Abstract
Intracranial volume reflects the maximally attained brain size during development, and remains stable with loss of tissue in late life. It is highly heritable, but the underlying genes remain largely undetermined. In a genome-wide association study of 32,438 adults, we discovered five novel loci for intracranial volume and confirmed two known signals. Four of the loci are also associated with adult human stature, but these remained associated with intracranial volume after adjusting for height. We found a high genetic correlation with child head circumference (ρgenetic=0.748), which indicated a similar genetic background and allowed for the identification of four additional loci through meta-analysis (Ncombined = 37,345). Variants for intracranial volume were also related to childhood and adult cognitive function, Parkinson’s disease, and enriched near genes involved in growth pathways including PI3K–AKT signaling. These findings identify biological underpinnings of intracranial volume and provide genetic support for theories on brain reserve and brain overgrowth.
The intricate genetic control of the human brain, complemented by environmental factors, leads to the observed variations in brain size in human populations1. Intracranial volume is closely related to brain volume in early life as the brain grows.2,3 However, it becomes stable after the brain has fully developed and remains unaffected by later age-related changes such as brain atrophy4,5, thus representing the maximal attained brain size. Discovering genetic variants that influence intracranial volume can contribute to our understanding of brain development and related diseases, but prior studies have only identified two influential genetic loci6–9.
Here, we performed genome-wide association studies in populations from the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE)10 and Enhancing NeuroImaging Genetics through Meta-Analysis (ENIGMA)11 consortia on intracranial volume measured by magnetic resonance imaging. Genotypes were imputed to the 1000 Genomes reference panel (phase 1, version 3). Meta-analysis revealed five novel loci associated with intracranial volume. We also discovered genome-wide overlap between intracranial volume and other key traits including height, cognitive ability, and Parkinson’s disease. Furthermore, we found relatively enriched patterns of association for certain functional categories of variants and near genes that are involved in specific pathways.
RESULTS
Genome-wide association studies
Detailed information on the population characteristics, image acquisition and processing, and genetic quality control can be found in the Online Methods and Supplementary Tables S1–3.
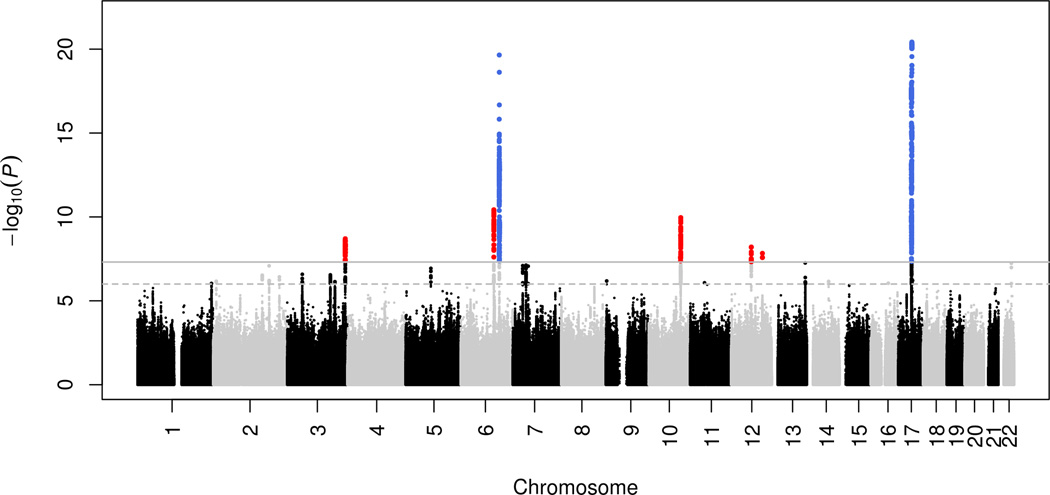
The discovery meta-analysis (N = 26,577) yielded seven genome-wide significant (p < 5 × 10−8) loci, five of them novel (Figures 1–2; Table 1). The quantile-quantile plot showed inflation (λ = 1.092; Supplementary Figure S1), which we determined to be mainly due to polygenicity rather than cryptic relatedness or population stratification using LD score regression12. Next we analyzed European samples (N = 2,362; not included in the discovery sample) and generalization samples with African (N = 938), Asian (N = 955), and Hispanic (N = 1,605) ancestries (Table 1). All variants had the same direction of effect in the additional European samples (sign test, P = 0.0078), and three variants replicated, at nominal significance. Although sample sizes were generally small for the non-Europeans, here too, the direction of effect was generally concordant with the discovery (sign test, P = 0.039). Five nominally significant associations were detected across all three ethnicities.
Figure 1. Common genetic variants associated with intracranial volume.
Manhattan plot where every point represents a single genetic variant plotted according to its genomic position (x-axis) and its –log10(p-value) for association with intracranial volume (y-axis). Variants in blue are genome-wide significant in a previously known locus, whereas red variants reach genome-wide significant for the first time in that locus. The dashed horizontal line represents a significance threshold of p-value < 10−6 and the full horizontal line represents genome-wide significance of p-value < 5 × 10−8. Variants surpassing these thresholds are indicated by larger points.
Figure 2. Regional association and functional annotation of novel genome-wide significant loci.
Regional association plots for the five novel genome-wide significant loci of intracranial volume with gene models below (GENCODE version 19). Annotation tracks below from the Roadmap Epigenomics Consortium57 highlight the genomic region that likely harbors the causal variant(s) (r2 > 0.8 from the top SNP). See Online Methods for detailed track information. Plots were generated using the LocusTrack software (http://gump.qimr.edu.au/general/gabrieC/LocusTrack/).
Table 1.
Association of genome-wide significant loci for intracranial volume in European, African, Asian, and Hispanic populations.
| European discovery (N=26,577) |
European replication (N=2,363) |
African generalization (N=938) |
Asian generalization (N=955) |
Hispanic generalization (N=1605) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genetic variant | Locus | Position | A1 | A2 | Freq | β | P | β | P | β | P | β | P | β | P |
| rs199525 | 17q21 | 44847834 | T | G | 0.80 | .102 | 3.8×10−21 | .024 | 0.407 | .358 | 1.3×10−3 | .264 | 0.406 | .035 | 0.493 |
| rs11759026 | 6q22 | 126792095 | A | G | 0.76 | −.095 | 2.2×10−20 | −.019 | 0.528 | −.131 | 0.194 | −.071 | 0.123 | −.046 | 0.209 |
| rs2022464 | 6q21 | 108945370 | A | C | 0.30 | −.063 | 3.7×10−11 | −.090 | 4.7×10−3 | −.060 | 0.233 | −.105 | 0.035 | −.088 | 0.013 |
| rs11191683 | 10q24 | 105170649 | T | G | 0.33 | .059 | 1.1×10−10 | .040 | 0.174 | .187 | 0.021 | .085 | 0.075 | −.005 | 0.911 |
| rs9811910 | 3q28 | 190670902 | C | G | 0.08 | .096 | 1.2×10−9 | .075 | 0.010 | .346 | 0.020 | .101 | 0.621 | −.148 | 0.187 |
| rs138074335 | 12q14 | 66374247 | A | G | 0.59 | .051 | 6.2×10−9 | .106 | 2.9×10−4 | −.016 | 0.735 | −.004 | 0.951 | .001 | 0.984 |
| rs2195243 | 12q23 | 102922986 | C | G | 0.22 | −.059 | 1.5×10−8 | −.044 | 0.132 | .037 | 0.585 | −.020 | 0.774 | −.093 | 0.101 |
Abbreviations: A1 = effect allele, A2 = reference allele, Freq = frequency of the effect allele, SE = standard error, N = sample size.
Next we were able to map the association to novel variants for two previously identified loci at chromosome 17q21 (rs199525; P = 3.8 × 10−21) and 6q22 (rs11759026; P = 2.2 × 10−20)6,7. The five novel loci were on chr 6q21 (rs2022464; P = 3.7 × 10−11), chr 10q24 (rs11191683; P = 1.1 × 10−10), chr 3q28 (rs9811910; P = 2.0 × 10−9), chr 12q14 (rs138074335/ rs7312464; P = 6.2 × 10−9), and chr 12q23 (rs2195243; P = 1.5 × 10−8). Functional annotation of the variants and those in LD (r2>0.8) can be found in Supplementary Table S4.
Height-adjusted analyses
Four of the seven loci for intracranial volume were previously discovered for height (17q21, 6q22, 6q21, and 12q14), prompting us to investigate genome-wide overlap between the two traits. As height and intracranial volume are correlated (weighted average Pearson’s r = 0.556; Supplementary Table S5) and this could drive association signals, we performed a GWAS of intracranial volume adjusted for height in the studies that had measured height (N = 21,875). Findings were compared to the corresponding subset of studies without adjustment (N = 22,378). Using LD score regression (Online Methods), we found that there is considerable genetic correlation between intracranial volume and height (ρgenetic = 0.241, P = 2.4 × 10−10), which disappears after adjusting for height (ρgenetic = 0.049, P = 0.21) (Table 2). The associations of the seven intracranial volume loci, however, remained significant after adjusting for height (Supplementary Table S6). To investigate whether more height loci were associated with intracranial volume independently of height, we analyzed all 697 genome-wide significant height variants13. An additional 73 variants (10.7%; 14 variants not available) showed nominally significant associations with intracranial volume but were not attenuated after adjustment for height, although none survived Bonferroni correction (Supplementary Table S7). For some variants, the direction of effect was discordant, i.e. positive for height and negative for intracranial volume. Furthermore, a polygenic score of the 697 variants predicted intracranial volume, and this was also the case after adjustment for height in a subset of the studies (Supplementary Table S8).
Table 2.
