Abstract
The salivary gland section in the 4th edition of the World Health Organization classification of head and neck tumors features the description and inclusion of several entities, the most significant of which is represented by (mammary analogue) secretory carcinoma. This entity was extracted mainly from acinic cell carcinoma based on recapitulation of breast secretory carcinoma and a shared ETV6-NTRK3 gene fusion. Also new is the subsection of “Other epithelial lesions,” for which key entities include sclerosing polycystic adenosis and intercalated duct hyperplasia. Many entities have been compressed into their broader categories given clinical and morphologic similarities, or transitioned to a different grouping as was the case with low-grade cribriform cystadenocarcinoma reclassified as intraductal carcinoma (with the applied qualifier of low-grade). Specific grade has been removed from the names of the salivary gland entities such as polymorphous adenocarcinoma, providing pathologists flexibility in assigning grade and allowing for recognition of a broader spectrum within an entity. Cribriform adenocarcinoma of (minor) salivary gland origin continues to be divisive in terms of whether it should be recognized as a distinct category. This chapter also features new key concepts such as high-grade transformation. The new paradigm of translocations and gene fusions being common in salivary gland tumors is featured heavily in this chapter.
Keywords: World Health Organization, Salivary gland, Translocations, Molecular, Correlative, Neoplasia, Classification
Introduction
Despite their rarity, salivary gland neoplasms show a diversity that is arguably unparalleled in comparison by any other organ. This extensive taxonomy is reflected even in earlier versions of the World Health Organization (WHO) classification. In the past decade since the most recent iteration (i.e. the 3rd edition of the “Blue Book”),[1] some new entities and variant morphologies have been described, some entities have been removed or collapsed into another category, and criterias for diagnosis of existing lesions have been modified. This edition deviates somewhat from the 3rd edition by discussing many of the non-neoplastic and epithelial proliferations as well. Entities within the soft tissue category have also been expanded slightly. As expected, ancillary immunohistochemical markers have continued to evolve. However, of note, the understanding of the molecular pathogenesis of salivary gland neoplasia has expanded rapidly, and several key defining alterations have made their way into clinical settings. The paradigm of defining translocations and gene fusions seen particularly in monomorphic salivary gland tumors [2] features heavily in the 4th edition given its importance to key tumor types such as adenoid cystic carcinoma, mucoepidermoid carcinoma, (mammary analogue) secretory carcinoma, and even pleomorphic adenoma. Other molecular alterations that are important diagnostically are summarized when relevant. While many of these developments have clarified and improved diagnosis of certain entities, some have led to controversy, particularly with respect to polymorphous (low-grade) adenocarcinoma. This review highlights the new entities, variants, changes in nomenclature and criteria for existing entities that are included in the 4th edition of the WHO classification of head and neck tumors as guidelines for diagnosis.
Newly Listed Entities and Variants
Secretory Carcinoma
Mammary analogue secretory carcinoma (MASC), first descibed in 2010, is a salivary gland malignancy that was essentially culled from acinic cell carcinomas and adenocarcinomas not otherwise specified [3, 4]. It is named for its recapitulation of secretory carcinoma of breast, along with its shared ETV6-NTRK3 gene fusion. Given this similarity and in an effort to standardize nomenclature across organ sites, the official designation for this entity is now simply “secretory carcinoma.”
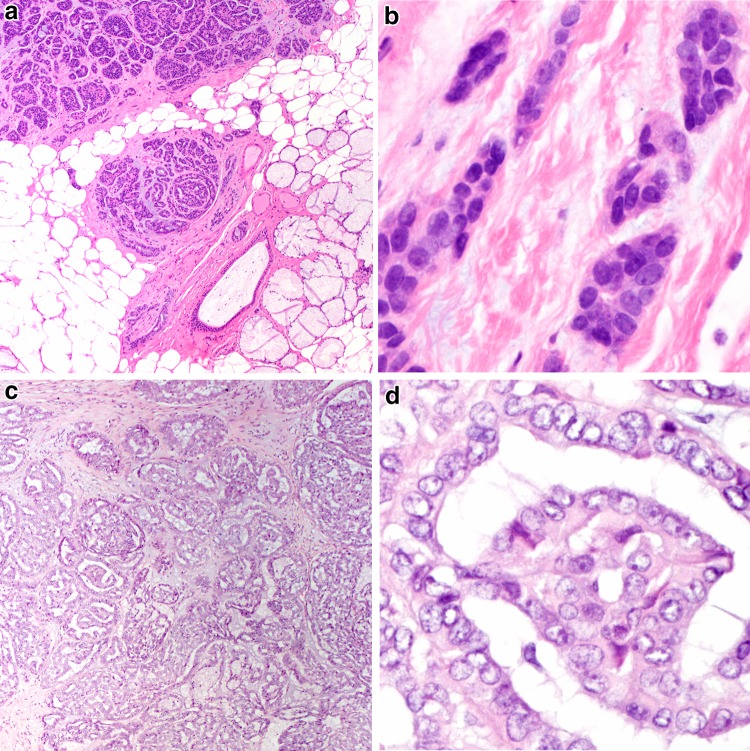
Acinic cell carcinoma serves as the best point of comparison for secretory carcinoma. Secretory carcinoma differs from acinic cell carcinoma epidemiologically in that the sex predilection is more equal, and secretory carcinoma arise in minor salivary sites more frequently than acinic cell carcinoma [3, 5]. Histologically, secretory carcinoma share nearly identical growth patterns to acinic cell carcinoma (Fig. 1a, b), but instead show a multivacuolated eosinophilic cytoplasm, often with luminal and intracytoplasmic mucin and no true zymogen granules. Papillary cystic architecture is now consider rare in true acinic cell carcinoma, being far more common in secretory carcinoma. Both secretory carcinoma and acinic cell carcinoma may show PAS positivity after diastase treatment, but the pattern in secretory carcinoma is globular (indicative of mucin) while that in acinic cell carcinoma is granular (Fig. 1a, b inset). Secretory carcinoma is S100, and mammaglobin positive, and typically negative for DOG1, while acinic cell carcinoma shows the opposite staining profile (Fig. 1c–f) [6].
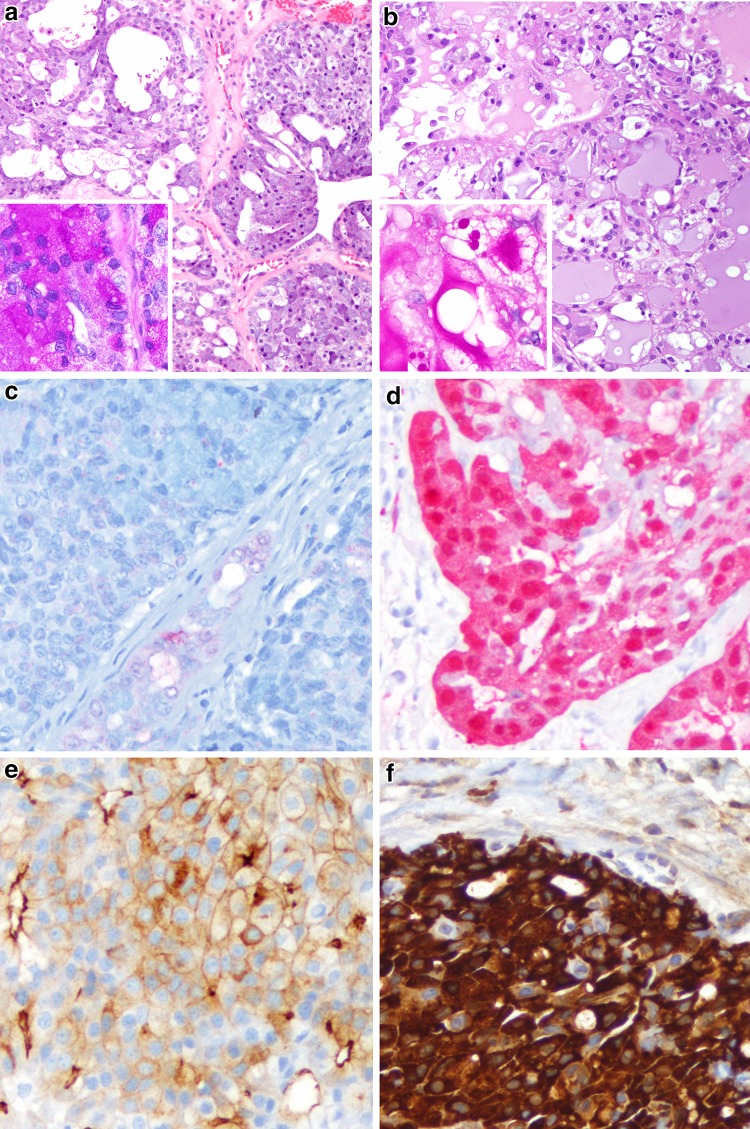
Fig. 1.
