Abstract
In Saccharomyces cerevisiae, Mec1/ATR plays a primary role in sensing and transducing checkpoint signals in response to different types of DNA lesions, while the role of the Tel1/ATM kinase in DNA damage checkpoints is not as well defined. We found that UV irradiation in G1 in the absence of Mec1 activates a Tel1/MRX-dependent checkpoint, which specifically inhibits the metaphase-to-anaphase transition. Activation of this checkpoint leads to phosphorylation of the downstream checkpoint kinases Rad53 and Chk1, which are required for Tel1-dependent cell cycle arrest, and their adaptor Rad9. The spindle assembly checkpoint protein Mad2 also partially contributes to the G2/M arrest of UV-irradiated mec1Δ cells independently of Rad53 phosphorylation and activation. The inability of UV-irradiated mec1Δ cells to undergo anaphase can be relieved by eliminating the anaphase inhibitor Pds1, whose phosphorylation and stabilization in these cells depend on Tel1, suggesting that Pds1 persistence may be responsible for the inability to undergo anaphase. Moreover, while UV irradiation can trigger Mec1-dependent Rad53 phosphorylation and activation in G1- and G2-arrested cells, Tel1-dependent checkpoint activation requires entry into S phase independently of the cell cycle phase at which cells are UV irradiated, and it is decreased when single-stranded DNA signaling is affected by the rfa1-t11 allele. This indicates that UV-damaged DNA molecules need to undergo structural changes in order to activate the Tel1-dependent checkpoint. Active Clb-cyclin-dependent kinase 1 (CDK1) complexes also participate in triggering this checkpoint and are required to maintain both Mec1- and Tel1-dependent Rad53 phosphorylation, suggesting that they may provide critical phosphorylation events in the DNA damage checkpoint cascade.
Eukaryotic cells have developed sophisticated surveillance mechanisms called checkpoints to ensure proper response to the presence of damaged or incompletely replicated DNA molecules (reviewed in references 49 and 72) and to alterations in the mitotic apparatus (reviewed in references 46 and 64). The failure of checkpoints causes accumulation of genetic changes and chromosome instability that may lead to cancer in multicellular eukaryotes (reviewed in reference 87).
DNA damage checkpoints are specialized in detecting abnormal DNA structures, serving at least two primary purposes: (i) to arrest the cell cycle in response to DNA damage, thereby coordinating cell cycle progression with DNA repair capacity (109); and (ii) to regulate transcription of DNA damage response genes, as well as to regulate activation and recruitment of various repair and recombination proteins that help cells survive genotoxic stress to sites of damage (reviewed in references 72 and 90). Therefore, DNA damage checkpoints are considered one of the main lines of defense against genomic instability. In fact, similar to mutations in recombination, replication, and repair genes, defective S-phase checkpoint genes increase the rate of gross chromosome rearrangements in Saccharomyces cerevisiae in the absence of exogenous DNA damage (40, 65, 66).
The DNA damage checkpoints are envisaged as signal transduction cascades, where upstream sensors monitor and detect altered DNA molecules, while central transducers act in a protein kinase cascade to regulate a myriad of downstream effectors (reviewed in references 72 and 90). Keystones of DNA damage checkpoints are protein kinases of a phosphoinositide 3-kinase-related family, which include S. cerevisiae Mec1 (55, 75, 110) and Tel1 (33, 62), Schizosaccharomyces pombe Rad3 and Tel1 (7), Drosophila melanogaster Mei-41 (35), and mammalian ATM (86) and ATR (7). These kinases respond to various DNA stresses by phosphorylating key proteins, thus regulating numerous processes, depending on the spectrum of their substrates (reviewed in reference 90).
Several lines of evidence indicate a functional distinction between ATM- and ATR-dependent pathways in both yeasts and mammals. In fact, in humans, ATR responds to UV-induced DNA damage, double-strand breaks (DSBs), and stalled replication forks, while ATM seems to respond primarily to DSBs (reviewed in reference 90). Similarly, S. cerevisiae Mec1 and S. pombe Rad3, more closely related to human ATR, are the prototype transducers of the DNA damage and replication stress signals (reviewed in references 49 and 72), while evidence regarding activation by DSBs of the S. cerevisiae and S. pombe ATM homologs, Tel1, is less extensive.
Budding yeast (S. cerevisiae) Mec1 plays a critical role in both sensing and transducing the checkpoint signals and is required to delay G1/S and G2/M transitions as well as to slow down DNA replication in the presence of damaged DNA molecules, depending on the cell cycle stage at which the damage occurs (reviewed in references 49 and 72). Mec1 functions in a complex with Ddc2/Lcd1/Pie1 (74, 81, 108), functionally related to S. pombe Rad26 and human ATRIP, which interact with Rad3 and ATR, respectively (15, 20). These complexes seem to respond directly to DNA insults. In particular, Mec1 and Ddc2 are recruited to sites of DNA damage independently of other checkpoint proteins (41, 58, 82), and in vivo Ddc2 and Rad26 phosphorylation does not require other known checkpoint factors but Mec1 and Rad3, respectively, suggesting a pivotal role for these kinases in sensing DNA alterations (15, 20, 74). Similarly, ATR colocalizes with ATRIP in nuclear foci after DNA damage, indicating that the ATR-ATRIP complex may also be recruited to damaged DNA (116). ATR does not localize at damaged sites in the absence of the single-stranded DNA (ssDNA) binding complex replication protein A (RPA), which also stimulates in vitro ATRIP binding to ssDNA in human cells, indicating that RPA-coated ssDNA is critical for recruiting the ATR-ATRIP complex (115). Similarly, Ddc2 is recruited to DSBs in an RPA-dependent manner (115).
In budding yeast, once DNA perturbations are sensed, checkpoint signals are propagated through the evolutionarily conserved protein kinases Rad53 and Chk1, which undergo Mec1-dependent phosphorylation after DNA damage (84, 85). While Rad53 is required for proper response to DNA damage in all cell cycle phases, Chk1 contributes only to the activation of the G2/M checkpoint in a Rad53-independent manner (85). The DNA damage-sensing functions are linked with the downstream effectors by Mec1-dependent phosphorylation of the Rad9 adaptor (21, 95, 106). In particular, this phosphorylation triggers Rad9 interaction with Rad53 and the subsequent release of active Rad53 kinase, thus indicating that Mec1 can regulate both sensing and transducing of checkpoint signals (31, 88).
The response to DSBs in humans occurs primarily through ATM and leads to phosphorylation of many targets critical for checkpoint activation, apoptosis, and DNA repair (reviewed in reference 90). ATM/Tel1 functions depend on a highly conserved complex, called MRN (Mre11-Rad50-Nbs1) in mammals and MRX (Mre11-Rad50-Xrs2) in S. cerevisiae, which is required for recombinational DNA repair, DSB end resection, telomere metabolism, and meiotic recombination (reviewed in reference 105). The phenotypes of cells defective for Mre11 or Nbs1 show significant overlap with those of ATM-deficient cells (112), raising the possibility that MRN deficiency may somehow affect ATM activation. Indeed, the MRN complex stimulates ATM kinase activity in vitro by facilitating stable substrate binding (44). Moreover, ATM phosphorylates Nbs1 in response to ionizing radiation (IR), and this phosphorylation is important for IR-induced cell cycle arrest (28, 48, 111, 112). This response is evolutionarily conserved, as certain types of DNA damage also stimulate Tel1-dependent phosphorylation of the S. cerevisiae Mre11 and Xrs2 proteins (17, 34). Finally, the complex is involved in checkpoint activation in response to DSB-inducing agents in both mammals and yeasts (10, 17, 34).
The hallmark of ATM's response to DSBs is a rapid increase in its kinase activity immediately after the formation of DSBs (5, 8). Although a portion of nuclear ATM is recruited to broken DNA soon after DNA damage (3), even a limited number of DSBs was shown to be sufficient to activate the majority of intracellular ATM molecules, suggesting that the signals for ATM activation might be chromatin structure perturbations, rather than direct ATM contact with the broken DNA (4).
Less clear is the role in DNA damage response of the S. cerevisiae ATM counterpart, Tel1. Although TEL1 deletion by itself does not confer sensitivity to DNA-damaging agents, Tel1 functions in the DNA damage response are inferred from the finding that its absence increases the sensitivity of mec1 mutants to genotoxic agents (62, 84), and it impairs the checkpoint response to phleomycin treatment during S phase (67). Tel1 is also responsible for the G2 arrest after DNA damage in a dominant-negative MEC3 mutant (29). Moreover, TEL1 overexpression can suppress both cell lethality and hypersensitivity to DNA-damaging agents of mec1Δ and ddc2Δ mutants, indicating that excess Tel1 can bypass the requirements for the Mec1-Ddc2 complex (11, 84). Finally, altering DSB processing by the absence of Sae2 or non-null mutations affecting Rad50 or Mre11 functions in mec1Δ cells triggers Tel1/MRX-dependent Rad53 phosphorylation and interaction with Rad9 after DNA damage, suggesting that these mutations may allow accumulation of DNA lesions that specifically activate Tel1 (104). Although it was shown that Tel1 association to DSBs does not necessarily result in activation of the Rad53 kinase, the finding that this association is mediated by an Xrs2-dependent mechanism supports the hypothesis that Tel1 may be activated by DSBs (68).
Altogether, the data obtained in both yeasts and humans highlight the functional distinction between ATM- and ATR-dependent pathways and indicate that their interrelationships may be less straightforward than merely serving the same purpose at different times. In particular, although mec1Δ cells seem to retain the ability to trigger a Tel1-dependent DNA damage checkpoint, further studies are required to clarify the molecular mechanisms and signals involved in this response, as well as the in vivo consequences of its activation.
In this context, we have found and analyzed in detail a Tel1/MRX-dependent G2/M checkpoint that is triggered by UV irradiation in the absence of Mec1. Unlike Mec1-dependent Rad53 phosphorylation, which occurs after UV treatment independently of cell cycle progression in wild-type cells, Tel1-dependent Rad53 phosphorylation occurs in mec1Δ cells only concomitantly with completion of UV-damaged DNA replication. Moreover, it requires both entry into S phase and active Clb-CDK complexes, and the resulting metaphase arrest can be relieved by eliminating the anaphase inhibitor Pds1, whose UV-induced phosphorylation and stabilization in mec1Δ cells depend on Tel1.
MATERIALS AND METHODS
S. cerevisiae strains and media.
The relevant genotypes of all S. cerevisiae strains used in this study are listed in Table 1. All strains were constructed during this study, and all were derivatives of W303 (K699) (MATa or MATα ade2-1 can1-100 his3-11,15 leu2-3,112 trp1-1 ura3 rad5-535). The rad5-535 mutation confers very slight sensitivity to methyl methanesulfonate (MMS) (23).
TABLE 1.
S. cerevisiae strains used in this study
| Strain | Relevant genotype |
|---|---|
| YLL1072 | MATaMRE11-HA::URA3 |
| DMP4225/4C | MATaMRE11-HA::URA3 sml1Δ::KANMX4 mec1Δ::HIS3 |
| DMP4226/1B | MATaMRE11-HA::URA3 tellΔ::HIS3 |
| DMP4239/8A | MATaMRE11-HA::URA3 sml1Δ::KANMX4 mec1Δ::HIS3 tel1Δ::HIS3 |
| DMP4290/27A | MATaleu2::LEU2::tetR-GFP ura3::URA3::224XtetO |
| DMP4290/13C | MATaleu2::LEU2::tetR-GFP ura3::URA3::224XtetO sml1Δ::KANMX4 mec1Δ::HIS3 |
| DMP4263/12B | MATahis3::HIS3::tetR-GFP ura3::URA3::336XtetO scc1Δ::HIS3 SCC1-TEV268-HA3::LEU2 GAL-NLS- myc9- TEV-NLS2::TRP1 |
| DMP4293/10D | MATahis3::HIS3::tetR-GFP ura3::URA3::336XtetO scc1Δ::HIS3 SCC1-TEV268-HA3::LEU2 GAL-NLS-myc9-TEV-NLS2::TRP1 sml1Δ::KANMX4 mec1Δ::HIS3 |
| DMP4062/19A | MATaleu2::LEU2::tetR-GFP ura3::URA3::224XtetO sml1Δ::KANMX4 tel1Δ::HIS3 |
| DMP4062/5C | MATaleu2::LEU2::tetR-GFP ura3::URA3::224XtetO sml1Δ::KANMX4 tel1Δ::HIS3 mec1Δ::HIS3 |
| DMP4290/3A | MATaleu2::LEU2::tetR-GFP ura3::URA3::224XtetO mre11Δ::HIS3 |
| DMP4290/10B | MATaleu2::LEU2::tetR-GFP ura3::URA3::224XtetO mre11Δ::HIS3 sml1Δ::KANMX4 mec1Δ::HIS3 |
| YLL839 | MATaCHK1-HA::URA3 |
| DMP3641/2C | MATaCHK1-HA::URA3 tel1Δ::HIS3 |
| DMP3642/8C | MATaCHK1-HA::URA3 sml1Δ::KANMX4 mec1Δ::HIS3 |
| DMP3815/3C | MATaCHK1-HA::URA3 mre11Δ::HIS3 |
| DMP3817/9A | MATaCHK1-HA::URA3 sml1Δ::KANMX4 tel1Δ::HIS3 mec1Δ::HIS3 |
| DMP3774/28A | MATaCHK1-HA::URA3 sml1Δ::KANMX4 mre11Δ::HIS3 mec1Δ::HIS3 |
| YLL490 | MATasml1Δ::KANMX4 mec1Δ::HIS3 |
| SP1070 | MATamad2Δ::TRP1 |
| DMP3249/14D | MATarad53Δ::HIS3 sml1Δ::KANMX4 mec1Δ::HIS3 |
| DMP3262/3C | MATamad2Δ::TRP1 sml1Δ::KANMX4 mec1Δ::HIS3 |
| DMP3274/1B | MATachk1Δ::HIS3 sml1Δ::KANMX4 mec1Δ::HIS3 |
| DMP4359/4C | MATarad53Δ::HIS3 chk1Δ::HIS3 sml1Δ::KANMX4 |
| DMP4359/6B | MATarad53Δ::HIS3 chk1Δ::HIS3 sml1Δ::KANMX4 mec1Δ::HIS3 |
| DMP4359/9A | MATarad53Δ::HIS3 chk1Δ::HIS3 sml1Δ::KANMX4 mad2Δ::HIS3 |
| DMP3390/17B | MATaPDS1-HA::LEU2 |
| DMP4292/4D | MATaPDS1-HA::LEU2 tel1Δ::HIS3 |
| DMP4291/4C | MATaPDS1-HA::LEU2 sml1Δ::KANMX4 mec1Δ::HIS3 |
| DMP4305/5C | MATaPDS1-HA::LEU2 sml1Δ::KANMX4 tel1Δ::HIS3 mec1Δ::HIS3 |
| SP2894 | MATaleu2::LEU2::tetR-GFP ura3::URA3::224XtetO pds1Δ::URA3 |
| DMP4290/27B | MATaleu2::LEU2::tetR-GFP ura3::URA3::224XtetO pds1Δ::URA3 sml1Δ::KANMX4 mec1Δ::HIS3 |
| YLL355 | MATarad14Δ::KANMX4 |
| DMP3942/6C | MATarad14Δ::KANMX4 sml1Δ::KANMX4 mec1Δ::HIS3 |
| DMP4313/11C | MATaleu2::LEU2::tetR-GFP ura3::URA3::224XtetO rfa1-t11 |
| DMP4313/7B | MATaleu2::LEU2::tetR-GFP ura3::URA3::224XtetO rfa1-t11 sml1Δ::KANMX4 mec1Δ::HIS3 |
| DMP4083/11C | MATaCHK1-HA::URA3 GAL-ubiCDC6::URA3 cdc6Δ::HISG sml1Δ::KANMX4 |
| DMP4083/6B | MATaCHK1-HA::URA3 GAL-ubiCDC6::URA3 cdc6Δ::HISG sml1Δ::KANMX4 mec1Δ::HIS3 |
| DMP4337/20C | MATaMRE11-HA::URA3 ura3::GAL-SIC1ΔNT-MYC-HIS::URA3 |
| DMP4337/14C | MATaMRE11-HA::URA3 ura3::GAL-SIC1ΔNT-MYC-HIS::URA3 sml1Δ::KANMX4 mec1Δ::HIS3 |
| DMP4340/5C | MATaMRE11-HA::URA3 clb2Δ::LEU2 trp1::GAL-CLB2::TRP1 sml1Δ::KANMX4 |
| DMP4340/1C | MATaMRE11-HA::URA3 clb2Δ::LEU2 trp1::GAL-CLB2::TRP1 sml1Δ::KANMX4 mec1Δ::HIS3 |
| DMP4341/10B | MATaMRE11-HA::URA3 clb2Δ::LEU2::7XGAL-CLB2::TRP1 sml1Δ::KANMX4 |
| DMP4341/6C | MATaMRE11-HA::URA3 clb2Δ::LEU2::7XGAL-CLB2::TRP1 sml1Δ::KANMX4 mec1Δ::HIS3 |
Standard yeast genetic techniques and media (80) were used. Cells were grown in YEP medium (1% yeast extract, 2% Bacto Peptone, 50 mg of adenine per liter) supplemented with 2% glucose (YEPD) or 2% raffinose (YEP+Raf) or 2% raffinose and 1% galactose (YEP+Raf+Gal).
