Abstract
Innate immunity is involved in regulating inflammatory and tissue repair responses to injury. In particular, humoral innate immunity plays functions related to wound clearance from tissue debris, and regulation of macrophage and stromal cell activities. PTX3, a component of humoral innate immunity, orchestrates tissue repair by interacting with plasminogen and fibrin. Fluid-phase molecules of innate immunity interact with elements of the extracellular matrix, and some of the latter display opsonic activity against certain bacterial species.
Thus, recognition of extracellular matrix and microbial components is a recurrent theme in the humoral arm of the innate immune system.
Keywords: Tissue repair, Innate immunity, Inflammation, Pattern recognition molecule, Pentraxin-3 (PTX3)
1. Introduction
The innate immune system represents a first line of resistance against pathogens and a key determinant of the activation and orientation of adaptive immunity, through the activity of its cellular and humoral arms [1], [2], [3]. In addition, innate immunity coordinates a complex series of events that lead to proper tissue repair [4] (Fig. 1 ). The cellular sensors of innate immunity play a crucial role in the initiation of the tissue repair process, through the regulation of the inflammatory response and the recruitment of immune cells into the injured site. The role of components of the humoral innate immunity includes their ability to opsonize tissue debris and apoptotic cells, activate complement and regulate inflammation. Recent studies indicate that these molecules play other roles that include direct regulation of macrophage activities and fibrocyte differentiation and contribute to the proliferation phase of repair. In addition, several lines of evidence show the interaction between components of the humoral arm of innate immunity and extracellular matrix elements, hence suggesting new roles of these molecules in the tissue repair process.
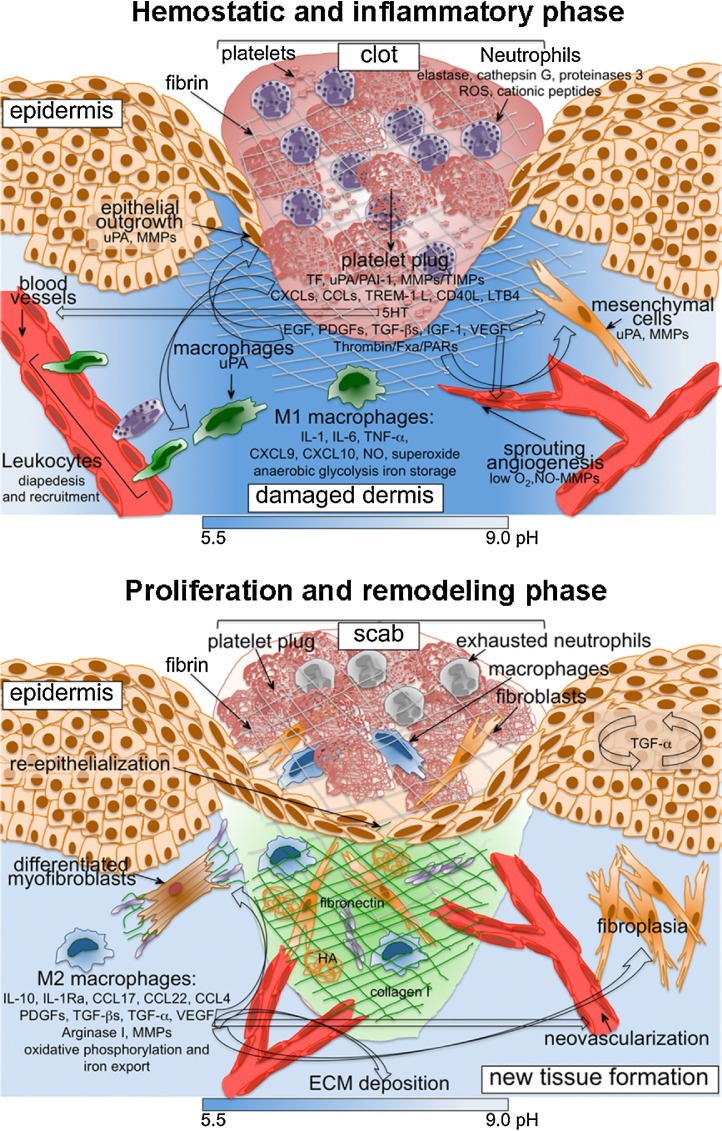
Fig. 1.
Schematic view of cellular and molecular mechanisms involved in inflammatory and proliferation phases of skin wound repair.
Soluble mediators released by platelets, neutrophils, macrophages and MSCs, and ECM components are depicted here and described in the text. In early phases, reduced oxygen tension causes a shift in cell metabolism towards an anaerobic glycolytic pathway and pH lowering in the wound site. (TXA2, thromboxane 2; LPA, lysophosphatidic acid).
Here we discuss the roles of cellular and molecular elements of the innate immune system in tissue repair, focusing on the function of the humoral innate immunity and in particular on the long pentraxin PTX3.
2. Cellular players in wound healing
Wounds heal efficiently through distinct but overlapping phases, hemostasis-inflammation, proliferation and remodeling, which involve specific cell types [4]. Platelets, neutrophils, macrophages and mesenchymal cells represent the major components of the healing response throughout the repair process.
Platelets recruited at site of injury in the coagulation process release clotting factors resulting in fibrin deposition, as well as several mediators and growth factors that sustain both the inflammatory and proliferative response [5], [6].
Neutrophils protect the host from invading microbes, begin debridement of non-functioning host cells and sustain the inflammatory response. In addition, neutrophils express proteins involved in fibrin clot breakdown and extracellular matrix (ECM) degradation, migration and proliferation of keratinocytes and fibroblasts, angiogenesis (e.g. uPA, VEGF, CXCL8, CXCL3) [7]. As inflammation resolves, neutrophils in the wound site diminish and the damaged tissue undergoes a lengthy period of resolution and remodeling [4]. Excessive neutrophil accumulation and persistence at the wound site contribute to altering the tissue repair process through the release of cytotoxic mediators, which cause increased tissue damage and persistent inflammation [8], as observed in chronic non-healing wounds.
Macrophages are involved in the promotion of effective healing through the regulation and resolution of inflammation and induction of ECM deposition [9]. Classically activated (M1) macrophages, which are induced by recognition of pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs) and have a proinflammatory phenotype, are abundant in the initial tissue damage response. They facilitate innate immune responses, removal of spent neutrophils and damaged tissue, digestion of previously deposited fibrin, and prepare the wound site for a phase of new tissue deposition [9] (Fig. 1). Alternative activated (M2) macrophages, which are induced by different stimuli (e.g., IL-4/IL-13, IL-10, TGF-β), are predominant in the late inflammatory response. They sustain resolution of inflammation and promote angiogenesis, fibroplasia, new ECM deposition and final tissue remodeling, through the production of several growth factors and proteases [9] (Fig. 1).
In the later proliferative phase, cells of mesenchymal origin are the predominant cell population in the wound site [4]. They are responsible for producing new ECM, including collagen needed to restore the structure and function of the damaged tissue. Fibroblasts within granulation tissue transform into contractile myofibroblast, which contribute to wound contraction [10]. New formed blood capillaries supply metabolically demanding wound tissues with nutrients and oxygen. After repair is complete, blood vessels regress and leukocytes and myofibroblasts undergo apoptosis. The last phase of wound healing includes maturation and remodeling of the new tissue with increase in tissue strength mediated by turnover and remodeling of ECM [4]. An important consequence of tissue repair, at least in wound healing of adults, is fibrosis and excessive scarring. Altered functions of macrophages are involved in the formation of pathological ulcers and hypertrophic scars and keloids [11].