A comparison of acinic cell carcinoma and (mammary analogue) secretory carcinoma. a This acinic cell carcinoma shows a solid to follicular patterned proliferation of lightly basophilic acinar type cells (H&E, 100x). Inset: PAS after diastase demonstrates a granular staining in keeping with zymogen granules (H&E, 400x). b This secretory carcinoma shows a follicular pattern but with eosinophilic vacuolated cells and mucinous luminal secretions (H&E, 100x). Inset: PAS after diastase highlights globular and luminal staining in keeping with mucin (H&E, 400x). c This acinic cell carcinoma is S100 negative (200x), while the d secretory carcinoma is S100 positive (200x). e Acinic cell carcinomas show consistent apical and membranous DOG-1 staining (200x). f Secretory carcinomas typically show strong mammaglobin staining (200x)
As noted above, secretory carcinoma usually harbors a t(12;15)(p13;q25) resulting in an ETV6-NTRK3 gene fusion [4]. A subset that are more infiltrative and sclerosing show ETV6 rearrangements with a yet unknown fusion partner [7]. Secretory carcinoma is typically indolent like acinic cell carcinoma, but may show a slightly higher lymph node metastatic rate (up to 25%) than true acinic cell carcinoma [8]. Prognostic features include stage and high-grade transformation (see below) [9]. With the expansion of selective TRK inhibitors, recognition of advanced stage cases of secretory carcinoma may eventually have direct therapeutic relevance [10].
Sclerosing Polycystic Adenosis
While sclerosing polycystic adenosis (SPAN) was described long before the 3rd edition of the salivary gland section,[11] it is a new entry and represents a major entity in the new “Other epithelial lesions” category in the 4th edition. SPAN is named for its resemblance to fibrocystic change and sclerosing adenosis of the breast. The age range is broad (typically 4th decade), with a slight female predilection. The vast majority are parotid lesions.
The characteristic appearance is that of a well circumscribed tubulocystic proliferation of glands within a sclerotic stroma (Fig. 2a). Duct morphology ranges from intercalated duct like to apocrine (Fig. 2b). A characteristic feature is the presence of acini with aberrant coarse red zymogen granules (Fig. 2c). SPAN is occasionally multifocal.
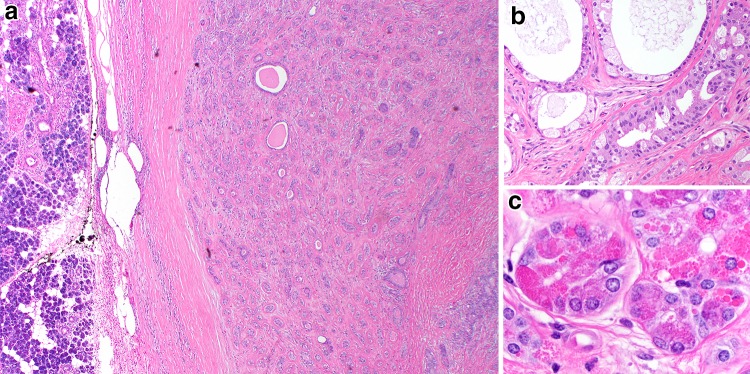
Fig. 2.
Sclerosing polycystic adenosis. a This lesion consists of a tubular to cystic proliferation of glands embedded in a highly sclerotic stroma (H&E, 20x). b Apocrine and secretory areas with histiocytes are typical (H&E, 100x), as are c acinar areas with abnormal red, coarse zymogen granules (H&E, 400x)
Studies of the X-chromosome inactivation pattern have suggested that SPAN is a clonal process suggesting that perhaps this entity is more appropriately placed in the neoplastic category [12]. The recurrence rate is as high as 11%, likely secondary to incomplete excision and multifocality. SPAN ductal components may become proliferative with an appearance similar to intraductal carcinoma [13]. However, only one case of a salivary duct carcinoma arising after a multiply recurrent SPAN has been described [14].
Remaining “Other Epithelial Lesions”
Intercalated duct hyperplasia (IDH) is a proliferation of intercalated duct caliber tubules that is mainly of interest given its reported association with other salivary gland tumors [15]. It is thought that IDH may represent a precursor lesion, particularly to basal cell adenoma and perhaps epithelial-myoepithelial carcinoma [15–17]. IDHs are most frequently parotid gland lesions, and are as a rule less than 1 cm. They are usually found incidentally, and only rarely present as masses. Subclassification of intercalated duct lesions has been proposed. Well demarcated/encapsulated nodular lesions are designated as intercalated duct adenoma (Fig. 3a) while the term IDH is retained for unencapsulated, expansile proliferations that retain the lobular architecture of the ductoacinar unit.
Fig. 3.
Select “other epithelial lesions.” a Intercalated duct lesion demonstrating a rounded well circumscribed proliferation of tubules with the same caliber as intercalated ducts. Such lesions are designated as “intercalated duct adenomas.” (H&E, 40x). b Clear cell oncocytosis demonstrating two well demarcated nodules (H&E, 20x). This lesion is reminiscent of metastatic renal cell carcinoma but is devoid of the vascularity and hemorrhage typical of this
Nodular oncocytic hyperplasia and lymphoepithelial lesion are well characterized entities that now have their own entries in the current edition. Nodular oncocytic hyperplasia is almost exclusively a lesion of the parotid and peaks in the 5th to 6th decade. It consists of multiple unencapsulated solid to tubulotrabecular patterned nodules of oncocytes, occasionally with marked clear cell change (Fig. 3b, so called clear cell oncocytosis), the latter being more frequently bilateral and prone to recurrence [18]. In contrast to oncocytoma, there is no dominant encapsulated lesion. Clear cell predominance may mimic the appearance of metastatic renal cell carcinoma, though nodular oncocytic hyperplasia does not show the hemorrhage or vascularity typically seen in renal cell carcinoma [19]. Lymphoepithelial lesions are pathognomonic for lymphoepithelial sialadenitis that is seen in Sjögren syndrome [20]. Both cystic and non-cystic versions may mimic a tumor. Lymphoepithelial lesions are characterized by metaplastic ducts infiltrated by lymphocytes. The lymphoid component effaces the surrounding acini and contains germinal centers. When cystic, lymphoepithelial lesions may overlap with the cystic lymphoid hyperplasia seen in immunosuppressed patients [21]. When malignant transformation occurs, it is most frequently the lymphoid component, resulting in MALT lymphoma [22].
Soft Tissue Lesions
In addition to hemangioma, which is the most common parotid tumor in infancy, the new edition now includes lipoma and nodular fasciitis. Lipomas are rare in salivary gland (<0.5%) and usually involve the parotid. They are well circumscribed and are designated as sialolipoma if they have an intratumoral ductal component. Oncocytic lipoadenoma appears to be a distinctive subtype of sialolipoma with oncocytes and sebaceous rests [23]. Nodular fasciitis may rarely involve the superficial parotid gland. As with other sites, these tend to occur in young adults (3rd to 4th decade) and show a characteristic rapid growth mimicking an aggressive neoplasm [23]. Histologically similar to nodular fasciitis at other sites, parotid lesions consist of a well demarcated but focally permeative myofibroblastic proliferation that is cellular but with little atypia. Cells vary from spindled to more stellate like. Nodular fasciitis is indolent and may regress spontaneously. The majority are now known to harbor an USP6 gene fusion [24]. Nodular fasciitis must be distinguished from more aggressive spindle cell sarcomas, as well as spindle cell myoepithelial cell rich neoplasms [23]. Other considerations in this region include schwannoma and solitary fibrous tumor.
Vanished/Collapsed Entities and Terminology Shifts
The removal of some entities represents a reworking of our conceptual framework (i.e., low-grade cribriform cystadenocarcinoma [25] to intraductal carcinoma, low-grade) and is discussed below. Other entities were more formally collapsed (i.e., sebaceous and non-sebaceous lymphadenomas,[26] inverted and intraductal papillomas [27] into their ICD-O based parent entities (lymphadenoma, and ductal papilloma)).