Genetic correlation between intracranial volume and other anthropometric traits, cognitive function, and neurodegenerative diseases.
| Intracranial volume Full sample (N=26,577) |
Intracranial volume Height subset (N=22,378) |
Intracranial volume Height adjusted (N=21,875) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phenotype | N total | N cases | Mean χ2 | ρgenetic | SE | P | ρgenetic | SE | P | ρgenetic | SE | P |
| Anthropometric traits | ||||||||||||
| Adult height | 253,280 | - | 2.98 | .249 | .037 | 1.4×10−11 | .241 | .038 | 2.4×10−10 | .049 | .039 | 0.21 |
| Child head circumference | 10,768 | - | 1.04 | .748 | .121 | 5.5×10−10 | .758 | .124 | 1.1×10−9 | .750 | .126 | 2.5×10−9 |
| Birth length | 28,459 | - | 1.07 | .296 | .087 | 6.7×10−4 | .278 | .087 | 1.3×10−3 | .192 | .088 | 0.029 |
| Birth weight | 26,836 | - | 1.06 | .285 | .081 | 4.4×10−4 | .219 | .082 | 7.9×10−3 | .160 | .086 | 0.062 |
| Neurological traits | ||||||||||||
| Childhood cognitive function | 12,441 | - | 1.08 | .277 | .090 | 2.2×10−3 | .277 | .091 | 2.5×10−3 | .257 | .090 | 4.2×10−3 |
| Adult cognitive function | 53,949 | - | 1.15 | .202 | .059 | 6.3×10−4 | .205 | .060 | 6.0×10−4 | .198 | .059 | 6.9×10−4 |
| Alzheimer’s Disease | 54,162 | 17,008 | 1.11 | −.070 | .097 | 0.47 | −.049 | .097 | 0.61 | −.043 | .098 | 0.66 |
| Parkinson’s Disease | 108,990 | 13,708 | 1.10 | .315 | .063 | 6.6×10−7 | .316 | .070 | 5.5×10−6 | .335 | .072 | 3.0×10−6 |
| White matter lesions | 17,936 | - | 1.07 | .112 | .075 | 0.13 | .111 | .078 | 0.16 | .096 | .079 | 0.23 |
| Psychiatric traits | ||||||||||||
| Autism | 10,263 | 4,949 | 1.07 | −.011 | .069 | 0.87 | −.036 | .074 | 0.63 | .026 | .071 | 0.72 |
| Bipolar disorder | 11,810 | 6,990 | 1.14 | .070 | .071 | 0.33 | .007 | .075 | 0.93 | −.004 | .076 | 0.95 |
| Major depressive disorder | 16,610 | 9,227 | 1.07 | .002 | .100 | 0.98 | .025 | .098 | 0.80 | .005 | .096 | 0.96 |
| Schizophrenia | 17,115 | 9,379 | 1.23 | .054 | .056 | 0.33 | .017 | .058 | 0.77 | −.009 | .058 | 0.87 |
| Extraversion | 63,030 | - | 1.08 | −.041 | .092 | 0.65 | −.101 | .095 | 0.29 | −.097 | .092 | 0.29 |
| Neuroticism | 63,661 | - | 1.06 | −.017 | .109 | 0.87 | .035 | .106 | 0.74 | .070 | .111 | 0.53 |
Genetic correlation between various phenotypes and intracranial volume in the complete discovery sample (“Full sample”), adjusted for height in the studies that had measured height (“Height adjusted”), and the corresponding subset of studies without adjustment (“Height subset”).
Abbreviations: SE = standard error.
Genetic correlation between various phenotypes and intracranial volume in the complete discovery sample (“Full sample”), adjusted for height in the studies that had measured height (“Height adjusted”), and the corresponding subset of studies without adjustment (“Height subset”).
Abbreviations: SE = standard error.
Genetic correlation
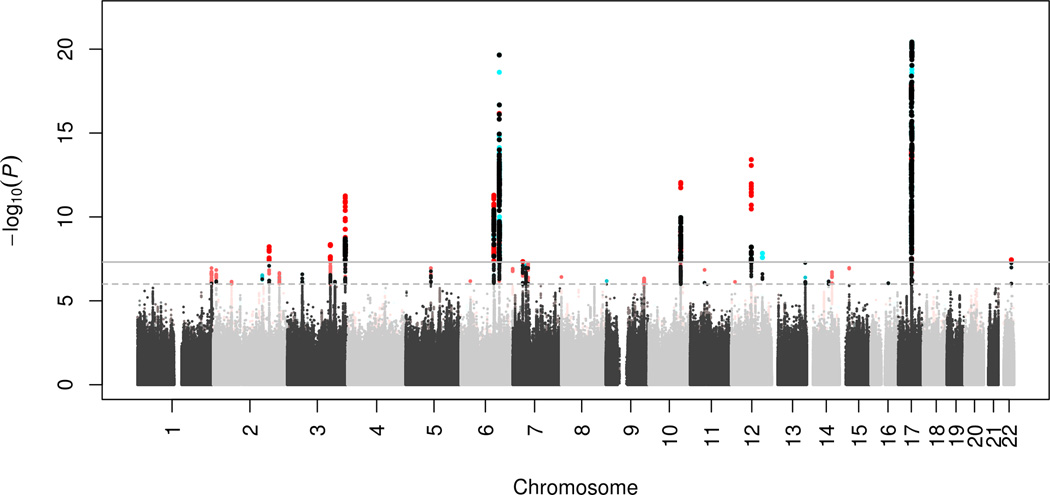
In addition to height, we examined the genome-wide genetic overlap between intracranial volume and other anthropometric traits, cognitive function, and neurodegenerative diseases (Table 2). We found a strong genetic correlation with child head circumference (ρgenetic = 0.748), which validates intracranial volume as a measure of brain growth during early development. Since this high correlation indicates that the genetic determinants of intracranial volume and child head circumference are largely shared, we aimed to leverage this information by performing a meta-analysis of both traits. The meta-analysis (combined N = 37,345) led to the identification of four novel loci (Figure 3; Supplementary Table S9).
Figure 3. Meta-analysis of intracranial volume and child head circumference.
A ‘twin’ Manhattan plot shows every variant twice: once for the discovery analysis and once for the combined discovery plus replication analysis. The least significant association of the variant-pair is plotted in grey (alternating light and dark between chromosomes). The most significant association of the variant-pair is plotted in red if is from the combined analysis (i.e., the association became more significant after meta-analyzing with the child head circumference GWAS) and in turquoise if it is from the discovery analysis (i.e., the association became less significant after meta-analyzing with the child head circumference GWAS). The dashed horizontal line represents a significance threshold of p-value < 10−6 and the full horizontal line represents genome-wide significance of p-value < 5 × 10−8. Variants surpassing these thresholds are indicated by larger and brighter points.
Weaker correlations were found with birth length and weight (ρgenetic < 0.3), which attenuated after adjusting for height. Additionally, intracranial volume was genetically correlated with cognitive function in childhood (ρgenetic = 0.277, P = 2.2×10−3) as well as general cognitive function in middle-aged and older adults (ρgenetic = 0.202, P = 6.3×10−4). Furthermore, we found a positive genetic correlation with Parkinson’s disease (ρgenetic = 0.315, P = 6.6 × 10−7), but there was no significant genetic overlap with Alzheimer’s disease, white matter lesions, and psychiatric traits.
Enrichment analyses
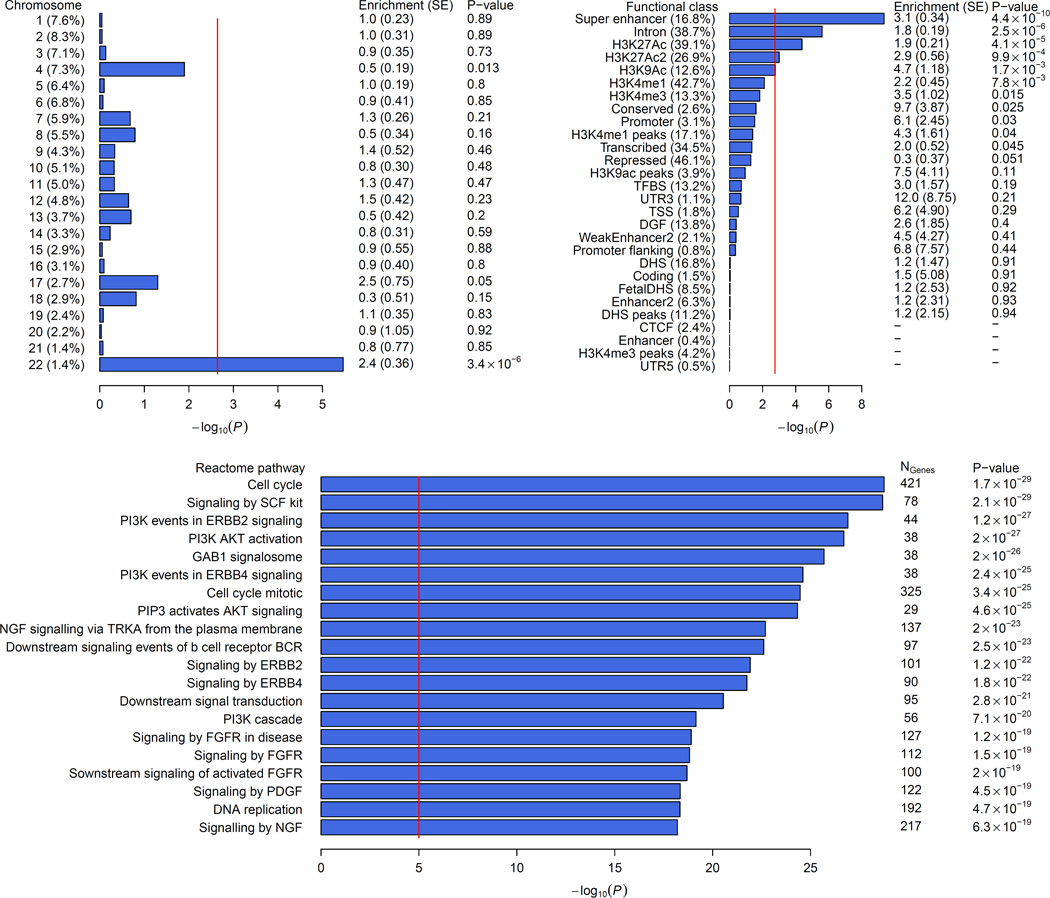
Next, we assessed whether particular subsets of genetic variants were enriched for association with intracranial volume using partitioned heritability and pathway analyses (Online Methods). Overall, we found that common variants genotyped from across the whole genome explained 25.42% (S.E. 2.73%) of the variation in intracranial volume. Partitioning heritability by chromosome showed that chromosome 22 contributed twofold more to variation in intracranial volume than would be expected by its size (Figure 4A), which was not seen for any of the other complex traits from the genetic correlation analysis (Supplementary Figure S2). Partitioning by functional elements showed an enrichment for introns and several histone codes that are found in actively transcribed promoters (Figure 4B). The enrichment for intronic variants was specific to intracranial volume, whereas the other functional classes were also enriched in other complex traits (Supplementary Figure S3). We also found that loci associated with intracranial volume cluster around genes involved in specific pathways, with 94 pathways significantly enriched (Figure 4C; full list in Supplementary Table S10). These pathways included all cell cycle components – the M-, G1-, S-, and G2-phases – and various growth factor signaling pathways, including PI3K–AKT.
Figure 4. Enrichment analyses of common variants associated with intracranial volume.
Enrichment of subsets of variants for association with intracranial volume: A) by chromosomes, B) by functional subtype, and C) by pathway. See Online Methods for additional information.