Mesenchymal Stromal Cells (MSCs) are multipotent stem cells characterized by a fibroblast-like morphology and the ability to differentiate into the mesengenic lineages, such as adipocytes, osteocytes and chondrocytes. MSCs play a beneficial effect on cutaneous wound healing [12], [13] they accelerate wound closure, enhance angiogenesis, regulate ECM remodeling and sustain skin regeneration. In addition, MSCs exert antimicrobial activity, which is fundamental for the clearance of bacterial infections [14] and significantly attenuate the inflammatory responses, through cell-to-cell interactions and production of soluble factors [15], [16]. More specifically, MSCs secrete indoleamine-2,3-dioxygenase (IDO), heme oxygenase-1, prostaglandin E2, human leukocyte antigen G (HLA-G5), and TNF-alpha-stimulated gene/protein 6 (TSG-6) [17]. Numerous studies demonstrated that the administration of MSCs attenuates excessive inflammation and generates therapeutic effects in a number of non-healing wounds resulting from burns, diabetes, vascular insufficiency, and Crohn's disease [12]. The underlying mechanisms include the role of MSCs in controlling the function of numerous cells of the immune system, including macrophages/monocytes, natural killer cells, neutrophils, dendritic cells and T and B lymphocytes [18]. MSCs inhibit host T cell proliferation, a crucial step for reducing wound bed inflammation [19], by inducing IL-10 in both native T cells and macrophages [20] and by increasing TGF-β activity. They modulate host TNF-α production and NK cell function, reducing IFN-γ activity in the process [15]. On the other hand, in the later stages of inflammation, TGF-α stimulates transplanted MSCs to produce several pro-healing growth factors, including VEGF. MSCs promote wound healing by limiting inflammatory cell recruitment, pro-inflammatory cytokine levels, such as IL-1 and TNF-α, or increasing the expression of TSG-6, NO, IDO, PGE2, and IL-10 [12]. In addition, MSCs cooperate with macrophages in improving wound healing [21] favoring the acquisition of an anti-inflammatory M2 phenotype [22] and promoting neovessel maturation and the expression of IL-10, VEGF-1, TGF-β1, and angiopoietin-1 [23]. In addition, MSC-derived let 7b-containing exosomes have been shown to favour M2 macrophage polarization [24]. Finally, MSCs produce antimicrobial peptides, necessary for wound resolution and bacterial clearance [14]. We recently reported that, after stimulation with inflammatory cytokines, BM-derived MSCs produce high amount of PTX3, and this production represents an essential requirement for MSC ability of tissue repair [25] (see below).
3. Pattern recognition molecules involved in tissue repair
Innate immunity consists of a fully integrated system composed by a cellular and a humoral arm [1]. Cell-associated pattern recognition molecules (PRMs) belong to different molecular classes and include Toll-like receptors (TLRs), NOD-like receptors, C-type lectins and scavenger receptors [1], [26]. In addition to sense pathogen-derived agonists, cellular PRMs also specifically recognize DAMPs released in injured tissues, such as ECM components, nuclear proteins and nucleic acids, leading to activation of the inflammatory response and initiating tissue repair processes. The humoral arm is constituted by fluid phase PRMs, which function as ancestors of antibodies. They are structurally diverse but share a common mode of action, regulating complement activation and opsonisation, and inducing microbe agglutination and neutralization [1]. Fluid phase PRMs include components of the complement cascade, ficolins, collectins and pentraxins [1], [27], [28].
The pentraxin family is an ancient group of evolutionarily conserved proteins. C reactive protein (CRP) and serum amyloid P (SAP) component constitute the short pentraxins [1] and represent the main liver-derived acute-phase reactants in human and mouse, respectively [27]. CRP was originally identified as a prototypic PRM recognizing various microorganisms (fungi, yeasts, bacteria and parasites) through common moieties such as phosphorylcholine (PC) and carbohydrate structures, promoting phagocytosis and therefore ensuring protection against infections [1], [27]. Similarly, SAP binds a wide range of microorganisms through structures present in their surface such as LPS, PC and terminal mannose or galactose glycan residues, inhibiting their infectivity [1]. SAP binds to chromatin and apoptotic cells favoring their clearance and is involved in promoting amyloid fibrils persistence [29]. CRP and SAP share several functional properties with antibodies, acting as opsonins, by interacting with C1q and ficolins, with the complement regulators factor H (FH) and C4-binding protein (C4BP), and with Fcγ receptors [1], [30].
PTX3, originally identified as a pro-inflammatory cytokine-inducible molecule, and pentraxin domain containing proteins, which were subsequently identified, constitute the long pentraxin family. Regulation, ligands and relevant in vivo functions of PTX3 will be described in detail in following sections.
3.1. Role of fluid-phase PRMs in tissue repair
Components of the humoral arm of innate immunity that play a role in tissue damage principally belong to the complement system, which in addition to defense from infections [31], also regulates migration and activation of immune cells involved in wound healing [32]. Abolition of complement activation in C3-deficient mice and defective leukocyte recruitment in C5a-deficient mice are associated with abrogation of inflammation in the wound site, leading to acceleration of wound closure [33]. In the wound site, C1q localizes without C4 and C3 on endothelium and stroma and affects in vivo blood vessel formation in the granulation tissue. C1q modulates in vitro EC functions including permeability, proliferation, migration and tube formation, contributing to skin healing [34]. Complement cascade components are also involved in activation and propagation of coagulation [32]. Mannose-binding lectin (MBL) is described to affect coagulation and to contribute to arterial thrombosis [35]. Upon binding to altered host cells in ischemia/reperfusion injury, MBL promotes the activation of the lectin complement pathway contributing to inflammation and hence tissue damage [35], [36].
Studies on the involvement of CRP in tissue damage are complicated by the low level of conservation among species in terms of regulation of expression (CRP is not an acute phase protein in the mouse) and functional activities (e.g. complement activation) [27]. A protective role of CRP was observed in tissue damage associated to autoimmunity. However, these anti-inflammatory effects of CRP have not been confirmed by other studies performed using highly pure native human CRP [37]. In models of atherosclerosis, cardiac ischemia/reperfusion and cerebral infarct, CRP was deposited in tissue lesions and activated the complement cascade, thus contributing to tissue damage [38]. Therapeutic inhibition of CRP appeared a promising approach to cardioprotection in acute myocardial infarction [39]. However, studies performed with transgenic human CRP in ApoE-deficient mice showed that CRP is not pro-inflammatory, pro-thrombotic or pro-atherogenic [40].
SAP has been show to control the inflammatory response to injury, by affecting neutrophil recruitment and activation, and exerting an anti-inflammatory effect [41]. By regulating activities of macrophages through FcγRs, SAP inhibits renal fibrosis in vivo [42]. SAP is also a component of normal tissues (e.g. skin and glomerular basement membrane) where it is covalently bound to collagens and other ECM proteins. Administration of SAP reduces pulmonary inflammation and fibrosis in animal models suggesting a role for SAP in anti-fibrotic therapy [43]. In a randomized, blinded, placebo controlled, human clinical trial, use of recombinant human SAP (PRM-151) reduced the number of fibrocytes in pulmonary fibrosis, and improved lung function in patients affected by idiopathic pulmonary fibrosis, by inhibiting alternative activation of macrophages and fibrocyte differentiation via interaction with FcγRs [44]. Recently it has been shown that SAP prevents fibrosis by interacting with DC-SIGN expressed on immune cells. A polycyclic aminothiazole DC-SIGN ligand and anti-DC-SIGN antibodies mimicked SAP effects in vitro and in an in vivo model of pulmonary fibrosis, by increasing the expression of IL-10 in lung epithelial cells and sustaining an anti-inflammatory and anti-fibrotic response [45]. Finally, SAP binds to amyloid fibrils present in tissue amyloid deposits in systemic amyloidosis, Alzheimer's disease and transmissible spongiform encephalopathy, and it contributes to pathogenesis by stabilizing amyloid deposits [29]. Administration in mice of antibodies directed against SAP within amyloid deposits was shown to trigger a potent, complement-dependent, macrophage-derived reaction that removed the massive visceral amyloid deposits [46]. The property of SAP to bind apoptotic cells, chromatin fragments and nuclear debris released under cell death, and to regulate the complement cascade [47] suggests the involvement in handling of injury-induced necrotic cells.
4. The long pentraxin PTX3 in tissue repair
4.1. Gene and protein
PTX3 belongs to a class of molecules identified in early 1990s called long pentraxins on the basis of their structure. The PTX3 gene is localized on the chromosome 3 and organized in three exons with the first and second coding the signal peptide and the N-terminal domain, and the third exon coding the C-terminal pentraxin domain. The proximal promoter includes numerous potential binding sites for transcription factors such as Pu1, AP-1, NF-κB, SP1 and NF-IL-6. PTX3 is a multimeric glycoprotein of eight identical protomers assembled to form an elongated octamer with a molecular weight of 344KDa stabilized by intermolecular disulphide bonds [1]. The C-terminal domain of PTX3 contains the pentraxin signature and is up to 57% homologous to CRP and SAP. In contrast, the N-terminal domain is unrelated with any known protein sequence. A unique N-linked glycosylation site is located in the C-terminal domain at Asn220 [1].
4.2. Regulation and sources
PTX3 is rapidly induced by several stimuli in different cell types, including cells of the myeloid lineage [e.g. monocytes, macrophages, dendritic cells (DCs)] and stromal cells [smooth muscle cells (SMCs), fibroblasts, chondrocytes, ECs, epithelial cells] in response to proinflammatory cytokines, DAMPs and agonists of TLRs, microbial components or intact microorganisms [1]. Neutrophils store PTX3 in secondary granules, and upon appropriate stimulation promptly release it [48].