Categorical Lumping
Both sebaceous and non-sebaceous lymphadenomas are benign non-Warthin lymphoid stroma rich tumors with variable mixtures of ductal, squamous, and myoepithelial elements; the main morphologic distinction is the presence or absence of sebaceous elements (Fig. 4a, b). While non-sebaceous lymphadenomas do have a stronger female predilection, they are overall biologically similar. Only rare cases of malignant transformation are described [28].
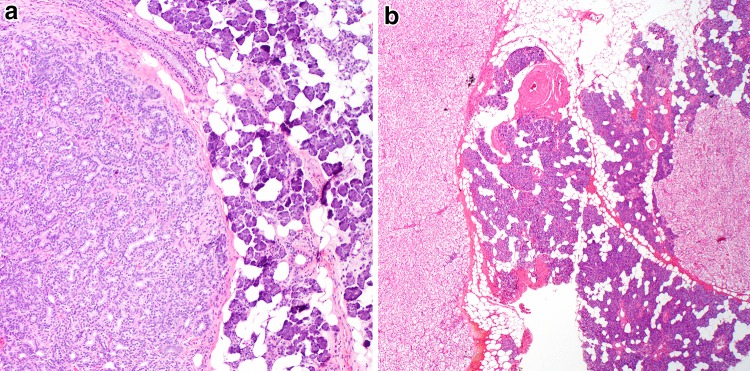
Fig. 4.
Lymphadenomas. a Sebaceous lymphadenomas consist of a well circumscribed solid and cystic proliferation of squamous and sebaceous elements (H&E, 100x). Similarly, non-sebaceous adenomas are also will demarcated with prominent lymphoid stroma. b They also demonstrate trabeculae of basaloid squamoid elements and may be variably microcystic, with the main distinction being the absence of sebaceous elements (H&E, 100x)
Inverted ductal papillomas appear to arise at the junction between minor salivary excretory duct and squamous mucosa and shows a nested transitional type epithelial proliferation with overlying surface ductal columnar epithelium, reminiscent of a Schneiderian type inverted papilloma. Intraductal papillomas appear to arise more distally in the duct and consist of papillary proliferations of bland columnar ductal cells [29].
With improved classification, the terms cystadenocarcinoma [30] and mucinous adenocarcinoma [31] have become diagnoses of exclusion and are now part of adenocarcinoma, not otherwise specified. Similarly, the exceptionally rare entity sebaceous lymphadenocarcinoma [32] has been subsumed under the malignant transformation portion of the “Lymphadenoma” chapter.
Metastasizing Pleomorphic Adenoma
The prior edition of this section classified metastasizing pleomorphic adenoma (MPA) under the “Malignant tumors” given its aggressive biologic behavior [32]. MPA is a tumor that is histologically identical to PA, but has metastasized regionally or distantly. It arises after multiple recurrences and typically spreads to lung and bone and as many as 40% of patients die with disease [33]. However, in the 4th edition, MPA has been relegated to subcategory status under the benign, “pleomorphic adenoma” section, since these are histologically indistinguishable. Thus, a word of caution regarding current edition is that despite this reorganization, MPA is still considered biologically aggressive.
Conceptual Changes and Controversies
Polymorphous Adenocarcinoma
Polymorphous low-grade adenocarcinoma, now shortened to polymorphous adenocarcinoma (PAC) is without a doubt the most contentious entity for this iteration of the WHO classification for salivary gland tumors. While described earlier, the term polymorphous low-grade adenocarcinoma was first used in 1984 by Evans and Batsakis [34] to describe an infiltrative salivary tumor with a variety of growth patterns but bland nuclei. The growth pattern and stromal characteristics mimic those of adenoid cystic carcinoma and have been historically diagnostically challenging to differentiate. The importance of distinction however lies in the much more indolent behavior of PAC as compared to adenoid cystic carcinoma.
PAC almost exclusively occur in minor salivary sites with palate being the most frequent. They predominate in the 5th decade and show a female predilection of 2:1 [35–37]. Histologically they are infiltrative and may show a spectrum of tubular, fascicular, cribriform, papillary or solid architecture. Classically they demonstrate considerable neurotropism and often show a targetoid appearance (Fig. 5a). However, as noted above, tumor cells are monomorphic and monotypic with a (terminal) ductal phenotype, consisting of uniform ovoid vesicular nuclei and scant to moderate lightly eosinophilic cytoplasm (Fig. 5b). S100 is diffusely strongly positive in these tumors,[35, 37] but they do not have a prominent basal or myoepithelial component. The frequent finding of p63 immunoreactivity may at times cause confusion. However, the staining is not patterned as seen with truly biphasic tumors such as adenoid cystic carcinoma and pleomorphic adenoma, and if necessary, the more myoepithelial cell specific ΔNp63 antibody, p40 will still be negative [38].
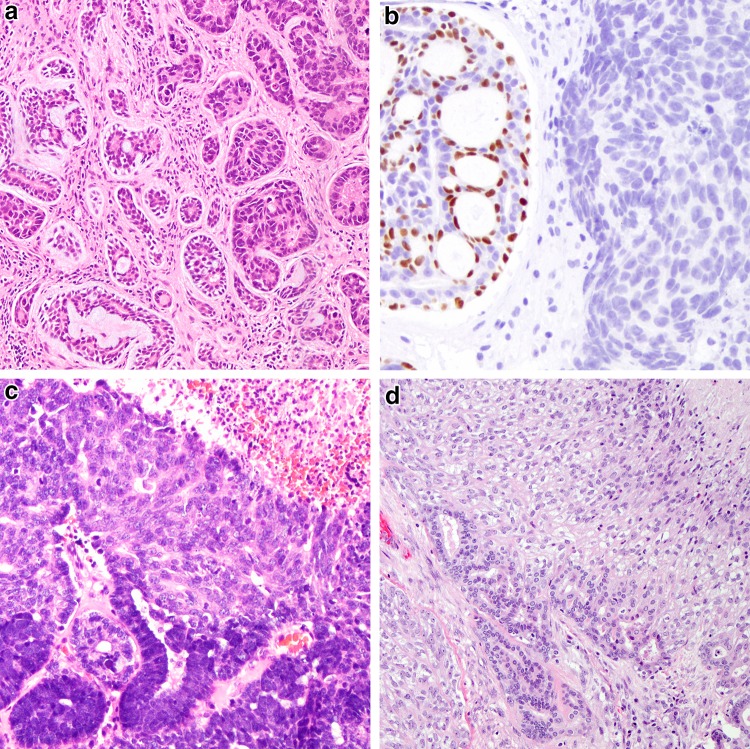
Fig. 5.
Polymorphous adenocarcinoma and cribriform adenocarcinoma of (minor) salivary gland. a Classic polymorphous adenocarcinoma, low-grade, demonstrates an infiltrative, neurotropic or targetoid pattern composed of tubulofascicular growth (H&E, 40x). b Tumor cells are phenotypically monomorphic and ductal, and composed of cells with ovoid vesicular nuclei with slight membrane irregularities and inconspicuous nuclei (H&E, 200x). c Cribriform adenocarcinoma of minor salivary gland actually consists of papillary glomeruloid structures as well as cribriform spaces (H&E, 40x). d Tumor cells are similarly monomorphic and ductal, but contain slightly larger nuclei and more markedly cleared chromatin remincent of (classic) papillary thyroid carcinoma nuclei
As a whole PAC show local recurrence rates of 10–33% and regional metastases in 9–15%. Distant metastases and death from disease are exceptionally rare [35–37]. However, even early on in the existence of this entity, papillary patterned tumors were thought to behave more aggressively with a greater capacity for regional and distant spread [36]. Additionally, at base of tongue sites a distinctive cribriform architecture was noted along with more overtly cleared (papillary thyroid carcinoma-like) nuclei initiating the proposal for reclassification of this subset of tumors as cribriform adenocarcinoma of tongue, and subsequently cribriform adenocarcinoma of (minor) salivary gland (CAMSG) (Fig. 5c, d) [39, 40]. Despite the presence of cribriform in the proposed name for this tumor, the growth pattern is actually mainly papillary glomeruloid, thus incorporating the previously noted morphologic feature of aggression. CAMSG morphology does tend to show more cytonuclear atypia and a greater capacity for regional and possibly distant metastasis, though no differences in survival have been documented to date. Aside from this morphology, overt high-grade transformation has been described as well (see also below) [41].