Deletions of the MEC1, RAD53, CHK1, SML1, TEL1, and RAD14 genes have been obtained as previously described (50, 51, 69, 74, 75). Deletion of MRE11 was generated from strain K699, where 2,111 bp of the MRE11 coding region was replaced by the HIS3 gene by one-step PCR disruption method (107). The rfa1-t11 mutant was kindly provided by R. D. Kolodner (University of California, La Jolla).
Strains carrying fully functional MRE11-HA3, CHK1-HA3, and PDS1-HA3 alleles at their corresponding chromosomal loci were constructed as previously described (6, 73, 75). W303-derived strains carrying either deletions of the PDS1 and MAD2 genes, or the GAL-CLB2 allele integrated in one copy at the TRP1 locus or in seven copies at the CLB2 locus (7XGAL-CLB2) (96), or a galactose-induced GAL-ubiCDC6 fusion at the CDC6 locus, were kindly provided by S. Piatti (University of Milano-Bicocca, Milan, Italy), while a W303-derived strain expressing the Scc1 version cleavable by the tobacco etch virus (TEV) protease (scc1Δ::HIS3 SCC1-TEV GAL-TEV) was kindly provided by F. Uhlmann (Cancer Research UK, London, United Kingdom). All these strains were used to disrupt MEC1 or TEL1 or both, as reported above. Strain YLL1437, carrying three copies of the GAL-SIC1ΔNT-MYC-HIS fusion integrated at the URA3 locus, was obtained by transforming strain K699 with ApaI-digested plasmid pLD1, kindly provided by J. Diffley (Clare Hall Laboratories, South Mimms, United Kingdom). The accuracy of integration was verified by Southern blot analysis.
The mec1Δ tel1Δ sml1Δ triple mutants were constructed by crossing mec1Δ sml1Δ and tel1Δ sml1Δ strains. The resulting diploid strain mec1Δ/MEC1 tel1Δ/TEL1 sml1Δ/sml1Δ was allowed to sporulate, and tetrads were dissected on YEPD plates. Forty-eight hours after tetrad dissection, segregant clones were streaked on YEPD plates and were used 24 h later for the experiments described in the text and concomitantly analyzed for the presence of the mec1Δ and tel1Δ markers.
Other techniques.
Synchronization experiments were performed as described previously (74). Mechloretamine (nitrogen mustard or HN2) and mononitrogen mustard (2-dimethylaminoethylchloride hydrochloride or HN1) were purchased from Sigma-Aldrich. Flow cytometric DNA analysis was performed on a Becton-Dickinson FACScan. Nuclear division on cells stained with propidium iodide was scored with a fluorescence microscope. tet operators integrated at the URA3 locus of chromosome V (35 kb from the centromere) using the GFP-tetR fusion were visualized as described previously (60). For Western blot analysis, protein extracts were prepared by trichloroacetic acid precipitation as previously described (51). Rad53 was detected using anti-Rad53 polyclonal antibodies kindly provided by J. Diffley (Clare Hall Laboratories), and Rad9 was detected using anti-Rad9 polyclonal antibodies kindly provided by N. Lowndes (University of Ireland, Galway). Secondary antibodies were purchased from Amersham, and proteins were visualized by an enhanced chemiluminescence system according to the manufacturer.
RESULTS
Cellular responses to different genotoxic agents in the absence of Mec1.
In order to gain new knowledge of the interrelationships between Mec1 and Tel1 in the DNA damage checkpoint response, we analyzed the abilities of mec1Δ cells to progress through the cell cycle and to activate Rad53 after treatment with different genotoxic agents. Rad53 activation was examined by monitoring its phosphorylation, which is known to be required for its activation as a kinase, and can be detected as an electrophoretic mobility shift (31, 84, 85, 95). It is worth pointing out that all strains carrying the mec1Δ allele also carried the sml1Δ allele (Table 1), which suppresses the lethality of MEC1 deletion, but not its effect on the DNA damage response (113).
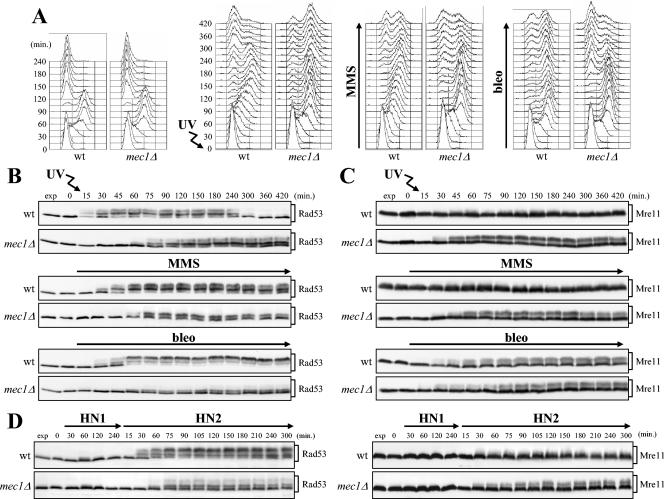
In the experiments shown in Fig. 1A, B, and C, G1-arrested wild-type and mec1Δ cells were either released into the cell cycle under unperturbed conditions or UV irradiated before release or released in the presence of the alkylating agent MMS or the radiomimetic drug bleomycin. When 95% of cells had budded after release, α-factor was added back in order to prevent cells from entering a second cell cycle. As expected, due to activation of the DNA damage checkpoint by the genotoxic treatments, damaged wild-type cells slowed down cell cycle progression and started cell division much later than the untreated cell cultures (Fig. 1A). Accordingly, phosphorylated Rad53 appeared in wild-type cells immediately after UV irradiation or concomitantly with S-phase entry in the presence of MMS and bleomycin (Fig. 1B). Interestingly, although both untreated and UV-, MMS-, and bleomycin-treated mec1Δ cells completed DNA replication with similar kinetics, the damaged mec1Δ cells consistently went through the subsequent cell cycle phases slower than the untreated cells did. Moreover, they accumulated phosphorylated Rad53, which was detected concomitantly with completion of damaged DNA replication about 60 min after genotoxic treatment (Fig. 1A and B).
FIG. 1.
Rad53 and Mre11 phosphorylation in response to different genotoxic treatments in mec1Δ cells. (A to C) Logarithmically growing wild-type (wt) (YLL1072) and mec1Δ (DMP4225/4C) cells were arrested in G1 with α-factor and released from the pheromone block in YEPD alone or YEPD supplemented with 10 mU of bleomycin (bleo) per ml or 0.02% MMS or were UV irradiated (30 J/m2) prior to release in YEPD. When 95% cells had budded after release, 3 μg of α-factor per ml was added back to all cultures to prevent them from entering a second cell cycle. Cell samples were collected at the indicated times after α-factor release to analyze the DNA content by FACS (A) and to detect the Rad53 (B) and Mre11 (C) proteins by Western blot analysis with anti-Rad53 and antihemagglutinin antibodies, respectively. (D) Logarithmically growing wild-type (YLL1072) and mec1Δ (DMP4225/4C) cells were arrested in G1 with α-factor and released from the pheromone block at time zero in YEPD containing HN1 (2 μM) or HN2 (0.5 μM). Extracts from cell samples collected at the indicated times after α-factor release were subjected to Western blot analysis as described above for panels B and C. Cell samples taken immediately before UV treatment were taken at time zero. exp, exponentially growing cells.
As shown in Fig. 1C, not only Rad53 but also Mre11 was phosphorylated in mec1Δ cells about 30 min after UV irradiation or bleomycin or MMS addition, whereas its phosphorylation in wild-type cells was detected only in the presence of bleomycin. Since Mre11 phosphorylation is known to be Tel1 dependent (17, 34) (see Fig. 3B), these data indicate that Tel1, which can substitute for the absence of Mec1 under specific circumstances, is activated in response to DNA damage in the absence of Mec1 and therefore might be responsible for the observed Mec1-independent phosphorylation events.
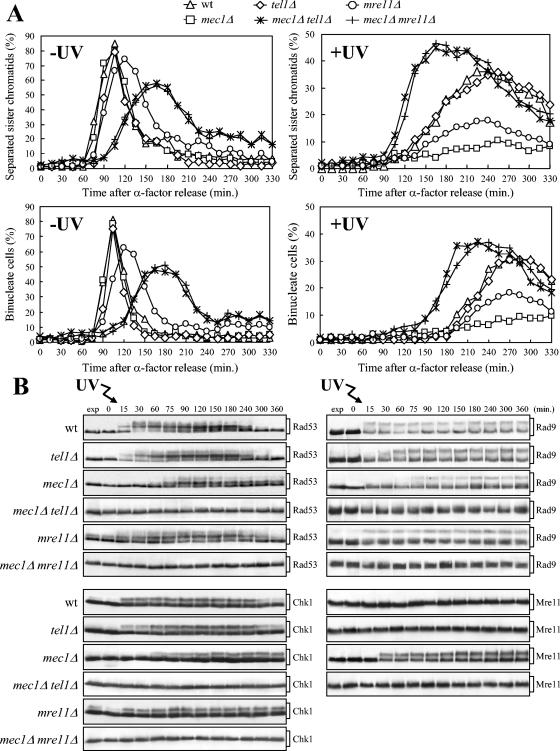
FIG. 3.
Tel1 and Mre11 are required for the response to UV irradiation in G1 in the absence of Mec1. (A) Cell cultures were arrested in G1 with α-factor and released from the G1 block either unirradiated (−UV) or after UV irradiation (30 J/m2) (+UV). As in Fig. 1, when 95% cells had budded after release, 3 μg of α-factor per ml was added back to all cultures. Samples of untreated (left) and UV-treated (right) wild-type (wt) (DMP4290/27A), tel1Δ (DMP4062/19A), mec1Δ (DMP4290/13C), mec1Δ tel1Δ (DMP4062/5C), mre11Δ (DMP4290/3A), and mec1Δ mre11Δ (DMP4290/10B) cell cultures collected at the indicated times after α-factor release were analyzed by fluorescence microscopy to determine the percentage of separated sister chromatids (top) and the percentage of binucleate cells (bottom). (B) Samples of wild-type (YLL839), tel1Δ (DMP3641/2C), mec1Δ (DMP3642/8C), mec1Δ tel1Δ (DMP3817/9A), mre11Δ (DMP3815/3C), and mec1Δ mre11Δ (DMP3774/28A) cells were treated as described above for panel A and analyzed to evaluate Rad53, Chk1, and Rad9 phosphorylation in the UV-irradiated cultures by Western blot analysis of protein extracts with anti-Rad53, antihemagglutinin, and anti-Rad9 antibodies, respectively. The DNA content by FACS and the percentage of binucleate cells were also analyzed (not shown). Samples of wild-type (YLL1072), tel1Δ (DMP4226/1B), mec1Δ (DMP4225/4C), and mec1Δ tel1Δ (DMP4239/8A) cells were treated as described above for panel A and analyzed to evaluate Mre11 phosphorylation in the UV-irradiated cultures by Western blot analysis with antihemagglutinin antibodies. Cell samples taken immediately before UV treatment were taken at time zero. exp, exponentially growing cells.
Although UV irradiation can cause replication fork stalling, like MMS and bleomycin (reviewed in reference 57), the highest level of Rad53 phosphorylation and the most pronounced delay in cell cycle progression in mec1Δ cells were induced by UV treatment, while MMS and bleomycin were more efficient than UV in causing these effects in wild-type cells (Fig. 1A and B). These results suggest that UV-induced DNA alterations might be more suitable than those caused by MMS and bleomycin of eliciting a Mec1-independent checkpoint response.
Besides inducing formation of cyclobutane pyrimidine dimers and pyrimidine-pyrimidone (6-4) photoproducts in DNA, UV irradiation can also generate strand breaks and cross-links between duplex DNA molecules (24), which constitute some of the most serious damage to DNA. Since previous studies have shown that DNA interstrand cross-links (ICLs) are capable of eliciting a checkpoint-dependent G2/M arrest in both S. pombe and humans (2, 43), we verified the ability of ICLs to induce Rad53 phosphorylation both in the presence and absence of Mec1. To this end, exponentially growing wild-type and mec1Δ cells were arrested in G1 with α-factor and released in the presence of the bifunctional alkylating agent nitrogen mustard HN2, which forms both monoadducts and cross-links (43, 83).
