Abstract
Astrocytes tile the entire CNS. They are vital for neural circuit function, but have traditionally been viewed as simple homogenous cells that serve the same essential supportive roles everywhere. Here, we summarize exciting breakthroughs that instead indicate astrocytes represent a population of complex and functionally diverse cells. Physiological diversity of astrocytes is apparent between different brain circuits and microcircuits, and individual astrocytes display diverse signaling within subcellular compartments. With respect to injury and disease, astrocytes undergo diverse phenotypic changes that may be protective or causative with regard to pathology in a context dependent manner. These new insights herald the concept that astrocytes represent a diverse population of genetically tractable cells that mediate neural circuit specific roles in health and disease.
Introduction and historical perspective
Glial cells exist throughout the central nervous system (CNS). Estimates regarding glia to neuron ratios vary1, 2. Most likely, glia are present in numbers that are at least equal to, or exceeding, neurons2 and ~20–40% of the total number of cells in mammalian brains are specialized glia called astrocytes2, with considerable variability between species and brain areas. Mammalian brain neuronal circuits are built within networks of astrocytes, but the ratio of astrocytes to neurons varies between areas such as cerebral cortex, where astrocytes outnumber neurons, and cerebellum, where neurons greatly outnumber astrocytes. Astrocytes are sparse in areas with a high density of neuronal cell bodies such as cerebellar granule and hippocampal pyramidal cell layers, whereas they are replete in areas with dendrites and axons. Hence, at a fundamental level there is regional diversity in astrocyte-to-neuron ratios, but the functional relevance and the regional differences in volumes occupied by astrocytes is not understood. It is remarkable how little we know about the functional diversity of astrocytes, especially as neuroscience seeks to map entire brain circuits3. Nonetheless, several functions are now well accepted and include roles for astrocytes in ion homeostasis, neurotransmitter clearance, synapse formation and removal as well as contributions to neurovascular coupling.
Early anatomical studies revealed a range of astrocyte morphologies, hinting at diversity and functional specializations4, and these observations have been extended with the latest imaging methods5. The distinction between protoplasmic and fibrous astrocytes is now well established based on morphological differences and their locations in gray and white matter, respectively6, 7. In contrast, studies of astrocyte function have lagged behind, and an appreciation of functional diversity has been slower to emerge. In large part, this reflects the fact that electrophysiology, which so advanced the understanding of neurons, is not ideally suited to study glia. Astrocytes8, like other glia, are largely electrically silent9, rarely deviate from the K+ equilibrium potential by more than a few millivolts10, and display a predictable9 57 mV depolarization for a 10-fold increase in extracellular K+. Astrocytes also have low membrane resistances and extensive small branchlets; the voltage changes in these distal structures may not be detected using somatic electrophysiology. In 1967, Stephen Kuffler, a pioneering glial researcher, perhaps recognizing their threadbare electrical signaling potential, wrote9
“Concerning the role of glial cells, a variety of mechanisms will probably emerge. Obvious gaps in our knowledge which prevent the formulation of more precise hypotheses relate to detailed information about the biochemical properties of various glial cells and the mechanisms of neuron-glia interactions”.
Much progress has been made in the years since, and a mechanistic understanding of astrocytes is emerging. Key advances have arisen with the use of modern imaging methods, physiological studies of astrocyte-neuron interactions, molecular genetics, protein engineering, transcriptomics and studies of how astrocytes respond to injury. We focus on some of the latest insights that shed new light on astrocyte functional dynamics, diversity and specialisations within neural circuits and microcircuits.
Readers should consider several general reviews on astrocyte heterogeneity11, 12 and development13, 14. In the current review, we seek to tackle astrocyte diversity from a different angle. In Box 1 we list questions that relate to functional diversity. We summarize evidence related to these and advance the concept that astrocytes represent a diverse population of cells with diverse dynamics and functions.
Box 1. Key questions that need to be addressed to demonstrate and exploit functional diversity of astrocytes.
Do astrocytes mediate distinct physiological responses in different brain circuits, or do they mediate the same functions throughout the brain, i.e. are astrocyte functions tailored to the functions of the circuits in which they reside?
Is there evidence for diverse astrocyte physiological responses within a given brain area, i.e. is there evidence of physiological specialization within local microcircuits?
Does signaling diversity exist within single astrocytes, i.e. do astrocytes contain signaling sub compartments that serve separable functions?
Is the response of a defined population of astrocytes to injury and disease homogenous or heterogeneous?
Can genetic markers and tools be exploited to study genetically specified populations of astrocytes and their functions in circuits?
Can astrocyte diversity be exploited to produce desirable effects within neural circuits in the context of CNS disorders?
How does slow and global signaling in astrocytes contribute to the functioning of specific neural circuits?
How do changes during hypertrophic reactive astrogliosis triggered by different insults impact on neural circuits?
Diversity of astrocyte molecules and markers
Glial fibrillary acidic protein (GFAP) was the first molecular marker to be robustly associated with astrocytes15, 16. Although GFAP is often regarded as the prototypical astrocyte marker, this status carries two important caveats. First, although upregulation of GFAP is a sensitive and reliable marker of astrocytes that are responding to CNS insults, in healthy tissue many astrocytes do not express GFAP in amounts detectable by immunohistochemistry. Thus, the absence of detectable GFAP does not indicate the absence of astrocytes that can be detected by ultrastructural evaluation or by other markers such as S100b, glutamine synthetase or Aldh1L1 (see Ref17). Astrocytes from different brain regions can exhibit pronounced differences in GFAP levels, including among locally intermingled astrocytes. Nearly all astrocytes in healthy hippocampus express readily detectable GFAP, whereas few astrocytes do so in thalamus. In cerebral cortex, many astrocytes in superficial and deep layers express detectable GFAP, whereas few astrocytes do so in middle layers. Similar differences exist in gray matter regions across the CNS, whereas most white matter astrocytes constitutively express detectable GFAP. Findings such as these were among the first to hint at astrocyte molecular diversity. It is also noteworthy that different isoforms and splice variants of GFAP (a,b,g,d and k) exist and may be variably expressed in different astrocytes18. The second major caveat regarding GFAP as an astrocyte marker is that it is expressed by other cell types in the CNS and peripheral organs17, 19. In the CNS, GFAP-expressing progenitor cells derived from radial glia appear in late development and generate neurons and glia in various CNS regions throughout life20, 21. Thus, detectable GFAP expression is neither required nor sufficient to identify a cell as a mature differentiated astrocyte.
As other molecules produced by astrocytes have been identified, examples of astrocyte molecular diversity have emerged, particularly across CNS regions. For example, astrocytes exhibit differences in glutamate transporters GLT-1 (EAAT2) and GLAST, such that GLT-1 (EAAT2) is higher in hippocampus, cerebral cortex and striatum, whilst GLAST is higher in cerebellum22. The gap junction protein connexin 30 (Cx30) is expressed by gray but not white matter astrocytes, and gray matter astrocytes in globus palidus express high Cx30 levels whereas those in neighboring striatum express low levels23. The potassium channel Kir4.1 is low in white matter astrocytes, and variable among gray matter astrocytes with particularly high levels in hippocampus and cerebellum24. Recent large-scale astrocyte transcriptome analyses have strengthened the notion of molecular diversity among astrocytes in different regions, and provided evidence that different stimuli can change astrocyte transcriptional profiles in different ways25–29.
There is also molecular heterogeneity among astrocytes locally interspersed within the same region. For example, a subpopulation of astrocytes in human cerebral cortex expresses the cell adhesion molecule, CD4430. Using genetic knock-in targeting strategies, Garcia et al showed that the sonic hedge hog (SHH) receptor, Gli1, is selectively expressed by scattered astrocytes in murine cerebral cortex, and that attenuation of neuronal SHH increased GFAP expression only in Gli1 expressing astrocytes, thereby demonstrating the capacity for selective signaling from neurons to a specific astrocyte subpopulation31. Later we consider how Sema3a expression identifies astrocyte subpopulations in the spinal cord32.
To date, most genetic targeting strategies directed at astrocytes have been based on molecular markers selected to target as broadly as possible, such as GFAP, GLAST, GLT-1 (EAAT2), Cx43 or Aldh1L1. Few targeting strategies exist that might target sub-populations of astrocytes, although potential candidates may be emerging. Genetic knock-in to the Gli1 locus discussed above targets reporter gene expression in the cerebral cortex selectively to scattered middle layer astrocytes that selectively respond to neuronal SHH signaling31. Other markers that target sub-populations of astrocytes may emerge from studies identifying signaling molecules that specify topographically restricted astrocyte lineages33.
Diversity and dynamics of astrocyte morphology
Gross astrocyte morphological differences between different brain areas and how astrocyte morphology changes in hypothalamus during lactation, parturition and dehydration34 have been reviewed35–37. We focus on dynamics of the finest processes of astrocytes that form close appositions to synapses. These fine processes have been described using several terms such as astrocyte lamellae, astrocyte sheets, veil-like lamellae, peripheral astrocyte processes, perisynaptic astrocyte processes, and astrocyte fingers. We refer to them collectively as astrocyte leaflets (Box 2). All of our discussion is based on studies of rodents. It should be noted that there is considerable species variability and it is well established that humans and non-human primates possess astrocytes that are far more complex than those in rodents in terms of size and complexity of processes5.
Box 2. Suggested general terminology for astrocyte sub compartments.
Emerging evidence, presented in the text, suggests that all astrocyte processes are not the same either functionally or morphologically. We suggest that the term astrocyte process should be replaced by several distinct terms.
Branches are the major processes emanating from the astrocyte soma. These probably do not number more than 8 per astrocyte and display diameters on the 1–2 micrometer scale and have also been called stem processes in the literature.
Branchlets are the finer secondary, tertiary and higher order structures that emanate from branches. The precise number of these is unknown, but they display diameters on the sub micrometer scale. Diffraction limited light microscopy is not ideal to study the finest branchlets, although they can be seen clearly as distinct from branches.
Leaflets are the very terminal extensions of astrocyte branchlets that contact synapses. The precise numbers of these per astrocyte is unknown, but based on electron microscopy studies they display dimensions on the hundreds of nanometer scale. Leaflets would be the same structures as those variously termed astrocyte lamellae, astrocyte sheets, veil like lamellae, peripheral astrocyte processes, perisynaptic astrocyte processes and astrocyte fingers. Leaflets cannot be imaged with diffraction-limited light microscopy.
End feet are specialized distal extensions of astrocytes that contact the vasculature. It is likely that each astrocyte bears only one or two branches with end feet. End feet appear to represent a uniquely specialized and polarized compartment of astrocytes.
