ABSTRACT
Both live attenuated influenza vaccines (LAIV) and inactivated influenza vaccines (IIV) induce protective immunity against influenza. There is evidence that LAIV induces superior protection in children, whereas IIV may induce superior protection in adults. The immune mechanisms responsible for these differences have not been identified. We previously compared LAIV and IIV in young children of 6 to 36 months of age, and we demonstrated that while both induced similar hemagglutination inhibition (HAI) antibody responses, only LAIV induced significant increases in T cell responses. In the present study, 37 healthy adult subjects of 18 to 49 years of age were randomized to receive seasonal influenza vaccination with LAIV or IIV. Influenza virus-specific HAI, T cell, and secretory IgA (sIgA) responses were studied pre- and postvaccination. In contrast to the responses seen in young children, LAIV induced only minimal increases in serum HAI responses in adults, which were significantly lower than the responses induced by IIV. Both LAIV and IIV similarly induced only transient T cell responses to replication-competent whole virus in adults. In contrast, influenza virus-specific sIgA responses were induced more strongly by LAIV than by IIV. Our previous studies suggest that LAIV may be more protective than IIV in young children not previously exposed to influenza virus or influenza vaccines due to increased vaccine-induced T cell and/or sIgA responses. Our current work suggests that in adults with extensive and partially cross-reactive preexisting influenza immunity, LAIV boosting of sIgA responses to hemagglutinin (HA) and non-HA antigenic targets expressed by circulating influenza virus strains may be an important additional mechanism of vaccine-induced immunity.
KEYWORDS: vaccine, influenza, LAIV, IIV, adults
INTRODUCTION
Seasonal influenza vaccinations are recommended in the United States for all persons of ≥6 months of age (1). Two types of influenza vaccines, live attenuated influenza vaccines (LAIV) and inactivated influenza vaccines (IIV) formulated with circulating type A H1N1 and H3N2 and type B antigens, are widely used (LAIV in persons of 2 to 49 years of age and IIV in persons of ≥6 months of age) and have been shown to be safe and effective (2, 3). IIV, which is administered intramuscularly, contains inactivated viral components, primarily purified hemagglutinin (HA) antigens. LAIV, which is administered intranasally, contains the full complement of viral components. LAIV replicates within epithelial cells in the nasopharynx, mimicking natural viral infection. Because of the mucosal administration route and the production of viral antigens within host antigen-presenting cells, LAIV may be more effective than IIV at inducing mucosal responses and/or overall T cell responses. On the other hand, preexisting cross-reactive influenza immunity may limit LAIV replication and therefore limit LAIV immunogenicity.
Previous phase III clinical trials demonstrated that both LAIV and IIV can induce significant protection in persons aged 2 to 50 years (4). IIV is licensed and protective (although somewhat less so) for young children aged 6 to 24 months and adults over 50 years old. Three randomized, controlled efficacy trials in children of >6 months and ≤18 years of age consistently demonstrated that LAIV was significantly more protective than IIV (4–7). Similarly, for adults older than 16 years, some comparative trials showed that LAIV was at least as effective as IIV (8–10). However, 2 adult trials showed that IIV was more protective than LAIV (11–13), and thus there is some controversy over whether LAIV can be as effective as a well-matched IIV seasonal vaccine for adults with previously extensive influenza virus exposure (2, 14).
Several factors are likely to affect the relative efficacies of LAIV and IIV in different populations, including the route of administration, the live versus inactivated antigens administered, the intrinsic immunogenicity of the vaccines, preexisting immunity, and the immune status of the vaccinee. Host status can have major effects on vaccine-induced responses. For example, preexisting immunity can inhibit the response to a vaccine that must infect and replicate for optimal immunogenicity (15, 16). Furthermore, relative immunodeficiencies can limit optimal vaccine-induced responses and may have a greater effect in reducing responses to IIV with fewer natural adjuvant properties. We previously showed that compared to IIV, LAIV induced similar serum hemagglutination inhibition (HAI) responses but significantly higher T cell responses in children of 6 to 36 months of age (17). In the current study, we evaluated three different influenza virus-specific immune responses induced in adult subjects given LAIV or IIV, as follows: serum antibody responses were examined by HAI assay, T cell responses by enzyme-linked immunosorbent spot (ELISPOT) and intracellular cytokine staining (ICS) assays, and secretory IgA (sIgA) responses by enzyme-linked immunosorbent assay (ELISA). The combination of all three of these immune responses as examined by state-of-the-art assays has not been studied in the same individuals before, which is the strength of this study. We now report for adults that (i) serum HAI responses induced by IIV are significantly higher than serum HAI responses induced by LAIV, (ii) LAIV and IIV induce comparable but only transient T cell responses, and (iii) LAIV is more effective than IIV at inducing sIgA responses. We hypothesize that LAIV has more intrinsic immunogenicity than IIV and that when preexisting heterotopic immunity does not reduce this immunogenicity, LAIV can induce larger T cell responses, promote similar serum HAI titers, and uniquely induce mucosal immune responses relevant for protective immunity. Furthermore, even in the absence of detectable increases in serum HAI titers, LAIV vaccination of adults may enhance protection by increasing influenza virus-specific secretory IgA responses.
RESULTS
Study subjects.
