Abstract
A major obstacle to a broadly neutralizing antibody (bnAb)-based HIV vaccine is the activation of appropriate B cell precursors. Germline-targeting immunogens must be capable of priming rare bnAb precursors in the physiological setting. We tested the ability of the VRC01-class bnAb germline-targeting immunogen eOD-GT8 60mer to activate appropriate precursors in mice transgenic for human immunoglobulin loci. Despite an average frequency of at most ~1 VRC01-class precursor per mouse, we found that at least 29% of singly-immunized mice produced a VRC01-class memory response, suggesting that priming generally succeeded when at least one precursor was present. The results demonstrate the feasibility of using germline targeting to prime specific and exceedingly rare bnAb precursor B cells within a human-like repertoire.
Main Text
It is widely thought that vaccine elicitation of sustained levels of potent broadly neutralizing antibodies (bnAbs) may protect humans against HIV (1, 2). HIV bnAbs from natural infection show extensive somatic mutation (1–9), and this maturation is required for cross-reactivity to diverse isolates: bnAb inferred-germline variants typically show detectable affinity for few if any HIV envelope protein (Env) antigens tested (3–5, 7, 10–21). Most wild-type Env proteins are therefore poor immunogens to prime bnAb responses (3–5, 17, 22, 23). Reliable vaccine initiation of bnAb responses will likely require design or discovery of “germline-targeting” priming immunogens with appreciable affinity for bnAb germline precursors (3–5, 10, 11, 17, 22–29). Furthermore, consistent bnAb priming may only be possible for bnAb precursors present at reasonable frequency in all or most vaccine recipients (27).
Proof of principle that a germline-targeting immunogen can prime relatively rare bnAb-precursor B cells was shown with the eOD-GT8 60 subunit self-assembling nanoparticle (60mer) that targets precursors of CD4-binding-site-directed VRC01-class bnAbs (26). VRC01-class precursors are defined by their use of a heavy chain VH1–2*02 (or *03 or *04) gene and light chains with unusually short complementarity determining region 3 (CDR3) loops of five amino acids (5, 30–32). In a transgenic mouse model expressing the germline-reverted VRC01 heavy chain paired with wild-type mouse light chains (VRC01 gH mouse), in which the frequency of VRC01-class precursor B cells was estimated to be higher than in humans by a factor of as little as ~5, a single immunization with eOD-GT8 60mer activated VRC01-class precursors and generated VRC01-class memory responses in nearly all immunized mice, while control immunogens presenting a native CD4 binding site failed to activate such precursors (26). The eOD-GT8 60mer activated target precursors less robustly in a different VRC01-class inferred-germline heavy chain transgenic mouse (25). Several properties of both mouse models lowered the bar for germline-targeting compared to the challenges confronted in a human: elevated bnAb precursor frequency, reduced competition from other B cell specificities, limited diversity of bnAb precursors owing to their uniform recombined heavy chains, and an affinity advantage conferred on bnAb precursors due to their mature H-CDR3s.