These developments raised two major issues with the term polymorphous low-grade adenocarcinoma. First, are these tumors always low-grade? Based on data summarized above, this is not necessarily the case, thus justifying removal of the term low-grade as a default. Thus on a case by case basis, PAC can be assigned a grade which in the vast majority of cases would still be low-grade, but this allows for recognition when a tumor deviates from the norm. The point of contention here, however, is that there is no structured evidence based grading scheme for PAC, unlike other tumor types such as mucoepidermoid carcinoma. Additionally, since the vast majority are still low-grade, the necessity of a name change is questionable.
The second issue raised is whether sufficient evidence exists to justify separating CAMSG as a distinct entity from PAC. Proponents for this separation point to the difference in site distribution, preponderance of papillary/glomeruloid or cribriform morphology, exaggerated nuclear features, and regional aggressiveness as sufficient evidence to establish this as a new entity. Furthermore, recent studies indicate that rearrangements of PRKD1-3, including ARID1A-PRKD1 and DDX3X-PRKD1 gene fusions, are seen in ~80% of cases with CAMSG morphology, and in less than 10% of cases with classic PAC morphology [42]. In contrast, PRKD1 E710D mutations are largely restricted to classic PAC, with only about 10% of CAMSG type tumors showing a mutation [43, 44]. The counterpoints to these arguments are that the findings in the literature are numerically insufficient, there is still some morphologic and genotypic overlap, and despite the regional aggressiveness, survival differences have not yet been established. Thus despite extensive debate, the decision was to retain a more conservative and unifying approach and leave CAMSG within the PAC subheading for this edition. This is also in line with the fact that both PAC and CAMSG are driven by genes in the same family, indicating that they are variants of one spectrum.
Grading in Mucoepidermoid Carcinoma
The 4th edition is less dogmatic about application of grading in mucoepidermoid carcinoma (MEC). In contrast to the 3rd edition,[45] in which the Goode et al. [46] (a.k.a AFIP) system was featured, the 4th edition does not endorse a specific grading scheme. Several grading schemes exist in the literature each with advantages and limitations, specifically with respect to intermediate-grade tumors [46–50]. Furthermore, studies are variably prone to misclassification artifact in the high-grade category as well. Given the lack of consensus on optimal grading criteria, only the general features of low-, intermediate-, and high-grade tumors are outlined.
High-Grade Transformation
New relative to the prior edition of this chapter is the term high-grade transformation (HGT). HGT is the preferred terminology (over dedifferentiation) for progression of a (usually) lower grade carcinoma with conventional morphology into a pleomorphic high-grade carcinoma [51]. The rationale for recognition of this morphology lies in its aggressive behavior and thus potential differences in clinical management. Tumors for which this phenomenon is well characterized include acinic cell carcinoma, adenoid cystic carcinoma, and epithelial-myoepithelial carcinoma (Fig. 6a–d). For acinic cell carcinoma with HGT a side by side comparison with conventional acinic cell carcinoma confirms a more aggressive behavior even when corrected for age. Secretory carcinoma and PAC have also been reported to rarely undergo high-grade transformation, which was part of the impetus to drop the term “low-grade” from PAC [9, 41].
Fig. 6.
High-grade transformation in carcinomas. a Adenoid cystic carcinoma with high-grade transformation showing a transition from a more typical cribriform growth pattern consisting of angulated, hyperchromatic but monomorphic nuclei (left) to a markedly pleomorphic adenocarcinoma (right) (H&E, 100x). b A hallmark of transformation of biphasic carcinomas like adenoid cystic carcinoma is ductal overgrowth and loss of biphasic cell composition as demonstrated by the loss of p63 staining in the transformed component (right) (200x). c Acinic cell carcinoma with high-grade transformation demonstrating transition from conventional acinic cell carcinoma (bottom) with deeply basophilic zymogen granules to a solid carcinoma with necrosis (H&E, 100x). d Epithelial myoepithelial carcinoma with high-grade transformation of myoepithelial cell component as demonstrated by the overgrowth of the abluminal clear cell component, pleomorphism and necrosis (top right) (H&E, 100x)
Acinic cell carcinoma, secretory carcinoma and PAC are monotypic/monophasic tumors that progress to nondescript pleomorphic cribriform to solid adenocarcinoma types. Adenoid cystic carcinoma and epithelial myoepithelial carcinomas are biphasic. To date adenoid cystic carcinoma with HGT reflects progression of the ductal component beyond solid growth pattern alone to an adenocarcinoma morphologically similar to the transformed components of the aforementioned carcinoma types. Given this overlap, distinction between these entities, and even adenocarcinoma not otherwise specified would require documentation of a conventional component, or a defining molecular alteration (i.e., ETV6-NTRK3 for secretory carcinoma with HGT). Epithelial myoepithelial carcinoma is somewhat unique in that the transformed phenotype may range from myoepithelial to ductal to undifferentiated [52]. Tumors with HGT may mimic salivary duct carcinoma, and in fact, most non-apocrine androgen receptor negative “salivary duct carcinomas” are in fact unrecognized HGT of another tumor type [53].
Carcinoma Ex Pleomorphic Adenoma
Carcinoma ex pleomorphic adenoma (CAxPA) represents a malignancy arising in a pleomorphic adenoma [54, 55]. It is a well-recognized phenomenon accounting for ~12% of all salivary carcinomas. Peak incidence is in the 6th to 7th decade (about 1–2 decades later than that of pleomorphic adenoma) [55, 56].
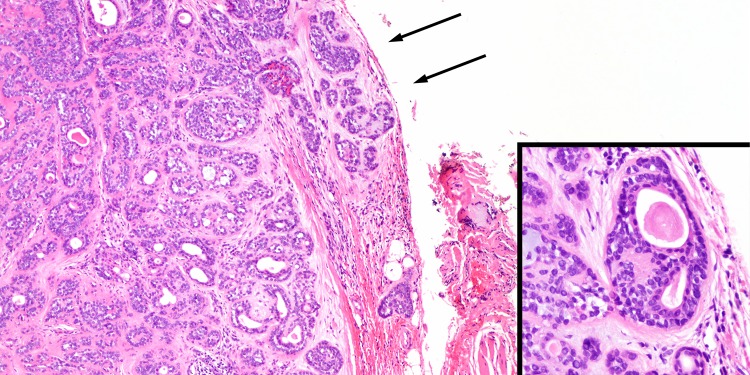
The major changes in this iteration of the salivary gland tumor classification center on how key features and concepts are framed. Importantly, in this edition it is explicitly stated that CAxPA should no longer be considered a standalone diagnosis, biology is determined by both extent and carcinoma subtype. While the majority are now recognized as salivary duct carcinomas, other morphologic subtypes including myoepithelial carcinoma and epithelial-myoepithelial carcinoma also exist and have less aggression than the prototypical CAxPA [57]. The concept of stratification by extent of invasion was previously introduced and expanded in this edition. CAxPA can be classified as intracapsular, minimally invasive and (widely)-invasive. Ironically with this edition, the definition of minimal invasion is less certain. The initial cut-off of 1.5 mm to delineate minimal invasion has been deemed arbitrary and too restrictive (Fig. 7), [58] as several studies have justified a 4–6 mm cut-off as still prognostically relevant [57, 59–61]. The current edition is realistic in the approach suggesting that the optimal cut-off to define minimal invasion requires further validation.
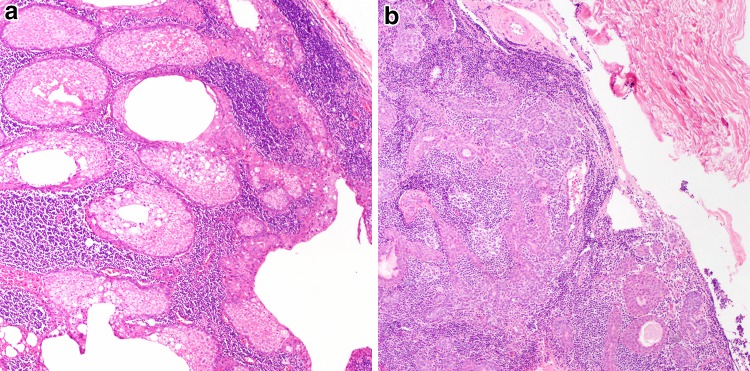
Fig. 7.
