Highlights
-
•
Arboviruses are still expanding their geographic distribution and causing significant public health impact around the world.
-
•
DENV, CHIKV and ZIKV are mosquito-transmitted pathogens posing a threat to human health in many regions of the world.
-
•
Global travel and trade have facilitated the emergence of vector-borne diseases.
-
•
Surveillance of areas close to the forest is important to monitor the emergence of pathogens from their sylvatic cycle.
Keywords: Arboviruses, Dengue, Zika, Chikungunya, Human transmission, Emergence
Abstract
Arthropod-borne viruses (arboviruses) present a substantial threat to human and animal health worldwide. Arboviruses can cause a variety of clinical presentations that range from mild to life threatening symptoms. Many arboviruses are present in nature through two distinct cycles, the urban and sylvatic cycle that are maintained in complex biological cycles. In this review we briefly discuss the factors driving the emergence of arboviruses, such as the anthropogenic aspects of unrestrained human population growth, economic expansion and globalization. Also the important aspects of viruses and vectors in the occurrence of arboviruses epidemics. The focus of this review will be on dengue, zika and chikungunya viruses, particularly because these viruses are currently causing a negative impact on public health and economic damage around the world.
1. Introduction
Emerging infectious diseases (EID) are defined as infections that have recently appeared in a population, and are quickly increasing in frequency or geographic range (Morse, 1995). For a disease to emerge, several factors are required, including the introduction of a pathogen and its spread into the human population, followed by its ability to be maintained in nature. Many pathogens require adaptation to emerge into a new environment, while for others adaptation is not necessary. Human behavior and ecology are two other factors that play a role in the emergence of diseases (Schrag and Wiener, 1995, Hahn et al., 2000, May et al., 2001). For example, the geographical expansion of human populations has facilitated the appearance of some emerging viruses, as well as the intensification of agriculture and the disturbance of habitats due to climate change or deforestation (Taylor et al., 2001, Jones et al., 2008).
Actually, only a few infectious agents are restricted to humans. The majority of emergent pathogens that affect humans are zoonotic agents that are maintained in enzootic cycles (Lloyd-Smith et al., 2009). During the past 70 years, emerging zoonoses have made up most of the emerging infectious diseases affecting people, and they have caused economic damage exceeding hundreds of billions of U.S. dollars (Jones et al., 2008, Newcomb et al., 2011, Karesh et al., 2012). Zoonotic diseases account for billions of cases of human illness and millions of deaths every year and constitute long-lasting health problems worldwide (I.L.R. Institute, 2012).
The host range expansion of the zoonotic agents requires multiple factors to establish transmission into the human population. Anthropogenic changes related to agriculture practices and deforestation are two factors that may bring humans in close contact with zoonotic reservoirs. Many wildlife species have been identified as reservoirs of pathogens that can be transmitted to humans (Levins et al., 1993, Morse, 1994). For example, bats represent a major source of zoonotic viruses (Calisher et al., 2006), including rabies, Nipah (NiV), SARS (SARS-CoV) and Ebola (EBOV) viruses (Taylor et al., 2001, Woolhouse et al., 2005).
Many other zoonotic viruses are transmitted to humans by hematophagous insects (mosquitoes, sandflies, biting midges and ticks) and are designated arthropod-borne viruses (arboviruses) (Higgs and Beaty, 2005). In recent years, the prevalence of vector-borne diseases has expanded considerably, due to intensification of human travel and transcontinental commerce. The number of cases has increased in endemic regions, but cases have also spread into new regions where the viruses never existed before (Gubler, 2002, Weaver and Reisen, 2010, Weaver, 2013, Weaver, 2014). Additionally, the development of mosquito resistance to insecticides has further complicated the control and eventual elimination of vector-borne diseases from specific areas (Saavedra-Rodriguez et al., 2012, Bisset et al., 2013).
1.1. Factors associated with the emergence of arboviruses
Arboviral diseases are caused by viruses that are maintained in transmission cycles between vertebrate hosts and blood-sucking arthropods such as mosquitoes, sandflies, midges and ticks. In order to complete the transmission cycle, the virus must produce a sufficiently high level of viremia in the vertebrate host for a susceptible arthropod to become infected while taking a blood meal (Karabatsos, 2001). There are at least 135 arboviruses that have been known to cause human disease. Arboviral infections can range from asymptomatic to fulminant fatal disease. The clinical symptoms are generally categorized as systemic febrile illness, hemorrhagic fever and invasive neurological disease (Gubler and Vasilakis, 2016). The vast majority of arboviruses are RNA viruses, belonging to the genera Alphavirus, Flavivirus, Orthobunyavirus, Nairovirus, Phlebovirus, Orbivirus, Vesiculovirus and Thogotovirus. Among DNA viruses, African swine fever virus (Asfivirus genus) represents the only DNA arbovirus (Calisher and Karabatsos, 1988, King et al., 2011).
In the past few decades, the total number of arboviral epidemics has significantly increased (Gubler and Vasilakis, 2016). In most cases, the emerging arboviral diseases were caused by viruses previously considered to be controlled or of little public health importance (Gubler and Vasilakis, 2016). Introduction of viruses into new geographic areas (i.e. WNV into the Americas), where naïve vertebrate and arthropod hosts were susceptible and able to sustain infection, also contributed to the occurrence of major outbreaks. In other cases, epidemics were associated with the regional spread of viruses previously considered restricted to a specific geographic area, e.g. Rift Valley fever, Ross River and chikungunya fevers, Japanese encephalitis and Venezuelan equine encephalitis.
One example of an arbovirus that has significantly expanded its geographic range and moved into new territories is chikungunya virus (CHIKV). CHIKV is a member of the genus Alphavirus, family Togaviridae; historically it was restricted to the Old World (Jupp and McIntosh, 1988). There are indications that the virus was originated in sub-Saharan Africa, where it is believed that CHIKV was maintained in an enzootic transmission cycle between non-human primates (NHP) and arboreal Aedes mosquitoes (Powers et al., 2000, Volk et al., 2010). Spillover transmission to nearby human populations probably occured multiple times, resulting a continuous transmission cycle between humans and anthropophilic mosquitoes, such as Ae. aegypti (Diallo et al., 1999, Diallo et al., 2012, Volk et al., 2010). In 2004, CHIKV emergence was reported in the costal area of Kenya (Chretien et al., 2007) following a global expansion to different regions of Africa, Asia, several islands in the Indian Ocean (Hochedez et al., 2006, Lanciotti et al., 2007, Taubitz et al., 2007) and temperate areas in Europe (Rezza et al., 2007, Grandadam et al., 2011). The contributing factor for the emergence of CHIKV was presumably via travelers who became infected in endemic/epidemic areas and returned home contributing to the establishment of autochthonous transmission (Hochedez et al., 2006, Lanciotti et al., 2007, Taubitz et al., 2007).
Four genotypes of CHIKV have been identified since its discovery in 1952: East-Central-South African (ECSA), West African, Asian, and the Indian Ocean Lineage (IOL) (Powers et al., 2000, Volk et al., 2010). The different CHIKV lineages can exhibit distinct patterns of infectivity and transmissibility in the mosquito vectors (Arias-Goeta et al., 2013, Vega-Rua et al., 2013). The acquisition of specific mutations in the E1 (Tsetsarkin et al., 2007, Vazeille et al., 2007) and E2 (Tsetsarkin and Weaver, 2011, Tsetsarkin et al., 2014) envelope glycoprotein of emerging IOL strains allowed virus adaptation and consequent increased transmission in the peridomestic mosquito Ae. albopictus. This adaptation may have contributed to the spread and continuous transmission of CHIKV in tropical urban areas where Ae. aegypti is abundant and also to peridomestic and/or temperate habitats where Ae. albopictus is more adapted (Leisnham et al., 2014).
Despite the presence of both Ae. aegypti and Ae. albopictus mosquito vectors and reports of imported cases from the 2006–2009 period (Lanciotti et al., 2007) in the Americas, local transmission of CHIKV was only been reported recently. In 2013, an Asian lineage of CHIKV was introduced into the Caribbean island of Saint Martin and established the first mosquito-human cycle in the Americas (Leparc-Goffart et al., 2014). Subsequently, cases of autochthonous transmission of CHIKV were reported throughout the Caribbean and Central America, South America and Florida (Weaver and Forrester, 2015). In Brazil, two different CHIKV lineages were detected (Nunes et al., 2015). The Asian lineage reported in North Brazil possibly originated from travelers coming from the Caribbean, while the index case for the ECSA lineage reported in the northeast region (Bahia state) probably was introduced from a resident returning from Angola (Nunes et al., 2015).
Zika virus (ZIKV) is another arbovirus of the Flaviviridae family, genus Flavivirus, that is rapidly expanding its geographic distribution and has been recently introduced into areas not previously reported. The disease is characterized by a broad range of clinical symptoms, including fever, rash, headache, retro-orbital pain, myalgia, arthritis or arthralgia, conjunctivitis and vomiting, which are clinical signs similar to dengue disease and many other diseases of viral (e.g chikungunya and Mayaro fevers) and parasitic (e.g. scrub typhus and leptospirosis) aetiologies (Macnamara, 1954, Olson et al., 1981, Duffy et al., 2009, Foy et al., 2011, Kutsuna et al., 2014). ZIKV was first isolated in 1947 from the blood of a sentinel rhesus monkey exposed in the canopy of Ziika Forest in Uganda during epidemiologic studies of yellow fever (Dick et al., 1952). Subsequent isolations of the virus were made from Aedes africanus, Ae. luteocephalus and Ae. furcifer (all tree-hole breeding mosquitoes implicated in the sylvan cycle of yellow fever virus) in Uganda, Senegal, Nigeria, Burkina Faso, Ivory Coast and the Central African Republic (Haddow et al., 2012). These reports were interpreted as evidence that ZIKV is maintained in forested areas of tropical Africa in a cycle similar to that of sylvan yellow fever (i.e. arboreal mosquitoes and non-human primates). ZIKV was first isolated from humans in 1954 from a 10 year old Nigerian female (Macnamara 1954). The virus was isolated from mice inoculated with the patient’s serum sample; two other human cases were also confirmed from the same country. In 1969, ZIKV was isolated for the first time outside the African continent from Ae. aegypti mosquitoes collected in Malaya (Marchette et al., 1969); and in 1977, the first human case was described in Indonesia (Olson et al., 1981). The factors associated with the emergence of ZIKV are not understood. On the island of Yap, in Micronesia, where the first large outbreak was reported in 2007, ZIKV was speculated to have been introduced by either viremic travelers or infected mosquitoes originating from the Philippines, since travel exchange between Yap state and Philippines is very frequent.
