Abstract
Chitin is the most abundant aminopolysaccharide polymer occurring in nature, and is the building material that gives strength to the exoskeletons of crustaceans, insects, and the cell walls of fungi. Through enzymatic or chemical deacetylation, chitin can be converted to its most well-known derivative, chitosan. The main natural sources of chitin are shrimp and crab shells, which are an abundant byproduct of the food-processing industry, that provides large quantities of this biopolymer to be used in biomedical applications. In living chitin-synthesizing organisms, the synthesis and degradation of chitin require strict enzymatic control to maintain homeostasis. Chitin synthase, the pivotal enzyme in the chitin synthesis pathway, uses UDP-N-acetylglucosamine (UDPGlcNAc), produce the chitin polymer, whereas, chitinase enzymes degrade chitin. Bacteria are considered as the major mediators of chitin degradation in nature. Chitin and chitosan, owing to their unique biochemical properties such as biocompatibility, biodegradability, non-toxicity, ability to form films, etc, have found many promising biomedical applications. Nanotechnology has also increasingly applied chitin and chitosan-based materials in its most recent achievements. Chitin and chitosan have been widely employed to fabricate polymer scaffolds. Moreover, the use of chitosan to produce designed-nanocarriers and to enable microencapsulation techniques is under increasing investigation for the delivery of drugs, biologics and vaccines. Each application is likely to require uniquely designed chitosan-based nano/micro-particles with specific dimensions and cargo-release characteristics. The ability to reproducibly manufacture chitosan nano/microparticles that can encapsulate protein cargos with high loading efficiencies remains a challenge. Chitosan can be successfully used in solution, as hydrogels and/or nano/microparticles, and (with different degrees of deacetylation) an endless array of derivatives with customized biochemical properties can be prepared. As a result, chitosan is one of the most well-studied biomaterials. The purpose of this review is to survey the biosynthesis and isolation, and summarize nanotechnology applications of chitin and chitosan ranging from tissue engineering, wound dressings, antimicrobial agents, antiaging cosmetics, and vaccine adjuvants.
Keywords: Chitin, chitosan, synthetic nanofiber, nanoparticle, biomedical nanotechnology, drug delivery, vaccine adjuvant
Introduction
Chitin (β-(1–4)-poly-N-acetyl-D-glucosamine) is widely distributed in nature and is the second most abundant polysaccharide after cellulose. Chitin, which occurs in nature as ordered macrofibrils, is the major structural component in the exoskeletons of the crustaceans, crabs and shrimps, as well as the cell walls of fungi. For biomedical applications chitin is usually converted to its deacetylated derivative, chitosan (1). Chitin and chitosan are both biocompatible, biodegradable, and non-toxic biopolymers. They are also antimicrobial and hydrating agents (2). Depending on the source of chitin, it occurs as two allomorphs, namely the α and β forms, that can be characterized by infrared and solid-state NMR spectroscopy, together with X-ray diffraction. γ-Chitin is a third allomorph that has also been described (3). The allomorphs differ in the orientation of the micro-fibrils. Chitin biosynthesis is catalyzed by a widely conserved enzyme in nature, called chitin synthase. This enzyme exists in every chitin-synthesizing organism. Chitin synthase remains bound to the growing polymer chain through many polymerization steps that sequentially adds single GlcNAc units to the non-reducing end of the extending chain (4, 5). The linear polymers of chitin that are first obtained, then spontaneously assemble into microfibrils of varying diameter and length. After polymer synthesis, the completed fibrils are transported to the extracellular space(4). Glycosyl hydrolases (GH) are a broad class of enzymes that degrade polysaccharides; they are currently classified into 130 families based on similarities in their amino acid sequences (6, 7). Chitinases are mostly classified in the GH-18 and GH-19 families, and have been found to be present in a wide range of organisms, including bacteria, fungi, insects, plants, and animals (8–11). Chitin and chitosan are used in a wide range of biomedical applications such as tissue engineering, drug and gene delivery, wound healing, and stem cell technology (12). These biopolymers can be easily processed into various products, including hydrogels (1), membranes (13–16), nanofibers (17–19), beads (20), micro/nanoparticles (1, 21), scaffolds (22, 23) and sponges (1). Tissue engineering is one of the most-studied fields in which chitin and chitosan have been successfully used to fabricate polymeric scaffolds for purposes of tissue repair and regeneration.
The most important characteristics of these biopolymers which have made them ideal candidates to fabricate polymeric tissue scaffolds are: high porosity; biodegradability; predictable degradation rate; structural integrity; non-toxicity to cells; and biocompatibility. Loading chitin-based nanoparticles with various drugs such as lamivudine and 5-fluorouracil, are just two examples of successful applications of chitin and chitosan in drug delivery. More recently, semiconductor nanocrystals (quantum dots) have been coated with chitosan for bioimaging applications in cancer diagnosis (24). The enhancement of immune responses against pathogenic microorganisms by using chitosan derivatives as vaccine adjuvants has been reported (25). Other applications of chitin and chitosan will be discussed in the following sections.
Chitin and chitosan: general characterization
Chitin, poly (β-(1–4)-N-acetyl-D-glucosamine), is a natural polysaccharide of major importance (3). The name ‘chitin’ is derived from the Greek word ‘chiton’, meaning a coat of mail (26). The use of chitin was first described by the French chemist, Henri Braconnot in 1811. The structure of chitin (C8H13O5N)n is similar to that of cellulose, but with 2-acetamido-2-deoxy-β-D-glucose (NAG) monomer units, which are attached to each other via β(1→4) linkages. Chitosan is the deacetylated form of chitin (that can have varying degrees of deacetylation), and it is soluble in acidic solutions (sometimes with difficulty) (27, 28). The conversion of chitin to chitosan is possible either by enzymatic preparations, or chemical hydrolysis (29–32). The worldwide natural production rate of chitin has been estimated to be approximately 1011 tons annually (9, 33). The material form of chitin is usually a white and hard nitrogenous polysaccharide which is inelastic. It has also been considered to be the major source of beach pollution in coastal areas (34). Chitin occurs as crystalline microfibrils in nature, that form structural elements of the exoskeletons of arthropods, and in fungal cell walls. For greater reinforcement and strength protection properties, chitin is also produced by a number of other living organisms in the lower plant and animal kingdoms (3, 35). (Fig.1)
Fig.1.
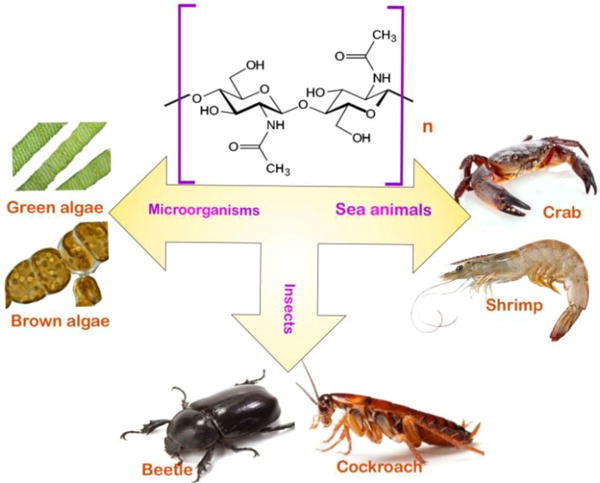
These organisms are united by the presence of chitin as an integral structural component; In some organisms (for example the snail) chitin is found in the mandible, as well as a supply of the chitin degrading enzyme, chitinase is present in the gut (36). The wide occurrence of chitin and chitosan in these creatures make them good natural sources of these biopolymers(3).
There have been no reports of quantitatively-significant long-term accumulation of chitin in nature, which implies that its production, degradation and turnover must be efficiently balanced. Alongside the abundance and ubiquity of chitin, chitin-degrading enzymes have also been detected in many organisms (37). Chitin owes its biodegradability to the action of chitinase enzymes that are widely distributed in nature (38).
The widespread occurrence of chitin in the biosphere and its insolubility, led to the idea that chitin should survive in fossils (37). There have indeed been reports of fossilized chitinous materials, e.g., in pogonophora (37, 39), and in insect wings preserved inside amber (37). The immunogenicity of chitin (in spite of presence of nitrogen in its structure) is exceptionally low. Chitin is a highly insoluble material that resembles cellulose in its low solubility and chemical non-reactivity. It may be regarded as a cellulose structure where the hydroxyl group at position C-2 has been replaced by an acetamido group. (Fig.2)
Fig.2.
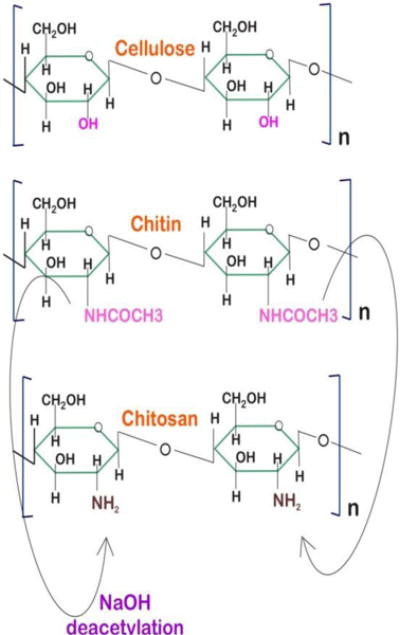
Structures of chitin, chitosan and cellulose.