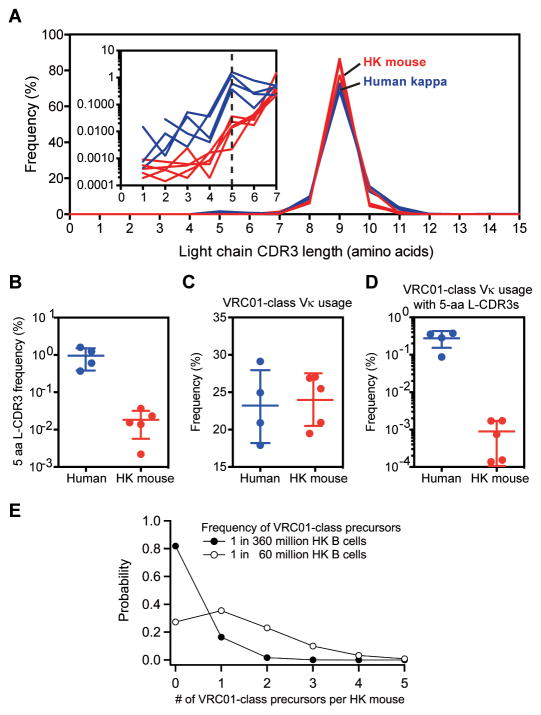
To better model the conditions for initiation of a VRC01-class bnAb response in humans, we investigated immunization with eOD-GT8 60mer in Kymab mice transgenic for the complete un-rearranged human antibody germline gene repertoire (33). Kymab mice contain human heavy chains paired with either human kappa light chains (HK mice) or human lambda light chains (HL mice), or both (HKL mice). To determine the level of difficulty for VRC01-class bnAb priming in Kymab mice, we first measured the frequency of VRC01-class precursors in the above mouse strains. Using B cell sorting methods, we previously detected eOD-GT8-specific VRC01-class precursors at a frequency of 1 in 2.4 million among human naïve B cells expressing kappa light chains (27). Using similar methods to probe a total of approximately 300 million naïve B cells from the spleens and lymph nodes of unimmunized HK and HL mice (N=3 each), we were unable to isolate any VRC01-class B cells, suggesting that the frequency of such B cells was considerably lower than in humans. The frequency of the VH1–2*02, *03, or *04 alleles among HK B cells was previously measured as 0.9% (33, 34), lower than the frequency in humans (2.9±1.3%) (fig. S1) (35, 36) by a factor of only ~3. Therefore, to explain the reduced frequency of VRC01-class B cells in Kymab mice, we analyzed the light chain variable gene (VL) usage and CDR3 length distributions from IgM+ or IgG+ B cells for five HK mice and four healthy humans by next-generation sequencing (NGS) (37). The kappa light chain-CDR3 (L-CDR3) length distributions were broadly similar in HK mice and humans (Fig. 1A), but the frequency of 5-amino acid L-CDR3s was lower in HK mice (0.018 ± 0.013%) than in humans (0.95 ± 0.57%) by a factor of approximately 50 (Fig. 1B). The cumulative frequency of Vκ genes used by known VRC01-class bnAbs (IGVK3–20, IGVK1–33 and IGVK3–15 (32, 38)) was 24.0 ± 3.5% in HK mice, similar to the 23.2 ± 4.9% that we measured in humans (Fig. 1C). However, the frequency of 5-amino acid L-CDR3s associated with known VRC01-class kappa chains was reduced by a factor of approximately 300 in HK mice (0.00089 ± 0.00079%) compared to humans (0.27 ± 0.13%) (Fig. 1D). Restricting the analysis to light chains with five or fewer VL nucleotide mutations produced similar conclusions (fig. S2). Whatever the cause, our data indicate that VRC01-class precursors are less frequent in HK mice than in humans by a factor of 150 to 900.
Fig. 1.
Frequency analysis of VRC01-class light chains and precursors in Kymab HK mice. (A) Light chain L-CDR3 length distributions in humans and HK mice. (B) Frequencies of 5-amino acid L-CDR3s in humans and HK mice. (C) Frequencies of known VRC01-class bnAb light chain Vκ genes in humans and HK mice. (D) Frequencies of known VRC01-class bnAb Vκ genes with 5-amino acid L-CDR3s in humans and HK mice. (E) Modeled distributions of the number of VRC01-class precursors per HK mouse for average precursor frequencies of 0.2 or 1.3 per HK mouse, corresponding to frequencies of 1 in 360 million or 1 in 60 million HK B cells, respectively. 1 in 60 million is likely to be an upper bound on the frequency, and so the distribution is likely to be shaped more like that shown for 1 in 360 million (see text). In (B)–(D), points represent frequencies for individual humans or mice (each sequenced once), and bars represent mean ± SD for N=4 humans or N=5 HK mice.