In 2013 a major epidemic of ZIKV was reported in French Polynesia, where human subjects were presenting dengue-like symptoms and rash. Interestingly, few of the affected patients presented severe neurological complications and non-vector borne transmission (sexual and transfusion-associated cases) were also described (Laigret et al., 1967, Cao-Lormeau et al., 2011, Musso et al., 2015). Although the total number of confirmed cases remains unknown, the number of patient consultations presenting symptoms of Zika fever was estimated to be about 28,000. A retrospective serosurvey, estimated the overall infection rate at 50–66% of the total population (Aubry et al., 2015). The virus strain involved in French Polynesia outbreak was phylogenetically closely related to strains isolated in Yap and in Cambodia, suggesting that ZIKV could have been introduced from these regions (Cao-Lormeau et al., 2014, Musso et al., 2014). In 2014, ZIKV cases were reported in New Caledonia in the South Pacific; unlike other Pacific regions where the virus source was unknown, in this outbreak the majority of the cases originated from individuals who have been in French Polynesia (ProMEDmail, 2014a, Dupont-Rouzeyrol et al., 2015). In Easter Island, a local festivity that happens every year may have facilitated the introduction of ZIKV through people who came from several Pacific regions including French Polynesia (ProMEDmail, 2014b, Musso, 2015). Following the introduction of imported cases from French Polynesia, other human infections were described and the presence of autochthonous cases of ZIKV was confirmed in the Cook Islands and on Easter Island in 2014 (ECDC, 2014, ProMEDmail, 2014b, WHO, 2015).
In 2015, ZIKV reached the Americas. The first country to report the virus was Brazil, where an outbreak of exanthematic disease was described and affected more than 6000 people in Northeast region of that country (ECDC, 2015b, ProMEDmail, 2015, Zanluca et al., 2015). The state of Bahia was the first state to report autochthonous transmission of ZIKV; however, the virus easily spread across the country, where 14 states described autochthonous transmission (PAHO, 2015, WHO, 2015). Several factors may have played a role in the emergence of ZIKV in Brazil. The abundance of Ae. aegypti and Ae. albopictus vectors probably facilitated the virus emergence. There is speculation that ZIKV was introduced in Brazil through people attending in the 2014 World Cup, although many countries with reported cases of ZIKV did not participate in the competition (Salvador and Fujita, 2015). Similarly athletes attending the World canoe championship, which took place in Rio de Janeiro, may also have been responsible for ZIKV’s introduction, as many represented countries had major epidemics at the time (e.g. French Polynesia, New Caledonia, Cook Island and Chile). Concurrent phylogenetic analysis identified the Brazilian ZIKV as an Asian strain, suggesting that the virus may indeed have been entered Brazil through Asia or the South Pacific (Musso, 2015). Since ZIKV introduction in Brazil, autochthonous transmission has been reported in 31 countries/territories in the Americas (PAHO/WHO, 2016).
1.2. Origin of dengue virus and dengue disease
The earliest evidence of dengue-like disease came from reports found in the Chinese medical encyclopedia dating back to AD 265-420 (further edited in AD 610 and AD 992) (Nobuchi, 1979). The disease was linked to the presence of water-associated flying insects and thus named ‘water poison’. Other reports of dengue-like disease were described in the West Indies in 1635 and in Panama in 1699 (Howe, 1977, McSherry, 1982). Following this period, numerous epidemics of disease resembling dengue were described in the continents of Asia, Africa and North America. Between 1779 and 1788, countries including Indonesia, Egypt, Spain and USA have reported dengue-like illness (Bylon, 1780, Christie, 1881, Hirsch, 1883, Pepper, 1941, Howe, 1977) characterizing the wide geographic distribution of the disease.
In Asia, dengue viruses probably first emerged into the human population during deforestation practices for the establishment of agricultural settlements in areas adjacent to the jungle. The peridomestic Ae. albopictus mosquito was likely the bridge vector in the transmission of DENV in these areas (Gubler, 2006). Consequently, human migration and trade facilitated introduction and establishment of DENV transmission into more populated areas of tropical Asia, where the Ae. albopictus and other peridomestic Stegomyia mosquito species were abundant (Gubler, 2006).
The introduction of the anthropophilic African mosquito Ae. aegypti aegypti in Asia, as well as in the New World, was facilitated by the sea-borne and slave trade. Beginning in the 17th century, a wide distribution of Ae. aegypti was present throughout the tropics, starting in port cities and expanding inwards into the continent as part of the human urbanization expansion. As a result, a favorable environment was established for the transmission of DENV and major dengue epidemics have occurred, which rapidly became pandemics following World War II and continuing until now (Leichtenstern, 1896, Halstead, 1992, Gubler, 1997). Also, following World War II, a new dengue-associated disease affecting predominantly children was described in endemic areas of Southeast Asia (Gubler, 1998). An initial outbreak in Manila in 1953/1954, followed by a larger outbreak in Bangkok in 1958, provided the first clinical description of dengue hemorrhagic fever (DHF) (Hammon et al., 1960).
In the Americas, DENV (and Yellow Fever virus) epidemics were restricted by a control campaign initiated in 1947 by the Pan American Health Organization (PAHO) aiming to eliminate Ae. aegypti from Central and South America. However, with the suspension of the control campaign in the 1970s, the region was reinfested with Ae. aegypti and the incidence of dengue started to rise again, reaching the pre-campaign levels by 1995. Since then the geographic distribution of dengue have increased not only in the Americas, but also in other regions of the world, from non-endemic to, in some circumstances, hyperendemic levels (Gubler and Clark, 1995, Gubler, 2002, Shepard et al., 2011).
1.3. DENV transmission cycles
Dengue viruses are maintained in nature through two evolutionary and ecologicaly distinct transmission cycles: a sylvatic cycle, where the virus is transmitted among non-human primates by several arboreal Aedes spp mosquitoes, and the urban/human cycle, where virus transmission occurs between humans and mainly the domestic Ae. aegypti mosquito (Vasilakis et al., 2011).
The human transmission cycle is by far the most important cycle, considering its impact to public health and by the fact that it is occurring throughout the tropics. Although the Ae. aegypti mosquito is the major vector, the peridomestic Ae. albopictus and Ae. polynesiensis can play a role as secondary vectors of transmission (Gubler et al., 1979, Gubler and Trent, 1994). Human-to-mosquito DENV transmission depends on the magnitude of human viremia necessary to infect mosquitoes and their vector competence (Vazeille-Falcoz et al., 1999, Bennett et al., 2002). Previous studies demonstrated that none or little transmission was achieved when the blood meal titer was below 103 viral RNA copies/ml and the level of transmission reached close to 100% when a dose was above 109 viral RNA copies/ml (Nguyen et al., 2013).
1.4. Emergence of dengue virus
The emergence of all four DENV serotypes from a common sylvatic ancestor occurred thousand years ago, congruent with the establishment of early human settlements large enough to sustain transmission and was associated with vector changing from arboreal Aedes to peridomestic/domestic Aedes spp. and human reservoir hosts (Wang et al., 2000). Emergence of the serotypes occurred independently and repeatedly in allopatric regions prior to their expansion in sympatric regions, using similar non-human primate hosts (Vasilakis et al., 2010, Vasilakis et al., 2011).
Phylogenetic studies demonstrated DENV was dispersed rapidly into new locations with the advent of air travel that enabled the movement of humans during the viremic phase of infection, resulting in the shift or extinction of local lineages (Rico-Hesse et al., 1997, Carrington et al., 2005, Myat Thu et al., 2005, Diaz et al., 2006). Ecological factors are also involved in the emergence of DENV. Deforestation is one of the major factors driving sylvatic DENV emergence. As people are exploring new resources deep into the forest, living in areas previously unexplored, the chances of sylvatic DENV emergence are also increasing (Patz et al., 2004). In regions of Asia and Africa, where rapid and uncontrolled urbanization takes place, the risk of sylvatic dengue emergence is high.
1.5. Antigenic relationship of dengue viruses
Historically, flaviviruses were classified into serocomplexes based on serologic relationships, such as the virus neutralization profile (Calisher et al., 1989). Following primary DENV infection, the monotypic immune response generates a full protection against homologous viruses, but partial and transient protection, lasting for only a few months, against heterologous DENV strains (Sabin, 1952). As a result, a single person can potentially be infected with all four DENV serotypes during her lifetime (Rothman, 2011).
