Abstract
Prolactin (PRL) is an important hormone with many diverse functions. Although it is predominantly produced by lactrotrophs of the pituitary there are a number of other organs, cells, and tissues in which PRL is expressed and secreted. The impact of this extrapituitary PRL (ePRL) on localized metabolism and cellular functions is gaining widespread attention. In 1996, a comprehensive review on ePRL was published. However, since this time, there have been a number of advancements in ePRL research. This includes a greater understanding of the components of the control elements located within the superdistal promoter of the ePRL gene. Furthermore, several new sites of ePRL have been discovered, each under unique control by a range of transcription factors and elements. The functional role of ePRL at each of the expression sites also varies widely leading to gender and site bias. This review aims to provide an update to the research conducted on ePRL since the 1996 review. The focus is on new data concerning the sites of ePRL expression, its regulation, and its function within the organs in which it is expressed.
Prolactin (PRL) is a helix bundle protein hormone expressed as a single copy on chromosome 1 in humans and chromosome 17 in rats. The primary source of PRL production is the lactotrophs of the anterior pituitary gland. PRL is highly versatile, with multiple biological functions in different species that fall into the following general categories: reproduction, pregnancy and lactation, growth and development, metabolism, immune modulation, electrolyte transport, regulation of the integument, behavior, and carcinogenesis. Hence, PRL has been widely studied and has been the subject of numerous reviews, providing much information on the distribution, regulation, function, and clinical aspects of this hormone.
Transcription, translation, and activity of extrapituitary PRL (ePRL) have been identified in many peripheral tissues and organs, with several key functional and regulatory differences from pituitary PRL (pPRL). Primary among those is an additional 150 bp in the ePRL transcript due to the presence of a 5′-noncoding exon (1a) (1) located 5.8 kb upstream of the ePRL start site, which is driven by a superdistal promoter (2). Thus, the regulation of ePRL is dissimilar to that of pPRL and is typically cell- or tissue specific. It should be emphasized, however, that the pPRL and ePRL proteins appear to be identical in terms of their primary, secondary, or tertiary structure, and both bind to the same receptor.
Unlike pPRL, expression of PRL in extrapituitary sites has not been extensively studied, with the last comprehensive review published in 1996 (3). Since this time, major advancements in ePRL research have occurred, and additional extrapituitary sites that produce and secrete PRL have been identified. In addition, there has been major progress in understanding the mechanism of ePRL regulation. The objectives of this review are to: 1) highlight the latest information with respect to the previous review; 2) provide an update on newly discovered organs, tissues, and cells that produce PRL; and 3) evaluate new regulatory elements.
Since the publication of the 1996 review (3), several excellent reviews have been published on ePRL in a variety of malignancies and other diseases. Thus, the reader is referred to these as follows: breast and mammary cancer (4–8), prostate cancer (5, 9, 10), autoimmune diseases (11, 12), skin and hair pathologies (13), and metabolic dysregulation and obesity (5, 14).
PRL Production by the Human Decidua
Most recent reports on the expression and regulation of ePRL focused on decidual PRL (dPRL). In mammals, the endometrial lining of the uterus becomes transformed into a secretory lining to enable embryo implantation. Following implantation, the lining further develops to form the decidua, which interacts with the placenta and participates in the exchange of nutrients, gas, and waste. PRL expression by decidual cells has long been recognized as a definitive marker for the onset of decidualization. Both the distribution and expression of PRL in the decidua exhibit spatiotemporal changes throughout the course of a pregnancy. Using labeled probes, Tanaka et al (15) observed more intense hybridization signals in tissues from early pregnancy than from term pregnancy. In early pregnancy, labeled cells in the decidual capsularis were more abundant near the amniotic cavity, whereas at term, they were concentrated closer to the maternal surface of the fetal membrane. In the decidua parietalis, almost all the cells were labeled, but no specific labeling was found in endometrial glands or capillary endothelium at either time point. In the decidua basalis, most decidual cells showed dPRL expression, whereas no hybridization was seen over trophoblast cells.
In terms of functions during pregnancy, dPRL has been shown to play an important role in repressing the expression of IL-6 and 20α-hydroxysteroid dehydrogenase (20α-HSD) at the level of transcription. The enzyme 20α-HSD catabolizes progesterone to its inactive form and is key for decreasing progesterone levels prior to parturition. However, during pregnancy, it is crucial that 20α-HSD remain silent both in the ovary (the source of progesterone) and the decidua (the major site of progesterone action), indicating the role of dPRL in these tissues during this period (16). Other postulated functions include autocrine activity to limit the extent of differentiation during decidualization (17). In all of the above functions, dPRL likely acts by binding to the PRL receptor (PRL-R), which is highly expressed in the decidua (18) as well as in the amnion and chorion (19). The increased expression of the PRL-R during labor and delivery in these compartments further supports an autocrine/paracrine role for dPRL in the peripartum.
Control of dPRL expression
Expression of dPRL is controlled by many cytokines, transcription factors, and signaling peptides that act either via well-defined regulatory pathways or by binding directly to putative control elements within the dPRL promoter regions (Figure 1). The cAMP-signaling cascade is one of the primary pathways that controls PRL release during in vitro decidualization (20). In endometrial stromal cells (ESCs), cAMP activates the dPRL promoter in 2 sequential steps: an initial weak induction within 12 hours, followed by a more pronounced induction at a later time (21). Deletion studies within the dPRL promoter identified a 332-bp segment (−332/0) sufficient for mediating full inducibility by 8-bromo-cAMP (8-Br-CAMP; a cAMP homolog) within 72 hours, indicating the presence of cAMP response elements (CREs). Mutation of this CRE abolished the early, but not the delayed, response. Additionally, the secondary induction was not seen using a control construct driven by CRE linked to a minimal promoter. Furthermore, dPRL is not expressed when transfected into a uterine cell line that does not express the endogenous dPRL gene (21). Collectively, these studies suggest that cAMP activation of the dPRL promoter is cell specific and indirect and is predominantly mediated by sequences outside the CRE. Subsequent studies found that the protein kinase A pathway induced the dPRL promoter in a delayed fashion via a −332/−270 region that contains 2 overlapping consensus binding sequences (−301/− 270 and −311/−291) for CCAAT/enhancer-binding proteins (C/EBP) (Figure 1b) (21, 22).
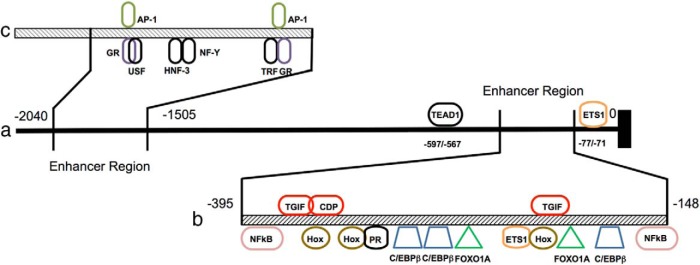
Figure 1.
Control elements within the superdistal promoter of the dPRL gene. The entire promoter length is more than 2 kb (panel a) and contains 2 enhancer regions. One of the regions (panel b) ranges from −148 bp to −395 from the transcriptional start site and contains several activator (bottom, various colors) and repressor (top, in red) binding sites (adapted from Lynch et al (33)). A second enhancer region (panel c) ranges from −1505 to −2040. GR, glucocorticoid receptor; HNF-3, hepatocyte nuclear factor 3; NF-Y, nuclear factor Y; USF, upstream stimulatory factor.
Using several decidual cell lines (stromal cells, decidual fibroblasts, and N5 endometrial cells), a 536-bp enhancer region within the superdistal promoter was identified at −2040/−1505 (Figure 1c) (23). Footprint analysis identified 3 protected regions (FP1–FP3), with both FP1 and FP3 containing a putative activator protein-1 (AP-1)-binding site. The transcription factors JunD and Fos-related antigen-2 (Fra-2) bind to these sites and activate transcription in decidual, but not in nondecidual, cell types. Mutations to either site reduced enhancer activity by 50%, whereas mutations in both sites reduced enhancer activity to near zero (23). Further analyses demonstrated the presence of a potential 35-bp activation element adjacent to FP1 at −1640/−1605, without which enhancer activity is eliminated (23).
