Abstract
Objective
The mechanism causing gastrointestinal intolerance to metformin treatment is unknown. We have previously shown that reduced-function alleles of organic cation transporter 1 (OCT1) are associated with increased intolerance to metformin. Considering recent findings that serotonin transporter (SERT) might also be involved in metformin intestinal absorption, and serotonin role in gastrointestinal physiology, in this study we investigated the association between a common polymorphism in SERT gene and metformin gastrointestinal intolerance.
Research Design and Methods
We explored the effect of composite SERT 5-HTTLPR/rs25531 genotypes, L*L* (LALA), L*S*(LALG, LAS), and S*S* (SS, SLG, LGLG), in 1,356 fully tolerant and 164 extreme metformin-intolerant patients by using logistic regression model, adjusted for age, sex, weight, OCT1 genotype, and concomitant use of medications known to inhibit OCT1 activity.
Results
The number of low-expressing SERT S* alleles increased the odds of metformin intolerance (OR=1.31, 95% CI 1.02-1.67, P=0.031). Moreover, a multiplicative interaction between the OCT1 and SERT genotypes was observed (P=0.003). In the analyses stratified by SERT genotype, the presence of two deficient OCT1 alleles was associated with over a nine-fold higher odds of metformin intolerance in patients carrying L*L* genotype (OR=9.25, 95% CI 3.18-27.0, P<10-4), however, it showed much smaller effect in L*S* carriers, and no effect in S*S* carriers.
Conclusions
Our results indicate that interaction between OCT1 and SERT genes might play an important role in metformin intolerance. Further studies are needed to replicate these findings and to substantiate the hypothesis that metformin gastrointestinal side-effects could be related to the reduced intestinal serotonin uptake.
Metformin is a first-line antihyperglycaemic agent, and the most widely used type 2 diabetes drug. It has major clinical advantages over other therapies due to its proven safety record, it does not induce hypoglycaemia or weight gain, and has possible cardiovascular benefits (1). The most common adverse effect of metformin treatment is gastrointestinal (GI) upset which occurs in approximately 30% of patients, limiting compliance. In 5% of patients treated with metformin, GI symptoms are intolerable and warrant the discontinuation of the drug (2). The mechanism of metformin GI side effects is not clear. Various pathophysiological hypotheses have been proposed, including metformin-induced release of serotonin in the intestinal mucosa (3), reduced absorption of bile salts (4), increase in glucagon-like peptide-1 (GLP-1) concentrations (5), and more recently, changes in the gut microbiome (6).
Metformin side effects might be related to high concentration of metformin in the gut after oral administration (7). We have recently shown that reduced-function alleles of organic cation transporter 1 (OCT1), as well as concomitant treatment with medications known to inhibit OCT1 activity, are risk factors for metformin intolerance in a large cohort of type 2 diabetes patients treated with metformin (8). OCT1 is one of the several cation-selective transporters expressed in the enterocytes, which could be involved in metformin absorption (9–11). Other potentially involved transporters are OCT3 and plasma membrane monoamine transporter (PMAT). Interestingly, a recent study showed that OCT1, PMAT, serotonin reuptake transporter (SERT, 5-HTT), and choline high-affinity transporter (CHT), and not OCT3, contribute to the apical uptake of metformin into Caco-2 cell monolayers, and thus, potentially to intestinal metformin absorption (11). The CHT is not expressed in human intestine (11), and there are no established common loss-of-function variants of PMAT. On the other hand, the expression of SERT is modulated by genetic variants, most notably the serotonin-transporter-linked polymorphic region (5-HTTLPR) variant, a well-established 43-base pair insertion/deletion polymorphism in the promoter region. Moreover, a recent study showed that metformin can inhibit serotonin uptake by OCT1, OCT3, and SERT, at concentrations that may be achieved in human intestine after oral administration (12). These findings contribute to the hypothesis of serotonin-mediated GI adverse effects following metformin treatment, as metformin inhibition of serotonin uptake could result in increased GI side effects (12).
Considering that different expression or activity of SERT might contribute to high inter-individual variability in GI intolerance to metformin, in this study we investigated the role of a common SERT tri-allelic 5-HTTLPR polymorphism in intolerance to metformin, and explored the potential interaction between SERT (SLC6A4) and OCT1 (SLC22A1) genes.