Morphological complexity
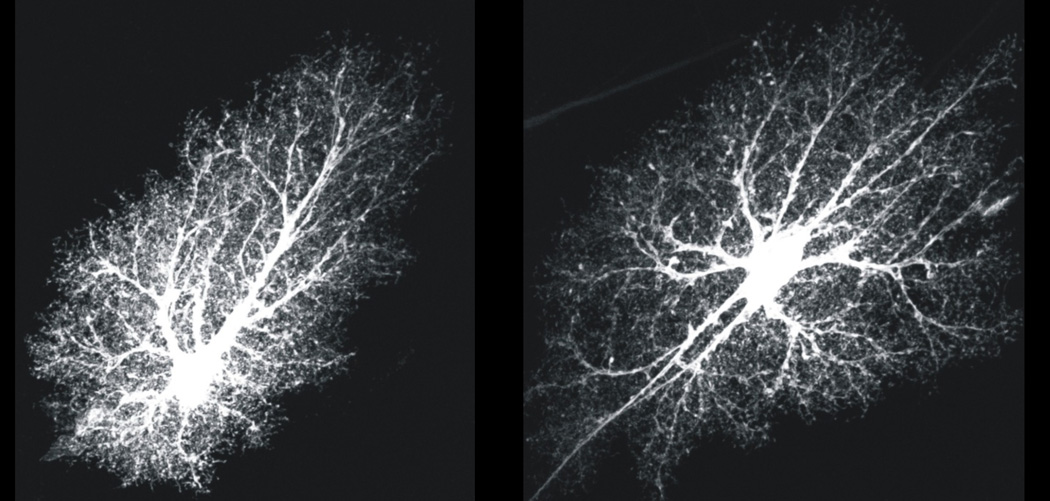
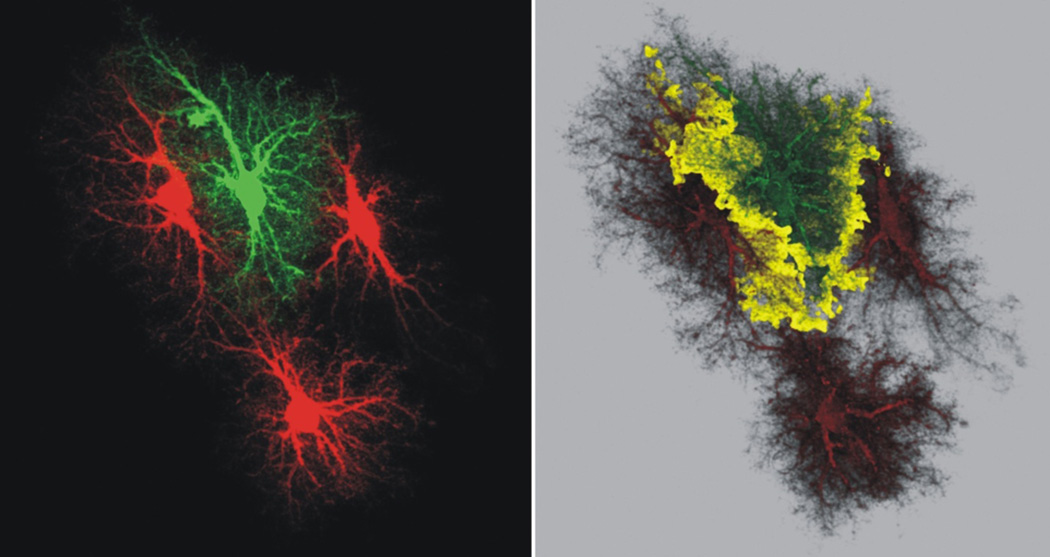
A rodent “typical” protoplasmic astrocyte consists of a soma, 4–10 major branches and thousands of branchlets and leaflets that lie close to synapses (Fig 1, Box 2). Astrocytes have highly complex spongiform shapes with diameters of ~40–60 µm and volumes of ~6.6 × 104 µm3 (refs38, 39). About 90–95% of an astrocyte’s area is formed by branches, branchlets and leaflets40 (Fig 1), and only ~15% can be observed by GFAP staining38, 40. Neighboring astrocytes display remarkably well delineated territories and interdigitate at the edges38 by as little as ~5% (Fig 2). Comparisons between light and electron microscopy show that leaflets cannot be imaged with conventional light microscopy, and that they display dimensions on the tens-to-hundred nanometer scale at sites of interactions with synapses41. Hence, astrocytes are remarkably complex cells (Fig 1, Box 2). The functional implications of this complexity and their regional differences are beginning to be explored.
Figure 1. Astrocytes are morphologically complex cells.
The images show hippocampal CA1 molecular layer protoplasmic astrocytes loaded with a fluorescent dye (Lucifer yellow). The images are from the Cell Centered Database38, 138, 139 at the National Center for Microscopy and Imaging Research (http://ccdb.ucsd.edu/index.shtm) and have Accession #1066, 1063. One can observe a soma, several major branches and thousands of branchlets and leaflets, which are discussed in the text
Figure 2. Morphologically complex astrocytes display well defined territories.
The left hand image shows four hippocampal CA1 molecular layer astrocytes loaded with fluorescent dyes to illustrate how they do not encroach in to each other territory. This feature is easily apparent when examining how the astrocyte filled with a green dye does not markedly overlap with those filled with a red dye. The panel on the right shows a reconstruction of a Z-series of images and the yellow areas indicate the regions when the red and green signals overlap. The region of overlap is on the order of ~5%. These images are from the Cell Centered Database38, 138, 139 at the National Center for Microscopy and Imaging Research (http://ccdb.ucsd.edu/index.shtm) and have Accession #28.
Astrocyte morphology, the number of synapses contained within individual astrocyte territories and the proximity of astrocyte leaflets to synapses varies between brain areas35, 36 and changes dramatically during disease and injury37. For example, in hippocampal stratum radiatum individual astrocytes form territories that contain ~140,000 synapses38, 39, and of these ~57% have juxtaposed astrocyte leaflets42. However, in neocortex this number may be as low as ~30%, whereas in cerebellum almost all synapses onto Purkinje neurons are contacted by leaflets of Bergmann glia36, a specialized radial astrocyte. Astrocyte leaflets are generally irregular, thin and sheet-like in shape with a clear cytoplasm that contains glycogen granules and ribosomes36. Astrocyte leaflets are devoid of GFAP, which is found only in major branches37, 40, and leaflets lack organelles such as mitochondria, microtubules, and endoplasmic reticulum.
Real-time dynamics of astrocyte leaflets
Studies of hippocampal stratum radiatum show that most dendritic spines are contacted by astrocyte leaflets, which impinge on about one third of the available perimeter of individual synapses42. Synapses with astrocyte leaflets at their perimeters tend to be larger than those without43, suggesting that interactions of astrocyte leaflets and synapses may be correlated with synaptic strength44. Recent studies using time-lapse imaging methods in situ and in vivo suggest that astrocyte leaflet motility increases in response to synaptic activity and is associated with increased synapse coverage and spine stability45. Hippocampal astrocyte leaflet motility was triggered by synaptic glutamate release and mediated by astrocyte metabotropic glutamate receptors and intracellular Ca2+ elevations45. Moreover, stimuli that caused long-term synaptic potentiation (LTP) of synapses also caused astrocyte leaflets to increase their motility (over ~10 min), which then stabilized after ~30 min around enlarged synapses45, recalling electron microscopy data46. Key observations were extended to in vivo imaging of astrocytes in the barrel cortex during whisker stimulation, demonstrating that observations made in situ were relevant in the context of an intact brain45. In accord, previous studies showed that whisker stimulation increased astrocyte coverage of synapses in the barrel cortex47. Broadly similar conclusions were reached using different methods48, however, these authors suggest that astrocyte leaflet structural changes may control astrocyte ability to regulate synapse function48. Thus astrocyte morphology and leaflet proximity to synapses is dynamic and regulated by neuron-to-astrocyte signaling.
An unexpected role for Cx30 in astrocyte morphology and leaflet-synapse proximity
Molecular mechanisms that determine astrocyte leaflet proximity to synapses are unclear, but may involve actin binding proteins profilin 1 and ezrin, which have been implicated in Ca2+ dependent astrocyte process outgrowth49, 50. Moreover, glutamate causes astrocyte filopodial outgrowth51. Recent experiments reveal a new mechanism involving connexin 30 (Cx30), a gap junction protein found postnatally within astrocytes52. By using Cx30-deletion mice, Pannasch et al., found a Cx30 function independent of its ion channel pore: loss of Cx30 resulted in significantly greater astrocyte process elongation and ramification in the neuropil52. The altered astrocyte morphology explains decreased excitatory synaptic function in Cx30 mice because the closer proximity of astrocyte leaflets to synapses increased glutamate transporter numbers near synapses. The ensuing downstream effects include decreased AMPA receptor mediated synaptic transmission, reduced hippocampal LTP and reduced contextual fear memory52. Hence, differences in astrocyte leaflet proximity to synapses have strong effects on synapses, circuits and behavior52. Additionally, astrocyte leaflet motility may be needed for maturation of dendritic spines during development53. Astrocyte morphology is controlled by FGF receptor signaling54 in Drosophila, and by mGluR5-dependent glutamate signaling in the case of rodent cortical, but not hypothalamic, astrocytes during development55.
Bergmann glia AMPA receptors and Purkinje cell synapses
Bergmann glia express GluA1 and GluA4 AMPA receptor subunits and functional AMPA receptors. Ca2+ permeable GluA1 containing AMPA receptors on Bergmann glia are needed for correct association of Bergmann glial processes with Purkinje cell synapses56. Genetic disruption of AMPA receptor Ca2+ permeability in Bergmann glia resulted in prolonged kinetics of glutamate synaptic transmission and multiple innervations of Purkinje cells by climbing fibers56. In adult mice, genetic deletion of Bergmann glia AMPA receptor subunits resulted in retraction of glial processes from the vicinity of synapses, altered synaptic transmission and changes in fine motor coordination of the mice57. Together, these studies provide compelling evidence for the physiological relevance of a specific type of AMPA receptor-mediated Ca2+ signaling mechanism in vitro and in vivo, underscoring astrocyte morphological dynamics and the realization that Ca2+ signals are diverse and not all mediated by intracellular release58. As far as we know, there is no evidence that cortical astrocytes express functional AMPA receptors.
Diversity and dynamics of intracellular Ca2+ signals within single astrocytes
Astrocyte Ca2+ signaling has attracted much interest59–62 because it represents a form of signaling that astrocytes may use for intercellular communication63–65. Hitherto, there has been little emphasis on work that provides evidence for Ca2+ signal diversity. Exploration of this issue has benefited from development of genetically encoded calcium indicators (GECIs)66–68, and there is now strong evidence that astrocytes display diverse Ca2+ signals within sub-compartments 69–73 (Box 2; Fig 3).
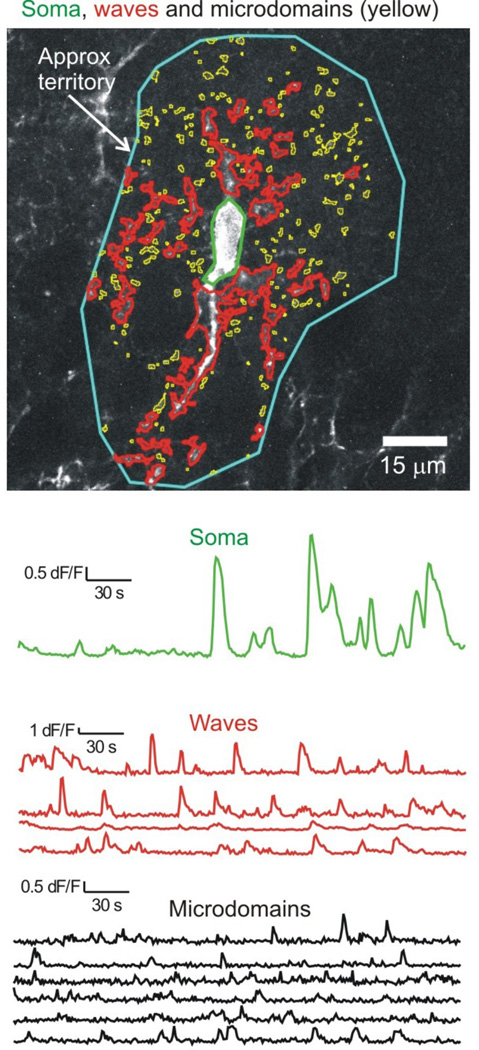
Figure 3. Single astrocytes display diverse Ca2+ signals within territories.