To determine the antigenic relationships among the DENV, it is common to represent their neutralization profile against a panel of several different sera know to react with specific DENV types (Vasilakis et al., 2008a). It has been demonstrated that sera obtained from humans during a primary infection or immunized with DENV exhibit strong homotypic neutralization against different urban and sylvatic DENV, where the heterotypic neutralization is absent or last for a short period of time (Vasilakis et al., 2008a, Vasilakis et al., 2008b). However, many times these analyses are difficult to interpret due the intrinsic variability among samples derived from different hosts or infection histories (Thomas et al., 2009, van Panhuis et al., 2010). More recently, the antigenic relationships of DENV have been studied using antigenic cartography to reduce some measurements errors of neutralization against multiple serotypes (Katzelnick et al., 2015). The analyses of a panel of human and non-human primate sera derived from experimental infection, as well vaccination and natural infection demonstrated that the majority of DENV isolates were clustered into each DENV type classification. However, a number of viruses were located more adjacent to another DENV type than its own type and the distance within and between types was similar. The neutralization profile of antisera demonstrated similar trend, with groups close to the homologous virus type, but also close to a heterologous DENV (Katzelnick et al., 2015).
1.6. Requirements for dengue emergence
Vector switching from arboreal primatophilic mosquito species to peridomestic mosquito vectors (Ae. aegypti and Ae. albopictus) may have facilitated the emergence of sylvatic strains into the urban transmission cycle (Wang et al., 2000). The expansion of non-human primates and human populations in different geographic areas allowed the sustained transmission of DENV into the major tropical regions of the world.
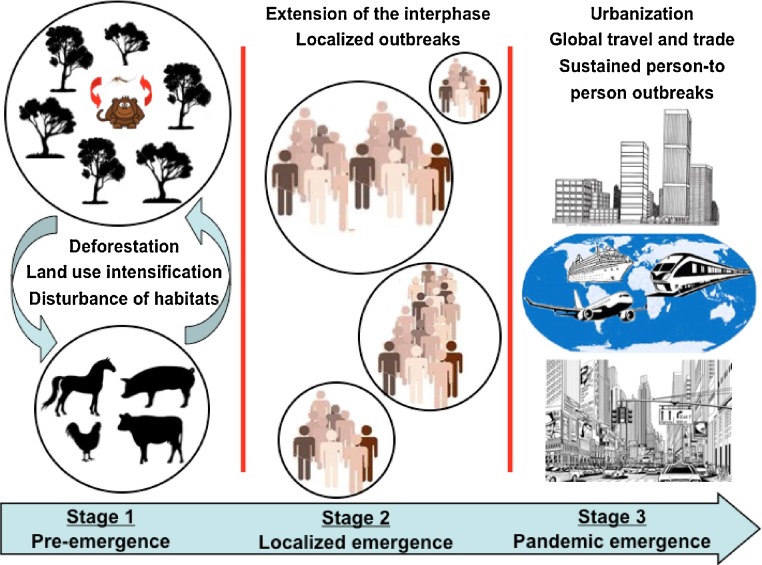
The possibility of sylvatic strains to enter the human transmission cycle was evaluated by both in vitro and in vivo human models of DENV replication. The purpose of those studies was to verify if any adaptation is required to sylvatic DENV strains been established in a new transmission cycle (Vasilakis et al., 2007). Replication of sylvatic DENV-2 in human monocytes-derived dendritic cells (moDCs) was comparable with human DENV-2 strains, suggesting they can promptly infect human hosts (Vasilakis et al., 2007). Other study using cell lines representing human (Huh-7), monkey (Vero) and mosquito (C6/36) hosts demonstrated that the human strains only have higher level of viral replication in the human cell, but virus titer were similar in the monkey and mosquito cell lines (Vasilakis et al., 2008b). Collectively, these studies demonstrate the ability of sylvatic DENV strains to replicate in a range of host cells, suggesting that their emergence in the human population is not dependent on adaptation to new hosts, but most dependent on the opportunity of the sylvatic virus to infect a wide range of hosts and eventually emerge into a human transmission cycle (Fig. 1 ).
Fig. 1.
The emergence of arboviral diseases from sylvan or rural habitats into urban areas. Distinct stages are involved in the introduction of arboviral diseases into human environments.
1.7. The impact of emerging infectious diseases
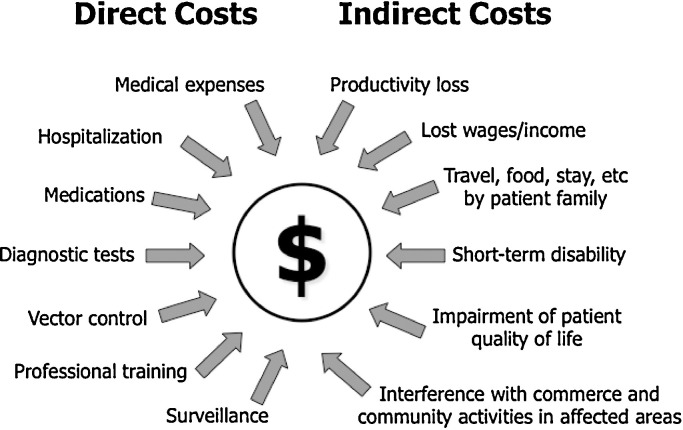
The impact of emerging infectious diseases (EIDs) is not only a public health threat, but also an economic burden, and has both direct and indirect consequences. The total investment for the development of tools for early detection of pathogens, as well as, sustainable surveillance for potential pathogens emerging into a population, are costs that must be considered as a direct consequence of EIDs economic impact (Fig. 2 ). These costs are incurred not only in diagnostic laboratory settings, but also directly in the field, in hospitals or other point-of-care (POC) health care facilities. Examples of indirect costs accountable for the economic burden of EIDs are productivity losses from work absence, short-term disability and impairment of patient quality of life (Fig. 2). Further steps following the introduction of an EID should be considered such as, training of health care and other professionals dealing with the emerging pathogen, reducing the possibility of transmission to a larger population, and treatment responses, if available (Table 1 ).
Fig. 2.
Economic impact of emerging infectious diseases. Representation of direct and indirect costs accounted for the total expenses of infectious diseases.
Table 1.
Examples of important arboviruses affecting humans.
| Virus | Family | Vector | Vertebrate hosts | Geographic distribution | References |
|---|---|---|---|---|---|
| Chikungunya | Togaviridae | Mosquitoes: Aedes and Culex spp. | Primates, birds, cattle, and rodents | Africa, Asia, Europe, Americas, Oceania | Busch and Erickson (2015), Staples et al. (2015), Vanlandingham et al. (2006) |
| Mayaro | Togaviridae | Mosquitoes: Haemagogus spp. | Primates, other mammals, birds | South and Central America | Stanton (1919), Tesh et al. (1999) |
| Ross River | Togaviridae | Mosquitoes: Aedes and Culex spp. | Marsupials, other mammals, birds | Oceania and Asia | Klapsing et al. (2005), PHAC (2014) |
| O’nyong-nyong | Togaviridae | Mosquitoes: Anopheles spp. | ? | Africa | PHAC (2011), Vanlandingham et al. (2006) |
| Sindbis | Togaviridae | Mosquitoes: Aedes, Culex, and Culiseta spp. | Birds | Europe, Africa, Oceania, Asia | ECDC (2015a), Kurkela et al. (2008) |
| Barmah Forest | Togaviridae | Mosquitoes: Aedes and Culex spp. | Birds? Marsupials, Others? | Oceania | Ehlkes et al. (2012), Naish et al. (2011) |
| Eastern equine encephalitis | Togaviridae | Mosquitoes: Culiseta, Aedes, Coquillettidia, and Culex spp. | Birds, horses, other mammals | Americas | CDC (2015a), CFSPH (2015), Weaver and Reisen (2010), Zacks and Paessler (2010) |
| Western equine encephalitis | Togaviridae | Mosquitoes: Culex, Aedes, Ochlerotatus, and Coquillettidia spp. | Birds, horses, other mammals | Americas | CFSPH (2015), Weaver and Reisen (2010), Zacks and Paessler (2010) |
| Venezuelan equine encephalitis | Togaviridae | Mosquitoes: Culex, Ochlerotatus, Anopheles, Mansonia, Psorophora, Aedes spp. and others | Horses, Rodents, Other mammals, Birds | Americas | CFSPH (2015), Weaver and Reisen (2010), Zacks and Paessler, (2010) |
| Dengue | Flaviviridae | Mosquitoes: Aedes spp | Primates | Asia, Americas, Africa, Europe, Oceania | CDC (2016a), Vasilakis and Cardosa et al. (2011) |
| Yellow Fever | Flaviviridae | Mosquitoes: Aedes and Haemogogus spp. | Primates | South America, Africa | Gershman and Staples (2015), Yactayo et al. (2015) |
| West Nile | Flaviviridae | Mosquitoes: Culex spp | Birds, Horses, Other Mammals | Africa, Asia, Europe, Oceania, Americas | CDC (2015c), Lanciotti et al. (1999), Mackenzie et al. (2004), Sambri et al. (2013) |
| Japanese encephalitis | Flaviviridae | Mosquitoes: Culex spp | Birds, Pigs | Asia, Oceania | Erlanger et al. (2009), Han et al. (2014), Huang et al. (2015), Mackenzie et al. (2004) |
| Murray Valley encephalitis | Flaviviridae | Mosquitoes: Culex spp | Birds | Oceania | CDC (2013a), Mackenzie et al. (2004), Selvey et al. (2014) |
| Zika virus | Flaviviridae | Mosquitoes: Aedes spp | Primates | Africa, Asia, Oceania, Central and South America | Campos et al. (2015), CDC (2016b); Hayes, (2009), WHO (2015) |
| Rocio | Flaviviridae | Mosquitoes: Psorophora and Aedes spp | Birds | South America | Medeiros et al. (2007), Mitchell et al. (1986), Silva et al. (2014) |
| St. Louis encephalitis | Flaviviridae | Mosquitoes: Culex spp | Birds, Bats, Other Mammals | Americas | CDC, (2010), Kopp et al., (2013), Reisen (2003) |
| Kyasanur Forest disease | Flaviviridae | Ticks: Hemaphysalis spp. | Primates, Rodents, Other Mammals | Asia | CDC (2014), Holbrook (2012) |
| Omsk hemorrhagic fever | Flaviviridae | Ticks: Dermacentor and Ixodes spp Mosquitoes:? |
Rodents, Volves, Other Mammals | Europe | CDC (2013b), Ruzek et al. (2010) |