Research Design and Methods
Study population and definition of intolerance
The study population was previously described in detail (8). Briefly, the study included patients with type 2 diabetes from the Genetics of Diabetes Audit and Research Tayside Study (GoDARTS), who were prescribed metformin for the first time in the period from 1st January 1994 to 1st June 2011. A surrogate phenotype of metformin intolerance was defined based on discontinuation of metformin within the first 6 months of treatment (immediate release form, IR), and switch to another oral hypoglycaemic agent, including metformin slow release forms, within 6 months of the last metformin IR prescription. Intolerant patients were compared with patients who were defined as tolerant based on treatment with ≥ 2000 mg of metformin IR form for more than 6 months.
Clinical cofactors, including anthropometric and biochemical parameters, metformin daily dose and use of OCT1-inhibiting medications were defined previously (8).
Genotyping
Genotyping of five OCT1 reduced-function variants (R61C, C88R, G401S, M420del, and G465R) and classification of individuals based on the number of haplotypes carrying reduced-function alleles were described in our previous study (8).
The 5-HTTLPR polymorphism in SERT gene (SLC6A4) is characterised by long (L) and short (S) alleles. The S allele has been associated with lower SERT expression and function (13). A single nucleotide polymorphism (SNP), rs25531 A>G, located within this region, further modulates SERT expression, with LA carriers having higher SERT expression, and LG lower SERT expression similar to that in S allele carriers (14). In this study, we predicted the 5-HTTLPR polymorphism based on published machine learning method of vertex discriminant analysis validated for Northern European populations (15). This method uses eight variants in partial linkage disequilibrium with 5-HTTLPR, to predict three genotypes, LL, SL and SS (15). Seven out of eight SNPs, and rs25531, were imputed from existing genome-wide data on 7,319 GoDARTS participants using the 1,000 Genome reference panel and software IMPUTE2. The imputation quality information values were between 0.88 and 1.00. All SNPs were in line with Hardy-Weinberg equilibrium (P > 0.05). Considering that LG allele has the same expression as the S allele, the tri-allelic 5-HTTLPR genotypes were coded as L*L* (LALA), L*S*(LALG, LAS), and S*S* (SS, SLG, LGLG).
Statistical analysis
Differences in quantitative variables between two groups were compared using a t test or Mann-Whitney U test, depending on the distribution normality, and categorical variables were compared using a χ2 test. For testing the significance of the additive genetic model, groups of quantitative variables were compared using ANOVA test for trend or Jonckheere's trend test, depending on the distribution normality, and categorical variables were compared using the Cochran-Armitage trend test. The logistic regression model was used to analyse the association of genotypes with metformin intolerance, with age, sex, weight and the concomitant use of OCT1-inhibiting medications as covariates (8). Based on our previous study, the effect of two deficient OCT1 alleles was assessed (recessive model) (8), and for the tri-allelic 5-HTTLPR polymorphism, an additive genetic model was used. The multiplicative interaction was assessed by adding an interaction term to the regression model. Statistical analyses were conducted using the SAS 9.3 software (SAS Institute Inc., Cary, NC), and statistical significance level was set to P < 0.05.
Results
A total of 1,356 tolerant and 164 intolerant patients with available OCT1 and SERT genotype data were included in the study (Table 1). Patients differed in baseline characteristics, in line with our previous study (8). The OCT1 or SERT genotypes were not associated with study participants’ baseline characteristics, with the exception of lower percentage of the antidiabetic drug-naïve patients in the group with two deficient OCT1 alleles compared to one or no deficient OCT1 allele carriers (Supplementary Table 1, P=0.003).
Table 1.
Baseline characteristics of metformin intolerant and tolerant group.
| Intolerant group (n=164) | Tolerant group (n=1356) | P | |
|---|---|---|---|
| Age (years) | 68.8 ± 9.7 | 58.4 ± 10.6 | <0.001 |
| Age at diagnosis (years) | 63.5 ± 9.9 | 55.2 ± 10.3 | <0.001 |
| Females/Males (Females %) | 94/70 (57.3%) | 545/811 (40.2%) | <0.001 |
| Weight (kg) | 81.7 ± 15.5 | 92.1 ± 18.3 | <0.001 |
| BMI (kg/m2) | 30.4 ± 5.4 | 32.6 ± 6.1 | <0.001 |
| HbA1c
(%) (mmol/mol) |
8.1 (7.7-9.2) 65 (61-77) |
8.8 (7.8-9.9) 73 (62-85) |
<0.001 |
| Creatinine (µmol/l) | 87.4 ± 14.4 | 87.2 ± 14.4 | 0.831 |
| Creatinine clearance (ml/min) | 74.4 (57.4-91.4) | 97.7 (77.0-120.7) | <0.001 |
| Antidiabetic drug-naive | 86 (52.4%) | 831 (61.3%) | 0.029 |
| Use of OCT1 inhibiting drugs* | 83 (50.6%) | 450 (33.2%) | <0.001 |
| Metformin daily dose (mg) | 1000 (1000-1000) | 1000 (1000-1500) | <0.001 |
P values refer to the significance of t test, Mann-Whitney U test or a χ2 test for data presented as means ± SD, medians (interquartile range), or numbers (percentages), respectively.