The image shows a CA1 region hippocampal astrocyte expressing cytosolic GCaMP6f. The colors indicate regions of interest that demarcate the soma (green), microdomains (yellow) and waves (red). The intensities of these regions are plotted as a function of time in the color coded traces (microdomains are shows in black for clarity). One can see that most Ca2+ signals occur in regions corresponding to branches and branchlets. The blue region demarcates the approximate territory, but such regions were not studied in detail. The figure and traces are reproduced from a published study73.
Diverse types of Ca2+ signals observed in astrocytes
The first type of numerically dominant astrocyte Ca2+ signal appears as spontaneous highly localised microdomains in branches and branchlets69–72, 74, 75. These signals are not triggered by action potential firing in neurons and only a component are mediated by Ca2+ release from IP3R2-dependent intracellular Ca2+ stores72.
The second type of Ca2+ signal is also a localised microdomain and has been observed in astrocytes from CA1 and dentate gyrus (DG) fields in hippocampus76, 77, but these signals are neuronally mediated. Similarly, in DG astrocytes a fraction of spontaneous Ca2+ signals are caused by spontaneous action potential firing within brain slice preparations77. This type of action-potential dependent spontaneous Ca2+ signal was not observed in CA3 astrocytes72.
The third type of Ca2+ signal is a local wave that usually encompasses large branches and occasionally the somata; these occur spontaneously and can spread a few tens of micrometers72. The fourth type of Ca2+ signal is a global wave that encompasses entire astrocytes, including their somata. This type of signal is largely reduced in IP3R2 deletion mice, and in CA3 can only be triggered by intense bursts of action potentials in mossy fiber axons that innervate the stratum lucidum72. In some instances, astrocyte somata may display spontaneous Ca2+elevations that are not part of a global wave and are not correlated with Ca2+ signals in branches or branchlets.
A fifth class of Ca2+ signal occurs in endfeet and is mediated by Ca2+ release from IP3R2-dependent stores78 and Ca2+ entry via TRPV4 channels79.
A sixth type of Ca2+ signal occurs in vivo during locomotion and startle responses: here the Ca2+ signals are extremely broad and cover essentially all astrocytes within the imaging field of view and are driven by volumetric release of neuromodulators73, 75, 80.
Finally, long lasting (~70 s) and highly localised Ca2+ twinkles have been recorded in cortical astrocyte branchlets in vivo81. Diverse Ca2+ signals have been observed in Bergmann glia82 of the cerebellum (described below).
In summary, a rich diversity of Ca2+ signals exists within single astrocytes. These are mediated by distinct mechanisms, and occur in distinct CNS locations.
Diverse functions of Ca2+ signals in astrocytes
What do diverse types of Ca2+ signals do within astrocytes? A complete answer to this question will require methods to mimic and block distinct types of Ca2+ signals precisely so that their consequences can be explored in specific circuits under specific settings60. Additionally, a generic tool to block the vast majority of Ca2+ signals in astrocytes is needed. There is unlikely to be a single physiological role for astrocyte Ca2+ signals that will apply to the whole CNS. It seems likely that distinct Ca2+ signals will regulate the release of signaling molecules via a variety of possible pathways83 (but see84), control blood vessel diameter85, control the release of synaptogenic/trophic factors1, 86–88, regulate gene expression, modulate K+ uptake89, regulate neurotransmitter uptake71, contribute to neuromodulation75, 80, 90, control movement of astrocyte leaflets45, 48, 49, contribute to axonal action potential broadening91, control neuronal synchronization92, contribute to UP states93 and perhaps control the release of injury related molecules and inflammatory mediators94. All of these phenomena are regulated by astrocyte Ca2+ signals and need to be explored with respect to the distinct types of recently discovered Ca2+ signals. It is implausible that all of these effects could be mediated by a single type of Ca2+ signal in a single type of astrocyte. From this perspective, the multitude of functional responses mediated by astrocytes provides the best indication yet for diversity within microcircuits. The key conceptual advance is that astrocytes display diverse Ca2+ signals in branches and branchlets that are spatially and temporally unrelated to those in the somata (Fig 2) and are distinct within circuits and microcircuits, emphasizing diversity of signalling within single cells and compartments. These insights73 challenge the view that astrocytes display a single type of intracellular Ca2+ signal58, 95.
In vivo and in situ tools to study Ca2+ signals in entire astrocytes
There has been tremendous progress in the generation and testing of strategies to express genetically-encoded Ca2+ indicators in astrocytes. These are now the tools of choice to study astrocyte Ca2+ signals (Box 3).
Box 3. Available and characterized genetic tools to study Ca2+ signals within entire astrocytes in situ and in vivo.
| Reagent | Areas where utility has been shown by imaging |
Targeting strategy | References |
|---|---|---|---|
| Cytosolic GCaMP3 and GCaMP6f virus |
hippocampus striatum cortex |
AAV 2/5 and GfaABC1D promoter |
69,72,73,98,140,141 |
| Membrane tethered GCaMP3 and GCaMP6f virus |
hippocampus striatum |
AAV 2/5 and GfaABC1D promoter |
69,72,98,140 |
| Cytosolic GCaMP3 mice |
cortex cerebellum |
Knock-in at the ROSA26 locus |
75,142 |
| Cytosolic GCaMP5G mice |
cortex | Knock-in at the Polr2a locus |
143 |
| Cytosolic YC-nano50 mice |
cortex | Knock-in using tetracycline transactivator (tTa)-tet operator (tetO) strategy at Actb locus |
81 |
| Cytosolic Yellow Cameleon 3.60 mice |
hippocampus cortex |
Transgenic mice driven by the S100β promoter |
144 |
| Cytosolic GCaMP6f and GCaMP6s mice |
hippocampus, cortex | Knock-in mice at the ROSA26 and TIGRE loci |
73,145 |
Diverse astrocyte responses within circuits and microcircuits
We adopt Shepherd and Grillner’s thoughtful definition96 of a brain microcircuit as: the way nerve cells (and associated cells such as glia) are organized to carry out specific operations within a region of the nervous system. We do not consider direct fast-acting gliotransmission, because the arguments for83 and against84 have been reviewed.
Hippocampus
High resolution imaging of Ca2+ signals in astrocyte branches using 2-photon microscopy provides evidence for diversity of astrocyte functional responses within different hippocampal fields. Astrocytes in CA1 stratum radiatum respond with an increase in Ca2+ in major astrocyte branches during minimal stimulation of Schaffer collateral fibers76. These responses are mediated by synaptic glutamate release acting on mGluR5 metabotropic glutamate receptors on astrocytes76. The resultant increase in astrocyte Ca2+ is proposed to evoke the release of gliotransmitters (e.g. ATP), which most likely, via the production of adenosine, act on presynaptic A2A receptors to regulate neurotransmitter release probability76. Astrocytes located in dentate gyrus (DG) display two types of Ca2+ signals that are termed focal and expanded; the latter are caused by action potential firing and the focal signals are mediated by spontaneous synaptic neurotransmitter release77. Focal and expanded Ca2+ signals are mediated by synaptic ATP because both types of Ca2+ signal are reduced by a P2Y1 receptor antagonist and both are mediated by Ca2+ release from IP3R2-dependent intracellular Ca2+ stores77. It is suggested that astrocyte Ca2+ signals in DG function to regulate the probability of excitatory neurotransmitter release onto granule cells perhaps via astrocytic release of a neuromodulator that acts on presynaptic nerve terminals77. Hence, astrocytes are proposed to act as rapid local detectors of neuronal activity, and to respond to activity changes by locally regulating neurotransmitter release from the same synapses.
In contrast, astrocytes located in CA3 stratum lucidum region only respond during intense bursts of action potentials evoked within mossy fiber axons, and display Ca2+ signals that are mediated largely by mGluR2/3 receptors and encompass large parts of the astrocytes72. Hence, CA3 astrocytes do not respond to sparse neuronal input and seem incapable of regulating synapses locally because their Ca2+ signals cover entire cells. Instead, they may control neuronal network synchronization92. Serial block face electron microscopy provides one explanation for differences between CA1 and CA3 region astrocytes. The fine leaflets of astrocytes are much closer to excitatory synapses in the CA1 region than the leaflets of CA3 astrocytes are to mossy fiber synapses72, illustrating that astrocyte sub-cellular anatomy and function varies within hippocampal fields.
There is additional evidence that distinct types of Ca2+ signals may serve distinct functions. Long-term synaptic potentiation (LTP) in CA1 pyramidal neurons is largely reduced when astrocytes are dialysed with Ca2+ chelators97, but is unaffected when intracellular store mediated astrocyte Ca2+ signals are reduced or increased95. These findings may be explained by the variable dependence of LTP on the source of astrocyte Ca2+. Hence, basal, rather than elevated, astrocyte Ca2+ signals may be important for LTP by regulating the constitutive release of D-serine98. The available data provide evidence that astrocyte functional roles are distinct within microcircuits that comprise the larger hippocampal circuit and are separable for distinct sources of Ca2+ within single astrocytes. These insights underscore the importance of studying specific and defined types of Ca2+ signal for specific neural responses under specified conditions.
Cerebellum
Bergmann glia display highly localised microdomain Ca2+ increases that can be evoked by electrical stimulation of afferent fibres99. Studies using in vivo imaging in awake head fixed mice free to rest or run on a freely moving ball100 revealed three types of Ca2+ signals within Bergmann glia of the cerebellum90. Two types of ongoing and spontaneous Ca2+ signals within the cerebellum of mice at rest were termed “sparkles” and “bursts”, whereas a third form called “flares” occurred in hundreds of Bergmann glia in a concerted manner during locomotor activity. Sparkles occurred mainly in processes and were reduced by TTX and glutamate receptor antagonists90. Bursts occurred spontaneously in mice at rest and appeared as radially expanding waves that encompassed ~55 Bergmann glia90 and a volume of ~86,000 µm3. These signals were not sensitive to TTX, but were abolished by an ATP receptor antagonist, implying they were due to endogenous ATP90 released from astrocytes themselves101. Flares occurred during voluntary running and encompassed hundreds of Bergmann glia and were abolished by TTX and a glutamate receptor antagonist90. Thus, a single type of astrocyte can display a variety of responses and mechanisms in vivo depending on the behavior of the mouse.
Bergmann glia and cortical astrocytes both respond during periods of enforced locomotion in ways that are indicative of arousal or heightened vigilance states, rather than locomotion per se75, 80. Under these circumstances, the responses were coincident in these anatomically distinct brain areas, were global because all imaged cells displayed Ca2+ signals (Fig 4), and in both brain areas the responses were mediated by noradrenaline acting on a1 adrenoceptors located on astrocytes and Bergmann glia75. Noradrenaline was likely released from varicose terminals of locus coeruleus neuron projections that extend throughout much of the brain, because cortical astrocytes displayed Ca2+ elevations when locus coeruleus neurons were electrically stimulated102. As such, astrocyte responses in cortex and cerebellum may be indicative of a global brain state associated with arousal or startle.