We previously estimated the true frequency of eOD-GT8-specific VRC01-class precursors as 1 in 400,000 naïve human kappa-chain B cells, accounting for cell sorter and PCR losses (27). Based on this number and the calculations above, we conclude that the frequency of VRC01-class precursors in HK mice is unlikely to be higher than 1 in 60 million (=150×400,000) B cells and might be as low as 1 in 360 million (=900×400,000) B cells. The spleens of HK and HL mice (7–18 weeks of age) contain 50 million B220+ B cells, of which ~60% are mature B cells (33) that are thought prepared to respond to antigen. Allowing for half that number again in the lymph nodes and periphery, we estimate that each mouse contains approximately 75 million B cells, of which 45 million are mature. Thus we expect a very low average frequency of 0.2 (≈75/360) to 1.3 (≈75/60) eOD-GT8-specific VRC01-class precursors per HK mouse at any given time. Modeling the number of precursors per mouse with Poisson distributions predicts that 27–82% of HK mice will have zero precursors and the remainder 1–4 precursors (Fig. 1E). Thus priming VRC01-class responses in HK mice appears substantially more difficult than in humans, as the precursor frequency is lower (by a factor of 150–900) and the number of precursors per individual is lower (by 3,000–30,000 precursors (27)). Frequency analysis in one HL mouse and two HKL mice reached similar conclusions that were only slightly more favorable for VRC01-class priming (table S1).
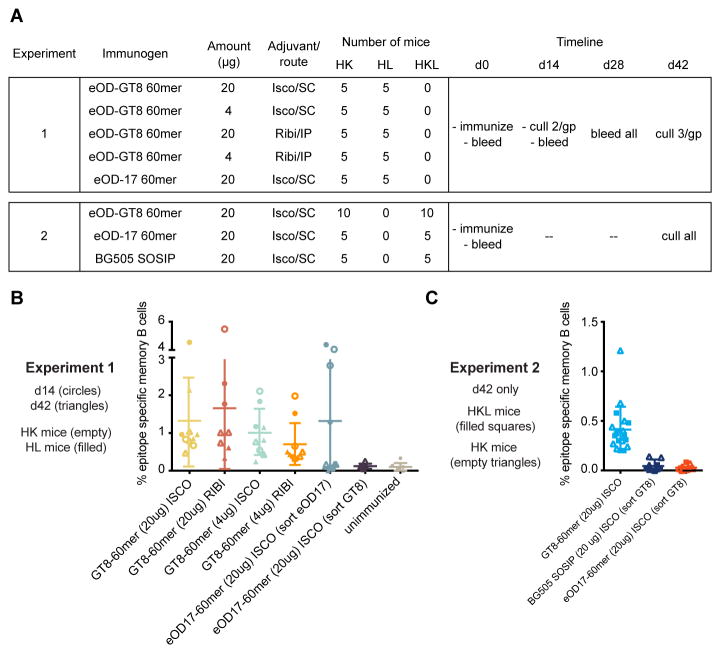
We conducted immunization experiments to determine if eOD-GT8 60mer could prime rare VRC01-class precursors in Kymab mice. Our first experiment (Fig. 2A), in both HK and HL mice (28 mice each), evaluated differences in antigen dose (20 versus 4 μg), adjuvant formulation and route (Iscomatrix/sub-cutaneous versus Sigma Adjuvant system also known as “Ribi”/intraperitoneal), and time point of analysis (14 days versus 42 days post-immunization). Unimmunized mice (3 HK and 3 HL) and mice immunized with 20 μg non-germline-targeting eOD-17 60mer in Iscomatrix were controls. Serum ELISA showed robust responses to eOD-GT8 (endpoint titers of [1–5]×10−5 and IC50s of [5–13]×10−4 at day 42) and lower responses to eOD-GT8 KO (fig. S3), a mutant with reduced binding to VRC01-class antibodies (26), indicating epitope-specific responses (fig. S4) (37). Spleens and lymph nodes from culled mice were processed, stained, and single-cell sorted for IgM-/IgD- memory B cells that bound to eOD-GT8 tetramers but not to eOD-GT8 KO tetramers (27, 37). Only eOD-GT8 60mer-immunized mice showed evidence of epitope-specific (eOD-GT8+/eOD-GT8 KO−) memory B cells (Fig. 2B). A control sort of eOD-17 60mer-immunized samples using eOD-17/eOD-17-KO probes identified memory B cells reactive with eOD-17 but not eOD-GT8.
Fig. 2.