Head growth trajectories
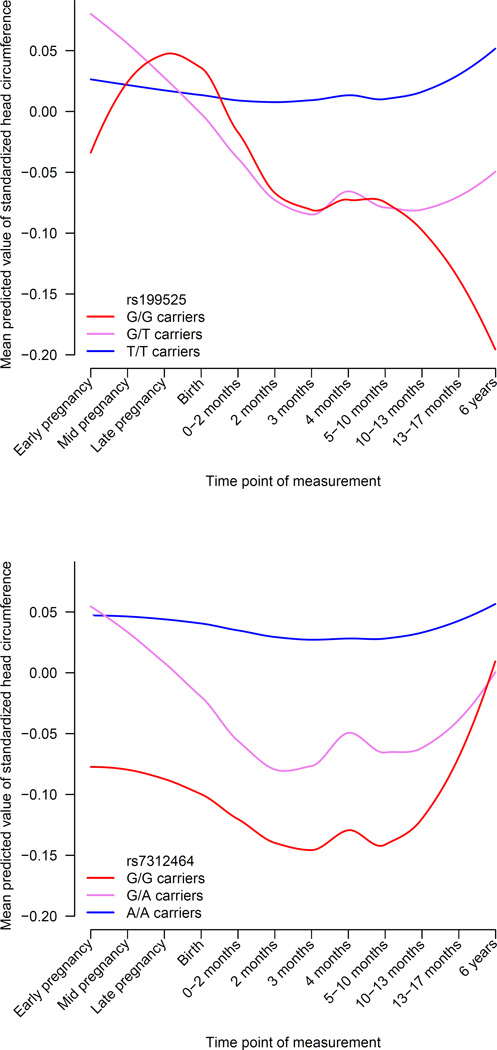
Although intracranial volume reflects brain development until maturation, and we identified influences of many growth-related processes contributing to its variation, all loci were still discovered via cross-sectional associations in adults. Therefore, we tested whether a polygenic score of the 7 loci could predict head growth in a longitudinal cohort of 2,824 children of European ancestry followed prenatally until 6 years of age (Online Methods). We found that a higher polygenic score, representing a genetically larger intracranial volume in adults, was also associated with a larger child head circumference (β = .031 per SD, P = 0.010). Furthermore, the effect of the polygenic score was age-dependent and more prominent in older children (β = 0.0080 per SD polygenic score per year age, Pinteraction = 0.0091). When investigating the individual loci separately, both 17q21 and 12q14 showed significant associations with child head circumference, but they influenced the trajectories of head growth differently (Figure 4A–B). For 17q21, the negative impact of the G allele on head circumference becomes apparent postnatally and increases towards six years, whereas the 12q14 locus exerts an effect from early pregnancy to one year of age, but is less prominent later in life.
DISCUSSION
Genes contributing to variation in the size of the human brain remain challenging to discover. In a worldwide project of unprecedented scale, we performed the largest-ever meta-analysis of genome-wide association studies of intracranial volume. We discovered five novel genetic loci associated with intracranial volume, and replicated two known signals. The discovery sample included Europeans only, but the direction of effect was similar in other ethnicities. The genes in these loci provide intriguing links between maximal brain size and various processes, including neural stem cell proliferation (FOXO3), neurodegeneration (MAPT), bone mineralization (CENPW), growth signaling (IGF1, HMGA2), DNA replication (GMNC), and rRNA maturation (PDCD). On a genome-wide scale, we discovered evidence of genetic correlation between intracranial volume and other key traits such as height and cognitive function, and also with Parkinson’s disease, indicating that the genes underlying brain development have far-reaching effects well beyond the initial years of life.
The 17q21 locus tags a 1Mb inversion that is under positive selection in Caucasians14. It contains multiple genes including the MAPT and KANSL1. The MAPT gene is consistently implicated in various neurodegenerative disorders including Parkinson’s disease, Alzheimer’s disease, and frontotemporal dementia15,16, and microduplications have been reported to cause microcephaly17. KANSL1 causes the reciprocal 17q21.31 microdeletion syndrome - a multisystem disorder with intellectual disability, hypotonia and distinctive facial features18. The signal at 6q22 is intergenic to CENPW and RSPO3, but now lies 172kb closer to CENPW. Interestingly, multiple variants at this locus independently influence bone mineral density19,20, and our signal particularly overlaps with the variant showing high specificity for the skull20.
The significant variants at chr 6q21 span FOXO3, a gene associated with longevity21, height13, and serum IGF1 levels22. FOXO3 regulates the proliferation of neural stem cells, and knockout mice show larger brains resulting from increased proliferation immediately after birth23, followed by a decrease in adult neural stem cell renewal23,24. The rs3800229 variant in strong LD with our top variant (r2 = 0.84) contains chromatin promoter marks in the fetal brain (Supplementary Table S4), and regulates serum IGF1 levels in infants25. This provides a link to the genome-wide significant locus on chr12q23 near IGF1, pointing to a potential mechanism through which these loci may affect brain growth. Chr12q23 lies 20Mb from one of two loci previously detected for head circumference in children26, but that region was not associated with intracranial volume in our study (rs7980687; P = 0.06). The other reported child head circumference locus, however, corresponded to our chr12q14 signal, with the top variant lying 14kb downstream of HMGA2, and already showed suggestive association with intracranial volume in a previous report7. It has also previously been associated with height13 and is essential for growth27. The chr10q24 LD-block covers multiple genes, but an intronic variant within PDCD11 is most significant. PDCD11 encodes an NF-kappa-B-binding protein required for rRNA maturation and generation of 18S rRNA28. A variant in LD (rs7894407) has recently been identified in a GWAS of cerebral white matter hyperintensities29. The top chr3q28 variant is located upstream of GMNC, which codes for the geminin coiled-coil domain-containing protein essential for DNA replication30.
Prior efforts to identify variants affecting intracranial volume were much smaller and critically did not adjust for height6–9. We found that 4 out of 7 loci were already discovered for height13, but also that over 10% of the known ‘height loci’ actually affect intracranial volume, even after regressing out height. Interestingly, some variants showed discordant associations for height and intracranial volume - in line with the recent finding that different height loci disproportionally affect either leg length or spine/head length31 and may be a marker for pathological development32. Also, height might thus serve as a proxy phenotype for intracranial volume, with the tenfold larger sample of the height GWAS giving greater power to detect associations. Neural genes are also enriched in pathway analyses of height13. However, to fully disentangle whether these identified genes are ‘height genes’, ‘brain volume genes’, or ‘growth genes’ (i.e., pleiotropic), a large collaborative effort is needed that examines the association of these variants with both intracranial volume and height under various models.
When investigating genome-wide overlap with other traits, we found a strong correlation with child head circumference, underlining that intracranial volume is valid measure for maximal attained brain size. We were able to leverage this genetic link by meta-analyzing both traits, which led to the identification of four additional loci (2q32.1, 3q23, 7p14.3, 22q13.2). The correlations with birth length and weight were weaker and decreased further after adjusting for height, so a similar phenotypic correlation between head size and body size at younger age may drive these correlations. Intracranial volume was also genetically associated with cognitive function in childhood as well as general cognitive function in middle-aged and older individuals. This indicates that variation in maximally attained brain size during development shares a genetic basis with cognitive ability later in life and supports intracranial volume as a measure of brain reserve5.
The brain reserve hypothesis states that premorbid brain size can modify resilience to age-related brain pathology33, but there was no indication of a genome-wide overlap with Alzheimer’s disease. However, we found a positive genetic correlation with Parkinson’s disease that rather points to a brain “overgrowth” hypothesis. Interestingly, the IGF1 and the PI3K–AKT pathways, key factors in both growth signaling and our current study of intracranial volume, are neuroprotective in a model system of Parkinson’s disease34. There were no correlations with other neurological or psychiatric traits, indicating that this finding might be specific to Parkinson’s disease. However, it is important to note that there is a certain extent of variation in the sample size and power of these studies, and larger GWAS might reveal genetic correlation with other traits as well.
It is not yet known if variance in intracranial volume, within the normal range, contributes to disease risk or brain reserve. There is no doubt that in the pathological extremes of the distribution, size can matter, as in disorders such as microcephaly or macrocephaly. Here we found evidence for a shared genetic background between intracranial volume and cognitive function, and risk of Parkinson’s disease. While not definitive, these are novel pieces of empirical evidence in the debate on whether or not whole brain size matters.
The pathway analyses highlight cellular growth and proliferation and included all components of the cell cycle (M-, G1-, S-, and G2-phase) and various growth factor signaling pathways. PI3K–AKT signaling has a well described role in brain overgrowth disorders35,36, and was the only significant pathway using a different pathway analysis method (Supplementary Table S11). Interestingly, AKT3 intronic variants showed suggestive evidence for association with intracranial volume (rs7538011; P = 9.2 × 10−7). Deletions of AKT3 cause microcephaly syndromes37, whereas duplications give rise to macrocephaly38. Similar to FOXO3, it is part of the IGF1 signaling pathway, which is important for human longevity39. The PI3K–AKT signaling pathway seems to have an important role in brain growth, not only in pathological extremes, but also for normal variation at a population level. Other pathways enriched for association with intracranial volume highlight neuronal functions such as neurotransmission and axon guidance.
We identified novel loci all influencing intracranial volume and, at a genome-wide level, there seem to be common pathways, but our longitudinal study reveals that their developmental effects are complex. The loci influenced trajectories of head growth differently; it also would be interesting to investigate whether their spatial profiles of effects are distinct, where certain loci promote growth of particular brain regions.
Here we identified key genetic loci implicated in intracranial volume within a global collaborative effort, followed by computational analyses to determine the important biological pathways and functional elements. While the majority of the genetic variants are yet to be discovered, it is clear that these will provide better insight into brain development, but also into related neuropsychiatric traits such as cognitive functioning and even for neurodegeneration late in life. Uncovering the remaining heritability will advance our understanding of the brain’s complex genetic architecture.
ONLINE METHODS
Study population
This study reports data on 32,438 subjects from 52 study sites that are part of the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE)10 consortium and Enhancing NeuroImaging Genetics through Meta-Analysis (ENIGMA)11 consortium. Briefly, the CHARGE consortium is a collaboration of predominantly population-based cohort studies that investigate the genetic and molecular underpinnings of age-related complex diseases, including those of the brain. The ENIGMA consortium brings together numerous studies, mainly with a case-control design, which performed neuroimaging in a range of neuropsychiatric or neurodegenerative diseases, as well as healthy normative populations. Studies participated in either the discovery cohort of European ancestry, the replication in European ancestry, or the generalization to other ethnicities. An overview of the demographics and type of contribution for each cohort is provided in Supplementary Table S1. Written informed consent was obtained from all participants. Each study was approved by the respective Institutional Review Board or Local Ethics Committee.