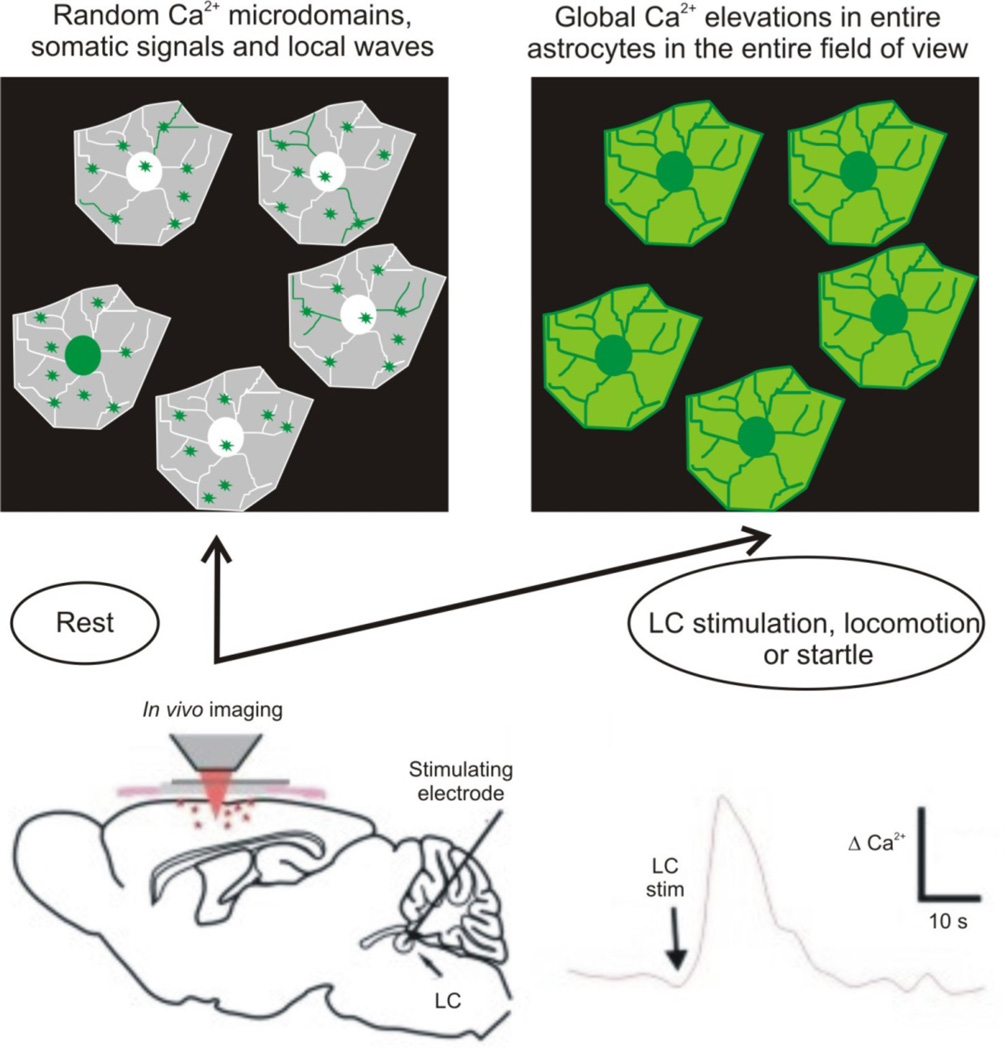
Figure 4. Astrocytes in vivo respond with global Ca2+ elevations during electrical stimulation of the locus coeruleus, during locomotion and during startle responses.
The lower cartoon illustrates the important experiment by Bekar and colleagues102: they electrically stimulated LC neurons while monitoring Ca2+ signals in cortical astrocytes102. They discovered global elevations in Ca2+ upon LC stimulation, which is illustrated in the upper cartoons. Similar global changes are seen when the mice are forced to walk or when they are startled75, 80. Hence, during such responses, which may indicate a brain state change, the mode astrocyte Ca2+ signaling shifts from random Ca2+ microdomains and waves to global Ca2+ elevations. These global changes are mediated by noradrenaline release from LC projections, as discussed in the text.
Thalamus
The thalamic reticular nucleus (nRT) is a source for endogenous benzodiazepine-mimicking peptides called endozepines103. Recent work shows that endozepine peptides are found within astrocytes of nRT, and use of a gliotoxin revealed that astrocytes are needed for their positive allosteric effects on synaptic inhibition104. The effect of gliotoxin was to reduce inhibition in nRT, because the toxin removed a source of endogenous endozepines within nRT104. However, the effect of gliotoxin in the ventrobasal nucleus (VB) was to increase inhibition via a mechanism that involved GABA transporters. Hence, astrocytes located in adjacent nRT and VB microcircuits have distinct effects on inhibitory synapses mediated by endozepines and GABA transporters, respectively.
Medulla
The retrotrapezoid nucleus (RTN) of the medulla is an important site of chemoreception allowing hypercapnia (an increase in blood CO2; pCO2) to increase breathing. A subset of neurons in RTN are highly sensitive to CO2/H+ and send excitatory glutamatergic projections to respiratory centers to regulate breathing. CO2/H+-evoked ATP release from astrocytes in the vicinity of RTN influences respiratory drive105 by regulating RTN neuron firing. Hence, astrocytes located near RTN are the cellular sources of ATP mediating the ATP component of the central chemosensory response to hypercapnia. Importantly, astrocytes within this area are particularly pH sensitive, responding with Ca2+ elevations and further release of ATP105. This study suggests that a vital step in central chemoreception involves ATP release from astrocytes located on the ventral medulla surface, that this signal is propagated by astrocyte ATP release, ultimately arriving at RTN neurons to depolarize them and thus regulate breathing105. As such, a potentially unique class of astrocytes within the respiratory chemoreceptor areas displays exquisite pH sensitivity and mediates direct effects on the ability to change breathing upon changes in pC02/H+.
Spinal cord
There is strong evidence for positionally distinct astrocyte subtypes in the spinal cord106, 107. Evidence that astrocytes in distinct domains are functionally diverse has been provided by examining differential gene expression between dorsal and ventral spinal cord astrocytes32. Sema3a was the most highly expressed ventral gene and this pattern was confirmed by protein expression32. Loss of Sema3a in ventral astrocytes had consequences for a-motor neurons: defective axon initial segment orientation, abnormal synapse formation and function, and a-motor neuron loss32. Cell culture studies suggested that the positional identity of astrocytes is intrinsic, as the relevant properties were retained in co-cultures independent of local environment. These studies raise the possibility that distinct populations of astrocytes serve distinct functions within microcircuits, and provide approaches to tackle this issue also in other circuits. Irrespectively, studies of dorsal-ventral differences in spinal cord astrocytes provide evidence for regional neural circuit diversity of molecular machinery and function32, 106.
Two broadly defined types of Ca2+ signal and responses in circuits – local and global
Recent in vivo studies represent a milestone because they provide a basis to explore and understand the physiological settings under which astrocytes respond to behaviors that are engaged within the intact brain. Although this is a nascent field, several general points are worth noting. First, during locomotion the responses of astrocytes in the cortex and Bergmann glia were correlated, implying that the underlying causes may be widespread75. Second, when astrocytes responded during locomotion, their responses were largely global and not localized to single cells or areas of single cells73, 75, 102. Third, astrocyte responses were mediated by a slow neuromodulator and not by a fast neurotransmitter75, 80. Together, these data suggest that astrocytes respond to slow neuromodulators that are released as volumetric transmitters80, and when astrocytes respond they do so over large parts of their territories. Moreover, in vivo responses and those in the CA3 region indicate that astrocytes may only modulate neuronal function on slow time scales and over large distances72, 73, 75, 80, 90, 102. These slow and global responses are distinct from local responses shown for CA1 and DG astrocytes76, 77. Notably, responses of layer 2/3 visual cortex astrocytes to light were enhanced markedly by enforced locomotion75, which implies that local and global signals act synergistically. Synergism for astrocyte Ca2+ signals evoked by noradrenaline and VIP receptors has been demonstrated in vitro108, and these experiments are relevant in the context of in vivo work where both noradrenaline and VIP are likely to be released during startle. There is also evidence that astrocytes display Ca2+ elevations during endogenous release of acetylcholine, which acts on astrocyte muscarinic receptors109, 110. It seems likely that astrocytes may respond to local signals and to volumetric signals depending on the particular conditions and brain region.
Diversity of astrocyte roles in CNS disorders
Astrocytes respond to all forms of CNS damage and disease by undergoing cellular, molecular and functional changes commonly referred to as reactive astrogliosis. In addition, recent observations indicate that astrocyte dysfunction can play causal roles in certain CNS disorders, and that the neurological consequences of astrocyte dysfunctions can precede the onset of detectable reactive astrogliosis. Thus, astrocyte roles in CNS disorders exhibit diversity and can be primary as well as reactive or secondary. A better understanding of this diversity has the potential to impact on the understanding and treatment of CNS injury and disease.
Reactive astrocyte changes associated with neuropathology and disease have been reviewed94, 111, 112 17. We focus on examples illustrating diversity of astrocyte functional roles in CNS disorders.
Astrocyte effects in Huntington’s disease (HD) models
HD is caused by an expanded chain of polyglutamines in the huntingtin protein (HTT) leading to intracellular accumulation and aggregation of mutant huntingtin (mHTT). HD patients and mouse models of HD exhibit nuclear inclusions of mHTT in striatal astrocytes113. Recent findings show that the onset and progression of neurological disease in HD mouse models is associated with specific astrocyte dysfunction in striatum, but not hippocampus, suggesting a neural circuit-specific nature of astrocyte roles in HD. Tong et al114 found, using two HD mouse models, that in comparison with pre-symptomatic stages, the onset of neurological symptoms was associated with a significant increase in astrocytes with mHTT nuclear inclusions and significant reductions in astrocyte functional proteins including GLT-1 (EAAT2) and Kir4.1. At symptom onset, and in spite of clear evidence of astrocyte dysfunction, there were no major phenotypic changes associated with astrocyte reactivity such as proliferation, hypertrophy and increased GFAP expression. These findings indicate that mHTT is associated with disruption of the expression of important astrocyte functional proteins that initially alter astrocyte function without noticeable astrogliosis. Loss of the glutamate transporter, GLT-1 (EAAT2), has been extensively studied in the context of HD115. Kir4.1 potassium channels regulate extracellular K+ levels and are expressed in the CNS primarily by astrocytes116. Loss of Kir4.1 currents in striatal astrocytes in HD mouse models114 led to higher ambient K+, which increased medium spiny neuron (MSN) excitability, a hallmark of HD mouse models117, and these deficits could be rescued by virus mediated delivery of Kir4.1-GFP channels selectively to astrocytes, thereby providing evidence that subtle increases in extracellular K+ due to astrocyte Kir4.1 loss can phenocopy MSN changes in HD mouse models. Together, these findings support the hypothesis that key aspects of altered MSN excitability in HD are caused by the specific primary dysfunction of a key astrocyte homeostatic function, maintenance of extracellular K+, and are not merely a generic or non-specific secondary consequence of astrocyte reactivity. This notion is relevant in the context of human HD, which displays a progressive increase in astrocyte reactivity over time. Thus, astrocyte contributions to underlying HD pathological mechanisms are diverse and change with the progression of disease, such that early stages of the disease are associated with functional deficits in Kir4.1 and GLT-1 (EAAT2), and later stages are also associated with increasing astrocyte reactivity. Therapeutic strategies targeting astrocytes need to embrace the concept that astrocytes display a range of specific dysfunctions as well as astrogliosis.