Minimally invasive carcinoma ex pleomorphic adenoma. a This tumor shows very limited i.e. <1.5 mm, extension beyond its capsule (arrows) (H&E, 40x). However, even extending the cut-off to 4–6 mm appears to still correlate with favorable outcome. b Tumors with minimal invasion may be difficult to subtype given the limited carcinomatous component, but here the biphasic appearance consisting of luminal eosinophilic ductal cells and clear abluminal myoepithelial cells justifies designation as epithelial-myoepithelial carcinoma
Intraductal Carcinoma
The entities, low-grade cribriform cystadenocarcinoma,[25] also known as “low-grade salivary duct carcinoma,” and (conventional) salivary duct carcinoma in-situ are now collectively categorized as intraductal carcinomas, low-grade and high-grade respectively. The bulk of this section focuses on low-grade intraductal carcinomas which, as a rule, are parotid tumors, and only rarely noted at other sites. They show a variety of growth patterns, both solid and cystic, ranging from cribriform to solid to micropapillary reminiscent of low-grade ductal carcinoma in situ or atypical ductal hyperplasia of the breast (Fig. 8a) [62]. Tumor cells are monomorphic and ovoid and evenly spaced with round nuclei and scant eosinophilic cytoplasm (Fig. 8b). Oncocytic and apocrine change and intermediate grade cytonuclear features are uncommon. Focal infiltration may be noted, but overall, low-grade intraductal carcinoma behaves as expected for a non-invasive tumor, with no reported recurrences. Intraductal carcinomas are delimited by a basal/myoepithelial layer, which is p63 and often muscle marker positive (Fig. 8c).
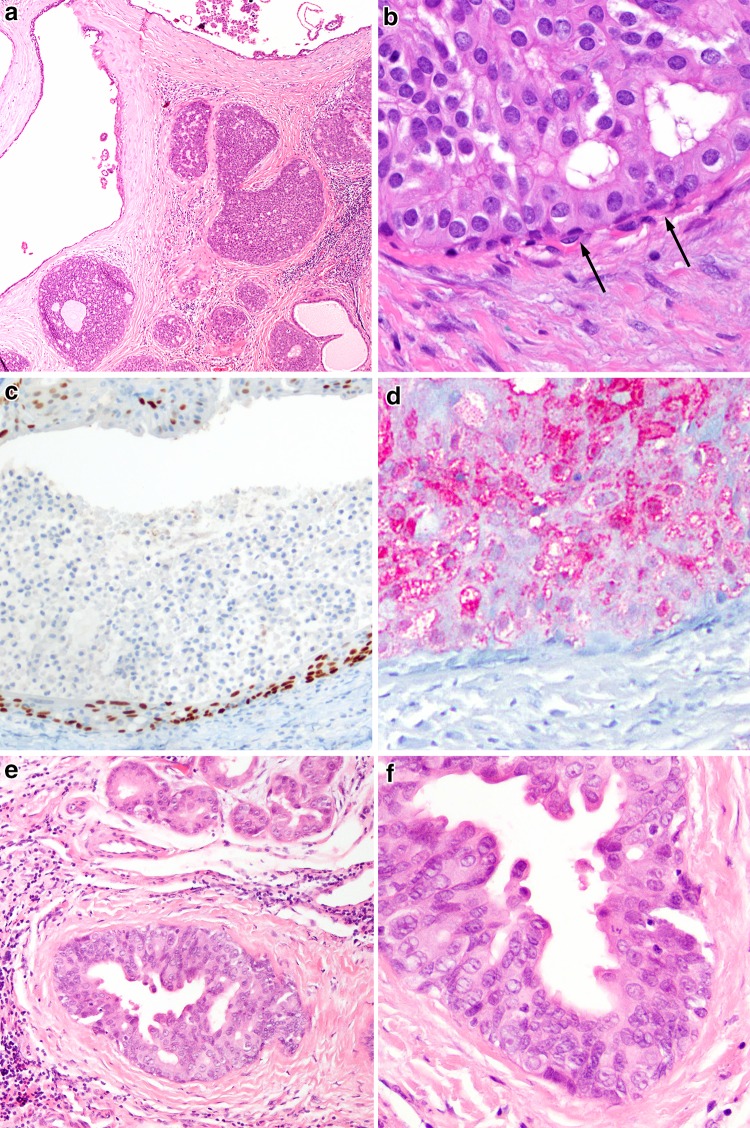
Fig. 8.
Intraductal carcinoma. a Intraductal carcinoma, low-grade demonstrating a typical cystic to solid/cribriform proliferation of cells reminiscent of low-grade ductal carcinoma in situ of breast (H&E, 40x). b Cells are monomorphic and demonstrate variable eosinophilic cytoplasm (H&E, 400x), and delimited by an attenuated basal/myoepithelial cell layer (arrows). c This layer can be readily demonstrated by a p63 immunostain (100x). d The actual lesional cells are S100 positive (100x). e In contrast, intraductal carcinoma, high-grade, demonstrates an appearance more reminiscent of salivary duct carcinoma. Cancerization of acini (top) is not uncommon (H&E, 100x). f The nuclear size variation, eosinophilic cytoplasm and abortive decapitation secretions are in keeping with an apocrine phenotype akin to salivary duct carcinoma (H&E, 200x)
While not explicitly stated, low-grade intraductal carcinomas and high-grade intraductal carcinomas are biologically distinct. Low-grade tumors are S100 positive (Fig. 8d), and typically negative for androgen receptor [62]. High-grade intraductal carcinomas, have the same phenotype as salivary duct carcinomas,[63] and have well developed apocrine features (Fig.8e, f) and are androgen receptor positive and S100 negative. There appears to be very little overlap between these phenotypes. Additionally, high-grade intraductal carcinoma should be diagnosed cautiously and on resections, thorough sampling must be performed to exclude an invasive component. Here, the stakes are potentially higher if invasion (i.e., salivary duct carcinoma) is noted.
Key Molecular Alterations
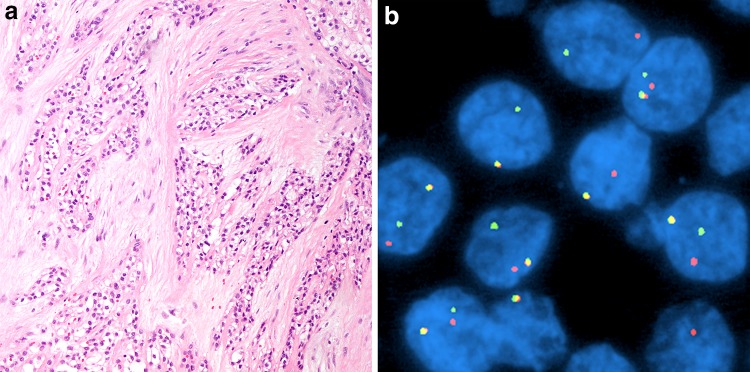
Key molecular alterations in salivary gland tumors are summarized in Table 1[4, 42–44, 64–68]. Of these the majority are mentioned in the new edition of this chapter. Currently, none of these molecular alterations are regarded necessary for diagnosis, prognosis or treatment of tumors. However, in many cases, when present, many molecular alterations, particularly the fusion genes are diagnosis defining. Importantly, the documentation of a defining EWSR1-ATF1 fusion in (hyalinizing) clear cell carcinoma along with the distinctive morphologic appearance (Fig. 9a, b) have effectively removed the previous suffix of “not otherwise specified”[69]. Moreover, recent studies using in particular next generation sequencing have identified several additional diagnosis defining gene fusions now making it possible to discern a gene fusion network in salivary gland carcinomas similar to what previously have been described in for example soft tissue sarcomas and hematological malignancies [68] (Table 1). Available data indicate that these fusions and other genomic alterations will become increasingly important as diagnostic biomarkers in the future.
Table 1.