Enrollment included 19 LAIV and 18 IIV recipients. Pre- and postvaccination samples were collected from 18 LAIV and 18 IIV recipients. No serious adverse events occurred during the 6-month follow-up period. One subject in the LAIV group did not return for follow-up.
Serum HAI responses.
The serum HAI geometric mean titers (GMT) detected in adults following IIV or LAIV vaccination are shown in Table 1. The results presented are for each group at 3 different time points prevaccination (day 0) to postvaccination (days 7 and 45), using each of the 3 HA antigens present in the vaccine strains. A major difference in vaccine-induced HAI responses was seen between the IIV and LAIV groups. In the IIV group, the HAI GMT progressively increased pre- to postvaccination, achieving levels 5- to 10-fold higher than baseline. In fact, the confidence intervals for IIV group prevaccination responses did not overlap the confidence intervals for the postvaccination visit 2 responses with any of the 3 HAI assay targets (H1, H3, and B HA antigens). In contrast, HAI responses in the LAIV group were similar at time points pre- to postvaccination. HAI GMT values were significantly different between the IIV and LAIV groups at both postvaccination time points. These results were not due to differences in baseline HAI; none of the prevaccination comparisons between IIV and LAIV HAI responses showed significant differences.
TABLE 1.
Comparison of serum hemagglutination inhibition antibody responsesa
| HAI assay target and time point | GMT (95% CI) |
P value |
|
|---|---|---|---|
| IIV (n = 18) | LAIV (n = 18) | ||
| Influenza A/H1N1 virus | |||
| Prevaccination | 11.76 (6.2, 22.4) | 11.11 (6.3, 19.9) | 0.89 |
| Postvaccination, day 7 | 47.03 (24.3, 91.1) | 9.33 (5.4, 16.3) | <0.001 |
| Postvaccination, day 45 | 101.59 (51.6, 200) | 12.70 (7.0, 23.2) | <0.0001 |
| Influenza A/H3N2 virus | |||
| Prevaccination | 6.35 (4.1, 9.9) | 7.44 (4.6, 12.0) | 0.63 |
| Postvaccination, day 7 | 17.96 (10.5, 30.8) | 7.13 (4.1, 11.7) | <0.02 |
| Postvaccination, day 45 | 32.00 (17.5, 58.4) | 9.70 (5.9, 16.0) | <0.01 |
| Influenza B virus | |||
| Prevaccination | 7.41 (5.2, 10.6) | 10.71 (6.6, 17.5) | 0.25 |
| Postvaccination, day 7 | 21.77 (14.2, 33.4) | 11.31 (6.8, 18.77) | 0.061 |
| Postvaccination, day 45 | 40.32 (24.4, 66.7) | 12.70 (7.9, 20.5) | <0.01 |
The P values for comparisons of HAI GMT values among treatment groups were calculated by the t test. CI, confidence interval.
IFN-γ ELISPOT responses.
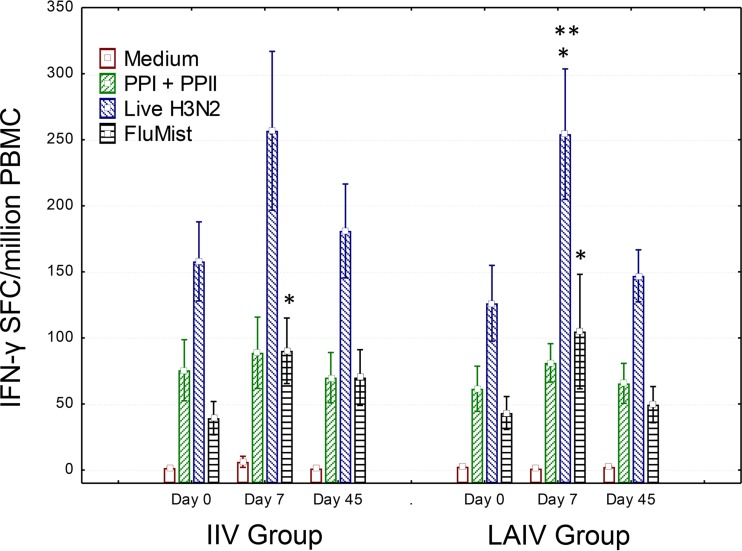
Overall influenza virus-specific T cell responses detected by a gamma interferon (IFN-γ) ELISPOT assay are shown in Fig. 1. As anticipated, both groups of volunteers had significant baseline responses to the conserved peptide pools, the heterotypic H3N2 virulent virus, and the LAIV components, consistent with previous priming due to previous influenza virus infections and/or vaccinations. Responses to LAIV components were significantly increased on day 7 postvaccination in both the LAIV and IIV vaccination groups, and LAIV induced a significant response to live heterologous H3N2 influenza virus on day 7 postvaccination. Both LAIV and IIV were able to boost, at least transiently, T cell responses to live H3N2 virus and to LAIV components in adults.
FIG 1.
