Abstract
Response to DNA damage and cell-cycle regulation differ markedly between embryonic stem (ES) cells and somatic cells. ES cells require exquisitely sensitive mechanisms to maintain genomic integrity and do so, in part, by suppressing spontaneous mutation. Spontaneous mutation frequency in somatic cells is ≈10-4 compared with 10-6 for ES cells. ES cells also lack a G1 checkpoint and are hypersensitive to IR and other DNA-damaging agents. These characteristics facilitate apoptosis and the removal of cells with a mutational burden from the population, thereby keeping the population free of damaged cells. Here, we identify signaling pathways that are compromised and lead to a natural absence of aG1 arrest in ES cells after DNA damage. The affected pathways are those mediated by p53 and p21 and by ATM, Chk2, Cdc25A, and Cdk2. In ES cells, Chk2 kinase is not intranuclear as in somatic cells but is sequestered at centrosomes and is unavailable to phosphorylate Cdc25A phosphatase and cause its degradation. Although ectopic expression of Chk2 does not rescue the p53/p21 pathway, its expression is sufficient to allow it to phosphorylate Cdc25A, activate downstream targets, restore a G1 arrest, and protect the cell from apoptosis.
Somatic cells have genomic requirements that are very different from those of germ cells or embryonic stem (ES) cells. Somatic cells have restricted patterns of gene expression characteristic of their specific differentiated lineages. ES cells retain the potential to produce any cell type in the body. The consequences of mutation in a somatic cell are limited to that particular cell lineage and may result in somatic diseases, e.g., cancer, but will not be passed on to the progeny. In contrast, mutation in ES cells potentially can compromise multiple cell lineages and affect the well-being of subsequent generations. Thus, ES cells have mechanisms, beyond those used by somatic cells, that protect the integrity of their genomes. One mechanism involves suppression of mutation and mitotic recombination. The spontaneous mutation frequency at the endogenous mouse adenine phophoribosyltransferase (Aprt) reporter gene in somatic cells in vivo is very high, approaching 10-4 (1, 2). In contrast, the mutation frequency in mouse ES cells is suppressed by about two orders of magnitude (3). A complementary mechanism to maintain genomic integrity is facilitated cell death that rids cells with a mutational burden from the population. Consistent with this proposition, mouse ES cells lack a G1 checkpoint (4, 5) and are hypersensitive to DNA damage (6, 7).
Two known pathways, both of which involve inhibition of Cdk2 activity, govern the G1/S checkpoint in somatic cells (8, 9). A rapid but transient response functions through Cdc25A degradation, whereas a p53/p21-mediated pathway supports a sustained but delayed response (10). In ES cells, p53 protein appears not to be fully functional because it is predominantly cytoplasmic and inefficiently translocated to the nucleus after DNA damage (4). Also, the Cdk inhibitors p21 and p27 are undetectable (11). In somatic cells, after DNA damage by ionizing radiation (IR), the ATM-Chk2-Cdc25A checkpoint pathway is initiated by autophosphorylation and activation of the phosphatidylinositol 3-kinase, ATM (12, 13). Activated ATM, in turn, phosphorylates Chk2, a serine/threonine kinase that phosphorylates several effector proteins such as BRCA1 and members of the Cdc25 family of phosphatases (14-16). In unperturbed cycling somatic cells, the Cdc25A phosphatase facilitates the transition from G1 to S phase by removing the inhibitory phosphates on the Cdk2/cyclin E complex (17, 18). After ionizing irradiation, the activated Chk2 phosphorylates Cdc25A on serine 123, causing its ubiquitin-mediated degradation (19). Thus, in the absence of functional Cdc25A, Cdk2 is not dephosphorylated and the cells arrest in G1. A second, but slower, response involves activation of p53 and induction of the Cdk inhibitor, p21.
This report establishes that the absence of a G1 checkpoint after IR exposure in ES cells is due to an altered intracellular localization of Chk2 kinase. In somatic cells, Chk2 is predominantly diffuse within the nucleus. Here we show that in ES cells, Chk2 localizes to centrosomes and is unavailable to phosphorylate some of its substrates. Furthermore, the data demonstrate that a G1 arrest can be restored by ectopic expression of Chk2 and that the restoration of a G1 arrest protects cells from apoptosis. We argue that natural absence of a G1 checkpoint in ES cells is beneficial to survival of the ES cell population and the species by facilitating removal of ES cells with damaged DNA and thus maintaining a pristine cell population. Conversely, acquisition of a G1 checkpoint by ES cells and protection from apoptosis would be disadvantageous.