| Tick-borne encephalitis | Flaviviridae | Ticks: Ixodes spp | Rodents, Goats, Sheep, Cows, Other Mammals, Birds? | Europe, Asia | Bogovic and Strle (2015), Fischer et al. (2015) |
| Sandfly fever | Bunyaviridae | Sandflies: Phlebotomus spp. |
Birds? Mammals? | Europe, Asia, Africa | Guler et al. (2012), Tufan and Tasyaran (2013) |
| Rift Valley fever | Bunyaviridae | Mosquitoes: Aedes, Ochlerotatus, Stegomyia, Anopheles, Culex, Neomelaniconion, Eretmapodites and others | Cows, Sheep, Camels, Goats and Other Mammals | Africa, Asia | Nanyingi et al. (2015), Olive et al., (2012), Tantely et al. (2015) |
| La Crosse encephalitis | Bunyaviridae | Mosquitoes: Aedes spp | Rodents | North America | CDC (2015b), Harris et al., (2015) |
| Crimean-Congo hemorrhagic fever | Bunyaviridae | Ticks: Hyalomma spp | Cows, Sheep, Goats, Hares and Other Mammals | Europe, Asia, Africa | Chinikar et al. (2008), Shayan et al., (2015), Tuncer et al. (2014), Whitehouse (2004) |
| Oropouche | Bunyaviridae | Midges: Culicoides sp | Primates? Sloths? Birds? | Central and South America | Anderson et al. (1961), Mourao et al. (2009), Nunes et al., (2005), Vasconcelos et al. (2011) |
| Severe febrile thrombocytopenia syndrome | Bunyaviridae | Ticks: Haemaphysalis sp | ? | Asia | Takahashi et al. (2014), Yu et al. (2011), Yun et al. (2013) |
| Chandipura | Rhabdoviridae | Sandflies: Phlebotomus and Sergentomyia spp. |
Hedgehogs, Others? |
Asia and Africa |
Fontenille et al. (1994), Maiti et al. (2014), Menghani et al. (2012), Rao et al. (2004), Tesh, (1988), Tesh and Modi, (1983) |
| Bluetongue | Reoviridae | Midges: Culicoides spp | Sheep, Cows, Other Mammals | Africa, Asia, Europe, Oceania, Americas (all except Antarctica) | Maclachlan (2011), OIE (2013) |
The World Economic Forum has listed the spread of EIDs as one of the top risk factors to cause potential economic loss to the world population (WEF, 2015). Although the economic impact of EIDs is difficult to be accurately determined, several studies have been conducted to estimate their economic burden to society (Newcomb, 2003, Zohrabian et al., 2004, Zohrabian et al., 2006, Barber et al., 2010). For example, during the emergence of severe acute respiratory syndrome (SARS) in 2003 in China, the virus rapidly spread to several countries in Asia, Europe and South and North America, in only a few months, affecting 8098 people resulting in 774 deaths (CDC, 2003). Its economic impact was estimated between 50 and 100 billion U.S. dollars (Newcomb, 2003). The economic impact of the 2002 outbreak of West Nile virus (WNV) in Louisiana, which resulted in 24 deaths of the total 329 reported cases, was estimated to cost approximately 20 million U.S. dollars. These costs included inpatient and outpatient visits, loss of work productivity, costs incurred by public health departments and mosquito control agencies (Zohrabian et al., 2004, Zohrabian et al., 2006). The spread of WNV in California and an outbreak in Sacramento County in 2005 resulted in 163 human cases, whose economic impact was estimated to be near 3 million U.S. dollars, which included medical visits and treatment, job productivity loss and mosquito control (Barber et al., 2010).
Additional studies have also attempted to anticipate the cost of potential outbreaks. In Australia, as one example of an isolated geographic area, the introduction of exotic diseases, as well as, pests and weeds could have a potential cost of over $1 billion Australian dollars (Murray et al., 2012). A study on the next influenza pandemic in the United States, estimated 89,000–207,000 deaths and an economic loss of 71.3–166.5 billion U.S. dollars. The cost was based on estimations for patient hospitalizations, outpatient visits and expenses for drug treatment and did not account for indirect costs interfering with commerce and community activities in affected areas (Meltzer et al., 1999). Overall, these examples highlight the impact EIDs can create for human populations and demonstrate the importance of controlling these diseases. One example would be the use of immunizations, when a vaccine is available.
2. Conclusions
The majority of viruses with potential to produce important epidemics are zoonotic, which means that they are originated in an animal hosts and are driven by several emergence forces, including changes in ecological and social behaviors, supporting the possibility to spill over into the human population. The understanding of the potential for spread of emerging infectious diseases is essential in the prevention and control of large-scale outbreaks and effective use of resources to combat them. Knowing the source and modes to human transmission allows the prediction of disease appearance and implementation of global measures to eliminate the risk. As example, the eradication campaigns initiated in the late 40’s to eliminate Ae. aegypti from Central and South America were effective during the time they were in effect because of government support, rigorous compliance and population support, which demonstrates the importance and effectiveness of coordinated global measures to combat diseases. Isolated prevention measures won’t have the same results, or even been completely unsuccessful. Nowadays, with the advance of new technologies for detection, treatment and control of diseases and quick and easy dissemination of information, prevention measures should be more effective and time of response should be much shorter. Based on the current knowledge, it seems that a successful program will require the integration of different segments involved in the detection, treatment and prevention of diseases including diagnostic labs, hospitals, and government agencies, among others.
In the case of dengue as one example of global human threat, to clearly comprehend and anticipate the occurrence of sylvatic DENV emergence is fundamental to clarify the ecological and epidemiological aspects related to this virus cycle. There is enough evidence to support the existence of endemic serotypes as a result of independent events through cross-species transmission of sylvatic DENV. However, there is clear indication that sylvatic DENV come into close contact with humans in Asia and Africa, and possibly in other parts of the world, originating sporadic severe dengue disease that can spillover in the urban environment. The sylvatic cycle of DENV has not being intensively explored and not considerable attention is given to the consequences involved in viruses coming from unexplored habitats. Additionally, different of what was proposed in the past, recent studies indicated that the emergence of sylvatic DENV represent a real threat to people considering the inexistence of an adaptation barrier to sylvatic viruses emerge into the human population. Moreover, the diversity of DENV strains and the emergence of new isolates have important consequences in the development of therapeutics, including vaccines currently in the developmental and clinical trial phases.
The establishment of preventive measures and surveillance of current and newly identified infectious diseases should be based on several factors, many of them discussed in this review, starting with the knowledge of the disease ecology, human behavior, socio-economic factors of a target population or area, among others. Policy-makers and the distribution of resources must consider not only short-term measures, but also long-term goals to maintain the infrastructure and research programs, from basic science through translational research. Nowadays, human travel and rapid transportation of products, live animals, insects, and so forth, not only locally, but around the world have the potential for quick dissemination or re-emergence of diseases. Anthropogenic land-use changes, especially intensification of agriculture and livestock production, increase the risk of pathogen spillover from wildlife hosts to the human population. Knowing the dynamic of diseases allows more effective surveillance and implementation of strategies that are critical for their control as well for the allocation of scarce financial resources.
Acknowledgements
This work was supported by grant 1U01AI115577 from the U.S. National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors have no conflicting financial interests.