Overall T cell responses induced by LAIV and IIV in healthy adults. PBMC from day 0 (prevaccination) and days 7 and 45 postvaccination were incubated overnight in wells of IFN-γ ELISPOT plates with medium alone, a highly conserved influenza virus-specific peptide pool containing CD4 and CD8 T cell epitopes predicted to cover all populations by 200% (PPI + PPII) (17), a live infectious 2004 H3N2 seasonal influenza virus strain (live H3N2), or LAIV components matching the LAIV used to vaccinate the LAIV group (FluMist). Numbers of IFN-γ spot-forming cells (SFC) per million PBMC were calculated. Similar but transient increases in overall influenza virus-specific T cells were seen for both the LAIV and IIV vaccination groups. Note that responses directed against the conserved influenza virus-specific predicted T cell epitopes were increased at baseline and increased only marginally postvaccination. Data are means and standard errors. *, P < 0.05 by the Wilcoxon matched-pairs test; **, P < 0.05 by repeated-measures ANOVA with the Tukey post hoc test (for differences from baseline within groups). For the IIV group, n = 18 for all visits; for the LAIV group, n = 19, 18, and 17 for visits 1, 2, and 3, respectively.
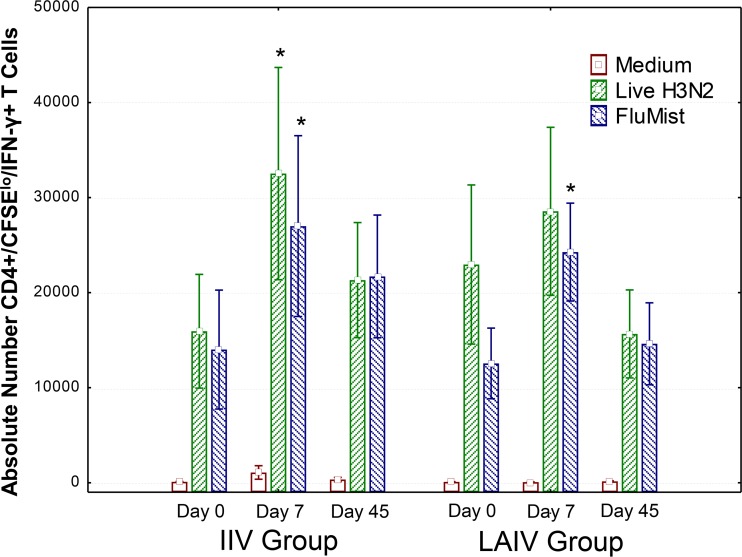
Flow cytometry-based CD4+ and CD8+ T cell proliferative and IFN-γ responses.
Flow cytometry-based intracellular cytokine staining assays were used to detect memory/effector T cells capable of both proliferating and producing the effector cytokine IFN-γ, as well as for identification of the T cell subsets producing antigen-specific responses. Similar to the results of the IFN-γ ELISPOT studies, CD4+ T cell responses increased after vaccination with both LAIV and IIV. Figure 2 shows that CD4+ T cells reactive to a previously circulating heterotypic H3N2 influenza virus strain, as well as to the matched LAIV components, were increased in both vaccine groups at day 7 postvaccination. However, CD4+ T cell responses induced by the conserved influenza virus peptide pools and CD8+ T cell responses induced by LAIV components, heterotypic H3N2 virus, and conserved influenza virus peptide pools were not significantly increased postvaccination in either group (data not shown). In general, the vaccine-induced CD4+ T cell responses were short-lived, falling to baseline levels by day 45.
FIG 2.
CD4+ memory/effector T cells reactive to heterotypic H3N2 virus and to all components of LAIV. PBMC from day 0 (prevaccination) and days 7 and 45 postvaccination were thawed and labeled with CSFE. These CFSE-labeled PBMC were incubated for 1 week with medium alone, a live infectious 2004 H3N2 seasonal influenza virus strain (live H3N2), or LAIV components matching the LAIV used to vaccinate the LAIV group (FluMist). After the 1-week stimulation cultures were complete, T cells were harvested and stained with surface markers and then intracellularly for IFN-γ. Absolute numbers of CFSElow IFN-γ+ CD4+ T cells (calculated by multiplying T cell subset percentages from fluorescence-activated cell sorting by the total number of viable cells recovered) are shown. These results are similar to the IFN-γ ELISPOT results shown in Fig. 1. Both LAIV and IIV induced similar but transient increases in CD4+ T cells. *, P < 0.05 for comparison of postvaccination with prevaccination responses by the Wilcoxon matched-pairs test for differences from baseline within groups. For the IIV group, n = 18 for all visits, and for the LAIV group, n = 19, 18, and 17 for visits 1, 2, and 3, respectively.
Secretory IgA responses.