As shown in Fig. 1D (left), partial Rad53 phosphorylation took place in mec1Δ cells 60 min after release from the G1 block in the presence of HN2, concomitantly with completion of S phase (data not shown), while its phosphorylation occurred in similarly treated wild-type cells 30 min after release as soon as cells initiated DNA replication (data not shown). Conversely, Rad53 phosphorylation was not observed when the same cell cultures were released in the presence of a monofunctional HN2 analogue, HN1, which causes only monoadduct formation (43, 83) (Fig. 1D, left). Although we could not rule out the possibility that HN2 formed monoadducts more efficiently than HN1 under our experimental conditions, these results suggest that HN2-dependent Rad53 phosphorylation might be induced by ICLs and not by monoadducts. Accordingly, HN2 treatment induced a delay in the G2/M transition in both wild-type and mec1Δ cells, while HN1 did not affect cell cycle progression at all (data not shown). Moreover, ICLs seem to trigger Tel1 activation in the absence of Mec1, since Mre11 phosphorylation was strongly induced by HN2 treatment in mec1Δ cells, while its level, if any, was very low in wild-type cells under the same conditions (Fig. 1D, right).
UV irradiation in the absence of Mec1 leads to Scc1-dependent metaphase arrest.
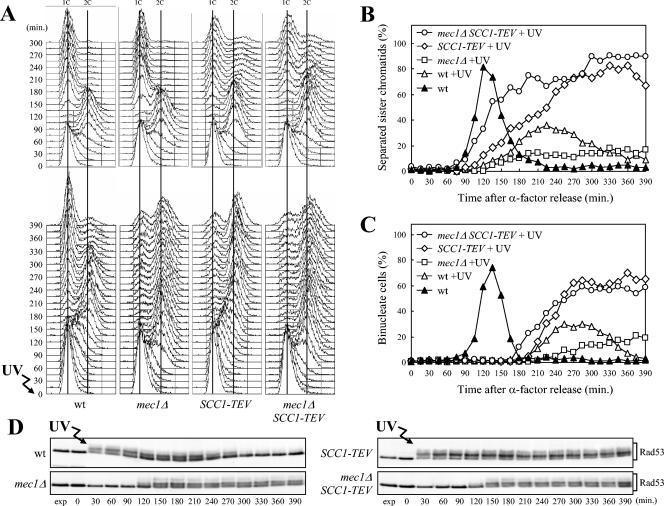
We analyzed the in vivo consequences of UV-induced DNA damage in the absence of Mec1 in more detail by monitoring the kinetics of progression through the cell cycle, separation of sister chromatids, and nuclear division of mec1Δ cells released from α-factor arrest either undamaged or after UV irradiation. All strains carried the tetR-GFP/tetO system that allows detection of the separation of sister chromatids on one arm of chromosome V (60). The kinetics of cell cycle progression, sister chromatid separation, and nuclear division after α-factor release were similar in untreated wild-type and mec1Δ cells (Fig. 2A, top, and data not shown). After UV irradiation, wild-type cells progressed through the S phase slower than the untreated cultures did, due to G1/S checkpoint activation, completing DNA replication within 150 min (Fig. 2A, bottom). As expected, when the checkpoint is turned off, the cells completed the cell cycle and started to undergo sister chromatid separation (Fig. 2B) and nuclear division (Fig. 2C) 150 and 210 min after UV irradiation, respectively, and arresting with 1C DNA content after cell division, due to the addition of α-factor. Conversely, UV-treated mec1Δ cells, which progressed through S phase much faster than similarly treated wild-type cells and completed DNA replication within 75 min (Fig. 2A, bottom), failed to undergo anaphase and arrested mostly with duplicated but unseparated chromosomes (Fig. 2B and C).
FIG. 2.
Scc1 cleavage relieves the inhibition of the metaphase-to-anaphase transition in UV-irradiated mec1Δ cells. Logarithmically growing wild-type (wt) (DMP4290/27A), mec1Δ (DMP4290/13C), SCC1-TEV (DMP4263/12B), and mec1Δ SCC1-TEV (DMP4293/10D) cells, carrying the tetR-GFP/tetO and GAL-TEV constructs, were arrested in G1 with α-factor in YEP+Raf and released from the pheromone block at time zero in YEP+Raf+Gal to induce the TEV protease production or were UV irradiated (30 J/m2) prior to the release in YEP+Raf+Gal. As in Fig. 1, when 95% cells had budded after release, 3 μg of α-factor per ml was added back to all cultures. Samples of untreated and UV-treated cell cultures were collected at the indicated times after α-factor release to analyze the DNA content by FACS in unirradiated (A, top) and UV-irradiated (A, bottom) cell cultures. The kinetics of sister chromatid separation using the tetR-GFP/tetO system was determined by fluorescence microscopy (B), and the kinetics of nuclear division was determined after propidium iodide staining (C). Rad53 protein was detected by Western blot analysis with anti-Rad53 antibodies in UV-treated cell extracts (D). exp, exponentially growing cells.
Sister chromatids are held together by cohesin, a multisubunit complex (reviewed in reference 100), and proteolytic cleavage of the Scc1 cohesin subunit at the metaphase-to-anaphase transition is essential for the separation of sister chromatids (102). If the metaphase arrest in UV-irradiated mec1Δ cells were due to some physical impediment, such as incompletely replicated DNA molecules not allowing sister chromatid separation, cleavage of Scc1 should not be sufficient to trigger anaphase in these cells. We took advantage of the fact that one Scc1 cleavage site can be exchanged with the recognition sequence for the TEV protease (101), and we generated isogenic wild-type and mec1Δ strains carrying both the SCC1-TEV allele at the SCC1 chromosomal locus and a galactose-induced GAL-TEV fusion at the TRP1 locus. Cell cultures were then arrested in G1 with α-factor and released from the α-factor block, either unirradiated or after UV irradiation, in medium containing galactose to induce TEV protease production (Fig. 2). As expected, both mec1Δ and mec1Δ SCC1-TEV cells completed DNA replication about 75 min after UV irradiation, faster than wild-type and SCC1-TEV cells under the same conditions (Fig. 2A, bottom). UV-irradiated mec1Δ cells then remained arrested with unseparated sister chromatids and single nuclei until the end of the experiment (Fig. 2B and C), whereas in mec1Δ SSC1-TEV cells, sister chromatids started to separate and nuclei started to divide 105 and 180 min after UV irradiation, respectively. It is worth pointing out that cell division in galactose-induced SCC1-TEV and mec1Δ SSC1-TEV cells was delayed compared to cell division in wild-type cells, as indicated by the accumulation of cells with 2C DNA content (Fig. 2A) and separated sister chromatids (Fig. 2B and data not shown), and that this delay was independent of UV treatment. This cell cycle delay might be due to activation of the spindle assembly checkpoint triggered by the lack of tension at kinetochores caused by Scc1 cleavage, since Scc1 cleavage has been shown to delay cell division in a Mad2-dependent manner (89, 92). Since Rad53 phosphorylation persisted in galactose-induced, UV-treated SCC1-TEV cells longer than in wild-type cells under the same conditions (Fig. 2D), it is also possible that Rad53 contributes to the cell division delay observed in these cells.
As shown in Fig. 2D, Scc1 cleavage did not seem to affect Rad53 phosphorylation or activation, since both the level and kinetics of Rad53 phosphorylation were similar in mec1Δ SCC1-TEV and mec1Δ cells after UV irradiation and release in medium containing galactose.
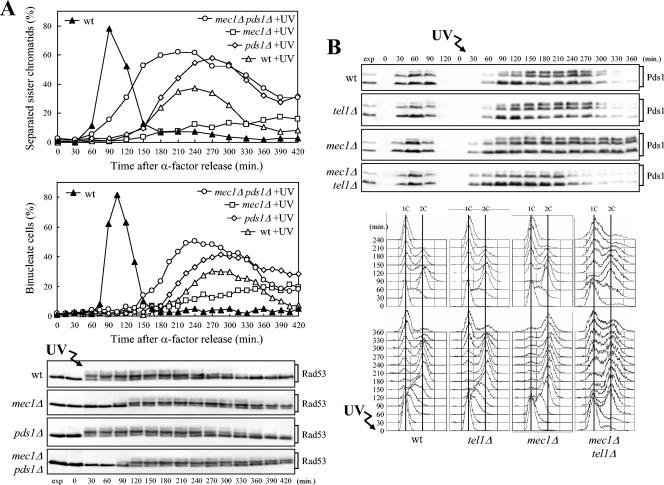
A Tel1/MRX-dependent checkpoint inhibits the metaphase-to-anaphase transition in UV-irradiated mec1Δ cells.
Since the inability to undergo anaphase of UV-irradiated mec1Δ cells requires the cohesins to hold sister chromatids together, this cell cycle arrest likely involves the activation of a checkpoint. Therefore, we asked whether Tel1 and the MRX complex, which were very good candidates for mediating such a checkpoint response, were required for mec1Δ cells to arrest in metaphase after UV irradiation in G1. Exponentially growing cells of mec1Δ tel1Δ and mec1Δ mre11Δ double mutant strains, together with the appropriate control strains, were arrested in G1, UV irradiated, and allowed to proceed through the cell cycle; α-factor was added back to the cultures when 95% of the cells had budded (Fig. 3). Unlike UV-treated mec1Δ cells, which did not undergo sister chromatid separation and nuclear division throughout the experiment, mec1Δ tel1Δ and mec1Δ mre11Δ double mutant cells started to exhibit sister chromatid separation and nuclear division about 105 and 150 min after UV irradiation, respectively (Fig. 3A); cell division followed (data not shown).
Both Tel1 and Mre11 were required in mec1Δ cells for UV-induced phosphorylation not only of Rad53 but also of the Rad9 adaptor and of the G2/M checkpoint kinase Chk1. In fact, phosphorylated forms of Rad53, Rad9, and Chk1, which were detected in mec1Δ cells 60 to 75 min after UV irradiation (Fig. 3B) concomitantly with completion of DNA replication (data not shown), were absent throughout the experiment in mec1Δ tel1Δ and mec1Δ mre11Δ double mutant cells under the same conditions. Thus, both these phosphorylation events and the inhibition of the metaphase-to-anaphase transition in UV-irradiated mec1Δ cells involve the activation of a Tel1/MRX-dependent checkpoint. Tel1 was also responsible for the phosphorylation of Mre11, which was detected in mec1Δ cells 30 min after UV irradiation (Fig. 3B), indicating that Tel1 is activated in the absence of Mec1 after UV-induced damage.
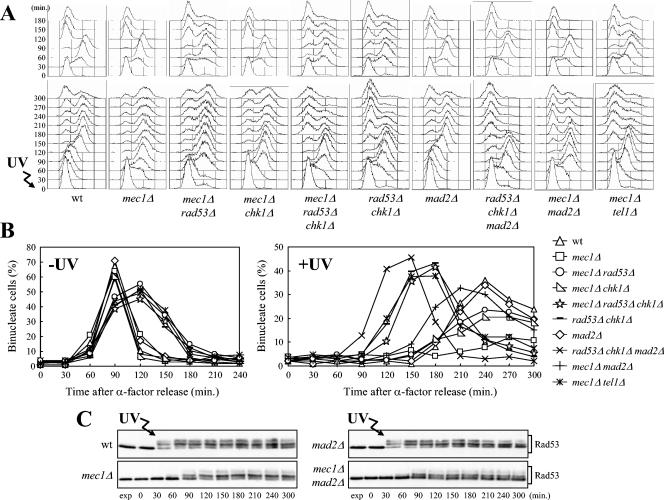
If Chk1 and Rad53, which act independently from one another to inhibit the G2/M transition after DNA damage in G2 (85), were responsible for the Tel1-dependent metaphase arrest in UV-irradiated mec1Δ cells, a rad53Δ chk1Δ double mutant should be unable to carry on the Tel1-dependent response and should therefore undergo anaphase even in the absence of Mec1 after UV irradiation in G1 and release into the cell cycle. As shown in Fig. 4A, both mec1Δ rad53Δ chk1Δ and rad53Δ chk1Δ cell cultures completed DNA replication about 90 min after UV irradiation in G1 and release into the cell cycle, similar to mec1Δ cells under the same conditions. They then started to exhibit nuclear separation about 120 min after UV irradiation (Fig. 4B), followed by cell division and arrest with 1C DNA content, due to α-factor readdition, while UV-treated mec1Δ cells remained arrested with undivided nuclei throughout the experiment (Fig. 4A and B). It is interesting that the kinetics of cell cycle progression and nuclear division in mec1Δ rad53Δ chk1Δ and rad53Δ chk1Δ cell cultures were similar to those of mec1Δ tel1Δ cells (Fig. 4A and B), indicating that Tel1 inhibits the metaphase-to-anaphase transition acting exclusively through Rad53 and Chk1.
FIG. 4.
Effects of RAD53, CHK1, and MAD2 deletions on cell cycle progression after UV irradiation in G1. Logarithmically growing wild-type (wt) (K699), mec1Δ (YLL490), mec1Δ rad53Δ (DMP3249/14D), mec1Δ chk1Δ (DMP3274/1B), mec1Δ rad53Δ chk1Δ (DMP4359/6B), rad53Δ chk1Δ (DMP4359/4C), mad2Δ (SP1070), rad53Δ chk1Δ mad2Δ (DMP4359/9A), mec1Δ mad2Δ (DMP3262/3C), and mec1Δ tel1Δ (DMP4062/5C) strains were arrested in G1 with α-factor and released from the pheromone block at time zero in YEPD or were UV irradiated (30 J/m2) prior to the release in YEPD. As in Fig. 1, when 95% cells had budded after release, 3 μg of α-factor per ml was added back to all cultures. Samples of untreated and UV-treated cell cultures were collected at the indicated times after α-factor release to analyze the DNA content by FACS (A). The kinetics of nuclear division after propidium iodide staining (B) and the kinetics of Rad53 phosphorylation by Western blot analysis with anti-Rad53 antibodies (C) were also determined. exp, exponentially growing cells.
Both Rad53 and Chk1 contributed to the metaphase arrest of UV-irradiated mec1Δ cells, since mec1Δ rad53Δ and mec1Δ chk1Δ cell cultures underwent nuclear division with similar kinetics and less efficiently than mec1Δ rad53Δ chk1Δ cells after UV irradiation (Fig. 4A and B). Thus, Chk1 and Rad53 are required to inhibit the metaphase-to-anaphase transition in UV-irradiated mec1Δ cells.
The spindle assembly checkpoint protein Mad2 contributes to the G2/M arrest of UV-irradiated mec1Δ cells.
Some delay in nuclear division was still apparent in cells carrying the mec1Δ tel1Δ, mec1Δ mre11Δ, mec1Δ rad53Δ chk1Δ, and rad53Δ chk1Δ deletions, when they were released into the cell cycle after UV irradiation in G1 (Fig. 3 and 4). These observations suggested that although the Tel1-dependent checkpoint seemed to have a major role in the UV-induced G2/M block in the absence of Mec1, other mechanisms might contribute to this arrest. We reasoned that replication of damaged DNA in the absence of Mec1 could lead to changes in kinetochores, which are assembled at centromeres and mediate spindle microtubule attachment. These changes might in turn activate the Mad2-dependent spindle assembly checkpoint that prevents the onset of anaphase in response to changes in the structure or tension of kinetochores (reviewed in reference 64).