Astrocyte changes in Alzheimer’s disease models
A mouse model of familial Alzheimer’s disease displayed elevated basal cortical astrocyte Ca2+ levels and increased Ca2+ transients termed hyperactive Ca2+ signaling118. Neither normal nor hyperactive Ca2+ signaling was the result of action potential firing in neurons119. Hyperactivity arose because reactive astrocytes near b-amyloid plaques displayed enhanced P2Y1-receptor mediated Ca2+ signaling119. The hyperactive phenotype could be rescued by blocking ATP release from connexin channels and by blocking P2Y1 receptors119. Together, these data indicate that astrocyte hyperactivity in Alzheimer’s disease may be caused by a combination of ATP release and/or increased P2Y1 receptor expression by a population of astrocytes near plaques, implying that blocking P2Y1 receptors represents a novel therapeutic strategy for Alzheimer’s disease. These observations also point towards diversity of astrocyte responses to different CNS insults. P2Y1 receptor mRNA expression and Ca2+ signaling was markedly reduced in in vitro models of astrocyte reactivity triggered by inflammatory mediators27, suggesting that the increase observed around β-amyloid plaques may represent a novel cellular phenotype specific to Alzheimer’s disease.
Diversity of astrocyte secondary responses to injury and disease
Considerable information has accrued regarding molecular and structural aspects of reactive astrogliosis17, 94, 111, 120, 121. Recent progress demonstrates that in contrast to long held beliefs, reactive astrogliosis is not a uniform set of stereotypic astrocyte changes regulated by a simple single genetic on-off switch. Instead, molecular dissection in vivo has revealed that diverse signaling events tailor astrocyte responses to specific CNS insults94, 111. For example, transcriptional changes induced by either ischemia or inflammation differ substantially27, 28, and trauma induced changes exhibit clear gradients that vary with distance from lesions and with injury intensity17, 122. Thus, reactive astrogliosis is both complex and heterogeneous and can be defined as a finely graded continuum of multiple potential changes that range from reversible alterations in gene expression and cell hypertrophy within preserved individual astrocyte domains, to scar formation that involves substantial cell proliferation and permanent rearrangement of tissue structure 17, 94, 111. Thus, reactive astrocytes exhibit substantial diversity at multiple levels, including with respect to gene expression, cell structure, cell signaling and cell function. This diversity exists not only across different CNS regions such as cerebral cortex, hippocampus and spinal cord, but also topographically with respect to distance from lesions and locally among neighboring cells 27, 122, 123.
Two functionally distinct categories of reactive astrocytes
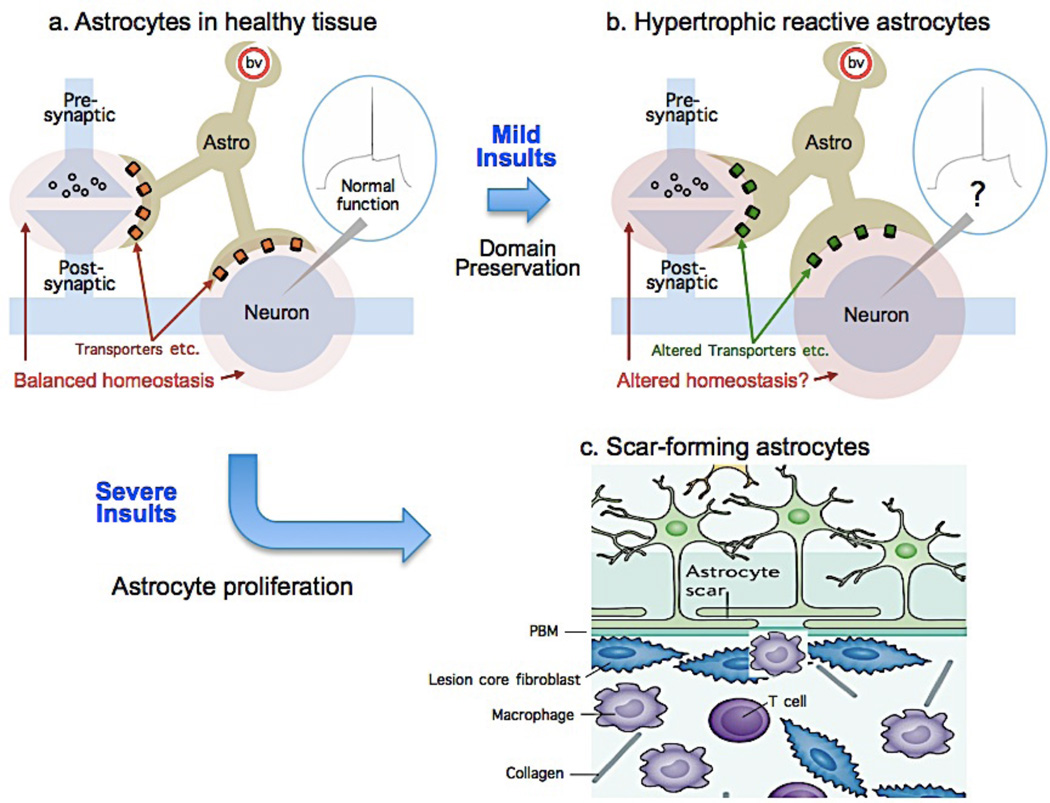
Much of the evidence that delineates heterogeneity among reactive astrocytes pertains to differences in molecular expression and cell structure. Although less is known about functional diversity, current information points towards a working model that recognizes at least two fundamentally distinct broad functional categories of reactive astrocytes, which we refer to as scar-forming reactive astrocytes and hypertrophic reactive astrocytes. (Fig. 5, Table 1).
Figure 5. Diverse astrocyte functional responses to tissue injury and disease.
Cartoons illustrate astrocytes in healthy tissue (a) and two different functional changes in response to mild or severe insults. b: Mild insults trigger hypertrophic reactive astrogliosis in which astrocytes remain in situ, retain their domains and interactions with functioning neural cells but hypertrophy and undergo molecular and functional changes the consequences of which are not well defined. c: Severe insults that cause overt tissue damage trigger astrocyte proliferation from multiple potential sources. Newly proliferated scar-forming astrocytes are motile, intertwine their processes, interact primarily with fibrotic and inflammatory cells, and form borders around damaged and inflamed tissue130. PBM, parenchymal basement membrane.
Table 1.
Defining phenotypic features of hypertrophic and scar-forming reactive astrocytes.
| Hypertrophic reactive astrocytes |
Scar-forming reactive astrocytes |
|
|---|---|---|
| Cell proliferation | No | Yes |
| Cell migration | No | Yes |
|
Do they exhibit individual territories? |
Yes | No |
|
How long do reactive changes last? |
Potentially reversible if stimuli resolve |
Permanent |
|
What are the primary other cell types that they interact with? |
Neural cells: neurons and oligodendrocytes (and blood vessels) |
Non-neural cells: inflammatory and fibrotic cells (and blood vessels) |
Scar-forming reactive astrocytes
In response to focal tissue damage or inflammation, reactive astrocytes in all CNS regions form scar borders that segregate damaged and inflamed tissue from adjacent viable and functioning neural tissue17, 112, 124. In forebrain, including cortex, hippocampus and thalamus125, 126, and spinal cord122, astrocyte scar-borders are comprised almost entirely of newly proliferated astrocytes that do not observe discrete individual cellular domains and have elongated processes that intertwine extensively122 (Fig. 5C). There is currently evidence for at least two different potential sources of newly proliferated scar-forming astrocytes. The first is from proliferation of local mature perivascular astrocytes as demonstrated by in vivo imaging of cerebral cortex127. A second source is from migrated progeny of peri-ependymal neural progenitors128, 129. The potentially diverse manner in which different sources of newly proliferated astrocytes contribute to scar formation or other injury responses in different CNS regions, are not yet defined. Nevertheless, an emerging overall function of scar-forming astrocytes in response to severe tissue damage after trauma, stroke or infection, is the formation of functional barriers that sharply demarcate tissue compartments within which robust inflammatory responses can occur that are essential to clear debris and neutralize microbial pathogens, while preserving immediately adjacent viable and functioning neural tissue94, 112, 124. Thus, the primary cellular interactions of scar-forming astrocytes are with multiple types of non-neural cells, including leukocytes, other inflammatory cells and fibrotic cells (Fig. 5C). Astrocyte scar formation is associated with permanent rearrangement of tissue architecture.
Hypertrophic reactive astrocytes
In response to mild or diffuse CNS insults, or in tissue adjacent to scar borders around focal lesions, astrocytes in all CNS regions undergo molecular, structural and functional changes that stop short of proliferation and scar formation, which we refer to collectively as hypertrophic reactive astrogliosis17, 94, 111. In these responses, hypertrophic reactive astrocytes (i) are comprised of non-proliferating resident astrocytes 112, 122, (ii) remain in their original locations and do not migrate 127, 130, (iii) exhibit a wide variety of potential changes in molecular expression 94, 111, (iv) exhibit varying degrees of cellular hypertrophy 94, 111, but (v) retain their processes more or less within their individual cellular domains in grey matter 131, and (vi) continue to interact with the same local cells as prior to the insult. Changes associated with hypertrophic astrogliosis have the potential to vary with the (i) nature of triggering insult, (ii) CNS region involved, (iii) distance of the astrocytes from an insult, and (iv) presence of comorbid factors such as peripheral infections, and most changes appear to be reversible in situations where insults resolve, for example after single event traumatic or ischemic injuries. Functionally, the primary cellular interactions of hypertrophic reactive astrocytes remain with the same neural cells that they interacted with in healthy tissue prior to the insult, either neurons or oligodendrocytes (Fig. 5A,B). Although reactive astrocytes undergo many molecular changes including in transporters critical for the extracellular homeostasis of ions, transmitters and water 111, 132, 133, little is known about the functional consequences of those changes (Fig. 5B). One early study on this topic reports that mild hypertrophic astrogliosis secondary to viral infection is associated with decreased neuronal inhibition in hippocampus 134. Even though hypertrophic astrogliosis is ubiquitous in all perimeter regions of functioning neural tissue around all forms of focal injury caused by stroke or trauma, as well as in all areas of chronic neurodegeneration in all CNS regions112, little is known about the effects of hypertrophic reactive astrogliosis on microcircuit function and whether, or how, these effects might differ in different regions or if they are detrimental or beneficial in differing circumstances.
Delineating these two broad functional categories of scar-forming and hypertrophic reactive astrocytes does not preclude additional levels of diversity within or in addition to those categories. Although all reactive astrocytes may not precisely fit, recognition of these categories will aid in experimental design, comparison of results, discussion and understanding. For example, reactive astrogliosis and scar formation are often regarded as synonymous, but this is not the case. The molecular mechanisms and functions of scar forming astrocytes that are newly proliferated and motile, and that function primarily to regulate inflammatory cells112, 130, will be fundamentally different from the molecular mechanisms and functions of hypertrophic reactive astrocytes that remain in situ and retain, but modify, their interaction with local neurons and synapses (Fig 5). Future investigations to dissect mechanisms and functions of reactive astrocytes will need to take into consideration and clearly define the type of reactive astrocyte that is being investigated, the nature of the triggering insult, the distance from that insult, and the CNS region being studied.
Distinguishing reactive astrogliosis from astrocyte dysfunction
Transgenic loss-of-function studies applied to in vivo models of CNS insults show that under normal circumstances, astrogliosis and scar formation serve essential functions that limit tissue damage, restrict inflammation and preserve function after CNS traumatic injury, stroke, infection, autoimmune attack and degenerative disease17, 94, 111, 112, 120, 121, 130. Emerging data also indicate that primary astrocyte dysfunctions can contribute to neuronal dysfunction and neurological symptoms prior to the onset of detectable astrogliosis114. Thus, astrocyte dysfunction and astrogliosis are not synonymous and can occur independently of each other. Together with the emergence of a concept of ‘normal astrogliosis’ critical for preservation of tissue and function after CNS insults, is the recognition that astrogliosis can also be prone to dysfunction as a result of genetic defects, genetic polymorphisms, autoimmune attack, or chronic exposure inflammatory stimuli caused by secondary infections94. Recognizing and understanding the differences between beneficial and dysfunctioning astrogliosis is likely to be fundamental to dissecting the cellular mechanisms underlying many CNS disorders.