B cell sorting analysis reveals reproducible epitope-specific responses to eOD-GT8 60mer prime. (A) Overview of two immunization experiments in Kymab mice. (B) Frequencies of epitope-specific memory B cells at days 14 or 42 after priming in experiment 1. (C) Same type of data as in (B) but for experiment 2 in which analysis was carried out at day 42 only. In (B) and (C), points represent frequencies measured for a single mouse, each measured once, and bars represent mean ± SD for all data in each column of the graph.
We performed a second experiment (Fig. 2A), in HK and HKL mice, with eOD-GT8 60mer, eOD-17 60mer, the native-like trimer BG505 SOSIP (39–44), and eOD-GT8 d41m3 60mer, a variant of eOD-GT8 60mer with disulfide-stabilization and modification of the underlying nanoparticle (fig. S5) the results for which are combined with those of eOD-GT8 60mer as the two were indistinguishable (fig. S6). Although epitope-specific memory B cell frequencies were slightly (factor of ~3) lower in the second experiment, potentially due to sorting with a different eOD-GT8 KO bait (eOD-GT8 KO2 [fig. S3]), comparison of frequencies between groups (Fig. 2C) supported the findings from the first experiment (Fig. 2B).
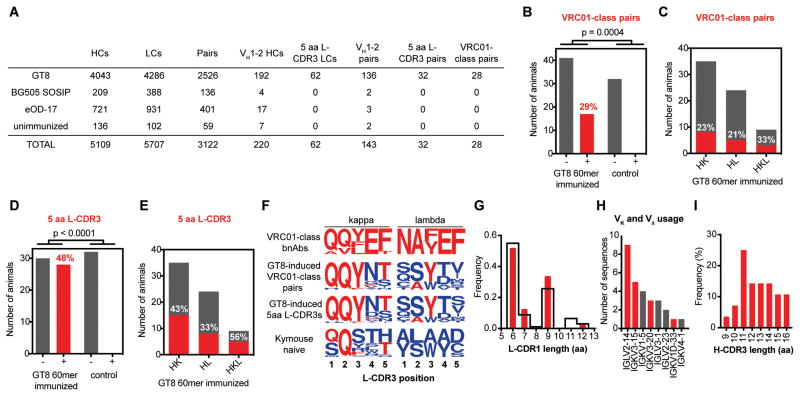
To determine whether or not the epitope-specific memory B cells generated by eOD-GT8 60mer immunization contained VRC01-class memory B cells, RNA from single eOD-GT8+/eOD-GT8 KO− sorted memory B cells was reverse transcribed and IgG variable genes were amplified and sequenced by NGS (37). The two experiments yielded a total of 10,816 wells from which unique heavy and/or light chain nucleotide sequences could be determined, and of these, 3,122 wells contained a heavy and light chain pair (2,526 pairs for eOD-GT8 60mer-immunized mice, 537 pairs for control-immunized mice and 60 pairs for unimmunized mice) (Fig. 3A and tables S2–S4). From these sequences, we identified 28 nucleotide-unique (26 amino acid-unique) VRC01-class heavy-light paired memory responses among 29% (17 of 58) of eOD-GT8 60mer-immunized mice (aggregating across different doses, adjuvants, timepoints, types of mice and the two experiments) (Figs. 3B and S7, table S5). In contrast, we detected no VRC01-class responses from 32 control mice. eOD-GT8 60mer-induced VRC01-class responses were found at approximately similar frequencies in HK, HL, and HKL mice (Fig. 3C).
Fig. 3.
Analysis of antibody sequences from epitope-specific memory B cells. (A) Summary of all sequence information obtained from experiments 1 and 2. Of the 33 VRC01-class pairs, 28 were identified as unique in nucleotide sequence. (B) Number of eOD-GT8 60mer-immunized or control mice from which at least one VRC01-class pair was isolated by B cell sorting (+) or no VRC01-class pairs were isolated (−). Data are aggregated from all animals and conditions in experiments 1 and 2, with a total of 90 mice. p-value was calculated using Fisher’s exact test. (C) Number of eOD-GT8 60mer-immunized HK, HL or HKL mice for which at least one VRC01-class pair was isolated (red, with percentages listed in white) or no VRC01-class pairs were isolated (gray). (D) and (E) same analysis as (B) and (C), respectively, but with 5-amino acid L-CDR3 light chains instead of VRC01-class pairs. (F) L-CDR3 sequence logos for VRC01-class bnAbs (top row), eOD-GT8 60mer-induced VRC01-class paired antibodies (second row), eOD-GT8 60mer-induced 5-amino acid L-CDR3s (third row), and naïve Kymab mice (bottom row), shown separately for kappa light chains (left column) and lambda light chains (right column). (G) L-CDR1 length distribution for eOD-GT8 60mer-induced VRC01-class antibodies (red) and all LCs in (A) (black). (H) Light chain Vκ and Vλ gene usage for eOD-GT8 60mer-induced VRC01-class antibodies. Red bars denote genes used by, or highly similar to those used by, known VRC01-class antibodies. (I) H-CDR3 length distribution for eOD-GT8 60mer-induced VRC01-class antibodies.