Genetics
Genotyping was performed using a variety of commercial arrays across the contributing sites. Both samples as well as variants underwent similar quality control procedures based on genetic homogeneity, call rate (less than 95%), minor allele frequency (MAF < 0.01), and Hardy-Weinberg Equilibrium (HWE p-value less than 1 × 10−6). Good quality variants were used as input for imputation to the 1000 Genomes reference panel (phase 1, version 3) using validated software packages (MaCH/minimac, IMPUTE2, BEAGLE, GenABLE). Variants that were poorly imputed (R2 < 0.5) or uncommon (MAF < 0.5%) were removed prior to meta-analysis. Full details on the site-specific genotyping and quality control may be found in Supplementary Table S2.
Imaging
Magnetic resonance imaging (MRI) was obtained from scanners with a diversity of manufacturers, field strengths, and acquisition protocols. Images were used to estimate milliliters of intracranial volume from automated segmentations generated by freely available or in-house methods that have been described and validated earlier. Most sites measured intracranial volume for each participant by multiplying the inverse of the determinant of the transformation matrix required to register the subject’s MRI scan to a common template by the template volume (1,948,105 mm3), using the FreeSurfer software. Visual inspections were performed to identify and remove poorly segmented images. Either all scans were visually inspected, or sites generated histogram plots to identify any outliers, which were defined as individuals with a volume more than three standard deviations away from the mean. Statistical outliers were only excluded if the segmentations were deemed improper‥ More site-specific information related to the imaging is available in Supplementary Table S3.
Genome-wide association studies
Genome-wide association studies of intracranial volume were performed for each site separately, controlling for age, sex, and, when applicable, age2, population stratification variables (MDS / principal components), study site (for multi-site studies only), diagnosis (for case-control studies only). Studies of unrelated individuals performed a linear regression analyses whereas studies of related individuals (ASPSFam, BrainSCALE, ERF, GeneSTAR, GOBS, NeuroIMAGE, NTR-Adults, OATS, QTIM, SYS) used linear mixed models to account for familial relationships. Summary statistics, including the effect estimates of the genetic variant with intracranial volume under an additive model, were exchanged to perform a fixed-effects meta-analysis weighting for sample size in METAL40. After the final meta-analysis, variants were excluded if they were only available for fewer than 5,000 individuals. Meta-analyses were stratified by race and done separately for discovery, replication, and generalization samples. Beta coefficients were recalculated from Z-scores, allele frequencies, and the sample, as described earlier41 Site-specific quantile-quantile plots were generated to inspect the presence of genomic inflation. The variance explained by all variants in the GWAS was estimated using LD score regression12,42. Sensitivity analyses were performed by excluding patients.
Functional annotation
All tracks of the regional association plots were taken from the UCSC Genome Browser Human hg19 assembly. SNPs (top 5%) shows the top 5% associated variants within the locus and are colored by their correlation to the top variant. Genes shows the gene models from GENCODE version 19. The tracks give the predicted chromatin states based on computational integration of ChIP-seq data for 12 chromatin marks in various human tissues derived from the Roadmap Epigenomics Consortium43. Additionally, we used HaploReg version 3 for annotation of the top variants and all variants in LD (> 0.80) (http://www.broadinstitute.org/mammals/haploreg/haploreg_v3.php).
Genetic correlation
The genetic correlation analyses were also performed using LD score regression. The GWAS meta-analysis of intracranial volume, as well as the height adjusted and height subset meta-analyses, were correlated with published GWAS of the following traits: Child head circumference26, birth weight44, birth length45, adult height13, childhood cognitive function46, adult cognitive function47, Alzheimer’s disease48, Parkinson’s disease49, white matter lesions50, psychiatric disorders51, neuroticism52, and extraversion53.
Enrichment analyses
To determine whether the intracranial volume association results were enriched for certain types of genetic variants, we employed two strategies: partitioned heritability and pathway analyses.
Partitioned heritability was calculated using a previously described method42. This was done by partitioning variants by chromosome and by 28 functional classes: coding, UTR, promoter, intron, histone marks H3K4me1, H3K4me3, H3K9ac5 and two versions of H3K27ac, open chromatin DNase I hypersensitivity Site (DHS) regions, combined chromHMM/Segway predictions, regions that are conserved in mammals, super-enhancers and active enhancers from the FANTOM5 panel of samples (Finucane et al. page 4)42. Multiple testing thresholds were calculated accordingly: Pthresh = 0.05/(22 chromosomes) = 2.27 × 10−3 for the chromosomes and Pthresh = 0.05/(28 classes) = 1.79 × 10−3 for the functional classes.
Pathway analyses were performed using the KGG2.554 and MAGENTA55 software packages. LD was calculated based with the 1000 Genomes Project European samples as a reference (see URLs). Variants were considered to be within a gene if they were within 5 kb of the 3’/5’ UTR based on chromosome positions (hg19) coordinates. Gene-based tests were done with the GATES test54 without weighting P-values by predicted functional relevance. Pathway analysis was performed using the HYST test of association56. A multiple testing threshold accounting for the number of pathways tested resulting in a significance threshold of Pthresh = 0.05/(671 pathways) = 7.45 × 10−5.
Head growth trajectories
Head growth trajectory analyses were done within the Generation R study, a longitudinal cohort study situated in Rotterdam, the Netherlands. For this analysis we included 2,824 children of European ancestry followed prenatally until 6 years of age. Head size was measured at the following points: prenatally (using echo) during the first, second, and third trimester, and postnatally (measuring head circumference) at 0–2 months, 2 months, 3 months, 4 months, 5–10 months, 10–13 months, 13–17 months, and 5 years of age. We tested whether a polygenic score of the 7 loci, as well as the 7 loci themselves separately, were related to head growth using linear mixed models and included an interaction term between time and the genetic score/variant (SAS software). Next, the predicted values were calculated for each person and plotted over time, stratified by genotype (0/1/2 risk alleles) using the R software package.
URLs
ftp://pricelab:pricelab@ftp.broadinstitute.org/LDSCORE/
http://enigma.ini.usc.edu/protocols/genetics-protocols/
Supplementary Material
Figure 5. Temporal trends of intracranial volume loci during pre- and postnatal brain development.
Mean predicted values of standardized head circumference using linear mixed models with age, sex, and the rs199525 or rs138074335 variants. The blue line represents children not carrying the risk allele, purple only a single risk allele, and red with two risk alleles. See Online Methods for additional information. Total sample size is 2,824.
Acknowledgments
CHARGE: Infrastructure for the CHARGE Consortium is supported in part by the National Heart, Lung, and Blood Institute grant HL105756 and for the neuroCHARGE phenotype working group through the National Institute on Aging grant AG033193.
ENIGMA: ENIGMA was supported in part by a Consortium grant (U54 EB020403 to PMT) from the NIH Institutes contributing to the Big Data to Knowledge (BD2K) Initiative, including the NIBIB and NCI.
Cohort-specific acknowledgements
Age, Gene/Environment Susceptibility-Reykjavik Study (AGES-Reykjavik): This study has been funded by NIH contracts N01-AG-1-2100 and 271201200022C, the NIA Intramural Research Program, Hjartavernd (the Icelandic Heart Association), and the Althingi (the Icelandic Parliament). The study is approved by the Icelandic National Bioethics Committee, VSN: 00-063. The researchers are indebted to the participants for their willingness to participate in the study.
Alzheimer’s Disease Neuroimaging Initiative (ADNI): Data collection and sharing for this project was funded by the ADNI (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; BioClinica, Inc.; Biogen Idec Inc.; Bristol-Myers Squibb Company; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; GE Healthcare; Innogenetics, N.V.; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Medpace, Inc.; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Synarc Inc.; and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory of Neuro Imaging at the University of Southern California.
ANM: AddNeuroMed was funded through the EU FP6 programme. HS: Academy of Finland, Research Council for Health, 258081, UEFBrain, University of Eastern Finland, VTR funding Kuopio University Hospital.
Atherosclerosis Risk In Communities Study (ARIC): The Atherosclerosis Risk in Communities study was performed as a collaborative study supported by National Heart, Lung, and Blood Institute (NHLBI) contracts (HHSN268201100005C, HSN268201100006C, HSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C), R01HL70825, R01HL087641, R01HL59367, and R01HL086694; National Human Genome Research Institute contract U01HG004402; and National Institutes of Health (NIH) contract HHSN268200625226C. Infrastructure was partly supported by grant No. UL1RR025005, a component of the NIH and NIH Roadmap for Medical Research. This project was also supported by NIH R01 grant NS087541 to MF.
Austrian Stroke Prevention Study Family (ASPS-Fam): The ASPS-Fam is funded by the Austrian Science Fund (FWF) project I904, the Medical University of Graz and the Steiermärkische Krankenanstalten Gesellschaft.
BETULA: This sample collection was supported by a Wallenberg Scholar grant from the Knut and Alice Wallenberg (KAW) foundation and a grant from Torsten and Ragnar Söderbergs Foundation to Lars Nyberg. Stephanie le Hellard was supported by a grant from HelseVest RHF (Grant 911554).
Bipolar Family Study (BFS): The Bipolar Family Study wishes to thank the Scottish Mental Health Research Network for research assistant support, the Brain Research Imaging Centre Edinburgh, a center in the Scottish Funding Council Scottish Imaging Network–A Platform for Scientific Excellence (SINAPSE) Collaboration, for image acquisition and the Wellcome Trust Clinical Research Facility for genotyping. Genotyping was supported by the National Alliance for Research on Schizophrenia and Depression (NARSAD) Independent Investigator Award (to A.M.M.), and data collection was supported by the Health Foundation Clinician Scientist Fellowship. The research leading to these results also receives funding from the European Community’s Seventh Framework Programme (FP7/2007– 2013) under grant agreements #602450 (IMAGEMEND) and ongoing support from the Wellcome Trust (Ref 104036/Z/14/Z).
Brain Imaging Genetics (BIG): This work makes use of the BIG database, first established in Nijmegen, The Netherlands, in 2007. This resource is now part of Cognomics (www.cognomics.nl), a joint initiative by researchers of the Donders Centre for Cognitive Neuroimaging, the Human Genetics and Cognitive Neuroscience departments of the Radboud university medical centre and the Max Planck Institute for Psycholinguistics in Nijmegen. The Board of the Cognomics Initiative consists of Barbara Franke, Simon Fisher, Guillen Fernandez, Peter Hagoort, Han Brunner, Jan Buitelaar, Hans van Bokhoven and David Norris. The Cognomics Initiative has received supported from the participating departments and centres and from external grants, i.e. the Biobanking and Biomolecular Resources Research Infrastructure (Netherlands) (BBMRI-NL), the Hersenstichting Nederland, and the Netherlands Organisation for Scientific Research (NWO). The research leading to these results also receives funding from the NWO Gravitation grant ‘Language in Interaction’, the European Community’s Seventh Framework Programme (FP7/2007– 2013) under grant agreements n° 602450 (IMAGEMEND), n°278948 (TACTICS), and n°602805 (Aggressotype) as well as from the European Community’s Horizon 2020 programme under grant agreement n° 643051 (MiND) and from ERC-2010-AdG 268800-NEUROSCHEMA. In addition, the work was supported by a grant for the ENIGMA Consortium (grant number U54 EB020403) from the BD2K Initiative of a cross-NIH partnership. We wish to thank all persons who kindly participated in the BIG research.