Number of individuals concomitantly treated with OCT1 inhibiting drugs, including proton pump inhibitors, tricyclic antidepressants, citalopram, verapamil, diltiazem, doxazosin, spironolactone, clopidogrel, rosiglitazone, quinine, tramadol and codeine.
The numbers of individuals in each genotype group are shown in Supplementary Table 2. In addition to the association of the two deficient OCT1 alleles with intolerance (recessive model, P=0.001) in line with our previous report (8), there was a significant difference in the SERT genotype frequencies between the intolerant and tolerant group (additive model, P=0.019).
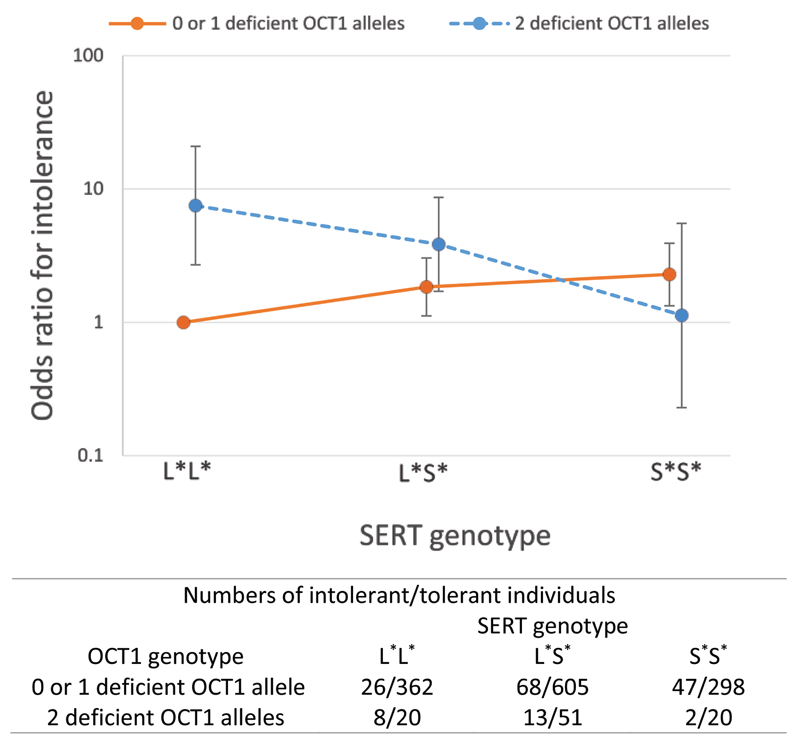
In the logistic regression analysis model adjusted for the clinical covariates age, sex and weight, the number of S* alleles was associated with higher odds of metformin intolerance (OR=1.28, 95% CI 1.01-1.63, P=0.040). This effect was greater after adding the OCT1 genotype and OCT1-inhibiting medications to the model (Table 2, OR=1.31, 95% CI 1.02-1.67, P=0.031). Furthermore, we tested the interaction between the OCT1 and SERT genotypes. A negative multiplicative interaction was observed between the two genes (P=0.003), which is visually presented in Figure 1. This shows the joint effects of OCT1 and SERT genotypes compared to the reference genotype group (the combination of one or no deficient OCT1 alleles and the L*L* genotype). In the analysis stratified by SERT genotypes, the presence of two deficient OCT1 alleles was associated with over a nine-fold higher odds of metformin intolerance (OR=9.25, 95% CI 3.18-27.0, P<10-4) in individuals with L*L* genotype, whereas there was no significant association in L*S* carriers (OR=2.11, 95% CI 0.99-4.50, P=0.054) and the S*S* genotype group (OR=0.45, 95% CI 0.09-2.20, P=0.325) (Table 3). On the other hand, when patients were stratified according to the OCT1 genotypes, the number of S* alleles increased intolerance in carriers of one or no deficient OCT1 allele (OR=1.48, 95% CI 1.15-1.92, P=0.003), but showed opposite effect in two deficient OCT1 allele carriers (OR=0.33, 95% CI 0.13-0.82, P=0.017) (Table 3).