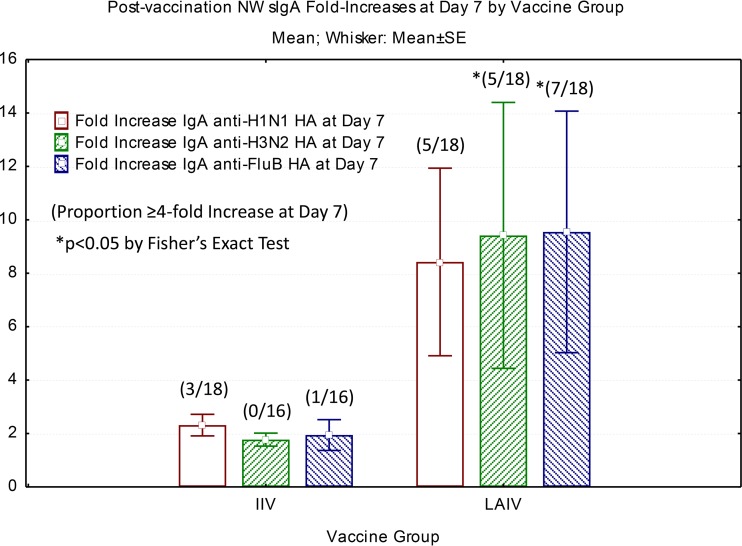
To compare mucosal immune responses induced by the 2 vaccines in adults, we measured influenza virus strain-specific nasal wash (NW) secretory IgA by ELISA. The most significant results were seen at day 45 post-LAIV vaccination. Table 2 presents the medians and ranges of sIgA titers for each vaccine group prevaccination and at day 45 postvaccination. The median titers for influenza virus H1, H3, and B HA-specific antibody responses modestly but significantly increased in LAIV recipients, with baseline medians ranging from 9.75 to 32.25 and postvaccination medians ranging from 16.75 to 57.25. In contrast, HA-specific sIgA binding titers 45 days after vaccination were similar to prevaccine levels for the IIV vaccination group. Figure 3 presents pre- to postvaccination mean fold increases in sIgA detected at 7 days postvaccination by vaccine group, as well as the proportion of subjects in each group mounting a 4-fold or greater increase above the baseline level. Mean fold increases for all 3 HA components were 8.4- to 9.5-fold for LAIV recipients, compared to 1.7- to 2.3-fold for IIV recipients. In general, LAIV recipients had higher average sIgA fold increases than IIV recipients, and they had significantly higher frequencies of 4-fold or greater sIgA responses than IIV recipients for 2 of 3 vaccine strains. Higher sIgA responses persisted at day 45 postvaccination in the LAIV group but not the IIV group.
TABLE 2.
Comparison of nasal wash hemagglutinin-specific sIgA responses
| ELISA target antigen | Vaccine group | Median (range) NW anti-HA sIgA endpoint titer |
P valuea (pre- vs day 45 postvaccination) | |
|---|---|---|---|---|
| Prevaccination | Day 45 postvaccination | |||
| Influenza A/H1N1 virus HA | IIV | 18 (1.25–64) | 20.75 (1.25–2328) | 0.717 |
| LAIV | 16.25 (3–41) | 28.5 (4–197) | 0.05 | |
| Influenza A/H3N2 virus HA | IIV | 26.5 (5–69) | 31.5 (3–476) | 0.064 |
| LAIV | 32.25 (1.25–88) | 57.25 (10–458) | 0.004 | |
| Influenza B virus HA | IIV | 16 (3–50) | 16.5 (1.25–77) | 0.754 |
| LAIV | 9.75 (3–47) | 16.75 (5–113) | 0.015 | |
Determined by the Wilcoxon matched-pair test. Day 0 (prevaccination) outliers (more than 1 standard error from the mean) were excluded.
FIG 3.
Fold changes in HA-specific nasal wash sIgA endpoint titers at 7 days postvaccination. Nasal wash specimens were collected prevaccination and at 7 days and 45 days postvaccination. Endpoint titers were determined for all samples, which were incubated in the wells of ELISA plates coated with H1N1 HA, H3N2 HA, and B HA (FluB HA), matching the 3 influenza virus components of LAIV and IIV. Data shown are average fold changes from baseline at day 7 postvaccination for all 3 HA antigens. Data in parentheses show the proportions of the groups mounting 4-fold or greater increases from baseline. *, P < 0.05 by Fisher's exact test for comparison of day 7 postvaccination responses of the LAIV and IIV groups.
DISCUSSION
We compared serum antibody, T cell, and secretory IgA immune responses in healthy adults, aged 18 to 49 years, who received either IIV or LAIV seasonal influenza vaccination. Each of these responses has been measured in previous vaccine trials comparing IIV and LAIV (14, 18–21), but the responses have not all been studied for the same group of adults. The seasonal vaccines administered contained antigens from equivalent influenza virus strains but were either inactivated and administered by the intramuscular route or live, attenuated, and administered by the mucosal route. Only subjects who received IIV had significant and sustained HAI responses to matched seasonal influenza A/H1N1, influenza A/H3N2, and influenza B virus antigens, whereas subjects who received LAIV had modest to no increase in HAI titer to any of the influenza virus antigens. Both LAIV and IIV induced similar but transient increases in influenza virus-specific memory/effector T cell responses to both seasonally matched influenza virus components and a live, previously circulating heterotypic strain of influenza A/H3N2 virus. Some of these T cell increases persisted for over 6 weeks. Boosted T cell responses included responses to highly conserved influenza virus class I and class II peptide epitopes relevant for induction of universal influenza immunity. Subjects who received LAIV were more likely to respond with 4-fold or greater increases 1 week following vaccination, and sIgA increases induced by LAIV were more likely to persist than those induced by IIV. The humoral and T cell responses reported here for adults are in contrast to previous results reported for young children (17), in whom both IIV and LAIV induced similar humoral immune responses but only vaccine regimens including LAIV induced influenza virus-specific CD4+, CD8+, and gamma delta T cell responses important for cell-mediated immune protection.