The expression of PTX3 is differently regulated on the basis of the cell type and/or and stimulus [1]. IL-1 receptor and MyD88 signaling are centrally involved in PTX3 induction both in sterile damage and infectious models [49], [50], [51]. In alveolar epithelial cells, TNF-α induces PTX3 through JNK. HIF-1α and C/EBPβ are involved in the regulation of PTX3 expression during cardiac surgery [52]. IFNγ inhibits PTX3 production induced by LPS in monocytes and IL-6 does not represent a stimulus to induce PTX3, in contrast with CRP and SAP. IL-10 induces PTX3 in myeloid cells and amplifies the production in inflammatory conditions, therefore suggesting that PTX3 is part of a genetic program activated by IL-10, which acts as an essential player in M2 macrophage-dependent resolution of inflammation in tissue damage [1]. PTX3 is epigenetically regulated in selected tumors (e.g., esophageal squamous cell carcinoma, leiomyosarcomas and colorectal cancer) due to hypermethylation of PTX3 gene promoter and of an enhancer [50].
4.3. Ligands and functions
PTX3 functions involve recognition and binding to different ligands, including microbial moieties, complement components, ECM proteins and growth factors [1], [53]. PTX3 is an essential component of the humoral innate response to microbes. PTX3 interacts with selected bacteria, fungi and viruses, including Klebsiella pneumonia, Pseudomonas aeruginosa, uropathogenic Escherichia coli, Aspergillus fumigatus, Paracoccidoides brasilienis, cytomegalovirus (CMV) and influenza virus (IV) type A [1], [53], [54]. PTX3, by acting as an opsonin for bacteria and fungi, activates a collaborative response between different humoral and cellular effector pathways that includes complement activation and FcγRII- and CD11b-dependent facilitated recognition and phagocytosis [55]. In humans, PTX3 single nucleotide polymorphisms (SNPs) associated in particular haplotypes are linked to susceptibility to aspergillosis, pulmonary tuberculosis, Pseudomonas aeruginosa infection in cystic fibrosis patients, fungal infections in solid organ transplanted patients, and urinary tract infections [1], [54], [56], [57].
PTX3 regulates inflammatory responses in infectious and sterile in vivo models, including acute kidney injury [58], severe acute respiratory syndrome or LPS-induced lung injury [59], atherosclerosis [60] and acute myocardial infarction [49], through multifaceted molecular mechanisms. A possible molecular mechanism is provided by binding of PTX3 to the adhesion molecule P-selectin through its N-linked glycosidic moiety, that leads to inhibition of leukocyte recruitment at damaged site [61]. In addition, PTX3 binds key elements of the complement cascade and modulates complement activation [62]. By interacting with C1q through its globular head, PTX3 modulates the classical pathway of complement. PTX3 interacts with the members of the lectin pathway ficolin-1, ficolin-2 and MBL and synergistically amplifies a complement-mediated innate response to Aspergillus fumigatus and Candida albicans [63]. As CRP and SAP, PTX3 also interacts with FH and C4BP [62] on the cell surface or in ECM, thus preventing alternative pathway-mediated exaggerated complement activation. This mechanism is of particular interest in cancer, where the interaction of PTX3 with FH is determinant in regulating complement-dependent tumor-promoting inflammation [50]. Moreover, PTX3 interacts with FGF2 and FGF8b and antagonizes their functions via its N-terminal domain and suppresses FGF2-mediated pro-angiogenic and pro-restenotic activity on ECs and SMCs respectively [64].
The first evidence of the involvement of PTX3 in ECM remodeling emerged from studies on the infertility of PTX3-deficient female mice [1], [65]. PTX3 produced by cumulus cells interacts with the ECM components TNFα-induced protein 6 (TNFAIP6 or TSG-6) and inter-α-trypsin inhibitor (IαI), which are essential to assembly a hyaluronan (HA)-rich matrix around the oocyte. PTX3-deficient female mice display infertility due to a defective assembly of this HA-rich matrix, essential for oocyte fertilization [65].
4.4. Role of PTX3 in tissue repair
The function of PTX3 in tissue repair was recently investigated in different models of non-infectious tissue damage (excisional skin wounding, chemically-induced liver and lung injury, arterial thrombosis) [51]. Results showed that after tissue damage, PTX3 contributed to the orchestration of tissue repair by interacting with plasminogen (Plg) and fibrin matrix, and promoting pericellular fibrinolysis. Local acidic pH, which normally occurs in the early phases of tissue injury [66], governs this interaction and sets PTX3 in a tissue repair mode, while retaining anti-microbial recognition and interaction with complement elements (Fig. 2 ).
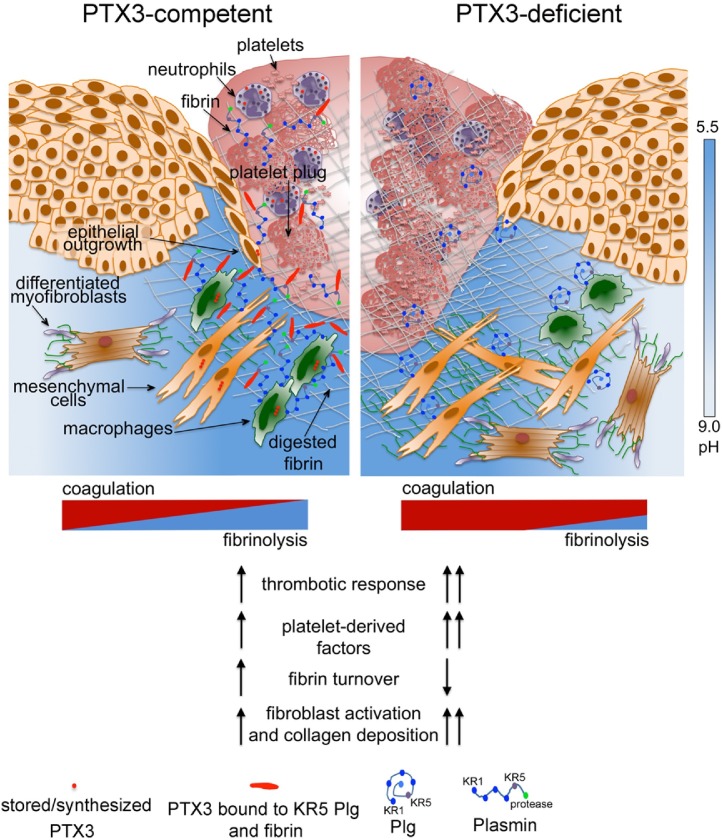
Fig. 2.
Role of PTX3 in skin wound repair.
PTX3 regulates the thrombotic response to injury by interacting with FG and Plg. PTX3 produced by invading remodeling macrophages and MSCs interacts with fibrin and Plg (through its KR5 domain) and this tripartite interaction promotes pericellular fibrinolysis by remodeling cells. In wounded skin, PTX3-deficient cells display defective fibrinolysis and directional migration toward the wound bed and clot, and re-epithelialization is delayed. The acidic pH governs the interaction of PTX3 with fibrin and Plg. PTX3-deficiency is associated with increased thrombotic response, augmented fibrin deposition and persistence, decreased Plg deposition and defective pericellular fibrinolysis, hyperactivation of fibroblastic cells and augmented collagen deposition.
PTX3 levels increased following skin injury in blood and locally, where it was detected for almost the entire duration of the repair process. PTX3 localized in the clot and bordered the pericellular ECM of aligned remodeling macrophages and MSCs collectively migrating towards the wound site, as well as the epithelial outgrowth. In later stages, PTX3 was scattered in the scab and newly formed tissue. Cell migration in cluster or ducts represents a hallmark of tissue remodeling events associated with wound repair and cancer invasion [67]. Several cell types were responsible of PTX3 production in the wound site in response to TLR activation and amplification by IL-1, including macrophages, MSCs and smooth muscle cells. Reactive MSCs, which are defined by PDGFRα and fibroblast activation protein (FAP) expression, were the most important producers of PTX3 in damaged skin. Neutrophils, which store preformed PTX3 in their granules [48], reasonably released it during clot formation. PTX3 deficiency was associated with an abnormal local hemostatic response to injury, as indicated by the presence of thicker clots, augmented fibrin deposition and persistence in damaged dermis, augmented content of factors derived from activated platelets and thrombin in wounds (Fig. 2). At later time points, PTX3-deficient wounds showed augmented expression of reactive fibroblasts and collagen deposition, and general signs of delay of mature tissue formation, including defective re-epithelialization and final epithelial hyperplasia. A premature wound contraction accompanied the initial impaired thrombotic response of PTX3-defcient mice. Differently from humans, a normal wound repair in mice includes a phase of wound contraction, which restricts the entry of microbes and facilitates wound closure. The most likely mechanism underlying the wound contraction in mice appears to be the interaction between contractile fibroblasts and the ECM [68], with the contribution of the panniculosus carnosus (a thin muscle layer found in humans only at the neck platysma) [68], [69]. Platelet-derived factors, such as PDGF, TGFβ, serotonin and thrombin have been implicated in wound contraction in animal models [68], [69]. Therefore, in PTX3-deficient wounds, an augmented content of factors reasonably derived from platelets during the clotting phase and the consequent higher expression of markers of differentiated/activated fibroblasts were possible mechanisms underlying the increased contraction and augmented collagen deposition. Administration of pharmacological inhibitors of coagulation and platelet activation acting on different molecular targets (Factor Xa, thrombin), abolished the defects observed in PTX3-deficient mice, including premature wound contraction and collagen accumulation, hence indicating that an altered hemostatic response was responsible for the phenotype observed. In agreement, in a mouse model of arterial thrombosis, PTX3 derived from vascular ECs localized within the thrombus and in the vessel wall, where it dampened thrombogenesis. By targeting fibrinogen (FG) through its N-terminal domain, PTX3 inhibited platelet adhesion and aggregation [70]. These phenotypes were independent from the interaction of PTX3 with P-selectin and leukocyte recruitment at the wound site [70]. Actually, the alterations in skin damage and arterial occlusion were identical in PTX3-deficient and double PTX3/P-selectin-deficient mice.