As shown in Fig. 4, Mad2 does not play a role in slowing down progression through the cell cycle in response to UV treatment when the DNA damage checkpoint is fully functional, since wild-type and mad2Δ cells exhibited delays in cell cycle progression and phosphorylated Rad53 with very similar kinetics after UV irradiation in G1 and release into the cell cycle. However, the absence of Mad2 advanced nuclear division of rad53Δ chk1Δ double mutants and partially relieved the metaphase arrest of mec1Δ cells after UV irradiation (Fig. 4A and B). In fact, UV-irradiated rad53Δ chk1Δ mad2Δ cells released from G1 arrest underwent nuclear division earlier than UV-treated rad53Δ chk1Δ cells did (Fig. 4B), while both cell cultures completed DNA replication at the same time (Fig. 4A). Moreover, unlike mec1Δ cells, which arrested with undivided nuclei after completion of UV-damaged DNA replication, mad2Δ mec1Δ cells underwent nuclear division, although much later than the untreated cultures, followed by mitotic exit and cell division (Fig. 4A and B). Rad53 phosphorylation was not affected by the absence of Mad2, since Rad53 was phosphorylated in mec1Δ mad2Δ cells after UV irradiation and release into the cell cycle (Fig. 4C).
Pds1/securin inactivation bypasses the metaphase arrest of UV-irradiated mec1Δ mutant.
At the onset of anaphase, a caspase-related protease (separase) destroys the link between sister chromatids by cleaving the cohesin subunit Scc1 (reviewed in reference 100). During most of the cell cycle, separase is kept inactive by binding to an inhibitory protein called Pds1/securin, which is destroyed by ubiquitin-mediated proteolysis shortly before the metaphase-to-anaphase transition (14, 25, 114). One of the mechanisms by which both the DNA damage and spindle assembly checkpoints prevent the metaphase-to-anaphase transition is the inhibition of Pds1 ubiquitination by the ubiquitin ligase anaphase-promoting complex (APC), thus stabilizing the securin. In particular, the spindle assembly checkpoint proteins Bub3, Mad2, and Mad3 physically interact with the APC regulatory subunit Cdc20, required for Pds1 ubiquitination, likely leading to Cdc20 inhibition (reviewed in reference 64). Conversely, Rad53 activation seems to affect the Pds1-Cdc20 interaction, whereas Chk1 is responsible for Pds1 phosphorylation, which in turn appears to inhibit the ubiquitination reaction itself (1, 13).
Therefore, we asked whether Pds1 was involved in the metaphase arrest of UV-irradiated mec1Δ cells. When cell cultures were UV irradiated in G1 and allowed to proceed through the cell cycle, UV-treated mec1Δ pds1Δ cells, which completed DNA replication faster than similarly treated wild-type and pds1Δ cells (data not shown), started to exhibit sister chromatid separation (Fig. 5A, top) and nuclear division (Fig. 5A, middle) about 90 and 150 min after release, respectively, while mec1Δ cells under the same conditions were still arrested with undivided sister chromatids and nuclei at the end of the experiment. Once the nuclei divided, mec1Δ pds1Δ cell cultures underwent cell division as indicated by fluorescence-activated cell sorting (FACS) analysis (data not shown) and by the decrease in the percentage of binucleate cells about 300 min after UV irradiation (Fig. 5A, middle). As expected, the absence of Pds1 did not prevent the Tel1-dependent Rad53 phosphorylation, which took place with similar kinetics in mec1Δ and mec1Δ pds1Δ UV-irradiated cells (Fig. 5A, bottom).
FIG. 5.
The Pds1 securin mediates the G2/M arrest and undergoes Tel1-dependent phosphorylation and stabilization in UV-treated mec1Δ cells. (A) Logarithmically growing cell cultures of wild-type (wt) (DMP4290/27A), mec1Δ (DMP4290/13C), pds1Δ (SP2894), and mec1Δ pds1Δ (DMP4290/27B) strains were arrested in G1 with α-factor and released from the pheromone block at time zero in YEPD or were UV irradiated (30 J/m2) prior to the release in YEPD. As in Fig. 1, when 95% cells had budded after release, 3 μg of α-factor per ml was added back to all cultures. Samples of UV-treated cell cultures were collected at the indicated times after α-factor release to analyze the DNA content by FACS (not shown). The kinetics of sister chromatid separation (top) and nuclear division (middle) were determined as described in the legends to Fig. 2B and C. The kinetics of Rad53 phosphorylation by Western blot analysis with anti-Rad53 antibodies (bottom) were also determined. The same procedure was applied to the unirradiated cell cultures (not shown, except for untreated wild type in panel A). (B) Logarithmically growing wild-type (DMP3390/17B), tel1Δ (DMP4292/4D), mec1Δ (DMP4291/4C), and mec1Δ tel1Δ (DMP4305/5C) cells, all expressing a Pds1-HA fusion, were treated as described above for panel A. (B) Samples of untreated and UV-treated cell cultures were collected at the indicated times after α-factor release to determine Pds1 level and phosphorylation by Western blot analysis with antihemagglutinin antibodies (top) and to analyze the DNA content by FACS (bottom). Cell samples taken immediately before UV treatment were taken at time zero. exp, exponentially growing cells.
We then monitored the phosphorylation and level of Pds1 of G1-arrested, UV-irradiated wild-type, tel1Δ, mec1Δ, and mec1Δ tel1Δ cells carrying a fully functional PDS1-HA tagged allele after the cells were allowed to proceed through the cell cycle. As previously reported (1), Pds1 appeared as a triplet in exponentially growing undamaged cells, with the fastest and constitutive migrating band exhibiting greater intensity than the two slower bands, one of which was weakly detected (Fig. 5B, top). After UV irradiation, the intensities of the two slower bands were expected to increase due to Mec1-dependent phosphorylation (1). As expected, Pds1 was absent in all α-factor-arrested cell cultures and increased in level when cells progressed through S phase. As shown in Fig. 5B, there was no change in the intensities of the two slower migrating bands of Pds1 in UV-irradiated mec1Δ tel1Δ double mutant cells. Conversely, the intensities of the two slower bands increased in similarly treated wild-type, tel1Δ, and mec1Δ cells (Fig. 5B, top), indicating that Tel1 contributed to the DNA damage-induced Pds1 phosphorylation. Moreover, UV-irradiated mec1Δ cells, which arrested with 2C DNA content (Fig. 5B, bottom), maintained constant Pds1 levels until the end of the experiment, while the securin was degraded after completion of DNA replication in wild-type, tel1Δ, and mec1Δ tel1Δ cells (Fig. 5B, top). Altogether, these data indicate that Pds1 is necessary for the Tel1-dependent metaphase arrest of UV-irradiated mec1Δ cells, where its phosphorylation and stabilization depend on Tel1, suggesting that its persistence may be responsible for the inability of these cells to undergo anaphase.
Signals triggering Tel1/MRX-dependent phosphorylation events in response to UV irradiation.
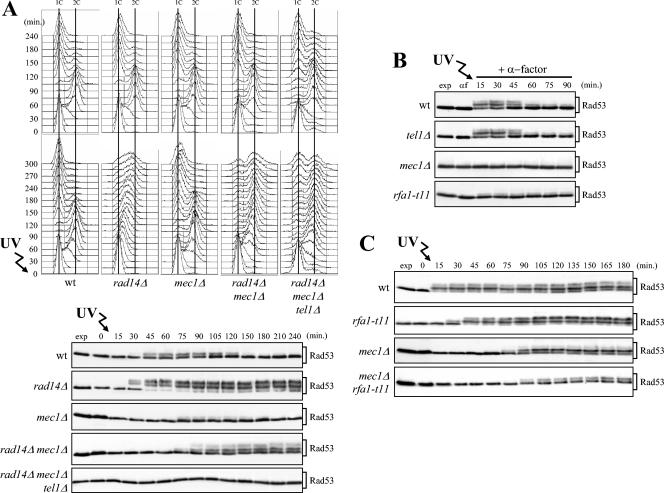
The pathway primarily responsible for removing UV-induced DNA lesions is the nucleotide excision repair (NER) pathway, which is also necessary for checkpoint activation after UV irradiation in both the G1 and G2 phases of the cell cycle (30, 69). In the absence of a functional NER pathway, not only is the removal of UV-induced lesions impaired (79), but replication fork stalling and accumulation of recombination intermediates are also observed (69). Therefore, NER inactivation by deletion of the RAD14 gene, acting at early stages of the pathway, might influence the generation of checkpoint signals leading to Tel1 activation in mec1Δ cells. Indeed, when G1-arrested cells were UV irradiated with a very low UV dose (5 J/m2) and then allowed to proceed through the cell cycle, Rad53 phosphorylation was detected in mec1Δ rad14Δ cells about 75 min after UV irradiation (Fig. 6A, bottom), concomitantly with completion of S phase in the presence of irreparable DNA damage (Fig. 6A, middle). Conversely, since small amounts of UV-induced DNA lesions are rapidly repaired when the NER pathway is functional, the extent of Rad53 phosphorylation under the same conditions was very low in wild-type cells and even lower in mec1Δ cells (Fig. 6A, bottom). As expected, this low UV dose led rad14Δ cells to arrest in S phase with high levels of phosphorylated Rad53 (Fig. 6A, middle and bottom) due to the activation of a Mec1-dependent checkpoint (69). Wild-type, mec1Δ, and rad14Δ mec1Δ cells under the same conditions completed DNA replication with similar kinetics (Fig. 6A, middle). Strikingly, rad14Δ mec1Δ cells then arrested with 2C DNA content (Fig. 6A, middle), persistent Rad53 phosphorylation (Fig. 6A, bottom), and undivided nuclei (data not shown), while wild-type and mec1Δ cells underwent nuclear and cell division, as indicated by the reappearance of cells with 1C DNA content (Fig. 6A, middle). Both Rad53 phosphorylation and the metaphase arrest of rad14Δ mec1Δ cells were dependent on Tel1, since similarly treated rad14Δ mec1Δ tel1Δ triple mutant cells did not phosphorylate Rad53 and underwent cell division (Fig. 6A). Thus, RAD14 deletion appears to amplify the UV-induced signals leading to Tel1 activation in the absence of Mec1. Since the absence of Rad14 causes the complete loss of NER proteins at the damaged site, possibly by affecting the structure of the complexes involved in lesion recognition (79), these data also indicate that NER processing of UV-induced lesions is not required to activate the Tel1-dependent checkpoint.
FIG. 6.
Effects of NER inactivation and defective ssDNA signaling on Tel1-dependent checkpoint response. (A) Logarithmically growing wild-type (wt) (K699), rad14Δ (YLL355), mec1Δ (YLL490), rad14Δ mec1Δ (DMP3942/6C), and rad14Δ mec1Δ tel1Δ (DMP3942/20B) cells were arrested in G1 with α-factor and released from the pheromone block at time zero in YEPD, either untreated or after irradiation with 5 J/m2 UV dose prior to the release in YEPD. As in Fig. 1, when 95% cells had budded after release, 3 μg of α-factor per ml was added back to all cultures. Cell samples were collected at the indicated times after α-factor release to analyze the DNA content by FACS in unirradiated (top) and UV-irradiated (middle) cell cultures and to evaluate Rad53 phosphorylation (bottom) in UV-treated cell extracts as described in the legend to Fig. 1B. (B) Logarithmically growing wild-type (DMP4290/27A), tel1Δ (DMP4062/19A), mec1Δ (DMP4290/13C), and rfa1-t11 (DMP4313/11C) cells were synchronized with α-factor (αf) and resuspended in YEPD containing 3 μg of α-factor per ml after UV irradiation (30 J/m2) (+ α-factor). Protein extracts prepared from cell samples collected at the indicated times were subjected to Western blot analysis with anti-Rad53 antibodies. (C) G1-arrested wild-type (DMP4290/27A), rfa1-t11 (DMP4313/11C), mec1Δ (DMP4290/13C), and rfa1-t11 mec1Δ (DMP4313/7B) cells were UV irradiated (30 J/m2) and released from the G1 block at time zero. As in Fig. 1, when 95% cells had budded after release, 3 μg of α-factor per ml was added back to all cultures. Cell samples were collected at the indicated times after α-factor release to evaluate Rad53 phosphorylation as described above for panel B. Cell samples taken immediately before UV treatment were taken at time zero. exp, exponentially growing cells.
Although it has been shown that RPA-coated ssDNA represents one of the signals activating the Mec1 kinase in response to DSBs induced by HO endonuclease (27, 45, 54, 115), it is still unclear whether UV-induced lesions in G1 need to be processed to ssDNA in order to be sensed by the checkpoint. To address this question, we used the rfa1-t11 mutant, because its alteration in the 70-kDa subunit of the RPA complex renders cells sensitive to UV radiation and impairs loading of checkpoint proteins onto damaged DNA, thus delaying checkpoint activation (103, 115). As shown in Fig. 6B, when G1-arrested cells were kept in G1 by α-factor addition after UV irradiation, rfa1-t11 cells exhibited defective phosphorylation of Rad53, which was normally phosphorylated in similarly treated wild-type cells, suggesting that ssDNA generation is necessary for the checkpoint machinery to detect UV-induced DNA lesions in G1. Moreover, Rad53 phosphorylation under these conditions was completely abolished in mec1Δ cells, while it still took place in tel1Δ cells (Fig. 6B), indicating that Mec1 is strictly required to respond to UV-induced lesions during the G1 phase.
In order to determine whether RPA-coated ssDNA is responsible for the Tel1-dependent Rad53 phosphorylation observed in UV-irradiated mec1Δ cells after the cells were allowed to proceed through the cell cycle, we released UV-irradiated mec1Δ rfa1-t11 double mutant cells and the appropriate control strains from G1 arrest. As shown in Fig. 6C, phosphorylated Rad53 appeared immediately in wild-type cells and 30 min after α-factor release in rfa1-t11 cells, while it was detected only 75 min after release in both rfa1-t11 mec1Δ and mec1Δ cells, concomitantly with S-phase completion (data not shown). Importantly, the level of phosphorylated Rad53 was significantly lower in the rfa1-t11 mec1Δ double mutant cells than in mec1Δ cells (Fig. 6C), suggesting that RPA-coated ssDNA may be part of the signals activating the Tel1-dependent G2/M checkpoint response.
Tel1-dependent checkpoint activation after UV irradiation requires DNA replication and occurs concomitantly with completion of the S phase.