It is interesting that IIV recipients developed serum HAI responses but not sIgA responses, while LAIV recipients developed sIgA responses but not serum HAI responses. Possible explanations for the apparent dissociation of mucosal and systemic antibody responses are the differences in vaccine formulations and in modes of vaccine delivery. Purified influenza virus protein antigens administered parenterally induce B and T cells with systemic homing molecules (e.g., cutaneous lymphatic antigen) important for recognition of molecular structures lining endothelial cells that are required for transpedesis into peripheral cutaneous tissues. Whereas the live attenuated whole viruses in LAIV presented intranasally provide for replication and prolonged antigen stimulation in the upper respiratory tract, increasing inflammation and stimulation in the mucosa, mucosal stimulation induces B cells that express mucosal homing molecules that are then upregulated, enhancing trafficking of memory immune T and B cells to the mucosa. For example, the α4β7 integrin complex is upregulated on the surfaces of lymphocytes activated in Peyer's patches. This integrin specifically binds to MadCAM1 on endothelial cells and triggers transendothelial migration from the vasculature into peripheral mucosal tissues (22, 23). LAIV induces B cells with mucosal trafficking/respiratory tract-resident cells. Circulating T cells may be induced differentially by LAIV to facilitate mucosal trafficking of vaccine-induced B cells activated by vaccination, and increased numbers of mucosa-resident T cells may directly recruit B cells to the upper respiratory tract via specific chemoattractants (22). In addition, it would be interesting in future studies to measure CCR5 and CCR7 expression on T cells induced by LAIV versus IIV, as these chemokine receptors have also been reported to facilitate lung trafficking of vaccine-induced T cells (24, 25).
The adult responses to LAIV and IIV reported here are in contrast to the responses previously reported for children. In addition to the striking differences in HAI responses between the two age groups, the T cell increases detected in this adult study were much less impressive than the LAIV-induced T cell responses detectable in children of 6 to 36 months of age (17). These differences may be due in part to preexisting immunity present in most adults due to multiple prior exposures to live circulating influenza virus strains and to previous influenza vaccinations. In a recent report, Barría et al. (15) evaluated HAI responses to H1N1 HA with respect to preexisting antibody titers and noted that the small subset of adults achieving a 4-fold or higher HAI response to H1N1 HA following LAIV vaccination had low to negative prevaccination HAI titers. The relative immunological naiveté of young children allows more prolonged replication of LAIV in the upper respiratory tract, which may result in greater stimulation of multiple T and B cell subsets. Inclusion of pathogen-associated molecular patterns (PAMPs) recognized by cells of the innate immune system facilitates robust immune responses in naive LAIV recipients. Conversely, adults with extensive cross-reactive influenza immunity substantially reduce the “take” or duration of replication of LAIV, thus preventing the stimulation of new systemic immune responses. IIV may induce only new B cell, new CD4+ T cell, and booster responses in memory/effector cells, while LAIV can induce new B cell, new CD4+ T cell, new CD8+ T cell, and booster responses in both B and T memory/effector cells.
Using the ferret model of intranasal influenza virus challenge, Cheng et al. (26) evaluated protective responses elicited by LAIV or IIV following upper respiratory challenge of seropositive ferrets. While both vaccines elicited humoral responses in the ferrets, only LAIV provided protection from respiratory challenge with a live heterologous influenza virus. Potential mechanisms of sIgA protection may include blocking of viral host cell attachment at the site of initial mucosal infection, intracellular uptake of sIgA with blocking of viral uncoating, or redirection for uptake of influenza virus particles by respiratory macrophages or other phagocytes that can mediate intracellular killing and inhibition of influenza virus replication (27). These protective mechanisms afforded by LAIV may explain the beneficial effects of LAIV vaccination in adults despite the absence of serum HAI responses.
Future studies should include the identification of epitopes most important in eliciting protective sIgA responses, including HA, HA stalk region, and other viral antigens that might confer cross-protective sIgA responses. In vitro protection assays to directly assess sIgA-mediated inhibition of viral replication in human macrophages would facilitate the understanding of sIgA protective capacity on a larger scale than is possible with the animal model. Studies are also needed to understand the differences in and clinical importance of sIgA in conferring protective immunity in children and adults, as well as the role of preexisting immunity in these populations. Intranasal vaccination with an inactivated whole influenza virus has been shown to induce HAI and neutralizing antibody responses in nasal secretions (28). Future studies should include evaluation of functional antibodies elicited by LAIV and IIV in nasal wash specimens from adults and children and investigation for correlations between sIgA and vaccine efficacy in these populations.
Previous studies suggested that LAIV may be more protective than IIV in young children who have not previously been vaccinated or exposed to influenza virus, due to enhanced T cell or sIgA responses (17). However, in adults with extensive preexisting influenza immunity, LAIV boosting of influenza virus-specific sIgA responses may be an important additional mechanism of vaccine-induced immunity. Influenza virus-specific sIgA and T cell responses may be important correlates of protection against heterotypic and emerging influenza virus strains and should be investigated as targets for next-generation influenza vaccines.
MATERIALS AND METHODS
Subjects and vaccines.