Chitin sometimes is considered to be a cellulose derivative, however it does not occur in cellulose producing organisms (40).There is no generally accepted nomenclature with respect to the degree of N-deacetylation of chitin and its derivatives. Chitin and chitosan have a high percentage of nitrogen (6.89%) compared to synthetically substituted cellulose derivatives that can only be prepared with a lower nitrogen content (1.25%). Most of the naturally occurring polysaccharides, e.g. cellulose, dextran, pectin, alginic acid, agar, agarose and carrageenan are neutral or acidic in nature, whereas chitosan is an examples of a highly basic polysaccharide. Other unique properties of chitin and chitosan include formation of polyoxysalts, ability to form films, biocompatibility, biodegradability, non-toxicity, molecular adsorption properties, etc. In spite of several reports describing the preparation of functionalized chitosan derivatives by chemical modification of the amino groups, very few of these have acceptable solubility in general organic solvents, or binary solvent systems. Some chemically modified chitin and chitosan derivatives possessing improved solubility in general organic solvents have been reported (41).
Chemical structure and properties
Chitin is poly-(1→4)-β-linked N-acetyl-D-glucosamine (42). The individual sugar units in its structure are rotated 180° with respect to each other, and each pair forms the disaccharide N,N′-diacetylchitobiose [(GlcNAc)2 (33, 43, 44). The individual polymer chains can be described as helices, in which each sugar unit is inverted with respect to its neighbors. Such a structure leads to high stability as the rigid ribbons are connected by 03-H→05 and 06-H→07 hydrogen bonds.
Chitin also has three different crystalline allomorphs: the α-, β- and γ-forms (32). These differ in the orientation of the micro-fibrils. The commonest form of chitin is α-chitin. Its unit cell is composed of two N,N′-diacetylchitobiose units forming two chains in an antiparallel arrangement. Therefore, adjacent polymer chains run in opposite directions, held together by 06-H→06 hydrogen bonds, and the chains are held in sheets by 07→H-N hydrogen bonds (45–47). This gives a statistical mixture of -CH2OH orientations, equivalent to half the oxygen atoms on each residue, being able to form inter- and intramolecular hydrogen bonds. This results in two different types of amide group; all are involved in forming the interchain C=O→H-N bonds, while half of the amide groups also serve as acceptors for 06-H→O=C intramolecular hydrogen bonds. Formation of these intermolecular hydrogen bonds leads to quite a stable structure. The polymer chains eventually give rise to microfibrils by self-assembly if they are allowed to crystallize (37).
β-chitin is a less common form of chitin, in which the unit cell is a N,N′-diacetylchitobiose unit, giving a polymer stabilized as a rigid ribbon, the same as α-chitin, by 03→05 intramolecular H-bonds (47, 48). The chains in this structure are held together in sheets by C=O→H-N H-bonds between the amide groups and by the -CH2OH side chains, which leads to formation of intersheet H-bonds to the carbonyl oxygens on the adjacent chains (06-H→07). This gives a structure of parallel poly-N-acetylglucoseamine chains with no intersheet H-bonds. The parallel arrangement of polymer chains in β-chitin allows for more flexibility than the antiparallel arrangement found in α-chitin, but the resultant polymer still has immense strength (49). γ-Chitin is the third allomorph, possessing mixed parallel and antiparallel orientations. It has been reported to occur in mushrooms (50, 51). Chitin is always found cross-linked to other structural components with the exception of the β-chitin found in diatoms. Chitin is found covalently bonded to glucans in fungal cell walls, either directly, as in Candida albicans (1) or via peptide bridges. Moreover, in insects and other invertebrates, chitin is always associated with specific proteins, with both covalent and noncovalent bonding. This association means it produces the observed ordered structures. There are also varying degrees of mineralization, such as calcification, and sclerotization, involving interactions with phenolic and lipid molecules (45). In organisms like fungi and invertebrates, there have been reported varying degrees of deacetylation, giving a continuum of structure between chitin (fully acetylated) and chitosan (fully deacetylated) (52). Although either acids or alkalis can be used to deacetylate chitin, the fact that glycosidic bonds are more susceptible to acid which would destroy the chain, the alkali deacetylation process is used more frequently (32, 53).
N-deacetylation of chitin can either by performed by heterogeneous or homogeneous reaction mixtures (32). The distinction between chitin and chitosan with different degrees of deactylation is not strict (25, 54). Putting a few exceptions aside, natural chitin occurs associated with other structural polymers like proteins or glucans, which often contribute more than 50% of the mass in chitin-containing tissue (55). Chitin can be N-deacetylated to such an extent that it becomes soluble in dilute acetic and formic acids. In chitin, the acetylated units prevail and the degree acetylation is typically 0.90, while chitosan is a fully or partially N-deacetylated derivative with a typical degree of deacetylation of more than 0.65. Many analytical tools have been used to determine this degree of deacetylation including IR spectroscopy, pyrolysis gas chromatography, gel permeation chromatography and UV-vis spectrophotometry, 1H NMR spectroscopy, 13C solid state NMR, thermal analysis, various titration schemes, acid hydrolysis, HPLC, separation spectrometry methods and more recently near-infrared spectroscopy (41).
Chitin biosynthesis
Chitin biosynthesis and cellular processing is a highly complex, multi-faceted and inter-connected series of events which starts intracellularly and ends in inclusion of chitin in exterior supra-macromolecular structures such as arthropod cuticles and fungi cell walls. (Fig.3)
Fig.3.
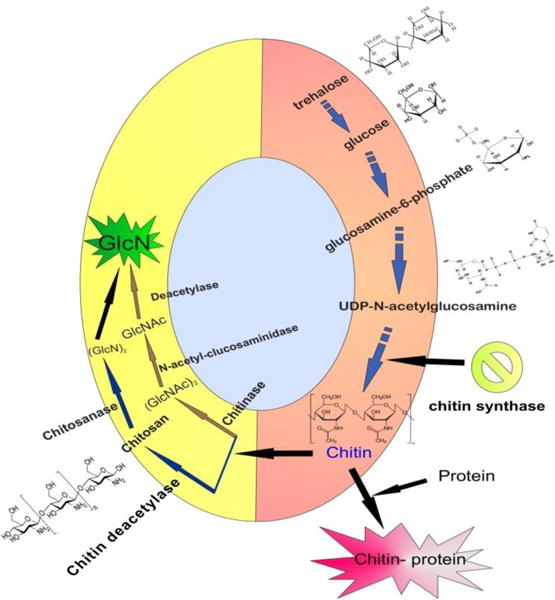
Events associated with chitin formation and degradation.
The whole process encompasses several individual steps:
Sequential biotransformation of sugars (mainly trehalose or glucose). This step includes biochemical reactions like phosphorylation, amination and also formation of the enzymic substrate, UDP-N-acetylglucosamine.
Chitin synthase (CS) synthesizes the chains. The CS unit is an enzyme which is a part of a protein/carbohydrate cluster including closely topologically packed molecules. Such an arrangement ensures coalescence of nascent chitin polymers into a crystalline fibril.
The orientation of chitin molecules which have long chains.
Polymer translocation across the plasma membrane.
Crystallization and formation of microfibrils by inter-chain hydrogen bonding.
Association with arthropod cuticular proteins or with other carbohydrates in fungal cell walls.
Chitosomes are cytoplasmic microvesicles with CS activity. These microvesicles have been identified through electron microscopy studies using fungal systems (56–58). The abundance of chitosomes at the hyphal tip implies their crucial role in CS trafficking to pre-determined locations (59). Chitosomes originate from organelles such as endoplasmic reticulum and Golgi. Chitosome vesicles contain zymogenic CS clusters. After eventually fusion of the chitosomes with the plasma membrane, the CS units become activated through proteolytic reactions(60). CS insertion into plasma membranes, involves the mediation of targeting and recognition proteins. The exact machinery of such fusion along with the proteins involved remains unresolved. Chitosome-like structures also have been reported in cell-free system derived from insects (61); however it has not been clarified whether they are involved in in vivo chitin formation. Moreover chitosome-like vesicles have not yet been described in intact insect epidermal cells(56).
Different forms of chitin are synthesized by the action of the enzyme, chitin synthase (UDP-N-acetyl-D-glucosamine: chitin4-β-N-acetylglucosaminyl-transferase; EC 2.4.1.16). This enzyme which is highly conserved is expressed in every organism possessing chitin synthesizing activity. Chitin synthase uses UDP-N-acetylglucosamine (UDPGlcNAc) as the activated sugar donor to produce the chitin polymer (62). Candy and Kilby (1962) first proposed a chitin synthesis biosynthetic pathway in insects. The proposed mechanism started with glucose and ended with UDP-GlcNAc. Jaworski et al. (1963) using cell-free extracts from the southern armyworm Spodoptera eridania, finally established the whole pathway from UDP-GlcNAc to chitin. Results of many subsequent studies conducted with preparations from various insects supported the molecular mechanism of this pathway.