Materials and Methods
Cell Culture. Mouse embryonic fibroblasts (MEFs) derived from 13-day-old embryos were cultured in DMEM with high glucose and 10% FBS. J11 ES cells were maintained on mitomycin C-treated MEF feeder cells in high-glucose DMEM supplemented with 15% ES-quality FBS/1× nonessential amino acids/1× GlutaMAX-I/100 units/ml penicillin-streptomycin/0.1 μM 2-mercaptoethanol/50 μM recombinant leukemia inhibitory factor in 10% CO2 at 37°C. To eliminate feeder cells, ES cells were seeded at 50% confluence in 10-cm tissue culture plates coated with 0.1% gelatin and in medium supplemented with 50 μM leukemia inhibitory factor.
Plasmid Construction, Transfection, Immunoprecipitation, and Western Blotting. The mouse GFP-Chk2 vector was constructed by PCR amplification of Chk2 from a Chk2/pBS plasmid (provided by David Johnson, M. D. Anderson Cancer Center, Houston) with subsequent cloning into the EcoRI and BamHI sites of pEGFP (Clontech). The Chk2 short interfering RNA (siRNA) vector was constructed by inserting the oligonucleotide sequence GCTGAAGCAACTGAAAGCC, complementary to the Chk2 mRNA sequence from nucleotides 169-187, into plasmid pSilencer 3.1-H1 hygro (Ambion, Austin, TX). Both constructs were confirmed by DNA sequencing. ES cells were trypsinized and transfected with the GFP-Chk2 fusion construct, a control GFP-histone H2b construct, or the Chk2-siRNA vector by using FuGENE 6 (Roche Diagnostics). After 24 h, cells were irradiated with 10-Gy x-rays (Faxitron X-ray, Wheeling, IL) and harvested at successive half-hour intervals. Cells were lysed in ice-cold RIPA buffer (20 mM Tris·HCl, pH 8.0/150 mM NaCl/1% Nonidet P-40/1% SDS/0.5% deoxycholic acid) supplemented with a protease inhibitor mixture (Sigma). SDS/PAGE was performed by using 40 μg of total protein from cell extracts that were fractionated on 8% polyacrylamide and probed with a Chk2 mAb (A-12, Santa Cruz Biotechnology), a Cdc25A mAb (F-6, Santa Cruz Biotechnology), a phosphospecific Cdc25A serine 123 Ab (from Helen Piwnica-Worms, Washington University, St. Louis), a p53 mAb (Ab-1, Oncogene), a phosphospecific polyclonal Ab directed at phosphorylated p53-Ser-23 (Cell Signaling Technology, Beverly, MA; listed as p53-Ser-20) and phosphorylated p53-Ser-18 (Cell Signaling Technology; listed as p53-Ser-15), or a p21 polyclonal Ab (C-19, Santa Cruz Biotechnology). The phosphorylation status of Cdk2 was determined by coimmunoprecipitation with anti-Cyclin E mAb (HE111, Santa Cruz Biotechnology). Cell lysates were incubated for 1 h with anti-Cyclin E Ab, followed by the addition of 20 μl of protein A/G plus agarose beads (Santa Cruz Biotechnology) and incubation for another 2 h. Beads were washed three times with lysis buffer (pH 8.0) and resuspended in 1× SDS/PAGE loading buffer (pH 7.2). The immunocomplexes were fractionated by 8% SDS/PAGE and probed with Ab to Cdk2 to show total Cdk2 present and with Cdk2 tyrosine 15 phosphospecific Ab (Cell Signaling Technology).
Immunofluorescence and Confocal Microscopy. Colonies of ES cells were grown overnight on gelatinized coverslips. Cells were fixed with Formalde-Fresh (Fisher) [4% formaldehyde (wt/vol)/1% methanol (wt/vol)], permeabilized in PBS containing 1% Nonidet P-40, and blocked with 10% rabbit serum for 1 h. The cells were coimmunostained with mAb to Chk2 (1:200 dilution) and γ-tubulin (1:700 dilution) or with Ab directed at p53-Ser-23 (1:200), washed with PBS, stained with secondary Ab (1:700 dilution), and coupled with Alexa Fluor 594 for Chk2 and Alexa Fluor 488 for γ-tubulin and p53-Ser-23 (Molecular Probes). Images were visualized on a Zeiss 510 confocal microscope with a ×60 oil objective lens and merged to highlight colocalization.
Cell-Cycle Analysis and Measure of Apoptosis. Wild-type J11 ES cells or J11 cells transfected with Chk2 cDNA were irradiated with 10 Gy of IR or left untreated. For comparison, MEFs were treated in the same manner. Cells were trypsinized 8 h after irradiation, resuspended in 1 ml of PBS, fixed in 4 ml of cold 100% ethanol, pelleted, and stained in 1 mg/ml propidium iodide containing 0.1 mg/ml RNase A. Stained cells were subjected to flow cytometry (Epics XL and system ii software, Version 3.0, Coulter) to determine cell distribution within the cell cycle. To assess the extent of apoptosis after irradiation, J11 ES cells were transfected with a Chk2 cDNA or mock-transfected with a control plasmid and treated with 10 Gy of IR or left untreated. Cells were harvested and resuspended in 1× binding buffer (10 mM Hepes, pH 7.4/140 mM NaCl/2.5 mM CaCl2), and cells were stained with 5 μl of annexin V-Cy5 (Pharmingen) and 2.5 μg/ml propidium iodide. The cell suspension was incubated for 15 min at room temperature and analyzed by flow cytometry.