Table 2.
Results of logistic regression model for metformin intolerance.
| OR (95% CI) | P | |
|---|---|---|
| Age | 1.11 (1.08-1.13) | <0.001 |
| Sex (Females vs. Males) | 1.82 (1.26-2.65) | 0.002 |
| Weight | 0.99 (0.97-1.00) | 0.031 |
| Use of OCT1 inhibiting drugs | 1.75 (1.22-2.49) | 0.002 |
| Two reduced-function OCT1 alleles | 2.27 (1.31-3.92) | 0.003 |
| Number of SERT S* alleles | 1.31 (1.02-1.67) | 0.031 |
OR, odds ratio for intolerance. Logistic regression analysis included 164 intolerant and 1356 tolerant patients.
Figure 1. Joint effects of OCT1 and SERT genotypes on metformin intolerance.
The combination one or no deficient OCT1 alleles/ L*L* is used as a reference group. The numbers in each genotype group are presented for the intolerant and tolerant individuals as ‘Intolerant/Tolerant’.
Table 3.
Stratified analyses according to OCT1 and SERT genotypes.
| Effect of two deficient OCT1 alleles - analysis stratified for SERT genotype | ||
|---|---|---|
| SERT genotype | OR (95% CI) | P |
| L*L* carriers* | 9.25 (3.18-27.0) | <0.0001 |
| L*S* carriers† | 2.11 (0.99-4.50) | 0.054 |
| S*S* carriers‡ | 0.45 (0.09-2.20) | 0.325 |
| Effect of the number of SERT S* alleles - analysis stratified for OCT1 genotype | ||
| OCT1 genotype | OR (95% CI) | P |
| 0 or 1 deficient alleles carriers§ | 1.48 (1.15-1.92) | 0.003 |
| 2 deficient alleles carriers|| | 0.33 (0.13-0.82) | 0.017 |
OR, odds ratio for intolerance. Analyses were adjusted for age, sex, weight, and use of OCT1-inhibiting medications.
34 intolerant and 382 tolerant patients
81 intolerant and 656 tolerant patients
49 intolerant and 318 tolerant patients
141 intolerant and 1265 tolerant patients
23 intolerant and 91 tolerant patients.
Conclusions
In the first study of genetic and phenotypic determinants of metformin intolerance, we showed that variants of highly polymorphic OCT1 gene are associated with severe intolerance leading to discontinuation of metformin therapy (8). We hypothesised this based on the possible role of OCT1 in metformin intestinal absorption. Our later prospective study demonstrated the relationship between OCT1 deficient alleles and common GI side effects of metformin, thus replicating earlier findings and extending them also to the milder intolerance phenotype (16). The mechanism for this association, however, was unclear. Based on the recent findings that metformin may alter serotonin uptake by gut transporters (12), and considering the role of serotonin in GI physiology, here we focused on the effect of a common and well-established SERT 5-HTTLPR functional polymorphism on metformin intolerance.
We found that the low-expressing S* allele of SERT gene is associated with increased intolerance to metformin, although this effect was smaller than that seen to be associated with two OCT1 deficient alleles. The 5-HTTLPR polymorphism has been extensively studied previously, and there is a possible association of 5-HTTLPR alleles with irritable bowel syndrome (17), psychiatric traits (18), and antidepressant drug response (19). Although results of the pharmacogenetic studies have been inconsistent, evidence from reviews and meta-analyses suggest that the L allele is a predictor of better response to selective serotonin reuptake inhibitors (SSRIs) in Caucasian populations (19). On the other hand, in the meta-analysis of nine studies, the S allele was significantly associated with more total adverse effects after SSRIs treatment, and showed a trend of association with GI side effects induced by SSRIs (20), in line with our results.