References
- Anderson C.R., Spence L. Oropouche virus: a new human disease agent from Trinidad, West Indies. Am. J. Trop. Med. Hyg. 1961;10:574–578. doi: 10.4269/ajtmh.1961.10.574. [DOI] [PubMed] [Google Scholar]
- Arias-Goeta C., Mousson L. Dissemination and transmission of the E1-226V variant of chikungunya virus in Aedes albopictus are controlled at the midgut barrier level. PLoS One. 2013;8(2):e57548. doi: 10.1371/journal.pone.0057548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubry M., Teissier A. Serosurvey of dengue, Zika and other mosquito-borne viruses in French Polynesia. ASTMH 64th Annual Meeting; Philadelphia, PA; 2015. (ASTMH) [Google Scholar]
- Barber L.M., Schleier J.J., 3rd Economic cost analysis of west nile virus outbreak, sacramento county, california, USA, 2005. Emerg. Infect. Dis. 2010;16(3):480–486. doi: 10.3201/eid1603.090667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett K.E., Olson K.E. Variation in vector competence for dengue 2 virus among 24 collections of Aedes aegypti from Mexico and the United States. Am. J. Trop. Med. Hyg. 2002;67(1):85–92. doi: 10.4269/ajtmh.2002.67.85. [DOI] [PubMed] [Google Scholar]
- Bisset J.A., Marin R. Insecticide resistance in two aedes aegypti (Diptera: culicidae) strains from Costa Rica. J. Med. Entomol. 2013;50(2):352–361. doi: 10.1603/me12064. [DOI] [PubMed] [Google Scholar]
- Bogovic P., Strle F. Tick-borne encephalitis: a review of epidemiology, clinical characteristics, and management. World J. Clin. Cases. 2015;3(5):430–441. doi: 10.12998/wjcc.v3.i5.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch M., Erickson G. An overview of Chikungunya virus. JAAPA. 2015;28(10):54–57. doi: 10.1097/01.JAA.0000470441.99693.e1. [DOI] [PubMed] [Google Scholar]
- Bylon D. Korte Aantekening, wegens eene Algemeene Ziekte: Doorgaans Genaamd de Knokkel-Koorts. Verhandelungen van het bataviaasch Genootschop der Konsten in Wetenschappen; Batavia; 1780. pp. 17–30. [Google Scholar]
- CDC Revised U.S. surveillance case definition for severe acute respiratory syndrome (SARS) and update on SARS cases–United States and worldwide, December 2003. MMWR. Morb. Mortal. Wkly. Rep. 2003;52(49):1202–1206. [PubMed] [Google Scholar]
- CDC . 2010. Saint Louis Encephalitis. (Retrieved December 5 2015 from http://www.cdc.gov/sle/) [Google Scholar]
- CDC . 2013. Murray Valley Encephalitis. (Retrieved November 19, 2015, from http://wwwnc.cdc.gov/travel/diseases/murray-valley-encephalitis-virus) [Google Scholar]
- CDC . 2013. Omsk Hemorrhagic Fever (OHF) (Retrieved December 4, 2015, from http://www.cdc.gov/vhf/omsk/index.html) [Google Scholar]
- CDC . 2014. Kyasanur Forest Disease (KFD) (Retrieved December 4, 2015, from http://www.cdc.gov/vhf/kyasanur/) [Google Scholar]
- CDC . 2015. Eastern Equine Encephalomyelitis. (Retrieved November 25, 2015, from http://www.cdc.gov/EasternEquineEncephalitis/index.html) [Google Scholar]
- CDC . 2015. La Crosse Encephalitis. (Retrieved November 18, 2015, from http://www.cdc.gov/lac/tech/virus.html) [Google Scholar]
- CDC . 2015. West Nile Virus. (Retrieved November 19, 2015, from http://www.cdc.gov/westnile/) [Google Scholar]
- CDC . 2016. Dengue. (Retrieved March 6, 2016, from http://www.cdc.gov/Dengue/) [Google Scholar]
- CDC . 2016. Zika Virus. (Retrieved March 6, 2016, from http://www.cdc.gov/zika/index.html) [Google Scholar]
- CFSPH . Iowa State University; 2015. Eastern, Western and Venezuelan Equine Encephalomyelitis. College of Veterinary Medicine. (Retrieved November 25, 2015, from http://www.cfsph.iastate.edu/Factsheets/pdfs/easter_wester_venezuelan_equine_encephalomyelitis.pdf) [Google Scholar]
- Calisher C.H., Karabatsos N. Arbovirus serogroups: definition and geographic distribution. In: Monath T.P., editor. vol. 1. CRC Press; Boca Raton, FL: 1988. pp. 19–57. (The Arboviruses: Epidemiology and Ecology). [Google Scholar]
- Calisher C.H., Karabatsos N. Antigenic relationships between flaviviruses as determined by cross-neutralization tests with polyclonal antisera. J. Gen. Virol. 1989;70(Pt. 1):37–43. doi: 10.1099/0022-1317-70-1-37. [DOI] [PubMed] [Google Scholar]
- Calisher C.H., Childs J.E. Bats: important reservoir hosts of emerging viruses. Clin. Microbiol. Rev. 2006;19(3):531–545. doi: 10.1128/CMR.00017-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos G.S., Bandeira A.C. Zika virus outbreak, Bahia, Brazil. Emerg. Infect. Dis. 2015;21(10):1885–1886. doi: 10.3201/eid2110.150847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao-Lormeau V.M., Roche C. Recent emergence of dengue virus serotype 4 in French Polynesia results from multiple introductions from other South Pacific Islands. PLoS One. 2011;6(12):e29555. doi: 10.1371/journal.pone.0029555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao-Lormeau V.M., Roche C. Zika virus, French polynesia, South pacific, 2013. Emerg. Infect. Dis. 2014;20(6):1085–1086. doi: 10.3201/eid2006.140138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington C.V., Foster J.E. Invasion and maintenance of dengue virus type 2 and type 4 in the Americas. J. Virol. 2005;79(23):14680–14687. doi: 10.1128/JVI.79.23.14680-14687.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinikar S. Surveillance and laboratory detection system of Crimean-Congo haemorrhagic fever in Iran. Transbound. Emerg. Dis. 2008;55(5–6):200–204. doi: 10.1111/j.1865-1682.2008.01028.x. [DOI] [PubMed] [Google Scholar]
- Chretien J.P., Anyamba A. Drought-associated chikungunya emergence along coastal East Africa. Am. J. Trop. Med. Hyg. 2007;76(3):405–407. [PubMed] [Google Scholar]
- Christie J. On epidemics of dengue fever: their diffusion and etiology. Glasg. Med. J. 1881;16:161–176. [PMC free article] [PubMed] [Google Scholar]
- Diallo M., Thonnon J. Vectors of Chikungunya virus in Senegal: current data and transmission cycles. Am. J. Trop. Med. Hyg. 1999;60(2):281–286. doi: 10.4269/ajtmh.1999.60.281. [DOI] [PubMed] [Google Scholar]
- Diallo D., Sall A.A. Landscape ecology of sylvatic chikungunya virus and mosquito vectors in southeastern Senegal. PLoS Negl. Trop. Dis. 2012;6(6):e1649. doi: 10.1371/journal.pntd.0001649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz F.J., Black W.C.t. Dengue virus circulation and evolution in Mexico: a phylogenetic perspective. Arch. Med. Res. 2006;37(6):760–773. doi: 10.1016/j.arcmed.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Dick G.W., Kitchen S.F. Zika virus. I: Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952;46(5):509–520. doi: 10.1016/0035-9203(52)90042-4. [DOI] [PubMed] [Google Scholar]
- Duffy M.R., Chen T.H. Zika virus outbreak on Yap island, Federated states of Micronesia. N. Engl. J. Med. 2009;360(24):2536–2543. doi: 10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]
- Dupont-Rouzeyrol M., O'Connor O. Co-infection with zika and dengue viruses in 2 patients, New Caledonia, 2014. Emerg. Infect. Dis. 2015;21(2):381–382. doi: 10.3201/eid2102.141553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECDC . 2014. Monitoring current threats: ECDC Communicable Disease Threats Report (CDTR), week 11/2014. (Retrieved December 20, 2015, from http://ecdc.europa.eu/en/press/news/_layouts/forms/News_DispForm.aspx?List=8db7286c-fe2d-476c-9133-18ff4cb1b568&ID=967) [Google Scholar]
- ECDC . 2015. Sindbis fever. European Centre for Disease Prevention and Control (ECDC) (Retrieved December 4, 2015, from http://ecdc.europa.eu/en/healthtopics/sindbis_fever/Pages/index.aspx) [Google Scholar]
- ECDC . 2015. Zika virus infection outbreak, Brazil and the Pacific region – 25 May 2015. (Retrieved December 20, 2015, from http://ecdc.europa.eu/en/publications/Publications/rapid-risk-assessment-Zika%20virus-south-america-Brazil-2015.pdf) [Google Scholar]
- Ehlkes L. Surveillance should be strengthened to improve epidemiological understandings of mosquito-borne Barmah Forest virus infection. West. Pac. Surveill. Response J. 2012;3(3):63–68. doi: 10.5365/WPSAR.2012.3.1.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlanger T.E. Past: present, and future of Japanese encephalitis. Emerg. Infect. Dis. 2009;15(1):1–7. doi: 10.3201/eid1501.080311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M., Rabe I.B., Rollin P.E. 2015. Tickborne Encephalitis. CDC Health Information for International Travel 2016. (Available from: http://wwwnc.cdc.gov/travel/yellowbook/2016/infectious-diseases-related-to-travel/tickborne-encephalitis) [Google Scholar]
- Fontenille D. First isolations of arboviruses from phlebotomine sand flies in West Africa. Am. J. Trop. Med. Hyg. 1994;50(5):570–574. doi: 10.4269/ajtmh.1994.50.570. [DOI] [PubMed] [Google Scholar]
- Foy B.D., Kobylinski K.C. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg. Infect. Dis. 2011;17(5):880–882. doi: 10.3201/eid1705.101939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershman M.D., Staples J.E. 2015. Yellow Fever. CDC Health Information for International Travel 2016. ([cited 2015 December 07]; Available from: http://wwwnc.cdc.gov/travel/yellowbook/2016/infectious-diseases-related-to-travel/yellow-fever) [Google Scholar]
- Grandadam M., Caro V. Chikungunya virus, southeastern France. Emerg. Infect. Dis. 2011;17(5):910–913. doi: 10.3201/eid1705.101873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler D.J., Clark G.G. Dengue/dengue hemorrhagic fever: the emergence of a global health problem. Emerg. Infect. Dis. 1995;1(2):55–57. doi: 10.3201/eid0102.952004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler D.J., Trent D.W. Emergence of epidemic dengue/dengue hemorrhagic fever as a public health problem in the Americas. Infect. Agents Dis. 1994;2:383–393. [PubMed] [Google Scholar]
- Gubler, D.J., Vasilakis, N., (2016). The Arboviruses: Quo Vadis? in preparation.