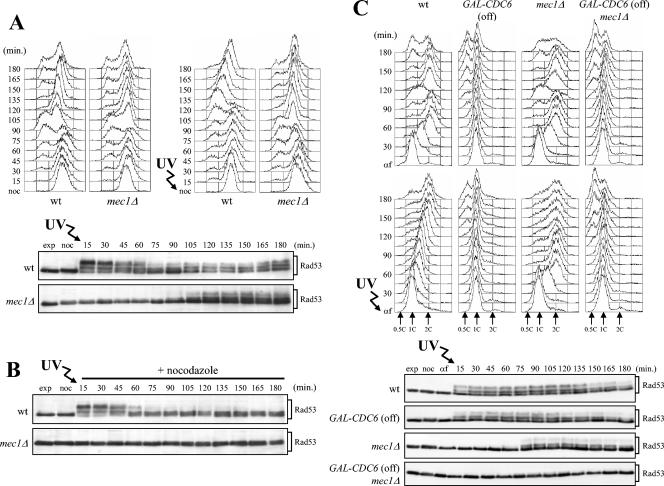
As shown in Fig. 7A, Rad53 phosphorylation could also be detected concomitantly with S-phase completion when mec1Δ cells were allowed to proceed through the cell cycle after UV irradiation in G2. Under these conditions, phosphorylated Rad53 appeared in mec1Δ cells about 105 min after release from the G2 block, when cells completed the subsequent DNA replication (Fig. 7A). This response was dependent on Tel1, since similarly treated mec1Δ tel1Δ cells did not undergo Rad53 phosphorylation at any time after release (data not shown). Moreover, these Tel1-dependent phosphorylation events required entry into the cell cycle. In fact, no phosphorylated Rad53 was detected when G2-arrested mec1Δ cells were held in G2 by the addition of nocodazole after UV irradiation (Fig. 7B), while wild-type cells were able to phosphorylate Rad53 immediately after UV irradiation in G2, independently of progression through the cell cycle (Fig. 7A and B).
FIG. 7.
Rad53 phosphorylation in mec1Δ cells requires DNA replication of UV-damaged DNA. (A and B) Logarithmically growing wild-type (wt) (K699) and mec1Δ (YLL490) cells were synchronized in G2 with nocodazole (noc) and UV irradiated (30 J/m2). Half of each culture was released from the G2 block in YEPD (A) or held in G2 by the addition of nocodazole (B). Cell samples were collected at the indicated times after UV irradiation to analyze the DNA content by FACS (A, top) and to evaluate Rad53 phosphorylation (A, bottom, and B) as described in the legend to Fig. 1B. (C) Logarithmically growing wild-type (K699), mec1Δ (YLL490), GAL-CDC6 (DMP4083/11C), and mec1Δ GAL-CDC6 (DMP4083/6B) cells, carrying a GAL-CDC6 fusion as the only source of Cdc6 protein, were grown in YEP+Gal and arrested in G2 with nocodazole. All arrested cultures were then transferred to YEPD in the presence of nocodazole for 1 h and then released from the G2 block into YEPD containing α-factor (G1 block). Finally, they were released from the α-factor block into YEPD, either unirradiated or after UV irradiation with 30 J/m2. Samples were taken at the indicated times after α-factor release (αf) to analyze the DNA content by FACS (C, top) and to evaluate Rad53 phosphorylation (C, bottom) in UV-treated cell extracts as described in the legend to Fig. 1. exp, exponentially growing cells.
The observation that Rad53 phosphorylation in UV-treated mec1Δ cells occurred concomitantly with the completion of damaged DNA replication, independently of whether the damage was generated in G1 or G2, suggested that S-phase entry and/or progression might be required for Tel1-dependent checkpoint activation. To further explore this possibility, we blocked initiation of DNA replication by preventing prereplicative complex (pre-RC) assembly through depletion of the Cdc6 protein (12, 77). To this end, isogenic wild-type and mec1Δ strains, both carrying an integrated copy of a galactose-induced GAL-CDC6 fusion (78) as the sole Cdc6 source, were blocked in G2 with nocodazole in medium containing galactose and transferred to medium containing nocodazole and glucose for 1 h to completely repress GAL-CDC6. Cell cultures were then released from the nocodazole arrest by transfer into fresh medium containing glucose in the presence of α-factor, followed by release from α-factor under glucose-repressed conditions of the G1-arrested cells, either unirradiated or after UV irradiation. As expected, GAL-CDC6 and mec1Δ GAL-CDC6 cells did not undergo DNA replication under these conditions independently of UV irradiation (Fig. 7C, top and middle), and the appearance of cells with less than 1C DNA content about 105 to 120 min after α-factor release indicated that they eventually underwent mitosis in the absence of DNA replication as previously described (77). Rad53 phosphorylation, which took place immediately after UV irradiation in wild-type and GAL-CDC6 cells and concomitantly with S-phase completion in UV-treated mec1Δ cells, was not detected in UV-irradiated mec1Δ GAL-CDC6 cells throughout the experiment (Fig. 7C, bottom).
Thus, UV-induced DNA damage requires entry into S phase to trigger Tel1-mediated Rad53 phosphorylation, suggesting that UV-damaged DNA needs to be replicated in order to be able to activate the Tel1-dependent checkpoint.
Links between cyclin-CDK complexes and Tel1-dependent Rad53 phosphorylation.
In both budding yeast (S. cerevisiae) and fission yeast (S. pombe), entry and progression into S and M phases require activation of the CDK1/Cdc28/Cdc2 cyclin-dependent kinase bound to S-phase- and M-phase-specific B-type cyclins (reviewed in reference 63). In budding yeast, the S-phase Clb5-CDK1 and Clb6-CDK1 complexes trigger the firing of origins that had previously formed pre-RCs, while activation of M-phase Clb1-, Clb2-, Clb3-, and Clb4-CDK1 complexes promote the formation of a mitotic spindle and APC activation. The observation that Tel1-dependent checkpoint activation in UV-irradiated mec1Δ cells occurs concomitantly with completion of S phase suggests that it may require Clb-CDK1 complexes specifically active at the end of S phase. If this were the case, ectopic Clb-CDK1 activation in G1 by overexpression of the major mitotic cyclin gene CLB2 might be expected to trigger premature Tel1-dependent Rad53 phosphorylation after UV irradiation.
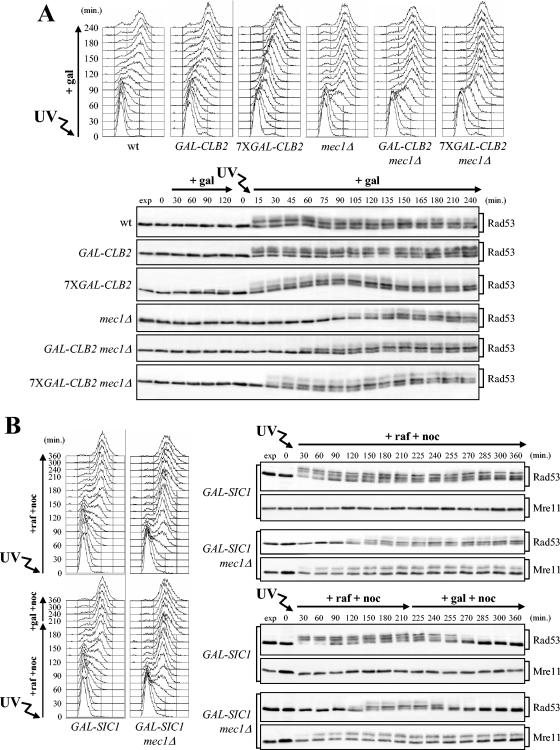
To explore this possibility, G1-arrested wild-type and mec1Δ cells, either lacking or carrying one (GAL-CLB2) or seven (7XGAL-CLB2) integrated copies of a galactose-induced GAL-CLB2 fusion (96), were UV irradiated and allowed to proceed through cell cycle in the presence of galactose to induce CLB2 expression from the GAL promoter. In all untreated cell cultures, CLB2 overexpression did not induce Rad53 phosphorylation (Fig. 8A, bottom). Phosphorylated Rad53 appeared immediately after UV irradiation in all strains expressing functional Mec1 (Fig. 8A, bottom), independent of the different kinetics of S-phase entry and progression, which were delayed by GAL-CLB2 expression (Fig. 8A, top). Conversely, all UV-irradiated mec1Δ strains completed DNA replication about 75 to 90 min after release, faster than the MEC1 strains (Fig. 8A, top). Strikingly, phosphorylated Rad53 was detected about 60 and 30 min after release in mec1Δ GAL-CLB2 and mec1Δ 7XGAL-CLB2 cells, respectively, while it was detected in similarly treated mec1Δ cells only after 90 min (Fig. 8A, bottom), when cells had completed DNA replication and reached the G2 phase (Fig. 8A, top). The advanced Rad53 phosphorylation induced by high levels of Clb2 was still dependent on Tel1, since both tel1Δ mec1Δ GAL-CLB2 and tel1Δ mec1Δ 7XGAL-CLB2 cells did not phosphorylate Rad53 in response to UV irradiation after induction of CLB2 overexpression (data not shown). Thus, ectopic CLB2 overexpression triggers UV-induced Rad53 phosphorylation in the absence of Mec1 even if cells have not yet completed replication of damaged DNA, indicating that these phosphorylation events are likely stimulated by a mitotic CDK.
FIG. 8.
Perturbations of Clb/CDK activity influence checkpoint response to UV irradiation. (A) Wild-type (wt) (YLL1072), GAL-CLB2 (DMP4340/5C), 7XGAL-CLB2 (DMP4341/10B), mec1Δ (DMP4225/4C), GAL-CLB2 mec1Δ (DMP4340/1C), and 7XGAL-CLB2 mec1Δ (DMP4341/6C) cells, exponentially growing in YEP+Raf, were arrested in G1 with α-factor, UV irradiated, and then released from α-factor in YEP+Raf+Gal to induce CLB2 overexpression (+ gal). Cell samples were collected at the indicated times after α-factor release to analyze the DNA content in UV-treated cell cultures by FACS (top) and to analyze Rad53 phosphorylation in both untreated and UV-treated cell cultures (bottom) as described in the legend to Fig. 1. (B) Logarithmically growing wild-type (DMP4337/20C) and mec1Δ (DMP4337/14C) cells, all carrying three copies of the GAL-SIC1ΔNT fusion integrated in the genome (GAL-SIC1), were arrested in G1 with α-factor and released from the pheromone block in medium containing raffinose and nocodazole (15 μg/ml) (+raf +noc) (top) either untreated (not shown) or after UV irradiation (30 J/m2). Galactose was added to half of each G2 nocodazole-arrested cell culture 210 min after α-factor release to induce GAL-SIC1ΔNT expression (+gal +noc) (B, bottom). Cell samples were collected at the indicated times after α-factor release for further analysis. The DNA content in both untreated (not shown) and UV-treated cell cultures was analyzed by FACS (B, left). Rad53 and Mre11 phosphorylation in both untreated (not shown) and UV-treated cell cultures was analyzed as described in the legends to Fig. 1B and C (B, right). No significant differences were detected in the FACS profiles in the different unirradiated cell cultures, which did not reveal any Rad53 and Mre11 phosphorylation throughout the experiment. exp, exponentially growing cells.
If unscheduled Tel1-dependent checkpoint activation caused by Clb2 overproduction required Clb/CDK1 kinase activity, Clb/CDK1 inhibition should influence UV-induced Rad53 and/or Mre11 phosphorylation. Previous studies have shown that overexpression of a truncated form of the Clb/CDK1 inhibitor Sic1 (Sic1ΔNT), lacking 50 N-terminal amino acids that target Sic1 for degradation, efficiently inhibits Clb/CDK1 activity (18, 71). Therefore, we analyzed the effects caused by overexpressing the galactose-induced GAL-SIC1ΔNT allele on UV-induced Rad53 and Mre11 phosphorylation in wild-type and mec1Δ cells (Fig. 8B). To this end, cell cultures exponentially growing in raffinose were arrested in G1 with α-factor, UV irradiated, and first released from G1 in medium containing raffinose in the presence of nocodazole, to allow DNA replication and Clb/CDK1 activation, followed by G2 arrest. After DNA replication was completed and cells were arrested in G2, SIC1ΔNT expression from the GAL promoter was induced in half of each cell culture by the addition of galactose. As shown in Fig. 8B, Rad53 was phosphorylated immediately after UV irradiation in wild-type cells, and 120 min later, concomitantly with S-phase completion, in mec1Δ cells. Phosphorylated Rad53 was still apparent after 300 min in both cell types in the absence of galactose (Fig. 8B, top), while it started to decrease with similar kinetics in both strains about 15 min after GAL-SIC1ΔNT induction (Fig. 8B, bottom). Thus, Clb/CDK1 activity is required to maintain Rad53 phosphorylation and activation both in the presence and absence of Mec1; therefore, some components of the Mec1- and Tel1-dependent checkpoint networks are likely activated by these CDKs. Interestingly, Clb/CDK1 inhibition did not affect Mre11 phosphorylation, which, as expected, was detected in UV-treated mec1Δ cells 30 min after release, concomitantly with S-phase entry, and persisted throughout the experiment independent of GAL-SIC1ΔNT induction (Fig. 8B).
DISCUSSION
In S. cerevisiae, Mec1 plays a primary role in sensing and transducing checkpoint signals in response to different types of DNA lesions, while Tel1 functions in the DNA damage response are inferred from the finding that its absence increases the sensitivity of mec1 mutants to genotoxic agents (62, 84), it impairs the checkpoint response to phleomycin treatment during S phase (67), and it allows the G2/M transition after DNA damage in a dominant-negative MEC3 mutant (29). Moreover, altering DSB processing in mec1Δ cells by deletion of SAE2 or non-null mutations in the RAD50 and MRE11 genes triggers Tel1/MRX-dependent Rad53 phosphorylation and interaction with Rad9 after MMS and hydroxyurea treatment, suggesting that accumulation of DNA lesions that specifically activate Tel1 might take place under these conditions (104). In this work, we have tried to gain insights into the roles of Tel1 in DNA damage checkpoint activation, as well as into the nature of the signals detected by this kinase.
Checkpoint-dependent metaphase arrest after UV irradiation in the absence of Mec1.
We found that UV irradiation in the absence of Mec1 leads to metaphase arrest and concomitant phosphorylation of the adaptor protein Rad9 and of the downstream checkpoint kinases Rad53 and Chk1. All these events require both Tel1 and Mre11, indicating that a Tel1/MRX-dependent checkpoint, whose activation results in the inhibition of the metaphase-to-anaphase transition, can be activated in response to UV irradiation in the absence of Mec1.