Thirty-seven healthy adults of 18 to 49 years of age, without symptoms of upper respiratory illness, were recruited to participate in this study. The study was approved by the Saint Louis University Institutional Review Board. After informed consent was obtained, subjects were randomized 1:1 to receive a single dose of either trivalent LAIV, known commercially as FluMist (MedImmune), or trivalent IIV, known commercially as Fluzone (Sanofi Pasteur). Both vaccines were licensed 2010-2011 trivalent seasonal products. For LAIV (FluMist), each 0.2-ml (intranasal) dose was formulated to contain 106.5 to 107.5 focus-forming units (FFU) of live attenuated influenza virus reassortants of three strains: A/California/7/2009 (H1N1), A/Perth/16/2009 (H3N2), and B/Brisbane/60/2008. For IIV (Fluzone), each 0.5-ml (intramuscular) dose contained 15 μg of each seasonal viral type, for a total dose of 45 μg. The strains used were A/California/07/2009, x-179A (H1N1), A/Victoria/210/2009, x-187 (an A/Perth/16/2009-like virus) (H3N2), and B/Brisbane/60/2008. Upon enrollment, a single dose of seasonal LAIV or IIV was administered to each study volunteer. Serum, peripheral blood mononuclear cell (PBMC), and nasal wash (NW) specimens were obtained on day 0 (prevaccination) and on days 7 and 45 to 51 (referred to as day 45 here) postvaccination.
Safety.
Since the study involved administration of licensed vaccines indicated for use in the enrolled population, detailed safety data were not collected. However, study subjects had 3 clinical visits and 2 follow-up telephone contacts, during which information on health status and any adverse events was solicited and documented.
Serum HAI tests.
Serum hemagglutination inhibition (HAI) tests were performed as previously described (29). Serum samples obtained prevaccination and at 7 days and 45 days postvaccination were tested in duplicate against seasonally matched influenza virus HAI test antigens obtained from the CDC. Test antigens were from beta-propiolactone-inactivated H1N1 (A/California/7/2009), H3N2 (A/Perth/16/2009), and B (B/Brisbane/60/2008) influenza virus strains.
CMI ELISPOT assays.
Cell-mediated immunity (CMI) ELISPOT assays detecting numbers of IFN-γ-producing cells were performed with PBMC obtained prevaccination and at 7 and 45 days postvaccination, using previously described methods (17). PBMC were stimulated in triplicate with medium alone, a live heterotypic influenza virus (A/H3N2/California/07/2004), influenza A virus peptide pool I (a pool of 35 class I peptides) and influenza A virus peptide pool II (a pool of 16 class II peptides) combined, or LAIV components (2010-2011 FluMist formulation).
Antigen-specific proliferation and production of IFN-γ by T cells.
Antigen-specific proliferation and production of IFN-γ by T cells were measured using a 7-day carboxyfluorescein succinimidyl ester (CFSE)-dilution intracellular cytokine staining (ICS) assay as previously described (17). CFSE-stained PBMC were stimulated with live influenza virus (A/H3N2/California/07/2004), influenza A virus peptide pool I (a pool of 35 class I peptides), influenza A virus peptide pool II (a pool of 16 class II peptides), influenza A virus peptide pools I and II combined, or LAIV components (2010-2011 FluMist formulation) or rested in medium alone for 1 week at 37°C with 5% CO2. Interleukin-2 (IL-2) was added to a concentration of 20 U/ml on the 4th day of incubation. Absolute numbers of CD4+ and CD8+ T cells that were CFSElow IFN-γ+ were determined by multiplying viable cell counts on day 7 by percentages of each T cell subset.
Secretory IgA antibodies.
Secretory IgA antibodies to hemagglutinin (HA) antigens from influenza A/H1N1 virus, influenza A/H3N2 virus, and influenza B virus were evaluated by ELISA, using nasal wash specimens obtained pre- and 7 and 45 days postvaccination. Microtiter plates were coated overnight with strain-matched, full-length glycosylated recombinant influenza virus HA proteins (obtained from Sino Biological, Inc., Beijing, People's Republic of China), i.e., H1N1 HA (A/California/07/2009), H3N2 HA (A/Perth/16/2009), and B HA (B/Brisbane/60/2008), at 1 μg/ml. Nasal wash specimens which had been sonicated and concentrated 5-fold were serially diluted in incubation buffer (phosphate-buffered saline [PBS], 0.1% Tween 20, 1% bovine serum albumin [BSA]), starting at a 1:2.5 dilution, and added to the washed plates together with controls. After overnight incubation, the plates were washed, and biotin-conjugated affinity-purified goat anti-human IgA (KPL) was added at 1:4,000. Plates were incubated for 2 h at 37°C, followed by a wash step and the addition of avidin-alkaline phosphatase (KPL) at 1:4,000. Following incubation, plates were washed and pNPP substrate added, and the absorbance was measured 1 h later by use of a spectrophotometer. After background subtraction, linear regression plots were generated (log absorbance versus log dilution) and endpoint titers (EPT) determined, using a cutoff of a 0.2 optical density (OD) unit.
Statistical methods.
Group antibody data were expressed as geometric mean titers. Mean baseline responses plus 2 standard errors were used to define cutoffs for positive responses. Transformed continuous data were compared by analysis of variance (ANOVA). Nontransformed continuous data were compared by the Wilcoxon matched-pairs test (pre- to postvaccination comparisons) or the Mann-Whitney U test (group comparisons at individual time points). Dichotomous responses were compared with 2-sided Fisher's exact test. Outliers among the sIgA data were defined as prevaccination nasal wash sIgA titers that were greater than the mean of all prevaccination levels plus 1 standard error.