Increased clotting and fibrin deposition followed by increased fibrosis were also observed in models of liver and lung sterile injury [51]. In these models, genetic deficiencies causing prothrombotic responses are associated with impaired tissue remodeling and fibrosis [71]. PTX3 was observed in necroinflammatory areas associated with neutrophils in acute injury, and in portal tracts associated with remodeling macrophages and MSCs in fibrotic liver. PTX3-deficiency caused increased centrolobular thrombosis and augmented deposits of fibrin in necroinflammatory areas, and augmented liver fibrosis. In line with these studies, PTX3 was shown to play a protective role in a mouse model of brain ischemic injury, being involved in edema resolution and glial scar formation [72].
The increased clotting and fibrin and collagen deposition associated with PTX3-deficiency in all these models suggested the interplay of PTX3 with the hemostatic system. In addition, these phenotypes are reminiscent of those obtained in mice with genetic defects in key molecules of the fibrinolytic system [73], [74], [75], [76]. Indeed, PTX3 was found to interact with FG, fibrin and Plg at acidic pH (optimal range from 6.5 to 5.5), while it did not bind other tested components of the coagulation or fibrinolytic cascade [51]. In agreement with these results, the interaction between PTX3 and members of the ficolin and collectin families via their FG domain and collagen domain was facilitated in an acidic milieu [62], [63], suggesting a conformational chance of the molecule, which potentially exposes new binding sites for specific ligands. In the first phases of skin wound healing, the extracellular pH is acidic and ranges from 5.7 to 6.1, while chronic and highly infected wounds are characterized by non-acidic pH [66]. Acidification of the wound site, which occurs as result of cell metabolic adaptation to trauma-induced tissue hypoperfusion, has functional relevance in the outcome of healing by affecting several processes including cell adhesion, migration and proliferation. An acidic pH is present in inflammatory phases of acute wounds that progress in healing, while chronic and highly infected wounds are characterized by non-acidic pH [66]. The interaction with fibrin and Plg was restricted to PTX3, since short pentraxins did not interact with these factors. Moreover, the second exon-encoded N-terminal domain of PTX3 was responsible for these interactions, whereas the C-terminal pentraxin domain showed a low affinity binding only with Plg. The use of specific antibodies directed against the N-terminal domain completely blocked PTX3 binding to fibrin. The in vivo administration of PTX3 rescued all the alterations associated with PTX3-deficiency, including premature wound contraction, altered clotting response and augmented collagen deposition, and the N-terminal domain of PTX3 alone recapitulated the activity of the whole molecule, suggesting therefore the primary involvement of this domain in tissue repair [51]. The interaction of PTX3 N-terminal domain with FG was also determinant in the attenuation of platelet aggregation [70]. As assessed by the use of selective antibodies and in competition experiments, PTX3 interaction with fibrin and Plg occurred via different sites in its N-terminal domain, and did not affect the interaction between fibrin with Plg. PTX3 was shown to interact with kringle (KR) 5 domain of Plg, which is involved in triggering the Plg conformational change leading to the open form, and not with Plg KR1 domain, which mediates the initial recruitment of the proenzyme to fibrin or cell surface. Ligands of l-lysine binding sites on the KR5 domain trigger the conformational changes of Plg that passes from a closed-inactive to an open-active form essential for its conversion to plasmin, which is central in fibrin matrix turnover and thrombus removal after tissue injury [75]. Whether the interaction of PTX3 with Plg through the KR5 domain has a functional relevance in the Plg activation is presently unknown. In cell-free fibrinolysis assays, the interaction of PTX3 with Plg resulted in potentiation of fibrin gel degradation mediated by plasmin and triggered by uPA and tissue plasminogen activator (tPA). In contrast, no potentiation was observed at near neutral pH, suggesting a critical role of acidic pH in governing this PTX3 function [51]. Plg activators uPA and tPA are neutral proteases and, accordingly, diminished cell-free fibrinolysis was observed at acidic pH compared to neutral pH. Therefore, the pH dependence of PTX3 binding to fibrin and Plg ensures that it would not occur in the circulation but rather at sites of tissue repair, where it sustains fibrin matrix resolution. The acidic environment likely acts as a “switch on” signal for this function of PTX3 in tissue repair. Use of confocal and two-photon microscopy, a pH sensitive probe and fluorophore-conjugated specific antibodies ascertained the occurrence of PTX3 interaction with fibrin and Plg preferentially in more acidic areas in the wound site [51].
At the beginning of new tissue formation, remodeling macrophages and MSCs enter the wound allowing the replacement of the provisional matrix by granulation tissue rich in type I collagen, as well as other ECM proteins [4]. These cells invade the fibrin matrix creating patent passages through pericellular fibrinolysis mainly mediated by Plg activation [77]. In wild type wounds, PTX3 and Plg colocalization bordered the pericellular matrix of these cells, whereas in PTX3-deficient wounds Plg was associated with cells dispersed in the fibrin matrix. In addition, PTX3-deficient macrophages and MSCs did not associate in bundles invading the injured tissue and failed to create passages within the fibrin matrix and showed defective in vitro fibrinolytic activity. Moreover, in PTX3-deficient wounds a higher content of intact fibrin, a lower content of Plg and plasmin associated with lower levels of D-dimer were observed. Therefore, these studies suggest that PTX3 contributes to a normal repair by promoting Plg-mediated pericellular fibrinolysis in the acidic wound site (Fig. 2). This model has been strengthened by a subsequent in vivo study showing that in bone marrow-derived MSC-dependent skin repair, PTX3 released from these cells promoted MSC pericellular fibrinolysis and migration within the fibrin rich wound site [25]. In this study, PTX3-deficient MSCs implanted into skin excisional wounds induced defective wound closure compared to wild type MSCs. Histological evaluation of skin samples treated with PTX3-deficient MSCs showed a reduction of the granulation tissue and excessive accumulation of fibrin. Accordingly, PTX3-deficient MSCs showed a defective ability to degrade the fibrin matrix both in vitro and in vivo. Thus, this study suggests that the fibrinolysis mediated by MSC-derived PTX3 is directly related to the dissolution of clot and remodeling of the fibrin-rich provisional matrix, and is required for the migration of these cells into the wound, which constitutes an important pre-requisite for appropriate wound healing in this model. Moreover, by creating passages into the fibrin-clot, the invading MSCs may promote the recruitment of other remodeling cells, thereby favoring the acceleration of repair processes, such as granulation tissue maturation. The capacity of MSC to reduce inflammation, in addition to their ability of inducing reepithelialization, promoting granulation tissue formation, increasing angiogenesis and regulating ECM remodeling, candidate these cells as an innovative therapy for cutaneous healing.
PTX3 administration was also observed to revert IL-6/STA3 dependent interstitial fibrosis in a mouse model of acute renal injury [78], and a recent in vitro study suggests a possible function of PTX3 in the promotion of human and murine fibrocyte differentiation via FcγRI interaction [79]. These results suggest therefore a potential therapeutic use of PTX3 as modulator of fibrinolysis in abnormal processes of tissue repair, for instance in human pathological hypertrophic scars.