Future outlook
Exploiting astrocyte diversity
The realization that astrocytes are diverse and mediate separable responses in the context of disease raises the possibility that this diversity could be exploited to derive therapeutic effects. One immediate way forward is to understand the full complement of proteins that astrocytes express; a good starting point is G-protein coupled receptors. Receptors with astrocyte specific expression could be exploited to produce neuromodulatory effects within circuits with potentially minimal effects on neurons. For example, a receptor pathway that leads to elevated Kir.4.1 expression may be useful for HD therapeutics and a P2Y1 antagonist may be of benefit in Alzheimer’s disease. Perhaps the release of endozepines from astrocytes in nRT can be exploited in the context of epilepsy. At this juncture what is needed is a deeper understanding of fundamental astrocyte biology and diversity.
Emerging questions
A first class of question concerns molecular mechanisms. What is the molecular makeup and signaling machinery of astrocytes in distinct circuits? By systematically comparing between different brain circuits it should be possible to assemble a set of molecular metrics with which to classify astrocytes as distinct or the same in different regions. Do astrocytes utilize signals other than Ca2+ for cellular communication? New families of highly specific genetically encoded indicators for neurotransmitters and neuromodulators are needed. We need also to measure the nature and amounts of substances released from astrocytes in specific brain areas and then explore the functions of key signaling species on a case-by-case basis in the context of specific circuits and behaviors.
A second class of question concerns how astrocytes contribute to the formation and function of synapses and circuits. Do individual astrocytes signal locally to single or small sets of synapses to allow formation or removal, or do single astrocytes signal globally to entire dendritic arbors within their territory or perhaps even to entire populations of neurons that reside within extended astrocyte volumes connected by gap junctions? Is synapse formation and removal triggered by activity-dependent and bidirectional signaling between astrocytes and neurons? If so, what chemical signals are involved, are they unique and can they be exploited pharmacologically? What do the various types of astrocyte Ca2+ signals encode within circuits? Is the main function of astrocytes to listen locally to synapses and then to regulate them locally, or is it to listen to widespread volumetric neuromodulator release and respond globally over large volumes of brain? Studies demonstrating motility of astrocyte leaflets are important because altering astrocyte proximity to synapses has the potential to alter synaptic and circuit function by altering ion and neurotransmitter homeostasis.
A third class of question concerns astrocyte functional diversity in CNS disorders. Astrocytes display a plethora of changes in CNS disorders, but which of these are correlative, beneficial or harmful will need to be determined on a case-by-case basis. Studies will need to explore astrocyte contributions to specific conditions in specific brain areas and at specific stages of pathology and avoid the temptation to generalize across the spectrum of disorders ranging from traumatic injury to neurodegenerative diseases. In this regard it is noteworthy that astrocytes perform specialized functions in higher primates135 and it is possible that astrocyte diversity is greater in humans than in rodents. It is likely that in vitro studies of astrocytes derived from patient induced pluripotent stem cells will be fruitful. Much could be learned about the regional and functional diversity of astrocytes and their contributions to pathology and neural circuit mechanisms within organoid systems as models for human disorders136.
Finally, how should one classify diverse astrocytes? We suggest that the answer will emerge from a broad understanding of functional diversity, rather than relying solely on single criteria such as morphology, connectivity, marker expression or gene profiles. Our emphasis is to define astrocyte diversity based on direct measurements of function, dynamics and molecular mechanisms in specific circuits. This captures recent progress with the classification of interneurons137.
Summary
We conclude by advancing the concept that astrocytes display a range of functional attributes that are astrocyte compartment, circuit, microcircuit and disease specific. As such, previous notions that astrocytes represent a homogenous population of cells throughout the CNS need to be revised. A deeper understanding of astrocyte diversity and its implications is necessary in order to fully understand neural circuits3 in health and disease, and to provide new opportunities to treat CNS disorders.
Acknowledgments
BSK was supported by NIH (NS060677, MH099559A, MH104069) and the CHDI Foundation. MVS was supported by NIH (NS084030), Wings for Life, Hilton Foundation, CHDI and Dr. Miriam and Sheldon B. Adelson Medical Research Foundation. Images in Figs 1 and 2 are from the The Cell Centered Database, which is supported by NIH grants from NCRR RR04050, RR RR08605 and the Human Brain Project DA016602 from the National Institute on Drug Abuse, the National Institute of Biomedical Imaging and Bioengineering and the National Institute of Mental Health.
Footnotes
Author contributions: BSK and MVS worked on subsections separately and drafted the final version together.
References
- 1.Allen NJ, Barres BA. Neuroscience: Glia - more than just brain glue. Nature. 2009;457:675–677. doi: 10.1038/457675a. [DOI] [PubMed] [Google Scholar]
- 2.Herculano-Houzel S. The glia/neuron ratio: how it varies uniformly across brain structures and species and what that means for brain physiology and evolution. Glia. 2014;62:1377–1391. doi: 10.1002/glia.22683. [DOI] [PubMed] [Google Scholar]
- 3.Insel TR, Landis SC, Collins FS. Research priorities. The NIH BRAIN Initiative. Science. 2013;340:687–688. doi: 10.1126/science.1239276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Felipe J. Cajal's butterflies of the soul: Science and art. Oxford University Press. 2010 [Google Scholar]
- 5.Oberheim NA, et al. Uniquely hominid features of adult human astrocytes. J Neurosci. 2009;29:3276–3287. doi: 10.1523/JNEUROSCI.4707-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kimelberg HK. Functions of mature mammalian astrocytes: a current view. Neuroscientist. 2010;16:79–106. doi: 10.1177/1073858409342593. [DOI] [PubMed] [Google Scholar]
- 7.Miller RH, Raff MC. Fibrous and protoplasmic astrocytes are biochemically and developmentally distinct. J Neurosci. 1984;4:585–592. doi: 10.1523/JNEUROSCI.04-02-00585.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mishima T, Hirase H. In vivo intracellular recording suggests that gray matter astrocytes in mature cerebral cortex and hippocampus are electrophysiologically homogeneous. J Neurosci. 2010;30:3093–3100. doi: 10.1523/JNEUROSCI.5065-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuffler SW. Neuroglial cells: physiological properties and a potassium mediated effect of neuronal activity on the glial membrane potential. Proc R Soc Lond B Biol Sci. 1967;168:1–21. doi: 10.1098/rspb.1967.0047. [DOI] [PubMed] [Google Scholar]
- 10.Orkand RK, Nicholls JG, Kuffler SW. Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. J Neurophysiol. 1966;29:788–806. doi: 10.1152/jn.1966.29.4.788. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Barres BA. Astrocyte heterogeneity: an underappreciated topic in neurobiology. Curr Opin Neurobiol. 2010;20:588–594. doi: 10.1016/j.conb.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Oberheim NA, Goldman SA, Nedergaard M. Heterogeneity of astrocytic form and function. Methods Mol Biol. 2012;814:23–45. doi: 10.1007/978-1-61779-452-0_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freeman MR. Specification and morphogenesis of astrocytes. Science. 2010;330:774–778. doi: 10.1126/science.1190928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freeman MR, Rowitch DH. Evolving concepts of gliogenesis: a look way back and ahead to the next 25 years. Neuron. 2013;80:613–623. doi: 10.1016/j.neuron.2013.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eng LF, Gerstl B, Vanderhaeghen JJ. A study of proteins in old multiple sclerosis plaques. Trans Am Soc Neurochem. 1970;1:42. [Google Scholar]
- 16.Eng LF, Ghirnikar RS, Lee YL. Glial fibrillary acidic protein: GFAP-thirty-one years (1969-2000) Neurochem Res. 2000;25:1439–1451. doi: 10.1023/a:1007677003387. [DOI] [PubMed] [Google Scholar]
- 17.Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Middeldorp J, Hol EM. GFAP in health and disease. Prog Neurobiol. 2011;93:421–443. doi: 10.1016/j.pneurobio.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Bush TG, et al. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell. 1998;93:189–201. doi: 10.1016/s0092-8674(00)81571-8. [DOI] [PubMed] [Google Scholar]
- 20.Garcia AD, Doan NB, Imura T, Bush TG, Sofroniew MV. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci. 2004;7:1233–1241. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- 21.Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lehre KP, Levy LM, Ottersen OP, Storm-Mathisen J, NC D. Differential expression of two glial glutamate transporters in the rat brain: quantitative and immunocytochemical observations. J Neurosci. 1995;15:1835–1583. doi: 10.1523/JNEUROSCI.15-03-01835.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagy JI, Patel D, Ochalski PA, Stelmack GL. Connexin30 in rodent, cat and human brain: selective expression in gray matter astrocytes, co-localization with connexin43 at gap junctions and late developmental appearance. Neuroscience. 1999;88:447–468. doi: 10.1016/s0306-4522(98)00191-2. [DOI] [PubMed] [Google Scholar]
- 24.Poopalasundaram S, et al. Glial heterogeneity in expression of the inwardly rectifying K(+) channel, Kir4.1, in adult rat CNS. Glia. 2000;30:362–372. doi: 10.1002/(sici)1098-1136(200006)30:4<362::aid-glia50>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 25.Bachoo RM, et al. Molecular diversity of astrocytes with implications for neurological disorders. Proc Natl Acad Sci USA. 2004;101:8384–8389. doi: 10.1073/pnas.0402140101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cahoy JD, et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamby ME, et al. Inflammatory mediators alter the astrocyte transcriptome and calcium signaling elicited by multiple g-protein-coupled receptors. J Neurosci. 2012;32:14489–14510. doi: 10.1523/JNEUROSCI.1256-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zamanian JL, et al. Genomic analysis of reactive astrogliosis. J Neurosci. 2012;32:6391–6410. doi: 10.1523/JNEUROSCI.6221-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y, et al. An RNA-Sequencing Transcriptome and Splicing Database of Glia, Neurons, and Vascular Cells of the Cerebral Cortex. J Neurosci. 2014;34:11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sosunov AA, et al. Phenotypic heterogeneity and plasticity of isocortical and hippocampal astrocytes in the human brain. J Neurosci. 2014;34:2285–2298. doi: 10.1523/JNEUROSCI.4037-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia AD, Petrova R, Eng L, Joyner AL. Sonic hedgehog regulates discrete populations of astrocytes in the adult mouse forebrain. J Neurosci. 2010;30:13597–13608. doi: 10.1523/JNEUROSCI.0830-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Molofsky AV, et al. Astrocyte-encoded positional cues maintain sensorimotor circuit integrity. Nature. 2014;509:189–194. doi: 10.1038/nature13161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bayraktar OA, Fuentealba LC, Alvarez-Buylla A, Rowitch DH. Astrocyte Development and Heterogeneity. Cold Spring Harb Perspect Biol. 2014 doi: 10.1101/cshperspect.a020362. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oliet SH, Bonfardin VD. Morphological plasticity of the rat supraoptic nucleus-- cellular consequences. Eur J Neurosci. 2010;32:1989–1994. doi: 10.1111/j.1460-9568.2010.07514.x. [DOI] [PubMed] [Google Scholar]