Although Tel1 and MRX can be activated in response to UV irradiation in the absence of Mec1, tel1Δ cells show neither sensitivity to DNA-damaging agents nor defects in Rad53 phosphorylation after UV irradiation in any cell cycle stage, suggesting that activation of the Tel1 kinase becomes apparent only in the absence of Mec1. Although Tel1 associates with DSBs in an Xrs2-dependent manner in the presence of Mec1 (68), it may be excluded from the sites of damage by Mec1 when processing of UV-induced lesions generates ssDNA, which is known to be recognized by the Mec1-Ddc2 complex (115). Consistent with this hypothesis, Tel1 and Mec1 are localized to telomeric ends in a reciprocal manner, with Mec1 preferentially bound to chromosomal ends when ssDNA was generated (97). In this view, Tel1 might be recruited to sites of UV-induced lesions only in the absence of Mec1, thus leading to Mre11, Rad9, Rad53, and Chk1 phosphorylation and checkpoint activation. Since Tel1-dependent Rad53 phosphorylation also occurs in mec1kd mutants with defective kinase (our unpublished observation), the absence of Mec1 kinase activity appears to be sufficient to allow Tel1/MRX activation, suggesting that Mec1 may regulate Tel1 association with damaged DNA through phosphorylation events.
However, since Mec1 is required to maintain the integrity of the replication forks under stress conditions, as well as for processive DNA replication in the presence of hydroxyurea and MMS (52, 91, 98), we cannot exclude the possibility that replication of damaged templates in the absence of functional Mec1 results in the production of potentially lethal events that trigger a Tel1-dependent checkpoint response. Indeed, the checkpoint pathway plays a role during DNA replication even during unperturbed conditions. In fact, defects in the checkpoint genes acting during S phase in the presence of DNA damage increased the rates of gross chromosome rearrangements, suggesting that one normal role of S-phase checkpoint functions is to facilitate nonmutagenic repair of the DNA damage that normally occurs during DNA replication (40, 65, 66).
We show that the Rad53 and Chk1 kinases are required for the Tel1-dependent metaphase arrest of UV-irradiated mec1Δ cells, since the kinetics of nuclear division of UV-treated mec1Δ tel1Δ, rad53Δ chk1Δ, and mec1Δ rad53Δ chk1Δ cells were similar. Nonetheless, UV-irradiated mec1Δ tel1Δ, rad53Δ chk1Δ, and mec1Δ rad53Δ chk1Δ cells still show some anaphase delay compared to the untreated cultures, suggesting that other factors may be responsible for this delay. Indeed, we found that the absence of the spindle assembly checkpoint Mad2 protein, which prevents the onset of anaphase in the presence of changes in the structure or tension of the kinetochore (reviewed in references 46 and 64), partially allows the bypass of inhibition of the metaphase-to-anaphase transition in UV-irradiated mec1Δ cells and advances nuclear division in rad53Δ chk1Δ cells under the same conditions. This suggests that replication of UV-damaged chromosomes in the absence of Mec1 may affect kinetochore assembly or structure, thus triggering the activation of the Mad2-dependent mitotic checkpoint. Alternatively, since Mad2 is known to prevent sister chromatid separation by inhibiting Cdc20/APC and the Mad2-Cdc20 complex is detected even during an unperturbed cell cycle (37), the absence of Mad2 may allow a portion of Cdc20 to be activated. This may in turn be sufficient to trigger the onset of anaphase when the DNA damage checkpoint is not fully functional. Whatever the mechanism, Mad2 appears to contribute to UV-induced metaphase arrest independently of the DNA damage checkpoint. In fact, the nuclei in rad53Δ chk1Δ mad2Δ cells divided earlier than in mec1Δ tel1Δ, rad53Δ chk1Δ, and mec1Δ rad53Δ chk1Δ cells after UV irradiation. Accordingly, Rad53 is still phosphorylated in UV-treated mad2Δ mec1Δ cells.
Cross talk between spindle assembly and DNA damage checkpoints in arresting the cell cycle has been reported in other studies. In fact, MAD2 deletion was shown to partially relieve the cell cycle arrest caused either by the yku70Δ allele (56) or by mutations affecting DNA replication (26). Moreover, cdc13-induced DNA damage triggers Rad53-dependent phosphorylation of the spindle orientation checkpoint protein Bfa1, which primarily acts together with Bub2 by inhibiting the Tem1 GTPase at the top of the mitotic exit network (36). Unlike the Mad2-dependent pathway, this branch of the mitotic checkpoint does not seem to be involved in the G2 arrest of UV-irradiated mec1Δ cells that persists even in the absence of Bub2 (our unpublished observation).
Like the MAD2-dependent checkpoint, the Tel1/MRX-dependent checkpoint appears to block the metaphase-to-anaphase transition in mec1Δ cells by preventing degradation of the anaphase inhibitor Pds1/securin, which does not allow cleavage of the cohesin Scc1 by keeping the caspase-related protease Esp1 inactive (reviewed in reference 100). In fact, we observed Tel1-dependent Pds1 phosphorylation and stabilization in UV-irradiated mec1Δ cells, which are allowed to undergo sister chromatid separation and nuclear division both by artificially cleaving the Scc1 cohesin and by eliminating Pds1.
Signals in the Mec1- and Tel1/MRX-dependent response to UV irradiation.
It has been proposed that ssDNA is a key structure for activation of the Mec1-Ddc2 complex in response to HO-induced DSB and cdc13-induced cell cycle arrest (27, 45, 54). This hypothesis is supported by the finding that RPA-coated ssDNA enables ATR/ATRIP association with DNA and stimulates target phosphorylation (115). Moreover, the Rfa1-t11 variant of the largest subunit of the RPA complex (103) has been shown to suppress the HO-induced cell cycle arrest and to cause defective recruitment of Mec1/Ddc2 to damaged DNA sites (45, 103, 115).
Our finding that UV-induced Rad53 phosphorylation or activation in G1 is Mec1 dependent but Tel1 independent indicates that only the Mec1 kinase can sense and transduce checkpoint signals in response to UV damage during the G1 phase. Since this Mec1-dependent Rad53 phosphorylation is dramatically reduced in rfa1-t11 cells, which were shown to exhibit defective recruitment of Mec1/Ddc2 to HO-induced DSBs, RPA-coated ssDNA appears to be responsible for the activation of the Mec1-dependent checkpoint in response to UV damage. This implies that in order to generate enough checkpoint signals, UV lesions in G1 need to be processed, for example, by DNA repair proteins. Consistent with this hypothesis, activation of the Mec1-dependent checkpoint in response to DNA damage outside of S phase depends entirely upon the NER pathway (30, 69).
While UV-induced lesions can trigger Mec1-dependent Rad53 phosphorylation and activation independent of S-phase entry and progression, UV-damaged DNA needs to be replicated in order to activate the Tel1/MRX-dependent checkpoint. In fact, Tel1-dependent Rad53 phosphorylation does not take place when initiation of DNA replication is prevented after UV irradiation in G1 by inhibiting pre-RC assembly, a condition that, on the other hand, allows cells with unreplicated DNA to accumulate normal mitotic cyclin levels and to undergo haploid mitosis (77, 89). Moreover, UV irradiation in G2 triggers Rad53 phosphorylation in mec1Δ cells only when they complete the subsequent S phase. These observations imply that the Tel1 kinase is not able to respond to unreplicated UV-induced DNA damage, even in the absence of Mec1. Indeed, UV-induced DNA lesions may undergo structural transformations and metabolic processing during DNA replication, thus generating secondary lesions that may amplify the signals able to trigger a checkpoint response. If UV-induced lesions are not repaired prior to initiation of DNA replication, a replication fork encountering a bulge in the template may stall and resume replication by repriming DNA synthesis downstream of the lesion (reviewed in reference 16). This mechanism would generate highly recombinogenic daughter strand gaps, thus converting primary lesions into intrinsically unstable ssDNA regions, which are promptly channeled into homologous recombination pathways (reviewed in references 39 and 57). Moreover, ssDNA accumulated during replication of UV-damaged template can be converted to DSBs under certain conditions (42, 61). Stalled replication forks, DNA strand breaks, ssDNA regions, and/or DNA recombination intermediates, which may result from replicating UV-damaged DNA, may activate Tel1. The requirement of DNA replication for signaling to the DNA damage checkpoint is also critical in other eukaryotes. In fact, the inhibition of DNA initiation in Xenopus egg extracts also prevents ATR-dependent checkpoint activation in response to UV irradiation (53, 93).
Whatever the structures resulting from replication of UV-damaged DNA are, the finding that the rfa1-t11 allele decreases Tel1-dependent Rad53 phosphorylation and activation suggests that ssDNA generated during replication of UV-damaged DNA is part of the signals that can be sensed by Tel1. In this view, the amount of ssDNA generated by NER-dependent processing of UV-induced lesions in G1 may not be sufficient to activate the Tel1-dependent response, while replication of the same lesions might allow reaching the ssDNA threshold required to activate Tel1. Since human ATM seems to respond primarily to DSBs (reviewed in reference 90) and Tel1 associates with DSBs through an Xrs2-dependent mechanism (68), Tel1 might also be activated by DSBs that can arise after replication of UV-damaged DNA and/or by ssDNA that can be generated by MRX-dependent DSB processing.
The requirement of a damage threshold for signaling to the Tel1/MRX-dependent checkpoint is also suggested by the finding that the absence of the NER protein Rad14 amplifies the signals for Rad53 activation in response to UV irradiation in mec1Δ cells. Since UV-treated NER-deficient cells accumulate Y-DNA intermediates, likely due to replication fork stalling at the site of damage and Rad52-dependent Holliday junction formation (69), all these structures may increase the amount of signals able to activate the Tel1-dependent checkpoint.
Cyclin-CDK1 complexes in DNA damage checkpoints.
Tel1/MRX-dependent Rad53 phosphorylation requires replication of UV-damaged DNA and this phosphorylation is detected after damaged DNA replication is completed, suggesting that it likely requires factors that are expressed or activated specifically at that cell cycle stage. Cell cycle-dependent checkpoint activation was also observed while studying checkpoints in response to a single DNA break in S. cerevisiae. In fact, there is no evident checkpoint kinase response to a single DSB in G1-arrested cells, whereas the G2/M-arrested cells respond to this kind of lesion, suggesting that DSBs may need to be processed by unidentified G2-specific factors in order to be detected by the checkpoint machinery (76).
Since the cell cycle transitions are controlled by the sequential action of cyclin-CDK complexes, possible candidates for a role in triggering Tel1-dependent Rad53 phosphorylation may be the mitotic CDKs Clb1-CDK1 to Clb4-CDK1 in budding yeast, which are active at the G2/M transition (reviewed in reference 63). Indeed, ectopic overexpression of the major mitotic cyclin gene, CLB2, leads to premature Rad53 phosphorylation in UV-irradiated mec1Δ cells that are still replicating their DNA, suggesting that Clb2-CDK1 activity may be required to transduce the checkpoint signals to the downstream kinases. This hypothesis is also supported by the finding that expressing high levels of the Clb-CDK1 inhibitor, Sic1, after UV-induced checkpoint activation leads to Rad53 dephosphorylation in both wild-type and mec1Δ cells, indicating that Clb-CDK1 activity is required at least to keep both the Mec1- and Tel1-dependent checkpoints active. Conversely, high levels of Sic1 do not affect the maintenance of Tel1-dependent Mre11 phosphorylation after UV irradiation. This observation, together with the fact that phosphorylated Mre11 is detected as soon as these cells initiate DNA replication and therefore earlier than Rad53 phosphorylation in the same cells, suggests that Clb-CDK1 complexes may influence only subsets of Tel1 functions.
A role for cyclins in the response to DNA damage is also observed in other systems. In fact, loss of the S-phase cyclins Clb5 and Clb6 renders yeast cells sensitive to MMS, UV, and IR (59). Moreover, the S. pombe cyclin B-CDK1 complex influences recombinational repair of radiation-induced DSBs during the G2 phase (9). In fact, defects in CDK1 kinase activity affect the formation of Rhp51 (Rad51 ortholog) foci in response to IR, and cyclin B-CDK1 activity is required, together with the checkpoint protein Crb2, to maintain topoisomerase III function (9). Finally, budding yeast telomerase-negative cells require Clb2-CDK1 to generate Rad50-dependent postsenescence survivors, suggesting that this kinase may control the Rad50-dependent homologous recombination pathway (32). Thus, cyclin B-CDK1 activity could regulate the expression of key repair or recombination enzymes and/or could directly phosphorylate proteins involved in the response to DNA damage. In fact, CDK-dependent phosphorylation has been reported for several proteins involved in DNA damage response, including human RPA (19, 70), the S. cerevisiae Srs2 helicase (47), and S. pombe Crb2 (22), as well as its S. cerevisiae ortholog Rad9 (99, 106). Since we found that Clb-CDK1 activity is required to keep the UV-induced checkpoint response active, it is possible that Clb-CDK1 complexes may influence the activity of proteins required to generate or maintain the checkpoint signals and/or to transduce the signals to the downstream kinases. Although no functions for RPA phosphorylation have been uncovered so far, a possible candidate for such regulation may be the RPA complex, which has been proposed to compete with Rad51 in generating RPA-ssDNA nucleofilaments that are in turn detected as checkpoint signals (38, 94). Moreover, since Rad9 is involved in the formation of ssDNA near telomeres in cdc13 mutants (27, 54) and is required to trigger Rad53 phosphorylation and activation (31, 104), Clb-CDK1 activity itself may exert a regulatory role on Rad9.
However, the finding that Rad53 cannot be phosphorylated when G1 or G2 UV-irradiated mec1Δ cells are prevented from entering the subsequent S phase without affecting cyclin/CDK activity indicates that active Clb-CDK1 complexes by themselves are not sufficient to trigger Tel1-dependent Rad53 phosphorylation, which still requires replication of UV-damaged DNA.
Altogether our data provide evidence that a Tel1/MRX-dependent checkpoint can be activated after UV irradiation and that its activation specifically triggers a metaphase arrest. Moreover, the mechanisms that couple DNA damage recognition to enhanced phosphorylation of substrates by Mec1 and Tel1 appear to be different for the two kinases, thus indicating that ATR/Mec1- and ATM/Tel1-dependent pathways are not redundant and highlighting the functional distinction between these pathways.
Acknowledgments
We thank J. F. X. Diffley, R. D. Kolodner, N. Lowndes, K. Nasmyth, S. Piatti, and F. Uhlmann for providing reagents; S. Piatti for helpful suggestions and critically reading the manuscript; and M. Foiani and all the members of our laboratory for useful discussions and criticisms.
This work was supported in part by grants from Telethon-Italy (E.1247) and the Associazione Italiana per la Ricerca sul Cancro and Cofinanziamento 2003 MIUR/Università di Milano-Bicocca to M.P.L. and grants from the Fondo per gli Investimenti della Ricerca di Base (FIRB) and Progetto Strategico MIUR-Legge 449/97 to G.L. V.B. was supported by a fellowship from Fondazione Italiana per la Ricerca sul Cancro.