In the model of vascular thrombosis, PTX3 limited the thrombotic response, and the N-domain of PTX3 was responsible of attenuation of FG-induced platelets aggregation, whereas the PTX3 C-terminal domain was shown to inhibit collagen-induced platelet activation [70]. The interaction of PTX3 and members of the ficolin and collectin family occurs via their FG-domain or collagen-domain, which are respectively recognized by the N-domain or C-domain of PTX3 [63], and results in amplification of microbial recognition and effector functions. Therefore, PTX3 has a dual role in defense against pathogens and in regulating thrombotic events and fibrinolysis in response to tissue damage, by interacting with domains conserved in molecules belonging to the innate immunity and hemostatic system, whose functions during evolution overlapped [80], [81]. Thus, PTX3 emerges as a molecule of the humoral innate immunity that plays a regulatory role in the interplay between hemostasis and tissue repair.
5. Interaction between humoral innate immunity and ECM molecules
Scattered lines of evidence show the interaction between components of fluid-phase innate immunity components with ECM molecules (Table 1 ). For instance, the interaction of collectins and ficolins with the Aα and Bβ chains of fibrin activates and enhances the lectin complement pathway [82], sustaining a collaborative response between lectins and coagulation in injury and inflammation. C1q binds fibrin with high affinity through its collagen-like and globular domains. C1q and collectins also interact with the ECM proteoglycans decorin and biglycan [83]. CRP interacts with fibronectin and laminin [84] and SAP interacts with several ECM components, including type IV collagen [85], laminin [86], fibronectin. On the other hand, several ECM components, such as fibronectin [87], mindin [88], osteopontin [89], and vitronectin [90], interact with microbes and have opsonic activity. Molecular phylogenetic analyses indicates that in invertebrates FG-domain-containing molecules are coupled to a variety of N-terminal domains such as immunoglobulin or collagen domains, providing possibility for diversification of the Ig molecule or adding new functional possibilities [80], [91]. Actually, in invertebrates the functions of FG-domain-containing molecules are linked to bacterial defense, agglutination and allorecognition, but not coagulation, suggesting that the latter role is acquired in vertebrates [80] (Table 2 ).
Table 1.
Interaction of fluid phase PRMs with ECM elements.
| PRM | ECM | Related function |
|---|---|---|
| Pentraxins | ||
| PTX3 | TSG-6/IαI | Organization of HA in the viscoelastic ECM of cumulus oophorus essential for female fertility |
| FG/fibrin (acidic pH-dependent) | Promotion of Plg-mediated pericellular fibrinolysis contributing to tissue repair; control of thrombogenesis inhibiting the FG-induced platelet aggregation | |
| collagen I | Control of thrombogenesis inhibiting the collagen-induced platelet aggregation | |
| SAP | fibronectin (no interaction in fluid phase) | Inhibition of cell adhesion and proinflamamtory activities of immune cells |
| collagen IV | n.d. | |
| HA and heparin sulfate | Inhibition of SAP-induced hemagglutination | |
| amyloid deposits | Stabilization of amyloid fibrosis contributing to pathogenesis | |
| laminin | Enhancement of laminin polymerization; no interference in laminin activity as cell adhesion substrate | |
| CRP | fibronectin (acidic pH-dependent; no interaction in fluid phase) | Inhibition of leukocyte adhesion |
| laminin | n.d. | |
| Collectins | ||
| C1q | FG/fibrin | n.d. |
| decorin | Inhibition of classical complement pathway activation and of production of proinflammatory cytokines induced by C1q/C1qR in ECs and leukocytes | |
| biglycan | Inhibition of classical complement pathway activation | |
| MBL | FG/fibrin | Amplification of lectin pathway activation on microbes and regulation of coagulation |
| decorin | n.d. | |
| biglycan | Inhibition of lectin pathway activation | |
| SP-D | decorin | n.d. |
| biglycan | n.d. | |
| Ficolins | ||
| Ficolin-1 | FG/fibrin | Amplification of lectin pathway activation on fibrin-coated microbes |
n.d., not determined.
Table 2.
Functions of FG domain- and collagen domain-containing molecules in evolution.
| Invertebrate | Vertebrate | |
|---|---|---|
| Coagulation | – | FG |
| CD | ||
| (MBL) | ||
| Defense, agglutination and allorecognition | FD (lectin-like) | CD(MBL, C1q, SP-A, SP-D) |
| FD coupled to Ig(s)-domain | FD coupled to CD (ficolin-1, 2, 3) | |
| FD coupled to CD | ||
| Wound healing and development | FD | FG collagen |
| CD | CD (MBL, C1q) |
Fibrinogen (FG) domain-containing proteins, (FD); collagen domain-containing proteins, (CD).
In vertebrates, several humoral PRMs, including C1q, collectins and ficolins, contain ancestral collagen- and FG-domains. Thus, recognition of both ECM and microbial components is a recurrent theme in the humoral arm of the innate immune system.
6. Conclusions
Innate immunity is primarily involved in coordinating a series of events leading to tissue repair, through the action of its cellular and humoral arms. The cellular sensors initiate the tissue repair process, by activating inflammation and leukocyte recruitment, whereas the humoral innate immunity is involved in activating complement and opsonizing tissue debris, regulate macrophage activities and fibrocyte differentiation. Recent studies discussed here show that the long pentraxin PTX3, a fluid phase molecule essential in anti-microbial resistance, emerges as a non-redundant player in tissue damage. PTX3, by interacting with Plg and fibrin in acidified wound sites, promotes fibrinolysis by macrophages and MSCs, which is crucial in the repair process. The finding that PTX3 plays a non-redundant role in damaged tissue repair further strengthens the functional interplay between innate immunity and ECM molecules in anti-microbial defense as well as in tissue repair [88], [90]. Furthermore, these data suggest that recognition of microbial moieties and ECM components are evolutionarily linked and represent related ancestral properties of humoral innate immunity.
Acknowledgements
This work was supported by the European Commission (ERC-PHII), Ministero della Salute (Ricerca Finalizzata, RF-2013-02355470), Cluster Alisei (MEDINTECH CTN01_00177_962865), Italian Cystic Fibrosis Research Foundation, and AIRC − Italian Association for Cancer Research, Progetto 5x1000.
References
- 1.Bottazzi B., Doni A., Garlanda C., Mantovani A. An integrated view of humoral innate immunity: pentraxins as a paradigm. Annu. Rev. Immunol. 2010;28:157–183. doi: 10.1146/annurev-immunol-030409-101305. [DOI] [PubMed] [Google Scholar]
- 2.Mantovani A., Cassatella M.A., Costantini C., Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011;11:519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 3.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 4.Singer A.J., Clark R.A. Cutaneous wound healing. N. Engl. J. Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 5.Clark R.A. Fibrin and wound healing. Ann. N. Y. Acad. Sci. 2001;936:355–367. doi: 10.1111/j.1749-6632.2001.tb03522.x. [DOI] [PubMed] [Google Scholar]
- 6.Nurden A.T. Platelets, inflammation and tissue regeneration. Thromb. Haemost. 2011;105(Suppl. 1):S13–33. doi: 10.1160/THS10-11-0720. [DOI] [PubMed] [Google Scholar]
- 7.Theilgaard-Monch K., Knudsen S., Follin P., Borregaard N. The transcriptional activation program of human neutrophils in skin lesions supports their important role in wound healing. J. Immunol. 2004;172:7684–7693. doi: 10.4049/jimmunol.172.12.7684. [DOI] [PubMed] [Google Scholar]
- 8.Martin P., Leibovich S.J. Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol. 2005;15:599–607. doi: 10.1016/j.tcb.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Mantovani A., Biswas S.K., Galdiero M.R., Sica A., Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 2013;229:176–185. doi: 10.1002/path.4133. [DOI] [PubMed] [Google Scholar]