- Gubler D.J., Nalim S. Variation in susceptibility to oral infection with dengue viruses among geographic strains of Aedes aegypti. Am. J. Trop. Med. Hyg. 1979;28(6):1045–1052. doi: 10.4269/ajtmh.1979.28.1045. [DOI] [PubMed] [Google Scholar]
- Gubler D.J. Human behaviour and cultural context in disease control. Trop. Med. Int. Health. 1997;2(11):A1–2. [PubMed] [Google Scholar]
- Gubler D.J. The global pandemic of dengue/dengue haemorrhagic fever: current status and prospects for the future. Ann. Acad. Med. Singapore. 1998;27(2):227–234. [PubMed] [Google Scholar]
- Gubler D.J. The global emergence/resurgence of arboviral diseases as public health problems. Arch. Med. Res. 2002;33(4):330–342. doi: 10.1016/s0188-4409(02)00378-8. [DOI] [PubMed] [Google Scholar]
- Gubler D.J. Dengue/dengue haemorrhagic fever: history and current status. Novartis Found. Symp. 2006;277:3–16. doi: 10.1002/0470058005.ch2. discussion 16–22, 71–3, 251–3. [DOI] [PubMed] [Google Scholar]
- Guler S. A sandfly fever virus outbreak in the East Mediterranean region of Turkey. Int. J. Infect. Dis. 2012;16(4):e244–6. doi: 10.1016/j.ijid.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Haddow A.D., Schuh A.J. Genetic characterization of Zika virus strains: geographic expansion of the Asian lineage. PLoS Negl. Trop. Dis. 2012;6(2):e1477. doi: 10.1371/journal.pntd.0001477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B.H., Shaw G.M. AIDS as a zoonosis: scientific and public health implications. Science. 2000;287(5453):607–614. doi: 10.1126/science.287.5453.607. [DOI] [PubMed] [Google Scholar]
- Halstead S.B. The XXth century dengue pandemic: need for surveillance and research. World Health Stat. Q. 1992;45(2–3):292–298. [PubMed] [Google Scholar]
- Hammon W.M., Rudnick A. Viruses associated with epidemic hemorrhagic fevers of the Philippines and Thailand. Science. 1960;131(3407):1102–1103. doi: 10.1126/science.131.3407.1102. [DOI] [PubMed] [Google Scholar]
- Han N., Adams J. Comparison of genotypes I and III in Japanese encephalitis virus reveals distinct differences in their genetic and host diversity. J. Virol. 2014;88(19):11469–11479. doi: 10.1128/JVI.02050-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M.C. La crosse virus field detection and vector competence of culex mosquitoes. Am. J. Trop. Med. Hyg. 2015;93(3):461–467. doi: 10.4269/ajtmh.14-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes E.B. Zika virus outside africa. Emerg. Infect. Dis. 2009;15(9):1347–1350. doi: 10.3201/eid1509.090442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs S., Beaty B.J. In: Natural Cycles of Vector-borne Pahogens Biology of Disease Vectors. Marquardt M.C., editor. Elsevier Academic Press; New York, NY, USA: 2005. pp. 167–185. [Google Scholar]
- Hirsch A. Dengue, a comparatively new desease: its symptoms. In: Creighton C., editor. Vol. I. Syndenham Society; 1883. pp. 55–81. (Handbook of Geographical and Historical Pathology). [Google Scholar]
- Hochedez P., Jaureguiberry S. Chikungunya infection in travelers. Emerg. Infect. Dis. 2006;12(10):1565–1567. doi: 10.3201/eid1210.060495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook M.R. Kyasanur forest disease. Antiviral Res. 2012;96(3):353–362. doi: 10.1016/j.antiviral.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe G.M. Academic Press; New York, NY: 1977. A World Geography of Human Disease. [Google Scholar]
- Huang Y.J. Susceptibility of a north american culex quinquefasciatus to Japanese encephalitis virus. Vector Borne Zoonotic Dis. 2015;15(11):709–711. doi: 10.1089/vbz.2015.1821. [DOI] [PubMed] [Google Scholar]
- I.L.R. Institute . International Livestock Research Institute; Nairobi, Kenya: 2012. Mapping of Poverty and Likely Zoonoses Hotspots. Zoonoses Project 4. Report to Department for International Development, UK. [Google Scholar]
- Jones K.E., Patel N.G. Global trends in emerging infectious diseases. Nature. 2008;451(7181):990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jupp P.G., McIntosh B.M. In: Monath T.P., editor. vol. II. CRC Press; Boca Raton, FL: 1988. pp. 137–157. (Chikungunya Virus Disease. The Arbovirus: Epidemiology and Ecology). [Google Scholar]
- Karabatsos N. International catalogue of arboviruses, including certain other viruses of vertebrates. Am. Soc. Trop. Med. Hyg. (San Antonio, TX, USA) 1985. 2001 doi: 10.4269/ajtmh.1978.27.372. (2001 update) [DOI] [PubMed] [Google Scholar]
- Karesh W.B., Dobson A. Ecology of zoonoses: natural and unnatural histories. Lancet. 2012;380(9857):1936–1945. doi: 10.1016/S0140-6736(12)61678-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzelnick L.C., Fonville J.M. Dengue viruses cluster antigenically but not as discrete serotypes. Science. 2015;349(6254):1338–1343. doi: 10.1126/science.aac5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A.M.Q., Lefkowitz E. Elsevier; 2011. Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses. [Google Scholar]
- Klapsing P., MacLean J.D. Ross river virus disease reemergence, Fiji, 2003–2004. Emerg. Infect. Dis. 2005;11(4):613–615. doi: 10.3201/eid1104.041070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp A., Gillespie T.R. Provenance and geographic spread of St. Louis encephalitis virus. MBio. 2013;4(3):e00313–e00322. doi: 10.1128/mBio.00322-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurkela S., Ratti O. Sindbis virus infection in resident birds, migratory birds, and humans, Finland. Emerg. Infect. Dis. 2008;14(1):41–47. doi: 10.3201/eid1401.070510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsuna S., Kato Y. Two cases of zika fever imported from French Polynesia to Japan, december 2013 to january 2014 [corrected] Euro Surveill. 2014;19(4) doi: 10.2807/1560-7917.es2014.19.4.20683. [DOI] [PubMed] [Google Scholar]
- Laigret J., Rosen L. On an epidemic of dengue occurring in Tahiti in 1964. Relations to the hemorrhagic fevers of Southeast Asia. Bull. Soc. Pathol. Exot. Filiales. 1967;60(14):339–353. [PubMed] [Google Scholar]
- Lanciotti R.S., Roehrig J.T. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science. 1999;286(5448):2333–2337. doi: 10.1126/science.286.5448.2333. [DOI] [PubMed] [Google Scholar]
- Lanciotti R.S., Kosoy O.L. Chikungunya virus in US travelers returning from India, 2006. Emerg. Infect. Dis. 2007;13(5):764–767. doi: 10.3201/eid1305.070015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leichtenstern O. In: Nothnagel H., editor. vol. IV. Alfred Holder; Wien: 1896. pp. 133–226. (Influenza and Dengue Specielle Pathologie Und Therapie). [Google Scholar]
- Leisnham P.T., LaDeau S.L. Spatial and temporal habitat segregation of mosquitoes in urban Florida. PLoS One. 2014;9(3):e91655. doi: 10.1371/journal.pone.0091655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leparc-Goffart I., Nougairede A. Chikungunya in the Americas. Lancet. 2014;383(9916):514. doi: 10.1016/S0140-6736(14)60185-9. [DOI] [PubMed] [Google Scholar]
- Levins R., Epstein P.R. Hantavirus disease emerging. Lancet. 1993;342(8882):1292. doi: 10.1016/0140-6736(93)92376-5. [DOI] [PubMed] [Google Scholar]
- Lloyd-Smith J.O., George D. Epidemic dynamics at the human-animal interface. Science. 2009;326(5958):1362–1367. doi: 10.1126/science.1177345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie J.S., Gubler D.J., Petersen L.R. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat. Med. 2004;10(12 Suppl):S98–109. doi: 10.1038/nm1144. [DOI] [PubMed] [Google Scholar]
- Maclachlan N.J. Bluetongue: history, global epidemiology, and pathogenesis. Prev. Vet. Med. 2011;102(2):107–111. doi: 10.1016/j.prevetmed.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Macnamara F.N. Zika virus: a report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans. R. Soc. Trop. Med. Hyg. 1954;48(2):139–145. doi: 10.1016/0035-9203(54)90006-1. [DOI] [PubMed] [Google Scholar]
- Maiti D., Halder P. Chandipura virus: another exotic tropical disease? J. Res. Med. Den. Sci. 2014;2(3):1–5. [Google Scholar]
- Marchette N.J., Garcia R. Isolation of Zika virus from Aedes aegypti mosquitoes in Malaysia. Am. J. Trop. Med. Hyg. 1969;18(3):411–415. doi: 10.4269/ajtmh.1969.18.411. [DOI] [PubMed] [Google Scholar]
- May R.M., Gupta S. Infectious disease dynamics: what characterizes a successful invader. Philos. Trans. R, Soc. Lond. B: Biol. Sci. 2001;356(1410):901–910. doi: 10.1098/rstb.2001.0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSherry J.A. Some medical aspects of the Darien scheme: was it dengue? Scott. Med. J. 1982;27(2):183–184. doi: 10.1177/003693308202700215. [DOI] [PubMed] [Google Scholar]
- Medeiros D.B., Nunes M.R. Complete genome characterization of Rocio virus (Flavivirus: flaviviridae), a Brazilian flavivirus isolated from a fatal case of encephalitis during an epidemic in Sao Paulo state. J. Gen. Virol. 2007;88(Pt. 8):2237–2246. doi: 10.1099/vir.0.82883-0. [DOI] [PubMed] [Google Scholar]