Industrial processing of chitin
The isolation of chitin from crustaceans such as crayfish, crab, shrimp, and other organisms such as fungi is a time-consuming process (63–65). It requires 17–72 h including 1–24h of treatment with HCl and 16–48h of NaOH processing. Lengthy procedures for chitin isolation require more energy, and in return increases the cost of production. Like crabs and shrimps, barnacles also belong to the Crustacea family. The shell structures of barnacle species are less crystalline and they are reported to contain more minerals than other members of the crustacean family (63). These minerals are mainly composed of calcite and calcium phosphate (66, 67).
It has been shown that these two mineral materials in the carapace can be easily removed from the carapace structure by using HCl. In such creatures, the protein only has a few weak bonds with the chitin so it can be removed from easily because of low-crystalline shell structure of the barnacle species. Therefore, it is expected that the isolation of chitin from barnacle species would be a comparatively quick process. Chelonibia patulais is a barnacle species in the subphylum Crustacea which lives epizoically on animals such as turtles, crabs, whales and mollusks, or on rock on the seashore where there is shallow water. In a procedure for the isolation of chitin from C. patula shells, they should be demineralized in 1M HCl for 10 min, and deproteinized in 2M NaOH for 20 min. The completion of the whole process takes only half an hour. It starts with dripping 1M HCl solution onto 10g of the dust obtained from ground C. patula shells followed by stirring for 10 min by means of a magnetic stirrer at room temperature. If the HCl is added too quickly a vigorous effervescence occurs that may led to overflowing. The samples then should be rinsed with distilled water until a neutral pH value is obtained. In a study, after drying the samples in an oven, 376 mg of material remained at the end of the process. Considering that the majority of the original mass consisted of minerals, these have been removed by means of HCl. Proteins are also found in shells and are removed by a deproteinization process (refluxing with a base for 20 min) which left 311 mg of dry chitin. (Fig.4) The chitin content in the shell was equivalent to 3.11% of the barnacle shell dry weight and the protein content of C. patula shell dust was low (63). Obtaining chitin from shrimp shells is associated with food industries such as shrimp processing, whereas the production of chitosan–glucan complexes from fungal mycelia is associated with fermentation processes such as that producing citric acid from Aspergillus niger. Generally, processing of crustacean shells involves the removal of proteins and then dissolution of calcium carbonate, which is present in the shells in high concentrations. Protocols for obtaining chitosan from these sources also involves deacetylation in 40% sodium hydroxide at 120°C for 1–3h. This treatment yields 70% deacetylated chitosan (41). Recently, a “green conversion” of agroindustrial wastes by the biological activity of Rhizopus arrhizus and Cunninghamella elegans strains has been reported to produce chitin and chitosan. Such industrial sources have significant advantages including avoiding allergic reactions in individuals susceptible to shellfish antigens and reduction in time and cost of production (68, 69).
Fig.4.
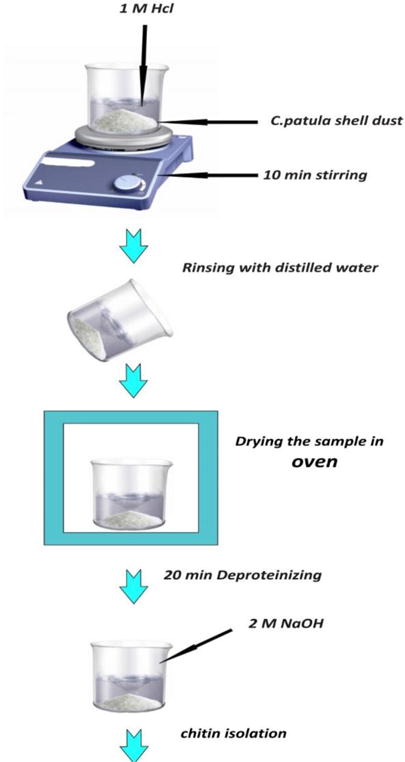
General steps of chitin processing from C. patula shell dust.
Chitin degradation
It is believed that bacteria are the major mediators of chitin degradation in nature. Their role can be demonstrated in both soil and water systems. In soil systems, the chitin hydrolysis rate correlates with bacterial population and abundance (70). The degradation in this case depends on factors such as temperature and pH. Not only the bacteria but also fungi may be considered quantitatively important agents in the chitin degradation process (44, 71). The results of plating experiments in aquatic systems demonstrated convincingly that bacteria are the main mediators of chitin degradation (72). Dense fungal colonization of the carapaces of chitinous zooplankton has been observed, and some diatoms were found to be able to hydrolyze chitin oligomers. Furthermore, enzymes released during molting of planktonic crustaceans are believed to be another source of enzymes that can metabolize chitin in aquatic systems (44). The degradation of chitin is a highly regulated process, and (depending on the organism under scrutiny) the hydrolytic enzymes have been found to be induced by the actual products of the chitin hydrolysis (GlcNAc (73) and soluble chitin oligomers (GlcNAc)2–6). In contrast to (GlcNAc)2, GlcNAc has also been reported to act as a suppressor of the expression of chitinase in a strain of Streptomyces (74). This suppressor activity may be connected with GlcNAc occurring in the murein found in fungal cell walls rather than in chitin (44). Despite the insoluble nature of chitin and its relatively resistance to degradation because of its complex crystalline structure, some soil and marine bacteria, such as Bacillus circulans WL-12 (75), Serratia marcescens (76), Streptomyces coelicolor A3 (77), Aeromonas caviae (78), Pseudoalteromonas sp. strain S91(79) and Vibrio harveyi (80) are able to degrade, transport, and utilize chitin as an energy source by the action of chitinases (EC 3.2.1.14). Itoh et al., (2013) studied Paenibacillus sp. strain FPU-7 and found cooperative degradation of chitin by extracellular and cell surface-expressed chitinases. This organism grew well in liquid medium and could completely hydrolyze chitin flakes from crab shells. (Fig.5)
Fig.5.
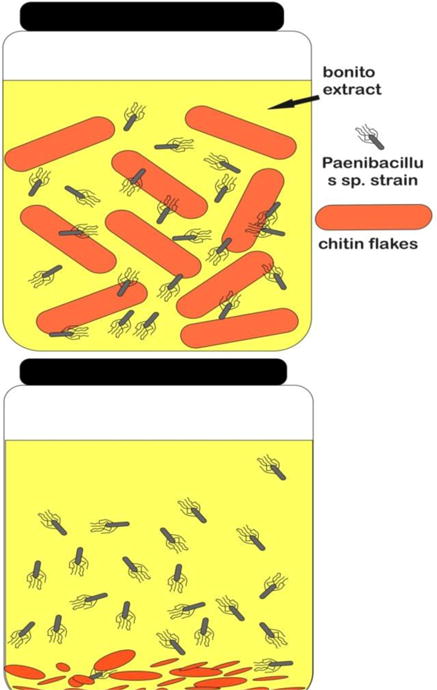
Chitin degrading ability of Strain FPU-7 was shown after culturing at 30°C for 5 days with shaking, in bonito extract medium containing 5.0% (wt/vol) crab shell chitin flakes. The solid matter (chitin flakes) in the flask disappeared after 5 days of incubation.
Possible chitin degradation pathways have been discussed by Davis and Eveleigh (81). The degradation process is best termed chitinoclastic (chitin breaking) when the degradation pathway is not exactly known, whereas it is best termed chitinolytic when the pathway involves the initial hydrolysis of the (1→4)-β-glycosidic bond. Hydrolysis of this bond is accomplished by chitinase. The exo-chitinase enzyme cleaves diacetylchitobiose units from the non-reducing end of the chitin chain, while the endo-chitinase enzyme cleaves glycosidic linkages randomly along the length of the chitin chain.
The common product of this enzyme is diacetylchitobiose, together with some triacetylchitotriose. The latter product may be slowly degraded to disaccharide and monosaccharide units. There may not always be a clear distinction between these two activities and the hydrolysis site strongly depends on the nature of substrate. For instance, Streptomyces chitinase only degrades the pure crystalline β-chitin of diatom spines from its end yielding only diacetylchitobiose, while colloidal chitin is degraded to oligomers and disaccharide (37).
Chitin and chitosan: biomedical and nanomedical applications
Tissue engineering
Tissue engineering involves the use of living cells, which are manipulated and controlled through their extracellular environment to fabricate tissue substitutes for implantation into the body (82, 83). The main purposes of tissue engineering can be categorized as to repair, replace, maintain, or enhance the function of a particular tissue or organ (84).
Chitin-based materials, which can be fabricated into tubular forms, can be successfully applied in tissue engineering of nerves and blood vessels as a template for cells (52, 85–87). Chitin-based scaffolds are versatile products and can be optimized for many regenerative purposes (88).
Chitin and chitosan have been successfully applied to fabricate polymer scaffolds in tissue engineering. Some basic requirements to design polymer scaffolds are: high porosity (with an appropriate pore size distribution); biodegradability (degradation rate should match the rate of neo-tissue formation); structural integrity (to prevent the pores of the scaffold from collapsing during neo-tissue formation); being non-toxic to cells; biocompatibility; interacting with the cells to promote cell adhesion; encouraging cell function (proliferation, migration and differentiation) (24).
There are several methods that have been used to produce chitosan scaffolds including: phase separation and lyophilization technique (88–94); particulate leaching techniques; freeze gelation technique; rapid prototyping technology; formation of microparticles and microspheres. (Fig.6)
Fig.6.
