, although the names of many appear in the citations, but I thank in particular my close colleagues in Cambridge, Profs. Michele Vendruscolo and Tuomas Knowles, who have contributed an incalculable amount to the work described in this article. This article is a substantially revised and updated version of one produced in conjunction with the 2014 Feltrinelli International Award from the Accademia Nazionale dei Lincei in Rome and published in the Proceedings of the Accademia (Dobson 2015a).
Footnotes
Editor: Stanley B. Prusiner
Additional Perspectives on Prion Biology available at www.cshperspectives.org
REFERENCES
- Alzheimer’s Association. 2015. 2015 Alzheimer’s disease facts and figures. Alzheimers Dement 11: 332–384. [DOI] [PubMed] [Google Scholar]
- Apetri MM, Maiti NC, Zagorski MG, Carey PR, Anderson VE. 2006. Secondary structure of α-synuclein oligomers: Characterization by Raman and atomic force microscopy. J Mol Biol 355: 63–71. [DOI] [PubMed] [Google Scholar]
- Arioso P, Vendruscolo M, Dobson CM, Knowles TP. 2014. Chemical kinetics for drug discovery to combat protein aggregation diseases. Trends Pharmacol Sci 35: 127–135. [DOI] [PubMed] [Google Scholar]
- Arosio P, Michaels TC, Linse S, Månsson C, Emanuelsson C, Presto J, Johansson J, Vendruscolo M, Dobson CM, Knowles TP. 2016. Kinetic analysis reveals the diversity of microscopic mechanisms through which molecular chaperones suppress amyloid formation. Nat Commun 7: 10948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer S, Meersman F, Dobson CM, Vendruscolo M. 2008. A generic mechanism of emergence of amyloid protofilaments from disordered oligomeric aggregates. PLoS Comput Biol 4: e1000222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch WE, Morimoto RI, Dillin A, Kelly JW. 2008. Adapting proteostasis for disease intervention. Science 319: 916–919. [DOI] [PubMed] [Google Scholar]
- Baldwin AJ, Knowles TP, Tartaglia G, Fitzpatrick A, Devlin G, Shammas S, Waudby CA, Mossuto MF, Gras SL, Christodoulou J, et al. 2011. Metastability of native proteins and the phenomenon of amyloid formation. J Am Chem Soc 133: 14160–14163. [DOI] [PubMed] [Google Scholar]
- Booth DR, Sunde M, Bellotti V, Robinson CV, Hutchinson WL, Fraser PE, Hawkins PN, Dobson CM, Radford SE, Blake CCF, et al. 1997. Instability, unfolding and aggregation of human lysozyme variants underlying amyloid fibrillogenesis. Nature 385: 787–793. [DOI] [PubMed] [Google Scholar]
- Bucciantini M, Giannoni E, Chiti F, Baroni F, Formigli L, Zurdo J, Taddei N, Ramponi G, Dobson CM, Stefani M. 2002. Inherent cytotoxicity of aggregates implies a common origin for protein misfolding diseases. Nature 416: 507–511. [DOI] [PubMed] [Google Scholar]
- Buell AK, Galvagnion C, Gasper R, Sparr E, Vendruscolo M, Knowles TP, Lines S, Dobson CM. 2014. Solution conditions determine the relative importance of nucleation and growth processes in α-synuclein aggregation. Proc Natl Acad Sci 111: 7671–7676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiti F, Dobson CM. 2006. Protein misfolding, functional amyloid, and human disease. Ann Rev Biochem 75: 333–366. [DOI] [PubMed] [Google Scholar]
- Chiti F, Webster P, Taddei N, Clark A, Stefani M, Ramponi G, Dobson CM. 1999. Designing conditions for in vitro formation of amyloid protofilaments and fibrils. Proc Natl Acad Sci 96: 3590–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SI, Vendruscolo M, Dobson CM, Knowles TP. 2012. From macroscopic measurements to microscopic mechanisms of protein aggregation. J Mol Biol 41: 160–171. [DOI] [PubMed] [Google Scholar]
- Cohen SI, Linse S, Luheshi LM, Hellstrand E, White DA, Rajah L, Otzen DE, Vendruscolo M, Dobson CM, Knowles TP. 2013. Proliferation of amyloid-β42 aggregates occurs through a secondary nucleation mechanism. Proc Natl Acad Sci 110: 9758–9763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SI, Rajah L, Yoon CH, Buell AK, White DA, Sperling RA, Vendruscolo M, Terentjev EM, Dobson CM, Weitz DA, et al. 2014. Spatial propagation of protein polymerization. Phys Rev Lett 112: 098101. [DOI] [PubMed] [Google Scholar]