- Meltzer M.I., Cox N.J. The economic impact of pandemic influenza in the United States: priorities for intervention. Emerg. Infect. Dis. 1999;5(5):659–671. doi: 10.3201/eid0505.990507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menghani S., Chikhale R. Chandipura Virus: an emerging tropical pathogen. Acta Trop. 2012;124(1):1–14. doi: 10.1016/j.actatropica.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Mitchell C.J., Forattini O.P., Miller B.R. Vector competence experiments with Rocio virus and three mosquito species from the epidemic zone in Brazil. Rev. Saude Publica. 1986;20(3):171–177. doi: 10.1590/s0034-89101986000300001. [DOI] [PubMed] [Google Scholar]
- Morse S.S. Hantaviruses and the hantavirus outbreak in the United States: a case study in disease emergence. Ann. N. Y. Acad. Sci. 1994;740:199–207. doi: 10.1111/j.1749-6632.1994.tb19870.x. [DOI] [PubMed] [Google Scholar]
- Morse S.S. Factors in the emergence of infectious diseases. Emerg. Infect. Dis. 1995;1(1):7–15. doi: 10.3201/eid0101.950102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourao M.P. Oropouche fever outbreak: manaus, Brazil, 2007–2008. Emerg. Infect. Dis. 2009;15(12):2063–2064. doi: 10.3201/eid1512.090917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray K.A., Skerratt L.F. Cooling off health security hot spots: getting on top of it down under. Environ. Int. 2012;48:56–64. doi: 10.1016/j.envint.2012.06.015. [DOI] [PubMed] [Google Scholar]
- Musso D., Nilles E.J. Rapid spread of emerging Zika virus in the Pacific area. Clin. Microbiol. Infect. 2014;20(10):O595–6. doi: 10.1111/1469-0691.12707. [DOI] [PubMed] [Google Scholar]
- Musso D., Roche C. Detection of Zika virus in saliva. J. Clin. Virol. 2015;68:53–55. doi: 10.1016/j.jcv.2015.04.021. [DOI] [PubMed] [Google Scholar]
- Musso D. Zika virus transmission from French Polynesia to Brazil. Emerg. Infect. Dis. 2015;21(10):1887. doi: 10.3201/eid2110.151125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myat Thu H., Lowry K. Lineage extinction and replacement in dengue type 1 virus populations are due to stochastic events rather than to natural selection. Virology. 2005;336(2):163–172. doi: 10.1016/j.virol.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Naish S., Hu W. Spatio-temporal patterns of Barmah forest virus disease in Queensland, Australia. PLoS One. 2011;6(10):e25688. doi: 10.1371/journal.pone.0025688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanyingi M.O., Munyua P. A systematic review of rift valley fever epidemiology 1931–2014. Infect. Ecol. Epidemiol. 2015;5:28024. doi: 10.3402/iee.v5.28024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb J., Harrington T. Bio Economic Research Associates; Cambridge, MA: 2011. The Economic Impact of Selected Infectious Disease Outbreaks. [Google Scholar]
- Newcomb J. Bio Economic Research Associates; Cambridge, MA: 2003. Biology and Borders: SARS and the New Economics of Biosecurity. [Google Scholar]
- Nguyen T.T., Lee S. In vitro evaluation of novel inhibitors against the NS2B-NS3 protease of dengue fever virus type 4. Molecules. 2013;18(12):15600–15612. doi: 10.3390/molecules181215600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobuchi H. The Symptoms of a dengue-like illness recorded in a Chinese medical encyclopedia (In Japanese) Kanpo no Rinsho. 1979;26:422–425. [Google Scholar]
- Nunes M.R., Martins L.C. Oropouche virus isolation, southeast Brazil. Emerg. Infect. Dis. 2005;11(10):1610–1613. doi: 10.3201/eid1110.050464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes M.R., Faria N.R. Emergence and potential for spread of Chikungunya virus in Brazil. BMC Med. 2015;13(102) doi: 10.1186/s12916-015-0348-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OIE . 2013. Bluetongue. (Retrieved November 12, 2015, from http://www.oie.int/fileadmin/Home/eng/Animal_Health_in_the_World/docs/pdf/Disease_cards/BLUETONGUE.pdf) [Google Scholar]
- Olive M.M., Goodman S.M., Reynes J.M. The role of wild mammals in the maintenance of Rift valley fever virus. J. Wildl. Dis. 2012;48(2):241–266. doi: 10.7589/0090-3558-48.2.241. [DOI] [PubMed] [Google Scholar]
- Olson J.G., Ksiazek T.G. Zika virus, a cause of fever in Central Java, Indonesia. Trans. R. Soc. Trop. Med. Hyg. 1981;75(3):389–393. doi: 10.1016/0035-9203(81)90100-0. [DOI] [PubMed] [Google Scholar]
- PAHO . 2015. Epidemiological Update. Zika Virus Infection 16 October 2015. (Retrieved December 20, 2015, from http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=32021&lang=en) [Google Scholar]
- PAHO/WHO . 2016. Zika − Epidemiological Update, 10 March 2016. (Retrieved May 13 2016 from www.paho.org) [Google Scholar]
- PHAC . 2011. O'Nyong-Nyong virus. (Retrieved December 4 2015 from http://www.phac-aspc.gc.ca/lab-bio/res/psds-ftss/msds130e-eng.php) [Google Scholar]
- PHAC . 2014. Ross river virus. (Retrieved December 4 2015 from http://www.phac-aspc.gc.ca/lab-bio/res/psds-ftss/msds170e-eng.php) [Google Scholar]
- Patz J.A., Daszak P. Unhealthy landscapes: policy recommendations on land use change and infectious disease emergence. Environ. Health Perspect. 2004;112(10):1092–1098. doi: 10.1289/ehp.6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper P. A note on David Bylon and dengue. Ann. Med. Hist. 1941;3:363–368. [PMC free article] [PubMed] [Google Scholar]
- Powers A.M., Brault A.C. Re-emergence of Chikungunya and O'nyong-nyong viruses: evidence for distinct geographical lineages and distant evolutionary relationships. J. Gen. Virol. 2000;81(Pt. 2):471–479. doi: 10.1099/0022-1317-81-2-471. [DOI] [PubMed] [Google Scholar]
- ProMEDmail . 2014. Zika virus − Pacific (03): New Caledonia. (Retrieved December 20, 2015, from http://promedmail.org/post/20140122.2224823) [Google Scholar]
- ProMEDmail . 2014. Zika virus − Pacific (07): Chile (Easter Island), French Polynesia. (Retrieved December 20, 2015, from http://www.promedmail.org/direct.php?id=2322907) [Google Scholar]
- ProMEDmail . 2015. Umdiagnosed Illness − Brazil: (Northeast, Rio De Janeiro) Zika Virus Suspected, Request For Information. (Retrieved December 20, 2015, from http://promedmail.chip.org/pipermail/promed/2015-May/007263.html) [Google Scholar]
- Rao B.L. A large outbreak of acute encephalitis with high fatality rate in children in Andhra Pradesh: india, in 2003, associated with Chandipura virus. Lancet. 2004;364(9437):869–874. doi: 10.1016/S0140-6736(04)16982-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisen W.K. Epidemiology of St. louis encephalitis virus. Adv. Virus Res. 2003;61:139–183. doi: 10.1016/s0065-3527(03)61004-3. [DOI] [PubMed] [Google Scholar]
- Rezza G., Nicoletti L. Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet. 2007;370(9602):1840–1846. doi: 10.1016/S0140-6736(07)61779-6. [DOI] [PubMed] [Google Scholar]
- Rico-Hesse R., Harrison L.M. Origins of dengue type 2 viruses associated with increased pathogenicity in the Americas. Virology. 1997;230(2):244–251. doi: 10.1006/viro.1997.8504. [DOI] [PubMed] [Google Scholar]
- Rothman A.L. Immunity to dengue virus: a tale of original antigenic sin and tropical cytokine storms. Nat. Rev. Immunol. 2011;11(8):532–543. doi: 10.1038/nri3014. [DOI] [PubMed] [Google Scholar]
- Ruzek D., Yakimenko V.V. Omsk haemorrhagic fever. Lancet. 2010;376(9758):2104–2113. doi: 10.1016/S0140-6736(10)61120-8. [DOI] [PubMed] [Google Scholar]
- Saavedra-Rodriguez K., Suarez A.F. Transcription of detoxification genes after permethrin selection in the mosquito Aedes aegypti. Insect Mol. Biol. 2012;21(1):61–77. doi: 10.1111/j.1365-2583.2011.01113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabin A.B. Research on dengue during world war II. Am. J. Trop. Med. Hyg. 1952;1(1):30–50. doi: 10.4269/ajtmh.1952.1.30. [DOI] [PubMed] [Google Scholar]
- Salvador F.S., Fujita D.M. Entry routes for Zika virus in Brazil after 2014 world cup: new possibilities. Travel Med. Infect. Dis. 2015;14(1):49–51. doi: 10.1016/j.tmaid.2015.10.004. [DOI] [PubMed] [Google Scholar]
- Sambri V., Capobianchi M. West Nile virus in Europe: emergence, epidemiology, diagnosis, treatment, and prevention. Clin. Microbiol. Infect. 2013;19(8):699–704. doi: 10.1111/1469-0691.12211. [DOI] [PubMed] [Google Scholar]
- Schrag S.J., Wiener P. Emerging infectious disease: what are the relative roles of ecology and evolution? Trends Ecol. Evol. 1995;10(8):319–324. doi: 10.1016/s0169-5347(00)89118-1. [DOI] [PubMed] [Google Scholar]
- Selvey L.A. The changing epidemiology of Murray Valley encephalitis in Australia: the 2011 outbreak and a review of the literature. PLoS Negl. Trop. Dis. 2014;8(1):e2656. doi: 10.1371/journal.pntd.0002656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shayan S., Bokaean M. Crimean-Congo hemorrhagic fever. Lab. Med. 2015;46(3):180–189. doi: 10.1309/LMN1P2FRZ7BKZSCO. [DOI] [PubMed] [Google Scholar]
- Shepard D.S., Coudeville L. Economic impact of dengue illness in the Americas. Am. J. Trop. Med. Hyg. 2011;84(2):200–207. doi: 10.4269/ajtmh.2011.10-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J.R., Romeiro M.F. A Saint Louis encephalitis and Rocio virus serosurvey in Brazilian horses. Rev. Soc. Bras. Med. Trop. 2014;47(4):414–417. doi: 10.1590/0037-8682-0117-2014. [DOI] [PubMed] [Google Scholar]