Whereas only 21% (28/136) of paired sequences with VH1–2*04 heavy chains had L-CDR3s of 5-amino acids, 88% (28/32) of paired sequences using a 5-amino acid L-CDR3 included a VH1–2*04 heavy chain (Fig. 3A), suggesting that 5-amino acid L-CDR3s in memory B cells may serve as a proxy for VRC01-like responses. Therefore, we examined the frequency of 5-amino acid L-CDR3s among all paired and unpaired light chain sequences. We identified 62 light chains with 5-amino acid L-CDR3s from 48% (28 of 58) of eOD-GT8 60mer-immunized mice, whereas we found no 5-amino acid L-CDR3s among control mice (Figs. 3A and 3D). eOD-GT8 60mer induced memory B cells with 5-amino acid L-CDR3s at substantial frequencies in all three types of mice (Fig. 3E). All combinations of dose, adjuvant and timepoint produced VRC01-class responses and 5-amino acid L-CDR3 responses (fig. S8). Given the very low frequency of VRC01-class precursors, these results indicate that VRC01-class priming by eOD-GT8 60mer was highly efficient and may have succeeded in all or most mice in which at least one precursor was present.
The VRC01-class antibodies induced by eOD-GT8 60mer shared other characteristic features of VRC01-class bnAbs in addition to the VH1–2 alleles and 5-amino acid L-CDR3. The L-CDR3 is a key site of affinity maturation in VRC01-class bnAbs (32), and the 28 VRC01-class antibodies showed clear signs of selection toward bnAb sequences in both kappa and lambda L-CDR3 (Figs. 3F). In addition, 21 of 28 VRC01-class pairs had L-CDR1 lengths matching those of the germline VL genes of known VRC01-class bnAbs (Fig. 3G)(32). Further, 18 of these 28 antibodies used known VRC01-class VL genes (Fig. 3H), and the H-CDR3 lengths among the VRC01-class pairs (9 to 16 amino acids) were similar to those of known VRC01-class bnAbs (12 to 18 amino acids) (Fig. 3I).
Consistent with a VRC01-class binding mode, all 20 VRC01-class antibodies that we expressed bound to eOD-GT8 but had no detectable affinity for either eOD-GT8 mutant, eOD-GT8 KO or eOD-GT8 KO2 (fig. S9). These VRC01-class antibodies had geometric mean (GM) affinity for eOD-GT8 of 134 nM (geometric standard deviation [GSD], 9.4), a factor of 25 higher than the geometric mean eOD-GT8 affinities of VRC01-class antibodies isolated from naïve human B cells (3.4 μM; GSD, 5.6)(27), possibly due to maturation of the immunogen-induced antibodies (mean±SD mutation levels were 0.8±1.1% in VH and 1.5±1.1% in VL). eOD-GT8 60mer-induced VRC01-class antibodies in the VRC01 gH mouse had similar mutation levels (VH: 1.3±3.2%; VL:2.1±1.9%) but higher GM affinity for eOD-GT8 by a factor of 22 (GM, 6.0 nM; GSD, 38.7)(26), probably due at least in part to the engineering of eOD-GT8 for ultra-high affinity (9 pM) to germline-reverted VRC01 (27). Thus the Kymab antibody affinities are likely better predictors of the affinities of human responses to a single immunization of eOD-GT8 60mer.