Results
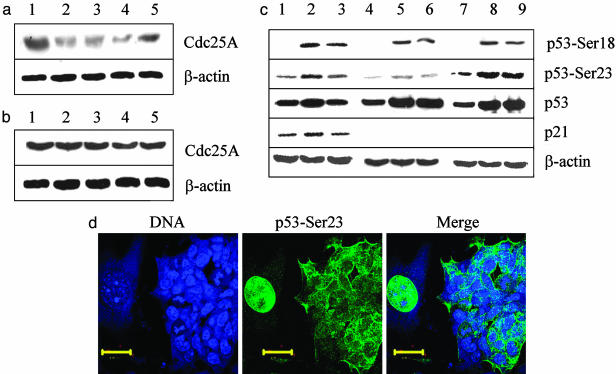
Cdc25A Phosphatase Is Degraded in Irradiated MEFs but Not in ES Cells. When MEFs are exposed to IR they manifest a transient degradation of Cdc25A (Fig. 1a), concomitant with a G1 arrest. To examine the behavior of Cdc25A in ES cells after irradiation, cells were subjected to 10 Gy of x-irradiation, and the level of Cdc25A protein was analyzed by Western blot at 30, 60, 90, and 120 min after treatment. In contrast to MEFs, the levels of Cdc25A remained unchanged in ES cells in response to challenge (Fig. 1b), and there was no G1 arrest (4, 5). The alternate pathway leading to a G1 arrest, involving p53 and p21, also was compromised in ES cells. In MEFs, phosphorylation of p53 on serine 23 (serine 20 in humans), a Chk2 target, was elevated after irradiation but was far less intense in ES cells than in MEFs (Fig. 1c, lanes 4-6 and 1-3, respectively), even though p53 protein was equally abundant. Phosphorylation of serine 18 (serine 15 in humans), an ATM target (20, 21), was induced equally in ES cells and MEFs and appeared to be of similar intensity (Fig. 1c). The p21 Cdk inhibitor was clearly evident in MEFs but undetectable in ES cells with or without irradiation (Fig. 1c). Phosphorylation of p53 on serine 23 coincided with its intranuclear localization in irradiated MEFs, whereas it remained cytoplasmic in irradiated Chk2-transfected ES cells on the same plate (Fig. 1d). The p53 protein was also cytoplasmic in untransfected, untreated ES cells (data not shown). Thus, neither overexpression of Chk2 nor phosphorylation of p53-Ser-23 is sufficient to restore the capacity of p53 to be translocated to the nucleus in ES cells, a finding that is consistent with the inability of these cells to induce p21 after IR.
Fig. 1.
The pathways mediated by Cdc25A and p53 that lead to a G1 arrest after exposure to IR are compromised in ES cells. (a) MEFs were irradiated with 10 Gy of x-ray, lysed with RIPA buffer, and immunoblotted with Cdc25A mAb at increasing times after IR treatment. Lane 1, untreated sample; lane 2, 30 min after irradiation; lane 3, 60 min after irradiation; lane 4, 90 min after irradiation; lane 5, 120 min after irradiation. (b) J11 ES cells were irradiated with 10 Gy, and cell extracts were subjected to Western blots with the Cdc25A mAb. Lane 1, untreated; lane 2, 30 min after irradiation; lane 3, 60 min after irradiation; lane 4, 90 min after irradiation; lane 5, 120 min after irradiation. (c) MEFs (lanes 1-3), ES cells (lanes 4-6), and ES cells transfected with GFP-Chk2 (lanes 7-9) were subjected to 10 Gy of x-ray and analyzed by Western blot with a phosphospecific Ab to p53-Ser-18, a phosphospecific Ab to p53-Ser-23, a mAb to p53, a polyclonal Ab to p21, and a polyclonal Ab to β-actin as a control. Lanes 1, 4, and 7, blots from cells that were left untreated; lanes 2, 5, and 8, blots from cells collected 2 h after irradiation; lanes 3, 6, and 9, blots from cells collected 4 h after irradiation. (d) Colonies of ES cells and MEFs were cocultured on gelatinized coverslips and transfected with GFP-Chk2. The cells were irradiated with 10 Gy, fixed after 2 h, and stained with DRAQ5 to stain nuclei (Left) and phosphospecific Ab to p53-Ser-23 (Center). (Right) A merged image of Left and Center. (Scale bars, 10 μm.)