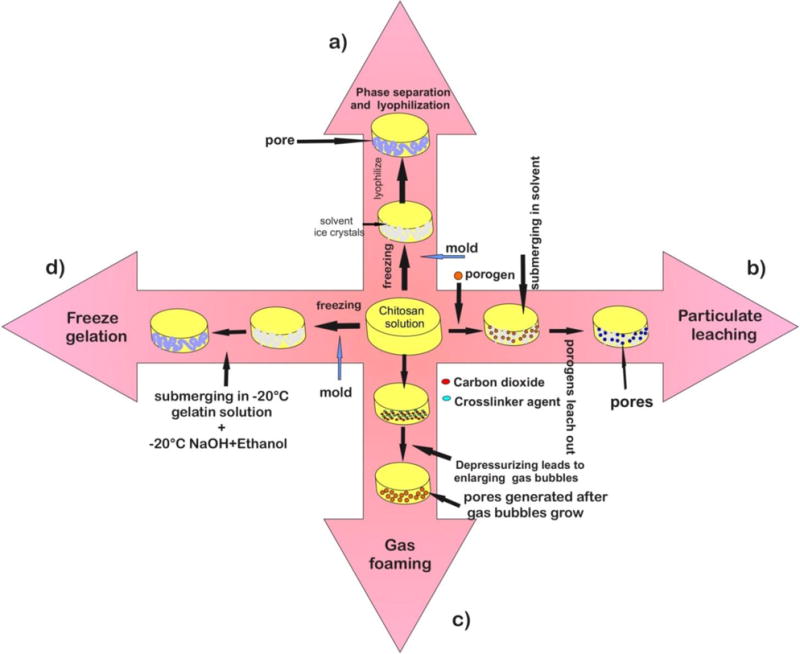
Four commonly used methods of chitosan scaffold fabrication. (a) Phase separation and lyophilization technique, a chitosan solution is introduced into a mold, then a freezing step makes it ready for phase separation with acetic acid solvent and chitosan acetate salt. Lyophilization is the final step. (b) Particulate leaching technique, is frequently used combined with phase separation. In this method, a porogen (i.e. gelatin) is mixed with chitosan solution prior to phase separation and lyophilization steps. Through submerging in a solvent, the resultant scaffold is prepared for porogen leaching. The obtained scaffolds would then possess additional porosity.
(c) In the gas foaming technique, a chitosan solution containing a cross-linker (glutaraldehyde) is supersaturated with carbon dioxide at high pressure to induce cross-linking. Gas bubbles are generated; for example by mixing a foaming or blowing agent such as sodium bicarbonate with a pre-polymer where gas is generated upon chemical decomposition (89, 95, 96). When the system is depressurized, thermodynamic instability leads to nucleation and gas bubble growth. Pores are generated while the bubbles escape the polymer solution. (d) Freeze gelation technique, which initially involves phase separation due to freezing. The obtained scaffold is placed in a gelation solution of sodium hydroxide and ethanol below the chitosan freezing temperature. The last step following the gelation, is scaffold air-drying to remove residual liquid (89).
Chitosan scaffolds also can be fabricated through the fusion or the sintering of chitosan microparticles or microspheres (97, 98). An electrospun water-soluble blend of carboxymethyl chitin (CMC) with PVA for tissue engineering applications was reported by Shalumon et al. (2009). In order to fabricate appropriate nanofibers, the concentration of CMC (7%) and PVA (8%) was optimized, blended in different ratios (0–100%) and electrospun. Fibers were made water-insoluble by cross-linking with glutaraldehyde vapors followed by thermal treatment. The nanofibers were found to be bioactive and biocompatible. Scaffold properties such as cytotoxicity and cell attachment were evaluated using human mesenchymal stem cells (hMSCs) by the MTT assay. These studies revealed ability of cells to attach and spread on the nanofibrous scaffolds. Peter et al. reported chitosan/gelatin/nanophase hydroxyapatite (nHAp) composite scaffolds produced by blending chitosan and gelatin with nHAp (24).
Wound healing
Madhumathi et al. (2010) developed α-chitin/nanosilver composite scaffolds for wound healing applications. These scaffolds were found to possess antibacterial activity against S. aureus and E. coli, as well as blood-clotting ability. Such properties have made them useful nanostructures for wound healing applications. Similarly, Sudheesh Kumar et al. (2010) developed and characterized β-chitin/nanosilver composite scaffolds for this application using β-chitin hydrogel containing silver nanoparticles. In addition, these scaffolds were evaluated for their cell adhesion properties using Vero cells, and the results revealed that nanosilver incorporated chitin scaffolds were ideal for wound healing applications (24).
Drug delivery
Water-soluble carboxymethyl chitin (CMC) was used for drug delivery applications. CMC nanoparticles were prepared through a cross-linking approach using CaCl2 and FeCl3. A spherical morphology was observed in SEM images of CMC nanoparticles, with diameters ranging from 200 to 250 nm. 5-fluorouracil (5-FU) drug-loaded nanoparticles also showed similar morphology. MTT assay results showed that they were non-toxic to normal fibroblast L929 mouse cells. The hydrophobic anticancer drug 5-FU was loaded into CMC nanoparticles via an emulsion cross-linking method, and these were found to have a controlled and sustained drug-release profile at pH-6.8. An anti-HIV drug delivery application was also reported by Dev, Binulal, et al. (2010) using poly (lactic acid) (PLA)/CS nanoparticles. In addition, lamivudine (a hydrophilic antiretroviral drug) was loaded into the PLA/CS nanoparticles. In this case absorption spectrophotometry was used to evaluate the encapsulation efficiency and the in vitro drug release behavior of drug loaded PLA/CS nanoparticles (24).
Cancer diagnosis
Semiconductor nanocrystals (or quantum dots) can be bioconjugated to a variety of biological recognition ligands, and can replace conventional organic fluorescent dyes in immunostaining applications and bioimaging of cancerous cells and tissues. However, quantum dots include the heavy metals, cadmium sulphide, cadmium selenide, zinc selenide, etc. and can be cytotoxic and even hazardous. A heavy-metal-free luminescent quantum dot (QD) based on doped-zinc sulphide (ZnS), conjugated with a cancer-targeting ligand, folic acid (FA) was developed for targeted cancer-imaging. Folate receptors are over-expressed on many cancer cells, and can mediate receptor-based endocytosis when they interact with folate-conjugated nanoparticles, providing intracellular uptake. Similarly, mannose receptors, have been used for cancer diagnosis (24). A novel FA-conjugated carboxymethyl chitosan (CMCS) coordinated to manganese doped zinc sulphide (ZnS:Mn) quantum dots (FA-CMCS-ZnS:Mn) forming composite nanoparticles was developed by Manjusha et al. (99). This multifunctional system could be used for targeting, controlled drug-delivery and cancer cell imaging. The selected anticancer drug was 5-FU, which is used for breast cancer treatment. L929 cells were used to confirm the non-toxicity of FA-CMCS-ZnS:Mn nanoparticles. Furthermore, the MCF-7 breast cancer cell line was used to study imaging, specific targeting and cytotoxicity of the drug loaded nanoparticles. The in vitro imaging of cancer cells with the nanoparticles was studied using fluorescence microscopy (100).
Chitin- and chitosan-based dressings
Sparkes and Murray (101) developed a chitosan–gelatin complex desu eb dluoc taht as a surgical dressing. The procedure involved dissolving the chitosan in water in the presence of a suitable acid in order to maintain the solution pH at about 2–3, followed by addition of gelatin dissolved in water. The ratio of chitosan and gelatin was between 3:1 and 1:3. A certain amount of a plasticizer such as glycerol or sorbitol could be added to the mixture to reduce its stiffness. A film was cast from this solution onto a flat plate and dried at room temperature so it could be used as a dressing. This experimental dressing displayed excellent adhesion to subcutaneous fat. The British Textile Technology Group (BTTG) patented a procedure for making a chitin-based fibrous dressing (102). In this method the chitin/chitosan fibers were obtained from micro-fungi (instead of shrimp shells) and were not fabricated by the traditional fiber-spinning technique. The procedure can be summarized as follows.
Preparation of micro-fungal mycelia from a culture of Mucor mucedo growing in a nutrient medium.
Washing and deproteinizing of the mycelial matt with NaOH to precipitate chitin/chitosan.
Bleaching and further washing.
Preparation and dispersion of the fibers using paper-making equipment.
Filtration and wet-laid preparation of a fiber matt; additional mechanical strength provided by mixing with other fibers.
Ophthalmology
Some of the particular characteristics of chitosan such as its optical clarity, mechanical stability, sufficient optical correction, gas permeability (particularly towards oxygen), wettability, and immunological compatibility make it ideal to be used for fabricating contact lenses for use in the eye. These lenses are made from partially depolymerized and purified chitosan isolated from squid pen and are clear, tough and possess other required physical properties such as Young’s modulus, tensile strength, tear strength, elongation, water content and oxygen permeability. The antimicrobial activity, film-forming capability, and wound-healing properties of chitosan also make it suitable for development of ocular bandage-lenses for traumatic injuries (59).