In humans, serotonin is predominantly synthesised in the enterochromaffin cells of the gut mucosa. Here it mediates many gastrointestinal functions, including motility, secretion and vasodilation by activating afferent neurons in the lamina propria (21). Serotonin has been involved in the pathophysiology of a number of GI disorders, and drugs targeting serotonin receptors have been used in the treatment of GI symptoms (21). Previously it has been shown that metformin can induce serotonin release from the intestinal mucosa, in dose-dependent manner, without effect on 5-HT3 receptors (3). In this study, the effect of metformin on serotonin reuptake was not explored (3). However, recent in vitro findings showed that metformin can inhibit serotonin uptake by SERT and other cation transporters (12). Thus, metformin could increase serotonin extracellular concentrations resulting in prolonged serotonergic signalling in the intestine and increased GI side effects (12). Although in this study, metformin inhibited serotonin uptake by OCT1 more strongly than by SERT (12), another in vitro study showed conversely that metformin is not a significant inhibitor of OCT1-mediated serotonin transport (22). Beside this, SERT has much higher expression than OCT1 in the human intestine (11, 23), implying that although metformin is a weak SERT inhibitor, it could inhibit SERT at the high concentrations achieved in the gut after oral administration (24). In addition, it has been proposed that inhibition of intestinal SERT may contribute to GI adverse effects commonly observed with SSRIs treatment (25), and possibly also to side effects of other drugs which may act as SERT inhibitors (26).
As we observed a significant interaction between OCT1 and SERT genotypes, we performed analyses stratified by each genotype. Interestingly, in the analyses stratified by SERT genotype, two OCT1 deficient alleles had high effect in patients carrying L*L* genotype, much smaller effect in L*S* carriers, and no effect in the S*S* genotype carriers. Furthermore, the low-expressing S* allele was associated with intolerance only in patients with one or no deficient OCT1 allele, and showed opposite direction in carriers of two deficient OCT1 alleles. It can be hypothesised that low activity of OCT1, possibly the main intestinal transporter of metformin, results in increased metformin concentrations in the gut, which can inhibit SERT and thus cause high extracellular serotonin levels and GI intolerance. On the other hand, although the number of low-expressing SERT S* alleles was associated with intolerance per se presumably also due to higher serotonin extracellular levels, the S* allele showed protective effect in the presence of two low-activity OCT1 alleles. This contradictory finding could be possibly explained by desensitization of serotonin receptors, which may occur as a consequence of greatly increased interstitial serotonin concentrations, in the case of low SERT expression (27) and high SERT inhibitor concentrations (28). However, the small numbers of patients especially in some of these genotype-stratified analyses preclude drawing strong conclusions about the observed interaction. SERT is expressed at both apical and basolateral membranes of the enterocytes, with predominant apical expression (11). However, there is ambiguity around the localisation of the OCT1 in the enterocytes, as it has been suggested to be located basolaterally (9, 29), and conversely, in a recent study, apically (10). Thus, it is unclear whether increased mucosal or luminal metformin concentrations could contribute to the GI adverse effects. Nevertheless, the results of our study suggest a plausible hypothesis for GI intolerance of metformin, which should be explored further.
In addition to the small sizes of groups in the stratified analyses, there are several other limitations of our study which need to be acknowledged. Firstly, we used a surrogate phenotype for metformin gastrointestinal intolerance based on discontinuation of metformin in the first months of treatment. We ensured that patients were switched to another oral hypoglycaemic agent, thus the cessation of metformin was not due to improvement in glycaemic control. However, there could be other reasons for stopping metformin, including other side effects or other reasons not related to drug intolerance. This could result in some imprecision in the definition of phenotype categories, although GI intolerance represents the most common adverse effect of metformin treatment. Furthermore, it would be interesting to explore the effect of concomitant treatment of SSRIs on metformin intolerance, and their interaction with SERT as well as OCT1 genotypes. However, we were not able to do this due to the small number of patients who were treated with SSRIs. In addition, SSRIs could also act as OCT1 inhibitors, and citalopram has been included in the overall OCT1-inhibiting drugs. Finally, considering the relatively small size of our study, the novel findings of our study should considered preliminary and require independent replication. Beside this, as clearly genetic studies alone cannot infer molecular mechanisms of drug effects, further in vitro and in vivo studies are needed to explore the proposed hypothesis of metformin GI intolerance.
In conclusion, our results indicate that SERT genotype and the interaction between OCT1 and SERT genes might play an important role in GI intolerance to metformin. Further studies are needed to replicate our preliminary findings as well to substantiate the proposed interaction between metformin and serotonin disposition in the intestine, and elucidate the exact mechanisms of GI intolerance to metformin.