The transcription factor Ets-1 is critical for basal expression of dPRL. Six Ets-1 sequence motifs are found within the proximal 1.5 kb of the dPRL promoter. However, as judged by mutational analysis, only one motif, located at −77/−71, is considered essential for basal gene expression (Figure 1a). Although Ets-1 induces PRL gene expression in decidualized cells, it is not sufficient to induce it in nondecidualized endometrial, stromal or fibroblast cells (24). Notably, direct physical associations between Ets and AP-1 transcription factors play an important role in regulating the expression of a number of mammalian genes (25). However, the closest AP-1-binding site is located more than 500 bp upstream, although it may come to close proximity by DNA looping.
Additional basal expression elements include a putative transcriptional enhancer activator domain (TEAD)-binding site located at −597/−567 (Figure 1a). Although TEAD family member 1 (TEAD1), the TEAD-binding protein, bound to the TEAD site and inhibited PRL expression, this may not be the mechanism of its inhibition, because absence of the TEAD-binding site failed to ameliorate the inhibitory effect of TEAD1, suggesting interaction with other transcriptional factors and quenching their activities (26). Other regulators include the transcription factor Nur77. Induction of Nur77 overexpression by a combination of 8-Br-cAMP and medroxyprogesterone acetate in human ESCs leads to a 14-fold increase in dPRL expression. In reporter systems containing the aforementioned −332/0 promoter element, Nur77 increased basal promoter activity more than 4-fold in a dose-dependent manner. Conversely, knockdown of Nur77 decreased dPRL expression following 8-Br-cAMP induction (27).
Additional cytokines, transcription factors, and inhibitors
The nitric oxide-cGMP pathway also affects dPRL, as supported by the ability of l-arginine, a nitric oxide precursor, and 8-bromo-cGMP, a cGMP homolog, to stimulate dPRL secretion (28). Antagonists for cAMP-mediated dPRL expression in primary ESC cultures include interferon-γ (IFNγ), which may act by inhibiting dPRL promoter activity. Although the mechanism of IFN inhibition is not fully understood, it may act via the signal transducer and activator of transcription-1 (Stat-1) pathway. However, the evidence for that is indirect, because IFNγ stimulates Stat-1 expression, phosphorylation, and translocation to the nucleus, and exogenously expressed Stat-1 represses dPRL promoter activation (29). Other cytokines that inhibit dPRL synthesis and release include IL-2, acting in an opposite effect to that seen for pPRL (30). Another antagonist is human chorionic gonadotropin (hCG), which is produced by the fertilized egg after conception no later than day 7 in the blastocyst stage. In cultured hESCs, high doses of recombinant hCG reduce dPRL protein and mRNA expression by more than 50% (31). Because both PRL and hCG appear to have direct effects on implantation, the purpose of PRL inhibition by hCG is not fully understood.
Regulation of dPRL by other factors is more complex. Forkhead box protein O1 (FOXO1) enhances protein kinase A-dependent activation of the dPRL promoter. Transcriptional augmentation by FOXO1 is affected through cooperation with C/EBPß and binding to a composite FKHR-C/EBPß response unit in the proximal promoter region (32). FOXO1 also augments the activity of a related protein, homeobox protein A-11 (HoxA-11). On its own, HoxA-11 does not affect PRL expression in undifferentiated hESCs, whereas its overexpression in differentiated hESCs leads to a marked increase in PRL expression (33). This indicates the necessity of a hormone-inducible cofactor for activation of dPRL. Reporter gene experiments showed that FOXO1A acts cooperatively with HoxA-11 to up-regulate expression through the dPRL enhancer and that HoxA-11 alone represses reporter gene activity. This suggests that its association with FOXO1A switches HoxA-11 from a repressor to an activator (33). This notion is supported by the fact that their respective binding sites lie adjacent to one another on the dPRL enhancer unit (Figure 1b).
Several studies appear to be contradictory, however, and require further clarification. For example, Yasui et al (34) found that the highly conserved PRL-releasing peptide (PrRP) was expressed in the decidua. PrRP consists of 2 distinct isoforms derived from the same preproprotein, a 20-amino acid isoform, as a C-terminal cleavage product of a larger 31-amino acid peptide (named PrRP20 and PrRP31, respectively) with downstream effects being modulated following binding to its receptor. The release of PRL by PrRP is dependent on the differential activation of both ERK and Jun N-terminal kinase, with both cascades being necessary to activate the PRL promoter in an Ets transcription factor-dependent mechanism (35). When PrRP was added to human stromal cells, PRL release did not increase, presumably because of the absence of PrRP receptors in the decidua (34). A later study by Reis et al (36) also detected PrRP in the first trimester of human decidua within both epithelial and stromal cells. Unlike Yasui et al, they showed coexpression of PrRP receptor in cultured cells and found that exposure to endogenously expressed PrRP increased dPRL levels.
PRL Expression in Mammary Tissue
The PRL protein has been found within mammary tissue and in milk. Sequestration from the circulation was initially assumed to be the only source of PRL in this tissue. However, later studies found that ePRL is expressed by mammary tissue from several species (3). In humans, ePRL mRNA is expressed at very low levels in normal and neoplastic breast tissue and in several mammary cell lines (37). De novo production of ePRL protein in both the stromal and glandular compartments of normal human breast tissue was confirmed by detecting metabolically labeled ePRL (ie, via incorporation of [35S]methionine and cysteine into PRL) in tissue extracts and conditioned media (38). Concentrations were measured using the Nb2 bioassay, which is based on a linear relationship increase in cell proliferation in response to PRL. Expression was driven by the superdistal promoter (37), and a time-dependent rise in ePRL release suggested removal of inhibitory control (38).
Regulation
Recent studies reported that the phosphatidylinositol 3-kinase − Akt pathway regulates production of autocrine PRL in the mouse mammary gland (39). Phosphatase and tensin analog catalyzes the dephosphorylation of phosphatidylinositol (3,4,5)-trisphosphate, resulting in the generation of phosphatidylinositol diphosphate and inhibition of the Akt-signaling pathway. Activation of the Akt pathway in the mammary epithelium of virgin mice by Akt1 overexpression or phosphatase and tensin deletion rapidly induced the production of autocrine PRL (39). Additionally, Akt-induced up-regulation occurs at the posttranscriptional level, suggesting yet another mechanism for ePRL protein expression (39). In addition to initiating lactation, autocrine ePRL production in mammary epithelial cells serves to induce terminal differentiation during late pregnancy. These processes are independent of ePRL production in mammary stromal cells or other tissues and cannot be compensated for by systemic pPRL (39).
Ovarian PRL
Several studies reported expression of ePRL at both the mRNA and protein levels in ovarian tissue from several species. In mouse ovaries, PRL mRNA levels showed a gradual decrease from day 10 to day 40, with lower levels maintained until day 80 (40). An age-related decline in ovarian PRL expression was also observed in humans, with both PRL mRNA and protein levels 4- to 5-fold lower in ovaries from postmenopausal than premenopausal women (41). PRL gene expression was detected in human ovarian follicles retrieved during in vitro fertilization (42), and immunoreactive PRL concentration was significantly higher in follicular fluid than in plasma. However, follicular PRL could come from 2 sources: local synthesis and release from mature follicles, as well as uptake and concentration from the circulation (43). In addition to the follicles, corpora lutea also express PRL, and most of the research was performed using bovine tissues (44, 45). It remains to be determined how follicular and/or luteal PRL expression and release is regulated. In addition, the relative roles of endocrine vs paracrine/autocrine PRL in the control of steroidogenesis, follicular maturation, ovulation, and luteal function await further investigation.
PRL in Male Reproductive Organs
Increasing evidence demonstrates the presence of ePRL, as well as its receptors, in male reproductive organs. ePRL mRNA was detected in the dorsal and lateral prostate of rats using Northern blotting and in situ hybridization. Immunostaining localized the protein to secretory granules of the apical cytoplasm of epithelial cells. Expression within the prostate was activated by testosterone, with castrated rats producing no ePRL whereas treatment with exogenous testosterone restored its production. This was confirmed in vitro using cultured prostatic cells, which express ePRL only in the presence of testosterone (46). PRL was detected by RT-PCR in both secretory epithelial (47) and smooth muscle cells (48) of the human prostate, which was supported by detecting the PRL protein by immunostaining. Unidentified seminal fluid factors induced PRL gene expression and release from cultured human prostatic smooth muscle cells, but, unlike the situation in the rat, PRL release was not affected by any sex steroid hormone tested (48). It remains to be determined whether autocrine PRL plays a significant role in the growth, differentiation, and secretory activity of the prostate gland.