Antibacterial properties
The growth of E. coli was inhibited in the presence of chitosan with a concentration greater than 0.025%. Chitosan is also able to inhibit the growth of other microbial species such as Fusarium, Alternaria and Helminthosporium. The cationic amino groups of chitosan probably bind to anionic groups of microorganisms and prevent their growth (41). Low-molecular-weight chitosan after penetrating into bacterial cell walls, can bind with DNA and inhibit DNA transcription and mRNA synthesis (103). The natural antimicrobial characteristics of chitosan derivatives have resulted in their use in commercial disinfectants and topical antimicrobials (104, 105).
Anti-thrombogenic and haemostatic materials
Blood clot formation is an area where chitosan may have completely opposite effects depending on its functional type and formulation. Chitosan has been shown to be a highly effective hemostatic agent when formulated as microspheres (106), as bandages or dressings (107), or as absorbable sponges (108). In fact a chitosan dressing was able to control an experimental arterial hemorrhage in dogs (109). The efficiency of chitosan as a hemostatic agent was shown to be dependent on its degree of hydration (110). However there are also reports that different chitosan preparations can be anti-thrombogenic and anti-coagulant. N-hexanoyl and N-octanoylchitosan fibers can be used as anti-thrombogenic materials (41). A phthalized chitosan derivative was used to prepare an anti-thrombogenic material for the fabrication of vascular grafts (111). Sulfate derivatives of chitin and chitosan have blood anticoagulant and lipoprotein lipase (LPL)-releasing activities. Chitin 3,6-sulfate showed about two-fold anticoagulant activity and also 0.1-fold LPL-releasing activity over those of heparin; the sulfate derivatives might be usable as heparinoids for artificial blood dialysis (112).
Antiaging cosmetics
Morganti et al. (113) developed block-copolymer nanoparticles (BPN) composed of phosphatidylcholine and linoleic acid nanocomplexed with hyaluronan and chitin nanofibrils (PHHYCN) Thee nanoconstructs were used to encapsulate cargos comprising cholesterol, creatine, caffeine, melatonin, vitamins E and C, and the amino acids glycine and arginine. The idea was to use these nanocarriers for skin rejuvenation, as all the individual ingredients had shown some activity in this regard. Subjects whose skin was treated with the active chitin nanofibrils containing loaded BPN were shown to be softer and more hydrated after one month of treatment. Both fine wrinkles and crease lines were reduced soon after the first 15 days of treatment with injectable active chitin nanofibril containing BPN, as well less presence of telangiectasia, so that the general appearance of the face was notably ameliorated during the regression period (113).
Antitumor activity
Chitosan and its derivatives have exhibited antitumor activity in both in vitro tests and in vivo animal models. Tokoro et al (114) suggested that this activity was due to an increase in secretion of interleukins-1 and 2 which caused maturation and infiltration into the tumor of cytolytic T-lymphocytes. It was further suggested by Dass and Choong (115) that chitosan could increase lymphokine production and enhance proliferation of cytolytic T-lymphocytes (103).
Vaccine Adjuvant
Studies in mice and guinea pigs have demonstrated that intranasal chitosan glutamate can significantly enhance antibody responses to a diphtheria antigen (a non-toxic cross-reacting derivative of diphtheria toxin, CRM197) (116). Mills et al. studied the impact of this chitosan adjuvant to a diphtheria vaccine in healthy human volunteers (117). Mann et al. conducted a live-virus challenge study in ferrets, which is a recognized preclinical model for human influenza. Both chitosan glutamate (CSN) and N,N,N-trimethylated chitosan (TM-CSN) were investigated as adjuvants for an inactivated NIBRG-14 (H5N1) subunit antigen (derived from influenza antigen A/Vietnam/1194) (25, 118).
Conclusion
The unique biochemical properties of chitin and chitosan suggest they could be seen as almost ideal biopolymers with numerous applications in biomedical research. These materials can be processed into various products and on the other hand it is possible to fabricate scaffolds and nanoparticles with increasing applications in the burgeoning field of nanomedicine. In this review we have tried to present an overview of the biosynthesis, isolation and technical applications of chitin and chitosan. However in the limited space available we have been able to cover only a fraction of the biomedical applications, and there is an even wider variety of applications in food packaging and processing, agriculture, biotechnology and cosmetics.
Acknowledgments
MR Hamblin was funded by US NIH grant R01AI050875.
References
- 1.Azuma K, Izumi R, Osaki T, Ifuku S, Morimoto M, Saimoto H, et al. Chitin, chitosan, and its derivatives for wound healing: old and new materials. Journal of functional biomaterials. 2015;6(1):104–42. doi: 10.3390/jfb6010104. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2.Jayakumar R, Prabaharan M, Sudheesh Kumar PT, Nair SV, Tamura H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnology advances. 2011;29(3):322–37. doi: 10.1016/j.biotechadv.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Rinaudo M. Chitin and chitosan: Properties and applications. Elsevier Ltd; 2006. [Google Scholar]
- 4.Merzendorfer H. Insect chitin synthases: a review. Journal of comparative physiology B, Biochemical, systemic, and environmental physiology. 2006;176(1):1–15. doi: 10.1007/s00360-005-0005-3. [DOI] [PubMed] [Google Scholar]
- 5.Imai T, Watanabe T, Yui T, Sugiyama J. The directionality of chitin biosynthesis: a revisit. The Biochemical journal. 2003;374(Pt 3):755–60. doi: 10.1042/BJ20030145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Itoh T, Hibi T, Fujii Y, Sugimoto I, Fujiwara A, Suzuki F, et al. Cooperative degradation of chitin by extracellular and cell surface-expressed chitinases from Paenibacillus sp. strain FPU-7. Applied and environmental microbiology. 2013;79(23):7482–90. doi: 10.1128/AEM.02483-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, Henrissat B. The Carbohydrate-Active EnZymes database (CAZy): an expert resource for Glycogenomics. Nucleic acids research. 2009;37:D233–8. doi: 10.1093/nar/gkn663. Database issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dahiya N, Tewari R, Hoondal GS. Biotechnological aspects of chitinolytic enzymes: a review. Applied microbiology and biotechnology. 2006;71(6):773–82. doi: 10.1007/s00253-005-0183-7. [DOI] [PubMed] [Google Scholar]
- 9.Eijsink V, Hoell I, Vaaje-Kolstada G. Structure and function of enzymes acting on chitin and chitosan. Biotechnology & genetic engineering reviews. 2010;27:331–66. doi: 10.1080/02648725.2010.10648156. [DOI] [PubMed] [Google Scholar]
- 10.Okazaki K, Yamashita Y, Noda M, Sueyoshi N, Kameshita I, Hayakawa S. Molecular cloning and expression of the gene encoding family 19 chitinase from Streptomyces sp. J-13-3. Bioscience, biotechnology, and biochemistry. 2004;68(2):341–51. doi: 10.1271/bbb.68.341. [DOI] [PubMed] [Google Scholar]
- 11.Ueda M, Kojima M, Yoshikawa T, Mitsuda N, Araki K, Kawaguchi T, et al. A novel type of family 19 chitinase from Aeromonas sp. No.10S-24. Cloning, sequence, expression, and the enzymatic properties. European journal of biochemistry/FEBS. 2003;270(11):2513–20. doi: 10.1046/j.1432-1033.2003.03624.x. [DOI] [PubMed] [Google Scholar]
- 12.Azuma K, Ifuku S, Osaki T, Okamoto Y, Minami S. Preparation and biomedical applications of chitin and chitosan nanofibers. Journal of biomedical nanotechnology. 2014;10(10):2891–920. doi: 10.1166/jbn.2014.1882. [DOI] [PubMed] [Google Scholar]
- 13.Yusof NL, Wee A, Lim LY, Khor E. Flexible chitin films as potential wound-dressing materials: wound model studies. Journal of biomedical materials research Part A. 2003;66(2):224–32. doi: 10.1002/jbm.a.10545. [DOI] [PubMed] [Google Scholar]
- 14.Marreco PR, da Luz Moreira P, Genari SC, Moraes AM. Effects of different sterilization methods on the morphology, mechanical properties, and cytotoxicity of chitosan membranes used as wound dressings. Journal of biomedical materials research Part B, Applied biomaterials. 2004;71(2):268–77. doi: 10.1002/jbm.b.30081. [DOI] [PubMed] [Google Scholar]