- 35.Reichenbach A, Wolburg H. Neuroglia. 3rd. Helmut Kettenmann & Bruce R. Ransom. Oxford University Press; 2013. Astrocytes and ependymal glia. Chapter 4. [Google Scholar]
- 36.Bernardinelli Y, Muller D, Nikonenko I. Astrocyte-synapse structural plasticity. Neural Plast. 2014;2014:232105. doi: 10.1155/2014/232105. Epub 2014 Jan 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun D, Jakobs TC. Structural remodeling of astrocytes in the injured CNS. Neuroscientist. 2012;18:567–588. doi: 10.1177/1073858411423441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bushong EA, Martone ME, Jones YZ, Ellisman MH. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci. 2002;22:183–192. doi: 10.1523/JNEUROSCI.22-01-00183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ogata K, Kosaka T. Structural and quantitative analysis of astrocytes in the mouse hippocampus. Neuroscience. 2002;113:221–233. doi: 10.1016/s0306-4522(02)00041-6. [DOI] [PubMed] [Google Scholar]
- 40.Reeves A, Shigetomi E, Khakh BS. Bulk loading of calcium indicator dyes to study astrocyte physiology: key limitations and improvements using morphological maps. J Neurosci. 2011;31:9353–9358. doi: 10.1523/JNEUROSCI.0127-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kosaka T, Hama K. Three-dimensional structure of astrocytes in the rat dentate gyrus. J Comp Neurol. 1986;249 doi: 10.1002/cne.902490209. [DOI] [PubMed] [Google Scholar]
- 42.Ventura R, Harris KM. Three-dimensional relationships between hippocampal synapses and astrocytes. J Neurosci. 1999;19:6897–6906. doi: 10.1523/JNEUROSCI.19-16-06897.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Witcher MR, Kirov SA, Harris KM. Plasticity of perisynaptic astroglia during synaptogenesis in the mature rat hippocampus. Glia. 2007;55:13–23. doi: 10.1002/glia.20415. [DOI] [PubMed] [Google Scholar]
- 44.Bourne JN, Harris KM. Balancing structure and function at hippocampal dendritic spines. Annu Rev Neurosci. 2008;31:47–67. doi: 10.1146/annurev.neuro.31.060407.125646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bernardinelli Y, et al. Activity-dependent structural plasticity of perisynaptic astrocytic domains promotes excitatory synapse stability. Curr Biol. 2014;24:1679–1688. doi: 10.1016/j.cub.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 46.Lushnikova I, Skibo G, Muller D, Nikonenko I. Synaptic potentiation induces increased glial coverage of excitatory synapses in CA1 hippocampus. Hippocampus. 2009;19:753–762. doi: 10.1002/hipo.20551. [DOI] [PubMed] [Google Scholar]
- 47.Genoud C, et al. Plasticity of astrocytic coverage and glutamate transporter expression in adult mouse cortex. PLoS Biol. 2006 Oct;4(11):e343. doi: 10.1371/journal.pbio.0040343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perez-Alvarez A, Navarrete M, Covelo A, Martin ED, Araque A. Structural and functional plasticity of astrocyte processes and dendritic spine interactions. J Neurosci. 2014;34:12738–12744. doi: 10.1523/JNEUROSCI.2401-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Molotkov D, Zobova S, Arcas JM, Khiroug L. Calcium-induced outgrowth of astrocytic peripheral processes requires actin binding by Profilin-1. Cell Calcium. 2013;53:338–348. doi: 10.1016/j.ceca.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 50.Lavialle M, et al. Structural plasticity of perisynaptic astrocyte processes involves ezrin and metabotropic glutamate receptors. Proc Natl Acad Sci U S A. 2011;108:12915–12919. doi: 10.1073/pnas.1100957108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cornell-Bell AH, Thomas PG, Smith SJ. The excitatory neurotransmitter glutamate causes filopodia formation in cultured hippocampal astrocytes. Glia. 1990;3:322–334. doi: 10.1002/glia.440030503. [DOI] [PubMed] [Google Scholar]
- 52.Pannasch U, et al. Connexin 30 sets synaptic strength by controlling astroglial synapse invasion. Nat Neurosci. 2014;17:549–558. doi: 10.1038/nn.3662. [DOI] [PubMed] [Google Scholar]
- 53.Nishida H, Okabe S. Direct astrocytic contacts regulate local maturation of dendritic spines. J Neurosci. 2007;27:331–340. doi: 10.1523/JNEUROSCI.4466-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stork T, Sheehan A, Tasdemir-Yilmaz OE, Freeman MR. Neuron-glia interactions through the Heartless FGF receptor signaling pathway mediate morphogenesis of Drosophila astrocytes. Neuron. 2014;83:388–403. doi: 10.1016/j.neuron.2014.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morel L, Higashimori H, Tolman M, Yang YV. GluT1+ neuronal glutamatergic signaling regulates postnatal developmental maturation of cortical protoplasmic astroglia. J Neurosci. 2014;34:10950–10962. doi: 10.1523/JNEUROSCI.1167-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iino M, et al. Glia-synapse interaction through Ca2+-permeable AMPA receptors in Bergmann glia. Science. 2001;292:926–929. doi: 10.1126/science.1058827. [DOI] [PubMed] [Google Scholar]
- 57.Saab AS, et al. Bergmann glial AMPA receptors are required for fine motor coordination. Science. 2012;337:749–753. doi: 10.1126/science.1221140. [DOI] [PubMed] [Google Scholar]
- 58.Petravicz J, Fiacco TA, McCarthy KD. Loss of IP3 receptor-dependent Ca2+ increases in hippocampal astrocytes does not affect baseline CA1 pyramidal neuron synaptic activity. J Neurosci. 2008;28:4967–4973. doi: 10.1523/JNEUROSCI.5572-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Agulhon C, et al. What is the role of astrocyte calcium in neurophysiology? Neuron. 2008;59:932–946. doi: 10.1016/j.neuron.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khakh BS, McCarthy KD. Astrocyte calcium signals: from observations to functions and the challenges therein. Cold Spring Harb Perspect Biol. 2014 doi: 10.1101/cshperspect.a020404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Agulhon C, et al. Calcium Signaling and Gliotransmission in Normal vs. Reactive Astrocytes. Front Pharmacol. 2012 doi: 10.3389/fphar.2012.00139. Epub 2012 Jul 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fiacco TA, Agulhon C, McCarthy KD. Sorting out astrocyte physiology from pharmacology. Annu Rev Pharmacol Toxicol. 2009;49:151–174. doi: 10.1146/annurev.pharmtox.011008.145602. [DOI] [PubMed] [Google Scholar]
- 63.Smith S. Neural signalling. Neuromodulatory astrocytes. Curr Biol. 1994;4:807–810. doi: 10.1016/s0960-9822(00)00178-0. [DOI] [PubMed] [Google Scholar]
- 64.Smith SJ. Do astrocytes process neural information? Prog Brain Res. 1992;94:119–136. doi: 10.1016/s0079-6123(08)61744-6. [DOI] [PubMed] [Google Scholar]
- 65.Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 1990;247:470–473. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- 66.Chen TW, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hires SA, Tian L, Looger LL. Reporting neural activity with genetically encoded calcium indicators. Brain Cell Biol. 2008;36:69–86. doi: 10.1007/s11068-008-9029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tian L, et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods. 2009;6:875–881. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shigetomi E, et al. Imaging calcium microdomains within entire astrocyte territories and endfeet with GCaMPs expressed using adeno-associated viruses. J Gen Physiol. 2013;141:633–647. doi: 10.1085/jgp.201210949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shigetomi E, Kracun S, Sofroniew MV, Khakh BS. A genetically targeted optical sensor to monitor calcium signals in astrocyte processes. Nature Neuroscience. 2010;13:759–766. doi: 10.1038/nn.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shigetomi E, Tong X, Kwan KY, Corey DP, Khakh BS. TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nature Neuroscience. 2011;15:70–80. doi: 10.1038/nn.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Haustein MD, et al. Conditions and constraints for astrocyte calcium signaling in the hippocampal mossy fiber pathway. Neuron. 2014;82:413–429. doi: 10.1016/j.neuron.2014.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Srinivasan R, et al. Ca2+ signaling in astrocytes from IP3R2-/- mice in brain slices and during startle responses in vivo. Nature Neuroscience. 2015 Apr 20; doi: 10.1038/nn.4001. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shigetomi E, Kracun S, Khakh BS. Monitoring astrocyte calcium microdomains with improved membrane targeted GCaMP reporters. Neuron Glia Biology. 2010;6:183–191. doi: 10.1017/S1740925X10000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Paukert M, et al. Norepinephrine controls astroglial responsiveness to local circuit activity. Neuron. 2014;82:1263–1270. doi: 10.1016/j.neuron.2014.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Panatier A, et al. Astrocytes are endogenous regulators of basal transmission at central synapses. Cell. 2011;146:785–798. doi: 10.1016/j.cell.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 77.Di Castro MA, et al. Local Ca2+ detection and modulation of synaptic release by astrocytes. Nat Neurosci. 2011;10:1276–1284. doi: 10.1038/nn.2929. [DOI] [PubMed] [Google Scholar]
- 78.Straub SV, Bonev AD, Wilkerson MK, Nelson MT. Dynamic Inositol Trisphosphate-mediated Calcium Signals within Astrocytic Endfeet Underlie Vasodilation of Cerebral Arterioles. J Gen Physiol. 2006;128:659–669. doi: 10.1085/jgp.200609650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dunn KM, Hill-Eubanks DC, Liedtke WB, Nelson MT. TRPV4 channels stimulate Ca2+-induced Ca2+ release in astrocytic endfeet and amplify neurovascular coupling responses. Proc Natl Acad Sci U S A. 2013;110:6157–6162. doi: 10.1073/pnas.1216514110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ding F, et al. α1-Adrenergic receptors mediate coordinated Ca(2+) signaling of cortical astrocytes in awake, behaving mice. Cell Calcium. 2013;54:387–394. doi: 10.1016/j.ceca.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kanemaru K, et al. In vivo visualization of subtle, transient, and local activity of astrocytes using an ultrasensitive Ca(2+) indicator. Cell Rep. 2014;8:311–318. doi: 10.1016/j.celrep.2014.05.056. [DOI] [PubMed] [Google Scholar]
- 82.Nimmerjahn A, Bergles DE. Large-scale recording of astrocyte activity. Curr Opin Neurobiol. 2015;32C:95–106. doi: 10.1016/j.conb.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Araque A, et al. Gliotransmitters travel in time and space. Neuron. 2014;81:728–739. doi: 10.1016/j.neuron.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hamilton NB, Attwell D. Do astrocytes really exocytose neurotransmitters? Nat Rev Neurosci. 2010;11:227–238. doi: 10.1038/nrn2803. [DOI] [PubMed] [Google Scholar]
- 85.Attwell D, et al. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Clarke LE, Barres BA. Emerging roles of astrocytes in neural circuit development. Nat Rev Neurosci. 2013;14:311–321. doi: 10.1038/nrn3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Eroglu C, Barres BA. Regulation of synaptic connectivity by glia. Nature. 2010;468:223–231. doi: 10.1038/nature09612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Allen NJ. Astrocyte regulation of synaptic behavior. Annu Rev Cell Dev Biol. 2014;30:439–463. doi: 10.1146/annurev-cellbio-100913-013053. [DOI] [PubMed] [Google Scholar]
- 89.Filosa JA, et al. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci. 2006;9:1397–1403. doi: 10.1038/nn1779. 2006 Nov;9(11):1397-1403. [DOI] [PubMed] [Google Scholar]