RT-PCR revealed expression of ePRL mRNA in mouse testes (40, 49). Like other extrapituitary sites, the level of expression was low compared with the pituitary, with expression detected in both Leydig and germ cells (49). However, both the localization and size of ePRL protein were different from those of the mRNA. The PRL protein in the testis and spermatozoa is cleaved, with the variants localized in the Golgi apparatus of spermatids and in spermatozoa tails. Western blots, using N-terminal PRL antibodies, show 16-kDa and 17-kDa proteins in the testis and 16-kDa and 18-kDa proteins in sperm; full-length ePRL was absent in both testis and sperm. It has been postulated that the cleaved PRL variants play a role in spermiogenesis and spermatogenesis and that their signal transduction and secretory mechanisms differ from those of intact ePRL (49). Similarly cleaved PRL fragments have been found in endothelial cells (discussed later in this review), raising the possibility that they also play a role in inhibiting endothelial cell proliferation.
Regulation
Currently, there are no data to support involvement of the superdistal promoter in the control of testicular ePRL expression. As stated earlier, PRL in rat prostate is affected by testosterone, but expressional control in the testis is less clear. Pit-1 is a known transcription factor of pPRL, but it also regulates the expression of GH, GH-releasing hormone receptor, TSHß, as well as its own Pit-1 gene (reviewed by Kerr et al (50)). Notably, Pit-1 also exists as several splice variants, which possess specific transactivation properties. One such variant, Pit-1w, which lacks exon 1, activates the ePRL promoter in mouse spermatocytes and spermatids but not the promoters of GH and TSHß (51). Although PRL exerts prosurvival actions on human sperm via the PRL receptors, which are localized to several regions along the spermatozoa and are linked to phosphatidylinositol 3-kinase /Akt pathway (52), there are no data on potential role of autocrine vs circulating PRL.
PRL in Endothelial Cells
The role and expression of ePRL in endothelial cells are somewhat convoluted. It had been known early on that a naturally occurring 16-kDa cleavage product of PRL existed in the pituitary (53). At this time it was not known what the exact functions (if any) of this fragment was. Later it was found that this 16-kDa fragment possessed binding sites on endothelial cells (54) and was a potent inhibitor of angiogenesis (55). In addition, it was found to induce apoptosis of endothelial cells through caspase activation (56).
Localized ePRL expression was subsequently found in vascular endothelial cells (57). Using bovine brain capillary endothelial cells, several differences to pPRL were found. In addition to full-length PRL transcript, a second transcript lacking the third exon of the gene was isolated. In addition, 3 ePRL immunoreactive proteins were found corresponding to molecular masses of 23, 21, and 14 kDa. A similar result was found in human umbilical vein endothelial cells (58) and rat retinal capillary endothelial cells (59). Both demonstrated multiple ePRL mRNA fragment lengths in addition to protein sizes relating to the full-sized ePRL of 23 kDa plus a number of other fragments ranging in size from 60 kDa to 14 kDa, which also included the 16-kDa cleavage product. The smaller fragments were later found to be N-terminal products of full-length PRL, created through peptide cleavage by either thrombin (60), matrix metalloproteases (61), cathepsin-D (62, 63), or bone morphogenetic protein 1 (64), depending on the tissue of origin. Similar to the pPRL cleaved products, these fragments were found to be biologically active with potent antiangiogenic properties and were subsequently renamed “vasoinhibins” (61, 65). Although they are transcribed by the same gene as PRL and are translated to produce a full-length product, they undergo posttranslational modifications and are now considered as separate entities to the parent PRL molecule and possess unique functions.
PRL in the Immune System
Pituitary PRL had been known to play a role in immunoregulation, and ePRL had been detected in isolated lymphocytes. Initially, the distribution of ePRL was largely unknown, but subsequent studies, using RT-PCR and in situ hybridization, have shown that it is expressed in all tissues of the human immune system: thymus, spleen, tonsil, and lymph nodes, as well as thymomas and lymphomas (66). PRL mRNA-expressing cells in the normal human thymus, tonsil, and lymph nodes were localized in the epithelium within the septa, in the subcapsular cortex, and in endothelium of blood vessels. In the spleen, ePRL mRNA was found in the white pulp, marginal zone, septum, and capsular areas. Granulocytes also express ePRL transcripts, yielding a high-molecular weight immunoreactive ePRL-like protein of 43 kDa, but the normal sized 23-kDa ePRL protein was absent (67). PRL protein and PRL-R mRNA were detected within intraepithelial lymphocytes of rats tested from 0–150 days of age, suggesting that these cells may be the source of intestinal ePRL (68). The importance of autocrine ePRL expression in the activation of T lymphocytes has been revealed by knockdown studies. Using the Nb2 PRL bioassay, it was shown that conditioned media obtained from Jurkat cells that had their PRL-R silenced through RNA interference resulted in an increase in cell proliferation compared with normal cells. This suggested that knockdown of PRL-R up-regulated the secretion of autocrine PRL in Jurkat cells. Conversely, the proliferation of the knockdown Jurkat cells was dramatically reduced and could not be reversed by the addition of hPRL. Furthermore the PRL-R(−) cells demonstrated reduced expression of phytohemagglutinin (PHA)-induced CD137 and CD154 and reduced secretion of PHA-induced IL-2 and IL-4 in addition to a reduction of proliferation. This suggests that autocrine PRL regulates these processes (69).
Regulation
The kinetics of promoter activation in lymphoid cells differs from that seen in ESCs. Reporter gene studies show that activation by 8-Br-cAMP occurs rapidly, peaking within 6 hours and then decreasing, with some reporter gene activity still seen at 24 hours (70). The induction by 8-Br-cAMP can be synergized by PHA or PHA together with phorbol myristate acetate in combination, but not by phorbol myristate acetate alone. Furthermore, this activation is dependent, in part, on an intact CRE motif and on binding of CRE-binding protein or a CRE-binding protein family member (70).
Calcitriol (the hormonal form of vitamin D) also regulates ePRL in peripheral blood mononuclear cells (PBMNCs). In resting PBMNCs, ePRL concentrations are significantly increased in its presence. However, PHA-activated PBMNCs secrete more ePRL than resting cells under basal conditions, whereas upon calcitriol stimulation, ePRL protein levels decrease in PHA-activated cell cultures (71).
During infection, ePRL mRNA expression in monocytes shows a delayed activation while exhibiting the presence of ePRL protein. This is suggestive of the presence of intracellular ePRL storage and is in contrast to most other reports that could not identify storage vesicles. Immunohistochemistry staining of PBMNCs revealed that intracellular ePRL is primarily localized in close vicinity to the nucleus. In addition, approximately 25% of the cells possessed vesicle-like deposits in the cytoplasm (72).
Brain PRL
Immunoreactive PRL has been detected in many regions of the brain, but its origin has been controversial. There is extensive evidence for its transport from the circulation via the choroid plexus or from retrograde blood flow from the pituitary, but also some early data on its de novo synthesis and release in vitro (reviewed by Ben-Jonathan et al (3)). More recently, immunoreactive PRL was localized to the paraventricular nuclei (PVN) and supraoptic nuclei of rats (73), exhibiting higher expression during estrus, when circulating estrogen levels are elevated (74). A large percentage of PRL-immunoreactive neurons in the PVN express estrogen receptor β (ERβ), supporting a direct effect of estrogen on PRL production (75). Expression of PRL in fetal and adult sheep brains was demonstrated by Western blots and RT-PCR, with in situ hybridization revealing wide distribution of PRL expression in the medial preoptic area, periventricular preoptic nucleus, bed nucleus of the stria terminalis, and the ventral PVN (76). A wide extrahypothalamic distribution of ePRL within the brain of the domestic turkey was recently reported, including the cerebellum, nucleus accumbens, and lateral septum (77).