Supplementary Material
Acknowledgements
We are grateful to all the participants who took part in this study, to the general practitioners, to the Scottish School of Primary Care for their help in recruiting the participants, and to the whole team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses.
Funding. The Wellcome Trust United Kingdom Type 2 Diabetes Case Control Collection (GoDARTS) cohort collection was funded by The Wellcome Trust and informatics support is provided by the Chief Scientist Office, Scotland. E.R.P. holds a Wellcome Trust New Investigator Award (102820/Z/13/Z).
Footnotes
Duality of Interest. We declare no conflict of interest.
Author Contributions. T.D. analysed and interpreted the data, and wrote the manuscript. K.Z., R.T., and C.N.A.P. analysed and interpreted the data, and critically assessed and reviewed the manuscript. E.R.P. designed the study, interpreted the data and wrote the manuscript. E.R.P. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of Hyperglycemia in Type 2 Diabetes, 2015: A Patient-Centered Approach: Update to a Position Statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–149. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]
- 2.DeFronzo RA. Pharmacologic therapy for type 2 diabetes mellitus. Ann Intern Med. 1999;131:281–303. doi: 10.7326/0003-4819-131-4-199908170-00008. [DOI] [PubMed] [Google Scholar]
- 3.Cubeddu LX, Bonisch H, Gothert M, Molderings G, Racke K, Ramadori G, Miller KJ, Schworer H. Effects of metformin on intestinal 5-hydroxytryptamine (5-HT) release and on 5-HT3 receptors. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:85–91. doi: 10.1007/s002109900152. [DOI] [PubMed] [Google Scholar]
- 4.Carter D, Howlett HC, Wiernsperger NF, Bailey CJ. Differential effects of metformin on bile salt absorption from the jejunum and ileum. Diabetes Obes Metab. 2003;5:120–125. doi: 10.1046/j.1463-1326.2003.00252.x. [DOI] [PubMed] [Google Scholar]
- 5.Mannucci E, Ognibene A, Cremasco F, Bardini G, Mencucci A, Pierazzuoli E, Ciani S, Messeri G, Rotella CM. Effect of metformin on glucagon-like peptide 1 (GLP-1) and leptin levels in obese nondiabetic subjects. Diabetes Care. 2001;24:489–494. doi: 10.2337/diacare.24.3.489. [DOI] [PubMed] [Google Scholar]
- 6.Forslund K, Hildebrand F, Nielsen T, Falony G, Le Chatelier E, Sunagawa S, Prifti E, Vieira-Silva S, Gudmundsdottir V, Krogh Pedersen H, Arumugam M, et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528:262–266. doi: 10.1038/nature15766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilcock C, Bailey CJ. Accumulation of metformin by tissues of the normal and diabetic mouse. Xenobiotica. 1994;24:49–57. doi: 10.3109/00498259409043220. [DOI] [PubMed] [Google Scholar]
- 8.Dujic T, Zhou K, Donnelly LA, Tavendale R, Palmer CN, Pearson ER. Association of Organic Cation Transporter 1 With Intolerance to Metformin in Type 2 Diabetes: A GoDARTS Study. Diabetes. 2015;64:1786–1793. doi: 10.2337/db14-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muller J, Lips KS, Metzner L, Neubert RH, Koepsell H, Brandsch M. Drug specificity and intestinal membrane localization of human organic cation transporters (OCT) Biochem Pharmacol. 2005;70:1851–1860. doi: 10.1016/j.bcp.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Han TK, Everett RS, Proctor WR, Ng CM, Costales CL, Brouwer KL, Thakker DR. Organic cation transporter 1 (OCT1/mOct1) is localized in the apical membrane of Caco-2 cell monolayers and enterocytes. Mol Pharmacol. 2013;84:182–189. doi: 10.1124/mol.112.084517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han TK, Proctor WR, Costales CL, Cai H, Everett RS, Thakker DR. Four cation-selective transporters contribute to apical uptake and accumulation of metformin in caco-2 cell monolayers. J Pharmacol Exp Ther. 2015;352:519–528. doi: 10.1124/jpet.114.220350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yee SW, Lin L, Merski M, Keiser MJ, Gupta A, Zhang Y, Chien HC, Shoichet BK, Giacomini KM. Prediction and validation of enzyme and transporter off-targets for metformin. J Pharmacokinet Pharmacodyn. 2015;42:463–475. doi: 10.1007/s10928-015-9436-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 14.Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, Xu K, Arnold PD, Richter MA, Kennedy JL, Murphy DL, et al. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am J Hum Genet. 2006;78:815–826. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu AT, Bakker S, Janson E, Cichon S, Cantor RM, Ophoff RA. Prediction of serotonin transporter promoter polymorphism genotypes from single nucleotide polymorphism arrays using machine learning methods. Psychiatr Genet. 2012;22:182–188. doi: 10.1097/YPG.0b013e328353ae23. [DOI] [PubMed] [Google Scholar]