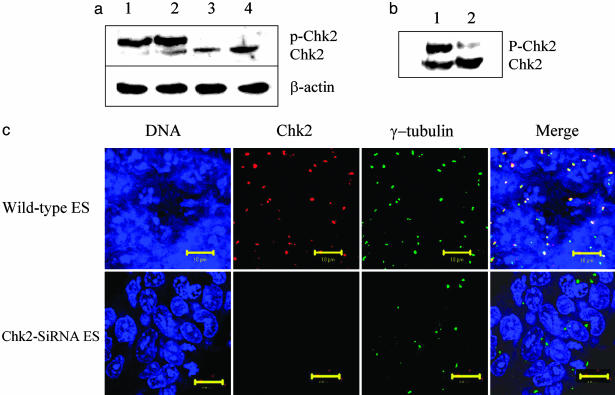
Chk2 in ES Cells Is Hyperphosphorylated and Colocalizes with γ-Tubulin in Centrosomes. Because Cdc25A is a substrate for Chk2, we examined the status of Chk2 in ES cells. When MEFs are lysed with buffer containing Nonidet P-40, Chk2 is predominantly in the soluble fraction (data not shown). When ES cells are lysed with the same buffer, Chk2 is not solubilized but remains with the insoluble fraction. Following with buffer containing SDS in addition to Nonidet P-40, Chk2 is found in the soluble fraction but migrates more slowly on SDS/PAGE than Chk2 derived from MEFs (Fig. 2a). The more slowly migrating form is due, in part, to phosphorylation because the upper band is lost after phosphatase treatment (Fig. 2b). Immunocytochemistry also shows that Chk2 in ES cells colocalizes with γ-tubulin in centrosomes (Fig. 2c), and it cofractionates with γ-tubulin (see Figs. 6-13, which are published as supporting information on the PNAS web site). Ab specificity to Chk2 is apparent from loss of immunofluorescence when ES cells are transfected with Chk2 siRNA (Fig. 2c) and from Western blots (Fig. 9). In aggregate, the data indicate that Chk2 is hyperphosphorylated in ES cells and that it is tethered in such a way that it may not be available to phosphorylate its Cdc25A substrate to effect a G1 arrest.
Fig. 2.
Stem cell Chk2 is hyperphosphorylated and colocalizes with γ-tubulin in centrosomes. (a) Lanes 1 and 3, untreated ES cells and MEFs were lysed with RIPA buffer containing 1% SDS and subjected to Western blot. Lanes 2 and 4, ES cells and MEFs were irradiated with 10 Gy and after 2 h were subjected to Western blot by using a mAb (A-12, Santa Cruz Biotechnology) directed at Chk2. Ab to β-actin was used as a control. (b) Aliquots of cell lysate from ES cells were subjected to Western blot analysis with (lane 2) or without (lane 1) treatment with calf intestinal alkaline phosphatase before electrophoretic fractionation. (c) Colonies of ES cells were grown overnight on gelatinized coverslips. Untreated cells (Upper) and cells transfected with Chk2-siRNA (Lower) were fixed with Formalde-Fresh and coimmunostained with Ab to detect endogenous Chk2 and γ-tubulin. In the leftmost panels, the ES cell nuclei were stained with DRAQ5. In the second panels from the left, cells were reacted with mAb to Chk2. In the third panels from the left, cells were reacted with Ab to γ-tubulin. In the rightmost panels, the images were merged to highlight colocalization. (Scale bars, 10 μm.)
IR Treatment of ES Cells Transfected with Chk2 Induces Phosphorylation of p53-Ser-23 but Does Not Induce p21. The amount of p53 protein appears comparable between MEFs and ES cells, and the level of p53 increases after irradiation, with or without ectopic Chk2 (Fig. 1c). When MEFs are irradiated, serine 23 of p53 becomes phosphorylated (Fig. 1c), presumably mediated by Chk2 (22). The basal level of phosphorylated p53-Ser-23 is significantly lower in ES cells than in MEFs. It is elevated in ES cells expressing ectopic Chk2 and further elevated after irradiation. Significantly, the increase in phosphorylated p53-Ser-23 and -Ser-18 does not translate into detectable p21, even after irradiation (Fig. 1c), consistent with an absent G1 arrest. The inability of IR to induce p21 in ES cells is likely due to an inability of p53 to translocate to the nucleus after challenge in these cells. In MEFs, virtually all of the p53 protein is intranuclear, whereas in ES cells it is cytoplasmic after DNA damage due to IR (Fig. 1d), consistent with absent p21 induction and the lack of a G1 checkpoint.