In addition to neurons, capillary endothelial cells from bovine brain express the full-length PRL mRNA as well as a shorter transcript, which lacks the third exon of the gene (57). In cultures, the aforementioned endothelial cells synthesize and secrete PRL-like immunoreactive proteins with apparent molecular masses of 23, 21, and 14 kDa. The PRL-like nature of these proteins is supported by induction of proliferation of Nb2-cells, a PRL-responsive cell line, upon coculture with brain endothelial cells, and this stimulation was neutralized with antibodies against PRL. Expression and production of PRL by human brain endothelial cells have recently been reported (78).
Function and regulation
In a 2002 review of the involvement of brain PRL in stress responses during the peripartum period, various regulatory and functional roles were discussed (79). Briefly, brain ePRL may act as an antistress modulator during lactation. This occurs by activation of the hypothalamic-pituitary-adrenal axis by brain ePRL, and the suppression of the responsiveness to oxytocin secretion during peripartum. Other mechanisms include alterations in stressor perception, reduction in activation by brainstem excitatory inputs to the hypothalamic CRH/vasopressin cells (the main ACTH secretagogues), reduced synthetic activity of CRH and vasopressin neurons, and alterations at the level of the pituitary corticotrophs. The overall effects are a decrease in anxiety and a reduction in behavioral responses to stress. The evolutionary advantage is to reduce the mother's fearfulness and neophobia to the pups during lactation, while increasing aggressive behavior to protect the offspring (80).
It has also been suggested that brain ePRL acts as a local neurotransmitter/neuromodulator (76, 81). This was demonstrated by submitting mice to various pharmacologic, physiological, or stressful stimuli, which cause an in vivo release of ePRL from neuronal structures within the PVN and medial preoptic area (81). Brain PRL may also act as a mitogen to increase the proliferation of astrocytes, and as a secretagogue, which induces expression of inflammatory cytokines such as TNF-α (82). Autocrine PRL has also been suggested to participate in the regulation of brain endothelial cell migration, invasion, and tube formation (78).
Very little is known in terms of ePRL's regulation in the brain. One study has shown that nitric oxide, which is part of a molecular network that regulates rapid eye movement during sleep, inhibits brainstem ePRL mRNA expression. However, the functional role of this is not fully understood (83). Another study found that constitutively active STAT5A, a downstream signaling transcription factor of the PRL-R, stimulates PRL release from brain endothelial cells, indicating the existence of a positive loop between STAT5 and PRL that promotes angiogenesis (78). Similar to its action on pPRL, estrogen increases ePRL in the brain (74), presumably via ERβ, which is expressed in PRL-expressing hypothalamic neurons (75), but the exact signaling pathway that mediates this effect remains to be determined.
PRL in Hair Follicles and Skin
One of the most recently discovered sites of ePRL expression in nonclassical organs is that of hair follicles and skin. As reviewed by Foitzik et al (84), systemic pPRL had long been known to play a critical role in hair cycle regulation in animals that exhibit seasonally-dependent cycles of pelage replacement such as sheep and mink, but also in those with seasonally-independent hair cycles such as mice and man (Figure 2). Recently, both PRL and its receptor have been located in inner root sheath and outer root sheath (ORS) keratinocytes of anagen and catagen hair follicles of mice (85). It was later found that follicular PRL regulates the timing of hair growth cycles in mice via a direct effect on the skin, being depressed during telogen and increasing until late anagen (86). Additional putative functions of ePRL include stimulation of keratinocyte proliferation, and modulation of cytokine/chemokine production in the keratinocytes (87).
Figure 2.
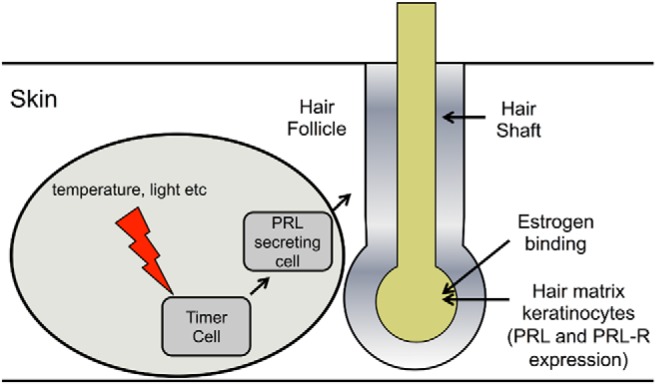
Activation and control of hair follicle PRL. Two mechanisms exist depending on the animal. For seasonal control (circled) timer cells stimulate PRL-secretory cells that effect the growth phases of the hair follicle. A second mechanism is dependent of the phase of the hair growth cycle. PRL and its receptor are expressed in hair matrix keratinocytes during different phases of the hair growth cycle. PRL inhibits hair-shaft elongation and hair-bulb keratinocyte proliferation. Additionally, it stimulates premature catagen development and increased hair-bulb keratinocyte apoptosis through autocrine/paracrine activity.
In humans, PRL transcripts and protein have been detected in both scalp skin and microdissected human hair follicles, in which ePRL protein was localized in a thin layer of keratinocytes between the inner and outer root sheath (88). However, whereas there was initially no evidence for the production of ePRL in corporal skin (89), it was postulated that extrinsic and/or intrinsic regulators of ePRL production stimulate its expression in dermal fibroblasts and skin immune cells, albeit in a gender- and/or site-dependent manner (13). Indeed, both mouse embryonic fibroblasts (64) and human dermal fibroblasts (90) cultured in vitro produce a 23-kDa PRL protein, which can be processed to cleaved products. Furthermore, detection of a larger transcript of PRL mRNA in human fibroblasts is consistent with the presence of the 5′-noncoding exon 1a, which is driven by the superdistal promoter.
Regulation
The 2 key hormones, estrogen and TRH, that control pPRL secretion also regulate ePRL and PRL-R expression in human scalp skin and hair follicles in vitro. Estrogen increases the expression of ePRL and PRL-R proteins in the epidermis, eccrine sweat glands and hair follicle ORS, corresponding to increased ePRL and PRL-R mRNA expression in ORS keratinocytes (87). The effect of TRH is more complex. In cultured human hair follicles, TRH increases ePRL mRNA and protein expression similar to estrogen. However, PRL-R expression at the protein level is decreased whereas PRL-R mRNA at the gene level is increased (87).
PRL in Adipose Tissue
As reviewed in Brandebourg et al (14), PRL production in human adipose tissue was serendipitously discovered upon studying PRL release by human breast explants. Intended for use as a negative control, breast adipose tissue was found to release 10–15 times more PRL than did glandular tissue (38). PRL release from glandular tissue was inhibited by progesterone, but neither estrogen nor progesterone affected PRL release from adipose tissue, indicating a dissimilar regulation in the 2 adjacent compartments. These unexpected findings raised several questions: 1) which cells within adipose tissue synthesize PRL and how is it regulated? 2) is PRL synthesized in other adipose depots and if so, what are the effects of obesity? and 3) what are the functions of local PRL?
ePRL was found to be produced de novo by breast, sc and visceral adipose depots. As illustrated in Figure 3, PRL release from both visceral and sc adipose explants in vitro increases in a time-dependent manner, indicating removal from inhibition (38, 91). Notably, PRL release from sc adipose explants from morbidly obese patients was significantly lower than that from lean patients, with no apparent difference between men and women (91). A relevant question is how much adipose PRL is released into the circulation? As human PRL binds to heparin (92), most of the released PRL is likely retained by proteoglycans near the producing cells, making it a true autocrine/paracrine factor. However, a study by Kok et al (93) reported that both basal and pulsatile PRL release are elevated in premenopausal women with visceral obesity compared with matched lean controls. These data suggest that adipose tissue is the source of some of the excess serum PRL levels in obesity.