REFERENCES
- 1.Agarwal, R., Z. Tang, H. Yu, and O. Cohen-Fix. 2003. Two distinct pathways for inhibiting Pds1 ubiquitination in response to DNA damage. J. Biol. Chem. 278:45027-45033. [DOI] [PubMed] [Google Scholar]
- 2.Akkari, Y. M. N., R. L. Bateman, C. A. Reifsteck, S. B. Olson, and M. Grompe. 2000. DNA replication is required to elicit cellular responses to psoralen-induced DNA interstrand cross-links. Mol. Cell. Biol. 20:8283-8289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andegeko, Y., L. Moyal, L. Mittelman, I. Tsarfaty, Y. Shiloh, and G. Rotman. 2001. Nuclear retention of ATM at sites of DNA double strand breaks. J. Biol. Chem. 276:38224-38230. [DOI] [PubMed] [Google Scholar]
- 4.Bakkenist, C. J., and M. B. Kastan. 2003. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 421:499-506. [DOI] [PubMed] [Google Scholar]
- 5.Banin, S., L. Moyal, S. Shieh, Y. Taya, C. W. Anderson, L. Chessa, N. I. Smorodinsky, C. Prives, Y. Reiss, Y. Shiloh, and Y. Ziv. 1998. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science 281:1674-1677. [DOI] [PubMed] [Google Scholar]
- 6.Baroni, E., V. Viscardi, H. Cartagena-Lirola, G. Lucchini, and M. P. Longhese. 2004. The functions of budding yeast Sae2 in the DNA damage response require Mec1- and Tel1-dependent phosphorylation. Mol. Cell. Biol. 24:4151-4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bentley, N. J., D. A. Holtzman, G. Flaggs, K. S. Keegan, A. DeMaggio, J. C. Ford, M. Hoekstra, and A. M. Carr. 1996. The Schizosaccharomyces pombe rad3 checkpoint gene. EMBO J. 15:6641-6651. [PMC free article] [PubMed] [Google Scholar]
- 8.Canman, C. E., D. S. Lim, K. A. Cimprich, Y. Taya, K. Tamai, K. Sakaguchi, E. Appella, M. B. Kastan, and J. D. Siliciano. 1998. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science 281:1677-1679. [DOI] [PubMed] [Google Scholar]
- 9.Caspari, T., J. M. Murray, and A. M. Carr. 2002. Cdc2-cyclin B kinase activity links Crb2 and Rqh1 topoisomerase III. Genes Dev. 16:1195-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chahwan, C., T. M. Nakamura, S. Sivakumar, P. Russell, and N. Rhind. 2003. The fission yeast Rad32 (Mre11)-Rad50-Nbs1 complex is required for the S-phase DNA damage checkpoint. Mol. Cell. Biol. 18:6564-6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clerici, M., V. Paciotti, V. Baldo, M. Romano, G. Lucchini, and M. P. Longhese. 2001. Hyperactivation of the yeast DNA damage checkpoint by TEL1 and DDC2 overexpression. EMBO J. 20:6485-6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cocker, J. H., S. Piatti, C. Santocanale, K. Nasmyth, and J. F. Diffley. 1996. An essential role for the Cdc6 protein in forming the pre-replicative complexes of budding yeast. Nature 379:180-182. [DOI] [PubMed] [Google Scholar]
- 13.Cohen-Fix, O., and D. Koshland. 1997. The anaphase inhibitor of Saccharomyces cerevisiae Pds1p is a target of the DNA damage checkpoint pathway. Proc. Natl. Acad. Sci. USA 94:14361-14366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen-Fix, O., J. M. Peters, M. W. Kirschner, and D. Koshland. 1996. Anaphase initiation in Saccharomyces cerevisiae is controlled by the APC-dependent degradation of the anaphase inhibitor Pds1p. Genes Dev. 10:3081-3093. [DOI] [PubMed] [Google Scholar]
- 15.Cortez, D., S. Guntuku, J. Qin, and S. J. Elledge. 2001. ATR and ATRIP: partners in checkpoint signaling. Science 294:1713-1716. [DOI] [PubMed] [Google Scholar]
- 16.Cox, M. M., M. F. Goodman, K. N. Kreuzer, D. J. Sherratt, S. J. Sandler, and K. J. Marians. 2000. The importance of repairing stalled replication forks. Nature 404:37-41. [DOI] [PubMed] [Google Scholar]
- 17.D'Amours, D., and S. P. Jackson. 2001. The yeast Xrs2 complex functions in S phase checkpoint regulation. Genes Dev. 15:2238-2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desdouets, C., C. Santocanale, L. S. Drury, G. Perkins, M. Foiani, P. Plevani, and J. F. X. Diffley. 1998. Evidence for a Cdc6-independent mitotic resetting event involving DNA polymerase α. EMBO J. 17:4139-4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dutta, A., and B. Stillman. 1992. Cdc2 family kinases phosphorylate a human cell DNA replication factor, RPA, and activate DNA replication. EMBO J. 11:2189-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edwards, R. J., N. J. Bentley, and A. M. Carr. 1999. A Rad3-Rad26 complex responds to DNA damage independently of other checkpoint proteins. Nat. Cell Biol. 1:393-398. [DOI] [PubMed] [Google Scholar]
- 21.Emili, A. 1998. MEC1-dependent phosphorylation of Rad9p in response to DNA damage. Mol. Cell 2:183-189. [DOI] [PubMed] [Google Scholar]
- 22.Esashi, F., and M. Yanagida. 1999. Cdc2 phosphorylation of Crb2 is required for re-establishing cell cycle progression after the damage checkpoint. Mol. Cell 4:167-174. [DOI] [PubMed] [Google Scholar]
- 23.Fan, H., K. K. Cheng, and H. L. Klein. 1996. Mutations in the RNA polymerase II transcription machinery suppress the hyperrecombination mutant hpr1Δ of Saccharomyces cerevisiae. Genetics 142:749-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedberg, E. C., G. C. Walker, and W. Siede. 1995. DNA repair and mutagenesis. ASM Press, Washington, D.C.
- 25.Funabiki, H., H. Yamano, K. Kumada, K. Nagao, T. Hunt, and M. Yanagida. 1996. Cut2 proteolysis required for sister-chromatid separation in fission yeast. Nature 381:438-441. [DOI] [PubMed] [Google Scholar]
- 26.Garber, P. M., and J. Rine. 2002. Overlapping roles of the spindle assembly and DNA damage checkpoints in the cell-cycle response to altered chromosomes in Saccharomyces cerevisiae. Genetics 161:521-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garvik, B., M. Carson, and L. Hartwell. 1995. Single-stranded DNA arising at telomeres in cdc13 mutants may constitute a specific signal for the RAD9 checkpoint. Mol. Cell. Biol. 15:6128-6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gatei, M., D. Young, K. M. Cerosaletti, A. Desai-Mehta, K. Spring, S. Kozlov, M. F. Lavin, R. A. Gatti, P. Concannon, and K. Khanna. 2000. ATM-dependent phosphorylation of nibrin in response to radiation exposure. Nat. Genet. 25:115-119. [DOI] [PubMed] [Google Scholar]
- 29.Giannattasio, M., E. Sommariva, R. Vercillo, F. Lippi-Boncambi, G. Liberi, M. Foiani, P. Plevani, and M. Muzi-Falconi. 2002. A dominant-negative MEC3 mutant uncovers new functions for the Rad17 complex and Tel1. Proc. Natl. Acad. Sci. USA 99:12997-13002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giannattasio, M., F. Lazzaro, M. P. Longhese, P. Plevani, and M. Muzi-Falconi. 2004. Physical and functional interactions between nucleotide excision repair and DNA damage checkpoint. EMBO J. 23:429-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilbert, C. S., C. M. Green, and N. F. Lowndes. 2001. Budding yeast Rad9 is an ATP-dependent Rad53 activating machine. Mol. Cell 8:129-136. [DOI] [PubMed] [Google Scholar]
- 32.Grandin, N., and M. Charbonneau. 2003. Mitotic cyclins regulate telomeric recombination in telomerase-deficient yeast cells. Mol. Cell. Biol. 23:9162-9177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greenwell, P. W., S. L. Kronmal, S. E. Porter, J. Gassenhuber, B. Obermaier, and T. D. Petes. 1995. TEL1, a gene involved in controlling telomere length in S. cerevisiae, is homologous to the human ataxia telangiectasia gene. Cell 82:823-829. [DOI] [PubMed] [Google Scholar]
- 34.Grenon, M., C. S. Gilbert, and N. F. Lowndes. 2001. Checkpoint activation in response to double-strand breaks requires the Mre11/Rad50/Xrs2 complex. Nat. Cell Biol. 3:844-847. [DOI] [PubMed] [Google Scholar]
- 35.Hari, K. L., A. Santerre, J. J. Sekelsky, K. S. McKim, J. B. Boyd, and R. S. Hawley. 1995. The mei-41 gene of D. melanogaster is a structural and functional homolog of the human ataxia telangiectasia gene. Cell 82:815-821. [DOI] [PubMed] [Google Scholar]
- 36.Hu, F., and S. J. Elledge. 2002. Bub2 is a cell cycle-regulated phospho-protein controlled by multiple checkpoints. Cell Cycle 1:351-355. [PubMed] [Google Scholar]
- 37.Hwang, L. H., L. F. Lau, D. L. Smith, C. A. Mistrot, K. G. Hardwick, E. S. Hwang, A. Amon, and A. W. Murray. 1998. Budding yeast Cdc20: a target of the spindle checkpoint. Science 279:1041-1044. [DOI] [PubMed] [Google Scholar]
- 38.Kantake, N., T. Sugiyama, R. D. Kolodner, and S. C. Kowalczykowski. 2003. The recombination-deficient mutant RPA (rfa1-t11) is displaced slowly from single-stranded DNA by Rad51 protein. J. Biol. Chem. 278:23410-23417. [DOI] [PubMed] [Google Scholar]
- 39.Kogoma, T. 1996. Recombination by replication. Cell 85:625-627. [DOI] [PubMed] [Google Scholar]
- 40.Kolodner, R. D., C. D. Putnam, and K. Myung. 2002. Maintenance of genome stability in Saccharomyces cerevisiae. Science 297:552-557. [DOI] [PubMed] [Google Scholar]
- 41.Kondo, T., T. Wakayama, T. Naiki, K. Matsumoto, and K. Sugimoto. 2001. Recruitment of Mec1 and Ddc1 checkpoint proteins to double-strand breaks through distinct mechanisms. Science 294:867-870. [DOI] [PubMed] [Google Scholar]
- 42.Kuzminov, A. 2001. Single-strand interruptions in replicating chromosomes cause double-strand breaks. Proc. Natl. Acad. Sci. USA 98:8241-8246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lambert, S., S. Mason, L. J. Barber, J. A. Hartley, J. A. Pearce, A. M. Carr, and P. J. McHugh. 2003. Schizosaccharomyces pombe checkpoint response to DNA interstrand cross-links. Mol. Cell. Biol. 23:4728-4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee, J. H., and T. T. Paull. 2004. Direct activation of the ATM protein kinase by the Mre11/Rad50/Nbs1 complex. Science 304:93-96. [DOI] [PubMed] [Google Scholar]
- 45.Lee, S. E., J. K. Moore, A. Holmes, K. Umezu, R. Kolodner, and J. E. Haber. 1998. Saccharomyces Ku70, Mre11/Rad50 and RPA proteins regulate adaptation to G2/M arrest after DNA damage. Cell 94:399-409. [DOI] [PubMed] [Google Scholar]
- 46.Lew, D. J., and D. J. Burke. 2003. The spindle assembly and spindle position checkpoints. Annu. Rev. Genet. 37:251-282. [DOI] [PubMed] [Google Scholar]
- 47.Liberi, G., I. Chiolo, A. Pellicioli, M. Lopes, P. Plevani, M. Muzi-Falconi, and M. Foiani. 2000. Srs2 DNA helicase is involved in checkpoint response and its regulation requires a functional Mec1-dependent pathway and Cdk1 activity. EMBO J. 19:5027-5038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lim, D. S., S. T. Kim, B. Xu, R. S. Maser, J. Lin, J. H. J. Petrini, and M. B. Kastan. 2000. ATM phosphorylates p95/nbs1 in an S-phase checkpoint pathway. Nature 404:613-617. [DOI] [PubMed] [Google Scholar]
- 49.Longhese, M. P., M. Clerici, and G. Lucchini. 2003. The S-phase checkpoint and its regulation in Saccharomyces cerevisiae. Mutat. Res. 532:41-58. [DOI] [PubMed] [Google Scholar]
- 50.Longhese, M. P., V. Paciotti, H. Neecke, and G. Lucchini. 2000. Checkpoint proteins influence telomeric silencing and length maintenance in budding yeast. Genetics 155:1577-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Longhese, M. P., V. Paciotti, R. Fraschini, R. Zaccarini, P. Plevani, and G. Lucchini. 1997. The novel DNA damage checkpoint protein Ddc1p is phosphorylated periodically during the cell cycle and in response to DNA damage in budding yeast. EMBO J. 16:5216-5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lopes, M., C. Cotta-Ramusino, A. Pellicioli, G. Liberi, P. Plevani, M. Muzi-Falconi, C. Newlon, and M. Foiani. 2001. The DNA replication checkpoint response stabilizes stalled replication forks. Nature 412:557-561. [DOI] [PubMed] [Google Scholar]
- 53.Lupardus, P. J., T. Byun, M. C. Yee, M. Hekmat-Nejad, and K. A. Cimprich. 2002. A requirement for replication in activation of the ATR-dependent DNA damage checkpoint. Genes Dev. 16:2327-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lydall, D., and T. Weinert. 1995. Yeast checkpoint genes in DNA damage processing: implications for repair and arrest. Science 270:1488-1491. [DOI] [PubMed] [Google Scholar]
- 55.Mallory, J. C., and T. D. Petes. 2000. Protein kinase activity of Tel1p and Mec1p, two Saccharomyces cerevisiae proteins related to the human ATM protein kinase. Proc. Natl. Acad. Sci. USA 97:13749-13754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maringele, L., and D. Lydall. 2002. EXO1-dependent single-stranded DNA at telomeres activates subsets of DNA damage and spindle checkpoint pathways in budding yeast yku70Δ mutants. Genes Dev. 16:1919-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McGlynn, P., and R. G. Lloyd. 2002. Recombinational repair and restart of damaged replication forks. Nat. Rev. Mol. Cell. Biol. 3:859-870. [DOI] [PubMed] [Google Scholar]
- 58.Melo, J. A., J. Cohen, and D. P. Toczyski. 2001. Two checkpoint complexes are independently recruited to sites of DNA damage in vivo. Genes Dev. 15:2809-2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meyn, M. A., and S. L. Holloway. 2000. S-phase cyclins are required for a stable arrest at metaphase. Curr. Biol. 10:1599-1602. [DOI] [PubMed] [Google Scholar]