Key genomic alterations in salivary gland tumors
| Tumor | Chromosomal rearrangement | Gene | Prevalence | |
|---|---|---|---|---|
| Pleomorphic adenoma | 8q12 translocations | PLAG1 fusions | > 50% | |
| 12q13-15 translocations | HMGA2 fusions | ~ 15% | ||
| Carcinoma ex pleomorphic adenoma | 8q12 translocations | PLAG1 fusions | NDA | |
| 12q13-15 translocations | HMGA2 fusions | NDA | ||
| 12q15 amplification | MDM2 | ~ 50% | ||
| TP53 mutation | ~ 25–50% | |||
| Epithelial myoepithelial carcinoma | HRAS mutation | 25% | ||
| Tubulotrabecular basal cell adenoma | CTTNB1 mutation | 60–70% | ||
| Membranous basal cell adenoma | 16q12-13 deletion | CYLD LOH/mutation | 75–80% | |
| Mucoepidermoid carcinoma | t (11;19) (q21;p13) | CRTC1-MAML2 | 40–80% | |
| t (11;15) (q21;q26) | CRTC3-MAML2 | ~ 5% | ||
| 9p21.3 | CDKN2A deletion | ~ 35% | ||
| Salivary duct carcinoma | TP53 mutation | 55% | ||
| 17q21.1 | ERBB2 amplification | ~ 40% | ||
| PIK3CA mutation | ~ 20% | |||
| Xq12 | AR copy gain | ~ 35% | ||
| Adenoid cystic carcinoma | 6q22-23 translocations | MYB fusion/activation# | ~ 80% | |
| 8q13 translocations | MYBL1 fusion/activation# | ~ 10% | ||
| NOTCH1 mutation | 5–10% | |||
| Secretory carcinoma (mammary analogue) | t (12;15) (p13;q25) | ETV6-NTRK3 | ~ 95–98% | |
| t (12;?) (p13;?) | ETV6-X | ~ 2–5% | ||
| Clear cell carcinoma (hyalinizing) | t (12;22) (q21;q12) | EWSR1-ATF1 | ~ 80–90% | |
| PAC/CAMSG | PRKD1 mutation | CAMSG: <10% | PAC: ~75% | |
| t (1;14) (p36.11;q12) | ARID1A-PRKD1 | |||
| t (X;14) (p11.4;q12) | DDX3X-PRKD1 | CAMSG: ~80% | PAC: <10% | |
| 19q13.32 | PRKD2 rearrangement | |||
| 2p21 | PRKD3 rearrangement | |||
NDA no data available, LOH loss of heterozygosity, MASC (mammary analogue)-secretory carcinoma, PAC polymorphous adenocarcinoma, CAMSG cribriform adenocarcinoma of (minor)-salivary gland origin
#Include cases with MYB and MYBL1 activation due to juxtaposition of genes (e.g. NFIB, RAD51B, and TGFBR3) with strong enhancer elements close to MYB or MYBL1
Fig. 9.
(Hyalinizing) clear cell carcinoma. a This is a clear cell tumor composed of monomorphic nests and cords of cells embedded in a hyalinized stroma (H&E, 100x). The peritumoral stroma is hyalinized while the intervening stroma is fibrocellular. b This tumor is characterized by an EWSR1-ATF1 fusion demonstratable by EWSR1 break-apart fluorescence in situ hybridization (separated red and green signals)
Conclusions
Salivary gland tumors remain diverse with new entities such as secretory carcinoma included in the 4th edition of the classification. The new category “other epithelial lesions,” adds tumor like lesions such as sclerosing polycystic adenosis and potential precursor lesion as IDH. Many entities have been combined into broader categories to streamline classification, including intraductal carcinomas. Specific grade has been removed from the names of salivary gland entities such as PAC, providing pathologists flexibility in assigning grade and allowing for recognition of a broader spectrum within an entity. Despite heated discussion, CAMSG remains within the spectrum of PAC. New key concepts such as high-grade transformation are now part of the discussion on the relevant entities. The new paradigm of translocations and gene fusions being common in salivary gland tumors is featured heavily in this chapter.
Compliance with Ethical Standards
Conflict of interest
The authors have no financial or other conflicts of interest to report.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Special Issue: World Health Organization Classification Update
References
- 1.Eveson JW, Auclair PL, Gnepp DR, El-Naggar AK. Tumours of the salivary gland. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. World Health Organization classification of tumours: pathology and genetics of head and neck tumours. Lyon: IARC; 2005. p. 164. [Google Scholar]
- 2.Stenman G. Fusion oncogenes in salivary gland tumors: molecular and clinical consequences. Head Neck Pathol. 2013;7(Suppl 1):S12–S19. doi: 10.1007/s12105-013-0462-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiosea SI, Griffith C, Assaad A, Seethala RR. The profile of acinic cell carcinoma after recognition of mammary analog secretory carcinoma. Am J Surg Pathol. 2012;36(3):343–350. doi: 10.1097/PAS.0b013e318242a5b0. [DOI] [PubMed] [Google Scholar]
- 4.Skalova A, Vanecek T, Sima R, Laco J, Weinreb I, Perez-Ordonez B, et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol. 2010;34(5):599–608. doi: 10.1097/PAS.0b013e3181d9efcc. [DOI] [PubMed] [Google Scholar]
- 5.Bishop JA, Yonescu R, Batista D, Eisele DW, Westra WH. Most nonparotid “acinic cell carcinomas” represent mammary analog secretory carcinomas. Am J Surg Pathol. 2013;37(7):1053–1057. doi: 10.1097/PAS.0b013e3182841554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chenevert J, Duvvuri U, Chiosea S, Dacic S, Cieply K, Kim J, et al. DOG1: a novel marker of salivary acinar and intercalated duct differentiation. Mod Pathol. 2012;25(7):919–929. doi: 10.1038/modpathol.2012.57. [DOI] [PubMed] [Google Scholar]
- 7.Skalova A, Vanecek T, Simpson RH, Laco J, Majewska H, Baneckova M, et al. Mammary analogue secretory carcinoma of salivary glands: molecular analysis of 25 ETV6 gene rearranged tumors with lack of detection of classical ETV6-NTRK3 fusion transcript by standard RT-PCR: report of 4 cases harboring ETV6-X gene fusion. Am J Surg Pathol. 2016;40(1):3–13. doi: 10.1097/PAS.0000000000000537. [DOI] [PubMed] [Google Scholar]
- 8.Chiosea SI, Griffith C, Assaad A, Seethala RR. Clinicopathological characterization of mammary analogue secretory carcinoma of salivary glands. Histopathology. 2012;61(3):387–394. doi: 10.1111/j.1365-2559.2012.04232.x. [DOI] [PubMed] [Google Scholar]
- 9.Skalova A, Vanecek T, Majewska H, Laco J, Grossmann P, Simpson RH, et al. Mammary analogue secretory carcinoma of salivary glands with high-grade transformation: report of 3 cases with the ETV6-NTRK3 gene fusion and analysis of TP53, beta-catenin, EGFR, and CCND1 genes. Am J Surg Pathol. 2014;38(1):23–33. doi: 10.1097/PAS.0000000000000088. [DOI] [PubMed] [Google Scholar]
- 10.Drilon A, Li G, Dogan S, Gounder M, Shen R, Arcila M, et al. What hides behind the MASC: clinical response and acquired resistance to entrectinib after ETV6-NTRK3 identification in a mammary analogue secretory carcinoma (MASC) Ann Oncol. 2016;27(5):920–926. doi: 10.1093/annonc/mdw042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith BC, Ellis GL, Slater LJ, Foss RD. Sclerosing polycystic adenosis of major salivary glands. A clinicopathologic analysis of nine cases. Am J Surg Pathol. 1996;20(2):161–170. doi: 10.1097/00000478-199602000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Skalova A, Gnepp DR, Simpson RH, Lewis JE, Janssen D, Sima R, et al. Clonal nature of sclerosing polycystic adenosis of salivary glands demonstrated by using the polymorphism of the human androgen receptor (HUMARA) locus as a marker. Am J Surg Pathol. 2006;30(8):939–944. doi: 10.1097/00000478-200608000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Skalova A, Michal M, Simpson RH, Starek I, Pradna J, Pfaltz M. Sclerosing polycystic adenosis of parotid gland with dysplasia and ductal carcinoma in situ. Report of three cases with immunohistochemical and ultrastructural examination. Virchows Arch. 2002;440(1):29–35. doi: 10.1007/s004280100481. [DOI] [PubMed] [Google Scholar]
- 14.Canas Marques R, Felix A. Invasive carcinoma arising from sclerosing polycystic adenosis of the salivary gland. Virchows Arch. 2014;464(5):621–625. doi: 10.1007/s00428-014-1551-4. [DOI] [PubMed] [Google Scholar]