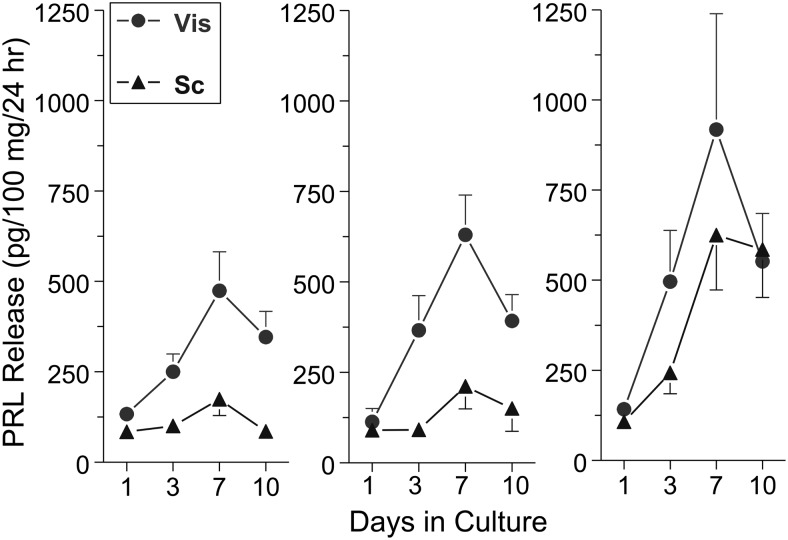
Figure 3.
The profile of PRL release from incubated visceral (Vis) and sc human adipose explants, as determined by the Nb2 bioassay. Left panel, obese women (BMI 48 ± 2; n= 22); middle panel, obese men (BMI 50 ± 1; n = 13); right panel, nonobese men and women (BMI 26 ± 1; n = 15). Note the dissimilar secretory profile of PRL by the 2 fat depots, the effects of obesity, and the progressive rise in PRL, suggesting removal from an inhibitory control (modified from Hugo et al (91)). BMI, body mass index.
Regulation
During differentiation of isolated breast preadipocytes, PRL expression was transiently increased during early adipogenesis (94). Both isoproterenol, a β-adrenergic receptor agonist, and pituitary adenylate cyclase activating peptide, increased PRL expression in the preadipocytes. This stimulation was suppressed by several protein kinase inhibitors, suggesting involvement of multiple signaling pathways. Transfection of the preadipocytes with a superdistal PRL promoter/luciferase reporter revealed 2 stimulatory domains and an inhibitory domain. These data establish the transcriptional regulation of adipocyte PRL by the superdistal PRL promoter, its transient expression during adipogenesis, and the stimulatory effect of catecholamines and pituitary adenylate cyclase activating peptide. In addition to altering expression, the cAMP-activating compounds also affected the release of locally produced PRL, as determined using the sensitive Nb2 bioassay for PRL.
The subsequent cloning of a novel human adipocyte cell line, named LS14, from a patient with liposarcoma (95), makes these cells an attractive model with which to study adipose PRL. These cells exhibit many properties of primary preadipocytes, including the ability to undergo terminal differentiation and expression of key adipocyte-specific genes. Similar to primary adipocytes, LS14 cells produce and respond to PRL. PRL production, both at the mRNA and protein levels, increases markedly during early differentiation, concomitant with down-regulation of the PRL-R.
PRL in the Cochlea
The most recent of ePRL discoveries is that of expression in the cochlea. Two separate microarray studies on mouse cochlea demonstrated a differential up-regulation of ePRL mRNA between postnatal mice and adult/aged mice (96, 97). Immunohistochemistry revealed the presence of ePRL protein within marginal cells of the stria vascularis and within cells of the spiral ganglion (96). Subsequent research also revealed that cochlea ePRL expression demonstrated a gender and age bias, in that it was expressed in female mice from 6–12 months but absent in male mice of the same age (98). In addition, ePRL expression in the cochlea also correlated to a loss of bone mineral density of the otic capsule and a loss of hearing (98), indicating that its presence may be related to an autocrine/paracrine disease pathology. This hypothesis is reinforced by the fact that PRL-R is located in most cells and organs of the cochlea (96) and is supported by studies of systemically increased levels of PRL, which show degeneration of cochlea bone and loss of hearing (99). However, the mechanism of cochlea PRL regulation or how it affects the surrounding cells and tissue is yet to be determined.
Conclusions
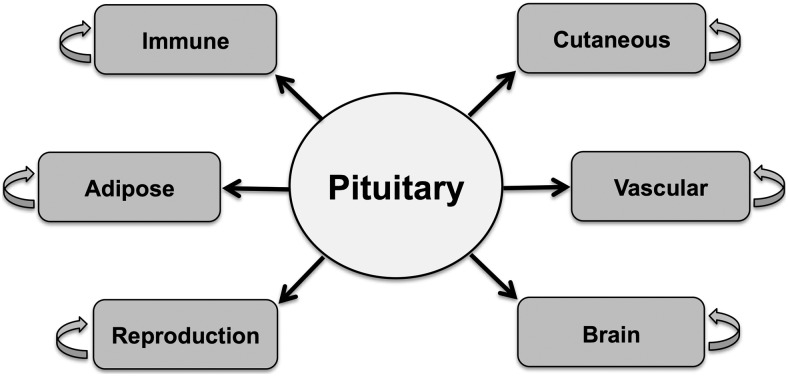
The functional properties of PRL and the number of extrapituitary expressing sites seem to be ever increasing. Since the most recent review, many new functions and sites of ePRL expression have been discovered. In addition, several novel functional elements have been discovered, along with new agonists and antagonists. Figure 4 illustrates the major tissues that express ePRL. The most widely studied tissues of ePRL expression still remain the decidua, brain, endothelial cells, and immune cells. However, other areas such as skin/hair follicles and adipose tissue are gaining more prominence as research subjects.
Figure 4.
Schematics of the major organs/tissues that express PRL. The pituitary gland serves as the largest source of circulating PRL, which affects all organs that express the PRL-R. The major organs/tissues that express ePRL and were covered in this review are shown in blue.
Previously, 5 areas of future research had been identified: 1) tissue-specific regulation of PRL gene expression; 2) biological functions of PRL variants; 3) characterization of PRL transporter/binding proteins; 4) PRL physiology in subsets of cells and within specific fluid compartments; and 5) ePRL as a factor in pathophysiological processes (3). Although there is still work to be done in these areas, especially in the more recently discovered extrapituitary sites, some of these shortfalls have been addressed with new data. In addition, biological functions of truncated PRL variants have been identified and characterized to the extent that they have been provided with the new name of vasoinhibins. Furthermore, specific ePRL-secretory cells have been identified for most tissues. However, more information is needed on the characterization or presence of transporter/binding proteins for ePRL.
Acknowledgments
Search Criteria: Articles were searched using publicly available databases (PubMed) using terms such as extrapituitary, prolactin, decidua prolactin, adipose prolactin, testes prolactin, brain prolactin. Papers were typically restricted to those published after 1996 (the time of the previous review).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AP-1
- activator protein-1
- 8-Br-cAMP
- 8-bromo-cAMP
- C/EBP
- CCAAT/enhancer-binding protein
- CRE
- cAMP response element
- dPRL
- decidual PRL
- ePRL
- extrapituitary PRL
- ERβ
- estrogen receptor β
- ESC
- endometrial stromal cell
- FOXO1
- forkhead box protein O1
- FP1
- footprint 1
- hCG
- human chorionic gonadotropin
- HoxA-11
- homeobox protein A-11
- 20α-HSD
- 20α-hydroxysteroid dehydrogenase
- IFNγ
- interferon-γ
- ORS
- outer root sheath
- PBMNC
- peripheral blood mononuclear cell
- PHA
- phytohemagglutinin
- pPRL
- pituitary PRL
- PRL
- prolactin
- PRL-R
- PRL receptor
- PrRP
- PRL-releasing peptide
- PVN
- paraventricular nuclei
- Stat-1
- signal transducer and activator of transcription-1
- TEAD
- transcriptional enhancer activator domain.