- 60.Michaelis, C., R. Ciosk, and K. Nasmyth. 1997. Cohesins: chromosomal proteins that prevent premature separation of sister chromatids. Cell 91:35-45. [DOI] [PubMed] [Google Scholar]
- 61.Michel, B., S. D. Ehrlich, and M. Uzest. 1997. DNA double-strand breaks caused by replication arrest. EMBO J. 16:430-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morrow, D. M., D. A. Tagle, Y. Shiloh, F. S. Collins, and P. Hieter. 1995. TEL1, an S. cerevisiae homolog of the human gene mutated in ataxia telangiectasia, is functionally related to the yeast checkpoint gene MEC1. Cell 82:831-840. [DOI] [PubMed] [Google Scholar]
- 63.Murray, A. W. 2004. Recycling the cell cycle: cyclins revisited. Cell 116:221-234. [DOI] [PubMed] [Google Scholar]
- 64.Musacchio, A., and K. G. Hardwick. 2002. The spindle checkpoint: structural insights into dynamic signalling. Nat. Rev. Mol. Cell. Biol. 3:731-741. [DOI] [PubMed] [Google Scholar]
- 65.Myung, K., A. Datta, and R. D. Kolodner. 2001. Suppression of spontaneous chromosomal rearrangements by S phase checkpoint functions in Saccharomyces cerevisiae. Cell 104:397-408. [DOI] [PubMed] [Google Scholar]
- 66.Myung, K., C. Chen, and R. D. Kolodner. 2001. Multiple pathways cooperate in the suppression of genome instability in Saccharomyces cerevisiae. Nature 411:1073-1076. [DOI] [PubMed] [Google Scholar]
- 67.Nakada, D., T. Shimomura, K. Matsumoto, and K. Sugimoto. 2003. The ATM-related Tel1 protein of Saccharomyces cerevisiae controls a checkpoint response following phleomycin treatment. Nucleic Acids Res. 31:1715-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakada, D., K. Matsumoto, and K. Sugimoto. 2003. ATM-related Tel1 associates with double-strand breaks through an Xrs2-dependent mechanism. Genes Dev. 16:1957-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Neecke, H., G. Lucchini, and M. P. Longhese. 1999. Cell cycle progression in the presence of irreparable DNA damage is controlled by a Mec1- and Rad53-dependent checkpoint in budding yeast. EMBO J. 18:4485-4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Niu, H., H. Erdjument-Bromage, Z. Q. Pan, S. H. Lee, P. Tempst, and J. Hurwitz. 1997. Mapping of amino acid residues in the p34 subunit of human single-stranded DNA-binding protein phosphorylated by DNA-dependent protein kinase and Cdc2 kinase in vitro. J. Biol. Chem. 272:12634-12641. [DOI] [PubMed] [Google Scholar]
- 71.Noton, E., and J. F. X. Diffley. 2000. CDK inactivation is the only essential function of the APC/C and the mitotic exit network proteins for origin resetting during mitosis. Mol. Cell 5:85-95. [DOI] [PubMed] [Google Scholar]
- 72.Nyberg, K. A., R. J. Michelson, C. W. Putnam, and T. A. Weinert. 2002. Toward maintaining the genome: DNA damage and replication checkpoints. Annu. Rev. Genet. 36:617-656. [DOI] [PubMed] [Google Scholar]
- 73.Paciotti, V., G. Lucchini, P. Plevani, and M. P. Longhese. 1998. Mec1p is essential for phosphorylation of the yeast DNA damage checkpoint protein Ddc1p, which physically interacts with Mec3p. EMBO J. 17:4199-4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Paciotti, V., M. Clerici, G. Lucchini, and M. P. Longhese. 2000. The checkpoint protein Ddc2, functionally related to S. pombe Rad26, interacts with Mec1 and is regulated by Mec1-dependent phosphorylation in budding yeast. Genes Dev. 14:2046-2059. [PMC free article] [PubMed] [Google Scholar]
- 75.Paciotti, V., M. Clerici, M. Scotti, G. Lucchini, and M. P. Longhese. 2001. Characterization of mec1 kinase-deficient mutants and of new hypomorphic mec1 alleles impairing subsets of the DNA damage response pathway. Mol. Cell. Biol. 21:3913-3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pellicioli, A., S. E. Lee, C. Lucca, M. Foiani, and J. E. Haber. 2001. Regulation of Saccharomyces Rad53 checkpoint kinase during adaptation from DNA damage-induced G2/M arrest. Mol. Cell 7:293-300. [DOI] [PubMed] [Google Scholar]
- 77.Piatti, S., C. Lengauer, and K. Nasmyth. 1995. Cdc6 is an unstable protein whose de novo synthesis in G1 is important for the onset of S phase and for preventing a “reductional” anaphase in the budding yeast Saccharomyces cerevisiae. EMBO J. 14:3788-3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Piatti, S., T. Bohm, J. H. Cocker, J. F. Diffley, and K. Nasmyth. 1996. Activation of S-phase-promoting CDKs in late G1 defines a “point of no return” after which Cdc6 synthesis cannot promote DNA replication in yeast. Genes Dev. 10:1516-1531. [DOI] [PubMed] [Google Scholar]
- 79.Prakash, S., and L. Prakash. 2000. Nucleotide excision repair in yeast. Mutat. Res. 451:13-24. [DOI] [PubMed] [Google Scholar]
- 80.Rose, M. D., F. Winston, and P. Hieter. 1990. Methods in yeast genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 81.Rouse, J., and S. P. Jackson. 2000. LCD1: an essential gene involved in checkpoint control and regulation of the MEC1 signaling pathway in Saccharomyces cerevisiae. EMBO J. 19:5801-5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rouse, J., and S. P. Jackson. 2002. Lcd1p recruits Mec1p to DNA lesions in vivo and in vitro. Mol. Cell 9:857-869. [DOI] [PubMed] [Google Scholar]
- 83.Ruhland, A., and M. Brendel. 1978. Mutagenesis by cytostatic alkylating agents in yeast strains of different repair capacities. Genetics 92:83-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sanchez, Y., B. A. Desany, W. J. Jones, Q. Liu, B. Wang, and S. J. Elledge. 1996. Regulation of RAD53 by the ATM-like kinases MEC1 and TEL1 in yeast cell cycle checkpoint pathways. Science 271:357-360. [DOI] [PubMed] [Google Scholar]
- 85.Sanchez, Y., J. Bachant, H. Wang, F. H. Hu, D. Liu, M. Tetzlaff, and S. J. Elledge. 1999. Control of the DNA damage checkpoint by Chk1 and Rad53 protein kinases through distinct mechanisms. Science 286:1166-1171. [DOI] [PubMed] [Google Scholar]
- 86.Savitsky, K., A. Bar-Shira, S. Gilad, G. Rotman, Y. Ziv, L. Vanagaite, D. A. Tagle, S. Smith, T. Uziel, S. Sfez, M. Ashkenazi, I. Pecker, M. Frydman, R. Harnik, S. R. Patanjali, A. Simmons, G. A. Clines, A. Sartiel, R. A. Gatti, L. Chessa, O. Sanal, M. F. Lavin, N. G. J. Jaspers, A. M. R. Taylor, C. F. Arlett, T. Miki, S. M. Weissman, M. Lovett, F. S. Collins, and Y. Shiloh. 1995. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science 268:1749-1753. [DOI] [PubMed] [Google Scholar]
- 87.Schultz, L. B., N. H. Chehab, A. Malikzay, R. A. DiTullio, E. S. Stavridi, and T. D. Halazonetis. 2000. The DNA damage checkpoint and human cancer. Cold Spring Harbor Symp. Quant. Biol. 65:489-498. [DOI] [PubMed] [Google Scholar]
- 88.Schwartz, M. F., J. K. Duong, Z. Sun, J. S. Morrow, D. Pradhan, and D. F. Stern. 2002. Rad9 phosphorylation sites couple Rad53 to the Saccharomyces cerevisiae DNA damage checkpoint. Mol. Cell 9:1055-1065. [DOI] [PubMed] [Google Scholar]
- 89.Severin, F., A. A. Hyman, and S. Piatti. 2001. Correct spindle elongation at the metaphase/anaphase transition is an APC-dependent event in budding yeast. J. Cell Biol. 155:711-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shiloh, Y. 2003. ATM and related protein kinases: safeguarding genome integrity. Nat. Rev. Cancer 3:155-168. [DOI] [PubMed] [Google Scholar]
- 91.Sogo, J. M., M. Lopes, and M. Foiani. 2002. Fork reversal and ssDNA accumulation at stalled replication forks owing to checkpoint defects. Science 297:599-602. [DOI] [PubMed] [Google Scholar]
- 92.Stern, B. M., and A. W. Murray. 2001. Lack of tension at kinetochores activates the spindle checkpoint in budding yeast. Curr. Biol. 11:1462-1467. [DOI] [PubMed] [Google Scholar]
- 93.Stokes, M. P., R. Van Hatten, H. D. Lindsay, and W. M. Michael. 2002. DNA replication is required for the checkpoint response to damaged DNA in Xenopus egg extracts. J. Cell Biol. 158:863-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sugiyama, T., and S. C. Kowalczykowski. 2002. Rad52 protein associates with replication protein A (RPA)-single-stranded DNA to accelerate Rad51-mediated displacement of RPA and presynaptic complex formation. J. Biol. Chem. 277:31663-31672. [DOI] [PubMed] [Google Scholar]
- 95.Sun, Z., J. Hsiao, D. S. Fay, and D. F. Stern. 1998. Rad53 FHA domain associated with phosphorylated Rad9 in the DNA damage checkpoint. Science 281:272-274. [DOI] [PubMed] [Google Scholar]
- 96.Surana, U., A. Amon, C. Dowzer, J. McGrew, B. Byers, and K. Nasmyth. 1993. Destruction of the CDC28/CLB mitotic kinase is not required for the metaphase to anaphase transition in budding yeast. EMBO J. 12:1969-1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Takata, H., Y. Kanoh, N. Gunge, K. Shirahige, and A. Matsuura. 2004. Reciprocal association of the budding yeast ATM-related proteins Tel1 and Mec1 with telomeres in vivo. Mol. Cell 14:515-522. [DOI] [PubMed] [Google Scholar]
- 98.Tercero, J. A., and J. F. X. Diffley. 2001. Regulation of DNA replication fork progression through damaged DNA by the Mec1/Rad53 checkpoint. Nature 412:553-557. [DOI] [PubMed] [Google Scholar]
- 99.Ubersax, J. A., E. L. Woodbury, P. N. Quang, M. Paraz, J. D. Blethrow, K. Shah, K. M. Shokat, and D. O. Morgan. 2003. Targets of the cyclin-dependent kinase Cdk1. Nature 425:859-864. [DOI] [PubMed] [Google Scholar]
- 100.Uhlmann, F. 2003. Chromosome cohesion and separation: from men and molecules. Curr. Biol. 13:104-114. [DOI] [PubMed] [Google Scholar]
- 101.Uhlmann, F., D. Wernic, M. A. Poupart, E. Koonin, and K. Nasmyth. 2000. Cleavage of cohesin by the CD clan protease separin triggers anaphase in yeast. Cell 103:375-386. [DOI] [PubMed] [Google Scholar]
- 102.Uhlmann, F., F. Lottspeich, and K. Nasmyth. 1999. Sister chromatid separation at anaphase onset is promoted by cleavage of the cohesin subunit Scc1p. Nature 400:37-42. [DOI] [PubMed] [Google Scholar]
- 103.Umezu, K., N. Sugawara, C. Chen, J. E. Haber, and R. D. Kolodner. 1998. Genetic analysis of yeast RPA1 reveals its multiple functions in DNA metabolism. Genetics 148:989-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Usui, T., H. Ogawa, and J. H. J. Petrini. 2001. A DNA damage response pathway controlled by Tel1 and the Mre11 complex. Mol. Cell 7:1255-1266. [DOI] [PubMed] [Google Scholar]
- 105.Van Den Bosch, M., R. T. Bree, and N. F. Lowndes. 2003. The MRN complex: coordinating and mediating the response to broken chromosomes. EMBO Rep. 9:844-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vialard, J. E., C. S. Gilbert, C. M. Green, and N. F. Lowndes. 1998. The budding yeast Rad9 checkpoint protein is subjected to Mec1/Tel1-dependent hyperphosphorylation and interacts with Rad53 after DNA damage. EMBO J. 17:5679-5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wach, A., A. Brachat, R. Pohlmann, and P. Philippsen. 1994. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast 13:1793-1808. [DOI] [PubMed] [Google Scholar]
- 108.Wakayama, T., T. Kondo, S. Ando, K. Matsumoto, and K. Sugimoto. 2001. Pie1, a protein interacting with Mec1, controls cell growth and checkpoint responses in Saccharomyces cerevisiae. Mol. Cell. Biol. 21:755-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Weinert, T., and L. Hartwell. 1989. Control of G2 delay by the RAD9 gene of Saccharomyces cerevisiae. J. Cell Sci. Suppl. 12:145-148. [DOI] [PubMed] [Google Scholar]
- 110.Weinert, T. A., G. L. Kiser, and L. H. Hartwell. 1994. Mitotic checkpoint genes in budding yeast and the dependence of mitosis on DNA replication and repair. Genes Dev. 8:652-665. [DOI] [PubMed] [Google Scholar]
- 111.Wu, X., V. Ranganathan, D. S. Weisman, W. F. Heine, D. N. Ciccone, T. B. O'Neill, K. E. Crick, K. A. Pierce, W. S. Lane, G. Rathbun, D. M. Livingston, and D. T. Weaver. 2000. ATM phosphorylation of Nijmegen breakage syndrome protein is required in a DNA damage response. Nature 405:477-482. [DOI] [PubMed] [Google Scholar]
- 112.Zhao, S., Y. C. Weng, S. S. Yuan, Y. T. Lin, H. C. Hsu, S. C. Lin, E. Gerbino, M. H. Song, M. Z. Zdzienicka, R. A. Gatti, J. W. Shay, Y. Ziv, Y. Shiloh, and E. Y. Lee. 2000. Functional link between ataxia-telangiectasia and Nijmegen breakage syndrome gene products. Nature 405:473-477. [DOI] [PubMed] [Google Scholar]
- 113.Zhao, X., E. G. D. Muller, and R. Rothstein. 1998. A suppressor of two essential checkpoint genes identifies a novel protein that negatively affects dNTP pools. Mol. Cell 2:329-340. [DOI] [PubMed] [Google Scholar]
- 114.Zou, H., T. J. McGarry, T. Bernal, and M. W. Kirschner. 1999. Identification of a vertebrate sister-chromatid separation inhibitor involved in transformation and tumorigenesis. Science 285:418-422. [DOI] [PubMed] [Google Scholar]
- 115.Zou, L., and S. J. Elledge. 2003. Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science 300:1542-1548. [DOI] [PubMed] [Google Scholar]
- 116.Zou, L., D. Cortez, and S. J. Elledge. 2002. Regulation of ATR substrate selection by Rad17 dependent loading of Rad9 complexes onto chromatin. Genes Dev. 16:198-208. [DOI] [PMC free article] [PubMed] [Google Scholar]