- 15.Weinreb I, Seethala RR, Hunt JL, Chetty R, Dardick I, Perez-Ordonez B. Intercalated duct lesions of salivary gland: a morphologic spectrum from hyperplasia to adenoma. Am J Surg Pathol. 2009;33(9):1322–1329. doi: 10.1097/PAS.0b013e3181a55c15. [DOI] [PubMed] [Google Scholar]
- 16.Montalli VA, Martinez E, Tincani A, Martins A, Abreu Mdo C, Neves C, et al. Tubular variant of basal cell adenoma shares immunophenotypical features with normal intercalated ducts and is closely related to intercalated duct lesions of salivary gland. Histopathology. 2014;64(6):880–889. doi: 10.1111/his.12339. [DOI] [PubMed] [Google Scholar]
- 17.Chetty R. Intercalated duct hyperplasia: possible relationship to epithelial-myoepithelial carcinoma and hybrid tumours of salivary gland. Histopathology. 2000;37(3):260–263. doi: 10.1046/j.1365-2559.2000.00976.x. [DOI] [PubMed] [Google Scholar]
- 18.Brandwein MS, Huvos AG. Oncocytic tumors of major salivary glands. A study of 68 cases with follow-up of 44 patients. Am J Surg Pathol. 1991;15(6):514–528. doi: 10.1097/00000478-199106000-00002. [DOI] [PubMed] [Google Scholar]
- 19.McHugh JB, Hoschar AP, Dvorakova M, Parwani AV, Barnes EL, Seethala RR. p63 immunohistochemistry differentiates salivary gland oncocytoma and oncocytic carcinoma from metastatic renal cell carcinoma. Head Neck Pathol. 2007;1(2):123–131. doi: 10.1007/s12105-007-0031-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daniels TE. Benign lymphoepithelial lesion and Sjögren’s syndrome. In: Ellis GL, Auclair PL, Gnepp DR, editors. Surgical pathology of the salivary glands. Philadelphia: W.B. Saunders Company; 1991. pp. 83–106. [Google Scholar]
- 21.Kreisel FH, Frater JL, Hassan A, El-Mofty SK. Cystic lymphoid hyperplasia of the parotid gland in HIV-positive and HIV-negative patients: quantitative immunopathology. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109(4):567–574. doi: 10.1016/j.tripleo.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Abbondanzo SL. Extranodal marginal-zone B-cell lymphoma of the salivary gland. Ann Diagn Pathol. 2001;5(4):246–254. doi: 10.1053/adpa.2001.26980. [DOI] [PubMed] [Google Scholar]
- 23.Agaimy A, Ihrler S, Markl B, Lell M, Zenk J, Hartmann A, et al. Lipomatous salivary gland tumors: a series of 31 cases spanning their morphologic spectrum with emphasis on sialolipoma and oncocytic lipoadenoma. Am J Surg Pathol. 2013;37(1):128–137. doi: 10.1097/PAS.0b013e31826731e0. [DOI] [PubMed] [Google Scholar]
- 24.Erickson-Johnson MR, Chou MM, Evers BR, Roth CW, Seys AR, Jin L, et al. Nodular fasciitis: a novel model of transient neoplasia induced by MYH9-USP6 gene fusion. Lab Invest. 2011;91(10):1427–1433. doi: 10.1038/labinvest.2011.118. [DOI] [PubMed] [Google Scholar]
- 25.Brandwein-Gensler MS, Gnepp DR. Low Grade Cribriform Cystadenocarcinoma. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. World Health Organization classification of tumours: pathology and genetics of head and neck tumours. Lyon: IARC; 2005. p. 233. [Google Scholar]
- 26.Gnepp DR, Cheuk W, Chan JKC, Nagao T. Lymphadenomas: Sebaceous and Non-Sebaceous. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. World Health Organization classification of tumours: pathology and genetics of head and neck tumours. Lyon: IARC; 2005. p. 269. [Google Scholar]
- 27.Brannon RB, Sciubba JJ. Ductal Papillomas. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. World Health Organization classification of tumours: pathology and genetics of head and neck tumours. Lyon: IARC; 2005. pp. 270–272. [Google Scholar]
- 28.Seethala RR, Thompson LD, Gnepp DR, Barnes EL, Skalova A, Montone K, et al. Lymphadenoma of the salivary gland: clinicopathological and immunohistochemical analysis of 33 tumors. Mod Pathol. 2012;25(1):26–35. doi: 10.1038/modpathol.2011.135. [DOI] [PubMed] [Google Scholar]
- 29.Brannon RB, Sciubba JJ, Giulani M. Ductal papillomas of salivary gland origin: a report of 19 cases and a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92(1):68–77. doi: 10.1067/moe.2001.115978. [DOI] [PubMed] [Google Scholar]
- 30.Auclair PL. Cystadenocarcinoma. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. World Health Organization classification of tumours: pathology and genetics of head and neck tumours. Lyon: IARC; 2005. p. 232. [Google Scholar]
- 31.Sun KH, Gao Y, Li TJ. Mucinous Adenocarcinoma. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. World Health Organization classification of tumours: pathology and genetics of head and neck tumours. Lyon: IARC; 2005. pp. 233–234. [Google Scholar]
- 32.Gnepp DR. Malignant Sebaceous Tumours. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. World Health Organization classification of tumours: pathology and genetics of head and neck tumours. Lyon: IARC; 2005. p. 231. [Google Scholar]
- 33.Bradley PJ. ‘Metastasizing pleomorphic salivary adenoma’ should now be considered a low-grade malignancy with a lethal potential. Curr Opin Otolaryngol Head Neck Surg. 2005;13(2):123–126. doi: 10.1097/01.moo.0000153450.87288.2a. [DOI] [PubMed] [Google Scholar]
- 34.Evans HL, Batsakis JG. Polymorphous low-grade adenocarcinoma of minor salivary glands. A study of 14 cases of a distinctive neoplasm. Cancer. 1984;53(4):935–942. doi: 10.1002/1097-0142(19840215)53:4<935::AID-CNCR2820530420>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 35.Castle JT, Thompson LD, Frommelt RA, Wenig BM, Kessler HP. Polymorphous low grade adenocarcinoma: a clinicopathologic study of 164 cases. Cancer. 1999;86(2):207–219. doi: 10.1002/(SICI)1097-0142(19990715)86:2<207::AID-CNCR4>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 36.Evans HL, Luna MA. Polymorphous low-grade adenocarcinoma: a study of 40 cases with long-term follow up and an evaluation of the importance of papillary areas. Am J Surg Pathol. 2000;24(10):1319–1328. doi: 10.1097/00000478-200010000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Seethala RR, Johnson JT, Barnes EL, Myers EN. Polymorphous low-grade adenocarcinoma: the University of Pittsburgh experience. Arch Otolaryngol Head Neck Surg. 2010;136(4):385–392. doi: 10.1001/archoto.2010.39. [DOI] [PubMed] [Google Scholar]
- 38.Rooper L, Sharma R, Bishop JA. Polymorphous low grade adenocarcinoma has a consistent p63+/p40- immunophenotype that helps distinguish it from adenoid cystic carcinoma and cellular pleomorphic adenoma. Head Neck Pathol. 2015;9(1):79–84. doi: 10.1007/s12105-014-0554-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skalova A, Sima R, Kaspirkova-Nemcova J, Simpson RH, Elmberger G, Leivo I, et al. Cribriform adenocarcinoma of minor salivary gland origin principally affecting the tongue: characterization of new entity. Am J Surg Pathol. 2011;35(8):1168–1176. doi: 10.1097/PAS.0b013e31821e1f54. [DOI] [PubMed] [Google Scholar]
- 40.Michal M, Skalova A, Simpson RH, Raslan WF, Curik R, Leivo I, et al. Cribriform adenocarcinoma of the tongue: a hitherto unrecognized type of adenocarcinoma characteristically occurring in the tongue. Histopathology. 1999;35(6):495–501. doi: 10.1046/j.1365-2559.1999.00792.x. [DOI] [PubMed] [Google Scholar]
- 41.Simpson RH, Pereira EM, Ribeiro AC, Abdulkadir A, Reis-Filho JS. Polymorphous low-grade adenocarcinoma of the salivary glands with transformation to high-grade carcinoma. Histopathology. 2002;41(3):250–259. doi: 10.1046/j.1365-2559.2002.01439.x. [DOI] [PubMed] [Google Scholar]