References
- 1. Hiraoka Y, Tatsumi K, Shiozawa M, et al. A placenta-specific 5′ non-coding exon of human prolactin. Mol Cell Endocrinol. 1991;75:71–80. [DOI] [PubMed] [Google Scholar]
- 2. Gellersen B, Kempf R, Telgmann R, DiMattia GE. Nonpituitary human prolactin gene transcription is independent of Pit-1 and differentially controlled in lymphocytes and in endometrial stroma. Mol Endocrinol. 1994;8:356–373. [DOI] [PubMed] [Google Scholar]
- 3. Ben-Jonathan N, Mershon JL, Allen DL, Steinmetz RW. Extrapituitary prolactin: distribution, regulation, functions, and clinical aspects. Endocr Rev. 1996;17:639–669. [DOI] [PubMed] [Google Scholar]
- 4. Arendt LM, Schuler LA. Transgenic models to study actions of prolactin in mammary neoplasia. J Mammary Gland Biol Neoplasia. 2008;13:29–40. [DOI] [PubMed] [Google Scholar]
- 5. Ben-Jonathan N, LaPensee CR, LaPensee EW. What can we learn from rodents about prolactin in humans? Endocr Rev. 2008;29:1–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clevenger CV, Plank TL. Prolactin as an autocrine/paracrine factor in breast tissue. J Mammary Gland Biol Neoplasia. 1997;2:59–68. [DOI] [PubMed] [Google Scholar]
- 7. Clevenger CV, Furth PA, Hankinson SE, Schuler LA. The role of prolactin in mammary carcinoma. Endocr Rev. 2003;24:1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Harvey PW. Hypothesis: prolactin is tumorigenic to human breast: dispelling the myth that prolactin-induced mammary tumors are rodent-specific. J Appl Toxicol. 2012;32:1–9. [DOI] [PubMed] [Google Scholar]
- 9. Goffin V, Hoang DT, Bogorad RL, Nevalainen MT. Prolactin regulation of the prostate gland: a female player in a male game. Nat Rev Urol. 2011;8:597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harvey PW, Everett DJ, Springall CJ. Adverse effects of prolactin in rodents and humans: breast and prostate cancer. J Psychopharmacol. 2008;22:20–27. [DOI] [PubMed] [Google Scholar]
- 11. Montgomery DW. Prolactin production by immune cells. Lupus. 2001;10:665–675. [DOI] [PubMed] [Google Scholar]
- 12. Orbach H, Shoenfeld Y. Hyperprolactinemia and autoimmune diseases. Autoimmun Rev. 2007;6:537–542. [DOI] [PubMed] [Google Scholar]
- 13. Langan EA, Foitzik-Lau K, Goffin V, Ramot Y, Paus R. Prolactin: an emerging force along the cutaneous-endocrine axis. Trends Endocrinol Metab. 2010;21:569–577. [DOI] [PubMed] [Google Scholar]
- 14. Brandebourg T, Hugo E, Ben-Jonathan N. Adipocyte prolactin: regulation of release and putative functions. Diabetes Obes Metab. 2007;9:464–476. [DOI] [PubMed] [Google Scholar]
- 15. Tanaka S, Koibuchi N, Ohtake H, et al. Regional comparison of prolactin gene expression in the human decidualized endometrium in early and term pregnancy. Eur J Endocrinol. 1996;135:177–183. [DOI] [PubMed] [Google Scholar]
- 16. Bao L, Tessier C, Prigent-Tessier A, et al. Decidual prolactin silences the expression of genes detrimental to pregnancy. Endocrinology. 2007;148:2326–2334. [DOI] [PubMed] [Google Scholar]
- 17. Eyal O, Jomain JB, Kessler C, Goffin V, Handwerger S. Autocrine prolactin inhibits human uterine decidualization: a novel role for prolactin. Biol Reprod. 2007;76:777–783. [DOI] [PubMed] [Google Scholar]
- 18. Jones RL, Critchley HO, Brooks J, Jabbour HN, McNeilly AS. Localization and temporal expression of prolactin receptor in human endometrium. J Clin Endocrinol Metab. 1998;83:258–262. [DOI] [PubMed] [Google Scholar]
- 19. Maaskant RA, Bogic LV, Gilger S, Kelly PA, Bryant-Greenwood GD. The human prolactin receptor in the fetal membranes, decidua, and placenta. J Clin Endocrinol Metab. 1996;81:396–405. [DOI] [PubMed] [Google Scholar]
- 20. Brar AK, Frank GR, Kessler CA, Cedars MI, Handwerger S. Progesterone-dependent decidualization of the human endometrium is mediated by cAMP. Endocrine. 1997;6:301–307. [DOI] [PubMed] [Google Scholar]
- 21. Telgmann R, Maronde E, Taskén K, Gellersen B. Activated protein kinase A is required for differentiation-dependent transcription of the decidual prolactin gene in human endometrial stromal cells. Endocrinology. 1997;138:929–937. [DOI] [PubMed] [Google Scholar]
- 22. Pohnke Y, Kempf R, Gellersen B. CCAAT/enhancer-binding proteins are mediators in the protein kinase A-dependent activation of the decidual prolactin promoter. J Biol Chem. 1999;274:24808–24818. [DOI] [PubMed] [Google Scholar]
- 23. Watanabe K, Kessler CA, Bachurski CJ, et al. Identification of a decidua-specific enhancer on the human prolactin gene with two critical activator protein 1 (AP-1) binding sites. Mol Endocrinol. 2001;15:638–653. [DOI] [PubMed] [Google Scholar]
- 24. Brar AK, Kessler CA, Handwerger S. An Ets motif in the proximal decidual prolactin promoter is essential for basal gene expression. J Mol Endocrinol. 2002;29:99–112. [DOI] [PubMed] [Google Scholar]
- 25. Butticè G, Duterque-Coquillaud M, Basuyaux JP, Carrère S, Kurkinen M, Stéhelin D. Erg, an Ets-family member, differentially regulates human collagenase1 (MMP1) and stromelysin1 (MMP3) gene expression by physically interacting with the Fos/Jun complex. Oncogene. 1996;13:2297–2306. [PubMed] [Google Scholar]
- 26. Kessler CA, Bachurski CJ, Schroeder J, Stanek J, Handwerger S. TEAD1 inhibits prolactin gene expression in cultured human uterine decidual cells. Mol Cell Endocrinol. 2008;295:32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jiang Y, Hu Y, Zhao J, Zhen X, Yan G, Sun H. The orphan nuclear receptor Nur77 regulates decidual prolactin expression in human endometrial stromal cells. Biochem Biophys Res Commun. 2011;404:628–633. [DOI] [PubMed] [Google Scholar]
- 28. Kumari M, Heffner LJ. The effects of L-arginine on the release of prolactin from decidual explants in vitro. Am J Obstet Gynecol. 2000;182:497–502. [DOI] [PubMed] [Google Scholar]
- 29. Christian M, Marangos P, Mak I, et al. Interferon-γ modulates prolactin and tissue factor expression in differentiating human endometrial stromal cells. Endocrinology. 2001;142:3142–3151. [DOI] [PubMed] [Google Scholar]
- 30. Kanda Y, Jikihara H, Markoff E, Handwerger S. Interleukin-2 inhibits the synthesis and release of prolactin from human decidual cells. J Clin Endocrinol Metab. 1999;84:677–681. [DOI] [PubMed] [Google Scholar]
- 31. Fluhr H, Krenzer S, Deperschmidt M, Zwirner M, Wallwiener D, Licht P. Human chorionic gonadotropin inhibits insulin-like growth factor-binding protein-1 and prolactin in decidualized human endometrial stromal cells. Fertil Steril. 2006;86:236–238. [DOI] [PubMed] [Google Scholar]
- 32. Christian M, Zhang X, Schneider-Merck T, et al. Cyclic AMP-induced forkhead transcription factor, FKHR, cooperates with CCAAT/enhancer-binding protein β in differentiating human endometrial stromal cells. J Biol Chem. 2002;277:20825–20832. [DOI] [PubMed] [Google Scholar]
- 33. Lynch VJ, Brayer K, Gellersen B, Wagner GP. HoxA-11 and FOXO1A cooperate to regulate decidual prolactin expression: towards inferring the core transcriptional regulators of decidual genes. PLoS One. 2009;4:e6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yasui Y, Yamaguchi M, Jikihara H, Yamamoto T, Kanzaki T, Murata Y. Expression of prolactin-releasing peptide in human placenta and decidua. Endocr J. 2001;48:397–401. [DOI] [PubMed] [Google Scholar]