- Stanton A.T. The mosquitoes of Far Eastern ports with special reference to the prevalence of Stegomyia fasciata. Bull. Entomol. Res. 1919;10:333–344. [Google Scholar]
- Staples J.E., Hills S.L. 2015. Chikungunya. CDC Health Information for International Travel 2016. ( http://wwwnc.cdc.gov/travel/yellowbook/2016/infectious-diseases-related-to-travel/chikungunya) [Google Scholar]
- Takahashi T. The first identification and retrospective study of severe fever with thrombocytopenia syndrome in Japan. J. Infect. Dis. 2014;209(6):816–827. doi: 10.1093/infdis/jit603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantely L.M., Boyer S. A review of mosquitoes associated with rift valley fever virus in Madagascar. Am. J. Trop. Med. Hyg. 2015;92(4):722–729. doi: 10.4269/ajtmh.14-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubitz W., Cramer J.P. Chikungunya fever in travelers: clinical presentation and course. Clin. Infect. Dis. 2007;45(1):e1–e4. doi: 10.1086/518701. [DOI] [PubMed] [Google Scholar]
- Taylor L.H., Latham S.M. Risk factors for human disease emergence. Philos. Trans. R Soc. Lond. B Biol. Sci. 2001;356(1411):983–989. doi: 10.1098/rstb.2001.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesh R.B., Modi G.B. Growth and transovarial transmission of Chandipura virus (Rhabdoviridae: vesiculovirus) in phlebotomus papatasi. Am. J. Trop. Med. Hyg. 1983;32(3):621–623. doi: 10.4269/ajtmh.1983.32.621. [DOI] [PubMed] [Google Scholar]
- Tesh R.B., Watts D.M. Mayaro virus disease: an emerging mosquito-borne zoonosis in tropical South America. Clin. Infect. Dis. 1999;28(1):67–73. doi: 10.1086/515070. [DOI] [PubMed] [Google Scholar]
- Tesh R.B. The genus Phlebovirus and its vectors. Annu. Rev. Entomol. 1988;33:169–181. doi: 10.1146/annurev.en.33.010188.001125. [DOI] [PubMed] [Google Scholar]
- Thomas S.J., Nisalak A. Dengue plaque reduction neutralization test (PRNT) in primary and secondary dengue virus infections: how alterations in assay conditions impact performance. Am. J. Trop. Med. Hyg. 2009;81(5):825–833. doi: 10.4269/ajtmh.2009.08-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsetsarkin K.A., Weaver S.C. Sequential adaptive mutations enhance efficient vector switching by Chikungunya virus and its epidemic emergence. PLoS Pathog. 2011;7(12):e1002412. doi: 10.1371/journal.ppat.1002412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsetsarkin K.A., Vanlandingham D.L. A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog. 2007;3(12):e201. doi: 10.1371/journal.ppat.0030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsetsarkin K.A., Chen R. Multi-peaked adaptive landscape for chikungunya virus evolution predicts continued fitness optimization in Aedes albopictus mosquitoes. Nat. Commun. 2014;5:4084. doi: 10.1038/ncomms5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufan Z.K., Tasyaran M.A. Sandfly fever. a mini review. Virol. Mycol. 2013;2 (p. 109) [Google Scholar]
- Tuncer P., Yesilbag K. Crimean-Congo hemorrhagic fever infection in domestic animals in marmara region, western Turkey. Ankara Üniv. Vet. Fak. Derg. 2014;61:49–53. [Google Scholar]
- Vanlandingham D.L., Tsetsarkin K. Determinants of vector specificity of o'nyong nyong and chikungunya viruses in Anopheles and Aedes mosquitoes. Am. J. Trop. Med. Hyg. 2006;74(4):663–669. [PubMed] [Google Scholar]
- van Panhuis W.G., Gibbons R.V. Inferring the serotype associated with dengue virus infections on the basis of pre- and postinfection neutralizing antibody titers. J. Infect. Dis. 2010;202(7):1002–1010. doi: 10.1086/656141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconcelos H.B. Molecular epidemiology of oropouche virus: Brazil. Emerg. Infect. Dis. 2011;17(5):800–806. doi: 10.3201/eid1705.101333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilakis N., Shell E.J. Potential of ancestral sylvatic dengue-2 viruses to re-emerge. Virology. 2007;358(2):402–412. doi: 10.1016/j.virol.2006.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilakis N., Durbin A.P. Antigenic relationships between sylvatic and endemic dengue viruses. Am. J. Trop. Med. Hyg. 2008;79(1):128–132. [PubMed] [Google Scholar]
- Vasilakis N., Fokam E.B. Genetic and phenotypic characterization of sylvatic dengue virus type 2 strains. Virology. 2008;377(2):296–307. doi: 10.1016/j.virol.2008.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilakis N., Hanley K.A. In: Dengue virus emergence from its sylvatic cycle. Frontiers in Dengue Virus Research. Hanley K.A., Weaver S.C., editors. Caister Academic Press; Norfolk, UK: 2010. pp. 183–217. [Google Scholar]
- Vasilakis N., Cardosa J. Fever from the forest: prospects for the continued emergence of sylvatic dengue virus and its impact on public health. Nat. Rev. Microbiol. 2011;9(7):532–541. doi: 10.1038/nrmicro2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazeille M., Moutailler S. Two Chikungunya isolates from the outbreak of La Reunion (Indian Ocean) exhibit different patterns of infection in the mosquito Aedes albopictus. PLoS One. 2007;2(11):e1168. doi: 10.1371/journal.pone.0001168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazeille-Falcoz M., Mousson L. Variation in oral susceptibility to dengue type 2 virus of populations of Aedes aegypti from the islands of Tahiti and Moorea, French Polynesia. Am. J. Trop. Med. Hyg. 1999;60(2):292–299. doi: 10.4269/ajtmh.1999.60.292. [DOI] [PubMed] [Google Scholar]
- Vega-Rua A., Zouache K. High efficiency of temperate Aedes albopictus to transmit chikungunya and dengue viruses in the Southeast of France. PLoS One. 2013;8(3):e59716. doi: 10.1371/journal.pone.0059716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk S.M., Chen R. Genome-scale phylogenetic analyses of chikungunya virus reveal independent emergences of recent epidemics and various evolutionary rates. J. Virol. 2010;84(13):6497–6504. doi: 10.1128/JVI.01603-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEF . 10th Edition. World Economic Forum; Geneva: 2015. Global Risks 2015. 69 p. [Google Scholar]
- WHO Zika virus outbreaks in the Americas. Wkly. Epidemiol. Rec. 2015;90(45):609–610. [PubMed] [Google Scholar]
- Wang E., Ni H. Evolutionary relationships of endemic/epidemic and sylvatic dengue viruses. J. Virol. 2000;74(7):3227–3234. doi: 10.1128/jvi.74.7.3227-3234.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver S.C., Forrester N.L. Chikungunya: evolutionary history and recent epidemic spread. Antiviral Res. 2015;120:32–39. doi: 10.1016/j.antiviral.2015.04.016. [DOI] [PubMed] [Google Scholar]
- Weaver S.C., Reisen W.K. Present and future arboviral threats. Antiviral Res. 2010;85(2):328–345. doi: 10.1016/j.antiviral.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver S.C. Urbanization and geographic expansion of zoonotic arboviral diseases: mechanisms and potential strategies for prevention. Trends Microbiol. 2013;21(8):360–363. doi: 10.1016/j.tim.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver S.C. Arrival of chikungunya virus in the new world: prospects for spread and impact on public health. PLoS Negl. Trop. Dis. 2014;8(6):e2921. doi: 10.1371/journal.pntd.0002921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse C.A. Crimean-Congo hemorrhagic fever. Antiviral Res. 2004;64(3):145–160. doi: 10.1016/j.antiviral.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Woolhouse M.E., Haydon D.T. Emerging pathogens: the epidemiology and evolution of species jumps. Trends Ecol. Evol. 2005;20(5):238–244. doi: 10.1016/j.tree.2005.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yactayo S., Pilar Ramón P. Yellow fever in africa and the americas, 2014. Wkly. Epidemiol. Rec. 2015;90(26):323–334. [PubMed] [Google Scholar]
- Yu X.J. Fever with thrombocytopenia associated with a novel bunyavirus in China. N. Engl. J. Med. 2011;364(16):1523–1532. doi: 10.1056/NEJMoa1010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun S.M., Lee W.G. Severe fever with thrombocytopenia syndrome virus in ticks collected from humans, South Korea, 2013. Emerg. Infect. Dis. 2013;20(8):1358–1361. doi: 10.3201/eid2008.131857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacks M.A., Paessler S. Encephalitic alphaviruses. Vet. Microbiol. 2010;140(3–4):281–286. doi: 10.1016/j.vetmic.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanluca C., de Melo V.C. First report of autochthonous transmission of Zika virus in Brazil. Mem. Inst. Oswaldo Cruz. 2015;110(4):569–572. doi: 10.1590/0074-02760150192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohrabian A., Meltzer M.I. West Nile virus economic impact, Louisiana, 2002. Emerg. Infect. Dis. 2004;10(10):1736–1744. doi: 10.3201/eid1010.030925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohrabian A., Hayes E.B. Cost-effectiveness of west Nile virus vaccination. Emerg. Infect. Dis. 2006;12(3):375–380. doi: 10.3201/eid1203.050782. [DOI] [PMC free article] [PubMed] [Google Scholar]