- 15.Jayakumar R, Nwe N, Tokura S, Tamura H. Sulfated chitin and chitosan as novel biomaterials. International journal of biological macromolecules. 2007;40(3):175–81. doi: 10.1016/j.ijbiomac.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 16.Madhumathi K, Binulal NS, Nagahama H, Tamura H, Shalumon KT, Selvamurugan N, et al. Preparation and characterization of novel beta-chitin-hydroxyapatite composite membranes for tissue engineering applications. International journal of biological macromolecules. 2009;44(1):1–5. doi: 10.1016/j.ijbiomac.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Gopalan Nair K, Dufresne A. Crab shell chitin whisker reinforced natural rubber nanocomposites. 1. Processing and swelling behavior. Biomacromolecules. 2003;4(3):657–65. doi: 10.1021/bm020127b. [DOI] [PubMed] [Google Scholar]
- 18.Fan Y, Saito T, Isogai A. Preparation of chitin nanofibers from squid pen beta-chitin by simple mechanical treatment under acid conditions. Biomacromolecules. 2008;9(7):1919–23. doi: 10.1021/bm800178b. [DOI] [PubMed] [Google Scholar]
- 19.Ifuku S, Nogi M, Abe K, Yoshioka M, Morimoto M, Saimoto H, et al. Preparation of chitin nanofibers with a uniform width as alpha-chitin from crab shells. Biomacromolecules. 2009;10(6):1584–8. doi: 10.1021/bm900163d. [DOI] [PubMed] [Google Scholar]
- 20.Yusof NL, Lim LY, Khor E. Preparation and characterization of chitin beads as a wound dressing precursor. Journal of biomedical materials research. 2001;54(1):59–68. doi: 10.1002/1097-4636(200101)54:1<59::aid-jbm7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 21.Prabaharan M. Review paper: chitosan derivatives as promising materials for controlled drug delivery. Journal of biomaterials applications. 2008;23(1):5–36. doi: 10.1177/0885328208091562. [DOI] [PubMed] [Google Scholar]
- 22.Prabaharan M, Jayakumar R. Chitosan-graft-beta-cyclodextrin scaffolds with controlled drug release capability for tissue engineering applications. International journal of biological macromolecules. 2009;44(4):320–5. doi: 10.1016/j.ijbiomac.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Maeda Y, Jayakumar R, Nagahama H, Furuike T, Tamura H. Synthesis, characterization and bioactivity studies of novel beta-chitin scaffolds for tissue-engineering applications. International journal of biological macromolecules. 2008;42(5):463–7. doi: 10.1016/j.ijbiomac.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Jayakumar R, M D, Manzoor K, Nair SV, Tamura H. Biomedical applications of chitin and chitosan based nanomaterials—A short review. Elsevier Ltd; 2010. [Google Scholar]
- 25.Smith A, Perelman M, Hinchcliffe M. Chitosan: a promising safe and immune-enhancing adjuvant for intranasal vaccines. Human vaccines & immunotherapeutics. 2014;10(3):797–807. doi: 10.4161/hv.27449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El-Diasty EMN, Eleiwa Z, Hoda AM. Aideia Using of Chitosan as Antifungal Agent in Kariesh Cheese. New York Science Journal. 2012 [Google Scholar]
- 27.Park BK, Kim MM. Applications of chitin and its derivatives in biological medicine. International journal of molecular sciences. 2010;11(12):5152–64. doi: 10.3390/ijms11125152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shahidi Fereidoon, JKV Aa, J Y-J. Food applications of chitin and chitosans. Elsevier Science Ltd; 1999. [Google Scholar]
- 29.Kafetzopoulos D, Martinou A, Bouriotis V. Bioconversion of chitin to chitosan: purification and characterization of chitin deacetylase from Mucor rouxii. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(7):2564–8. doi: 10.1073/pnas.90.7.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aiba S. Preparation of N-acetylchitooligosaccharides by hydrolysis of chitosan with chitinase followed by N-acetylation. Carbohydrate research. 1994;265(2):323–8. doi: 10.1016/0008-6215(94)00243-6. [DOI] [PubMed] [Google Scholar]
- 31.Tokuyasu K, Mitsutomi M, Yamaguchi I, Hayashi K, Mori Y. Recognition of chitooligosaccharides and their N-acetyl groups by putative subsites of chitin deacetylase from a deuteromycete, Colletotrichum lindemuthianum. Biochemistry. 2000;39(30):8837–43. doi: 10.1021/bi0005355. [DOI] [PubMed] [Google Scholar]
- 32.Younes I, Rinaudo M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Marine drugs. 2015;13(3):1133–74. doi: 10.3390/md13031133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Merzendorfer H, Zimoch L. Chitin metabolism in insects: structure, function and regulation of chitin synthases and chitinases. The Journal of experimental biology. 2003;206(Pt 24):4393–412. doi: 10.1242/jeb.00709. [DOI] [PubMed] [Google Scholar]
- 34.Tripathi VS, D PK, Da J. chitin and chitosan: chemistry, properties and applications. Journal of scientific & Industrial Research. 2004 [Google Scholar]
- 35.Noishiki Y, Nishiyama Y, Wada M, Okada S, Kuga S. Inclusion complex of beta-chitin and aliphatic amines. Biomacromolecules. 2003;4(4):944–9. doi: 10.1021/bm034024k. [DOI] [PubMed] [Google Scholar]
- 36.Finney NS, Jay In Memoriam: Albert Hofmann (1906–2008) CHIMIA International Journal for Chemistry. 2008 [Google Scholar]
- 37.Gooday GW. The Ecology of Chitin Degradation. Advances in Microbial Ecology. 1990 [Google Scholar]
- 38.Sashiwa H, Saimoto H, Shigemasa Y, Ogawa R, Tokura S. Lysozyme susceptibility of partially deacetylated chitin. International journal of biological macromolecules. 1990;12(5):295–6. doi: 10.1016/0141-8130(90)90016-4. [DOI] [PubMed] [Google Scholar]
- 39.Carlisle DB. Chitin in a Cambrian fossil, Hyolithellus. The Biochemical journal. 1964;90(2):1C–2C. doi: 10.1042/bj0900001c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lodhi G, Kim YS, Hwang JW, Kim SK, Jeon YJ, Je JY, et al. Chitooligosaccharide and its derivatives: preparation and biological applications. BioMed research international. 2014;2014:654913. doi: 10.1155/2014/654913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumar MNVR. A review of chitin and chitosan applications. Elsevier Science BV. 2000 [Google Scholar]
- 42.Jolanta Kumirska, W MX, Thöming Jorg, Stepnowski Piotr. Biomedical Activity of Chitin/Chitosan Based Materials-Influence of Physicochemical Properties Apart from Molecular Weight and Degree of N-Acetylation. Polymers. 2011 [Google Scholar]
- 43.Schaefer J, Kramer KJ, Garbow JR, Jacob GS, Stejskal EO, Hopkins TL, et al. Aromatic cross-links in insect cuticle: detection by solid-state 13C and 15N NMR. Science (New York, NY) 1987;235(4793):1200–4. doi: 10.1126/science.3823880. [DOI] [PubMed] [Google Scholar]
- 44.Beier S, Bertilsson S. Bacterial chitin degradation-mechanisms and ecophysiological strategies. Frontiers in microbiology. 2013;4:149. doi: 10.3389/fmicb.2013.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Friedman AJ, Phan J, Schairer DO, Champer J, Qin M, Pirouz A, et al. Antimicrobial and anti-inflammatory activity of chitosan-alginate nanoparticles: a targeted therapy for cutaneous pathogens. The Journal of investigative dermatology. 2013;133(5):1231–9. doi: 10.1038/jid.2012.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raafat D, Sahl HG. Chitosan and its antimicrobial potential–a critical literature survey. Microbial biotechnology. 2009;2(2):186–201. doi: 10.1111/j.1751-7915.2008.00080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pusateri AE, McCarthy SJ, Gregory KW, Harris RA, Cardenas L, McManus AT, et al. Effect of a chitosan-based hemostatic dressing on blood loss and survival in a model of severe venous hemorrhage and hepatic injury in swine. The Journal of trauma. 2003;54(1):177–82. doi: 10.1097/00005373-200301000-00023. [DOI] [PubMed] [Google Scholar]
- 48.Badwan AA, Rashid I, Omari MM, Darras FH. Chitin and chitosan as direct compression excipients in pharmaceutical applications. Marine drugs. 2015;13(3):1519–47. doi: 10.3390/md13031519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yen Ming-Tsung, Y J-H, Mau Jeng-Leun. Physicochemical characterization of chitin and chitosan from crab shells. Elsevier Ltd; 2009. [Google Scholar]
- 50.Ospina Alvarez SP, Ramirez Cadavid DA, Escobar Sierra DM, Ossa Orozco CP, Rojas Vahos DF, Zapata Ocampo P, et al. Comparison of extraction methods of chitin from Ganoderma lucidum mushroom obtained in submerged culture. BioMed research international. 2014;2014:169071. doi: 10.1155/2014/169071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bowman SM, Free SJ. The structure and synthesis of the fungal cell wall. BioEssays: news and reviews in molecular, cellular and developmental biology. 2006;28(8):799–808. doi: 10.1002/bies.20441. [DOI] [PubMed] [Google Scholar]