Brain Genomics Superstruct Project (GSP): Data were provided [in part] by the Brain Genomics Superstruct Project of Harvard University and the Massachusetts General Hospital, with support from the Center for Brain Science Neuroinformatics Research Group, the Athinoula A. Martinos Center for Biomedical Imaging, and the Center for Human Genetic Research. 20 individual investigators at Harvard and MGH generously contributed data to GSP. This work was made possible by the resources provided through P.H.L is supported by NIMH grants K99 MH101367 (PHL), R01-MH079799 (JWS), K24MH094614 (JWS), and K01MH099232 (AJH).
Brainscale and NTR-Adults: We would like to thank all twin participants from the Netherlands Twin Register. The NTR-adult and Brainscale studies were supported by the Netherlands Organization for Scientific Research NWO [MW904-61-193 (E.d.G & D.B), MaGW-nr: 400-07-080 (D. v’t E.), MagW 480-04-004 (D.B), (51.02.060 (H.H.), 668.772 (D.B. & H.H.); NWO/SPI 56-464-14192 (D.B.), the European Research Council (ERC-230374) (D.B.), High Potential Grant Utrecht University (H.H.), NWO Brain and Cognition 433-09-220 (H.H.) and the Neuroscience Campus Amsterdam (NCA).
Cardiovascular Health Study (CHS): This research was supported by NHLBI contracts HHSN268201200036C, HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086; and NHLBI grants U01HL080295, R01HL087652, R01HL105756, R01HL103612, and R01HL120393 with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided through R01AG023629, R01AG15928, R01AG20098, R01AG027002, R01AG05133, and R01AG027058 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org. The provision of genotyping data was supported in part by the National Center for Advancing Translational Sciences, CTSI grant UL1TR000124, and the National Institute of Diabetes and Digestive and Kidney Disease Diabetes Research Center (DRC) grant DK063491 to the Southern California Diabetes Endocrinology Research Center.
CHAP: This research was funded by grants from the National Institute of Health (AG011101 and AG030146) and the International Alzheimer’s Association (NIRP-14-302587). DNA samples were collected during clinical evaluations and population interviews, and analyzed at the Broad Institute.
Epidemiology of Dementia in Singapore (EDIS): The Singapore Malay Eye Study (SiMES) and the Singapore Chinese Eye. Study (SCES) are funded by National Medical Research Council (grants 0796/2003, IRG07nov013, IRG09nov014, STaR/0003/2008 and CG/SERI/2010) and Biomedical Research Council (grants 09/1/35/19/616), Singapore. The Genome Institute of Singapore, Agency for Science, Technology and Research, Singapore provided services for genotyping. The Epidemiology of Dementia in Singapore study is supported by the National Medical Research Council, Singapore (NMRC/CG/NUHS/2010 [Grant no: R-184-006-184-511]). MKI received additional funding from the Singapore Ministry of Health’s National Medical Research Council (NMRC/CSA/038/2013).
EPIGEN: Work from the London Cohort was supported by research grants from the Wellcome Trust (grant 084730 to S.M.S.), University College London (UCL)/University College London Hospitals (UCLH) NIHR Biomedical Research Centre/Specialist Biomedical Research Centres (CBRC/SBRC) (grant 114 to S.M.S.), the European Union Marie Curie Reintegration (to M. Matarin and S.M.S.), the UK NIHR (08-08-SCC), the Comprehensive Local Research Network (CLRN) Flexibility and Sustainability Funding (FSF) (grant CEL1300 to S.M.S.), The Big Lottery Fund, the Wolfson Trust and the Epilepsy Society. This work was undertaken at UCLH/UCL, which received a proportion of funding from the UK Department of Health’s NIHR Biomedical Research Centres funding scheme. Work from the Royal College of Surgeons in Ireland was supported by research grants from the Science Foundation Ireland (Research Frontiers Programme award 08/RFP/GEN1538) and Brainwave–the Irish Epilepsy Association. M. Matarin is funded by Epilepsy Research UK (grant F1206).
Erasmus Rucphen Family study (ERF) The ERF study as a part of EUROSPAN (European Special Populations Research Network) was supported by European Commission FP6 STRP grant number 018947 (LSHG-CT-2006-01947) and also received funding from the European Community’s Seventh Framework Programme (FP7/2007-2013)/grant agreement HEALTH-F4-2007-201413 by the European Commission under the programme “Quality of Life and Management of the Living Resources” of 5th Framework Programme (no. QLG2-CT-2002-01254). High-throughput analysis of the ERF data was supported by joint grant from Netherlands Organization for Scientific Research and the Russian Foundation for Basic Research (NWO-RFBR 047.017.043). We are grateful to all study participants and their relatives, general practitioners and neurologists for their contributions and to P. Veraart for her help in genealogy, J. Vergeer for the supervision of the laboratory work and P. Snijders for his help in data collection. Najaf Amin is supported by the Netherlands Brain Foundation (project number F2013(1)-28). The ERF study genome-wide array data and phenotype data (age and gender) is archived in European Genome-Phenome Database (EGA). The study is archived in the DAC named Erasmus Rucphen Family Study with the accession code: EGAS00001001134. Researchers who wish to use other phenotypic data of the Erasmus Rucphen Family Study must seek approval from the management team of the Erasmus Rucphen Family study. They are advised to contact the study PI, professor Cornelia van Duijn (c.vanduijn@erasmusmc.nl).
Framingham Heart Study (FHS): This work was supported by the dedication of the Framingham Study participants, the National Heart, Lung and Blood Institute’s Framingham Heart Study (Contract No. HHSN268201500001I), and by grants from the National Institute of Health (AG08122, AG033193, AG010129, NS017950, and U01AG49505).
Generation R: The Generation R Study is conducted by the Erasmus Medical Centre in close collaboration with the Municipal Health Service Rotterdam area, and the Stichting Trombosedienst & Artsenlaboratorium Rijnmond (STAR), Rotterdam. We gratefully acknowledge the contribution of general practitioners, hospitals, midwives, and pharmacies in Rotterdam. Additional support for neuroimaging is supported by ZonMw TOP 40-00812-98-11021.
GeneSTAR: was supported by grants from the National Institutes of Health National Institute of Neurological Disorders and Stroke (R01NS062059), the National Institutes of Health National Heart, Lung, and Blood Institute (U01 HL72518, HL097698) and the National Institutes of Health/National Center for Research Resources (M01-RR000052) to the Johns Hopkins General Clinical Research Center. We would like to thank the participants and families of GeneSTAR and our dedicated staff for all their sacrifices.
GIG: The GIG (Genomic Imaging Göttingen) sample was established at the Center for Translational Research in Systems Neuroscience and Psychiatry at Göttingen University. We thank Maria Keil, Esther Diekhof, Tobias Melcher and Ilona Henseler for assistance in MRI data acquisition, and Elisabeth Binder and Holger Mohr for their valuable help with genotyping. We are grateful to all persons who kindly participated in the GIG study.
GOBS: We acknowledge the ultimate source of our data, the Mexican American community of San Antonio and surrounding areas. Financial support for this study was provided by grants from the National Institute of Mental Health MH0708143 (Principal Investigator [PI]: DC Glahn), MH078111 (PI: J Blangero), and MH083824 (PI: Glahn & Blangero). Theoretical development of SOLAR is supported by MH59490 (PI: Blangero). This investigation was conducted, in part, in facilities constructed with support from Research Facilities Improvement Program Grant Numbers C06 RR13556 and C06 RR017515 from the National Center for Research Resources, NIH. Some of this work was performed at Texas Biomedical Research Institute where Dr. Blangero began this investigator-initiated competitively publicly funded work.
HUBIN: This study was financed by the Swedish Research Council (K2007-62X-15077-04-1, K2008-62P-20597-01-3. K2010-62X-15078-07-2, K2012-61X-15078-09-3), the regional agreement on medical training and clinical research between Stockholm County Council and the Karolinska Institutet, the Knut and Alice Wallenberg Foundation, and the HUBIN project. Genotyping was performed by the SNP&SEQ Technology Platform in Uppsala. The platform is part of Science for Life Laboratory at Uppsala University and supported as a national infrastructure by the Swedish Research Council.
IMAGEN: IMAGEN was supported by the European Union-funded FP6 Integrated Project IMAGEN (Reinforcement- related behaviour in normal brain function and psychopathology) (LSHM-CT- 2007-037286), the FP7 projects IMAGEMEND (602450) and MATRICS (603016), and the Innovative Medicine Initiative Project EU-AIMS (115300-2), the Medical Research Council Programme Grant “Developmental pathways into adolescent substance abuse” (93558), as well as the NIHR-biomedical Research Center “Mental Health”. Further support was provided by the Swedish Research Council FORMAS, and the German Federal Ministry for Education and Research BMBF (eMED SysAlc 01ZX1311A; Forschungsnetz AERIAL; 1EV0711) and the National Institutes of Health, U.S.A. (Axon, Testosterone and Mental Health during Adolescence; MH085772-01A1).
IMpACT: This study was funded by a grant from the Brain & Cognition Excellence Program of the Netherlands Organization for Scientific Research (NWO, grant 433-09-229) and in part by the Netherlands Brain Foundation (grant number, 15F07[2]27). B. Franke is supported by a Vici grant from the Netherlands Organisation for Scientific Research (NWO; grant n° 016.130.669). The research leading to these results also receives funding from the European Community’s Seventh Framework Programme (FP7/2007– 2013) under grant agreements n° 602450 (IMAGEMEND), n°278948 (TACTICS), and n°602805 (Aggressotype) as well as from the European Community’s Horizon 2020 programme under grant agreement n° 643051 (MiND). In addition, the work was supported by a grant for the ENIGMA Consortium (grant number U54 EB020403) from the BD2K Initiative of a cross-NIH partnership.