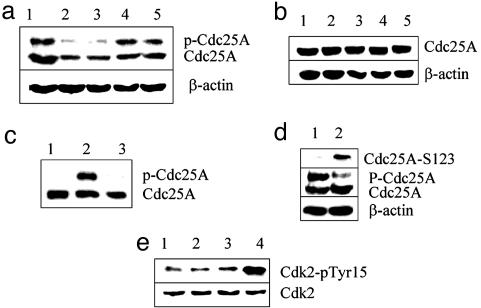
IR Treatment of ES Cells Transfected with Chk2 Induces Cdc25A Instability and the Accumulation of Phosphorylated Cdk2. Because endogenous Chk2 in ES cells is sequestered at centrosomes and does not phosphorylate Cdc25A after IR exposure, we asked whether ectopically expressed Chk2 is functional. ES cells were transfected with GFP-Chk2 or with GFP-histone H2b as a control. Unlike endogenous Chk2, the overexpressed GFP-Chk2 appeared diffuse within the nuclei (Fig. 12). Cdc25A became phosphorylated only after Chk2 transfection (Fig. 3a, lane1), and phosphorylation was confirmed by phosphatase treatment (Fig. 3c). Furthermore, irradiated ES cells transfected with GFPChk2, but not with control GFP-histone H2b plasmid, displayed transiently degraded Cdc25A (Fig. 3a) similar to that seen in MEFs (Fig. 1a). Transfection with Chk2 and consequent phosphorylation of Cdc25A alone, however, was not sufficient to cause Cdc25A degradation (Fig. 3a, lane 1) but also required irradiation. Because phosphorylation of serine 123 on Cdc25A is critical to its degradation after DNA damage (19), the status of serine 123 phosphorylation was assessed with a phosphospecific Ab. As shown in Fig. 3d, serine 123 became phosphorylated only after irradiation, and only after irradiation was there a transient degradation of Cdc25A.
Fig. 3.
Ectopic expression of Chk2 induces Cdc25A instability and accumulation of Cdk2 phosphorylated on tyrosine 15 after IR treatment. ES cells were trypsinized and transfected with a GFP-Chk2 fusion construct (a) or a control GFP-H2b construct (b) by using FuGENE 6 to effect the transfection. After 24 h, cells were irradiated with 10 Gy and harvested at half-hour intervals, and cell lysates were fractionated by SDS/PAGE and probed with a mAb to Cdc25A. In both a and b, the lane 1 sample was unirradiated, and samples in lanes 2, 3, 4, and 5 were obtained 30, 60, 90, and 120 min after irradiation, respectively. (c) Cell lysates from ES cells were fractionated by SDS/PAGE and probed by Western blot with mAb to Cdc25A. Lane 1, nontransfected J11 ES cells; lane 2, J11 ES cells transfected with a GFP-Chk2 cDNA construct; lane 3, J11 ES cells transfected with a GFP-Chk2 cDNA construct in which the cell lysate was treated with calf intestine alkaline phosphatase. (d) The phosphorylation status of Cdc25A serine 123 was assessed with a phosphospecific Ab after transfection with Chk2 minus irradiation (lane 1) and 1 h after irradiation (lane 2). The same blots were probed with mAb to Cdc25A (Cdc25A) and with Ab to β-actin as a loading control (β-actin). (e) The phosphorylation status of Cdk2 was determined by immunoprecipitation of Cyclin E with Ab from untreated (lanes 1 and 3) and IR-treated (lanes 2 and 4) ES cells. The extracts in lanes 3 and 4 are from ES cells transfected with Chk2. Cell lysates were fractionated by SDS/PAGE and probed with Ab to Cdk2 to show total Cdk2 present and with mAb directed at phosphorylated tyrosine 15 of Cdk2.
These data predict that with diminished Cdc25A activity, there should be an increase in phosphorylated Cdk2. To test this proposition, cyclin E was immunoprecipitated from cell extracts, and the immunoprecipitated complex was probed with a phosphospecific Ab to phosphotyrosine 15 of Cdk2. The level of phosphoprotein was elevated severalfold after IR treatment of ES cells transfected with Chk2 but not of untransfected ES cells (Fig. 3e), indicating that Cdc25A phosphatase retains its capacity to be inactivated by Chk2 in ES cells. This inactivation, however, is not manifest unless Chk2 kinase activity is available to phosphorylate Cdc25A and induce its turnover.