- 52.Yang TL. Chitin-based materials in tissue engineering: applications in soft tissue and epithelial organ. International journal of molecular sciences. 2011;12(3):1936–63. doi: 10.3390/ijms12031936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hajji S, Younes I, Ghorbel-Bellaaj O, Hajji R, Rinaudo M, Nasri M, et al. Structural differences between chitin and chitosan extracted from three different marine sources. International journal of biological macromolecules. 2014;65:298–306. doi: 10.1016/j.ijbiomac.2014.01.045. [DOI] [PubMed] [Google Scholar]
- 54.Amidi M, Mastrobattista E, Jiskoot W, Hennink WE. Chitosan-based delivery systems for protein therapeutics and antigens. Advanced drug delivery reviews. 2010;62(1):59–82. doi: 10.1016/j.addr.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 55.Xu Q, Wang CH, Pack DW. Polymeric carriers for gene delivery: chitosan and poly(amidoamine) dendrimers. Current pharmaceutical design. 2010;16(21):2350–68. doi: 10.2174/138161210791920469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cohen E. Chitin synthesis and inhibition: a revisit. Pest management science. 2001;57(10):946–50. doi: 10.1002/ps.363. [DOI] [PubMed] [Google Scholar]
- 57.Bracker CE, Ruiz-Herrera J, Bartnicki-Garcia S. Structure and transformation of chitin synthetase particles (chitosomes) during microfibril synthesis in vitro. Proceedings of the National Academy of Sciences of the United States of America. 1976;73(12):4570–4. doi: 10.1073/pnas.73.12.4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ruiz-Herrera J, Lopez-Romero E, Bartnicki-Garcia S. Properties of chitin synthetase in isolated chitosomes from yeast cells of Mucor rouxii. The Journal of biological chemistry. 1977;252(10):3338–43. [PubMed] [Google Scholar]
- 59.Sietsma JH, Beth Din A, Ziv V, Sjollema KA, Yarden O. The localization of chitin synthase in membranous vesicles (chitosomes) in Neurospora crassa. Microbiology (Reading, England) 1996;142(Pt 7):1591–6. doi: 10.1099/13500872-142-7-1591. [DOI] [PubMed] [Google Scholar]
- 60.Suresh K, Subramanyam C. A putative role for calmodulin in the activation of Neurospora crassa chitin synthase. FEMS microbiology letters. 1997;150(1):95–100. doi: 10.1111/j.1574-6968.1997.tb10355.x. [DOI] [PubMed] [Google Scholar]
- 61.Cohen E. In vitro chitin synthesis in an insect: formation and structure of microfibrils. European journal of cell biology. 1982;26(2):289–94. [PubMed] [Google Scholar]
- 62.Glaser L, Brown DH. The synthesis of chitin in cell-free extracts of Neurospora crassa. The Journal of biological chemistry. 1957;228(2):729–42. [PubMed] [Google Scholar]
- 63.Kaya M, Karaarslan M, Baran T, Can E, Ekemen G, Bitim B, et al. The quick extraction of chitin from an epizoic crustacean species (Chelonibia patula) Natural product research. 2014;28(23):2186–90. doi: 10.1080/14786419.2014.927469. [DOI] [PubMed] [Google Scholar]
- 64.Liu S, Sun J, Yu L, Zhang C, Bi J, Zhu F, et al. Extraction and characterization of chitin from the beetle Holotrichia parallela Motschulsky. Molecules (Basel, Switzerland) 2012;17(4):4604–11. doi: 10.3390/molecules17044604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kaya M, Sargin I, Tozak KO, Baran T, Erdogan S, Sezen G. Chitin extraction and characterization from Daphnia magna resting eggs. International journal of biological macromolecules. 2013;61:459–64. doi: 10.1016/j.ijbiomac.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 66.Raman S, Kumar R. Construction and nanomechanical properties of the exoskeleton of the barnacle, Amphibalanus reticulatus. Journal of structural biology. 2011;176(3):360–9. doi: 10.1016/j.jsb.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 67.Reid DG, Mason MJ, Chan BK, Duer MJ. Characterization of the phosphatic mineral of the barnacle Ibla cumingi at atomic level by solid-state nuclear magnetic resonance: comparison with other phosphatic biominerals. Journal of the Royal Society, Interface/the Royal Society. 2012;9(72):1510–6. doi: 10.1098/rsif.2011.0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fai AE, Stamford TC, Stamford-Arnaud TM, Santa-Cruz PD, da Silva MC, Campos-Takaki GM, et al. Physicochemical characteristics and functional properties of chitin and chitosan produced by Mucor circinelloides using yam bean as substrate. Molecules (Basel, Switzerland) 2011;16(8):7143–54. doi: 10.3390/molecules16087143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Berger LR, Stamford TC, Stamford-Arnaud TM, de Alcantara SR, da Silva AC, da Silva AM, et al. Green conversion of agroindustrial wastes into chitin and chitosan by Rhizopus arrhizus and Cunninghamella elegans strains. International journal of molecular sciences. 2014;15(5):9082–102. doi: 10.3390/ijms15059082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kielak AM, Cretoiu MS, Semenov AV, Sorensen SJ, van Elsas JD. Bacterial chitinolytic communities respond to chitin and pH alteration in soil. Applied and environmental microbiology. 2013;79(1):263–72. doi: 10.1128/AEM.02546-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Manucharova NA, Vlasenko AN, Men’ko EV, Zviagintsev DG. Specificity of the chitinolytic microbial complex of soils incubated at different temperatures. Mikrobiologiia. 2011;80(2):219–29. [PubMed] [Google Scholar]
- 72.Aumen NG. Microbial succession on a chitinous substrate in a woodland stream. Microbial ecology. 1980;6(4):317–27. doi: 10.1007/BF02010494. [DOI] [PubMed] [Google Scholar]
- 73.Techkarnjanaruk S, Pongpattanakitshote S, Goodman AE. Use of a promoterless lacZ gene insertion to investigate chitinase gene expression in the marine bacterium Pseudoalteromonas sp. strain S9. Applied and environmental microbiology. 1997;63(8):2989–96. doi: 10.1128/aem.63.8.2989-2996.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miyashita K, Fujii T, Saito A. Induction and repression of a Streptomyces lividans chitinase gene promoter in response to various carbon sources. Bioscience, biotechnology, and biochemistry. 2000;64(1):39–43. doi: 10.1271/bbb.64.39. [DOI] [PubMed] [Google Scholar]
- 75.Watanabe T, Oyanagi W, Suzuki K, Tanaka H. Chitinase system of Bacillus circulans WL-12 and importance of chitinase A1 in chitin degradation. Journal of bacteriology. 1990;172(7):4017–22. doi: 10.1128/jb.172.7.4017-4022.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fuchs RL, McPherson SA, Drahos DJ. Cloning of a Serratia marcescens Gene Encoding Chitinase. Applied and environmental microbiology. 1986;51(3):504–9. doi: 10.1128/aem.51.3.504-509.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saito A, Fujii T, Yoneyama T, Miyashita K. glkA is involved in glucose repression of chitinase production in Streptomyces lividans. Journal of bacteriology. 1998;180(11):2911–4. doi: 10.1128/jb.180.11.2911-2914.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sitrit Y, Vorgias CE, Chet I, Oppenheim AB. Cloning and primary structure of the chiA gene from Aeromonas caviae. Journal of bacteriology. 1995;177(14):4187–9. doi: 10.1128/jb.177.14.4187-4189.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Techkarnjanaruk S, Goodman AE. Multiple genes involved in chitin degradation from the marine bacterium Pseudoalteromonas sp. strain S91. Microbiology (Reading, England) 1999;145(Pt 4):925–34. doi: 10.1099/13500872-145-4-925. [DOI] [PubMed] [Google Scholar]
- 80.Montgomery MT, Kirchman DL. Role of Chitin-Binding Proteins in the Specific Attachment of the Marine Bacterium Vibrio harveyi to Chitin. Applied and environmental microbiology. 1993;59(2):373–9. doi: 10.1128/aem.59.2.373-379.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Davis B, D E. Chitosanases: Occurrence, Production and Immobilization. In: Zikakis JP, editor. Chitin, Chitosan, and Related Enzymes. Orlando, FL: Academic Press; 1984. [Google Scholar]
- 82.Suh JK, Matthew HW. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: a review. Biomaterials. 2000;21(24):2589–98. doi: 10.1016/s0142-9612(00)00126-5. [DOI] [PubMed] [Google Scholar]
- 83.Venkatesan J, Vinodhini PA, Sudha PN, Kim SK. Chitin and chitosan composites for bone tissue regeneration. Advances in food and nutrition research. 2014;73:59–81. doi: 10.1016/B978-0-12-800268-1.00005-6. [DOI] [PubMed] [Google Scholar]
- 84.Jayakumar R, N SV, Furuike T, Tamura H. Perspectives of Chitin and Chitosan Nanofibrous Scaffolds in Tissue Engineering. Intech [Google Scholar]
- 85.Freier T, Montenegro R, Shan Koh H, Shoichet MS. Chitin-based tubes for tissue engineering in the nervous system. Biomaterials. 2005;26(22):4624–32. doi: 10.1016/j.biomaterials.2004.11.040. [DOI] [PubMed] [Google Scholar]
- 86.Wang W, Itoh S, Matsuda A, Ichinose S, Shinomiya K, Hata Y, et al. Influences of mechanical properties and permeability on chitosan nano/microfiber mesh tubes as a scaffold for nerve regeneration. Journal of biomedical materials research Part A. 2008;84(2):557–66. doi: 10.1002/jbm.a.31536. [DOI] [PubMed] [Google Scholar]