- Cohen SI, Arosio P, Presto J, Kurudenkandy FR, Biverstål H, Dolfe L, Dunning C, Yang X, Frohm B, Vendruscolo M, et al. 2015. A molecular chaperone breaks the catalytic cycle that generates toxic Aβ oligomers. Nat Struct Mol Biol 22: 207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins SR, Douglass A, Vale RD, Weissman JS. 2004. Mechanism of prion propagation: Amyloid growth occurs by monomer addition. PLoS Biol 2: e321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremades N, Cohen SI, Deas E, Abramov AY, Chen AY, Orte A, Sandal M, Clarke RW, Dunne P, Aprile FA, et al. 2012. Direct observation of the interconversion of normal and toxic forms of α-synuclein. Cell 149: 1048–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyriam P, Kundra R, Morimoto RI, Dobson CM, Vendruscolo M. 2015. Supersaturation is a major driving force for protein aggregation in neurodegenerative diseases. Trends Pharmacol Sci 36: 72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson CM. 1992. Unfolded proteins, compact states and molten globules. Curr Opin Struct Biol 2: 6–12. [Google Scholar]
- Dobson CM. 1999. Protein misfolding, evolution and disease. Trends Biochem Sci 24: 329–332. [DOI] [PubMed] [Google Scholar]
- Dobson CM. 2002. Getting out of shape—Protein misfolding diseases. Nature 418: 729–730. [DOI] [PubMed] [Google Scholar]
- Dobson CM. 2003. Protein folding and misfolding. Nature 426: 884–890. [DOI] [PubMed] [Google Scholar]
- Dobson CM. 2008. Protein folding and misfolding: From atoms to organisms. In Physical biology: From atoms to medicine (ed. Zewail A), pp. 289–335. Imperial College Press, London. [Google Scholar]
- Dobson M. 2013. The story of medicine: From bloodletting to biotechnology. Quercus, London. [Google Scholar]
- Dobson CM. 2015a. Alzheimer’s disease: Addressing a twenty-first century plague. Rendiconti Lincei 26: 251–262. [Google Scholar]
- Dobson M. 2015b. Murderous contagion: A human history of disease. Quercus, London. [Google Scholar]
- Dobson CM, Dobson MJ. 2017. Plagues and history: From the Black Death to Alzheimer’s disease (ed. Heeney J, Friedemann S), pp. 32–65. Cambridge University Press, Cambridge. [Google Scholar]
- Fändrich M, Dobson CM. 2002. The behaviour of polyamino acids reveals an inverse side chain effect in amyloid structure formation. EMBO J 21: 5682–5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick AW, Debelouchina GT, Bayro MJ, Clare DK, Caparoni MA, Bajaj VS, Jaroniec CP, Wang L, Ladizhansky V, Muller SA, et al. 2013. Atomic-resolution structure of a cross-β amyloid fibril. Proc Natl Acad Sci 110: 5468–5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- G8 Dementia Summit. 2013. UK Prime Minister’s Speech 11 December. [Google Scholar]
- Galvagnion C, Buell AK, Meisl G, Michaels TC, Vendruscolo M, Knowles TPJ, Dobson CM. 2015. Lipid vesicles trigger α-synuclein aggregation by stimulating primary nucleation. Nat Chem Biol 11: 229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guijarro JI, Sunde M, Jones JA, Campbell ID, Dobson CM. 1998. Amyloid fibril formation by an SH3 domain. Proc Natl Acad Sci 95: 4224–4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habchi J, Arosio P, Perni M, Costa AR, Yagi-Utsumi M, Joshi P, Chia S, Cohen SI, Müller MB, Linse S, et al. 2016. An anticancer drug suppresses the primary nucleation reaction that initiates the production of the toxic Aβ42 aggregates linked with Alzheimer's disease. Sci Adv 2: e1501244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines MR, Steckel RH. 2014. A population history of North America. Cambridge University Press, Cambridge. [Google Scholar]
- Hartl FU, Hayer-Hartl M. 2009. Converging concepts of protein folding in vitro and in vivo. Nat Struct Mol Biol 16: 574–581. [DOI] [PubMed] [Google Scholar]