ACKNOWLEDGMENTS
We thank all volunteers for their participation, as well as the nurses and staff who assisted with this study.
This work was funded by a MedImmune Investigator-Initiated Award to Daniel F. Hoft.
Robert B. Belshe has served as a consultant to MedImmune and a speaker for MedImmune, Sanofi, and Merck.
REFERENCES
- 1.Armstrong C. 2015. ACIP releases recommendations for influenza vaccination, 2015–2016. Am Fam Physician 92:732–740.26554415 [Google Scholar]
- 2.Beyer WE, Palache AM, de Jong JC, Osterhaus AD. 2002. Cold-adapted live influenza vaccine versus inactivated vaccine: systemic vaccine reactions, local and systemic antibody response, and vaccine efficacy. A meta-analysis. Vaccine 20:1340–1353. [DOI] [PubMed] [Google Scholar]
- 3.Clements ML, Murphy BR. 1986. Development and persistence of local and systemic antibody responses in adults given live attenuated or inactivated influenza A virus vaccine. J Clin Microbiol 23:66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ambrose CS, Levin MJ, Belshe RB. 2011. The relative efficacy of trivalent live attenuated and inactivated influenza vaccines in children and adults. Influenza Other Respir Viruses 5:67–75. doi: 10.1111/j.1750-2659.2010.00183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashkenazi S, Vertruyen A, Aristegui J, Esposito S, McKeith DD, Klemola T, Biolek J, Kuhr J, Bujnowski T, Desgrandchamps D, Cheng SM, Skinner J, Gruber WC, Forrest BD, CAIV-T Study Group. 2006. Superior relative efficacy of live attenuated influenza vaccine compared with inactivated influenza vaccine in young children with recurrent respiratory tract infections. Pediatr Infect Dis J 25:870–879. doi: 10.1097/01.inf.0000237829.66310.85. [DOI] [PubMed] [Google Scholar]
- 6.Fleming DM, Crovari P, Wahn U, Klemola T, Schlesinger Y, Langussis A, Oymar K, Garcia ML, Krygier A, Costa H, Heininger U, Pregaldien JL, Cheng SM, Skinner J, Razmpour A, Saville M, Gruber WC, Forrest B, CAIV-T Asthma Study Group. 2006. Comparison of the efficacy and safety of live attenuated cold-adapted influenza vaccine, trivalent, with trivalent inactivated influenza virus vaccine in children and adolescents with asthma. Pediatr Infect Dis J 25:860–869. doi: 10.1097/01.inf.0000237797.14283.cf. [DOI] [PubMed] [Google Scholar]
- 7.Belshe RB, Edwards KM, Vesikari T, Black SV, Walker RE, Hultquist M, Kemble G, Connor EM, CAIV-T Comparative Efficacy Study Group. 2007. Live attenuated versus inactivated influenza vaccine in infants and young children. N Engl J Med 356:685–696. doi: 10.1056/NEJMoa065368. [DOI] [PubMed] [Google Scholar]
- 8.Treanor JJ, Kotloff K, Betts RF, Belshe R, Newman F, Iacuzio D, Wittes J, Bryant M. 1999. Evaluation of trivalent, live, cold-adapted (CAIV-T) and inactivated (TIV) influenza vaccines in prevention of virus infection and illness following challenge of adults with wild-type influenza A (H1N1), A (H3N2), and B viruses. Vaccine 18:899–906. doi: 10.1016/S0264-410X(99)00334-5. [DOI] [PubMed] [Google Scholar]
- 9.Eick AA, Wang Z, Hughes H, Ford SM, Tobler SK. 2009. Comparison of the trivalent live attenuated vs. inactivated influenza vaccines among U.S. military service members. Vaccine 27:3568–3575. doi: 10.1016/j.vaccine.2009.03.088. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z, Tobler S, Roayaei J, Eick A. 2009. Live attenuated or inactivated influenza vaccines and medical encounters for respiratory illnesses among US military personnel. JAMA 301:945–953. doi: 10.1001/jama.2009.265. [DOI] [PubMed] [Google Scholar]
- 11.Ohmit SE, Victor JC, Rotthoff JR, Teich ER, Truscon RK, Baum LL, Rangarajan B, Newton DW, Boulton ML, Monto AS. 2006. Prevention of antigenically drifted influenza by inactivated and live attenuated vaccines. N Engl J Med 355:2513–2522. doi: 10.1056/NEJMoa061850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohmit SE, Victor JC, Teich ER, Truscon RK, Rotthoff JR, Newton DW, Campbell SA, Boulton ML, Monto AS. 2008. Prevention of symptomatic seasonal influenza in 2005–2006 by inactivated and live attenuated vaccines. J Infect Dis 198:312–317. doi: 10.1086/589885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monto AS, Ohmit SE, Petrie JG, Johnson E, Truscon R, Teich E, Rotthoff J, Boulton M, Victor JC. 2009. Comparative efficacy of inactivated and live attenuated influenza vaccines. N Engl J Med 361:1260–1267. doi: 10.1056/NEJMoa0808652. [DOI] [PubMed] [Google Scholar]