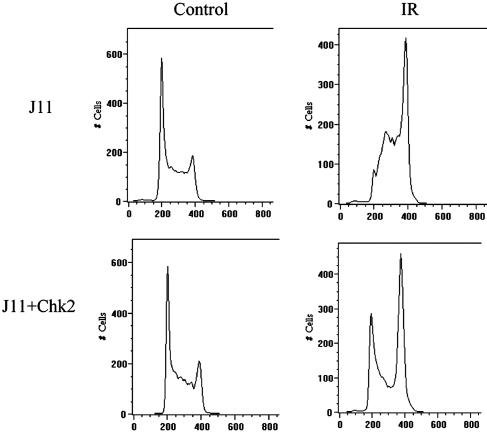
Ectopic Expression of Chk2 in ES Cells Is Sufficient to Establish a G1 Arrest. Reduction of Cdc25A should result in increased phosphorylation of the Cdk2/cyclin E complex and in an accumulation of cells in G1. This possibility was examined by flow cytometry of wild-type ES cells and ES cells transfected with GFP-Chk2 after IR. As previously reported (4), ≈65% of untreated ES cells are in the S phase. A similar cell-cycle distribution was seen in untreated ES cells transfected with Chk2 (Fig. 4). The distribution of cells in the cell cycle changed dramatically after irradiation. In wild-type ES cells, there was no apparent G1 checkpoint. A large number of cells were arrested in G2, consistent with an active G2 checkpoint (5), and a majority of cells were in S phase, consistent with an S-phase arrest. In contrast, ES cells transfected with Chk2 and subjected to IR accumulated in G1 as well as in G2 and showed far fewer cells in S phase (Fig. 4). Thus, ectopic expression of Chk2 is sufficient to reconstitute a G1 arrest by means of phosphorylation of Cdc25A in ES cells after DNA damage by IR. Furthermore, there was no evidence of an S-phase arrest, suggesting that the absence of available functional Chk2 in ES cells facilitates an accumulation of cells stalled in S and that expression of ectopic Chk2 clears these cells from the S phase.
Fig. 4.
Ectopic expression of Chk2 restores the G1/S checkpoint in stem cell. Wild-type J11 ES cells or J11 ES cells transfected with Chk2 were subjected to 10 Gy of IR (Right) or left untreated (Left). Eight hours after irradiation, cells were trypsinized, resuspended in PBS, and stained with 1 mg/ml propidium iodide containing 0.1 mg/ml RNase A. Stained cells were subjected to flow cytometry (system ii software, Version 3.0) to cell-cycle distribution of cells.
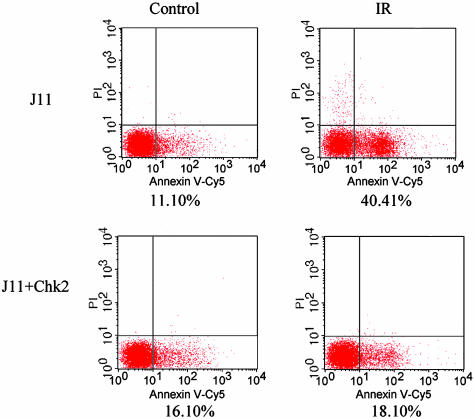
Restoration of a G1 Arrest Protects ES Cells from Apoptosis. The G1 checkpoint provides somatic cells time to repair damaged DNA and to prevent cells with damaged DNA from entering S phase. One consequence of a checkpoint arrest is that cells with repaired DNA should be less subject to apoptosis. Thus, restoration of a G1 checkpoint in ES cells predicts that these cells would be protected from cell death. This proposition was tested by staining wild-type ES cells and cells transfected with Chk2 cDNA for annexin V as a marker of apoptosis before and after irradiation. The cells also were stained with propidium iodide before fixation to identify dead cells. As shown in Fig. 5, 40% of wild-type ES cells were annexin V-positive 16 h after exposure to IR, whereas only 18% of ES cells transfected with GFP-Chk2 stained for annexin V. These data are in marked contrast with those obtained with thymocytes and neurons in the developing brain of Chk2-/- mice, which are more radioresistant than their Chk2 wild-type counterparts (23). These differences in cellular response to irradiation are intriguing and likely represent a further manifestation of the unique regulatory controls that distinguish somatic and ES cells.
Fig. 5.
Restoration of a G1 checkpoint by ectopic Chk2 expression protects ES cells from apoptosis. J11 ES cells transfected with Chk2 or mock-transfected ES cells were treated with 10 Gy of IR (Right) or left untreated (Left). Sixteen hours after treatment, both cell populations were harvested, stained with annexin V-Cy5 and 2.5 μg/ml propidium iodide, and analyzed by flow cytometry. Cells above the horizontal line are positive for propidium iodide staining and represent dead cells. Those displaced to the right of the vertical line are positive for annexin V-Cy5 and represent early apoptotic cells.