Ultimate elicitation of bnAbs will probably require sequential boosting with more native-like epitope variants to select sufficient bnAb-like mutations (1, 4, 5, 10, 16, 17, 22, 23, 25, 26). Consistent with this expectation and with results of eOD-GT8 60mer priming experiments in knock-in mice (25, 26), the primed VRC01-class antibodies here showed no affinity for the native-like trimer BG505 SOSIP (fig. S10) and no neutralizing activity against the VRC01-sensitive HXB2 HIV strain from which eOD-GT8 was derived (fig. S11). The fact that only ~1% (28/2526) of epitope-specific antibodies were VRC01-class (Fig. 3A) suggests that immuno-focusing strategies may be needed to suppress competing responses during boosting (1, 45), though in humans the higher VRC01-class precursor frequency may mitigate this challenge.
Germline targeting is a promising vaccine strategy, but developing suitable model systems to evaluate targeting of human germline B cells is difficult. Kymab human immunoglobulin loci transgenic mice offer a more stringent and human-like model compared to most knock-in mice. Although Kymab mice under-represent the frequency of VRC01-class precursor B cells compared to humans and possess an average of at most 1.3 such precursors per mouse, the eOD-GT8 60mer still proved capable of priming. The seemingly high targeting efficiency of eOD-GT8 60mer in this mouse model encourages human testing wherein the VRC01-class precursor frequency among B cells and the number of precursors per individual are more favorable for bnAb priming. The results here should also encourage germline targeting for other bnAbs with lower human precursor frequencies.
Supplementary Material
Acknowledgments
We thank P. Kellam for comments on the manuscript. This work was partially funded by IAVI with the generous support of USAID, Ministry of Foreign Affairs of the Netherlands, and the Bill & Melinda Gates Foundation; a full list of IAVI donors is available at www.iavi.org (W.R.S., D.R.B). This work was also supported by the Bill and Melinda Gates Foundation (G.A.F., A.B.), the Ragon Institute of MGH, MIT and Harvard (D.R.B. and W.R.S.), the Helen Hay Whitney Foundation (J.G.J.), and National Institute of Allergy and Infectious Diseases grants: P01 AI094419 (W.R.S.), CHAVI-ID 1UM1AI100663 (W.R.S., D.R.B.). The data presented in this manuscript are tabulated in the main paper and in the supplementary materials. All mice were maintained, and all procedures carried out under United Kingdom Home Office License 70/8718 and with the approval of the Sanger Institute Animal Welfare and Ethical Review Body. The VRC01-class paired sequences described in this study were deposited in Genbank under accession numbers: KX814864 to KX814919.
Footnotes
References and Notes
- 1.Mascola JR, Haynes BF. HIV-1 neutralizing antibodies: understanding nature’s pathways. Immunological reviews. 2013;254:225–244. doi: 10.1111/imr.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burton DR, Hangartner L. Broadly Neutralizing Antibodies to HIV and Their Role in Vaccine Design. Annu Rev Immunol. 2016;34:635–659. doi: 10.1146/annurev-immunol-041015-055515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiao X, et al. Germline-like predecessors of broadly neutralizing antibodies lack measurable binding to HIV-1 envelope glycoproteins: implications for evasion of immune responses and design of vaccine immunogens. Biochemical and biophysical research communications. 2009;390:404–409. doi: 10.1016/j.bbrc.2009.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dimitrov DS. Therapeutic antibodies, vaccines and antibodyomes. mAbs. 2010;2:347–356. doi: 10.4161/mabs.2.3.11779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou T, et al. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science. 2010;329:811–817. doi: 10.1126/science.1192819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu X, et al. Focused evolution of HIV-1 neutralizing antibodies revealed by structures and deep sequencing. Science. 2011;333:1593–1602. doi: 10.1126/science.1207532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheid JF, et al. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science. 2011;333:1633–1637. doi: 10.1126/science.1207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker LM, et al. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonsignori M, et al. Analysis of a clonal lineage of HIV-1 envelope V2/V3 conformational epitope-specific broadly neutralizing antibodies and their inferred unmutated common ancestors. J Virol. 