- 87.Zhang L, Ao Q, Wang A, Lu G, Kong L, Gong Y, et al. A sandwich tubular scaffold derived from chitosan for blood vessel tissue engineering. Journal of biomedical materials research Part A. 2006;77(2):277–84. doi: 10.1002/jbm.a.30614. [DOI] [PubMed] [Google Scholar]
- 88.Madihally SV, Matthew HW. Porous chitosan scaffolds for tissue engineering. Biomaterials. 1999;20(12):1133–42. doi: 10.1016/s0142-9612(99)00011-3. [DOI] [PubMed] [Google Scholar]
- 89.Levengood SL, Zhang M. Chitosan-based scaffolds for bone tissue engineering. Journal of materials chemistry B, Materials for biology and medicine. 2014;2(21):3161–84. doi: 10.1039/C4TB00027G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Seol YJ, Lee JY, Park YJ, Lee YM, Young K, Rhyu IC, et al. Chitosan sponges as tissue engineering scaffolds for bone formation. Biotechnology letters. 2004;26(13):1037–41. doi: 10.1023/B:BILE.0000032962.79531.fd. [DOI] [PubMed] [Google Scholar]
- 91.VandeVord PJ, Matthew HW, DeSilva SP, Mayton L, Wu B, Wooley PH. Evaluation of the biocompatibility of a chitosan scaffold in mice. Journal of biomedical materials research. 2002;59(3):585–90. doi: 10.1002/jbm.1270. [DOI] [PubMed] [Google Scholar]
- 92.Zhang Y, Zhang M. Cell growth and function on calcium phosphate reinforced chitosan scaffolds. Journal of materials science Materials in medicine. 2004;15(3):255–60. doi: 10.1023/b:jmsm.0000015485.94665.25. [DOI] [PubMed] [Google Scholar]
- 93.Kavya KC, Jayakumar R, Nair S, Chennazhi KP. Fabrication and characterization of chitosan/gelatin/nSiO2 composite scaffold for bone tissue engineering. International journal of biological macromolecules. 2013;59:255–63. doi: 10.1016/j.ijbiomac.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 94.Park H, Choi B, Nguyen J, Fan J, Shafi S, Klokkevold P, et al. Anionic carbohydrate-containing chitosan scaffolds for bone regeneration. Carbohydrate polymers. 2013;97(2):587–96. doi: 10.1016/j.carbpol.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ju YM, Park K, Son JS, Kim JJ, Rhie JW, Han DK. Beneficial effect of hydrophilized porous polymer scaffolds in tissue-engineered cartilage formation. Journal of biomedical materials research Part B, Applied biomaterials. 2008;85(1):252–60. doi: 10.1002/jbm.b.30943. [DOI] [PubMed] [Google Scholar]
- 96.Nam YS, Yoon JJ, Park TG. A novel fabrication method of macroporous biodegradable polymer scaffolds using gas foaming salt as a porogen additive. Journal of biomedical materials research. 2000;53(1):1–7. doi: 10.1002/(sici)1097-4636(2000)53:1<1::aid-jbm1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 97.Chesnutt BM, Viano AM, Yuan Y, Yang Y, Guda T, Appleford MR, et al. Design and characterization of a novel chitosan/nanocrystalline calcium phosphate composite scaffold for bone regeneration. Journal of biomedical materials research Part A. 2009;88(2):491–502. doi: 10.1002/jbm.a.31878. [DOI] [PubMed] [Google Scholar]
- 98.Jiang T, Abdel-Fattah WI, Laurencin CT. In vitro evaluation of chitosan/poly(lactic acid-glycolic acid) sintered microsphere scaffolds for bone tissue engineering. Biomaterials. 2006;27(28):4894–903. doi: 10.1016/j.biomaterials.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 99.Manjusha EM, Mohan JC, Manzoor K, Nair SV, Tamura HJ, J R. Folate conjugated carboxymethyl chitosan–manganese doped zinc sulphide nanoparticles for targeted drug delivery and imaging of cancer cells. Carbohydrate polymers. 2010;80(2):442–8. [Google Scholar]
- 100.Manjusha Elizabeth, Mathew JCM, Manzoor K, Nair SV, Tamura H, Jayakumar R. Folate conjugated carboxymethyl chitosan–manganese doped zinc sulphide nanoparticles for targeted drug delivery and imaging of cancer cells. Elsevier Ltd; 2009. [Google Scholar]
- 101.Sparkes BG, Murray DG. Chitosan based wound dressing materials. 4572906. US Patent. 1986
- 102.Sagar B, Hamlyn P, Wales D. Wound dressing. 0291587. European Patent. 1994
- 103.Cheung RC, Ng TB, Wong JH, Chan WY. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Marine drugs. 2015;13(8):5156–86. doi: 10.3390/md13085156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chung YC, Wang HL, Chen YM, Li SL. Effect of abiotic factors on the antibacterial activity of chitosan against waterborne pathogens. Bioresource technology. 2003;88(3):179–84. doi: 10.1016/s0960-8524(03)00002-6. [DOI] [PubMed] [Google Scholar]
- 105.Chen CY, Chung YC. Antibacterial effect of water-soluble chitosan on representative dental pathogens Streptococcus mutans and Lactobacilli brevis. Journal of applied oral science: revista FOB. 2012;20(6):620–7. doi: 10.1590/S1678-77572012000600006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shi X, Fang Q, Ding M, Wu J, Ye F, Lv Z, et al. Microspheres of carboxymethyl chitosan, sodium alginate and collagen for a novel hemostatic in vitro study. Journal of biomaterials applications. 2015 doi: 10.1177/0885328215618354. [DOI] [PubMed] [Google Scholar]
- 107.Bennett BL, Littlejohn L. Review of new topical hemostatic dressings for combat casualty care. Mil Med. 2014;179(5):497–514. doi: 10.7205/MILMED-D-13-00199. [DOI] [PubMed] [Google Scholar]
- 108.Huang X, Sun Y, Nie J, Lu W, Yang L, Zhang Z, et al. Using absorbable chitosan hemostatic sponges as a promising surgical dressing. International journal of biological macromolecules. 2015;75:322–9. doi: 10.1016/j.ijbiomac.2015.01.049. [DOI] [PubMed] [Google Scholar]
- 109.Szatmari V. Chitosan hemostatic dressing for control of hemorrhage from femoral arterial puncture site in dogs. J Vet Sci. 2015;16(4):517–23. doi: 10.4142/jvs.2015.16.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wu S, Huang Z, Yue J, Liu D, Wang T, Ezanno P, et al. The efficient hemostatic effect of Antarctic krill chitosan is related to its hydration property. Carbohydrate polymers. 2015;132:295–303. doi: 10.1016/j.carbpol.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 111.Qiu Y, Zhang N, Kang Q, An Y, Wen X. Fabrication of permeable tubular constructs from chemically modified chitosan with enhanced antithrombogenic property. Journal of biomedical materials research Part B, Applied biomaterials. 2009;90(2):668–78. doi: 10.1002/jbm.b.31333. [DOI] [PubMed] [Google Scholar]
- 112.Hirano S, Tanaka Y, Hasegawa M, Tobetto K, Nishioka A. Effect of sulfated derivatives of chitosan on some blood coagulant factors. Carbohydrate research. 1985;137:205–15. doi: 10.1016/0008-6215(85)85161-2. [DOI] [PubMed] [Google Scholar]
- 113.Morganti P, Palombo P, Palombo M, Fabrizi G, Cardillo A, Svolacchia F, et al. A phosphatidylcholine hyaluronic acid chitin-nanofibrils complex for a fast skin remodeling and a rejuvenating look. Clinical, cosmetic and investigational dermatology. 2012;5:213–20. doi: 10.2147/CCID.S29664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tokoro A, Tatewaki N, Suzuki K, Mikami T, Suzuki S, Suzuki M. Growth-inhibitory effect of hexa-N-acetylchitohexaose and chitohexaose against Meth-A solid tumor. Chemical & pharmaceutical bulletin. 1988;36(2):784–90. doi: 10.1248/cpb.36.784. [DOI] [PubMed] [Google Scholar]
- 115.Lin SY, Chan HY, Shen FH, Chen MH, Wang YJ, Yu CK. Chitosan prevents the development of AOM-induced aberrant crypt foci in mice and suppressed the proliferation of AGS cells by inhibiting DNA synthesis. Journal of cellular biochemistry. 2007;100(6):1573–80. doi: 10.1002/jcb.21152. [DOI] [PubMed] [Google Scholar]
- 116.McNeela EA, O’Connor D, Jabbal–Gill I, Illum L, Davis SS, Pizza M, et al. A mucosal vaccine against diphtheria: formulation of cross reacting material (CRM(197)) of diphtheria toxin with chitosan enhances local and systemic antibody and Th2 responses following nasal delivery. Vaccine. 2000;19(9–10):1188–98. doi: 10.1016/s0264-410x(00)00309-1. [DOI] [PubMed] [Google Scholar]
- 117.Mills KH, Cosgrove C, McNeela EA, Sexton A, Giemza R, Jabbal-Gill I, et al. Protective levels of diphtheria-neutralizing antibody induced in healthy volunteers by unilateral priming-boosting intranasal immunization associated with restricted ipsilateral mucosal secretory immunoglobulin a. Infect Immun. 2003;71(2):726–32. doi: 10.1128/IAI.71.2.726-732.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mann AJ, Noulin N, Catchpole A, Stittelaar KJ, de Waal L, Veldhuis Kroeze EJ, et al. Intranasal H5N1 vaccines, adjuvanted with chitosan derivatives, protect ferrets against highly pathogenic influenza intranasal and intratracheal challenge. PLoS One. 2014;9(5):e93761. doi: 10.1371/journal.pone.0093761. [DOI] [PMC free article] [PubMed] [Google Scholar]