Discussion
The differences in mutation frequency and DNA damage response between somatic and ES cells are striking. We and others (1, 2, 7) have reported previously that mutation frequency in vivo at a locus heterozygous for Aprt is as high as 10-4. Up to 80% of the mutational events involved loss of heterozygosity mediated by recombination between chromosome homologs. In ES cells, mutation frequency and mitotic recombination are both ≈100-fold lower (3). If one assumes that mutation frequency in somatic cells is similar for all autosomes, that loss of heterozygosity affects multiple heterozygous loci, and that there are at least 10 heterozygous loci between the crossover point and the reporter gene, the overall somatic mutation frequency will approach 1 in 50. Such a high mutant frequency is clearly detrimental to the individual because it promotes a high risk of somatic disease. A high somatic mutation frequency, however, is advantageous to the species. Usually, multiple mutations are necessary for the development of somatic disease, and a latency period of 20 years is not uncommon. By the time somatic disease manifests, the individual should have contributed to the gene pool and become irrelevant to further propagation of the species (24). In contrast, a high mutation frequency in germ cells, and by extrapolation in ES cells, would be detrimental, not only to the individual but also to the species because all mutations would be passed on to progeny and affect all cells of the body. Consequently, such cells exploit multiple mechanisms to maintain exquisite genomic integrity. In 1975, Cairns (25) argued that during DNA replication, it was the daughter strand that incurred replicative errors, whereas the template parent strand did not. He postulated that when stem cells divide asymmetrically to yield a self-renewing stem cell and a daughter cell committed to differentiation, the DNA strands segregate in such a way that the stem cell daughter receives both parental template strands, whereas the cell committed to differentiation receives the two daughter strands (25). In this way, the self-renewing daughter stem cell would receive DNA with no replicative errors. Recently, two laboratories have provided independent evidence in support of this hypothesis (26, 27).
A second mechanism for maintaining genomic integrity would be to suppress mutation and mitotic recombination. Indeed, ES cells have a spontaneous mutation frequency that is ≈100-fold below that of isogenic adult somatic cells and MEFs (3). Both point mutation and recombination between chromosome homologs are suppressed, allowing loss of heterozygosity and uniparental disomy, as a consequence of nondisjunction, to emerge as the principal form of mutation. A mechanism complementary to suppression of mutation is the facilitation of apoptosis, which would eliminate cells that have accumulated mutations from the population, leaving the remaining cells with genomes that have been minimally affected. Here, we show that the last of these strategies is also operative in mouse ES cells and that these cells have elaborate mechanisms that compromise the G1 checkpoint and facilitate cell death. In mouse ES cells, the two signaling pathways that lead to a G1 arrest after DNA damage are not fully functional. The rapid response pathway involving p53 and p21 is defective in allowing translocation of p53 to the nucleus after exposure to IR, thereby interfering with the induction of the Cdk2 inhibitor p21, even in the presence of ectopic Chk2. The more rapid response pathway involving ATM, Chk2, Cdc25A, and Cdk2 appears to be compromised because of the lack of available functional Chk2, which is sequestered at centrosomes and unavailable to phosphorylate Cdc25A phosphatase. The association of Chk2 with centrosomes, although previously unreported for ES cells, has been described in early Drosophila embryos after DNA damage (28) and during early mitosis of cultured mammalian cells (29). It is unlikely that any single mechanism would be sufficient to effectively protect the ES cell genome. In combination, however, these cellular strategies should provide a cell population that is genetically pristine and that is able to give rise to all of the cells of an organism, including germ cells that harbor a minimum of new mutations.
Supplementary Material
Acknowledgments
We thank J. Tischfield, J. Groden, Y. Sanchez, T. Doetschman, and E. Knudsen for valuable comments; David Johnson for providing us with the murine Chk2 cDNA; Helen Piwnica-Worms for providing the Ab to Cdc25A serine 123; and Sandra Schwemberger and George Babcock for the excellent technical help with flow cytometry. This work was supported by National Institutes of Health Grants R01-CA90934 and U01-ES11038 and Center for Environmental Genetics Grant P30-ES06096.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: ES, embryonic stem; IR, ionizing radiation; MEF, mouse embryonic fibroblast.