- 14.He XS, Holmes TH, Sasaki S, Jaimes MC, Kemble GW, Dekker CL, Arvin AM, Greenberg HB. 2008. Baseline levels of influenza-specific CD4 memory T-cells affect T-cell responses to influenza vaccines. PLoS One 3:e2574. doi: 10.1371/journal.pone.0002574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barría MI, Garrido JL, Stein C, Scher E, Ge Y, Engel SM, Kraus TA, Banach D, Moran TM. 2013. Localized mucosal response to intranasal live attenuated influenza vaccine in adults. J Infect Dis 207:115–124. doi: 10.1093/infdis/jis641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ilyushina NA, Haynes BC, Hoen AG, Khalenkov AM, Housman ML, Brown EP, Ackerman ME, Treanor JJ, Luke CJ, Subbarao K, Wright PF. 2015. Live attenuated and inactivated influenza vaccines in children. J Infect Dis 211:352–360. doi: 10.1093/infdis/jiu458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoft DF, Babusis E, Worku S, Spencer CT, Lottenbach K, Truscott SM, Abate G, Sakala IG, Edwards KM, Creech CB, Gerber MA, Bernstein DI, Newman F, Graham I, Anderson EL, Belshe RB. 2011. Live and inactivated influenza vaccines induce similar humoral responses, but only live vaccines induce diverse T-cell responses in young children. J Infect Dis 204:845–853. doi: 10.1093/infdis/jir436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sasaki S, Jaimes MC, Holmes TH, Dekker CL, Mahmood K, Kemble GW, Arvin AM, Greenberg HB. 2007. Comparison of the influenza virus-specific effector and memory B-cell responses to immunization of children and adults with live attenuated or inactivated influenza virus vaccines. J Virol 81:215–228. doi: 10.1128/JVI.01957-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He XS, Holmes TH, Zhang C, Mahmood K, Kemble GW, Lewis DB, Dekker CL, Greenberg HB, Arvin AM. 2006. Cellular immune responses in children and adults receiving inactivated or live attenuated influenza vaccines. J Virol 80:11756–11766. doi: 10.1128/JVI.01460-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moldoveanu Z, Clements ML, Prince SJ, Murphy BR, Mestecky J. 1995. Human immune responses to influenza virus vaccines administered by systemic or mucosal routes. Vaccine 13:1006–1012. doi: 10.1016/0264-410X(95)00016-T. [DOI] [PubMed] [Google Scholar]
- 21.Sasaki S, He XS, Holmes TH, Dekker CL, Kemble GW, Arvin AM, Greenberg HB. 2008. Influence of prior influenza vaccination on antibody and B-cell responses. PLoS One 3:e2975. doi: 10.1371/journal.pone.0002975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kunkel EJ, Butcher EC. 2002. Chemokines and the tissue-specific migration of lymphocytes. Immunity 16:1–4. doi: 10.1016/S1074-7613(01)00261-8. [DOI] [PubMed] [Google Scholar]
- 23.Hoft DF, Brusic V, Sakala IG. 2011. Optimizing vaccine development. Cell Microbiol 13:934–942. doi: 10.1111/j.1462-5822.2011.01609.x. [DOI] [PubMed] [Google Scholar]
- 24.Kohlmeier JE, Miller SC, Smith J, Lu B, Gerard C, Cookenham T, Roberts AD, Woodland DL. 2008. The chemokine receptor CCR5 plays a key role in the early memory CD8+ T cell response to respiratory virus infections. Immunity 29:101–113. doi: 10.1016/j.immuni.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sridhar S, Begom S, Bermingham A, Hoschler K, Adamson W, Carman W, Bean T, Barclay W, Deeks JJ, Lalvani A. 2013. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat Med 19:1305–1312. doi: 10.1038/nm.3350. [DOI] [PubMed] [Google Scholar]
- 26.Cheng X, Zengel JR, Suguitan AL Jr, Xu Q, Wang W, Lin J, Jin H. 2013. Evaluation of the humoral and cellular immune responses elicited by the live attenuated and inactivated influenza vaccines and their roles in heterologous protection in ferrets. J Infect Dis 208:594–602. doi: 10.1093/infdis/jit207. [DOI] [PubMed] [Google Scholar]
- 27.van Riet E, Ainai A, Suzuki T, Hasegawa H. 2012. Mucosal IgA responses in influenza virus infections; thoughts for vaccine design. Vaccine 30:5893–5900. doi: 10.1016/j.vaccine.2012.04.109. [DOI] [PubMed] [Google Scholar]
- 28.Ainai A, Tamura S, Suzuki T, van Riet E, Ito R, Odagiri T, Tashiro M, Kurata T, Hasegawa H. 2013. Intranasal vaccination with an inactivated whole influenza virus vaccine induces strong antibody responses in serum and nasal mucus of healthy adults. Hum Vaccin Immunother 9:1962–1970. doi: 10.4161/hv.25458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.King JC Jr, Lagos R, Bernstein DI, Piedra PA, Kotloff K, Bryant M, Cho I, Belshe RB. 1998. Safety and immunogenicity of low and high doses of trivalent live cold-adapted influenza vaccine administered intranasally as drops or spray to healthy children. J Infect Dis 177:1394–1397. doi: 10.1086/517822. [DOI] [PubMed] [Google Scholar]