- 16.Dujic T, Causevic A, Bego T, Malenica M, Velija-Asimi Z, Pearson ER, Semiz S. Organic cation transporter 1 variants and gastrointestinal side effects of metformin in patients with Type 2 diabetes. Diabet Med. 2016;33:511–514. doi: 10.1111/dme.13040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang ZF, Duan ZJ, Wang LX, Yang D, Zhao G, Zhang L. The serotonin transporter gene polymorphism (5-HTTLPR) and irritable bowel syndrome: a meta-analysis of 25 studies. BMC Gastroenterol. 2014;14:23. doi: 10.1186/1471-230X-14-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gelernter J. SLC6A4 polymorphism, population genetics, and psychiatric traits. Hum Genet. 2014;133:459–461. doi: 10.1007/s00439-013-1412-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fabbri C, Minarini A, Niitsu T, Serretti A. Understanding the pharmacogenetics of selective serotonin reuptake inhibitors. Expert Opin Drug Metab Toxicol. 2014;10:1093–1118. doi: 10.1517/17425255.2014.928693. [DOI] [PubMed] [Google Scholar]
- 20.Kato M, Serretti A. Review and meta-analysis of antidepressant pharmacogenetic findings in major depressive disorder. Mol Psychiatry. 2010;15:473–500. doi: 10.1038/mp.2008.116. [DOI] [PubMed] [Google Scholar]
- 21.Mawe GM, Hoffman JM. Serotonin signalling in the gut--functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2013;10:473–486. doi: 10.1038/nrgastro.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boxberger KH, Hagenbuch B, Lampe JN. Common drugs inhibit human organic cation transporter 1 (OCT1)-mediated neurotransmitter uptake. Drug Metab Dispos. 2014;42:990–995. doi: 10.1124/dmd.113.055095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meier Y, Eloranta JJ, Darimont J, Ismair MG, Hiller C, Fried M, Kullak-Ublick GA, Vavricka SR. Regional distribution of solute carrier mRNA expression along the human intestinal tract. Drug Metab Dispos. 2007;35:590–594. doi: 10.1124/dmd.106.013342. [DOI] [PubMed] [Google Scholar]
- 24.Proctor WR, Bourdet DL, Thakker DR. Mechanisms underlying saturable intestinal absorption of metformin. Drug Metab Dispos. 2008;36:1650–1658. doi: 10.1124/dmd.107.020180. [DOI] [PubMed] [Google Scholar]
- 25.Hu XZ, Rush AJ, Charney D, Wilson AF, Sorant AJ, Papanicolaou GJ, Fava M, Trivedi MH, Wisniewski SR, Laje G, Paddock S, et al. Association between a functional serotonin transporter promoter polymorphism and citalopram treatment in adult outpatients with major depression. Arch Gen Psychiatry. 2007;64:783–792. doi: 10.1001/archpsyc.64.7.783. [DOI] [PubMed] [Google Scholar]
- 26.Martel F. Recent advances on the importance of the serotonin transporter SERT in the rat intestine. Pharmacol Res. 2006;54:73–76. doi: 10.1016/j.phrs.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Drago A, De Ronchi D, Serretti A. Pharmacogenetics of antidepressant response: an update. Hum Genomics. 2009;3:257–274. doi: 10.1186/1479-7364-3-3-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wade PR, Chen J, Jaffe B, Kassem IS, Blakely RD, Gershon MD. Localization and function of a 5-HT transporter in crypt epithelia of the gastrointestinal tract. J Neurosci. 1996;16:2352–2364. doi: 10.1523/JNEUROSCI.16-07-02352.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang DS, Jonker JW, Kato Y, Kusuhara H, Schinkel AH, Sugiyama Y. Involvement of organic cation transporter 1 in hepatic and intestinal distribution of metformin. J Pharmacol Exp Ther. 2002;302:510–515. doi: 10.1124/jpet.102.034140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.