References
- 1.Stambrook, P. J., Shao, C., Stockelman, M., Boivin, G., Engle, S. J. & Tischfield, J. A. (1996) Environ. Mol. Mutagen. 28, 471-482. [DOI] [PubMed] [Google Scholar]
- 2.Shao, C., Deng, L., Hehegariu, O., Liang, L., Stambrook, P. J. & Tischfield, J. A. (2000) Proc. Natl. Acad. Sci. USA 97, 7405-7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cervantes, R. B., Stringer, J. R., Shao, C., Tischfield J. A. & Stambrook, P. J. (2002) Proc. Natl. Acad. Sci. USA 99, 3586-3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aladjem, M., Spike, B. T., Rodewald, L. W., Hope, T. J., Klemm, M., Jaenisch, R. & Wahl, G. M. M. (1998) Curr. Biol. 8, 145-155. [DOI] [PubMed] [Google Scholar]
- 5.Hirao, A., Kong, Y.-Y., Matsuoka, S., Wakeham, A., Ruland, J., Yoshida, H., Liu, D., Elledge, S. J. & Mak, T. W. (2000) Science 289, 1824-1827. [DOI] [PubMed] [Google Scholar]
- 6.de Waard, H., de Wit, J., Gorgels, T. G., van den Aardweg, G., Andressoo, J. O., Vermeij, M., van Steeg, H., Hoeijimakers, J. H. & van der Horst, G. T. (2003) DNA Repair 2, 13-25. [DOI] [PubMed] [Google Scholar]
- 7.Van Sloun, P. P., Jansen, J. G., Weeda, G., Mullenders, L. H., van Zeeland, A. A., Lohman, P. H. & Vrieling, H. (1999) Nucleic Acids Res. 27, 3276-3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elledge, S. J. (1996) Science 274, 1664-1672. [DOI] [PubMed] [Google Scholar]
- 9.Weinert, T. (1998) Cell 94, 555-558. [DOI] [PubMed] [Google Scholar]
- 10.Bartek, J. & Lukas, J. (2001) FEBS Lett. 490, 117-122. [DOI] [PubMed] [Google Scholar]
- 11.Stead, E., White, J., Fast, R., Conn, S., Goldstone, S., Rathjen, J., Dhinggra, U., Rathjen, P., Walker, D. & Dalton, S. (2002) Oncogene 21, 8320-8333. [DOI] [PubMed] [Google Scholar]
- 12.Kastan, M. B. & Lim, D. S. (2000) Nat. Rev. Mol. Cell Biol. 1, 179-186. [DOI] [PubMed] [Google Scholar]
- 13.Bakkenist, C. J. & Kastan, M. B. (2003) Nature 421, 499-506. [DOI] [PubMed] [Google Scholar]
- 14.Bartek, J., Falck, J. & Lukas, J. (2001) Nat. Rev. Mol. Cell Biol. 2, 877-886. [DOI] [PubMed] [Google Scholar]
- 15.Lee, J. S., Collins, K. M., Brown, A. L., Lee, C.-H. & Chung, J. H. (2000) Nature 404, 201-204. [DOI] [PubMed] [Google Scholar]
- 16.Peng, C.-Y., Graves, P. R., Thoma, R. S., Wu, Z., Show, A. S. & Piwnica-Worms, H. (1997) Science 277, 1501-1505. [DOI] [PubMed] [Google Scholar]
- 17.Hoffmann, I., Draetta, G. & Karsenti, E. (1994) EMBO J. 13, 4302-4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jinno, S., Suto, K., Nagata, A., Igarashi, M., Kanaoka, Y., Nojima, H. & Okayam, H. (1994) EMBO J. 13, 1549-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Falck, J., Mailand, N., Syljuasen, R. G., Bartek, J. & Lukas, J. (2001) Nature 410, 842-847. [DOI] [PubMed] [Google Scholar]
- 20.Banin, S., Moyal, L., Shieh, S., Taya, Y., Anderson, C. W., Chessa, L., Smorodinsky, N. I., Prives, C., Reiss, Y., Shiloh, Y. & Ziv, Y. (1998) Science 281, 1674-1677. [DOI] [PubMed] [Google Scholar]
- 21.Canman, C. E., Lim, D. S., Cimpri, K. A., Taya, Y., Tamai, K., Sakaguchi, K., Appella, E., Kastan, M. B. & Siliciano, J. D. (1998) Science 281, 1677-1679. [DOI] [PubMed] [Google Scholar]
- 22.Shieh, S. Y., Ahn, J., Tamai, K. I., Taya, Y. & Prives, C. (2000) Genes Dev. 14, 289-300. [PMC free article] [PubMed] [Google Scholar]
- 23.Takai, H., Naka, K., Okada, Y., Watanabe, M., Harada, N., Saito, S., Anderson, C. W., Appella, E., Nakanishi, M., Suzuki, H., et al. (2002) EMBO J. 21, 5195-5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weill, J. C. & Radman, M. (2004) Philos. Trans. R. Soc. London B 359, 95-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cairns, J. (1975) Nature 255, 197-200. [DOI] [PubMed] [Google Scholar]
- 26.Potten, C. S., Owen, G. & Booth, D. (2002) J. Cell Sci. 115, 2381-2388. [DOI] [PubMed] [Google Scholar]
- 27.Merok, J. R., Lansita, J. A., Tunstead, J. R. & Aherley, J. L. (2002) Cancer Res. 62, 6791-6795. [PubMed] [Google Scholar]
- 28.Takada, S., Kelkar, A. & Theurkauf, W. E. (2003) Cell 113, 87-99. [DOI] [PubMed] [Google Scholar]
- 29.Tsvetkov, L., Xu, X., Li, J. & Stern, D. F. (2003) J. Biol. Chem. 278, 8467-8475. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.