LBC1936: We thank the LBC1936 participants and the members of the LBC1936 research team who collected and collated the phenotypic and genotypic data. This work was undertaken as part of the Cross Council and University of Edinburgh Centre for Cognitive Ageing and Cognitive Epidemiology (CCACE; http://www.ccace.ed.ac.uk). This work was supported by a Research into Ageing programme grant (to I.J.D.) and the Age UK-funded Disconnected Mind project (http://www.disconnectedmind.ed.ac.uk; to I.J.D. and J.M.W.), with additional funding from the UK Medical Research Council (MRC; to I.J.D., J.M.W. and M.E.B.). J.M.W. is supported by the Scottish Funding Council through the SINAPSE Collaboration (http://www.sinapse.ac.uk). M.V.M. is supported by the Row Fogo Charitable Trust. CCACE (MRC MR/K026992/1) is funded by the UK Biotechnology and Biological Sciences Research Council (BBSRC) and the UK MRC. Genotyping was supported by a grant from the BBSRC (BB/F019394/1).The image acquisition and analysis was performed at the Brain Research Imaging Centre, University of Edinburgh (http://www.bric.ed.ac.uk).
Leiden Longevity Study (LLS): The Leiden Longevity Study was supported by a grant from the Innovation-Oriented Research Program on Genomics [SenterNovem IGE05007] and the Netherlands Consortium for Healthy Ageing [grant number 050-060-810].
Mind Clinical Imaging Consortium (MCIC): Data used in the preparation of this work were obtained from the Mind Clinical Imaging Consortium database through the Mind Research Network (www.mrn.org). The MCIC project was supported by the Department of Energy under Award Number DE-FG02-08ER64581. MCIC is the result of efforts of co-investigators from University of Iowa, University of Minnesota, University of New Mexico, and Massachusetts General Hospital.
MooDS: The establishment of the MooDS sample was funded by the German Federal Ministry of Education and Research (BMBF) through the Integrated Genome Research Network (IG) MooDS (Systematic Investigation of the Molecular Causes of Major Mood Disorders and Schizophrenia; grant 01GS08144 to Markus M. Nöthen and Sven Cichon, grant 01GS08147 to Marcella Rietschel and Andreas Meyer-Lindenberg and grant 01GS08148 to Andreas Heinz),) under the auspices of the National Genome Research Network plus (NGFNplus), and through the Integrated Network IntegraMent (Integrated Understanding of Causes and Mechanisms in Mental Disorders), under the auspices of the e:Med Programme (grant 01ZX1314A to Markus M. Nöthen, grant 01ZX1314C to Hendrik Walter, grant 01ZX1314G to Marcella Rietschel).
MPIP: The MPIP Munich Morphometry Sample comprises images acquired as part of the Munich Antidepressant Response Signature Study and the Recurrent Unipolar Depression (RUD) Case-Control study performed at the MPIP, and control subjects acquired at the Ludwig-Maximilians-University, Munich, Department of Psychiatry. We thank Eva Meisenzahl and Dan Rujescu for providing MRI and genetical data for inclusion into the MPIP Munich Morphometry sample. We wish to acknowledge Anna Olynyik and radiographers Rosa Schirmer, Elke Schreiter, Reinhold Borschke and Ines Eidner for image acquisition and data preparation. We thank Dorothee P. Auer for local study management in the initial phase of the RUD study. We are grateful to GlaxoSmithKline for providing the genotypes of the Recurrent Unipolar Depression Case-Control Sample. We thank the staff of the Center of Applied Genotyping (CAGT) for generating the genotypes of the MARS cohort. The study is supported by a grant of the Exzellenz-Stiftung of the Max Planck Society. This work has also been funded by the Federal Ministry of Education and Research (BMBF) in the framework of the National Genome Research Network (NGFN), FKZ 01GS0481.
NCNG: this sample collection was supported by grants from the Bergen Research Foundation and the University of Bergen, the Dr Einar Martens Fund, the K.G. Jebsen Foundation, the Research Council of Norway, to SLH, VMS and TE.
NESDA: Funding was obtained from the Netherlands Organization for Scientific Research (Geestkracht program grant 10-000-1002); the Center for Medical Systems Biology (CSMB, NWO Genomics), Biobanking and Biomolecular Resources Research Infrastructure (BBMRI-NL), VU University’s Institutes for Health and Care Research (EMGO+) and Neuroscience Campus Amsterdam, University Medical Center Groningen, Leiden University Medical Center, National Institutes of Health (NIH, R01D0042157-01A, MH081802, Grand Opportunity grants 1RC2 MH089951 and 1RC2 MH089995). Part of the genotyping and analyses were funded by the Genetic Association Information Network (GAIN) of the Foundation for the National Institutes of Health.Computing was supported by BiG Grid, the Dutch e-Science Grid, which is financially supported by NWO.
NeuroIMAGE: The NeuroIMAGE project was supported by NIH Grant R01MH62873 (to Stephen V. Faraone), NWO Large Investment Grant 1750102007010 (to Jan Buitelaar), and by grants from Radboud University Nijmegen Medical Center, University Medical Center Groningen and Accare, and VU University Amsterdam. The work contributing to this result also receives support from the European Community’s Seventh Framework Programme (FP7/2007– 2013) under grant agreements n° 602450 (IMAGEMEND), n°278948 (TACTICS) and n°602805 (Aggressotype) as well as from the European Community’s Horizon 2020 programme under grant agreement n° 643051 (MiND). In addition, the work was supported by a grant for the ENIGMA Consortium (grant number U54 EB020403) from the BD2K Initiative of a cross-NIH partnership.
NIMH-IRP: Supported in part by the NIMH Intramural Research Program (ZIAMH002810; Z01MH002792; Z01MH002790)
Older Australian Twins Study (OATS): We would like to acknowledge and thank the OATS participants, their supporters and respective Research Teams. This work was supported by a number of sources. OATS is supported by the NHMRC/Australian Research Council Strategic Award 401162 and NHMRC Project Grant 1045325 to P. Sachdev and colleagues. OATS was facilitated through access to the Australian Twin Registry, a national research resource supported by the NHMRC Enabling Grant 310667, administered by the University of Melbourne. DNA was extracted by Genetic Repositories Australia, an Enabling Facility supported by the NHMRC Grant 401184. OATS genotyping was partly funded by a Commonwealth Scientific and Industrial Research Organisation Flagship Collaboration Fund Grant. Henry Brodaty is supported by the Australian Government funded Dementia Collaborative Research Centre (DCRC), UNSW. Nicola Armstrong was supported by the NHMRC Project Grant 525453 and Karen Mather is supported by an Alzheimer’s Australia Dementia Research Foundation Postdoctoral Fellowship and the NHMRC Capacity Building Grant 568940.
Osaka: This study was supported, in part, by research grants from the Japanese Ministry of Health, Labor and Welfare; the Japanese Ministry of Education, Culture, Sports, Science and Technology (MEXT) KAKENHI and Scientific Research on Innovative Areas (Comprehensive Brain Science Network)]; and the Brain Sciences Project of the Center for Novel Science Initiatives (CNSI), the National Institutes of Natural Sciences (NINS), and the Brain Mapping by Integrated Neurotechnologies for Disease Studies (Brain/MINDS) from Japan Agency for Medical Research and development, AMED.
PAFIP: The PAFIP study was supported by Instituto de Salud Carlos III, FIS00/3095, 01/3129, PI020499, PI060507, PI10/00183, PI14/00639, the SENY Fundació Research Grant CI 2005-0308007, and the Fundación Marqués de Valdecilla API07/011. PAFIP wish to acknowledge WTCCC2 (Wellcome Trust Case Control Consortium 2) for DNA Genotyping, Valdecilla Biobank for providing the biological samples and associated data included in this study and Idival Neuroimaging Unit for its help in the technical execution of this work. Diana Tordesillas-Gutiérrez is funded by a contract from the Carlos III Health Institute (CA12/00312).
PROSPER: The PROSPER study was supported by an investigator initiated grant obtained from Bristol-Myers Squibb. Prof. Dr. J. W. Jukema is an Established Clinical Investigator of the Netherlands Heart Foundation (grant 2001 D 032). Support for genotyping was provided by the seventh framework program of the European commission (grant 223004) and by the Netherlands Genomics Initiative (Netherlands Consortium for Healthy Aging grant 050-060-810).
QTIM: DPH, NJ, CRKC, and PMT are supported, in part, by NIH grants R01 NS080655, R01AG040060, R01 EB008432, R01 MH097268, U01 AG024904, R01 MH085667, R01 MH089722, P41 EB015922, and R01 MH094343. RKW is supported by National Science Foundation (BCS-1229450). JLS was supported by the NIMH (K99MH102357) and Autism Speaks. SEM and GZ are supported by Future Fellowships (FT110100548, FT0991634) from the Australian Research Council, and GWM is supported by a National Health and Medical Research Council (NHMRC), Australia, Fellowship (619667). The QTIM study is supported by grants from NIH (R01 HD050735) and the NHMRC (389875, 486682, 1009064). We thank the twins and siblings for their participation, Marlene Grace and Ann Eldridge for twin recruitment, Aiman Al Najjar and other radiographers for scanning, Kerrie McAloney and Daniel Park for research support, and Anjali Henders and staff for DNA sample processing and preparation.
ROS and MAP: The clinical, genomic, and neuroimaging data for the Religious Orders Study and the Rush Memory and Aging Project was funded by NIH grants P30AG10161, RF1AG15819, R01AG17917, R01AG30146, R01AG40039, and the Translational Genomics Research Institute.
Rotterdam Study: The generation and management of GWAS genotype data for the Rotterdam Study are supported by the Netherlands Organisation of Scientific Research NWO Investments (nr. 175.010.2005.011, 911-03-012). This study is funded by the Research Institute for Diseases in the Elderly (014-93-015; RIDE2), the Netherlands Genomics Initiative (NGI)/Netherlands Organisation for Scientific Research (NWO) project nr. 050-060-810. The Rotterdam Study is funded by Erasmus Medical Center and Erasmus University, Rotterdam, Netherlands Organization for the Health Research and Development (ZonMw), the Research Institute for Diseases in the Elderly (RIDE), the Ministry of Education, Culture and Science, the Ministry for Health, Welfare and Sports, the European Commission (DG XII), and the Municipality of Rotterdam. This research is supported by the Dutch Technology Foundation STW, which is part of the NWO, and which is partly funded by the Ministry of Economic Affairs. MAI is supported by ZonMW grant number 916.13.054. HHHA is supported by the Van Leersum Grant of the Royal Netherlands Academy of Arts and Sciences.
Saguenay Youth Study (SYS): The Saguenay Youth Study project is funded by the Canadian Institutes of Health Research (TP, ZP), Heart and Stroke Foundation of Quebec (ZP), and the Canadian Foundation for Innovation (ZP). TP is supported by the Tanenbaum Chair in Population Neuroscience at the Rotman Research Institute, University of Toronto.