- 42.Weinreb I, Zhang L, Tirunagari LM, Sung YS, Chen CL, Perez-Ordonez B, et al. Novel PRKD gene rearrangements and variant fusions in cribriform adenocarcinoma of salivary gland origin. Genes Chromosomes Cancer. 2014;53(10):845–856. doi: 10.1002/gcc.22195. [DOI] [PubMed] [Google Scholar]
- 43.Weinreb I, Piscuoglio S, Martelotto LG, Waggott D, Ng CK, Perez-Ordonez B, et al. Hotspot activating PRKD1 somatic mutations in polymorphous low-grade adenocarcinomas of the salivary glands. Nat Genet. 2014;46(11):1166–1169. doi: 10.1038/ng.3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weinreb I, Chiosea SI, Seethala RR, Reis-Filho JS, Weigelt B, Piscuoglio S, et al. Genotypic and phenotypic comparison of polymorphous and cribriform adenocarcinomas of salivary gland. Mod Pathol. 2015;28(S2):333. [Google Scholar]
- 45.Goode RK, El-Naggar AK. Mucoepidermoid Carcinoma. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. World Health Organization classification of tumours: pathology and genetics of head and neck tumours. Lyon: IARC; 2005. pp. 219–220. [Google Scholar]
- 46.Goode RK, Auclair PL, Ellis GL. Mucoepidermoid carcinoma of the major salivary glands: clinical and histopathologic analysis of 234 cases with evaluation of grading criteria. Cancer. 1998;82(7):1217–1224. doi: 10.1002/(SICI)1097-0142(19980401)82:7<1217::AID-CNCR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 47.Brandwein MS, Ivanov K, Wallace DI, Hille JJ, Wang B, Fahmy A, et al. Mucoepidermoid carcinoma: a clinicopathologic study of 80 patients with special reference to histological grading. Am J Surg Pathol. 2001;25(7):835–845. doi: 10.1097/00000478-200107000-00001. [DOI] [PubMed] [Google Scholar]
- 48.Batsakis JG, Luna MA. Histopathologic grading of salivary gland neoplasms: I. Mucoepidermoid carcinomas. Ann Otol Rhinol Laryngol. 1990;99(10 Pt 1):835–838. doi: 10.1177/000348949009901015. [DOI] [PubMed] [Google Scholar]
- 49.Katabi N, Ghossein R, Ali S, Dogan S, Klimstra D, Ganly I. Prognostic features in mucoepidermoid carcinoma of major salivary glands with emphasis on tumour histologic grading. Histopathology. 2014;65(6):793–804. doi: 10.1111/his.12488. [DOI] [PubMed] [Google Scholar]
- 50.Seethala RR, Dacic S, Cieply K, Kelly LM, Nikiforova MN. A reappraisal of the MECT1/MAML2 translocation in salivary mucoepidermoid carcinomas. Am J Surg Pathol. 2010;34(8):1106–1121. doi: 10.1097/PAS.0b013e3181de3021. [DOI] [PubMed] [Google Scholar]
- 51.Costa AF, Altemani A, Hermsen M. Current concepts on dedifferentiation/high-grade transformation in salivary gland tumors. Patholog Res Int. 2011;2011:325965. doi: 10.4061/2011/325965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roy P, Bullock MJ, Perez-Ordonez B, Dardick I, Weinreb I. Epithelial-myoepithelial carcinoma with high grade transformation. Am J Surg Pathol. 2010;34(9):1258–1265. doi: 10.1097/PAS.0b013e3181e366d2. [DOI] [PubMed] [Google Scholar]
- 53.Williams L, Thompson LD, Seethala RR, Weinreb I, Assaad AM, Tuluc M, et al. Salivary duct carcinoma: the predominance of apocrine morphology, prevalence of histologic variants, and androgen receptor expression. Am J Surg Pathol. 2015;39(5):705–713. doi: 10.1097/PAS.0000000000000413. [DOI] [PubMed] [Google Scholar]
- 54.Lewis JE, Olsen KD, Sebo TJ. Carcinoma ex pleomorphic adenoma: pathologic analysis of 73 cases. Hum Pathol. 2001;32(6):596–604. doi: 10.1053/hupa.2001.25000. [DOI] [PubMed] [Google Scholar]
- 55.LiVolsi VA, Perzin KH. Malignant mixed tumors arising in salivary glands. I. Carcinomas arising in benign mixed tumors: a clinicopathologic study. Cancer. 1977;39(5):2209–2230. doi: 10.1002/1097-0142(197705)39:5<2209::AID-CNCR2820390540>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 56.Katabi N, Ghossein R, Ho A, Dogan S, Zhang L, Sung YS, et al. Consistent PLAG1 and HMGA2 abnormalities distinguish carcinoma ex-pleomorphic adenoma from its de novo counterparts. Hum Pathol. 2015;46(1):26–33. doi: 10.1016/j.humpath.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Katabi N, Gomez D, Klimstra DS, Carlson DL, Lee N, Ghossein R. Prognostic factors of recurrence in salivary carcinoma ex pleomorphic adenoma, with emphasis on the carcinoma histologic subtype: a clinicopathologic study of 43 cases. Hum Pathol. 2010;41(7):927–934. doi: 10.1016/j.humpath.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 58.Gnepp DR, Brandwein-Gensler MS, el-Naggar AK, Nagao T. Carcinoma ex Pleomorphic Adenoma. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. World Health Organization classification of tumours: pathology and genetics of head and neck tumours. Lyon: IARC; 2005. pp. 242–243. [Google Scholar]
- 59.Di Palma S. Carcinoma ex pleomorphic adenoma, with particular emphasis on early lesions. Head Neck Pathol. 2013;7(Suppl 1):S68–S76. doi: 10.1007/s12105-013-0454-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Griffith CC, Thompson LD, Assaad A, Purgina BM, Lai C, Bauman JE, et al. Salivary duct carcinoma and the concept of early carcinoma ex pleomorphic adenoma. Histopathology. 2014;65(6):854–860. doi: 10.1111/his.12454. [DOI] [PubMed] [Google Scholar]
- 61.Weiler C, Zengel P, van der Wal JE, Guntinas-Lichius O, Schwarz S, Harrison JD, et al. Carcinoma ex pleomorphic adenoma with special reference to the prognostic significance of histological progression: a clinicopathological investigation of 41 cases. Histopathology. 2011;59(4):741–750. doi: 10.1111/j.1365-2559.2011.03937.x. [DOI] [PubMed] [Google Scholar]
- 62.Brandwein-Gensler M, Hille J, Wang BY, Urken M, Gordon R, Wang LJ, et al. Low-grade salivary duct carcinoma: description of 16 cases. Am J Surg Pathol. 2004;28(8):1040–1044. doi: 10.1097/01.pas.0000128662.66321.be. [DOI] [PubMed] [Google Scholar]
- 63.Simpson RH, Desai S, Di Palma S. Salivary duct carcinoma in situ of the parotid gland. Histopathology. 2008;53(4):416–425. doi: 10.1111/j.1365-2559.2008.03135.x. [DOI] [PubMed] [Google Scholar]
- 64.Persson M, Andren Y, Mark J, Horlings HM, Persson F, Stenman G. Recurrent fusion of MYB and NFIB transcription factor genes in carcinomas of the breast and head and neck. Proc Natl Acad Sci USA. 2009;106(44):18740–18744. doi: 10.1073/pnas.0909114106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tonon G, Modi S, Wu L, Kubo A, Coxon AB, Komiya T, et al. t(11;19)(q21;p13) translocation in mucoepidermoid carcinoma creates a novel fusion product that disrupts a Notch signaling pathway. Nat Genet. 2003;33(2):208–213. doi: 10.1038/ng1083. [DOI] [PubMed] [Google Scholar]
- 66.Jo VY, Sholl LM, Krane JF. Distinctive patterns of CTNNB1 (beta-Catenin) alterations in salivary gland basal cell adenoma and basal cell adenocarcinoma. Am J Surg Pathol. 2016;40(8):1143–1150. doi: 10.1097/PAS.0000000000000669. [DOI] [PubMed] [Google Scholar]
- 67.Persson F, Andren Y, Winnes M, Wedell B, Nordkvist A, Gudnadottir G, et al. High-resolution genomic profiling of adenomas and carcinomas of the salivary glands reveals amplification, rearrangement, and fusion of HMGA2. Genes Chromosomes Cancer. 2009;48(1):69–82. doi: 10.1002/gcc.20619. [DOI] [PubMed] [Google Scholar]
- 68.Andersson MK, Stenman G. The landscape of gene fusions and somatic mutations in salivary gland neoplasms - Implications for diagnosis and therapy. Oral Oncol. 2016;57:63–69. doi: 10.1016/j.oraloncology.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 69.Antonescu CR, Katabi N, Zhang L, Sung YS, Seethala RR, Jordan RC, et al. EWSR1-ATF1 fusion is a novel and consistent finding in hyalinizing clear-cell carcinoma of salivary gland. Genes Chromosomes Cancer. 2011;50(7):559–570. doi: 10.1002/gcc.20881. [DOI] [PubMed] [Google Scholar]