- 35. Lin SH. Prolactin-releasing peptide. Results Probl Cell Differ. 2008;46:57–88. [DOI] [PubMed] [Google Scholar]
- 36. Reis FM, Viganò P, Arnaboldi E, Spritzer PM, Petraglia F, Di Blasio AM. Expression of prolactin-releasing peptide and its receptor in the human decidua. Mol Hum Reprod. 2002;8:356–362. [DOI] [PubMed] [Google Scholar]
- 37. Shaw-Bruha CM, Pirrucello SJ, Shull JD. Expression of the prolactin gene in normal and neoplastic human breast tissues and human mammary cell lines: promoter usage and alternative mRNA splicing. Breast Cancer Res Treat. 1997;44:243–253. [DOI] [PubMed] [Google Scholar]
- 38. Zinger M, McFarland M, Ben-Jonathan N. Prolactin expression and secretion by human breast glandular and adipose tissue explants. J Clin Endocrinol Metab. 2003;88:689–696. [DOI] [PubMed] [Google Scholar]
- 39. Chen CC, Stairs DB, Boxer RB, et al. Autocrine prolactin induced by the Pten-Akt pathway is required for lactation initiation and provides a direct link between the Akt and Stat5 pathways. Genes Dev. 2012;26:2154–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Imaoka T, Matsuda M, Mori T. Expression of prolactin messenger ribonucleic acid in the mouse gonads during sexual maturation. Life Sci. 1998;63:2251–2258. [DOI] [PubMed] [Google Scholar]
- 41. Schwärzler P, Untergasser G, Hermann M, Dirnhofer S, Abendstein B, Berger P. Prolactin gene expression and prolactin protein in premenopausal and postmenopausal human ovaries. Fertil Steril. 1997;68:696–701. [DOI] [PubMed] [Google Scholar]
- 42. Phelps JY, Bugg EM, Shamblott MJ, Vlahos NP, Whelan J, Zacur HA. Prolactin gene expression in human ovarian follicular cells. Fertil Steril. 2003;79:182–185. [DOI] [PubMed] [Google Scholar]
- 43. Romão GS, Ferriani RA, Moura MD, Martins AR. Screening for prolactin isoforms in the follicular fluid of patients undergoing in vitro fertilization. Gynecol Obstet Invest. 2002;54:46–49. [DOI] [PubMed] [Google Scholar]
- 44. Erdmann S, Ricken A, Merkwitz C, et al. The expression of prolactin and its cathepsin D-mediated cleavage in the bovine corpus luteum vary with the estrous cycle. Am J Physiol Endocrinol Metab. 2007;293:E1365–E1377. [DOI] [PubMed] [Google Scholar]
- 45. Shibaya M, Murakami S, Tatsukawa Y, Skarzynski DJ, Acosta TJ, Okuda K. Bovine corpus luteum is an extrapituitary site of prolactin production. Mol Reprod Dev. 2006;73:512–519. [DOI] [PubMed] [Google Scholar]
- 46. Nevalainen MT, Valve EM, Ahonen T, Yagi A, Paranko J, Härkönen PL. Androgen-dependent expression of prolactin in rat prostate epithelium in vivo and in organ culture. FASEB J. 1997;11:1297–1307. [DOI] [PubMed] [Google Scholar]
- 47. Nevalainen MT, Valve EM, Ingleton PM, Nurmi M, Martikainen PM, Harkonen PL. Prolactin and prolactin receptors are expressed and functioning in human prostate. J Clin Invest. 1997;99:618–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Untergasser G, Rumpold H, Plas E, Witkowski M, Berger P. Seminal plasma factors induce in vitro PRL secretion in smooth muscle cells of the human prostate. J Clin Endocrinol Metab. 2001;86:5577–5584. [DOI] [PubMed] [Google Scholar]
- 49. Ishida M, Yoshida M, Fukuta S, et al. Analysis of prolactin gene expression and cleaved prolactin variants in the mouse testis and spermatozoa. J Reprod Dev. 2010;56:567–574. [DOI] [PubMed] [Google Scholar]
- 50. Kerr J, Wood W, Ridgway EC. Basic science and clinical research advances in the pituitary transcription factors: Pit-1 and Prop-1. Curr Opin Endocrinol Diabetes Obes. 2008;15:359–363. [DOI] [PubMed] [Google Scholar]
- 51. Maeda K, Taniuchi S, Takahashi S, Takeuchi S. Pit-1w may regulate prolactin gene expression in mouse testis. Gen Comp Endocrinol. 2012;178:180–184. [DOI] [PubMed] [Google Scholar]
- 52. Pujianto DA, Curry BJ, Aitken RJ. Prolactin exerts a prosurvival effect on human spermatozoa via mechanisms that involve the stimulation of Akt phosphorylation and suppression of caspase activation and capacitation. Endocrinology. 2010;151:1269–1279. [DOI] [PubMed] [Google Scholar]
- 53. Clapp C. Analysis of the proteolytic cleavage of prolactin by the mammary gland and liver of the rat: characterization of the cleaved and 16K forms. Endocrinology. 1987;121:2055–2064. [DOI] [PubMed] [Google Scholar]
- 54. Clapp C, Weiner RI. A specific, high affinity, saturable binding site for the 16-kilodalton fragment of prolactin on capillary endothelial cells. Endocrinology. 1992;130:1380–1386. [DOI] [PubMed] [Google Scholar]
- 55. Clapp C, Martial JA, Guzman RC, Rentier-Delure F, Weiner RI. The 16-kilodalton N-terminal fragment of human prolactin is a potent inhibitor of angiogenesis. Endocrinology. 1993;133:1292–1299. [DOI] [PubMed] [Google Scholar]
- 56. Martini JF, Piot C, Humeau LM, Struman I, Martial JA, Weiner RI. The antiangiogenic factor 16K PRL induces programmed cell death in endothelial cells by caspase activation. Mol Endocrinol. 2000;14:1536–1549. [DOI] [PubMed] [Google Scholar]
- 57. Clapp C, López-Gómez FJ, Nava G, et al. Expression of prolactin mRNA and of prolactin-like proteins in endothelial cells: evidence for autocrine effects. J Endocrinol. 1998;158:137–144. [DOI] [PubMed] [Google Scholar]
- 58. Corbacho AM, Macotela Y, Nava G, et al. Human umbilical vein endothelial cells express multiple prolactin isoforms. J Endocrinol. 2000;166:53–62. [DOI] [PubMed] [Google Scholar]
- 59. Ochoa A, Montes de Oca P, Rivera JC, et al. Expression of prolactin gene and secretion of prolactin by rat retinal capillary endothelial cells. Invest Ophthalmol Vis Sci. 2001;42:1639–1645. [PubMed] [Google Scholar]
- 60. Khurana S, Liby K, Buckley AR, Ben-Jonathan N. Proteolysis of human prolactin: resistance to cathepsin D and formation of a nonangiostatic, C-terminal 16K fragment by thrombin. Endocrinology. 1999;140:4127–4132. [DOI] [PubMed] [Google Scholar]
- 61. Macotela Y, Aguilar MB, Guzmán-Morales J, et al. Matrix metalloproteases from chondrocytes generate an antiangiogenic 16 kDa prolactin. J Cell Sci. 2006;119:1790–1800. [DOI] [PubMed] [Google Scholar]
- 62. Clapp C, Thebault S, Martínez de la Escalera G. Hormones and postpartum cardiomyopathy. Trends Endocrinol Metab. 2007;18:329–330. [DOI] [PubMed] [Google Scholar]
- 63. Cruz-Soto ME, Cosío G, Jeziorski MC, et al. Cathepsin D is the primary protease for the generation of adenohypophyseal vasoinhibins: cleavage occurs within the prolactin secretory granules. Endocrinology. 2009;150:5446–5454. [DOI] [PubMed] [Google Scholar]
- 64. Ge G, Fernández CA, Moses MA, Greenspan DS. Bone morphogenetic protein 1 processes prolactin to a 17-kDa antiangiogenic factor. Proc Natl Acad Sci USA. 2007;104:10010–10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Clapp C, González C, Macotela Y, et al. Vasoinhibins: a family of N-terminal prolactin fragments that inhibit angiogenesis and vascular function. Front Horm Res. 2006;35:64–73. [DOI] [PubMed] [Google Scholar]
- 66. Wu H, Devi R, Malarkey WB. Expression and localization of prolactin messenger ribonucleic acid in the human immune system. Endocrinology. 1996;137:349–353. [DOI] [PubMed] [Google Scholar]