- 10.Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J. Pathol. 2003;200:500–503. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- 11.Adamson R. Role of macrophages in normal wound healing: an overview. J. Wound Care. 2009;18:349–351. doi: 10.12968/jowc.2009.18.8.43636. [DOI] [PubMed] [Google Scholar]
- 12.Duscher D., Barrera J., Wong V.W., Maan Z.N., Whittam A.J., Januszyk M., Gurtner G.C. Stem cells in wound healing: the future of regenerative medicine? A mini-review. Gerontology. 2016;62:216–225. doi: 10.1159/000381877. [DOI] [PubMed] [Google Scholar]
- 13.Nuschke A. Activity of mesenchymal stem cells in therapies for chronic skin wound healing. Organogenesis. 2014;10:29–37. doi: 10.4161/org.27405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krasnodembskaya A., Song Y., Fang X., Gupta N., Serikov V., Lee J.W., Matthay M.A. Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells. 2010;28:2229–2238. doi: 10.1002/stem.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aggarwal S., Pittenger M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 16.Singer N.G., Caplan A.I. Mesenchymal stem cells: mechanisms of inflammation. Annu. Rev. Pathol. 2011;6:457–478. doi: 10.1146/annurev-pathol-011110-130230. [DOI] [PubMed] [Google Scholar]
- 17.Sala E., Genua M., Petti L., Anselmo A., Arena V., Cibella J., Zanotti L., D'Alessio S., Scaldaferri F., Luca G., Arato I., Calafiore R., Sgambato A., Rutella S., Locati M., Danese S., Vetrano S. Mesenchymal stem cells reduce colitis in mice via release of TSG6, independently of their localization to the intestine. Gastroenterology. 2015;149(163–76):e20. doi: 10.1053/j.gastro.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 18.Uccelli A., Moretta L., Pistoia V. Immunoregulatory function of mesenchymal stem cells. Eur. J. Immunol. 2006;36:2566–2573. doi: 10.1002/eji.200636416. [DOI] [PubMed] [Google Scholar]
- 19.Beyth S., Borovsky Z., Mevorach D., Liebergall M., Gazit Z., Aslan H., Galun E., Rachmilewitz J. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005;105:2214–2219. doi: 10.1182/blood-2004-07-2921. [DOI] [PubMed] [Google Scholar]
- 20.Yang S.H., Park M.J., Yoon I.H., Kim S.Y., Hong S.H., Shin J.Y., Nam H.Y., Kim Y.H., Kim B., Park C.G. Soluble mediators from mesenchymal stem cells suppress T cell proliferation by inducing IL-10. Exp. Mol. Med. 2009;41:315–324. doi: 10.3858/emm.2009.41.5.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Q.Z., Su W.R., Shi S.H., Wilder-Smith P., Xiang A.P., Wong A., Nguyen A.L., Kwon C.W., Le A.D. Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells. 2010;28:1856–1868. doi: 10.1002/stem.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mao H.P.F., Guo H., Bu F., Xin T., Chen S., Guo Y. Feedback mechanisms between M2 macrophages and Th17 cells in colorectal cancer patients. Tumour Biol. 2016 doi: 10.1007/s13277-016-5085-z. (in press) [DOI] [PubMed] [Google Scholar]
- 23.Shohara R., Yamamoto A., Takikawa S., Iwase A., Hibi H., Kikkawa F., Ueda M. Mesenchymal stromal cells of human umbilical cord Wharton's jelly accelerate wound healing by paracrine mechanisms. Cytotherapy. 2012;14:1171–1181. doi: 10.3109/14653249.2012.706705. [DOI] [PubMed] [Google Scholar]
- 24.Ti D., Hao H., Tong C., Liu J., Dong L., Zheng J., Zhao Y., Liu H., Fu X., Han W. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J. Transl. Med. 2015;13:308. doi: 10.1186/s12967-015-0642-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cappuzzello C., Doni A., Dander E., Pasqualini F., Nebuloni M., Bottazzi B., Mantovani A., Biondi A., Garlanda C., D'Amico G. Mesenchymal stromal cell-derived PTX3 promotes wound healing via fibrin remodeling. J. Invest. Dermatol. 2016;136:293–300. doi: 10.1038/JID.2015.346. [DOI] [PubMed] [Google Scholar]
- 26.Rakoff-Nahoum S., Medzhitov R. Toll-like receptors and cancer. Nat. Rev. Cancer. 2009;9:57–63. doi: 10.1038/nrc2541. [DOI] [PubMed] [Google Scholar]
- 27.Pepys M.B., Hirschfield G.M. C-reactive protein: a critical update. J. Clin. Invest. 2003;111:1805–1812. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujita T. Evolution of the lectin-complement pathway and its role in innate immunity. Nat. Rev. Immunol. 2002;2:346–353. doi: 10.1038/nri800. [DOI] [PubMed] [Google Scholar]
- 29.Botto M., Hawkins P.N., Bickerstaff M.C., Herbert J., Bygrave A.E., McBride A., Hutchinson W.L., Tennent G.A., Walport M.J., Pepys M.B. Amyloid deposition is delayed in mice with targeted deletion of the serum amyloid P component gene. Nat. Med. 1997;3:855–859. doi: 10.1038/nm0897-855. [DOI] [PubMed] [Google Scholar]
- 30.Lu J., Marnell L.L., Marjon K.D., Mold C., Du Clos T.W., Sun P.D. Structural recognition and functional activation of FcgammaR by innate pentraxins. Nature. 2008;456:989–992. doi: 10.1038/nature07468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ricklin D., Hajishengallis G., Yang K., Lambris J.D. Complement: a key system for immune surveillance and homeostasis. Nat. Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oikonomopoulou K., Ricklin D., Ward P.A., Lambris J.D. Interactions between coagulation and complement-their role in inflammation. Semin. Immunopathol. 2012;34:151–165. doi: 10.1007/s00281-011-0280-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rafail S., Kourtzelis I., Foukas P.G., Markiewski M.M., DeAngelis R.A., Guariento M., Ricklin D., Grice E.A., Lambris J.D. Complement deficiency promotes cutaneous wound healing in mice. J. Immunol. 2015;194:1285–1291. doi: 10.4049/jimmunol.1402354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bossi F., Tripodo C., Rizzi L., Bulla R., Agostinis C., Guarnotta C., Munaut C., Baldassarre G., Papa G., Zorzet S., Ghebrehiwet B., Ling G.S., Botto M., Tedesco F. C1q as a unique player in angiogenesis with therapeutic implication in wound healing. Proc. Natl. Acad. Sci. U. S. A. 2014;111:4209–4214. doi: 10.1073/pnas.1311968111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Genster N., Takahashi M., Sekine H., Endo Y., Garred P., Fujita T. Lessons learned from mice deficient in lectin complement pathway molecules. Mol. Immunol. 2014;61:59–68. doi: 10.1016/j.molimm.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 36.Orsini F., Villa P., Parrella S., Zangari R., Zanier E.R., Gesuete R., Stravalaci M., Fumagalli S., Ottria R., Reina J.J., Paladini A., Micotti E., Ribeiro-Viana R., Rojo J., Pavlov V.I., Stahl G.L., Bernardi A., Gobbi M., De Simoni M.G. Targeting mannose-binding lectin confers long-lasting protection with a surprisingly wide therapeutic window in cerebral ischemia. Circulation. 2012;126:1484–1494. doi: 10.1161/CIRCULATIONAHA.112.103051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carlucci F., Cook H.T., Garg A., Pepys M.B., Botto M. Lack of effect of a single injection of human C-reactive protein on murine lupus or nephrotoxic nephritis. Arthritis Rheum. 2010;62:245–249. doi: 10.1002/art.27232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gill R., Kemp J.A., Sabin C., Pepys M.B. Human C-reactive protein increases cerebral infarct size after middle cerebral artery occlusion in adult rats. J. Cereb. Blood Flow Metab. 2004;24:1214–1218. doi: 10.1097/01.WCB.0000136517.61642.99. [DOI] [PubMed] [Google Scholar]
- 39.Pepys M.B., Hirschfield G.M., Tennent G.A., Gallimore J.R., Kahan M.C., Bellotti V., Hawkins P.N., Myers R.M., Smith M.D., Polara A., Cobb A.J., Ley S.V., Aquilina J.A., Robinson C.V., Sharif I., Gray G.A., Sabin C.A., Jenvey M.C., Kolstoe S.E., Thompson D., Wood S.P. Targeting C-reactive protein for the treatment of cardiovascular disease. Nature. 2006;440:1217–1221. doi: 10.1038/nature04672. [DOI] [PubMed] [Google Scholar]