SHIP and TREND: The SHIP datasets are part of the Community Medicine Research net (CMR) of the University of Greifswald, which is funded by the German Federal Ministry of Education and Research and the German Ministry of Cultural Affairs, as well as by the Social Ministry of the Federal State of Mecklenburg–West Pomerania (grants no. 01ZZ9603, 01ZZ0103, and 01ZZ0403), and the network ‘Greifswald Approach to Individualized Medicine (GANI_MED)’ funded by the Federal Ministry of Education and Research (grant 03IS2061A). Genome-wide data and MRI scans were supported by the Federal Ministry of Education and Research (grant no. 03ZIK012) and a joint grant from Siemens Healthcare, Erlangen, Germany, and the Federal State of Mecklenburg–West Pomerania. The University of Greifswald is a member of t the Caché Campus Program of the InterSystems GmbH.
Sydney Memory and Ageing Study (Sydney MAS): We would like to thank the Sydney MAS participants, their supporters and respective Research Teams. Sydney MAS was supported by the Australian National Health and Medical Research Council (NHMRC) Program Grants 350833 and 568969 to P Sachdev, H Brodaty and G Andrews. DNA was extracted by Genetic Repositories Australia, an Enabling Facility supported by the NHMRC Grant 401184. Henry Brodaty is supported by the Australian Government funded Dementia Collaborative Research Centre (DCRC), UNSW. Nicola Armstrong was supported by the NHMRC Project Grant 525453 and Karen Mather is supported by an Alzheimer’s Australia Dementia Research Foundation Postdoctoral Fellowship. Both Simone Reppermund and Karen Mather are supported by the NHMRC Capacity Building Grant 568940.
Tasmanian Study of Gait and Cognition (TASCOG): The Tasmanian Study of Gait and Cognition is supported by project grants from the National Health and Medical Research Council of Australia (NHMRC; 403000,491109, and 606543) and a grant from the Wicking Dementia Education and Research Centre, Hobart. V.S. is supported by a cofunded NHMRC Career Development Fellowship (1061457) and a Heart Foundation Future Leader Fellowship (ID 100089).
Three City Dijon Study: The 3-City Study is conducted under a partnership agreement among the Institut National de la Santé et de la Recherche Médicale (INSERM), the Victor Segalen–Bordeaux II University, and Sanofi-Aventis. The Fondation pour la Recherche Médicale funded the preparation and initiation of the study. The 3C Study is also supported by the Caisse Nationale Maladie des Travailleurs Salariés, Direction Générale de la Santé, Mutuelle Générale de l’Education Nationale (MGEN), Institut de la Longévité, Conseils Régionaux of Aquitaine and Bourgogne, Fondation de France, and Ministry of Research–INSERM Programme “Cohortes et collections de données biologiques.” Christophe Tzourio and Stéphanie Debette are supported by a grant from the Fondation Leducq.
TOP: The study was supported by the Research Council of Norway (#213837, #223273, #229129), South-East Norway Health Authority (#2013-123) and KG Jebsen Foundation.
UCLA_NL_BP: Data collection and genotyping was made possible with (NIH/NIMH) R01 MH090553 to R.A.O.th-East Norway Health Authority (#2013-123) and KG Jebsen Foundation.
UMCU: UMCU acknowledgement data: This work was supported by 917.46.370 (H.H.) and 908-02-123 (H.H.) from the Netherlands Organisation for Health Research and Development ZonMW.
WHICAP: This study was supported by a grant from the NIH (5R01AG037212).
Published GWASs used for genetic correlation analysis
CHARGE consortium: See Davies et al.47 for the general cognitive function GWAS, and Verhaaren et al.50 for the white matter lesion GWAS.
Early Growth Genetics (EGG) consortium: Data on head circumference, birth weight, and birth length have been contributed by the EGG Consortium and has been downloaded from www.egg-consortium.org.
Genetic Investigation of ANthropometric Traits (GIANT) consortium: See Wood et al.13
Genetics of Personality Consortium: See De Moor et al.52 for the neuroticism GWAS and Van den Berg et al.53 for the extraversion GWAS.
IGAP: We thank the International Genomics of Alzheimer’s Project (IGAP) for providing summary results data for these analyses. The investigators within IGAP contributed to the design and implementation of IGAP and/or provided data but did not participate in analysis or writing of this report. IGAP was made possible by the generous participation of the control subjects, the patients, and their families. The i–Select chips was funded by the French National Foundation on Alzheimer’s disease and related disorders. EADI was supported by the LABEX (laboratory of excellence program investment for the future) DISTALZ grant, Inserm, Institut Pasteur de Lille, Université de Lille 2 and the Lille University Hospital. GERAD was supported by the Medical Research Council (Grant n° 503480), Alzheimer’s Research UK (Grant n° 503176), the Wellcome Trust (Grant n° 082604/2/07/Z) and German Federal Ministry of Education and Research (BMBF): Competence Network Dementia (CND) grant n° 01GI0102, 01GI0711, 01GI0420. CHARGE was partly supported by the NIH/NIA grant R01 AG033193 and the NIA AG081220 and AGES contract N01–AG–12100, the NHLBI grant R01 HL105756, the Icelandic Heart Association, and the Erasmus Medical Center and Erasmus University. ADGC was supported by the NIH/NIA grants: U01 AG032984, U24 AG021886, U01 AG016976, and the Alzheimer’s Association grant ADGC–10–196728.
International Parkinson’s Disease Genomics Consortium (IPDGC): See Nalls et al.49
Psychiatric Genomics Consortium: See Cross-Disorder Group of the Psychiatric Genomics paper. 51
Social Science Genetic Association Consortium (SSGAC): See Benyamin et al.46 for the childhood cognitive function GWAS.
Footnotes
COMPETING FINANCIAL INTERESTS STATEMENT
The authors declare no competing financial interest related to any of the work described in this manuscript.
AUTHOR CONTRIBUTIONS
Conceived of the study and drafted the manuscript: H.H.H.A., D.P.H., V.C., J.L.S., M.E.R., S.T., A.A., P.N, V.G., G.S., M.F., B.F., S.D., S.E.M., M.A.I., P.M.T.
Performed statistical analyses: H.H.H.A., D.P.H., V.C., J.L.S., M.E.R., S.T., A.A., Sy.D., A.H.B., N.J., K.W., Lu.A., N.A., M.A., B.S.A., N.J.A., La.A., A.B., M.B., J.C.B., L.M.E.B., S.H.B., M.M.B., Ja.B., O.C., M.M.C., Ga.C., Q.C., C.R.K.C., G.C., Nh.D., St.E., Ti.G., Su.G., A.L.G., C.U.G., Ol.G., M.E.G., T.G., Jo.H., U.K.H., S.H., E.H., M.H., D.J., T.J., N.K., D.K., S.K., M.K., B.K., P.H.L., J.L., D.C.M.L., L.M.L., M.L., Ch.M., Su.M., An.M., Ma.M., M.M., Be.M., D.R.M., R.M., Y.M., R.L.M., K.N., L.M.O., J.O., Ma.P., I.P., L.P., S.P., B.P., K.B.R., A.R., J.S.R., S.L.R., R.R., Na.R., N.A.R., T.R., C.L.S., Li.S., An.J.S., L.S., J.S., A.V.S., E.S., L.T.S., Al.T., Ro.T., Di.T., R.T., D.T., Dh.V., J.V., S.J.V., D.vdM., M.M.J.V., K.R.V., D.vR., Es.W., L.T.W., A.M.W., G.W., C.W., Th.W., L.R.Y., J.Y., M.P.Z., A.M.D., I.O.F., B.M., T.E.N., J.A.T., B.X., Sa.A., A.M.B., A.dB., A.J.H., A.C.N., P.G.S., C.D.W., S.M.B., R.M.B., G.D., J.G., O.G., R.K., C.M., M.A.N., D.V., B.N.V., T.W., E.J.R.
Acquired data: P.N., Su.S., K.A.A., T.A., M.P.B., Ir.F., R.F.G., D.H., K.A.M., Em.S., B.G.W., A.Z., I.A., N.T.A., L.A., D.A., P.A., O.A.A., S.A., A.A.A., M.E.B., D.M.B., J.T.B., D.A.B., J.B., H.v.a.B., D.I.B., H.B., H.G.B., R.L.B., J.K.B., K.B.B., W.C., V.D.C., D.M.C., G.L.C., C-Y.C., C.C., S.C., M.R.C., A.C., B.C., J.E.C., M.C., G.E.D., E.J.C.D., P.L.D., G.I.D., N.D., Ch.D., A.DeS., A.D., Sr.D., W.C.D., R.D., T.D.D., S.E., T.E., D.A.E., G.F., L.F., S.E.F., D.A.F., I.F., T.M.F., P.T.F., C.F., Ma.F., D.C.G., R.L.G., H.H.H.G., H.J.G., R.C.G., S.G., N.K.H., J.H., C.A.H., R.H., K.H., An.H., S.L.e.H., D.G.H., D.J.H., B.H., P.J.H., W.H., A.H., F.H., G.H., N.H., J-J.H., H.E.H., M.I., M.K.I., C.R.J., R.J., E.G.J., J.J., R.S.K., I.K., D.S.K., P.K., J.B.K., L.J.L., S.M.L., H.L., X.L., D.L.L., W.L., O.L.L., S.L., O.M., J.M., V.S.M., A.M.M., F.J.M., K.L.M., P.M., I.M., A.M., S.M., G.W.M., D.W.M., T.H.M., T.W.M., M.N., W.J.N., M.M.N., L.N., K.O., R.L.O., R.A.O., M.P., T.P., Z.P., B.W.J.P., G.P., S.G.P., B.M.P., S.R., Ma.R., J.L.R., N.R., J.I.R., M.R., R.L.S., P.S.S., A.J.S., He.S., P.R.S., S.S., A.S., S.M.S., C.S., J.W.S., H.S., V.S., V.M.S., D.J.S., J.E.S., A.T., H.T., A.W.T., B.T., J.T., C.T., A.G.U., M.C.V., M.vdB., A.V., N.J.A.V., C.M.V., N.E.M.V., M.V., D.J.V., M.W.V., H.V., H.W., J.M.W., T.H.W., M.E.W., D.R.W., M.W.W., W.W., E.W., T.Y.W., C.B.W., R.H.Z., A.B.Z., I.J.D., C.D., R.S., N.G.M., A.J.M.D., M.J.W., V.G., G.S., M.F., B.F., S.D., S.E.M., M.A.I., P.M.T., A.Sim., Sa.A., A.M.B., A.dB., A.J.H., A.C.N., P.G.S., C.D.W., S.M.B., R.M.B., G.D., J.G., O.G., R.K., C.M., M.A.N., D.V., B.N.V., T.W., E.J.R.
All authors critically reviewed the manuscript for important intellectual content.