- 90.Nimmerjahn A, Mukamel EA, Schnitzer MJ. Motor behavior activates Bergmann glial networks. Neuron. 2009;62:400–412. doi: 10.1016/j.neuron.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sasaki T, Matsuki N, Ikegaya Y. Action-potential modulation during axonal conduction. Science. 2011;331:599–601. doi: 10.1126/science.1197598. [DOI] [PubMed] [Google Scholar]
- 92.Sasaki T, et al. Astrocyte calcium signalling orchestrates neuronal synchronization in organotypic hippocampal slices. J Physiol. 2014;592:2771–2783. doi: 10.1113/jphysiol.2014.272864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Poskanzer KE, Yuste R. Astrocytic regulation of cortical UP states. Proc Natl Acad Sci U S A. 2011;45:18453–18458. doi: 10.1073/pnas.1112378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sofroniew MV. Astrogliosis. Cold Spring Harb Perspect Biol. 2014 doi: 10.1101/cshperspect.a020420. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Agulhon C, Fiacco TA, McCarthy KD. Hippocampal short- and long-term plasticity are not modulated by astrocyte Ca2+ signaling. Science. 2010;327:1250–1254. doi: 10.1126/science.1184821. [DOI] [PubMed] [Google Scholar]
- 96.Shepherd GM, Grillner S. Introduction (page xvii) Handbook of brain microcircuits. Editors Grillner and Shepherd. Oxford University Press. 2010 [Google Scholar]
- 97.Henneberger C, Papouin T, Oliet SH, Rusakov DA. Long-term potentiation depends on release of D-serine from astrocytes. Nature. 2010;463:232–236. doi: 10.1038/nature08673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shigetomi E, Jackson-Weaver O, Huckstepp RT, O'Dell TJ, Khakh BS. TRPA1 channels are regulators of astrocyte basal calcium levels and long-term potentiation via constitutive D-serine release. J Neurosci. 2013;33:10143–10153. doi: 10.1523/JNEUROSCI.5779-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Grosche J, et al. Microdomains for neuron-glia interaction: parallel fiber signaling to Bergmann glial cells. Nat Neurosci. 1999;2 doi: 10.1038/5692. [DOI] [PubMed] [Google Scholar]
- 100.Kerr JN, Nimmerjahn A. Functional imaging in freely moving animals. Curr Opin Neurobiol. 2012;22:45–53. doi: 10.1016/j.conb.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 101.Kang J, et al. Connexin 43 hemichannels are permeable to ATP. J Neurosci. 2008;28:4702–4711. doi: 10.1523/JNEUROSCI.5048-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bekar LK, He W, Nedergaard M. Locus coeruleus alpha-adrenergic-mediated activation of cortical astrocytes in vivo. Cereb Cortex. 2008;18:2789–2795. doi: 10.1093/cercor/bhn040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Christian CA, et al. Endogenous positive allosteric modulation of GABA(A) receptors by diazepam binding inhibitor. Neuron. 2013;78:1063–1074. doi: 10.1016/j.neuron.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Christian CA, Huguenard JR. Astrocytes potentiate GABAergic transmission in the thalamic reticular nucleus via endozepine signaling. Proc Natl Acad Sci U S A. 2013;110:20278–20283. doi: 10.1073/pnas.1318031110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gourine AV, et al. Astrocytes control breathing through pH-dependent release of ATP. Science. 2010;329:571–575. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tsai HH, et al. Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science. 2012;337:358–362. doi: 10.1126/science.1222381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hochstim C, Deneen B, Lukaszewicz A, Zhou Q, Anderson DJ. Identification of positionally distinct astrocyte subtypes whose identities are specified by a homeodomain code. Cell Calcium. 2008;133:510–522. doi: 10.1016/j.cell.2008.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fatatis A, Holtzclaw LA, Avidor R, Brenneman DE, Russell JT. Vasoactive intestinal peptide increases intracellular calcium in astroglia: synergism with alphaadrenergic receptors. Proc Natl Acad Sci U S A. 1994;91:2036–2040. doi: 10.1073/pnas.91.6.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Takata N, et al. Astrocyte calcium signaling transforms cholinergic modulation to cortical plasticity in vivo. J Neurosci. 2011;31:18155–18165. doi: 10.1523/JNEUROSCI.5289-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Navarrete M, et al. Astrocytes mediate in vivo cholinergic-induced synaptic plasticity. PLoS Biol. 2012;10(2):e1001259. doi: 10.1371/journal.pbio.1001259. Epub 2012 Feb 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Burda JE, Sofroniew MV. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron. 2014;81:229–248. doi: 10.1016/j.neuron.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bradford J, et al. Expression of mutant huntingtin in mouse brain astrocytes causes agedependent neurological symptoms. Proc Natl Acad Sci U S A. 2009;106:22480–22485. doi: 10.1073/pnas.0911503106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tong X, et al. Astrocyte Kir4.1 ion channel deficits contribute to neuronal dysfunction in Huntington's disease model mice. Nat Neurosci. 2014;17:694–703. doi: 10.1038/nn.3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Estrada-Sánchez AM, Rebec GV. Corticostriatal dysfunction and glutamate transporter 1 (GLT1) in Huntington's disease: interactions between neurons and astrocytes. Basal Ganglia. 2012;2:57–66. doi: 10.1016/j.baga.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kofuji P, Newman EA. Potassium buffering in the central nervous system. Neuroscience. 2004;129:1045–1056. doi: 10.1016/j.neuroscience.2004.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cepeda C, Cummings DM, André VM, Holley SM, Levine MS. Genetic mouse models of Huntington's disease: focus on electrophysiological mechanisms. ASN Neuro. 2010 Apr 7;2(2):e00033. doi: 10.1042/AN20090058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kuchibhotla KV, Lattarulo CR, Hyman BT, Bacskai BJ. Synchronous hyperactivity and intercellular calcium waves in astrocytes in Alzheimer mice. Science. 2009;323:1211–1215. doi: 10.1126/science.1169096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Delekate A, et al. Metabotropic P2Y1 receptor signalling mediates astrocytic hyperactivity in vivo in an Alzheimer's disease mouse model. Nat Commun. 2014 Nov 19;5:5422. doi: 10.1038/ncomms6422. [DOI] [PubMed] [Google Scholar]
- 120.Kang W, Hebert JM. Signaling pathways in reactive astrocytes, a genetic perspective. Mol Neurobiol. 2011;43:147–154. doi: 10.1007/s12035-011-8163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pekny M, Pekna M. Astrocyte Reactivity and Reactive Astrogliosis: Costs and Benefits. Physiol Rev. 2014;94:1077–1098. doi: 10.1152/physrev.00041.2013. [DOI] [PubMed] [Google Scholar]
- 122.Wanner IB, et al. Glial scar borders are formed by newly proliferated, elongated astrocytes that interact to corral inflammatory and fibrotic cells via STAT3-dependent mechanisms after spinal cord injury. J Neurosci. 2013;33:12870–12886. doi: 10.1523/JNEUROSCI.2121-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Anderson MA, Ao Y, Sofroniew MV. Heterogeneity of reactive astrocytes. Neurosci Lett. 2014;565C:23–29. doi: 10.1016/j.neulet.2013.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sofroniew MV. Astrocyte barriers to neurotoxic inflammation. Nat Rev Neursci. 2015 doi: 10.1038/nrn3898. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bush TG, et al. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron. 1999;23:297–308. doi: 10.1016/s0896-6273(00)80781-3. [DOI] [PubMed] [Google Scholar]
- 126.Myer DJ, Gurkoff GG, Lee SM, Hovda DA, Sofroniew MV. Essential protective roles of reactive astrocytes in traumatic brain injury. Brain. 2006;129:2761–2772. doi: 10.1093/brain/awl165. [DOI] [PubMed] [Google Scholar]
- 127.Bardehle S, et al. Live imaging of astrocyte responses to acute injury reveals selective juxtavascular proliferation. Nat Neurosci. 2013;16:580–586. doi: 10.1038/nn.3371. [DOI] [PubMed] [Google Scholar]
- 128.Benner EJ, et al. Protective astrogenesis from the SVZ niche after injury is controlled by Notch modulator Thbs4. Nature. 2013;497:369–373. doi: 10.1038/nature12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Barnabe-Heider F, et al. Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell. 2010;7:470–482. doi: 10.1016/j.stem.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 130.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 131.Wilhelmsson U, et al. Redefining the concept of reactive astrocytes as cells that remain within their unique domains upon reaction to injury. Proc. Natl. Acad. Sci. USA. 2006;103:17513–17518. doi: 10.1073/pnas.0602841103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rao VL, Bowen KK, Dempsey RJ. Transient focal cerebral ischemia downregulates glutamate transporters GLT-1 and EAAC1 expression in rat brain. Neurochem Res. 2001;26:497–502. doi: 10.1023/a:1010956711295. [DOI] [PubMed] [Google Scholar]
- 133.Olsen ML, Sontheimer H. Functional implications for Kir4.1 channels in glial biology: from K+ buffering to cell differentiation. J Neurochem. 2008;107:589–601. doi: 10.1111/j.1471-4159.2008.05615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ortinski PI, et al. Selective induction of astrocytic gliosis generates deficits in neuronal inhibition. Nat Neurosci. 2010;13:584–591. doi: 10.1038/nn.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Han X, et al. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell. 2013;12:342–353. doi: 10.1016/j.stem.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lancaster MA, Knoblich JA. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 2014 Jul 18;345(6194):1247125. doi: 10.1126/science.1247125. Epub 2014 Jul 17., 1247. [DOI] [PubMed] [Google Scholar]
- 137.Kepecs A, Fishell G. Interneuron cell types are fit to function. Nature. 2014;505:318–326. doi: 10.1038/nature12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Martone ME, et al. A cell centered database for electron tomographic data. J. Struct. Biology. 2002;138:145–155. doi: 10.1016/s1047-8477(02)00006-0. [DOI] [PubMed] [Google Scholar]
- 139.Bushong EA, Martone ME, Ellisman MH. Maturation of astrocyte morphology and the establishment of astrocyte domains during postnatal hippocampal development. Int J Dev Neurosci. 2004;22:73–86. doi: 10.1016/j.ijdevneu.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 140.Bonder DE, McCarthy KD. Astrocytic Gq-GPCR-Linked IP3R-Dependent Ca2+ Signaling Does Not Mediate Neurovascular Coupling in Mouse Visual Cortex In Vivo. J Neurosci. 2014;34:13139–13150. doi: 10.1523/JNEUROSCI.2591-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jiang R, Haustein MD, Sofroniew MV, Khakh BS. Imaging Intracellular Ca2+ Signals in Striatal Astrocytes from Adult Mice Using Genetically-encoded Calcium Indicators. J Vis Exp. 2014;(93):e51972. doi: 10.3791/51972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zariwala HA, et al. A Cre-Dependent GCaMP3 Reporter Mouse for Neuronal Imaging In Vivo. J Neurosci. 2012;32:2131–2141. doi: 10.1523/JNEUROSCI.4469-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gee JM, et al. Imaging activity in neurons and glia with a Polr2a-based and credependent GCaMP5G-IRES-tdTomato reporter mouse. Neuron. 2014;83:1058–1072. doi: 10.1016/j.neuron.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Atkin SD, et al. Transgenic mice expressing a cameleon fluorescent Ca2+ indicator in astrocytes and Schwann cells allow study of glial cell Ca2+ signals in situ and in vivo. J Neursci Methods. 2009;181:212–226. doi: 10.1016/j.jneumeth.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Madisen L, et al. Transgenic mice for intersectional targeting of neural sensors and effectors with high specificity and performance. Neuron. 2015;85:942–958. doi: 10.1016/j.neuron.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]