- Jiménez JL, Guijarro JI, Orlova E, Zurdo J, Dobson CM, Sunde M, Saibil HR. 1999. Cryo-electron microscopy structure of an SH3 amyloid fibril and model of the molecular packing. EMBO J 18: 815–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi P, Chia S, Habchi J, Knowles TP, Dobson CM, Vendruscolo MA. 2016. Fragment-based method of creating small-molecule libraries to target the aggregation of intrinsically disordered proteins. ACS Comb Sci 18: 144–153. [DOI] [PubMed] [Google Scholar]
- Knowles TPJ, Fitzpatrick AW, Meehan S, Mott HR, Vendruscolo M, Dobson CM, Welland ME. 2007. Role of intermolecular forces in defining material properties of protein nanofibrils. Science 318: 1900–1903. [DOI] [PubMed] [Google Scholar]
- Knowles TPJ, Waudby CA, Devlin GL, Cohen SI, Aguzzi A, Vendruscolo M, Terentjev EM, Welland ME, Dobson CM. 2009. An analytical solution to the kinetics of breakable filament assembly. Science 326: 1533–1537. [DOI] [PubMed] [Google Scholar]
- Knowles TP, White DA, Abate AR, Agresti JJ, Cohen SI, Sperling RA, De Genst EJ, Dobson CM, Weitz DA. 2011. Observation of spatial propagation of amyloid assembly from single nuclei. Proc Natl Acad Sci 108: 14746–14751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles TPJ, Vendruscolo M, Dobson CM. 2014. The amyloid state and its association with protein misfolding diseases. Nat Rev Mol Cell Biol 15: 384–396. [DOI] [PubMed] [Google Scholar]
- Luheshi LM, Tartaglia GG, Brorsson AC, Pawar AP, Watson IE, Chiti F, Vendruscolo M, Lomas DA, Dobson CM, Crowther DC. 2007. Systematic in vivo analysis of the intrinsic determinants of amyloid β pathogenicity. PLoS Biol 5: e290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minton AP. 2000. Implications of macromolecular crowding for protein assembly. Curr Opin Struct Biol 10: 34–39. [DOI] [PubMed] [Google Scholar]
- Möller HJ, Graeber MB. 1998. The case described by Alois Alzheimer in 1911. Eur Arch Psychiatry Clin Neurosci 248: 111–122. [DOI] [PubMed] [Google Scholar]
- Nelson R, Sawaya MR, Balbirnie M, Madsen AØ, Riekel C, Grothe R, Eisenberg D. 2005. Structure of the cross-β spine of amyloid-like fibrils. Nature 455: 773–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, Hayflick L, Butler RN, Allison DB, Ludwig DS. 2005. A potential decline in life expectancy in the United States in the 21st century. New Engl J Med 352: 1138–1145. [DOI] [PubMed] [Google Scholar]
- Peacock S. 2014. Health care: Bring microbial sequencing to hospitals. Nature 509: 557–559. [DOI] [PubMed] [Google Scholar]
- Pepys MB, Hawkins PN, Booth DR, Vigushin DM, Tennant GA, Soutar AK, Totty N, Nguyen O, Blake CC, Terry CJ, et al. 1993. Human lysozyme gene mutations cause hereditary systemic amyloidosis. Nature 362: 553–557. [DOI] [PubMed] [Google Scholar]
- Prusiner SB. 1998. Prions. Proc Natl Acad Sci 95: 13363–13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner SB. 2012. A unifying role for prions in neurodegenerative diseases. Science 336: 1511–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radford SE, Dobson CM, Evans PA. 1992. The folding of hen lysozyme involves partially structured intermediates and multiple pathways. Nature 358: 302–307. [DOI] [PubMed] [Google Scholar]
- Riley JC. 2005. Estimates of regional and global life expectancy 1800–2001. Pop Dev Rev 31: 537–543. [Google Scholar]
- Rubinsztein DC. 2006. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature 443: 780–786. [DOI] [PubMed] [Google Scholar]
- Sunde M, Blake CCF. 1997. The structure of amyloid fibrils by electron microscopy and X-ray diffraction. Adv Protein Chem 50: 123–159. [DOI] [PubMed] [Google Scholar]
- Tartaglia GG, Pechmann S, Dobson CM, Vendruscolo M. 2007. Life on the edge: A link between gene expression levels and aggregation rates of human proteins. Trends Biochem Sci 32: 204–206. [DOI] [PubMed] [Google Scholar]
- Wright MA, Aprile FA, Arosio P, Vendruscolo M, Dobson CM, Knowles TPJ. 2015. Biophysical approaches for the study of interactions between molecular chaperones and protein aggregates. Chem Commun 51: 14425–14434. [DOI] [PMC free article] [PubMed] [Google Scholar]