2011;85:9998–10009. doi: 10.1128/JVI.05045-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pancera M, et al. Crystal structure of PG16 and chimeric dissection with somatically related PG9: structure-function analysis of two quaternary-specific antibodies that effectively neutralize HIV-1. J Virol. 2010;84:8098–8110. doi: 10.1128/JVI.00966-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma BJ, et al. Envelope deglycosylation enhances antigenicity of HIV-1 gp41 epitopes for both broad neutralizing antibodies and their unmutated ancestor antibodies. PLoS Pathog. 2011;7:e1002200. doi: 10.1371/journal.ppat.1002200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ota T, et al. Anti-HIV B Cell lines as candidate vaccine biosensors. J Immunol. 2012;189:4816–4824. doi: 10.4049/jimmunol.1202165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mouquet H, et al. Complex-type N-glycan recognition by potent broadly neutralizing HIV antibodies. Proc Natl Acad Sci U S A. 2012;109:E3268–3277. doi: 10.1073/pnas.1217207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoot S, et al. Recombinant HIV envelope proteins fail to engage germline versions of anti-CD4bs bNAbs. PLoS Pathog. 2013;9:e1003106. doi: 10.1371/journal.ppat.1003106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein F, et al. Somatic mutations of the immunoglobulin framework are generally required for broad and potent HIV-1 neutralization. Cell. 2013;153:126–138. doi: 10.1016/j.cell.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liao HX, et al. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature. 2013;496:469–476. doi: 10.1038/nature12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jardine J, et al. Rational HIV immunogen design to target specific germline B cell receptors. Science. 2013;340:711–716. doi: 10.1126/science.1234150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sok D, et al. The effects of somatic hypermutation on neutralization and binding in the PGT121 family of broadly neutralizing HIV antibodies. PLoS Pathog. 2013;9:e1003754. doi: 10.1371/journal.ppat.1003754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doria-Rose NA, et al. Developmental pathway for potent V1V2-directed HIV-neutralizing antibodies. Nature. 2014;509:55–62. doi: 10.1038/nature13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrabi R, et al. Identification of Common Features in Prototype Broadly Neutralizing Antibodies to HIV Envelope V2 Apex to Facilitate Vaccine Design. Immunity. 2015;43:959–973. doi: 10.1016/j.immuni.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorman J, et al. Structures of HIV-1 Env V1V2 with broadly neutralizing antibodies reveal commonalities that enable vaccine design. Nat Struct Mol Biol. 2016;23:81–90. doi: 10.1038/nsmb.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haynes BF, Kelsoe G, Harrison SC, Kepler TB. B-cell-lineage immunogen design in vaccine development with HIV-1 as a case study. Nature biotechnology. 2012;30:423–433. doi: 10.1038/nbt.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGuire AT, et al. Engineering HIV envelope protein to activate germline B cell receptors of broadly neutralizing anti-CD4 binding site antibodies. The Journal of experimental medicine. 2013;210:655–663. doi: 10.1084/jem.20122824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGuire AT, et al. HIV antibodies. Antigen modification regulates competition of broad and narrow neutralizing HIV antibodies. Science. 2014;346:1380–1383. doi: 10.1126/science.1259206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dosenovic P, et al. Immunization for HIV-1 Broadly Neutralizing Antibodies in Human Ig Knockin Mice. Cell. 2015;161:1505–1515. doi: 10.1016/j.cell.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jardine JG, et al. HIV-1 VACCINES. Priming a broadly neutralizing antibody response to HIV-1 using a germline-targeting immunogen. Science. 2015;349:156–161. doi: 10.1126/science.aac5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jardine JG, et al. HIV-1 broadly neutralizing antibody precursor B cells revealed by germline-targeting immunogen. Science. 2016;351:1458–1463. doi: 10.1126/science.aad9195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGuire AT, et al. Specifically modified Env immunogens activate B-cell precursors of broadly neutralizing HIV-1 antibodies in transgenic mice. Nat Commun. 