- 67. Kooijman R, Gerlo S, Coppens A, Hooghe-Peters EL. Growth hormone and prolactin expression in the immune system. Ann NY Acad Sci. 2000;917:534–540. [DOI] [PubMed] [Google Scholar]
- 68. Urtishak SL, McKenna EA, Mastro AM. Prolactin and prolactin receptor expression in rat, small intestine, intraepithelial lymphocytes during neonatal development. Dev Immunol. 2001;8:319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Xu D, Lin L, Lin X, Huang Z, Lei Z. Immunoregulation of autocrine prolactin: suppressing the expression of costimulatory molecules and cytokines in T lymphocytes by prolactin receptor knockdown. Cell Immunol. 2010;263:71–78. [DOI] [PubMed] [Google Scholar]
- 70. Reem GH, Ray DW, Davis JR. The human prolactin gene upstream promoter is regulated in lymphoid cells by activators of T-cells and by cAMP. J Mol Endocrinol. 1999;22:285–292. [DOI] [PubMed] [Google Scholar]
- 71. Diaz L, Martínez-Reza I, García-Becerra R, González L, Larrea F, Méndez I. Calcitriol stimulates prolactin expression in non-activated human peripheral blood mononuclear cells: breaking paradigms. Cytokine. 2011;55:188–194. [DOI] [PubMed] [Google Scholar]
- 72. Cejkova P, Chroma V, Cerna M, et al. Monitoring of the course of sepsis in hematooncological patients by extrapituitary prolactin expression in peripheral blood monocytes. Physiol Res. 2012;61:481–488. [DOI] [PubMed] [Google Scholar]
- 73. Mejía S, Morales MA, Zetina ME, Martínez de la Escalera G, Clapp C. Immunoreactive prolactin forms colocalize with vasopressin in neurons of the hypothalamic paraventricular and supraoptic nuclei. Neuroendocrinology. 1997;66:151–159. [DOI] [PubMed] [Google Scholar]
- 74. Torner L, Nava G, Dueñas Z, et al. Changes in the expression of neurohypophyseal prolactins during the estrous cycle and after estrogen treatment. J Endocrinol. 1999;161:423–432. [DOI] [PubMed] [Google Scholar]
- 75. Suzuki S, Handa RJ. Estrogen receptor-β, but not estrogen receptor-α, is expressed in prolactin neurons of the female rat paraventricular and supraoptic nuclei: comparison with other neuropeptides. J Comp Neurol. 2005;484:28–42. [DOI] [PubMed] [Google Scholar]
- 76. Roselli CE, Bocklandt S, Stadelman HL, Wadsworth T, Vilain E, Stormshak F. Prolactin expression in the sheep brain. Neuroendocrinology. 2008;87:206–215. [DOI] [PubMed] [Google Scholar]
- 77. Chaiseha Y, Ngernsoungnern P, Sartsoongnoen N, Prakobsaeng N, El Halawani ME. Presence of prolactin mRNA in extra-pituitary brain areas in the domestic turkey. Acta Histochem. 2012;114:116–121. [DOI] [PubMed] [Google Scholar]
- 78. Yang X, Meyer K, Friedl A. STAT5 and Prolactin Participate in a Positive Autocrine Feedback Loop That Promotes Angiogenesis. J Biol Chem. 2013;288:21184–21196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Torner L, Neumann ID. The brain prolactin system: involvement in stress response adaptations in lactation. Stress. 2002;5:249–257. [DOI] [PubMed] [Google Scholar]
- 80. Bosch OJ. Maternal nurturing is dependent on her innate anxiety: the behavioral roles of brain oxytocin and vasopressin. Horm Behav. 2011;59:202–212. [DOI] [PubMed] [Google Scholar]
- 81. Torner L, Maloumby R, Nava G, Aranda J, Clapp C, Neumann ID. In vivo release and gene upregulation of brain prolactin in response to physiological stimuli. Eur J Neurosci. 2004;19:1601–1608. [DOI] [PubMed] [Google Scholar]
- 82. DeVito WJ, Stone S, Mori K. Low concentrations of ethanol inhibits prolactin-induced mitogenesis and cytokine expression in cultured astrocytes. Endocrinology. 1997;138:922–928. [DOI] [PubMed] [Google Scholar]
- 83. Chen L, Taishi P, Duricka D, Krueger JM. Brainstem prolactin mRNA is enhanced in mice with suppressed neuronal nitric oxide synthase activity. Brain Res Mol Brain Res. 2004;129:179–184. [DOI] [PubMed] [Google Scholar]
- 84. Foitzik K, Langan EA, Paus R. Prolactin and the skin: a dermatological perspective on an ancient pleiotropic peptide hormone. J Invest Dermatol. 2009;129:1071–1087. [DOI] [PubMed] [Google Scholar]
- 85. Foitzik K, Krause K, Nixon AJ, et al. Prolactin and its receptor are expressed in murine hair follicle epithelium, show hair cycle-dependent expression, and induce catagen. Am J Pathol. 2003;162:1611–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Craven AJ, Nixon AJ, Ashby MG, et al. Prolactin delays hair regrowth in mice. J Endocrinol. 2006;191:415–425. [DOI] [PubMed] [Google Scholar]
- 87. Langan EA, Ramot Y, Hanning A, et al. Thyrotropin-releasing hormone and oestrogen differentially regulate prolactin and prolactin receptor expression in female human skin and hair follicles in vitro. Br J Dermatol. 2010;162:1127–1131. [DOI] [PubMed] [Google Scholar]
- 88. Foitzik K, Krause K, Conrad F, Nakamura M, Funk W, Paus R. Human scalp hair follicles are both a target and a source of prolactin, which serves as an autocrine and/or paracrine promoter of apoptosis-driven hair follicle regression. Am J Pathol. 2006;168:748–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Slominski A, Malarkey WB, Wortsman J, Asa SL, Carlson A. Human skin expresses growth hormone but not the prolactin gene. J Lab Clin Med. 2000;136:476–481. [DOI] [PubMed] [Google Scholar]
- 90. Richards RG, Hartman SM. Human dermal fibroblast cells express prolactin in vitro. J Invest Dermatol. 1996;106:1250–1255. [DOI] [PubMed] [Google Scholar]
- 91. Hugo ER, Borcherding DC, Gersin KS, Loftus J, Ben-Jonathan N. Prolactin release by adipose explants, primary adipocytes, and LS14 adipocytes. J Clin Endocrinol Metab. 2008;93:4006–4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Khurana S, Kuns R, Ben-Jonathan N. Heparin-binding property of human prolactin: a novel aspect of prolactin biology. Endocrinology. 1999;140:1026–1029. [DOI] [PubMed] [Google Scholar]
- 93. Kok P, Roelfsema F, Frölich M, Meinders AE, Pijl H. Prolactin release is enhanced in proportion to excess visceral fat in obese women. J Clin Endocrinol Metab. 2004;89:4445–4449. [DOI] [PubMed] [Google Scholar]
- 94. McFarland-Mancini M, Hugo E, Loftus J, Ben-Jonathan N. Induction of prolactin expression and release in human preadipocytes by cAMP activating ligands. Biochem Biophys Res Commun. 2006;344:9–16. [DOI] [PubMed] [Google Scholar]
- 95. Hugo ER, Brandebourg TD, Comstock CE, Gersin KS, Sussman JJ, Ben-Jonathan N. LS14: a novel human adipocyte cell line that produces prolactin. Endocrinology. 2006;147:306–313. [DOI] [PubMed] [Google Scholar]
- 96. Marano RJ, Tickner J, Redmond SL. Age related changes in gene expression within the cochlea of C57BL/6J mice. Aging Clin Exp Res. 2012;24:603–611. [DOI] [PubMed] [Google Scholar]
- 97. Smeti I, Assou S, Savary E, Masmoudi S, Zine A. Transcriptomic analysis of the developing and adult mouse cochlear sensory epithelia. PLoS One. 2012;7:e42987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Marano RJ, Tickner J, Redmond SL. Prolactin expression in the cochlea of aged BALB/c mice is gender biased and correlates to loss of bone mineral density and hearing loss. PLoS One. 2013;8:e63952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Horner KC. The effect of sex hormones on bone metabolism of the otic capsule–an overview. Hear Res. 2009;252:56–60. [DOI] [PubMed] [Google Scholar]