REFERENCES
- 1.Bartley AJ, Jones DW, Weinberger DR. Genetic variability of human brain size and cortical gyral patterns. Brain. 1997;120:257–269. doi: 10.1093/brain/120.2.257. [DOI] [PubMed] [Google Scholar]
- 2.Davis PJM, Wright EA. A new method for measuring cranial cavity volume and its application to the assessment of cerebral atrophy at autopsy. Neuropathology and applied neurobiology. 1977;3:341–358. [Google Scholar]
- 3.Sgouros S, Goldin JH, Hockley AD, Wake MJ, Natarajan K. Intracranial volume change in childhood. Journal of neurosurgery. 1999;91:610–616. doi: 10.3171/jns.1999.91.4.0610. [DOI] [PubMed] [Google Scholar]
- 4.Buckner RL, et al. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 5.Farias ST, et al. Maximal brain size remains an important predictor of cognition in old age, independent of current brain pathology. Neurobiology of aging. 2012;33:1758–1768. doi: 10.1016/j.neurobiolaging.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ikram MA, et al. Common variants at 6q22 and 17q21 are associated with intracranial volume. Nat Genet. 2012;44:539–544. doi: 10.1038/ng.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stein JL, et al. Identification of common variants associated with human hippocampal and intracranial volumes. Nat Genet. 2012;44:552–561. doi: 10.1038/ng.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hibar DP, et al. Common genetic variants influence human subcortical brain structures. Nature. 2015 doi: 10.1038/nature14101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paus T, et al. KCTD8 gene and brain growth in adverse intrauterine environment: a genome-wide association study. Cerebral Cortex. 2012;22:2634–2642. doi: 10.1093/cercor/bhr350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Psaty BM, et al. Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) consortium design of prospective meta-analyses of genome-wide association studies from 5 cohorts. Circulation: Cardiovascular Genetics. 2009;2:73–80. doi: 10.1161/CIRCGENETICS.108.829747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson PM, et al. The ENIGMA Consortium: large-scale collaborative analyses of neuroimaging and genetic data. Brain imaging and behavior. 2014;8:153–182. doi: 10.1007/s11682-013-9269-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bulik-Sullivan BK, et al. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet. 2015;47:291–295. doi: 10.1038/ng.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wood AR, et al. Defining the role of common variation in the genomic and biological architecture of adult human height. Nat Genet. 2014;46:1173–1186. doi: 10.1038/ng.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stefansson H, et al. A common inversion under selection in Europeans. Nature genetics. 2005;37:129–137. doi: 10.1038/ng1508. [DOI] [PubMed] [Google Scholar]
- 15.Spillantini MG, Goedert M. Tau pathology and neurodegeneration. The Lancet Neurology. 2013;12:609–622. doi: 10.1016/S1474-4422(13)70090-5. [DOI] [PubMed] [Google Scholar]
- 16.Desikan R, et al. Genetic overlap between Alzheimer’s disease and Parkinson’s disease at the MAPT locus. Molecular psychiatry. 2015 doi: 10.1038/mp.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirchhoff M, Bisgaard AM, Duno M, Hansen FJ, Schwartz M. A 17q21.31 microduplication, reciprocal to the newly described 17q21.31 microdeletion, in a girl with severe psychomotor developmental delay and dysmorphic craniofacial features. European journal of medical genetics. 2007;50:256–263. doi: 10.1016/j.ejmg.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Koolen DA, et al. Mutations in the chromatin modifier gene KANSL1 cause the 17q21 31 microdeletion syndrome. Nature genetics. 2012;44:639–641. doi: 10.1038/ng.2262. [DOI] [PubMed] [Google Scholar]
- 19.Estrada K, et al. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nature genetics. 2012;44:491–501. doi: 10.1038/ng.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kemp JP, et al. Phenotypic dissection of bone mineral density reveals skeletal site specificity and facilitates the identification of novel loci in the genetic regulation of bone mass attainment. PLoS genetics. 2014;10:e1004423. doi: 10.1371/journal.pgen.1004423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Broer L, et al. GWAS of Longevity in CHARGE Consortium Confirms APOE and FOXO3 Candidacy. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2015;70:110–118. doi: 10.1093/gerona/glu166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaplan RC, et al. A genome-wide association study identifies novel loci associated with circulating IGF-I and IGFBP-3. Human molecular genetics. 2011;20:1241–1251. doi: 10.1093/hmg/ddq560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paik J-h, et al. FoxOs cooperatively regulate diverse pathways governing neural stem cell homeostasis. Cell stem cell. 2009;5:540–553. doi: 10.1016/j.stem.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Renault VM, et al. FoxO3 regulates neural stem cell homeostasis. Cell stem cell. 2009;5:527–539. doi: 10.1016/j.stem.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rzehak P, et al. Associations of IGF-1 gene variants and milk protein intake with IGF-I concentrations in infants at age 6months—Results from a randomized clinical trial. Growth hormone & IGF research. 2013;23:149–158. doi: 10.1016/j.ghir.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 26.Taal HR, et al. Common variants at 12q15 and 12q24 are associated with infant head circumference. Nat Genet. 2012;44:532–538. doi: 10.1038/ng.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lynch SA, et al. The 12q14 microdeletion syndrome: six new cases confirming the role of HMGA2 in growth. European Journal of Human Genetics. 2011;19:534–539. doi: 10.1038/ejhg.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sweet T, Yen W, Khalili K, Amini S. Evidence for involvement of NFBP in processing of ribosomal RNA. Journal of cellular physiology. 2008;214:381–388. doi: 10.1002/jcp.21204. [DOI] [PubMed] [Google Scholar]
- 29.Verhaaren BF, et al. Multi-Ethnic Genome-Wide Association Study of Cerebral White Matter Hyperintensities on MRI. Circulation: Cardiovascular Genetics. 2015 doi: 10.1161/CIRCGENETICS.114.000858. CIRCGENETICS. 114.000858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balestrini A, Cosentino C, Errico A, Garner E, Costanzo V. GEMC1 is a TopBP1-interacting protein required for chromosomal DNA replication. Nature cell biology. 2010;12:484–491. doi: 10.1038/ncb2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan Y, et al. Genome-wide Analysis of Body Proportion Classifies Height-Associated Variants by Mechanism of Action and Implicates Genes Important for Skeletal Development. The American Journal of Human Genetics. 2015 doi: 10.1016/j.ajhg.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fukumoto A, et al. Head circumference and body growth in autism spectrum disorders. Brain and Development. 2011;33:569–575. doi: 10.1016/j.braindev.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. The Lancet Neurology. 2012;11:1006–1012. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quesada A, Lee BY, Micevych PE. PI3 kinase/Akt activation mediates estrogen and IGF-1 nigral DA neuronal neuroprotection against a unilateral rat model of Parkinson’s disease. Developmental neurobiology. 2008;68:632–644. doi: 10.1002/dneu.20609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hevner RFin. Seminars in perinatology. Elsevier; pp. 36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rivière J-B, et al. De novo germline and postzygotic mutations in AKT3, PIK3R2 and PIK3CA cause a spectrum of related megalencephaly syndromes. Nature genetics. 2012;44:934–940. doi: 10.1038/ng.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boland E, et al. Mapping of deletion and translocation breakpoints in 1q44 implicates the serine/threonine kinase AKT3 in postnatal microcephaly and agenesis of the corpus callosum. The American Journal of Human Genetics. 2007;81:292–303. doi: 10.1086/519999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang D, Zeesman S, Tarnopolsky MA, Nowaczyk MJ. Duplication of AKT3 as a cause of macrocephaly in duplication 1q43q44. American Journal of Medical Genetics Part A. 2013;161:2016–2019. doi: 10.1002/ajmg.a.35999. [DOI] [PubMed] [Google Scholar]
- 39.Pawlikowska L, et al. Association of common genetic variation in the insulin/IGF1 signaling pathway with human longevity. Aging cell. 2009;8:460–472. doi: 10.1111/j.1474-9726.2009.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
METHODS-ONLY REFERENCES
- 40.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26:2190–2191. doi: 10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chauhan G, et al. Association of Alzheimer disease GWAS loci with MRI-markers of brain aging. Neurobiology of aging. 2015 doi: 10.1016/j.neurobiolaging.2014.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Finucane HK, et al. Partitioning heritability by functional category using GWAS summary statistics. 2015 doi: 10.1038/ng.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roadmap Epigenomics C, et al. Integrative analysis of 111 reference human epigenomes. Nature. 2015;518:317–330. doi: 10.1038/nature14248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Horikoshi M, et al. New loci associated with birth weight identify genetic links between intrauterine growth and adult height and metabolism. Nature genetics. 2013;45:76–82. doi: 10.1038/ng.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van der Valk RJP, et al. A novel common variant in DCST2 is associated with length in early life and height in adulthood. Human molecular genetics. 2015;24:1155–1168. doi: 10.1093/hmg/ddu510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benyamin B, et al. Childhood intelligence is heritable, highly polygenic and associated with FNBP1L. Molecular Psychiatry. 2014;19:253–258. doi: 10.1038/mp.2012.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davies G, et al. Genetic contributions to variation in general cognitive function: a meta-analysis of genome-wide association studies in the CHARGE consortium (N = 53 949) Molecular Psychiatry. 2015 doi: 10.1038/mp.2014.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lambert J-C, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nature genetics. 2013;45:1452–1458. doi: 10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nalls MA, et al. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nature genetics. 2014 doi: 10.1038/ng.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Verhaaren BFJ, et al. Multi-ethnic genome-wide association study of cerebral white matter hyperintensities on MRI. Circulation: Cardiovascular Genetics. 2015 doi: 10.1161/CIRCGENETICS.114.000858. CIRCGENETICS. 114.000858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cross-Disorder Group of the Psychiatric Genomics and C. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. The Lancet. 2013;381:1371–1379. doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Moor MHM, et al. Genome-wide association study identifies novel locus for neuroticism and shows polygenic association with Major Depressive Disorder. JAMA psychiatry. 2015;72:642. doi: 10.1001/jamapsychiatry.2015.0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Den Berg SM, et al. Meta-analysis of Genome-Wide Association Studies for Extraversion: Findings from the Genetics of Personality Consortium. Behavior genetics. 2016;46:170–182. doi: 10.1007/s10519-015-9735-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li MX, Gui HS, Kwan JS, Sham PC. GATES: a rapid and powerful gene-based association test using extended Simes procedure. American journal of human genetics. 2011;88:283–293. doi: 10.1016/j.ajhg.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Segrè AV, et al. Common inherited variation in mitochondrial genes is not enriched for associations with type 2 diabetes or related glycemic traits. PLoS genetics. 2010;6:e1001058. doi: 10.1371/journal.pgen.1001058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li MX, Kwan JS, Sham PC. HYST: a hybrid set-based test for genome-wide association studies, with application to protein-protein interaction-based association analysis. American journal of human genetics. 2012;91:478–488. doi: 10.1016/j.ajhg.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kundaje A, et al. Integrative analysis of 111 reference human epigenomes. Nature. 2015;518:317–330. doi: 10.1038/nature14248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.