2016;7:10618. doi: 10.1038/ncomms10618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jardine JG, et al. Minimally Mutated HIV-1 Broadly Neutralizing Antibodies to Guide Reductionist Vaccine Design. PLoS Pathog. 2016;12:e1005815. doi: 10.1371/journal.ppat.1005815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu X, et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010;329:856–861. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.West AP, Jr, Diskin R, Nussenzweig MC, Bjorkman PJ. Structural basis for germ-line gene usage of a potent class of antibodies targeting the CD4-binding site of HIV-1 gp120. Proc Natl Acad Sci U S A. 2012;109:E2083–2090. doi: 10.1073/pnas.1208984109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou T, et al. Multidonor analysis reveals structural elements, genetic determinants, and maturation pathway for HIV-1 neutralization by VRC01-class antibodies. Immunity. 2013;39:245–258. doi: 10.1016/j.immuni.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee EC, et al. Complete humanization of the mouse immunoglobulin loci enables efficient therapeutic antibody discovery. Nature biotechnology. 2014;32:356–363. doi: 10.1038/nbt.2825. [DOI] [PubMed] [Google Scholar]
- 34.Among human VH1–2 alleles, Kymab mice only contain VH1–2*04. This was misreported as *02 in Lee, et al. Nat Biotech. 2014
- 35.Arnaout R, et al. High-resolution description of antibody heavy-chain repertoires in humans. Plos One. 2011;6 doi: 10.1371/journal.pone.0022365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeKosky BJ, et al. In-depth determination and analysis of the human paired heavy- and light-chain antibody repertoire. Nature medicine. 2015;21:86–91. doi: 10.1038/nm.3743. [DOI] [PubMed] [Google Scholar]
- 37.See supplementary materials on Science Online.
- 38.Georgiev IS, et al. Delineating antibody recognition in polyclonal sera from patterns of HIV-1 isolate neutralization. Science. 2013;340:751–756. doi: 10.1126/science.1233989. [DOI] [PubMed] [Google Scholar]
- 39.Sanders RW, et al. A next-generation cleaved, soluble HIV-1 Env trimer, BG505 SOSIP.664 gp140, expresses multiple epitopes for broadly neutralizing but not non-neutralizing antibodies. PLoS Pathog. 2013;9:e1003618. doi: 10.1371/journal.ppat.1003618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Julien JP, et al. Crystal structure of a soluble cleaved HIV-1 envelope trimer. Science. 2013;342:1477–1483. doi: 10.1126/science.1245625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lyumkis D, et al. Cryo-EM structure of a fully glycosylated soluble cleaved HIV-1 envelope trimer. Science. 2013;342:1484–1490. doi: 10.1126/science.1245627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pancera M, et al. Structure and immune recognition of trimeric pre-fusion HIV-1 Env. Nature. 2014;514:455–461. doi: 10.1038/nature13808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Do Kwon Y, et al. Crystal structure, conformational fixation and entry-related interactions of mature ligand-free HIV-1 Env. Nat Struct Mol Biol. 2015;22:522–531. doi: 10.1038/nsmb.3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanders RW, et al. HIV-1 VACCINES. HIV-1 neutralizing antibodies induced by native-like envelope trimers. Science. 2015;349:aac4223. doi: 10.1126/science.aac4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kong L, Sattentau QJ. Antigenicity and Immunogenicity in HIV-1 Antibody-Based Vaccine Design. J AIDS Clin Res. 2012;S8:3. doi: 10.4172/2155-6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tiller T, et al. Efficient generation of monoclonal antibodies from single human B cells by single cell RT-PCR and expression vector cloning. J Immunol Methods. 2008;329:112–124. doi: 10.1016/j.jim.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sok D, et al. Recombinant HIV envelope trimer selects for quaternary-dependent antibodies targeting the trimer apex. Proc Natl Acad Sci U S A. 2014;111:17624–17629. doi: 10.1073/pnas.1415789111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin M. Cutadapt Removes Adapter Sequences From High-Throughput Sequencing Reads. EMBnet.journal. 2011;17:10–12. [Google Scholar]
- 49.Masella AP, Bartram AK, Truszkowski JM, Brown DG, Neufeld JD. PANDAseq: paired-end assembler for illumina sequences. BMC Bioinformatics. 2012;13:31. doi: 10.1186/1471-2105-13-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Briney B, Le K, Zhu J, Burton DR. Clonify: unseeded antibody lineage assignment from next-generation sequencing data. Sci Rep. 2016;6:23901. doi: 10.1038/srep23901. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.