- 40.Tennent G.A., Hutchinson W.L., Kahan M.C., Hirschfield G.M., Gallimore J.R., Lewin J., Sabin C.A., Dhillon A.P., Pepys M.B. Transgenic human CRP is not pro-atherogenic, pro-atherothrombotic or pro-inflammatory in apoE−/− mice. Atherosclerosis. 2008;196:248–255. doi: 10.1016/j.atherosclerosis.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 41.Cox N., Pilling D., Gomer R.H. Serum amyloid P: a systemic regulator of the innate immune response. J. Leukoc. Biol. 2014;96:739–743. doi: 10.1189/jlb.1MR0114-068R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Castano A.P., Lin S.L., Surowy T., Nowlin B.T., Turlapati S.A., Patel T., Singh A., Li S., Lupher M.L., Jr., Duffield J.S. Serum amyloid P inhibits fibrosis through Fc gamma R-dependent monocyte-macrophage regulation in vivo. Sci. Transl. Med. 2009;1:5ra13. doi: 10.1126/scitranslmed.3000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pilling D., Gomer R.H. Persistent lung inflammation and fibrosis in serum amyloid P component (APCs−/−) knockout mice. PLoS One. 2014;9:e93730. doi: 10.1371/journal.pone.0093730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dillingh M.R., van den Blink B., Moerland M., van Dongen M.G., Levi M., Kleinjan A., Wijsenbeek M.S., Lupher M.L., Jr., Harper D.M., Getsy J.A., Hoogsteden H.C., Burggraaf J. Recombinant human serum amyloid P in healthy volunteers and patients with pulmonary fibrosis. Pulm. Pharmacol. Ther. 2013;26:672–676. doi: 10.1016/j.pupt.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 45.Cox N., Pilling D., Gomer R.H. DC-SIGN activation mediates the differential effects of SAP and CRP on the innate immune system and inhibits fibrosis in mice. Proc. Natl. Acad. Sci. U. S. A. 2015;112:8385–8390. doi: 10.1073/pnas.1500956112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bodin K., Ellmerich S., Kahan M.C., Tennent G.A., Loesch A., Gilbertson J.A., Hutchinson W.L., Mangione P.P., Gallimore J.R., Millar D.J., Minogue S., Dhillon A.P., Taylor G.W., Bradwell A.R., Petrie A., Gillmore J.D., Bellotti V., Botto M., Hawkins P.N., Pepys M.B. Antibodies to human serum amyloid P component eliminate visceral amyloid deposits. Nature. 2010;468:93–97. doi: 10.1038/nature09494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Du Clos T.W., Mold C. Pentraxins (CRP, SAP) in the process of complement activation and clearance of apoptotic bodies through Fcgamma receptors. Curr. Opin. Organ Transplant. 2011;16:15–20. doi: 10.1097/MOT.0b013e32834253c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jaillon S., Peri G., Delneste Y., Fremaux I., Doni A., Moalli F., Garlanda C., Romani L., Gascan H., Bellocchio S., Bozza S., Cassatella M.A., Jeannin P., Mantovani A. The humoral pattern recognition receptor PTX3 is stored in neutrophil granules and localizes in extracellular traps. J. Exp. Med. 2007;204:793–804. doi: 10.1084/jem.20061301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salio M., Chimenti S., De Angelis N., Molla F., Maina V., Nebuloni M., Pasqualini F., Latini R., Garlanda C., Mantovani A. Cardioprotective function of the long pentraxin PTX3 in acute myocardial infarction. Circulation. 2008;117:1055–1064. doi: 10.1161/CIRCULATIONAHA.107.749234. [DOI] [PubMed] [Google Scholar]
- 50.Bonavita E., Gentile S., Rubino M., Maina V., Papait R., Kunderfranco P., Greco C., Feruglio F., Molgora M., Laface I., Tartari S., Doni A., Pasqualini F., Barbati E., Basso G., Galdiero M.R., Nebuloni M., Roncalli M., Colombo P., Laghi L., Lambris J.D., Jaillon S., Garlanda C., Mantovani A. PTX3 is an extrinsic oncosuppressor regulating complement-dependent inflammation in cancer. Cell. 2015;160:700–714. doi: 10.1016/j.cell.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 51.Doni A., Musso T., Morone D., Bastone A., Zambelli V., Sironi M., Castagnoli C., Cambieri I., Stravalaci M., Pasqualini F., Laface I., Valentino S., Tartari S., Ponzetta A., Maina V., Barbieri S.S., Tremoli E., Catapano A.L., Norata G.D., Bottazzi B., Garlanda C., Mantovani A. An acidic microenvironment sets the humoral pattern recognition molecule PTX3 in a tissue repair mode. J. Exp. Med. 2015;212:905–925. doi: 10.1084/jem.20141268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liangos O., Domhan S., Schwager C., Zeier M., Huber P.E., Addabbo F., Goligorsky M.S., Hlatky L., Jaber B.L., Abdollahi A. Whole blood transcriptomics in cardiac surgery identifies a gene regulatory network connecting ischemia reperfusion with systemic inflammation. PLoS One. 2010;5:e13658. doi: 10.1371/journal.pone.0013658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Magrini E., Mantovani A., Garlanda C. The dual complexity of PTX3 in health and disease: a balancing act. Trends Mol. Med. 2016;22:497–510. doi: 10.1016/j.molmed.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jaillon S., Moalli F., Ragnarsdottir B., Bonavita E., Puthia M., Riva F., Barbati E., Nebuloni M., Cvetko Krajinovic L., Markotic A., Valentino S., Doni A., Tartari S., Graziani G., Montanelli A., Delneste Y., Svanborg C., Garlanda C., Mantovani A. The humoral pattern recognition molecule PTX3 is a key component of innate immunity against urinary tract infection. Immunity. 2014;40:621–632. doi: 10.1016/j.immuni.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 55.Moalli F., Doni A., Deban L., Zelante T., Zagarella S., Bottazzi B., Romani L., Mantovani A., Garlanda C. Role of complement and Fc{gamma} receptors in the protective activity of the long pentraxin PTX3 against Aspergillus fumigatus. Blood. 2010;116:5170–5180. doi: 10.1182/blood-2009-12-258376. [DOI] [PubMed] [Google Scholar]
- 56.Cunha C., Aversa F., Lacerda J.F., Busca A., Kurzai O., Grube M., Loffler J., Maertens J.A., Bell A.S., Inforzato A., Barbati E., Almeida B., Santos e Sousa P., Barbui A., Potenza L., Caira M., Rodrigues F., Salvatori G., Pagano L., Luppi M., Mantovani A., Velardi A., Romani L., Carvalho A. Genetic PTX3 deficiency and aspergillosis in stem-cell transplantation. N. Engl. J. Med. 2014;370:421–432. doi: 10.1056/NEJMoa1211161. [DOI] [PubMed] [Google Scholar]
- 57.Wojtowicz A., Lecompte T.D., Bibert S., Manuel O., Rueger S., Berger C., Boggian K., Cusini A., Garzoni C., Hirsch H., Khanna N., Mueller N.J., Meylan P.R., Pascual M., van Delden C., Bochud P.Y. Swiss transplant cohort S. PTX3 polymorphisms and invasive mold infections after solid organ transplant. Clin. Infect. Dis. 2015;61:619–622. doi: 10.1093/cid/civ386. [DOI] [PubMed] [Google Scholar]
- 58.Lech M., Rommele C., Grobmayr R., Eka Susanti H., Kulkarni O.P., Wang S., Grone H.J., Uhl B., Reichel C., Krombach F., Garlanda C., Mantovani A., Anders H.J. Endogenous and exogenous pentraxin-3 limits postischemic acute and chronic kidney injury. Kidney Int. 2013;83:647–661. doi: 10.1038/ki.2012.463. [DOI] [PubMed] [Google Scholar]
- 59.Han B., Ma X., Zhang J., Zhang Y., Bai X., Hwang D.M., Keshavjee S., Levy G.A., McGilvray I., Liu M. Protective effects of long pentraxin PTX3 on lung injury in a severe acute respiratory syndrome model in mice. Lab. Invest. 2012;92:1285–1296. doi: 10.1038/labinvest.2012.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Norata G.D., Marchesi P., Pulakazhi Venu V.K., Pasqualini F., Anselmo A., Moalli F., Pizzitola I., Garlanda C., Mantovani A., Catapano A.L. Deficiency of the long pentraxin PTX3 promotes vascular inflammation and atherosclerosis. Circulation. 2009;120:699–708. doi: 10.1161/CIRCULATIONAHA.108.806547. [DOI] [PubMed] [Google Scholar]
- 61.Deban L., Russo R.C., Sironi M., Moalli F., Scanziani M., Zambelli V., Cuccovillo I., Bastone A., Gobbi M., Valentino S., Doni A., Garlanda C., Danese S., Salvatori G., Sassano M., Evangelista V., Rossi B., Zenaro E., Constantin G., Laudanna C., Bottazzi B., Mantovani A. Regulation of leukocyte recruitment by the long pentraxin PTX3. Nat. Immunol. 2010;11:328–334. doi: 10.1038/ni.1854. [DOI] [PubMed] [Google Scholar]
- 62.Doni A., Garlanda C., Bottazzi B., Meri S., Garred P., Mantovani A. Interactions of the humoral pattern recognition molecule PTX3 with the complement system. Immunobiology. 2012;217:1122–1128. doi: 10.1016/j.imbio.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 63.Ma Y.J., Doni A., Skjoedt M.O., Honore C., Arendrup M., Mantovani A., Garred P. Heterocomplexes of mannose-binding lectin and the pentraxins PTX3 or serum amyloid P component trigger cross-activation of the complement system. J. Biol. Chem. 2011;286:3405–3417. doi: 10.1074/jbc.M110.190637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ronca R., Giacomini A., Di Salle E., Coltrini D., Pagano K., Ragona L., Matarazzo S., Rezzola S., Maiolo D., Torrella R., Moroni E., Mazzieri R., Escobar G., Mor M., Colombo G., Presta M. Long-pentraxin 3 derivative as a small-Molecule FGF trap for cancer therapy. Cancer Cell. 2015;28:225–239. doi: 10.1016/j.ccell.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 65.Salustri A., Garlanda C., Hirsch E., De Acetis M., Maccagno A., Bottazzi B., Doni A., Bastone A., Mantovani G., Beck Peccoz P., Salvatori G., Mahoney D.J., Day A.J., Siracusa G., Romani L., Mantovani A. PTX3 plays a key role in the organization of the cumulus oophorus extracellular matrix and in vivo fertilization. Development. 2004;131:1577–1586. doi: 10.1242/dev.01056. [DOI] [PubMed] [Google Scholar]
- 66.Schneider L.A., Korber A., Grabbe S., Dissemond J. Influence of pH on wound-healing: a new perspective for wound-therapy. Arch. Dermatol. Res. 2007;298:413–420. doi: 10.1007/s00403-006-0713-x. [DOI] [PubMed] [Google Scholar]
- 67.Friedl P., Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat. Rev. Mol. Cell Biol. 2009;10:445–457. doi: 10.1038/nrm2720. [DOI] [PubMed] [Google Scholar]
- 68.Tomasek J.J., Gabbiani G., Hinz B., Chaponnier C., Brown R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 69.Wong V.W., Sorkin M., Glotzbach J.P., Longaker M.T., Gurtner G.C. Surgical approaches to create murine models of human wound healing. J. Biomed. Biotechnol. 2011;2011:969618. doi: 10.1155/2011/969618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bonacina F., Barbieri S.S., Cutuli L., Amadio P., Doni A., Sironi M., Tartari S., Mantovani A., Bottazzi B., Garlanda C., Tremoli E., Catapano A.L., Norata G.D. Vascular pentraxin 3 controls arterial thrombosis by targeting collagen and fibrinogen induced platelets aggregation. Biochim. Biophys. Acta. 2016;1862:1182–1190. doi: 10.1016/j.bbadis.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Idell S. Coagulation, fibrinolysis, and fibrin deposition in acute lung injury. Crit. Care Med. 2003;31:S213–20. doi: 10.1097/01.CCM.0000057846.21303.AB. [DOI] [PubMed] [Google Scholar]
- 72.Rodriguez-Grande B., Swana M., Nguyen L., Englezou P., Maysami S., Allan S.M., Rothwell N.J., Garlanda C., Denes A., Pinteaux E. The acute-phase protein PTX3 is an essential mediator of glial scar formation and resolution of brain edema after ischemic injury. J. Cereb. Blood Flow Metab. 2014;34:480–488. doi: 10.1038/jcbfm.2013.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.de Giorgio-Miller A., Bottoms S., Laurent G., Carmeliet P., Herrick S. Fibrin-induced skin fibrosis in mice deficient in tissue plasminogen activator. Am. J. Pathol. 2005;167:721–732. doi: 10.1016/S0002-9440(10)62046-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bugge T.H., Kombrinck K.W., Flick M.J., Daugherty C.C., Danton M.J., Degen J.L. Loss of fibrinogen rescues mice from the pleiotropic effects of plasminogen deficiency. Cell. 1996;87:709–719. doi: 10.1016/s0092-8674(00)81390-2. [DOI] [PubMed] [Google Scholar]
- 75.Ploplis V.A., Carmeliet P., Vazirzadeh S., Van Vlaenderen I., Moons L., Plow E.F., Collen D. Effects of disruption of the plasminogen gene on thrombosis, growth, and health in mice. Circulation. 1995;92:2585–2593. doi: 10.1161/01.cir.92.9.2585. [DOI] [PubMed] [Google Scholar]
- 76.Lund L.R., Green K.A., Stoop A.A., Ploug M., Almholt K., Lilla J., Nielsen B.S., Christensen I.J., Craik C.S., Werb Z., Dano K., Romer J. Plasminogen activation independent of uPA and tPA maintains wound healing in gene-deficient mice. EMBO J. 2006;25:2686–2697. doi: 10.1038/sj.emboj.7601173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fleetwood A.J., Achuthan A., Schultz H., Nansen A., Almholt K., Usher P., Hamilton J.A. Urokinase plasminogen activator is a central regulator of macrophage three-dimensional invasion, matrix degradation, and adhesion. J. Immunol. 2014;192:3540–3547. doi: 10.4049/jimmunol.1302864. [DOI] [PubMed] [Google Scholar]
- 78.Xiao Y., Yang N., Zhang Q., Wang Y., Yang S., Liu Z. Pentraxin 3 inhibits acute renal injury-induced interstitial fibrosis through suppression of IL-6/Stat3 pathway. Inflammation. 2014;37:1895–1901. doi: 10.1007/s10753-014-9921-2. [DOI] [PubMed] [Google Scholar]
- 79.Pilling D., Cox N., Vakil V., Verbeek J.S., Gomer R.H. The long pentraxin PTX3 promotes fibrocyte differentiation. PLoS One. 2015;10:e0119709. doi: 10.1371/journal.pone.0119709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hanington P.C., Zhang S.M. The primary role of fibrinogen-related proteins in invertebrates is defense, not coagulation. J. Innate Immun. 2011;3:17–27. doi: 10.1159/000321882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Loof T.G., Schmidt O., Herwald H., Theopold U. Coagulation systems of invertebrates and vertebrates and their roles in innate immunity: the same side of two coins. J. Innate Immun. 2011;3:34–40. doi: 10.1159/000321641. [DOI] [PubMed] [Google Scholar]
- 82.Endo Y., Nakazawa N., Iwaki D., Takahashi M., Matsushita M., Fujita T. Interactions of ficolin and mannose-binding lectin with fibrinogen/fibrin augment the lectin complement pathway. J. Innate Immun. 2010;2:33–42. doi: 10.1159/000227805. [DOI] [PubMed] [Google Scholar]
- 83.Groeneveld T.W., Oroszlan M., Owens R.T., Faber-Krol M.C., Bakker A.C., Arlaud G.J., McQuillan D.J., Kishore U., Daha M.R., Roos A. Interactions of the extracellular matrix proteoglycans decorin and biglycan with C1q and collectins. J. Immunol. 2005;175:4715–4723. doi: 10.4049/jimmunol.175.7.4715. [DOI] [PubMed] [Google Scholar]
- 84.Tseng J., Mortensen R.F. The effect of human C-reactive protein on the cell-attachment activity of fibronectin and laminin. Exp. Cell Res. 1989;180:303–313. doi: 10.1016/0014-4827(89)90059-1. [DOI] [PubMed] [Google Scholar]
- 85.Zahedi K. Characterization of the binding of serum amyloid P to type IV collagen. J. Biol. Chem. 1996;271:14897–14902. doi: 10.1074/jbc.271.25.14897. [DOI] [PubMed] [Google Scholar]
- 86.Zahedi K. Characterization of the binding of serum amyloid P to laminin. J. Biol. Chem. 1997;272:2143–2148. [PubMed] [Google Scholar]
- 87.Proctor R.A. Fibronectin: an enhancer of phagocyte function. Rev. Infect. Dis. 1987;9(Suppl 4):S412–9. doi: 10.1093/clinids/9.supplement_4.s412. [DOI] [PubMed] [Google Scholar]
- 88.He Y.W., Li H., Zhang J., Hsu C.L., Lin E., Zhang N., Guo J., Forbush K.A., Bevan M.J. The extracellular matrix protein mindin is a pattern-recognition molecule for microbial pathogens. Nat. Immunol. 2004;5:88–97. doi: 10.1038/ni1021. [DOI] [PubMed] [Google Scholar]
- 89.Schack L., Stapulionis R., Christensen B., Kofod-Olsen E., Skov Sorensen U.B., Vorup-Jensen T., Sorensen E.S., Hollsberg P. Osteopontin enhances phagocytosis through a novel osteopontin receptor, the alphaXbeta2 integrin. J. Immunol. 2009;182:6943–6950. doi: 10.4049/jimmunol.0900065. [DOI] [PubMed] [Google Scholar]
- 90.Gerold G., Abu Ajaj K., Bienert M., Laws H.J., Zychlinsky A., de Diego J.L. A Toll-like receptor 2-integrin beta3 complex senses bacterial lipopeptides via vitronectin. Nat. Immunol. 2008;9:761–768. doi: 10.1038/ni.1618. [DOI] [PubMed] [Google Scholar]
- 91.Dushay M.S. Insect hemolymph clotting. Cell. Mol. Life Sci. 2009;66:2643–2650. doi: 10.1007/s00018-009-0036-0. [DOI] [PMC free article] [PubMed] [Google Scholar]